Abstract
Laminin α1 (LAMA1), a subunit of the laminin-111 basement membrane component, has been implicated in various biological functions in vivo and in vitro. Although LAMA1 is present in kidney, its roles in the kidney are unknown because of early embryonic lethality. Herein, we used a viable conditional knockout mouse model with a deletion of Lama1 in the epiblast lineage (Lama1CKO) to study the role of LAMA1 in kidney development and function. Adult Lama1CKO mice developed focal glomerulosclerosis and proteinuria with age. In addition, mesangial cell proliferation was increased, and the mesangial matrix, which normally contains laminin-111, was greatly expanded. In vitro, mesangial cells from Lama1CKO mice exhibited significantly increased proliferation compared with those from controls. This increased proliferation was inhibited by the addition of exogenous LAMA1-containing laminin-111, but not by laminin-211 or laminin-511, suggesting a specific role for LAMA1 in regulating mesangial cell behavior. Moreover, the absence of LAMA1 increased transforming growth factor (TGF)-β1–induced Smad2 phosphorylation, and inhibitors of TGF-β1 receptor I kinase blocked Smad2 phosphorylation in both control and Lama1CKO mesangial cells, indicating that the increased Smad2 phosphorylation occurred in the absence of LAMA1 via the TGF-β1 receptor. These findings suggest that LAMA1 plays a critical role in kidney function and kidney aging by regulating the mesangial cell population and mesangial matrix deposition through TGF-β/Smad signaling.
Laminins comprise a family of heterotrimeric extracellular matrix (ECM) proteins consisting of α, β, and γ chains1,2 that regulate cell attachment, proliferation, and differentiation.3,4 During early embryogenesis, laminin α1 (LAMA1) first appears at the 16-cell stage and is later present in the two basement membranes (BMs) formed before gastrulation, the embryonic BM, and Reichert’s membrane. In the developing kidney, temporal changes in laminin isoform expression occur as formation of glomeruli, the filtering unit of the kidney, proceeds. The earliest precursor of the glomerular BM (GBM) contains laminin-111 (LM-111; α1β1γ1). In contrast, the GBM contains LM-521 (α5β2γ1) at later developmental stages and in adulthood. Although LAMA1 is absent from the mature GBM, it is present in the glomerular mesangial matrix, an amorphous matrix made by mesangial cells that is one of the few prominent sites in which laminins are present outside of a definitive BM.5 The glomerular capillary wall consists of podocytes with interdigitated foot processes bridged by slit diaphragms, glomerular endothelial cells, and the intervening GBM6 that these two cell types together produce.7 Mesangial cells (MCs), the third cell type of the glomerulus, comprise approximately one-third of the glomerular tuft cell population. MCs bind the GBM at the base of the glomerular capillary loops to establish and maintain the structural architecture of the glomerular capillaries,6 similar to the function of certain microvascular pericytes. These cells also contribute to mesangial matrix homeostasis, regulate filtration surface area and capillary blood pressure, and phagocytose apoptotic cells and immune complexes formed at or delivered to the glomerular capillaries. Cell biological and biochemical studies have characterized MC responses to hormones, cytokines, growth factors, and metabolic, inflammatory, and immune mediators that are highly relevant to primary glomerular diseases or to systemic diseases that target glomerular cells.8
Because inactivation of the Lama1 gene in mice results in a failure of assembly of Reichert’s membrane and developmental arrest shortly after implantation,9 in vivo studies of LAMA1 function have been limited. Herein, we used a conditional Lama1 knockout (KO) mouse model (Lama1CKO) with specific deletion of Lama1 in the epiblast lineage using Sox2-Cre to study the role of LAMA1 in kidney development and function. We found that the absence of LAMA1 delayed glomerular development, and adult Lama1CKO mice developed focal glomerulosclerosis and proteinuria with age. MCs from Lama1CKO mice showed increased proliferation, resulting in expansion of the mesangial cell compartment. Thus, LAMA1 plays a critical role in MC homeostasis and kidney function.
Materials and Methods
Animal Experiments
Conventional Lama1 KO (Lama1del/del) mice die at approximately embryonic day 7 because of lack of Reichert’s membrane.9,10 In a previous study, floxed Lama1 (Lama1flox/flox) mice and heterozygous Lama1 null mice carrying the Sox2-Cre transgene (Lama1del/+; Sox2-Crecre/+) were generated.11 Lama1CKO (Lama1floxed/del; Sox2-Crecre/+) mice with a conditional Lama1 deficiency, specifically in the epiblast, and its derivatives, which comprise the entire embryo, were then generated by crossing the two lines. Genotypes were confirmed by PCR of tail-snip DNA.11 Animals examined included male and female mice, aged 1 day to 24 months. Lama1flox/flox [wild-type (WT)] mice and Lama1flox/del (heterozygous) mice were used as normal controls.
Immunofluorescence
Kidneys from 7-month-old mice were fixed with 4% PFA in 0.1 mol/L PBS, pH 7.4, overnight at 4°C and then cryoprotected in 30% sucrose in PBS for 72 hours at 4°C. Sections (4 μm thick) were cut with a cryostat and mounted onto glass slides. For immunostaining, the frozen sections were air dried and washed with PBS. The sections were incubated with 0.1% Triton X-100 (Polysciences, Inc., Warrington, UK) in PBS for 15 minutes. After washing, they were blocked with blocking buffer (5% normal donkey serum and 2% bovine serum albumin in PBS) for 30 minutes and incubated with dilutions of the primary antibody in the blocking buffer for 1 hour. The following primary antibodies were used: rabbit anti-laminin α1 (LAMA1)12; rabbit anti-laminin α2 (LAMA2), rabbit anti-laminin α3 (LAMA3), rabbit anti-laminin α4 (LAMA4), and rabbit anti-laminin α5 (LAMA5) (gifts from Dr. Takako Sasaki); rat anti-laminin γ1 (LAMC1) (Chemicon International, Inc., Temecula, CA); mouse anti-synaptopodin (Progen Biotechnik, Heidelberg, Germany); rat anti-platelet endothelial cell adhesion molecule (PECAM; BD Pharmingen, Erembodegem, Belgium); and rabbit anti-smooth muscle actin (Abcam, Cambridge, MA). The slides were washed with PBS and incubated with the secondary antibodies, Alexa 488 donkey anti-rabbit (Molecular Probes, Eugene, OR), Cy3 donkey anti-mouse, and Cy 5–conjugated donkey anti-rat (Jackson ImmunoResearch Laboratories, Baltimore, MD) for 1 hour. In each experiment, several sections were incubated without the primary antibody to serve as controls. When the primary antibody was omitted from the staining, no immunoreactivity was observed.
Histological and Morphometric Data
For histological data, mice were perfused with 4% paraformaldehyde (PFA) under anesthesia. Kidney pieces from 2- to 24-month-old mice were fixed in 4% PFA and embedded in paraffin. Sections (4 μm thick) were deparaffinized with xylene, hydrated in a graded series of ethanol, and washed with PBS.
For morphometric analysis of glomeruli, sections were stained with either PAS or toluidine blue. Mesangial area was quantified by a blind observer (L.N.). Fifteen glomeruli cut at the vascular pole were randomly selected in PAS-stained sections from each animal in each experimental group. The increase in mesangial matrix (defined as mesangial area) was determined by the presence of PAS-positive and nuclei-free areas in the mesangium; the glomerular area was also traced along the outline of capillary loops using Imaging System KS400 (Imaging Associates, Thame, UK).
For electron microscopy, small pieces of kidney cortex from neonatal and 2- to 16-month-old mice were fixed in 2.5% glutaraldehyde in PBS. Tissues were dehydrated and embedded in plastic, and ultrathin sections were viewed by transmission electron microscopy, as previously described.13 GBM thickness at 15 months of age in three Lama1CKO mice and three heterozygous mice was determined by using the orthogonal intercept method.14 The degree of foot process effacement was evaluated by foot process width. A foot process was defined as any connected epithelial segment butting on the basement membrane between two neighboring filtration pores or slits. From each image, the arithmetic mean of the foot process width was calculated, as described previously,15 using the following equation:
| (1) |
where ∑ foot process is the total number of foot processes counted in each image, ∑ GBM length is the total GBM length measured in each image, and the correction factor of π/4 serves to correct for presumed random variation in the angle of section relative to the long axis of the podocyte.
Urine Albumin and Creatinine Assays
At monthly intervals beginning at 4 months of age, mice were placed in metabolic cages, and urine was collected for 24 hours. In some cases, 10 μL of urine in loading buffer was analyzed on SDS-PAGE gels stained with Coomassie Brilliant Blue. The urinary albumin-creatinine ratio was determined by immunoassay (DCA Vantage Analyzer; Siemens Healthcare Diagnostics, Deerfield, IL).
Cell Culture
Glomeruli were isolated through the injection of magnetic beads, which become trapped within glomerular capillaries.16 Briefly, anesthetized 2-month-old mice were perfused with 8 × 107 Dynabeads M-450 (Invitrogen, Carlsbad, CA). The kidneys were removed and minced on ice, followed by digestion at 37°C with 1 mg/mL collagenase and 100 U/mL DNase I for 30 minutes, then filtered twice with 100-μm Falcon cell strainers (BD Falcon, Bedford, MA). The tissue was pelleted by gentle centrifugation (200 × g, 5 minutes), and glomeruli containing Dynabeads were collected using a magnetic particle concentrator (Dynal, Oslo, Norway) and washed in HBSS three times. Isolated glomeruli were cultured on type I collagen-coated culture dishes (BD BIOCOAT, Bedford, MA) in Dulbecco’s modified Eagle’s medium supplemented with 20% heat-inactivated fetal bovine serum, 100 μg/mL streptomycin, and 100 U/mL penicillin at 37°C in a humidified 95%/5% air/CO2 atmosphere. When primary glomerular explant cultures reached day 30, cells were washed with PBS and removed from dishes by trypsinization (0.05% trypsin). Cells at passage 5 to 8 were characterized and used for the experiments. MCs cultured on chamber slides were fixed in 4% PFA and processed for immunofluorescence microscopy using antibodies for podocytes, endothelial cells, and MCs, as described previously.17
Analysis of Cell Proliferation
MCs were seeded in 8-well chamber slides (5 × 103 cells per well) and cultured with or without 5 μg/mL LM-111, LM-211, or LM-511 (Biolamina, Sundbyberg, Sweden). After 48 hours of culture, cell proliferation was assessed by measuring the incorporation of 5-bromo-2′-deoxyuridine (BrdU) into cellular DNA by an immunofluorescence assay (Kit 1-1296736; Roche, East Sussex, UK). Cells were incubated with 10 μmol/L BrdU (final concentration) for 60 minutes before fixation. The number of BrdU-positive cells was counted and expressed as a percentage of the total number of cells counted per field. At least five separate fields per well were examined. A negative control in which the anti-BrdU primary antibody was omitted was also assayed in each trial.
RT-PCR and Real-Time PCR Analysis
Total RNA was extracted from the MCs with TRIzol reagent, according to the manufacturer’s instructions (Invitrogen). cDNA was synthesized from 2 μg of total RNA using oligo dT primers (Invitrogen) and Superscript III Reverse Transcriptase (Invitrogen). Real-time quantitative PCR (qPCR) was performed using SYBR Green PCR Master MIX and an ABI Prism 7500 Fast Sequence Detection System (Applied Biosystems, Santa Clara, CA). The primers used for transforming growth factor (TGF)-β1, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) amplification were as follows: TGF-β1 5′-ACCTTGGTAACCGGCTGC-3′ (forward) and 5′-TCCTTGGTTCAGCCACTGC-3′ (reverse); and GAPDH 5′-ACGGCAAATTCAACGGCACAG-3′ (forward) and 5′-AGACTCCACGACATACTCAGCAC-3′ (reverse). Each sample was run in triplicate and normalized to GAPDH expression.
ELISA Data
A sample of 1 N HCl (20 μL) was added to 100 μL of cell culture supernatant and incubated for 10 minutes to activate latent TGF-β1. After the supernatants were neutralized with 1.2 N NaOH/0.5 mol/L HEPES, the samples were analyzed with a commercial TGF-β1 sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, Minneapolis, MN), according to the manufacturer’s recommendations. A standard curve was constructed using serial dilutions of ultrapure human TGF-β1 (R&D Systems). The amount of secreted TGF-β1 was normalized using total cellular proteins. Each sample was measured in duplicate.
Western Blot Analysis
MCs were seeded at a density of 3 × 105 cells per dish, grown to 70% confluence, and cultured in the absence or presence of 5 μg/mL LM-111 for 24 hours. After serum starvation for 24 hours in the absence or presence of 5 μg/mL LM-111, cells were treated with 10 ng/mL TGF-β1 (WAKO, Osaka, Japan) for 30 minutes. To study the effect of TGF-β1 receptor I (TβRI) kinase inhibitors on Smad2 phosphorylation, cells were serum starved and treated with increasing concentrations of SB-431542 (0.1, 1, 5, and 10 μmol/L) or dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO) in the presence of 10 ng/mL TGF-β1 for 30 minutes.18 Protein extracts were prepared in lysis buffer (Cell Signaling, Danvers, MA) containing 1 mmol/L phenylmethylsulfonyl fluoride, 10 mmol/L Na3VO4, and 2 mmol/L NaF, as well as phosphatase inhibitor cocktail. After incubation at 4°C for 30 minutes, nuclear and cellular debris were removed by centrifugation at 20,000 × g for 15 minutes at 4°C. Protein was quantified by BCA assay (Thermo Scientific, Rockford, IL) and 10 μg of protein per lane was separated by SDS-PAGE. Western blot analysis was performed as described previously.19 The primary antibody used was anti–phospho-Smad2 (Ser465/467) (1:1000; Cell Signaling). The blots were subsequently reprobed with anti-Smad2/3 (1:1000; Cell Signaling). For the TβRI and collagen IV (COLIV) expression analysis, primary antibodies for TβRI (1:1000; Abcam) and COLIV (1:1000; Abcam) were used. The blots were subsequently reprobed with anti–β-actin antibody (1:1000; Santa Cruz Biotechnology, Inc., Santa Cruz, CA). Horseradish peroxidase–labeled secondary antibody to rabbit IgG or mouse IgG (1:1000) was purchased from GE Healthcare (Piscataway, NJ). Super Signal West Dura Extended Duration Substrate (Thermo Scientific) was used for detection of signals, and the images were captured using LAS-3000 mini (Fujifilm, Tokyo, Japan). Densitometry analysis of band intensity was performed using Multi Gauge version 3.0 (Fujifilm).
Transfection
FuGENE HD (Promega, Madison, WI) was used for transfection. Mesangial cells were plated on 6-well plates, grown to 50% to 60% confluence, and then transfected with either pcDNA3.1-Lama1 or control pcDNA3.1. FuGENE HD Transfection Reagent (3 μL) was added to the DNA solution (1 μg in 100 μL Optimem). The mixture was incubated for 15 minutes at room temperature, then added to the cells. The cells were treated with TGF-β1 for 24 hours after transfection.
Statistical Analysis
Data are expressed as means ± SEM. A two-sided Student’s t-test was used for comparisons between two groups. P < 0.05 was considered statistically significant.
Results
Delayed Kidney Development in Lama1-Deficient (Lama1CKO) Mice
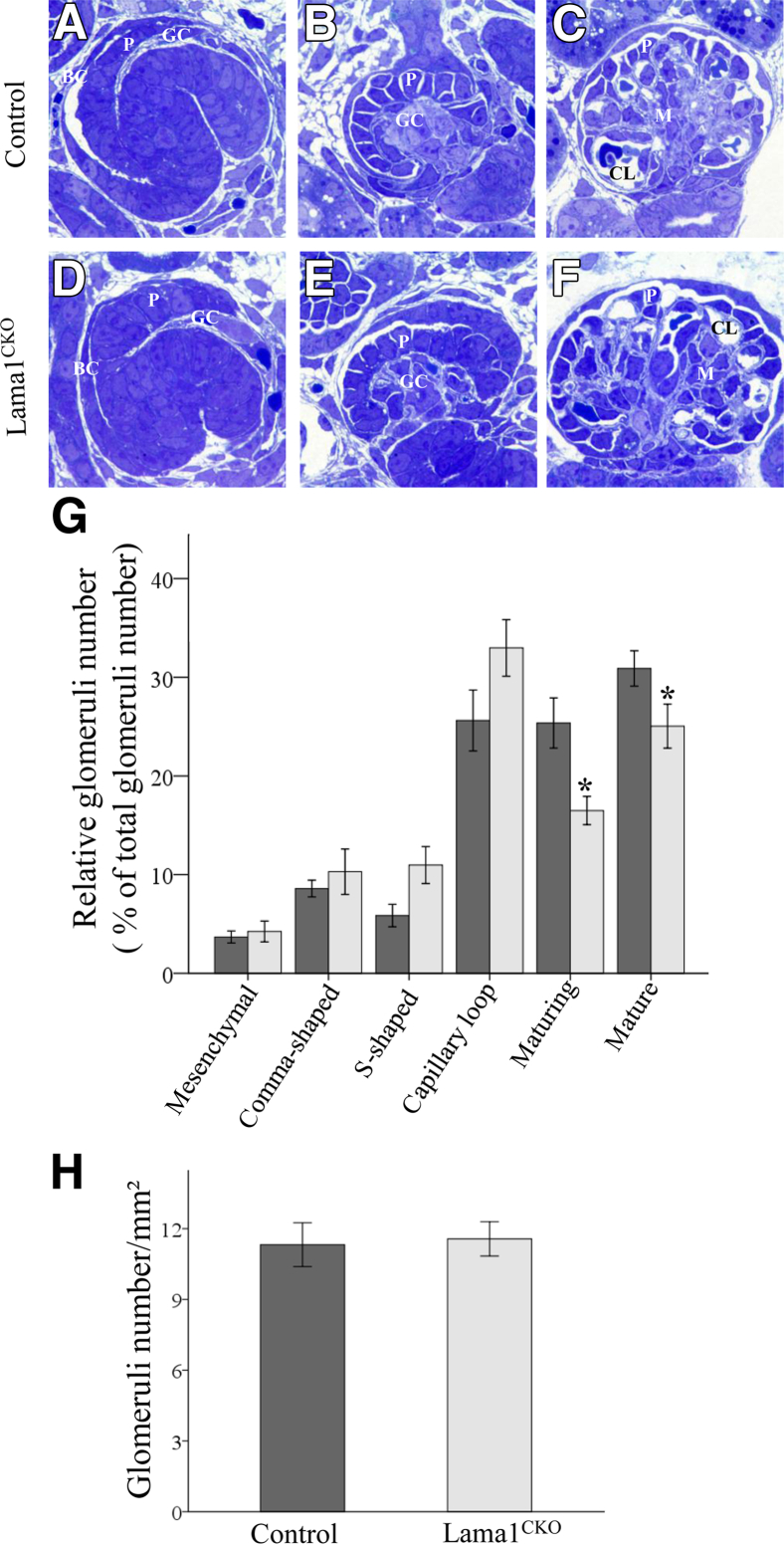
LAMA1 has been implicated in kidney development on the basis of results from organotypic cultures.20,21 However, the in vivo function of LAMA1 in kidney is unknown, primarily because Lama1 KO mice die at early embryonic stages. Herein, we used a viable conditional KO mouse (Lama1CKO) model with a Sox2-Cre–mediated deletion of Lama1 specifically in the epiblast lineage, which produces all embryonic tissues.22 This enabled us to study the role of LAMA1 in kidney development and function. We first examined developing nephrons in newborn Lama1CKO mice because all stages of nephrogenesis can be visualized in the newborn cortex.22 We observed all appropriate developmental stages in both control and Lama1CKO mice, including mesenchymal condensates and comma-shaped bodies (data not shown), S-shaped bodies (Figure 1, A and D), capillary loop stages with forming glomerular capillaries (Figure 1, B and E), and maturing glomeruli with blood cells in the capillaries (Figure 1, C and F). We next quantitated the extent of nephron/glomerular development for each developmental stage in control and Lama1CKO mice as percentages of the total (Figure 1G). The percentage of maturing and fully mature glomeruli was significantly decreased in Lama1CKO mice compared with controls. These results showed that glomerular development was delayed in Lama1CKO mice. To investigate whether the delay in glomerular development caused fewer nephrons in Lama1CKO mice, we measured numbers of glomeruli in Lama1CKO and control kidneys from 2-month-old mice. We found that there was no significant difference in numbers of glomeruli between control and Lama1CKO mice (Figure 1H).
Figure 1.
Histological characteristics of glomerular development. A–F: Toluidine blue–stained sections from newborn (post-natal day 1) mouse kidneys. A and D: S-shaped body. A capillary loop is present in the cleft. B and E: Capillary loop stage glomeruli showing the cup-shaped arrangement of the podocytes. C and F: Mature glomeruli. The percentage of glomeruli at each developmental stage in control and Lama1CKO mice. G: Significantly lower percentages of maturing and mature glomeruli are observed in Lama1CKO kidneys (light gray bars) compared with the controls (dark gray bars). H: There is no significant difference in the number of glomeruli observed in Lama1CKO versus control kidneys at 2 months of age. Data represent means ± SEM. *P < 0.05 versus control at same glomerular developmental stage. Original magnification, ×1000 (A–F). BC, Bowman’s capsule; CL, capillary loop; GC, glomerular cleft; M, mesangial cells; P, podocyte progenitors.
Renal Abnormalities in Adult Lama1CKO Mice
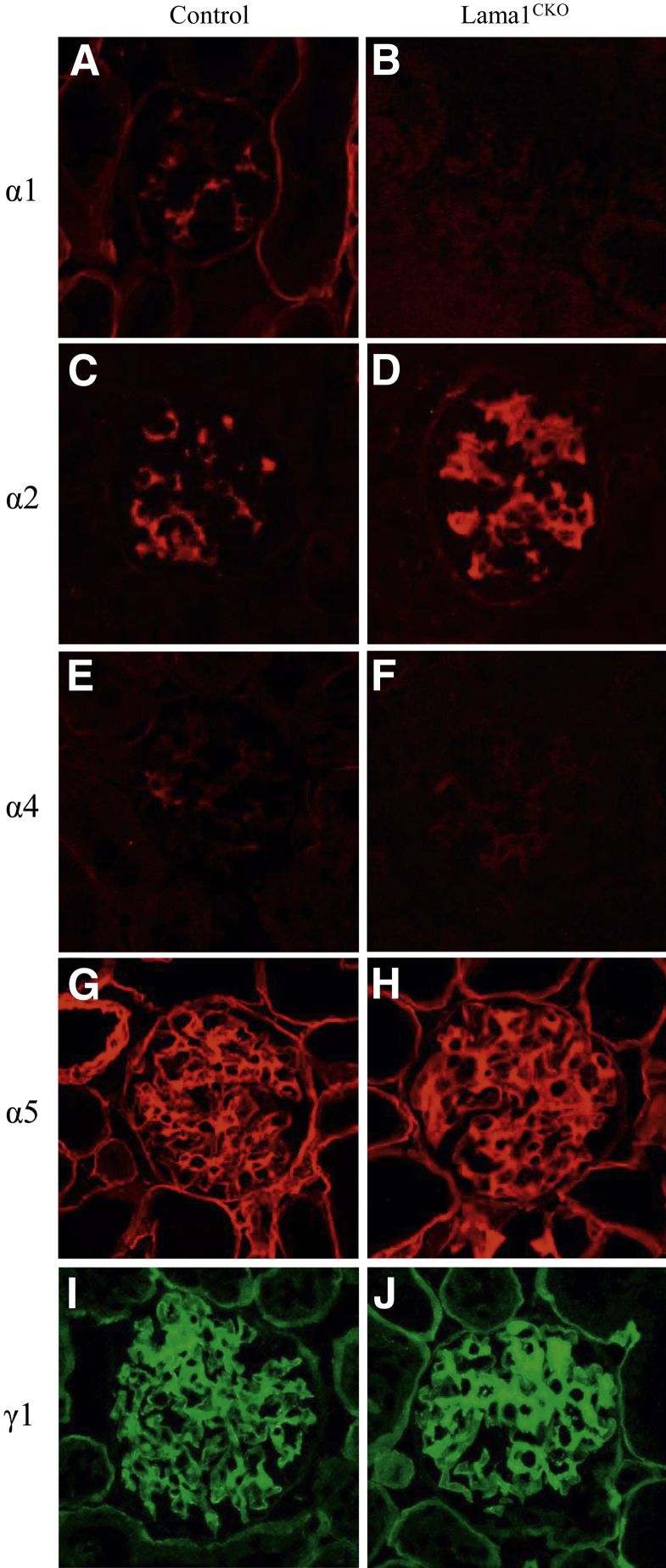
Laminin isoform composition differs in GBM and in the mesangial matrix, and it changes with glomerular development.5,23 In some renal diseases, the normal transition and final composition are altered.22,24–28 Immunostaining revealed that, in Lama1CKO mice, LAMA1 was absent from the mesangium (Figure 2B), whereas it was expressed in control mice (Figure 2A), as shown previously.5 LAMA2 was detected in the mesangium at a higher level in mutant versus control (Figure 2, C and D), and LAMA4 was also detected weakly in glomeruli of both control and Lama1CKO mice (Figure 2, E and F). LAMA5 was detected in virtually all GBM segments (Figure 2, G and H), in agreement with previous studies.5 LAMC1 (γ1) was detected in both the GBM and the mesangium in control and in Lama1CKO kidneys, but increased in the Lama1CKO mesangium (Figure 2, I and J). These data show that even though LAMA1 was absent from glomeruli, the distribution of the other laminin α chains in glomeruli was similar to those of normal mice, except for stronger immunostaining for LAMA2 and LAMC1 in the mesangium of Lama1CKO mice.
Figure 2.
Immunofluorescence microscopic analysis of laminin chain deposition. Basement membranes and mesangial matrix were stained with anti-laminin α1, α2, α4, and α5 (red), and anti-laminin γ1 (green) antibodies, as indicated. LMα1 is detected in the mesangial matrix in control mice (A) and is absent in Lama1CKO mice (B). There are no detectable differences in laminin α chain immunolocalization (C–J), but LMα2 and LMγ1 increase in the mesangial matrix in Lama1CKO mice (D and J). Original magnification, ×400 (A–J).
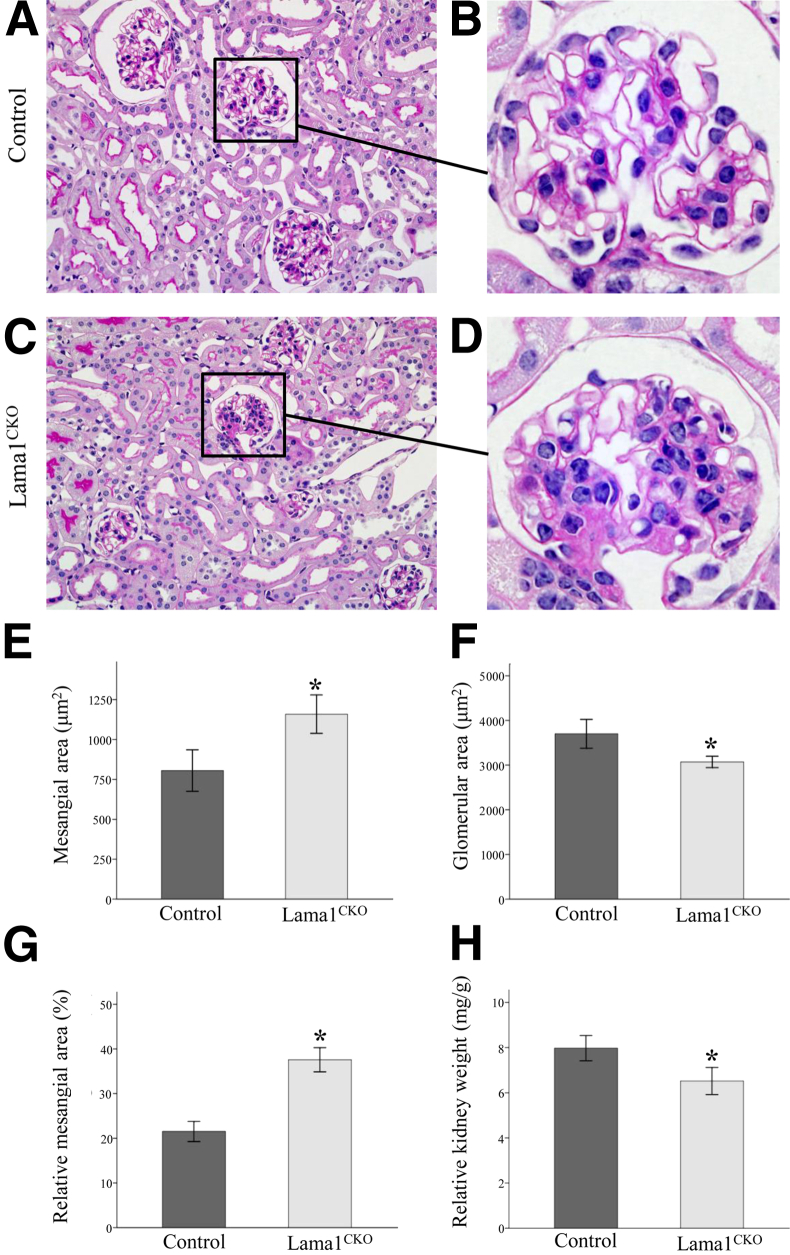
Increased levels of LAMA2 and LAMC1 (as well as perlecan; data not shown) in the mesangium indicated a possible expansion of the mesangial compartment in Lama1CKO kidneys. Histological examination by light microscopy revealed abnormalities in Lama1CKO mice compared with the control exclusively in glomeruli. In agreement with the immunofluorescence, dividing kidneys into sections (9 heterozygous, 1 WT, and 11 Lama1CKO females at 7 months), followed by PAS staining, showed mesangial expansion in Lama1CKO mice (Figure 3, A–D). Mesangial expansion was quantitated by a morphometric analysis; the PAS-positive and nuclei-free mesangial area in the glomeruli of Lama1CKO mice was 44% more than that in control mice (Figure 3E). Total glomerular area was defined by tracing along the outline of the capillary loops and was decreased in Lama1CKO mice compared with control mice (Figure 3F). Thus, the relative mesangial area, as calculated by determining the mesangial area/total glomerular area ratio, was increased by 67% in the Lama1CKO mice (Figure 3G). Taken together, these findings indicate that LAMA1 plays a role in mesangial cell homeostasis and that other laminin α chains cannot substitute for Lama1 in mediating this function.
Figure 3.
Light microscopy reveals renal abnormalities in adult Lama1CKO mice. A–D: Representative images of PAS-stained kidney specimens from 6- to 8-month-old control and Lama1CKO mice. C and D: Glomerular size reduction and mesangial expansion are observed in Lama1CKO mice. The graphs depict quantitative measurements of mesangial area (E), glomerular area (F), and relative mesangial matrix area (G) of control (dark gray bars) versus Lama1CKO (light gray bars) mice. H: The average kidney weight relative to total body weight is shown. Data represent means ± SEM. *P < 0.05 versus control. Original magnifications: ×400 (A and C); ×1000 (B and D).
In addition to the abnormalities in the mesangium, a significant difference in kidney weight between Lama1CKO mice and control mice was observed, with the kidney weight of Lama1CKO mice being lower than that of controls at 7 months of age (data not shown). These differences remained significant when values were normalized to body weight (Figure 3H).
Lama1CKO Mice Develop Glomerulosclerosis with Increased MC Proliferation and Mesangial Matrix Expansion with Age
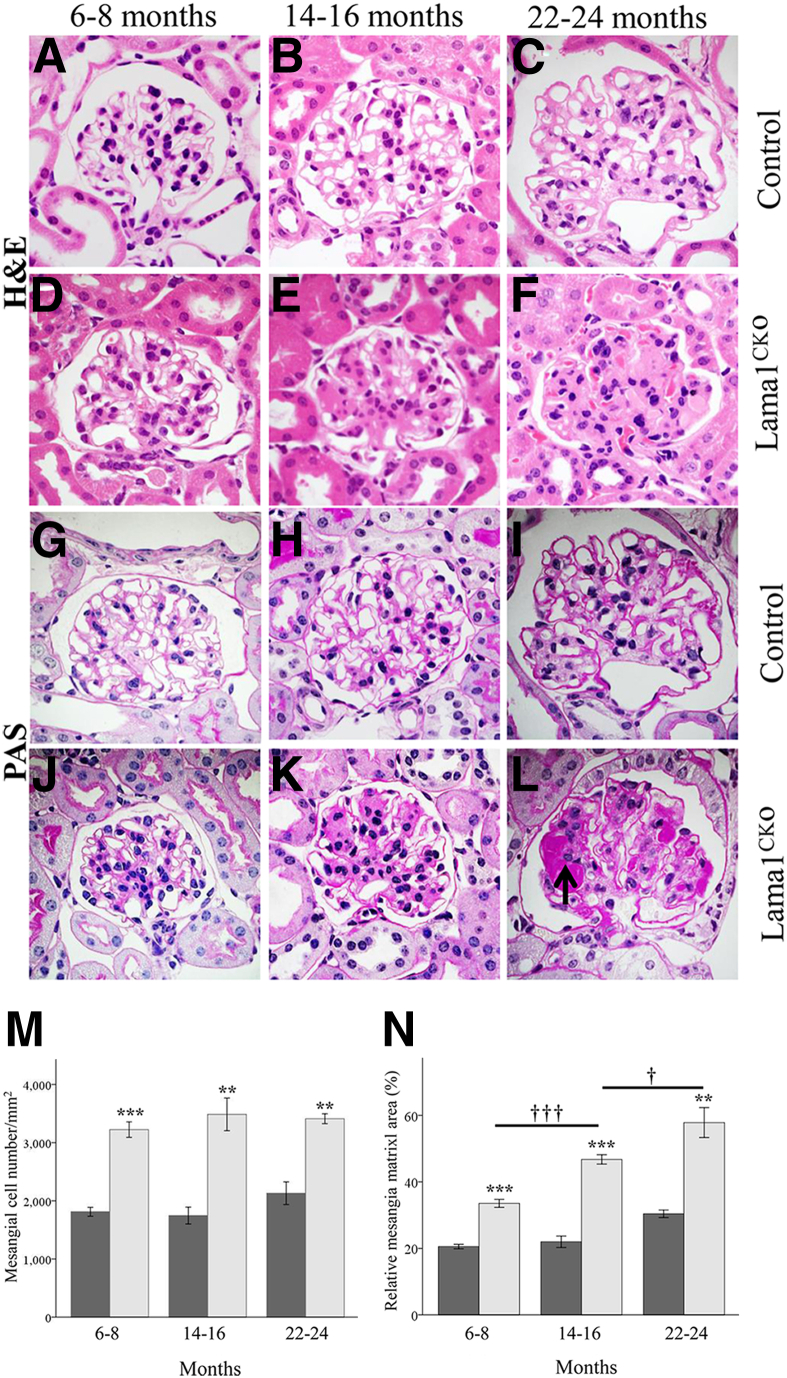
We next examined aging-associated glomerular pathological features in Lama1CKO mice at 2 to 24 months of age. Before 6 months of age, no significant renal histopathological changes were observed in either control or Lama1CKO mice (data not shown). However, after 6 months of age, Lama1CKO mice showed mesangial cell proliferation and mesangial matrix expansion compared with control mice (Figure 4, D–F and J–L). As Lama1CKO mice aged further, mesangial cell numbers stayed the same, but mesangial matrix expansion increased (Figure 4, M and N). By 6 to 8 months of age, Lama1CKO mice showed a moderate mesangial matrix expansion, but they did not exhibit glomerulosclerosis (Figure 4, D and J). By 14 to 16 months of age, 35% of Lama1CKO mice showed numerous segmentally sclerosed glomeruli (Figure 4E) associated with GBM thickening (Figure 4K), whereas no control mice showed glomerulosclerosis with GBM thickening (Figure 4, B and H). By 22 to 24 months of age, most Lama1CKO mice had many globally and segmentally sclerosed glomeruli (Figure 4, F and L), and PAS+ nodular mesangial sclerosis was observed (Figure 4L). Control mice also showed moderate mesangial expansion and GBM thickening at this age, but only rarely showed glomerulosclerosis (Figure 4, C and I). Glomerular abnormalities were characterized in more detail by electron microscopy in mice aged 14 to 16 months (Figure 5). In agreement with light microscopy, there were more MCs and an increased amount of mesangial matrix in old Lama1CKO mice compared with controls (Figure 5B). Many areas of GBM thickening were also observed (Figure 5D). These data show that older Lama1CKO mice exhibit a greater degree of MC and matrix irregularities versus controls, presumably associated with aging.
Figure 4.
Light microscopy reveals progressive development of glomerulosclerosis in Lama1CKO mice. Representative micrographs of PAS- and H&E-stained glomeruli in control and Lama1CKO mice aged 7, 15, and 23 months (as indicated). H&E staining shows matrix expansion in the Lama1CKO mouse (D–F) compared with the control (A–C). E: At 14 to 16 months, the Lama1CKO mice showed moderate mesangial sclerosis. F: As the Lama1CKO mice aged, mesangial sclerosis became more conspicuous. PAS staining revealed increased mesangial cell proliferation and GBM thickening in the Lama1CKO (J–L) compared with the control (G–I) mice. L: PAS+ nodular mesangial sclerosis was observed in Lama1CKO mice at 22 to 24 months of age (arrow). M and N: Quantitative measurements of mesangial cell number and relative mesangial matrix area (per total glomerular tuft cross-sectional area) for control (dark gray bars) and Lama1CKO (light gray bars) mice. Data represent means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 versus control at same age; †P < 0.05 versus 14- to 16-month-old mice; †††P < 0.001 versus 6- to 8-month-old mice. Original magnification, ×1000 (A–L).
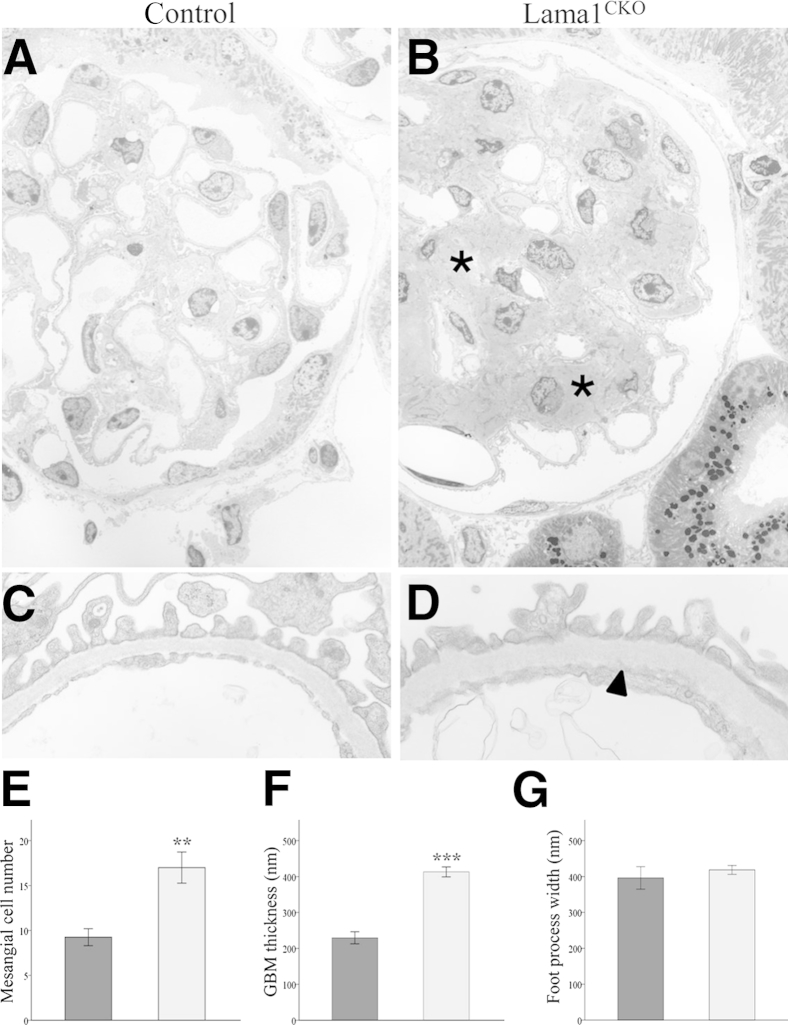
Figure 5.
Electron microscopic analysis of glomeruli. Representative electron micrographs of control (A and C) and Lama1CKO (B and D) glomeruli at 14 to 16 months of age. Lama1CKO mice had significant mesangial expansion (asterisks) and GBM thickening (arrowhead). E–G: Quantitation of the indicated features in control (dark gray bars) and Lama1CKO (light gray bars) mice show significant increases in mesangial cell number and GBM thickness but no significant difference was observed in podocyte foot process width. Data represent means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001 versus control. Original magnifications: ×1000 (A and B); ×10,000 (C and D).
Defects in Renal Function of Lama1CKO Mice
Because glomerular structure can influence glomerular filtration,6 the glomerular abnormalities in Lama1CKO mice led us to look for alterations in renal function. Urine from Lama1CKO and control mice was collected every 2 months and analyzed (Figure 6). Coomassie Blue staining of urine samples from Lama1CKO mice showed an increased level of albumin compared with controls (Figure 6A). We also consistently detected higher urinary albumin/creatinine ratios in Lama1CKO mice at 6 to 24 months of age, and the difference increased as the mice aged (Figure 6B). These results demonstrate a progressive defect in the kidney’s ability to handle albumin in Lama1CKO mice.
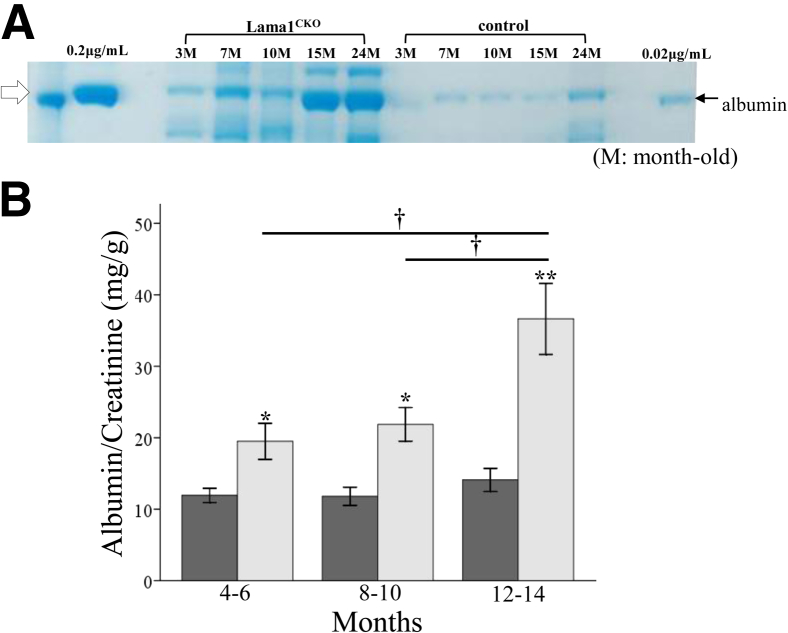
Figure 6.
Analysis of renal function. A: Urine from 3- to 24-month-old (M) Lama1CKO and control mice was collected and analyzed by SDS-PAGE and Coomassie Blue staining. The arrow denotes approximately 66 KDa, the size of excreted albumin. Lama1CKO mice have a higher concentration of albumin as they age compared with control mice. B: Urine was collected for 24 hours from control (dark gray bars) and Lama1CKO (light gray bars) mice and analyzed for albumin/creatinine ratio. Ratios in Lama1CKO mice are higher than in controls. Data represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01 versus control; †P < 0.05 versus 4- to 6-month-old mice and 8- to 10-month-old mice.
MCs from Lama1CKO Mice Show Increased Proliferation in Vitro
In vivo studies have provided evidence that LAMA1 deficiency promotes MC proliferation. To investigate this further, we examined MC proliferation in vitro. Primary cultures derived from mouse glomeruli yielded a homogeneous population of MCs (Figure 7, A–C). These were characterized by their stellate/spindle-shaped morphological features, positive staining for α-smooth muscle actin, and negative staining for synaptopodin and PECAM, which are specific for podocytes and endothelial cells, respectively. MCs from Lama1CKO mice exhibited significantly increased proliferation compared with those from control mice (Figure 7D).
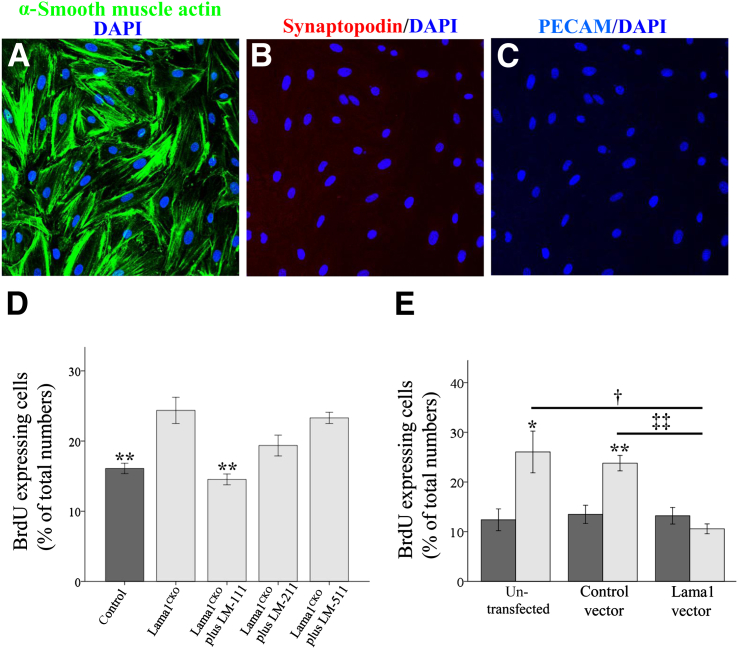
Figure 7.
Analysis of primary mesangial cells. Mesangial cells are elongated, arranged in multiple layers, and stained positively for smooth muscle α-actin (A), but not synaptopodin (B) or PECAM (C). D: Proliferation was assessed by immunofluorescence assay for BrdU-positive cells as a percentage of the total number of cells, averaged from five separate fields of view. Mesangial cells from Lama1CKO mice exhibit significantly increased proliferation compared with control cells. This increase is inhibited by exogenous LAMA1-containing LM-111, but not by LAMA2- or LAMA5-containing isoforms. E: Mesangial cell proliferation determined in cells transfected with vector alone or with a LAMA1 expression vector. Control (dark gray bars) and Lama1CKO (light gray bars) cells were cultured on slide chambers for 24 hours and transfected for 48 hours before proliferation analysis. Restoration of LAMA1 expression by Lama1CKO cells suppresses their proliferation. Data represent means ± SEM. ∗P < 0.05, ∗∗P < 0.01 versus control; †P < 0.05 versus untransfected cells; ‡‡P < 0.01 versus control vector transfected cells.
After this, we tested MC proliferation by treatment with LM-111, LM-211, or LM-511. The increase in MC proliferation was inhibited by exogenous LM-111, but not by either LM-211 or LM-511 (Figure 7D). We also tested whether LAMA1 deficiency is directly involved in this increased MC proliferation by transfection of Lama1CKO MCs with a Lama1 expression vector. RT-qPCR analysis showed the expression of Lama1 mRNA in Lama1CKO MCs transfected with the Lama1 vector (Supplemental Figure S1B). We found that the expression of exogenous Lama1 abrogated the increased proliferation of Lama1CKO MCs, whereas the control empty vector did not (Figure 7E), suggesting that LAMA1/LM-111 plays a specific role in regulating MC homeostasis.
Loss of LAMA1 Increases TGF-β/Smad Signaling in Lama1CKO MCs
TGF-β/Smad signaling is a major pathway involved in renal fibrosis.29 Binding of TGF-β1 to TβRII activates TβRI kinase, which phosphorylates Smad2 and Smad3. The phosphorylated Smad2 (p-Smad2) and p-Smad3 then bind to Smad4 and form the Smad complex, which translocates into the nucleus and regulates target gene transcription.29 Smad7 is an inhibitory Smad that blocks TGF-β–induced Smad-dependent fibrosis. Because TGF-β1 stimulates the synthesis and inhibits the degradation of extracellular matrix molecules,30 and is associated with increased mesangial matrix in several glomerular diseases,30–32 we investigated the effect of the absence of LAMA1 on TGF-β signaling. We first examined the level of TGF-β1 mRNA and protein secreted in Lama1CKO MCs. We did not detect significant differences compared with control MCs (Figure 8, A–C), indicating that the absence of LAMA1 does not alter the levels of TGF-β1 expression. Next, we examined the levels of p-Smad2 in the absence of LAMA1. The addition of TGF-β1 increased p-Smad2 levels to a greater extent in Lama1CKO cells than in control cells (Figure 8D), suggesting that the absence of LAMA1 increases TGF-β/Smad signaling. The basal phosphorylation level of Smad2 in Lama1CKO MCs was higher than that in control MCs (Supplemental Figure S2) when cells were cultured without serum starvation. In addition, LM-111 treatment rescued the increased TGF-β1–induced Smad2 phosphorylation in Lama1CKO cells, implying that LAMA1 likely plays a direct role in dampening the levels of TGF-β1–mediated Smad2 phosphorylation in MCs. Furthermore, we sought to elucidate the mechanism of increased Smad2 phosphorylation in the absence of LAMA1. MCs were pretreated with SB431542, a specific and potent inhibitor of TGF-β–induced phosphorylation of Smad2 by TβRI.33 SB431542 was added to the control and Lama1CKO cells at varying doses, and cells were treated for 30 minutes with TGF-β1. Cell lysates were collected and processed for Western blot analysis of p-Smad2 levels. Decreased or loss of Smad2 phosphorylation was observed in a dose-dependent manner for both control and Lama1CKO cells (Figure 8E). These data show that all of the phosphorylation of Smad2 occurs via the activity of TβRI, regardless of the presence or absence of LAMA1. This indicates that LAMA1 plays a direct role in limiting TGF-β/Smad signaling. Finally, because reduced Smad7 results in enhanced activation of TGF-β signaling,34 we also investigated Smad7 expression in MCs. There was no significant difference in the level of Smad7 protein in control and in Lama1CKO cells (data not shown).
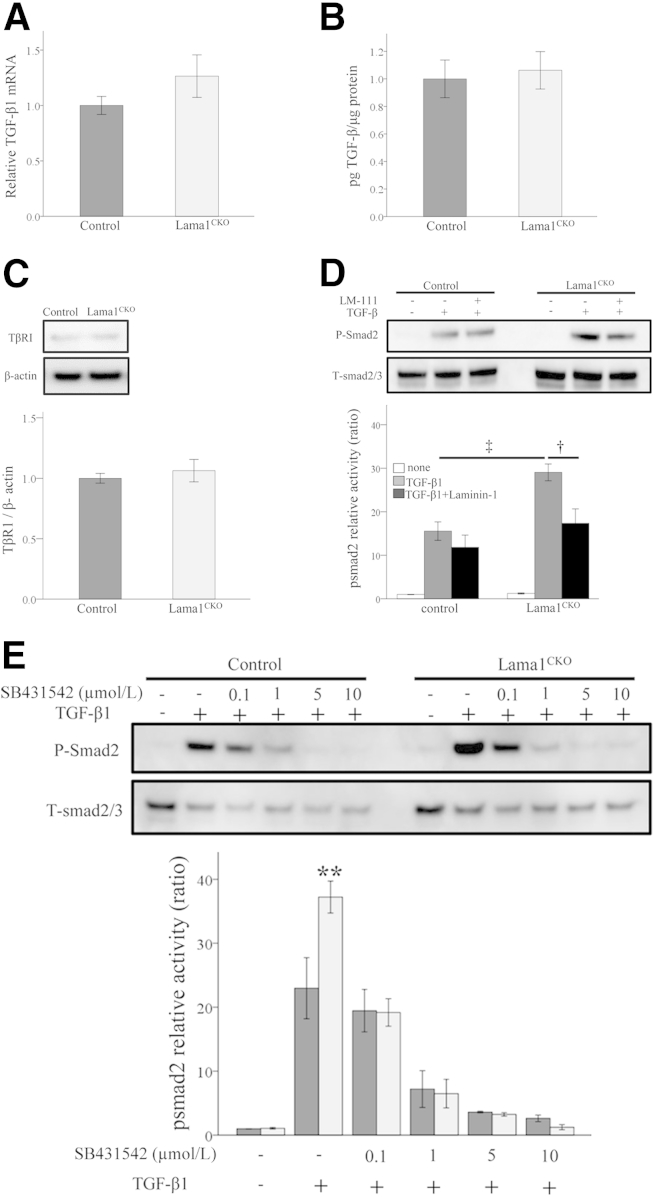
Figure 8.
TGF-β1/Smad signaling in mesangial cells from Lama1CKO and control mice. A and B: TGF-β1 mRNA expression and secretion in mesangial cells. MCs were cultured for 48 hours and serum starved for an additional 48 hours before total mRNA extraction and culture media collection. A: TGF-β1 mRNA expression levels measured by RT-qPCR. The bar graph represents the ratio between the TGF-β1 and GAPDH levels. B: Immunoreactive TGF-β1 synthesis measured by ELISA. C: Western blot analysis of TβRI protein levels in MCs. D and E: Smad2 phosphorylation determined by using Western blot analysis. The intensity of signal was analyzed by Multi Gauge version 3.0 (Fujifilm). Relative activity, expressed as the ratio of activated phospho-Smad2/total Smad2/3, was quantified as the fold increase relative to non-stimulation conditions (defined as 1). MCs from control and Lama1CKO mice were cultured in the absence or presence of 5 μg/mL LM-111 for 24 hours. D: After serum starvation for 24 hours, cells were treated with 10 ng/mL TGF-β1 (WAKO) for 30 minutes. MCs from control and Lama1CKO mice were serum starved for 24 hours, followed by 1-hour treatment with increasing concentrations of SB-431542 (0.1, 1, 5, and 10 μmol/L) or dimethyl sulfoxide (DMSO), and then stimulated for 30 minutes with 10 ng/mL TGF-β. E: Loss of Smad2 phosphorylation is observed in an SB-431542 dose-dependent manner for both control (dark gray bars) and Lama1CKO (light gray bars) cells. Data represent means ± SEM. ∗∗P < 0.01 versus control; †P < 0.05 versus laminin-1 treatment cells; ‡P < 0.01 versus control cells.
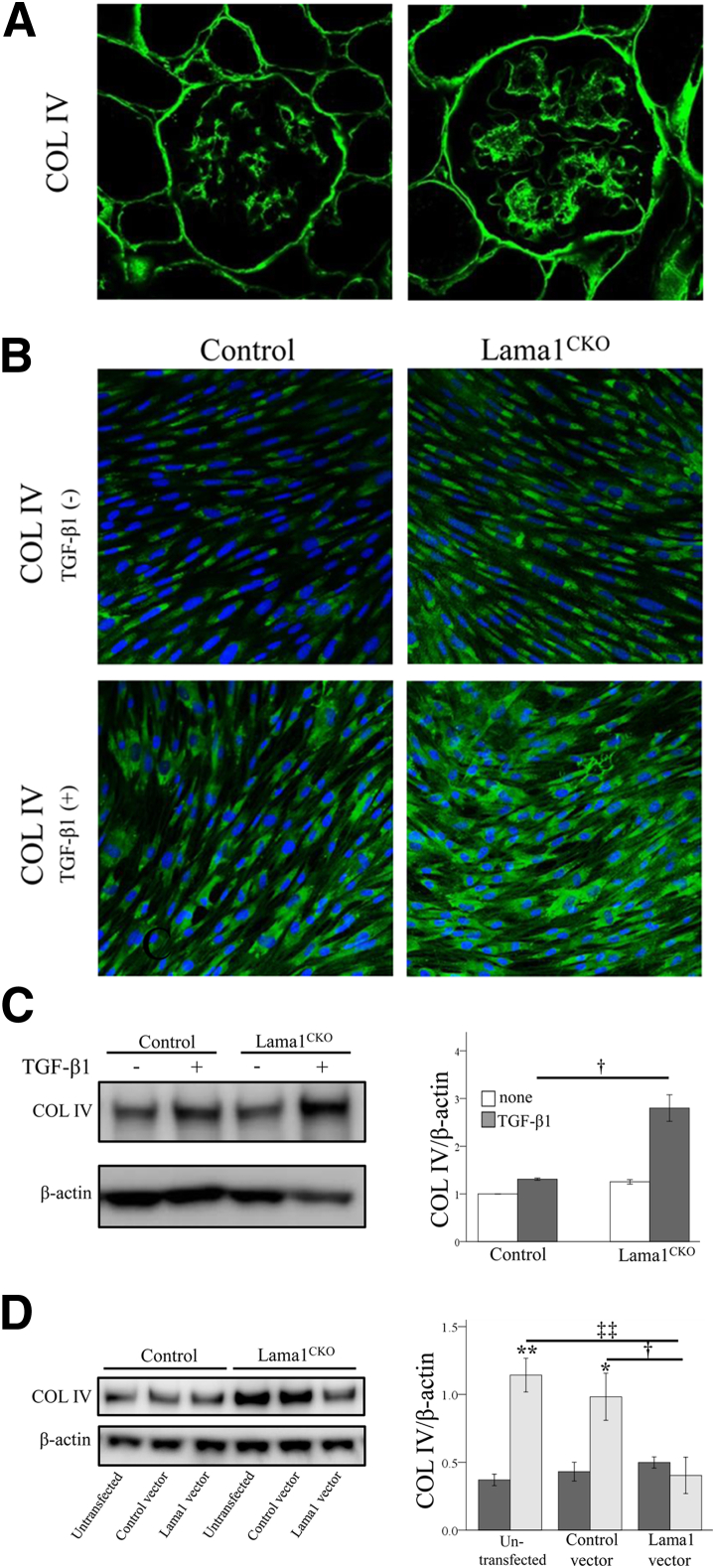
TGF-β1–Induced Type Ⅳ Collagen Expression Is Increased in the Absence of LAMA1
Because Lama1CKO mice showed a thickened GBM (Figure 5D), we examined the expression of COLIV, one of the major mesangial matrix components.35 Immunostaining analysis revealed more COLIV deposition in the mesangium of Lama1CKO mice than of control mice (Figure 9A). Previous studies have shown that TGF-β1 induces COLIV expression in mouse MCs.36 Therefore, we examined the effect of TGF-β1 on COLIV expression in MCs prepared from Lama1CKO mice. We found that TGF-β1 induced COLIV expression in both control and Lama1CKO MCs, but the level of COLIV expression was significantly higher in Lama1CKO MCs than in control cells (Figure 9, B and C). This higher COLIV expression level in Lama1CKO MCs was abrogated by transfection with the LAMA1 expression vector, but not by transfection with the empty control vector (Figure 9D). These results suggest that LAMA1 is involved in the regulation of mesangial matrix production.
Figure 9.
Expression of COLIV. A: The deposition of COLIV in the glomeruli of control and Lama1CKO mice. Immunofluorescence staining for COLIV was performed in frozen tissue sections from 7-month-old mice. B–D: Effect of TGF-β1 on COLIV synthesis by MCs. Control and Lama1CKO MCs were cultured on slide chambers for 24 hours, serum deprived for an additional 24 hours, and then treated with TGF-β1 for 24 hours. Immunofluorescence (B) and Western blot analysis (C) were performed to analyze COLIV synthesis and deposition. Values in C are expressed as COLIV/β-actin and quantified as the fold increase relative to control non-stimulation conditions (defined as 1). Control and Lama1CKO MCs were cultured on 6-well plates for 24 hours and transfected for 48 hours before a 24-hour TGF-β1 treatment. †P < 0.05 versus TGF-β1 treatment control cells. D: COLIV protein levels were then determined by using Western blot analysis. β-Actin was used as a control. The intensity of signal was analyzed by Multi Gauge version 3.0 (Fujifilm). The absence of LAMA1 increases TGF-β1–induced COLIV expression. This increase was abrogated by transfection of Lama1CKO (light gray bars) MCs with the LAMA1 expression vector; control MCs are represented by dark gray bars. ∗P < 0.05, ∗∗P < 0.01 versus control; †P < 0.05 versus control vector transfected cells; ‡‡P < 0.01 versus untransfected cells. Original magnification, ×400 (A).
Discussion
Our interest in LM-111 function in the kidney was initially stimulated by the observation that Lama1 has a rather restricted expression pattern. LAMA1 is found transiently in the developing GBM, is absent in the mature GBM, and is present in the glomerular mesangium in adult mice.5 Herein, we demonstrated that Lama1 is critical for mesangial homeostasis and kidney function.
An impediment to elucidating kidney LAMA1 function had been that Lama1 disruption results in lethality at an early embryonic stage.1 We previously described the generation of a conditional KO of Lama1 with selective deletion only in embryonic cells, wherein the early embryonic death observed in total Lama1 KO mice was overcome. Our results demonstrate important functional roles for LAMA1, which are exemplified by delayed glomerular development, abnormal renal histological features, and impaired renal function. Increased MC proliferation and mesangial matrix expansion are remarkable features observed in Lama1CKO mice.
Diabetic nephropathy is histologically characterized by thickening of the GBM and mesangial expansion.37–39 The earliest morphological change in diabetic nephropathy is mesangial expansion due to increased mesangial matrix deposition and a mild increase in mesangial cellularity, as well as hypertrophy of mesangial cells. The characteristic histological changes of diabetic nephropathy are diffuse and nodular glomerulosclerosis, along with afferent and efferent hyaline arteriolosclerosis. A reduction in podocyte number is associated with foot process effacement.40 In the present study, we observed GBM thickening, mesangial expansion, and increased mesangial cell proliferation from 6-month-old Lama1CKO mice. As the mice aged, there was increased mesangial expansion due to glomerulosclerosis, which was accompanied by progressive proteinuria. These pathological conditions are similar to those seen in diabetic nephropathy, but we did not observe differences between Lama1CKO and control mice in terms of podocyte number or foot process effacement. Although proteinuria was detectable in Lama1CKO mice, this was at a lower level than in most kidney diseases. Because there is no or little LAMA1 in the GBM, podocytes would not be expected to have abnormalities. The proteinuria in the later stages was likely due to mesangial cell defects, which led to profibrotic cross talk that affected the podocytes, or perhaps due to some unknown mechanism.
Many studies have indicated that activated mesangial cells are the major cells responsible for the expression of interstitial matrix components, such as fibronectin and type I collagen, which directly result in mesangial expansion leading to glomerulosclerosis.41 The present study demonstrated that the deletion of LAMA1 results in age-dependent mesangial expansion, which develops into focal glomerulosclerosis. These observations lead us to hypothesize that LAMA1 may be an endogenous negative regulator that specifically suppresses mesangial cell activation.
TGF-β is the most potent cytokine inducing mesangial cell activation both in vitro and in vivo.42 It stimulates the expression of ECM proteins, including collagens, laminin, and fibronectin, while suppressing the synthesis of ECM protease inhibitors.43 The Smad pathway is known to mediate the functions of TGF-β on renal fibrogenesis and subsequent ECM accumulation in diabetic nephropathy.44 In the present study, we were unable to detect increased TGF-β1 mRNA expression in Lama1CKO MCs. However, an alternative mechanism is suggested from the increased TGF-β1–induced Smad2 phosphorylation. In addition, inhibitors of TβRI kinase blocked Smad2 phosphorylation in both control and Lama1CKO MCs. This supports the hypothesis that LAMA1 suppresses mesangial cell activation via inhibition of the TGF-β/Smad pathway.
Laminins not only function as structural components, but also bind cell surface receptors, including integrins and α-dystroglycan.45 We demonstrated that LM-111 treatment abrogated the increased TGF-β1–induced Smad2 phosphorylation in Lama1CKO cells. Furthermore, the restoration of LAMA1 expression in Lama1CKO MCs by transfection with the LAMA1 expression vector inhibited the increased MC proliferation and TGF-β1–induced COLIV expression. We, therefore, speculate that LM-111 synthesized by WT mesangial cells inhibits TGF-β1 signaling. TGF-β1 signals through its type I and type II serine/threonine kinase receptors,42 and this pathway is tightly controlled by multiple positive and negative regulator proteins.46 Negative regulation of TGF-β signaling is accomplished by the rapid attenuation or even inhibition of TβRI/II and/or Smad activities.47,48 In extracellular compartments, decorin, a proteoglycan associated with matrix components, binds to active TGF-β1 and prevents it from engaging with its receptors.49 In cell membranes, integrin signaling is involved in negatively regulating TGF-β signaling, because adhesion reduces TGF-β–induced Smad2 phosphorylation.50 Laminins regulate cellular behavior through interactions with cell surface receptors, including integrins, syndecans, and α-dystroglycan.1,51 In the cytoplasm, inhibitory Smad7 either competes with receptor-regulated Smad to bind to activated type I receptors or interacts with growth arrest and DNA damage protein, a regulatory subunit of the protein phosphatase 1 holoenzyme, which subsequently recruits catalytic subunit of PP1 to dephosphorylate and inactivate the TGF-β type I receptor.52
The present study demonstrated that excess Smad2 phosphorylation occurred in the absence of Lama1 via TβRI and the absence of LAMA1 did not change the expression of Smad7. In addition, the Biacore analysis and solid-phase binding assays showed that the active form of TGF-β bound to LM-111 (Supplemental Figure S3 and Supplemental Table S1). This suggests that LAMA1 may play a direct role in inhibiting TGF-β signaling by binding to active TGF-β1 to prevent it from engaging with its receptors or by interaction with integrin signaling, which is involved in negatively regulating TGF-β signaling.
Taken together, our results suggest that LAMA1 plays a role in negatively regulating TGF-β/Smad signaling. This may be important for maintaining mesangial cell populations and mesangial matrix deposition.
Acknowledgments
We thank Glenn Longenecker and Ashok B. Kulkarni for help generating mutant mice and Dr. Yasuo Uchiyama, Takashi Ueno, and Tsutomu Fujimura (Juntendo University, Tokyo, Japan) for useful advice.
Footnotes
Supported by a Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan, Grant-in-Aid for Young Scientists (B) 24790860 (L.N.) and grants 17082008 and 2230023 (E.A.-H.), NIH Intramural Program of the National Institute of Dental and Craniofacial Research grant (Y.Y.), NIH grant R01DK078314 (J.H.M.), and MEXT–Supported Program for the Strategic Research Foundation at Private Universities (2011 to 2015).
Disclosures: None declared.
Supplemental Data
A: Immunofluorescence reveals expression of LAMA1 in mesangial cells from control mice, no expression in mesangial cells from Lama1CKO mice, and expression of LAMA2 in mesangial cells from control and Lama1CKO mice. B: RT-PCR analysis of mRNA isolated from mesangial cells reveals expression of Lama1 in mesangial cells from control mice and no expression in mesangial cells from Lama1CKO mice. However, Lama1 mRNA expression can be reconstituted by the Lama1 expression vector transfection in Lama1CKO mice.
MCs from Lama1CKO mice exhibit significantly increased TGF-β1–induced Smad2 phosphorylation compared with control cells. The basal phosphorylation of Smad2 in MCs from Lama1CKO mice is also higher than that in MCs from control mice. MCs were cultured for 2 days without starvation before TGF-β1 stimulation. *P < 0.05, **P < 0.01.
A solid-phase binding assay reveals that TGF-β1 has a higher binding affinity for LM-111 than for LM-211. The x axis is the concentrations of TGF-β1. ∗P < 0.05, ∗∗P < 0.01. BSA, bovine serum albumin.
References
- 1.Miner J.H., Yurchenco P.D. Laminin functions in tissue morphogenesis. Annu Rev Cell Dev Biol. 2004;20:255–284. doi: 10.1146/annurev.cellbio.20.010403.094555. [DOI] [PubMed] [Google Scholar]
- 2.Aumailley M., Bruckner-Tuderman L., Carter W.G., Deutzmann R., Edgar D., Ekblom P., Engel J., Engvall E., Hohenester E., Jones J.C., Kleinman H.K., Marinkovich M.P., Martin G.R., Mayer U., Meneguzzi G., Miner J.H., Miyazaki K., Patarroyo M., Paulsson M., Quaranta V., Sanes J.R., Sasaki T., Sekiguchi K., Sorokin L.M., Talts J.F., Tryggvason K., Uitto J., Virtanen I., von der Mark K., Wewer U.M., Yamada Y., Yurchenco P.D. A simplified laminin nomenclature. Matrix Biol. 2005;24:326–332. doi: 10.1016/j.matbio.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Ryan M.C., Christiano A.M., Engvall E., Wewer U.M., Miner J.H., Sanes J.R., Burgeson R.E. The functions of laminins: lessons from in vivo studies. Matrix Biol. 1996;15:369–381. doi: 10.1016/s0945-053x(96)90157-2. [DOI] [PubMed] [Google Scholar]
- 4.Kleinman H.K., Weeks B.S., Schnaper H.W., Kibbey M.C., Yamamura K., Grant D.S. The laminins: a family of basement membrane glycoproteins important in cell differentiation and tumor metastases. Vitam Horm. 1993;47:161–186. doi: 10.1016/s0083-6729(08)60446-x. [DOI] [PubMed] [Google Scholar]
- 5.Miner J.H., Patton B.L., Lentz S.I., Gilbert D.J., Snider W.D., Jenkins N.A., Copeland N.G., Sanes J.R. The laminin alpha chains: expression, developmental transitions, and chromosomal locations of alpha1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel alpha3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miner J.H. Building the glomerulus: a matricentric view. J Am Soc Nephrol. 2005;16:857–861. doi: 10.1681/ASN.2004121139. [DOI] [PubMed] [Google Scholar]
- 7.Wang R., Moorer-Hickman D., St John P.L., Abrahamson D.R. Binding of injected laminin to developing kidney glomerular mesangial matrices and basement membranes in vivo. J Histochem Cytochem. 1998;46:291–300. doi: 10.1177/002215549804600302. [DOI] [PubMed] [Google Scholar]
- 8.Abboud H.E. Mesangial cell biology. Exp Cell Res. 2012;318:979–985. doi: 10.1016/j.yexcr.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 9.Miner J.H., Li C., Mudd J.L., Go G., Sutherland A.E. Compositional and structural requirements for laminin and basement membranes during mouse embryo implantation and gastrulation. Development. 2004;131:2247–2256. doi: 10.1242/dev.01112. [DOI] [PubMed] [Google Scholar]
- 10.Alpy F., Jivkov I., Sorokin L., Klein A., Arnold C., Huss Y., Kedinger M., Simon-Assmann P., Lefebvre O. Generation of a conditionally null allele of the laminin alpha1 gene. Genesis. 2005;43:59–70. doi: 10.1002/gene.20154. [DOI] [PubMed] [Google Scholar]
- 11.Ichikawa-Tomikawa N., Ogawa J., Douet V., Xu Z., Kamikubo Y., Sakurai T., Kohsaka S., Chiba H., Hattori N., Yamada Y., Arikawa-Hirasawa E. Laminin alpha1 is essential for mouse cerebellar development. Matrix Biol. 2012;31:17–28. doi: 10.1016/j.matbio.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sasaki T. Expression and distribution of laminin α1 and α2 chains in embryonic and adult mouse tissues: an immunochemical approach. Exp Cell Res. 2002;275:185–199. doi: 10.1006/excr.2002.5499. [DOI] [PubMed] [Google Scholar]
- 13.Jarad G., Cunningham J., Shaw A.S., Miner J.H. Proteinuria precedes podocyte abnormalities in Lamb2-/- mice, implicating the glomerular basement membrane as an albumin barrier. J Clin Invest. 2006;116:2272–2279. doi: 10.1172/JCI28414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen E.B., Gundersen H.J., Osterby R. Determination of membrane thickness distribution from orthogonal intercepts. J Microsc. 1979;115:19–33. doi: 10.1111/j.1365-2818.1979.tb00149.x. [DOI] [PubMed] [Google Scholar]
- 15.Koop K., Eikmans M., Baelde H.J., Kawachi H., De Heer E., Paul L.C., Bruijn J.A. Expression of podocyte-associated molecules in acquired human kidney diseases. J Am Soc Nephrol. 2003;14:2063–2071. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- 16.Takemoto M., Asker N., Gerhardt H., Lundkvist A., Johansson B.R., Saito Y., Betsholtz C. A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yaoita E., Kurihara H., Sakai T., Ohshiro K., Yamamoto T. Phenotypic modulation of parietal epithelial cells of Bowman’s capsule in culture. Cell Tissue Res. 2001;304:339–349. doi: 10.1007/s004410100380. [DOI] [PubMed] [Google Scholar]
- 18.Nyati S., Schinske K., Ray D., Nyati M.K., Ross B.D., Rehemtulla A. Molecular imaging of TGFbeta-induced Smad2/3 phosphorylation reveals a role for receptor tyrosine kinases in modulating TGFbeta signaling. Clin Cancer Res. 2011;17:7424–7439. doi: 10.1158/1078-0432.CCR-11-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hammerschmidt E., Loeffler I., Wolf G. Morg1 heterozygous mice are protected from acute renal ischemia-reperfusion injury. Am J Physiol Renal Physiol. 2009;297:F1273–F1287. doi: 10.1152/ajprenal.00204.2009. [DOI] [PubMed] [Google Scholar]
- 20.Klein G., Langegger M., Timpl R., Ekblom P. Role of laminin A chain in the development of epithelial cell polarity. Cell. 1988;55:331–341. doi: 10.1016/0092-8674(88)90056-6. [DOI] [PubMed] [Google Scholar]
- 21.Sorokin L.M., Conzelmann S., Ekblom P., Battaglia C., Aumailley M., Timpl R. Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp Cell Res. 1992;201:137–144. doi: 10.1016/0014-4827(92)90357-e. [DOI] [PubMed] [Google Scholar]
- 22.Abrahamson D.R., St John P.L. Loss of laminin epitopes during glomerular basement membrane assembly in developing mouse kidneys. J Histochem Cytochem. 1992;40:1943–1953. doi: 10.1177/40.12.1280666. [DOI] [PubMed] [Google Scholar]
- 23.Miner J.H. Developmental biology of glomerular basement membrane components. Curr Opin Nephrol Hypertens. 1998;7:13–19. doi: 10.1097/00041552-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Peutz-Kootstra C.J., Hansen K., De Heer E., Abrass C.K., Bruijn J.A. Differential expression of laminin chains and anti-laminin autoantibodies in experimental lupus nephritis. J Pathol. 2000;192:404–412. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH707>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.Abrahamson D.R., Prettyman A.C., Robert B., St John P.L. Laminin-1 reexpression in Alport mouse glomerular basement membranes. Kidney Int. 2003;63:826–834. doi: 10.1046/j.1523-1755.2003.00800.x. [DOI] [PubMed] [Google Scholar]
- 26.St John P.L., Wang R., Yin Y., Miner J.H., Robert B., Abrahamson D.R. Glomerular laminin isoform transitions: errors in metanephric culture are corrected by grafting. Am J Physiol Renal Physiol. 2001;280:F695–F705. doi: 10.1152/ajprenal.2001.280.4.F695. [DOI] [PubMed] [Google Scholar]
- 27.Kashtan C.E., Kim Y., Lees G.E., Thorner P.S., Virtanen I., Miner J.H. Abnormal glomerular basement membrane laminins in murine, canine and human Alport syndrome: aberrant laminin alpha2 deposition is species-independent. J Am Soc Nephrol. 2001;12:252–260. doi: 10.1681/ASN.V122252. [DOI] [PubMed] [Google Scholar]
- 28.Cosgrove D., Rodgers K., Meehan D., Miller C., Bovard K., Gilroy A., Gardner H., Kotelianski V., Gotwals P., Amatucci A., Kalluri R. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in Alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am J Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lan H.Y., Chung A.C. TGF-beta/Smad signaling in kidney disease. Semin Nephrol. 2012;32:236–243. doi: 10.1016/j.semnephrol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Ziyadeh F.N. Different roles for TGF-beta and VEGF in the pathogenesis of the cardinal features of diabetic nephropathy. Diabetes Res Clin Pract. 2008;82(Suppl 1):S38–S41. doi: 10.1016/j.diabres.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 31.Ziyadeh F.N., Sharma K., Ericksen M., Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshioka K., Takemura T., Murakami K., Okada M., Hino S., Miyamoto H., Maki S. Transforming growth factor-beta protein and mRNA in glomeruli in normal and diseased human kidneys. Lab Invest. 1993;68:154–163. [PubMed] [Google Scholar]
- 33.Inman G.J., Nicolas F.J., Callahan J.F., Harling J.D., Gaster L.M., Reith A.D., Laping N.J., Hill C.S. SB-431542 is a potent and specific inhibitor of transforming growth factor-beta superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- 34.Heldin C.H., Miyazono K., ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- 35.Kikkawa R., Togawa M., Isono M., Isshiki K., Haneda M. Mechanism of the progression of diabetic nephropathy to renal failure. Kidney Int Suppl. 1997;62:S39–S40. [PubMed] [Google Scholar]
- 36.Chai Q., Krag S., Miner J.H., Nyengaard J.R., Chai S., Wogensen L. TGF-beta1 induces aberrant laminin chain and collagen type IV isotype expression in the glomerular basement membrane. Nephron Exp Nephrol. 2003;94:e123–e136. doi: 10.1159/000072496. [DOI] [PubMed] [Google Scholar]
- 37.Falk R.J., Scheinman J.I., Mauer S.M., Michael A.F. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32(Suppl 2):34–39. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- 38.Adler S. Structure-function relationships associated with extracellular matrix alterations in diabetic glomerulopathy. J Am Soc Nephrol. 1994;5:1165–1172. doi: 10.1681/ASN.V551165. [DOI] [PubMed] [Google Scholar]
- 39.Mauer S.M., Steffes M.W., Ellis E.N., Sutherland D.E., Brown D.M., Goetz F.C. Structural-functional relationships in diabetic nephropathy. J Clin Invest. 1984;74:1143–1155. doi: 10.1172/JCI111523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Alsaad K.O., Herzenberg A.M. Distinguishing diabetic nephropathy from other causes of glomerulosclerosis: an update. J Clin Pathol. 2007;60:18–26. doi: 10.1136/jcp.2005.035592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mason R.M., Wahab N.A. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- 42.Dai C., Liu Y. Hepatocyte growth factor antagonizes the profibrotic action of TGF-beta1 in mesangial cells by stabilizing Smad transcriptional corepressor TGIF. J Am Soc Nephrol. 2004;15:1402–1412. doi: 10.1097/01.asn.0000130568.53923.fd. [DOI] [PubMed] [Google Scholar]
- 43.Schnaper H.W., Kopp J.B., Poncelet A.C., Hubchak S.C., Stetler-Stevenson W.G., Klotman P.E., Kleinman H.K. Increased expression of extracellular matrix proteins and decreased expression of matrix proteases after serial passage of glomerular mesangial cells. J Cell Sci. 1996;109(Pt 10):2521–2528. doi: 10.1242/jcs.109.10.2521. [DOI] [PubMed] [Google Scholar]
- 44.Schnaper H.W., Hayashida T., Poncelet A.C. It’s a Smad world: regulation of TGF-beta signaling in the kidney. J Am Soc Nephrol. 2002;13:1126–1128. doi: 10.1681/ASN.V1341126. [DOI] [PubMed] [Google Scholar]
- 45.Higaki Y., Schullery D., Kawata Y., Shnyreva M., Abrass C., Bomsztyk K. Synergistic activation of the rat laminin gamma1 chain promoter by the gut-enriched Kruppel-like factor (GKLF/KLF4) and Sp1. Nucleic Acids Res. 2002;30:2270–2279. doi: 10.1093/nar/30.11.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.ten Dijke P., Miyazono K., Heldin C.H. Signaling inputs converge on nuclear effectors in TGF-beta signaling. Trends Biochem Sci. 2000;25:64–70. doi: 10.1016/s0968-0004(99)01519-4. [DOI] [PubMed] [Google Scholar]
- 47.Di Guglielmo G.M., Le Roy C., Goodfellow A.F., Wrana J.L. Distinct endocytic pathways regulate TGF-beta receptor signalling and turnover. Nat Cell Biol. 2003;5:410–421. doi: 10.1038/ncb975. [DOI] [PubMed] [Google Scholar]
- 48.Feng X.H., Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- 49.Border W.A., Noble N.A., Yamamoto T., Harper J.R., Yamaguchi Y., Pierschbacher M.D., Ruoslahti E. Natural inhibitor of transforming growth factor-beta protects against scarring in experimental kidney disease. Nature. 1992;360:361–364. doi: 10.1038/360361a0. [DOI] [PubMed] [Google Scholar]
- 50.Thannickal V.J., Lee D.Y., White E.S., Cui Z., Larios J.M., Chacon R., Horowitz J.C., Day R.M., Thomas P.E. Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J Biol Chem. 2003;278:12384–12389. doi: 10.1074/jbc.M208544200. [DOI] [PubMed] [Google Scholar]
- 51.Gullberg D., Ekblom P. Extracellular matrix and its receptors during development. Int J Dev Biol. 1995;39:845–854. [PubMed] [Google Scholar]
- 52.Shi W., Sun C., He B., Xiong W., Shi X., Yao D., Cao X. GADD34-PP1c recruited by Smad7 dephosphorylates TGFbeta type I receptor. J Cell Biol. 2004;164:291–300. doi: 10.1083/jcb.200307151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Immunofluorescence reveals expression of LAMA1 in mesangial cells from control mice, no expression in mesangial cells from Lama1CKO mice, and expression of LAMA2 in mesangial cells from control and Lama1CKO mice. B: RT-PCR analysis of mRNA isolated from mesangial cells reveals expression of Lama1 in mesangial cells from control mice and no expression in mesangial cells from Lama1CKO mice. However, Lama1 mRNA expression can be reconstituted by the Lama1 expression vector transfection in Lama1CKO mice.
MCs from Lama1CKO mice exhibit significantly increased TGF-β1–induced Smad2 phosphorylation compared with control cells. The basal phosphorylation of Smad2 in MCs from Lama1CKO mice is also higher than that in MCs from control mice. MCs were cultured for 2 days without starvation before TGF-β1 stimulation. *P < 0.05, **P < 0.01.
A solid-phase binding assay reveals that TGF-β1 has a higher binding affinity for LM-111 than for LM-211. The x axis is the concentrations of TGF-β1. ∗P < 0.05, ∗∗P < 0.01. BSA, bovine serum albumin.