Abstract
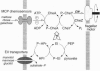
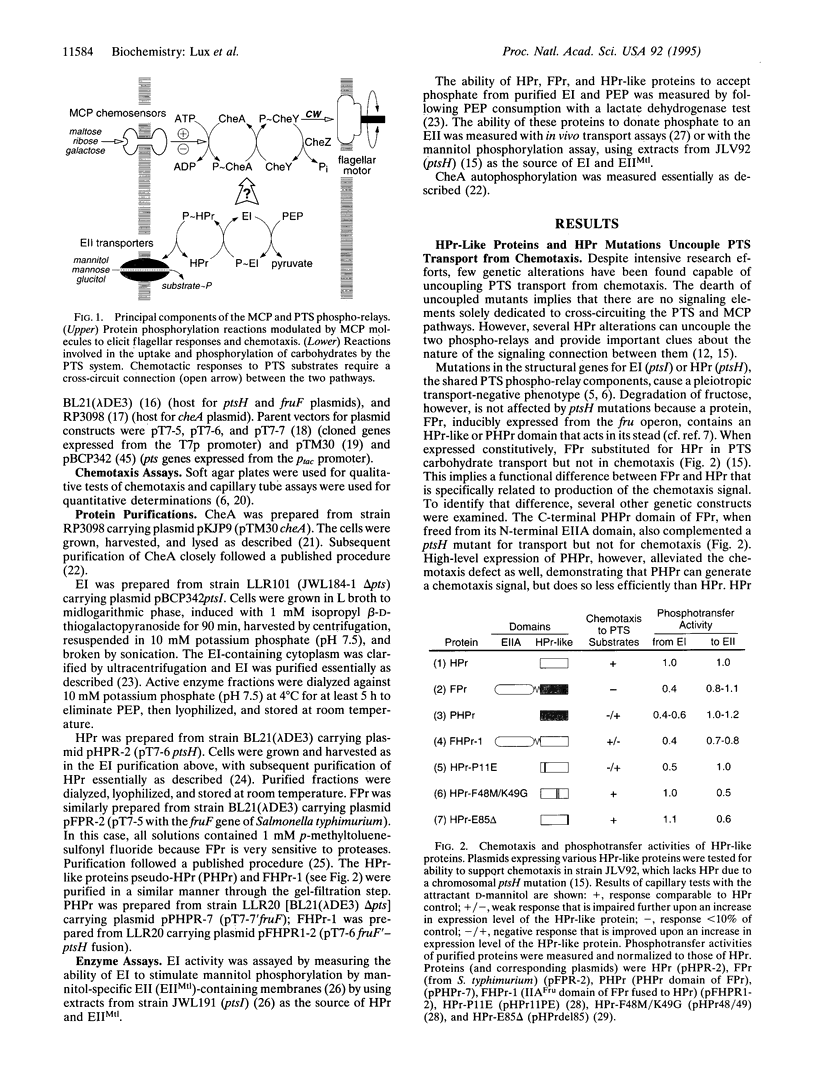
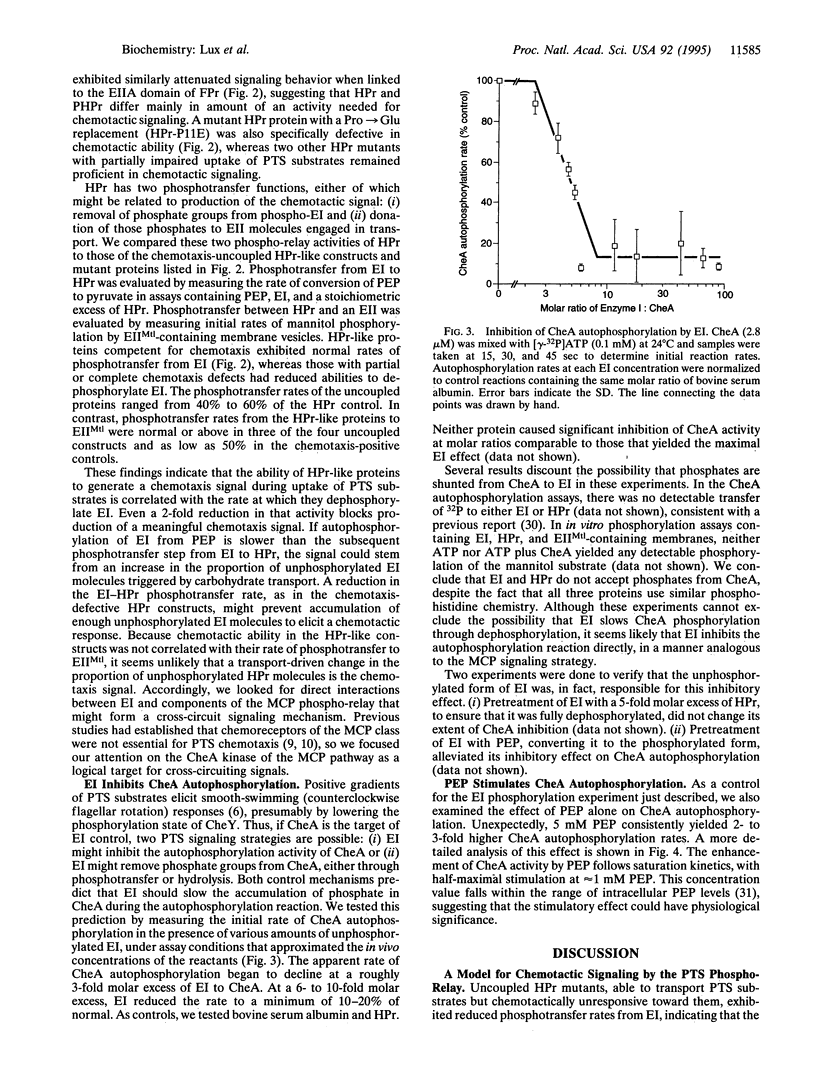
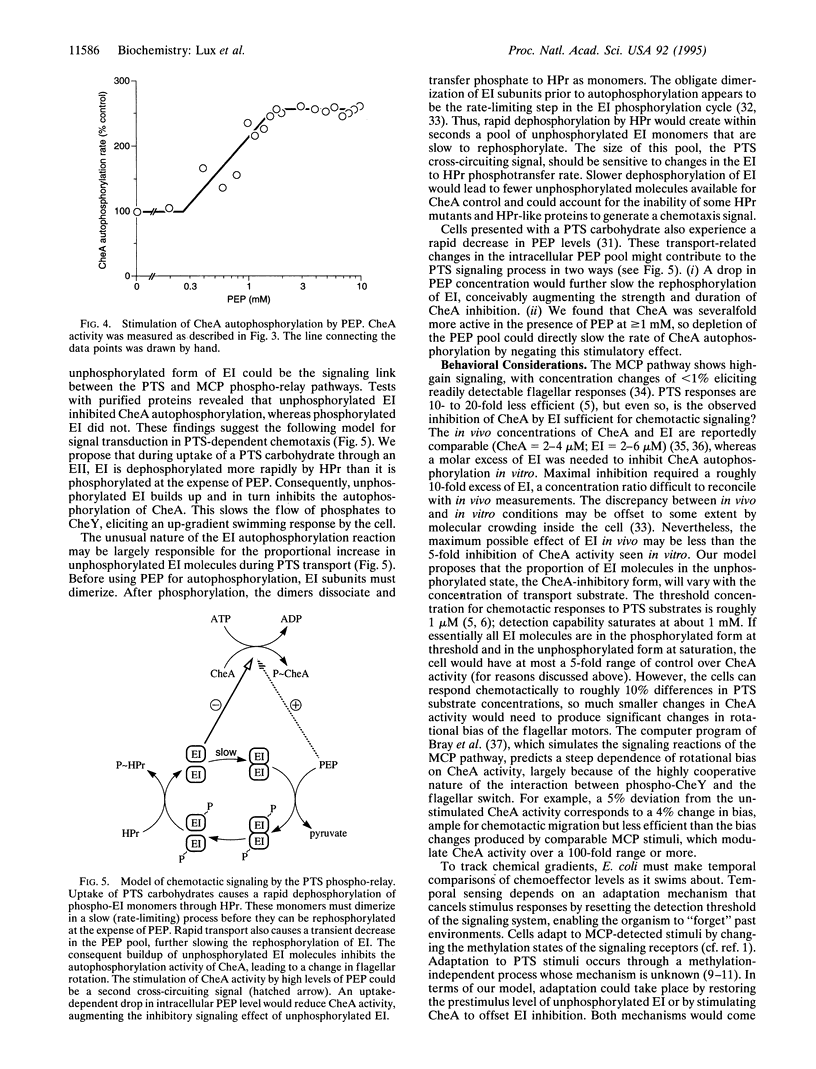
Chemotactic responses in Escherichia coli are typically mediated by transmembrane receptors that monitor chemoeffector levels with periplasmic binding domains and communicate with the flagellar motors through two cytoplasmic proteins, CheA and CheY. CheA autophosphorylates and then donates its phosphate to CheY, which in turn controls flagellar rotation. E. coli also exhibits chemotactic responses to substrates that are transported by the phosphoenolpyruvate (PEP)-dependent carbohydrate phosphotransferase system (PTS). Unlike conventional chemoreception, PTS substrates are sensed during their uptake and concomitant phosphorylation by the cell. The phosphoryl groups are transferred from PEP to the carbohydrates through two common intermediates, enzyme I (EI) and phosphohistidine carrier protein (HPr), and then to sugar-specific enzymes II. We found that in mutant strains HPr-like proteins could substitute for HPr in transport but did not mediate chemotactic signaling. In in vitro assays, these proteins exhibited reduced phosphotransfer rates from EI, indicating that the phosphorylation state of EI might link the PTS phospho-relay to the flagellar signaling pathway. Tests with purified proteins revealed that unphosphorylated EI inhibited CheA autophosphorylation, whereas phosphorylated EI did not. These findings suggest the following model for signal transduction in PTS-dependent chemotaxis. During uptake of a PTS carbohydrate, EI is dephosphorylated more rapidly by HPr than it is phosphorylated at the expense of PEP. Consequently, unphosphorylated EI builds up and inhibits CheA autophosphorylation. This slows the flow of phosphates to CheY, eliciting an up-gradient swimming response by the cell.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. A method for measuring chemotaxis and use of the method to determine optimum conditions for chemotaxis by Escherichia coli. J Gen Microbiol. 1973 Jan;74(1):77–91. doi: 10.1099/00221287-74-1-77. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames P., Parkinson J. S. Constitutively signaling fragments of Tsr, the Escherichia coli serine chemoreceptor. J Bacteriol. 1994 Oct;176(20):6340–6348. doi: 10.1128/jb.176.20.6340-6348.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. W., Bhanot P., Georges F., Klevit R. E., Waygood E. B. Involvement of the carboxy-terminal residue in the active site of the histidine-containing protein, HPr, of the phosphoenolpyruvate:sugar phosphotransferase system of Escherichia coli. Biochemistry. 1991 Oct 8;30(40):9601–9607. doi: 10.1021/bi00104a006. [DOI] [PubMed] [Google Scholar]
- Barak R., Eisenbach M. Fumarate or a fumarate metabolite restores switching ability to rotating flagella of bacterial envelopes. J Bacteriol. 1992 Jan;174(2):643–645. doi: 10.1128/jb.174.2.643-645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyreuther K., Raufuss H., Schrecker O., Hengstenberg W. The phosphoenolpyruvate-dependent phosphotransferase system of Staphylococcus aureus. 1. Amino-acid sequence of the phosphocarrier protein HPr. Eur J Biochem. 1977 May 2;75(1):275–286. doi: 10.1111/j.1432-1033.1977.tb11527.x. [DOI] [PubMed] [Google Scholar]
- Bourret R. B., Borkovich K. A., Simon M. I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- Bray D., Bourret R. B., Simon M. I. Computer simulation of the phosphorylation cascade controlling bacterial chemotaxis. Mol Biol Cell. 1993 May;4(5):469–482. doi: 10.1091/mbc.4.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvin F., Brand L., Roseman S. Sugar transport by the bacterial phosphotransferase system. Characterization of the Escherichia coli enzyme I monomer/dimer transition kinetics by fluorescence anisotropy. J Biol Chem. 1994 Aug 12;269(32):20270–20274. [PubMed] [Google Scholar]
- Gegner J. A., Dahlquist F. W. Signal transduction in bacteria: CheW forms a reversible complex with the protein kinase CheA. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):750–754. doi: 10.1073/pnas.88.3.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegner J. A., Graham D. R., Roth A. F., Dahlquist F. W. Assembly of an MCP receptor, CheW, and kinase CheA complex in the bacterial chemotaxis signal transduction pathway. Cell. 1992 Sep 18;70(6):975–982. doi: 10.1016/0092-8674(92)90247-a. [DOI] [PubMed] [Google Scholar]
- Grisafi P. L., Scholle A., Sugiyama J., Briggs C., Jacobson G. R., Lengeler J. W. Deletion mutants of the Escherichia coli K-12 mannitol permease: dissection of transport-phosphorylation, phospho-exchange, and mannitol-binding activities. J Bacteriol. 1989 May;171(5):2719–2727. doi: 10.1128/jb.171.5.2719-2727.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grübl G., Vogler A. P., Lengeler J. W. Involvement of the histidine protein (HPr) of the phosphotransferase system in chemotactic signaling of Escherichia coli K-12. J Bacteriol. 1990 Oct;172(10):5871–5876. doi: 10.1128/jb.172.10.5871-5876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess J. F., Oosawa K., Kaplan N., Simon M. I. Phosphorylation of three proteins in the signaling pathway of bacterial chemotaxis. Cell. 1988 Apr 8;53(1):79–87. doi: 10.1016/0092-8674(88)90489-8. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W. Polyhydric alcohol transport by bacteria. Methods Enzymol. 1986;125:473–485. doi: 10.1016/s0076-6879(86)25037-5. [DOI] [PubMed] [Google Scholar]
- Lengeler J. W., Vogler A. P. Molecular mechanisms of bacterial chemotaxis towards PTS-carbohydrates. FEMS Microbiol Rev. 1989 Jun;5(1-2):81–92. doi: 10.1016/0168-6445(89)90011-9. [DOI] [PubMed] [Google Scholar]
- Lengeler J., Auburger A. M., Mayer R., Pecher A. The phosphoenolpyruvate-dependent carbohydrate: phosphotransferase system enzymes II as chemoreceptors in chemotaxis of Escherichia coli K 12. Mol Gen Genet. 1981;183(1):163–170. doi: 10.1007/BF00270156. [DOI] [PubMed] [Google Scholar]
- Lengeler J. Mutations affecting transport of the hexitols D-mannitol, D-glucitol, and galactitol in Escherichia coli K-12: isolation and mapping. J Bacteriol. 1975 Oct;124(1):26–38. doi: 10.1128/jb.124.1.26-38.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LiCalsi C., Crocenzi T. S., Freire E., Roseman S. Sugar transport by the bacterial phosphotransferase system. Structural and thermodynamic domains of enzyme I of Salmonella typhimurium. J Biol Chem. 1991 Oct 15;266(29):19519–19527. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Maddock J. R., Shapiro L. Polar location of the chemoreceptor complex in the Escherichia coli cell. Science. 1993 Mar 19;259(5102):1717–1723. doi: 10.1126/science.8456299. [DOI] [PubMed] [Google Scholar]
- Mattoo R. L., Waygood E. B. Determination of the levels of HPr and enzyme I of the phosphoenolpyruvate-sugar phosphotransferase system in Escherichia coli and Salmonella typhimurium. Can J Biochem Cell Biol. 1983 Jan;61(1):29–37. doi: 10.1139/o83-005. [DOI] [PubMed] [Google Scholar]
- Morrison T. B., Parkinson J. S. Liberation of an interaction domain from the phosphotransfer region of CheA, a signaling kinase of Escherichia coli. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5485–5489. doi: 10.1073/pnas.91.12.5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwano M., Taylor B. L. Novel sensory adaptation mechanism in bacterial chemotaxis to oxygen and phosphotransferase substrates. Proc Natl Acad Sci U S A. 1982 Jan;79(1):11–15. doi: 10.1073/pnas.79.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson J. S., Kofoid E. C. Communication modules in bacterial signaling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- Parkinson J. S. Signal transduction schemes of bacteria. Cell. 1993 Jun 4;73(5):857–871. doi: 10.1016/0092-8674(93)90267-t. [DOI] [PubMed] [Google Scholar]
- Pertierra A. G., Cooper R. A. Pyruvate formation during the catabolism of simple hexose sugars by Escherichia coli: studies with pyruvate kinase-negative mutants. J Bacteriol. 1977 Mar;129(3):1208–1214. doi: 10.1128/jb.129.3.1208-1214.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postma P. W., Lengeler J. W., Jacobson G. R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993 Sep;57(3):543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S., Meadow N. D. Signal transduction by the bacterial phosphotransferase system. Diauxie and the crr gene (J. Monod revisited). J Biol Chem. 1990 Feb 25;265(6):2993–2996. [PubMed] [Google Scholar]
- Segall J. E., Block S. M., Berg H. C. Temporal comparisons in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8987–8991. doi: 10.1073/pnas.83.23.8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. A., Parkinson J. S. Overlapping genes at the cheA locus of Escherichia coli. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5370–5374. doi: 10.1073/pnas.77.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Sutrina S. L., Chin A. M., Esch F., Saier M. H., Jr Purification and characterization of the fructose-inducible HPr-like protein, FPr, and the fructose-specific enzyme III of the phosphoenolpyruvate: sugar phosphotransferase system of Salmonella typhimurium. J Biol Chem. 1988 Apr 15;263(11):5061–5069. [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. A bacteriophage T7 RNA polymerase/promoter system for controlled exclusive expression of specific genes. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1074–1078. doi: 10.1073/pnas.82.4.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waygood E. B., Steeves T. Enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system of Escherichia coli. Purification to homogeneity and some properties. Can J Biochem. 1980 Jan;58(1):40–48. doi: 10.1139/o80-006. [DOI] [PubMed] [Google Scholar]
- Weigel N., Kukuruzinska M. A., Nakazawa A., Waygood E. B., Roseman S. Sugar transport by the bacterial phosphotransferase system. Phosphoryl transfer reactions catalyzed by enzyme I of Salmonella typhimurium. J Biol Chem. 1982 Dec 10;257(23):14477–14491. [PubMed] [Google Scholar]
- Weng Q. P., Elder J., Jacobson G. R. Site-specific mutagenesis of residues in the Escherichia coli mannitol permease that have been suggested to be important for its phosphorylation and chemoreception functions. J Biol Chem. 1992 Sep 25;267(27):19529–19535. [PubMed] [Google Scholar]
- van der Vlag J., van't Hof R., van Dam K., Postma P. W. Control of glucose metabolism by the enzymes of the glucose phosphotransferase system in Salmonella typhimurium. Eur J Biochem. 1995 May 15;230(1):170–182. doi: 10.1111/j.1432-1033.1995.0170i.x. [DOI] [PubMed] [Google Scholar]