Abstract
The atp operon is highly conserved among eubacteria, and it has been considered a molecular marker as an alternative to the 16S rRNA gene. PCR primers were designed from the consensus sequences of the atpD gene to amplify partial atpD sequences from 12 Bifidobacterium species and nine Lactobacillus species. All PCR products were sequenced and aligned with other atpD sequences retrieved from public databases. Genes encoding the subunits of the F1F0-ATPase of Bifidobacterium lactis DSM 10140 (atpBEFHAGDC) were cloned and sequenced. The deduced amino acid sequences of these subunits showed significant homology with the sequences of other organisms. We identified specific sequence signatures for the genus Bifidobacterium and for the closely related taxa Bifidobacterium lactis and Bifidobacterium animalis and Lactobacillus gasseri and Lactobacillus johnsonii, which could provide an alternative to current methods for identification of lactic acid bacterial species. Northern blot analysis showed that there was a transcript at approximately 7.3 kb, which corresponded to the size of the atp operon, and a transcript at 4.5 kb, which corresponded to the atpC, atpD, atpG, and atpA genes. The transcription initiation sites of these two mRNAs were mapped by primer extension, and the results revealed no consensus promoter sequences. Phylogenetic analysis of the atpD genes demonstrated that the Lactobacillus atpD gene clustered with the genera Listeria, Lactococcus, Streptococcus, and Enterococcus and that the higher G+C content and highly biased codon usage with respect to the genome average support the hypothesis that there was probably horizontal gene transfer. The acid inducibility of the atp operon of B. lactis DSM 10140 was verified by slot blot hybridization by using RNA isolated from acid-treated cultures of B. lactis DSM 10140. The rapid increase in the level of atp operon transcripts upon exposure to low pH suggested that the ATPase complex of B. lactis DSM 10140 was regulated at the level of transcription and not at the enzyme assembly step.
Bifidobacteria and lactobacilli are gram-positive bacteria, and these groups include large numbers of different taxa that colonize various environments. Bifidobacteria and lactobacilli are important residents of the gastrointestinal microflora and have been the subjects of intense and growing interest due to their possible role in the maintenance of gastrointestinal health (4). The ability of these microorganisms to grow in this environment is also linked to their ability to resist its harsh conditions; the gastric pH is less than 2.0 in healthy humans (29). Changes in pH in the environment have been reported to influence the expression of many genes (34), most of which are involved in maintaining the pH at values around 7.0 (21). It has been demonstrated that in Lactobacillus acidophilus NCK 56 the F1F0-ATPase is an important element in the response and tolerance to low pH (21). A similar situation has been found in Enterococcus faecalis (17), in Salmonella enterica serovar Typhimurium (9, 10), and in Clostridium (7). The F1F0-ATPase is encoded by the atp operon, which in many bacteria consists of eight genes (atpB, atpE, atpF, atpH, atpA, atpG, atpD, and atpC, encoding the a, c, b, δ, α, γ, β, and ɛ subunits, respectively). The atp operon of Escherichia coli includes an additional gene, designated atpI, whose function is unknown and which precedes the other eight atp genes (14, 15). The F1F0-ATPase consists of two subcomplexes, a membrane-extrinsic F1 part and a membrane-intrinsic F0 part. In organisms with a respiratory chain, the primary role of this enzyme is to couple the electrochemical potential difference for H+ across the inner membrane to synthesis of ATP from ADP and phosphate. Conversely, in bacteria that lack a respiratory chain, its role is to create a proton gradient, and this process is then driven by ATP hydrolysis. This is the case for streptococci (3, 20), E. faecalis (17), L. acidophilus (21), and Lactococcus lactis (18, 19). In all these bacteria the activity of the F1F0-ATPase increases as the pH of the growth media decreases. However, the regulation of this pH-inducible phenotype has not been clearly established at the molecular level. In fact, while in L. acidophilus transcription of the atp operon is pH regulated, this is not the case for Streptococcus faecalis and other streptococci. In addition, in S. faecalis, the control seems to act at the level of ATPase enzyme assembly (2). The atp genes are included in the category of housekeeping genes; in fact, their presence in the bacterial genome is considered essential for the survival of these microorganisms. Especially due to its ubiquitous distribution, functional constancy, and conservation, the gene encoding the β-subunit of the atp operon (atpD) is considered to be a suitable molecular marker for bacterial phylogenetic investigations (22, 23).
So far, the evolutionary relationships among lactic acid bacteria (LAB) have been determined by comparing the sequences of rRNA genes, mainly 16S rRNA genes, because of their ubiquity and their resistance to evolutionary changes. The low rate of 16S rRNA evolution is responsible for the failure of this molecule to provide multiple diagnostic sites for closely related but ecologically distinct taxa. The rates of evolutionary substitution in protein-encoding genes are an order of magnitude higher than those in 16S rRNA genes. Thus, some pairs of ecologically distinct taxa may have had time to accumulate neutral sequence divergence at rapidly evolving loci but not at the 16S rRNA level yet (11). Therefore, the importance of comparing the sequences of several genes to evaluate a comprehensive bacterial phylogeny has been stressed (35). Consequently, in the present study we investigated the value of another gene, atpD, which is recognized as a valid molecular marker (22, 23), in order to supplement the classification scheme for some bifidobacteria and LAB species. To date, little is known about the atp genes of bifidobacterial species. In this report we describe the atp locus whose genes code for various subunits of the F1F0-ATPase in Bifidobacterium lactis DSM 10140. We tested the pH inducibility of this operon by Northern blot hybridization and primer extension experiments. Moreover, we partially sequenced of the gene coding for the β subunit of the atp operon from 12 bifidobacterial species and nine Lactobacillus species in order to provide a new molecular marker for phylogenetic analysis of bifidobacteria and LAB species.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains and their origins are summarized in Table 1. All Bifidobacterium strains were grown anaerobically in MRS (Difco, Detroit, Mich.). In order to preserve the anaerobic conditions all media were supplemented with 0.05% l-cysteine-HCl and incubated at 37°C for 16 h. Lactobacillus strains were grown aerobically in MRS (Difco) and incubated at 37°C for 16 h.
TABLE 1.
Strains, origins, and atpD and 16S rDNA sequence accession numbers
| Species | Straina | Origin | atpD accession no.b | 16S rDNA accession no.b |
|---|---|---|---|---|
| Bifidobacterium lactis | DSM 10140T | Yoghurt | AY487153 | X89513 |
| Bifidobacterium animalis | ATCC 25527T | Rat feces | AY487152 | X70971 |
| Bifidobacterium coryneforme | ATCC 25911T | Honeybee hindgut | AY487148 | M58733 |
| Bifidobacterium breve | ATCC 15700T | Intestine of infant | AY487154 | AB006658 |
| Bifidobacterium adolescentis | ATCC 15703T | Intestine of adult | AY487144 | M58729 |
| Bifidobacterium choerinum | ATCC 27686T | Swine feces | AY487147 | D86186 |
| Bifidobacterium longum | NCC 2705 | Intestine of adult | NC_004307 | NC_004307 |
| Bifidobacterium infantis | ATCC 15697T | Intestine of infant | AY487150 | D86184 |
| Bifidobacterium suis | ATCC 27533T | Swine feces | AY487151 | M58743 |
| Bifidobacterium bifidum | ATCC 29521T | Infant feces | AY487145 | M84777 |
| Bifidobacterium dentium | ATCC 27534T | Dental caries | AY487149 | D86183 |
| Bifidobacterium catenulatum | ATCC 27539T | Intestine of adult | AY487146 | M58732 |
| Lactobacillus gallinarum | ATCC 33199T | Chicken crop | AY487160 | AJ242968 |
| Lactobacillus crispatus | NCTC 4 | Human vagina | AY487158 | AJ242969 |
| Lactobacillus amylolyticus | DSM 11614T | Beer wort | AY487155 | Y17361 |
| Lactobacillus gasseri | DSM 20243T | Human | AY487156 | M58820 |
| Lactobacillus johnsonii | ATCC 33200T | Human blood | AY487162 | AJ002515 |
| Lactobacillus acidophilus | ATCC 4356T | Human | AY487161 | M58802 |
| Lactobacillus amylovorus | DSM 20531T | Cattle waste corn fermentation | AY487157 | M58805 |
| Lactobacillus rhamnosus | DSM 20021 | Unknown | AY487159 | M58815 |
| Lactobacillus hilgardii | DSM 20051 | Wine | AJ508914 | M58821 |
| Lactobacillus helveticus | NCDO 2712T | Cheese | AY487163 | X61141 |
| Lactobacillus brevis | DSM 2647 | Human feces | AJ508913 | D37785 |
| Lactobacillus plantarum | WCFS1 | Human oral cavity | NC_004567 | NC_004567 |
| Lactococcus lactis subsp. lactis | IL-1403 | Unknown | NC_002662 | NC_002662 |
| Oenococcus oeni | IOB84.13 | Wine | AJ491851 | NA |
| Oenococcus oeni | JCM 6125 | Wine | NA | AB022924 |
| Leuconostoc mesenteroides | NCDO 523 (= DSM 20343) | Fermenting olives | AJ508912 | X95978 |
| Streptococcus thermophilus | ATCC 19258 | Cheese | NA | AY188354 |
| Streptococcus thermophilus | CHCC2136 | Unknown | AY090612 | NA |
| Streptococcus pyogenes | M1 GAS | Nasal cavity | NC_002737 | NC_002737 |
| Listeria momocytogenes | EGD-e | Food | NC_003210 | NC_003210 |
| Pediococcus parvulus | JCM 5889 (= DSM 20332) | Silage | AJ515143 | D88528 |
| Pediococcus damnosus | DSM 20331 | Beer | AJ515142 | AJ318414 |
| Mycobacterium leprae | TN | Human | NC_002677 | NC_002677 |
| Escherichia coli | K-12 | NC_000913 | NC_000913 | |
| Streptomyces coelicolor | A3 | NC_003888 | NC_003888 | |
| Bacterioides thetaiotaomicron | VP1 5482 | Human feces | NC_004663 | NC_004663 |
| Enterococcus faecalis | V583 | NC_004668 | NC_004668 | |
| Clostridium perfringens | Str. 13 | Human | NC_003366 | NC_003366 |
T = type strain. ATCC, American Type Culture Collection; DSM, Deutsche Sammlung von Mikroorganismen; NCC, Nestlé Culture Collection; JCM, Japanese Collection of Microorganisms; NCDO, National Collection of Dairy Organisms; NCTC, National Collection of Type Cultures; VPI, Virginia Polytechnic Institute.
For the strains whose genome sequences are available the atpD gene and 16S rDNA sequences were retrieved from the complete bacterial genome, and the accession numbers are indicated. NA, not available.
DNA isolation.
Genomic DNA was extracted by using the protocol described in a previous study (50).
DNA amplification and cloning of the atpD gene.
PCR was used to amplify the atpD gene in all Bifidobacterium strains investigated. DNA fragments that were approximately 1,100 bp long and corresponded to the atpD gene were amplified by using oligonucleotides atBIF-1 (5′-CACCCTCGAGGTCGAAC-3′) and atBIF-2 (5′-CTGCATCTTGTGCCACTTC-3′), while the atpD fragment of Lactobacillus strains was amplified by employing oligonucleotides AT-1 (5′-CTNGAAGTTNCNCTNGAAC-3′) and AT-2 (5′-ACGGAANGCATCTTCTGG-3′).
Each PCR mixture (50 μl) contained 20 mM Tri-HCl, 50 mM KCl, each deoxynucleoside triphosphate at a concentration of 200 μM, 50 pmol of each primer, 1.5 mM MgCl2, 1 U of Taq DNA polymerase (Gibco BRL, Paisley, United Kingdom), and 50 ng of DNA template. Each PCR cycling profile consisted of an initial denaturation step of 3 min at 95°C, followed by amplification for 30 cycles of denaturation for 30 s at 95°C, annealing for 30 s at 50°C, and extension for 2 min at 72°C. The PCR was completed with an elongation phase consisting of 10 min at 72°C. The resulting amplicons were separated on a 1% agarose gel, and this was followed by ethidium bromide staining. PCR fragments were purified by using a PCR purification kit (Qiagen, West Sussex, United Kingdom) and were subsequently cloned in the pGEM-T Easy plasmid vector (Promega, Southampton, United Kingdom) by following the supplier's instructions.
DNA sequencing and phylogeny study.
DNA was extracted from the clones with a Quiaprep kit by following the instructions of the supplier (Promega). Nucleotide sequencing of both strands of cloned DNA was performed with a fluorescence-labeled primer cycle sequencing kit (Amersham Buchler, Braunschweig, Germany) by following the supplier's instructions. The primers used were atBIF-1, atBIF-2, AT-1, and AT-2, labeled with IRD800 (MWG Biotech, Ebersberg, Germany). The atpD genes of all Bifidobacterium and Lactobacillus strains examined in this study, as well as those available in the GenBank database, were used for comparison. Sequence alignments were performed by using the MultiAlign program and Clustal W (45). Phylogenetic trees were constructed by using the neighbor-joining program from the PHYLIP software package (version 3.5c) (8). Dendrograms from gene sequences were also drawn by using the Clustal X program (National Center for Biotechnology Information) and were visualized with the TreeView program.
Reference sequences used.
The genome accession numbers for the sequences of the atp operons used in this study were as follows: Bifidobacterium longum NCC 2705, NC_004307; Lactobacillus plantarum WCFS1, AL_004567; L. acidophilus NCK56, AF098522; L. lactis subsp. lactis IL-1403, NC_002662; and E. coli K-12, NC_000913.
RNA isolation and Northern blot analysis.
B. lactis DSM 10140 was grown to an optical density at 560 nm of 0.7, and then the pH of culture was adjusted to 6.0, 5.5, 4.0, or 3.5 with concentrated HCl. RNA was isolated from B. lactis at 100 min after exposure to pH 6.0, 5.5 and 4.0 or at 0, 10, 20, and 100 min after exposure to pH 3.5. Total RNA was isolated by resuspending bacterial cell pellets in TRIzol (GibcoBRL), adding 106-μm-diameter glass beads (Sigma, St. Louis, Mo.), and shearing the slurry with a Mini-Beadbeater-8 cell disruptor (Biospec Products, Bartlesville, Okla.) as described by Ventura et al. (49). The initial Northern blot analysis of Bifidobacterium atpD gene activity was carried out with 15-μg aliquots of RNA isolated from 10-ml portions of Bifidobacterium strains collected after 8 or 18 h of growth under the conditions described above. The RNA was separated in a 1.5% agarose-formaldehyde denaturing gel, transferred to a Zeta-Probe blotting membrane (Bio-Rad, Hertfordshire, United Kingdom) as described by Sambrook and Russell (39), and fixed by UV cross-linking by using a Stratalinker 1800 (Stratagene, La Jolla, Calif.). PCR amplicons obtained with primers atBIF-1 and atBIF-2 were radiolabeled (39). Prehybridization and hybridization were carried out at 65°C in a mixture containing 0.5 M NaHPO4 (pH 7.2), 1.0 mM EDTA and 7.0% sodium dodecyl sulfate (SDS). Following 18 h of hybridization, the membrane was rinsed twice for 30 min at 65°C in 0.1 M NaHPO4 (pH 7.2)-1.0 mM EDTA-1% SDS and twice for 30 min at 65°C in 0.1 mM NaHPO4 (pH 7.2)-1.0 mM EDTA-0.1% SDS and exposed to X-OMAT autoradiography film (Eastman Kodak, Rochester, N.Y.). Autoradiographs were analyzed with ImaGene 5.1 (BioDiscovery).
Primer extension analysis.
The 5′ ends of the RNA transcripts were determined as follows. Separate primer extension reactions were conducted with 15-μg aliquots of RNA isolated as described above and mixed with 1 pmol of primer labeled with IRD800 (MWG Biotech) and 2 μl of buffer H [2 M NaCl, 50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES); pH 6.4]. The mixture was denatured at 90°C for 5 min and then hybridized for 60 min at 42°C. After addition of 5 μl of 1 M Tris-HCl (pH 8.2), 10 μl of 0.1 M dithiothreitol, 5 μl of 0.12 M MgCl2, 20 μl of a mixture containing each deoxynucleoside triphosphate at a concentration of 2.5 mM, 0.4 μl (5 U) of reverse transcriptase (Sigma), and 49.6 μl of double-distilled water, the enzymatic reaction mixture was incubated at 42°C for 2 h. The reaction was stopped by adding 250 μl of an ethanol-acetone mixture (1:1), and the reaction mixture was incubated at −70°C for 15 min and then centrifuged at 10,000 × g for 15 min. The pellets were dissolved in 4 μl of distilled water and mixed with 2.4 μl of loading buffer from a sequencing kit (ThermoSequenase, fluorescence labeled; Amersham, Piscataway, N.J.). The cDNA was separated on an 8% polyacrylamide-urea gel along with mixtures from sequencing reactions which were conducted with the same primers that were used for the primer extension reactions and detected with a LiCor sequencer machine (MWG Biotech). The synthetic oligonucleotides used were met-prom (5′-GTCTCGTCTCGATCTTCTTC-3′), atp-B-prom (5′-CACGAAGATGATGCGGTTGATTG-3′), and atp-A-prom (5′-CAAAGGTCAGCAGTTCGTTG-3′).
Factorial correspondence analysis.
Factorial correspondence analysis was performed with the assistance of the GCUA software (version 1.0) (28).
Nucleotide sequence accession numbers.
The GenBank accession numbers for partial atpD gene sequences generated in this study are as follows: Lactobacillus gallinarum ATCC 33199, AY487160; Lactobacillus crispatus NCTC 4, AY487158; Lactobacillus amylolyticus DSM 11614, AY487155; Lactobacillus gasseri DSM 20243, AY487156; Lactobacillus johnsonii ATCC 33200, AY487162; Lactobacillus amylovorus DSM 20531, AY487157; Lactobacillus rhamnosus DSM 20021, AY487159; Lactobacillus helveticus NCDO 2712, AY487163; L. acidophilus ATCC 4356, AY487161; Bifidobacterium bifidum ATCC 29521, AY487145; Bifidobacterium infantis ATCC 15697, AY487150; Bifidobacterium catenulatum ATCC 27539, AY487146; Bifidobacterium adolescentis ATCC 15703, AY487144; Bifidobacterium breve ATCC 15700, AY487154; Bifidobacterium animalis ATCC 25527, AY487152; B. lactis DSM 10140, AY487153; Bifidobacterium suis ATCC 27533, AY487151; Bifidobacterium choerinum ATCC 27686, AY487147; Bifidobacterium coryneforme ATCC 25911, AY487148; and Bifidobacterium dentium ATCC 27534, AY487149. The nucleotide sequence data for the atp operons reported in this paper have been deposited in the GenBank database under accession numbers AY488175 (B. lactis DSM 10140) and AY488174 (B. breve NCIMB 8807).
RESULTS
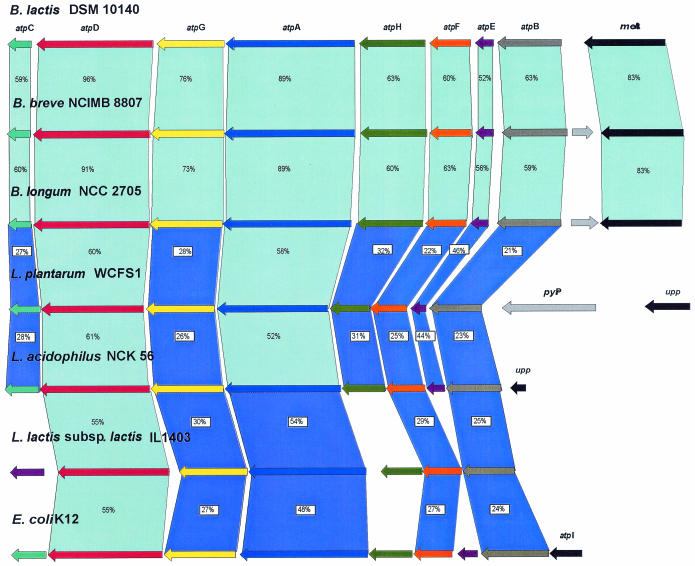
Analysis of the protein sequences from a random clone library of B. lactis DSM 10140 revealed the presence of a clone with sequences which exhibited significant amino acid homology with the gene products of atpD of B. longum NCC 2705 (41) and L. acidophilus NCK 56 (21). By using a PCR-based chromosome-walking strategy we cloned the regions surrounding the atpD gene. The complete nucleotide sequence of the atp operon was determined, and the proposed organization of the operon is shown in Fig. 1. The atp operon was composed of eight open reading frames (ORFs) corresponding to a 7.3-kb DNA segment. The gene order of the atp operon was atpBEFHAGDC, which was identical to that observed previously in other bacteria (21, 40). The deduced amino acid sequences encoded by the B. lactis DSM 10140 atp operon were aligned with those of B. longum NCC 2705, B. breve NCIMB 8807, L. acidophilus NCK 56, L. plantarum WCFS1, L. lactis subsp. lactis IL-1403, and E. coli K-12 (Fig. 1). Protein comparison (Fig. 1) showed that the proteins most similar to the B. lactis ATPase subunits were those from B. breve and B. longum. Levels of identity of >21% were observed for the eight subunits and the subunits of lactobacilli, while the comparison of the atp operon genes of B. lactis with the genes of the phylogenetically distant taxa (L. lactis subsp. lactis and E. coli) revealed consistent identities for only a few atp genes. In general, greater homology was observed for the atpA and atpD gene products corresponding to the cytoplasmic domain. The consensus nucleotide-binding domains, Walker motifs A (GXXXXGKT) and B (l-hydrophobic-hydrophobic-hydrophobic-d) (1, 51), were also conserved in the deduced sequences of the α and β subunits. Significantly lower levels of homology were observed for the atpB, atpE, and atpF gene products comprising the membrane-binding domain, F0. The atpG and atpF genes appeared to use UUG and GTG, respectively, as the initiation codons instead of the more frequently used AUG start codon; it was reported previously that L. lactis and L. acidophilus each use this alternative start codon in one of the genes of the atp operon (19, 21).
FIG. 1.
Comparison of the atp operon of B. lactis DSM 10140 with the corresponding operons of different bacteria. The putative function of the protein is indicated above each arrow. Related proteins are linked by light blue shading (≥52%) and dark blue shading (≤51%) according to the different levels of amino acid similarity; the levels of amino acid identity (expressed as percentages) are indicated. The length of each arrow is proportional to the length of the predicted ORF. Corresponding genes are indicated by arrows that are the same color.
Notably, the first gene of the atp operon of B. lactis, B. breve, and B. longum was preceded by an ORF which exhibited homology to bacterial metA coding for homoserine O-succinyltransferase, while in L. acidophilus the initial atpB gene of the atp operon is preceded by a homologue of the upp gene, which codes for uracil phosphoribosyltransferase (21). Interestingly, in the L. plantarum genome a gene encoding for the pyrophosphate hydrolase is inserted between the atpB and upp genes. The atp operons of bifidobacteria and lactobacilli (21) do not contain the atpI gene, which is not present in any of the bacteria investigated so far (42, 43).
The gene order of all of the Lactobacillus regions investigated was found to be identical to that of the B. longum NCC 2705 region. The atpC gene of B. lactis DSM 10140 is preceded by two ORFs, ORF1 and ORF2, while the metA gene is followed by two other ORFs, ORF3 and ORF4 (Fig. 2). BLAST analysis of ORF1 and ORF2 gave positive matches with a conserved hypothetical protein of B. longum (accession number AE014654_4; 72% identity) and with a putative drug-binding lipoprotein of B. longum (accession number AE014654_3; 53% identity), respectively. ORF3 showed 51% identity with a multidrug transporter of L. lactis subsp. lactis (accession number AL646AE006394_2), whereas RF4 exhibited 66% identity with an inosine-uridine nucleoside N-ribohydrolase of Corynebacterium glutamicum (accession number BAB98757.1).
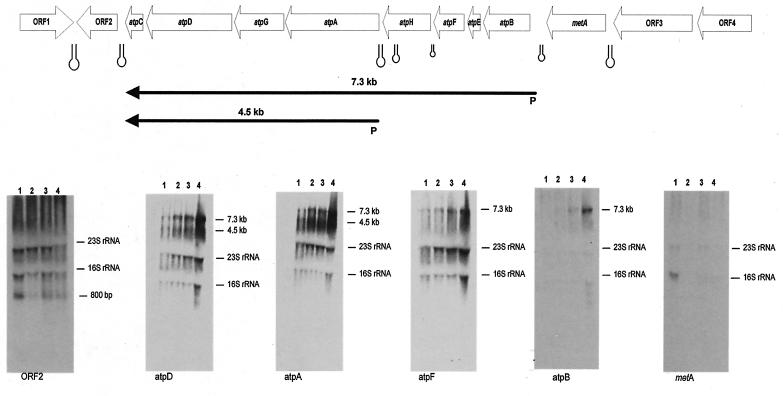
FIG. 2.
Northern hybridization analysis of B. lactis RNA and transcription unit mapping of the atp operon. All predicted ORFs are indicated and are annotated with their database matches. The transcripts are indicated by arrows, and the arrows point toward the 3′ end of the mRNA. The estimated size of the mRNA is indicated. Hairpins indicate possible rho-independent terminators. The transcripts are positioned with respect to the genome map shown at the top. The gels show the results of Northern blot hybridization of RNA isolated from B. lactis DSM 10140 upon exposure to low pH at 100 min. Lane 1, RNA isolated from a culture after exposure to pH 6.0; lane 2, RNA isolated from a culture after exposure to pH 5.5; lane 3, RNA isolated from a culture after exposure to pH 4.0; lane 4, RNA isolated from a culture after exposure to pH 3.5. Hybridization was performed by using the probes corresponding to the ORFs shown in the gene map at the top. The molecular size calculated for the hybridization signal is indicated next to each autoradiograph.
Transcriptional analysis of the atp operon.
In order to verify that the genes belonging to the atp operon are expressed, we probed total RNA extracted from B. lactis DSM 10140 cells with DNA probes targeting different genes of the atp operon. It appeared that initially long transcripts were synthesized, which were subsequently degraded into smaller products, as indicated by the presence of smears in the blots (Fig. 2). In the background of the blots hybridized to the atpC, atpD, atpG, and atpA probes (corresponding to the genes encoding the F1 subunit of the ATP synthase) we found one pronounced band of a smaller transcript corresponding to a 4.5-kb mRNA (Fig. 2). The maximum size of the transcripts was approximately 7.3 kb, suggesting that all eight genes are transcribed into one single polycistronic mRNA (Fig. 2). The presence of a background in Northern blots of atp mRNA has also been reported for E. coli and is probably due to specific endonucleolytic cleavage of the transcripts of the corresponding genes (26, 27). When the Northern blots were probed with the metA and recB genes, no transcripts or 700-bp mRNA species were detected, suggesting that the metA and the recB genes are not part of the atp operon (Fig. 2). An inverted repeat was observed in the region immediately downstream of the atpC gene, which may serve as the terminator sequence. A similar palindromic sequence was noticed in the intergenic region downstream of the metA gene. Other secondary structures that might act as weak terminator sequences were also found within the operon (Fig. 2).
Primer extension analysis showed that the metA gene does not possess a promoter region and confirmed that this gene is not included in the atp operon. Primer extension analysis was attempted for the atp operon in order to elucidate the transcriptional start site of the two transcripts identified. The transcriptional start sites were identified upstream of the assumed start codons of the atpB and atpA genes (Fig. 3a). Once the start sites were identified, sequence analysis of the regions immediately upstream suggested that there was a putative Pribnow box, while no canonical −35 region sequences were observed (Fig. 3a). Primer extension experiments were conducted with RNA extracted from cultures in which the medium pH was shifted from pH 6 to 3.5. While the abundance of transcripts appeared to increase in response to pH 3.5, the transcription start site did not change (data not shown).
FIG. 3.
(a) Primer extension analysis and comparison of the putative promoter sequences for the atp operon. Boldface type and underlining indicate −10 and −35 putative hexamers; the box indicates a TG doublet of an extended −10 sequence; and the asterisks indicate transcription start points. The start codon is at the right end of the sequence. (b) Slot blot hybridization of mRNA from cells incubated at pH 3.5 for various times. Each slot contained 20 μg of RNA, and all slots were probed with 32P-labeled PCR products corresponding to the atpD gene. The graph shows the results of two different experiments. The numbers above the slot blot indicate the times of exposure of the B. lactis DSM 10140 culture at pH 3.5.
Induction of ATPase activity under acidic growth conditions.
Previous data have shown that in other LAB the ATPase is up-regulated under acidic conditions (21). Maximal induction of activity was seen at pH 3.5. To examine the issue more directly, RNA was isolated from acid-treated cultures of B. lactis and used as a target in an RNA slot blot analysis in which the atpD, atpH, and atpB genes were used as probes (Fig. 3b and data not shown). The level of the mRNAs increased upon exposure to pH 3.5 and was almost 15-fold greater after 100 min.
Sequencing and phylogenetic analysis of the atpD gene.
The atpD sequences from a number of selected bacterial species were aligned and compared. Two highly conserved regions were identified, and a pair of primers (AT-1 and AT-2) amplifying a 1,114- to 1,120-bp region was designed. These primers allowed amplification of atpD sequences from a wide variety of bacteria, including 12 Bifidobacterium species and nine Lactobacillus species.
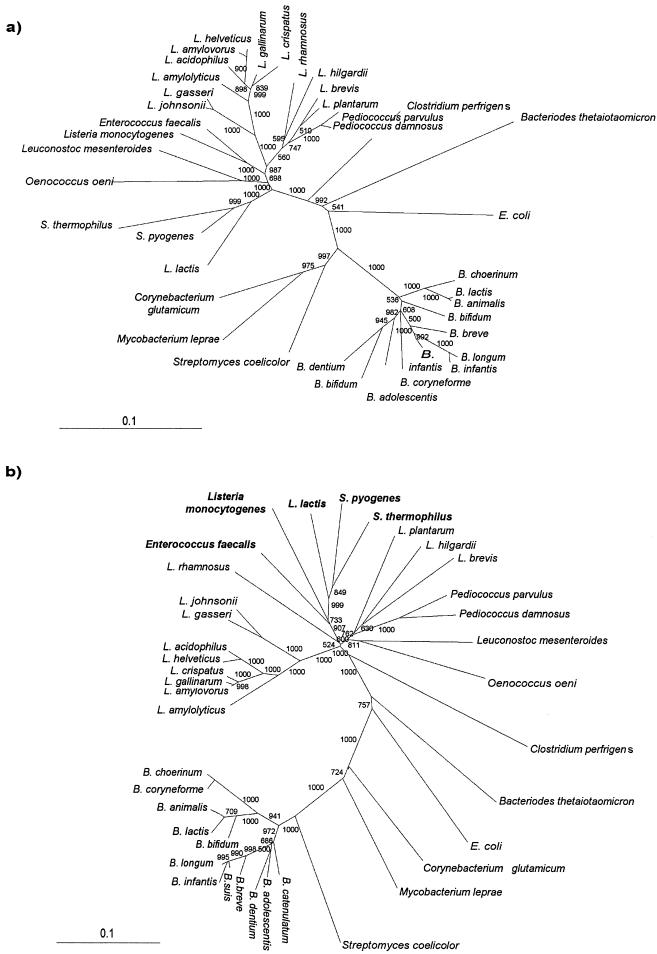
We performed a phylogenetic analysis based on the atpD sequences of bifidobacteria and lactobacilli using the neighbor-joining method (Fig. 4). For completeness we included in our analysis the atpD DNA sequences of other strains belonging to different genera representing the LAB group (e.g., Lactococcus, Streptococcus, Pediococcus, Oenococcus, Leuconostoc, and Enterococcus) and other gram-positive and gram-negative bacteria with high and low G+C contents, including some Actinomycetales (e.g., Corynebacterium, Streptomyces, and Mycobacterium). A phylogenetic tree was also generated by using the 16S ribosomal DNA (rDNA) sequences from the same set of strains that was used for the atpD gene-based tree (except for Streptococcus thermophilus, Oenococcus oeni, and L. acidophilus) (Fig. 4a). The reliability of the trees generated was supported by bootstrap analysis. Both trees showed that there was a clear division between the low-G+C-content bacteria (Lactobacillus, Lactococcus, Streptococcus, Pediococcus, Oenococcus, Leuconostoc, and Enterococcus) and the high-G+C-content bacteria (Bifidobacterium, Mycobacterium, and Streptomyces). Interestingly, all bifidobacterial strains clustered together at the right end of both trees (Fig. 4), whereas an apparent shuffling of lactobacilli in the atpD gene-based tree was observed (Fig. 4b). In fact, Lactococcus, Streptococcus, Enterococcus, and Listeria clustered within the genus Lactobacillus. This could have been due to extensive gene transfer among bacteria and/or independent sequence divergence. In addition, the G+C contents of the Lactococcus, Streptococcus, Enterococcus, and Listeria atpD genes were between 3.12- and 6.8-fold higher than the average G+C contents of the genomes of these organisms, whereas the G+C content of the atpD gene of Lactobacillus was nearly identical to that of the rest of the genome. Moreover, the factorial correspondence analysis of codon usage in Lactococcus, Streptococcus, and Listeria ORFs revealed a very different codon usage bias for the atpD gene (Fig. 5). In fact, as shown in Fig. 5, the dots corresponding to the atpD genes of Lactococcus, Streptococcus, and Listeria are located at some distance from the rest of the cloud of dots representing all of the rest of the genomic genes. Notably, closely related strains which have nearly identical 16S rRNA sequences, like the L. acidophilus B group (L. gasseri and L. johnsonii), as well as B. animalis and B. lactis, clearly branch separately in the atpD sequence-based tree (Fig. 4b).
FIG. 4.
Neighbor-joining phylogenetic trees obtained by using the 16S rRNA genes (a) and the atpD genes (b). The scale bars indicate phylogenetic distances. Bootstrap values are indicated at the nodes for a total of 1,000 replicates. Boldface type indicates species in which horizontal gene transfer of the atpD gene is suspected. The strains and the accession numbers of the atpD genes and 16S rDNA sequences are shown in Table 1.
FIG. 5.
Factorial correspondence analysis of codon usage in various ORFs in Streptococcus (a), Lactococcus (b), and Listeria (c). The position of atpD is indicated by a cross.
Most of the base substitutions in the atpD genes were synonymous; i.e., they did not result in amino acid changes. Alignment of the atpD-encoded protein sequences of all of the strains investigated led to identification of 16 conserved signatures that appeared to be specific for the genus Bifidobacterium (Table 2), whereas no amino acid signatures have been detected for the genus Lactobacillus. Interestingly, comparative analysis of the atpD nucleotide sequences revealed four amino acid signatures which are found in B. lactis or B. animalis strains, as well as eight amino acid signatures which are found in the L. johnsonii and L. gasseri strains, but which are not found in any of the other microorganisms investigated. These sequence signatures can be used directly to design specific PCR primers or as targets for specific restriction enzymes, providing species-specific restriction fragment length polymorphism patterns. Other closely related species, like B. longum, B. suis, and B. infantis, have identical atpD sequences, providing new evidence that these bacteria could belong to the same taxon (38).
TABLE 2.
atpD-amino acid and nucleotide signatures of Bifidobacterium and Lactobacillus taxa
| Taxon | Position(s)a | atpD amino acid signatureb | atpD nucleotideb signature |
|---|---|---|---|
| Bifidobacterium | 38 | I | ATT |
| Bifidobacterium | 41-42 | KK | AAGAAG |
| Bifidobacterium | 58-59 | NP | AACCCG |
| Bifidobacterium | 84 | Q | CAG |
| Bifidobacterium | 106-107 | QR | CAGCGA |
| Bifidobacterium | 137-138 | IG | ATCGGC |
| Bifidobacterium | 158 | Q | CAG |
| Bifidobacterium | 167 | P | CCG |
| Bifidobacterium | 182 | N | AAT |
| Bifidobacterium | 304 | N | AAC |
| Bifidobacterium | 308 | A | GCG |
| Bifidobacterium | 344 | Q | CAG |
| Bifidobacterium | 347 | G | GGC |
| Bifidobacterium | 351 | Y | TAC |
| Bifidobacterium | 355 | K | AAG |
| B. lactis (B. animalis) | 12 | I (V) | ATC (GTC) |
| B. lactis (B. animalis) | 49 | V (E) | GTG (GAG) |
| B. lactis (B. animalis) | 286 | A (S) | GCC (TCC) |
| B. lactis (B. animalis) | 337 | T (I) | ACA (ATA) |
| L. johnsonii (L. gasseri) | 17 | G (A) | GGT (GCT) |
| L. johnsonii (L. gasseri) | 163-164 | GK (AT) | GGTAAG (GCCACC) |
| L. johnsonii (L. gasseri) | 252 | T (P) | ACA (CCA) |
| L. johnsonii (L. gasseri) | 254-255 | LS (PA) | CTTTCA (CCAGCA) |
| L. johnsonii (L. gasseri) | 305 | K (Q) | AAG (CAA) |
| L. johnsonii (L. gasseri) | 359 | I (L) | ATT (CTT) |
Position in the amino acid sequence.
The residues in parentheses are the residues in the species in parentheses.
DISCUSSION
In the present work we genetically characterized the operon encoding the F1F0-ATPase of B. lactis, which consists of eight subunits. In contrast to other bacterial atp operons, there is no atpI gene in B. lactis or in B. breve or B. longum, and the overall order of the genes is atpB-atpE-atpF-atpH-atpA-atpG-atpD-atpD-atpC, which is similar to the order in L. acidophilus (21) and in E. faecalis (17). The inferred amino acid sequences encoded by these genes exhibited homology with the sequences of other ATPases. In particular, there was strong identity between the subunits of B. lactis and those of B. breve, B. longum, L. plantarum, and L. acidophilus. The operon is transcribed in two separate mRNAs, an mRNA covering all subunits, similar to that reported for the equivalent operon of other bacterial species (12, 42), and a shorter mRNA corresponding to the last four genes of the operon. The transcription start sites of these mRNAs were experimentally determined, and no consensus promoter sequences were identified. The fact that no definitive consensus sequence could be determined from these motifs may have been due to the fact that the RNA polymerase recognition sites can tolerate a considerable amount of degeneration or to the fact that the sequences investigated here were not representative of typical −10 and −35 hexamers. So far, there have been only two other reports of use of the primer extension technique for determination of a transcriptional start site in Bifidobacterium spp. (24, 37), and neither of them identified the classical recognition sites of the vegetative RNA polymerase.
Interestingly, the last gene of the operon was followed by a long palindromic sequence, which might play the role of a rho-independent terminator for the operon. Other short palindromic sequences were detected in the operon sequences, at positions corresponding to those described in the L. acidophilus and E. coli atp operons (36, 44). In the ATPases complexes of these bacteria these palindromic sequences have been found to increase the mRNA stability and thus enhance the translational efficiency of the corresponding genes (21).
The bacterial acid response is a very complicated process that involves synthesis of a large arsenal of proteins (33, 34). For many bacteria it has been shown that treatment with acid and a subsequent decrease in the intracellular pH are accompanied by an increase in the amount of the F1F0-ATPase (2, 19, 21, 30, 31). This phenomenon has been studied most extensively in S. faecalis (2, 17), in L. lactis (18, 19), and in L. acidophilus (21), in which the cytoplasmic pH is maintained by the amount and activity of the H+-ATPase, which catalyzes the ATP-driven translocation of protons from the cytoplasm. In S. faecalis it has been demonstrated that the transcriptional activity of the atp operon is not pH regulated and that the increase in the enzyme level is pH regulated at the enzyme assembly step. Conversely, the abundance of atp transcripts of B. lactis increased upon exposure to a low pH, suggesting that there is regulation at the level of transcription or mRNA degradation. This mechanism of regulation is similar to that described for L. acidophilus and L. lactis strains.
The discovery of genes whose expression responds to changes in pH is of immense importance for bifidobacteria. The growth of these bacteria is associated with generation of acidic products, which accumulate during fermentation. Similarly, the acidity of the stomach is a strong barrier against passage and survival of bacteria in the gastrointestinal tract. Maus and Ingham (25) showed that a B. lactis strain was capable of surviving in synthetic human gastric fluid at a pH of 3.5, especially after application of a sublethal acidic stress during culture production (e.g., exposure to low pH in a yogurt sample). Acids do pass passively through the bifidobacterial membrane and are rapidly dissociated into their impermeable constituent protons and charged derivatives. The maintenance of cytoplasmic pH requires that these protons be neutralized or expelled from the cytosol. In this respect, the proton-translocating ATPase may play an important role in the success of B. lactis in environments with low pH values. In this respect, knowledge about the genetic basis of this acid resistance for gastrointestinal or industrially useful bacteria that resist extreme acidic conditions during passage through the human stomach is very important.
Our results indicate that the atp operon is essential for growth of bifidobacteria under acidic conditions, which is in agreement with the observation that the activity of the F1F0-ATPase in related anaerobic bacteria is enhanced at a low external pH (19, 21). The genes of the atp operon, especially the atpD gene, have all of the prerequisites to be suitable phylogenetic markers, such as very high genetic stability and a wide distribution (22, 23). This operon has already been used to infer phylogeny in the genera Salmonella (6) and Rhizobium (13). This alternative molecular marker might corroborate and help complete the evolutionary history of various LAB species. The use of atpD genes in LAB species as an alternative or complement to the 16S rDNA marker provides sequence signatures that can be used to distinguish closely related species (e.g., B. animalis and B. lactis). These sequence signatures can be used directly to design specific PCR primers or might be targets for specific restriction enzymes, providing species-specific restriction fragment length polymorphism patterns. The failure of 16S rDNA sequences to provide multiple diagnostic sites could stem from the unusually low rate of evolution of this molecule. The rates of evolutionary substitution in 16S rRNA genes are an order of magnitude lower than those in protein-encoding genes, such as the atpD gene (32, 52). Interestingly, there were some differences in the phylogenies obtained by using atpD genes and 16S rRNA genes. These differences are linked to insertion of the genera Enterococcus, Lactococcus, Streptococcus, and Listeria into the Lactobacillus lineage. Analysis of the G+C contents of these organisms and the bias of their codon usage support the hypothesis that there was probably horizontal transfer of the atpD genes. Notably, these organisms showed a reshuffling of the atp operon; in fact, the F0 gene order of Lactococcus and Streptococcus is atpEBF, compared to the most common order, atpBEF (18, 20). The atpD- and 16S rRNA gene-based trees were generated from the same set of strains with only two exceptions for the 42 strains. This should not decrease the accuracy of the analysis since the differences in the tree topology were observed in more than one species of the same genus (e.g., Streptococcus). The genome sequence analysis showed that horizontal gene transfer is an event that also occurs frequently in housekeeping genes, like the tuf gene (16), and in the S14 gene encoding the ribosomal protein (5).
Recently, polyphasic taxonomy (46) has been recognized by the International Committee on Systematic Bacteriology as a new tool for description of species and for revision of the current nomenclature of some bacterial groups. In view of its demonstrated effectiveness, sequence analysis of protein-encoding genes (such as atpD) as alternative phylogenetic markers could be added to the arsenal of rRNA sequence databases and to the relatively small groEL and recA sequence databases.
In conclusion, analysis of atpD might enable us to resolve phylogenetic lineages of bifidobacteria at species and subspecies levels. In fact, the comparison of the atpD genes of B. lactis and B. animalis supported previously published data (47, 48, 49, 51) and provided new evidence that B. lactis and B. animalis are clearly members of two separate lineages. Moreover, analysis of the atpD gene also supports the recent decision of the International Committee on Systematic Bacteriology to unify B. suis, B. longum, and B. infantis into one unique taxon since no clear genetic (DNA-DNA hybridization, ribotyping patterns) or physiological differences could be detected (39), whereas caution should be used in any subsequent interpretation of bacterial evolution based on the atpD gene, in which horizontal gene transfer events might have occurred (e.g., in the genera Lactococcus, Streptococcus, Enterococcus, and Listeria).
Furthermore, the growing picture of pH-regulated catabolism has obvious significance for questions of survival of industrially useful bacteria, like B. lactis, in the human body, as well as in fermented foods with low pH values. Our study revealed various avenues of investigation for examining metabolic components of, for example, pH stress responses.
Acknowledgments
Part of this work was financially supported by Enterprise Ireland (grant BR/1998/202), by the Higher Education Authority Programme for Research in Third Level Institutions, by the Science Foundation Ireland Centre for Science Engineering and Technology, and by a Marie Curie Development Host Fellowship (HPMD-2000-00027) to M.V.
REFERENCES
- 1.Abrahams, J. P., A. G. W. Laslie, R. Lutter, and J. E. Walker. 1994. Structure at 2.8 A resolution of F1-ATPase from bovine heart mitochondria. Nature 370:621-628. [DOI] [PubMed] [Google Scholar]
- 2.Arikado, E., H. Ishihara, T. Ehara, C. Shibata, H. Saito, T. Kakegawa, K. Igarashi, and H. Kobayashi. 1999. Enzyme level of enterococcal F1F0-ATPase is regulated by pH at the step of assembly. Eur. J. Biochem. 259:262-268. [DOI] [PubMed] [Google Scholar]
- 3.Bender, G., S. Sutton, and R. Marquis. 1986. Acid tolerance proton permeabilities and membrane ATPases of oral streptococci. Infect. Immun. 53:331-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bengmark, S. 1998. Ecological control of the gastrointestinal tract. The role of probiotic flora. Gut 42:2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brochier, C., H. Philippe, and D. Moreira. 2000. The evolutionary history of ribosomal protein RpS14: horizontal gene transfer at the heart of the ribosome. Trends Genet. 16:529-533. [DOI] [PubMed] [Google Scholar]
- 6.Christensen, H., and J. E. Olsen. Phylogenetic relationships of Salmonella based on DNA sequence comparison of atpD encoding the β subunit of ATP synthase. FEMS Microbiol. Lett. 161:89-96. [DOI] [PubMed]
- 7.Das, A., and L. G. Ljungdahl. 1997. Composition and primary structure of the F1F0 ATP synthase from the obligately anaerobic bacterium Clostridium thermoacetium. J. Bacteriol. 179:3746-3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felsenstein, J. 1993. PHYLIP (phylogeny inference package), version 3.5c. Department of Genetics, University of Washington, Seattle, Wash.
- 9.Foster, J. W., and H. K. Hall. 1990. Adaptive acidification tolerance response of Salmonella typhimurium. J. Bacteriol. 172:771-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster, J. W., and H. K. Hall. 1991. Inducible pH homeostasis and the acid tolerance response of Salmonella typhimurium. J. Bacteriol. 173:5129-5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fox, G. E., J. D. Wisotzkey, and P. Jurtshuk. 1992. How close is close: 16S rRNA sequence identity may not be sufficient to guarantee species identity. Int. J. Syst. Bacteriol. 42:166-170. [DOI] [PubMed] [Google Scholar]
- 12.Galiano, A. J. M., M. J. Ferrandiz, and A. G. de la Campa. 2001. The promoter of the operon encoding the F0F1 ATPase of Streptococcus pneumoniae is inducible by pH. Mol. Microbiol. 41:1327-1338. [DOI] [PubMed] [Google Scholar]
- 13.Gaunt, M. W., S. L. Turner, L. Rigottier-Gois, S. A. Lloyd-Macgilp, and J. P. W. Young. 2001. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 51:2037-2048. [DOI] [PubMed] [Google Scholar]
- 14.Gunsalus, R. P., W. S. A. Brusilow, and R. D. Simoni. 1982. Gene order and gene-polypeptide relationships of the proton translocating ATPase operon (unc) of Escherichia coli. Proc. Natl. Acad. Sci. 79:320-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kasimoglu, E., S. J. Park, J. Malek, C. P. Tseng, and R. P. Gunsalus. 1996. Transcriptional regulation of the proton-translocating ATPase (atpIBEFHAGDC) operon of Escherichia coli: control by cell growth rate. J. Bacteriol. 178:5563-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ke, D., M. Boissinot, A. Huletsky, F. J. Picard, J. Frenette, M. Ouellette, P. H. Roy, and M. G. Bergeron. 2000. Evidence for horizontal gene transfer in evolution of elongation factor Tu in enterococci. J. Bacteriol. 182:6913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobayashi, H., T. Suzuki, N. Kinoshita, and T. Unemoto. 1984. Amplification of the Streptococcus faecalis proton-translocating ATPase by a decrease in cytoplasmic pH. J. Bacteriol. 158:1157-1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koebmann, B. J., C. Solem, M. B. Pedersen, D. Nilsson, and P. R. Jensen. 2002. Expression of genes encoding F1-ATPase results in uncoupling of glycolysis from biomass production in Lactococcus lactis. Appl. Environ. Microbiol. 68:4274-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koebmann, B. J., D. Nilson, O. P. Kuipers, and P. R. Jensen. 2000. The membrane-bound H-ATPase complex is essential for growth of Lactococcus lactis. J. Bacteriol. 182:4738-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuhnert, W. L., and R. G. Quivey. 2003. Genetic and biochemical characterization of the F-ATPase operon from Streptococcus sanguis 10904. J. Bacteriol. 185:1525-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullen, M. J., and T. R. Klaenhammer. 1999. Identification of the pH-inducible, proton-translocating F1F0-ATPase (atpBEFHAGDC) operon of Lactobacillus acidophilus by differential display: gene structure, cloning and characterization. Mol. Microbiol. 33:1152-1161. [DOI] [PubMed] [Google Scholar]
- 22.Ludwig, W., and K. H. Schleifer. 1999. Phylogeny of bacteria beyond the 16S rRNA standard. ASM News 65:752-757. [Google Scholar]
- 23.Ludwig, W., J. Neumaier, N. Klugbauer, E. Brockmann, C. Roller, S. Jilg, K. Reetz, I. Schachtner, A. Ludvigsen, M. Bachleitner, U. Fischer, and K. H. Schleifer. 1993. Phylogenetic relationships of bacteria based on comparative sequence analysis of elongation factor Tu and ATP-synthase beta subunit genes. Antonie Leeuwenhoek. 64:285-305. [DOI] [PubMed] [Google Scholar]
- 24.MacConaill, L. E., D. Butler, M. O'Connel-Montherway, G. F. Fitzgerald, and D. van Sinderen. 2003. Identification of two component regulatory systems in Bifidobacterium infantis by functional complementation and degenerate PCR approaches. Appl. Environ. Microbiol. 69:4219-4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maus, J. E., and S. C. Ingham. 2003. Employment of stressful conditions during culture production to enhance subsequent cold- and acid-tolerance of bifidobacteria. J. Appl. Microbiol. 95:146-154. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy, J. E. G., B. Schauder, and P. Ziemke. 1988. Post-transcriptional control in Escherichia coli: translation and degradation of the atp operon mRNA. Gene 72:131-139. [DOI] [PubMed] [Google Scholar]
- 27.McCarthy, J. E. G., G. Gerstel, B. Surin, U. Wiedemann, and P. Ziemke. 1991. Differential expression from Escherichia coli atp operon mediated by segmental differences in mRNA stability. Mol. Microbiol. 5:2447-2458. [DOI] [PubMed] [Google Scholar]
- 28.McInerney, J., and D. Kooij. 1997. Economic analysis of alternative AD control programmes. Vet. Microbiol. 55:113-121. [DOI] [PubMed] [Google Scholar]
- 29.McLauchlan, G., G. M. Fullarton, G. P. Crean, and K. E. McColl. 1998. Comparison of gastric body and antral pH: a 24 hour ambulatory study in health volunteers. Gut 30:573-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miwa, T., H. Esaki, J. Umemori, and T. Hino. 1997. Activity of H(+)-ATPase in ruminal bacteria with special reference to acid tolerance. Appl. Environ. Microbiol. 63:2155-2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nannen, N. L., and R. W. Hutkins. 1991. Proton-translocating adenosine triphosphatase activity in lactic acid bacteria. J. Dairy Sci. 74:747-751. [Google Scholar]
- 32.Ochman, H., and A. C. Wilson. 1987. Evolution in bacteria: evidence for a universal substitution rate in cellular genomes. J. Mol. Evol. 26:74-86. [DOI] [PubMed] [Google Scholar]
- 33.O'Driscoll, B., C. G. M. Gahan, and C. Hill. 1997. Two-dimensional polyacrylamide gel electrophoresis analysis of the tolerance response in Listeria monocytogenes LO28. Appl. Environ. Microbiol. 63:2679-2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olson, E. R. 1993. Influence of pH on bacterial gene expression. Mol. Microbiol. 8:5-14. [DOI] [PubMed] [Google Scholar]
- 35.Palys, T., L. K. Nakamura, and F. M. Cohan. 1997. Discovery and classification of ecological diversity in the bacterial world: the role of DNA sequence data. Int. J. Syst. Bacteriol. 47:1145-1156. [DOI] [PubMed] [Google Scholar]
- 36.Porter, A. C. G., W. S. A. Brusilow, and R. D. Simoni. 1983. Promoter for the unc operon of Escherichia coli. J. Bacteriol. 155:1271-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rossi, M., L. Altomare, A. Gonzales Vara y Rodriguez, P. Brigidi, and D. Matteuzzi. 2000. Nucleotide sequence, expression and transcriptional analysis of the Bifidobacterium longum MB219 lacZ gene. Arch. Microbiol. 174:74-80. [DOI] [PubMed] [Google Scholar]
- 38.Sakata, S., M. Kitahara, M. Sakamoto, H. Hayashi, M. Fukuyama, and Y. Benno. 2002. Unification of Bifidobacterium infantis and Bifidobacterium suis as Bifidobacterium longum. Int. J. Syst. Evol. Microbiol. 52:1945-1951. [DOI] [PubMed] [Google Scholar]
- 39.Sambrook, J., and D. W. Russel. 2001. Molecular cloning: a laboratory manual, 3rd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 40.Santana, M., M. S. Ionescu, A. Vertes, R. Longin, F. Kunst, A. Danchin, and P. Glaser. 1994. Bacillus subtilis F0F1 ATPase: DNA sequence of the atp operon and characterization of atp mutants. J. Bacteriol. 176:6802-6811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schell, M. A., M. Karmirantzou, B. Snel, D. Vilanova, G. Pessi, M. C. Zwahlen, F. Desiere, P. Bork, M. Delley, and F. Arigoni. 2002. The genome sequence of Bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. 99:14422-14427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata, C., T. Ehara, K. Tomura, K. Igarashi, and H. Kobayashi. 1992. Gene structure of Enterococcus hirae (Streptococcus faecalis) F1F0-ATPase, which functions as a regulator of cytoplasmic pH. J. Bacteriol. 174:6117-6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith, A. J., R. G. Quivey, and R. C. Faustoferri. 1996. Cloning and sequence analysis of the Streptococcus mutans membrane-bound, proton-translocating ATPase operon. Gene 183:87-96. [DOI] [PubMed] [Google Scholar]
- 44.Stancik, L. M., D. M. Stancik, B. Schmidt, D. M. Barnhart, Y. N. Yoncheva, and J. L. Slonczewski. 2002. pH-dependent expression of periplasmic proteins and amino acid catabolism in Escherichia coli. J. Bacteriol. 184:4246-4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic. Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandamme, P., B. Pot, M. Gillis, P. de Vos, K. Kersters, and J. Swings. 1996. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol. Rev. 60:407-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ventura, M., V. Meylan, and R. Zink. 2003. Identification and tracing of Bifidobacterium species by enterobacterial repetitive intergenic consensus sequences. Appl. Environ. Microbiol. 69:4296-4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ventura, M., and R. Zink. 2002. Rapid identification, differentiation, and proposed new taxonomic classification of Bifidobacterium lactis. Appl. Environ. Microbiol. 68:6429-6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ventura, M., I. Jankovic, D. C. Walker, R. D. Pridmore, and R. Zink. 2002. Identification and characterization of novel surface proteins in Lactobacillus johnsonii and Lactobacillus gasseri. Appl. Environ. Microbiol. 68:6172-6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ventura, M., R. Reniero, and R. Zink. 2001. Specific identification and targeted characterization of Bifidobacterium lactis from different environmental isolates by a combined multiplex-PCR approach. Appl. Environ. Microbiol. 67:2760-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Walker, J. E., M. Saraste, J. Runswick, and N. J. Gay. 1982. Distantly related sequences in the alpha and beta subunits of ATP synthase, myosin, kinases and other ATP requiring enzymes and a common nucleotide binding fold. EMBO J. 1:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamamoto, S., and S. Harayama. 1998. Phylogenetic relationship of Pseudomonas putida strains deduced from the nucleotide sequences of gyrB, rpoD and 16S rRNA genes. Int. J. Syst. Bacteriol. 48:813-819. [DOI] [PubMed] [Google Scholar]