Abstract
Genetically modified auxotrophic mutants of different fish pathogens have been used as live vaccines in laboratory experiments, but the behavior of the strains after release into aquatic ecosystems has not been characterized. We previously constructed and characterized an aroA mutant of Aeromonas hydrophila and studied the protection afforded by this mutant as a live vaccine in rainbow trout. In this work, we describe the survival of this strain in aquatic microcosms prepared from fish water tanks. The aroA mutant disappeared rapidly in nonfiltered, nonautoclaved fish tank water, declining below detection levels after 15 days, suggesting an inhibitory effect of the autochthonous microflora of the water. When the aroA strain was used to inoculate sterilized water, its culturability was lower than that of wild-type strain A. hydrophila AG2; after long periods of incubation, aroA cells were able to enter a viable but nonculturable state. Entry into this nonculturable state was accompanied by changes in the cell morphology from rods to spheres, but the cells appeared to remain potentially viable, as assessed by the preservation of cell membrane integrity. Supplementation of the culture medium with sodium pyruvate favored the culturability and resuscitation of the two A. hydrophila strains at low temperatures (6 and 16°C). These results contribute to a better understanding of the behavior of the aroA strain in natural environments and suggest that the inactivation of the aroA gene may be beneficial for the safety of this live vaccine for aquacultures.
Aeromonas hydrophila is a gram-negative enterobacterium widely distributed in aquatic environments (22, 24, 26, 45), and it has long been known as a pathogen of amphibians, reptiles, and fish (4, 19, 52, 55-57, 66). Also, this bacterial species has been reported to cause a wide variety of human infections (2, 28, 29, 42, 70). Diseases caused by A. hydrophila (hemorrhagic septicemia, fin-tail rot, and epizootic ulcerative syndrome) have a major impact in aquacultures (3, 5). At present, no vaccines for the protection of farmed fish against A. hydrophila infections are commercially available, although several studies have proved that various vaccine formulations may provide protection (15, 33, 37, 51, 59).
The enzyme 5-enolpyruvylshikimate-3-phosphate synthase (EC 2.5.1.19) is present in bacteria, where it has a function in the biosynthesis of aromatic amino acids (58). Dysfunction of the aroA gene, encoding this enzyme, leads to auxotrophy of the organism for 4-aminobenzoic acid, 2,3-dihydroxybenzoic acid, and the aromatic amino acids phenylalanine (Phe), tyrosine (Tyr), and tryptophan (Trp). Making use of this dysfunction is finding widespread applications for the development of vaccines for human diseases, such a cholera, tetanus, leishmaniasis, and typhoid (27, 36, 63, 68, 76), and has also been applied to protection against various fish diseases (71, 73).
Hernanz Moral et al. previously constructed and characterized a nutritional derivative of a virulent A. hydrophila strain (AG2) for use as a live vaccine in rainbow trout (Oncorhynchus mykiss) (25). This method blocks the aromatic amino acid pathway, such that the strain cannot grow unless provided with a metabolite not available in fish tissues. Blocking of aromatic amino acid biosynthesis by mutation of the aroA gene causes a loss of virulence, so that the intraperitoneal injection of 108 CFU ml−1 in trout produces no ill effects, whereas the 50% lethal dose of the virulent wild-type strain is 106 CFU ml−1 (25).
The use of aroA mutant strains in fish vaccination by different routes of administration may facilitate the spread of these bacteria into aquatic environments. Because licensing regulations require assessing the risks associated with the release of genetically modified microorganisms, the persistence and behavior of mutant strains in natural environments need to be carefully studied. Several studies concerning the survival of A. hydrophila strains in aquatic environments, such as distilled water (44), mineral water (18, 45, 47), tap water (43), and seawater (40), have been published. Microcosms are often used in these studies, most of them applied to assess the health impact of the presence of motile Aeromonas species in drinking water, because the production of a wide range of virulence factors is a common feature of virulent strains (10, 13, 53, 54, 64).
Under adverse conditions, such as those present in some natural aquatic environments, A. hydrophila strains are able to enter a viable but nonculturable (VBNC) or an active nonculturable (ANC) state (40, 44, 75), from which they may be resuscitated under permissive conditions (40, 75). A VBNC or an ANC state allows microorganisms long-term permanence in the environment and makes their isolation for diagnostic procedures difficult (9, 31).
Interestingly, it was reported that A. hydrophila can remain in aquatic environments for prolonged periods in the VBNC state when cultured in a 0.35% NaCl solution at pH 7.5 and at 25°C for 50 days, although cells in this state were not pathogenic to goldfish, Carassius auratus (60). Because of their importance for the safety of an aroA live vaccine, the behavior and culturability of aroA cells in different water microcosms were compared with those of wild-type strain AG2 in this work. The morphological modifications and cell viability shown by the nonculturable bacteria and resuscitation from the VBNC or ANC state in culture medium supplemented with sodium pyruvate were studied. The effect on culturability of the growth phase of the vaccine and the effect of fish-associated autochthonous microflora present in water were also investigated.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
This study was performed with a previously described (25) A. hydrophila aroA mutant and wild-type strain A. hydrophila AG2, which were identified by PCR amplification of the aroA gene of A. hydrophila (14). Stock cultures were frozen at −80°C with 20% (vol/vol) glycerol, and the strains were routinely cultured on tryptic soy agar (TSA) or in tryptic soy broth (TSB) (Cultimed, Barcelona, Spain) at 22°C.
Survival assays in fish tank water microcosms and bacterial counts.
Thirty rainbow trout, O. mykiss (20 to 35 g), were placed in a 100-liter plastic tank supplied with running well water at 16°C, maintained under constant photoperiod conditions (12 h of light and 12 h of darkness), and fed every other day with commercial trout pellets (Trouw, Burgos, Spain). After 14 days, when the autochthonous aquatic microflora was well developed (reaching about 104 CFU ml of water−1; see Fig. 1), water samples from the fish tank were collected in 1-liter sterile glass bottles and split into subsamples of 200 ml that were used for different purposes in each of the following experiments: (i) nonautoclaved, nonfiltered (i.e., nonsterilized) water without any inoculation, to observe the behavior of the autochthonous microflora; (ii) nonsterilized water inoculated (see below) with the aroA strain; and (iii) autoclaved (121°C for 15 min), filtered (bottle-top 0.2-μm-pore-size cellulose acetate filter [Corning]) (i.e., sterilized) water inoculated with strain AG2 or with the aroA mutant. The bottles were incubated at 6, 16, or 20°C without shaking and in the dark.
FIG. 1.
Growth of autochthonous bacteria in nonsterilized (nonautoclaved, nonfiltered) fish tank water at 16°C (open squares) and 20°C (filled squares). Each point in the curve represents the mean of two independent experiments.
For survival assays, the A. hydrophila strains were grown in TSB with orbital shaking (150 rpm) at 25°C. This culture medium was selected for the survival studies because it allows the easy growth of Aeromonas species (5) and, in particular, aroA cells grown in TSB provided significant protection in vaccination-challenge studies with trout (25, 74). Inocula were prepared from cells harvested from earlier (14-h) and later (24-h) stationary-growth-phase cultures. The cells were harvested by centrifugation (2,000 × g, 30 min, 4°C) and washed three times in phosphate-buffered saline (PBS) to eliminate the possibility of carryover of nutrients; the pellets were resuspended in the same type of water to be inoculated. The fish water tank microcosms were inoculated to give a final concentration of approximately 104 CFU ml−1 in 200 ml of water.
Culturable bacterial counts were determined from an undiluted sample and from four samples serially diluted in PBS by the standard plate count technique immediately after inoculation of water and at regular intervals postinoculation. As the A. hydrophila aroA strain contains a stable intrachromosomical kanamycin resistance cassette and natural ampicillin resistance, samples containing autochthonous microflora and the aroA strain were plated on TSA containing ampicillin (200 μg/ml; A2804 σ) and kanamycin (50 μg/ml; K0879 σ) to inhibit the growth of autochthonous microorganisms; CFU were enumerated after 36 h of incubation at 22°C. Samples from sterilized water inoculated only with strain AG2 or the aroA strain were plated on TSA without antibiotics; CFU were enumerated as described above. Water samples from bottles containing the autochthonous microflora of the fish tank were plated on TSA; populations were determined after 7 days of incubation at 22°C. All platings were done in triplicate, the experiments were independently repeated two times, and the mean log CFU were calculated.
Solid medium resuscitation experiments and effects of sodium pyruvate on recovery of strains.
During the experiments, samples from the various microcosms were serially diluted and used to inoculate TSA amended with sodium pyruvate (0.1% [wt/vol]) as described by Mizunoe et al. (48) and Wai et al. (75). Sodium pyruvate was added directly to TSA prior to autoclaving.
SEM and detection of viable cells with a BacLight viability kit.
For scanning electron microscopy (SEM) studies, samples of bacteria were obtained from the inocula (time zero) and from the various microcosms at 90 days postinoculation. Cells at earlier (14-h) and later (24-h) stationary growth phases in TSB were harvested by centrifugation (2,000 × g, 30 min, 4°C), washed three times in PBS, and collected by filtration of the cell suspensions through 0.2-μm-pore-size sterile filters (Corning). Bacteria from the various microcosms were harvested by filtration of water through the same type of filters. The filters were fixed with 2% (vol/vol) glutaraldehyde (Polysciences, Warrington, Pa.) in PBS at 4°C for 1 h, dehydrated in a graded ethanol series, and then coated with gold in a sputter coater (Balzers SCD004). The samples were examined in a JEOL JSM 6100 scanning electron microscope at 20 kV.
For detection of bacteria with intact or damaged cell membranes, cells were stained with a LIVE/DEAD BacLight viability kit (L-7007; Molecular Probes). Samples of aroA cells incubated in sterilized water at 6°C for 70 days were prepared by filtration of 30 ml of such water through Isopore 0.2-μm-pore-size black polycarbonate filters (Millipore). The filters were stained with the kit dyes according to the manufacturer's instructions, placed over a slide, immersed in mounting oil, and covered with a coverslip. The samples were observed in an inverted microscope (Eclipse TE-300; Nikon) equipped with an epifluorescence attachment, and separate band-pass filter sets were used to observe fluorescence from SYTO 9 dye (B-2 filter; Nikon) and from propidium iodide (G-2A filter; Nikon). The images were captured with a cooled charge-coupled device camera (DX 30; Kappa) and KAPPA ImageBase software at a ×40 objective.
RESULTS
Figures 1 to 4 show the growth of the autochthonous microflora in the fish tank water, the survival of the A. hydrophila aroA strain in nonsterilized water, and the survival of strain AG2 and the aroA strain in sterilized water at various temperatures. In nonsterilized water, five to seven different bacterial phenotypes were observed, and these microflora grew actively after bottling to reach approximately 106 CFU ml−1 in the first week at 16 and 20°C (Fig. 1).
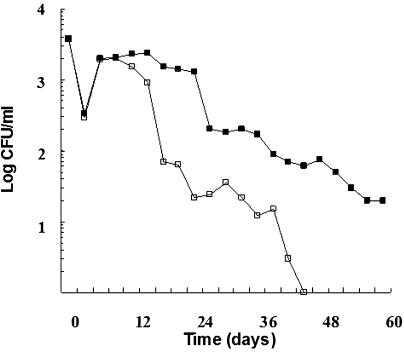
FIG. 4.
Culturability of the A. hydrophila aroA strain in sterilized fish tank water at 6°C. CFU were determined on TSA plates (open squares) or on TSA supplemented with sodium pyruvate (filled squares). Each point in the curve represents the mean of two independent experiments.
The behavior of the aroA strain at 16 and 20°C in nonsterilized water was initially characterized by a remarkable increase in the numbers of CFU, independently of the growth phase at which the cells were collected for the inocula (Fig. 2). In sterilized water, only strain AG2 showed such an initial increase (Fig. 3), and the culturability of the aroA strain declined from the time of inoculation, a phenomenon that was more prominent at 6°C (Fig. 4).
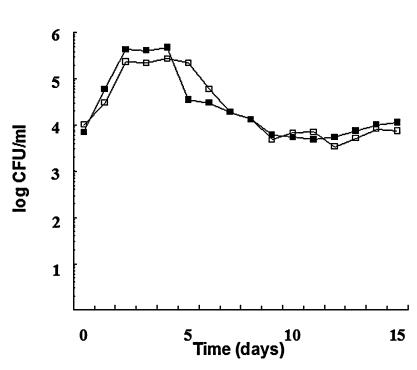
FIG. 2.
Culturability of A. hydrophila aroA in the presence of autochthonous microflora in nonsterilized fish tank water at 16°C (a) and 20°C (b). Open squares represent CFU for inocula prepared from 24-h-old cultures, and filled squares represent CFU for inocula prepared from 14-h-old cultures. Each point in the curve represents the mean of two independent experiments.
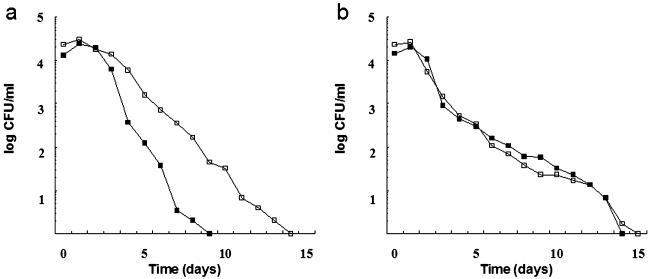
FIG. 3.
Culturability of the A. hydrophila aroA strain (a) and of wild-type strain AG2 (b) in sterilized (filtered and autoclaved) fish tank water. CFU were determined on TSA plates (open symbols) or on TSA supplemented with sodium pyruvate (filled symbols) at 16°C (squares) and 20°C (circles). Each point in the curve represents the mean of two independent experiments.
After the initial growth, the aroA strain disappeared rapidly in nonsterilized fish tank water (Fig. 2) and declined to below the detection level after 15 days. The culturability of the aroA strain in nonsterilized water was higher for inocula prepared from late-stationary-growth-phase cultures in TSB for experiments carried out at 16°C (Fig. 2a), but there were no differences at 20°C (Fig. 2b).
In sterilized water at 16 and 20°C, both strains showed prolonged culturability periods, but strain AG2 was able to survive much longer than the mutant strain (Fig. 3). The aroA strain became nonculturable more rapidly in sterilized water at 6°C, declining to below the detection level on TSA after 42 days of incubation (Fig. 4). When TSA was supplemented with sodium pyruvate, a positive effect on culturability was clearly observed at 6°C (aroA strain) and 16°C (aroA strain and strain AG2) but not at 20°C (Fig. 3). After incubation times at which the aroA strain was nonculturable on TSA (i.e., after 42 to 45 days at 6°C, 95 days at 16°C, and 115 days at 20°C in sterilized water), resuscitation of the mutant strain on sodium pyruvate-amended TSA was observed (Fig. 3 and 4).
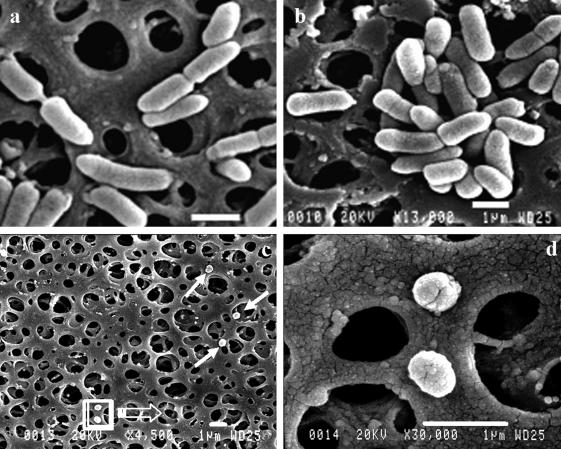
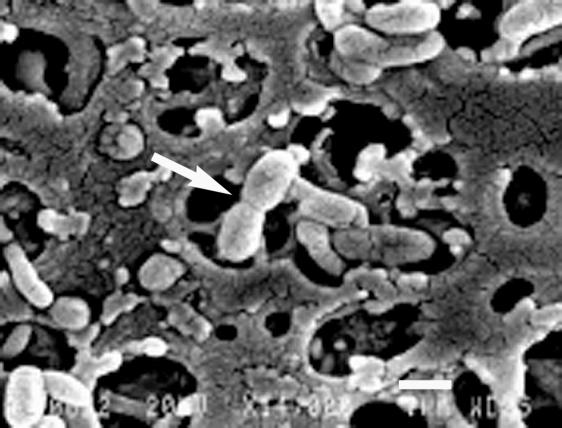
SEM studies demonstrated remarkable changes in the morphology of the aroA cells after long incubation periods in sterilized water. aroA cells collected at time zero from either early- or late-stationary-growth-phase cultures in TSB showed typical rod shapes, with average lengths of 1.5 to 2.5 μm and average diameters of 0.6 to 0.8 μm (Fig. 5a and b). AG2 cells collected from TSB cultures at the same growth phases showed a similar morphology (data not shown). After incubation in sterilized water for 90 days, aroA cells became coccoid or spheroid in shape, with a remarkable reduction in length and with diameters of ca. 0.5 μm (Fig. 5c and d). Most AG2 cells maintained coccobacillary shapes, and some dividing AG2 cells were observed (Fig. 6).
FIG. 5.
SEM images of aroA cells collected from earlier (14-h) (a) and later (24-h) (b) stationary-growth-phase cultures in TSB and after 90 days of incubation in sterilized fish tank water at 20°C (c and d). Arrows in panel c indicate coccoid cells, and the boxed field is magnified in panel d. Bars, 1 μm.
FIG. 6.
SEM image of AG2 cells after 90 days of permanence in sterilized fish tank water at 20°C. The arrow shows a dividing cell. Bar, 1 μm.
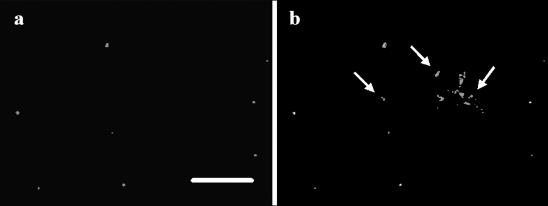
Staining of aroA cells sampled from sterilized water at 70 days of incubation at 6°C demonstrated the presence of cells with damaged (Fig. 7a) and intact (Fig. 7b) cell membranes. The use of separate filter sets to observe fluorescence for the two dyes in the bacterial viability kit on the same microscopic field allowed us to clearly distinguish cells stained with propidium iodide, which penetrates only cells with damaged membranes, from those stained with SYTO 9 dye, which labels all bacteria.
FIG. 7.
Fluorescence microscopy of a sample of aroA cells collected from a sterilized fish tank water microcosm after 70 days of incubation at 6°C. The cells were stained with the LIVE/DEAD BacLight viability kit. The images show the same field of a sample viewed for propidium iodide fluorescence (a) and for SYTO 9 fluorescence (b). The arrows in panel b indicate cells that were not stained for propidium iodide. Bar, 5 μm.
DISCUSSION
The safe use of live vaccines and/or genetically modified microorganisms requires evaluating important questions about potential hazards related to release, spread, and survival in natural environments. Our study reveals the effect of the aroA mutation on the behavior of the A. hydrophila live vaccine in freshwater at temperatures (16 to 20°C) at which the efficacy of this vaccine has been tested (74) and that are similar to those at which vaccination in fish farms is usually carried out (38) or at a temperature that can be found in salmonid rivers (6°C).
In the present study, the aroA strain was able to start to grow at 16 and 20°C, reaching a maximum cell density at 24 to 36 h; afterward, however, it rapidly lost its culturability in a microcosm containing water and fish-associated autochthonous microflora. The initial rapid growth seen with the A. hydrophila aroA strain after inoculation of nonsterilized water microcosms has also been reported for other A. hydrophila strains (10, 18) as well as for other species, such as Vibrio vulnificus (11), Pseudomonas aeruginosa (35, 69), and Photobacterium damselae subsp. damselae (21), and appears to be correlated with the water volume and the recipient surface (34).
The decline in the culturability of the aroA strain in nonsterilized fish tank water suggests an inhibitory effect of the natural aquatic microflora on the growth and survival of the mutant. A similar decline in culturability has been reported for other A. hydrophila strains in tap water (32, 43) and seawater (40) microcosms. In fish farms and natural aquatic environments, introduced or resident bacteria may be regulated by biotic or abiotic factors, such as protozoan predation (16, 23, 65, 67). When considering the effect of the growth phase at which the aroA cells were harvested to prepare the inocula, we observed differences in survival rates in nonfiltered, nonautoclaved water (higher for the cells collected from the late stationary growth phase) only at 16°C and not at 20°C; this result might have been due to the rapid growth of the autochthonous microflora at 20°C. Similar results have been reported for A. hydrophila incubated in filter-sterilized seawater (40), where differences in resistance to seawater stress between starved cells and those from the exponential growth phase were evident at 5°C but not at 20°C.
In sterilized water, wild-type strain AG2 showed a clear fitness advantage over the aroA mutant strain, probably due to the effect of the inactivation of the aroA gene. Thus, at 16 and 20°C, strain AG2 survived in sterilized water for a longer period than did the mutant strain; after 45 days of incubation, CFU counts for the wild-type strain were higher than those for the aroA strain. Maximum cell densities found in this study for wild-type strain AG2 in sterilized water were of the same order of magnitude as those previously reported for other A. hydrophila strains in various types of water (43, 61, 72).
A decline in culturability after long periods of incubation in sterilized water may be due either to cell death or to entry into a VBNC or an ANC state (6, 30, 41, 77). Rahman et al. (60) reported that A. hydrophila cultured in a 0.35% NaCl solution at pH 7.5 and 25°C for 50 days showed a VBNC state characterized by the presence of viable but nonculturable bacteria. In our study, the culturability of the aroA strain declined more rapidly at 6°C than at the higher temperatures, and it has been reported that low temperatures seem to favor entry into the VBNC or ANC state (21, 75). To clarify this point, we tested a method for in vitro resuscitation, studied the morphological changes, and assessed the viability of the nonculturable A. hydrophila cells by using an indirect labeling method to distinguish cells with damaged and intact cell membranes.
After 90 days of incubation in sterilized water, when the culturability of the aroA cells on TSA was at its lowest level, we found significant differences in cell morphology between the mutant strain and the wild-type strain. Similarly, in oligotrophic and starvation microcosms, similar changes in cell size and morphology have been described for various bacteria (20, 30). It has been proposed that reductions in cell size and in metabolic activity confer better resistance to environmental stress (8, 50).
Although indirect labeling methods to assess the viability of nonculturable bacteria are not always reliable (7), nonculturable aroA cells appearing as coccoid or round forms in our study appeared to be in a true VBNC or ANC state, as some of them were not stained for propidium iodide; this result indicates that they retained intact cell membranes. A similar pattern of staining for fluorescein diacetate and ethidium bromide was used to assess the presence of viable A. hydrophila cells in the VBNC state after prolonged incubation in a 0.35% NaCl solution (60). Moreover, aroA cells could be resuscitated on TSA supplemented with sodium pyruvate. Sodium pyruvate and H2O2-degrading agents seem to be effective compounds for recovering the physiological state after cell damage or a loss of culturability (1, 12, 46, 48, 49). In our study, amendment of TSA with sodium pyruvate notably improved the culturability of the aroA and AG2 cells. This effect was greater at 6 and 16°C than at 20°C. A similar effect of temperature on the resuscitation of A. hydrophila strain HR7 was reported by Wai et al. (75). This effect may be explained by the fact that 20°C is closer to the optimal growth temperature of the bacteria.
Therefore, attenuation of the live vaccine, which is related to the decreased ability of the bacteria to persist in host tissues (25, 74), may also modulate survival in water. Other authors have reported that mutations in strains used as live vaccines may have consequences for resistance to various environmental stresses (17, 62). The mutation in the A. hydrophila aroA gene which blocks the aromatic amino acid pathway may hinder the ability of the mutant to utilize a wide range of nutrients found in aquatic environments; this scenario would favor the disappearance of A. hydrophila aroA cells by nutrient competition in the presence of other species. This phenomenon has been demonstrated in fresh and salty waters for A. hydrophila strain NCTC 8049 transformed with plasmid pVL1013 (39).
In conclusion, this study suggests that the aroA live vaccine has a lower survival potential in water than wild-type strain AG2, and it is of particular interest that the mutant disappeared rapidly in a microcosm similar to the natural aquatic environment of fish farms. Further studies, such as those considering the effects of other culture media that would be more appropriate for the commercial production of the vaccine, are needed. However, our results contribute to a better understanding of this type of genetically modified vaccine and suggest that the inactivation of the aromatic amino acid pathway, which allows the development of attenuated live vaccines that retain a high level of antigenicity, may also affect the survival of the strain in water and contribute to the safety of these vaccines when released into natural environments.
Acknowledgments
This work was cofunded by the EU FEDER Programme and Spanish Plan Nacional de I+D project no. 1FD97-0467.
We thank Marta Fernández Caso for excellent technical assistance.
REFERENCES
- 1.Alonso, J. L., A. Soriano, O. Carbajo, I. Amorós, and H. Garelick. 1999. Comparison and recovery of Escherichia coli and thermotolerant coliforms in water with a chromogenic medium incubated at 41 and 44.5°C. Appl. Environ. Microbiol. 65:3746-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altwegg, M., and H. K. Geiss. 1989. Aeromonas as a human pathogen. Crit. Rev. Microbiol. 16:253-286. [DOI] [PubMed] [Google Scholar]
- 3.Angka, S. L., T. J. Lam, and Y. M. Sin. 1995. Some virulence characteristics of Aeromonas hydrophila in walking catfish (Clarias gariepinus). Aquaculture 130:103-112. [Google Scholar]
- 4.Austin, B., and C. Adams. 1996. Fish pathogens, p. 197-243. In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. John Wiley & Sons, Ltd., Chichester, England.
- 5.Austin, B., and D. A. Austin. 1999. Bacterial fish pathogens. Diseases of farmed and wild fish, 3rd ed. Springer Praxis, Chichester, England.
- 6.Baffone, W., B. Citterio, E. Vittoria, A. Casaroli, R. Campana, L. Falzano, and G. Donelli. 2003. Retention of virulence in viable but non-culturable halophilic Vibrio spp. Int. J. Food Microbiol. 89:31-39. [DOI] [PubMed] [Google Scholar]
- 7.Barer, M. R., and C. R. Harwood. 1999. Bacterial viability and culturability. Adv. Microb. Physiol. 41:93-137. [DOI] [PubMed] [Google Scholar]
- 8.Benjamin, M. M., and A. R. Datta. 1995. Acid tolerance of enterohemorrhagic Escherichia coli. Appl. Environ. Microbiol. 61:1669-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogosian, G., and E. V. Bourneuf. 2001. A matter of bacterial life and death. EMBO Rep. 2:770-774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brandi, G., M. Sisti, F. Giardini, F. F. Schiavano, and A. Albano. 1999. Survival ability of cytotoxic strains of motile Aeromonas spp. in different types of water. Lett. Appl. Microbiol. 29:211-215. [DOI] [PubMed] [Google Scholar]
- 11.Bryan, P. J., R. J. Steffan, A. DePaola, J. W. Foster, and A. K. Bej. 1999. Adaptive response to cold temperatures in Vibrio vulnificus. Curr. Microbiol. 38:168-175. [DOI] [PubMed] [Google Scholar]
- 12.Calabrese, J. P., and G. K. Bissonnette. 1990. Improved detection of acid mine water stressed coliform bacteria on media containing catalase and sodium pyruvate. Can. J. Microbiol. 36:544-550. [DOI] [PubMed] [Google Scholar]
- 13.Cascón, A., J. Yugueros, A. Temprano, M. Sánchez, C. Hernanz Moral, J. M. Luengo, and C. Naharro. 2000. A major secreted elastase is essential for pathogenicity of Aeromonas hydrophila. Infect. Immun. 68:3233-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascón Soriano, A., J. Anguita Castillo, C. Hernanz Moral, M. Sanchez Salazar, J. Yugueros Marcos, and G. Naharro Carrasco. 1997. RFLP-PCR analysis of the aroA gene as a taxonomic tool for the genus Aeromonas. FEMS Microbiol. Lett. 156:199-204. [DOI] [PubMed] [Google Scholar]
- 15.Chandran, M. R., B. W. Aruna, S. M. Logambal, and M. R. Dinakaran. 2002. Immunisation of Indian major carps against Aeromonas hydrophila by intraperitoneal injection. Fish Shellfish Immunol. 13:1-9. [DOI] [PubMed] [Google Scholar]
- 16.Christoffersen, K., O. Nybroe, K. Jürgens, and M. Hansen. 1997. Measurement of bacterivory by heterotrophic nanoflagellates using immunofluorescence labelling of ingested cells. Aquat. Microb. Ecol. 13:127-134. [Google Scholar]
- 17.Coynault, C., V. Robbe-Saule, and F. Norel. 1996. Virulence and vaccine potential of Salmonella typhimurium mutants deficient in the expression of the RpoS (σs) regulon. Mol. Microbiol. 22:149-160. [DOI] [PubMed] [Google Scholar]
- 18.Croci, L., S. Di Pasquale, L. Cozzi, and L. Toti. 2001. Behaviour of Aeromonas hydrophila in bottled mineral waters. J. Food Prot. 64:1836-1840. [DOI] [PubMed] [Google Scholar]
- 19.Cunningham, A. A., T. E. Langton, P. M. Bennett, J. F. Lewin, S. E. Drury, R. E. Gough, and S. K. Macgregor. 1996. Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Philos. Trans. R. Soc. Lond. Ser. B 351:1539-1557. [DOI] [PubMed] [Google Scholar]
- 20.Federighi, M., J. L. Tholozan, J. M. Cappelier, J. P. Tissier, and J. L. Jouve. 1998. Evidence of non-coccoid viable but non-culturable Campylobacter jejuni cells in microcosm water by direct viable count, CTC-DAPI double staining, and scanning electron microscopy. Food Microbiol. 15:539-550. [Google Scholar]
- 21.Fouz, B., A. E. Toranzo, E. Marco-Noales, and C. Amaro. 1998. Survival of fish-virulent strains of Photobacterium damselae subsp. damselae in seawater under starvation conditions. FEMS Microbiol. Lett. 168:181-186. [DOI] [PubMed] [Google Scholar]
- 22.González, C. J., J. A. Santos, M. L. García López, and A. Otero. 2002. Virulence markers in Aeromonas hydrophila and Aeromonas veronii biovar sobria isolates from freshwater fish and from a diarrhoea case. J. Appl. Microbiol. 93:414-419. [DOI] [PubMed] [Google Scholar]
- 23.González, J. M. 1999. Bacterivory rate estimates and fraction of active bacterivores in natural protist assemblages from aquatic systems. Appl. Environ. Microbiol. 65:1463-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazen, T. C., C. B. Fliermans, R. P. Hirsch, and G. W. Esch. 1978. Prevalence and distribution of Aeromonas hydrophila in the United States. Appl. Environ. Microbiol. 36:731-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernanz Moral, C., E. Flaño, P. López Fierro, A. Villena, J. Anguita, A. Cascón, M. Sánchez Salazar, B. Razquin Peralta, and G. Naharro Carrasco. 1998. Molecular characterization of the Aeromonas hydrophila aroA gene and potential use of an auxotrophic aroA mutant as a live attenuated vaccine. Infect. Immun. 66:1813-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmes, P., L. M. Niccolls, and D. P. Sartory. 1996. The ecology of mesophilic Aeromonas in the aquatic environment, p. 127-150. In B. Austin, M. Altwegg, P. J. Gosling, and S. W. Joseph (ed.), The genus Aeromonas. John Wiley & Sons, Ltd., Chichester, England.
- 27.Holmgren, J., J. Clemens, D. A. Sack, and A. M. Svennerholm. 1989. New cholera vaccines. Vaccine 7:94-96. [DOI] [PubMed] [Google Scholar]
- 28.Janda, J. M. 1991. Recent advances in the study of the taxonomy, pathogenicity, and infectious syndromes associated with the genus Aeromonas. Clin. Microbiol. Rev. 4:397-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janda, J. M., and P. S. Duffey. 1988. Mesophilic Aeromonas in human disease: current taxonomy, laboratory identification, and infectious disease spectrum. Rev. Infect. Dis. 10:980-997. [DOI] [PubMed] [Google Scholar]
- 30.Jiang, X., and T. J. Chai. 1996. Survival of Vibrio parahaemolyticus at low temperatures under starvation conditions and subsequent resuscitation of viable, nonculturable cells. Appl. Environ. Microbiol. 62:1300-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joux, F., and P. Lebaron. 2000. Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microb. Infect. 2:1523-1535. [DOI] [PubMed] [Google Scholar]
- 32.Kersters, I., G. Huys, H. Van Duffel, M. Vancanneyt, K. Kersters, and W. Verstraete. 1996. Survival potential of Aeromonas hydrophila in freshwaters and nutrient-poor waters in comparison with other bacteria. J. Appl. Bacteriol. 80:266-276. [DOI] [PubMed] [Google Scholar]
- 33.Lamers, C. H. J., and W. B. Van Muiswinkel. 1986. Natural and acquired agglutinins to Aeromonas hydrophila in carp (Cyprinus carpio). Can. J. Fish. Aquat. Sci. 43:619-624. [Google Scholar]
- 34.Leclerc, H., and A. Moreau. 2002. Microbiological safety of natural mineral water. FEMS Microbiol. Rev. 26:207-222. [DOI] [PubMed] [Google Scholar]
- 35.Legnani, P., E. Leoni, S. Rapuano, D. Turin, and C. Valenti. 1999. Survival and growth of Pseudomonas aeruginosa in natural mineral water: a 5-year study. Int. J. Food Microbiol. 53:153-158. [DOI] [PubMed] [Google Scholar]
- 36.Liew, F. Y. 1991. The effector mechanism and vaccination against cutaneous leishmaniasis. Behring Inst. Mitt. 88:239-243. [PubMed] [Google Scholar]
- 37.Loghothetis, P. N., and B. Austin. 1994. Immune response of rainbow trout (Oncorhynchus mykiss, Walbaum) to Aeromonas hydrophila. Fish Shellfish Immunol. 4:239-254. [DOI] [PubMed] [Google Scholar]
- 38.Loghothetis, P. N., and B. Austin. 1996. Variations in antigenicity of Aeromonas hydrophila strains in rainbow trout (Oncorhynchus mykiss, Walbaum): an association with surface characteristics. Fish Shellfish Immunol. 6:47-55. [Google Scholar]
- 39.Lowcock, D., and C. Edwards. 1994. Survival of genetically-marked Aeromonas hydrophila in water. Lett. Appl. Microbiol. 19:121-123. [Google Scholar]
- 40.Maalej, S., M. Denis, and S. Dukan. 2004. Temperature and growth-phase effects on Aeromonas hydrophila survival in natural seawater microcosms: role of protein synthesis and nucleic acid content on viable but temporarily nonculturable response. Microbiology 150:181-187. [DOI] [PubMed] [Google Scholar]
- 41.Magariños, B., J. L. Romalde, J. L. Barja, and A. E. Toranzo. 1994. Evidence of a dormant but infective state of the fish pathogen Pasteurella piscicida in seawater and sediment. Appl. Environ. Microbiol. 60:180-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mani, S., M. Sadish, and V. T. Andriole. 1995. Clinical spectrum of Aeromonas hydrophila infections: report of 11 cases in a community hospital and review. Infect. Dis. Clin. Pract. 4:79-86. [Google Scholar]
- 43.Mary, P., G. Buchet, C. Defives, and J. P. Hornez. 2001. Growth and survival of clinical vs. environmental species of Aeromonas in tap water. Int. J. Food Microbiol. 69:191-198. [DOI] [PubMed] [Google Scholar]
- 44.Mary, P., N. E. Chihib, O. Charafeddine, C. Defives, and J. P. Hornez. 2002. Starvation survival and viable but nonculturable states in Aeromonas hydrophila. Microb. Ecol. 43:250-258. [DOI] [PubMed] [Google Scholar]
- 45.Massa, S., C. Altieri, and A. D′Angela. 2001. The occurrence of Aeromonas spp. in natural mineral water and well water. Int. J. Food Microbiol. 63:169-173. [DOI] [PubMed] [Google Scholar]
- 46.McDonald, L. C., C. R. Hackney, and B. Ray. 1983. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl. Environ. Microbiol. 45:360-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Messi, P., E. Guerrieri, and M. Bondi. 2002. Survival of an Aeromonas hydrophila in an artificial mineral water microcosm. Water Res. 36:3410-3415. [DOI] [PubMed] [Google Scholar]
- 48.Mizunoe, Y., S. Wai, A. Takade, and S. Yoshida. 1999. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch. Microbiol. 172:63-67. [DOI] [PubMed] [Google Scholar]
- 49.Mizunoe, Y., S. Wai, T. Ishikawa, A. Takade, and S. Yoshida. 2000. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol. Lett. 186:115-120. [DOI] [PubMed] [Google Scholar]
- 50.Morita, R. Y. 1993. Bioavailability of energy and the starvation state, p. 81-102. In S. Kjelleberg (ed.), Starvation in bacteria. Plenum Press, New York, N.Y.
- 51.Newman, S. G. 1993. Bacterial vaccines for fish. Annu. Rev. Fish Dis. 3:145-185. [Google Scholar]
- 52.Ogara, W. O., P. G. Mbuthia, H. F. A. Kaburia, H. Sørum, D. K. Kagunya, D. I. Nduthu, and D. Colquhoun. 1998. Motile aeromonads associated with rainbow trout (Oncorhynchus mykiss) mortality in Kenya. Bull. Eur. Assoc. Fish Pathol. 18:7-9. [Google Scholar]
- 53.Ormen, O., and O. Ostensvik. 2001. The occurrence of aerolysin-positive Aeromonas spp. and their cytotoxicity in Norwegian water sources. J. Appl. Microbiol. 90:797-802. [DOI] [PubMed] [Google Scholar]
- 54.Paniagua, C., O. Rivero, J. Anguita, and G. Naharro. 1990. Pathogenicity factors and virulence for rainbow trout (Salmo gairdneri) of motile Aeromonas spp. isolated from a river. J. Clin. Microbiol. 28:350-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pathiratne, A., and R. P. P. K. Jayasinghe. 2001. Environmental influence on the occurrence of epizootic ulcerative syndrome (EUS) in freshwater fish in Bellanwila-Attidia wetlands, Sri Lanka. J. Appl. Ichthyol. 17:30-34. [Google Scholar]
- 56.Pathiratne, A., G. S. Widanpathirana, and W. H. S. Chandrakanthi. 1994. Association of Aeromonas hydrophila with epizootic ulcerative syndrome (EUS) of freshwater fish in Sri Lanka. J. Appl. Ichthyol. 10:204-208. [Google Scholar]
- 57.Pearson, M. D., I. Hirono, T. Aoki, R. Miranda, and V. Inglis. 2000. Virulence properties of motile aeromonads isolated from farmed frogs Rana tigerina and R. rugulosa. Dis. Aquat. Org. 40:185-193. [DOI] [PubMed] [Google Scholar]
- 58.Pittard, A. J. 1987. Biosynthesis of the aromatic amino acids, p. 368-394. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 59.Rahman, M. H., and K. Kawai. 2000. Outer membrane proteins of Aeromonas hydrophila induce protective immunity in goldfish. Fish Shellfish Immunol. 10:379-382. [DOI] [PubMed] [Google Scholar]
- 60.Rahman, M. H., S. Suzuki, and K. Kawai. 2001. Formation of viable but non-culturable state (VBNC) of Aeromonas hydrophila and its virulence in goldfish, Carassius auratus. Microbiol. Res. 156:103-106. [DOI] [PubMed] [Google Scholar]
- 61.Rippey, S. R., M. A. Troy, and V. J. Cabelli. 1994. Growth kinetics of Aeromonas hydrophila in freshwaters supplemented with various organic and inorganic nutrients. World J. Microbiol. Biotechnol. 10:159-164. [DOI] [PubMed] [Google Scholar]
- 62.Robbe-Saule, V., C. Coynault, and F. Norel. 1995. The live oral typhoid vaccine Ty21a is a rpoS mutant and is susceptible to various environmental stresses. FEMS Microbiol. Lett. 126:171-176. [DOI] [PubMed] [Google Scholar]
- 63.Roberts, M., J. Li, A. Bacon, and S. Chatfield. 1998. Oral vaccination against tetanus: comparison of the immunogenicities of Salmonella strains expressing fragment C from the nirB and htrA promoters. Infect. Immun. 66:3080-3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Santos, J. A., C. J. González, A. Otero, and M. L. García López. 1999. Hemolytic activity and siderophore production in different Aeromonas species isolated from fish. Appl. Environ. Microbiol. 65:5612-5614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sherr, E. B., and B. F. Sherr. 1994. Bacterivory and hervibory: key roles of phagotrophic protists in pelagic food webs. Microb. Ecol. 28:223-235. [DOI] [PubMed] [Google Scholar]
- 66.Shotts, E. B., J. L. Gaines, L. Martin, and A. K. Prestwood. 1972. Aeromonas-induced deaths among fish and reptiles in an eutrophic inland lake. J. Am. Vet. Med. Assoc. 161:603-607. [PubMed] [Google Scholar]
- 67.Sibille, I., T. Sime-Ngando, L. Mathieu, and J. C. Block. 1998. Protozoan bacterivory and Escherichia coli survival in drinking water distribution systems. Appl. Environ. Microbiol. 64:197-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stocker, B. A. 1988. Auxotrophic Salmonella typhi as live vaccine. Vaccine 6:141-145. [DOI] [PubMed] [Google Scholar]
- 69.Tamagnini, L. M., and R. D. Gonzalez. 1997. Bacteriological stability and growth kinetics of Pseudomonas aeruginosa in bottled water. J. Appl. Microbiol. 83:91-94. [DOI] [PubMed] [Google Scholar]
- 70.Thornley, J. P., I. E. Shaw, I. A. Gryllos, and A. Eley. 1997. Virulence properties of clinically significant Aeromonas species: evidence for pathogenicity. Rev. Med. Microbiol. 8:61-72. [Google Scholar]
- 71.Thune, R. L., D. H. Fernandez, and J. R. Battista. 1999. An aroA mutant of Edwardsiella ictaluri is safe and efficacious as a live, attenuated vaccine. J. Aquat. Anim. Health 11:358-372. [Google Scholar]
- 72.Tsai, G. J., and S. C. Yu. 1997. Microbiological evaluation of bottled uncarbonated mineral water in Taiwan. Int. J. Food Microbiol. 37:137-143. [DOI] [PubMed] [Google Scholar]
- 73.Vaughan, L. M., P. R. Smith, and T. J. Foster. 1993. An aromatic-dependent mutant of the fish pathogen Aeromonas salmonicida is attenuated in fish and is effective as a live vaccine against the salmonid disease furunculosis. Infect. Immun. 61:2172-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vivas J., J. Riaño, B. Carracedo, B. E. Razquin, P. López-Fierro, G. Naharro, and A. J. Villena. 2003. The auxotrophic aroA mutant of Aeromonas hydrophila as a live attenuated vaccine against A. salmonicida infections in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 16:193-206. [DOI] [PubMed] [Google Scholar]
- 75.Wai, S. N., Y. Mizunoe, A. Takade, A., and S. Yoshida. 2000. A comparison of solid and liquid media for resuscitation of starvation- and low-temperature-induced nonculturable cells of Aeromonas hydrophila. Arch. Microbiol. 173:307-310. [DOI] [PubMed] [Google Scholar]
- 76.Ward, S. J., G. Douce, D. Figueiredo, G. Dougan, and B. W. Wren. 1999. Immunogenicity of a Salmonella typhimurium aroA aroD vaccine expressing a nontoxic domain of Clostridium difficile toxin A. Infect. Immun. 67:2145-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Whitesides, M. D., and J. D. Oliver. 1997. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl. Environ. Microbiol. 63:1002-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]