Abstract
Vascular remodeling is a feature of sustained inflammation in which capillaries enlarge and acquire the phenotype of venules specialized for plasma leakage and leukocyte recruitment. We sought to determine whether neutrophils are required for vascular remodeling in the respiratory tract by using Mycoplasma pulmonis infection as a model of sustained inflammation in mice. The time course of vascular remodeling coincided with the influx of neutrophils during the first few days after infection and peaked at day 5. Depletion of neutrophils with antibody RB6-8C5 or 1A8 reduced neutrophil influx and vascular remodeling after infection by about 90%. Similarly, vascular remodeling after infection was suppressed in Cxcr2−/− mice, in which neutrophils adhered to the endothelium of venules but did not extravasate into the tissue. Expression of the venular adhesion molecule P-selectin increased in endothelial cells from day 1 to day 3 after infection, as did expression of the Cxcr2-receptor ligands Cxcl1 and Cxcl2. Tumor necrosis factor α (TNFα) expression increased more than sixfold in the trachea of wild-type and Cxcr2−/− mice, but intratracheal administration of TNFα did not induce vascular remodeling similar to that seen in infection. We conclude that neutrophil influx is required for remodeling of capillaries into venules in the airways of mice with Mycoplasma infection and that TNFα signaling is necessary but not sufficient for vascular remodeling.
Neutrophils are key effector cells of innate immunity that rapidly arrive at sites of tissue injury to kill bacteria and interact with macrophages and other cells to orchestrate a coordinated immune cell and cytokine response to injury.1–4 Neutrophils are involved in many inflammatory diseases of the airways and lung, including pneumonia, acute lung injury, sepsis, asthma, cystic fibrosis, bronchitis, and chronic obstructive lung disease,5 also contribute to tissue damage in inflammatory conditions of other organs, and play a role in arterial remodeling in atherosclerosis.4
The signals and events that bring neutrophils to sites of inflammation are well characterized.6–8 These include expression of endothelial cell adhesion molecules to induce rolling and firm attachment, followed by extravasation into tissues where they release cytokines and other products that can kill bacteria and promote tissue remodeling. The dominant mechanism driving neutrophil influx may be organ-specific.9,10 Blood vessels of the microcirculation undergo numerous changes in sustained inflammation, and these include structural and functional remodeling of endothelial cells and pericytes.11–14 Among these changes, capillaries transform into venules that support plasma leakage and leukocyte influx. The contribution of neutrophils to this remodeling is not well understood. Circumferential vessel enlargement is a prominent feature of vascular remodeling–sustained airway inflammation15–23 and is distinct from more familiar and better-documented types of sprouting angiogenesis.24
We asked whether incoming neutrophils contribute to the vascular remodeling, with the thought that the initial wave of leukocyte influx could render blood vessels more efficient for leukocyte adhesion and transmigration. Although leukocyte influx is known to accompany blood vessel remodeling,15,18,22 it is unknown whether there is a causal relationship and, if so, what is the underlying mechanism? Neutrophils are attractive candidates for contributing to vascular remodeling because they are among the first leukocytes to enter inflamed tissues4,6,25 and can produce cytokines, growth factors, proteases, and reactive oxygen species that have profound vascular effects.2–4,26
With this background, we sought to determine whether neutrophils are essential for the vascular remodeling that occurs soon after Mycoplasma pulmonis infection, when capillaries transform into venules. In particular, we asked whether neutrophil influx coincides spatially and temporally with vascular remodeling, can vascular remodeling be prevented by neutrophil depletion, and if Cxcr2 signaling is required for the neutrophil influx that accompanies vascular remodeling?
To address these questions we examined the relationship between neutrophil influx and vascular remodeling during the first week after M. pulmonis infection of the respiratory tract of mice. The approach was to compare the time course of neutrophil influx and vascular remodeling in the trachea and then determine whether the remodeling was blocked by neutrophil depletion by either of two different antineutrophil antibodies: RB6-8C5 or 1A8. We also tested whether vascular remodeling was prevented by genetic deletion of Cxcr2, which mediates the actions of the chemotactic chemokines Cxcl1 and Cxcl2, which bring neutrophils into inflamed tissues. Because previous studies have shown that vascular remodeling was inhibited by blocking tumor necrosis factor α (TNFα) signaling,19 we asked whether TNFα expression was increased in wild-type and Cxcr2−/− mice and whether intratracheal administration of TNFα was sufficient to induce vascular remodeling similar to that seen after infection. Other studies examined the expression of the Cxcr2 ligands, Cxcl1 and Cxcl2. Together, the experiments showed that neutrophil influx was required for vascular remodeling after M. pulmonis infection, and that TNF signaling was necessary but not sufficient for vascular remodeling.
Materials and Methods
Mice
We used adult female wild-type C57BL/6 mice and Cxcr2−/− mice27 (C57BL/6 background, strain #006848; The Jackson Laboratory, Bar Harbor, ME) of either sex. Mice were genotyped by PCR analysis of genomic tail DNA, housed under pathogen-free conditions, and had free access to food and water. For experiments, mice were anesthetized i.m. with 85 mg/kg ketamine and 15 mg/kg xylazine.19 The Institutional Animal Care and Use Committee of the University of California at San Francisco approved all experimental procedures.
M. pulmonis Infection
Anesthetized mice were infected by intranasal inoculation of 50 μL broth containing 106 colony-forming units of M. pulmonis organisms of strain CT7.28 Infection severity was assayed by quantitative RT-PCR (RT-qPCR) for bacterial 16S bacterial rRNA.22
RT-qPCR
After perfusion of anesthetized pathogen-free or infected mice with PBS, tracheas were removed, RNA was extracted, and cDNA was prepared.19 Samples of 1 ng cDNA were subjected to RT-qPCR using SYBR GreenER qPCR SuperMix Universal (Invitrogen, Carlsbad, CA) and measured in duplicate with a Bio-Rad MyiQ detection system (Hercules, CA) using gene-specific primers (Table 1). Gene expression values were normalized to β-actin, and the results were presented as fold-changes in comparison with pathogen-free controls or as copy number relative to β-actin.
Table 1.
Sequence of Primers Used for RT-qPCR
| Name | Forward primer | Reverse primer |
|---|---|---|
| β-actin | 5′-GGCTGTATTCCCCTCCATCG-3′ | 5′-CCAGTTGGTAACAATGCCATGT-3′ |
| 16S rRNA | 5′-CGGTACAGGAAACTGTTGCTAATACCG-3′ | 5′-CCATTTCAAAGTGAAGCAAACG-3′ |
| S100a9 | 5′-GGAGCGCAGCATAACCACCATC-3′ | 5′-GCCATCAGCATCATACACTCCTCA-3′ |
| Cxcr2 | 5′-ATGCCCTCTATTCTGCCAGAT-3′ | 5′-GGTGCTCCGGTTGTATAAGATGA-3′ |
| Cxcl1 | 5′-CCGAAGTCATAGCCACACTCAA-3′ | 5′-GCAGTCTGTCTTCTTTCTCCGTTAC-3′ |
| Cxcl2 | 5′-AGACAGAAGTCATAGCCACTCTCAAG-3′ | 5′-CCTCCTTTCCAGGTCAGTTAGC-3′ |
| P-selectin | 5′-GCATACTCATGGAATAACTCACG-3′ | 5′-GACGTCATTGAGGTGAGCG-3′ |
| TNFα | 5′-CCACCACGCTCTTCTGTCTAC-3′ | 5′-AGGGTCTGGGCCATAGAACT-3′ |
Measurement of Tissue TNFα
Tracheas were removed from anesthetized pathogen-free or infected mice and frozen at liquid nitrogen temperature. Tissues were homogenized and TNFα protein levels were determined using a mouse TNFα enzyme-linked immunosorbent assay kit (eBioscience, San Diego, CA) according to the manufacturer’s instructions. The sensitivity of the assay was 8 pg/mL. Pilot experiments showed that the amount of TNFα in individual tracheas was near the detection limit so four to five tracheas were pooled for each experimental group to yield enough protein for analysis. Amounts of TNFα per trachea were expressed as picograms of TNFα per milligram of protein.
Intratracheal TNFα Administration
Recombinant murine TNFα (catalog #315-01A; PeproTech, Rocky Hill, NJ) was administered daily to anesthetized mice for 7 days at a dose of 50, 100, 200, or 500 ng per mouse in 30 μL sterile 0.9% NaCl. The aliquot was delivered via a pipette inserted into the back of the mouth near the trachea by a technique developed for efficient, atraumatic delivery of substances to the mouse respiratory tract.29 The dose range of TNFα was based on published reports of TNFα administered intratracheally to induce changes in mouse lungs.30,31 At 7 days, tracheas were removed and vascular remodeling and neutrophil influx were assessed in tissue whole mounts processed for immunohistochemistry.
Blood Leukocyte Counting
Neutrophils in fresh peripheral blood removed via the tail vein were counted using a Hemavet 850 automated veterinary hemocytometer (Drew Scientific, Dallas, TX).
Neutrophil Depletion
Neutrophils were depleted by daily intraperitoneal injections of 250 μg rat monoclonal anti-mouse neutrophil antibodies 1A832 or RB6-8C5,33 starting 1 day before infection and continuing for 7 days. Changes in neutrophils in blood and tissues were assessed by counting neutrophils in blood with a hemocytometer or in immunohistochemically stained whole-mount preparations of the trachea.
Tissue Preparation and Immunohistochemistry
Tracheas of anesthetized mice were preserved by vascular perfusion of fixative (1% paraformaldehyde in PBS, pH 7.4) for 2 minutes. Tracheas were removed and immersed in fixative for 1 hour at room temperature, washed, and then stained immunohistochemically by incubation as whole mounts in one or more primary antibodies (Table 2) diluted at 1 μg/mL, as described previously.28 Antibodies to S100a8, S100a9, or myeloperoxidase were confirmed to stain mouse neutrophils and to be suitable for multiple labeling studies of paraformaldehyde-fixed tracheal whole mounts (Supplemental Figure S1). Primary antibodies were detected by secondary antibodies labeled with Alexa488, Cy3, or Cy5 (Jackson ImmunoResearch, West Grove, PA). Specimens were imaged with a Zeiss (Thornwood, NY) LSM510 confocal microscope using Zeiss AIM software version 4.0.
Table 2.
Antibodies Used for Immunohistochemistry
| Antigen | Host species | Antibody type | Vendor | Catalog number |
|---|---|---|---|---|
| Pecam1 (CD31) | Armenian hamster | Monoclonal | Thermo/Fisher | MA3105 |
| Pecam1 (CD31) | Rabbit | Polyclonal | Abnova | PAB11924 |
| P-selectin (CD62P) | Rat | Monoclonal | BD Biosciences | 550289 |
| S100a8 | Goat | Polyclonal | R&D Systems | AF3059 |
| S100a9 | Rat | Polyclonal | Abcam | Ab105472 |
| Myeloperoxidase | Rabbit | Polyclonal | Dako (Carpinteria, CA) | A0398 |
| Cxcl1 | Rabbit | Polyclonal | PeproTech | 500-P115 |
| Cxcl2 | Goat | Polyclonal | R&D Systems | AF452NA |
| E-cadherin | Rat | Monoclonal | Invitrogen | 131900 |
Morphometric Measurements
Blood vessels were measured in trachea images stained for platelet-endothelial cell adhesion molecule 1 (Pecam1) using a digitizing tablet linked to a video camera on a Zeiss Axiophot microscope.28 Blood vessels and neutrophils were measured in regions of mucosa overlying the tracheal cartilage rings, where the greatest changes occurred after infection.
Statistical Analysis
Normally distributed values are presented as means ± SEM, with 3 to 12 mice per group. Differences between means were assessed by analysis of variance followed by the Bonferroni test. P < 0.05 was considered significant. The Pearson correlation coefficient r was calculated to assess the relationship between the two variables concerned. Frequency distributions were compared with the Kolmogorov-Smirnov two-sample test.
Results
Temporal and Spatial Relationship of Neutrophil Influx and Vascular Remodeling
In pathogen-free mice, blood vessels over tracheal cartilage rings had a mean diameter of 9.2 ± 0.3 μm and consisted of one or two endothelial cells (Figure 1A). After infection, these vessels were slightly larger at 3 days (Figure 1B) and were more than twice normal size at 7 days, with a mean diameter of 24.7 ± 2.3 μm (Figure 1, C and D). Other blood vessels in the tracheal mucosa were similarly enlarged.19
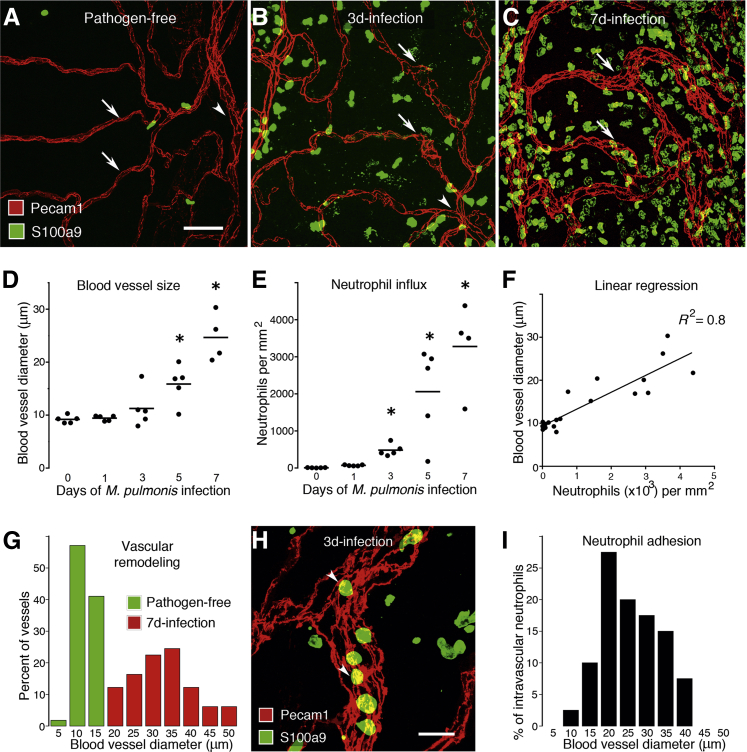
Figure 1.
Time course of neutrophil influx and vascular remodeling after M. pulmonis infection. A–C: Whole mounts of mouse trachea stained for endothelial cells (Pecam1, red) and neutrophils (S100a9, green). In a pathogen-free mouse, capillaries (arrows) span a cartilage ring and join a venule (arrowhead) between cartilages; neutrophils are sparse (A). B: There were a moderate number of neutrophils on day 3 of infection. Capillaries were mostly narrow but had focal enlargements (arrows) and joined a venule (arrowhead). C: Neutrophils were abundant on day 7. Vessels over cartilage were enlarged (arrows). D and E: Values per mouse and group means show the time course after infection of enlargement of capillaries over cartilage rings (D) and influx of S100a9-immunoreactive neutrophils (E). N = 4 to 5 mice per group; ∗P < 0.05 compared with the pathogen-free group. F: Linear regression of blood vessel size versus the number of neutrophils in the trachea from 0 to 7 days after infection. N = 4 to 5 mice per group, P < 0.001. G: Distribution of the diameter of blood vessels located over tracheal cartilage in pathogen-free and 7-day-infected mice. Capillaries (green) in pathogen-free mice were remodeled into larger venules (red) by day 7 of infection. N = 3 mice per group, P < 0.05; Kolmogorov-Smirnov test. H: Neutrophils (arrowheads) inside a venule located between cartilage rings on day 3 of infection. Scattered extravascular neutrophils also were present. I: Size distribution of blood vessels (median, 22.5 μm) that contain adherent neutrophils on day 3 of infection. N = 3 mice. Scale bars: 50 μm (A–C); 20 μm (H).
Few neutrophils were found in the tracheal mucosa of pathogen-free mice (Figure 1A). Neutrophils were significantly more abundant at day 3 of infection (Figure 1B), and at day 7 were >3000-fold more abundant than at baseline (Figure 1, C and E). The amount of vascular remodeling correlated closely with the severity of neutrophil influx in the trachea (R2 = 0.8; P < 0.001) (Figure 1F). During the first week of infection, the number of circulating neutrophils in blood also increased and peaked on day 5 at approximately three times the pathogen-free value (Supplemental Figure S2A). During this period, the blood vessels overlying the cartilage were converted from capillaries—the only type of vessels normally found in this location—to venules (Figure 1G).
Intravascular neutrophils (diameter, 9.2 ± 0.3 μm) were most abundant in postcapillary venules and collecting venules (median diameter, 22.5 μm) (Figure 1, H and I). On days 1 and 3 after infection, most of the neutrophil-containing vessels were venules located between the cartilage and the capillaries over the cartilage did not support neutrophil adhesion. However, from day 5 onward, some neutrophils also were found in vessels over the cartilage. The presence of neutrophils in these vessels was another indication that these capillaries underwent remodeling into venules capable of supporting leukocyte adhesion.
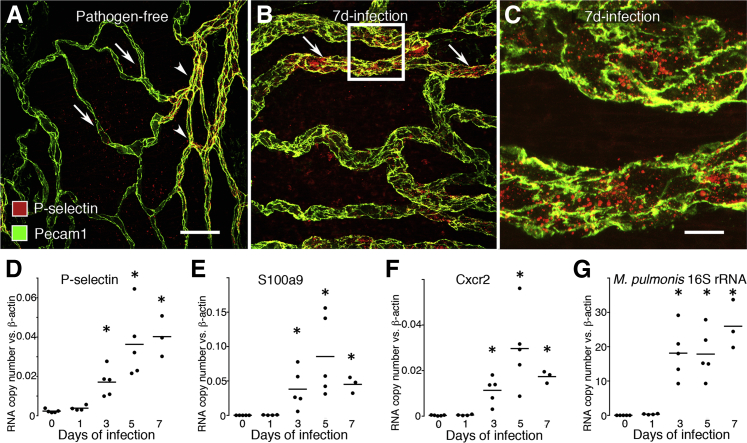
Evidence of remodeling of capillaries into venules was supported further by the appearance of P-selectin immunoreactivity, which normally was absent in capillaries over cartilage, and only present in venules between cartilage (Figure 2A). P-selectin was present in the remodeled vessels overlying cartilage on day 7 after infection (Figure 2B). P-selectin staining in these vessels had a granular appearance (Figure 2C), consistent with the presence of P-selectin in Weibel-Palade bodies.34 The increase in P-selectin immunoreactivity coincided with increases in expression of P-selectin mRNA assessed by RT-qPCR. P-selectin expression was twice the baseline value at 1 day after infection, 6 times baseline at 3 days, and 15 times baseline at 7 days (Figure 2D).
Figure 2.
Time course of expression of P-selectin and neutrophil markers after M. pulmonis infection. Whole mounts of mouse trachea stained for P-selectin (red) and endothelial cells (Pecam1, green). A: In a pathogen-free mouse, P-selectin immunoreactivity was faint or absent in capillaries (arrows), but was moderate in venules (arrowheads) located between cartilage rings. B: At day 7 after infection, P-selectin was moderate to strong in enlarged remodeled vessels (arrows) over cartilage. C: Enlargement of boxed region in B. Most P-selectin immunoreactivity was in granules in endothelial cells of remodeled vessels. Pecam1 staining at endothelial cell borders of remodeled vessel was stronger, wider, and more irregular than in capillaries (A). D–G: Time course of expression of P-selectin (D), the neutrophil marker S100a9 (E), Cxcr2 (F), and the severity of infection reflected by expression of M. pulmonis 16S rRNA (G) assessed by RT-qPCR. Black circles indicate values for individual mice and the lines show the group mean. N = 3 to 5 mice per group. ∗P < 0.05 compared with the pathogen-free group. Scale bars: 50 μm in (A and B); 10 μm (C).
The increase in P-selectin in the trachea was accompanied by the accumulation of neutrophils in the tracheal mucosa, reflected by increased expression of the neutrophil cytosolic protein S100a9 (Figure 2E) and the neutrophil chemokine receptor Cxcr2 (Figure 2F). Expression of P-selectin and S100a9 were relatively low at baseline, increased with the duration of infection, and correlated positively with one another (R2 = 0.6, P < 0.001). Expression of mRNA for P-selectin, S100a9, and Cxcr2 peaked on day 5 after infection. The amount of neutrophil influx, vascular remodeling, and the expression of the earlier-mentioned adhesion molecule, neutrophil cytosolic protein, and receptor driving neutrophil chemotaxis all increased with the progression of infection, as reflected by expression of M. pulmonis 16S rRNA in the trachea (Figure 2G).
Dependence of Vascular Remodeling on Neutrophil Influx
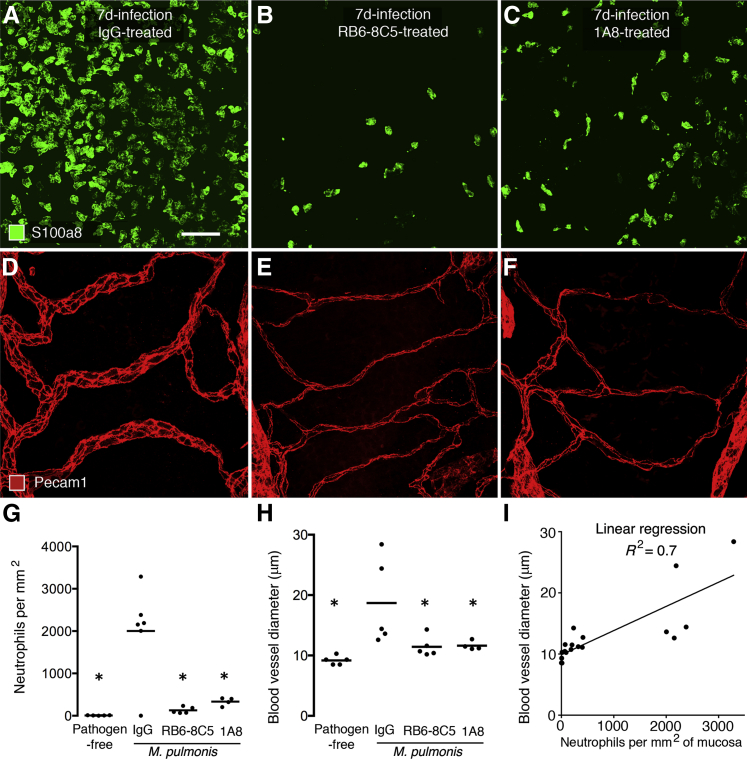
To determine whether the coincidence of neutrophil influx and vascular remodeling reflected a causal relationship, we tested the effect of two different antineutrophil antibodies, RB6-8C5 and 1A8, on the amounts of neutrophil influx and vascular remodeling. As documented in previous reports,32,33 administration of antibody RB6-8C5 or 1A8 was accompanied by a significant reduction in neutrophils in the bloodstream (Supplemental Figure S2B). Similarly, fewer neutrophils were present in tracheas of mice that received either of the two antibodies throughout a 7-day period of M. pulmonis infection (Figure 3, A–C). By comparison, neutrophil influx was unimpeded after infection of mice that received a nonspecific IgG as a control antibody. The suppression of neutrophil influx in mice treated with RB6-8C5 or 1A8 during the infection was paralleled by much less enlargement of blood vessels over cartilage rings (Figure 3, D–F). Measurements showed 95% fewer neutrophils and 91% less vascular enlargement in tracheas of infected mice treated with RB6-8C5, and 86% fewer neutrophils and 89% less vascular enlargement in mice treated with antibody 1A8 (Figure 3, G and H). A significant correlation between the amounts of neutrophil influx and vascular enlargement was evident by linear regression analysis (R2 = 0.7, P < 0.001) (Figure 3I).
Figure 3.
Effect of neutrophil-depleting antibodies on neutrophil influx and vascular remodeling after M. pulmonis infection. Whole mounts of mouse trachea comparing the number of neutrophils (S100a8, green) (A–C) and size of blood vessels (Pecam1, red) (D–F) in mucosa over a cartilage ring in mice given control IgG (A and D), RB6-8C5 (B and E), or 1A8 (C and F) throughout the 7-day infection. Neutrophil influx and vascular enlargement after infection are much less after RB6-8C5 or 1A8 than after the control IgG. Measurements of neutrophils (G) and blood vessel diameter (H) in tracheal mucosa over cartilage rings on day 7 of infection after antibody treatment. N = 4 to 6 mice per group; ∗P < 0.05 compared with IgG group. I: Linear regression of blood vessel size versus number of neutrophils on day 7 of infection for all mice in the four treatment groups. N = 4 to 5 mice per group, P < 0.001. Capillary enlargement was correlated positively to the number of neutrophils and was not present in the absence of neutrophil influx in pathogen-free mice or in infected mice after neutrophil depletion. Scale bar = 50 μm (A–F).
Dependence of Neutrophil Influx on Cxcr2 Signaling
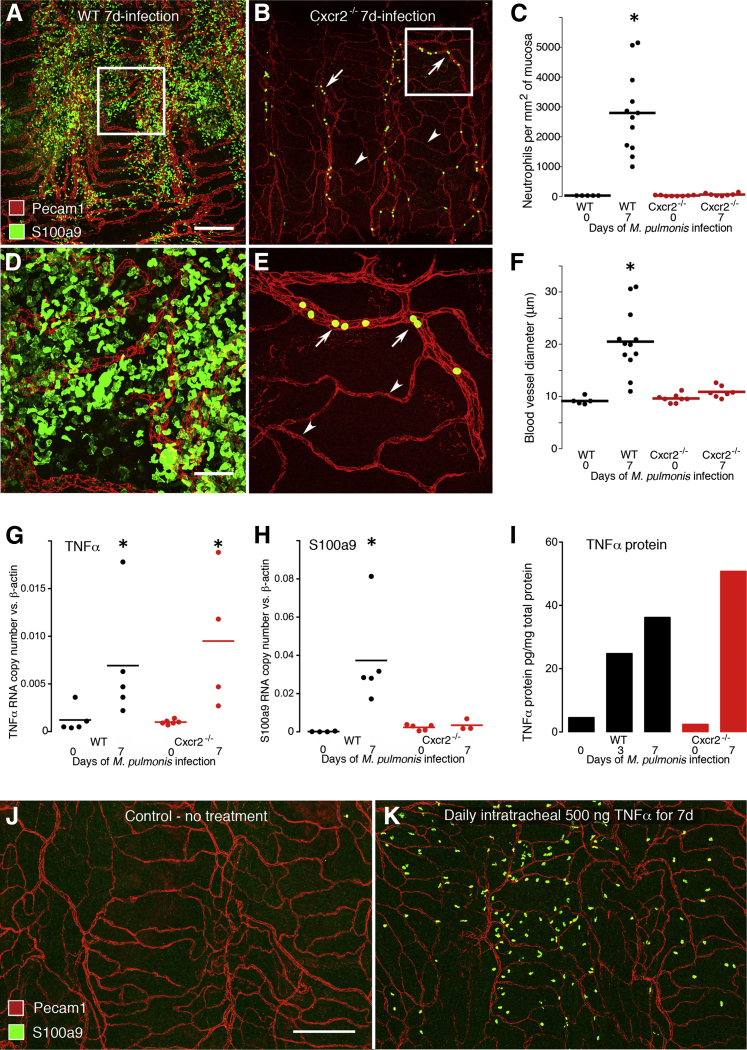
As an independent approach to test the dependency of vascular remodeling on neutrophil influx and to elucidate the underlying mechanism, we next asked whether one or both could be blocked by deletion of Cxcr2, a key receptor for neutrophil chemotactic chemokines. In particular, we compared the amount of neutrophil influx and vascular remodeling after M. pulmonis infection in wild-type mice and Cxcr2−/− mice, which have severely impaired neutrophil chemotaxis.35,36 We found that neutrophil influx in the trachea at day 7 after infection was 97% less in Cxcr2−/− mice than in corresponding wild-type mice (Figure 4, A–C). Similarly, vascular enlargement after infection was 85% less in Cxcr2−/− mice than in wild-type mice (Figure 4, D–F).
Figure 4.
Changes in TNFα and dependence of neutrophil influx and vascular remodeling on Cxcr2 signaling. Whole mounts of mouse trachea comparing the number and distribution of neutrophils (S100a9, green) in relation to blood vessels (Pecam1, red) in wild-type (WT) and Cxcr2−/− mice at day 7 of infection (A, B, D, and E). A and B: Low-magnification view comparing widespread neutrophils in a WT mouse and sparse neutrophils in a Cxcr2−/− mouse. Most of the latter are inside venules (arrows) but not in capillaries (arrowheads). C: Measurements per mouse and group means show extensive neutrophil influx after infection of WT mice but not of Cxcr2−/− mice. N = 5 to 12 mice per group; ∗P < 0.05 compared with the pathogen-free group. D and E: Higher magnification view of boxed areas in A and B show abundant neutrophils after infection in a WT mouse and sparse neutrophils in a Cxcr2−/− mouse. F: Blood vessel diameters show prominent enlargement at day 7 of infection in WT mice but not in Cxcr2−/− mice. N = 5 to 12 mice per group; ∗P < 0.05 compared with the pathogen-free group. G and H: TNFα (G) and S100a9 (H) mRNA expression assayed by RT-qPCR in pathogen-free and 7-day M. pulmonis–infected WT mice (black circles) and Cxcr2−/− mice (red circles). After infection of WT mice, the expression of both TNFα and S100a9 were significantly greater than in pathogen-free controls, but in infected Cxcr2−/− mice, TNFα was greater but S100a9 was not different from the corresponding pathogen-free value. N = 4 to 6 mice per group, ∗P < 0.05. I: TNFα protein levels in tracheas of pathogen-free mice and M. pulmonis–infected WT mice assayed by enzyme-linked immunosorbent assay. Tracheas were pooled (from N = 5 to 6 mice/group) to yield enough protein for analysis. J and K: Confocal images of whole mounts of tracheas, stained for blood vessels (Pecam1, red) and neutrophils (S100a9, green), from pathogen-free mice that were untreated (J) or given 500 ng of recombinant mouse TNFα (K) daily for 7 days by intratracheal instillation. No evidence for vascular remodeling was evident in the TNFα-challenged trachea, although neutrophils were more numerous than in untreated controls. Scale bars: 200 μm (A, B, J, and K); 50 μm (D and E).
Unlike wild-type mice in which >95% of neutrophils were extravascular on day 7 of infection (Figure 4D), a distinctive feature of infected Cxcr2−/− mice was that most neutrophils in the trachea were intravascular (Figure 4E). The finding of almost no neutrophil extravasation in Cxcr2−/− mice infected with M. pulmonis was in sharp contrast to the fivefold higher neutrophil concentration in peripheral blood than found in wild-type mice (Supplemental Figure S2A).
Dependence of Vascular Remodeling on TNFα
Because TNFα is a key inflammatory mediator that is up-regulated after M. pulmonis infection and is required for vascular remodeling after infection,19 we sought to learn whether the suppression of neutrophil influx and vascular remodeling after infection of Cxcr2−/− mice was accompanied by reduced expression of TNFα. For this purpose, we measured TNFα mRNA and protein levels. We also measured S100a9 mRNA levels as a marker of neutrophil influx. Expression of TNFα mRNA in tracheas at day 7 of infection was sixfold greater in wild-type mice and 15-fold greater in Cxcr2−/− mice than in corresponding pathogen-free controls (Figure 4G). In the same tracheas, S100a9 expression was 370-fold greater after infection in wild-type mice compared with a 50% increase in Cxcr2−/− mice (Figure 4H). After infection for 7 days, the amount of TNFα protein was eightfold greater in wild-type mice and 21-fold greater in Cxcr2−/− mice than in controls (Figure 4I). Despite the contribution of low baseline values in pathogen-free mice to some of the large fold increases in TNFα and S100a9 after infection, the findings indicate that TNFα increased after infection in the absence of much neutrophil influx and are consistent with TNFα being necessary but not sufficient for vascular remodeling after M. pulmonis infection.
To use another approach to test whether the increase in TNFα expression was not only necessary but also sufficient for vascular remodeling, we determined whether local administration of recombinant mouse TNFα could mimic the neutrophil influx and vascular remodeling accompanying M. pulmonis infection. TNFα was administered intratracheally to pathogen-free wild-type mice at daily doses of 50, 100, 200, or 500 ng for 7 days, based on doses reported in the literature.30,31 The 50 ng dose had no detectable effect on vascular remodeling or neutrophil influx (data not shown). Daily TNFα doses of 200 or 500 ng resulted in modest neutrophil influx into the trachea but no noticeable vascular remodeling (Figure 4, J and K). Despite the limited effect of the 500 ng TNFα dose on neutrophil influx and no apparent vascular remodeling in the trachea, this dose exceeded the maximal tolerated dose because only 1 of 7 mice survived the full 7-day course of intratracheal TNFα administration. All of the lower doses were well tolerated.
Distribution of Cxcr2 Ligands
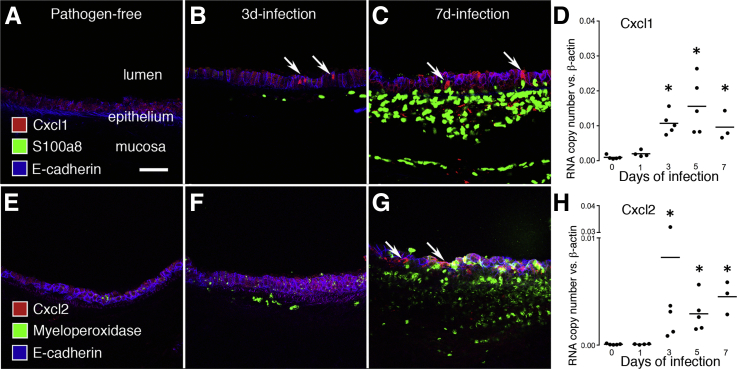
To further explore the roles of neutrophils in vascular remodeling, we determined the amount, location, and time course of expression of two important Cxcr2 ligands, Cxcl1 and Cxcl2, which are made by epithelial cells and other cell types.7,8 Cxcl1 immunoreactivity increased in the tracheal epithelium after infection (Figure 5, A–C). Similarly, RT-qPCR measurements of Cxcl1 expression increased after infection (Figure 5D). Cxcl1 expression at day 7 was approximately 10-fold the baseline value in pathogen-free mice. Staining for Cxcl2 also increased in the epithelium after infection (Figure 5, E–G), and Cxcl2 expression at day 7 was nearly 50-fold the baseline value (Figure 5H).
Figure 5.
Cxcl1 and Cxcl2 expression in trachea after M. pulmonis infection. A–C: Cross-sections of mouse trachea stained for Cxcl1 (red), neutrophils (S100a8, green), and epithelial cells (E-cadherin, blue). A: No epithelial Cxcl1 staining or neutrophils in pathogen-free trachea. B: Scattered epithelial cells with Cxcl1 staining (arrows) and some neutrophils at day 3 of infection. C: More epithelial cells with Cxcl1 staining (arrows) and abundant neutrophils on day 7 of infection. D: Time course of Cxcl1 expression after infection measured in trachea by RT-qPCR. N = 3 to 5 mice per group; ∗P < 0.05 compared with the pathogen-free group. E–G: Cross-sections of mouse trachea stained for Cxcl2 (red), neutrophils (myeloperoxidase, green), and epithelial cells (E-cadherin, blue). E: No epithelial Cxcl2 staining was visible in pathogen-free trachea. F: No Cxcl2 staining and few neutrophils in mucosa were visible on day 3 of infection. G: Cxcl2 staining in epithelium (arrows) and abundant neutrophils at day 7 of infection. H: Time course of Cxcl2 expression after infection measured in trachea by RT-qPCR. N = 3 to 5 mice per group; ∗P < 0.05 compared with the pathogen-free group. Scale bars: 50 μm (A–C, E–G).
Discussion
We sought to determine whether neutrophils are essential for the vascular remodeling that occurs in sustained inflammation and is manifested by transformation of capillaries into venules.15,16,23 Importantly, these changes support leukocyte trafficking and plasma leakage, which are essential components of the inflammatory response. Our experiments, using M. pulmonis infection of the respiratory tract in mice as a model, produced complementary lines of evidence linking neutrophil influx and vascular remodeling. First, neutrophil influx coincided in timing and location with vascular remodeling. Second, pretreatment with either of two antineutrophil antibodies (RB6-8C5 or 1A8) reduced neutrophils in the bloodstream and in the tissue and prevented vascular remodeling. Third, neutrophil influx and vascular remodeling both were reduced sharply in the absence of Cxcr2, a key receptor on neutrophils mediating actions of chemotactic chemokines.
Time Course of Neutrophil Influx and Vascular Remodeling
Respiratory tract infection by M. pulmonis in mice results in sustained and progressive inflammation in the airways and lung, accompanied by infiltration by neutrophils and mononuclear cells, exaggerated mucus production, and fibrosis.37,38 M. pulmonis in mice resembles lung infection by M. pneumoniae in mice,39 but M. pulmonis infection is more prolonged, and has features of M. pneumoniae infection in humans, which can cause community-acquired pneumonia and is known to exacerbate asthma and chronic obstructive pulmonary disease.40–43
The present study showed that vascular remodeling in the airway mucosa coincides spatially and temporally with neutrophil influx during the first week after M. pulmonis infection. Few, if any, neutrophils were present in the airway mucosa under baseline conditions. Capillaries in the mucosa over cartilage rings of the normal trachea, similar to capillaries elsewhere, lacked endothelial P-selectin immunoreactivity and adherent neutrophils. By comparison, after infection, P-selectin increased in endothelial cells, neutrophils adhered to the endothelium and migrated into the airway mucosa, and capillaries enlarged and acquired a venular phenotype. Vessel size, P-selectin expression, and neutrophil influx peaked around day 7. Vascular enlargement results from endothelial cell proliferation, which peaks at day 5 after infection.17 The amount of neutrophil influx assessed by S100a8, S100a9, or myeloperoxidase immunoreactivity fit with RT-qPCR measurements of S100a9 that were very low at baseline but increased rapidly after infection through day 7. During this period, the appearance of P-selectin in endothelial cells, luminal adherence of neutrophils, and increase in vessel size were consistent with transformation of capillaries into venules.
Suppression of Vascular Remodeling by Neutrophil Depletion
To test the mechanistic relevance of the spatial and temporal coincidence of neutrophil influx and vascular remodeling, we depleted neutrophils by using two different antineutrophil antibodies. Initially, we used the neutrophil blocking antibody RB6-8C5. For confirmation of the result we then used antibody 1A8. Both antibodies decreased neutrophils in the bloodstream and neutrophil influx into the airways after infection, and both also reduced vascular remodeling by about 90%. Antibody RB6-8C5 resulted in somewhat greater neutrophil depletion in the blood and tissue (95%) than 1A8 (86%), but is known to be less neutrophil-specific than 1A8 because RB6-8C5 also can reduce Ly6C-positive monocytes/macrophages, dendritic cells, and lymphocytes.32 The antibody was injected daily, but neutrophils in blood began to return to baseline levels toward the end of the week-long infection, as more neutrophils were released from bone marrow. As a result, the neutrophil depletion was more complete at the outset than at the end. However, vascular remodeling almost completely was prevented despite the diminished reduction toward the end.
Suppression of Vascular Remodeling by Deletion of Cxcr2
To extend the results of the neutrophil-depletion experiments, we examined the effects of blocking neutrophil recruitment into the tissue. The approach was to determine the amount of vascular remodeling during the first week of infection in mice lacking the chemotactic chemokine receptor Cxcr2. Cxcr2−/− mice are known to have defective neutrophil chemotaxis owing to impaired responses to Cxcr2 ligands Cxcl1 and Cxcl2.27,36 However, the Cxcr2−/− mice had five times the normal level of neutrophils in the bloodstream and more severe infections. This exaggeration of disease severity reflected the adaptive value of the normal inflammatory response and the health impact of impaired neutrophil function in bacterial infections.44
The use of staining for S100a8 or S100a9 and Pecam1 in tracheal whole mounts showed that some neutrophils were present in the airways of Cxcr2−/− mice, but they were intravascular and did not migrate into the tissue. The absence of vascular remodeling in these mice suggests that adherent intravascular neutrophils were not sufficient to promote the transformation of capillaries into venules. The two complementary experimental approaches, antibody depletion of neutrophils and prevention of neutrophil chemotaxis, both had the same overall effect of blocking vascular remodeling, but by different actions on neutrophils. In the antibody-blocking studies, the overall number of neutrophils was reduced greatly, but those present were able to migrate into the tissue. In contrast, in Cxcr2−/− mice neutrophils were increased greatly in number in the blood, but could not migrate into the tissue. We interpret these findings to indicate that neutrophil accumulation is necessary for vascular remodeling to occur.
Potential Mechanisms of Neutrophil-Mediated Vascular Remodeling
As innate immune effectors and early responders to bacterial infection, neutrophils produce many inflammatory mediators. In addition to proteases,2,45 neutrophils produce leukotriene LTB4,7,46 free radicals, and histamine.47 TNFα is also among the attractive candidates for mediators involved in vascular remodeling that are produced by neutrophils. Although many types of cells can secrete TNFα,48 neutrophils have been reported as a major source in sustained inflammation.2,49 The abundance of neutrophils at the sites of vascular remodeling would position these cells strategically for delivery of mediators to the remodeling vessels. TNFα up-regulates the expression of many adhesion molecules, including P-selectin, E-selectin, intercellular adhesion molecule 1, vascular cell adhesion molecule 1, and Cxcl1 and Cxcl2 ligands for Cxcr2 receptors.50,51 TNFα also promotes phosphorylation of vascular endothelial cadherin, loosens endothelial junctions to increase endothelial permeability,52 and promotes endothelial proliferation.53 Involvement of TNFα in vascular remodeling after M. pulmonis is evident from the significant reduction in remodeling after pharmacologic inhibition of TNFα or genetic deletion of TNF receptor-1.19 TNFα expression increases rapidly after infection before the peak of vascular remodeling occurs.19
Consistent with our previous findings,19 TNFα mRNA expression and protein were increased significantly in the trachea after M. pulmonis infection, both in wild-type mice and in Cxcr2−/− mice. This finding is consistent with the known role of TNFα in the pathophysiology of airway changes after mycoplasmal infection.19 However, because the large increase in TNFα after infection was accompanied by little or no vascular remodeling in Cxcr2−/− mice, TNFα appears to be necessary but not sufficient to promote vascular remodeling. The angiopoietins are among the other factors that can contribute to vascular remodeling after M. pulmonis infection.20,21
Daily administration of recombinant mouse TNFα for 7 days resulted in mild neutrophil influx but no apparent vascular remodeling, even though the 500-ng daily dose had lethal consequences in most mice over the 7-day period. This could be explained by only a small fraction of the dose penetrating into the tracheal wall en route to the lungs. However, vascular remodeling after M. pulmonis infection is likely to result from the combined actions of multiple cytokines acting synergistically. The finding that TNFα increased after infection at least as much in Cxcr2−/− mice as in wild-type mice, regardless of whether vascular remodeling occurred, is consistent with a multihit mechanism, in which other factors are required in addition to TNFα.
The effects of CXC chemokines and CXCR2 signaling have long been recognized in other forms of angiogenesis, including in tumors,54 but have not been associated previously with the type of vascular remodeling studied here. The dependence of mouse neutrophil chemotaxis on Cxcr2 signaling provides a ready explanation for why Cxcr2−/− mice have less vascular remodeling and point to a key role of neutrophils in driving the changes. Although the factors that drive the vascular remodeling are yet to be defined, angiopoietin-2 is another attractive candidate,55 although vascular endothelial growth factor does not seem to be involved.28 Angiopoietin-2 increases after M. pulmonis infection, with a time course that parallels vascular remodeling21,22 and promotes blood vessel destabilization during the remodeling process.55,56 TNFα could serve as a link between neutrophils and the effects of angiopoietin-2 in vascular remodeling56 by up-regulating angiopoietin-2 expression in endothelial cells.57–59
Vascular Remodeling as a Feature of Sustained Inflammation
A prominent feature of vascular remodeling after M. pulmonis infection is the transformation of capillaries into venules. This change involves endothelial cell proliferation, circumferential vessel enlargement, and structural and functional alterations in endothelial cell phenotype.15–17 Accordingly, vascular remodeling differs from vasodilatation and other physiological changes in vessel caliber12,14,17 and is distinct from sprouting angiogenesis, where new vessels grow from existing ones.24 However, angiogenesis and vascular remodeling occur together in conditions in which expansion of the vascular network accompanies the change in vessel phenotype.60
Vascular remodeling is found in many settings of sustained inflammation,60–63 albeit the relevant literature is challenging to decipher because the term is used to describe different phenomena, and capillary to venule transformation is not always designated as vascular remodeling. Historically, enlarged dilated or congested capillaries, as the remodeled vessels have been described, were recognized features of asthma.64 Vascular remodeling also has been described in inflammatory bowel disease, psoriasis,65 gingivitis,66 and sterile necrosis, in which blood vessels expand in response to nearby dying cells.13,14 Vascular remodeling observed in mouse tracheas also occurs in intrapulmonary airways supplied by the bronchial circulation (unpublished data), but different mechanisms are likely to apply to the specialized vasculature of the pulmonary circulation.67
The population of venules in inflamed tissues expands as a consequence of vascular remodeling, which similarly expands the part of the microvasculature specialized for plasma leakage and leukocyte influx.68,69 From a therapeutic perspective, the vascular remodeling after M. pulmonis infection resolves after treatment with anti-inflammatory steroids or antibiotics is important.22
In summary, this study showed that neutrophils are required for the vascular remodeling that occurs in early stages of the inflammatory response to M. pulmonis infection of mouse airways. The vascular remodeling is suppressed either by depletion of neutrophils from the blood or by impairment of Cxcr2-mediated neutrophil chemotaxis into airway tissues. Findings of increased TNFα, which is known to be essential for vascular remodeling, in infected Cxcr2−/− null mice that lacked vascular remodeling fit with the concept that the cytokine is necessary but not sufficient for vascular remodeling under these conditions. These findings build on earlier evidence elucidating the links between the initial inflammatory response and vascular remodeling that enables the response to become sustained. Remodeled capillaries acquire the phenotype of venules that support plasma leakage and leukocyte influx typical of established inflammation. This work highlights neutrophils as potential therapeutic targets for suppression of vascular remodeling as a key step in the progression of the inflammatory response. Elucidation of the consequences of airway and lung infection by M. pulmonis in mice provides insight into the complex pathophysiology of M. pneumoniae infection in humans.
Acknowledgments
We thank Oishee Bose for genotyping mice and preparing stocks of M. pulmonis and McDonald laboratory members for helpful discussions.
P.B. and D.M.M. conceived and wrote the manuscript, and P.B., K.P., L.-C.Y., A.A., and M.N. performed experiments and analyzed data.
Footnotes
Supported in part by grants from the NIH/National Heart, Lung, and Blood Institute (P01 HL024136 and R01 HL059157) and by funding from AngelWorks Foundation and from the Leducq Foundation (D.M.M.).
Disclosures: None declared.
Supplemental Data
Colocalization of myeloperoxidase, S100a8, and S100a9 in neutrophils. Whole-mount preparation of trachea of a wild-type mouse infected with M. pulmonis for 3 days and stained for myeloperoxidase (red, A), S100a9 (green, B), and S100a8 (blue, C). Merged image shows all three markers together (D) and enlarged (E). Most cells have some staining for all three markers. Myeloperoxidase has a granular distribution and is found without S100a9 or S100a8 in some patches (arrows), which could be dead cells. S100a8 and S100a9 have nearly complete correspondence and appear cytoplasmic. Scale bars: 20 μm (A); 10 μm (E).
Peripheral blood neutrophil concentration measured by a veterinary hemocytometer. A: Neutrophils in pathogen-free mice and wild-type (WT, black) and Cxcr2−/− (red) mice after M. pulmonis infection for 7 days (A). Cxcr2−/−mice had neutrophilia even before infection, which became even more pronounced after infection (n = 6 to 10 mice per group, ∗P < 0.05 compared with the pathogen-free group). B: Effect of neutrophil-depleting antibodies 1A8 or RB6-8C5 or control rat IgG on peripheral blood neutrophils during the first 7 days after M. pulmonis infection in WT mice (n = 4 to 8 mice per group). Both antibodies resulted in a reduction in circulating neutrophils compared with control IgG, but antibody RB6-8C5 resulted in a somewhat greater and more sustained reduction than 1A8. ∗P < 0.05 compared with mice treated with IgG.
References
- 1.Soehnlein O., Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10:427–439. doi: 10.1038/nri2779. [DOI] [PubMed] [Google Scholar]
- 2.Mantovani A., Cassatella M.A., Costantini C., Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. 2011;11:519–531. doi: 10.1038/nri3024. [DOI] [PubMed] [Google Scholar]
- 3.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13:159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 4.Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–1299. doi: 10.1084/jem.20122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mason R., Broaddus V., Martin T., King T.J., Schraufnagel D., Murray J., Nadel J. ed 5. Saunders Elsevier; Philadelphia: 2010. Murray and Nadel's textbook of respiratory medicine. [Google Scholar]
- 6.Ley K., Laudanna C., Cybulsky M.I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 7.Sadik C.D., Kim N.D., Luster A.D. Neutrophils cascading their way to inflammation. Trends Immunol. 2011;32:452–460. doi: 10.1016/j.it.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Williams M.R., Azcutia V., Newton G., Alcaide P., Luscinskas F.W. Emerging mechanisms of neutrophil recruitment across endothelium. Trends Immunol. 2011;32:461–469. doi: 10.1016/j.it.2011.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grommes J., Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossaint J., Zarbock A. Tissue-specific neutrophil recruitment into the lung, liver, and kidney. J Innate Immun. 2013;5:348–357. doi: 10.1159/000345943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fromer C.H., Klintworth G.K. An evaluation of the role of leukocytes in the pathogenesis of experimentally induced corneal vascularization. III. Studies related to the vasoproliferative capability of polymorphonuclear leukocytes and lymphocytes. Am J Pathol. 1976;82:157–170. [PMC free article] [PubMed] [Google Scholar]
- 12.Sholley M.M., Cavallo T., Cotran R.S. Endothelial proliferation in inflammation. I. Autoradiographic studies following thermal injury to the skin of normal rats. Am J Pathol. 1977;89:277–296. [PMC free article] [PubMed] [Google Scholar]
- 13.Joris I., Cuenoud H.F., Doern G.V., Underwood J.M., Majno G. Capillary leakage in inflammation. A study by vascular labeling. Am J Pathol. 1990;137:1353–1363. [PMC free article] [PubMed] [Google Scholar]
- 14.Joris I., Cuenoud H.F., Majno G. Capillary remodeling in acute inflammation: a form of angiogenesis. FASEB J. 1992;6:A938. [Google Scholar]
- 15.Thurston G., Murphy T.J., Baluk P., Lindsey J.R., McDonald D.M. Angiogenesis in mice with chronic airway inflammation: strain-dependent differences. Am J Pathol. 1998;153:1099–1112. doi: 10.1016/S0002-9440(10)65654-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thurston G., Maas K., Labarbara A., McLean J.W., McDonald D.M. Microvascular remodelling in chronic airway inflammation in mice. Clin Exp Pharmacol Physiol. 2000;27:836–841. doi: 10.1046/j.1440-1681.2000.03342.x. [DOI] [PubMed] [Google Scholar]
- 17.Ezaki T., Baluk P., Thurston G., La Barbara A., Woo C., McDonald D.M. Time course of endothelial cell proliferation and microvascular remodeling in chronic inflammation. Am J Pathol. 2001;158:2043–2055. doi: 10.1016/S0002-9440(10)64676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aurora A.B., Baluk P., Zhang D., Sidhu S.S., Dolganov G.M., Basbaum C., McDonald D.M., Killeen N. Immune complex-dependent remodeling of the airway vasculature in response to a chronic bacterial infection. J Immunol. 2005;175:6319–6326. doi: 10.4049/jimmunol.175.10.6319. [DOI] [PubMed] [Google Scholar]
- 19.Baluk P., Yao L.C., Feng J., Romano T., Jung S.S., Schreiter J.L., Yan L., Shealy D.J., McDonald D.M. TNF-alpha drives remodeling of blood vessels and lymphatics in sustained airway inflammation in mice. J Clin Invest. 2009;119:2954–2964. doi: 10.1172/JCI37626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fuxe J., Lashnits E., O'Brien S., Baluk P., Tabruyn S.P., Kuhnert F., Kuo C., Thurston G., McDonald D.M. Angiopoietin/Tie2 signaling transforms capillaries into venules primed for leukocyte trafficking in airway inflammation. Am J Pathol. 2010;176:2009–2018. doi: 10.2353/ajpath.2010.090976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tabruyn S.P., Colton K., Morisada T., Fuxe J., Wiegand S.J., Thurston G., Coyle A.J., Connor J., McDonald D.M. Angiopoietin-2-driven vascular remodeling in airway inflammation. Am J Pathol. 2010;177:3233–3243. doi: 10.2353/ajpath.2010.100059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yao L.C., Baluk P., Feng J., McDonald D.M. Steroid-resistant lymphatic remodeling in chronically inflamed mouse airways. Am J Pathol. 2010;176:1525–1541. doi: 10.2353/ajpath.2010.090909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McDonald D.M., Yao L.C., Baluk P. Dynamics of airway blood vessels and lymphatics: lessons from development and inflammation. Proc Am Thorac Soc. 2011;8:504–507. doi: 10.1513/pats.201102-022MW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carmeliet P., Jain R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307. doi: 10.1038/nature10144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nourshargh S., Hordijk P.L., Sixt M. Breaching multiple barriers: leukocyte motility through venular walls and the interstitium. Nat Rev Mol Cell Biol. 2010;11:366–378. doi: 10.1038/nrm2889. [DOI] [PubMed] [Google Scholar]
- 26.Bergers G., Benjamin L.E. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 27.Cacalano G., Lee J., Kikly K., Ryan A.M., Pitts-Meek S., Hultgren B., Wood W.I., Moore M.W. Neutrophil and B cell expansion in mice that lack the murine IL-8 receptor homolog. Science. 1994;265:682–684. doi: 10.1126/science.8036519. [DOI] [PubMed] [Google Scholar]
- 28.Baluk P., Tammela T., Ator E., Lyubynska N., Achen M.G., Hicklin D.J., Jeltsch M., Petrova T.V., Pytowski B., Stacker S.A., Yla-Herttuala S., Jackson D.G., Alitalo K., McDonald D.M. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J Clin Invest. 2005;115:247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su X., Looney M., Robriquet L., Fang X., Matthay M.A. Direct visual instillation as a method for efficient delivery of fluid into the distal airspaces of anesthetized mice. Exp Lung Res. 2004;30:479–493. doi: 10.1080/01902140490476382. [DOI] [PubMed] [Google Scholar]
- 30.Bachurski C.J., Pryhuber G.S., Glasser S.W., Kelly S.E., Whitsett J.A. Tumor necrosis factor-alpha inhibits surfactant protein C gene transcription. J Biol Chem. 1995;270:19402–19407. doi: 10.1074/jbc.270.33.19402. [DOI] [PubMed] [Google Scholar]
- 31.Busse P.J., Zhang T.F., Srivastava K., Lin B.P., Schofield B., Sealfon S.C., Li X.M. Chronic exposure to TNF-alpha increases airway mucus gene expression in vivo. J Allergy Clin Immunol. 2005;116:1256–1263. doi: 10.1016/j.jaci.2005.08.059. [DOI] [PubMed] [Google Scholar]
- 32.Daley J.M., Thomay A.A., Connolly M.D., Reichner J.S., Albina J.E. Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. J Leukoc Biol. 2008;83:64–70. doi: 10.1189/jlb.0407247. [DOI] [PubMed] [Google Scholar]
- 33.Xu X., Zhang D., Zhang H., Wolters P.J., Killeen N.P., Sullivan B.M., Locksley R.M., Lowell C.A., Caughey G.H. Neutrophil histamine contributes to inflammation in mycoplasma pneumonia. J Exp Med. 2006;203:2907–2917. doi: 10.1084/jem.20061232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McEver R.P., Beckstead J.H., Moore K.L., Marshall-Carlson L., Bainton D.F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989;84:92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Devalaraja R.M., Nanney L.B., Du J., Qian Q., Yu Y., Devalaraja M.N., Richmond A. Delayed wound healing in CXCR2 knockout mice. J Invest Dermatol. 2000;115:234–244. doi: 10.1046/j.1523-1747.2000.00034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reutershan J., Morris M.A., Burcin T.L., Smith D.F., Chang D., Saprito M.S., Ley K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J Clin Invest. 2006;116:695–702. doi: 10.1172/JCI27009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindsey J.R., Cassell H. Experimental Mycoplasma pulmonis infection in pathogen-free mice. Models for studying mycoplasmosis of the respiratory tract. Am J Pathol. 1973;72:63–90. [PMC free article] [PubMed] [Google Scholar]
- 38.McDonald D.M. Infections intensify neurogenic plasma extravasation in the airway mucosa. Am Rev Respir Dis. 1992;146:S40–S44. doi: 10.1164/ajrccm/146.5_Pt_2.S40. [DOI] [PubMed] [Google Scholar]
- 39.Hardy R.D., Jafri H.S., Olsen K., Hatfield J., Iglehart J., Rogers B.B., Patel P., Cassell G., McCracken G.H., Ramilo O. Mycoplasma pneumoniae induces chronic respiratory infection, airway hyperreactivity, and pulmonary inflammation: a murine model of infection-associated chronic reactive airway disease. Infect Immun. 2002;70:649–654. doi: 10.1128/iai.70.2.649-654.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kraft M., Cassell G.H., Pak J., Martin R.J. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 41.Smith L.G. Mycoplasma pneumonia and its complications. Infect Dis Clin North Am. 2010;24:57–60. doi: 10.1016/j.idc.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y., Zhu Z., Church T.D., Lugogo N.L., Que L.G., Francisco D., Ingram J.L., Huggins M., Beaver D.M., Wright J.R., Kraft M. SHP-1 as a critical regulator of Mycoplasma pneumoniae-induced inflammation in human asthmatic airway epithelial cells. J Immunol. 2012;188:3371–3381. doi: 10.4049/jimmunol.1100573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Atkinson T.P., Waites K.B. Mycoplasma pneumoniae Infections in Childhood. Pediatr Infect Dis J. 2014;33:92–94. doi: 10.1097/INF.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 44.Beyrau M., Bodkin J.V., Nourshargh S. Neutrophil heterogeneity in health and disease: a revitalized avenue in inflammation and immunity. Open Biol. 2012;2:120134. doi: 10.1098/rsob.120134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassatella M.A., Gasperini S., Russo M.P. Cytokine expression and release by neutrophils. Ann N Y Acad Sci. 1997;832:233–242. doi: 10.1111/j.1749-6632.1997.tb46251.x. [DOI] [PubMed] [Google Scholar]
- 46.Lammermann T., Afonso P.V., Angermann B.R., Wang J.M., Kastenmuller W., Parent C.A., Germain R.N. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature. 2013;498:371–375. doi: 10.1038/nature12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fiedler U., Scharpfenecker M., Koidl S., Hegen A., Grunow V., Schmidt J.M., Kriz W., Thurston G., Augustin H.G. The Tie-2 ligand angiopoietin-2 is stored in and rapidly released upon stimulation from endothelial cell Weibel-Palade bodies. Blood. 2004;103:4150–4156. doi: 10.1182/blood-2003-10-3685. [DOI] [PubMed] [Google Scholar]
- 48.Ware C.F. The TNF receptor super family in immune regulation. Immunol Rev. 2011;244:5–8. doi: 10.1111/j.1600-065X.2011.01065.x. [DOI] [PubMed] [Google Scholar]
- 49.Xing Z., Kirpalani H., Torry D., Jordana M., Gauldie J. Polymorphonuclear leukocytes as a significant source of tumor necrosis factor-alpha in endotoxin-challenged lung tissue. Am J Pathol. 1993;143:1009–1015. [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Z., Miner J.J., Yago T., Yao L., Lupu F., Xia L., McEver R.P. Differential regulation of human and murine P-selectin expression and function in vivo. J Exp Med. 2010;207:2975–2987. doi: 10.1084/jem.20101545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Griffin G.K., Newton G., Tarrio M.L., Bu D.X., Maganto-Garcia E., Azcutia V., Alcaide P., Grabie N., Luscinskas F.W., Croce K.J., Lichtman A.H. IL-17 and TNF-alpha sustain neutrophil recruitment during inflammation through synergistic effects on endothelial activation. J Immunol. 2012;188:6287–6299. doi: 10.4049/jimmunol.1200385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Angelini D.J., Hyun S.W., Grigoryev D.N., Garg P., Gong P., Singh I.S., Passaniti A., Hasday J.D., Goldblum S.E. TNF-alpha increases tyrosine phosphorylation of vascular endothelial cadherin and opens the paracellular pathway through fyn activation in human lung endothelia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1232–L1245. doi: 10.1152/ajplung.00109.2006. [DOI] [PubMed] [Google Scholar]
- 53.Frater-Schroder M., Risau W., Hallmann R., Gautschi P., Bohlen P. Tumor necrosis factor type alpha, a potent inhibitor of endothelial cell growth in vitro, is angiogenic in vivo. Proc Natl Acad Sci U S A. 1987;84:5277–5281. doi: 10.1073/pnas.84.15.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strieter R.M., Burdick M.D., Gomperts B.N., Belperio J.A., Keane M.P. CXC chemokines in angiogenesis. Cytokine Growth Factor Rev. 2005;16:593–609. doi: 10.1016/j.cytogfr.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Kim H., Koh G.Y. Ang2, the instigator of inflammation. Blood. 2011;118:4767–4768. doi: 10.1182/blood-2011-09-377333. [DOI] [PubMed] [Google Scholar]
- 56.Augustin H.G., Koh G.Y., Thurston G., Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 57.Kim I., Kim J.H., Ryu Y.S., Liu M., Koh G.Y. Tumor necrosis factor-alpha upregulates angiopoietin-2 in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2000;269:361–365. doi: 10.1006/bbrc.2000.2296. [DOI] [PubMed] [Google Scholar]
- 58.Chen J.X., Chen Y., DeBusk L., Lin W., Lin P.C. Dual functional roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H187–H195. doi: 10.1152/ajpheart.01058.2003. [DOI] [PubMed] [Google Scholar]
- 59.Fiedler U., Reiss Y., Scharpfenecker M., Grunow V., Koidl S., Thurston G., Gale N.W., Witzenrath M., Rosseau S., Suttorp N., Sobke A., Herrmann M., Preissner K.T., Vajkoczy P., Augustin H.G. Angiopoietin-2 sensitizes endothelial cells to TNF-alpha and has a crucial role in the induction of inflammation. Nat Med. 2006;12:235–239. doi: 10.1038/nm1351. [DOI] [PubMed] [Google Scholar]
- 60.Majno G. Chronic inflammation: links with angiogenesis and wound healing. Am J Pathol. 1998;153:1035–1039. doi: 10.1016/S0002-9440(10)65648-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sholley M.M., Cotran R.S. Endothelial proliferation in inflammation. II. Autoradiographic studies in x-irradiated leukopenic rats after thermal injury to the skin. Am J Pathol. 1978;91:229–242. [PMC free article] [PubMed] [Google Scholar]
- 62.Peirce S.M., Skalak T.C. Microvascular remodeling: a complex continuum spanning angiogenesis to arteriogenesis. Microcirculation. 2003;10:99–111. doi: 10.1038/sj.mn.7800172. [DOI] [PubMed] [Google Scholar]
- 63.Skalak T.C. Angiogenesis and microvascular remodeling: a brief history and future roadmap. Microcirculation. 2005;12:47–58. doi: 10.1080/10739680590895037. [DOI] [PubMed] [Google Scholar]
- 64.Li X., Wilson J.W. Increased vascularity of the bronchial mucosa in mild asthma. Am J Respir Crit Care Med. 1997;156:229–233. doi: 10.1164/ajrccm.156.1.9607066. [DOI] [PubMed] [Google Scholar]
- 65.Hern S., Mortimer P.S. In vivo quantification of microvessels in clinically uninvolved psoriatic skin and in normal skin. Br J Dermatol. 2007;156:1224–1229. doi: 10.1111/j.1365-2133.2007.07889.x. [DOI] [PubMed] [Google Scholar]
- 66.Zoellner H., Chapple C.C., Hunter N. Microvasculature in gingivitis and chronic periodontitis: disruption of vascular networks with protracted inflammation. Microsc Res Tech. 2002;56:15–31. doi: 10.1002/jemt.10009. [DOI] [PubMed] [Google Scholar]
- 67.Mitzner W., Wagner E.M. Vascular remodeling in the circulations of the lung. J Appl Physiol. 2004;97:1999–2004. doi: 10.1152/japplphysiol.00473.2004. [DOI] [PubMed] [Google Scholar]
- 68.McDonald D.M. Endothelial gaps and permeability of venules in rat tracheas exposed to inflammatory stimuli. Am J Physiol. 1994;266:L61–L83. doi: 10.1152/ajplung.1994.266.1.L61. [DOI] [PubMed] [Google Scholar]
- 69.He P. Leucocyte/endothelium interactions and microvessel permeability: coupled or uncoupled? Cardiovasc Res. 2010;87:281–290. doi: 10.1093/cvr/cvq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Colocalization of myeloperoxidase, S100a8, and S100a9 in neutrophils. Whole-mount preparation of trachea of a wild-type mouse infected with M. pulmonis for 3 days and stained for myeloperoxidase (red, A), S100a9 (green, B), and S100a8 (blue, C). Merged image shows all three markers together (D) and enlarged (E). Most cells have some staining for all three markers. Myeloperoxidase has a granular distribution and is found without S100a9 or S100a8 in some patches (arrows), which could be dead cells. S100a8 and S100a9 have nearly complete correspondence and appear cytoplasmic. Scale bars: 20 μm (A); 10 μm (E).
Peripheral blood neutrophil concentration measured by a veterinary hemocytometer. A: Neutrophils in pathogen-free mice and wild-type (WT, black) and Cxcr2−/− (red) mice after M. pulmonis infection for 7 days (A). Cxcr2−/−mice had neutrophilia even before infection, which became even more pronounced after infection (n = 6 to 10 mice per group, ∗P < 0.05 compared with the pathogen-free group). B: Effect of neutrophil-depleting antibodies 1A8 or RB6-8C5 or control rat IgG on peripheral blood neutrophils during the first 7 days after M. pulmonis infection in WT mice (n = 4 to 8 mice per group). Both antibodies resulted in a reduction in circulating neutrophils compared with control IgG, but antibody RB6-8C5 resulted in a somewhat greater and more sustained reduction than 1A8. ∗P < 0.05 compared with mice treated with IgG.