Abstract
Background
ClinicalTrials.gov is an NIH-sponsored registry of federally and privately funded trials. We sought to determine fundamental characteristics of registered pediatric cardiovascular trials (PCVTs).
Methods
A data set including 68134 interventional clinical trials was downloaded from ClinicalTrials.gov and entered into a relational database. Aggregate data from PCVTs were compared with other trial specialties. Multivariable logistic regression was used to evaluate factors associated with improved trial quality metrics including blinding and randomization.
Results
Between 7/01/2005 and 9/27/2010, 5035 (7%) registered trials targeted pediatric populations, including 213 PCVTs (4.2%), 1176 pediatric infectious disease trials (23%), 664 pediatric mental health trials (13%) and 346 pediatric hematology/oncology trials (7%). Median (IQR) PCVT enrollment was 65 subjects (36–186) and median study duration was 2.3 years (1.3–3.7). The most common PCVTs targeted acquired diseases including hypertension (n=41, 14%), obesity (n=26, 9%), pulmonary hypertension (n=25, 9%) and dyslipidemia (n=19, 7%). Important factors associated with improved quality metrics included: NIH as opposed to industry funding (OR=1.9, p<0.0001); trial location (trials with both U.S.and foreign enrollment vs. trials with US only or foreign only enrollment, p=0.02) and trials restricted to younger children as opposed to trials including adolescents (OR=1.4, p<0.0001).
Conclusion
PCVTs represent a small proportion of clinical trials relative to other pediatric sub-specialties. Most PCVTs tend to parallel adult morbidities while there is a relative paucity of trials focused on congenital heart disease. These data may be useful to stakeholders in informing decisions regarding the conduct of PCVTs, and to provide insight into mechanisms to advance PCVT infrastructure.
Keywords: Pediatrics, Clinical Trial, Congenital Heart Disease, Trial Quality, Funding
Introduction
Nearly 40,000 children are born with congenital heart defects each year and an estimated 1.6 million children and adults are living in the United States with congenital heart disease. This latter number is expected to increase by 1–5% annually due to improved survival.1 Furthermore, childhood acquired cardiovascular diseases, such as hypertension and obesity, have quadrupled in prevalence over the preceding several decades.2 Thus childhood cardiovascular diseases are an important and increasing contributor to the societal health care burden.1, 3, 4
Clinical trials are the most effective means for evaluating preventive, diagnostic and therapeutic strategies and therefore reducing the health care burden.5 For decades pediatric clinical trials have been neglected with pediatrics described as the “therapeutic orphan”.6 While congressional passage of the Best Pharmaceuticals for Children Act (BPCA) created mandates and incentives that have stimulated a much-needed commitment to pediatric clinical trials, the incentive structure favors study of blockbuster adult agents and not necessarily the most needed pediatric agents.7–9 This has sparked recent debate regarding whether the pediatric clinical trial landscape appropriately represents the burden of pediatric disease.10, 11
ClinicalTrials.gov is a registry of clinical trials that was mandated by Congress and implemented in 2000 by the National Library of Medicine, NIH. Since July 1, 2005, the International Committee of Medical Journal Editors (ICMJE) has required registration of interventional trials in a public trials registry (such as ClinicalTrials.gov) as a condition for publication.12, 13 In addition, in 2007, Congress passed legislation (Section 801 of the U.S. Food and Drug Administration [FDA] Amendments Act) expanding the legal requirements to register trials and report key data elements and basic trial results at ClinicalTrials.gov.14
We sought to describe the pediatric cardiovascular trial (PCVT) portfolio using ClinicalTrials.gov data. Secondary aims included comparing PCVT characteristics with other major pediatric sub-specialties and with adult cardiovascular trials, to describe trends in PCVTs over time, and to assess factors associated with trial quality metrics.
Methods
Data Source
A data set of 96 346 clinical studies registered at ClinicalTrials.gov was downloaded on September 27, 2010. The data set was locked, and a relational database was subsequently designed to facilitate analysis.15
Study Selection
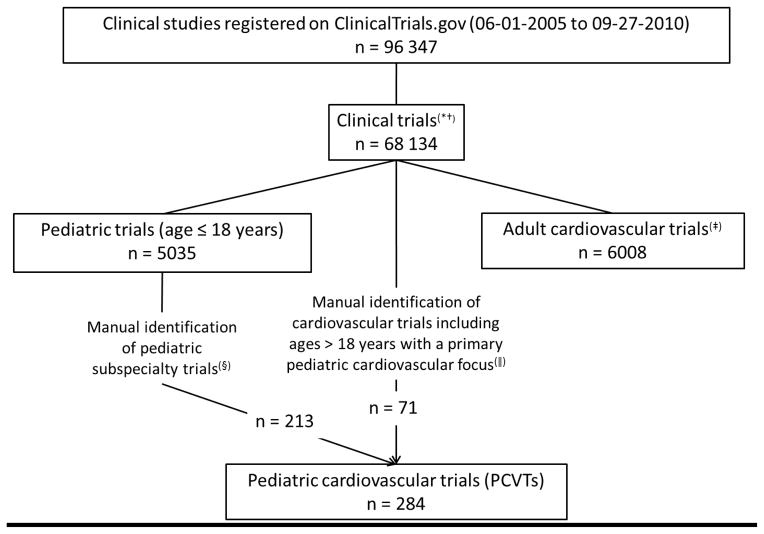
FIGURE I outlines criteria and methods for study selection. All trials entered as a study type of “interventional” registered from July 1, 2005 (the date ICMJE guidelines took effect) to September 27, 2010, were eligible for inclusion. PCVTs enrolling only those ≤ 18 years of age were compared with other pediatric specialty trials as well as with adult cardiovascular trials (those restricting enrollment to ages ≥ 18 years).
Figure I.
Consortium diagram: Inclusion criteria and methods for study selection.
*Clinical Trials refers to those trials registered as “interventional studies” on ClinicalTrials.gov. Interventional studies are defined by ClinicalTrials.gov as those in which an investigator assigns an intervention (including diagnostic, therapeutic or other types of interventions) based on a protocol.16
† Non-interventional or observational studies were excluded because there are no current registration requirements at ClinicalTrials.gov.
‡Methods for identification of adult cardiovascular trials described previously. These trials exclude trials enrolling participants < 18 years.15
§ Trials classified into pediatric subspecialties based on manual review of the trial characteristics as previously reported.11
||A pediatric cardiologist reviewed all adult cardiovascular trials that also enrolled subjects < 18 years of age and identified those that focused on pediatric cardiovascular diseases or conditions.
Data Collection
Trial data are reported by trial sponsors or investigators using a Web-based system.12 Each record contains a set of data elements describing the study’s purpose, recruitment/enrollment, design, eligibility criteria, location, sponsor, and other protocol information; standard definitions are used.16 Publication of main trial results was assessed based on review of publications reported on ClinicalTrials.gov. When this data field was incomplete, a PubMed review using the ClinicalTrials.gov identifier was conducted by a single physician to identify publications containing trial results. If no publications were identified then an additional search was conducted using the listed principal investigator as well key study identifiers.
Derived Variables
For pediatric trials, age eligibility was defined using the submitted minimum and maximum age criteria. Funding source was derived using information about the lead sponsor (the organization that oversees implementation of the study and data analysis) and collaborators (organizations that provide support including funding). Funding source was defined as NIH if the lead sponsor or any collaborators were from NIH, and the lead sponsor was not from industry. Funding source was defined as industry if the lead sponsor was from industry or if any collaborators were from industry and there was no NIH involvement. For the remaining studies, funding source was defined as from non-NIH, non-industry sources. The primary purpose of the trial was described as Treatment, Prevention or Diagnostic as entered in the database, with Supportive Care, Screening, Health Services Research, and Basic Science grouped with Other. Trials were classified as early phase (phase 0, 1, 1/2, or 2), late phase (phase 2/3, 3, or 4), or phase not applicable. Each trial was assigned to a mutually exclusive group based on the interventions listed: Procedure/Device if the trial included a procedure or device intervention, Drug/Biological if the trial included a drug or biological intervention, but not a procedure/device intervention, Behavioral if the trial included a behavioral intervention but not a Procedure/Device/Drug/Biological intervention, and remaining studies with dietary supplement, genetic, radiation, or other interventions were classified as having Other interventions. The question about appointment of a data monitoring committee (DMC) became available in April 2007, and is not a required field. Studies for which this information was missing were classified as DMC Unknown, and included in analysis.
Analysis
Trial characteristics were described using standard summary statistics. Categorical variables were reported as proportions and continuous variables as medians and interquartile ranges. Missing values were excluded from analysis unless indicated. To describe trends in time, pediatric studies were evaluated over two time periods; 06-2005 to 09-2007 (era 1, before the FDA Amendments Act registry requirements were implemented), and 10-2007 to 09-2010 (era 2, after implementation). Period was derived using the date the trial was first registered with ClinicalTrials.gov. DMC use was summarized only for studies registered from 10-2007 to 09-2010. Due to the descriptive nature of the study, formal statistical comparisons were not made between trial types. However prior to data review, meaningful differences were defined including: 1) for categorical variables, a difference of at least 5 percentage points, or a relative increment of at least 1.5; and 2) for enrollment, a shift in median enrollment of at least 25 participants. Multivariable logistic regression was used to evaluate factors associated with simultaneous use of blinding (single or double blind) and randomization. These metrics have been previously reported as reasonable measures of trial quality in the ClinicalTrials.gov dataset.17 This regression analysis was restricted to pediatric trials enrolling those ≤ 18 years. Factors selected a priori for inclusion in the regression model included: therapeutic area (PCVTs versus all other pediatric trials), funding source, study phase, enrollment, intervention type, registration period, primary purpose, U.S. / non-U.S. location of trial sites, and adolescent eligibility. Significant interactions between therapeutic area and other covariates were identified by backward variable selection, and retained in the model if the Wald chi-square p-value for the interaction term was less than 0.05. A 2-tailed p value < 0.05 was considered statistically significant, and associations between covariates and simultaneous use of randomization and blinding were quantified with odds ratios and 95% confidence intervals.
Results
PCVT characteristics and comparison across age groups
From July 1, 2005 to September 27, 2010, 68 134 trials were registered at ClinicalTrials.gov. Of these, 5035 were pediatric trials and of the pediatric trials, 213 (4.2%) focused on pediatric cardiovascular interventions. After manual review, an additional 71 studies that enrolled both children and adults were classified as PCVTs. (FIGURE I).
Baseline PCVT characteristics and comparisons by age groups eligible for enrollment are presented in TABLE I with additional details in eTABLE I. Median trial enrollment was 65 subjects with 6/284 (2%) trials enrolling > 1000 subjects. Median time to trial completion was 2.3 years. Trial interventions included drugs/biologics (61%), procedures/devices (20%) and behavioral interventions (11%). Overall there were relatively few differences across age ranges. Notable exceptions were that: 1) more early phase trials were conducted in neonates/infants; and 2) trials restricted to pediatric subjects (age ≤ 18 years) more often tested drugs or biologics while a higher proportion of trials that included adult subjects tested procedures and devices.
Table I.
Characteristics of pediatric cardiovascular trials by age eligibility
| All PCVTs | Neonates/infants (*)(0–1 yr) | Child (*)(1–12 yrs) | Adolescent (*)(12–18 yrs) | Adult (*,†)(> 18 yrs) | |
|---|---|---|---|---|---|
|
| |||||
| (N = 284) | (N = 164) | (N = 210) | (N = 174) | (N = 71) | |
| Enrollment (No. of subjects) (‡) | |||||
| Median (25th, 75th) | 65 (36, 186) | 60 (35, 120) | 75 (36, 200) | 80 (40, 210) | 60 (32, 200) |
| Study duration (yrs) (‡) | |||||
| Median (25th, 75th) | 2.3 (1.3, 3.7) | 2.5 (1.5, 3.8) | 2.4 (1.3, 3.8) | 2.4 (1.3, 4.0) | 2.9 (1.3, 4.0) |
| Study duration missing, n (%) | 75 (26%) | 42 (26%) | 53 (25%) | 47 (27%) | 20 (28%) |
| Phase, n (%) | |||||
| Early (<= 2) | 80 (28%) | 58 (35%) | 57 (27%) | 49 (28%) | 16 (23%) |
| Late phase (>= 2/3) | 101 (36%) | 49 (30%) | 80 (38%) | 65 (37%) | 24 (34%) |
| NA | 103 (36%) | 57 (35%) | 73 (35%) | 60 (35%) | 31 (44%) |
| Intervention type, n (%) | |||||
| Procedure/Device | 58 (20%) | 41 (25%) | 50 (24%) | 47 (27%) | 33 (47%) |
| Drug/Biological | 173 (61%) | 106 (65%) | 124 (59%) | 97 (56%) | 29 (41%) |
| Behavioral | 30 (11%) | 6 (4%) | 20 (10%) | 17 (10%) | 8 (11%) |
| Other (§) | 23 (8%) | 11 (7%) | 16 (8%) | 13 (8%) | 1 (1%) |
| Endpoint classification, n (%) | |||||
| Safety | 15 (6%) | 13 (9%) | 11 (7%) | 10 (7%) | 3 (5%) |
| Efficacy | 84 (36%) | 41 (29%) | 62 (37%) | 55 (38%) | 18 (31%) |
| Safety/Efficacy | 116 (49%) | 71 (51%) | 82 (48%) | 71 (49%) | 37 (63%) |
| Pharmacometrics | 21 (9%) | 15 (11%) | 15 (9%) | 8 (6%) | 2 (2%) |
| Endpoint classification missing | 48 (17%) | 24 (15%) | 40 (19%) | 30 (17%) | 12 (17%) |
Studies may enroll in > 1 age group or be included in multiple columns.
Open to adult and pediatric participants.
Includes both actual and anticipated duration or enrollment for completed and ongoing studies, respectively. Data rounded to nearest %, may sum to <> 100%.
including trials with interventions identified as radiation, genetic (including gene transfer, stem cell and recombinant DNA), or dietary supplement (e.g., vitamins, minerals).
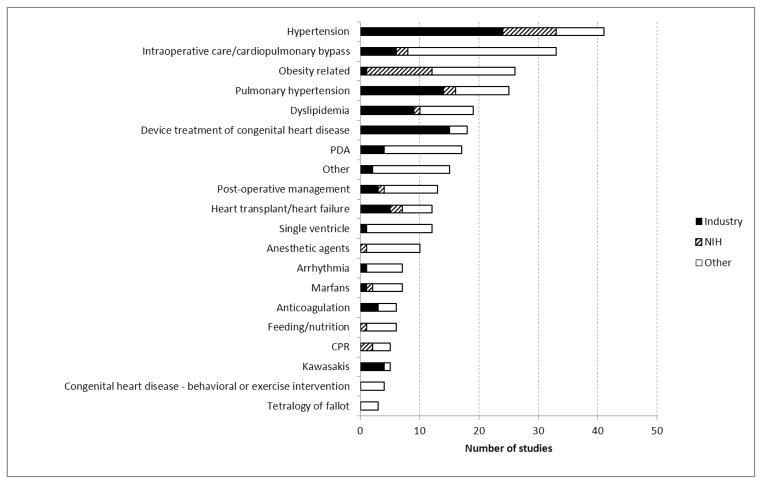
FIGURE II shows the broad diagnostic categories of trial focus segregated by trial sponsor. Diagnostic categories with more than 20 reported trials included: hypertension (n=41, 14%), intraoperative / bypass (n=33, 12%), obesity (n=26, 9%) and pulmonary hypertension (n=25, 9%). Industry support was more common for hypertension (n=24), device (n=15), pulmonary hypertension (n=14) and dyslipidemia (n=9) trials, while trials more commonly supported by NIH included obesity (n=11) and hypertension trials (n=9) (FIGURE 2).
Figure II.
Pediatric Cardiovascular Trial intervention categories and funding source. PDA = Patent Ductus Arteriosus; CPR = Cardiopulmonary Resuscitation; CHD = Congenital Heart Disease.
Comparison with adult cardiovascular (CV) and other pediatric sub-specialties
TABLE II (with additional details in eTABLE 2) compares selected characteristics of PCVTs that restricted enrollment to age ≤ 18 years with adult CV trials (enrollment restricted to age > 18 years) and also with other major pediatric trial specialties including pediatric infectious disease (ID), mental health and hematology / oncology. There were > 25 times as many adult CV trials as PCVTs. Adult CV trials were also larger (median enrollment 115 versus 68 subjects), more frequently sponsored by industry (45% versus 32%) and more often conducted in foreign only sites (59% versus 37%). Compared to trials in other major pediatric sub-specialties, PCVTs were also less frequent with > 5 times as many registered pediatric ID trials (n=1176), > 3 times as many registered mental health trials (n=664) and almost twice as many pediatric hematology / oncology trials (n=346). This relationship persists regardless of funding source.
Table II.
Pediatric sub-specialty trial characteristics
| Pediatric (*) CV | Adult (*) CV | Pediatric (*) ID | Pediatric (*) Hematology/ oncology | Pediatric (*) Mental health | Pediatric (*) Other therapeutic areas | |
|---|---|---|---|---|---|---|
|
| ||||||
| (N = 213) | (N=5256) | (N = 1176) | (N = 346) | (N = 664) | (N = 2636) | |
| Age eligible for enrollment (†), n (%) | ||||||
| Neonates/infants (0–1 yr) | 130 (61%) | NA | 839 (71%) | 236 (68%) | 23 (4%) | 1122 (43%) |
| Children (1–12 yrs) | 144 (68%) | NA | 835 (71%) | 304 (88%) | 601 (91%) | 2038 (77%) |
| Adolescents (12–18 yrs) | 103 (48%) | NA | 288 (25%) | 233 (67%) | 405 (61%) | 1247 (47%) |
| Study duration (yrs) (‡) | ||||||
| Median (25th, 75th) | 2.2 (1.4, 3.3) | 2.0 (1.1, 3.1) | 1.3 (0.7, 2.3) | 2.9 (1.6, 4.7) | 2.4 (1.4, 3.9) | 1.9 (1.0, 3.2) |
| Study duration missing, n (%) | 55 (26%) | 1278 (24.3%) | 314 (27%) | 89 (26%) | 162 (24%) | 625 (24%) |
| Enrollment (No. of subjects) (‡) | ||||||
| Median (25th, 75th) | 68 (36, 186) | 115 (46, 304) | 305 (120, 751) | 60.0 (26, 205) | 80.0 (40.0, 192.0) | 88 (40, 200) |
| 0 to 100 | 133 (64%) | 2463 (48%) | 260 (22%) | 206 (62%) | 372 (57%) | 1461(57%) |
| 101 to 500 | 62 (30%) | 1804 (35%) | 487 (43%) | 92 (28%) | 253 (39%) | 855 (33%) |
| 501 to 1000 | 8 (4%) | 393 (7%) | 177 (16%) | 14 (4%) | 15 (2%) | 113 (4%) |
| >1000 | 4 (1.9%) | 454 (9%) | 218 (19%) | 20 (6%) | 10 (2%) | 142 (6%) |
| Enrollment missing | 6 (3%) | 142 (3%) | 34 (3%) | 14 (4%) | 14 (2%) | 65 (3%) |
| Phase, n (%) | ||||||
| Early (<= 2) | 64 (30%) | 1232 (23%) | 351 (30%) | 139 (40%) | 180 (27%) | 615 (23%) |
| Late phase (>= 2/3) | 77 (36%) | 2412 (46%) | 676 (58%) | 111 (32%) | 309 (47%) | 1063 (40%) |
| NA | 72 (34%) | 1612 (31%) | 149 (13%) | 96 (28%) | 175 (26%) | 958 (36%) |
| Intervention type, n (%) | ||||||
| Procedure/Device | 25 (12%) | 1858 (35%) | 63 (5%) | 76 (22%) | 24 (4%) | 458 (17%) |
| Drug/Biological | 144 (68%) | 2559 (49%) | 1030 (88%) | 224 (65%) | 366 (55%) | 1320 (50%) |
| Behavioral | 22 (10%) | 387 (7%) | 32 (3%) | 19 (6%) | 215 (32%) | 438 (17%) |
| Other (||) | 22 (10%) | 452 (9%) | 51 (4%) | 27 (8%) | 59 (9%) | 420 (16%) |
| Endpoint classification, n (%) | ||||||
| Safety | 12 (7%) | 283 (6.3%) | 142 (13%) | 15 (6%) | 27 (5%) | 135 (6%) |
| Efficacy | 66 (37%) | 1693 (37.7%) | 321 (30%) | 92 (38%) | 283 (49%) | 1001 (46%) |
| Safety/Efficacy | 79 (45%) | 2294 (51.1%) | 539 (50%) | 120 (49%) | 250 (44%) | 912 (42%) |
| Pharmacometrics | 20 (11%) | 223 (5%) | 68 (6%) | 18 (7%) | 14 (2%) | 135 (6%) |
| Endpoint classification missing | 36 (17%) | 763 (15%) | 106 (9%) | 101 (29%) | 90 (14%) | 453 (17%) |
| Location of study facilities, n (%) | ||||||
| U.S. only | 84 (44%) | 1621 (34%) | 227 (21%) | 132 (42%) | 408 (69%) | 1154 (48%) |
| Foreign only | 71 (37%) | 2824 (59%) | 769 (72%) | 150 (48%) | 162 (27%) | 1110 (47%) |
| Both U.S. and Foreign | 38 (20%) | 383 (8%) | 69 (7%) | 34 (11%) | 23 (4%) | 121 (5%) |
| Locations unknown | 20 (9%) | 428 (8%) | 111 (9%) | 30 (9%) | 71 (11%) | 251 (10%) |
| Derived funding source (§), n (%) | ||||||
| Industry | 69 (32%) | 2356 (45%) | 708 (60%) | 83 (24%) | 227 (34%) | 839 (32%) |
| NIH | 24 (11%) | 295 (6%) | 84 (7%) | 57 (17%) | 149 (22%) | 305 (12%) |
| Other | 120 (56%) | 2605 (50%) | 384 (33%) | 206 (60%) | 288 (43%) | 1492 (57%) |
Restricted to trials excluding participants > 18 years for pediatric trials and trials excluding patients <18 years for adult CV trials.
Studies may enroll participants in > 1 age group and could be included in multiple columns.
Includes actual and anticipated duration or enrollment for completed and ongoing studies, respectively.
Funding source derived from submitted lead sponsor and collaborator information.
Other interventions represent radiation, genetic (including gene transfer, stem cell and recombinant DNA), or dietary supplement (e.g., vitamins, minerals), as well as trials where interventions were identified as “other” (i.e., not any of the other listed intervention types.
Data are rounded to the nearest % and may sum to <> 100%.
Size and duration of PCVTs were similar to the other pediatric trial specialties with the exception of ID trials which were larger (median enrollment 305 versus 60–80 for the other subspecialties) and shorter in duration (median 1.3 years versus 2.2–2.9 years for other specialties). ID trials were also more likely to focus on prevention (63%) while trials in the other pediatric specialties were predominantly treatment-oriented (74–84%, depending on specialty). Overall 37% of PCVTs were conducted only in foreign sites and 44% were conducted only in the U.S. In comparison, ID trials were commonly conducted only in foreign sites (72%) while mental health trials were largely conducted only in the U.S. (69%). Compared to other trial specialties, PCVTs were more likely to include both U.S. and foreign sites (20% versus 4–11% for the other specialties), however there was an appreciable increase in foreign only PCVTs in the later years of trial reporting (33% foreign only trials in era 1 versus 45% in era 2). Many of the other differences in trial characteristics across pediatric specialties were anticipated including differences in eligible age ranges and trial interventions.
Funding of PCVTs: trends over time and comparison with other pediatric sub-specialties and adult cardiovascular trials
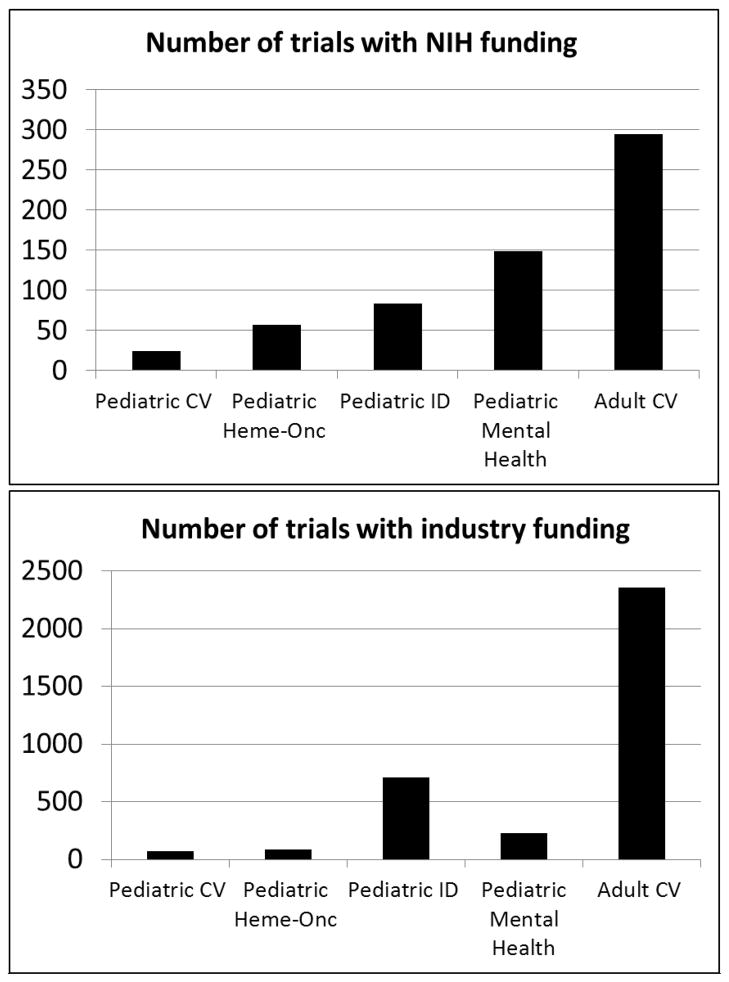
FIGURE III demonstrates the number of industry and NIH funded PCVTs in comparison to other pediatric specialties and adult cardiovascular trials. Overall industry and NIH-funded PCVTs account for 3.6% (n=69/2545) and 3.9% (n=24/619) of all industry and NIH-funded pediatric trials respectively, substantially less than any of the other pediatric subspecialties that were evaluated. Compared to cardiovascular trials restricting enrollment to adults (n=5256), PCVTs were less likely to be funded by industry or NIH (44% funding for PCVTs versus 50% for adult cardiovascular trials). The number of PCVTs funded by either NIH or industry decreased by 40% in era 2 of the analysis (31 versus 19 registered trials with funding / year for the earlier versus later eras respectively). This change in funding has been most noticeable for NIH-funded trials, with a 76% decline in the average annual number of NIH-funded PCVTs. While a similar decline was seen among registered adult cardiovascular trials, the relative change was not as substantial (19% decline between eras).
Figure III.
Industry and NIH funded pediatric cardiovascular trials compared to other pediatric sub-specialties and adult cardiovascular trials. Funding source is derived from the submitted lead sponsor and collaborator information. Pediatric cardiovascular trials restricted to those excluding participants > 18 years while adult cardiovascular trials restricted to those excluding participants < 18 years.
Factors influencing trial quality
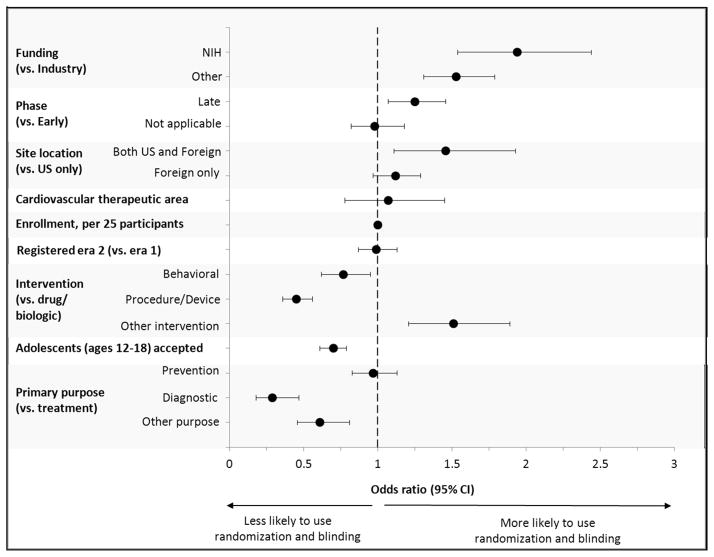
For PCVTs that restricted enrollment to age ≤ 18 years, the majority reported randomization (75%) and blinding (52%). A DMC was used in 54% of PCVTs entered into the registry after this question was introduced in 2007 (eTABLE II). Multivariable regression analyses of quality metrics (use of a randomized, blinded design) in pediatric trials are shown in FIGURE IV. There was no difference in these quality metrics when comparing PCVTs to all other pediatric trials. Also, there were no significant interactions between therapeutic area and factors in terms of their association with randomization and blinding. For the subset of all pediatric trials, important factors associated with improved quality metrics included: NIH funding (OR=1.9, [95% CI=1.5–2.4] compared to industry); trial location (trials with U.S. and foreign enrollment > trials with U.S. only or foreign only enrollment, p=0.02) and trials restricted to younger children (OR=1.4, [95% CI 0.6–0.8] compared to trials including adolescents). Other factors associated with these quality metrics could be anticipated and included: later phase trials (OR 1.3 [95% CI=1.1–1.5] compared to early phase); intervention type (drug / biologic trials > behavioral trials > procedure / device trials, p<0.001); and primary purpose (treatment and prevention trials > diagnostic trials, p<0.001). A sensitivity analysis, excluding all device trials demonstrated no significant change in the findings (results not shown). Among adult and pediatric cardiovascular trials, similar factors were associated with use of randomization and blinding (results not shown), although adult cardiovascular trials were more likely (OR=1.6, p=0.002) to have these quality metrics than pediatric cardiovascular trials, after accounting for other factors.
Figure IV.
Multivariable logistic regression for use of randomization and blinding in pediatric trials. Era 1: July05–Sep07 (before the FDA Amendments Act registry requirements were implemented), and era 2: Oct07–Sep10 (after implementation)
Publication of completed trials
Overall 90 PCVTs were listed as “completed” as of 09-27-2010. Although only 24% (n=21) of these trials included results on the ClinicalTrials.gov registry, a PubMed citation reporting the main results was located for 73% (n=66) of these trials. Time to publication could be determined for 53 of these trials with 30 (33% of completed trials) published within 2 years of the listed study completion date.
Discussion
This analysis provides a descriptive assessment of the pediatric cardiovascular clinical trials landscape, and a comparison with adult cardiovascular trials and trials within other pediatric specialties. Thus this analysis presents a unique opportunity to evaluate the PCVT trial landscape and identify areas of relative strength or weakness. With respect to PCVTs, the ClinicalTrials.gov data raises several concerning issues.
Similar to prior ClinicalTrials.gov analyses in adults and in the broader subset of pediatric trials,11, 17 most PCVTs are smaller trials and a majority are not funded by either industry or NIH. Perhaps most concerning, however, is that the number of registered pediatric cardiovascular trials is relatively low - substantially smaller than the number of adult cardiovascular trials and also smaller than for other pediatric sub-specialties. This does not seem to be representative of the relative public health burden. Congenital heart diseases account for more life years lost than leukemia and asthma combined and acquired heart diseases of childhood (e.g. hypertension, dyslipidemia) are increasing contributors to childhood morbidities.1, 3, 4
Surprisingly, PCVTs rarely focused on specific congenital malformations and more commonly focused on diseases and conditions typically considered high impact adult cardiovascular diseases. Hypertension, pulmonary hypertension and dyslipidemia trials combined accounted for 1/3rd of all PCVTs. There are several potential explanations for this finding. First there are unique challenges to conducting trials in children with congenital heart disease. These diseases are rare, heterogeneous conditions that often require procedural interventions. This introduces unique challenges related to trial design, patient enrollment and end point determination. These difficulties incur added expense and can prolong trials, potentially serving as a disincentive to trial conduct. Secondly, the 2002 BPCA financial incentive structure for industry favors trials focused on blockbuster drugs9, 18. These drugs have often been developed to treat high impact adult cardiovascular conditions. According to the FDA website 24/31 pediatric cardiovascular drugs that have been granted exclusivity under the BPCA program are agents used to treat hypertension (n=17) and dyslipidemia (n=7).19 Approval of each of these agents typically requires several trials, potentially accounting for many of the registered industry-sponsored trials in our analysis. Pulmonary hypertension was added to the FDA priority list only in 2008 therefore these trials may be registered but have not yet been completed and granted exclusivity. In support of this potential explanation, trials focused on hypertension, dyslipidemia and pulmonary hypertension were largely supported by industry rather than NIH or other sponsor sources.
Overall relatively few PCVTs were funded by industry or NIH – a combined total of 93 trials over the 5-year time period studied. By comparison there were 792 industry or NIH funded ID trials and 2651 industry or NIH funded adult cardiovascular trials. Furthermore the annual number of NIH funded PCVTs declined by 76% (from 34 / year to 19 / year) in the latter era of reporting. To assess whether this might be an isolated temporal finding, we subsequently reviewed the number of NIH funded PCVTs reported between 10/2010 and 08/2013. Although this analysis was conducted post-hoc we adhered to the same criteria for defining PCVTs and derived NIH sponsorship. The declining trend in NIH-funding persists with even fewer (n=23 total, average annual number = 8) registered NIH-sponsored PCVTs in this reporting time frame. The exact reason for this decline is unclear but we speculate that this reflects the financial climate associated with the great recession and the more recent sequestration of federal funds. Across pediatric and adult specialties, the annual number of NIH funded awards decreased by ~ 5% between 2005 and 2010 with more than 2300 fewer total NIH grants awarded in 2010 versus 2005.20 Pediatric cardiovascular trials appear to have been disproportionately affected by this decline. This is particularly concerning as NIH-funded trials more frequently demonstrate positive trial quality metrics when compared to other funding sources.
We also identified several reassuring findings. Pediatric cardiovascular trials performed relatively well with respect to trial quality metrics. Use of randomization and a blinded design have been used in prior analyses to assess overall quality of the clinical trials landscape, and specific factors associated with trial quality.17 These measures are certainly not required for all high quality trials and certain trials (e.g. early phase trials, device trials, pharmacokinetic trials) may be readily, or out of necessity, conducted without blinding or randomization. Nonetheless it is reassuring that 75% of PCVTs reported using a randomized design and more than half used blinding and a data monitoring committee. Interestingly foreign only trials demonstrated improved quality metrics when compared to U.S. only trials. This may represent a registration bias with lower quality U.S. trials more likely to be registered than lower quality foreign trials because FDA amendments act registration requirements do not necessarily apply to early phase trials conducted at non-U.S. sites.
A second positive finding is that a relatively large percentage of completed PCVTs have already been published in the peer review literature. Historically there has been a bias towards publication of only positive trial results.21–23 The 73% track record of publication of completed PCVTs that we demonstrate is a significant improvement over prior analyses focused on pediatric trials.24
There are important limitations to this analysis. First, there is a significant amount of missing or unsubmitted data for certain data fields. This limits the comprehensiveness of analyses that can be performed with these data. Second, temporal differences in reporting requirements could affect analyses of trends over time; however, we restricted our analysis of trends to comparison with adult cardiovascular trials, anticipating that reporting requirements would equally affect PCVTs and adult cardiovascular trials. Third, no standard or comprehensive classification scheme is used for pediatric disease type or therapeutic area (e.g. cardiovascular versus other pediatric sub-specialties), such that we characterized this factor through manual evaluation of the key words and conditions entered for the study. Fourth, we did not include studies in this analysis that reported that age criteria were not applicable for enrollment. Finally, there are undoubtedly some trials that are not registered in ClinicalTrials.gov or any other publicly accessible registry, and these studies were not included in our evaluation. In particular, current federal guidelines do not require registration of Phase I trials, trials not involving a drug, biological, or device, and trials not under U.S. jurisdiction.
In conclusion, we found that the number of PCVTs seems disproportionately small relative to disease burden in comparison with trials in other pediatric sub-specialties. Most PCVTs are well-conducted, meeting standard quality metrics, but as industry and NIH funding for PCVTs has declined in recent years, concerns arise about future quality. Importantly PCVT focus does not seem to broadly affect the overall pediatric cardiovascular disease burden and may be influenced by inherent difficulties associated with conduct of PCVTs, as well as an incentive structure that favors study of high impact adult cardiovascular drugs. Looking forward, it will be critically important to focus PCVTs on diseases and conditions with the highest disease burden.
Acknowledgments
Funding: Financial support for this work was provided by cooperative agreement U19 FD003800 awarded by the U.S. Food and Drug Administration to Duke University in support of the Clinical Trials Transformation Initiative. Drs Li and Califf received support from the NIH sponsored Duke Clinical and Translational Science Award (UL1TR001117).
Footnotes
disclosures: The views expressed in this manuscript do not necessarily reflect those of the NHLBI or the NIH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125(1):e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sorof JM, Lai D, Turner J, et al. Overweight, ethnicity, and the prevalence of hypertension in school-aged children. Pediatrics. 2004;113(3 Pt 1):475–82. doi: 10.1542/peds.113.3.475. [DOI] [PubMed] [Google Scholar]
- 3.Improved national prevalence estimates for 18 selected major birth defects--United States, 1999–2001. MMWR Morb Mortal Wkly Rep. 2006;54(51):1301–5. [PubMed] [Google Scholar]
- 4.Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects--United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(2):25–9. [PubMed] [Google Scholar]
- 5.Fair tests of treatments in health care. The James Lind Library; [Accessed December 29, 2012.]. http://www.jameslindlibrary.org. [Google Scholar]
- 6.Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104(3 Pt 2):585–90. [PubMed] [Google Scholar]
- 7.Food and Drug Administration. [Accessed December 29, 2012];Status Report to Congress on the Pediatric Exclusivity Provision. 2001 http://www.fda.gov/downloads/drugs/developmentapprovalprocess/developmentresources/ucm049915.pdf.
- 8.Field M, Boat TF, editors. Safe and Effective Medicines for Children. Pediatric Studies Conducted Under the Best Pharmaceuticals for Children Act and the Pediatric Research Equity Act. 1. Washington, DC: The National Academies Press; 2012. [PubMed] [Google Scholar]
- 9. [Accessed December 29, 2012.];Full text of Best Pharmaceuticals for Children Act. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/ucm148011.htm.
- 10.Bourgeois FT, Murthy S, Pinto C, et al. Pediatric versus adult drug trials for conditions with high pediatric disease burden. Pediatrics. 2012;130(2):285–92. doi: 10.1542/peds.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquali SK, Lam WK, Chiswell K, et al. Status of the pediatric clinical trials enterprise: an analysis of the US ClinicalTrials.gov registry. Pediatrics. 2012;130(5):e1269–77. doi: 10.1542/peds.2011-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zarin DA, Tse T, Williams RJ, et al. The ClinicalTrials.gov results database--update and key issues. N Engl J Med. 2011;364(9):852–60. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Angelis C, Drazen JM, Frizelle FA, et al. Clinical trial registration: a statement from the International Committee of Medical Journal Editors. N Engl J Med. 2004;351(12):1250–1. doi: 10.1056/NEJMe048225. [DOI] [PubMed] [Google Scholar]
- 14. [Accessed December 29, 2012.];Full Text of Food and Drug Administration Amendments Act of 2007. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/SignificantAmendmentstotheFDCAct/FoodandDrugAdministrationAmendmentsActof2007/FullTextofFDAAALaw/default.htm.
- 15.Tasneem A, Aberle L, Ananth H, et al. The database for aggregate analysis of ClinicalTrials.gov (AACT) and subsequent regrouping by clinical specialty. PLoS One. 2012;7(3):e33677. doi: 10.1371/journal.pone.0033677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. [Accessed October 2, 2012.];Protocol Data Element Definitions. https://www.trialstransformation.org/ctdotgov%20high%20level%20data%20dictionary%20V2.0.pdf.
- 17.Califf RM, Zarin DA, Kramer JM, et al. Characteristics of clinical trials registered in ClinicalTrials.gov, 2007–2010. JAMA. 2012;307(17):1838–47. doi: 10.1001/jama.2012.3424. [DOI] [PubMed] [Google Scholar]
- 18.Li JS, Eisenstein EL, Grabowski HG, et al. Economic return of clinical trials performed under the pediatric exclusivity program. JAMA. 2007;297(5):480–8. doi: 10.1001/jama.297.5.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. [Accessed January 8, 2013.];List of dugs granted pediatric exclusivity. http://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM223058.pdf.
- 20. [Accessed January 8, 2013.];Summary of NIH funding. http://report.nih.gov/budget_and_spending/index.aspx.
- 21.Ioannidis JP. Effect of the statistical significance of results on the time to completion and publication of randomized efficacy trials. JAMA. 1998;279(4):281–6. doi: 10.1001/jama.279.4.281. [DOI] [PubMed] [Google Scholar]
- 22.Bhandari M, Busse JW, Jackowski D, et al. Association between industry funding and statistically significant pro-industry findings in medical and surgical randomized trials. CMAJ. 2004;170(4):477–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Easterbrook PJ, Berlin JA, Gopalan R, et al. Publication bias in clinical research. Lancet. 1991;337(8746):867–72. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 24.Benjamin DK, Jr, Smith PB, Murphy MD, et al. Peer-reviewed publication of clinical trials completed for pediatric exclusivity. JAMA. 2006;296(10):1266–73. doi: 10.1001/jama.296.10.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]