Abstract
Introduction:
Narcolepsy is associated with obesity though it is uncertain whether this is caused by changes in glucose and fat metabolism. Therefore, we performed a detailed analysis of systemic energy homeostasis in narcolepsy patients, and additionally, investigated whether it was affected by three months of sodium oxybate (SXB) treatment.
Methods:
Nine hypocretin deficient patients with narcolepsy-cataplexy, and nine healthy sex, age, and BMI matched controls were enrolled. A hyperinsulinemic-euglycemic clamp combined with stable isotopes ([6,6-2H2]-glucose and [2H5]- glycerol) was performed at baseline. In seven patients a second study was performed after three months of SXB treatment.
Results:
Glucose disposal rate (GDR) per unit serum insulin was significantly higher in narcolepsy patients compared to matched controls (1.6 ± 0.2 vs. 1.1 ± 0.3 μmol/kgFFM/min/mU×L; P = 0.024), whereas β-cell function was similar (P = 0.50). Basal steady state glycerol appearance rate tended to be lower in narcolepsy patients (5.2 ± 0.4 vs. 7.5 ± 1.3 μmol/kgFM/min; P = 0.058), suggesting a lower rate of lipolysis. SXB treatment induced a trend in reduction of the GDR (1.4 ± 0.1 vs. 1.1 ± 0.2 μmol/kgFFM/min/mU×L; P = 0.063) and a reduction in endogenous glucose production (0.24 ± 0.03 vs. 0.16 ± 0.03 μmol/kgFFM/min/mU×L: P = 0.028) per unit serum insulin. After SXB treatment lipolysis increased (4.9 ± 0.4 vs. 6.5 ± 0.6 μmol/kgFM/min; P = 0.018), and body weight decreased in narcolepsy patients (99.2 ± 6.0 vs. 94.0 ± 5.4 kg; P = 0.044).
Conclusion:
We show that narcolepsy patients are more insulin sensitive and may have a lower rate of lipolysis than matched controls. SXB stimulated lipolysis in narcolepsy patients, possibly accounting for the weight loss after treatment. While sodium oxybate tended to decrease systemic insulin sensitivity, it increased hepatic insulin sensitivity, suggesting tissue-specific effects.
Citation:
Donjacour CE; Aziz NA; Overeem S; Kalsbeek A; Pijl H; Lammers GJ. Glucose and fat metabolism in narcolepsy and the effect of sodium oxybate: a hyperinsulinemic-euglycemic clamp study. SLEEP 2014;37(4):795-801.
Keywords: Hypocretin, orexin, insulin, diabetes, weight loss
INTRODUCTION
Narcolepsy is a sleep disorder that is characterized by excessive daytime sleepiness, cataplexy, hypnagogic hallucinations, sleep paralysis, disturbed nocturnal sleep and obesity.1 Both obesity and disturbed nocturnal sleep are important risk factors for the development of type 2 diabetes mellitus (T2DM).2 Narcolepsy is caused by a loss of hypocretin (orexin) neurons in the hypothalamus.3,4 Hypocretins are neuropeptides known to be involved in sleep-wake regulation, feeding behavior as well as body weight and temperature regulation.5,6 Furthermore hypocretins are important hypothalamic regulators of glucose homeostasis. Disturbed activation of hypocretin neurons during sleep deprivation may lead to increased basal endogenous glucose production and a decreased insulin sensitivity in rats.7 Moreover, hypocretin deficiency leads to an age-related defective glucose tolerance and insulin resistance.8 Intracerebroventricular infusions of hypocretin-1 increase hepatic glucose production in rats,9,10 but intracerebral injections of hypocretin-1 in the ventromedial hypothalamus increase glucose uptake and promote insulin induced glucose uptake and glycogen synthesis in skeletal muscle of mice.11 Taken together, these data imply that the absence of hypocretin in human narcolepsy may disturb glucose homeostasis.
In the sixties and eighties of the previous century, some reports suggested a higher incidence of T2DM in narcolepsy patients.12–14 Another study confirmed a higher risk of metabolic syndrome independent of body mass index (BMI) when comparing patients with narcolepsy to those with idiopathic hypersomnia.15 However, in a recent study comparing narcolepsy patients with healthy controls matched for BMI, no differences in insulin resistance could be detected using the homeostatic model assessment (HOMA),16 a method which estimates β-cell function and insulin resistance from a single pair of fasting glucose and insulin measurements.17 Similarly, another more recent study could not find differences in glucose tolerance and β-cell function between 17 narcolepsy patients and healthy controls.18 So far, there have been no studies using a hyperinsulinemic-euglycemic clamp to estimate insulin sensitivity in narcolepsy patients.
Metabolic syndrome and insulin resistance may lead to T2DM within several years if lifestyle is not adapted.19 Under normal circumstances pancreatic islet β-cells compensate for insulin resistance by increasing insulin release. However, when these compensatory mechanisms fail T2DM will develop.20 The most accurate method available for measuring insulin sensitivity is the hyperinsulinemic-euglycemic clamp technique.21 During a clamp a fixed dose of insulin is continuously infused along with a variable amount of glucose so as to maintain euglycemia, i.e., the plasma glucose levels are “clamped” at a predefined level. Thus, the amount of glucose infused necessary to keep plasma glucose levels constant can be used as a measure of peripheral insulin sensitivity since relatively lower amounts of glucose will be needed in case of insulin resistance.21
Sodium oxybate (SXB), also known as γ-hydroxybutyrate (GHB), is an effective treatment of narcolepsy. It reduces cataplexy, improves nocturnal sleep fragmentation and in higher doses it may also reduce excessive daytime sleepiness.22 SXB activates dopaminergic circuits in the brain.23 As diminished dopamine (D2) receptor mediated signal transduction appears to induce insulin resistance, the metabolic syndrome and T2DM,24 it is conceivable that SXB might be protective against developing T2DM. Additionally, there are indications that SXB might reduce body weight,25 which could also decrease the risk of T2DM.
The abovementioned discrepancies between the findings from earlier reports regarding potential disturbances in glucose metabolism in narcolepsy patients may be due to suboptimal assessment of insulin sensitivity. Moreover, to our knowledge, fat metabolism, which is obviously key to systemic energy homeostasis, has not been scrutinized before in narcolepsy patients. Therefore, in the present study we applied the gold standard for measuring insulin sensitivity, i.e., the hyperinsulinemic-euglycemic clamp, complemented with a stable isotope technique to assess both glucose and fat metabolism in a group of 9 hypocretin deficient narcolepsy with cataplexy patients and 9 individually matched healthy controls. In addition, we assessed the effect of three months of treatment with SXB on glucose and fat metabolism in narcolepsy patients.
METHODS
Subjects
Nine narcolepsy with cataplexy patients and nine, individually age, sex, BMI, fat percentage, and waist-to-hip ratio (WHR) matched healthy controls were enrolled. All patients fulfilled the ICSD-2 criteria for narcolepsy with cataplexy.26 All were HLA DQB1*06:02positive, and CSF hypocretin-1 deficient. None of the controls used medication. Seven patients were drug naive, one discontinued methylphenidate 2 weeks prior to the study. The last patient was tapered off antidepressants and methylphenidate and did not take any drugs in the 2 weeks prior to the start of the study. None of the patients used any other medication. Subjects were eligible for study after exclusion of hypertension, any known (history of) pituitary, psychiatric, or neurological disease (other than narcolepsy), alcohol or drug abuse, recent weight change (> 3 kg weight change within the last 3 months), or a sleep disorder history (controls). Routine laboratory tests were performed to rule out overt diabetes, thyroid disease, anemia, and hepatic and/or renal disease. The study was approved by the ethics committee of the Leiden University Medical Centre. Written informed consent was obtained from all subjects.
Clinical Protocol
All studies started at 08:30 after an overnight fast. Alcohol consumption and excessive exercise on the day preceding the study was not allowed. Caffeinated drinks were also not allowed from 18:00 prior to and on the day of study, and smoking was prohibited until the end of the measurements. Height, weight, BMI, and waist-to-hip ratio were measured according to the WHO recommendations.27 Bioelectrical impedance analysis (Bodystat, Douglas, Isle of Man, UK) was used to estimate lean body mass and fat percentage. Subjects were requested to lie down on a bed in a semi-supine position. A polyethylene catheter was inserted into an antecubital vein for infusion of test substances. Another catheter was inserted into a contralateral dorsal hand vein for blood sampling; this hand was kept in a heated box (60°C) throughout the test to obtain arterialized blood.28 Samples were taken for measurement of basal levels of glucose, insulin, total cholesterol, LDL cholesterol, HDL-cholesterol, triglycerides, glycerol and background enrichment of [6,6-2H2]-glucose and [2H5]-glycerol. At 08:30 (t = 0 min), an adjusted primed (17.6 μmol/ kg×actual plasma glucose concentration (mmol/L)) continuous (0.33 μmol/kg/min) infusion of [6,6-2H2]-glucose (enrichment 99.9%; Cambridge Isotopes, Cambridge, Massachusetts, USA) was started and continued throughout the study. After 60 min, a primed (1.6 μmol/kg) continuous (0.11 μmol/kg/min) infusion of [2H5]-glycerol (Cambridge Isotopes) was started and continued throughout the study. Subsequently, a primed continuous infusion of insulin (Actrapid, Novo Nordisk Pharma BV, Alphen aan de Rijn, The Netherlands; 40 mU/m2/min) was started (t = 120 min). Exogenous glucose 20% enriched with 3% [6,6-2H2]-glucose was infused at a variable rate to maintain the plasma glucose level at 5.0 mmol/L. From t = 210 to 240 min, blood was drawn every 10 min for determination of [6,6-2H2]-glucose and [2H5]-glycerol specific activities, glucose, insulin, and glycerol. While blood in the serum samples was allowed to clot, plasma samples were immediately put on ice. Within 60 min of sampling, all samples were centrifuged at 1,610 g at 4°C for 20 min and then stored at -80°C until assay.
Sodium Oxybate
Narcolepsy patients started SXB treatment after completion of the first clamp session. SXB was given in a regular starting dose of 2 nighttime doses of 2.25 grams. Subsequently, the dose was titrated up to an optimum for each individual patient, although never exceeded the maximum dose of 9 grams per night. When there was satisfactory symptom control for 3 months, according to the patient and the treating neurologist, patients were allowed to embark on the second clamp session. The same neurologist (GJL) evaluated all cases. No other drugs were allowed during these 3 months.
Assays
Serum insulin concentrations were measured by enzyme-labelled chemiluminescent immunometric assay (Immulite 2500; Siemens, Munich, Germany) with an intra-assay coefficient of variation (CV) of 4%. Enrichment of plasma [6,6-2H2]-glucose was determined in a single analytical run using gas chromatography coupled to mass spectrometry, as described previously.29 All isotope enrichments were measured on a gas chromatograph mass spectrometer (model 6890/5973; Hewlett-Packard, Palo Alto, CA). Serum cholesterol, high-density lipoprotein (HDL) and triglycerides (TG) were measured with a fully automated P-800 module (Roche, Almere, The Netherlands). For both TG and total cholesterol (TC) the CV was less than 2%. For HDL the CV was less than 3%. Low-density lipoprotein (LDL) cholesterol was calculated according to the Friedewald equation.30
Calculations
In all clamp sessions a physiological steady state was achieved during the last 30 min of both the basal as well as the hyperinsulinemic period; therefore, the rates of appearance and disappearance for glucose and glycerol were calculated as the tracer infusion rate divided by the tracer to tracee ratio.31 Under steady-state conditions the rate of appearance equals the rate of disappearance, or the disposal rate. Glucose flux rates were expressed per kg fat free mass (FFM), whereas glycerol flux rates were normalized per kg fat mass (FM). Endogenous glucose production (EGP) during the basal steady state is equal to the rate of appearance of glucose whereas EGP during the clamp was calculated as the difference between the rates of glucose appearance and infusion. Since the fasting plasma insulin concentration is a strong inhibitory stimulus for EGP, the basal hepatic insulin resistance index (μmol/min/kgFFM/ pmol×L) was calculated as the product of fasting EGP and fasting plasma insulin concentration.21 The metabolic clearance rate of insulin was calculated as the constant infusion rate of insulin divided by the steady state serum insulin concentration corrected for endogenous insulin secretion. β-cell function was estimated using the HOMA as described previously.17
Statistical Analysis
Results are expressed as mean ± standard error (SEM) unless otherwise specified. The nonparametric Mann-Whitney U test was used to assess differences in medians between patients and controls, whereas the Wilcoxon signed ranks test was applied to assess differences between baseline and SXB conditions. The nonparametric Spearman rank correlation coefficient was used to assess correlations between changes in weight and other metabolic parameters. A paired-samples t-test was used if applicable. All tests were two-tailed, and significance level was set at P < 0.05. Statistical analyses were performed using SPSS for Windows (release 18.0, SPSS, Inc.).
RESULTS
Subjects
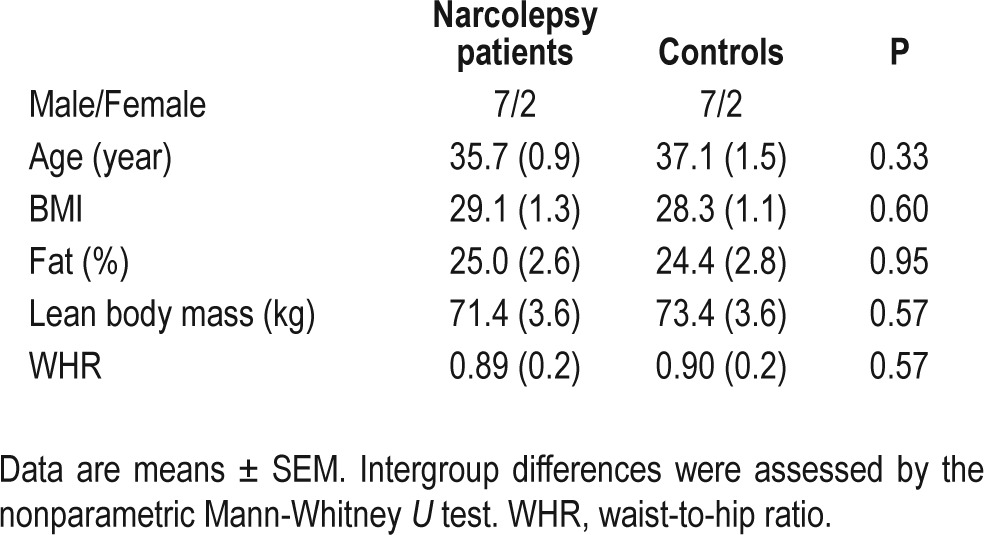
Narcolepsy patients did not differ from controls with respect to sex, age, BMI, WHR, lean body mass, or fat percentage (all P ≥ 0.33, Table 1). Moreover baseline glucose, insulin, total cholesterol (4.7 ± 0.23 vs. 4.7 ± 0.26 mmol/L), LDL cholesterol (3.1 ± 0.18 vs. 3.0 ± 0.23 mmol/L), HDL-cholesterol (0.98 ± 0.06 vs. 1.2 ± 0.11 mmol/L), and triglycerides (1.4 ± 0.26 vs. 1.0 ± 0.14 mmol/L) did not differ between the 2 groups (all P ≥ 0.10). SXB was well tolerated except in one patient who discontinued due to an unacceptable increase in sleep paralysis. Another patient with excessive daytime sleepiness as major complaint could not be assessed after treatment due to the fact that he insisted on taking stimulants. Thus 9 patients and controls were measured at baseline, and 7 patients were assessed for a second time. Narcolepsy patients lost an average amount of 5.2 kg of their body weight after 3 months of treatment with SXB (99.2 ± 6.0 vs. 94.0 ± 5.4 kg; P = 0.044).
Table 1.
Demographics and body composition
Glucose Metabolism in Narcolepsy Patients versus Controls
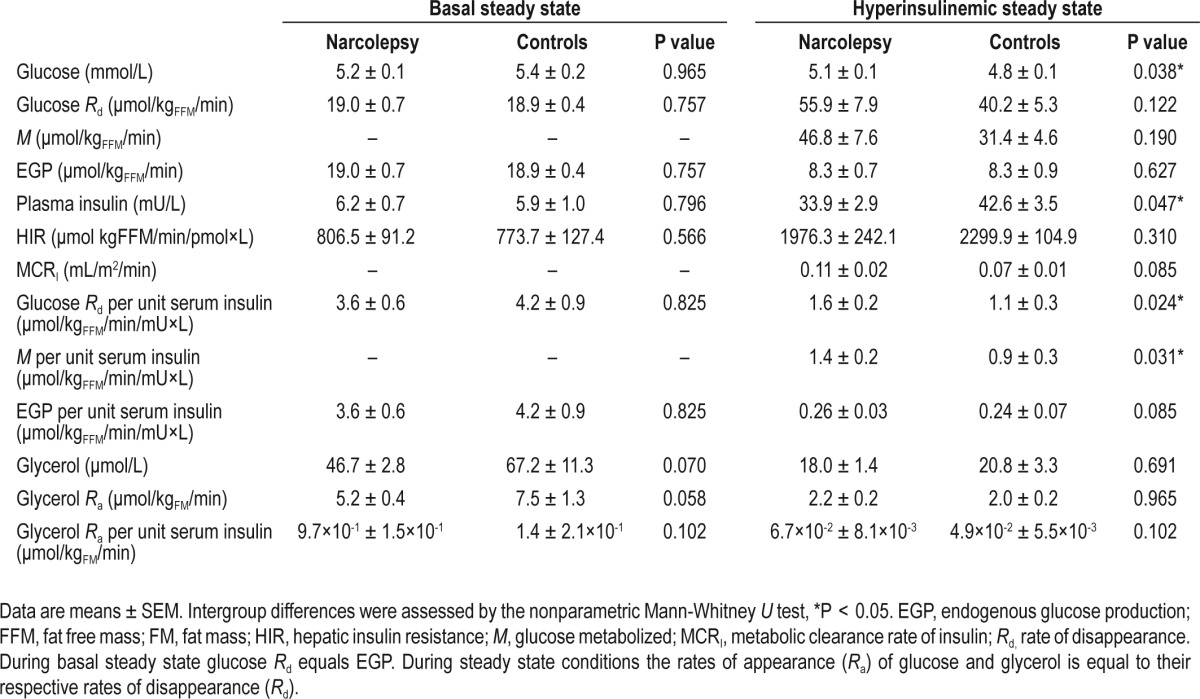
An overview of all metabolic parameters in narcolepsy patients and controls during both the basal steady state and the hyperinsulinemic steady state is presented in Table 2. During the basal steady state there were no significant differences in glucose disposal rate (Glucose Rd) between patients and controls. In addition, during the hyperinsulinemic steady state the glucose disposal rate was similar in narcolepsy patients and controls (55.9 ± 7.9 vs. 40.2 ± 5.3 μmol/kgFFM/min, P = 0.122), despite significantly lower steady state plasma insulin levels (33.9 ± 2.9 vs. 42.6 ± 3.5 mU/L, P = 0.047). When adjusted for the differences in plasma insulin levels, the glucose disposal rate (1.6 ± 0.2 vs. 1.1 ± 0.3 μmol/kgFFM/min/mU×L, P = 0.024) was significantly higher in patients, whereas the rate of endogenous glucose production (0.26 ± 0.03 vs. 0.24 ± 0.07 μmol/kgFFM/min/mU×L, P = 0.085) did not differ between both groups. In addition, β-cell function as assessed using the baseline glucose and insulin levels was equal in the two groups (8.7 vs. 10.3 Mann-Whitney U = 33, Z = -0.66, P = 0.50).
Table 2.
Metabolic parameters in nine patients with narcolepsy and nine matched controls during the basal steady state and during the hyperinsulinemic steady state
Lipid Metabolism in Narcolepsy Patients versus Controls
During the basal steady state plasma glycerol levels (46.7 ± 2.8 vs. 67.2 ± 11.3 μmol/L, P = 0.070), as well as the rate of glycerol appearance (5.2 ± 0.4 vs. 7.5 ± 1.3 μmol/kgFM/min, P = 0.058) tended to be lower in narcolepsy patients compared to controls, suggesting a lower rate of lipolysis in narcolepsy patients. However, these trends disappeared during the hyperinsulinemic steady state, indicating an intact insulin mediated suppression of lipolysis in narcolepsy patients. These findings remained unchanged when adjusted for plasma insulin levels (Table 2).
Glucose Metabolism in Narcolepsy Patients before and after SXB
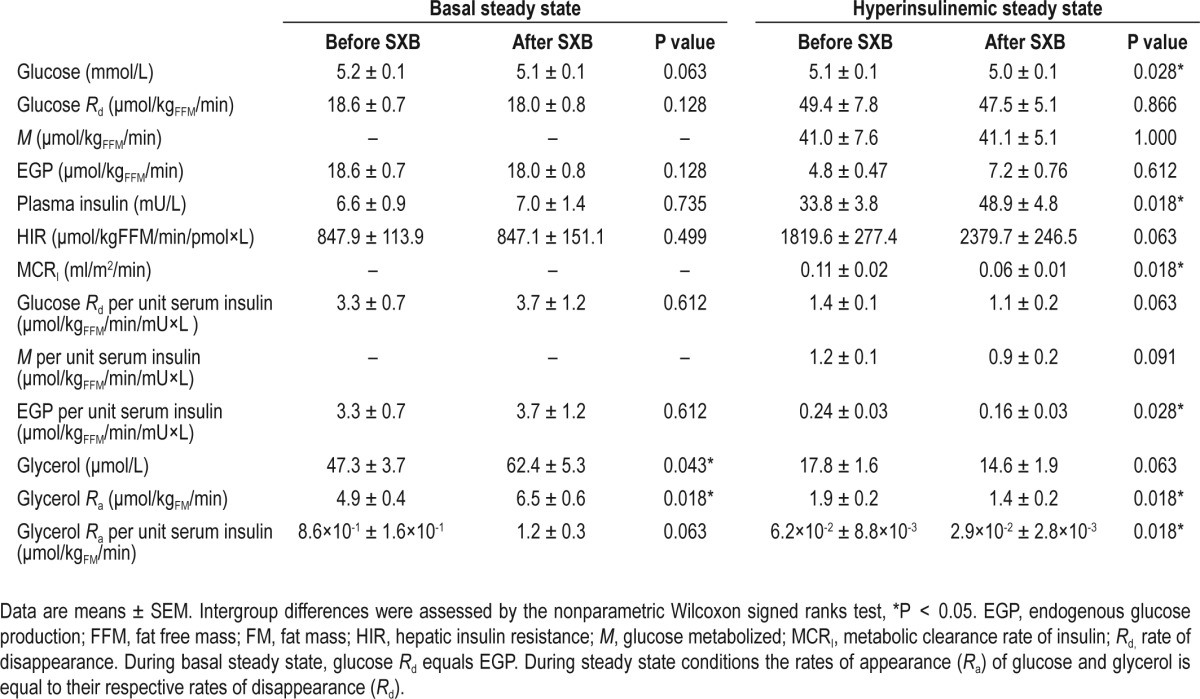
β-cell function did not change after SXB treatment (P = 0.61). SXB had no obvious effects on the basal steady state glucose metabolism (Table 3). After SXB treatment the steady state plasma insulin levels were significantly higher during the hyperinsulinemic conditions (33.8 ± 3.8 vs. 48.9 ± 4.8 mU/L, P = 0.018), perhaps due to a significantly lower metabolic clearance rate of insulin (0.11 ± 0.02 vs. 0.06 ± 0.01 mL/ m2/min, P = 0.018). When adjusted for these differences in plasma insulin levels, SXB treatment induced a trend towards a reduced rate of glucose disposal (1.4 ± 0.1 vs. 1.1 ± 0.2 μmol/ kgFFM/min/mU×L, P = 0.063). In addition there was a signifi-cant reduction in endogenous glucose production (0.24 ± 0.03 vs. 0.16 ± 0.03 μmol/kgFFM/min/mU×L, P = 0.028) during the hyperinsulinemic condition. Thus, SXB had a tendency to decrease peripheral (primarily muscle) insulin sensitivity, while it increased hepatic insulin sensitivity (Table 3).
Table 3.
Metabolic parameters in seven patients with narcolepsy before and after sodium oxybate (SXB) treatment
Lipid Metabolism in Narcolepsy Patients before and after SXB
SXB treatment significantly increased both basal levels of glycerol (47.3 ± 3.7 vs. 62.4 ± 5.3 μmol/L, P = 0.043) and the rate of glycerol appearance (4.9 ± 0.4 vs. 6.5 ± 0.6 μmol/kgFM/ min, P = 0.018), indicating that SXB treatment increases lipolysis in narcolepsy patients (Table 3). After SXB treatment the rate of lipolysis became lower during hyperinsulinemic conditions as evidenced by a trend towards lower plasma glycerol levels (17.8 ± 1.6 vs. 14.6 ± 1.9 μmol/L, P = 0.063) and a lower rate of glycerol appearance (1.9 ± 0.2 vs. 1.4 ± 0.2 μmol/kgFM/min, P = 0.018), suggesting that SXB increases the sensitivity of fat tissue to the inhibitory effects of insulin on lipolysis (Table 3). These findings remained largely unchanged when adjusted for plasma insulin levels (Table 3).
Metabolic Parameters in Relation to Weight Change
The change in body weight after SXB treatment was strongly associated with alterations in glucose disposal rate per unit serum insulin (r = -0.93, P = 0.003; Figure 1), but not with changes in endogenous glucose production per unit serum insulin (r = 0.29, P = 0.535).
Figure 1.
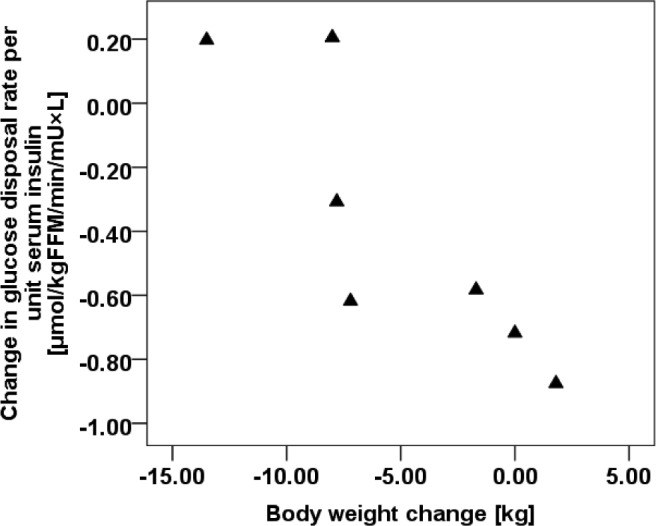
Correlations in body weight change and glucose disposal rate.
DISCUSSION
The aim of this study was to accurately assess both glucose and fat metabolism in narcolepsy patients, as well as the metabolic effects of SXB treatment using the most accurate method available, i.e. a combination of a hyperinsulinemic-euglycemic clamp and stable isotope techniques. Surprisingly, we found increased peripheral insulin sensitivity in narcolepsy patients, whereas hepatic insulin sensitivity and β-cell function were not different from matched healthy controls. The higher insulin sensitivity in narcolepsy patients was reflected by a higher rate of insulin-mediated glucose uptake in peripheral tissues, of which skeletal muscle is the most important. Lipolysis tended to be lower in narcolepsy patients, which could be due to insulin sensitivity of adipose tissue. This finding may at least partly account for comorbid overweight in narcolepsy, which is seen in two thirds of the patients.32 Also the higher insulin sensitivity itself might contribute.
Furthermore, we found that SXB treatment increased hepatic but tended to decrease whole body insulin sensitivity. Moreover, SXB stimulated lipolysis, which might be one of the reasons why patients lose weight while on this drug. In our study, patients lost on average 5.2 kg of weight after 3 months of SXB treatment which is in line with an earlier report that SXB treatment is associated with weight loss.25 The mechanisms through which hypocretin-deficiency and SXB treatment affect glucose and fat metabolism might involve modulation of autonomic nervous system activity, which recently has been demonstrated to be critically implicated in the regulation of energy homeostasis.33 SXB appears to hamper insulin mediated glucose disposal in patients who maintain a stable body weight during treatment, but it simultaneously induces weight loss in other patients, which clearly compensates for the reduction in insulin sensitivity (Figure 1). On average, glucose disposal per unit of circulating insulin was reduced by SXB (Table 3). The impact of SXB on insulin action appears to be tissue specific: while glucose disposal is diminished, the capacity of insulin to suppress glucose production is reinforced by SXB (Table 3). The mechanism underlying the effects of SXB is unknown. As glucose disposal rate is primarily determined by glucose uptake in muscle tissue, and given the fact that the capacity of insulin to suppress lipolysis was not affected by SXB treatment, our findings suggest that the systemic effects of SXB on insulin sensitivity are chiefly mediated by changes in muscle, rather than fat, insulin sensitivity.
Since peripheral insulin sensitivity is increased and pancreatic β-cell function is normal in narcolepsy patients, our data do not support the notion that the risk of T2DM is increased in narcolepsy, which is in contrast to some earlier reports.12–15 We found no differences in pancreatic β-cell function, which is in line with previous observations.16,18 However, increased insulin sensitivity has not been previously reported in narcolepsy. Several explanations might account for these differences. Firstly, to our knowledge we are the first to apply the hyper-insulinemic-euglycemic clamp to assess insulin sensitivity, whereas previous studies used indirect and derived measures of insulin sensitivity such as the homeostasis model assessment and minimal model analysis.18 Although the method used by Beitinger et al. highly correlates with the clamp technique, it is not the same and less accurate.34 Secondly, in both earlier studies patients continued to be on their usual medication which might have confounded their results. For example, antidepressants, which are used as anticataplectic drugs, may induce insulin resistance.35 Thirdly, all our patients were proven to be hypocretin deficient and HLA-DQB1*06:02 positive which makes our group more homogeneous than the ones in earlier reports. There is a growing body of evidence that hypocretin plays a fundamental role in glucose metabolism.7,33,36 However, the precise role of hypocretin in systemic glucose homeostasis is likely to be complex and seems even paradoxical since it can have both hyperglycemic9 as well as hypoglycemic effects.33 This dual effect of hypocretin in glucose homeostasis is probably related to its decisive role in the control of sleep/ wake and circadian rhythms, i.e., at awakening hypocretin not only stimulates arousal but also glucose production as well as glucose uptake.37
One limitation of this study is the relatively small number of subjects. However, this limitation is partly offset by the application of very sensitive techniques, which would have been unfeasible in larger groups of subjects due to the expensive and arduous nature of the experiments.
In conclusion, our findings show that narcolepsy patients are more insulin sensitive and tend to have a lower rate of lipolysis than weight matched controls. SXB stimulated lipolysis in narcolepsy patients, possibly accounting for the observed weight loss after treatment. While SXB had a tendency to decrease systemic insulin sensitivity, it increased hepatic insulin sensitivity, suggesting tissue-specific effects.
DISCLOSURE STATEMENT
This study is supported by a grant from UCB Pharma, but was designed, performed, analysed, and written without any involvement of UCB pharma. Dr. Donjacour, Dr. Lammers, and Dr. Overeem received lecture fees and conference travel support from UCB Pharma. Dr. Lammers is a member of the international advisory board on narcolepsy of UCB Pharma. Dr. Overeem has served as a paid member of the UCB advisory board and also received lecture fees from, Novartis, and Boeh-ringer Ingelheim. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank all volunteers for participating in this study. In addition we are greatly indebted to P. P. Buijserd-Amesz, S.T. Walma and EJM Ladan-Eygenraam for technical assistance during the study. We also thank T. Streefland for determinations of stable isotope enrichment.
REFERENCES
- 1.Overeem S, Mignot E, VAN Dijk JG, Lammers GJ. Narcolepsy: clinical features, new pathophysiologic insights, and future perspectives. J Clin Neurophysiol. 2001;18:78–105. doi: 10.1097/00004691-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Spiegel K, Knutson K, Leproult R, Tasali E, Cauter van E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99:2008–19. doi: 10.1152/japplphysiol.00660.2005. [DOI] [PubMed] [Google Scholar]
- 3.Thannickal TC, Moore RY, Nienhuis R, et al. Reduced number of hypocretin neurons in human narcolepsy. Neuron. 2000;27:469–74. doi: 10.1016/s0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peyron C, Faraco J, Rogers W, et al. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med. 2000;6:991–7. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- 5.Willie JT, Chemelli RM, Sinton CM, Yanagisawa M. To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci. 2001;24:429–58. doi: 10.1146/annurev.neuro.24.1.429. [DOI] [PubMed] [Google Scholar]
- 6.Taylor MM, Samson WK. The other side of the orexins: endocrine and metabolic actions. Am J Physiol Endocrinol Metab. 2003;284:E13–7. doi: 10.1152/ajpendo.00359.2002. [DOI] [PubMed] [Google Scholar]
- 7.Girault EM, Yi CX, Fliers E, Kalsbeek A. Orexins, feeding, and energy balance. Prog Brain Res. 2012;198:47–64. doi: 10.1016/B978-0-444-59489-1.00005-7. [DOI] [PubMed] [Google Scholar]
- 8.Tsuneki H, Murata S, Anzawa Y, et al. Age-related insulin resistance in hypothalamus and peripheral tissues of orexin knockout mice. Diabetologia. 2008;51:657–67. doi: 10.1007/s00125-008-0929-8. [DOI] [PubMed] [Google Scholar]
- 9.Yi CX, Serlie MJ, Ackermans MT, et al. A major role for perifornical orexin neurons in the control of glucose metabolism in rats. Diabetes. 2009;58:1998–2005. doi: 10.2337/db09-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yi CX, Sun N, Ackermans MT, et al. Pituitary adenylate cyclase-activating polypeptide stimulates glucose production via the hepatic sympathetic innervation in rats. Diabetes. 2010;59:1591–600. doi: 10.2337/db09-1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiuchi T, Haque MS, Okamoto S, et al. Hypothalamic orexin stimulates feeding-associated glucose utilization in skeletal muscle via sympathetic nervous system. Cell Metab. 2009;10:466–80. doi: 10.1016/j.cmet.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 12.Honda Y, Doi Y, Ninomiya R, Ninomiya C. Increased frequency of non-insulin-dependent diabetes mellitus among narcoleptic patients. Sleep. 1986;9:254–9. doi: 10.1093/sleep/9.1.254. [DOI] [PubMed] [Google Scholar]
- 13.Roberts HJ. The syndrome of narcolepsy and diabetogenic (“functional”) hyperinsulinism. Observations on 190 patients, with emphasis upon its relationship to obesity, diabetes mellitus and cerebral dysrhythmias. J Fla Med Assoc. 1963;50:355–66. [PubMed] [Google Scholar]
- 14.Roberts HJ. The syndrome of narcolepsy and diabetogenic (“functional”) hyperinsulinism, with special reference to obesity, diabetes, idiopathic edema, cerebral dysrhythmias and multiple sclerosis (200 patients) J Am Geriatr Soc. 1964;12:926–76. doi: 10.1111/j.1532-5415.1964.tb00642.x. [DOI] [PubMed] [Google Scholar]
- 15.Poli F, Plazzi G, Di DG, et al. Body mass index-independent metabolic alterations in narcolepsy with cataplexy. Sleep. 2009;32:1491–7. doi: 10.1093/sleep/32.11.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engel A, Helfrich J, Manderscheid N, et al. Investigation of insulin resistance in narcoleptic patients: dependent or independent of body mass index? Neuropsychiatr Dis Treat. 2011;7:351–6. doi: 10.2147/NDT.S18455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 18.Beitinger PA, Fulda S, Dalal MA, et al. Glucose tolerance in patients with narcolepsy. Sleep. 2012;35:231–6. doi: 10.5665/sleep.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cowie CC, Rust KF, Byrd-Holt DD, et al. Prevalence of diabetes and high risk for diabetes using A1C criteria in the U.S. population in 1988-2006. Diabetes Care. 2010;33:562–8. doi: 10.2337/dc09-1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahn SE. Clinical review 135: The importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001;86:4047–58. doi: 10.1210/jcem.86.9.7713. [DOI] [PubMed] [Google Scholar]
- 21.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 22.The Xyrem International Study Group. A double-blind, placebo-controlled study demonstrates sodium oxybate is effective for the treatment of excessive daytime sleepiness in narcolepsy. J Clin Sleep Med. 2005;1:391–7. [PubMed] [Google Scholar]
- 23.Carter LP, Koek W, France CP. Behavioral analyses of GHB: receptor mechanisms. Pharmacol Ther. 2009;121:100–14. doi: 10.1016/j.pharmthera.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pijl H. Reduced dopaminergic tone in hypothalamic neural circuits: expression of a “thrifty” genotype underlying the metabolic syndrome? Eur J Pharmacol. 2003;480:125–31. doi: 10.1016/j.ejphar.2003.08.100. [DOI] [PubMed] [Google Scholar]
- 25.Husain AM, Ristanovic RK, Bogan RK. Weight loss in narcolepsy patients treated with sodium oxybate. Sleep Med. 2009;10:661–3. doi: 10.1016/j.sleep.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 26.American Academy of Sleep Medicine. International classification of sleep disorders. diagnostic and coding manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005. American Sleep Association. [Google Scholar]
- 27.Andersson BL, Bjorntorp P, Seidell JC. Measuring obesity- classification and description of anthropometric data report on a WHO consultation on epidemiology of obesity. 1988;125:1–22. [Google Scholar]
- 28.Liu D, Moberg E, Kollind M, Lins PE, Adamson U, Macdonald IA. Arterial, arterialized venous, venous and capillary blood glucose measurements in normal man during hyperinsulinaemic euglycaemia and hypoglycaemia. Diabetologia. 1992;35:287–90. doi: 10.1007/BF00400932. [DOI] [PubMed] [Google Scholar]
- 29.Jazet IM, Pijl H, Frolich M, Romijn JA, Meinders AE. Two days of a very low calorie diet reduces endogenous glucose production in obese type 2 diabetic patients despite the withdrawal of blood glucose-lowering therapies including insulin. Metabolism. 2005;54:705–12. doi: 10.1016/j.metabol.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 31.Steele ER. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–30. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 32.Kok SW, Overeem S, Visscher TL, et al. Hypocretin deficiency in narcoleptic humans is associated with abdominal obesity. Obes Res. 2003;11:1147–54. doi: 10.1038/oby.2003.156. [DOI] [PubMed] [Google Scholar]
- 33.Tsuneki H, Wada T, Sasaoka T. Role of orexin in the central regulation of glucose and energy homeostasis. Endocr J. 2012;59:365–74. doi: 10.1507/endocrj.ej12-0030. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–70. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 35.Khoza S, Barner JC, Bohman TM, Rascati K, Lawson K, Wilson JP. Use of antidepressant agents and the risk of type 2 diabetes. Eur J Clin Pharmacol. 2012;68:1295–302. doi: 10.1007/s00228-011-1168-3. [DOI] [PubMed] [Google Scholar]
- 36.Venner A, Karnani MM, Gonzalez JA, Jensen LT, Fugger L, Burdakov D. Orexin neurons as conditional glucosensors: paradoxical regulation of sugar sensing by intracellular fuels. J Physiol. 2011;589:5701–8. doi: 10.1113/jphysiol.2011.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kalsbeek A, Yi CX, La Fleur SE, Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab. 2010;21:402–10. doi: 10.1016/j.tem.2010.02.005. [DOI] [PubMed] [Google Scholar]


