Abstract
STAT4, which is activated mainly by IL-12, promotes inflammatory responses by inducing Th1 and Th2 cytokines. Recent genome-wide association studies indicate that STAT4 gene variants are associated with risk of various types of liver diseases, but how STAT4 contributes to liver disease pathogenesis remains obscure. In this study, STAT4 activation was detected in liver immune cells from patients with viral hepatitis and autoimmune hepatitis, as well as in a mouse model of concanavalin A (Con A)–induced hepatitis. Such STAT4 activation was detected mainly in T cells, natural killer T cells, and macrophages and Kupffer cells, and was diminished in Il12a−/− and Il12b−/− mice. As expected, disruption of the Stat4 gene reduced production of Th1 and Th2 cytokines, but surprisingly exacerbated Con A–induced liver injury. Similarly, disruption of Il12a or Il12b also augmented Con A–induced hepatocellular damage. Further studies showed that hepatic natural killer T (NKT) cells from Con A–treated Stat4−/− mice had higher levels of FasL expression and increased cytotoxicity against hepatocytes than those from Con A–treated WT mice. In vitro, blocking FasL attenuated Stat4−/− NKT cytotoxicity against hepatocytes. In conclusion, despite up-regulation of proinflammatory cytokines, STAT4 protects against acute T-cell hepatitis, which is mediated by direct or indirect down-regulation of FasL expression on NKT cells.
Immune-mediated liver injury contributes to the pathogenesis of a variety of liver diseases, including viral hepatitis, autoimmune hepatitis (AIH), alcoholic hepatitis, nonalcoholic steatohepatitis, and drug-induced liver injury.1–4 However, the molecular and cellular mechanisms underlying immune-induced hepatocellular damage are not fully understood.5 Over the last two decades, genome-wide association studies have identified that genetic variants of several inflammation-related genes are associated with the risk of various types of liver diseases, findings that not only enhance our understanding of liver disease pathogenesis, but also provide novel therapeutic targets for the treatment of liver diseases.6–8 For example, several recent genome-wide association studies have demonstrated that signal transducer and activator of transcription 4 (STAT4) gene variants are closely associated with the increased risk of hepatitis B–related liver cancer,9 primary biliary cirrhosis,10,11 and hepatitis C–induced fibrosis.12 However, how these STAT4 variants affect STAT4 gene expression and how STAT4 affects liver injury and inflammation remain largely unknown.
STAT proteins are a group of transcription factors that transmit signals from the extracellular milieu of cells to the nucleus. The seven known mammalian STAT family members are STATs 1, 2, 3, 4, 5A, 5B, and 6. In the liver, the functions of STATs 1, 3, and 6 have been extensively investigated in many models of liver diseases,13 including concanavalin A (Con A)–induced T-cell hepatitis. Injection of mice with a single dose of Con A rapidly activates T cells and natural killer T (NKT) cells and subsequently causes hepatitis and liver injury, closely resembling the pathogenesis of AIH and viral hepatitis.1 With this model, we and others have demonstrated that interferon γ (IFN-γ) and IL-4 play a critical role in promoting hepatocellular damage via activation of STAT114 and STAT6,15 respectively, whereas IL-6 and IL-22 are important hepatoprotective cytokines that protect against liver injury via activation of STAT3.14,16 In contrast to the well-documented hepatic functions of STATs 1, 3, and 6, the roles of STAT4 in liver injury and inflammation have not been carefully examined.
STAT4, which is activated mainly by IL-12,17–19 and to a lesser extent by IFN-α/β,17 IL-2,18 and IL-17,20 plays critical roles in regulating inflammatory responses in various types of diseases.21 IL-12 is a heterodimeric 70-kDa glycoprotein consisting of a 40-kDa subunit (encoded by human Il12b or mouse Il12b) and a 35-kDa subunit (encoded by Il12a or Il12a). On binding to an IL-12 receptor complex consisting of IL-12R1 and IL-12R2, IL-12 predominantly activates STAT4, and also weakly activates STAT1 and STAT3. IL-12 plays an important role in promoting inflammation and antitumor immunity by inducing Th1 differentiation and IFN-γ production.19 The proinflammatory and antitumor effects of IL-12 have also been documented in several animal models of liver injury.22–25 However, reports on the functions of STAT4 in liver injury and inflammation have been controversial. Shen et al26 found that STAT4 knockout (KO) mice were resistant to liver ischemia–reperfusion injury, but later Kato et al27 found that STAT4 KO mice and wild-type (WT) mice had equal liver injury after ischemia–reperfusion. The reason for the discrepancy between these two studies remains obscure.
In the present study, STAT4 activation was examined in liver samples from patients with chronic viral hepatitis and AIH, as well in a mouse model of Con A–induced T-cell hepatitis. The role of IL-12 and STAT4 in the pathogenesis of T-cell hepatitis was further investigated in Stat4−/−, Il12a−/−, and Il12b−/−mice. Our findings reveal that STAT4 promotes production of Th1 and Th2 cytokines, but inhibits expression of FasL in NKT cells. STAT4 thus plays a dual role in controlling T-cell hepatitis.
Materials and Methods
Materials
Anti-STAT4 and anti–p-STAT4 (Tyr693) antibodies were obtained from Cell Signaling Technology (Danvers, MA). Neutralizing FasL antibody (clone MFL3) was obtained from BD Biosciences (San Jose, CA). The sources of other antibodies were as indicated below.
Liver Tissue Samples from Human Subjects
Cirrhotic liver samples (stage 3 to 4 fibrosis) were collected from donor livers during liver transplantation from the Liver Tissue Cell Distribution System, University of Minnesota (Minneapolis, MN).
Mice
Male WT BALB/c mice, Stat4 KO (Stat4−/−) mice on a BALB/c background, WT C57BL/6J mice, Il12b KO (Il12b−/−) mice, and Il12a KO (Il12a−/−) mice on a C57BL/6J background were obtained from the Jackson Laboratory (Bar Harbor, ME). Colonies were maintained and bred in the NIH National Institute on Alcohol Abuse and Alcoholism (NIAAA) animal facility. Mice used in experiments were 8 to 12 weeks of age. Handling of mice and experimental procedures were in accordance with institutional guidelines, and all animal experiments were approved by the Institutional Animal Care and Use Committee of the NIAAA.
Induction of Mouse Hepatitis Model Induced by Con A
Con A (Sigma-Aldrich, St. Louis, MO) was dissolved in PBS and injected intravenously through the tail vein at a dose of 15 mg/kg for BALB/c mice and 12 mg/kg for C57BL/6J mice. Serum from individual mice was obtained at 0, 1, 2, 3, 6, 9, and 24 hours after Con A injection. Serum alanine aminotransferase (ALT) activities were determined using an IDEXX chemistry analyzer system (IDEXX Laboratories, Westbrook, ME).
Immunohistochemistry
Liver sections from both human and mouse samples were immunostained for p-STAT4 using an anti–p-STAT4 antibody (Cell Signaling Technology). The number of p-STAT4+ cells in the liver sections was counted in 10 randomly selected fields (×200), and the average of the 10 fields was calculated.
Isolation and Culture of Primary Mouse Hepatocytes
Mice weighing 20 to 25 g were anesthetized intraperitoneally with 30 mg/kg pentobarbital sodium, and the portal vein was cannulated under aseptic conditions. The liver was subsequently perfused with an EGTA solution [5.4 KCl, 0.44 KH2PO4, 140 NaCl, 0.34 Na2HPO4, 0.5 EGTA, and 25 tricine (pH 7.2), all in mmol/L] and Dulbecco's modified Eagle's medium (Life Technologies, Carlsbad, CA) and digested with 0.075% collagenase solution. Isolated mouse hepatocytes (5 × 104 per well) were then cultured in Dulbecco's modified Eagle's medium containing 10% fetal calf serum and 2 mmol/L penicillin–streptomycin on rat-tail collagen-coated plates for 2 hours, then in serum-free Dulbecco's modified Eagle's medium. The hepatocytes were used to assay for NKT cytotoxicity or Jo2 cytotoxicity.
Isolation of Hepatic Mononuclear Cells
The isolation of hepatic mononuclear cells (MNCs) was performed as described previously.28 In brief, mouse livers were removed and pressed through a 70-μm cell strainer. The liver cells were suspended in PBS and centrifuged at 50 × g for 5 minutes. Supernatants containing MNCs were collected, washed in PBS, and resuspended in 40% Percoll medium (GE Healthcare, Little Chalfont, UK). The cell suspension was gently overlaid onto 70% Percoll and was centrifuged for 30 minutes at 750 × g. MNCs were collected from the interphase, washed twice in PBS, and resuspended in PBS for fluorescence-activated cell sorting analysis or cytotoxicity assays.
Flow Cytometry Analysis
Single-cell suspension of liver or spleen MNCs was washed in PBS containing 1% bovine serum albumin. The cells were surface-stained with fluorochrome-conjugated monoclonal antibody for 30 minutes on ice. The antibodies used were anti-CD3, anti-NK1.1, anti–Gr-1, anti-CD11b, anti-FasL, and anti-CD69 (eBioscience, San Diego, CA). CD1d tetramer was obtained from the NIH Tetramer Core Facility at Emory University (Atlanta, GA). For p-STAT4 staining, cells with surface staining were fixed and permeabilized according to the manufacturer's instructions and then were incubated with p-STAT4 antibody (BD Biosciences) at room temperature for 30 minutes. Samples were acquired on a FACSCalibur flow cytometer (BD Biosciences) and the data were analyzed using FlowJo software version 7.6.5 (TreeStar, Ashland, OR).
Western Blotting
Tissues were homogenized in lysis buffer (30 mmol/L Tris at pH 7.5, 150 mmol/L sodium chloride, 1 mmol/L fluoride, 1 mmol/L sodium orthovanadate, 1% Nonidet P-40, 10% glycerol) at 4°C, vortexed, and centrifuged at 15,700 × g at 4°C for 10 minutes. The supernatants were mixed in Laemmli loading buffer, boiled for 10 minutes, and then subjected to SDS-PAGE. After electrophoresis on 4% to 12% Bis–Tris gel (Life Technologies), proteins were transferred onto nitrocellulose membranes and blotted against primary antibody overnight under shaking conditions. Membranes were washed with 0.05% (v/v) Tween 20 in PBS (pH 7.4) and incubated with a 1:5000 dilution of horseradish peroxidase–conjugated secondary antibody for 1 hour. Protein bands were visualized by enhanced chemiluminescence reaction (Thermo Scientific, Waltham, MA).
H&E Staining of Liver Sections
After fixation with 10% formalin–PBS, livers were paraffin-embedded, sliced, and stained with H&E.
TUNEL Staining
TUNEL-positive cells in sections of mouse livers were detected using an in situ apoptosis detection kit (Millipore, Billerica, MA) according to the manufacturer's instructions.
Cytokine Measurement
Serum cytokine levels were measured using a cytometry bead array (BD Biosciences) according to the manufacturer's protocol. The analysis was performed using a conventional flow cytometer (FACSCalibur; BD Biosciences).
Cytotoxicity Assay
For Jo2–induced cytotoxicity, hepatocytes were cultured in serum-free Dulbecco's modified Eagle's medium and incubated with different concentration of Jo2 antibody. For hepatic MNC-induced cytotoxicity, hepatocytes were cocultured with hepatic MNCs isolated from Con A–treated mice for 4 hours with or without 10 μg/mL FasL neutralizing antibody. Hepatocyte death was quantified by measuring the activity of released aspartate transaminase (AST) in culture medium. The specific cytotoxicity was calculated as [(ASTexperimental − ASTspontaneous)/(ASTmaximum − ASTspontaneous)] × 100.
Statistical Analysis
Student's two-sample t-test was performed to compare values from two groups. To compare values from three or more groups, one-factor analysis of variance was used, followed by Tukey's post hoc test. P values of <0.05 were considered significant.
Results
STAT4 Is Activated in Inflammatory Cells in Livers from Patients with Chronic Liver Diseases and in a Mouse Model of T-Cell Hepatitis
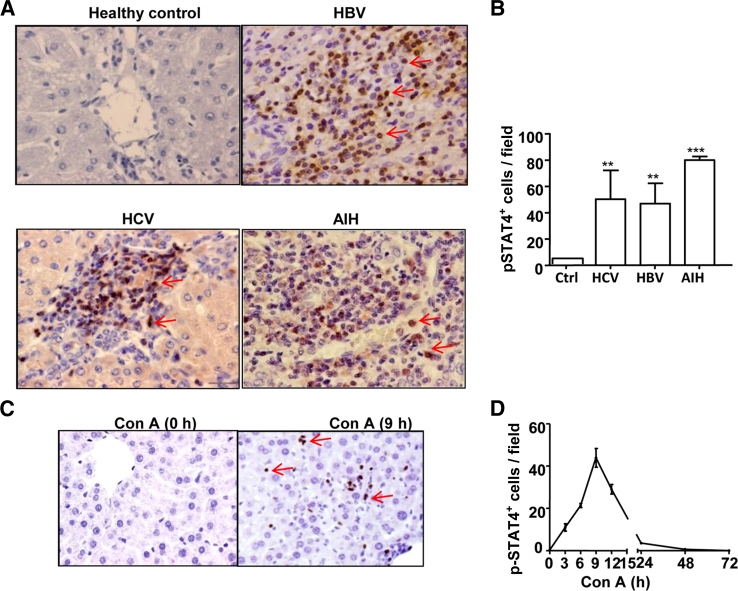
To investigate the roles of STAT4 in the pathogeneses of liver diseases, we first performed immunohistochemistry analyses of phosphorylated STAT4 (p-STAT4) in liver sections from patients with viral hepatitis B (HBV), viral hepatitis C (HCV), or AIH. Very few p-STAT4+ cells were detected in normal healthy control livers, but strong p-STAT4 staining was found in inflammatory cells from livers of HBV, HCV, and AIH patients (Figure 1A). p-STAT4 staining was not observed in hepatocytes. The total number of p-STAT4+ cells was significantly higher in livers from patients with HBV, HCV, and AIH than in healthy control livers (Figure 1B).
Figure 1.
Activation of STAT4 in human liver diseases and in mouse T-cell hepatitis. A: Representative images of liver sections from normal healthy control subjects and from patients with HBV, HCV, and AIH. Sections were immunostained with an anti–p-STAT4 antibody. B: p-STAT4+ cells were counted in 10 randomly selected high-power fields. C: Representative images of liver sections from Con A–treated mice. Sections were immunostained with an anti–p-STAT4 antibody; brown indicates positive staining. D: p-STAT4+ cells were counted in 10 randomly selected high-power fields at various time points from baseline to 72 hours after Con A injection. Data are expressed as means ± SEM. n = 3 to 10 patients per group (B). ∗∗P < 0.01; ∗∗∗P < 0.001. Original magnification, ×400.
Next, we performed immunohistochemistry analyses of p-STAT4 in a mouse model of inflammatory liver injury induced by Con A administration. p-STAT4+ cells were not observed in the livers from mice without Con A treatment (Figure 1C). After Con A injection, p-STAT4+ cells were detected in the livers, with peak effects occurring 9 hours after injection, and disappeared 48 and 72 hours after Con A injection. In agreement with the immunostaining findings from human samples, p-STAT4 was detected in inflammatory cells but not in hepatocytes.
STAT4 Is Activated in Multiple Types of Liver Inflammatory Cells from Con A–Treated Mice, Including T Cells, NKT Cells, and Macrophages, but the Activation Is Diminished in Il12a−/− and Il12b−/− Mice
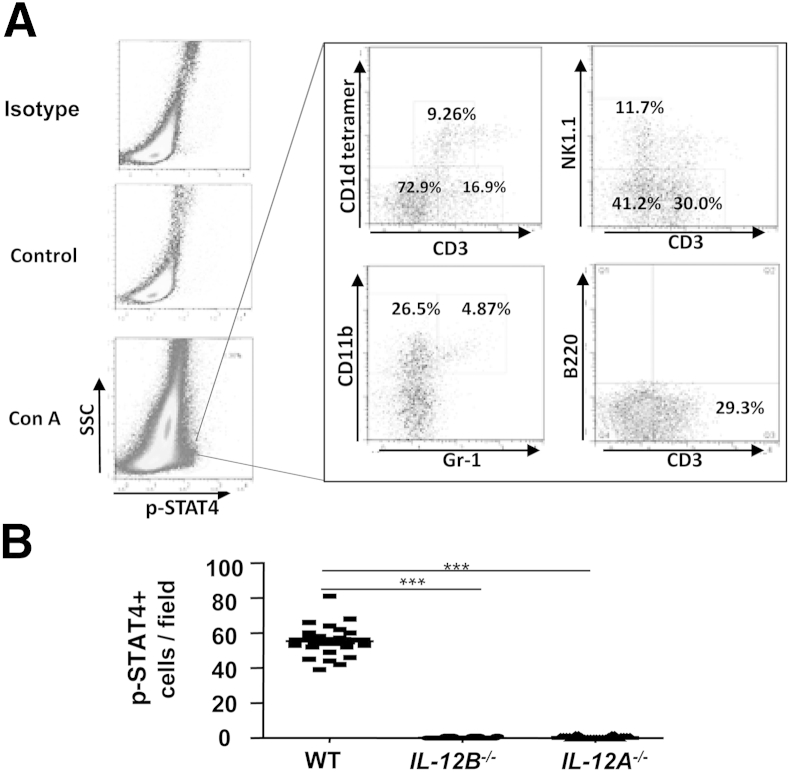
To further define the p-STAT4+ cell types in the liver and spleen, flow cytometric analyses were used to analyze MNCs isolated from mice at 9 hours after Con A treatment or saline treatment. We chose the 9-hour time point because peak levels of p-STAT4 activation occurred at this time (Figure 1). Mice without Con A treatment had very few p-STAT4+ MNCs in the liver; however, approximately 1% to 2% of hepatic MNCs from Con A–treated mice were p-STAT4+ (Figure 2A). Of these p-STAT4+ cells, approximately 9% were NKT cells (CD1d tetramer+CD3+), 12% were NK cells (NK1.1+CD3−), and 30% were T cells (NK1.1−CD3+). Approximately 26% of the p-STAT4+ cells were CD11b+Gr-1low cells (macrophages and Kupffer cells), and 5% were CD11b+Gr1high cells (neutrophils). These findings suggest that STAT4 is activated in multiple types of liver immune cells from Con A–treated mice. Similarly, STAT4 was also activated in multiple immune cells (eg, T cells and NKT cells) in the spleen after Con A injection (Supplemental Figure S1A).
Figure 2.
STAT4 is activated in multiple types of liver immune cells from Con A–treated mice, including T cells, NKT cells, and macrophages; the effect is diminished in Il12a−/− and Il12b−/− mice. A: Mice were injected with Con A and liver lymphocytes were isolated at 9 hours after Con A administration. Expression of p-STAT4 and surface expression of CD3+CD1d tetramer+ NKT cells, NK1.1+CD3+ NKT cells, CD3+CD1d tetramer− T cells, NK1.1−CD3+ T cells, CD11b+Gr-1low macrophages, CD3−B220+ B cells, and CD11b+Gr-1high neutrophils were analyzed by flow cytometry. Data are representative of three independent experiments. B: WT, Il12b−/−, and Il12a−/− mice were injected with 12 μg/g Con A. At 9 hours after injection, liver tissues were collected for p-STAT4 staining. p-STAT4+ cells were counted in 10 randomly selected fields (×200). Data are expressed as means of the 10 fields. ∗∗∗P < 0.001. IL-12A-/-, Il12a−/−; IL-12B-/-, Il12b−/−; SSC, side scatter.
STAT4 is activated mainly by IL-12, and to a lesser extent by IFN-α/β, IL-12, and IL-17.17–20,29–32 To test whether IL-12 is a major factor responsible for activation of STAT4 in the liver after administration of Con A, we used WT and Il12a−/−or Il12b−/− mice. Liver tissues were collected for p-STAT4 immunohistochemistry staining. p-STAT4+ cells were detected in livers of WT mice, but not of Il12a−/− or Il12b−/− mice (Figure 2B and Supplemental Figure S1B). These findings indicate that STAT4 activation in the Con A–induced hepatitis model is IL-12 dependent.
Stat4−/− Mice Have Lower Serum Levels of Cytokines after Con A Injection than WT Mice
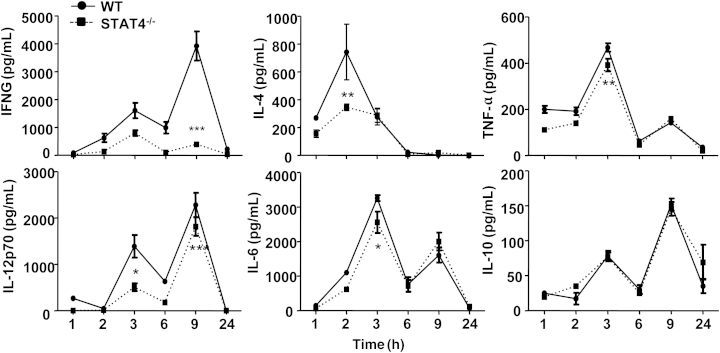
Given that STAT4 plays a critical role in the control of Th1 and Th2 cytokine production,21 we hypothesized that cytokine production would be attenuated in Stat4−/− mice, compared with WT mice, in the Con A–mediated hepatitis model. As expected, Con A injection–induced elevation of IFN-γ, IL-4, and IL-12 was significantly attenuated in Stat4−/− mice, compared with WT mice (Figure 3). Elevation of serum IL-6 and TNF-α was slightly (but significantly) inhibited in Stat4−/− mice, compared with WT mice, after Con A injection (Figure 3). However, serum levels of IL-10, MCP-1, and IL-22 were similar in Con A–treated WT and Stat4−/− mice (data not shown).
Figure 3.
Stat4−/− mice have lower serum levels of cytokines after Con A injection, compared with WT mice. WT and Stat4−/−mice were injected with Con A, and serum cytokine levels were determined using cytometric bead array at various time points from 1 to 24 hours after injection. Data are expressed as means ± SEM. n = 4 or 5 mice. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.0001 versus corresponding Con A–treated WT group. STAT4-/-, Stat4−/−.
Stat4−/−, Il12a−/−, and Il12b−/− Mice Are More Susceptible to Con A–Induced Murine Hepatitis than WT Mice
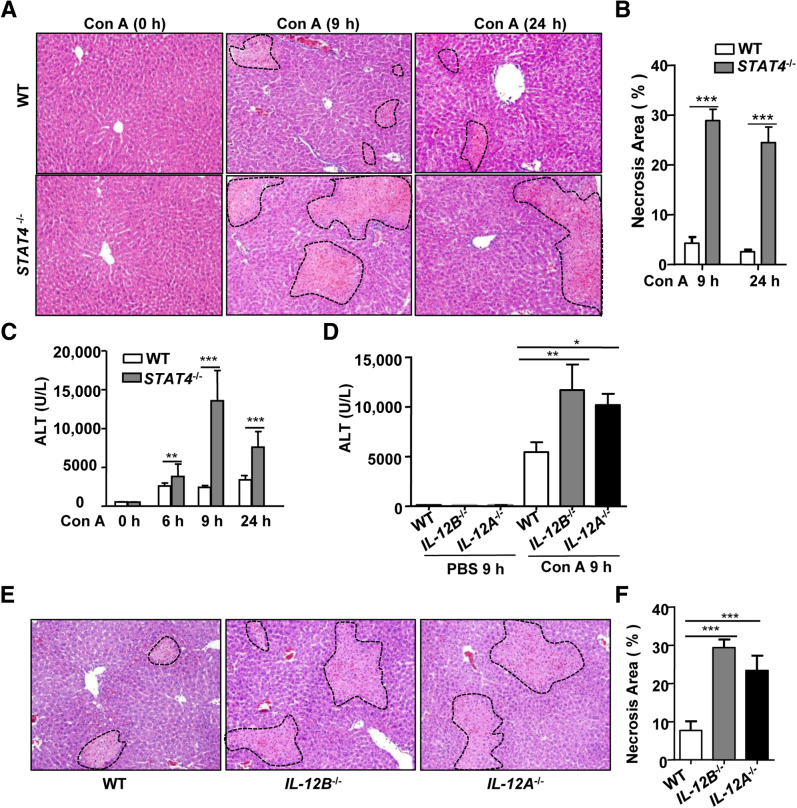
Because IFN-γ and IL-4 play critical roles in Con A–induced liver injury,14,15 we hypothesized that Stat4−/− mice (which have reduced production of both cytokines) would have reduced liver injury after Con A injection. Surprisingly, however, Stat4−/− mice had larger areas of necrosis and higher levels of serum ALT than WT mice after Con A injection (Figure 4, A–C).
Figure 4.
Stat4−/−, Il12a−/−, and Il12b−/− mice are more susceptible to Con A–induced murine hepatitis. A and B: WT and Stat4−/− mice were injected with 15 μg/g Con A. H&E-stained sections were analyzed for necrosis (outlined areas) at baseline and at 9 and 24 hours after injection (A) and Percentage necrotic area in Stat4−/− and WT mice at 9 and 24 hours after Con A injection (B). C: Serum ALT levels in Stat4−/− and WT mice were determined at 0, 6, 9, and 24 hours. D–F: WT, Il12b−/−, and Il12a−/− mice were injected with 12 μg/g Con A. D: Serum ALT activity was determined at 9 hours after injection. E and F: H&E-stained sections were analyzed for necrosis (outlined areas) (E) and the necrotic area was calculated as a percentage (F). Representative images are shown. Data are expressed as means ± SEM. n = 4 to 12 mice. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnification, ×100.
Because STAT1 and STAT3 are known to play important roles in regulating liver regeneration,13 we wondered whether STAT4 also regulates liver regeneration. Western blot analysis revealed that hepatic activation of both p-STAT1 and p-STAT3 was lower in Stat4−/− mice than in WT mice (Supplemental Figure S2C), which is in agreement with the finding that Stat4−/− mice had lower levels of IFN-γ and IL-6 after Con A injection, compared with WT mice (Figure 3). Despite lower levels of hepatic STAT3 activation, Con A–treated Stat4−/− mice had greater numbers of hepatocytes positive for bromodeoxyuridine (BrdU+), compared with Con A–treated WT mice (Supplemental Figure S2, B and C). This finding suggests that the increased liver injury observed in Stat4−/− mice was not due to impaired liver regeneration in these mice.
Next, we investigated Con A–induced liver injury in Il12a−/− and Il12b−/− mice; livers of these mice had very few p-STAT4+ cells after Con A treatment (Figure 2B and Supplemental Figure S1B). Serum levels of ALT were higher in Con A–treated Il12a−/− and Il12b−/− mice, compared with WT mice (Figure 4D). Liver histology analyses also confirmed that Il12a−/− and Il12b−/− mice had larger areas of necrosis than WT mice after Con A injection (Figure 4, E and F). These findings confirm the protective role of STAT4 in T-cell hepatitis.
NKT Cells from Stat4−/− Mice Have Higher Levels of FasL and Greater Cytotoxicity against Hepatocytes than Those from WT Mice
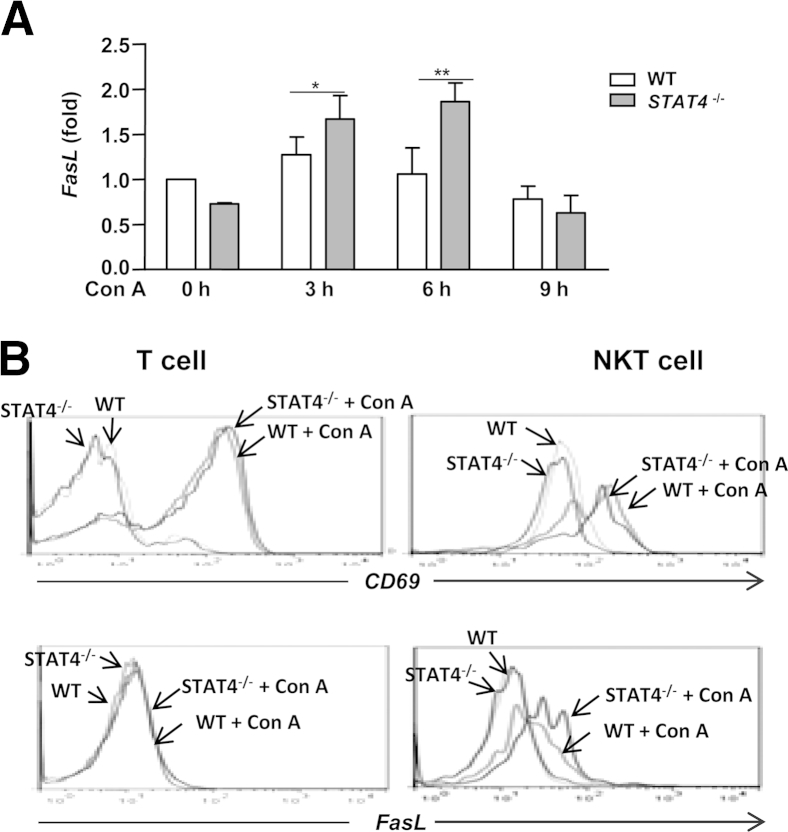
Our finding that Stat4−/− mice have reduced cytokine production after Con A injection, compared with WT mice, cannot explain the exacerbated liver injury in Stat4−/− mice. Because NKT cell killing of hepatocytes plays an important role in promoting Con A–induced hepatitis,33 we measured mRNA levels of cytotoxicity-associated genes (eg, perforin, granzyme B, and FasL) in WT and Stat4−/− mice after Con A injection. No significant difference was found in the expression levels of perforin and granzyme B (data not shown). However, expression levels of hepatic FasL mRNA were significantly higher in Stat4−/− mice after Con A administration, compared with WT mice (Figure 5A).
Figure 5.
FasL levels are higher in NKT cells from Stat4−/− mice than from WT mice after Con A injection. A and B: Hepatic MNCs isolated from Con A–injected WT and Stat4−/− mice were subjected to real-time quantitative PCR analyses (A) and flow cytometric analyses of CD69 and FasL in T cells and NKT cells (B). Representative flow cytometric graphs from 3 hours Con A–treated mice are shown. Fold-change data are expressed as means ± SEM. ∗P < 0.05; ∗∗P < 0.01.
Next, we performed flow cytometric analyses to determine which cell types express FasL. Consistent with previous report,33 Con A injection increased expression of FasL in NKT cells but not in T cells, and expression of the activation marker CD69 was up-regulated in both T cells and NKT cells (Figure 5B). Expression of CD69 was similar in WT and Stat4−/− mice after Con A injection, whereas expression of FasL protein was higher in NKT cells from Con A–treated Stat4−/− mice, compared with WT mice (Figure 5B).
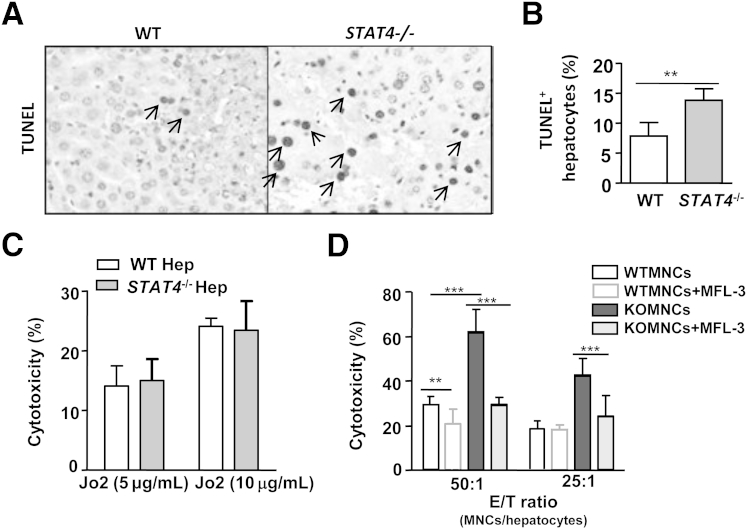
TUNEL analyses revealed that, after Con A injection, Stat4−/− mouse livers had greater numbers of TUNEL+ hepatocytes than did WT mouse livers (Figure 6, A and B), suggesting that Con A injection induces more hepatocyte apoptosis in Stat4−/− mice than in WT mice.
Figure 6.
Increased cytotoxicity against hepatocytes of NKT cells from Stat4−/− mice after Con A injection, relative to NKT cells from WT mice. A: WT and Stat4−/− mice were treated with Con A; at 9 hours after injection, liver tissues were subjected to TUNEL immunostaining. Arrows indicate TUNEL+ hepatocytes. B: TUNEL+ hepatocyte percentage. C: Hepatocytes were isolated from WT and Stat4−/− mice and treated with Jo2 anti-Fas antibody; cell death was then determined. D: WT mouse hepatocytes were incubated with hepatic MNCs that were isolated from 2-hour Con A–treated WT mice, with or without neutralizing FasL monoclonal antibody MFL3; cytotoxicity was then determined. Data are expressed as means ± SEM. ∗∗P < 0.01; ∗∗∗P < 0.001. E/T, effector (MNCs)/target (hepatocytes); Hep, hepatocytes.
Although there was no indication that STAT4 was activated in hepatocytes, we could not rule out the possibility that Stat4−/− hepatocytes are more sensitive to FasL-induced cell death than are WT hepatocytes. To address this question, we isolated hepatocytes from WT and Stat4−/− mice and incubated the cells with 5 μg/mL or 10 μg/mL anti-Fas antibody Jo2 for 4 hours to mimic FasL-induced hepatocyte apoptosis, and then determined the cytotoxicity. We found no difference between the sensitivity of WT hepatocytes and Stat4−/− hepatocytes to Jo2-induced cell death (Figure 6C).
We also wanted to determine whether Stat4−/− NKT cells have greater cytotoxicity against hepatocytes than do WT NKT cells. Because NKT cells are the major cell type responsible for liver MNC-mediated cytotoxicity against hepatocytes, we used MNCs as a proxy for NKT cell cytotoxicity. In addition, a marked depletion of liver NKT cells was observed 4 to 20 hours after intravenous injection of Con A into WT mice.33 We therefore isolated liver MNCs at an early time point (2 hours) after Con A injection. MNCs isolated from Con A–treated Stat4−/− mice exhibited enhanced cytotoxicity against hepatocytes, compared with WT mice (Figure 6D). The enhanced cytotoxicity in the Stat4−/− MNC group was remarkably reduced when a FasL neutralizing monoclonal antibody was added.
Discussion
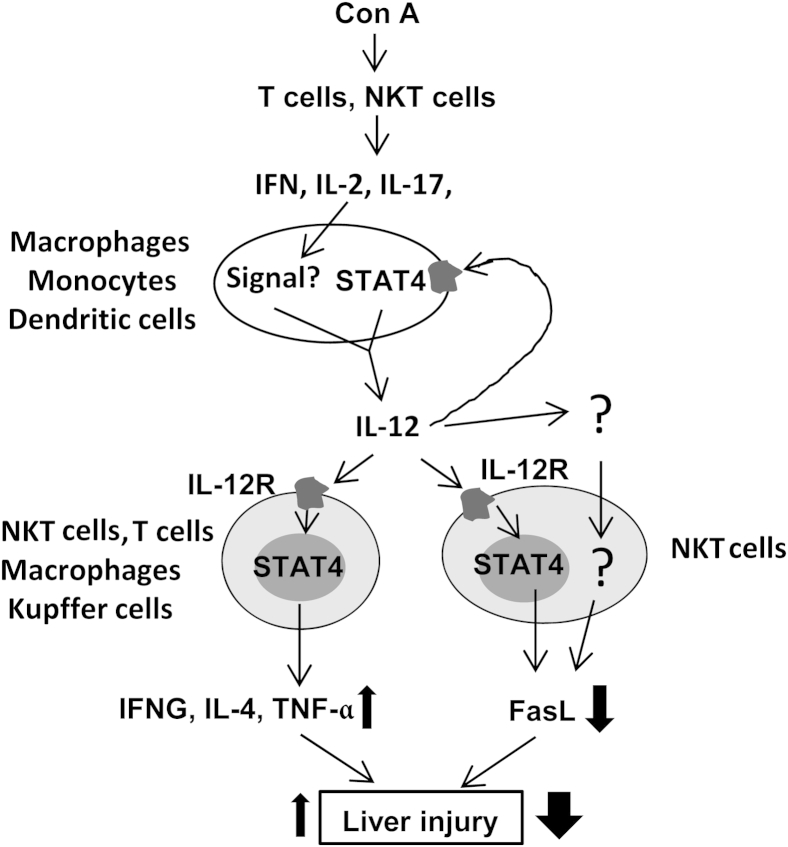
With the present study, we have demonstrated that STAT4 is activated by IL-12 in T cells, NKT cells, and macrophages and Kupffer cells in Con A–induced T-cell hepatitis. We have also demonstrated that disruption of the STAT4 gene reduces the production of Th1 and Th2 cytokines but, surprisingly, exacerbates hepatocellular damage, suggesting that IL-12 activation of STAT4 in T cells, NKT cells, and macrophages plays a role in ameliorating Con A–induced liver injury. Further studies suggest that the protective effect of STAT4 is mediated by down-regulating the expression of FasL in NKT cells. We have integrated all of these findings into a model (Figure 7) describing the complex role of STAT4 in control of Con A–induced hepatitis by regulating the balance of inhibition of Fas/FasL-mediated hepatotoxicity and by up-regulating proinflammatory cytokines.
Figure 7.
A complex role of IL-12 and STAT4 in pathogenesis of Con A–induced liver injury. Con A activates T cells and NKT cells to produce a variety of cytokines (eg, IFN, IL-2, and IL-17), and these cytokines stimulate immune cells (eg, monocytes, macrophages, and dendritic cells) to produce IL-12. In a positive feedback loop, IL-12 stimulates its own production via activation of STAT4. IL-12 also activates STAT4 in NKT cells and in macrophages and Kupffer cells to produce IFN-γ, IL-4, and TNF-α, thereby exacerbating Con A–induced hepatitis. By contrast, activation of STAT4 by IL-12 indirectly or directly down-regulates FasL in NKT cells and protects against Con A–induced hepatitis. STAT4-mediated down-regulation of FasL may dominate over STAT4-mediated up-regulation of proinflammatory cytokines in the Con A–induced T-cell hepatitis model, leading to the observed protection of STAT4 against liver injury in this model.
IL-12 Is Essential for STAT4 Activation in Inflammatory Cells, and STAT4 Partially Contributes to IL-12 Production in Con A–Induced Hepatitis
STAT4 is activated mainly by IL-1217–19 and to a much lesser degree by IFN-α/β,17 IL-2,18 and IL-17.20 Serum levels of IL-12 elevation (Figure 3) correlated well with p-STAT4 activation in liver MNCs (Figure 1), with both peak effects occurring 9 hours after Con A injection. Furthermore, disruption of the Il12a or Il12b gene completely abolished STAT4 activation in Con A–induced hepatitis (Figure 2 and Supplemental Figure S1B), suggesting that IL-12 plays an essential role in inducing STAT4 activation in this model. IL-12 is produced mainly by monocytes, macrophages, and dendritic cells.34 Injection of Con A–induced elevation of IL-12p70, with one peak at 3 hours after injection and another at 9 hours. Disruption of the Stat4 gene markedly prevented elevation of IL-12p70 at 3 hours after Con A injection and also, to a lesser extent, at 9 hours after injection. These findings indicate that STAT4 contributes partially to IL-12p70 production, with a more important role at early time points (eg, 3 hours) and a less important role at later time points (eg, 9 hours) after Con A injection. Taken together, our findings suggest that IL-12 acts in a positive feedback loop to induce its own production via activation of STAT4 in immune cells such as monocytes, macrophages, and dendritic cells. In addition to STAT4, other signaling pathways activated by IFN-α/β,17 IL-2,18 and IL-1720 likely also contribute to IL-12 production.
IL-12 Activation of STAT4 Plays a Protective Role in Con A–Induced Hepatitis via Inhibition of FasL Expression in NKT Cells
IL-12 activation of STAT4 plays an important role in promoting Th1 (IFN-γ) and Th2 (IL-4) cytokine production.19 In accord with this notion, serum levels of IFN-γ, IL-4, and TNF-α were lower in Stat4−/− mice than in WT mice (Figure 3). All of these cytokines have been shown to exacerbate Con A–induced T-cell hepatitis.1 Surprisingly, however, Con A–induced liver injury was markedly enhanced in Stat4−/− mice, relative to WT mice (Figure 4). Similarly, disruption of the Il12a or Il12b gene also exacerbated Con A–induced elevation of serum ALT and liver necrosis (Figure 4). Interestingly, our present findings are in contrast to earlier reports on the role of IL-12 in Con A–induced liver injury. In an early study, treatment of mice with IL-12 exacerbated Con A–induced liver injury, and blocking IL-12 with a neutralizing antibody reduced the injury; however, Con A–induced liver injury was similar in WT and Il12b−/− mice.35 In a later study, Il12b−/− mice were less susceptible to Con A–induced T-cell hepatitis than were WT mice.24 The discrepancies among these studies and our present study may be explained by differences in the doses used (20 μg/g Con A in the two previous studies versus 12 μg/g in the present study) and to the dual roles of IL-12 and STAT4 in controlling Con A–induced hepatitis (discussed below).
In contrast to the reduced serum cytokine levels, FasL expression in NKT cells from Con A–treated Stat4−/− mice was significantly higher than that from Con A–treated WT mice (Figure 5). It is not clear whether the elevated FasL expression in NKT cells from Stat4−/− mice was due to a compensatory mechanism in KO mice, nor whether STAT4 directly or indirectly down-regulates FasL expression in NKT cells. IL-4 treatment has been reported to up-regulate expression of FasL in NKT cells.36 Our present findings, that IL-4 levels were lower but FasL levels were higher in Con A–treated Stat4−/− mice than in WT mice, suggest that STAT4-mediated down-regulation of FasL expression on NKT cells is not mediated by the stimulation of IL-4. Further studies are required to identify the mechanisms for reduced FasL expression in NKT cells of Stat4−/− mice, compared with WT mice.
The next question is whether up-regulation of FasL expression in NKT cells contributes to the enhanced liver injury in Con A–treated Stat4−/− mice. In one study, caspase inhibition did not prevent Con A–induced liver injury, suggesting that Con A treatment induces a caspase-independent necrotic death.37 Several other studies, however, suggest that FasL-mediated apoptosis contributes to Con A–induced liver injury. First, FasL- and Fas-deficient mice are resistant to Con A–induced liver injury.36,38,39 Second, administration of a FasL-neutralizing monoclonal antibody reduces Con A–induced T-cell hepatitis.38 Third, although Con A–induced liver injury is associated predominantly with necrosis,39,40 significant hepatocyte apoptosis was detected in the liver after Con A injection (Figure 6A). These findings suggest that both apoptosis and necrosis contribute to Con A–induced hepatocyte damage. However, how apoptotic injury elicited by FasL translates into necrosis in this model is not clear. In the present study, up-regulation of FasL expression was detected as early as 3 hours after Con A injection, with higher levels in Stat4−/− mice than in WT mice (Figure 5). This upregulation may contribute to the higher levels of ALT elevation in Stat4−/− mice at early time points (eg, 6 hours) after Con A injection, compared with WT mice (Figure 4).
In addition to elevated FasL, which induces hepatocyte apoptosis, other factors may also contribute to the increased susceptibility of Stat4−/− mice to Con A–induced liver injury, because necrosis also significantly contributes to Con A–induced hepatocellular damage.37 In the present study, Stat4−/− mice had greater necrosis than WT mice after Con A injection. Given the well-documented hepatoprotective function of IL-6 in ameliorating Con A–induced necrosis,14 lower levels of IL-6 in Con A–treated Stat4−/− mice likely also contribute to the higher levels of liver injury in these mice, compared with WT mice.
In summary, given the complex pathogenesis of Con A–induced T-cell hepatitis, which is controlled both by interaction among T cells, NKT cells, and macrophages and Kupffer cells and by interaction between pro- and anti-inflammatory cytokines,1 STAT4 likely plays a multifaceted role in controlling T-cell hepatitis by targeting several types of immune cells and regulating expression of various cytokines, including IL-12 (Figure 7).
STAT4 Activation in Liver Diseases
STAT4 gene variants have been implicated in pathogenesis of various types of liver diseases.9–12 However, how these variants affect STAT4 expression or activation and how STAT4 contributes to liver injury and inflammation remain largely unknown. In the present study, immunohistochemistry analyses revealed strong STAT4 activation in inflammatory cells (but not in hepatocytes) in the liver tissues from patients with chronic HBV, HCV, and AIH, conditions in which T cell–mediated immune responses play a central role in hepatocellular injury. Thus, activation of STAT4 in viral hepatitis and AIH likely promotes production of Th1 and Th2 cytokines and subsequently exacerbates liver inflammation and injury; however, such activation may also down-regulate FasL expression in NKT cells, thereby reducing hepatocellular damage. The net effect of STAT4 activation on the progression of liver injury is determined by the balance between inhibition of FasL expression and up-regulation of proinflammatory cytokines. Further studies are needed to clarify the multiple functions of STAT4 in the liver and to translate these findings into clinical practice and therapy.
Footnotes
Supported by the intramural program of the NIH–National Institute on Alcohol Abuse and Alcoholism (B.G.), the China Scholarship Council (Y.W.), the Wu Mengchao Medical Science Foundation (Y.W.), and National Natural Science Foundation of China grant 81300312 (Y.W.). The Liver Tissue Cell Distribution System, University of Minnesota, Minneapolis, MN, is supported by NIH contract N01-DK-7-0004/HHSN267200700004C.
Supplemental Data
STAT4 is activated in multiple types of spleen immune cells (including T cells, NKT cells, and macrophages) from Con A–treated mice, which is diminished in Il12a−/− and Il12b−/− mice. A: Mice were injected with Con A, and splenocytes were isolated 9 hours after injection. Expression of p-STAT4 and surface expression of CD3+CD1d tetramer+ NKT cells, NK1.1+CD3+ NKT cells, CD3+CD1d tetramer− T cells, NK1.1−CD3+ T cells, CD11b+Gr-1low macrophages, and CD11b+Gr-1high neutrophils were analyzed by flow cytometry Data are representative of three independent experiments. B: WT, Il12b−/−, and Il12a−/− mice were injected with 12 μg/g Con A. At 9 hours after injection, liver tissues were collected for staining. p-STAT4+ cells are marked by arrows. Representative images are shown. Original magnification, ×200. SS, side scatter.
Stat4−/− mice have higher levels of liver regeneration than WT mice after Con A injection. A: Mice were injected with Con A, and liver tissues were isolated and subjected to Western blot analysis. B: Mice were injected with Con A and euthanized at 48 hours or 72 hours after injection. BrdU was injected at 2 hours before euthanization. BrdU+ hepatocytes are marked by arrows. Representative images are shown. C: BrdU+ hepatocyte percentage. Data are expressed as means ± SEM. ∗∗P < 0.01; ∗∗∗P < 0.001.
References
- 1.Tiegs G. Cellular and cytokine-mediated mechanisms of inflammation and its modulation in immune-mediated liver injury. Z Gastroenterol. 2007;45:63–70. doi: 10.1055/s-2006-927397. [DOI] [PubMed] [Google Scholar]
- 2.Xu R., Zhang Z., Wang F.S. Liver fibrosis: mechanisms of immune-mediated liver injury. Cell Mol Immunol. 2012;9:296–301. doi: 10.1038/cmi.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kubes P., Mehal W.Z. Sterile inflammation in the liver. Gastroenterology. 2012;143:1158–1172. doi: 10.1053/j.gastro.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Szabo G., Csak T. Inflammasomes in liver diseases. J Hepatol. 2012;57:642–654. doi: 10.1016/j.jhep.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 5.Adams D.H., Ju C., Ramaiah S.K., Uetrecht J., Jaeschke H. Mechanisms of immune-mediated liver injury. Toxicol Sci. 2010;115:307–321. doi: 10.1093/toxsci/kfq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimmer V., Lammert F. Genetics in liver disease: new concepts. Curr Opin Gastroenterol. 2011;27:231–239. doi: 10.1097/MOG.0b013e3283444862. [DOI] [PubMed] [Google Scholar]
- 7.Krawczyk M., Müllenbach R., Weber S.N., Zimmer V., Lammert F. Genome-wide association studies and genetic risk assessment of liver diseases. Nat Rev Gastroenterol Hepatol. 2010;7:669–681. doi: 10.1038/nrgastro.2010.170. [DOI] [PubMed] [Google Scholar]
- 8.Karlsen T.H., Melum E., Franke A. The utility of genome-wide association studies in hepatology. Hepatology. 2010;51:1833–1842. doi: 10.1002/hep.23564. [DOI] [PubMed] [Google Scholar]
- 9.Jiang D.K., Sun J., Cao G., Liu Y., Lin D., Gao Y.Z. Genetic variants in STAT4 and HLA-DQ genes confer risk of hepatitis B virus-related hepatocellular carcinoma. Nat Genet. 2013;45:72–75. doi: 10.1038/ng.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mells G.F., Floyd J.A.B., Morley K.I., Cordell H.J., Franklin C.S., Shin S.Y., Heneghan M.A., Neuberger J.M., Donaldson P.T., Day D.B., Ducker S.J., Muriithi A.W., Wheater E.F., Hammond C.J., Dawwas M.F., Richardson P., Nasr I., Aspinall R.J., UK PBC Consortium; Wellcome Trust Case Control Consortium 3. Jones D.E., Peltonen L., Alexander G.J., Sandford R.N., Anderson C.A. Genome-wide association study identifies 12 new susceptibility loci for primary biliary cirrhosis. Nat Genet. 2011;43:329–332. doi: 10.1038/ng.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirschfield G.M., Chapman R.W., Karlsen T.H., Lammert F., Lazaridis K.N., Mason A.L. The genetics of complex cholestatic disorders. Gastroenterology. 2013;144:1357–1374. doi: 10.1053/j.gastro.2013.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eurich D., Boas-Knoop S., Struecker B., Neuhaus R., Neuhaus P., Bahra M. Genetic variants of STAT-4 affect the development of graft fibrosis after liver transplantation for HCV-induced liver disease. Transplantation. 2013;95:203–208. doi: 10.1097/TP.0b013e318277e2f6. [DOI] [PubMed] [Google Scholar]
- 13.Gao B., Wang H., Lafdil F., Feng D. STAT proteins–key regulators of anti-viral responses, inflammation, and tumorigenesis in the liver. J Hepatol. 2012;57:430–441. doi: 10.1016/j.jhep.2012.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hong F., Jaruga B., Kim W.H., Radaeva S., El-Assal O.N., Tian Z., Nguyen V.A., Gao B. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaruga B., Hong F., Sun R., Radaeva S., Gao B. Crucial role of IL-4/STAT6 in T cell-mediated hepatitis: up-regulating eotaxins and IL-5 and recruiting leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 16.Radaeva S., Sun R., Pan H.N., Hong F., Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 17.Cho S.S., Bacon C.M., Sudarshan C., Rees R.C., Finbloom D., Pine R., O'Shea J.J. Activation of STAT4 by IL-12 and IFN-alpha: evidence for the involvement of ligand-induced tyrosine and serine phosphorylation. J Immunol. 1996;157:4781–4789. [PubMed] [Google Scholar]
- 18.Bacon C.M., Cho S.S., O'Shea J.J. Signal transduction by interleukin-12 and interleukin-2. A comparison and contrast. Ann N Y Acad Sci. 1996;795:41–59. doi: 10.1111/j.1749-6632.1996.tb52654.x. [DOI] [PubMed] [Google Scholar]
- 19.Morinobu A., Gadina M., Strober W., Visconti R., Fornace A., Montagna C., Feldman G.M., Nishikomori R., O'Shea J.J. STAT4 serine phosphorylation is critical for IL-12-induced IFN-gamma production but not for cell proliferation. Proc Natl Acad Sci USA. 2002;99:12281–12286. doi: 10.1073/pnas.182618999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Subramaniam S.V., Cooper R.S., Adunyah S.E. Evidence for the involvement of JAK/STAT pathway in the signaling mechanism of interleukin-17. Biochem Biophys Res Commun. 1999;262:14–19. doi: 10.1006/bbrc.1999.1156. [DOI] [PubMed] [Google Scholar]
- 21.Kaplan M.H. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-Galan M.C., Reynolds D., Correa S.G., Iribarren P., Watanabe M., Young H.A. Coexpression of IL-18 strongly attenuates IL-12-induced systemic toxicity through a rapid induction of IL-10 without affecting its antitumor capacity. J Immunol. 2009;183:740–748. doi: 10.4049/jimmunol.0804166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsuda M., Zhang W., Yang G.X., Tsuneyama K., Ando Y., Kawata K., Park O., Leung P.S., Coppel R.L., Ansari A.A., Ridgway W.M., Gao B., Lian Z.X., Flavell R., He X.S., Gershwin M.E. Deletion of interleukin (IL)-12p35 induces liver fibrosis in dominant-negative TGFbeta receptor type II mice. Hepatology. 2013;57:806–816. doi: 10.1002/hep.25829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu R., Diem S., Araujo L.M., Aumeunier A., Denizeau J., Philadelphe E., Damotte D., Samson M., Gourdy P., Dy M., Schneider E., Herbelin A. The pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J Immunol. 2007;178:5435–5442. doi: 10.4049/jimmunol.178.9.5435. [DOI] [PubMed] [Google Scholar]
- 25.Chang C.J., Chen Y.H., Huang K.W., Cheng H.W., Chan S.F., Tai K.F., Hwang L.H. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology. 2007;45:746–754. doi: 10.1002/hep.21560. [DOI] [PubMed] [Google Scholar]
- 26.Shen X.D., Ke B., Zhai Y., Gao F., Anselmo D., Lassman C.R., Busuttil R.W., Kupiec-Weglinski J.W. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 27.Kato A., Graul-Layman A., Edwards M.J., Lentsch A.B. Promotion of hepatic ischemia/reperfusion injury by IL-12 is independent of STAT4. Transplantation. 2002;73:1142–1145. doi: 10.1097/00007890-200204150-00023. [DOI] [PubMed] [Google Scholar]
- 28.Wang H., Feng D., Park O., Yin S., Gao B. Invariant NKT cell activation induces neutrophil accumulation and hepatitis: opposite regulation by IL-4 and IFN-γ. Hepatology. 2013;58:1474–1485. doi: 10.1002/hep.26471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leonard W.J., O'Shea J.J. Jaks and STATs: biological implications. Annu Rev Immunol. 1998;16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 30.Nishikomori R., Usui T., Wu C.Y., Morinobu A., O'Shea J.J., Strober W. Activated STAT4 has an essential role in Th1 differentiation and proliferation that is independent of its role in the maintenance of IL-12R beta 2 chain expression and signaling. J Immunol. 2002;169:4388–4398. doi: 10.4049/jimmunol.169.8.4388. [DOI] [PubMed] [Google Scholar]
- 31.Bacon C.M., Petricoin E.F., 3rd, Ortaldo J.R., Rees R.C., Larner A.C., Johnston J.A., O'Shea J.J. Interleukin 12 induces tyrosine phosphorylation and activation of STAT4 in human lymphocytes. Proc Natl Acad Sci USA. 1995;92:7307–7311. doi: 10.1073/pnas.92.16.7307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miyagi T., Gil M.P., Wang X., Louten J., Chu W.M., Biron C.A. High basal STAT4 balanced by STAT1 induction to control type 1 interferon effects in natural killer cells. J Exp Med. 2007;204:2383–2396. doi: 10.1084/jem.20070401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takeda K., Hayakawa Y., Van Kaer L., Matsuda H., Yagita H., Okumura K. Critical contribution of liver natural killer T cells to a murine model of hepatitis. Proc Natl Acad Sci USA. 2000;97:5498–5503. doi: 10.1073/pnas.040566697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gee K., Guzzo C., Che Mat N.F., Ma W., Kumar A. The IL-12 family of cytokines in infection, inflammation and autoimmune disorders. Inflamm Allergy Drug Targets. 2009;8:40–52. doi: 10.2174/187152809787582507. [DOI] [PubMed] [Google Scholar]
- 35.Nicoletti F., Di Marco R., Zaccone P., Salvaggio A., Magro G., Bendtzen K., Meroni P. Murine concanavalin A-induced hepatitis is prevented by interleukin 12 (IL-12) antibody and exacerbated by exogenous IL-12 through an interferon-gamma-dependent mechanism. Hepatology. 2000;32:728–733. doi: 10.1053/jhep.2000.17701. [DOI] [PubMed] [Google Scholar]
- 36.Kaneko Y., Harada M., Kawano T., Yamashita M., Shibata Y., Gejyo F., Nakayama T., Taniguchi M. Augmentation of Valpha14 NKT cell-mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A-induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ni H.M., Chen X., Ding W.X., Schuchmann M., Yin X.M. Differential roles of JNK in ConA/GalN and ConA-induced liver injury in mice. Am J Pathol. 2008;173:962–972. doi: 10.2353/ajpath.2008.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seino K., Kayagaki N., Takeda K., Fukao K., Okumura K., Yagita H. Contribution of Fas ligand to T cell-mediated hepatic injury in mice. Gastroenterology. 1997;113:1315–1322. doi: 10.1053/gast.1997.v113.pm9322527. [DOI] [PubMed] [Google Scholar]
- 39.Tagawa Y., Kakuta S., Iwakura Y. Involvement of Fas/Fas ligand system-mediated apoptosis in the development of concanavalin A-induced hepatitis. Eur J Immunol. 1998;28:4105–4113. doi: 10.1002/(SICI)1521-4141(199812)28:12<4105::AID-IMMU4105>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 40.Fujino M., Kawasaki M., Funeshima N., Kitazawa Y., Kosuga M., Okabe K., Hashimoto M., Yaginuma H., Mikoshiba K., Okuyama T., Suzuki S., Li X.K. CrmA gene expression protects mice against concanavalin-A-induced hepatitis by inhibiting IL-18 secretion and hepatocyte apoptosis. Gene Ther. 2003;10:1781–1790. doi: 10.1038/sj.gt.3302067. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
STAT4 is activated in multiple types of spleen immune cells (including T cells, NKT cells, and macrophages) from Con A–treated mice, which is diminished in Il12a−/− and Il12b−/− mice. A: Mice were injected with Con A, and splenocytes were isolated 9 hours after injection. Expression of p-STAT4 and surface expression of CD3+CD1d tetramer+ NKT cells, NK1.1+CD3+ NKT cells, CD3+CD1d tetramer− T cells, NK1.1−CD3+ T cells, CD11b+Gr-1low macrophages, and CD11b+Gr-1high neutrophils were analyzed by flow cytometry Data are representative of three independent experiments. B: WT, Il12b−/−, and Il12a−/− mice were injected with 12 μg/g Con A. At 9 hours after injection, liver tissues were collected for staining. p-STAT4+ cells are marked by arrows. Representative images are shown. Original magnification, ×200. SS, side scatter.
Stat4−/− mice have higher levels of liver regeneration than WT mice after Con A injection. A: Mice were injected with Con A, and liver tissues were isolated and subjected to Western blot analysis. B: Mice were injected with Con A and euthanized at 48 hours or 72 hours after injection. BrdU was injected at 2 hours before euthanization. BrdU+ hepatocytes are marked by arrows. Representative images are shown. C: BrdU+ hepatocyte percentage. Data are expressed as means ± SEM. ∗∗P < 0.01; ∗∗∗P < 0.001.