Abstract
We successfully isolated a novel aerobic chemolithotrophic sulfur-oxidizing bacterium, designated strain SO07, from wastewater biofilms growing under microaerophilic conditions. For isolation, the use of elemental sulfur (S0), which is the most abundant sulfur pool in the wastewater biofilms, as the electron donor was an effective measure to establish an enrichment culture of strain SO07 and further isolation. 16S rRNA gene sequence analysis revealed that newly isolated strain SO07 was affiliated with members of the genus Halothiobacillus, but it was only distantly related to previously isolated species (89% identity). Strain SO07 oxidized elemental sulfur, thiosulfate, and sulfide to sulfate under oxic conditions. Strain SO07 could not grow on nitrate. Organic carbons, including acetate, propionate, and formate, could not serve as carbon and energy sources. Unlike other aerobic sulfur-oxidizing bacteria, this bacterium was sensitive to NaCl; growth in medium containing more than 150 mM was negligible. In situ hybridization combined with confocal laser scanning microscopy revealed that a number of rod-shaped cells hybridized with a probe specific for strain SO07 were mainly present in the oxic biofilm strata (ca. 0 to 100 μm) and that they often coexisted with sulfate-reducing bacteria in this zone. These results demonstrated that strain SO07 was one of the important sulfur-oxidizing populations involved in the sulfur cycle occurring in the wastewater biofilm and was primarily responsible for the oxidation of H2S and S0 to SO42− under oxic conditions.
The sulfur cycle, driven by sulfate reduction and sulfide oxidation, is an important cycle of minerals in various sulfur- and sulfide-rich environments (e.g., hot spring microbial mats, lake sediments, marine sediments, and wastewater biofilms), and it is closely linked with other cycles (e.g., carbon, oxygen, and nitrogen). For instance, up to 50% of organic matter can be mineralized by sulfate reduction in marine sediments (18). Successional development of sulfate reduction and subsequent sulfide oxidation were precisely determined within wastewater biofilms with a typical thickness of approximately 1,000 μm, which accounted for a substantial part of oxygen consumption and mineralization of organic matter (25, 33).
The sulfur cycle in wastewater biofilms is mainly driven by the close association of sulfate-reducing bacteria and sulfur-oxidizing bacteria, both of which comprise phylogenetically diverse groups of microorganisms adapted to wastewater conditions, e.g., neutral pH and low dissolved oxygen concentrations. The diversity and in situ ecophysiology of sulfate-reducing bacteria in the wastewater biofilms have been intensively investigated by a 16S rRNA gene approach and microelectrodes (16, 17, 32, 33, 35), while the diversity and ecological importance of sulfur-oxidizing bacteria involved in the sulfur cycle are still largely unknown. Recently, novel sulfur-oxidizing bacteria belonging to the γ and ɛ subclasses of the Proteobacteria have been discovered and isolated from various habitats, e.g., marine mud (7, 8), oil field waters (12, 23), and a hydrothermal vent system (36). Moreover, sulfur-oxidizing bacteria originally classified as Thiobacillus species were reclassified into the α, β, and γ subclasses of the Proteobacteria based on their phylogenetic relatedness and their physiological similarities (14, 19, 21, 22, 29). These reports clearly indicate that the phylogenetic and physiological diversity of sulfur-oxidizing bacteria is greater than has been expected.
Only a few members of the genus Thiobacillus, the genus Beggiatoa, and the genus Thiothrix have been described as typical sulfur-oxidizing bacterial species inhabiting wastewater environments in the last few decades (28, 30, 38). Detailed analysis of the sulfur-oxidizing bacterial community structure in wastewater biofilms via modern culture-independent approaches is more poorly documented than that of extreme environments (e.g., hydrothermal vents and hypersaline mats). In addition, the physiological characteristics of sulfur-oxidizing bacteria are very diverse and highly dependent on various environmental factors such as pH, temperature, NaCl concentration, utilization of organic compounds, and availability of sulfur compounds (i.e., S2−, S0, and S2O32−). However, either thiosulfate or sulfide is generally used as an electron donor to enrich and isolate sulfur-oxidizing bacteria from wastewater habitats (24, 28, 30), even though the most abundant sulfur pool is elemental sulfur (S0) in wastewater biofilms (17, 33). The ecophysiology and phylogenic diversity of predominant sulfur-oxidizing bacteria in wastewater environments as determined by culture-based approaches are largely unknown at present.
We preliminarily analyzed the sulfur-oxidizing bacterial community structure of wastewater biofilms by 16S rRNA gene cloning-based analysis and frequently obtained an unidentifiable clone sequence (referred to as SO07 in this paper) affiliated with a novel sulfur-oxidizing bacterial cluster in the γ subclass of the Proteobacteria (T. Ito, K. Sugita, H. Satoh, and S. Okabe, unpublished data). To further characterize the physiology and metabolic function of the sulfur-oxidizing bacterium represented by this novel clone sequence (designated strain SO07) in the wastewater biofilm, we attempted to isolate strain SO07 from the wastewater biofilm with elemental sulfur as an electron donor. During this isolation, molecular techniques (PCR and fluorescent in situ hybridization with specific primers and oligonucleotide probes) were used to monitor the success of isolation. In this paper, we report the isolation, partial characterization, and in situ detection of a novel aerobic chemolithoautotrophic sulfur-oxidizing bacterium inhabiting wastewater biofilms.
MATERIALS AND METHODS
Biofilm samples.
The wastewater biofilm sample used for isolation of the sulfur-oxidizing bacterium was obtained from a sewer line that transports the primary settling tank effluent at the Soseigawa municipal wastewater treatment plant, Sapporo, Japan. The average concentrations of dissolved organic carbon, dissolved oxygen, ammonia, nitrate, and sulfate in the primary settling tank effluent were 1,800, 180, 900, 36, and 290 μM, respectively.
Culture medium.
A slightly modified medium for neutrophilic Thiobacillus spp. (24), designated SOB medium in this study, was used for enrichment and isolation. SOB medium contained 2.0 g of NaHCO3, 1.0 g of KH2PO4, 1.0 g of K2HPO4, 1.0 g of NH4Cl, 0.1 g of CaCO3, and 0.2 g of MgSO4 liter−1 and 1 ml of trace element solution (24). Either elemental sulfur (41.6 to 416 mg liter−1) or thiosulfate (6.5 mM) was used as the sole electron donor. Since elemental sulfur is generally insoluble, the amount of elemental sulfur added to the medium is expressed as milligrams per liter in this paper. The medium was sterilized by autoclaving and then cooled. The pH was adjusted to 7.0. The plate SOB medium contained 3% Gelrite (Wako, Osaka, Japan).
Isolation.
Biofilm samples were homogenized and subjected to enrichment and isolation. Enrichment cultivation was carried out with 100-ml serum vials containing 50 ml of SOB medium containing elemental sulfur (41.6 to 416 mg liter−1) at 30°C in the dark and under oxic conditions with shaking at 60 rpm. Subsamples of these enrichment cultures were transferred into fresh SOB medium (1% [vol/vol]) and incubated for a few days. This cultivation was repeated at least three times. Cell growth in the cultures was monitored by epifluorescence microscopy after they were stained with 4′,6-diamidino-2-phenylindole (DAPI) and filtered onto a black 0.2-μm-pore-size 25-mm-diameter polycarbonate filter (15).
The purity of strain SO07 in the cultures was checked by in situ hybridization with fluorescently labeled probes specific for SO07 and most Bacteria (EUB338) (3) (see below). After several consecutive passages, the enrichment culture was streaked on plate SOB medium containing 6.5 mM thiosulfate. These plates were incubated at 30°C in the dark under oxic conditions. The colonies formed on the plates were picked and further purified by restreaking on plate SOB medium several times. A small number of colonies were subjected to PCR amplification with the SO07-specific primer for screening strain SO07 as described below. In addition, several SO07-positive colonies were picked up with sterilized needles, and fluorescent in situ hybridization was performed with the SO07 and EUB338 probes to check the identity and purity of each colony.
DNA extraction from colonies and PCR amplification for screening.
The method of Hiraishi (13) was slightly modified and used to directly extract DNA from colonies and PCR amplification for screening colonies of strain SO07. The approximately 300-bp-long 16S rRNA gene was amplified by PCR with the SO07-specific primer as the reverse primer, the same sequence as that of the fluorescent in situ hybridization probe (see below), combined with bacterial primer 341f (31) as the forward primer. The PCR products were evaluated on a 1% (wt/vol) agarose gel. For further confirmation, the amplified DNA products were purified with the PCR Preps DNA purification system (Promega) and sequenced as described below. The partial 16S rRNA gene sequence (about 300 bp) was compared with that of clone SO07 obtained from the wastewater biofilms in a previous study (T. Ito, K. Sugita, H. Satoh, and S. Okabe, unpublished data).
DNA extraction, 16S rRNA gene sequencing, and phylogenetic analysis of a pure culture.
We subjected 1 ml of pure culture (approximately 108 cells ml−1) to DNA extraction. Genomic DNA was extracted with the Fast DNA spin kit (Bio 101). The nearly full-length 16S rRNA gene was amplified by PCR with the bacterial universal primer set 27f and 1492r (26). The PCR product was purified with the Wizard PCR Preps DNA purification system (Promega). The purified PCR product was sequenced with the Taq dideoxy terminator cycle sequencing kit (Applied Biosystems) and a model 310 genetic analyzer (Applied Biosystems).
The sequences obtained were compared with those available in public databases (GenBank and DDBJ) with the BLAST system (1). Phylogenetic inferences were also made with the 16S rRNA gene database associated with the ARB software package (http://www.arb-home.de). Clone sequences that were not included in the ARB database were added from GenBank (6) with a parsimony insertion tool. The ARB_align tool was used for sequence alignment. Phylogenetic trees were then constructed with application of the ARB neighbor-joining algorithms. Partial sequences were inserted into the reconstructed tree by applying the parsimony criteria without allowing for changes in the overall tree topology. Bootstrap analysis was performed to establish a confidence level for any node. Similarity values of the 16S rRNA gene sequences were calculated by the Similarity_Matrix program in the Ribosomal Database Project II (10).
Probe design and in situ hybridization.
The 16S rRNA-targeted oligonucleotide probe specific for strain SO07, S-*-SO07-0655-a-A-19 (5′-CCCCCATCCTCTTGCACAC-3′) was designed with the ARB program in this study. In addition to the SO07 probe, the following oligonucleotide probes were used: (i) EUB338 probe for the domain Bacteria (3), (ii) GAM42a probe for γ-Proteobacteria (27), and (iii) SRB385 and SRB385Db probe mixture for most sulfate-reducing bacteria of the δ-Proteobacteria (4, 34). The oligonucleotide probes were 5′ labeled with either tetramethylrhodamine-5-isothiocyanate (TRITC), fluorescein isothiocyanate (FITC), or hydrophilic sulfoindocyanine fluorescent dye (Cy5) (TaKaRa, Shiga, Japan). The stringency of hybridization was adjusted by adding formamide to the hybridization buffer (40% for S-*-SO07-0655-a-A-19). For determination of the specificity of S-*-SO07-0655-a-A-19, the following reference organisms were used: Halothiobacillus neapolitanus (DSM 15147), Acidithiobacillus thiooxidans (DSM 14887), and strain SO07. Whole-cell hybridization for Halothiobacillus neapolitanus and Acidithiobacillus thiooxidans gave no fluorescent signals with probe S-*-SO07-0655-a-A-19 at a formamide concentration of 40%, though they were positive with probe GAM42a.
Cells in the enrichment and pure cultures were fixed in 4% paraformaldehyde solution, resuspended in a 1:1 mixture of phosphate-buffered saline and ethanol, and stored at −20°C (2). Intact biofilm samples were fixed in 4% paraformaldehyde solution, rinsed twice with phosphate-buffered saline, embedded in Tissue-Tek OCT compound (Sakura Finetechnical), and frozen at −20°C. Vertical thin sections (20-μm thick) of the fixed biofilm were prepared by use of a cryostat (Reicher-Jung Cryocut 1800; Leica). For in situ hybridization, dispersed cells and biofilm thin sections were immobilized on glass slides coated with gelatin (2). Hybridization was carried out as described previously (2, 33). Samples hybridized with probes were mounted with the Slow Fade Light antifading kit (Molecular Probes).
Fluorescent and phase-contrast images were recorded with an LSM 510 confocal laser scanning microscope (Zeiss) equipped with an argon laser (488 nm) and two HeNe lasers (543 nm and 633 nm, respectively). Image combining, processing, and analysis were performed with the standard software package provided by Zeiss.
Physiological tests.
Cells grown in SOB medium with thiosulfate (6.5 mM) were used for physiological tests unless otherwise specified. All of the following tests were performed at least in duplicate. Effects of temperature, pH, and salinity (NaCl concentration) on bacterial growth were determined in SOB medium containing thiosulfate (6.5 mM) as the electron donor under oxic conditions. Bacterial growth was determined by monitoring optical density at 540 nm. Aerobic growth was examined in SOB medium supplemented with either sulfide (13 mM), thiosulfate (6.5 mM), elemental sulfur (416 mg liter−1), methanol (1 mM), acetate (1 mM), formate (1 mM), or propionate (1 mM) as the electron donor. Anaerobic growth was examined in SOB medium containing the same electron donors as the aerobic growth test, with nitrate (0.74 mM) as an electron acceptor. In the anaerobic growth test, the headspace gas consisted of N2 (99.99% [vol/vol]).
Fermentative growth was examined in SOB medium containing the same electron donors as the aerobic growth test under anoxic conditions without nitrate. Substrate utilization was determined by measuring each substrate concentration and by DAPI direct counting as described by Hobbie et al. (15). The concentrations of sulfate, thiosulfate, nitrate, nitrite, acetate, propionate, and formate in the culture solutions were determined with an ion chromatograph equipped with an IonPac AS9-HCC column for sulfate, thiosulfate, nitrate and nitrite and with an ICE-AS1 column for acetate, propionate, and formate (model DX-100; Nippon Dionex, Osaka, Japan). The concentration of elemental sulfur was not determined in this study. The concentration of total dissolved sulfide (H2S, HS−, and S2−) was determined by the methylene blue method (9).
Nucleotide sequence accession number.
The 16S rRNA gene sequence of strain SO07 has been deposited in the EMBL, GenBank, and DDBJ nucleotide sequence databases under accession no. AB118236.
RESULTS
Enrichment and isolation of strain SO07.
For enrichment of strain SO07, biofilm samples were gently washed, homogenized, and incubated under oxic conditions in liquid SOB medium containing 41.6 to 416 mg of elemental sulfur liter−1 as the sole electron donor. Sulfate production was observed after a 12-h incubation. The culture was successively inoculated into fresh medium (1% [vol/vol]) every 3 days. After three successive transfers, the culture produced sulfate constantly, and concomitant cell growth was confirmed. Interestingly, when thiosulfate was used as the electron donor, enrichment of the SO07 population often became unstable during consecutive transfers. The use of elemental sulfur as an electron donor was effective for recovering the SO07 population.
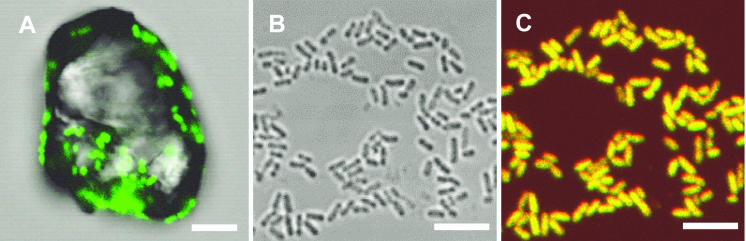
At this point, we examined the purity of the enrichment cultures by 16S rRNA-based molecular approaches (i.e., PCR amplification and fluorescent in situ hybridization). PCR amplification with the primer set specific for SO07 clearly showed the presence of strain SO07 in the cultures. The partial sequence (about 300 bp) of the PCR-amplified 16S rRNA gene fragment was identical to the sequence of clone SO07 obtained previously (100% sequence similarity). Furthermore, fluorescent in situ hybridization showed that the cells that hybridized with the SO07-specific probe accounted for more than 90% of the total bacterial cells that hybridized with EUB338. Attached growth of the SO07 probe-hybridized cells on elemental sulfur particles was frequently found (Fig. 1A).
FIG. 1.
(A) Fluorescent micrograph of chemolithoautotrophic sulfur-oxidizing bacterial strain SO07 attached to sulfur granules, which were hybridized with the FITC-labeled SO07 probe (green). (B and C) Photomicrographs of a pure culture of strain SO07 grown on thiosulfate. A phase-contrast micrograph (B) and a double-stained fluorescent micrograph (C) with the TRITC-labeled SO07 probe (red) and FITC-labeled EUB338 probe (green) of the same field. Bars, 5 μm.
The results of these molecular analyses indicated that the SO07 probe-hybridized cells dominated the enrichment cultures. Thus, we attempted to isolate SO07 on plate SOB medium containing 13 mM thiosulfate under oxic conditions. Once the enrichment culture of strain SO07 was obtained, thiosulfate could be used as an electron donor instead of elemental sulfur. Small colonies that were white, lens shaped, and 0.5 to 1.0 mm in diameter formed after a 24-h incubation. These microcolonies were randomly picked and further purified by restreaking on plates several times. The identity of the purified strain was checked by fluorescent in situ hybridization with the SO07-specific probe and sequence analysis of the 16S rRNA gene amplified with the bacterial universal primer set 27f and 1492r. In situ hybridization revealed that the purified strain hybridized with the SO07-specific probe and that the culture was highly pure (Fig. 1B and C). Furthermore, the nearly full-length 16S rRNA gene sequence of the purified strain was completely identical to that of clone SO07 detected in the previous enrichment culture (100% sequence similarity). These results indicated that a novel sulfur-oxidizing bacterium, strain SO07, was successfully isolated in pure culture.
Phylogenetic analysis of strain SO07.
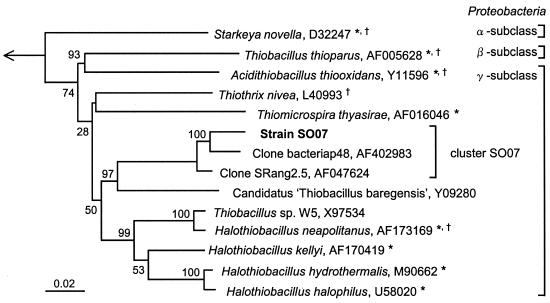
To precisely determine the phylogenetic position of strain SO07, we compared the 16S rRNA gene sequence with those of cultured and uncultured microorganisms belonging to the cluster originally classified as Thiobacillus and other sulfur-oxidizing bacteria. The phylogenetic affiliation of strain SO07 is shown in Fig. 2. Comparison of the nearly complete 16S rRNA gene sequence revealed that strain SO07 belonged to the γ subclass of the Proteobacteria, with less than 89% similarity to previously isolated bacteria in the genus Halothiobacillus (22) such as Halothiobacillus neapolitanus (type species), Halothiobacillus kellyi (36), Halothiobacillus hydrothermalis (11), and Halothiobacillus halophilus (39) (sequence similarity, 87, 89, 89, and 88%, respectively). The SO07 sequence was closely related to environmental clones from various sulfurous environments, i.e., clone bacteriap48 (37), clone SRang2.5 (5), and Candidatus “Thiobacillus baregensis” (accession number Y09280) with sequence similarities of 97, 98, and 90%, respectively (Fig. 2). These clones formed a unique clade which contained no cultured representatives. The 16S rRNA gene sequence analysis of strain SO07 in this study strongly indicated that the cluster including these environmental clones (designated cluster SO07) comprised chemolithotrophic sulfur-oxidizing bacteria.
FIG. 2.
Phylogenetic tree of strain SO07, originally classified among Thiobacillus species and other sulfur-oxidizing bacteria in the class Proteobacteria, based on a distance matrix analysis of 16S rRNA gene sequences (neighbor-joining tree). Aquifex pyrophilus in the class Aquificae was used as the out group. The accession numbers of the sequences retrieved from the database are given following the species names. The scale bar represents the number of nucleotide changes per sequence position. The numbers at each node show the bootstrap values (percent) obtained with 1,000 resamplings. *, species originally classified in the genus Thiobacillus. †, type species.
Morphological and physiological characteristics of strain SO07.
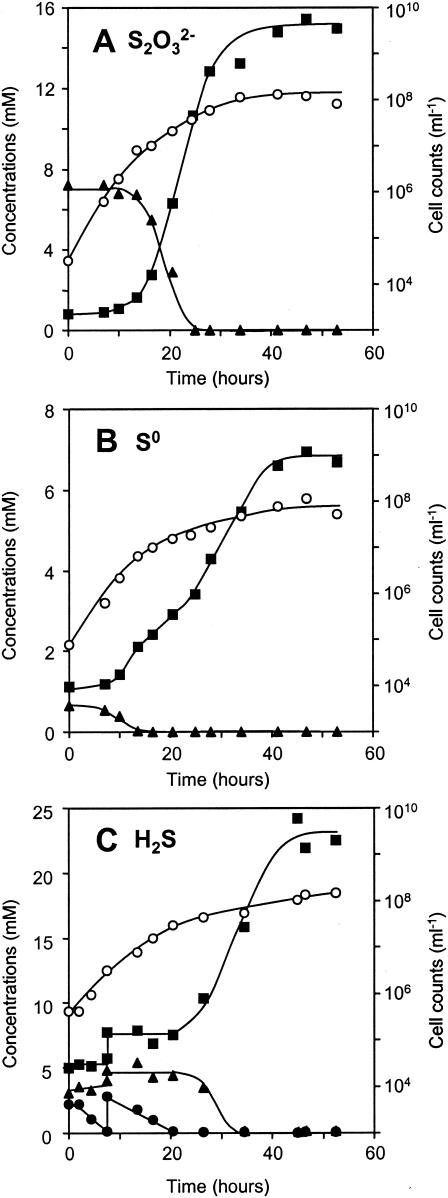
The cells of strain SO07 were rod shaped, 1.0 to 2.0 μm long, and 0.5 to 0.8 μm wide. Gram staining was negative. They occurred mostly in pairs and some singles (Fig. 1B and C). Typical growth patterns of strain SO07 in SOB medium with either thiosulfate, elemental sulfur, or sulfide as the sole electron donor under oxic conditions are shown in Fig. 3. Sterile controls indicated no significant chemical oxidation of thiosulfate, elemental sulfur, and sulfide during the incubation. When grown on thiosulfate, strain SO07 produced sulfate with a molar ratio of thiosulfate consumed to sulfate produced of 1:2, which is in agreement with the stoichiometric equation S2O32− + 2 O2 + H2O → 2 SO42− + 2 H+ (Fig. 3A). When grown on elemental sulfur, strain SO07 oxidized elemental sulfur to sulfate after complete utilization of thiosulfate, which was chemically oxidized (Fig. 3B). In cultures containing elemental sulfur, some of the cells attached and grew on the surface of elemental sulfur particles. We may have underestimated the cell number to some extent because only suspended cells were measured in this culture. It is speculated that strain SO07 grown on sulfide oxidized sulfide to sulfate via elemental sulfur (HS− + 0.5 O2 → S0 + H2O and S0 + 1.5 O2 + H2O → SO42− + 2H+), which accumulated during the initial 20-h incubation (Fig. 3C). Strain SO07 subsequently oxidized thiosulfate, and elemental sulfur accumulated during the initial 20 h to sulfate after the added sulfide was completely depleted. However, since we did not measure elemental sulfur concentrations, an exact stoichiometric evaluation cannot be made.
FIG. 3.
Changes in concentrations of sulfate, thiosulfate, and sulfide and in cell numbers during chemolithoautotrophic growth of strain SO07 in SOB medium supplemented with bicarbonate at 30°C under oxic conditions. The medium contained 6.5 mM thiosulfate (A), 416 mg of sulfur liter−1 (B), or 13 mM sulfide (C) as the electron donor. ○, cell number; ▴, thiosulfate; ▪, sulfate; •, dissolved sulfide. Cell numbers were determined by DAPI direct counting (15). The experiments were repeated twice and gave similar results.
Strain SO07 grew chemolithoautotrophically on sulfide, thiosulfate, and elemental sulfur, reducing the pH to a minimum of 6.0. The growth of strain SO07 ceased at cell counts of about 108 cells ml−1. This strain did not show a typical exponential growth pattern, probably due to its two-step oxidation of sulfide, i.e., oxidation of sulfide to sulfate via elemental sulfur. The maximum specific growth rates on thiosulfate, elemental sulfur, and sulfide were found to be 0.41, 0.08, and 0.30 h−1, respectively.
As for the physiological characteristics of strain SO07, heterotrophic growth was not observed when strain SO07 was tested on acetate, formate, propionate, and methanol. Anoxic growth did not occur in the presence of any organic or inorganic substrates when nitrate was used as an electron acceptor. Fermentative growth was negative. Growth of strain SO07 occurred over a temperature range of 15 to 42°C, with the optimum growth temperature being about 30 to 34°C, and within a pH range from 6.0 to 9.0, with an optimal pH of 7.0 to 8.0. Growth in medium containing more than 150 mM NaCl was negligible. These data clearly illustrate that strain SO07 is a chemolithoautotrophic sulfur-oxidizing bacterium.
Spatial distribution of strain SO07 in wastewater biofilms.
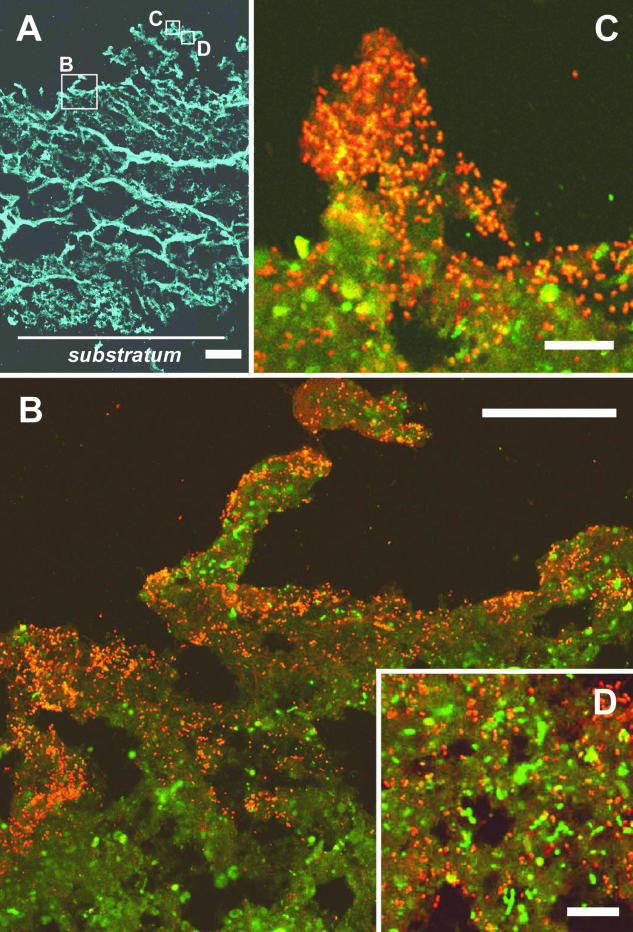
To elucidate the spatial distribution of the newly isolated strain SO07 in the wastewater biofilm, in situ hybridization with the TRITC-labeled SO07 probe, Cy5-labeled EUB338 probe, and FITC-labeled probe mixture of SRB385 and SRB385Db combined with confocal laser scanning microscopy was applied to vertical thin sections of the wastewater biofilm. Figure 4 shows composite cross-section images of the wastewater biofilm, showing a significant heterogeneous structure consisting of discrete biomass and interstitial voids. The fluorescent signal derived from the Bacteria-specific EUB338 probe-stained cells was detected throughout the biofilm (thickness, approximately 1,400 μm) (Fig. 4A). In situ hybridization showed that probe SO07-hybridized rod-shaped cells, morphologically similar to those of the pure culture of strain SO07 (Fig. 1B), were mainly detected in the oxic surface biofilm, while they were hardly detected in the deeper biofilm stratum (below ca. 100 μm) (Fig. 4B and C). Both probe SO07-hybridized cells and probe SRB385- and SRB385Db-hybridized sulfate-reducing bacterium-like cells were present in the surface oxic biofilm stratum (Fig. 4D).
FIG. 4.
In situ hybridization of vertical sections (20 μm thick) of a wastewater biofilm viewed by confocal laser scanning microscopy. (A) Fluorescent micrograph with the Cy5-labeled EUB338 probe (blue). (B, C, and D) Close-up views of the microscopic fields enclosed by boxes in panel A. Images were taken after in situ hybridization with the TRITC-labeled SO07 probe (red) combined with the FITC-labeled SRB385 and SRB385Db probes (green). Weak yellow signals show autofluorescence. The biofilm surface is at the top of the panels. Bars: A, 200 μm; B, 50 μm; C, 10 μm; D, 10 μm.
DISCUSSION
Isolation of strain SO07.
The combination of conventional isolation methods with genetic screening (i.e., PCR with the specific primer and fluorescent in situ hybridization with the specific probe) was the key to success in isolating strain SO07. Strain SO07 was able to grow in both liquid and plate SOB medium. One of the difficulties in establishing the enrichment culture of SO07 was that the population became unstable in liquid medium with thiosulfate as the electron donor, in which SO07 was outcompeted by other sulfur-oxidizing bacteria. The use of elemental sulfur instead of thiosulfate was effective in recovering the SO07 population and making it dominant, probably because strain SO07 grows faster on elemental sulfur than other sulfur-oxidizing bacteria, though the specific growth rate of strain SO07 on thiosulfate was lower than those of sulfur-oxidizing bacteria (11, 22). In general, thiosulfate has been used as the electron donor for isolation of sulfur-oxidizing bacteria because it is more readily soluble in water than elemental sulfur. This might be the main reason why no cultured representatives have been obtained in the cluster SO07 until now, even though many environmental clones closely related to strain SO07, e.g., clone SRang2.5 (5) and clone bacteriap48 (37), have recently been retrieved from sulfurous environments.
Comparison of strain SO07 with other known sulfur-oxidizing bacteria.
The physiological characteristics of strain SO07 were compared with the characteristics of other sulfur-oxidizing bacteria belonging to the α-, β-, and γ-Proteobacteria: Starkeya novella (21) in the α-Proteobacteria; Thiobacillus thioparus (20) in the β-Proteobacteria; and Halothiobacillus neapolitanus (22), Halothiobacillus hydrothermalis (11), Halothiobacillus halophilus (22), and Acidithiobacillus thiooxidans (22) in the γ-Proteobacteria. It should be noted that all these bacteria had originally been classified as Thiobacillus but were recently reclassified by Kelly et al. (21) and Kelly and Wood (22).
Strain SO07 differed from the other species in its inability to grow at low pH (no growth below pH 6.0). Furthermore, strain SO07 showed distinctive features compared with Starkeya novella in its inability to grow on some organic substrates and ability of grow on sulfur (21). Strain SO07 is the first isolate of clone cluster SO07 to be obtained. Judging from the 16S rRNA gene sequence analysis shown in Fig. 2, strain SO07 seems to be par of a large group, including members of the genus Halothiobacillus. The 16S rRNA gene sequences of strain SO07 and previously identified Halothiobacillus species were, however, 89% similar at most. The most notable difference between the genus Halothiobacillus and strain SO07 is that strain SO07 cannot grow under high NaCl concentrations (no growth above 150 mM NaCl), while the genus Halothiobacillus requires 400 to 1,000 mM NaCl and can tolerate up to 4,000 mM NaCl (22, 36). Therefore, it seems inappropriate to class strain SO07 as a species of Halothiobacillus. We propose that strain SO07 should be classified as a novel genus.
Ecophysiology of strain SO07 in wastewater biofilms.
The in situ spatial organization of newly isolated strain SO07 within the biofilm was successfully visualized by fluorescent in situ hybridization (Fig. 4). We found that strain SO07 was mainly present in high abundance in the oxic biofilm strata (approximately 0 to 100 μm deep). This spatial distribution of obligate aerobic strain SO07 in the biofilm corresponded well with the oxygen microprofiles determined with microelectrodes and the vertical concentration profiles of elemental sulfur determined in our previous studies (17, 33). These studies demonstrated that oxygen could penetrate only about 100 μm from the surface of the biofilm and coexist with sulfide in this region (33). In addition, elemental sulfur was the most abundant sulfur pool at a depth of 0 to 400 μm from the surface, and the production of elemental sulfur was certainly due to biological reoxidation of sulfide produced by sulfate-reducing bacteria below the oxic-anoxic interface. In this study, close coexistence of strain SO07 and sulfate-reducing bacteria was also found in the surface biofilm (Fig. 4D), which might suggest efficient sequential utilization of sulfide produced by sulfate-reducing bacteria.
In summary, we successfully isolated and partially characterized a novel chemolithoautotrophic sulfur-oxidizing bacterium, strain SO07, that inhabits wastewater biofilms and that grows under neutral pH, mesophilic, and microaerophilic conditions. We were also able to visualize the in situ spatial distribution of strain SO07 in the wastewater biofilm with a specific oligonucleotide probe. These results indicated that strain SO07 was one of the significant populations involved in the internal sulfur cycle occurring in the wastewater biofilm and might be responsible for the oxidation of sulfide and/or elemental sulfur to sulfate under oxic conditions. More details about the growth and physiological properties of strain SO07 are needed to better understand its in situ ecophysiology in complex wastewater biofilm communities.
Acknowledgments
This work was partially supported by a grant-in-aid (no. 13650593) for developmental scientific research from the Ministry of Education, Science and Culture of Japan. This study was also carried out as a part of the Project for Development of Technologies for Analyzing and Controlling the Mechanism of Biodegrading and Processing, which was entrusted to us by the New Energy and Industrial Technology Development Organization (NEDO).
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amann, R. I. 1995. In situ identification of micro-organisms by whole-cell hybridization with rRNA-targeted nucleic acid probes, p. 1-15. In A. D. L. Akkerman, J. D. van Elsas, and F. J. de Bruijn (ed.), Molecular microbial ecology manual. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 3.Amann, R. I., L. Krumholz, and D. A. Stahl. 1990. Fluorescent-oligonucleotide probing of whole cells for determinative, phylogenetic, and environmental studies in microbiology. J. Bacteriol. 172:762-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amann, R. I., J. Stromley, R. Devereux, R. Key, and D. A. Stahl. 1992. Molecular and microscopic identification of sulfate-reducing bacteria in multispecies biofilms. Appl. Environ. Microbiol. 58:614-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angert, E. R., D. E. Northup, A.-L. Reysenbach, A. S. Peek, B. M. Goebel, and N. R. Pace. 1998. Molecular phylogenetic analysis of a bacterial community in Sulphur River, Parker Cave, Kentucky. Am. Mineral. 83:1583-1592. [Google Scholar]
- 6.Benson, D. A., I. Karsch-Mizrachi, D. J. Lipman, J. Ostell, and D. L. Wheeler. 2003. GenBank. Nucleic Acids Res. 31:23-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brinkhoff, T., G. Muyzer, C. O. Wirsen, and J. Kuever. 1999. Thiomicrospira kuenenii sp. nov. and Thiomicrospira frisia sp. nov., two mesophilic obligately chemolithoautotrophic sulfur-oxidizing bacteria isolated from an intertidal mud flat. Int. J. Syst. Bacteriol. 49:385-392. [DOI] [PubMed] [Google Scholar]
- 8.Brinkhoff, T., G. Muyzer, C. O. Wirsen, and J. Kuever. 1999. Thiomicrospira chilensis sp. nov., a mesophilic obligately chemolithoautotrophic sulfur-oxidizing bacterium isolated from a Thioploca mat. Int. J. Syst. Bacteriol. 49:875-879. [DOI] [PubMed] [Google Scholar]
- 9.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 10.Cole, J. R., B. Chai, T. L. Marsh, R. J. Farris, Q. Wang, S. A. Kulam, S. Chandra, D. M. McGarrell, T. M. Schmidt, G. M. Garrity, and J. M. Tiedje. 2003. The Ribosomal Database Project (RDP-II): previewing a new autoaligner that allows regular updates and the new prokaryotic taxonomy. Nucleic Acids Res. 31:442-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durand, P., A. L. Reysenbach, D. Prieur, and N. Pace. 1993. Isolation and characterization of Thiobacillus hydrothermalis sp. nov., a mesophilic obligately chemolithotrophic bacterium isolated from a deep-sea hydrothermal vent in Fiji Basin. Arch. Microbiol. 159:39-44. [Google Scholar]
- 12.Gevertz, D., A. J. Telang, G. Voordouw, and G. E. Jenneman. 2000. Isolation and characterization of strains CVO and FWKO B, two novel nitrate-reducing, sulfur-oxidizing bacteria isolated from oil field brine. Appl. Environ. Microbiol. 66:2491-2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15:210-213. [DOI] [PubMed] [Google Scholar]
- 14.Hiraishi, A., K. V. P. Nagashima, K. Matsuura, K. Shimada, S. Takaichi, N. Wakao, and Y. Katayama. 1998. Phylogeny and photosynthetic features of Thiobacillus acidophilus and related acidophilic bacteria: its transfer to the genus Acidiphilum as Acidiphilum acidophilum comb. nov. Int. J. Syst. Bacteriol. 48:1389-1398. [DOI] [PubMed] [Google Scholar]
- 15.Hobbie, J. E., R. J. Daley, and S. Jasper. 1977. Use of Nuclepore filters for counting bacteria by fluorescence microscopy. Appl. Environ. Microbiol. 33:1225-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito, T., J. L. Nielsen, S. Okabe, Y. Watanabe, and P. H. Nielsen. 2002. Phylogenetic identification and substrate uptake patterns of sulfate-reducing bacteria inhabiting an oxic-anoxic sewer biofilm determined by combining microautoradiography and fluorescent in situ hybridization. Appl. Environ. Microbiol. 68:356-364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito, T., S. Okabe, H. Satoh, and Y. Watanabe. 2002. Successional development of sulfate-reducing bacterial populations and their activities in a wastewater biofilm growing under microaerophilic conditions. Appl. Environ. Microbiol. 68:1392-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jørgensen, B. B. 1982. Mineralization of organic matter in the sea bed—the role of sulphate reduction. Nature 296:643-645. [Google Scholar]
- 19.Katayama, Y., A. Hiraishi, and H. Kuraishi. 1995. Paracoccus thiocyanatus sp. nov., a new species of thiocyanate-utilizing facultative chemolithotroph, and transfer of Thiobacillus versutus to the genus Paracoccus as Paracoccus versutus comb. nov. with emendation of the genus. Microbiology 141:1469-1477. [DOI] [PubMed] [Google Scholar]
- 20.Kelly, D. P., and A. H. Harrison. 1989. Genus Thiobacillus, p. 1842-1858. In J. T. Staley, M. P. Bryant, N. Pfennig, and J. G. Holt (ed.), Bergey's manual of systematic bacteriology, 1st ed., vol. 3. Williams & Wilkins, Baltimore, Md.
- 21.Kelly, D. P., I. R. McDnald, and A. P. Wood. 2000. Proposal for the reclassification of Thiobacillus novellus as Starkeya novella gen. nov., comb. nov., in the alpha-subclass of the Proteobacteria. Int. J. Syst. Evol. Microbiol. 50:1797-1802. [DOI] [PubMed] [Google Scholar]
- 22.Kelly, D. P., and A. P. Wood. 2000. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov. and Thermithiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 50:511-516. [DOI] [PubMed] [Google Scholar]
- 23.Kodama, Y., and K. Watanabe. 2003. Isolation and characterization of a sulfur-oxidizing chemolithotroph growing on crude oil under anaerobic conditions. Appl. Environ. Microbiol. 69:107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuenen, J. G., L. A. Robertson, and O. H. Tuovinen. 1991. The genera Thiobacillus, Thiomicrospira, and Thiosphaera, p. 2638-2657. In A. Balows, H. G. Truper, M. Dworkin, W. Harder, and K.-H. Schleifer (ed.), The prokaryotes, 2nd ed., vol. 3. Springer-Verlag, New York, N.Y.
- 25.Kühl, M., and B. B. Jørgensen. 1992. Microsensor measurement of sulfate reduction and sulfide oxidation in compact microbial communities of aerobic biofilms. Appl. Environ. Microbiol. 58:1164-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lane, D. J. 1991. 16S/23S rRNA sequencing, p. 115-175. In E. Stackebrandt and M. Goodfellow (ed.), Nucleic acid techniques in bacterial systematics. John Wiley & Sons, Chichester, England.
- 27.Manz, W., R. Amann, W. Ludwig, M. Wagner, and K.-H. Schleifer. 1992. Phylogenetic oligonucleotide probes for the major subclass of proteobacteria: problems and solutions. Syst. Appl. Microbiol. 15:593-600. [Google Scholar]
- 28.Milde, K., W. Sand, W. Wolff, and E. Bock. 1983. Thiobacilli of the corroded concrete walls of the Hamburg sewer system. J. Gen. Microbiol. 129:1327-1333. [Google Scholar]
- 29.Moreira, D., and R, Amils. 1997. Phylogeny of Thiobacillus cuprinus and other mixotrophic Thiobacilli: proposal for Thiomonas gen. nov. Int. J. Syst. Bacteriol. 47:522-528. [DOI] [PubMed] [Google Scholar]
- 30.Mori. T., M. Koga, Y. Hikosaka, T. Nonaka, F. Mishima, Y. Sakai, and J. Koizumi. 1991. Microbial corrosion of concrete sewer pipes, H2S production from sediments and determination of corrosion rate. Water Sci. Technol. 23:1275-1282. [Google Scholar]
- 31.Muyzer, G., E. C. de Waal, and A. G. Uitterlinden. 1993. Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl. Environ. Microbiol. 59:695-700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okabe, S., T. Ito, and H. Satoh. 2003. Sulfate-reducing bacterial community structure and their contribution to carbon mineralization in a wastewater biofilm growing under microaerophilic conditions. Appl. Microbiol. Biotechnol. 63:322-334. [DOI] [PubMed]
- 33.Okabe, S., T. Itoh, H. Satoh, and Y. Watanabe. 1999. Analyses of spatial distributions of sulfate-reducing bacteria and their activity in aerobic wastewater biofilms. Appl. Environ. Microbiol. 65:5107-5116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rabus, R., M. Fukui, H. Wilkers, and F. Widdel. 1996. Degradative capacities and 16S rRNA-targeted whole-cell hybridization of sulfate-reducing bacteria in an aerobic enrichment culture utilizing alkylbenzenes from crude oil. Appl. Environ. Microbiol. 62:3605-3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santegoeds, C. M., T. G. Ferdelman, G. Muyzer, and D. de Beer. 1998. Structure and functional dynamics of sulfate-reducing populations in bacterial biofilms. Appl. Environ. Microbiol. 64:3731-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sievert, S. M., T. Heidorn, and J. Kuever. 2000. Halothiobacillus kellyi sp. nov., a mesophilic, obligately chemolithoautotrophic, sulfur-oxidizing bacterium isolated from a shallow-water hydrothermal vent in the Aegean sea, and emended description of the genus Halothiobacillus. Int. J. Syst. Evol. Microbiol. 50:1229-1237. [DOI] [PubMed] [Google Scholar]
- 37.Sunna, A., and P. L. Bergquist. 2003. A gene encoding a novel extremely thermostable 1,4-β-xylanase isolated directly from an environmental DNA sample. Extremophiles 7:63-70. [DOI] [PubMed] [Google Scholar]
- 38.Williams, T. M., and R. F. Unz. 1985. Filamentous sulfur bacteria of activated sludge: characterization of Thiothrix, Beggiatoa, and Eikelboom type 021N strains. Appl. Environ. Microbiol. 49:887-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wood, A. P., and D. P. Kelly. 1991. Isolation and characterisation of Thiobacillus halophilus sp. nov., a sulphur-oxidizing autotrophic eubacterium from a Western Australian hypersline lake. Arch. Microbiol. 156:277-280. [Google Scholar]