Abstract
To conditionally inactivate genes in the retinal pigment epithelium (RPE) transgenic mouse strains have been developed, in which Cre recombinase (Cre) expression is driven by an RPE-specific gene promoter. The RPE is a quiescent epithelium, and continuous expression of Cre could affect its function. Here, we tested the hypothesis that continuous postnatal Cre expression in the RPE may lead to cellular abnormalities, which may depend on both age and Cre gene dosage. We therefore examined the eyes of homozygous and heterozygous VMD2-Cre mice at various ages. In VMD2-Cre heterozygous mice variable progressive age-dependent RPE abnormalities were noticed, including attenuation of phalloidin and cytoplasmic active β-catenin staining, reduced cell size, and loss of the typical honeycomb pattern of RPE morphology in those RPE cells that stained for Cre. These morphological RPE abnormalities were not noticed in Cre-negative RPE cells in VMD2-Cre or age-matched control mice. In addition, an abnormal number and morphology of cell nuclei were noticed in a subset of Cre-expressing RPE cells in aged heterozygous VMD2-Cre mice, whereas more severe nuclear abnormalities were observed already in young homozygous VMD2-Cre mice. Thus, continuous postnatal expression of Cre causes abnormalities in the RPE in an age- and Cre gene dosage-dependent manner, which needs to be considered in the interpretation of gene targeting studies in the RPE.
The retinal pigment epithelium (RPE) is a nonregenerative single layer epithelium in the adult eye that is essential for the visual cycle and photoreceptor function.1 Degenerative changes in the RPE affect retinal function and cause several ocular diseases. The fact that this epithelium does not continuously regenerate postnatally could make this epithelium more susceptible to accumulative genetic alterations that affect its overall function and morphology.
Because of its critical role in vision and for the maintenance of photoreceptor function, understanding biological processes in the RPE are fundamentally important. Conditional gene inactivation studies have been undertaken to determine RPE-specific functions of certain genes. Several transgenic mouse strains have been developed that express Cre recombinase (Cre) in the RPE driven by a gene promoter that is active in this epithelium. These mice allow targeted inactivation of a gene in the RPE when crossed with mice that carry floxed alleles for the gene of interest.2–7 Most RPE-specific Cre strains that have been reported show an incomplete Cre expression pattern in the RPE, with patches of Cre-positive RPE cells adjacent to Cre-negative RPE cells. Thus, it is important to correlate phenotypic changes in the RPE with the Cre expression pattern. Furthermore, the extent of Cre expression is variable, and it has been reported that mice of the same transgenic strain show a high degree of variability of Cre expression, with the observation of complete silencing of Cre expression in some mice that carry the Cre allele.2 Cre-mediated RPE toxicity has been reported in Trp1-Cre transgenic mice,3,8 raising the question whether this is a strain-specific observation or whether Cre expression in the RPE results in toxicity as a general phenomenon that may depend on the level and duration of cellular Cre expression in the RPE. The Trp1-Cre strain shows RPE-specific Cre expression already at embryonic day 10.5, which suggests that RPE-specific abnormalities in these mice may result from developmental abnormalities due to Cre expression at the onset of RPE morphogenesis. Whether Cre expression in the RPE postnatally would affect its viability and function is not known.
In several other cell types Cre-mediated cell toxicity has been reported, with degenerative changes that correlated to the degree of Cre expression.9–11 This Cre toxicity may result from genetic alterations due to sequences in the genome that may be targeted by Cre. Cre catalyzes recombination between two loxP recognition sites, which is characterized by a short recognition sequence.12,13 Reports have shown that Cre expression can lead to DNA cleavage in mammalian cells at sites with homology to the loxP recognition sites (pseudo-loxP sites), which results in genetic alterations in mammalian cells that can significantly affect cell function.14,15
It is likely that the RPE is particularly susceptible to Cre-mediated toxicity, because it is a nonregenerative epithelium that would accumulate genetic changes with progressive age. To test this hypothesis, VMD2-Cre mice (also named BEST1-Cre mice) that express Cre in the RPE after postnatal day 10 were examined for Cre-mediated RPE abnormalities.2 If Cre expression by itself leads to phenotypic changes in the RPE, we hypothesized that these changes would increase with progressive age and the amount of Cre expression in the RPE. Indeed, we show that RPE abnormalities in VMD2-Cre mice progressed with age and were more pronounced in mice that were homozygous for the Cre allele than in heterozygous mice. These findings have important implications for conditional inactivation studies in the RPE, and they suggest that the RPE is susceptible to Cre-mediated cellular dysfunction. Phenotypes due to RPE-specific conditional inactivation of a target gene need to be interpreted in the context of Cre-mediated cellular abnormalities, and in all studies mice heterozygous for Cre with age-matched heterozygous Cre control mice should be used. The conclusions that can be made in RPE-specific gene inactivation studies that use these mice, and likely with other RPE-specific Cre strains as well, need to carefully consider the observed variability of RPE abnormalities even among Cre heterozygous mice.
Materials and Methods
Animals
The generation of VMD2-Cre mice (BEST1-Cre mice) was previously reported.2 These mice were maintained on the original background and crossed with either C57BL/6J or VMD2-Cre mice to generate either homozygous or heterozygous VMD2-Cre mice. The rd1 and rd8 mutation was excluded in these mice. Mice heterozygous and homozygous for VMD2-Cre and control littermates were compared up to 13 months of age. Experimental groups of mice at ages 1, 4, or 7 months of age were used for quantitative analyses (n = 3 mice per group). In addition, aged wild-type C57BL/6J mice up to 20 months of age were examined. For all animal studies institutional approval by Massachusetts General Hospital was granted. Zygosity for Cre was determined by real-time quantitative PCR (Real Time Laboratories, Carrollton, TX).
Immunolabeling, Microscopy, and Quantification of RPE Abnormalities
Eyes were enucleated and fixed in 4% paraformaldehyde overnight at 4°C or for shorter durations at room temperature. The observed RPE abnormalities occurred independently of the fixation protocol. Because Cre expression occurs in patches in VMD2-Cre mice, Cre-negative RPE cells served as an internal control for the observed abnormalities and the immunolabeling experiments. In addition, for each experimental mouse group littermate wild-type mice were used as controls. Eyes were dissected along the ora serrata to remove the anterior chamber, iris, and lens. The retina was removed, and the remaining posterior eyecup (choroid/RPE tissue) was used for subsequent analysis. For choroidal flat mount staining, posterior eyecups (choroid/RPE tissue without retina) were permeabilized in 0.5% Triton X and subsequently blocked with 5% goat serum. Primary antibodies were incubated overnight at 4°C. The following primary antibodies were used: mouse anti-Cre (clone 2D8; Millipore, Billerica, MA), monoclonal anti-mouse non-phospho (active) β-catenin (Ser33/37/Thr41; D13A1; Cell Signaling Technology Inc, Danvers, MA), and Alexa 488-conjugated phalloidin (Life Technologies). Secondary antibodies used were Alexa 555 or Alexa 647 (Life Technologies, Carlsbad, CA). Colabeling experiments as well as single-labeling experiments were performed, and experiments that omitted either the primary or the secondary antibodies were used to distinguish immunolabeling from autofluorescence. Nuclei were labeled with DAPI (Life Technologies). After immunolabeling posterior eyecups were flat mounted on cover slides with four radial incisions, and Prolong Gold mounting medium was used (Life Technologies). Images were acquired with a Nikon Eclipse confocal microscope (Nikon, Melville, NJ) or a Zeiss Axiovert epifluorescence microscope (Carl Zeiss, Thornwood, NY) at ×10 and ×20 magnification.
At least five images of choroidal flat mounts were acquired from each eye to cover the entire fundus. From representative microscopic images taken at ×20 magnification, 20 Cre-positive and Cre-negative RPE cells were randomly selected and analyzed for cell size and cytoplasmic active β-catenin immunolabeling intensity. Cell size was determined by outlining cell membranes of RPE cells by using Axiovision software version 4.8.2. Average cell size per mouse was measured, and statistically significant differences in each experimental group were determined with a two-tailed Student’s t-test.
A scoring system was established to determine cytoplasmic active β-catenin levels. Normal cytoplasmic active β-catenin levels as seen in Cre-negative RPE cells were scored 3.0, whereas reduced staining for cytoplasmic active β-catenin was scored 2.0, and absence of cytoplasmic active β-catenin labeling was scored 1.0. Statistically significant differences in each experimental group were determined with a two-tailed Student’s t-test. P values < 0.05 were considered statistically significant.
Results
Progressive Age-Dependent Abnormalities in the RPE in Heterozygous VMD2-Cre Mice
We hypothesized that continuous chronic exposure of RPE cells to Cre would result in cellular abnormalities and would thus increase with progressive age. Therefore, we examined whether heterozygous VMD2-Cre mice showed abnormalities of the RPE with progressive age. We found that a sensitive method to analyze morphological abnormalities in the RPE was by labeling posterior eyecups (after removing the retina) with fluorescein-labeled phalloidin, which labeled the actin cytoskeleton of cells and highlighted the cell membranes of RPE cells. Fluorescein-phalloidin labeling showed regular honeycomb pattern morphology of RPE cells and uniform cytoplasmic labeling in control mice. In the labeling experiments of posterior eyecups we also included immunolabeling for active β-catenin, which showed strong staining of the RPE cell membranes (because β-catenin is a component of the adherens junctions of RPE cells) and in addition uniform labeling of the cytoplasm. Progressive aging did not affect the regular RPE cell morphology, phalloidin staining, and active β-catenin labeling (control mice up to 20 months old were examined) (Figure 1E).
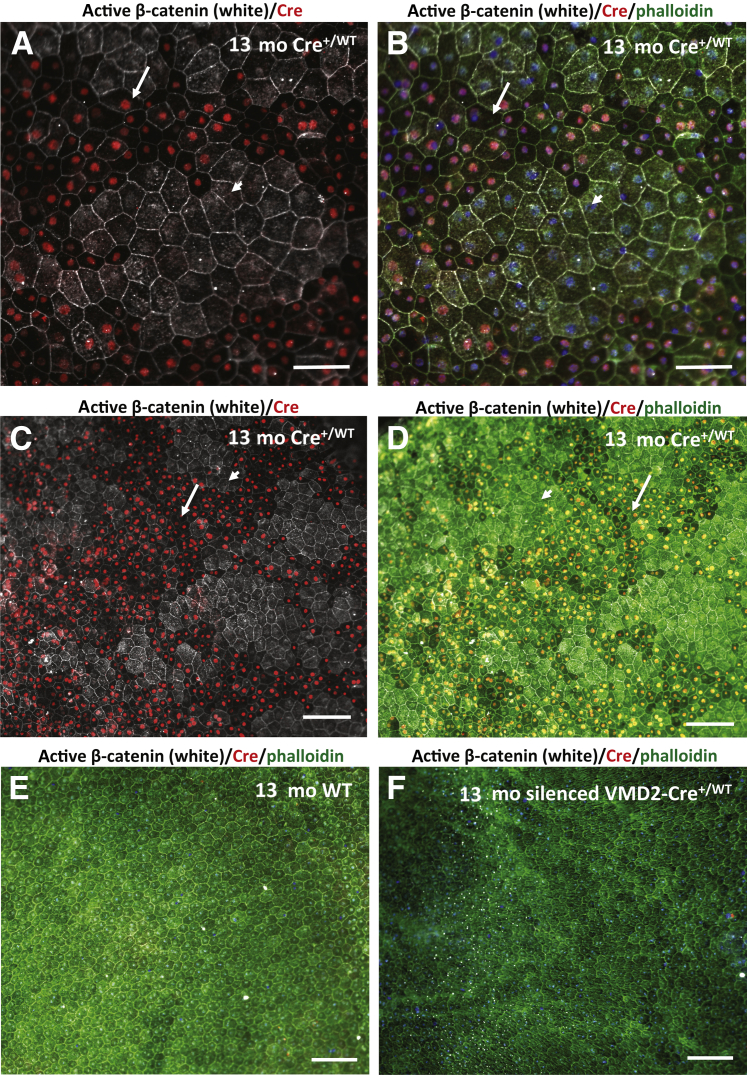
Figure 1.
Cre expression in the RPE correlates with abnormal RPE cell morphology and attenuation of cytoplasmic β-catenin and phalloidin staining in VMD2-Cre+/WT mice. A: Immunolabeling of a choroidal flat mount in a 13-month-old VMD2-Cre+/WT mouse shows that Cre-expressing (Cre+/WT) cells (long arrow) are smaller and have attenuated cytoplasmic active β-catenin labeling, compared with adjacent Cre-negative cells (short arrow). B: Cre-expressing (Cre+/WT) (long arrow) cells also show reduced phalloidin labeling compared with Cre-negative cells (short arrow) in the same choroidal flat mount. C and D: Smaller magnification images from same choroidal flat mount indicate that Cre expression in RPE cells is associated with reduced phalloidin and cytoplasmic active β-catenin staining and with smaller RPE cell size (long arrows), compared with Cre-negative RPE cells (short arrows). E: Choroidal flat mount staining for active β-catenin, phalloidin, and Cre in a 13-month-old wild-type (Cre-negative) age-matched littermate shows normal RPE cells. F: In a 13-month-old VMD2-Cre+/WT mouse with silenced Cre expression in the RPE (no Cre expression was detected by immunolabeling for Cre), no RPE abnormalities were found, indicating that RPE abnormalities correlate with Cre expression in this mouse strain. Blue nuclei are because of DAPI staining. Scale bars: 50 μm (A and B); 100 μm (C–F).
We used posterior eyecups from groups of heterozygous VMD2-Cre mice that were 1, 4, 7, or 13 months old for phalloidin and active β-catenin labeling. To correlate morphological changes of RPE cells with Cre expression, we included immunolabeling for Cre in these experiments (strongly labeling Cre-positive nuclei in VMD2-Cre mice).
These VMD2-Cre mice show Cre expression at approximately postnatal day 10; thus, RPE cells of 1-month-old VMD2-Cre mice were exposed to Cre only for a few weeks. In these 1-month-old heterozygous VMD2-Cre mice (Cre+/WT) we did not observe significant changes in RPE cell morphology, and phalloidin and active β-catenin labeling were not significantly reduced compared with adjacent Cre-negative cells (or age-matched control mice) (Figure 2, A–C, E, and G).
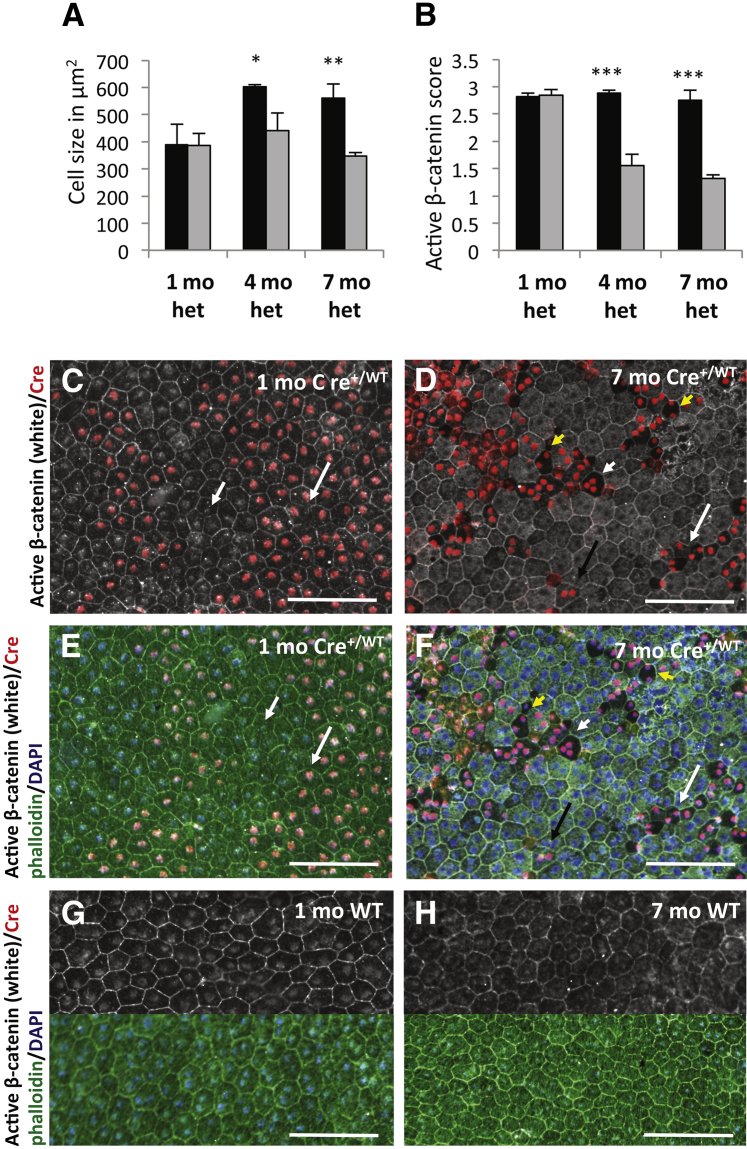
Figure 2.
Progressive age-dependent RPE abnormalities in heterozygous VMD2-Cre mice (Cre+/WT). A: Quantitative analysis shows that RPE cell size of Cre-expressing (Cre+/WT; gray bars) cells compared with Cre-negative (black bars) cells is unchanged in 1-month-old heterozygous VMD2-Cre mice (het), whereas Cre-expressing (Cre+/WT) RPE cells in 4- and 7-month-old heterozygous VMD2-Cre mice show a significant reduction in cell size. B: Scoring cytoplasmic active β-catenin labeling in Cre-expressing (Cre+/WT) and Cre-negative RPE cells reveals a significant attenuation of cytoplasmic active β-catenin with progressive age in heterozygous VMD2-Cre mice. C: One-month-old heterozygous VMD2-Cre mice show Cre-expressing (Cre+/WT) RPE cells with normal cell morphology and normal cytoplasmic active β-catenin staining (long arrow), compared with adjacent Cre-negative RPE cells (short arrow). D: A representative image of a choroidal flat mount from a 7-month-old heterozygous VMD2-Cre mouse (Cre+/WT). Although Cre-negative cells show normal cell size and honeycomb morphology with uniform cytoplasmic active β-catenin staining, Cre-expressing (Cre+/WT) cells show loss of cytoplasmic active β-catenin staining and atypically formed small RPE cells (white arrow). Notably, some Cre-expressing (Cre+/WT) cells appear normal (black arrow). A subset of Cre-expressing (Cre+/WT) cells show a displacement of cell nuclei toward the periphery, opposed to the normal central or paracentral localization of RPE cell nuclei (yellow short arrows). Although normal RPE cells show one or two cell nuclei, few Cre-expressing (Cre+/WT) cells show three nuclei (short white arrow). E and F: Phalloidin labeling of the flat mounts shown in C and D. Although 1-month-old heterozygous VMD2-Cre mice show normal phalloidin labeling of Cre-expressing (Cre+/WT) cells (E, long arrow) as seen in adjacent Cre-negative cells (E, short arrow), 7-month-old heterozygous VMD2-Cre mice show loss of phalloidin labeling in Cre-expressing (Cre+/WT) cells, while adjacent Cre-negative RPE cells show normal phalloidin labeling (arrows as in D). G and H: Age-matched 1- or 7-month-old wild-type control mice do not show these RPE abnormalities and instead reveal a uniform labeling pattern for phalloidin (lower part of images) and cytoplasmic active β-catenin (upper part of images), which is maintained with increasing age of the mice. Data are expressed as means ± SD. n = 3 mice per group (A and B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars: 100 μm.
However, in heterozygous VMD2-Cre mice that were 4 or 7 months old, Cre-positive (CreWT/+) RPE cells showed significantly altered cell morphology with a progressive age-dependent reduction of cell size, reduced phalloidin labeling, and diminished cytoplasmic active β-catenin labeling (Figure 2, A, B, D, and F). Loss of cytoplasmic active β-catenin labeling was the first observed change and the most sensitive method to determine Cre-induced RPE abnormalities, and it was used to quantitate age-dependent Cre-mediated cell abnormalities (Figure 2B). In contrast to 1-month-old heterozygous VMD2-Cre mice that showed normal labeling for cytoplasmic active β-catenin, 4- and 7-month-old mice showed a highly significant and progressive loss of cytoplasmic active β-catenin labeling in Cre-positive (CreWT/+) cells compared with Cre-negative cells. In age-matched control mice no abnormalities of RPE cell morphology, phalloidin labeling, or cytoplasmic active β-catenin labeling was observed (Figure 2, G and H). Consistent with normal electroretinograms in these mice, no major morphological photoreceptor abnormalities were detected (not shown).2
Importantly, some Cre-expressing (CreWT/+) RPE cells showed normal cell morphology and phalloidin and cytoplasmic active β-catenin labeling, indicating a degree of variability of RPE cell abnormalities in VMD2-Cre mice (Figure 2, D and F). Notably, in a subset of Cre-expressing (CreWT/+) RPE cells in 7-month-old VMD2-Cre mice we observed atypical nuclei (Figure 2F). Although normal RPE cells show either one or two nuclei that are located in a central or paracentral position within RPE cells, some Cre-expressing (CreWT/+) RPE cells showed either three nuclei or an atypical localization of nuclei closer toward the cell membranes (Figure 2, D and F).
The observed changes in heterozygous VMD2-Cre mice became even more apparent with advanced age. Choroidal flat mount staining in 13-month-old heterozygous VMD2-Cre mice consistently showed that Cre-expressing (CreWT/+) RPE cells, compared with Cre-negative RPE cells, have a smaller cell size and showed weaker staining for phalloidin and active β-catenin (Figure 1, A–D). These changes were not observed in eyes from control wild-type littermate mice or VMD2-Cre heterozygous mice with silenced Cre expression (no Cre-labeling was observed) (Figure 1, E and F), further confirming that the observed abnormalities are due to cellular Cre expression in the RPE.
Homozygosity for Cre Results in Highly Irregular RPE Cell Nuclei
Our observation that cellular abnormalities of the RPE in heterozygous VMD2-Cre mice increase with age suggested that long-term expression of Cre results in progressive accumulation of cytopathic effects. This suggests that the amount and duration of Cre expression was directly correlated to the RPE abnormalities in VMD2-Cre mice. Thus, we speculated that mice that are homozygous for VMD2-Cre (VMD2-Cre+/+) should manifest RPE abnormalities earlier or with a higher degree of severity than age-matched heterozygous VMD2-Cre (VMD2-Cre+/WT) mice.
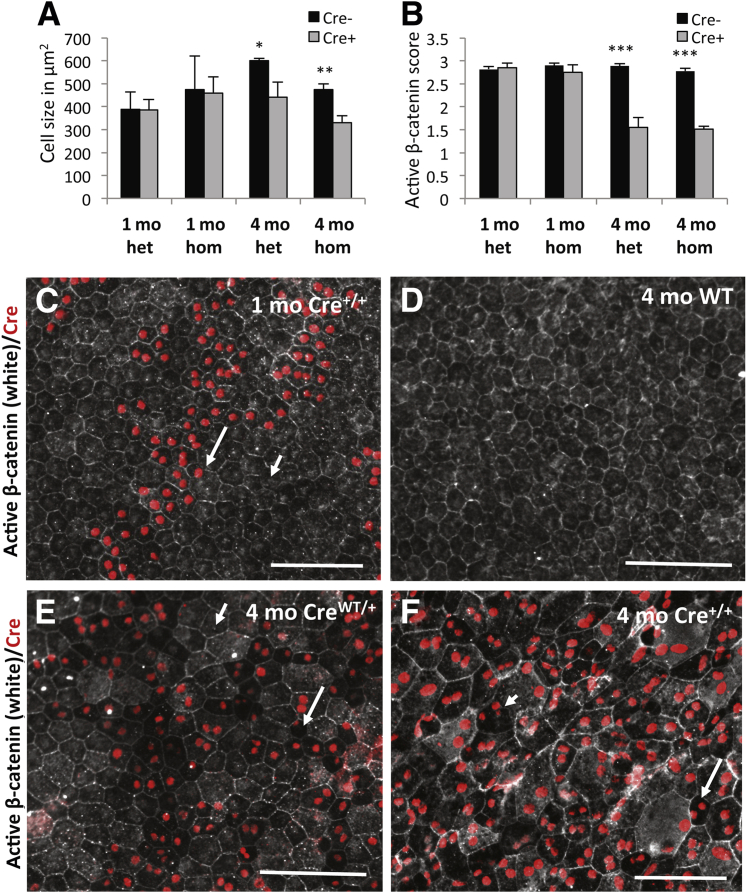
We used posterior eyecups of 1- and 4-month-old homozygous VMD2-Cre mice and compared them directly to age-matched heterozygous and control mice in our labeling experiments for phalloidin, Cre, and active β-catenin (Figure 3, A–F). As with heterozygous VMD2-Cre mice, Cre-positive RPE cells in 1-month-old homozygous VMD2-Cre mice did not show a significant reduction of RPE cell size or cytoplasmic active β-catenin labeling compared with Cre-negative RPE cells (Figure 3, A–C). However, similarly to heterozygous VMD2-Cre mice, 4-month-old homozygous VMD2-Cre mice showed significantly reduced cell size and cytoplasmic active β-catenin labeling of Cre-positive RPE cells compared with Cre-negative RPE cells (Figure 3, A and B). Notably, cell size and cytoplasmic active β-catenin labeling was not significantly different in Cre-positive RPE cells between heterozygous or homozygous VMD2-Cre mice (P > 0.05), indicating that the presence of a single Cre allele is already sufficient to reduce RPE cell size and cytoplasmic active β-catenin. Importantly, although only a few nuclei in Cre-expressing (CreWT/+) RPE cells appeared abnormal in number, shape, or intracellular localization in 4- or 7-month-old heterozygous VMD2-Cre mice (Figures 2D and 3E), 3- or 4-month-old homozygous mice already showed highly increased RPE cell nuclear abnormalities (Figures 3F and 4, A–D). Although no more than three nuclei were observed in 4- or 7-month-old heterozygous Cre+ RPE cells (Figures 2D and 4E), Cre-expressing (Cre+/+) RPE cells in 4-month-old homozygous VMD2-Cre mice showed often more than three nuclei with highly irregular size, shape, and intracellular distribution (Figure 4, A–D). Some RPE cells in homozygous VMD2-Cre mice showed ring-like structured nuclei that stained for Cre (Figure 4C) or several Cre-negative DAPI-labeled nuclei within the same cell that contained Cre-positive DAPI-labeled nuclei (Figure 4D).
Figure 3.
RPE abnormalities increase in homozygous VMD2-Cre (Cre+/+) mice. A and B: Like heterozygous VMD2-Cre mice (het), 1-month-old homozygous VMD2 Cre mice (hom) show no statistically significant reduction in cell size or cytoplasmic active β-catenin labeling. However, 4-month-old homozygous VMD2-Cre mice show a significantly reduced cell size and loss of cytoplasmic active β-catenin labeling, seen as well in 4-month-old heterozygous VMD2-Cre mice. C: Cre expression in 1-month-old homozygous VMD2-Cre (Cre+/+) mice does not cause marked morphological RPE abnormalities and shows normal active β-catenin staining (long arrow points to an RPE cell with normal appearance and cytoplasmic active β-catenin labeling), compared with adjacent Cre-negative RPE cells (short arrow). D: Four-month-old control wild-type mouse shows normal active β-catenin staining and RPE cell size. E: In 4-month-old heterozygous VMD2-Cre mice (CreWT/+) RPE cells that stain for Cre often show reduced cell size and staining for active β-catenin (long arrow), whereas Cre-negative cells appear normal (short arrow). F: Four-month-old homozygous VMD2-Cre mice (Cre+/+) show highly irregular Cre-expressing RPE cells with variable cell size. Some Cre-expressing (Cre+/+) RPE cells show loss of cytoplasmic active β-catenin labeling (long arrow), and some Cre+/+ RPE cells show an increased number of cell nuclei with irregular size and form (short arrow). Data are expressed as means ± SD, n = 3 mice per group (A and B). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Scale bars: 100 μm.
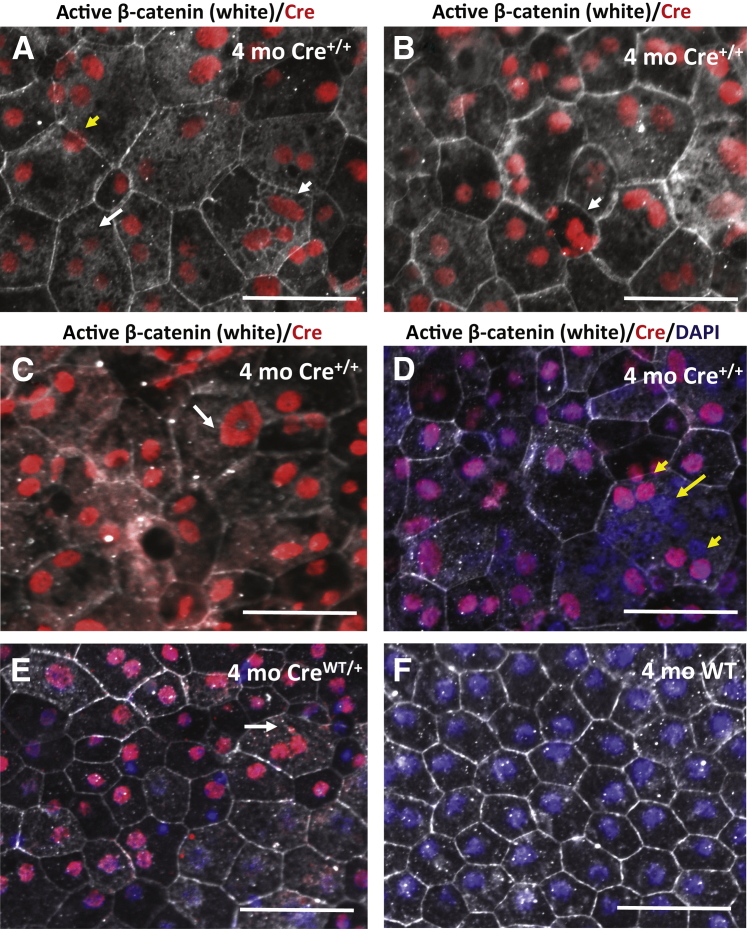
Figure 4.
Nuclear RPE abnormalities increase in homozygous VMD2-Cre (Cre+/+) mice. A–D: Higher magnification images of RPE cells from a 4-month-old homozygous VMD2-Cre mouse (Cre+/+). A: Increased numbers of cell nuclei in Cre-expressing Cre+/+ RPE cells (short white arrow) are observed much more frequently than in aged heterozygous VMD2-Cre mice, and some cells have more than three nuclei (long white arrow) (which was not observed in heterozygous VMD2-Cre mice). Cre+/+ cell nuclei are often atypically displaced in a peripheral intracellular localization (yellow arrow). B: Some Cre+/+ RPE cells in 4-month-old homozygous VMD2-Cre mice show a large number of cell nuclei with highly variable size and form (white arrow). C: Atypical forms of Cre+/+ nuclei are observed in RPE cells of these homozygous VMD2-Cre mice, some appearing as ring-like nuclei (white arrow). D: DAPI staining binds to DNA and labels nuclei. In irregular RPE cells with an increased number of Cre-expressing Cre+/+ cell nuclei (short yellow arrows) additional DAPI+ nuclei that were negative for Cre (long yellow arrow) are observed. E: In age-matched 4-month-old heterozygous VMD2-Cre mice (CreWT/+) only a few cells show more than two nuclei or other nuclear abnormalities (arrow). F: These nuclear changes are not observed in wild-type control mice of the same age. Scale bars: 50 μm.
These highly irregular nuclear abnormalities were observed in young (3 to 4 months old) homozygous VMD2-Cre mice (Cre+/+) but not even in 13-month-old heterozygous VMD2-Cre mice (Figure 1, A–D), suggesting that increased Cre-gene dosage in homozygous mice causes more severe nuclear abnormalities in the RPE. Four-month-old heterozygous VMD2-Cre mice showed only a few nuclear abnormalities with some RPE cells having three nuclei (Figure 4E), whereas these nuclear abnormalities were not observed in wild-type control mice (Figure 4F).
Discussion
Expression of Cre in the RPE in VMD2-Cre mice results in age-dependent progressive abnormalities of the RPE, and nuclear abnormalities are more pronounced in mice that are homozygous for the Cre allele than in heterozygous mice. This observation is consistent with a role of Cre in inducing cellular abnormalities in the RPE that depend on the amount of Cre and the duration of exposure of the cell to this recombination-catalyzing enzyme. Similarly, severe morphological abnormalities in RPE cells have been reported for Trp1-Cre mice that express Cre in the RPE from embryonic day 10.5 on.3,8
In VMD2-Cre mice Cre expression occurs postnatally (approximately postnatal day 10), whereas in Trp1-Cre mice Cre expression has been reported to start at approximately embryonic day 10.5, which may explain the less severe Cre-mediated RPE abnormalities in heterozygous VMD2-Cre mice compared with those observed in Trp1-Cre mice. The occurrence of RPE defects in both Trp1-Cre mice and VMD2-Cre mice, and the increasing RPE abnormalities with age and Cre gene dosage in VMD2-Cre mice, suggests that these RPE abnormalities are a direct consequence of the amount and duration of Cre expression in the RPE and may be a general phenomenon that is not limited to a single RPE-specific Cre strain.
Notably, RPE changes were already noticed in 3- to 4-month-old mice that were heterozygous for Cre (VMD2-Cre+/WT mice), and these changes varied among different mice of the same genotype. This observation indicates that phenotypes in mice with conditional inactivation of genes in the RPE by using the Cre/loxP system must be carefully compared with age-matched heterozygous Cre control mice, and phenotypic changes must be evaluated in this context. Overall, the variability of Cre-mediated RPE abnormalities in VMD2-Cre mice need to be considered when drawing conclusions from conditional gene targeting studies in the RPE, particularly when the observed phenotypes are mild or when analyzing aged mice.
Several studies have investigated Cre-induced cytopathic effects.9,14,16 For example, Cre expression in basal keratinocytes in mouse epidermis was shown to induce DNA damage and tetraploidy in keratinocytes.16 In that same study it was shown that Cre expression in a chromosomally stable cell line (HCT116 cells) induced DNA damage, genomic instability and tetraploidy in vitro. Notably, homozygosity for Cre in basal keratinocytes (K5Cre+/+ mice) was associated with an accelerated formation of skin cancer in a chemical model of skin carcinogenesis.10 The skin phenotype with abnormal whiskers and hair coat (and abnormal mammary glands with impaired lactation) was noticed only in homozygous K5-Cre mice but not in heterozygotes. This observation is consistent with a dose-dependent cytopathic effect of Cre.
Previously, it was shown that Cre toxicity can develop in a dose-dependent manner and in the absence of loxP target sites. These Cre-induced abnormalities lead to growth arrest, chromosomal abnormalities, and cell death in vitro.17 Notably, generation of Cre mutants that lack the ability to cleave DNA showed no cytotoxic effects in vitro.17 Use of a self-excising retroviral vector that allowed limiting the duration and intensity of Cre expression resulted also in reduced cell toxicity.17
It has been speculated that Cre causes these cellular abnormalities due to recombinations that occur at pseudo-loxP sites in the mammalian genome and lead to an accumulation of chromosomal aberrations.11,14,18 Notably, cellular abnormalities induced by Cre occur after extended periods of Cre activity; thus, there seems to be a more pronounced cytotoxic effect of Cre with increased dose and duration of Cre exposure.9
These previous studies are consistent with the observation of Cre-induced abnormalities in RPE cells in VMD2-Cre mice, because this epithelium is a postmitotic nonregenerative epithelium, and continuous Cre expression in the RPE would thus be predicted to result in cellular abnormalities in an age- and gene dosage-dependent manner. Particularly, the observation of highly irregular RPE cell nuclei in homozygous VMD2-Cre mice suggests that one mechanism through which Cre induces cytopathic changes in the RPE of these mice may indeed be by causing DNA damage and polyploidy.
A previous study suggested that short-term exposure to Cre can significantly reduce its cytopathic effects.19 Self-deleting Cre expression vectors or inducible Cre mouse strains (eg, tamoxifen or doxycycline-inducible Cre expression systems) may permit temporal control of Cre expression that result in recombination at loxP sites, while limiting the unwanted cytopathic effects of prolonged Cre expression in mammalian cells.
Footnotes
This work was supported by NIH National Eye Institute grant R01-EY019297 (A.G.M.).
Disclosures: None declared.
References
- 1.Kiser P.D., Golczak M., Maeda A., Palczewski K. Key enzymes of the retinoid (visual) cycle in vertebrate retina. Biochim Biophys Acta. 2012;1821:137–151. doi: 10.1016/j.bbalip.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iacovelli J., Zhao C., Wolkow N., Veldman P., Gollomp K., Ojha P., Lukinova N., King A., Feiner L., Esumi N., Zack D.J., Pierce E.A., Vollrath D., Dunaief J.L. Generation of Cre transgenic mice with postnatal RPE-specific ocular expression. Invest Ophthalmol Vis Sci. 2011;52:1378–1383. doi: 10.1167/iovs.10-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mori M., Metzger D., Garnier J.M., Chambon P., Mark M. Site-specific somatic mutagenesis in the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2002;43:1384–1388. [PubMed] [Google Scholar]
- 4.Aydin I.T., Beermann F. A mart-1::Cre transgenic line induces recombination in melanocytes and retinal pigment epithelium. Genesis. 2011;49:403–409. doi: 10.1002/dvg.20725. [DOI] [PubMed] [Google Scholar]
- 5.Le Y.Z., Zheng W., Rao P.C., Zheng L., Anderson R.E., Esumi N., Zack D.J., Zhu M. Inducible expression of cre recombinase in the retinal pigmented epithelium. Invest Ophthalmol Vis Sci. 2008;49:1248–1253. doi: 10.1167/iovs.07-1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Longbottom R., Fruttiger M., Douglas R.H., Martinez-Barbera J.P., Greenwood J., Moss S.E. Genetic ablation of retinal pigment epithelial cells reveals the adaptive response of the epithelium and impact on photoreceptors. Proc Natl Acad Sci U S A. 2009;106:18728–18733. doi: 10.1073/pnas.0902593106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guyonneau L., Rossier A., Richard C., Hummler E., Beermann F. Expression of Cre recombinase in pigment cells. Pigment Cell Res. 2002;15:305–309. doi: 10.1034/j.1600-0749.2002.02039.x. [DOI] [PubMed] [Google Scholar]
- 8.Thanos A., Morizane Y., Murakami Y., Giani A., Mantopoulos D., Kayama M., Roh M.I., Michaud N., Pawlyk B., Sandberg M., Young L.H., Miller J.W., Vavvas D.G. Evidence for baseline retinal pigment epithelium pathology in the Trp1-Cre mouse. Am J Pathol. 2012;180:1917–1927. doi: 10.1016/j.ajpath.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt-Supprian M., Rajewsky K. Vagaries of conditional gene targeting. Nat Immunol. 2007;8:665–668. doi: 10.1038/ni0707-665. [DOI] [PubMed] [Google Scholar]
- 10.Chan E.L., Peace B.E., Toney K., Kader S.A., Pathrose P., Collins M.H., Waltz S.E. Homozygous K5Cre transgenic mice have wavy hair and accelerated malignant progression in a murine model of skin carcinogenesis. Mol Carcinog. 2007;46:49–59. doi: 10.1002/mc.20192. [DOI] [PubMed] [Google Scholar]
- 11.Higashi A.Y., Ikawa T., Muramatsu M., Economides A.N., Niwa A., Okuda T., Murphy A.J., Rojas J., Heike T., Nakahata T., Kawamoto H., Kita T., Yanagita M. Direct hematological toxicity and illegitimate chromosomal recombination caused by the systemic activation of CreERT2. J Immunol. 2009;182:5633–5640. doi: 10.4049/jimmunol.0802413. [DOI] [PubMed] [Google Scholar]
- 12.Rajewsky K., Gu H., Kuhn R., Betz U.A., Muller W., Roes J., Schwenk F. Conditional gene targeting. J Clin Invest. 1996;98:600–603. doi: 10.1172/JCI118828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton D.L., Abremski K. Site-specific recombination by the bacteriophage P1 lox-Cre system. Cre-mediated synapsis of two lox sites. J Mol Biol. 1984;178:481–486. doi: 10.1016/0022-2836(84)90154-2. [DOI] [PubMed] [Google Scholar]
- 14.Loonstra A., Vooijs M., Beverloo H.B., Allak B.A., van Drunen E., Kanaar R., Berns A., Jonkers J. Growth inhibition and DNA damage induced by Cre recombinase in mammalian cells. Proc Natl Acad Sci U S A. 2001;98:9209–9214. doi: 10.1073/pnas.161269798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schmidt E.E., Taylor D.S., Prigge J.R., Barnett S., Capecchi M.R. Illegitimate Cre-dependent chromosome rearrangements in transgenic mouse spermatids. Proc Natl Acad Sci U S A. 2000;97:13702–13707. doi: 10.1073/pnas.240471297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janbandhu V.C., Moik D., Fassler R. Cre recombinase induces DNA damage and tetraploidy in the absence of LoxP sites. Cell Cycle. 2013;13:462–470. doi: 10.4161/cc.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silver D.P., Livingston D.M. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- 18.Semprini S., Troup T.J., Kotelevtseva N., King K., Davis J.R., Mullins L.J., Chapman K.E., Dunbar D.R., Mullins J.J. Cryptic loxP sites in mammalian genomes: genome-wide distribution and relevance for the efficiency of BAC/PAC recombineering techniques. Nucleic Acids Res. 2007;35:1402–1410. doi: 10.1093/nar/gkl1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baba Y., Nakano M., Yamada Y., Saito I., Kanegae Y. Practical range of effective dose for Cre recombinase-expressing recombinant adenovirus without cell toxicity in mammalian cells. Microbiol Immunol. 2005;49:559–570. doi: 10.1111/j.1348-0421.2005.tb03753.x. [DOI] [PubMed] [Google Scholar]