Abstract
Development of novel strategies to treat noninfectious posterior uveitis is an ongoing challenge, in part because of limited availability of animal models that mimic the naturally occurring disease in humans. Mice deficient in the autoimmune regulatory gene Aire develop a spontaneous T-cell and macrophage-mediated autoimmune uveitis that closely recapitulates human endogenous uveitis and thus provide a useful model for mechanistic and therapeutic investigations. Lymphocytic and mononuclear infiltration of the retina in Aire knockout (KO) mice triggers the onset of uveitis from initial retinal inflammation to eventual destruction of the neuroretina with loss of photoreceptors. The C-C chemokine receptor type 2 protein (CCR2) functions in directing monocyte and macrophage migration to inflamed tissues via interaction with monocyte chemotactic proteins. Using the Aire KO mouse model, we demonstrated an essential role for CCR2 in the pathogenesis of autoimmune-mediated uveitis. Loss of functional CCR2 effectively reduced immune cell infiltration and rescued the retina from destruction. CCR2-dependent migration of bone marrow–derived cells provided the driving force for retinal inflammation, with CCR2-expressing mononuclear cells contributing to retinal damage via recruitment of CD4+ T cells. These studies identify the CCR2 pathway as a promising therapeutic target that may prove an effective approach to treat uveitis associated with autoimmunity.
Uveitis ranks third among causes of blindness in the United States; however, the mechanisms of pathogenesis are largely unknown. Clinically, the most common form of sight-threatening uveitis is posterior uveitis associated with systemic autoimmune disease, in which noninfectious intraocular inflammation is characterized by severe infiltration by T cells and macrophages.1,2 Because current approaches for treating posterior uveitis rely on general suppression of the immune system, there is urgent need for more specific treatments with fewer adverse effects. Identifying therapeutic targets (ie, specific immune mediators involved in the pathogenesis of autoimmune uveitis) is key to discovering effective treatments.
Monocyte recruitment to the inflamed tissue is a common manifestation and essential cause of many inflammatory diseases. The C-C chemokine receptor type 2 protein (CCR2) plays a critical role in regulation of monocyte trafficking into inflamed tissues, through interactions with ligands belonging to the family of proinflammatory monocyte chemotactic proteins (MCPs).3 CCR2 is reported to be critical in the efficient recruitment of monocytes in experimental autoimmune encephalomyelitis,4 thioglycollate-induced peritonitis,5,6 and atherosclerosis.7 CCR2 has also been implicated in neuroinflammatory processes of degenerative central nervous system diseases such as Alzheimer disease,8 ischemic neuropathy,9 and multiple sclerosis.10 In the eye, macrophage infiltration has been reported to contribute to the pathogenesis of choroidal neovascularization,11 retinitis pigmentosa,12 and dry eye associated with autoimmune disease.13 Blockade of macrophage recruitment using the CCR2 antagonist INCB3344 effectively inhibited the formation of choroidal neovascularization in mice,14 and CCR2 knockdown alleviated photoreceptor death in a mouse model of inherited retinal degeneration.12
We investigated the contribution and functional significance of CCR2 in directing monocyte and T-cell recruitment to provoke retinal inflammation in a spontaneous mouse model of autoimmune-mediated uveitis. Current murine models used to mimic immune-mediated uveitis in humans involve experimental induction by immunization with retinal autoantigens, such as interphotoreceptor retinoid-binding protein (IRBP), S-arrestin (retinal S-antigen), or endotoxin lipopolysaccharide. The IRBP-induced experimental autoimmune uveitis (EAU) model in mice is a recognized and extensively studied model of human posterior uveitis.15,16 Dagkalis et al17 found that CCR2 knockdown did not reduce the severity of disease or the percentage of monocytes recruited to the site of retinal inflammation. Despite a significant number of infiltrating macrophages in the retinal tissues of EAU mice, the authors concluded that CCR2 does not have a primary role in the recruitment of monocytes to the inflammatory site across the blood–retina barrier in well-developed EAU.
In the present study, we revisited the role of CCR2-expressing cells in the pathogenesis of noninfectious posterior uveitis, using an alternative model of ocular autoimmune disease. Mice deficient in the autoimmune regulator gene Aire provide a model of spontaneous, organ-specific, CD4+ T-cell–mediated autoimmune uveitis that closely mimics human endogenous uveitis. Aire knockout (Aire KO) mice develop immune infiltrates in the posterior chamber of the eye that are reminiscent of those seen in the induced rodent model of EAU.18 Aire-deficient mice develop uveitis as a result of loss of thymic expression of a single eye antigen, IRBP. Lack of IRBP expression solely in the thymus, even in the presence of Aire expression, is sufficient to trigger spontaneous eye-specific autoimmunity. Autoantibodies specific to IRBP are found within the photoreceptor layer of the retina,19 where they initiate or trigger retinal inflammation and photoreceptor destruction. Alongside the equine recurrent uveitis model20 and the recently developed IRBP T-cell receptor transgenic mouse (R161H),21 Aire KO mice represent a unique, spontaneous animal model of autoimmune uveitis and thereby provide an efficient model to recapitulate clinical and immunopathological aspects of the human disease.
In the present study, we demonstrate monocytic and lymphocytic infiltration of the neuroretina in the eyes of Aire KO mice. Our findings from genetic knockdown and bone-marrow chimera models suggest that the recruitment of monocytes and T cells to the inflamed retina of Aire KO mice is CCR2 dependent. Accordingly, CCR2-deficient bone marrow cells effectively rescue the neuroretina from destruction in Aire KO chimeras. These results provide evidence that CCR2-expressing bone marrow–derived mononuclear cells induce uveitis through the recruitment of autoreactive CD4+ T cells. Delineating the underlying immune events that provoke uveitis in the setting of autoimmune disease is an essential step toward developing novel treatments. Our findings point to CCR2-expressing cells as promising therapeutic targets in the management of autoimmune uveitis.
Materials and Methods
Mice and Reagents
Mouse strains with targeted disruption of Ccr2 gene6 [C57BL/6 × J129 (C57/J129) back-crossed eight times onto a BALB/c background] were provided by Dr. John Osterholzer of the University of Michigan. Mouse strains with targeted mutation in the Aire gene19 [BALB/c background (Aire KO)] were crossed with BALB/c mice deficient in functional Ccr2 (Ccr2 KO) to generate BALB/c Aire−/−Ccr2−/− [Aire–Ccr2 double KO (DKO)] mice. Mice were housed in a pathogen-free barrier facility at the University of California, San Francisco. Genomic DNA isolated from tail clippings was genotyped for the Aire and Ccr2 mutations by PCR. Primers for Aire were forward 5′-AGACTAGGTGTTCCCTCCCAACCTCAG-3′ and reverse 5′-GTCATGTTGACGGATCCAGGGTAGAAAGT-3′. PCR products in WT and Aire KO mice were 1150 bp and 690 bp, respectively. Primers used for detecting WT Ccr2 were WAK 0 forward 5′-TGGGGATACTGCTTAAATGGCGCAA-3′ and WAK 134 reverse 5′-TCAGAGATGGCCAAGTTGAGCAGA-3′; primers used for detecting mutant Ccr2 were WAK 134 forward 5′-TCAGAGATGGCCAAGTTGAGCAGA-3′ and WAK 121 reverse 5′-TTCCATTGCTCAGCGGTGCT-3′. PCR products in WT and CCR2 KO mice were 400 bp and 450 bp, respectively. The PCR reaction was performed for 5 minutes at 94°C, followed by 35 cycles of 30 seconds at 94°C, 75 seconds at 60°C, and 1 minute at 72°C for amplification, with 5 minutes at 72°C to finish. Representative genotyping results are presented in Supplemental Figure S1. All experimental procedures adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
DAPI was obtained from Life Technologies, (Carlsbad, CA). F4/80 antibody was from AbD Serotec (Raleigh, NC). MC-21 (anti-CCR2)22 antibody was gift from Dr. Matthias Mack (Regensburg, Germany). Antibody to ionized calcium-binding adapter molecule 1 (Iba1) [recommended name: allograft inflammatory factor 1 (AIF-1)] was from WAKO Chemicals (Richmond, VA). CD4 antibody was from BD Pharmingen (San Diego, CA). A 3,3′-diaminobenzidine staining kit was from Vector Laboratories (Burlingame, CA). Horseradish peroxidase (HRP)-conjugated donkey anti-rat secondary antibody was from Jackson ImmunoResearch Laboratories (West Grove, PA). Alexa Fluor 488–conjugated donkey anti-rat IgG and Alexa Fluor 594 donkey anti-rabbit IgG were from Life Technologies.
Bone-Marrow Chimeras
Bone marrow was harvested from the femur, humerus, and tibia of donor mice and CD4+ or CD8+ T cells were removed by complement depletion. In brief, cells were incubated with anti-CD4 (clone GK1.5) and anti-CD8 (clone YTS-169), followed by rabbit complement (Sigma-Aldrich, St. Louis, MO). Efficiency for complement depletion of CD4+ and CD8+ T cells was confirmed by flow cytometry (Supplemental Figure S2). Recipients were irradiated in two doses with 550 rads (5.5 Gy) per dose, delivered at least 3 hours apart. Bone marrow (5 × 106 CD4+ depleted, CD8+ depleted) was injected via the tail vein into BALB/c Aire KO and Aire–Ccr2 DKO mice. Animals were sacrificed at 7 weeks after bone marrow transplantation.
Immunohistochemistry and Immunofluorescence Quantification
Immune cell subtypes were visualized by immunohistochemistry using an antibody specific for CD4 (BD Pharmingen), a donkey anti-rat secondary antibody conjugated to HRP, and a 3,3′-diaminobenzidine staining kit. In brief, enucleated eyes were embedded in Tissue-Tek optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA) for freezing. Sections (7 μm thick) were prepared from these tissues using a cryostat (Leica Microsystems, Wetzlar, Germany) and were mounted on SuperFrost Plus slides (Fisher Scientific, Pittsburgh, PA). Sections were fixed for 10 minutes in acetone at −20°C, washed in PBS for 5 minutes, and then blocked with 5% normal goat serum for 1 hour at room temperature. After blocking, slides were incubated in 3% hydrogen peroxide to inactivate endogenous peroxidases, then incubated with primary CD4 antibody diluted 1:50 in blocking agent overnight at 4°C, washed three times with PBS for 5 minutes each, and incubated with secondary antibody diluted 1:100 in blocking agent for 30 minutes at room temperature. After three additional 5-minute washes in PBS, slides were developed with substrate. For immunofluorescence detection of cells expressing F4/80 and Iba1, sections were incubated with primary antibodies (1:25 and 1:500 dilution, respectively) at 4°C overnight. After a wash, the appropriate secondary antibody (1:400 dilution) was added, and nuclei were stained with DAPI. Immunofluorescent staining of cells was photographed with a Nikon (Tokyo, Japan) Eclipse Ti-E microscope. The images were analyzed with NIS Elements Advanced Research software version 3.10 (Nikon) to estimate the number of nucleus-associated Iba1+ cells across the entire retina. Iba1+ cells and CD4+ cells were manually counted with their associated nuclei. Data are reported as the total number of nucleus-associated Iba1+ and CD4+ cells.
Histology and Scoring
Eyes were enucleated from mice, snap-frozen in Tissue-Tek OCT compound (Sakura Finetek), cryosectioned (7 μm), and stained with H&E. Retina histology was scored on a four-point scale, in which 0 indicates no histological infiltrate, normal retinal architecture, clear separation between the inner and outer nuclear layer, and intact photoreceptors; 1 indicates narrowing or loss of the outer plexiform layer and intact photoreceptors; 2 indicates narrowing or loss of the OPL and photoreceptor loss; and 3 indicates retinal atrophy, including photoreceptor loss, thinning of the nerve fiber layer, loss of ganglion cells, and decreased cell numbers in the inner and outer nuclear layers.
Retina Examination and Retinal Fluorescein Angiography
For mice in the autoimmune uveitis experiments, fundus examination was performed using a Micron III retinal imaging microscope (Phoenix Research Labs, Pleasanton, CA). All mice had their whiskers trimmed using curved, blunt-tipped scissors; this was followed by application of one to two drops of cyclopentolate to the eye to dilate the pupils and application of proparacaine to anesthetize the cornea. The animals were anesthetized with ketamine–xylazine, to stabilize the animal for imaging. Each retina was analyzed with fluorescein angiography immediately after fundus examination, while pupils were still dilated. A single intraperitoneal injection of sodium fluorescein at 2 μL/g body weight (approximately 50 μL for an adult mouse) was administered with a 30-gauge needle to each mouse.23 After approximately 30 seconds, the animal was gently restrained, and the retinas were imaged approximately 5 times per minute for 3 minutes.
Transcriptional Profiling of MCP-1 in the Retina Using TaqMan PCR
Total RNA was extracted from retinal tissues using an RNeasy RNA isolation mini kit (Qiagen, Valencia, CA). Total RNA was eluted from minicolumns with 30 μL of RNase-free water. cDNA (120 ng) was synthesized using TaqMan reverse transcription reagent containing random hexamer, RNase inhibitor, dNTP mixture, and MultiScribe Reverse Transcriptase (Life Technologies). The reaction was performed for 10 minutes at 25°C, 15 minutes at 42°C, and 5 minutes at 95°C. To compare the relative abundance of MCP-1 transcripts, a TaqMan probe gene expression assay with exon boundary-crossing primers was used. Real-time quantitative PCR was performed with an ABI Prism 7500 PCR system (Life Technologies), with thermal cycling conditions of 95°C for 10 minutes, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C for amplification. All assays were performed in three technical replicates and were normalized to GAPDH as a housekeeping gene. CT values were derived from three mice in each of the two groups of mice. After normalization, the fold change derived from ΔΔCT of each experimental group versus control was examined by analysis of variance. Positive and negative quality controls for reproducibility, reverse transcription, and genomic DNA contamination were assessed and found to be acceptable.
ELISA
MCP-1 levels were measured using a mouse enzyme-linked immunosorbent assay (ELISA) kit (ELM-MCP1-001C; RayBiotech, Norcross, GA). Retinas were removed from 8-week-old WT and Aire KO mice. Each test sample was made up of two whole retinas. The retinas were homogenized in lysis buffer containing 150 mmol/L NaCl, 100 mmol/L Tris (pH 7.4), 1 mmol/L EGTA, 1 mmol/L EDTA, 0.5% sodium deoxycholate, 1% Triton X-100, and protease inhibitors. Total protein concentration was determined with a Bradford assay. Individual mouse retinal lysates (300 μg) were assayed using ELISA, according to the manufacturer's guidelines. The assay sensitivity for MCP-1 is 3 pg/mL.
Radioligand Binding Assay
Full-length mouse IRBP cDNA (MMM1013; Thermo Fisher Scientific) was in vitro transcribed and translated with [35S]methionine using a TnT transcription and translation system kit (Promega, Madison, WI). The 35S-radiolabeled proteins immunoprecipitated with serum or positive control antibodies to mouse IRBP (sc-25787; Santa Cruz Biotechnology, Dallas, TX) were aliquoted in triplicate in 96-well polyvinylidene difluoride filtration plates (Millipore, Billerica, MA). In each well, 35S-radiolabeled proteins [20,000 counts per minute (cpm)] were used for immunoprecipitation. The radioactivity of the immunoprecipitated material was evaluated with the use of a liquid scintillation counter (Beckman Coulter, Brea, CA). The autoantibody index was calculated as (cpmsample − cpmnegative control)/(cpmpositive standard − cpmnegative standard).
Statistical Analysis
We used t-test (two-tailed) or nonparametric Kruskal–Wallis test, depending on data distribution, to compare macrophage and CD4+ T-cell infiltration, mouse MCP-1 protein concentration, retinal histology score, mIRBP autoantibody index, and qPCR. We used analysis of variance to perform comparisons among the three chimera groups while adjusting for multiple comparisons using the Bonferroni correction. All analyses were performed using Stata software version 9.0 (Stata Corp, College Station, TX) for MacIntosh. P < 0.05 was considered statistically significant. Data are expressed as means ± SEM.
Results
Histological Alteration of the Retina during Autoimmune-Mediated Uveitis
Aire KO mice developed a spontaneous, CD4+ T cell–mediated autoimmune uveitis characterized by destruction of retinal tissue. By 7 weeks of age, Aire KO mice demonstrated immunopathological signs of uveitis, as evidenced by infiltration of inflammatory cells throughout the retina and choroid. By 8 weeks, retinal pathology was well established and reflected a full spectrum of disease development, from initial inflammation of the retina to eventual destruction of the neuroretina (Figure 1A).
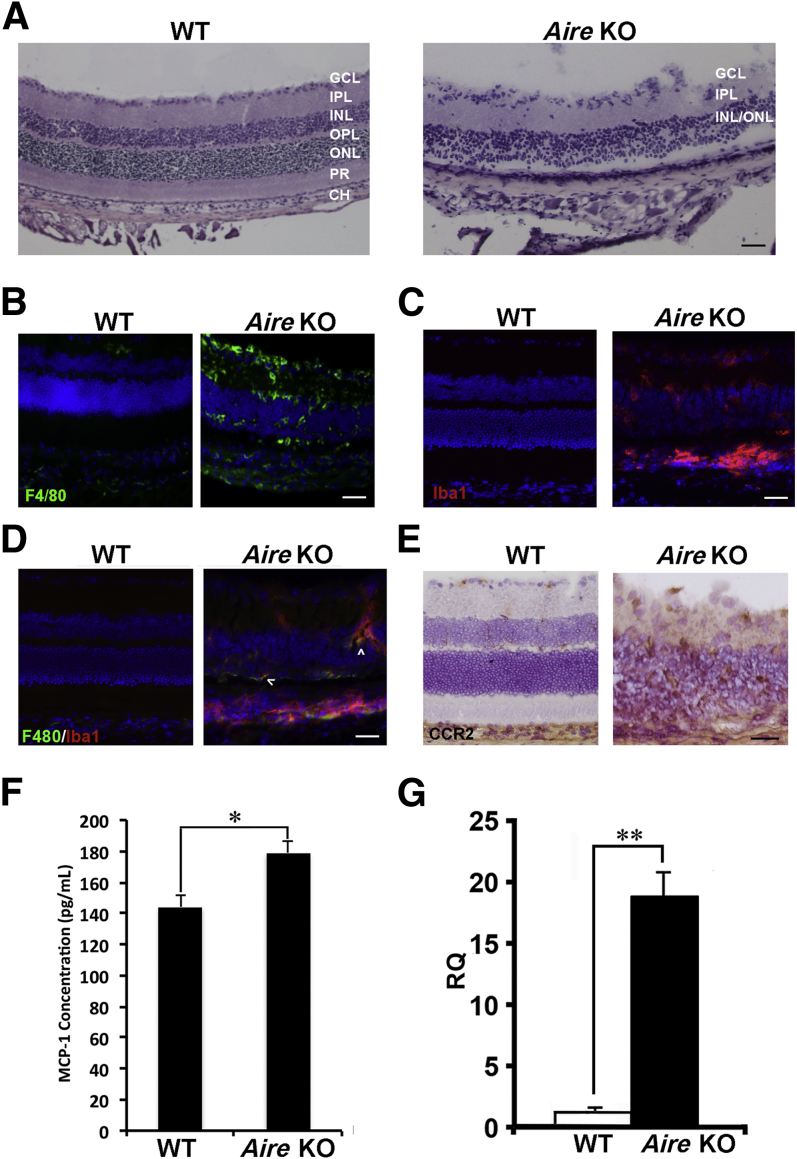
Figure 1.
Autoimmune-mediated inflammation of the neuroretina in Aire KO mice. A: H&E-stained sections of retinas obtained from 8- to 10-week-old BALB/c WT and Aire KO mice. Normal retina consists of the ganglion cell layer (GCL), inner plexiform (IPL), inner nuclear (INL), outer plexiform (OPL), outer nuclear layer (ONL), photoreceptors (PR), retinal pigment epithelium (not labeled) and choroid (CH). B: Mononuclear infiltration of the retina was assessed by immunofluorescence using antibody directed against F4/80. C: Retina-specific macrophages and microglia were detected using antibody directed against Iba1. D: Colocalization (arrowheads) of F4/80+ and Iba1+ macrophages was detected in the Aire KO retina. E: Immunohistochemistry using the monoclonal antibody MC-21 delineated CCR2+ cells (brown) in the retina. F and G: MCP-1 protein was evaluated by ELISA (F), and MCP-1 mRNA expression was evaluated by quantitative real-time PCR (G) in WT and Aire KO retina. For RQ values, MCP-1 transcript in WT retina was set as the onefold reference value. Data are expressed as means ± SEM and are representative of at least four independent tests. n = 4 per group. ∗P < 0.05; ∗∗P < 0.01. Scale bar = 100 μm.
Aire KO mice exhibited a striking retinal pathology that included thinning of the plexiform layers, loss of cell bodies in the inner and outer nuclear layers, and progressive destruction of the photoreceptors. This retinal phenotype was characteristic of the target organ–specific autoimmune destruction that occurs in Aire-deficient mice in which loss of Aire gene expression in medullary epithelial cells of the thymus prevents the proper removal of autoreactive lymphocytes directed against numerous tissue-specific self-antigens, including IRBP.18,19 In previous studies of Aire KO mice, our research group found a large number of mononuclear cells infiltrating the anterior segment of the eye, where they played an essential role in mediating inflammatory ocular surface disease.13 In the present study, we noted a similar significant infiltration of F4/80+ mononuclear cells throughout the posterior segment, with a substantial number populating the neuroretina and choroid (Figure 1B). We also observed an increased presence of Iba1+ cells within the Aire KO retina (Figure 1C). Iba1 is a microglia- and macrophage-specific marker whose expression is up-regulated in microglia after activation with macrophage colony-stimulating factor.24 Interestingly, a substantial number of F4/80+ cells in the Aire KO retina were colocalized with Iba1+ cells (Figure 1D). The monocyte receptor CCR2 functions in monocyte chemotaxis to provoke recruitment of monocytes and macrophages to inflamed tissues, where they function in antigen presentation and phagocytosis. In Aire KO mice, CCR2+ cells were found throughout the retina (Figure 1E). In accord, monocyte chemoattractant protein 1 (MCP-1; alias CCL2), a principal ligand of CCR2, was significantly increased in retinas of Aire KO mice relative to WT controls at both the protein level (179 ± 7.928 versus 144 ± 7.862 pg/mL; P < 0.05) (Figure 1F) and the transcript level [28.069 ± 1.296 versus 0.331 ± 0.199 relative quantitation units (RQ); P < 0.01] (Figure 1G).
Mononuclear and CD4+ T-Cell Infiltration of the Retina Is Attenuated in the Absence of CCR2
MCP-1–CCR2 signaling is crucial to monocyte recruitment during inflammation. In a previous study, our research group discovered an essential role for macrophages in linking CD4+ T cells to ocular surface inflammation in mice with CD4+ T cell–mediated, aqueous-deficient, dry eye disease.13 To examine the functional role of CCR2 in recruiting monocytes to the retina in Aire KO mice, we crossed Aire and Ccr2 KO mice to generate Aire–Ccr2 DKO mice. Interestingly, genetic knockdown of Ccr2 in Aire KO mice failed to attenuate immune cell infiltration of the lacrimal gland and cornea, and severe aqueous-deficient dry eye and lacrimal gland exocrinopathy persisted in Aire–Ccr2 DKO mice (Supplemental Figure S1, B and C). In contrast, immune cell infiltration of the posterior uveal tract in DKO mice was significantly reduced, with a corresponding rescue of the neuroretina. This retina-specific effect of CCR2 depletion clearly demonstrated the tissue specificity of Aire-mediated autoimmunity and directed our further investigations.
First, we compared the retinal histopathology of Aire KO mice with and without functional Ccr2 (Figure 2A), using antibodies directed against CD4+ T cells and Iba1+ macrophages and microglia. Retinal CD4+ T cells and Iba1+ mononuclear cells were counted in WT, Aire KO, and Aire–Ccr2 DKO mice (Figure 2B). Severe destruction of retinal tissues was accompanied by persistent mononuclear and CD4+ T-cell infiltration in Aire KO, relative to WT mice (236.2 ± 21.84 versus 8 ± 1.62 Iba1+ cells, P < 0.01; 154 ± 26.99 versus no infiltrating CD4+ cells, P < 0.05), with Iba1-expressing cells largely distributed throughout the retina, along with a notable accumulation in the subretinal space. By contrast, mononuclear and CD4+ T-cell infiltrates were significantly reduced in retinas of Aire–Ccr2 DKO mice, relative to Aire KO mice (108.5 ± 20.04 versus 236.2 ± 21.84 Iba1+ cells, P < 0.01; 16.67 ± 7.37 versus 154 ± 26.99 CD4+ cells, P < 0.05).
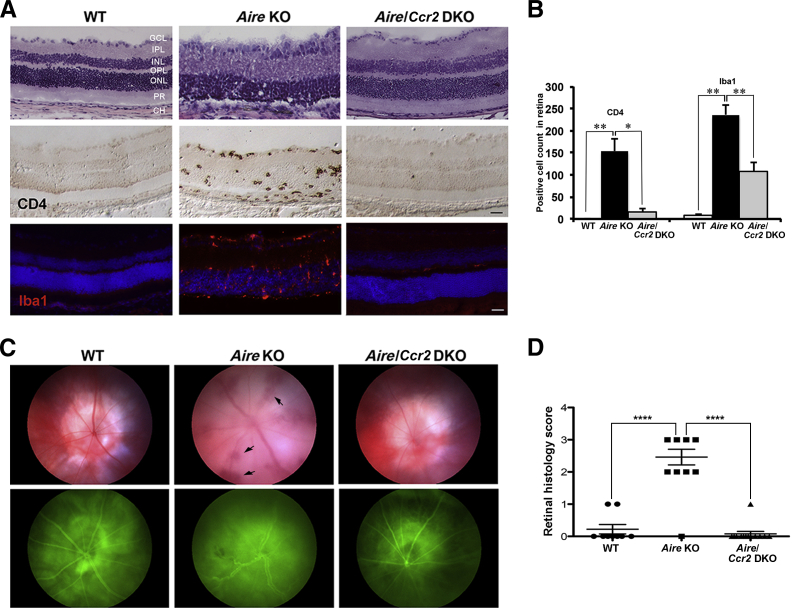
Figure 2.
Histological rescue and reduced immune cell infiltration of the retina in Aire–Ccr2 DKO mice. H&E staining (top row) of retinal cross sections reveals histological destruction of the retina in Aire KO mice, but not in WT or Aire–Ccr2 DKO mice; the damage is confirmed by immunostaining of CD4+ T cells (middle row) and Iba1+ microglia (bottom row). B: Quantitative analysis of CD4+ T cells and Iba1+ microglia across the entire length of the retina of WT, Aire KO, and Aire–Ccr2 DKO mice. C:In vivo imaging of the retina in WT, Aire KO, and Aire–Ccr2 DKO using fundoscopy (top row) reveals inflammation of the uveal tract and vascular hemorrhage (arrows), and fluorescein angiography (bottom row) reveals retinal vessel damage, perivascular leakage, and intraretinal hemorrhage. D: Retinal histology score (on a scale of 0 to 3, as described under Materials and Methods) was used to quantify retinal tissue damage. Retinal scores of WT and Aire–Ccr2 DKO mice were significantly lower than those of Aire KO mice. Data are expressed as means ± SEM. n = 3 to 5 (B), n = 3 (C), and n = 9 to 13 (D) mice per group. Individual data points (D) represent the mean of at least two retinal sections from a single animal. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001. Scale bar = 100 μm.
In vivo imaging was used to visualize and compare intraocular inflammation and retinal vasculature in Aire KO mice with and without the Ccr2 gene (Figure 2C). Fundoscopy and fluorescein angiography together revealed intraretinal hemorrhage, perivascular leakage, and retinal vessel disruption in Aire KO mice, but not in WT or Aire–Ccr2 DKO mice. Retinal histology scores were derived by grading structural damage and loss of photoreceptors on a scale of 0 to 3 (Figure 2D). Notably, the retina of Aire–Ccr2 DKO mice retained the structural details of a healthy retina with a histology score significantly less than that of Aire KO mice (0.077 ± 0.077 DKO score versus 2.27 ± 0.30 Aire KO score; P < 0.0001). Decreased immune cell infiltration and maintenance of normal retinal architecture in Aire–Ccr2 DKO mice suggested that CCR2 signaling was essential in recruiting the macrophages and CD4+ T cells that ultimately provoke retinal tissue damage.
Transfer of CCR2+ Bone Marrow Cells Promotes Retinal Autoimmunity
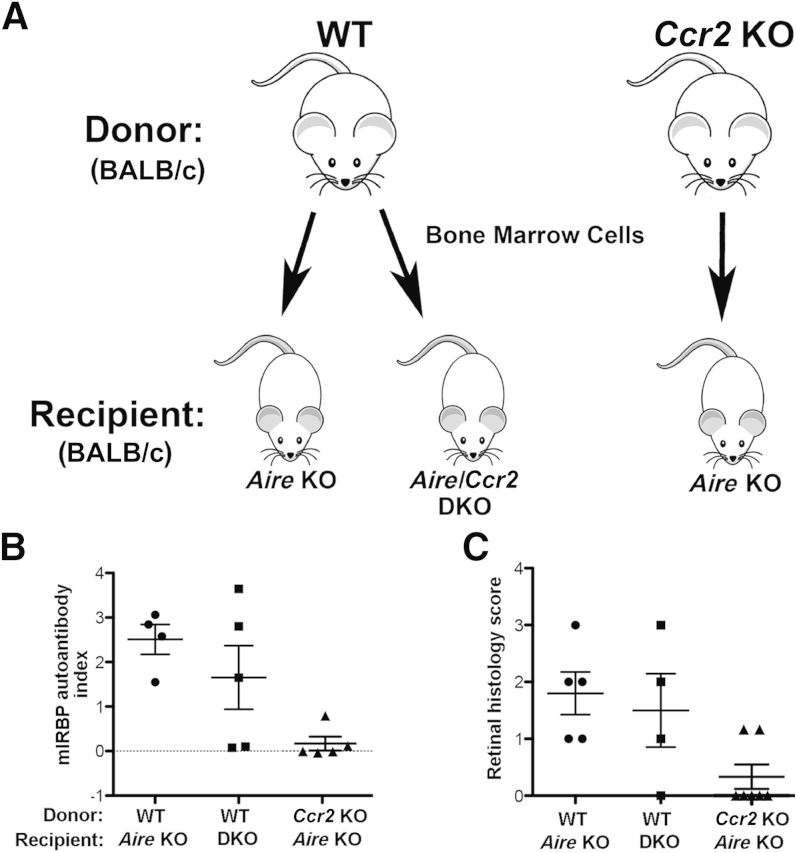
Bone-marrow chimeric mice were generated to elucidate the contribution and functional significance of CCR2 in provoking intraocular inflammation and destruction of the neuroretina. Bone marrow cells from two different donor phenotypes, WT (Aire+/+Ccr2+/+) and Ccr2 KO (Aire+/+Ccr2−/−), were depleted of CD4+ and CD8+ lymphocytes (Supplemental Figure S2) and were transferred to Aire KO (Aire−/−Ccr2+/+) or Aire–Ccr2 DKO (Aire−/−Ccr2−/−) recipients, yielding three chimera groups: WT→Aire KO, WT→Aire–Ccr2 DKO, and Ccr2 KO→Aire KO (Figure 3A). After 7 weeks of reconstitution, chimeras were screened for development of uveitis as evidenced by the presence of IRBP autoantibody in the serum. Our research group has previously identified IRBP as the dominant antigen in the eye that induces uveitis in Aire KO mice. Absence of IRBP within the thymus was sufficient to induce spontaneous autoimmune uveitis regardless of functional Aire.18 Furthermore, autoantibody-targeted organs correlated well with those targeted by infiltrates,19 and thus autoantibody reactivity against IRBP provided a reasonable disease index. The mIRBP autoantibody index was computed relative to positive control Aire KO serum and was compared among the chimera groups (Figure 3B). Mice that received WT bone marrow developed uveitis and exhibited strong autoantibody reactivity against mIRBP. In contrast, Aire KO mice that received Ccr2 KO bone marrow cells failed to develop uveitis, and demonstrated a low mIRBP autoantibody index (2.508 ± 0.335 WT→Aire KO index versus 0.473 ± 0.341 Ccr2 KO→Aire KO index; P < 0.01). Interestingly, we did not observe this difference in the WT bone marrow to Aire–Ccr2 DKO condition, suggesting a pathogenic role for CCR2+ bone marrow–derived cells in the development of autoimmune uveitis. A study in equine recurrent uveitis revealed significantly increased autoantigen IRBP levels in the uveitic retinas, which was believed to be responsible for initiating or inducing recurrent uveitis.25 The absence of IRBP autoantibodies in the serum of Aire KO recipients of Ccr2 KO bone marrow thus suggests that intolerance to the retinal autoantigen is CCR2 dependent.
Figure 3.
Development of CD4+ T cell–mediated retinitis in Aire KO mice is CCR2 dependent. A: Cells obtained from bone marrow of adult WT and Ccr2 KO mice were transferred to 4- to 6-week-old Aire KO and Aire–Ccr2 DKO recipients to generate bone-marrow chimeras. B: Serum from individual chimeric mice was analyzed for the presence of IRBP and autoantibody reactivity by radioimmunoassay at 49 days after transfer. Serum from adult Aire KO mice was used as a positive control and was assigned an mIRBP autoantibody index of 1. C: Retinal histology scores for each animal in the three chimera groups. Data are expressed as means ± SEM and as individual data points. n = 4 to 7 animals per group. Individual data points represent the average index of multiple measurements on a single animal (B) or the average score of an animal's two eyes with a minimum of three retinal sections graded per eye (C).
CCR2-Expressing Bone Marrow–Derived Cells Promote Uveitis
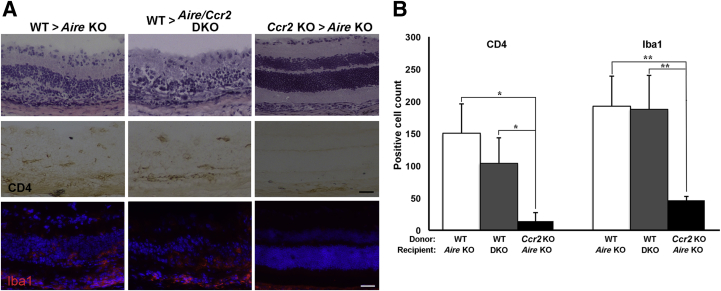
Retinal histopathology was evaluated by scoring on a disease severity scale from 0 to 3. Scores of individual chimeras from each of the three groups were plotted, and score distribution was compared across groups (Figure 3C). The retinal structure of Aire KO recipients reconstituted with Ccr2 KO bone marrow remained intact, as indicated by lower histology scores than for Aire KO recipients reconstituted in WT bone marrow (0.4 ± 0.24 versus 2 ± 0.41; P = 0.0096, t-test) (Figure 3C). Changes in retinal histology closely paralleled immune cell infiltration (Figure 4A). Retinas of Aire KO recipients reconstituted with Ccr2 KO bone marrow were nearly free of infiltrating CD4+ T cells, unlike those of Aire KO recipients of WT bone marrow (14.143 ± 13.094 versus 150.25 ± 45.728 CD4+ cells; P = 0.01). Similarly, retinal macrophage Iba1+ microglial cells were significantly reduced in Aire KO recipients of Ccr2 KO bone marrow, compared with Aire KO recipients of WT bone marrow (46 ± 6.913 versus 192.75 ± 45.885 Iba1+ cells; P < 0.01) (Figure 4B). The transfer of WT bone marrow cells to Aire–Ccr2 DKO recipients led to retinal disease reminiscent of that in untreated Aire KO mice, with significant accumulation of Iba1+ microglia and CD4+ T cells (Figure 4A). Taken together, our results strongly suggest that CCR2+ bone marrow–derived cells are key immune mediators triggering the immunopathological response associated with autoimmune-mediated uveitis.
Figure 4.
CCR2-expressing bone marrow–derived cells participate in recruitment of microglia and CD4+ T cells to the retinas of Aire KO mice. A: Representative images of H&E-stained retinal sections from the three bone-marrow chimera groups (top row). Immunostaining of retinal cross sections using antibodies directed against CD4+ T cells (middle row) and Iba1+ microglia (bottom row). B: Quantitative analysis of CD4+ T cells and Iba1+ microglia in the retina of the three bone-marrow chimera groups. Data are expressed as means ± SEM. n = 4 to 6 mice per group. ∗P < 0.05, ∗∗P < 0.01. Scale bar = 100 μm.
Discussion
Uveitis is a leading cause of blindness in the United States, but the specific immune events that provoke uveitis in the setting of autoimmune disease are largely unknown, and treatment options are limited. This is due, in part, to the lack of animal models for studying disease mechanisms and evaluating new therapies. It has become clear over recent years that existing models (eg, EAU) do not closely recapitulate the clinical features of uveitis in patients. The unmet clinical need and the high potential for significant vision-threatening consequences highlight the importance of research efforts aimed at understanding the molecular mechanisms of uveitis pathogenesis.
Macrophages play dynamic roles in the pathogenesis of autoimmune-mediated inflammatory diseases, serving as scavengers monitoring the local microenvironment in tissues under steady state or amplifying inflammation in a pathological state. Because of their heterogeneity and multifunctional capacity, macrophages are classified into two distinct subsets: M1 proinflammatory macrophages and M2 anti-inflammatory macrophages. Evidence suggests that macrophages of the proinflammatory M1 subset participate in various chronic inflammatory and autoimmune diseases, causing extensive collateral tissue damage to the host,26 and it has been reported that activation of macrophages is essential for coordinating T cell–mediated autoimmune disease in experimental uveitis.27 In the present study, we used the Aire KO mouse model to explore the functional contribution of CCR2-expressing cells as a driving force in the immunopathology of spontaneous autoimmune uveitis. Our results suggest that CCR2-expressing bone marrow–derived cells provoke the infiltration and activation of macrophages and autoreactive CD4+ T cells, which together trigger inflammation and progressive destruction of the neuroretina.
Studies of chemokine-induced monocyte recruitment in various inflammatory and autoimmune processes revealed the pathogenic role of CCR2 in disease development. In the present study, Aire KO mice with a nonfunctional Ccr2 gene (Aire–Ccr2 DKO mice) had significantly reduced infiltration of mononuclear cells into the retina, despite persistent choroidal infiltration. These findings suggest an essential role for CCR2 in the pathogenesis of organ-specific T cell–mediated and monocyte-mediated inflammatory disease, which is consistent with previous reports that CCR2 is required for efficient recruitment of peripheral monocytes to the inflamed tissues in peritonitis,5 autoimmune encephalitis,4 tuberculosis,28 and atherosclerosis.7
To decipher the mechanism whereby CCR2 elicits its pathogenic effects in autoimmune uveitis, we sought to identify the origin of CCR2-expressing cells that provoke retinal destruction in Aire KO mice. CCR2-expressing monocytes from the bone marrow are linked to inflammation and can be selectively recruited and rapidly migrate to sites of inflammation.29,30 To elucidate the functional contribution of CCR2-expressing bone marrow–derived cells in the recruitment of macrophage and CD4+ T-cell to the inflamed retina, we adoptively transferred WT or Ccr2 KO bone marrow to Aire KO mice. Reconstitution with Ccr2 KO bone marrow resulted in reduced macrophage and CD4+ T-cell infiltration of the retina and effectively rescued the retinal phenotype. Alternatively, reconstitution with WT bone marrow resulted in severe uveitis, evidenced by cellular infiltration and destruction of the retina. These results suggest that CCR2 signaling in bone marrow–derived cells directs migration of immune cells to the inflamed Aire KO retina. The role of CCR2 has recently been implicated in monocyte egress from the bone marrow, where Ccr2 knockdown inhibits the exit of monocytes from the bone marrow to the blood.31 The marked reduction of mononuclear infiltrates in the retinas of Aire KO mice reconstituted with Ccr2 KO bone marrow supports the probable theory that the vast majority of monocytes failed to exit the bone marrow compartment. It cannot be ruled out, however, that knockdown of Ccr2 in Aire KO mice reduced monocyte trafficking to the retina by suppressing their migration from the bloodstream to the inflamed tissue.
It is important to note that the CCR2-dependent migration of bone marrow–derived cells was retina specific, with disruption of CCR2 alone in the Aire KO model rescuing the retina from immune-mediated destruction. Inflammation of the ocular surface was unaltered, in that both mononuclear and CD4+ T-cell infiltration of the anterior eye remained unchanged. This was surprising, in light of a previous study in which we discovered reduced ocular surface disease in Aire KO mice after macrophage depletion with clodronate liposome; although macrophage infiltration was effectively reduced after clodronate injection, infiltrating CD4+ T cells persisted at the ocular surface.13 By contrast, in the present study we observed a compelling reduction in both mononuclear and T-cell infiltration in the Aire KO retina after the genetic disruption of CCR2 expression. The absence of infiltrating CD4+ T cells was restricted to the retinas of Aire KO mice reconstituted with Ccr2 KO bone marrow, in accord with the absence of photoreceptor-specific autoantibodies reacting against IRBP in serum collected from the same mice. DeVoss et al18 established IRBP as the primary antigen and autoreactive CD4+ T cells as the principal effector cells driving uveitis in Aire KO mice. Nonetheless, it is unclear how IRBP peptide is presented to autoreactive T cells and where the priming of IRBP-specific T cells occurs. Our present results provide essential evidence supporting the role of CCR2 in promoting autoimmunity and driving the immune-mediated processes that provoke posterior uveitis in Aire KO mice. Our findings strongly suggest the importance of CCR2-expressing bone marrow–derived cells in provoking retinal infiltration with autoreactive T cells that set off the destructive cycle of autoimmunity. Although further investigation is needed, our results support a potential functional role for CCR2 in promoting monocyte access to the retina through the blood–retina barrier.
Another immunopathological feature of autoimmune uveitis in the Aire KO model is the increased presence of Iba1+ mononuclear cells in the retina. Microglia, the mononuclear phagocytes of the retina most closely related to bone marrow–derived macrophages, are key players in inflammatory processes of the central nervous system.32 Although we could not differentiate retinal microglia from infiltrating macrophages using Iba1 and F4/80 markers, we cannot exclude the increased presence of microglia in the setting of retinal inflammatory diseases and their potential contribution to retinal tissue damage. Microglia represent the primary type of antigen-presenting cell in the retina and can become efficient antigen-presenting cells to T cells when they become activated and acquire MHC class II proteins.33,34
In vitro studies have demonstrated an essential role for MCP-1–CCR2 signaling in the recruitment of microglia to inflammatory sites in the central nervous system, including the retina.35,36 We therefore hypothesize that the rescue or attenuation of retinal infiltration in Aire KO recipients of Ccr2 KO bone marrow resulted from the failure of CCR2-deficient bone marrow cells to produce a pool of CCR2-expressing monocytes capable of crossing the blood–retina barrier and differentiating into proinflammatory macrophages that subsequently facilitate CD4+ T-cell recruitment. Alternatively, it is possible that, after infiltrating the retina, CCR2-expressing bone marrow–derived monocytes act as potent antigen-presenting cells that direct the presentation of IRBP. In either scenario, the autoreactive CD4+ T cells can, once presented with antigen, extravasate the retina and coordinate with macrophages or resident microglia to mediate tissue damage.
Precedent for the functional role of activated microglia as antigen-presenting cells capable of T-cell activation is found in multiple sclerosis.27 Moreover, activated microglia have been shown to influence the adaptive immune response through activation of naïve T cells and the production of proinflammatory cytokines that induce differentiation of effector T cells.32 Recruitment of activated microglia has also been shown to trigger photoreceptor cell death even in the absence of CD4+ T cells in models of light-induced retinal degeneration.37 Finally, another important consequence of activated resident microglia is their capacity to proliferate locally and give rise to populations of mature proinflammatory macrophages that provoke host-tissue damage.38 Although the specific identity of mononuclear cells in the Aire KO retina and whether they are infiltrating macrophages or activated microglia are topics of ongoing investigation, one could speculate that the infiltration of CCR2+ bone marrow–derived cells (most likely macrophages) triggers the activation of retinal microglia to promote retinal inflammation.
The beneficial effect of Ccr2 knockdown in preserving the neuroretina of Aire KO mice was in contrast to findings from similar studies in the EAU model, in which Ccr2 knockdown failed to attenuate retinal inflammation. The immunological events that induce disease and regulate the immune response differ considerably between the spontaneous (Aire KO) and experimentally induced (EAU) autoimmune models. For example, an interesting aspect of the EAU model is self-limiting disease that resolves with resistance to relapse. This is believed to result from the re-establishment of ocular immune privilege, which is a topic of great interest and intense investigation. Although understanding the protective regulatory immunity that occurs in EAU mice may provide important insight for deciphering the pathogenesis of immune-mediated processes, this model does not closely recapitulate the human condition of autoimmune-mediated uveitis, in which the disease is often chronic and/or recurrent.
As an alternative, Aire KO mice provide a model that mimics the clinical development of human autoimmune disease, in which the release of immune mediators, infiltration of macrophages, and the effector function of CD4+ T cells have been established in the pathogenesis of uveitis. Aire KO mice also share prominent histopathological findings noted in the retinas of patients, including inflammatory cellular infiltration, vasculitis, and loss of photoreceptors. It is worth considering that differences observed between the Aire KO and EAU models of uveitis could be, in part, background dependent, because genetic variation influences the overall penetrance of immune phenotype. Unlike the EAU model, Aire KO mice on the BALB/c background are more susceptible to autoimmune disease, compared with the C57BL/6 background,39 although both strains are susceptible to autoimmune uveitis.18 Another distinguishing feature of Aire KO mice is their development of spontaneous, tissue-specific autoimmune disease, rather than a generalized systemic immune response characteristic of some induced models. Although the specific mechanism or mechanisms whereby CCR2 governs macrophage and microglia activation, retinal inflammation, and neuroretinal damage is a topic of ongoing investigation, we believe that the use of a CCR2 antagonist to specifically target CCR2+ bone marrow–derived cells or activated microglial cells in the retina could provide a promising and directed approach to manage uveitis in patients with autoimmune disease.
Footnotes
Supported by NIH grants NEIEY016203 (N.A.M.) and EY02162 (UCSF Ophthalmology Core).
Disclosures: None declared.
Supplemental Data
Representative genotyping results of Aire and Ccr2 mutations. A: PCR products from genomic DNA were run on a 1.8% agarose gel. Expected PCR products in WT and Aire KO mice are 1150 bp and 690 bp, respectively; the presence of both bands is indicative of Aire heterozygosity. Expected PCR products in WT and Ccr2 KO mice are 450 bp and 400 bp, respectively. Reduced retinal inflammation after Ccr2 knockdown was tissue-specific. B and C: Spontaneous immune cell infiltration of the ocular surface (B) and lacrimal gland (C) was increased in Aire KO and Aire–Ccr2 DKO mice, compared with WT controls. Thus, unlike the retina, knockdown of Ccr2 did not reduce ocular surface or lacrimal gland disease severity in Aire KO mice.
Confirmation of CD4+ and CD8+ T-cell depletion by flow cytometry. Representative plots are shown for CD4-depleted, CD8-depleted and undepleted bone marrow are shown. Lymph node and thymus served as positive controls. Numbers within plots indicate the percentage of each cell type identified in the gated population.
References
- 1.Xu H., Koch P., Chen M., Lau A., Reid D.M., Forrester J.V. A clinical grading system for retinal inflammation in the chronic model of experimental autoimmune uveoretinitis using digital fundus images. Exp Eye Res. 2008;87:319–326. doi: 10.1016/j.exer.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 2.Hauck S.M., Dietter J., Kramer R.L., Hofmaier F., Zipplies J.K., Amann B., Feuchtinger A., Deeg C.A., Ueffing M. Deciphering membrane-associated molecular processes in target tissue of autoimmune uveitis by label-free quantitative mass spectrometry. Mol Cell Proteomics. 2010;9:2292–2305. doi: 10.1074/mcp.M110.001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Charo I.F., Taubman M.B. Chemokines in the pathogenesis of vascular disease. Circ Res. 2004;95:858–866. doi: 10.1161/01.RES.0000146672.10582.17. [DOI] [PubMed] [Google Scholar]
- 4.Izikson L., Klein R.S., Charo I.F., Weiner H.L., Luster A.D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR2) J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boring L., Gosling J., Chensue S.W., Kunkel S.L., Farese R.V., Jr., Broxmeyer H.E., Charo I.F. Impaired monocyte migration and reduced type 1 (Th1) cytokine responses in C-C chemokine receptor 2 knockout mice. J Clin Invest. 1997;100:2552–2561. doi: 10.1172/JCI119798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuziel W.A., Morgan S.J., Dawson T.C., Griffin S., Smithies O., Ley K., Maeda N. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci USA. 1997;94:12053–12058. doi: 10.1073/pnas.94.22.12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boring L., Gosling J., Cleary M., Charo I.F. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 8.Hickman S.E., El Khoury J. Mechanisms of mononuclear phagocyte recruitment in Alzheimer's disease. CNS Neurol Disord Drug Targets. 2010;9:168–173. doi: 10.2174/187152710791011982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dimitrijevic O.B., Stamatovic S.M., Keep R.F., Andjelkovic A.V. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 10.Mahad D.J., Ransohoff R.M. The role of MCP-1 (CCL2) and CCR2 in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE) Semin Immunol. 2003;15:23–32. doi: 10.1016/s1044-5323(02)00125-2. [DOI] [PubMed] [Google Scholar]
- 11.Tsutsumi C., Sonoda K.H., Egashira K., Qiao H., Hisatomi T., Nakao S., Ishibashi M., Charo I.F., Sakamoto T., Murata T., Ishibashi T. The critical role of ocular-infiltrating macrophages in the development of choroidal neovascularization. J Leukoc Biol. 2003;74:25–32. doi: 10.1189/jlb.0902436. [DOI] [PubMed] [Google Scholar]
- 12.Guo C., Otani A., Oishi A., Kojima H., Makiyama Y., Nakagawa S., Yoshimura N. Knockout of ccr2 alleviates photoreceptor cell death in a model of retinitis pigmentosa. Exp Eye Res. 2012;104:39–47. doi: 10.1016/j.exer.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 13.Zhou D., Chen Y.T., Chen F., Gallup M., Vijmasi T., Bahrami A.F., Noble L.B., van Rooijen N., McNamara N.A. Critical involvement of macrophage infiltration in the development of Sjögren's syndrome-associated dry eye. Am J Pathol. 2012;181:753–760. doi: 10.1016/j.ajpath.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie P., Kamei M., Suzuki M., Matsumura N., Nishida K., Sakimoto S., Sakaguchi H., Nishida K. Suppression and regression of choroidal neovascularization in mice by a novel CCR2 antagonist, INCB3344. PLoS One. 2011;6:e28933. doi: 10.1371/journal.pone.0028933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Avichezer D., Silver P.B., Chan C.C., Wiggert B., Caspi R.R. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest Ophthalmol Vis Sci. 2000;41:127–131. [PubMed] [Google Scholar]
- 16.Caspi R.R., Roberge F.G., Chan C.C., Wiggert B., Chader G.J., Rozenszajn L.A., Lando Z., Nussenblatt R.B. A new model of autoimmune disease. Experimental autoimmune uveoretinitis induced in mice with two different retinal antigens. J Immunol. 1988;140:1490–1495. [PubMed] [Google Scholar]
- 17.Dagkalis A., Wallace C., Xu H., Liebau S., Manivannan A., Stone M.A., Mack M., Liversidge J., Crane I.J. Development of experimental autoimmune uveitis: efficient recruitment of monocytes is independent of CCR2. Invest Ophthalmol Vis Sci. 2009;50:4288–4294. doi: 10.1167/iovs.09-3434. [DOI] [PubMed] [Google Scholar]
- 18.DeVoss J., Hou Y., Johannes K., Lu W., Liou G.I., Rinn J., Chang H., Caspi R.R., Fong L., Anderson M.S. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–2735. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson M.S., Venanzi E.S., Klein L., Chen Z., Berzins S.P., Turley S.J., Boehmer von H., Bronson R., Dierich A., Benoist C., Mathis D. Projection of an immunological self shadow within the thymus by the Aire protein. Science. 2002;298:1395–1401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 20.Deeg C.A., Hauck S.M., Amann B., Pompetzki D., Altmann F., Raith A., Schmalzl T., Stangassinger M., Ueffing M. Equine recurrent uveitis–a spontaneous horse model of uveitis. Ophthalmic Res. 2008;40:151–153. doi: 10.1159/000119867. [DOI] [PubMed] [Google Scholar]
- 21.Horai R., Silver P.B., Chen J., Agarwal R.K., Chong W.P., Jittayasothorn Y., Mattapallil M.J., Nguyen S., Natarajan K., Villasmil R., Wang P., Karabekian Z., Lytton S.D., Chan C.C., Caspi R.R. Breakdown of immune privilege and spontaneous autoimmunity in mice expressing a transgenic T cell receptor specific for a retinal autoantigen. J Autoimmun. 2013;44:21–33. doi: 10.1016/j.jaut.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mack M., Cihak J., Simonis C., Luckow B., Proudfoot A.E., Plachý J., Brühl H., Frink M., Anders H.J., Vielhauer V., Pfirstinger J., Stangassinger M., Schlöndorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- 23.Hawes N.L., Smith R.S., Chang B., Davisson M., Heckenlively J.R., John S.W. Mouse fundus photography and angiography: a catalogue of normal and mutant phenotypes. Mol Vis. 1999;5:22. [PubMed] [Google Scholar]
- 24.Ohsawa K., Imai Y., Sasaki Y., Kohsaka S. Microglia/macrophage-specific protein Iba1 binds to fimbrin and enhances its actin-bundling activity. J Neurochem. 2004;88:844–856. doi: 10.1046/j.1471-4159.2003.02213.x. [DOI] [PubMed] [Google Scholar]
- 25.Deeg C.A., Hauck S.M., Amann B., Kremmer E., Stangassinger M., Ueffing M. Major retinal autoantigens remain stably expressed during all stages of spontaneous uveitis. Mol Immunol. 2007;44:3291–3296. doi: 10.1016/j.molimm.2007.02.027. [DOI] [PubMed] [Google Scholar]
- 26.Sindrilaru A., Peters T., Wieschalka S., Baican C., Baican A., Peter H., Hainzl A., Schatz S., Qi Y., Schlecht A., Weiss J.M., Wlaschek M., Sunderkötter C., Scharffetter-Kochanek K. An unrestrained proinflammatory M1 macrophage population induced by iron impairs wound healing in humans and mice. J Clin Invest. 2011;121:985–997. doi: 10.1172/JCI44490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broekhuyse R.M., Huitinga I., Kuhlmann E.D., Rooijen N.V., Winkens H.J. Differential effect of macrophage depletion on two forms of experimental uveitis evoked by pigment epithelial membrane protein (EAPU), and by melanin-protein (EMIU) Exp Eye Res. 1997;65:841–848. doi: 10.1006/exer.1997.0396. [DOI] [PubMed] [Google Scholar]
- 28.Peters W., Scott H.M., Chambers H.F., Flynn J.L., Charo I.F., Ernst J.D. Chemokine receptor 2 serves an early and essential role in resistance to Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 2001;98:7958–7963. doi: 10.1073/pnas.131207398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swirski F.K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J.L., Kohler R.H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T.R., Libby P., Weissleder R., Pittet M.J. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geissmann F., Jung S., Littman D.R. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 31.Tsou C.L., Peters W., Si Y., Slaymaker S., Aslanian A.M., Weisberg S.P., Mack M., Charo I.F. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saijo K., Glass C.K. Microglial cell origin and phenotypes in health and disease. Nat Rev Immunol. 2011;11:775–787. doi: 10.1038/nri3086. [DOI] [PubMed] [Google Scholar]
- 33.Hickey W.F., Kimura H. Perivascular microglial cells of the CNS are bone marrow-derived and present antigen in vivo. Science. 1988;239:290–292. doi: 10.1126/science.3276004. [DOI] [PubMed] [Google Scholar]
- 34.Mack C.L., Vanderlugt-Castaneda C.L., Neville K.L., Miller S.D. Microglia are activated to become competent antigen presenting and effector cells in the inflammatory environment of the Theiler's virus model of multiple sclerosis. J Neuroimmunol. 2003;144:68–79. doi: 10.1016/j.jneuroim.2003.08.032. [DOI] [PubMed] [Google Scholar]
- 35.Conductier G., Blondeau N., Guyon A., Nahon J.L., Rovère C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010;224:93–100. doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 36.Jiang X.S., Ni Y.Q., Liu T.J., Zhang M., Ren H., Jiang R., Huang X., Xu G.Z. CCR2 overexpression promotes the efficient recruitment of retinal microglia in vitro. Mol Vis. 2012;18:2982–2992. [PMC free article] [PubMed] [Google Scholar]
- 37.Zeng H., Green W., Tso M. Activation of microglia in human retinitis pigmentosa (abstract) ARVO Meet Abstr. 2005;46:513. [Google Scholar]
- 38.Ajami B., Bennett J.L., Krieger C., Tetzlaff W., Rossi F.M.V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10:1538–1543. doi: 10.1038/nn2014. [DOI] [PubMed] [Google Scholar]
- 39.Jiang W. Modifier loci condition autoimmunity provoked by Aire deficiency. J Exp Med. 2005;202:805–815. doi: 10.1084/jem.20050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative genotyping results of Aire and Ccr2 mutations. A: PCR products from genomic DNA were run on a 1.8% agarose gel. Expected PCR products in WT and Aire KO mice are 1150 bp and 690 bp, respectively; the presence of both bands is indicative of Aire heterozygosity. Expected PCR products in WT and Ccr2 KO mice are 450 bp and 400 bp, respectively. Reduced retinal inflammation after Ccr2 knockdown was tissue-specific. B and C: Spontaneous immune cell infiltration of the ocular surface (B) and lacrimal gland (C) was increased in Aire KO and Aire–Ccr2 DKO mice, compared with WT controls. Thus, unlike the retina, knockdown of Ccr2 did not reduce ocular surface or lacrimal gland disease severity in Aire KO mice.
Confirmation of CD4+ and CD8+ T-cell depletion by flow cytometry. Representative plots are shown for CD4-depleted, CD8-depleted and undepleted bone marrow are shown. Lymph node and thymus served as positive controls. Numbers within plots indicate the percentage of each cell type identified in the gated population.