Abstract
Study Objectives:
Interspecific variation in sleep measured in captivity correlates with various physiological and environmental factors, including estimates of predation risk in the wild. However, it remains unclear whether prior comparative studies have been confounded by the captive recording environment. Herein we examine the effect of predation pressure on sleep in sloths living in the wild.
Design:
Comparison of two closely related sloth species, one exposed to predation and one free from predation.
Setting:
Panamanian mainland rainforest (predators present) and island mangrove (predators absent).
Participants:
Mainland (Bradypus variegatus, five males and four females) and island (Bradypus pygmaeus, six males) sloths.
Interventions:
None.
Measurements and Results:
Electroencephalographic (EEG) and electromyographic (EMG) activity was recorded using a miniature data logger. Although both species spent between 9 and 10 h per day sleeping, the mainland sloths showed a preference for sleeping at night, whereas island sloths showed no preference for sleeping during the day or night. Standardized EEG activity during nonrapid eye movement (NREM) sleep showed lower low-frequency power, and increased spindle and higher frequency power in island sloths when compared to mainland sloths.
Conclusions:
In sloths sleeping in the wild, predation pressure influenced the timing of sleep, but not the amount of time spent asleep. The preference for sleeping at night in mainland sloths may be a strategy to avoid detection by nocturnal cats. The pronounced differences in the NREM sleep EEG spectrum remain unexplained, but might be related to genetic or environmental factors.
Citation:
Voirin B; Scriba MF; Martinez-Gonzalez D; Vyssotski AL; Wikelski M; Rattenborg NC. Ecology and neurophysiology of sleep in two wild sloth species. SLEEP 2014;37(4):753-761.
Keywords: Benzodiazepine, EEG, predation, NREM sleep, REM sleep, sloth, spindle, phasing, wild
INTRODUCTION
Sleep is an important part of an animal's life, yet the actual function of sleep remains actively debated.1–5 Every organism studied has been found to sleep in some manner,6 even though the reduction in environmental awareness can be dangerous.7 Mammals engage in two types of sleep: rapid eye movement (REM) sleep and non-rapid eye movement (NREM) sleep. REM sleep is characterized by low-amplitude, high-frequency activity in the electroencephalogram (EEG) similar to that occurring during wakefulness.8 REM sleep is distinguished from wakefulness by a marked reduction in skeletal muscle tone, as well as behavioral signs of sleep, including eye closure and an increased arousal threshold.8 NREM sleep is characterized by high-amplitude, low-frequency EEG waves (typically 0.5-4.5 Hz) and thalamocortical spindles,9 intermittent bursts of waxing and waning (i.e., spindle-shaped) activity. Although the general characteristics of spindles are similar, the frequency of the oscillations varies across species (e.g., echidna, 6-8 Hz10; opossum, 8-11 Hz11; armadillo, 12 Hz12; sloth, 6-7 Hz13; guinea pig, 13-15 Hz14; cat, 7-14 Hz15; dog, 12-15 Hz16; human, 12-15 Hz17).
The amount of time spent sleeping, as well as the relative time spent in NREM and REM sleep, varies greatly between species.18–20 It has been proposed that predation pressure can partially explain the diversity of sleep patterns observed in mammals7,21,22 and other animals.23–25 When compared with NREM sleep, REM sleep appears to be particularly sensitive to predation risk, perhaps because higher arousal thresholds during REM sleep might render an animal more vulnerable to predation.19,22,26 In interspecific comparisons of sleep in captive mammals, prey species that sleep exposed to predators in the wild spend a lower percentage of their total sleep time in REM sleep (percent REM sleep) than those that sleep in low-risk settings in the wild.21,22 In addition, in laboratory experiments, rodents exposed to simulated predation attacks exhibit a pronounced reduction in percent REM sleep,27,28 particularly when the threat is inescapable.29 However, it remains unclear whether these relationships exist in the wild or simply reflect an artifact of the captive environment.
Until recently, EEG investigations of sleep in animals were confined to captivity. However, as technological advances allow more biological processes to be studied in the wild, it is becoming apparent that studies involving wild and captive individuals of the same species often yield largely contradictory results. For example, circadian rhythms, immune function, and reproductive physiology are influenced by captivity.30–32 In addition, in the first EEG-based study of sleep in an animal in the wild, we found that brown-throated three-toed sloths (Bradypus variegatus) in the rainforest slept more than 6 h less than ones in captivity.13,33 This finding high-lighted persistent concerns that prior comparative studies based on sleep durations in captive animals may have been confounded by the captive environment.22,34 Although the reasons for more sleep in captive sloths remain unclear, one possible explanation is that they do not face the risk of predation as do their wild counterparts, and therefore are free to sleep longer.
Herein, we examined sleep in two closely related species of three-toed sloths living under different predation pressure situations. One population lives in the tropical rainforest with natural predators (mainly nocturnal cats), and the other lives on an isolated island with no apparent predators.35 Based on the hypothesis that the lower amount of sleep in wild sloths reflects a response to increased predation pressure, we predicted that sloths living in the high predation habitat would (1) sleep less, (2) show reduced REM sleep as a percent of total sleep time, and (3) show a preference for sleeping during the day, when nocturnal cats are sleeping.36
METHODS
Ethics Statement
This study and all methods used, including animal capture, handling, and logger attachment, were fully approved by the Smithsonian Tropical Research Institute's Animal Care and Use Committee (IACUC). The fieldwork was conducted under research permit SA/E 21-09, granted by La Autoridad Nacional del Ambiente (ANAM).
Animals, Locations, and Environmental Conditions
Bradypus variegatus: The brown-throated three-toed sloth is an arboreal mammal common throughout tropical rainforests in Central America and South America. They feed on a variety of tree and liana species.37 B. variegatus is vulnerable to a number of natural predators, including nocturnal cats38,39 and possibly owls,40 and diurnal harpy eagles (Harpia harpyja)41,42; although harpy eagles became extinct in the study area in the last century.43 Average body weight is 4 kg, and their average home range in Panama is 1.5 hectares.37
Bradypus pygmaeus: The pygmy sloth is endemic to Isla Escudo de Veraguas, Panama (9° 5'58.78”N, 81°33'33.60”W). It is closely related to B. variegatus, and has been separated geographically for only 8,900 years.35 B. pygmaeus is found mainly in patches of red mangroves (Rhizophora mangle), and is thought to eat predominantly, if not solely, mangrove leaves,44,45 although this feeding habit has not been systematically studied. They face no natural predators on the island. Their average weight is 3 kg, and their population and home range are unknown. B. variegatus (hereafter referred to as mainland sloths) were studied in the rainforests near Almirante, in Bocas del Toro, Panama (9°19'2.29”N, 82°26'29.91”W) between April 7-17, 2009. During this period sunrise ranged from 06:18-06:23 and sunset was at 18:39. Human settlements are scattered throughout the forest. Sloths are abundant, as are their main nocturnal feline predators, ocelots (Leopardus pardalis), pumas (Puma concolor), and margays (Leopardus wiedii).46,47
B. pygmaeus (hereafter referred to as island sloths) were studied in the mangroves of Isla Escudo de Veraguas. The island is 4.3 km2 and is 17.6 km offshore from the Valiente Peninsula. Recordings were done between April 20-29, 2009. During this period, sunrise occurred from 06:11-06:14 and sunset from 18:37-18:38. Consequently, day length differed between the recording periods for each sloth species by less than 10 min.
Weather data was obtained from Bocas del Toro (9°20'45.69”N, 82°15'11.74”W), the closest weather station to the two study sites. The station is approximately 21 and 80 km from the mainland and island sloth populations, respectively; the distance between the two populations is approximately 100 km. The minimum (min) and maximum (max) temperatures during the recording periods for the two sloth species were similar (mean ± standard error of the mean [SEM]; mainland sloths, min 24.3 ± 0.2°C, max 29.7 ± 0.3°C; island sloths, min 24.6 ± 0.3°C, max 29.1 ± 0.4°C; unpaired two-tailed t-tests; min, P = 0.40 and max, P = 0.17). Rain was reported (but not quantified) on 72.3% and 70.0% of the days during the recording period for the mainland and island sloths, respectively. Although these data suggest that the weather conditions were similar during the two recording periods, we cannot rule out the possibility that very local differences in weather occurred during the two recording periods.
Animal Capture and Recapture
Fifteen adult sloths (mainland: five males and four females; island: six males; sex determined by pelage) were caught using single rope-climbing technique and a snare pole,37 or when possible, by hand. On the ground, the sloths were held while an EEG logger (Neurologger 248, www.vyssotski.ch/neurologger2) was attached to their head (see next paragraphs). In addition, sloths were fitted with a radio collar (www.ATS-Tracking.com) to relocate them. After attaching the equipment, the sloths were placed at the base of the tree where they were caught.
EEG and Electromyogram Recording
The EEG logger attachment procedure that was used previously on sloths was followed.33 A patch of hair was trimmed directly over the cranium. The scalp was disinfected with alcohol wipes and sprayed with an analgesic (Gingicain® aerosol, Sanofi-Aventis GmbH, Frankfurt, Germany). The electrode placement along the anterior-posterior axis was standardized across sloths of different sizes using the distance between a palpable anterior cranial concavity over bregma and a posterior concavity over lambda (Figure 1). The distance between bregma and lambda was divided into segments of 25%. For each hemisphere, an anterior electrode (silver wire, 7/40 AG, Sigmund Cohn, Mt Vernon, NY) was inserted under the skin with a 22-gauge needle, at a point 25% posterior to bregma. A second wire was inserted 25% anterior to lambda. Each wire was equidistant from the midline and the most dorsal point of attachment of the temporalis muscle, also palpable through the skin. Because the EEG electrodes were close to major points of muscle attachment (Figure 1), they were also sensitive to changes in muscle tone. The ground was centered between the other electrodes. The wires were fixed in place using adhesive glue, and were then connected to the logger. The logger was housed in a modified film canister, which was then glued to the top of the head (Figure 2). The logger was powered by a 3.6-V battery (Saft, model LS-14250, Bagnolet, France). The total weight of the logger, battery, and housing (18 g), plus the radio collar (56 g) was less than 3% of the animal's weight. All research equipment was completely removed from the animal at the end of the study. The sloths showed no signs of scratching at or otherwise being bothered by the equipment. Bipolar EEG signals from each hemisphere were sampled and recorded at 100 Hz. The recordings lasted between 212 and 241 h.
Figure 1.
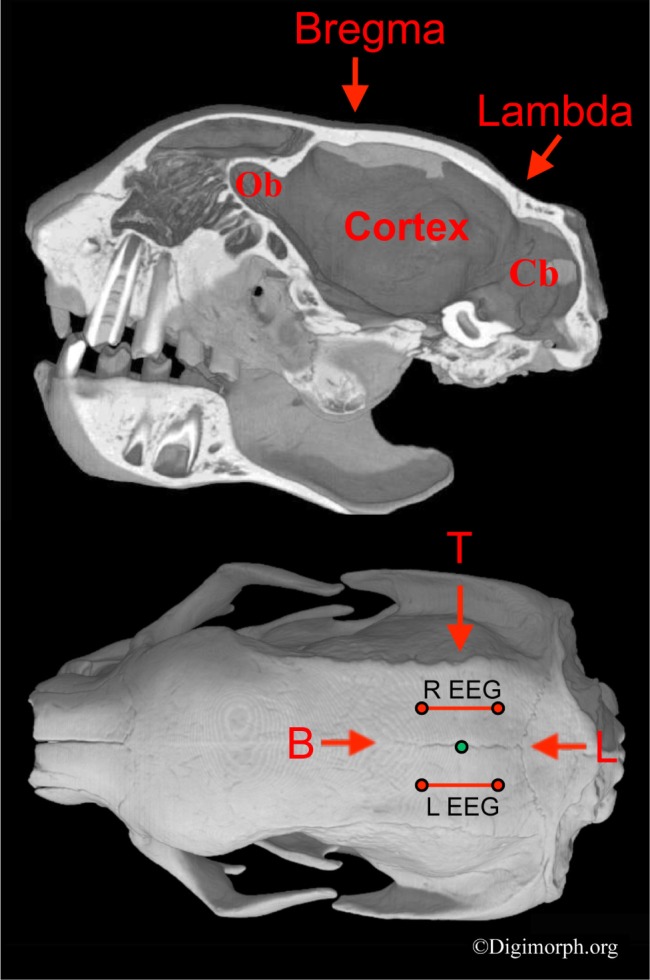
Sagittal cross section (top) and dorsal view (bottom) of Bradypus variegatus' (CT image) skull showing electrode placement. Electroencephalogram (EEG) electrodes (red dots) and bipolar EEG derivations (red lines) are shown relative to bregma (B), lambda (L), and the most dorsal point of attachment of the temporalis muscle (T). The green dot shows the ground electrode. The sagittal cross-section through the midline shows the concavities overlying the cranial sutures bregma and lambda, and their positions relative to the underlying brain case. These surface concavities were palpable through the skin and used as landmarks. Given that the skull structure of B. pygmaeus and B. variegatus are very similar,35 the same landmarks were used to position the electrodes in both species. The electrode placement was scaled to the size of the individual's skull. Ob, olfactory bulb; Cb, cerebellum. CT images provided with permission from Digimorph.org.
Figure 2.

Brown-throated three-toed sloth equipped with an encephalo-graphic logger (black hat).
State Scoring
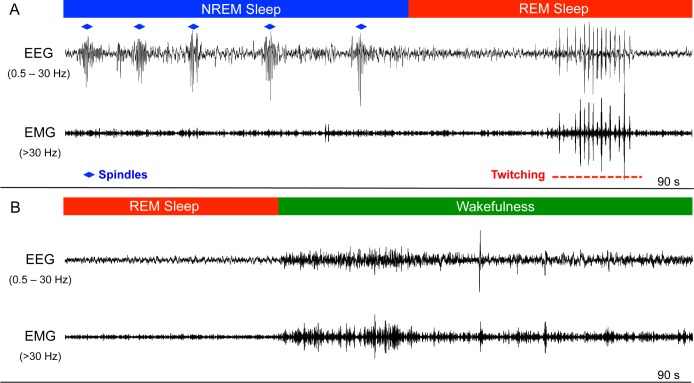
The EEG data was downloaded and imported into Somnologica Science Software (MedCare Corporation, Newport Beach, California, USA) for scoring and analysis. During scoring, each signal was visualized with the filters set for EEG activity (0.5-30 Hz pass) and again with the filters set for electromyogram (EMG) activity (> 30 Hz pass) (Figure 3). For each sloth, the state (wakefulness, NREM sleep, and REM sleep) was scored in 10-sec epochs for the last 3 full recording days, sunrise to sunrise. NREM sleep was characterized by high-amplitude, low-frequency EEG activity (≈ 0.8-4.0 Hz). In addition, brief bursts of 6.0-8.0 Hz activity occurred intermittently during NREM sleep, but not wakefulness or REM sleep (Figure 3). Given this pattern of occurrence, and the fact that such activity usually had a spindle shape (Figure 3A), it apparently reflects thalamocortical spindling.13 Spindles typically occurred simultaneously in both hemispheres, and were particularly apparent in the pygmy sloths (see next paragraph). As in the previous studies of captive13 and wild33 sloths, REM sleep was characterized by low muscle artifact and the onset of low-amplitude, high-frequency activity arising from a bout of NREM sleep (Figure 3A). Additionally, in all periods of REM sleep, distinctive rhythmic waxing and waning bursts of twitching were observed (Figure 3A) that resembled artifacts associated with feeding,13 but with drastically lower amplitude. This activity may reflect rhythmic, chewing-like movements similar to those recently described in guinea pigs during REM sleep.49 As in captive sloths,13 EMG activity either declined from prior NREM sleep levels, or if already low (the typical condition), showed no further changes during tonic REM sleep. The exact point of REM sleep onset was defined as the end of the last spindle, or the point at which the EMG artifact reduced if this occurred after the last spindle. The end of a bout of REM sleep was marked by either the resumption of spindles, or in most cases, an awakening characterized by an abrupt and tonic increase in EMG activity (Figure 3B). Epochs with more than one state were scored according to the predominant state. During wakefulness, time spent feeding (i.e., chewing) was scored for each sloth based on large, stereotypical rhythmic artifacts (≈ 1.0 Hz).13,33
Figure 3.
Electroencephalographic (EEG) recordings from Bradypus pygmaeus. (A) EEG recording (90 sec) showing a period of nonrapid eye movement (NREM) sleep transitioning into rapid eye movement (REM) sleep. NREM sleep was characterized by frequent high-amplitude spindles (blue diamonds) and slow waves. During REM sleep, a stereotypical and rhythmic “twitching” artifact is visible. (B) Transition from REM sleep to wakefulness characterized by an abrupt increase in tonic electromyogram (EMG) artifact in the EEG signal. The EMG signal was obtained by high-pass (> 30 Hz) filtering the EEG signal.
The following variables were calculated and averaged across the three scoring days for each sloth: percent time spent in each state (wakefulness, NREM sleep, and REM sleep) per 24-h day, percent of total sleep time spent in REM sleep, percent time spent in each state for each hour of the 24-h day, and percent time spent in each state during the day (sunrise to sunset) and night (sunset to sunrise). Reported values for all variables are the mean of these individual sloth means. We calculated the mean duration of bouts of each state in a similar manner. A bout was defined as a sequence of consecutive epochs of one state. For statistical tests, we used the mean value for each variable from each sloth. As a result, the sample sizes were nine and six for mainland and island sloths, respectively. Because the sample sizes for male (n = 5) and female (n = 4) mainland sloths were deemed too small for a statistical comparison, we only report mean values for each sex.
EEG Spectral Analysis
We calculated power density for each state using Somnologica's fast Fourier transform function (0.8-25 Hz, in 0.4-Hz frequency bins). Only artifact-free periods of each state were used for the analysis. To account for potential differences in EEG amplitude related to interspecific differences in skull thickness and brain size, the spectral data for each sloth was standardized as a percentage of the 24-h NREM sleep mean across all frequency bins. Because EEG activity in the two hemispheres was similar, we randomly chose the left EEG for spectral analysis. In the few cases where the quality of the right EEG signal was markedly better than the left, we analyzed that signal instead.
RESULTS
The total sleep time for both sloth species was similar, with mainland sloths sleeping 9.60 ± 0.25 h (mean ± SEM) and island sloths sleeping 9.69 ± 0.4 h (unpaired, two-tailed t-test, P = 0.86; Figure 4). The amount of time spent in NREM sleep also did not differ significantly between the species (unpaired, two-tailed t-test, P = 0.25). The time spent in REM sleep showed a trend (unpaired, two-tailed t-test, P = 0.095) for more REM sleep in mainland sloths that was significant (unpaired, two-tailed t-test, P = 0.037) when REM sleep was expressed as a percentage of total sleep time. For mainland sloths, males and females spent 9.47 ± 0.10 h and 9.77 ± 0.15 h asleep, respectively. Males spent 8.05 ± 0.03 h in NREM sleep and 1.41 ± 0.07 h in REM sleep. Females spent 8.13 ± 0.08 h in NREM sleep and 1.64 ± 0.09 h in REM sleep. REM sleep as a percentage of total sleep time was 15.02 ± 0.55 % and 16.76 ± 0.75% for males and females, respectively.
Figure 4.
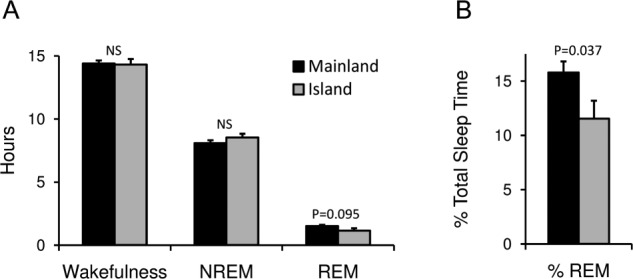
Time spent in each state. (A) Number of hours spent in wakefulness, nonrapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep in mainland (black) and island (gray) sloths. (B) REM sleep as a percentage of total sleep time for the two sloth species. Error bars show standard error of the mean.
The mean bout durations for wakefulness, NREM sleep, and REM sleep were similar for both sloth species. Bouts of wakefulness lasted 264.62 ± 26.90 sec and 279.43 ± 40.94 sec for mainland and island sloths, respectively (unpaired, two-tailed t-test, P = 0.76). Bouts of NREM sleep lasted 149.75 ± 14.46 sec and 168.86 ± 25.11 sec for mainland and island sloths, respectively (unpaired, two-tailed t-test, P = 0.49). Bouts of REM sleep lasted 407.95 ± 40.52 sec and 449.81 ± 60.24 sec for mainland and island sloths, respectively (unpaired, two-tailed t-test, P = 0.56). For male and female mainland sloths, respectively, bouts of wakefulness were 246.94 ± 26.62 sec and 286.73 ± 53.54 sec, bouts of NREM sleep were 137.98 ± 11.28 sec and 164.45 ± 30.21 sec, and bouts of REM sleep were 435.42 ± 50.36 sec and 373.62 ± 70.12 sec.
Mainland sloths showed a preference for being awake during the day and asleep at night (unpaired, two-tailed t-test, P < 0.002), whereas island sloths showed no preference for day or night (unpaired, two-tailed t-test, P > 0.3; Figures 5 and 6). In addition, mainland sloths showed peak sleep amounts around sunrise and sunset, whereas only a single peak in sleep was observed in island sloths during the last third of the night. These differences in the timing of sleep do not appear to be attributable to differences in the composition of males and females in the two samples (i.e., all male in the island sloths), as male and female mainland sloths showed similar patterns (Figure 7); males slept 3.07 ± 0.26 h and 6.39 ± 0.34 h during the day and night, respectively, and females slept 3.86 ± 0.75 h and 5.91 ± 0.66 h during the day and night, respectively. In both species, time spent feeding paralleled hourly changes in time spent awake (Figure 5), and reached their lowest levels during the last third of the night.
Figure 5.
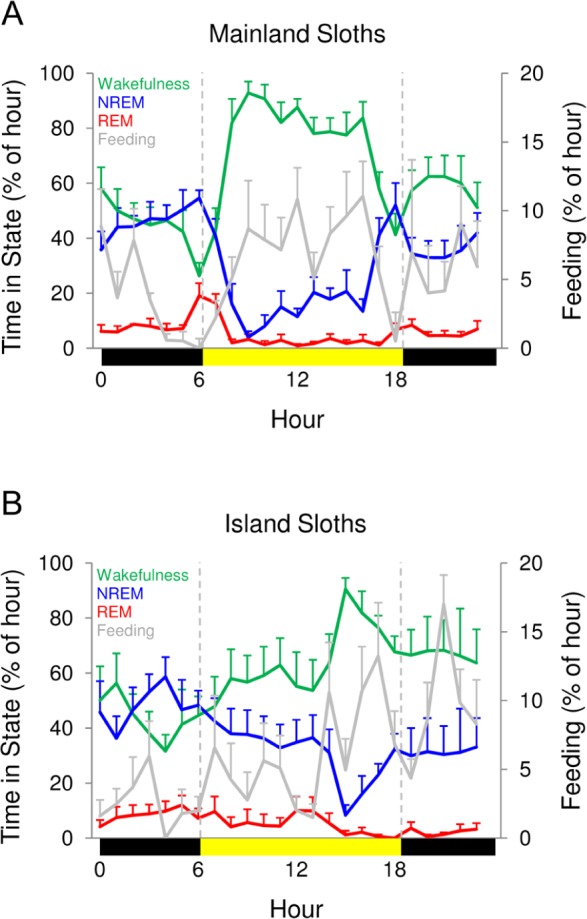
Hourly time spent in each state. The percentage of time spent in wakefulness (green), nonrapid eye movement (NREM) sleep (blue), and rapid eye movement (REM) sleep (red) for each hour for (A) mainland and (B) island sloths. The bars on the x-axis mark day (sunrise to sunset, yellow) and night (sunset to sunrise, black). Data reflect averages of all 3 days for each sloth. Percentage of time spent feeding (gray) is plotted on the secondary y-axis. Values for a given hour are plotted at the start of the hour. Error bars show standard error of the mean.
Figure 6.
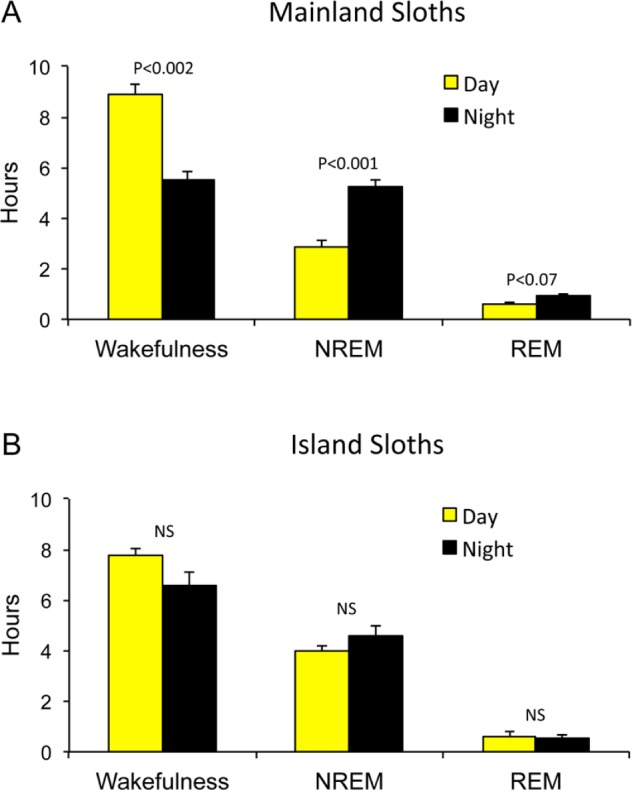
Time spent in the three states during the day and night. Time spent in wakefulness, nonrapid eye movement (NREM) sleep, and rapid eye movement (REM) sleep during the day (sunrise to sunset, yellow) and night (sunset to sunrise, black) for (A) mainland and (B) island sloths. Error bars show standard error of the mean.
Figure 7.
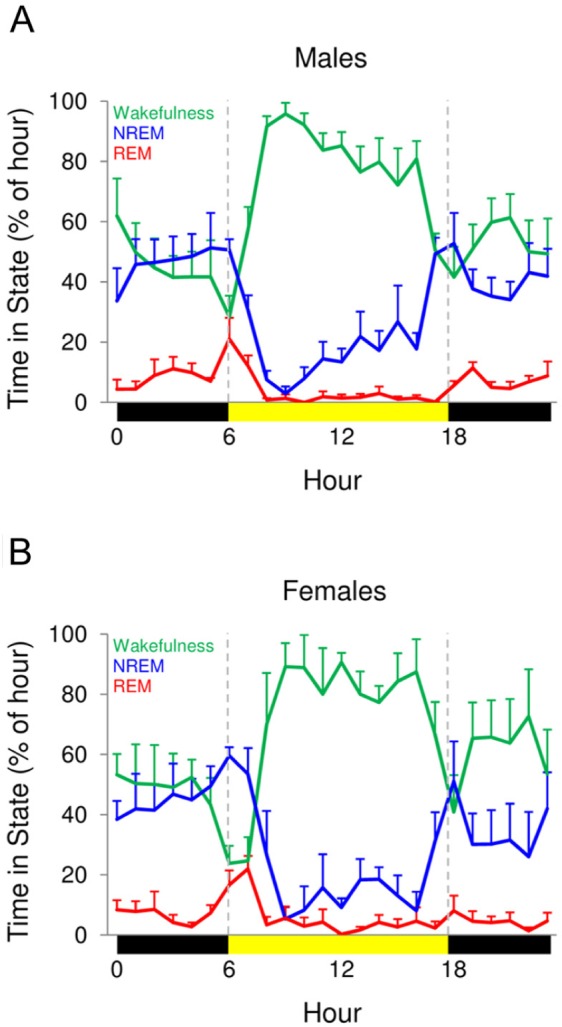
Hourly time spent in each state for male and female mainland sloths. The percentage of time spent in wakefulness (green), nonrapid eye movement (NREM) sleep (blue), and rapid eye movement (REM) sleep (red) for each hour for (A) male and (B) female mainland sloths. The bars on the x-axis mark day (sunrise to sunset, yellow) and night (sunset to sunrise, black). Data reflects averages of all 3 days for each sloth. Values for a given hour are plotted at the start of the hour. Error bars show standard error of the mean.
In both species, when compared to wakefulness and REM sleep, NREM sleep was characterized by increased power in low (0.8-5.0 Hz), spindle (6.0-8.0 Hz), and some higher frequencies (Figures 8A and 8B; see figure for statistics). The most striking differences between the species were found during NREM sleep (presented together with statistics in Figure 8C). In island sloths, standardized low-frequency (0.8-4.0 Hz) power was lower, and spindle (6.0-8.0 Hz) and higher frequency (13.0-18.0 Hz) power were higher than in mainland sloths. Even at sleep onset, the ratio of spindle to low-frequency power was noticeably greater in island sloths. The spectral power was similar for male and female mainland sloths.
Figure 8.
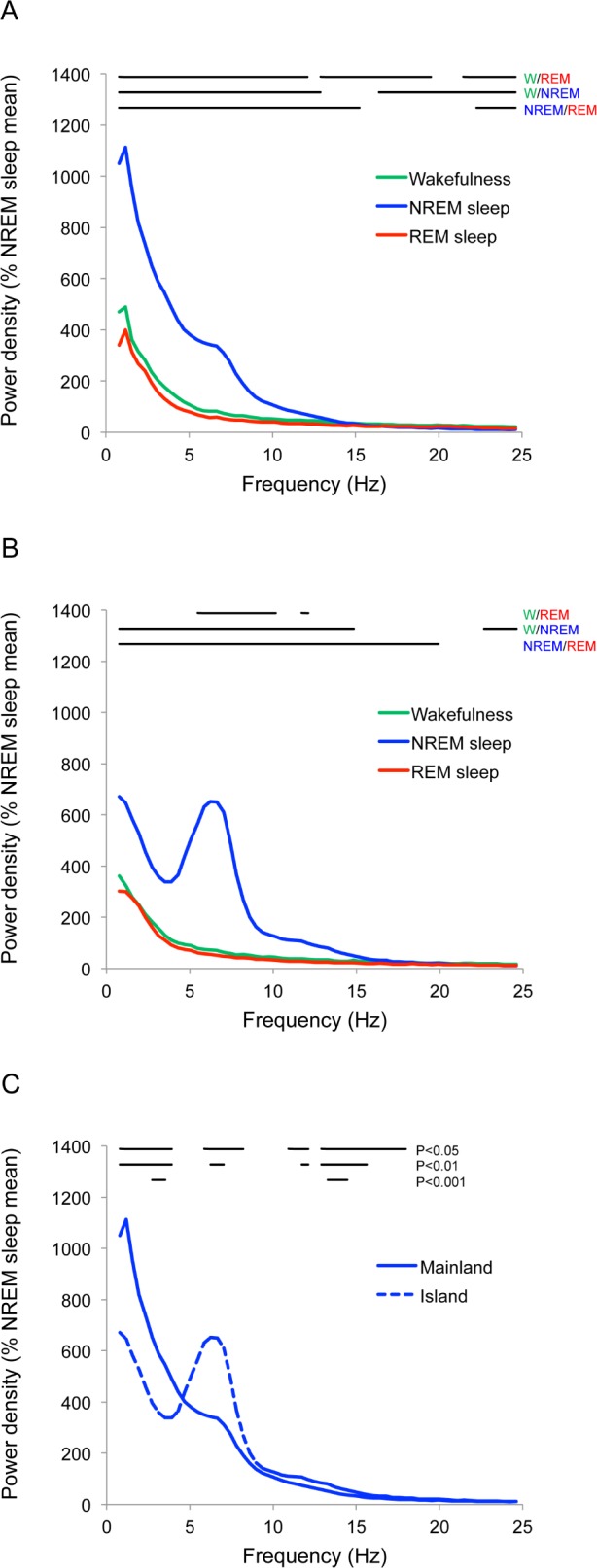
Standardized EEG power density for each state. (A) Mainland sloths and (B) island sloths during wakefulness (green), nonrapid eye movement (NREM) sleep (blue), and rapid eye movement (REM) sleep (red). Lines at the top show significant (paired, two-tailed t-test, P < 0.05) differences between states. (C) Standardized NREM sleep power density from (A) and (B) plotted together to illustrate the differences between mainland (solid line) and island (dashed line) sloths. Lines at the top of the graph show significant differences (P < 0.05, P < 0.01, and P < 0.001). Power for each 0.4 Hz frequency bin is plotted at the start of the bin.
DISCUSSION
Both pygmy and brown-throated three-toed sloths slept just over 9.5 h per day. This finding is consistent with that of our initial study of sleep in three wild brown-throated three-toed sloths from a different part of mainland Panama,33 which slept 9.6 h. Thus, this appears to be the typical sleep amount for wild three-toed sloths, at least those living in Panama. These findings are consistent with the observation that closely related species exhibit similar sleep durations.18
Based on the hypothesis that wild sloths sleep less than captive sloths due to a difference in predation pressure, we expected mainland sloths living with nocturnal predators to sleep less, engage in less REM sleep as a percent of total sleep time, and show a preference for sleeping during the day when compared to island sloths living without predators. However, the absence of a difference in total sleep time, the higher percent REM sleep, and the preference for sleeping at night in mainland sloths were all contrary to our predictions. These results suggest that factors other than predation, such as increased foraging time in the wild, may account for the differences in sleep amounts between captive and wild sloths.33
Our interpretation of these findings is dependent on an accurate assessment of the relative risk of predation during sleep and wakefulness in sloths. For sloths, sleeping may actually be safer than being actively awake. Because sloths are slow and lack significant antipredator defenses,50 they may be particularly vulnerable to detection and capture by predators when moving. In contrast to the ineffective fight or flight responses that might render sloths vulnerable to predation while active, their preference for sleeping sites high in the vegetation and their cryptic pelage (including green algae growing in their hair51,52) may make sleeping safer than being actively awake. Collectively, these factors may explain why mainland sloths, exposed to nocturnal predators, were primarily active during the day. In addition to the preference for sleeping at night, the fact that sleep was maximal around dusk and dawn when the activity of nocturnal cats is maximal53,54 also supports the notion that the timing of sleep and wakefulness in mainland sloths reflects an antipredator strategy. A similar shift toward being inactive when predators are active has been reported in Norway rats (Rattus norvegicus), which sleep safely in burrows.55 In addition, golden hamsters (Mesocricetus auratus), which also sleep in burrows, are nocturnally active in captivity, but diurnally active in the wild, possibly to avoid predation by owls.32 Moreover, a recent evolutionary strategic model predicts that species that are safer when sleeping should sleep when their predators are active, whereas species that are more vulnerable when sleeping should sleep when their predators sleep.56
The higher REM sleep as a percentage of total sleep time in sloths exposed to predators may also be related to safety during sleep. If the risk of predation during sleep is low in both mainland and island sloths, the potential influence of other, perhaps physiological, factors might play a greater role in determining the proportion of sleep dedicated to REM sleep. However, given that this difference was only marginally significant, and not significant when REM sleep was expressed as a percentage of recording time, we are reluctant to draw any further conclusions. Ultimately, a larger sample of sloths and other species sleeping in the wild is needed to effectively identify factors influencing REM sleep.
Although sleep can be safer than being actively awake, this does not necessarily support the idea that the function of sleep per se is to enforce immobility at times of the day when it is either dangerous or unproductive to be awake (i.e., the immobilization hypothesis).57 Indeed, three-toed sloths are very adept at remaining still and cryptic while awake, and usually resorted to this strategy when we approached them. Similarly, Montgomery and Sunquist37 found that three-toed sloths did not react to a human attempting to catch them unless they were physically touched. Given that sloths do not need sleep to “enforce” immobility, sleep must serve other functions.4,7
Factors other than predation not assessed in this study might have also influenced sleep and its timing differently between the two species. For example, comparative studies based on captive mammals suggest that species feeding on low quality food spend less time sleeping.18,58 Consequently, dietary differences between the sloths species may have masked differences in sleep duration related to other ecological factors such as predation risk. However, it is unclear how food quality or its spatial distribution relative to sleeping sites36 might have contributed to the differences in the timing of sleep given that both species fed during the day and night, and sleep in the same trees in which they forage (personal observation).
The spectral differences in standardized NREM sleep-related EEG activity observed between the mainland and island sloths were unexpected. The island sloths showed significantly lower low-frequency power (or slow wave activity, SWA) and increased spindle and higher frequency power compared to mainland sloths. Based on the inverse relationship between SWA and spindles, and the positive relationship between SWA and sleep depth observed in humans,59 this difference could reflect differences in average sleep intensity between the two species. However, we observed that even at sleep onset when the first spindles occurred, spindle power relative to SWA was markedly greater in the island sloths. Consequently, differences in sleep intensity do not appear to fully explain the marked differences in NREM sleep EEG spectral power.
Based on research in humans, it is conceivable that the differences in spindling reflect different cognitive abilities and/ or demands in mainland and island sloths. For example, spindling is positively correlated with IQ (intelligence quotient).60,61 However, in the case of sloths, it is unclear what pressures would select for this trait only in island sloths. Given that spindles increase following learning, and the degree to which they increase predicts post-sleep cognitive performance,62–64 it is possible that increased spindling in island sloths reflects a response to waking experiences. However, differences in the sloths' waking activity and challenges that might account for this difference in spindling are not readily apparent. Moreover, given the slower frequency of spindles in sloths (6-8 Hz), it is unclear whether they perform the same function as faster (12-15 Hz) spindles in humans.
Alternatively, the differences in NREM sleep EEG activity could simply reflect a selectively neutral artifact linked to the small size of the island population. For example, if the island population became unusually small in the past, and by chance genes responsible for increased spindles were disproportionally represented in the remaining population, this genotype would be more prevalent in the subsequent island population than in the mainland population.65 Consistent with this scenario is the fact that EEG traits are highly heritable.66
Although speculative, it is conceivable that the lower standardized SWA, higher standardized spindle power, and lower percent REM sleep seen in island sloths could be linked to diet. These characteristics of sleep mimic those observed following the consumption of benzodiazepines in humans and other mammals.67–70 Naturally occurring benzodiazepines or benzodiazepine-like compounds, with similar potentiating effects on γ-aminobutyric acidA (GABAA) receptors,71 have been isolated from a variety of plants, or their endophytic fungi, including those growing in marine-mangrove habitats.72–77 For example, benzalphthalides—a group of compounds with benzodiazepine-like anxiolytic effects78—have been isolated from the fungi Guignardia species growing on the mangrove tree Kandelia candel in China.79,80 In addition to naturally occurring compounds, human-made benzodiazepines have been found as pollutants in water systems81 at levels shown to alter vertebrate behavior, presumably via their effect on brain neuro-chemistry.82 Interestingly, whereas mainland sloths are known to feed on at least 31 plant species,37 island sloths are thought to feed mainly on the leaves of red mangroves or other plants growing in this habitat.35 Indeed, over the 10-day study period, no island sloths moved over 20 m from their initial mangrove tree of capture, strongly suggesting that they did not leave the mangroves, and therefore presumably ate the leaves of plants growing in this habitat. Consequently, if the level of naturally occurring or human-made benzodiazepine-like compounds in the diet was higher in island sloths than in mainland sloths, this finding might explain many of the differences in sleep observed between the species. Although this hypothesis is speculative, other mammals are known to regularly consume plants containing medicinal and/or psychoactive compounds.83,84 Ultimately, a full characterization of the sloths' diets and the level of benzodiazepine-like compounds found therein is needed to test this hypothesis.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank La Autoridad Nacional del Ambiente for granting us research permits to conduct fieldwork in Panama, and Sebastian Codas and Christian Lundeberg for their help catching sloths. We thank the Smithsonian Tropical Research Institute for logistical and administrative support, the Smithsonian Fellowship program, Gabriel Jacome and the entire staff of STRI Bocas station, and the International Max Planck Research School for Organismal Research. This research was funded by the Max Planck Society.
REFERENCES
- 1.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–71. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima SL, Rattenborg NC. A behavioural shutdown can make sleeping safer: a strategic perspective on the function of sleep. Anim Behav. 2007;74:189–97. [Google Scholar]
- 3.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–26. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 4.Rattenborg NC, Lesku JA, Martinez-Gonzalez D, Lima SL. The nontrivial functions of sleep. Sleep Med Rev. 2007;11:405–9. doi: 10.1016/j.smrv.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Mignot E. Why we sleep: the temporal organization of recovery. PLoS Biol. 2008;6:e106. doi: 10.1371/journal.pbio.0060106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cirelli C, Tononi G. Is sleep essential? PLoS Biol. 2008;6:e216. doi: 10.1371/journal.pbio.0060216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lima SL, Rattenborg NC, Lesku JA, Amlaner CJ. Sleeping under the risk of predation. Anim Behav. 2005;70:723–36. [Google Scholar]
- 8.Siegel JM. REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. 5th ed. Philadelphia: WB Saunders; 2011. pp. 92–111. [Google Scholar]
- 9.Steriade M. Grouping of brain rhythms in corticothalamic systems. Neurosci. 2006;137:1087–106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Nicol SC, Andersen NA, Phillips NH, Berger RJ. The echidna manifests typical characteristics of rapid eye movement sleep. Neurosci Lett. 2000;283:49–52. doi: 10.1016/s0304-3940(00)00922-8. [DOI] [PubMed] [Google Scholar]
- 11.Van Twyver H, Allison T. Sleep in the opossum, Didelphis marsupialis. Electroenceph Clin Neurophysiol. 1970;29:181–9. doi: 10.1016/0013-4694(70)90121-5. [DOI] [PubMed] [Google Scholar]
- 12.Affanni JM, Cervino CO, Marcos HJ. Absence of penile erections during paradoxical sleep. Peculiar penile events during wakefulness and slow wave sleep in the armadillo. J Sleep Res. 2001;10:219–28. doi: 10.1046/j.1365-2869.2001.00259.x. [DOI] [PubMed] [Google Scholar]
- 13.de Moura Filho AG, Huggins SE, Lines SG. Sleep and waking in the three-toed sloth, Bradypus tridactylus. Comp Biochem Physiol. 1983;76:345–55. doi: 10.1016/0300-9629(83)90336-5. [DOI] [PubMed] [Google Scholar]
- 14.Pellet J. Etude electropolygraphique et comportementale des etas de veile et de sommeil chez le cobaye (Cavia porcellus) C R Senac Soc Biol. 1966;169:1476–82. [PubMed] [Google Scholar]
- 15.Paré D, Steriade M, Deschênes M, Oakson G. Physiological characteristics of anterior thalamic nuclei, a group devoid of inputs from reticular thalamic nucleus. J Neurophysiol. 1987;57:1669–85. doi: 10.1152/jn.1987.57.6.1669. [DOI] [PubMed] [Google Scholar]
- 16.Jeserevics J, Viitmaa R, Cizinauskas S, et al. Electroencephalography findings in healthy and Finnish Spitz dogs with epilepsy: visual and background quantitative analysis. J Vet Intern Med. 2007;21:1299–306. doi: 10.1892/06-285.1. [DOI] [PubMed] [Google Scholar]
- 17.Dijk DJ, Czeisler C. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Capellini I, Barton RA, Mcnamara P, Preston BT, Nunn CL. Phylogenetic analysis of the ecology and evolution of mammalian sleep. Evolution. 2008;62:1764–76. doi: 10.1111/j.1558-5646.2008.00392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campbell SS, Tobler I. Animal sleep: a review of sleep duration across phylogeny. Neurosci Biobehav Rev. 1984;8:269–300. doi: 10.1016/0149-7634(84)90054-x. [DOI] [PubMed] [Google Scholar]
- 20.Lesku JA, Roth TC, Amlaner CJ, Lima SL. A phylogenetic analysis of sleep architecture in mammals: the integration of anatomy, physiology, and ecology. Am Nat. 2006;168:441–53. doi: 10.1086/506973. [DOI] [PubMed] [Google Scholar]
- 21.Allison T, Cicchetti D. Sleep in mammals: ecological and constitutional correlates. Science. 1976;194:732–4. doi: 10.1126/science.982039. [DOI] [PubMed] [Google Scholar]
- 22.Lesku JA, Roth TC, Rattenborg NC, Amlaner CJ, Lima SL. History and future of comparative analyses in sleep research. Neurosci Biobehav Rev. 2009;33:1024–36. doi: 10.1016/j.neubiorev.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Lendrem DW. Sleeping and vigilance in birds. I. Field observations of the mallard (Anas platyrhynchos) Anim Behav. 1983;31:532–8. [Google Scholar]
- 24.Rattenborg NC, Lima SL, Amlaner CJ. Half-awake to the risk of predation. Nature. 1999;397:397–8. doi: 10.1038/17037. [DOI] [PubMed] [Google Scholar]
- 25.Roth TC, Lesku JA, Amlaner CJ, Lima SL. A phylogenetic analysis of the correlates of sleep in birds. J Sleep Res. 2006;15:395–402. doi: 10.1111/j.1365-2869.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 26.Bradley C, Meddis R. Arousal thresholds in dreaming sleep. Physiol Psychol. 1974;2:109–10. [Google Scholar]
- 27.Lesku JA, Bark RJ, Martinez-Gonzalez D, et al. Predator-induced plasticity in sleep architecture in wild-caught Norway rats (Rattus norvegicus) Behav Brain Res. 2008;189:298–305. doi: 10.1016/j.bbr.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 28.Sanford LD, Tang X, Ross RJ, Morrison AR. Influence of shock training and explicit fear-conditioned cues on sleep architecture in mice: strain comparison. Behav Gen. 2003;33:43–58. doi: 10.1023/a:1021051516829. [DOI] [PubMed] [Google Scholar]
- 29.Sanford LD, Yang L, Wellman LL, Liu X, Tang X. Differential effects of controllable and uncontrollable footshock stress on sleep in mice. Sleep. 2010;33:621–30. doi: 10.1093/sleep/33.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calisi RM, Bentley GE. Lab and field experiments: Are they the same animal? Horm Behav. 2009;56:1–10. doi: 10.1016/j.yhbeh.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 31.Daan S. How and why? The lab versus the field. Sleep Biol Rhythms. 2011;9:1–2. [Google Scholar]
- 32.Gattermann R, Johnston RE, Yigit N, et al. Golden hamsters are nocturnal in captivity but diurnal in nature. Biol Lett. 2008;4:253–5. doi: 10.1098/rsbl.2008.0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rattenborg NC, Voirin B, Vyssotski AL, et al. Sleeping outside the box: electroencephalographic measures of sleep in sloths inhabiting a rainforest. Biol Lett. 2008;4:402–5. doi: 10.1098/rsbl.2008.0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horne J. Why we sleep: the function of sleep in humans and other mammals. Oxford, UK: Oxford University Press; 1988. [Google Scholar]
- 35.Anderson RP, Handley CO. A new species of three-toed sloth (Mammalia: Xenarthra) from Panama, with a review of the genus Bradypus. Proc Biol Soc Washington. 2001;114:1–33. [Google Scholar]
- 36.Acerbi A, McNamara P, Nunn CL. To sleep or not to sleep: the ecology of sleep in artificial organisms. BMC Ecol. 2008;8:10. doi: 10.1186/1472-6785-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Montgomery G, Sunquist M. Impact of sloths on Neotropical forest energy flow and nutrient cycling. In: Golley FB, Medina E, editors. Tropical ecology systems trends in terrestrial and aquatic research (Vol 11) New York: Springer-Verlag; 1975. pp. 69–98. [Google Scholar]
- 38.Moreno R, Kays R, Samudio R. Competitive release in diets of ocelot (Leoparadus pardalis) and puma (Puma concolor) after jaguar (Pathera onca) decline. J Mammal. 2006;87:808–16. [Google Scholar]
- 39.Hayssen V. Bradypus variegatus (Pilosa: Bradypodidae) Mamm Species. 2010;42:19–32. [Google Scholar]
- 40.Voirin JB, Kays R, Lowman MD, Wikelski M. Evidence for three-toed sloth (Bradypus variegatus) predation by spectacled owl (Pulsatrix perspicillata) Edentata. 2009;8:15–20. [Google Scholar]
- 41.Galetti M, Carvalho O., Jr Sloths in the diet of a Harpy Eagle nestling in eastern Amazon. Wils Bull. 2000;112:535–6. [Google Scholar]
- 42.Springer MT, Nielsen CK, Carver AD, Correa NJ. Harpy Eagle (Harpia harpyja) feeding behavior on a brown-throated three-toed sloth (Bradypus variegates) J Raptor Res. 2011;45:100–3. [Google Scholar]
- 43.Touchton J, Hsu Y, Palleroni A. Foraging ecology of reintroduced captive-bred subadult harpy eagles (Harpia harpyja) on Barro Colorado Island, Panama. Ornitologia Neotropical. 2002;13:365–79. [Google Scholar]
- 44.Anderson RP, Handley CO., Jr Dwarfism in insular sloths: biogeography, selection, and evolutionary rate. Evolution. 2002;56:1045–58. doi: 10.1111/j.0014-3820.2002.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 45.Superina M, Plese T, Moraes-Barros N, Abba A. The 2010 sloth red list assessment. Edentata. 2010;11:115–34. [Google Scholar]
- 46.Sunquist M, Sunquist F. Wild cats of the world. Chicago, IL: The University of Chicago Press; 2002. [Google Scholar]
- 47.Reid F. A field guide to the mammals of central America and southeast Mexico. Oxford, UK: Oxford University Press; 2009. [Google Scholar]
- 48.Vyssotski AL, Dell'Omo G, Dell'Ariccia G, et al. EEG responses to visual landmarks in flying pigeons. Curr Biol. 2009;19:1159–66. doi: 10.1016/j.cub.2009.05.070. [DOI] [PubMed] [Google Scholar]
- 49.Kato T, Nakamura N, Masuda Y, et al. Phasic bursts of the antagonistic jaw muscles during REM sleep mimic a coordinated motor pattern during mastication. J Appl Physiol. 2013;114:316–28. doi: 10.1152/japplphysiol.00895.2012. [DOI] [PubMed] [Google Scholar]
- 50.Montgomery G, Sunquist M. Habitat selection and use by two-toed and three-toed sloths. In: Montgomery G, editor. Ecology of arboreal folivores. Washington, DC: Smithsonian Institute Press; 1978. pp. 329–59. [Google Scholar]
- 51.Weber-van Bosse A. Étude sur les algues parasites des Paresseux. Natuurk Verh Holl Maatsch Wetensch Haarlem III. 1887;5:10. [Google Scholar]
- 52.Suutari M, Majaneva M, Fewer DP, et al. Molecular evidence for a diverse green algal community growing in the hair of sloths and a specific association with Trichophilus welckeri (Chlorophyta, Ulvophyceae) BMC Evol Biol. 2010;10:86. doi: 10.1186/1471-2148-10-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ludlow M, Sunquist M. Ecology and behavior of ocelots in Venezuela. Natl Geogr Res. 1987;3:447–61. [Google Scholar]
- 54.Weller SH, Bennett CL, Zoo D, Frwy RLT. Twenty-four hour activity budgets and patterns of behavior in captive ocelots (Leopardus pardalis) Appl Anim Behav Sci. 2001;71:67–79. doi: 10.1016/s0168-1591(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 55.Fenn M, MacDonald D. Use of middens by red foxes: risk reverses rhythms of rats. J Mammal. 1995;76:130–6. [Google Scholar]
- 56.Acerbi A, Nunn C. Predation and the phasing of sleep: an evolutionary individual-based model. Anim Behav. 2011;81:801–11. [Google Scholar]
- 57.Meddis R. On the function of sleep. Anim Behav. 1975;23:676–91. doi: 10.1016/0003-3472(75)90144-x. [DOI] [PubMed] [Google Scholar]
- 58.Elgar MA, Pagel MD, Harvey PH. Sleep in mammals. Anim Behav. 1988;36:1407–19. [Google Scholar]
- 59.Dijk DJ, Hayes B, Czeisler C. Dynamics of electroencephalographic sleep spindles and slow wave activity in men: effect of sleep deprivation. Brain Res. 1993;626:190–9. doi: 10.1016/0006-8993(93)90579-c. [DOI] [PubMed] [Google Scholar]
- 60.Fogel SM, Smith CT. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci Biobehav Rev. 2011;35:1154–65. doi: 10.1016/j.neubiorev.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 61.Geiger A, Huber R, Kurth S, et al. The sleep EEG as a marker of intellectual ability in school age children. Sleep. 2011;34:181–9. doi: 10.1093/sleep/34.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schabus M, Hödlmoser K, Gruber G, et al. Sleep spindle-related activity in the human EEG and its relation to general cognitive and learning abilities. Eur J Neurosci. 2006;23:1738–46. doi: 10.1111/j.1460-9568.2006.04694.x. [DOI] [PubMed] [Google Scholar]
- 63.Fogel SM, Nader R, Cote KA, Smith CT. Sleep spindles and learning potential. Behav Neurosci. 2007;121:1–10. doi: 10.1037/0735-7044.121.1.1. [DOI] [PubMed] [Google Scholar]
- 64.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and Stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 65.Frankham R. Do island populations have less genetic variation than mainland populations? Heredity. 1997;78:311–27. doi: 10.1038/hdy.1997.46. [DOI] [PubMed] [Google Scholar]
- 66.Tafti M, Franken P. Invited review: genetic dissection of sleep. J Appl Physiol. 2002;92:1339–47. doi: 10.1152/japplphysiol.00834.2001. [DOI] [PubMed] [Google Scholar]
- 67.Borbély AA, Mattmann P, Loepfe M, Strauch I, Lehmann D. Effect of benzodiazepine hypnotics on all-night sleep EEG spectra. Hum Neurobiol. 1985;4:189–94. [PubMed] [Google Scholar]
- 68.Lancel M. Role of GABAA receptors in the regulation of sleep: initial sleep responses to peripherally administered modulators and agonists. Sleep. 1999;22:33–42. doi: 10.1093/sleep/22.1.33. [DOI] [PubMed] [Google Scholar]
- 69.Aeschbach D, Dijk DJ, Trachsel L, Brunner DP, Borbély AA. Dynamics of slow-wave activity and spindle frequency activity in the human sleep EEG: effect of midazolam and zopiclone. Neuropsychopharmacol. 1994;11:237–44. doi: 10.1038/sj.npp.1380110. [DOI] [PubMed] [Google Scholar]
- 70.Kopp C, Rudolph U, Löw K, Tobler I. Modulation of rhythmic brain activity by diazepam: GABA(A) receptor subtype and state specificity. Proc Natl Acad Sci U S A. 2004;101:3674–9. doi: 10.1073/pnas.0306975101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mubassara S, Hossain S, Ahmed F, et al. Potentiation of the responses of GABAA receptors by Bangladeshi medicinal plants. Food Sci Technol Res. 2009;15:315–24. [Google Scholar]
- 72.Medina J, Paladini A, Izquierdo I. Naturally occurring benzodiazepines and benzodiazepine-like molecules in brain. Behav Brain Res. 1993;58:1–8. doi: 10.1016/0166-4328(93)90085-5. [DOI] [PubMed] [Google Scholar]
- 73.Sand P, Kavvadias D, Feineis D, et al. Naturally occurring benzodiazepines: current status of research and clinical implications. Eur Arch Psychiatry Clin Neurosci. 2000;250:194–202. doi: 10.1007/s004060070024. [DOI] [PubMed] [Google Scholar]
- 74.Ren L, Wang F, Xu Z, Chan W. GABA A receptor subtype selectivity underlying anxiolytic effect of 6-hydroxyflavone. Biochem Pharmacol. 2010;79:1337–44. doi: 10.1016/j.bcp.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 75.Cui C, Li X, Li C, Sun H. Benzodiazepine alkaloids from marine derived endophytic fungus Aspergillus ochraceus. Helv Chim Acta. 2009;92:1366–70. [Google Scholar]
- 76.Seelan J, Ali A, Muid S. Aspergillus species isolated from mangrove forests in Borneo Island, Sarawak, Malaysia. J Threat Taxa. 2009;1:344–6. [Google Scholar]
- 77.Bennett J. An overview of the genus Aspergillus. In: Machda M, Gomi K, editors. Aspergillus. Molecular biology and genomics. Norfolk, UK: Caister Academic Press; 2010. pp. 1–17. [Google Scholar]
- 78.Zamilpa A, Herrera-Ruiz M, del Olmo E, et al. Anxiolytic effects of benzalphthalides. Bioorg Medi Chem Lett. 2005;15:3483–6. doi: 10.1016/j.bmcl.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 79.Xia XK, Huang HR, She ZG, et al. Structural and biological properties of vermistatin and two new vermistatin derivatives isolated from the marine-mangrove endophytic fungus Guignardia sp. No. 4382. Helv Chim Acta. 2007;90:1925–31. [Google Scholar]
- 80.Nicoletti R, Manzo E, Ciavatta ML. Occurrence and bioactivities of funicone-related compounds. Int J Mol Sci. 2009;10:1430–44. doi: 10.3390/ijms10041430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Calisto V, Esteves VI. Chemosphere psychiatric pharmaceuticals in the environment. Chemosphere. 2009;77:1257–74. doi: 10.1016/j.chemosphere.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 82.Brodin T, Fick J, Jonsson M, Klaminder J. Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science. 2013;339:814–5. doi: 10.1126/science.1226850. [DOI] [PubMed] [Google Scholar]
- 83.Wiens F, Zitzmann A, Lachance MA, et al. Chronic intake of fermented floral nectar by wild treeshrews. Proc Natl Acad Sci U S A. 2008;105:10426–31. doi: 10.1073/pnas.0801628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.de Roode JC, Lefèvre T, Hunter MD. Ecology. Self-medication in animals. Science. 2013;340:150–1. doi: 10.1126/science.1235824. [DOI] [PubMed] [Google Scholar]