Abstract
To evaluate the denitrification abilities of many Bradyrhizobium field isolates, we developed a new 15N-labeled N2 detection methodology, which is free from interference from atmospheric N2 contamination. 30N2 (15N15N) and 29N2 (15N14N) were detected as an apparent peak by a gas chromatograph equipped with a thermal conductivity detector with N2 gas having natural abundance of 15N (0.366 atom%) as a carrier gas. The detection limit was 0.04% 30N2, and the linearity extended at least to 40% 30N2. When Bradyrhizobium japonicum USDA110 was grown in cultures anaerobically with 15NO3−, denitrification product (30N2) was detected stoichiometrically. A total of 65 isolates of soybean bradyrhizobia from two field sites in Japan were assayed by this method. The denitrification abilities were partly correlated with filed sites, Bradyrhizobium species, and the hup genotype.
Denitrification is anaerobic respiration using nitrate (NO3−), nitrite (NO2−), nitric oxide (NO), and nitrous oxide (N2O) as terminal electron acceptors. Denitrifying species are distributed over a broad variety of bacteria, including Proteobacteria spp., gram-positive bacteria, and archaea (31). Most denitrifying bacteria reduce the electron acceptors sequentially as NO3− → NO2− → NO → N2O → N2, and each step of these reactions is catalyzed by specific respective reductases (31). To evaluate denitrification capability, the acetylene inhibition assay (30) has been widely used in pure cultures (13) as well as for environmental samples such as soils (8, 14, 27) and water (24) because of sensitivity and atmospheric N2 contamination (13). In this assay, the excess of N2O emission in the presence of acetylene over the level of N2O emission in the absence of acetylene is regarded as the N2 evolution (because acetylene inhibits N2O reductase). N2O can be detected by a gas chromatograph (GC) equipped with a 63Ni electron-capture detector possessing high-level sensitivity for N2O. The amount of N2O is calculated on the basis of the measured headspace concentration and corrected for dissolved gas by using the Bunsen coefficient (29).
However, the acetylene inhibition assay has some drawbacks for evaluating bacterial denitrification. The most serious disadvantage is that it is impossible to measure N2O reductase activity directly. For this reason, the activities and kinetic parameters of N2O reductase have been studied directly by measurement of the N2O decrease (4) or by spectrophotometric assay of reduced benzyl viologen for nitrous oxide reductase activity in vitro (12, 23). Moreover, the underestimation of denitrification capability may occur because of incomplete blockage of N2O reductase at a low nitrate concentration or in the presence of sulfide (24). In practice, two determinations of N2O concentration per sample—in the presence and absence of acetylene—are required for estimation of the amount of N2 evolved by N2O reductase.
Acetylene inhibition assays have revealed the diversity of the denitrification activities of Bradyrhizobium species (1, 2, 28). Bradyrhizobium japonicum USDA110, which possesses a full set of denitrification genes on the chromosome (11), consistently shows denitrification from nitrate to N2 (2, 28). However, soybean bradyrhizobia often show diverse denitrification patterns. Their denitrification end products are N2O and NO2− as well as N2 when nitrate is supplied to media under anaerobic conditions, while a few strains are unable to denitrify at all (1, 2, 28). This polymorphism is probably due to truncation of the processes of anaerobic nitrate respiration (31). It has been shown that indigenous populations of soybean bradyrhizobia show significant field site variation in terms of DNA fingerprints according to insertion sequences, species level, and hydrogen uptake phenotype and genotype (7, 16, 17, 21). Similarly, we wanted to determine whether the denitrification patterns of indigenous populations of soybean bradyrhizobia differed in accordance with their field sites.
We therefore developed a new methodology for determination of 30N2 levels by using a GC equipped with a thermal conductivity detector (TCD). This method, which we refer to as the 15N/TCD method, enables 15N tracer analysis without the need for isotope ratio determination by mass spectrometry and will overcome some of the problems of the acetylene inhibition method when large numbers of field isolates are analyzed.
MATERIALS AND METHODS
Bacterial strains.
Strain USDA110 was used as a standard strain of B. japonicum (11). A total of 65 field isolates of Bradyrhizobium were isolated from the soils of the Nakazawa fields at the Niigata Agricultural Experiment Station (Nagaoka, Niigata, Japan) and the Tokachi field at the Tokachi Agricultural Station (Memuro, Tokachi, Hokkaido, Japan) as described previously (9, 15, 18).
Standard gases.
A gas mixture composed of 40% (vol/vol) 30N2 (15N15N) (15N, 99.7 atom%), 40% (vol/vol) Ar, and 20% (vol/vol) O2 (Shoko Co., Ltd., Tokyo, Japan) was used as a 30N2 original standard gas. A standard gas of 29N2 (15N14N) was prepared by using codenitrification of Fusarium solani IFO9425 anaerobically grown in cultures under He with Na15NO2 according to the method of Shoun et al. (22) and Tanimoto et al. (26). The amount of 29N2 was determined by using a GC-mass spectrometer (GC-MS QP5050; Shimadzu, Kyoto, Japan).
Growth media and growth conditions.
Bradyrhizobium cells were aerobically grown to full growth at 30°C in 15-ml HM salts medium (5) supplemented with 0.1% arabinose and 0.025% (wt/vol) yeast extract (Difco, Detroit, Mich.). For anaerobic growth for denitrification, HM medium was further supplemented with 0.55 μM Na2MoO42̇H2O, 1 μM FeCl2, and 1 μM CuSO42̇H2O, which were required for nitrate, nitrite, and nitrous oxide reductase activities, respectively (31). Filter-sterilized K15NO3 (15N, 99.6 atom%) (Shoko Co., Ltd.) or K14NO3 was added to the medium at a final concentration of 2 mM.
We used two bottle systems for denitrification experiments: 70 ml of medium in a 123-ml airtight specimen vial with a butyl rubber stopper and an aluminum seal (Maruemu, Osaka, Japan), and 15 ml of medium in a 27-ml test tube with a double butyl rubber stopper. Full-grown cells of bradyrhizobia were inoculated to the media to achieve an initial optical density of 0.05 at 660 nm. Just after the vials and test tubes were sealed, the overhead space gas was replaced several times with N2 or He in a vacuum line. Gas samples were collected 10 days after incubation at 30°C. For the acetylene inhibition assay, 14NO3− was used as a substrate of denitrification in the presence and absence of 10% (vol/vol) acetylene.
Gas chromatography.
For 28N2, 29N2, and 30N2 analyses, gas samples (1 ml or 0.5 ml) were injected into a GC (GC-7A; Shimadzu) equipped with a TCD and a Molecular Sieve 5A column (80/100 mesh, 0.3-mm diameter by 2-m length). The carrier gas used was He or commercially available N2 gas that has a natural abundance of 15N (0.366 atom%). The flow rate of the carrier gas was 30 ml/min. Throughout the TCD GC analyses, the temperatures of injection, column, and detector were 100, 50, and 100°C, respectively. Determination of N2O levels was carried out by the TCD GC system described above except for use of a PorapakQ column (80/100 mesh, 0.3-mm diameter by 2-m length) and He carrier gas (flow rate, 65 ml/min). The amount of N2O emission was calculated on the basis of the measured headspace concentration and corrected for the presence of dissolved gas by using the Bunsen coefficient (29). Peak areas were calculated from chromatograms by an integrator (ChromatoPack C-R18; Shimadzu).
Nitrate assay.
The concentration of nitrate in the culture was measured colorimetrically (3).
Statistics.
Regression analysis was carried out with Microsoft Excel X for Mac software (Microsoft Co., Redmond, Wash.).
RESULTS
Detection of 15N-N2 by TCD gas chromatography.
We have often used a gas mixture composed of 40% (vol/vol) 30N2 (15N 99.7 atom%), 40% (vol/vol) Ar, and 20% O2 (vol/vol) to determine nitrogenase activity of rhizobia and diazotrophic endophytes associated with plants. When we analyzed, by chance, the gas mixture with a TCD GC using a Molecular Sieve 5A column and N2 carrier gas, an obvious peak appeared at a retention time after Ar and O2 had already eluted (data not shown). Although TCD senses any gaseous materials that have thermal conductivity different from that of carrier gas (6, 10, 20), it is surprising that chemical species identical to the carrier gas might be able to form a peak on the gas chromatogram. Thus, we wanted to examine whether the peak is really derived from 15N-labeled N2 (15N-N2) gas and whether this detection could be utilized for denitrification studies.
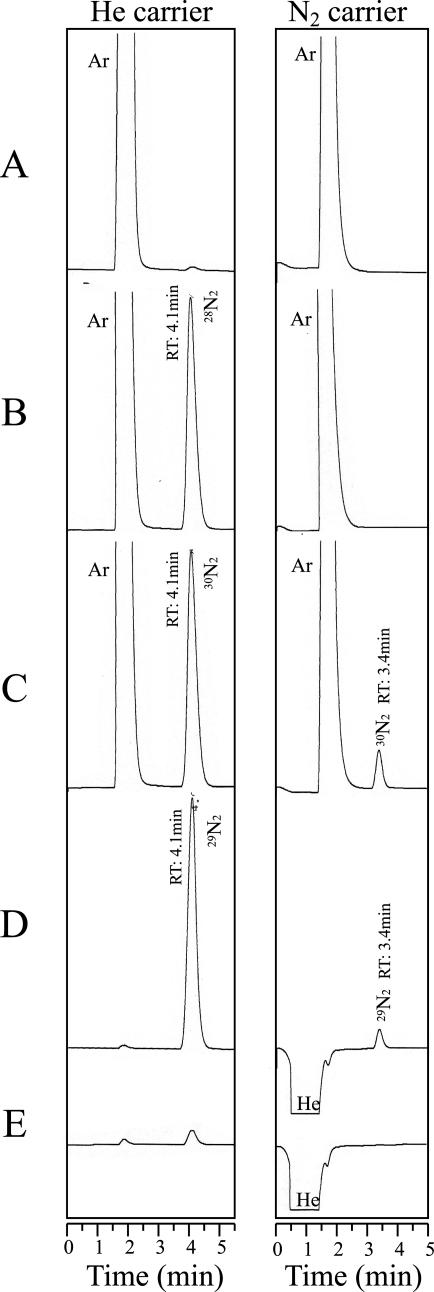
Three kinds of 4% (vol/vol) N2 gas, 28N2 (14N14N), 29N2 (15N14N), and 30N2 (15N15N), and their balance gases (a mixture of Ar and O2 gas for 28N2 and 30N2 and He for 29N2) were measured by using 0.5-ml injection and He or N2 as a carrier gas (Fig. 1). When He was used as a carrier, 28N2, 29N2, and 30N2 were detected at similar retention times of 4.1 min with similar peak sizes. When N2 gas was used as a carrier, on the other hand, 28N2 was not detected at all (Fig. 1B); however, the use of 30N2 gave rise to an apparent peak at a retention time of 3.4 min, as expected (Fig. 1C). Moreover, 29N2 gas injection showed a peak at the same retention time as 30N2 gas injection. The size of the 29N2 peak was about half (43%) of that of the 30N2 peak on a molar basis (Fig. 1D). Since the retention times of 28N2, 29N2, and 30N2 molecules were identical when He and N2 carriers were used (Fig. 1), a Molecular Sieve 5A column did not separate these molecules. The thermal conductivity values of 15N-N2 molecules are likely different from those of normal N2 with a natural abundance of 15N, however, which enabled us to observe a peak (3.4 min) specific for 15N-N2 in the chromatogram. Interestingly, 15N-N2 behaved as if the chemical species of 15N-N2 differed from that of the N2 carrier gas. These results demonstrated that the system of TCD gas chromatography is able to detect 15N-N2 gases including 30N2 and 29N2. In this work, we refer to this 15N-N2 detection system as the “15N/TCD method.”
Fig. 1.
Gas chromatograms of 15N-N2 standards and reference gases with He or N2 as a carrier gas. (A) 80% Ar and 20% O2; (B) 4.0% 28N2, 77% Ar, and 19% O2; (C) 4.0% 30N2, 76% Ar, and 20% O2; (D) 4.3%29N2 in He; (E) He. Gas samples (0.5 ml) were injected into a GC (GC-7A, Shimadzu) equipped with a TCD and a Molecular Sieve 5A column (80/100 mesh, 0.3-mm diameter by 2-m length). The carrier gas used was He or commercially available N2 gas that has a natural abundance of 15N of 0.366 atom%. The flow rate of the carrier gases was 30 ml/min. 29N2 gas was generated by codenitrification culture of F. solani IFO9425 under He atmosphere conditions (22, 26), and the amount of evolved 29N2 was determined by gas chromatography-mass spectrometry (GC-MS QP5050; Shimadzu). RT, retention time of the corresponding peak on gas chromatograms. The intensity of the 29N2 peak was about half (43%) of that of the 30N2 peak on a molar basis when the gas was eluted with N2 carrier.
Quantification of 30N2 by the 15N/TCD method.
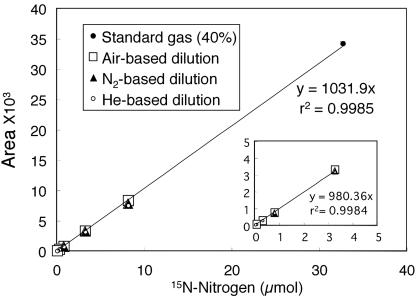
We further investigated the usefulness of this 15N/TCD method for quantification. A standard gas of 30N2 was diluted with air, N2, or He to make concentrations of 30N2 from 0.01 to 40% (vol/vol). A total of 1 ml of each gas sample was injected. Fig. 2 shows a standard curve between the amounts of 30N2 and the areas of peaks. The limit of detection was 0.04% (32.7 nmol of 15N). A best-fit curve without an intercept was calculated by linear regression. The equation of the resultant curve was given by y = 1,031.9x (r2 = 0.9985). From the best-fit curve, the quantitative range was 0.1% (81.8 nmol of 15N) to 40% (32.7 μmol of 15N). Changes in the dilution medium (air, atmospheric N2, or He) had no effect on the measurements.
Fig. 2.
Standard curve for 30N2 quantification by the 15N/TCD method. Gas samples (1 ml) were injected into the TCD GC with a Molecular Sieve 5A column and N2 as a carrier gas. Areas were calculated from a peak specific for 15N-N2 (retention time, 3.4 min) as described for Fig. 1. Standard gas (solid circles) contains 40% 30N2, 20% O2, and 40% Ar. Dilution series were prepared with air (open squares), N2 gas with a natural abundance of 15N (solid triangles), or He (open circles). The best-fit curve was found by linear regression analysis (y = 1,031.9x, r2 = 0.9985). The interior figure shows an enlarged area representing the results seen with 0 to 5 μmol of 15N.
Detection of 15N-N2 generated from B. japonicum cells grown in cultures under denitrification conditions.
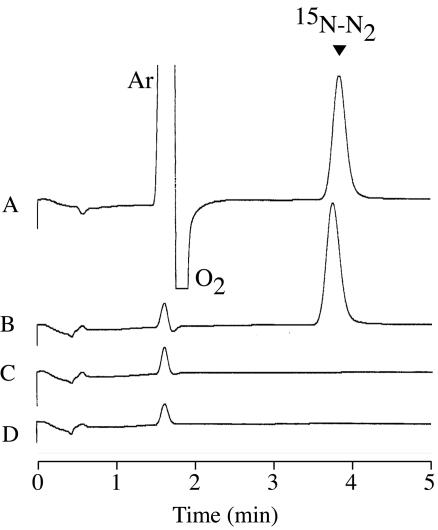
After anaerobic cultivation of B. japonicum USDA110 for 10 days with 2 mM 15NO3− or 2 mM 14NO3− in a 123-ml airtight specimen vial (70 ml of culture and 53 ml of headspace), 1 ml of headspace gas was taken and introduced to the GC. When the headspace gas of the vial with 15NO3− was measured, the peak of 15N-N2 was observed (Fig. 3B) in the same retention time (3.8 min) as for 30N2 standard gas (Fig. 3A). The slight delay of retention time compared to the result shown in Fig. 1 (3.4 min) was possibly derived from a decrease in the flow rate of carrier gas by manual adjustments. In contrast, no peak was detected at this position when 2 mM 14NO3− was supplied (Fig. 1C). Therefore, it is apparent that 15N-N2 was generated from 15NO3− by cells of B. japonicum USDA110. Recently, the process of anaerobic ammonia oxidation, which converts nitrogen from NH4+ and NO2− to N2 by the activity of a microorganism, was discovered to be another route of anaerobic N2 generation (25). We thus examined whether anaerobic N2 generation by B. japonicum occurs exclusively through classical denitrification. Because NH4Cl is the sole nitrogen source of the HM medium, we replaced 6 mM 14NH4Cl with 15NH4Cl (99.7 atomic percentage) (Shoko Co., Ltd.) and added 2 mM 14NO3− as a denitrification substrate. No 15N-N2 peak value was detected from the gas phase that was sampled from the anaerobic culture with 15NH4Cl and 14NO3− (Fig. 3D). Therefore, the production of 15N-N2 observed in the culture of B. japonicum USDA110 was exclusively a consequence of classical denitrification.
Fig. 3.
Gas chromatograms of 15N-N2 produced in the B. japonicum USDA110 culture cultivated under denitrifying conditions in the presence of 2 mM 15NO3− (B), 2 mM 14NO3− (C), or 2 mM 14NO3− and 6 mM 15NH4Cl (D). (A) To identify the position of 30N2 elution, a gas mixture of 4% 30N2, 21% O2, and 5% Ar was analyzed. The arrowhead indicates the position of a 15N-N2 peak. The retention time of the 30N2 peak was slightly longer than that seen in a previous analysis of TCD gas chromatography (Fig. 1), probably because the flow rate of N2 gas was slightly reduced compared to the value of 30 ml/min obtained earlier. O2 was partially coeluted with Ar and showed a peak in the minus direction at a retention time of about 1.8 min, because the gas contained as much as 21% of O2. Gas samples (1 ml) were injected into the TCD GC with a Molecular Sieve 5A column and N2 as a carrier gas.
Stoichiometric conversion of NO3− to N2 observed using the 15N/TCD method.
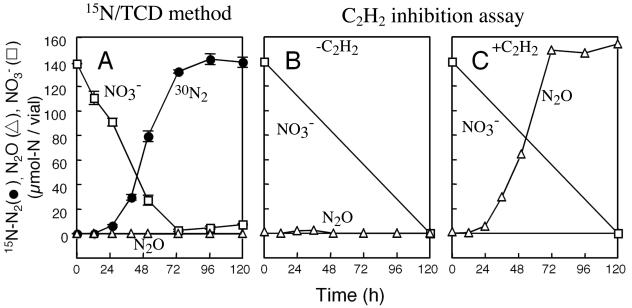
To examine whether the conversion of 15NO3− occurs stoichiometrically or not, the nitrogen amounts of NO3−, N2O, and 15N-N2 were monitored in an anaerobic culture of B. japonicum USDA110 supplemented with 2 mM 15NO3− in a 123-ml airtight specimen vial (70 ml of culture and 53 ml of headspace). We periodically sampled 0.1 ml of liquid culture for NO3−, 0.2 ml of gas for N2O, and 0.5 ml of gas for 15N-N2 determinations. Fig. 4A shows that the final amount of 15N-N2 was 140 μmol of N/vial, which was equal to the amount of 15NO3− initially supplied. This result clearly indicates that all nitrogen supplied as 15NO3− was transformed into 30N2.
Fig. 4.
Comparison of the 15N/TCD method (A) and the acetylene inhibition assay (B and C) in the detection of NO3− conversion via denitrification by B. japonicum USDA110. Concentrations of 15N-N2 (solid circles), N2O (open triangles), and NO3− (open squares) in culture were determined periodically as described in the text. B. japonicum USDA110 was cultivated anaerobically in the presence of 2 mM 15NO3− (A) or 2 mM 14NO3− with (C) or without (B) 10% (vol/vol) acetylene. Results are shown as means of triplicate determinations. Bars indicate standard deviations.
Comparison with the acetylene inhibition method.
To compare the 15N/TCD method with an acetylene inhibition assay, conversion of 14NO3− to N2O-N and N2-N were observed using two anaerobic cultures of B. japonicum USDA110 in the presence and absence of C2H2. Almost no evolution of N2O-N was detected in the culture without C2H2 (Fig. 4B), whereas the evolution of N2O-N in the culture with C2H2 (Fig. 4C) corresponded to that of 30N2-N in the culture without C2H2 (Fig. 4A). Moreover, the pattern of 30N2 evolution (Fig. 4A) was in accordance with that of N2O evolution (Fig. 4C). These results indicate that the 15N/TCD method is compatible with the conventional denitrification assay by acetylene inhibition. In addition, 15N/TCD method is a useful assay for direct and simple analysis for bacterial denitrification.
Denitrification abilities of indigenous soybean bradyrhizobia.
We used 65 Bradyrhizobium isolates from soybeans inoculated with the soils from the Nakazawa and Tokachi field sites. The species, hup genotype, and insertion sequence accumulation of the isolates had been previously characterized (9, 15, 18). The production of 30N2 from anaerobic culture of the isolates with 15NO3− was examined by the 15N/TCD method. A total of 28 isolates from the Nakazawa field emitted an amount of 30N2 equivalent to the amount of 15NO3− supplied in the medium, whereas no evolution of 30N2 was detected from the other isolates (Table 1). To examine whether these were truncated variants lacking N2O reductase (2, 28, 31), we determined the N2O concentration 7 days after inoculation in the medium supplemented with 14NO3− (2 mM). N2O accumulated from 3 isolates from the Nakazawa field and from 18 isolates from the Tokachi field (Table 1). The amount of N2O-N was approximately equivalent to that of the added NO3−-N (even in the test tube assay).
TABLE 1.
Denitrification capability of soybean bradyrhizobia isolated from two field sites in Japan by the 15N/GC methoda
| Field site (no. of isolates) | Denitri- fication end productb
|
Species | hupc | HRSd | No. of isolates | |
|---|---|---|---|---|---|---|
| N2 | N2O | |||||
| Nakazaka (n = 42) | + | − | B. japonicum | + | − | 22e |
| + | − | B. japonicum | − | − | 6f | |
| − | + | B. japonicum | − | − | 3g | |
| − | − | B. japonicum | + | + | 2h | |
| − | − | B. elkanii | − | − | 9i | |
| Tokachi (n = 23) | − | + | B. japonicum | − | − | 17j |
| − | + | B. japonicum | − | + | 1k | |
| − | − | B. japonicum | − | − | 1l | |
| − | − | B. japonicum | − | + | 4m | |
Soybean bradyrhizobia previously isolated from Nakazawa and Tokachi field soils were characterized for species, uptake hydrogenase, and repeated sequence fingerprints (9, 18).
The N2 column indicates completion of the full process of denitrification from 15NO3− (2 mM) to 30N2 with from 98 to 110% recovery after 7 days (+) except for a low recovery (62%) of isolate NC34a. −, the isolate evolved no 30N2 (<0.03% [vol/vol]). The N2O column indicates positive (+) and negative (−) N2O accumulation in the presence of 14NO3− (2 mM) after 7 days. 30N2 and N2O were determined at least in duplicate.
HRS, highly reiterated sequence-possessing strains carrying high copy numbers of insertion sequences (9, 15, 18, 21).
Isolates NC4a, NC5a, NC6a, NC10a, NC11a, NC12a, NC13a, NC14a, NC15a, NC16a, NC17a, NC18a, NC20a, NC21a, NC22a, NC24a, NC26a, NC29a, NC34a, NC35a, NC37a, and NC41a.
Isolates NC2a, NC19b, NC27a, NC28a, NC38a, and NC39a.
Isolates NC8a, NC33b, and NC36a.
Isolates NC3a and NC32a.
Isolates NC7a, NC23a, NC25a, NC31b, NC42a, NC43a, NC44a, NC45a, and NC46a.
Isolates T1, T3, T4, T5, T6, T7, T8a, T9, T10a, T11, T12, T13, T20, T27, T29, T37, and T39.
Isolate T25.
Isolate T40.
Isolates T2, T15, T22, and T31.
DISCUSSION
We developed a new method of GC measurement of 30N2 generated through denitrification by bacterial pure cultures. As far as we know, this is a first report of 15N-N2-specific determination by TCD GCs (Fig. 1). The sensitivity of TCD is directly proportional to the differences in thermal conductivity between the sample and the carrier gases (20). The elute profiles of 28N2, 29N2, and 30N2 (Fig. 1) showed that a 15N-N2 peak appeared in the direction opposite from that of the peak of He with a higher thermal conductivity in the chromatogram eluted with N2 carrier (20). This suggests that the thermal conductivities of 30N2 and 29N2 are slightly lower than that of 28N2. Recently, it has been shown that the absolute response of TCD to the eluting component cannot be interpreted simply but is involved in a number of heat-transfer factors such as flow rate, molar heat capacity, convection, and radiation (6, 10). Thus, it has so far been difficult to predict theoretically molar response factors of 30N2 and 29N2 for TCD.
The advantages of this 15N/TCD method are (i) use of a more commonly available piece of equipment (a TCD GC) rather than a mass spectrometer, (ii) no interference with 30N2 determination by atmospheric N2 contamination, (iii) simultaneous analysis of many cultures, and (iv) presumptive application to N2O reductase activity assay by product measurement. Although field isolates of soybean bradyrhizobia were analyzed in this work, the 15N/TCD method is equally applicable to other bacteria in their corresponding media by using compounds highly enriched with 15N such as NO3−, NO2−, and N2O.
Use of the 15N/TCD method rapidly demonstrated that the Bradyrhizobium isolates can apparently be categorized into three denitrification types: (a) full denitrifier (up to N2), (b) truncated denitrifier (up to N2O), and (c) nondenitrifier. No full denitrifiers was found among the Tokachi isolates, interestingly, whereas the majority of Nakazawa isolates (67%) showed full denitrification from nitrate to N2 (Table 1). In contrast, truncated denitrifiers (up to N2O) accounted for 78% of the Tokachi isolates (Table 1). If soybean bradyrhizobia contribute to N2O emission from field soils, then an indigenous population dominated by Bradyrhizobium full denitrifiers would reduce N2O emission from agricultural soils. When the three types of denitrification were compared with previously characterized traits, we found some relationships among the tested isolates (Table 1). All of the isolates with the ability to take up hydrogen (hydrogen uptake-positive isolates) fell into the full-denitrifier category. All of the B. elkanii isolates were found to be nondenitrifiers by this assay. Highly reiterated sequence-possessing isolates carrying high copy numbers of insertion sequences tended to belong to the nondenitrifiers. To confirm these relationships, examination of additional field isolates and appropriate standard strains of Bradyrhizobium spp. (together with analyses of their denitrification genes) would be required.
To apply this method to environmental samples such as soil and water we must consider 29N2 (15N14N) evolution, because denitrification produces 30N2 (15N15N), 29N2(15N14N), and 28N2 (14N14N) through random isotope pairing (19) and because anaerobic ammonia oxidation and codenitrification contribute to 29N2(15N14N) production (22, 25, 26). Since 29N2 gave rise to a level of signal intensity about half of that seen with 30N2 (Fig. 1C and D), 15N incorporation into N2 molecule can be monitored by the 15N/TCD method as a first screening assay for environmental samples. Afterwards, mass spectrometry analysis would be required to specify 30N2 (15N15N) and 29N2(15N14N) molecules or their ratios for the environmental samples.
Finally, we summarize the recommended procedure of the 15N/TCD method for denitrification assays of microbial pure cultures. A gas mixture of 4% (vol/vol) 30N2 (15N, more than 99 atom%) and 96% (vol/vol) Ar was recommended as an ideal standard gas, because the Ar peak was oriented in the same direction as the 30N2 peak and because the Ar peak is smaller than the He peak (Fig. 1). Commercially available N2 gas that has a natural abundance of 15N (0.366 atom%) should be used as a carrier gas. The recommended conditions of TCD GC are as follows: flow rate of carrier gas, 30 ml/min; column, Molecular Sieve 5A (80/100 mesh, 0.3-mm diameter by 2-m length); detector temperature, 100°C: column temperature, 50°C; injection volume, 0.5 to 1.0 ml.
Acknowledgments
This work was supported in part by grants 13007988-00 and 14360037 to R. Sameshima and K. Minamisawa from the Ministry of Education, Science, Sports and Culture of Japan. We thank PROBRAIN (Japan) for supporting the research of K. Minamisawa.
We acknowledge H. Shoun and T. Toya (Tokyo University) for production of 29N2 gas and analysis by gas chromatography-mass spectrometry.
REFERENCES
- 1.Asakawa, S. 1993. Denitrifying ability of indigenous strains of Bradyrhizobium japonicum isolated from fields under paddy-upland rotation. Biol. Fertil. Soils 15:196-200. [Google Scholar]
- 2.Breitenbeck, G. A., and J. M. Bremner. 1989. Ability of free-living cells of Bradyrhizobium japonicum to denitrify in soils. Biol. Fertil. Soils 7:219-224. [Google Scholar]
- 3.Cataldo, D. A., M. Haroon, L. E. Schrader, and V. L. Youngs. 1975. Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun. Soil Sci. Plant Anal. 6:71-80. [Google Scholar]
- 4.Chan, Y.-K., and R. Wheatcroft. 1993. Detection of a nitrous oxide reductase structural gene in Rhizobium meliloti strains and its location on the nod megaplasmid of JJ1c10 and SU47. J. Bacteriol. 175:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cole, M. A., and G. H. Elkan. 1973. Transmissible resistance to penicillin G, neomycin, and chloramphenicol in Rhizobium japonicum. Antimicrob. Agents Chemother. 4:248-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gislason, J., and S. M. Wharry. 2000. Relative molar response factors for thermal conductivity detectors. J. Chromatograph. Sci. 38:129-132. [DOI] [PubMed] [Google Scholar]
- 7.Hartmann, A., M. Gomez, J. J. Giraud, and C. Revellin. 1996. Repeated sequence RSα is diagnostic for Bradyrhizobium japonicum and Bradyrhizobium elkanii. Biol. Fertil. Soils 23:15-19. [Google Scholar]
- 8.Hashimoto, T., and H. Niimi. 2001. Seasonal and vertical changes in denitrification activity and denitrifying bacterial populations in surface and subsurface upland soils with slurry application. Soil Sci. Plant Nutr. 47:503-510. [Google Scholar]
- 9.Isawa, T., R. Sameshima, H. Mitsui, and K. Minamisawa. 1999. IS1631 occurrence in Bradyrhizobium japonicum highly reiterated sequence-possessing strains with high copy numbers of repeated sequences RSα and RSβ. Appl. Environ. Microbiol. 65:3493-3501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jalali-Heravi, and M. H. Fatemi. 2000. Prediction of thermal conductivity detection response factors using an artificial neural network. J. Chromatogr. A 897:227-235. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 12.Kristjansson, J. K., and T. C. Hollocher. 1980. First practical assay for soluble nitrous oxide reductase of denitrifying bacteria and a partial kinetic characterization. J. Biol. Chem. 255:704-707. [PubMed] [Google Scholar]
- 13.Mahne, I., and J. M. Tiedje. 1995. Criteria and methodology for identifying respiratory denitrifiers. Appl. Environ. Microbiol. 61:1110-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mergel, A., O. Schmitz, T. Mallmann, and B. Hermann. 2001. Relative abundance of denitrifying and dinitrogen-fixing bacteria in layers of a forest soil. FEMS Microbiol. Ecol. 36:33-42. [DOI] [PubMed] [Google Scholar]
- 15.Minamisawa, K., T. Isawa, Y. Nakatsuka, and N. Ichikawa. 1998. New Bradyrhizobium japonicum strains that possess high copy numbers of the repeated sequence RSα. Appl. Environ. Microbiol. 64:1845-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Minamisawa, K., Y. Nakatsuka, and T. Isawa. 1999. Diversity and field site variation of indigenous populations of soybean bradyrhizobia in Japan by fingerprints with repeated sequences RSα and RSβ. FEMS Microbiol. Ecol. 29:171-178. [Google Scholar]
- 17.Minamisawa, K., and H. Mitsui. 2000. Genetic ecology of soybean bradyrhizobia, p. 349-377. In J. Bollag and G. Stotzky (ed.), Soil biochemistry, vol. 10. Marcel Dekker, New York, N.Y.
- 18.Minamisawa, K., T. Seki, S. Onodera, M. Kubota, and T. Asami. 1992. Genetic relatedness of Bradyrhizobium japonicum field isolates as revealed by repeated sequences and various other characteristics. Appl. Environ. Microbiol. 58:2832-2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nielsen, L. P. 1992. Denitrification in sediment determined from nitrogen isotope paring. FEMS Microbiol. Ecol. 86:357-362. [Google Scholar]
- 20.Rey, N. H. 1958. Effect of the carrier gas on the sensitivity of a thermal conductivity detector in gas chromatography. Nature 182:1663. [DOI] [PubMed] [Google Scholar]
- 21.Sameshima, R., T. Isawa, M. J. Sadowsky, T. Hamada, H. Kosai, A. Shutsrirung, H. Mitsui, and K. Minamisawa. 2003. Phylogeny and distribution of extra-slow-growing Bradyrhizobium japonicum harboring high copy numbers of RSα, RSβ and IS1631. FEMS Microbiol. Ecol. 44:191-202. [DOI] [PubMed] [Google Scholar]
- 22.Shoun, H., D. H. Kim, H. Uchiyama, and J. Sugiyama. 1992. Denitrification by fungi. FEMS Microbiol. Lett. 94:277-282. [DOI] [PubMed] [Google Scholar]
- 23.SooHoo, C. K., and T. C. Hollocher. 1991. Purification and characterization of nitrous oxide reductase from Pseudomonas aeruginosa strain P2. J. Biol. Chem. 266:2203-2209. [PubMed] [Google Scholar]
- 24.Steingruber, S. M., J. Friedrich, R. Gachter, and B. Wehrli. 2001. Measurement of denitrification in sediments with the 15N isotope pairing technique. Appl. Environ. Microbiol. 67:3771-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strous, M., J. A. Fuerst, E. H. M. Kramer, S. Logemann, G. Muyzer, K. T. van de Pas-Schoonen, R. Webb, J. G. Kuenen, and M. S. M. Jetten. 1999. Missing lithotroph identified as new planctomycete. Nature 400:446-449. [DOI] [PubMed] [Google Scholar]
- 26.Tanimoto, T., K. Hatano, D. H. Kim, H. Uchiyama, and H. Shoun. 1992. Co-denitrification by the denitrifying system of the fungus Fusarium oxysporum. FEMS Microbiol. Lett. 93:177-180. [Google Scholar]
- 27.Tiedje, J. M. 1982. Denitrification. p. 1011-1026. In A. L. Page, R. H. Miller, and D. R. Keeney (ed.), Methods of soil analysis, part 2: chemicals and microbiological properties, 2nd ed. American Society of Agronomy, Madison, Wis.
- 28.van Berkum, P., and H. H. Keyser. 1985. Anaerobic growth and denitrification among different serogroups of soybean rhizobia. Appl. Environ. Microbiol. 49:772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilheim, E. R., R. Battino, and R. J. Wilcock. 1977. Low-pressure solubility of gases in liquid water. Chem. Rev. 77:219-262. [Google Scholar]
- 30.Yoshinari, T., and R. Knowles. 1976. Acetylene inhibition of nitrous oxide reduction by denitrifying bacteria. Biochem. Biophys. Res. Commun. 69:705-710. [DOI] [PubMed] [Google Scholar]
- 31.Zumft, W. G. 1997. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 61:533-616. [DOI] [PMC free article] [PubMed] [Google Scholar]