Abstract
IL-27 is a heterodimeric cytokine composed of the subunits p28 and Epstein-Barr virus induced gene (EBI)-3 and is known for its effects on T-cell function and differentiation. IL-27 signals through the widely expressed IL-27 receptor (IL-27R), composed of the ligand-specific IL-27Rα chain and gp130. Engagement of the IL-27R activates STAT1 signaling, induces the expression of the type 1 helper T-cell (Th1) cytokine, interferon γ, and suppresses the differentiation of Th2 and Th17 cells. This study investigates the role of IL-27 signaling in respiratory syncytial virus (RSV) infection using IL-27Rα–deficient mice (IL-27rKO). Analysis of lungs from RSV-infected IL-27rKO mice showed exacerbation of mucus secretion compared with wild type, as well as enhanced expression of Muc5ac and Gob5 mRNA, markers of goblet cell metaplasia/hyperplasia. When compared with wild-type mice, RSV-challenged IL-27rKO mice had enhanced expression of Th17-associated cytokine IL-17a and an imbalance between Th1 and Th2 cytokine levels. Neutralization of IL-17 in RSV-infected IL-27rKO mice resulted in a significant decrease in the pulmonary mucus response and inhibition of the Th2-associated cytokines. Interestingly, IL-17 blockage led to an increase in the expression of IL-27 subunits p28 and EBI-3 in the lungs and lymph nodes of RSV-infected mice. Thus, IL-27 functions as a regulatory cytokine during RSV pathogenesis by suppressing the development of Th17 cells, but it also appears to be regulated by IL-17 induced by the virus.
IL-27, a member of the IL-6/IL-12 family of cytokines, is a heterodimeric cytokine composed of Epstein-Barr virus induced gene (EBI)-3 and p28 subunits. It signals through a receptor composed of WSX-1 [IL-27 receptor (IL-27R) α], a class I cytokine receptor with homology to the IL-12 receptor, and gp130, the common receptor chain used by several cytokines.1 IL-27 is produced by antigen presenting cells (APCs), especially dendritic cells (DCs), and its receptor is found in memory, regulatory, and effector CD4+ T cells.2,3 In T cells, engagement of the IL-27R activates members of the STAT family, predominantly STAT1 and STAT3,2,4,5 and leads to the up-regulation of T-bet and IL-12 receptor β2 expression, supporting type 1 helper T-cell (Th1) responses.4 However, studies conducted with several infectious and autoimmune inflammatory diseases have shown that, although the role of IL-27 in developing Th1 responses may be redundant,6,7 it exerts a regulatory function in the immune system, because IL-27R–deficient mice (IL-27rKO) are prone to dysregulated T-cell responses and immune pathological features.8–11 Accordingly, IL-27 activation of STAT1 and T-bet suppresses GATA3 and the development of Th2 cells.4 A study with Trichuris muris showed that IL-27rKO mice control larvae infestation much faster than wild-type (WT) mice because of the augmentation in Th2 cell differentiation.8 In addition, IL-27 not only suppressed Th2 development but also inhibited the production of IL-5 and IL-13 by differentiated Th2 cells in a dose-dependent manner.12 In experimental asthma, a disease directly associated with Th2 response, mice lacking IL-27R had exacerbation of pulmonary lesions when compared with WT mice. Conversely, intranasal administration of IL-27 inhibited signs of asthma severity, including airway hyperresponsiveness (AHR), goblet cell hyperplasia, and airway eosinophil infiltration.12 IL-27 also inhibits IL-6, IL-23, RAR-related orphan receptor (ROR)-γt, and Th17 differentiation.13 In a study of autoimmune encephalitis, IL-27R-deficient mice developed a hyperinflammatory phenotype with enhanced differentiation and infiltration of Th17 cells. IL-27 regulated the disease by suppressing the development of Th17 cell differentiation driven by IL-6 and transforming growth factor-β in an STAT1-dependent and an interferon (IFN)-γ–independent way.14
Respiratory syncytial virus (RSV) infection leads to differentiation of Th cells away from Th1 and toward Th2 and Th17 subsets. Lung inflammation is a feature of RSV infection, which is the single most important virus worldwide, leading to respiratory tract infections during childhood.15 Severe RSV infection is associated with decreased IFNγ production, suggesting a Th1-type response is involved in the viral clearance.16,17 Moreover, Th2 cytokines play crucial roles in RSV-induced airway responses and lung inflammation. IL-13 is known to induce goblet cell hyperplasia and mucus production,18 whereas IL-5–dependent eosinophilia has been implicated in RSV-induced AHR.19 Our laboratory showed that IL-17 participates in the pathogenesis of RSV-induced disease.20 Mice inoculated with RSV were found to display significant up-regulation of IL-17 in the lungs and peribronchial lymph nodes (LNs). In addition, there was an increase in the transcript levels of IL-6 and IL-23p19, which are involved in the differentiation and maintenance of Th17 cells. Furthermore, IL-17 was shown to up-regulate mucus production and to inhibit CD8+ T-cell effector functions, thereby reducing viral clearance.
Because of the role that IL-27 plays in the Th phenotype and in cell balance, we investigated its effects on RSV pathogenesis in IL-27rKO mice. We found that IL-27rKO mice showed exacerbation of RSV-induced disease, including mucus secretion, enhanced expression of the Th17-related cytokine IL-17a and Th2-related cytokines IL-5 and IL-13, and inhibition of the Th1-associated cytokine IFNγ. Neutralization of IL-17 in the RSV-infected IL-27rKO mice resulted in a significant decrease in the pulmonary mucogenic response and inhibition of the Th2 cytokines IL-5, IL-4, and IL-13. Moreover, IL-17 blockage led to a significant increase in the transcripts of IL-27 subunits p28 and EBI-3 in the lungs and peribronchial LNs of RSV-infected mice. Thus, IL-27 functions not only as a regulatory cytokine during RSV pathogenesis by suppressing the development of Th17 cells but also appears to be regulated by the high levels of IL-17 induced by the virus.
Materials and Methods
Animals
The WT C57BL/6 controls were purchased from Taconic Farms (Germantown, NY). IL-27rα knockout mice (IL-27rKO) were kindly provided by Amgen Inc (Thousand Oaks, CA) and bred in-house. All mice were housed at the University Laboratory Animal Facility at the University of Michigan Medical School (Ann Arbor, MI). All animal experiments were conducted according to animal protocols approved by The Animal Use Committee at the University of Michigan.
Respiratory Tract Syncytial Infection
The RSV strain 2-20, kindly provided by Dr. Martin Moore (Emory University, Atlanta, GA), was originally isolated from a severely ill RSV-infected infant21 and propagated in our laboratory in HEp-2 cells (ATCC, Manassas, VA). Mice were anesthetized, and the virus was administered intratracheally at 1 × 105 plaque-forming units (PFUs)22 on day 0. Lungs were harvested at 1, 2, 4, 6, 8, and 12 days after infection (dpi) for the time course study. For all other studies, lungs were harvested at the time specified.
Plaque Assay
Plaque assays were performed on RSV-infected lungs, as previously described,20 at 3 days after infection. For the plaque assay experiments, mice were infected with the RSV strain line 19 A2,23 kindly provided by Dr. Martin Moore (Emory University). After 3 days, viral plaques were visualized using goat anti-RSV polyclonal antibody (Ab; Millipore, Temecula, CA).
Primary Cell Isolation and Stimulation
Peribronchial LNs were enzymatically digested using 1 mg/mL collagenase A (Roche, Indianapolis, IN) and 25 U/mL DNase I (Sigma-Aldrich, St. Louis, MO) in RPMI 1640 medium with 10% fetal calf serum for 1 hour. LNs were broken down mechanically using 18-gauge needles. Cells were passed through a 40-μm strainer, and cell numbers were quantified. A total of 5 × 105 LN cells per well were cultured in a 96-well plate in complete RPMI 1640 medium (10% fetal calf serum, penicillin/streptomycin, l-glutamine, nonessential amino acids, Na-pyruvate, and 50 mmol/L β-mercaptoethanol) and restimulated with RSV [for 48 hours; multiplicity of infection (MOI) = 0.5] for RNA isolation and cytokine measurements in the culture supernatant.
Lung tissue was processed via enzymatic and mechanical digestion, as previously described. Different cell populations were accessed by flow cytometry using an FACSAria II cell sorter (BD Biosciences).
Bone marrow was flushed from femur and tibia bones. Bone marrow–derived dendritic cells (BMDCs) and bone marrow–derived macrophages (BMDMs) were grown as described previously.24 BMDCs and BMDMs were infected with RSV, 20 μg/mL polyinosinic/polycytidylic acid (poly I:C), 1 μg/mL imiquimod, or 100 ng/mL lipopolysaccharide (LPS) for 24 hours for RNA extraction and measurement of protein in the culture supernatant. When specified, 0.25 μg/mL of recombinant IL-17 (rIL-17) was added to the culture.
Neutralization of IL-17
Mice were pretreated i.p. with 2.5 mg of purified polyclonal anti-mouse IL-17 antibody 2 hours before RSV infection on day 0 and then every other day until day 6.20 The control group similarly received 2.5 mg of anti-mouse IgG antibody (cab).
mRNA Extraction, Reverse Transcription, and Real-Time PCR
mRNA was isolated from LN cell cultures and lung tissues using TRIzol reagent (Invitrogen, Carlsbad, CA), according to the manufacturer’s instructions. RNA (1 μg per sample) was reverse transcribed using murine leukemia virus RTase (Invitrogen). Expression levels of IL-2, IL-4, IL-5, IL-6, IL-13, IL-17a, IFN-γ, Foxp3, IL-10, IL-23, IL-27p28, EBI-3, Gata-3, T-bet, and ROR-γt (Applied Biosystems, Grand Island, NY) were analyzed with TaqMan gene expression assays (Applied Biosystems) using the 7500 Real-Time PCR System (Applied Biosystems). Primers for RSV protein F (RSV-F) and RSV protein G (RSV-G) were custom-made. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase and expressed as fold change over the gene expression in control mice.
Cytokine Assay
Cytokine levels of IL-4, IL-5, IL-13, IFN-γ, IL-17, and IL-10 were determined from cell culture supernatants using a Bio-Plex assay (Bio-Rad, Hercules, CA). IL-27p28 was analyzed in culture supernatants using an enzyme-linked immunosorbent assay kit (R&D Systems Inc., Minneapolis, MN).
Flow Cytometry
Lung cells were stained for flow cytometry as previously described.24 Briefly, cells were FcR blocked and stained with anti-CD11c (N418), anti-Ly6C (HK1.4), and anti-Ly6G (1A8) from Biolegend (San Diego, CA); anti-CD11b (M1/70), anti-CD103 (2E7), and anti-F4/80 (BM8) from eBioscience (San Diego, CA); and anti-GR1 (RBB6-8C5), anti-IL5rα (T21), anti-CD86 (GL-1), and anti–MHC-II/I Ab (AF6-120.1) from BD Biosciences (San Jose, CA). Macrophages were gated as autofluorescent+CD11chiF4/80mid; CD11b+DCs were defined as low-autofluorescent CD11chiMHCII+CD11bhiCD103− cells; CD103+DCs were defined as low-autofluorescent CD11chiMHCII+CD11bloCD103+ cells. Inflammatory neutrophils were gated as low-autofluorescent CD11cloCD11bhiLy6C+Ly6G+; eosinophils were analyzed as low-autofluorescent CD11cloCD11bhiGr1+IL5rα cells.
For intracellular cytokine staining, LN cell suspensions were restimulated for 6 hours in complete medium containing 0.5 μL/mL GolgiPlug, 0.5 μL/mL GolgiStop (BD Biosciences), 0.5 ng/mL phorbol 12-myristate 13-acetate, and 500 ng/mL ionomycin. Subsequently, LN cells were first stained for surface markers with anti-CD3 (17A2), anti-CD4 (RM4-5), and anti-CD8 Abs (16-10A1), all from eBioscience or BD Biosciences. Cells were then fixed/permeabilized (BD Biosciences Fix/Perm Kit) and labeled with anti–IFN-γ Ab (XMG1.2), anti-IL5 (TRFK8), anti-IL13 (ebio13A), and anti-IL17a (ebio17b7) from eBioscience, and anti-IL4 (11B11) from Biolegend.
Histological Characteristics
Lungs were perfused with 4% formaldehyde for fixation and embedded in paraffin using standard histological techniques. Lung sections were stained with H&E for analysis of inflammatory cell infiltration and PAS to detect mucus production. Cellular infiltration and goblet cell hyperplasia in the airway and lung tissues were evaluated using light microscopy by an independent blinded observer (N.W.L.). The lung sections were scored using the following scoring system: 0, absent; 1, minimal; 2, slight; 3, moderate; and 4, severe.
Statistical Analysis
Reported values are expressed as means ± SEM. Data were analyzed and graphs were generated using GraphPad Prism software 6 (GraphPad Software Inc, San Diego, CA). Statistical significance was assessed by a two-tailed Student’s t-test for single-group analysis or one-way analysis of variance, followed by a Student Newman-Keuls test as a post hoc test for multiple comparisons. P < 0.05 was considered significant.
Results
RSV Infection Induces Early But Transitory IL-27 Expression by DCs
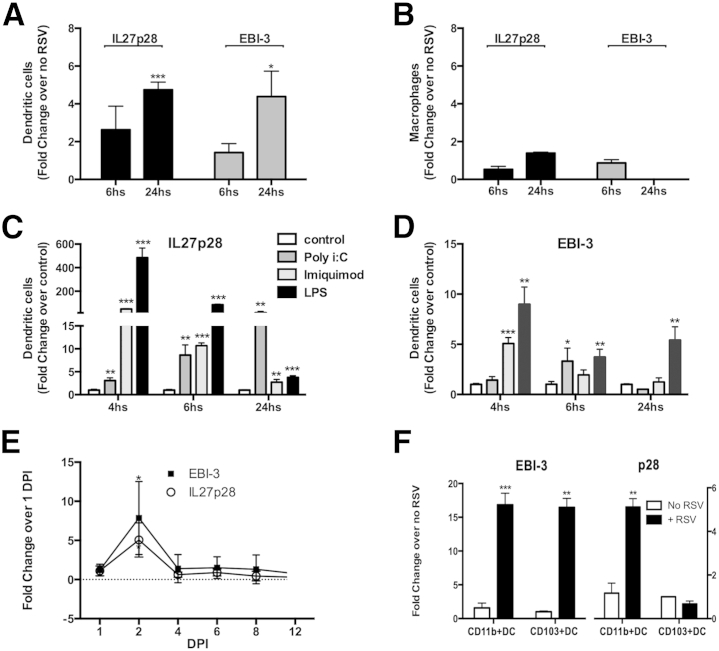
Because IL-27 subunits are expressed predominately by macrophages and DCs,1 studies were designed to determine whether RSV induces IL-27 in myeloid cell populations. BMDCs and BMDMs were infected with RSV, and IL-27 expression was assessed by quantitative RT-PCR (RT-qPCR). The results showed that RSV modestly up-regulates the expression of both subunits of IL-27 (p28 and EBI-3) in BMDCs (Figure 1A), but not in BMDMs (Figure 1B), 24 hours after infection. This up-regulation was transitory because 48 hours after stimulation, no difference in IL-27 expression was detected between RSV-infected and RSV-noninfected cells (data not shown). In addition, because multiple toll-like receptor (TLR) pathways contribute to the generation of the anti-RSV response,25 the effect of TLR signaling on the expression levels of IL-27 was examined. BMDCs were cultured in the presence of TLR3/poly I:C, TLR7/imiquimod, and TLR4/LPS26 stimulus, each for 4, 6, and 24 hours. The results demonstrated that the TLR3 ligand poly I:C up-regulated the IL-27p28 subunit in a time-dependent manner (Figure 1C), whereas the expression levels of the subunit EBI-3 were modestly up-regulated at 6 hours (Figure 1D). In addition, BMDC stimulation by the TLR7 ligand imiquimod or by the TLR4 ligand LPS induced high expression of both IL-27 subunits p28 and EBI-3 early after activation, followed by a decrease in the expression levels over time (Figure 1, C and D) when compared with the unstimulated cell control group. To determine the effect of RSV on IL-27 production in vivo, WT mice were infected with 1.6 × 105 PFU RSV and a kinetic analysis of IL-27 mRNA in the lungs was performed. The results revealed an increase in IL-27p28 and EBI-3 expression at 2 dpi, followed by a rapid decrease at 4, 6, 8, and 12 dpi (Figure 1E). Moreover, we analyzed which DC population was responsible for the production of IL-27 in the lungs using flow cytometry. CD11c+CD11b+DCs and CD11c+CD103+DCs were cell sorted from lungs of naïve WT mice and either stimulated with RSV or left unstimulated for 48 hours in vitro. After mRNA isolation, RT-qPCR analysis showed that both CD11c+CD11b+ and CD11c+CD103+ up-regulated EBI-3 expression on RSV infection compared with unstimulated cells, whereas IL-27p28 expression was found to be up-regulated only in CD11b+DCs (Figure 1F). Thus, these results show that RSV infection induces an early IL-27 response by DCs in the lungs, most likely by CD11c+CD11b+DCs, and this response deteriorates during the progression of the infection.
Figure 1.
RSV infection induces an early, but transitory, IL-27 expression by DCs. BMDCs (A) and BMDMs (B) were infected with RSV (MOI = 0.5) for 6 and 24 hours. Cells were isolated, mRNA was extracted, and transcripts of IL-27 subunits p28 and EBI were measured by RT-qPCR. BMDCs were stimulated with TLR ligands (poly I:C, imiquimod, and LPS) or kept unstimulated (control). At 4, 6, and 24 hours, cells were harvested, mRNA was isolated, and expression of the IL-27 subunits p28 (C) and EBI-3 (D) was measured by RT-qPCR. E: Expression of IL-27p28 and EBI-3 in the lungs at day 1 to day 12 after RSV infection. Data represent three independent experiments and are presented as means ± SEM (n = 4 to 5 per group). F: CD11b+DCs and CD103+DCs were sorted, using a fluorescently activated cell sorter, from lungs of naïve WT mice and infected in vitro with RSV for 48 hours. mRNA was extracted, and expression of the IL-27 subunits p28 and EBI-3 was measured by RT-qPCR. Cells were sorted from the lungs of 10 mice, and data are presented as means ± SEM of triplicate wells. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
IL-27 Signaling Impairment Results in Exacerbation of RSV-Related Lung Pathological Features
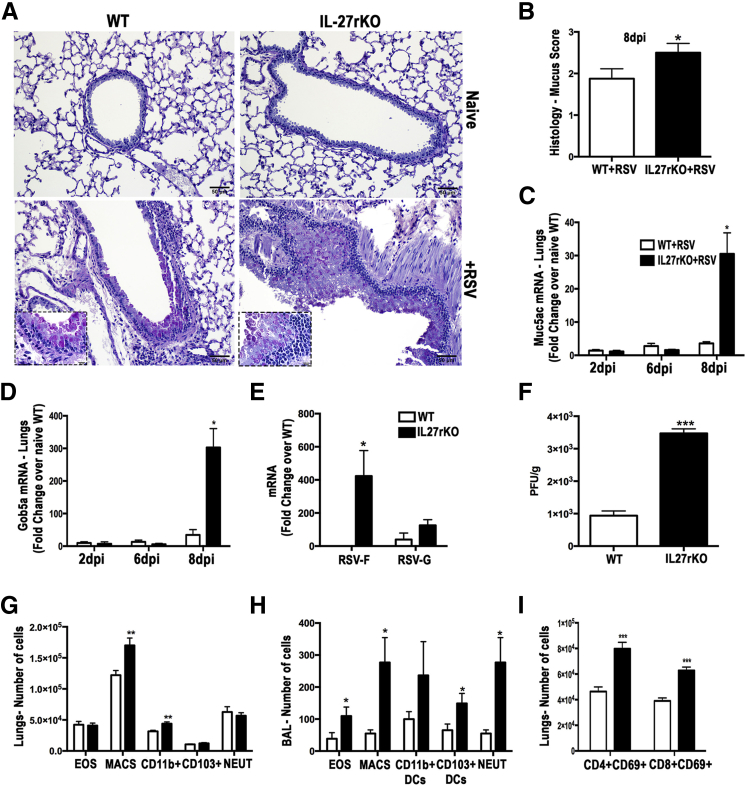
The importance of IL-27 has been investigated in several infectious and noninfectious diseases,26 and recent studies suggest that it has an immunoregulatory function.7–10 To determine the importance of IL-27 in the pathogenesis of RSV infection, IL-27rKO and WT mice were infected with RSV and the histopathological pulmonary changes were assessed. At 8 dpi, PAS staining of lung sections from RSV-infected IL-27rKO animals showed worse goblet cell hyperplasia and exacerbation of mucus secretion in the large airways compared with the WT animals (Figure 2, A and B). These animals also showed enhanced expression of the mucus-associated genes Muc5ac (Figure 2C) and Gob5a (Figure 2D). Because virus persistence and clearance are important aspects of RSV-associated pathological features, we then investigated whether virus persistence was contributing to the disease-associated pathological exacerbation observed in IL-27rKO mice. A kinetic analysis of RSV-F and RSV-G mRNA in the lungs of IL-27rKO mice at 2, 3, 6, and 8 dpi showed that 3 dpi was the peak of viral titers (Supplemental Figure S1). Thus, differences in viral persistence and clearance between RSV-infected WT and IL-27rKO animals were examined at 3 dpi. A significant increase in the expression of RSV-F was detected in the lungs of IL-27rKO when compared with WT mice (Figure 2E). Although RSV-G was also increased in the lungs of IL-27rKO mice, the difference did not reach statistical significance. To confirm this finding, we measured viral load by assessing PFUs from lungs of RSV-infected IL-27rKO and WT mice. Results showed that, at 3 dpi, IL-27rKO had higher viral titers compared with WT mice (Figure 2F). Moreover, airway inflammation was evaluated by measuring cell infiltration in lung homogenates of RSV-infected mice at 8 dpi by flow cytometry. Compared with WT mice, lungs from RSV-infected IL-27rKO mice exhibited significantly more macrophages and CD11c+CD11b+DCs (Figure 2G). When analyzing the bronchoalveolar lavage from these mice, in addition to CD11c+CD11b+DCs and macrophages, RSV-infected IL-27rKO mice also had higher levels of CD11c+CD103+DCs, eosinophils, and neutrophils compared with WT mice (Figure 2H). When assessing the infiltration of lymphocytic populations, the proportion of activated (CD69+) CD4+ and CD8+ T cells was significantly higher in lungs of RSV-infected IL-27rKO mice compared with WT mice (Figure 2I). Thus, results suggest that RSV infection in mice with IL-27r deficiency leads to exacerbation of lung pathological characteristics and increase in mucus production, virus persistency, and inflammatory infiltration.
Figure 2.
RSV-infected IL-27rKO mice show exacerbation of mucogenic responses, goblet cell hyperplasia, and pulmonary inflammation. A: PAS staining of lung sections of naïve or RSV-infected WT or IL-27rKO mice at 8 dpi, analyzed by light microscopy. B: Bar graph demonstrating histological score of mucus assessed in lung sections of RSV-infected WT and IL-27rKO mice. The expression levels of mucus-associated genes Muc5ac (C) and Gob5 (D) were determined by RT-qPCR of whole lung homogenates at 2, 6, and 8 dpi. E: mRNA was extracted from whole lungs of RSV-infected WT and IL-27rKO mice at 3 dpi, and expression levels of the RSV proteins F and G were quantified by RT-qPCR. F: Viral PFU per wet weight of lung were measured in RSV-infected WT and IL-27rKO at 3 dpi. G: Infiltration of eosinophils (EOS), macrophages (MACS), CD11b+DCs, CD103+DCs, and neutrophils (NEUT) in the lungs of RSV-infected WT and IL-27rKO mice at 8 dpi was assessed by flow cytometry. H: Same as in G, but cells were isolated from bronchoalveolar lavage. I: Infiltration of CD4+CD69+ and CD8+CD69+ T cells in the lungs of RSV-infected WT and IL-27rKO mice at 8 dpi assessed by flow cytometry. Data represent three independent experiments and are presented as means ± SEM (n = 4 to 5 per group). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnifications: ×20 (A); ×100 (insets, bottom panels, A).
IL-27 Regulates Th17 Cell Development during RSV Infection
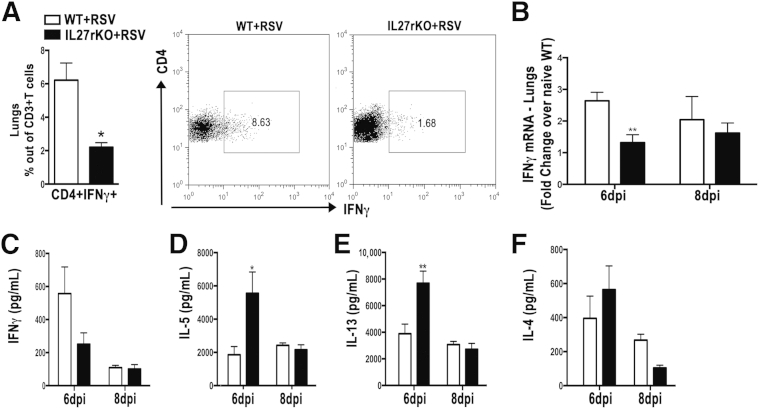
Because of the key role of IL-17a in exacerbation of RSV infection20,25,27,28 and the importance of IL-27 in regulating Th17 cells by inhibiting IL-17a production in an STAT1-dependent manner,14,29–31 we asked whether IL-27r deficiency would affect IL-17a production and the infiltration of Th17 cells during RSV infection. By using flow cytometry, lungs from RSV-infected IL-27rKO mice at 8 dpi showed higher infiltration of Th17 (CD4+IL17a+) cells compared with RSV-infected WT mice (Figure 3A). Also, mRNA isolated from lung homogenates showed that RSV-infected IL-27rKO mice had a significant increase in the expression levels of IL-17a when compared with WT mice (Figure 3B). To further confirm these findings, we measured IL-17a production by peribronchial LN cells isolated from RSV-infected IL-27rKO and WT mice restimulated in vitro with RSV for 48 hours. Results showed that LN cells from RSV-infected IL-27rKO mice at 6 dpi, when restimulated with RSV, produce significantly higher IL-17 compared with WT mice (Figure 3C). Next, because a recent study showed that IL-27 acts on Th17 cell development by inhibiting Th17 lineage-specific transcription factor ROR-γt,31 we assessed its expression in the peribronchial LNs of RSV-infected IL-27rKO and WT mice. Although not statistically significant, IL-27rKO mice tended to have higher expression levels of ROR-γt (Supplemental Figure S2A). More important, because IL-6 and IL-23 have been shown to induce Th17 development, they were assessed. We found that the expression levels of IL-6 tended to be higher in the LNs of IL-27rKO mice compared with WT mice; however, the difference did not reach statistical significance. Also, no difference was found in the expression levels of IL-23 (Supplemental Figure S2B). Thus, IL-27r deficiency affects RSV-related IL-17 production in an IL-6/IL-23–independent manner, most likely by having the effect of STAT1 stimulation by IL-27 counteracted with the absence of the IL-27 signaling, which may contribute to the exacerbated pulmonary pathological characteristics found in IL-27rKO mice.
Figure 3.
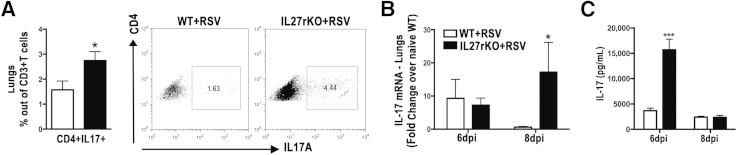
IL-27 regulates IL-17 production during RSV infection. A: Lung cells were recovered by enzymatic digestion from RSV-infected WT and IL-27rKO mice at 8 dpi, and the infiltration of Th17 (CD3+CD4+IL17+) was determined by flow cytometry. mRNA was extracted from the whole lungs of naïve and RSV-infected WT and IL-27rKO at 6 and 8 dpi. B: Expression levels of IL-17a were assessed by RT-qPCR. Peribronchial LNs were harvested from RSV-infected WT and IL-27rKO mice at 6 and 8 dpi. Single-cell suspensions were obtained via enzymatic digestion, and cells were restimulated in vitro with RSV (MOI = 0.5) for 48 hours. C: IL-17 cytokine production was determined by Luminex (Austin, TX) assay. Data represent three independent experiments and are presented as means ± SEM (n = 5 to 6 per group). ∗P < 0.05, ∗∗∗P < 0.001.
IL-27 Regulates Th1 and Th2 Cell Balance in the Lungs during RSV Infection
Previous studies demonstrated that IL-27 promotes Th1 and inhibits Th2 cells, subsets shown to be important in the pathogenesis of RSV infection.18,19 Thus, we asked whether mRNA and protein levels of Th1- and Th2-associated cytokines were altered in the lungs of RSV-infected IL-27rKO mice. By using flow cytometry, we found that lungs from IL-27rKO mice had significantly lower infiltration of CD4+IFNγ+ Th1 cells compared with WT (Figure 4A) at 8 dpi. Gene expression of IFNγ was also significantly decreased in the lungs of IL-27rKO mice (Figure 4B) compared with WT mice at 6 dpi. To further verify our findings, we assessed the protein levels of IFNγ, IL-4, IL-5, and IL-13 in the peribronchial LNs of RSV-infected IL-27rKO and WT mice. In contrast to the results with lungs, LN cells from RSV-infected IL-27rKO mice showed no significant decrease in the production of IFNγ compared with WT mice (Figure 4C). However, we observed a significant increase in the production of IL-5 (Figure 4D) and IL-13 (Figure 4E) by LN cells derived from RSV-infected IL-27rKO compared with WT mice at 6 dpi. However, no significant change in IL-4 production was detected (Figure 4F). Next, we assessed the expression of the Th1 and Th2 lineage-specific transcription factors, T-bet and Gata-3, in the lungs of RSV-infected IL-27rKO and WT mice (Supplemental Figure S2, C and D). Accordingly, T-bet expression was decreased, a result that correlated with the lower expression levels of IFNγ found in the lungs of RSV-infected IL-27rKO mice. No significant difference in Gata-3 expression was detected when comparing lungs from IL-27rKO and WT mice infected with RSV (Supplemental Figure S2D). Although IL-27 has been described to be important in the regulation of regulatory T cells (Tregs), production of IL-10,32 and expression of the Treg-specific transcription factor Foxp3,33–35 no changes in the gene expression of IL-10 (Supplemental Figure S3A) and Foxp3 (Supplemental Figure S3B) were observed in the lungs of RSV-infected IL-27rKO compared with WT mice. Furthermore, no difference was seen when analyzing the infiltration of CD4+CD25+Foxp3+ Treg cells in the lungs of these mice by flow cytometry (Supplemental Figure S3C). Also, no change in IL-10 production was detected in supernatants derived from peribronchial LN cells of RSV-infected IL-27rKO and WT mice restimulated in vitro with RSV (Supplemental Figure S3D). Thus, data demonstrate that RSV infection in mice that are IL27r deficient lead to an imbalance between Th1 and Th2, favoring the production of pathogenic Th2 cytokines IL-5 and IL-13 in the LNs and inhibiting the anti-viral Th1-associated cytokine IFNγ. These factors likely contribute to the more severe lung pathological features found in the IL-27rKO mice in a Treg cell–independent way.
Figure 4.
Deficiency in IL-27 signaling alters Th1 and Th2 cytokines during RSV infection. A: Results of flow cytometric determination of the infiltration of Th1 (CD3+CD4+IFNγ+) in the lungs. B: mRNA was extracted from the whole lungs of naïve and RSV-infected WT and IL-27rKO at 6 and 8 dpi, and expression levels of IFNγ were detected by RT-qPCR. Peribronchial LN cells were isolated from RSV-infected WT and IL-27rKO mice at 6 and 8 dpi and restimulated in vitro with RSV (MOI = 0.5) for 48 hours. IFNγ (C), IL-5 (D), IL-13 (E), and IL-4 (F) cytokine production was determined by Luminex assay. Data represent three independent experiments and are presented as means ± SEM (n = 5 to 6 per group). ∗P < 0.05, ∗∗P < 0.01.
Anti–IL-17 Increases IL-27 Response and Attenuates Lung Pathological Features
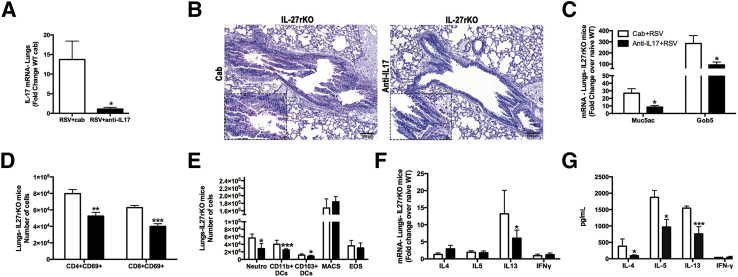
Previous studies indicated that IL-17 could directly induce mucus production from pulmonary epithelial cells.20,36 Given the high levels of IL-17 found in the LNs and lungs of RSV-infected IL-27rKO mice, studies were designed to determine whether IL-17 played a role in the exacerbation of the RSV infection observed in IL-27rKO mice. To address this issue, IL-17 was neutralized in vivo using passive immunization of IL-27rKO mice with anti–IL-17 Ab or with control IgG (cab) during RSV infection. Our results show that RSV-infected IL-27rKO mice that received anti–IL-17 had a significant inhibition in the expression levels of IL-17a in the lungs compared with mice that received cab (Figure 5A). Lung sections from RSV-infected cab or anti–IL-17–treated IL-27rKO mice were stained with PAS to assess goblet cell hyperplasia and mucus production. Microscopic analysis of lung sections showed that anti–IL-17–treated IL-27rKO mice had less goblet cell hyperplasia, mucus production, and inflammatory cell infiltration compared with the cab treatment (Figure 5B). In addition, lungs from RSV-infected anti–IL-17–treated IL-27rKO mice had significantly decreased mucus production, as assessed by the expression of the mucus-associated genes Muc5ac and Gob5 when compared with the cab-treated group (Figure 5C). No difference in viral gene expression was observed on anti–IL-17 treatment. In addition, we found that the anti–IL-17 treatment significantly reduced the infiltration of CD4+ and CD8+ activated (CD69+) T cells (Figure 5D), as well as CD11b+DCs, CD103+DCs, and neutrophils (Figure 5E) in the lungs of RSV-infected IL-27rKO mice compared with cab-treated mice. Interestingly, IL-13 mRNA transcripts were significantly lower when RSV-infected IL-27rKO mice were treated with anti–IL-17 (Figure 5F) compared with cab treatment. Moreover, the cytokine profile in the peribronchial LNs of these animals was examined by restimulating single LN cell suspensions from cab- or anti–IL-17–treated RSV-infected IL-27rKO mice with RSV in vitro for 48 hours. Compared with the cab-treated group, anti–IL-17–treated IL-27rKO LN cells had significantly lower production of the Th2-associated cytokines IL-4, IL-5, and IL-13 (Figure 5G). The production of IFNγ was not changed by the treatment with anti–IL-17 (Figure 5G). Furthermore, the lungs of anti–IL-17–treated RSV-infected IL-27rKO mice had significantly higher expression levels of the IL-27 subunits p28 and EBI-3 compared with cab-treated mice (Figure 6A), suggesting that by neutralizing IL-17, IL-27 production could be up-regulated. To further investigate the effect of IL-17 on IL-27 production, mRNA was extracted from LNs of cab- or anti–IL-17–treated RSV-infected IL-27rKO mice rechallenged in vitro with RSV. Our data showed that both subunits of IL-27 (EBI-3 and p28) were increased when IL-17 was neutralized; however, the increase seen in IL-27p28 did not reach statistical significance. Next, to further characterize the IL-17 regulation of IL-27 production by DCs during RSV infection, BMDCs were infected with RSV for 4, 8, and 24 hours in the presence or absence of rIL-17. Our data showed that during RSV infection, the addition of rIL-17 inhibited the expression of both IL-27 p28 and EBI-3 by BMDCs at 24 hours after stimulation (Figure 6C). In addition, to verify which component of the anti-RSV response was involved in the down-regulation of IL-27 production by BMDCs when activated with rIL-17, we stimulated BMDCs with multiple TLR ligands. The results showed that BMDCs stimulated with the TLR3 ligand poly I:C, in the presence of IL-17, produced significantly lower levels of IL-27 (Figure 6D). No significant differences were seen when cells were stimulated with control, imiquimod, or LPS (Figure 6D). Thus, these data suggest that during RSV infection, in the absence of IL-27r signaling, an overactivation of the IL-17 pathway occurs, leading to a more robust production of IL-17, which augments Th2-related cytokine secretion by peribronchial LNs and pulmonary mucus production. In addition, results suggest that overproduction of IL-17, induced during RSV infection, inhibits IL-27 production by DCs in a TLR3-specific manner, contributing to exacerbated lung pathological features, which might explain the low and transitory levels of IL-27 produced during RSV infection.
Figure 5.
Neutralization of IL-17 attenuates lung pathological features in IL-27rKO mice. mRNA was extracted from lung homogenates of cab- or anti–IL-17–treated RSV-infected IL-27rKO mice at 8 dpi. A: Expression levels of the IL-17a were measured by RT-qPCR. B: PAS staining of lung sections of cab- or anti–IL-17–treated RSV-infected IL-27rKO mice at 8 dpi analyzed by light microscopy. C: Mucus-associated genes Muc5ac and Gob5 were measured by RT-qPCR. Lung cells were recovered by enzymatic digestion from anti–IL-17 or cab-treated, RSV-infected IL-27rKO mice at 8 dpi and the infiltration of activated CD4+CD69+ and CD8+CD69+ T cells (D), and neutrophils (neutro), dendritic cells (DCs), macrophages (MACS), and eosinophils (EOS) were determined by flow cytometry (E). F: mRNA levels of the Th1- and Th2-associated cytokines in the lungs were assessed by RT-qPCR. G: Peribronchial LN cells from cab- or anti–IL-17–treated RSV-infected IL-27rKO mice at 8 dpi. Single-cell suspensions were obtained via enzymatic digestion, and cells were restimulated in vitro with RSV (MOI = 0.5) for 48 hours. IL-4, IL-5, IL-13, and IFNγ production levels were determined by a Luminex assay. Data are presented as means ± SEM of four replicate wells (n = 5 to 6 per group). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnifications: ×10 (B); ×40 (B, insets).
Figure 6.
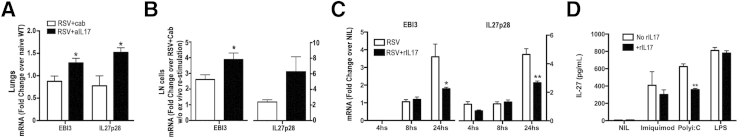
IL-17 potentially regulates IL-27 production by RSV-infected DCs. mRNA was extracted from lung homogenates of cab- or anti–IL-17–treated RSV-infected IL-27rKO mice at 8 dpi. A: Gene expression of IL-27 subunits p28 and EBI-3 was assessed by RT-qPCR. Peribronchial LN cells were harvested from cab- or anti–IL-17–treated RSV-infected IL-27rKO mice at 8 dpi and restimulated in vitro with RSV (MOI = 0.5) for 48 hours. B: mRNA was extracted, and the expression levels of IL-27 subunits p28 and EBI-3 were measured by RT-qPCR. BMDCs were stimulated with RSV in the presence or absence of rIL-17 for 4, 8, and 24 hours. C: Cells were harvested at each time point, mRNA was extracted, and gene expression of IL-27 subunits p28 and EBI-3 was measured by RT-qPCR. DMDCs were stimulated with the TLR ligands imiquimod (TLR7), poly I:C (TLR3), or LPS (TLR4), or kept unstimulated (NIL) in the presence or absence of rIL-17. D: After 48 hours, supernatants were harvested and the production of IL-27p28 (pg/mL) was measured by an enzyme-linked immunosorbent assay. Data are presented as means ± SEM of triplicate wells and represent two independent experiments (n = 5 to 6 per group). ∗P < 0.05, ∗∗P < 0.01.
Discussion
The clearance of virus in the lungs depends on a balanced Th cell response to promote minimal pulmonary damage. However, RSV-specific T-cell responses can often result in severe pulmonary inflammation, partially due to an altered immune environment.37–39 Herein, we report that, even though the induction of IL-27 production by RSV is transitory, IL-27 signaling plays an important role in controlling the severity of RSV infection by suppressing Th17 cells and altering the Th cell response in the lungs. Because IL-27 is produced by APCs,40 we first analyzed whether RSV would induce IL-27 production by BMDCs and BMDMs. Our studies demonstrated that RSV induces modest expression levels of the IL-27 subunits p28 and EBI-3 by BMDCs, but not by BMDMs, and was further confirmed using a kinetic analysis of the expression profile of IL-27 p28 and EBI-3 subunits in the lungs of RSV-infected mice.
In addition, RSV-F protein binds and activates the LPS receptor TLR4,41 the activation of the chemokines chemokine ligand 5 and CXCL10 can be regulated by the RSV-related activation of TLR3,22 and TLR7 appears to regulate IL-17–mediated responses.25 Likewise, this study indicated that all TLR3, TLR4, and TLR7 agonists resulted in increased expression of the IL-27 subunits EBI-3 and p28 by BMDCs, whereas reports showed that stimulation of TLR2 and TLR7/8 had only modest effects.40
By sorting distinct populations of DCs (CD11c+CD11b+ and CD11c+CD103+) from the lungs of naïve WT mice and infecting them with RSV in vitro, our data revealed that the CD11b+DCs are the primary source of IL-27 during RSV infection, confirming a study in a model of imiquimod-induced psoriasis, which showed colocalization of IL-27 in DCs.42 Our in vivo data also showed that at 8 dpi, IL-27rKO mice have increased infiltration of DCs and macrophages in the lungs compared with WT mice, suggesting a role of these cells in modulating the T-cell immunity against RSV and perpetuating the inflammatory response in IL-27rKO mice. Interestingly, a previous study reported a role for IL-27 in the functional suppression of DCs and macrophages.43 Thus, the increased infiltration of DCs and macrophages observed in the lungs of IL-27rKO mice may be accompanied by up-regulation of cytokine production and expression of accessory molecules by them, which may affect the T-cell–mediated inflammation on RSV infection.
Accordingly, the studies performed herein verified that the exacerbation of the inflammatory response observed in the lungs of IL-27rKO was related to altered T-cell responses, as indicated by more activated CD4+ and CD8+ T cells in RSV-infected IL-27rKO mice compared with WT mice. Moreover, it has been proposed that the regulatory role of IL-27 is the result of its suppressive effect on IL-2, a cytokine known to induce cell proliferation and activation.44,45 Although we did not find evidence of increased IL-2 production in RSV-infected IL-27rKO mice during our study (data not shown), it is possible that IL-2 was transiently expressed early during RSV infection, thus contributing to the increased infiltration of activated T cells in the lungs.
A key finding in these studies was the significant increase in production of IL-17 in the lungs and peribronchial LNs of RSV-infected IL-27rKO mice compared with WT mice. Indeed, it has been shown that IL-27 is capable of inhibiting the development of Th17 cells in vitro.30,46 Studies focusing on the role of IL-27 in the development of Th17 cells using IL-27rKO mice chronically infected with Toxoplasma gondii or immunized with myelin oligodendrocyte glycoprotein peptide to induce experimental autoimmune encephalomyelitis have revealed that IL-27 is a natural antagonist of Th17 activity.14,29,31 In these studies, the lack of IL-27 signaling resulted in more Th cells and increased IL-17 production. In a study of RSV vaccine-enhanced disease, mice co-immunized with pcDNA3–IL-27 and a recombinant vaccine candidate (G1F/M2) did not develop vaccine-enhanced inflammatory responses or pulmonary disease after RSV challenge because the IL-27 plasmid caused suppression in both Th2 and Th17 responses.39 In addition, treatment of mice with rIL-27 suppressed the development of experimental autoimmune encephalomyelitis, and was associated with decreased infiltration of Th17 cells and IL-17 production in the central nervous system.29 In agreement with previous reports, the increase in Th17 cell infiltration found in IL-27rKO mice infected with RSV was accompanied by worse lung pathological features when compared with WT mice. Because IL-27 activation signals through STAT12,14 and RSV-infected STAT1KO mice have increased production of IL-17,47 we speculate that the increased IL-17 production found in the lungs and LNs of RSV-infected IL-27r–deficient mice might be STAT1 dependent.
Exacerbation of RSV-related pathological features, mucus secretion, and AHR are associated with the production of the Th2-related cytokines, especially IL-13.48 We demonstrated that IL-27r–deficient mice have exacerbated mucus production and increased levels of mucus-associated genes Muc5ac and Gob5. Correspondingly, peribronchial LNs in IL-27rKO mice had increased production of the Th2 cytokines IL-5 and IL-13 compared with WT mice. In agreement with our results, a study using T. muris showed that IL-27rKO mice have an augmentation in Th2 cell differentiation with an increase in the production of Th2-associated cytokines, exaggerated goblet cell hyperplasia, mastocytosis, and early clearance of larvae burden in the intestinal track.8 In experimental asthma, mice lacking IL-27r had exacerbation of pulmonary lesions when compared with WT mice, with increased goblet cell hyperplasia at the bronchiole and enhanced expression of Muc5ac.10 Conversely, intranasal administration of IL-27 inhibited signs of asthma severity, including AHR, goblet cell hyperplasia, and airway eosinophilic infiltration.12 The mechanism by which IL-27 inhibits Th2 cells may be, in part, due to its capacity to activate STAT1 and inhibit Gata-3, a critical transcription factor for Th2 development.4 Although the present study did not detect a significant increase of GATA3 in the IL-27rKO mice, a significant decrease in T-bet was observed relating to the altered Th cell phenotype. These results are in agreement with studies showing the importance of IL-27 in Th1 cell development through activation of STAT1 and T-bet.4 In addition, IL-27r–deficient mice infected with Leishmania major7 and Listeria monocytogenes49 also showed impaired IFNγ production compared with WT mice. On the basis of our findings and the role of IFNγ in virus clearance,16,17 we suggest that the lack of IL-27r during RSV infection and inhibition of IFNγ could favor viral propagation and persistency, as demonstrated by the increase in RSV-F gene expression and enhanced virus titers in the lungs.
To determine whether IL-17 was responsible for the exacerbated RSV infection found in IL-27rKO mice, blockage of IL-17 during RSV infection was performed, which reduced the severity of disease in RSV-infected IL-27rKO mice. The infiltration of activated CD4+CD69+ and CD8+CD69+ T cells also was reduced, suggesting inhibition of the inflammatory response. Moreover, the number of neutrophils in the lungs of RSV-infected anti–IL-17–treated IL-27rKO was decreased, in agreement with previous studies relating IL-17 overproduction with the induction of CXC chemokines, increased neutrophilic infiltration, and consequent exacerbation of tissue damage and mucus production.50–52 These results further confirm a previous study showing that IL-17 alters the epithelial cell response that controls mucus production.53 Also, peribronchial LNs from anti–IL-17 or cab-treated RSV-infected IL-27rKO mice produced significantly less IL-4, IL-5, and IL-13, in agreement with previous reports showing that neutralization or the absence of IL-17 alters Th2-associated cytokines.13,20,54 Strikingly, by neutralizing IL-17 during RSV infection, we found that IL-27 p28 and EBI-3 subset transcripts were up-regulated, suggesting a regulatory role of IL-17 in the production of IL-27. Because DCs express the IL-17 receptor and are able to respond to IL-17,55 we further investigated the effect of IL-17 on the expression of IL-27 by BMDCs infected with RSV. Results confirmed that, in rIL-17–stimulated RSV-infected DCs, the expression levels of IL-27 p28 and EBI-3 subunits are both inhibited. In addition, by studying the effect of IL-17 on RSV-related activation of TLRs in BMDCs, data showed that IL-17 inhibition of IL-27 production was verified during activation of TLR3. Because IL-17 did not inhibit other TLR-mediated IL-27 production, it may be that the TIR-domain—containing adapter-inducing IFN-β adapter pathway is specifically altered in the presence of IL-17, which would further reduce IL-27 production. In macrophages, IFNγ and the TLR4 agonist LPS synergistically induce the IL-27 constituent gene p28, in an MyD88-, c-Rel–, interferon regulatory factor 1–, and NF-κB–dependent manner.56 To the best of our knowledge, this is the first report suggesting a suppressive role of IL-17 and TLR3 signaling cascade in the production of IL-27. IL-17 has been shown to activate NF-κB,57 and the proximal activation of NF-κB in the IL-17RA signaling pathway has been related to TNF receptor associated factor 6,58 an important adaptor protein in the TLR signaling cascades; however, TRIF has been shown to be unnecessary for IL-17–mediated signaling.57 The mechanism involved in the TLR3–IL-17 regulation of IL-27 needs to be further investigated. Thus, results suggest that the RSV-dependent IL-17 overproduction inhibits IL-27 p28 and EBI-3 subunit expression by DCs, perhaps by a feedback inhibition of the IL-17–IL-17 receptor interaction. This could explain the low and transitory IL-27 expression levels in the lungs of RSV-infected mice.
In summary, the data presented herein demonstrate that IL-27 functions as a regulatory cytokine during RSV pathogenesis primarily by controlling Th17 cell development, Th2-related cytokines, mucus production, and lung pathological features.
Acknowledgments
We thank Andrew Rasky, Susan Morris, Ana Lucia Coelho, Lisa Riggs Johnson, and Pamela Lincoln for technical assistance; Dr. Judith Connett for editing the manuscript; Amgen, Inc., for kindly providing the IL-27rKO mice; and Dr. Martin Moore (Emory University, Atlanta, GA) for RSV strain 2-20 and Line 19 A2.
Footnotes
Supported by NIH grants AI073876 (N.W.L.) and T32 AI007413 (D.E.d.A.N.).
Disclosures: Amgen, Inc., provided the IL-27rKO mice.
Supplemental Data
During RSV infection, the peak of viral load in the lungs occurs at day 3. A kinetic analysis of RSV-F and RSV-G mRNA in the lungs of IL-27rKO at 2, 3, 6, and 8 dpi measured by RT-qPCR. Data are presented as means ± SEM (n = 5 to 6 per group).
RSV-infected IL-27rKO mice have decreased expression of T-bet in the lungs. mRNA was extracted from the whole lungs of naïve and RSV-infected WT and IL-27rKO mice at 8 dpi. Expression levels of ROR-γt (A), IL-6 and IL-23 (B), T-bet (C), and Gata-3 (D) were assessed by RT-qPCR. Data are presented as means ± SEM (n = 5 to 6 per group). ∗P < 0.05.
The suppressive effect of the IL-27 signaling during RSV infection is independent of Tregs and IL-10. mRNA was extracted from lungs of RSV-infected IL-27rKO mice at 2, 6, and 8 dpi. Expression levels of IL-10 (A) and Foxp3 (B) were measured by RT-qPCR. Lung cells were recovered by enzymatic digestion from RSV-infected IL-27rKO mice at 8 dpi, and the percentage of infiltrating Treg cells (CD4+CD25+Foxp3+) from the total CD3+ cell population was determined by flow cytometry. C: Cells had been previously gated on CD3+ cells. Peribronchial LN cells were harvested from RSV-infected WT and IL-27rKO mice at 8 dpi. Single-cell suspensions were obtained via enzymatic digestion, and cells were restimulated in vitro with RSV (MOI = 0.5) for 48 hours. D: IL-10 production was determined by Luminex assay. Data are presented as means ± SEM (n = 5 to 6 per group).
References
- 1.Pflanz S., Timans J.C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W.M., Mattson J.D., Wagner J.L., To W., Zurawski S., McClanahan T.K., Gorman D.M., Bazan J.F., de Waal Malefyt R., Rennick D., Kastelein R.A. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- 2.Hibbert L., Pflanz S., De Waal Malefyt R., Kastelein R.A. IL-27 and IFN-alpha signal via Stat1 and Stat3 and induce T-Bet and IL-12Rbeta2 in naive T cells. J Interferon Cytokine Res. 2003;23:513–522. doi: 10.1089/10799900360708632. [DOI] [PubMed] [Google Scholar]
- 3.Pflanz S., Hibbert L., Mattson J., Rosales R., Vaisberg E., Bazan J.F., Phillips J.H., McClanahan T.K., de Waal Malefyt R., Kastelein R.A. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- 4.Lucas S., Ghilardi N., Li J., de Sauvage F.J. IL-27 regulates IL-12 responsiveness of naive CD4+ T cells through Stat1-dependent and -independent mechanisms. Proc Natl Acad Sci U S A. 2003;100:15047–15052. doi: 10.1073/pnas.2536517100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda A., Hamano S., Yamanaka A., Hanada T., Ishibashi T., Mak T.W., Yoshimura A., Yoshida H. Cutting edge: role of IL-27/WSX-1 signaling for induction of T-bet through activation of STAT1 during initial Th1 commitment. J Immunol. 2003;170:4886–4890. doi: 10.4049/jimmunol.170.10.4886. [DOI] [PubMed] [Google Scholar]
- 6.Holscher C., Holscher A., Ruckerl D., Yoshimoto T., Yoshida H., Mak T., Saris C., Ehlers S. The IL-27 receptor chain WSX-1 differentially regulates antibacterial immunity and survival during experimental tuberculosis. J Immunol. 2005;174:3534–3544. doi: 10.4049/jimmunol.174.6.3534. [DOI] [PubMed] [Google Scholar]
- 7.Yoshida H., Hamano S., Senaldi G., Covey T., Faggioni R., Mu S., Xia M., Wakeham A.C., Nishina H., Potter J., Saris C.J., Mak T.W. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- 8.Artis D., Villarino A., Silverman M., He W., Thornton E.M., Mu S., Summer S., Covey T.M., Huang E., Yoshida H., Koretzky G., Goldschmidt M., Wu G.D., de Sauvage F., Miller H.R., Saris C.J., Scott P., Hunter C.A. The IL-27 receptor (WSX-1) is an inhibitor of innate and adaptive elements of type 2 immunity. J Immunol. 2004;173:5626–5634. doi: 10.4049/jimmunol.173.9.5626. [DOI] [PubMed] [Google Scholar]
- 9.Hamano S., Himeno K., Miyazaki Y., Ishii K., Yamanaka A., Takeda A., Zhang M., Hisaeda H., Mak T.W., Yoshimura A., Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- 10.Miyazaki Y., Inoue H., Matsumura M., Matsumoto K., Nakano T., Tsuda M., Hamano S., Yoshimura A., Yoshida H. Exacerbation of experimental allergic asthma by augmented Th2 responses in WSX-1-deficient mice. J Immunol. 2005;175:2401–2407. doi: 10.4049/jimmunol.175.4.2401. [DOI] [PubMed] [Google Scholar]
- 11.Yamanaka A., Hamano S., Miyazaki Y., Ishii K., Takeda A., Mak T.W., Himeno K., Yoshimura A., Yoshida H. Hyperproduction of proinflammatory cytokines by WSX-1-deficient NKT cells in concanavalin A-induced hepatitis. J Immunol. 2004;172:3590–3596. doi: 10.4049/jimmunol.172.6.3590. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T., Yoshimoto T., Yasuda K., Mizuguchi J., Nakanishi K. IL-27 suppresses Th2 cell development and Th2 cytokines production from polarized Th2 cells: a novel therapeutic way for Th2-mediated allergic inflammation. J Immunol. 2007;179:4415–4423. doi: 10.4049/jimmunol.179.7.4415. [DOI] [PubMed] [Google Scholar]
- 13.Yang J., Yang M., Htut T.M., Ouyang X., Hanidu A., Li X., Sellati R., Jiang H., Zhang S., Li H., Zhao J., Ting A.T., Mayer L., Unkeless J.C., Labadia M.E., Hodge M., Li J., Xiong H. Epstein-Barr virus-induced gene 3 negatively regulates IL-17, IL-22 and RORgamma t. Eur J Immunol. 2008;38:1204–1214. doi: 10.1002/eji.200838145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Batten M., Li J., Yi S., Kljavin N.M., Danilenko D.M., Lucas S., Lee J., de Sauvage F.J., Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- 15.Borchers A.T., Chang C., Gershwin M.E., Gershwin L.J. Respiratory syncytial virus: a comprehensive review. Clin Rev Allergy Immunol. 2013;45:331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schauer U., Hoffjan S., Rothoeft T., Bartz H., Konig S., Fuchs E., Bittscheidt J., Kochling A., Stephan V. Severe respiratory syncytial virus infections and reduced interferon-gamma generation in vitro. Clin Exp Immunol. 2004;138:102–109. doi: 10.1111/j.1365-2249.2004.02582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Semple M.G., Dankert H.M., Ebrahimi B., Correia J.B., Booth J.A., Stewart J.P., Smyth R.L., Hart C.A. Severe respiratory syncytial virus bronchiolitis in infants is associated with reduced airway interferon gamma and substance P. PLoS One. 2007;2:e1038. doi: 10.1371/journal.pone.0001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–190. doi: 10.1111/j.0105-2896.2004.00215.x. [DOI] [PubMed] [Google Scholar]
- 19.Schwarze J., Cieslewicz G., Joetham A., Ikemura T., Hamelmann E., Gelfand E.W. CD8 T cells are essential in the development of respiratory syncytial virus-induced lung eosinophilia and airway hyperresponsiveness. J Immunol. 1999;162:4207–4211. [PubMed] [Google Scholar]
- 20.Mukherjee S., Lindell D.M., Berlin A.A., Morris S.B., Shanley T.P., Hershenson M.B., Lukacs N.W. IL-17-induced pulmonary pathogenesis during respiratory viral infection and exacerbation of allergic disease. Am J Pathol. 2011;179:248–258. doi: 10.1016/j.ajpath.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stokes K.L., Chi M.H., Sakamoto K., Newcomb D.C., Currier M.G., Huckabee M.M., Lee S., Goleniewska K., Pretto C., Williams J.V., Hotard A., Sherrill T.P., Peebles R.S., Jr., Moore M.L. Differential pathogenesis of respiratory syncytial virus clinical isolates in BALB/c mice. J Virol. 2011;85:5782–5793. doi: 10.1128/JVI.01693-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudd B.D., Smit J.J., Flavell R.A., Alexopoulou L., Schaller M.A., Gruber A., Berlin A.A., Lukacs N.W. Deletion of TLR3 alters the pulmonary immune environment and mucus production during respiratory syncytial virus infection. J Immunol. 2006;176:1937–1942. doi: 10.4049/jimmunol.176.3.1937. [DOI] [PubMed] [Google Scholar]
- 23.McKimm-Breschkin J.L. A simplified plaque assay for respiratory syncytial virus: direct visualization of plaques without immunostaining. J Virol Methods. 2004;120:113–117. doi: 10.1016/j.jviromet.2004.02.020. [DOI] [PubMed] [Google Scholar]
- 24.Demoor T., Petersen B.C., Morris S., Mukherjee S., Ptaschinski C., De Almeida Nagata D.E., Kawai T., Ito T., Akira S., Kunkel S.L., Schaller M.A., Lukacs N.W. IPS-1 signaling has a nonredundant role in mediating antiviral responses and the clearance of respiratory syncytial virus. J Immunol. 2012;189:5942–5953. doi: 10.4049/jimmunol.1201763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lukacs N.W., Smit J.J., Mukherjee S., Morris S.B., Nunez G., Lindell D.M. Respiratory virus-induced TLR7 activation controls IL-17-associated increased mucus via IL-23 regulation. J Immunol. 2010;185:2231–2239. doi: 10.4049/jimmunol.1000733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jankowski M., Kopinski P., Goc A. Interleukin-27: biological properties and clinical application. Arch Immunol Ther Exp (Warsz) 2010;58:417–425. doi: 10.1007/s00005-010-0098-6. [DOI] [PubMed] [Google Scholar]
- 27.Bystrom J., Al-Adhoubi N., Al-Bogami M., Jawad A.S., Mageed R.A. Th17 lymphocytes in respiratory syncytial virus infection. Viruses. 2013;5:777–791. doi: 10.3390/v5030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee S., Allen R.M., Lukacs N.W., Kunkel S.L., Carson W.F., 4th STAT3-mediated IL-17 production by postseptic T cells exacerbates viral immunopathology of the lung. Shock. 2012;38:515–523. doi: 10.1097/SHK.0b013e31826f862c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald D.C., Ciric B., Touil T., Harle H., Grammatikopolou J., Das Sarma J., Gran B., Zhang G.X., Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- 30.Neufert C., Becker C., Wirtz S., Fantini M.C., Weigmann B., Galle P.R., Neurath M.F. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- 31.Stumhofer J.S., Laurence A., Wilson E.H., Huang E., Tato C.M., Johnson L.M., Villarino A.V., Huang Q., Yoshimura A., Sehy D., Saris C.J., O’Shea J.J., Hennighausen L., Ernst M., Hunter C.A. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- 32.Batten M., Kljavin N.M., Li J., Walter M.J., de Sauvage F.J., Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 33.Hall A.O., Beiting D.P., Tato C., John B., Oldenhove G., Lombana C.G., Pritchard G.H., Silver J.S., Bouladoux N., Stumhofer J.S., Harris T.H., Grainger J., Wojno E.D., Wagage S., Roos D.S., Scott P., Turka L.A., Cherry S., Reiner S.L., Cua D., Belkaid Y., Elloso M.M., Hunter C.A. The cytokines interleukin 27 and interferon-gamma promote distinct Treg cell populations required to limit infection-induced pathology. Immunity. 2012;37:511–523. doi: 10.1016/j.immuni.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wojno E.D., Hosken N., Stumhofer J.S., O’Hara A.C., Mauldin E., Fang Q., Turka L.A., Levin S.D., Hunter C.A. A role for IL-27 in limiting T regulatory cell populations. J Immunol. 2011;187:266–273. doi: 10.4049/jimmunol.1004182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carrier Y., Whitters M.J., Miyashiro J.S., LaBranche T.P., Ramon H.E., Benoit S.E., Ryan M.S., Keegan S.P., Guay H., Douhan J., Collins M., Dunussi-Joannopoulos K., Medley Q.G. Enhanced GITR/GITRL interactions augment IL-27 expression and induce IL-10-producing Tr-1 like cells. Eur J Immunol. 2012;42:1393–1404. doi: 10.1002/eji.201142162. [DOI] [PubMed] [Google Scholar]
- 36.Newcomb D.C., Boswell M.G., Sherrill T.P., Polosukhin V.V., Boyd K.L., Goleniewska K., Brody S.L., Kolls J.K., Adler K.B., Peebles R.S., Jr. IL-17A induces signal transducers and activators of transcription-6-independent airway mucous cell metaplasia. Am J Respir Cell Mol Biol. 2013;48:711–716. doi: 10.1165/rcmb.2013-0017OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gonzalez P.A., Prado C.E., Leiva E.D., Carreno L.J., Bueno S.M., Riedel C.A., Kalergis A.M. Respiratory syncytial virus impairs T cell activation by preventing synapse assembly with dendritic cells. Proc Natl Acad Sci U S A. 2008;105:14999–15004. doi: 10.1073/pnas.0802555105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voges B., Vallbracht S., Zimmer G., Bossow S., Neubert W.J., Richter K., Hobeika E., Herrler G., Ehl S. Recombinant Sendai virus induces T cell immunity against respiratory syncytial virus that is protective in the absence of antibodies. Cell Immunol. 2007;247:85–94. doi: 10.1016/j.cellimm.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Zeng R., Zhang H., Hai Y., Cui Y., Wei L., Li N., Liu J., Li C., Liu Y. Interleukin-27 inhibits vaccine-enhanced pulmonary disease following respiratory syncytial virus infection by regulating cellular memory responses. J Virol. 2012;86:4505–4517. doi: 10.1128/JVI.07091-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stumhofer J.S., Hunter C.A. Advances in understanding the anti-inflammatory properties of IL-27. Immunol Lett. 2008;117:123–130. doi: 10.1016/j.imlet.2008.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haynes L.M., Moore D.D., Kurt-Jones E.A., Finberg R.W., Anderson L.J., Tripp R.A. Involvement of toll-like receptor 4 in innate immunity to respiratory syncytial virus. J Virol. 2001;75:10730–10737. doi: 10.1128/JVI.75.22.10730-10737.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shibata S., Tada Y., Asano Y., Yanaba K., Sugaya M., Kadono T., Kanda N., Watanabe S., Sato S. IL-27 activates Th1-mediated responses in imiquimod-induced psoriasis-like skin lesions. J Invest Dermatol. 2013;133:479–488. doi: 10.1038/jid.2012.313. [DOI] [PubMed] [Google Scholar]
- 43.Wang S., Miyazaki Y., Shinozaki Y., Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- 44.Owaki T., Asakawa M., Kamiya S., Takeda K., Fukai F., Mizuguchi J., Yoshimoto T. IL-27 suppresses CD28-mediated [correction of medicated] IL-2 production through suppressor of cytokine signaling 3. J Immunol. 2006;176:2773–2780. doi: 10.4049/jimmunol.176.5.2773. [DOI] [PubMed] [Google Scholar]
- 45.Villarino A.V., Stumhofer J.S., Saris C.J., Kastelein R.A., de Sauvage F.J., Hunter C.A. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- 46.Yoshimura T., Takeda A., Hamano S., Miyazaki Y., Kinjyo I., Ishibashi T., Yoshimura A., Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
- 47.Hashimoto K., Durbin J.E., Zhou W., Collins R.D., Ho S.B., Kolls J.K., Dubin P.J., Sheller J.R., Goleniewska K., O’Neal J.F., Olson S.J., Mitchell D., Graham B.S., Peebles R.S., Jr. Respiratory syncytial virus infection in the absence of STAT 1 results in airway dysfunction, airway mucus, and augmented IL-17 levels. J Allergy Clin Immunol. 2005;116:550–557. doi: 10.1016/j.jaci.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 48.Lukacs N.W., Tekkanat K.K., Berlin A., Hogaboam C.M., Miller A., Evanoff H., Lincoln P., Maassab H. Respiratory syncytial virus predisposes mice to augmented allergic airway responses via IL-13-mediated mechanisms. J Immunol. 2001;167:1060–1065. doi: 10.4049/jimmunol.167.2.1060. [DOI] [PubMed] [Google Scholar]
- 49.Chen Q., Ghilardi N., Wang H., Baker T., Xie M.H., Gurney A., Grewal I.S., de Sauvage F.J. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- 50.Hoshino H., Laan M., Sjostrand M., Lotvall J., Skoogh B.E., Linden A. Increased elastase and myeloperoxidase activity associated with neutrophil recruitment by IL-17 in airways in vivo. J Allergy Clin Immunol. 2000;105:143–149. doi: 10.1016/s0091-6749(00)90189-1. [DOI] [PubMed] [Google Scholar]
- 51.Laan M., Cui Z.H., Hoshino H., Lotvall J., Sjostrand M., Gruenert D.C., Skoogh B.E., Linden A. Neutrophil recruitment by human IL-17 via C-X-C chemokine release in the airways. J Immunol. 1999;162:2347–2352. [PubMed] [Google Scholar]
- 52.Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., Shellito J.E., Bagby G.J., Nelson S., Charrier K., Peschon J.J., Kolls J.K. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hashimoto K., Graham B.S., Ho S.B., Adler K.B., Collins R.D., Olson S.J., Zhou W., Suzutani T., Jones P.W., Goleniewska K., O’Neal J.F., Peebles R.S., Jr. Respiratory syncytial virus in allergic lung inflammation increases Muc5ac and gob-5. Am J Respir Crit Care Med. 2004;170:306–312. doi: 10.1164/rccm.200301-030OC. [DOI] [PubMed] [Google Scholar]
- 54.Nakae S., Komiyama Y., Nambu A., Sudo K., Iwase M., Homma I., Sekikawa K., Asano M., Iwakura Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 55.Antonysamy M.A., Fanslow W.C., Fu F., Li W., Qian S., Troutt A.B., Thomson A.W. Evidence for a role of IL-17 in organ allograft rejection: IL-17 promotes the functional differentiation of dendritic cell progenitors. J Immunol. 1999;162:577–584. [PubMed] [Google Scholar]
- 56.Liu J., Guan X., Ma X. Regulation of IL-27 p28 gene expression in macrophages through MyD88- and interferon-gamma-mediated pathways. J Exp Med. 2007;204:141–152. doi: 10.1084/jem.20061440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaffen S.L. Structure and signalling in the IL-17 receptor family. Nat Rev Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schwandner R., Yamaguchi K., Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
During RSV infection, the peak of viral load in the lungs occurs at day 3. A kinetic analysis of RSV-F and RSV-G mRNA in the lungs of IL-27rKO at 2, 3, 6, and 8 dpi measured by RT-qPCR. Data are presented as means ± SEM (n = 5 to 6 per group).
RSV-infected IL-27rKO mice have decreased expression of T-bet in the lungs. mRNA was extracted from the whole lungs of naïve and RSV-infected WT and IL-27rKO mice at 8 dpi. Expression levels of ROR-γt (A), IL-6 and IL-23 (B), T-bet (C), and Gata-3 (D) were assessed by RT-qPCR. Data are presented as means ± SEM (n = 5 to 6 per group). ∗P < 0.05.
The suppressive effect of the IL-27 signaling during RSV infection is independent of Tregs and IL-10. mRNA was extracted from lungs of RSV-infected IL-27rKO mice at 2, 6, and 8 dpi. Expression levels of IL-10 (A) and Foxp3 (B) were measured by RT-qPCR. Lung cells were recovered by enzymatic digestion from RSV-infected IL-27rKO mice at 8 dpi, and the percentage of infiltrating Treg cells (CD4+CD25+Foxp3+) from the total CD3+ cell population was determined by flow cytometry. C: Cells had been previously gated on CD3+ cells. Peribronchial LN cells were harvested from RSV-infected WT and IL-27rKO mice at 8 dpi. Single-cell suspensions were obtained via enzymatic digestion, and cells were restimulated in vitro with RSV (MOI = 0.5) for 48 hours. D: IL-10 production was determined by Luminex assay. Data are presented as means ± SEM (n = 5 to 6 per group).