Abstract
Although the toxic effect of heavy metals on soil microorganism activity is well known, little is known about the effects on different organism groups. The influence of heavy metal addition on total, bacterial, and fungal activities was therefore studied for up to 60 days in a laboratory experiment using forest soil contaminated with different concentrations of Zn or Cu. The effects of the metals differed between the different activity measurements. During the first week after metal addition, the total activity (respiration rate) decreased by 30% at the highest level of contamination and then remained stable during the 60 days of incubation. The bacterial activity (thymidine incorporation rate) decreased during the first days with the level of metal contamination, resulting in a 90% decrease at the highest level of contamination. Bacterial activity then slowly recovered to values similar to those of the control soil. The recovery was faster when soil pH, which had decreased due to metal addition, was restored to control values by liming. Fungal activity (acetate-in-ergosterol incorporation rate) initially increased with the level of metal contamination, being up to 3 and 7 times higher than that in the control samples during the first week at the highest levels of Zn and Cu addition, respectively. The positive effect of metal addition on fungal activity then decreased, but fungal activity was still higher in contaminated than in control soil after 35 days. This is the first direct evidence that fungal and bacterial activities in soil are differently affected by heavy metals. The different responses of bacteria and fungi to heavy metals were reflected in an increase in the relative fungal/bacterial ratio (estimated using phospholipid fatty acid analysis) with increased metal load.
Toxic effects of heavy metals on soil microorganisms have been extensively studied in the past (3, 19, 40), and almost every group of organisms has been studied in this respect. Fungi and bacteria constitute the main components of the soil microbial biomass. It has often been stated that fungi are more tolerant of heavy metals as a group than bacteria (15, 21). This was initially inferred by comparison of metal tolerance of pure culture isolates of soil microorganisms (9, 22). Similar conclusions have been made using phospholipid fatty acid (PLFA) analysis to differentiate between the fungal and bacterial components of the soil microbial biomass (18, 23). In addition, different biomass measurements or plate counting techniques have also indicated that heavy metals affect bacteria and fungi in soil differently (21, 24, 26, 30). However, a reduction in the fungal marker PLFA (18:2ω6,9) in coniferous forest soils near metal smelters was reported by Pennanen et al. (35). The authors concluded that this was probably not due to direct effects of metals on fungi. High loads of metals disturbed tree growth, and this induced effects in the mycorrhiza fungi. Thus, to compare heavy metal effects on the saprotrophic part of the fungal and bacterial communities, experiments should be performed without the involvement of plants. Furthermore, measurements of activity would be the most direct way of comparing these two groups of microorganisms, since this is a more sensitive measure than biomass measurements.
Bacterial activity and growth rate have usually been determined in situ by the thymidine or leucine incorporation technique. Both methods were initially developed for use in aquatic environments, but are nowadays frequently used in terrestrial habitats (e.g., see references 4, 11, 20, 27, and 39). In a similar way fungal activity and growth rate in aquatic habitats have been estimated by the acetate-in-ergosterol incorporation technique (31, 32). However, this method has only recently been applied to the soil environment (6, 34).
In the present study, the effects of Cu and Zn on bacterial and fungal activity, as well as on total microbial activity (respiration), were compared in a laboratory experiment using artificially contaminated soil. First, the short-term effect was monitored for a week. Our hypothesis was that fungal activity would be less negatively affected by heavy metals than bacterial activity, while total activity would be between these two measurements. Second, the long-term effect of metal additions was monitored over a 60-day period. The objective was to compare the rates of recovery of the different activities over time. The effect of metals on microbial activity was also compared with changes in microbial biomass and community structure.
MATERIALS AND METHODS
Soil sampling and experimental design.
Soil was collected from a spruce forest in southern Sweden. The organic humus horizon (A00/A01) was taken in November 2000, sieved (2-mm mesh size), stored overnight at 4°C, and then used in the experiment. The organic matter content was 79%, the pH (H2O) was 4.0, and the water content was adjusted to 270% water of the dry weight of the soil (approximately 60% water-holding capacity). In a pilot experiment, soil from the same forest, stored for 6 months at 4°C, was used.
The soil was distributed into 16 jars (70 g dry weight in each). The soil samples were contaminated by adding Cu (as CuSO4) or Zn (as ZnSO4 · 7H2O) to give one series of Cu- and one of Zn-contaminated soils, with 0, 2, 4, 8, 16, 32, 64, and 128 mmol of metal kg (dry weight) of soil−1. The jars were sealed with plastic lids and incubated at room temperature (22°C). The soil samples were aerated intermittently, and the moisture content was maintained at a constant level throughout the incubation period. Samples were taken for measurements (see below) after 1, 2, 4, 7, 11, 21, 35, and 60 days. In the pilot experiment, the same contamination levels were used, but measurements were made after 2 days only.
The pH of the four most-contaminated soils decreased by 0.8 and 1 U, respectively. After 16 days of incubation, 20 g (dry weight) of soil was removed from each jar, transferred to another set of jars, and amended with different amounts of CaCO3. This resulted in a pH in all samples ranging between 3.9 and 4.3 during the remaining period of the experiment. Samples were taken from these limed samples 23, 28, 35, and 60 days after metal contamination.
Microbial activity.
The total microbial activity was estimated as respiration at 22°C. One gram (wet weight) of soil was transferred to a 20-ml glass vial, which was sealed, and the headspace concentration of CO2 was determined after 20 h using gas chromatography. The first measurement started 1 day (day 1) after metal addition.
Bacterial activity was estimated using the thymidine incorporation technique on bacteria extracted from soil (4), with the modifications introduced by Bååth et al. (8). Briefly, 1 g (wet weight) of soil was mixed with 40 ml of water and treated on a shaker (200 rpm) for 15 min, followed by low-speed centrifugation (1,000 × g for 10 min). From the supernatant, 1.5 ml of the bacterial suspension was transferred to Eppendorf tubes and 5 μl of [methyl-3H]thymidine (925 GBq mmol−1; Amersham) was added. Incorporation, washing of excess tracer, and measurement of radioactivity incorporated by actively growing bacterial cells were described in detail by Bååth et al. (8).
Fungal activity was measured using the acetate-in-ergosterol technique devised by Newell and Fallon (32) and modified for use in the soil habitat by Bååth (6). In short, 0.25 g (wet weight) of soil was transferred in test tubes and 0.03 ml of 1,2-[14C]acetic acid (sodium salt, 2.07 GBq mmol−1; Amersham), 0.47 ml of 1 mM nonradioactive acetate, and 1.5 ml of distilled water or buffer were added. After incubating the mixture for 16 h at room temperature, 1 ml of 5% formalin was added, the test tubes were centrifuged, and the supernatant (with nonincorporated acetate) was discarded. The ergosterol was then extracted and measured using high-performance liquid chromatography. The ergosterol peak was collected into scintillation vials, and the amount of radioactivity incorporated into ergosterol was then measured on a scintillator. The first measurement started 1 day after metal addition. Samples from day 60 were lost due to apparatus failure.
On the first two sampling occasions, 1.5 ml of distilled water was added along with acetate. However, the reduction in soil pH in various degrees due to metal contamination may affect acetate incorporation. Therefore, to avoid such interference with pH, on day 4, a citrate phosphate buffer (pH 5.0, 10.7 mM K2HPO4, 5.2 mM citric acid) was used instead of distilled water. We compared the results of using buffer with those of using distilled water for the uncontaminated controls and the highest levels of metal addition at this sampling occasion. Although no differences in acetate incorporation were found when using water or buffer, buffer was used on subsequent measurement occasions. Thus, the data from days 1 and 2 are without buffer, while later measurements were made with buffer.
Microbial community structure.
The phospholipid fatty acid pattern was determined according to Frostegård et al. (17) on 0.5 g (wet weight) of soil. This was carried out on nonlimed samples 30 days after the start of the experiment. We calculated a relative fungal/bacterial ratio by using the PLFA 18:2ω6,9 as an indicator of fungi and the sum of 12 bacterial PLFAs as an indicator of bacteria (16).
The catabolic diversity was also estimated 30 days after the start of the experiment on nonlimed soils as a way of indicating changes in the community. This analysis was performed using the technique described by Degens and Harris (12), with some modifications. A total of 15 substrates were used in this assay. Substrates were chosen from those used by Degens and Harris (12) to get a subset of amino acids, sugars, and carboxylic acids. The substrates were d-glutamine, l-asparagine, l(−)-glutamine, l(−)-alanine, l-leucine at 15 mM each; trisodium-citrate-dihydrate, d-mannose, d-glucose, and l-sorbose at 75 mM each; and succinic acid, l-ascorbic acid, d-gluconic acid, maleic acid, disodium dl-malate, and d-mannitol at 100 mM each. The pH of the solutions was adjusted to 5.0 to 5.4. Two milliliters of substance solution was added to 1 g (wet weight) of soil, and the respiration during 0.5 to 3 h was determined with a gas chromatograph as described above. The data from d-glucose-amended soils (four replicates) were also used to calculate substrate-induced respiration (SIR), which indicates the total microbial biomass (2).
Plate counts of bacteria and fungi were performed 30 days after the start of the experiment. Soils from noncontaminated controls and with the lowest and two highest levels of metal contamination were studied. Bacteria were counted after 2 weeks at room temperature, using 1/10-strength Trypton Soya agar, while fungi were counted after 1 week on 1% malt extract agar.
Statistical analysis.
In the short-term study (1 week; Fig. 1), the means of measurements performed during this time were calculated (except for respiration, where the mean of days 2 to 11 was used). The long-term study (up to 60 days of incubation) was not intended to compare the effects of Zn and Cu toxicity. Instead, the different metals were treated statistically as a replication of metal effects. Thus, when effects of liming and time were compared using the paired t test, the four highest levels of metals for the two different metals were used as replicates, giving n = 8, except when samples were lost. The standard error of the no-effect level was calculated at each sampling occasion using the noncontaminated controls and the lowest level of metal contamination for Zn and Cu, where no negative effect of the metals was found, giving n = 4.
FIG. 1.
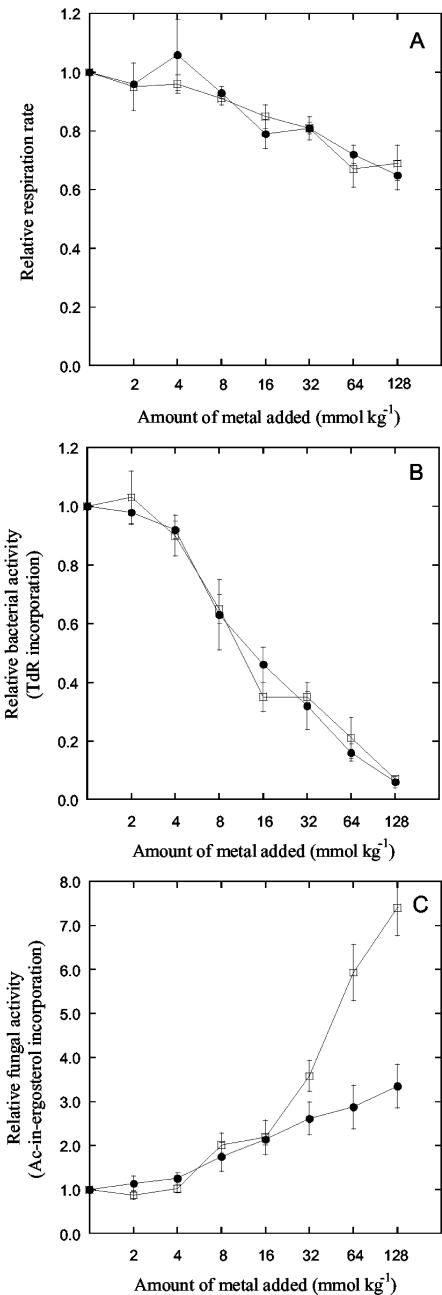
Short-term effects of Zn (solid symbols) and Cu (open symbols) addition on soil respiration rate (A), bacterial activity (thymidine [TdR] incorporation rate) (B), and fungal activity (acetate-in-ergosterol incorporation rate) (C). The values were normalized to that of the control soil. Each point is the mean ± standard error of four measurements during days 1 to 7 (three measurements during days 2 to 11 for respiration). Note the logarithmic x axes.
The PLFA data (in moles percent) were subjected to a principal component analysis after scaling to unit variance. The respiration rates after adding different substrates (catabolic diversity measurements) were also analyzed by principal component analysis after scaling to unit variance.
RESULTS
Short-term effects of metals.
The soil respiration rate was only slightly affected by the metal contamination, although a clear dose-response effect was seen at added metal concentrations above 4 mmol kg−1 (Fig. 1A). The highest level of Cu and Zn resulted in a 30% reduction in soil respiration rate compared with the control soil. The metal effect on soil respiration rate did not differ between Zn and Cu contamination. In the pilot experiment respiration rate decreased in a similar manner as in the main experiment, with 38% at the highest level of Cu and with 34% at the highest level of Zn addition.
Bacterial activity, measured as the thymidine incorporation rate, decreased linearly with the logarithm of the metal addition above a metal concentration of 2 mmol kg−1 (Fig. 1B). No differences were observed between effects due to Zn and Cu contamination. The highest levels of metals decreased bacterial activity to less than 10% of that in the control samples. Fifty percent effective dose values (the ecological dose resulting in a 50% decrease in activity) were around 10 mmol of added metal kg−1. In the pilot experiment, bacterial activity decreased in 2 days to less than 20% of the control at the highest level of both Cu and Zn addition.
Fungal activity, measured as acetate-in-ergosterol incorporation, increased with the added soil metal concentration above 4 mmol kg−1 (Fig. 1C). The increase was most evident for the Cu-contaminated soil, where the highest level of Cu addition increased fungal activity seven times. In the Zn-contaminated soil, fungal activity in the soil with the highest level of contamination was three times higher than that in the control samples. In the pilot study, fungal activity also increased with metal levels after 2 days, with about three times at the highest level of Cu and with two times at the corresponding level of Zn addition.
Changes in activity over time and effect of pH.
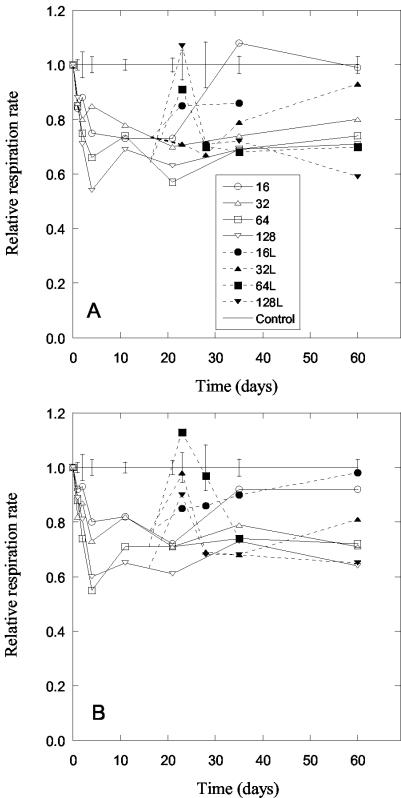
Soil respiration decreased in the noncontaminated control soil over the 60-day incubation period. To compensate for this variation, the effect of the metal addition was compared each sampling day with that of the control. The soil respiration rate in the metal-contaminated soils decreased gradually compared with the noncontaminated control samples during the first 3 days after metal addition, after which soil respiration became stable (Fig. 2). Respiration rates after 10 and 60 days of incubation did not differ significantly (paired t test, n = 8). Similar effects were observed for both Zn and Cu contamination.
FIG. 2.
Long-term effects of Zn (A) and Cu (B) addition on soil respiration rates. Only the four highest levels of contamination (16 to 128 mmol kg−1) are shown, normalized to that of the control soil. Bars indicate standard error for each sampling date, calculated using the controls and lowest metal levels (no negative effect of metal addition), giving n = 4. Open symbols indicate soil with only metals added, and solid symbols indicate soil that was limed (L) 16 days after metal addition to give the same soil pH throughout the experiment.
A short increase in respiration rate in the contaminated soils was evident following the application of lime. After that levels similar to those in the nonlimed samples were found (paired t tests of samples from limed and unlimed samples after 60 days not significantly different, n = 8).
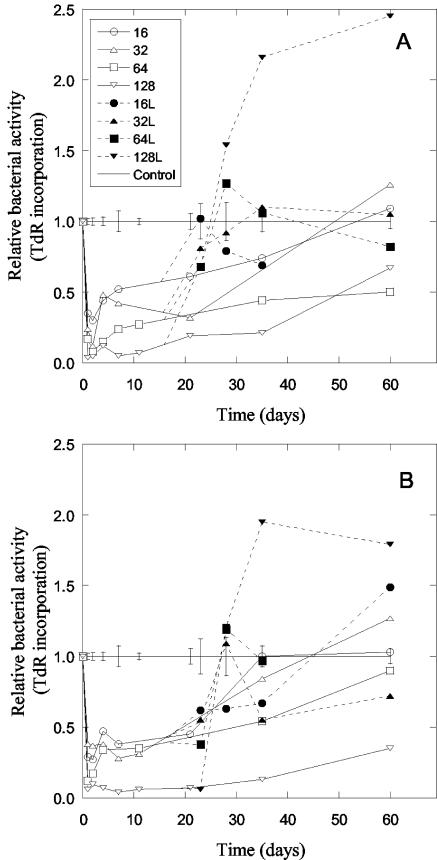
The bacterial activity was similarly affected by the addition of Zn and Cu (Fig. 3). Bacterial activity in metal-contaminated samples decreased compared with the control soil to low levels within a day after the addition of metals. Slow recovery of bacterial activity then followed, resulting in most samples, except those with the highest levels of contamination, having similar bacterial activities to the noncontaminated control soil after 60 days. The bacterial activity in the samples to which the highest level of Zn and Cu had been added were still around half of that in the control samples after 60 days.
FIG. 3.
Long-term effects of Zn (A) and Cu (B) addition on bacterial activity (thymidine [TdR] incorporation rates). Only the four highest levels of contamination (16 to 128 mmol kg−1) are shown, normalized to that of the control soil. Bars indicate standard error for each sampling date, calculated using the controls and lowest metal levels (no negative effect of metal addition), giving n = 4. Open symbols indicate soil with only metals added, and solid symbols indicate soil that was limed (L) 16 days after metal addition to give the same soil pH throughout the experiment.
Liming of the contaminated samples after 16 days increased the rate of recovery of the bacterial activity (Fig. 3). As a result, 35 days after starting the experiment, the bacterial activity in limed contaminated samples was significantly higher than in unlimed ones (paired t test, n = 7). The bacterial activity in the most contaminated samples actually increased to more than twice that in the control samples, in both Zn- and Cu-contaminated soil 35 and 60 days after adding the metals.
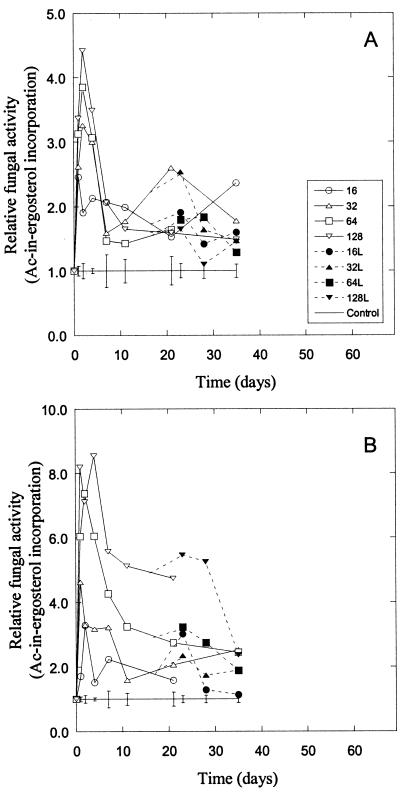
The fungal activities in metal-contaminated soil increased within 2 to 3 days to their highest levels (Fig. 4). Peak levels of activity were higher in Cu-contaminated (Fig. 4B) than in Zn-contaminated (Fig. 4A) samples. Thereafter, the fungal activity started to decrease, but even on day 35, the fungal activity in the most contaminated samples was significantly higher than in the noncontaminated control soil, both for Zn and Cu addition.
FIG. 4.
Long-term effects of Zn (A) and Cu (B) addition on fungal activity (acetate-in-ergosterol incorporation rates). Only the four highest levels of contamination (16 to 128 mmol kg−1) are shown, normalized to that of the control soil. Bars indicate the standard error for each sampling date, calculated using the controls and lowest metal levels (no negative effect of metal addition), giving n = 4. Open symbols indicate soil with only metals added, and solid symbols indicate soil that was limed (L) 16 days after metal addition to give the same soil pH throughout the experiment.
Liming of the contaminated soil samples after 16 days to restore the pH to levels similar to those in the control soil did not affect the fungal activity (Fig. 4). There were no differences between limed and unlimed soils in this respect after 35 days (paired t test, n = 5 due to several samples being lost on the last sampling occasion).
Effects on community composition and biomass.
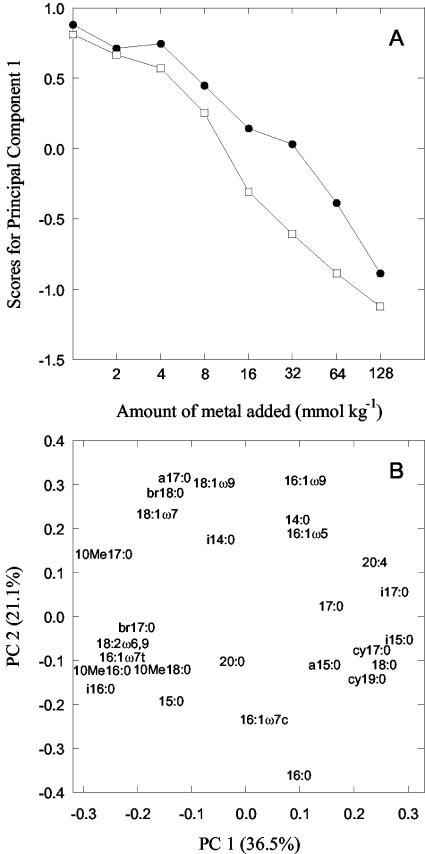
Principal component analysis of the PLFA data indicated that 36.5% of the variation was accounted for by the first principal component (PC 1). This component was related to the levels of metal added. Plotting the scores of PC 1 against metal addition levels showed that the PLFA pattern after 30 days was linearly affected by the logarithm of the metal concentration above 4 mmol kg−1 (Fig. 5A). Cu affected the PLFA pattern slightly more than the corresponding levels of Zn. The second principal component accounted for 21.1% of the variation but only differentiated two of the low-Zn-contaminated soils from all the other soil samples.
FIG. 5.
Effect of Zn (solid symbols) and Cu (open symbols) addition on the microbial community structure estimated after 30 days using PLFA analysis. The data were analyzed by principal component analysis. The scores of PC 1 (indicating metal effects) were plotted against the logarithm of the amount of metal added to the soil (A), and the loadings for individual PLFAs were plotted along the first two principal components (B). Values within parentheses indicate variation explained by the principal component.
The PLFAs increasing most after metal addition are the ones having large negative loading values along PC 1 (Fig. 5B). These PLFAs were 10Me16:0 (decreasing from 5.5 to 6.2 mol% in control soils to 8.5 to 9.7% in the most-metal-polluted samples), 10Me17:0 (from 1.1 to 1.3% to 2.0 to 2.6%), i16:0 (from 4.1 to 5.2% to 6.2 to 8.4%), br17:0 (from 0.23 to 0.26% to 0.40 to 0.44%), br18:0 (from 2.9 to 3.0% to 3.9 to 5.1%), and 18:2ω6,9 (from 2.9% to 3.9 to 5.1%). The PLFAs having positive loading values along PC 1 decreased in metal-polluted soils: e.g., i15:0 (from 8.8 to 10.1% to 5.2 to 6.3%), i17:0 (from 1.9 to 2.1% to 1.2 to 1.3%), cy17:0 (from 1.8 to 2.0% to 1.5%), cy19:0 (from 6.7 to 7.9% to 4.4 to 4.9%), and 20:4 (from 1.5 to 1.7% to 0.5 to 1.1%).
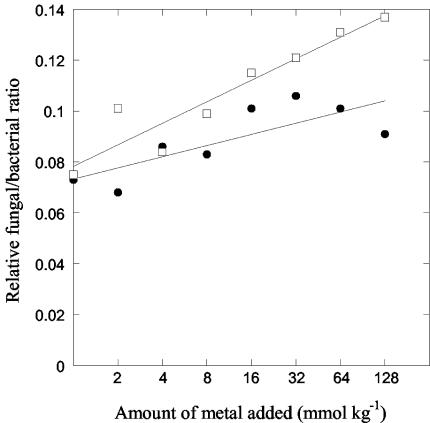
The relative fungal/bacterial ratio increased with increasing metal load (Fig. 6; linear regression significant at P < 0.05 and P < 0.001 for Zn and Cu, respectively). The increase was largest for the Cu-amended soils, where the ratio increased from around 0.075 in the control to almost 0.14 in the most-polluted soils. Corresponding values for the Zn-amended soil were from 0.075 to around 0.10.
FIG. 6.
Effect of Zn (solid symbols) and Cu (open symbols) addition on the relative fungal/bacterial ratios calculated using the PLFA 18:2ω6,9 as an indicator of fungal biomass and the sum of 12 bacterium-specific PLFAs as an indicator of bacterial biomass. Linear regression for Zn had R2 = 0.61, and that for Cu had R2 = 0.89.
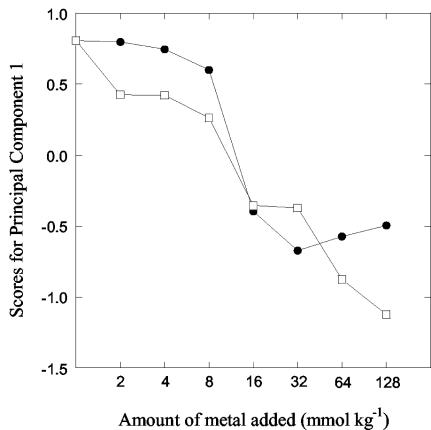
Principal component analysis of the catabolic diversity data indicated that PC 1 accounted for 45.1% of the variation. This component was related to metal level, since the scores of PC 1 were related to metal addition, with effects in samples treated with ≥16 mmol of metal kg−1 or more (Fig. 7). No apparent differences were found between Zn and Cu addition. The respiration rate decreased for most additives in metal-contaminated soil compared to the control soil, except for ascorbic acid. Adding this substance increased respiration more in contaminated soil than in the control soil.
FIG. 7.
Effect of Zn (solid symbols) and Cu (open symbols) addition on microbial catabolic diversity. The data were analyzed by principal component analysis, and the scores of the first principal component (indicating metal effects) are plotted against the logarithm of the metal added to the soil.
Microbial biomass, estimated using SIR, decreased only slightly in soils contaminated with metal levels above 8 mmol kg−1 (data not shown). The highest levels of metal decreased the microbial biomass to 72% ± 3% and 82% ± 2% of that in the noncontaminated control soil for Cu and Zn, respectively. The microbial biomass, estimated as the total amount of PLFAs, was not affected by the metal addition (data not shown).
Bacterial plate counts were drastically reduced in the two most-contaminated samples (64 and 128 mmol kg−1), becoming less than 0.2% of that in the control soil for both Cu and Zn contamination. Fungal plate counts were not significantly affected by metal addition.
DISCUSSION
The short-term effects on microbial activity clearly indicate that bacteria and fungi were affected differently by the addition of the metals (Fig. 1B and C). This study provides the first direct evidence on a differential response using activity measurements. That bacterial activity, measured by the thymidine incorporation technique, is very sensitive to metal pollution had been shown earlier in both laboratory (13, 14) and field (35) studies. Our finding that fungal activity was less affected was in accordance with earlier studies indicating that fungi were less sensitive to heavy metals than bacteria (21, 24, 25, 26, 30). The opposite effect of heavy metals on these groups of organisms was further evident by the drastic decrease in CFU for bacteria but not for fungi found in the present study and by the increase in the relative fungal/bacterial ratio with increasing metal levels (Fig. 6).
The fungal activity actually increased with metal load within a few days after metal addition (Fig. 3C), which was unexpected. This increase was most evident in the Cu-contaminated soils, which also had the largest increase in relative fungal/bacterial ratio (Fig. 6). One explanation may be that fungal growth, like that of other soil microorganisms, is carbon limited (1). The extra carbon released from dead bacteria would thus trigger increased fungal growth, overriding the negative impact of the heavy metals. Bacteria, which were negatively affected by the metals (Fig. 1B), apparently could not take advantage of this extra carbon. The observation may also, of course, partly be due to problems with the techniques. The acetate-in-ergosterol technique for measuring fungal activity has been much less used than has the thymidine (or the similar leucine) incorporation technique for measuring bacterial activity. Unknown technical factors may affect the former method, resulting in erroneous results. One such factor might be pH of the soil slurry. pH was earlier found to directly affect leucine but not thymidine incorporation rate measurements, indicating the importance of this factor (5). Similarly, acetate incorporation in the slurries of the contaminated soils took place at lower pH than in slurries from control samples. However, comparisons made between buffered and nonbuffered soil slurries did not indicate any effects of pH on acetate incorporation. Neither were there any differences between fungal activity in limed and unlimed soil. A direct pH effect in the soil, where the lower pH in contaminated soils enhanced the negative effect on bacteria and the positive effect on fungi, however, cannot be excluded. Earlier studies have shown both that acidification can explain part of the effect of heavy metals, at least in laboratory studies (13, 38), and that fungi are apparently favored in acidified soils (34).
The total activity (respiration rate) was only slightly affected by metal addition, even at very high levels (Fig. 1A). This was found earlier in different types of soils (17, 33, 38). In general, respiration should reflect both bacterial and fungal activities. The almost complete disappearance of bacterial activity (Fig. 1B) could therefore have been counteracted by the increased fungal activity (Fig. 1C). This explanation does not, however, explain the long-term effects. At a time when bacterial activity had recovered completely, especially after liming (Fig. 3), and fungal activity was unchanged or higher in contaminated soils than that in the controls (Fig. 4), respiration values were still lower in metal-contaminated soils (Fig. 2). Thus, it appears that at this time the respiration rate did not directly reflect the activity of the main part of the soil microbial community (i.e., fungi and bacteria). Since the methods used for estimating fungal and bacterial activities in soil are very new, systematic comparisons of these activities and respiration under different environmental conditions are not yet available.
It has previously been shown that bacterial activity, measured as the thymidine or leucine incorporation rate, recovers after heavy metal addition. For example, the addition of Zn at a rate of 256 mmol kg−1 to a high-pH (pH 7.8) agricultural soil initially resulted in almost complete inhibition of thymidine incorporation, but bacterial growth recovered rapidly, resulting in activity levels similar to or higher than those in the noncontaminated soil after 16 days (13). Cu addition to the same soil resulted in bacterial activity that recovered to levels more than twice that in the control soil after 1 month (14). However, in the present study, bacterial activity only recovered slowly in the most-contaminated soils during the first month (Fig. 3). One reason for this disparity compared to the earlier studies might have been the low pH of contaminated soils in our study, which could have retarded the regrowth of bacteria. It is well known that regrowth of microorganisms after fumigation or heating (to kill off a large part of the community) is slow in acidic soils compared with soils with more neutral pH (41). Further evidence of the negative effect of low pH on bacterial growth could be the effect of liming in the present study, since the recovery increased drastically when the contaminated soils were limed to the initial soil pH (Fig. 3). Within 2 weeks, the most-contaminated soils (both Zn and Cu) had thymidine incorporation rates higher than the control soil. However, liming is known to increase soluble organic carbon (36, 37). Accordingly, the faster recovery of bacterial activity could partly be attributed to increased substrate availability after liming. A third explanation is that the addition of lime decreases the availability of the heavy metals, thus decreasing the level of toxicity.
Both measurements of community structure indicated that the heavy metals had an effect. For the PLFA measurements, effects were seen at metal levels above 4 mmol kg−1 (Fig. 5A). Thus, both community composition and the bacterial growth rate (Fig. 1B) were affected approximately at the same metal level. At this level, heavy metal-sensitive bacteria are probably responsible for the decrease in bacterial activity and the competitive advantage of more tolerant ones resulted in a change in community composition. This phenomenon parallels the coupling between changes in pollution-induced community tolerance and changes in species composition (10). In a previous study conducted with a similar forest humus soil, effects of Zn and Cu addition on the PLFA pattern were observed at 4 and 8 mmol kg−1, respectively (17).
The individual PLFAs were affected in the same way in the earlier study (17) as in the present one. For example, an increase in the relative concentrations (moles percent) of the PLFAs 10Me16:0, 10Me17:0, i16:0, br17:0, br18:0, and 18:2ω6,9 and a decrease in the PLFAs i15:0, i17:0, cy17:0, cy19:0, and 20:4 were found in both studies. Especially the increase in the PLFAs i16:0, br17:0, and br18:0 and the decrease in 20:4 appear to be a common result of metal pollution of forest humus soil in both laboratory (17) and field (7, 35) studies. The former three PLFAs are all associated with gram-positive bacteria, indicating that specific gram-positive bacteria are more common in metal-polluted forest soils.
There was a decrease in microbial biomass 30 days after adding metals, as measured using the SIR technique, but not when microbial biomass was estimated as the total amount of PLFAs. One reason for this difference might be that the SIR technique estimates active microorganisms able to directly respire the added glucose, while the total amount of PLFAs would include PLFAs from recently dead organisms, where the PLFA is still intact, which will obscure any changes in living biomass. This is similar to an earlier study (17) in which, 6 months after a metal amendment, an activity-based biomass method (ATP) had decreased to less than half of the initial values, while total PLFAs only decreased by around 20%. Despite the fact that no differences in the total amounts of PLFAs were found, clear changes in the PLFA patterns were found to be due to metals (Fig. 5), showing that individual PLFAs (indicating different organism groups) were affected differently by the heavy metals.
Regarding the catabolic diversity, an effect was found only at 16 mmol kg−1 and higher (Fig. 5B). The variation also appeared to be higher than for the PLFA data. In comparison to a larger number of substrates used in the original method (12), only 15 substrates were used in our study. This might be one reason for the observed low sensitivity of the method. However, all substrates (except ascorbic acid) covaried in their response to heavy metals, indicating that a larger set of substrates would not improve the detection level. The observation that the addition of ascorbic acid increased the respiration rate more in contaminated than in control soils was unexpected. One reason for this could be that the decomposition of this substrate was mainly due to fungi. Therefore, respiration may have increased, since fungal growth was not negatively affected by heavy metals 1 month after addition (Fig. 3). Another reason may be the low pH in more contaminated soils, which might have rendered ascorbic acid more available to enzymes releasing CO2.
Recent studies in different ecosystems have indicated that fungi and bacteria can have antagonistic relationships: that is, an increase in activity of one organism group results in a decrease in the activity of the other. This phenomenon was reported in a study on decomposing aquatic plant litter (28) and in another on decomposing beech leaves in soil (29). The decrease in fungal activity after the first few days (Fig. 4) might be due to increased competition caused by the recovering bacterial community. However, the decrease could also be explained by exhaustion of the substrate released by the bacteria that were initially killed by the heavy metals. Besides, a further decrease in fungal activity was not evident in limed soils, where bacterial activity increased rapidly (compare Fig. 3 and 4). Therefore, our experiment does not provide evidence supporting hypotheses on fungal-bacterial antagonism.
This is the first study in which the direct effects of metals on fungal and bacterial growth rates in soil have been studied. The acetate-in-ergosterol technique has not been much used in soil systems, and it is more time-consuming and costly and less reproducible than the techniques used for estimating bacterial growth rates. However, our results show that their simultaneous use can contribute significantly to our understanding of community dynamics of fungi and bacteria in situ. Not only could the contrasting effects of heavy metals on these two groups of organisms be clearly demonstrated, but this technique also helped in explaining respiration data as a combination of the activity of these two groups. Thus, the combination of methods indicating total, bacterial, and fungal activities appears to be a promising tool for elucidating the effects of other environmental factors, such as nutrient addition and physical or chemical factors.
Acknowledgments
This study was supported by grants from the Swedish Research Council to E.B.; from the Personnel Development Project of the Ministry of Science and Technology, Sri Lanka, to R.M.C.P.R.; and by a student grant from TEMPUS (EU) to M.A.T.-K.
REFERENCES
- 1.Aldén, L., F. Demoling, and E. Bååth. 2001. Rapid method of determining factors limiting bacterial growth in soil. Appl. Environ. Microbiol. 67:1830-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. P. E., and K. H. Domsch. 1978. A physiological method for the quantitative measurement of microbial biomass in soil. Soil Biol. Biochem. 10:215-221. [Google Scholar]
- 3.Bååth, E. 1989. Effects of heavy metals in soil on microbial processes and populations (a review). Water Air Soil Pollut. 47:335-379. [Google Scholar]
- 4.Bååth, E. 1992. Thymidine incorporation into macromolecules of bacteria extracted from soil by homogenization-centrifugation. Soil Biol. Biochem. 24:1157-1165. [Google Scholar]
- 5.Bååth, E. 1998. Growth rates of bacterial communities in soils at varying pH: a comparison of the thymidine and leucine incorporation techniques. Microb. Ecol. 36:316-327. [DOI] [PubMed] [Google Scholar]
- 6.Bååth, E. 2001. Estimation of fungal growth rates in soil using 14C-acetate incorporation into ergosterol. Soil Biol. Biochem. 33:2011-2018. [Google Scholar]
- 7.Bååth, E., Å. Frostegård, M. Díaz-Raviña, and A. Tunlid. 1998. Microbial community-based measurements to estimate heavy metal effects in soil: the use of phospholipid fatty acid patterns and bacterial community tolerance. Ambio 27:58-61. [Google Scholar]
- 8.Bååth, E., M. Pettersson, and K. H. Söderberg. 2001. Adaptation of a rapid and economical microcentrifugation method to measure thymidine and leucine incorporation by soil bacteria. Soil Biol. Biochem. 33:1571-1574. [Google Scholar]
- 9.Babich, H., and G. Stotzky. 1978. Toxicity of zinc to fungi, bacteria, and coliphages: influence of chloride ions. Appl. Environ. Microbiol. 36:906-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blanck, H. 2002. A critical review of procedures and approaches used for assessing pollution-induced community tolerance (PICT) in biotic communities. Hum. Ecol. Risk Assess. 8:1003-1034. [Google Scholar]
- 11.Christensen. H., R. Rønn, F. Ekelund, and S. Christensen. 1994. Bacterial production determined by [3H]thymidine incorporation in field rhizospheres as evaluated by comparison to rhizodeposition. Soil Biol. Biochem. 27:93-99. [Google Scholar]
- 12.Degens, B. P., and J. A. Harris. 1997. Development of a physiological approach to measuring the catabolic diversity of soil microbial communities. Soil Biol. Biochem. 29:1309-1320. [Google Scholar]
- 13.Díaz-Raviña, M., and E. Bååth. 1996. Development of metal tolerance in soil bacterial communities exposed to experimentally increased metal levels. Appl. Environ. Microbiol. 62:2970-2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Díaz-Raviña, M., and E. Bååth. 1996. Thymidine and leucine incorporation into bacteria from soils experimentally contaminated with heavy metals. Appl. Soil Ecol. 3:225-234. [Google Scholar]
- 15.Doelman, P. 1985. Resistance of soil microbial communities to heavy metals, p. 369-384. In V. Jensen, A. Kjøller, and L. H. Sørensen (ed.), Microbial communities in soil. Elsevier, London, United Kingdom.
- 16.Frostegård, Å., and E. Bååth. 1996. The use of phospholipid fatty acid analysis to estimate bacterial and fungal biomass in soil. Biol. Fertil. Soils 22:59-65. [Google Scholar]
- 17.Frostegård, Å., A. Tunlid, and E. Bååth. 1993. Phospholipid fatty acid composition, biomass, and activity of microbial communities from two soil types experimentally exposed to different heavy metals. Appl. Environ. Microbiol. 59:3605-3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frostegård, Å., A. Tunlid, and E. Bååth. 1996. Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biol. Biochem. 28:55-63. [Google Scholar]
- 19.Giller, K. E., E. Witter, and S. P. McGrath. 1998. Toxicity of heavy metals to microorganisms and microbial processes in agricultural soil: a review. Soil Biol. Biochem. 30:1389-1414. [Google Scholar]
- 20.Harris, D., and E. A. Paul. 1994. Measurement of bacterial growth rates in soil. Appl. Soil Ecol. 1:277-290. [Google Scholar]
- 21.Hiroki, M. 1992. Effects of heavy metal contamination on soil microbial populations. Soil Sci. Plant Nutr. 38:141-147. [Google Scholar]
- 22.Jordan, M. J., and M. P. Lechevalier. 1975. Effects of zinc-smelter emissions on forest soil microflora. Can. J. Microbiol. 21:1855-1865. [DOI] [PubMed] [Google Scholar]
- 23.Kelly, J. J., M. Häggblom, and R. L. Tate III. 1999. Changes in soil microbial communities over time resulting from one time application of zinc: a laboratory microcosm study. Soil Biol. Biochem. 31:1455-1465. [Google Scholar]
- 24.Khan, M., and J. Scullion. 2002. Effects of metal (Cd, Cu, Ni, Pb or Zn) enrichment of sewage-sludge on soil micro-organisms and their acitivities. Appl. Soil Ecol. 20:145-155. [Google Scholar]
- 25.Korthals, G. W., A. D. Alexiev, T. M. Lexmond, J. E. Kammenga, and T. Bongers. 1996. Long-term effects of copper and pH on the nematode community in an agroecosystem. Environ. Toxicol. Chem. 15:979-985. [Google Scholar]
- 26.Maliszewska, W., S. Dec, H. Wierzbicka, and A. Woźniakowska. 1985. The influence of various heavy metal compounds on the development and activity of soil micro-organisms. Environ. Pollut. 37:195-215. [Google Scholar]
- 27.Michel, P. H., and J. Bloem. 1993. Conversion factors for estimation of cell production rates of soil bacteria from [3H]thymidine and [3H]leucine incorporation. Soil Biol. Biochem. 25:943-950. [Google Scholar]
- 28.Mille-Lindblom, C., and L. J. Tranvik. 2003. Antagonism between bacteria and fungi on decomposing aquatic plant litter. Microb. Ecol. 45:173-182. [DOI] [PubMed] [Google Scholar]
- 29.Møller, J., M. Miller, and A. Kjøller. 1999. Fungal-bacterial interaction on beech leaves: influence on decomposition and dissolved organic carbon quality. Soil Biol. Biochem. 31:367-374. [Google Scholar]
- 30.Müller, A. K., K. Westergaard, S. Christensen, and S. J. Sørensen. 2001. The effect of long-term mercury pollution on the soil microbial community. FEMS Microbiol. Ecol. 36:11-19. [DOI] [PubMed] [Google Scholar]
- 31.Newell, S. Y. 1996. The [14C]acetate-to-ergosterol method: factors for conversion from acetate incorporation to organic fungal mass synthesized. Soil Biol. Biochem. 28:681-683. [Google Scholar]
- 32.Newell, S. Y., and R. D. Fallon. 1991. Towards a method for measuring instantaneous fungal growth rates in field samples. Ecology 72:1547-1559. [Google Scholar]
- 33.Niklinska, M., R. Laskowski, and M. Maryanski. 1998. Effect of heavy metals and storage time on two types of forest litter: basal respiration and exchangeable metals. Ecotoxicol. Environ. Saf. 41:8-18. [DOI] [PubMed] [Google Scholar]
- 34.Pennanen, T., H. Fritze, P. Vanhala, O. Kiikkilä, S. Neuvonen, and E. Bååth. 1998. Structure of a microbial community in soil after prolonged addition of low levels of simulated acid rain. Appl. Environ. Microbiol. 64:2173-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pennanen, T., Å. Frostegård, H. Fritze, and E. Bååth. 1996. Phospholipid fatty acid composition and heavy metal tolerance of soil microbial communities along two heavy metal-polluted gradients in coniferous forests. Appl. Environ. Microbiol. 62:420-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Persson, T., A. Wirén, and S. Andersson. 1991. Effects of liming on carbon and nitrogen mineralization in coniferous forests. Water Air Soil Pollut. 54:351-364. [Google Scholar]
- 37.Pettersson, M., and E. Bååth. 2003. The rate of change of a soil bacterial community after liming as a function of temperature. Microb. Ecol. 46:177-186. [DOI] [PubMed] [Google Scholar]
- 38.Speir, T. W., H. A. Kettles, H. J. Percival, and A. Parshotam. 1999. Is soil acidification the cause of biochemical responses when soils are amended with heavy metal salts? Soil Biol. Biochem. 31:1953-1961. [Google Scholar]
- 39.Uhlířová, E., and H. Šantručková. 2003. Growth rate of bacteria is affected by soil texture and extraction procedure. Soil Biol. Biochem. 35:217-224. [Google Scholar]
- 40.Van Beelen, P., and P. Doelman. 1997. Significance and application of microbial toxicity tests in assessing ecotoxicological risks of contamination in soil and sediment. Chemosphere 34:455-499. [Google Scholar]
- 41.Vance, E. D., P. C. Brookes, and D. S. Jenkinson. 1987. Microbial biomass measurements in forest soils—determination of kc values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol. Biochem. 19:689-696. [Google Scholar]