Abstract
Study Objectives:
To compare pregabalin versus placebo and pramipexole for reducing restless legs syndrome (RLS)-related sleep disturbance.
Design:
Randomized, double-blinded, crossover trial.
Setting:
Twenty-three US sleep centers.
Participants:
Eighty-five individuals with moderate to severe idiopathic RLS and associated sleep disturbance.
Interventions:
Participants were randomized across 6 treatment sequences comprising three 4-week periods on pregabalin 300 mg/day (n = 75), pramipexole 0.5 mg/day (n = 76), or placebo (n = 73).
Measurements and Results:
Polysomnography was conducted over 2 nights at the end of each period. Primary (wake after sleep onset [WASO], pregabalin vs placebo) and key secondary endpoints were analyzed for statistical significance, with descriptive statistics for other endpoints. Pregabalin improved sleep maintenance, demonstrated by reductions in WASO (-27.1 min vs placebo [P < 0.0001]; -26.9 vs pramipexole) and number of awakenings after sleep onset (-2.7 vs placebo; -7.9 vs pramipexole [P < 0.0001]) by polysomnography, and an increase in subjective total sleep time (30.8 min vs placebo [P < 0.0001]; 26.8 vs pramipexole). Pregabalin also increased slow wave sleep duration (20.9 min vs placebo; 32.1 vs pramipexole [P < 0.0001]). Reduction in periodic limb movement arousal index (PLMAI) with pregabalin was similar to pramipexole and greater than placebo (-3.7 PLMA/h [P < 0.0001]), although reduction in total PLM in sleep was less than for pramipexole.
Conclusions:
This study demonstrated improvements in objective and subjective measures of sleep maintenance and sleep architecture with pregabalin compared with placebo and pramipexole. Effects of pregabalin on periodic limb movement arousal index were comparable to pramipexole.
Trial Registration:
ClinicalTrials.gov identifier, NCT00991276; http://clinicaltrials.gov/show/NCT00991276
Citation:
Garcia-Borreguero D; Patrick J; DuBrava S; Becker PM; Lankford A; Chen C; Miceli J; Knapp L; Allen RP. Pregabalin versus pramipexole: effects on sleep disturbance in restless legs syndrome. SLEEP 2014;37(4):635-643.
Keywords: Restless legs syndrome, sleep, polysomnography, clinical trial
INTRODUCTION
Restless legs syndrome (RLS; Willis-Ekbom disease) is a common neurological condition which can have a significant impact on daily function and quality of life.1–3 For patients with RLS, sleep disturbance is reported as the most common factor affecting quality of life and the main reason for seeking treatment.4–7 Development of therapies to address sleep disturbance is thus an important clinical goal.
Dopamine agonists are first-line treatment for RLS, and are effective for the improvement of RLS symptoms and reduction of periodic limb movements (PLM).8–10 However, rates of PLM in sleep (PLMS) do not correlate strongly with degree of sleep disturbance in RLS11; thus, the benefits of dopamine agonists on PLMS cannot be assumed to translate to improvements in sleep. Indeed, while polysomnography (PSG) studies have observed trends toward improvement in sleep maintenance with dopamine agonists, improvements in the critical measure of total sleep time (TST) were rarely statistically significant.12–20
Two prior small studies have investigated pregabalin, an α2δ ligand, as a potential RLS treatment.21,22 One study demonstrated a dose-dependent reduction in RLS symptoms, and improvements in actigraphy measures of TST and sleep efficiency (SE), with pregabalin.21 Another demonstrated significant improvements in RLS symptoms, PSG measures of sleep maintenance and architecture, and subjective sleep measures, as well as reductions in PLM compared with placebo.22 In the current multicenter crossover trial, the efficacy of pregabalin for reducing sleep disturbance in moderate to severe RLS was compared with placebo and the dopamine agonist pramipexole.
METHODS
Study Design
This was a randomized, double-blinded, placebo-controlled, 3-way crossover trial conducted in 23 centers in the United States from December 2009 to June 2011, involving participants with moderate to severe idiopathic RLS with associated sleep disturbance (ClinicalTrials.gov identifier: NCT00991276).
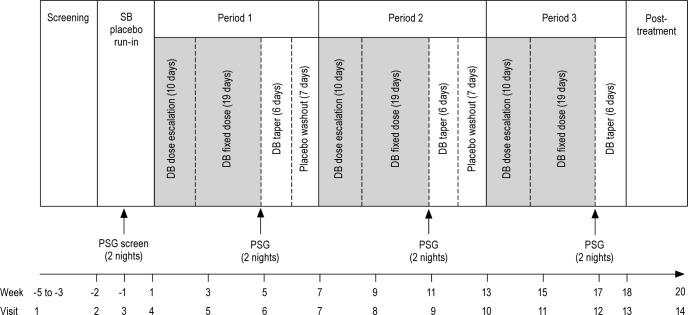
Participants meeting screening criteria entered a 14-day single-blind placebo run-in period, with PSG screening on 2 consecutive nights (beginning day 7) to determine eligibility. Eligible participants were randomized across 6 treatment sequences, each comprising 3 double-blind treatment periods with pregabalin 300 mg/day, pramipexole 0.5 mg/day, and placebo. Each treatment period included 10 days' dose escalation and 19 days' fixed-dose treatment, followed by PSG testing on 2 consecutive nights (days 28-30). Participants then entered a 6-day taper and 7-day placebo washout phase (periods 1 and 2 only) (Figure 1).
Figure 1.
Study design. DB, double-blind; PSG, polysomnography; SB, single-blind.
The study was conducted in accordance with the Declaration of Helsinki and International Conference on Harmonization Good Clinical Practice Guidelines. The protocol and informed consent documents were approved by the relevant Institutional Review Boards. All participants provided written informed consent.
Study Participants
Participants were aged ≥ 18 years and had a diagnosis of moderate to severe idiopathic RLS1 with predominantly evening symptoms. At screening, participants were required to have an International RLS Study Group Rating Scale (IRLS) total score ≥ 15 and a score ≥ 2 on Item 4, indicating moderate to very severe sleep disturbance secondary to RLS. Participants also had a history of symptom-related sleep interference over ≥ 6 months and, on screening, met PSG criteria for sleep interference (mean wake after sleep onset [WASO] ≥ 60 min over 2 nights with WASO < 30 min on neither; mean PLM Index ≥ 10; mean TST ≥ 3.0 and < 6.5 h). Participants were excluded for secondary RLS, sleep/circadian rhythm disorders, conditions that might have confounded assessments (e.g., muscle fasciculation or attention deficit hyperactivity disorder), employment in night or shift work, regular napping for > 30 min or regular napping after 6 PM, serum ferritin < 10 μg/L, or improvement of > 50% in RLS symptoms during the placebo run-in.
No concomitant medications for the treatment of RLS were permitted; stable doses/regimens of medications for chronic conditions were allowed. Medications/substances that may have interfered with efficacy of the study drugs or sleep were either not permitted (e.g., selective serotonin reuptake inhibitors, selective norepinephrine reuptake inhibitors) or limited (e.g., caffeine, tobacco, alcohol). At least 7 days or > 5 half-lives (whichever was greater) was required for washout of prohibited medications prior to the start of single-blind placebo and the screening PSG.
Randomization and Blinding
Randomization was based on a 6-sequence Williams' crossover design balanced for first order carryover effects. Study medication was dispensed through an interactive voice recognition system according to a randomization code generated by Pfizer Inc. Study medication was supplied as masked capsules of pregabalin (75 mg, 150 mg, and 300 mg), pramipexole 0.125 mg and 0.5 mg, and matching placebos for a triple-dummy design.
Administration of Study Medication
Study medication was administered orally once daily, 1 to 3 h before the participant's bedtime (2 h before bedtime during PSG testing). The maintenance dose of pregabalin 300 mg/day was selected based on a prior dose-finding study.21 The maintenance dose of pramipexole 0.5 mg/day is the maximum recommended for treating RLS in the United States.23 Pregabalin dosing began at 75 mg/day, with dose escalation to 150 mg/day (day 6) and 300 mg/day (day 11). Pramipexole dosing began at 0.125 mg/day, with dose escalation to 0.25 mg/day (day 6) and 0.5 mg/day (day 11). Following each treatment period the drug dosage was tapered over 6 days.
Efficacy Assessments
PSG recordings were performed for an 8-h period in a sleep laboratory and were carried out in accordance with the Rechtschaffen and Kales manual24 (as modified by American Academy of Sleep Medicine 2007 guidelines25). Readings were averaged over 2 consecutive nights. All sleep laboratories were certified by a central laboratory, and all PSG recordings were evaluated in a blinded fashion by the central laboratory.
PSG measures reported include: WASO, the number of minutes the participant was awake after onset of persistent sleep (10 min of non-wake on electroencephalogram); NAASO, the number of awakenings after sleep onset following onset of persistent sleep; the total number of awakenings of ≥ 1 30-sec epoch (to be counted, each awakening had to be separated by a stage 2 NREM (N2), N3 or R [REM] epoch); TST, total minutes of sleep during the PSG recording; SE (%), TST as a percentage of time in bed; minutes of each sleep stage (N1, N2, N3 [slow wave sleep; SWS] and R); latency to persistent sleep (LPS), minutes from the start of the PSG recording to onset of 10 consecutive min of sleep;26 arousal index (AI), number of arousals divided by hours of sleep from onset of persistent sleep to the end of the PSG recording (arousals were defined as shifts from a stage N2-3 or R epoch to a stage N1 epoch); PLM arousal index (PLMAI), the number of PLM leading to arousal/h TST; and PLM in sleep index (PLMSI), the number PLM/h of TST.
During the 7 days prior to a PSG visit, participants completed the Subjective Sleep Questionnaire (SSQ)27 within 30 min of waking and the RLS-Next Day Impact (RLS-NDI) questionnaire28 at the end of each day. At each PSG visit, the Clinical Global Impressions–Improvement (CGI-I) questionnaire, a physician-administered 7-point scale, was completed to assess improvement in RLS compared with the start of study medication. Participants also completed the IRLS29 and RLS-Quality of Life (QoL) questionnaires,30 and the Medical Outcomes Study (MOS)–Sleep Scale (SS),31 with a recall period of the prior week.
Primary and Key Secondary Endpoints
The primary efficacy endpoint was WASO for pregabalin compared with placebo. Key secondary endpoints were: PLMAI, subjective TST (sTST) derived from the SSQ, and RLS-NDI for pregabalin compared with placebo; and SWS and NAASO for pregabalin compared with pramipexole.
Safety and Tolerability
Safety and tolerability were assessed by monitoring adverse events (AEs), physical examination findings, clinical laboratory tests, vital signs, concomitant medications, and suicidality. Suicidal ideation and behavior was assessed using the Columbia-Classification Algorithm of Suicide Assessment (C-CASA) derived from the Sheehan-Suicidality Tracking Scale (S-STS).
Statistical Analysis
A sample size of 84 participants (14 per treatment sequence) was estimated to detect clinically significant differences in key variables (considered as a mean [standard deviation] difference of: 20 [60] min for WASO; 2 [5] PLMA/h for PLMAI; 25 [70] min for sTST; 12 [35] min for SWS). Assuming a 20% discontinuation rate, the randomization target was 105 participants. The protocol specified sample size re-estimation procedure to assess for the need to increase the required sample size concluded that no such increase was recommended. Enrollment in the study was stopped, with 85 participants randomized, based on an administrative decision for business reasons (i.e., a strategic decision by Pfizer Inc to deprioritize RLS as a development program and discontinue further investigation of pregabalin in RLS). The observed standard deviation for WASO was 37.5, providing > 99% power with 85 participants to detect clinically significant differences.
Efficacy analyses were conducted for the intent-to-treat (ITT) population, defined as participants who received ≥ 1 dose of study medication and had ≥ 1 postrandomization efficacy assessment. The safety population included all participants who received ≥ 1 dose of study medication. Least-squares (LS) means, confidence intervals (CIs), and treatment group comparisons were estimated from a mixed model including fixed effects for sequence, period, and treatment. Participant nested within sequence was included as a random effect. Statistical inferences for treatment group comparisons for the primary and key secondary endpoints were performed using a 2-sided model based t test of LS mean differences at the 5% level of significance.
A prespecified step-down testing procedure was employed to preserve the experiment-wise type 1 error < 0.05 over the multiple tests. The steps were: (1) WASO (pregabalin vs placebo); (2) PLMAI (pregabalin vs placebo); (3) sTST (pregabalin vs placebo); (4) SWS (pregabalin vs pramipexole); (5) NAASO (pregabalin vs pramipexole); and (6) RLS-NDI (pregabalin vs placebo). The treatment comparison at each step had to reach statistical significance (P < 0.05) for testing to proceed to the next step. Only the endpoints included in the step-down testing procedure were analyzed for statistical significance; for all other assessments, only descriptive statistics are reported (without P values).
RESULTS
Study Population
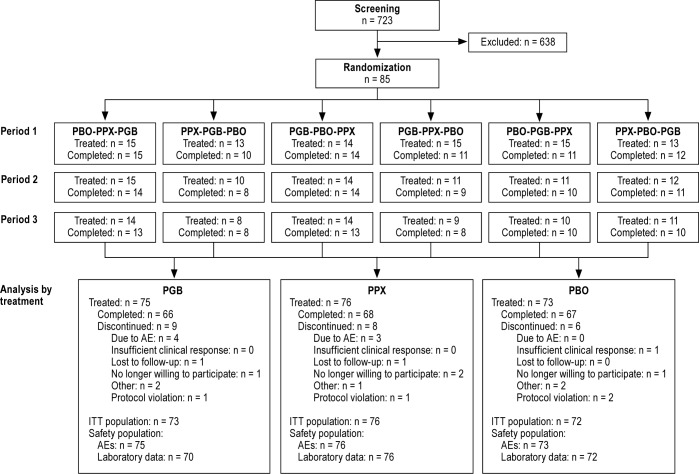
A total of 85 participants were randomized; all received ≥ 1 dose of study drug and 62 completed all 3 periods of the trial. Approximately equal numbers received each treatment (75 pregabalin, 76 pramipexole, 73 placebo). Completion and discontinuation rates were broadly comparable across treatments (Figure 2). The mean age of the overall study population was 50.3 to 57.4 years (range across treatment groups) and 36% were male. The mean time since RLS onset was 2.0 to 11.9 years (range across treatment groups). Participant characteristics were broadly comparable across each of the 6 treatment sequences with differences in the ratio of males to females (from 20% to 46.2% male) and age distribution, arising from the relatively low numbers of participants per treatment sequence (Table 1).
Figure 2.
Participant disposition. AE, adverse event; ITT, intent-to-treat; PBO, placebo; PGB, pregabalin; PPX, pramipexole.
Table 1.
Participant demographics by treatment sequence
Polysomnography
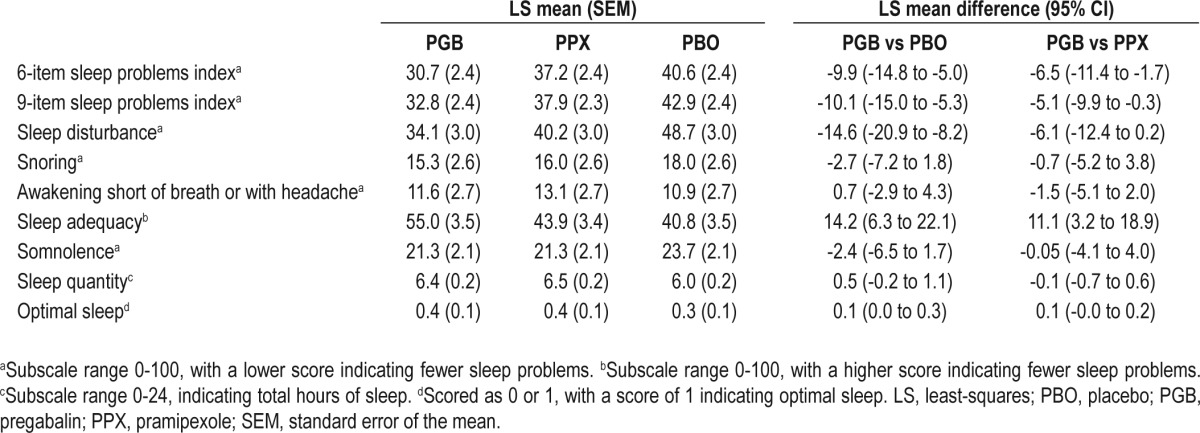
Measures of sleep maintenance improved more with pregabalin than with either placebo or pramipexole (Table 2). The primary endpoint, WASO, improved by 27.1 minutes with pregabalin compared with placebo (P < 0.0001) and 26.9 minutes compared with pramipexole. Sensitivity analyses for the primary endpoint did not indicate any carry-over effect between treatment periods. The key secondary endpoint of NAASO was also reduced by 2.7 awakenings with pregabalin compared with placebo and 7.9 awakenings compared with pramipexole (P < 0.0001; Table 2).
Table 2.
Polysomnography endpoints
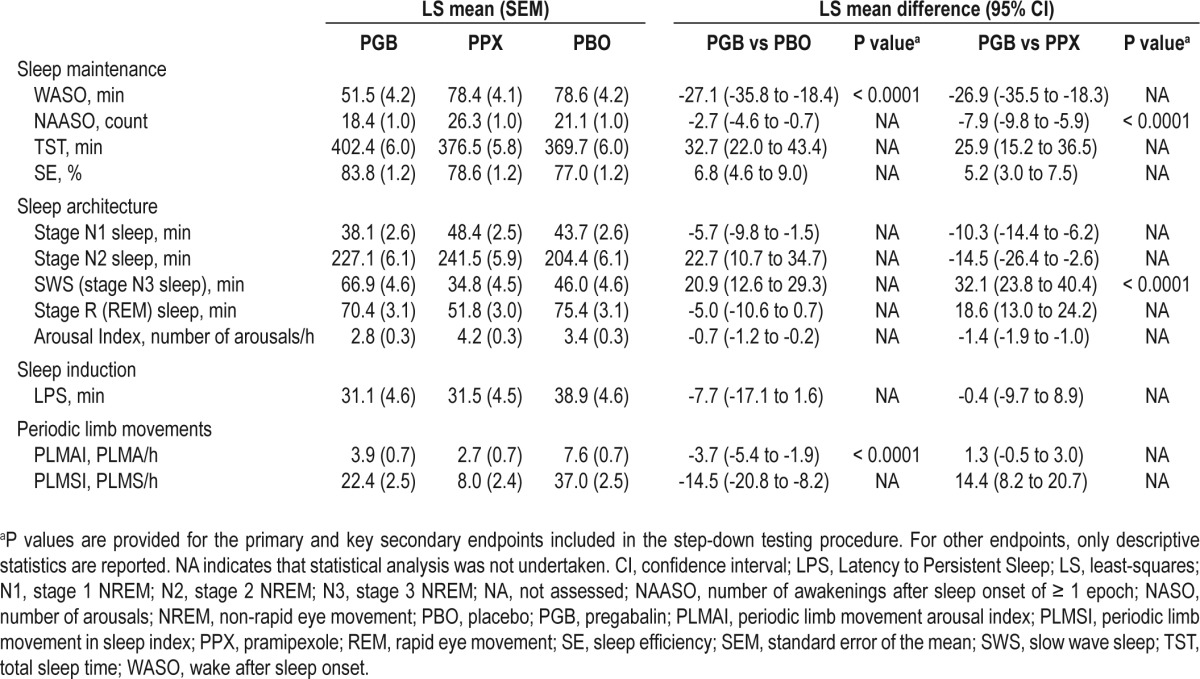
The key secondary sleep architecture endpoint of SWS increased by 20.9 minutes with pregabalin compared with placebo and 32.1 min compared with pramipexole (P < 0.0001). Stage N1 sleep decreased with pregabalin compared with both placebo and pramipexole, while stage N2 sleep increased with both pregabalin and pramipexole compared with placebo (Table 2). The frequency of arousals also decreased with pregabalin compared with placebo and pramipexole. Sleep induction, as measured by LPS, was reduced by 7.7 min with pregabalin compared with placebo, which was comparable to the change with pramipexole (Table 2).
With respect to motor function, the key secondary endpoint of PLMAI improved by 3.7 PLMA/h with pregabalin compared with placebo (P < 0.0001), which was comparable to the improvement with pramipexole. PLMSI also improved with pregabalin compared with placebo; however, the reduction was less than with pramipexole (Table 2).
Participant-Reported Measures
IRLS total score (range, 0-40) improved with pregabalin compared with placebo (-6.1; 95% CI, -8.1 to -4.1) and pramipexole (-3.1; -5.1 to -1.1). A higher proportion of participants also reported that their RLS symptoms were “very much improved” or “much improved” on the CGI-I scale with pregabalin (61.2%) compared with placebo (33.3%) and pramipexole (50.0%). No participants reported worsening of symptoms while receiving pregabalin (Figure 3).
Figure 3.
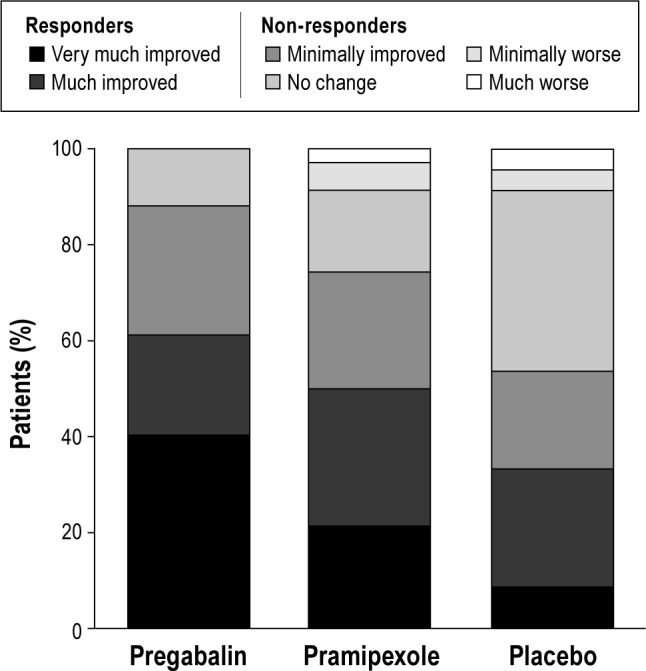
Clinical Global Impressions–Improvement. Responders were classified as participants reporting that RLS symptoms had “very much improved” or “much improved” (61.2% with pregabalin, 50.0% with pramipexole, and 33.3% with placebo).
Subjective measures of sleep maintenance reported on the SSQ improved with pregabalin compared with placebo and pramipexole (Table 3). In particular, the key secondary endpoint of sTST improved by 30.8 min with pregabalin compared with placebo (P < 0.0001) and 26.8 min compared with pramipexole. Subjective sleep latency was reduced with pregabalin compared with placebo and was similar to that with pramipexole. The composite 6-item and 9-item sleep problems indices of the MOS-SS were also improved with pregabalin compared with placebo and pramipexole (Table 4).
Table 3.
Subjective Sleep Questionnaire (SSQ) endpoints
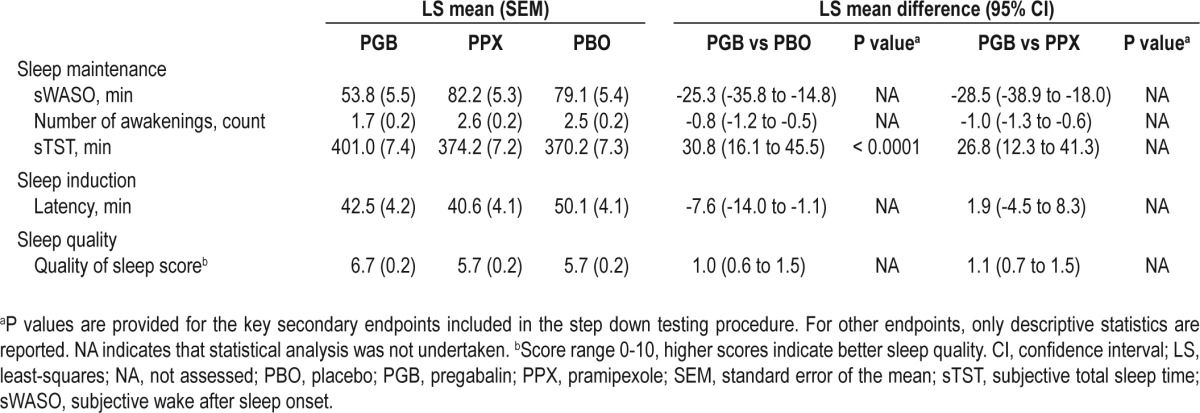
Table 4.
Medical Outcomes Study (MOS)–Sleep Scale (SS)
With respect to the impact of RLS on daily living, the key secondary endpoint of RLS-NDI total score (range, 0-140) significantly improved with pregabalin compared with placebo (-5.4; 95% CI, -10.4 to -0.3; P = 0.0396). RLS-QoL score (range, 0-100) also improved with pregabalin compared with placebo (5.3; 2.0 to 8.6). RLS-NDI and RLS-QoL scores both showed a trend towards improvement with pregabalin compared with pramipexole; however, the 95% CIs spanned zero (RLS-NDI, -4.9 [95% CI, -10.0 to 0.1]; RLS-QoL, 3.3 [-0.01 to 6.5]).
Safety and Tolerability
Treatment-emergent AEs (TEAEs) were experienced by 46 (61.3%) participants during pregabalin treatment, 40 (52.6%) during pramipexole treatment, and 27 (37.0%) during placebo treatment. The most frequently reported TEAEs during pregabalin treatment were dizziness and somnolence (24.0% and 17.3% of participants, respectively), and during pramipexole treatment were somnolence, headache, and nausea (each in 7.9% of participants; Table 5).
Table 5.
Summary of treatment-emergent (all causality) adverse events
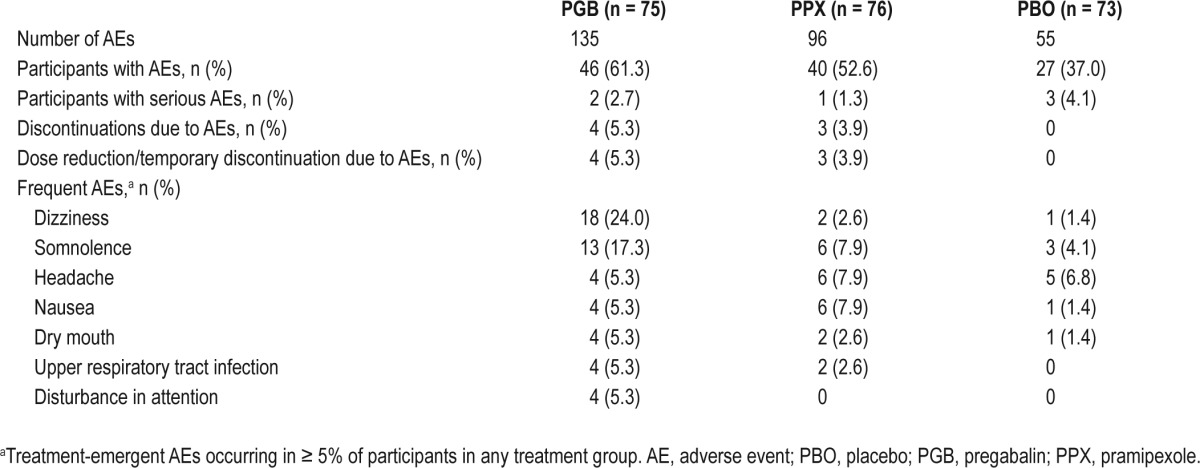
Five participants experienced treatment-emergent serious AEs (SAEs). Suicidal ideation in 1 participant, which began on placebo and continued during pramipexole treatment, was considered treatment-related. SAEs of gastroenteritis and hypokalemia during placebo treatment, as well as deep vein thrombosis and decreased blood pressure/postural dizziness during pregabalin treatment, were not considered related to treatment. Seven participants discontinued due to TEAEs: 4 during pregabalin treatment (3 due to dizziness/balance disorder and 1 hypertension) and 3 during pramipexole treatment (1 due to suicidal ideation, 1 nausea, and 1 agitation). All of these AEs resolved following treatment discontinuation.
DISCUSSION
In this double-blinded, randomized, crossover study, pregabalin 300 mg/day was more effective than placebo and pramipexole 0.5 mg/day in improving sleep disturbance, as assessed by objective and subjective measures of sleep maintenance and sleep architecture, following 4 weeks' double-blind treatment. Furthermore, pregabalin 300 mg/day improved subjective RLS symptoms (measured by IRLS and CGI-I) more than placebo or pramipexole 0.5 mg/day. The effect of pregabalin on arousals associated with PLM was similar to that of pramipexole, despite smaller overall reductions in PLMS. Pregabalin and pramipexole were generally well tolerated. TEAEs appeared to be more frequent with pregabalin than pramipexole (61.3% compared with 52.6%), with the most common TEAEs with pregabalin (dizziness and somnolence) occurring more frequently than the most common TEAEs with pramipexole (nausea, headache, and somnolence).
Improvements in objective measures of sleep were matched by similar improvements in participants' perception of their sleep. For example, subjective and objective reductions in WASO were 25.3 and 27.1 min, respectively, with pregabalin compared with placebo, and 28.5 and 26.9 min compared with pramipexole. Pregabalin also improved depth of sleep by increasing SWS and reducing the number of arousals compared with placebo and pramipexole. These results are consistent with PSG data from prior pregabalin trials in patients with RLS,22 fibromyalgia,32 and epilepsy,33 and in healthy volunteers,34 which similarly demonstrated increases in SWS. An increase in SWS has also been observed with the α2δ ligands gabapentin and gabapentin enacarbil, suggesting that this may be a class effect.35,36 In contrast, prior trials with dopamine agonists have demonstrated decreases in SWS, consistent with results in this study.15,17,18,20 Of interest for future study would be the effects of pregabalin on sleep microstructure, which has been shown by analysis of cyclic alternating patterns (CAP) to differ between RLS patients and controls.14 Acute treatment with pramipexole has been shown to fail to correct abnormalities in sleep micro-structure in RLS, despite significantly reducing PLMS.14
Overall rates of PLM in sleep were much decreased with pramipexole compared with placebo, with a smaller decrease with pregabalin compared with placebo. Nonetheless, arousals due to PLM were similar for both drugs. Thus, it seems likely that the effects of pregabalin on sleep in RLS patients are partially independent of improvements in PLM. This apparent disconnect between improvements in motor function and sleep is suggestive of differences in underlying brain function relating to these characteristics of RLS, which may be affected differently by pregabalin (potentially affecting the arousal system) and pramipexole (motor functions). This may also explain the observation that while studies consistently show a benefit of dopamine agonists on PLM and subjective sleep measures, significant improvements in objective sleep measures have not generally been observed.12–20 This disconnect between objective sleep measures and the PLM rates and subjective sleep measures with pramipexole could be further evaluated. At the same time, improvements in RLS symptoms, as assessed by IRLS and CGI-I, were greater with pregabalin suggesting that improvement in symptoms correlates better with objective measures of sleep.
Improvements in sleep architecture seen in this study have also been demonstrated in previous placebo-controlled, crossover trials of other α2δ ligands.35,36 In these trials, gabapentin was shown to improve TST by approximately 30 minutes (compared with 32.7 min in this study); however, PLMA/h were not significantly changed.35 In contrast, gabapentin enacarbil was shown to improve PLMA/h (mean treatment difference of -3.1, compared with -3.7 in this study) together with similar improvements in TST (32.7 min) and WASO (28.5 min)36 to those seen in this study.
A task force recently established by the International RLS Study Group identified pregabalin as effective for the treatment of RLS for up to 1 year (with Level A evidence), while pramipexole, ropinirole and rotigotine were established as effective for up to 6 months (Level A evidence).37 A number of other therapies were identified as effective for durations of up to 5 years with Level B evidence, these were: gabapentin enacarbil, pramipexole, and ropinirole (1 year); levodopa (2 years); and rotigotine (5 years).37 The task force recommended either dopa-mine agonists or α2δ ligands as first-line treatment, dependent on patient characteristics (e.g., dopamine agonists for patients with severe RLS symptoms or excess weight and α2δ ligands for patients with severe sleep disturbance or comorbid pain).37
In this study, the data suggest that pregabalin may lead to greater improvements in measures of quality of live and subjective sleep than pramipexole. Comparisons of pregabalin with studies of other RLS therapies are challenging due to differences in trial design but ropinirole38 and rotigotine39,40 have also been shown to improve quality of life and subjective sleep. The International RLS Study Group task force indicated that both dopamine agonists and α2δ ligands were “somewhat likely” to improve quality of life while α2δ ligands were more likely than dopamine agonists to improve subjective measures of sleep.37
Overall, the results of this study, together with prior research,21,22 indicate that pregabalin could represent a possible alternative to dopamine agonists for the effective treatment of RLS, and that it may offer greater improvements in sleep maintenance and quality, key clinical treatment goals in RLS. Further investigations should continue to evaluate the relative benefits and risks of both treatments for patients with RLS. Short-term AE rates appear to be higher for pregabalin than pramipexole, particularly for dizziness and somnolence, but this may not be the case for AEs over longer-term treatment. In the case of long-term use, dopamine agonists are often limited by the development of augmentation (an increase in the severity of RLS symptoms during treatment),41 and it would be of interest to determine whether pregabalin, due to its nondopaminergic mechanism of action, is associated with a lower augmentation risk. The design and short duration of the current study did not permit evaluation of effects on augmentation; however, this could represent another area of future research.
Another limitation of this study was the requirement for a relatively large number of participants to be screened in order to identify 85 participants. This was principally due to the requirement that participants meet the sleep parameter inclusion criteria. Participants were required to have WASO ≥ 60 minutes on the 2 PSG screening nights (with neither night having WASO < 30 min). This restriction likely also contributed to the lower than expected variance in WASO. Other studies with similar sleep parameter-based inclusion criteria have had similar screening failure rates.32 While this study was limited to participants with significant sleep disturbance, we expect that the results could also extend to other patients.
This study demonstrated improvements in objective and subjective measures of sleep maintenance and sleep architecture with pregabalin compared with placebo and pramipexole in patients with RLS. Decreases in PLMAI with pregabalin were comparable to those with pramipexole. Pregabalin also improved RLS symptoms compared with placebo and pramipexole.
DISCLOSURE STATEMENT
This study was sponsored by Pfizer Inc. Mr. Patrick, Ms. DuBrava, Dr. Chen, and Dr. Knapp are employees of Pfizer Inc. Dr. Miceli was an employee of Pfizer Inc at the time the study was conducted. Dr. Garcia-Borreguero has received research funding from Pfizer Inc and UCB Pharma and has served as a consultant/advisory board participant for Pfizer Inc, UCB Pharma, Xenoport, Otsuka Pharmaceuticals, and Laboratoires Urgo. Dr. Becker has served as a consultant for Pfizer Inc, UCB, and Impax, has received research support from UCB, Apnicure, and Aerial, and has acted as an investigator for Pfizer Inc. Dr. Lankford has received research funding from Actelion, Apni-cure, Arena, Cephalon, Evotec, Fisher Paykel, GlaxoSmith-Kline, Lilly, Merck, Neurim, Neurocrine, Neurogen, Organon, Pfizer Inc, Respironics, Sanofi-Aventis, Schering-Plough, Sepracor, Somaxon, Sunovion, Takeda, Transcept, UCB, Ventus, and Vanda; has served as a consultant/advisory board participant for Actelion, Apnicure, Cephalon, Pfizer Inc., and Somaxon; and has participated in speaking engagements for Jazz Pharmaceuticals, Sanofi-Aventis, Somaxon, and Purdue. Dr. Allen has served as a consultant for Pfizer Inc, GlaxoSmith-Kline, UCB, Xenoport, Impax, Pharmacosmos, and Luipold and has received research support from GlaxoSmithKline and Pharmacosmos.
ACKNOWLEDGMENTS
The authors thank the study investigators and centers who participated in this trial: Paul Wylie, Arkansas Center for Sleep Medicine, Little Rock, AR; Alan Lankford, Sleep Disorders Center of Georgia, Atlanta, GA; Carl Griffin, Lynn Health Science Institute, Oklahoma City, OK; Antoinette Pragalos, Community Research, Cincinnati, OH; Milton Erman, Dormir Clinical Trials Inc, San Diego, CA; Stephen Thein, Pacific Research Network, San Diego, CA; Howard Schwartz, Miami Research Associates, Miami, FL; Richard Bogan, SleepMed of South Carolina, Columbia, SC; Mansoor Ahmed, Cleveland Sleep Research Center, Middleburg Heights, OH; Charles Wells Jr, Sleepmed of Central Georgia, Macon, GA; Derek Loewy, REM Medical Clinical Research, Tucson, AZ; Philip Becker, Sleep Medicine Associates of Texas, Dallas, TX; Jon Freeman, Clinilabs Inc, New York, NY; Beth Safirstein, MD Clinical, Hallandale Beach, FL; Markus Schmidt, Ohio Sleep Medicine and Neuroscience Institute Inc, Dublin, OH; Lawrence Scrima, Sleep Alertness Disorders Center Inc, Aurora, CO; Michael DeSantis, Clinical Trials Of America Inc, Hickory, NC; Brock Summers, Southwestern Research Inc, Pasadena, CA; John Murphy, Southwestern Research Inc, Santa Ana, CA; Ashokkumar Patel, Sleep Lab of Northeastern Pennsylvania, Clarks Summit, PA; John Schwab, Louisiana Sleep Foundation LLC, Baton Rouge, LA; June Fry, Center for Sleep Medicine, Lafayette Hill, PA; William Kohler, Florida Sleep Institute, Spring Hill, FL.
Medical writing support was provided by Elizabeth Harvey PhD, of Engage Scientific Solutions, and funded by Pfizer Inc.
REFERENCES
- 1.Allen RP, Picchietti D, Hening WA, Trenkwalder C, Walters AS, Montplaisir J. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4:101–19. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 2.Hening W, Walters AS, Allen RP, Montplaisir J, Myers A, Ferini-Strambi L. Impact, diagnosis and treatment of restless legs syndrome (RLS) in a primary care population: the REST (RLS epidemiology, symptoms, and treatment) primary care study. Sleep Med. 2004;5:237–46. doi: 10.1016/j.sleep.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 3.Allen RP, Walters AS, Montplaisir J, et al. Restless legs syndrome prevalence and impact: REST general population study. Arch Intern Med. 2005;165:1286–92. doi: 10.1001/archinte.165.11.1286. [DOI] [PubMed] [Google Scholar]
- 4.Winkelman JW, Redline S, Baldwin CM, Resnick HE, Newman AB, Gottlieb DJ. Polysomnographic and health-related quality of life correlates of restless legs syndrome in the Sleep Heart Health Study. Sleep. 2009;32:772–8. doi: 10.1093/sleep/32.6.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saletu B, Anderer P, Saletu M, Hauer C, Lindeck-Pozza L, Saletu-Zyhlarz G. EEG mapping, psychometric, and polysomnographic studies in restless legs syndrome (RLS) and periodic limb movement disorder (PLMD) patients as compared with normal controls. Sleep Med. 2002;3(Suppl):S35–42. doi: 10.1016/s1389-9457(02)00147-8. [DOI] [PubMed] [Google Scholar]
- 6.Hornyak M, Feige B, Voderholzer U, Philipsen A, Riemann D. Polysomnography findings in patients with restless legs syndrome and in healthy controls: a comparative observational study. Sleep. 2007;30:861–5. doi: 10.1093/sleep/30.7.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushida CA, Allen RP, Atkinson MJ. Modeling the causal relationships between symptoms associated with restless legs syndrome and the patient-reported impact of RLS. Sleep Med. 2004;5:485–8. doi: 10.1016/j.sleep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Hornyak M, Trenkwalder C, Kohnen R, Scholz H. Efficacy and safety of dopamine agonists in restless legs syndrome. Sleep Med. 2012;13:228–36. doi: 10.1016/j.sleep.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Trenkwalder C, Hening WA, Montagna P, et al. Treatment of restless legs syndrome: an evidence-based review and implications for clinical practice. Mov Disord. 2008;23:2267–302. doi: 10.1002/mds.22254. [DOI] [PubMed] [Google Scholar]
- 10.Hening WA. Current guidelines and standards of practice for restless legs syndrome. Am J Med. 2007;120:S22–7. doi: 10.1016/j.amjmed.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Montplaisir J, Boucher S, Poirier G, Lavigne G, Lapierre O, Lesperance P. Clinical, polysomnographic, and genetic characteristics of restless legs syndrome: a study of 133 patients diagnosed with new standard criteria. Mov Disord. 1997;12:61–5. doi: 10.1002/mds.870120111. [DOI] [PubMed] [Google Scholar]
- 12.Manconi M, Ferri R, Zucconi M, et al. First night efficacy of pramipexole in restless legs syndrome and periodic leg movements. Sleep Med. 2007;8:491–7. doi: 10.1016/j.sleep.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Manconi M, Ferri R, Zucconi M, et al. Pramipexole versus ropinirole: polysomnographic acute effects in restless legs syndrome. Mov Disord. 2011;26:892–5. doi: 10.1002/mds.23543. [DOI] [PubMed] [Google Scholar]
- 14.Ferri R, Manconi M, Arico D, et al. Acute dopamine-agonist treatment in restless legs syndrome: effects on sleep architecture and NREM sleep instability. Sleep. 2010;33:793–800. doi: 10.1093/sleep/33.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allen R, Becker PM, Bogan R, et al. Ropinirole decreases periodic leg movements and improves sleep parameters in patients with restless legs syndrome. Sleep. 2004;27:907–14. doi: 10.1093/sleep/27.5.907. [DOI] [PubMed] [Google Scholar]
- 16.Tagaya H, Wetter TC, Winkelmann J, et al. Pergolide restores sleep maintenance but impairs sleep EEG synchronization in patients with restless legs syndrome. Sleep Med. 2002;3:49–54. doi: 10.1016/s1389-9457(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 17.Partinen M, Hirvonen K, Jama L, et al. Efficacy and safety of pramipexole in idiopathic restless legs syndrome: a polysomnographic dose-finding study--the PRELUDE study. Sleep Med. 2006;7:407–17. doi: 10.1016/j.sleep.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Saletu M, Anderer P, Saletu-Zyhlarz G, Hauer C, Saletu B. Acute placebo-controlled sleep laboratory studies and clinical follow-up with pramipexole in restless legs syndrome. Eur Arch Psychiatry Clin Neurosci. 2002;252:185–94. doi: 10.1007/s00406-002-0380-7. [DOI] [PubMed] [Google Scholar]
- 19.Montplaisir J, Nicolas A, Denesle R, Gomez-Mancilla B. Restless legs syndrome improved by pramipexole: a double-blind randomized trial. Neurology. 1999;52:938–43. doi: 10.1212/wnl.52.5.938. [DOI] [PubMed] [Google Scholar]
- 20.Jama L, Hirvonen K, Partinen M, et al. A dose-ranging study of pramipexole for the symptomatic treatment of restless legs syndrome: polysomnographic evaluation of periodic leg movements and sleep disturbance. Sleep Med. 2009;10:630–6. doi: 10.1016/j.sleep.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Allen R, Chen C, Soaita A, et al. A randomized, double-blind, 6-week, dose-ranging study of pregabalin in patients with restless legs syndrome. Sleep Med. 2010;11:512–9. doi: 10.1016/j.sleep.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Borreguero D, Larrosa O, Williams AM, et al. Treatment of restless legs syndrome with pregabalin: a double-blind, placebo-controlled study. Neurology. 2010;74:1897–904. doi: 10.1212/WNL.0b013e3181e1ce73. [DOI] [PubMed] [Google Scholar]
- 23.Mirapex (pramipexole dihydrochloride) [Prescribing Information] Ridgefield, CT: Boehringer Ingelheim Pharmaceuticals, Inc.; 2011. [accessed 1 May 2012]. Available at: http://bidocs.boehringer-ingelheim.com/BIWebAccess/ViewServlet.ser?docBase=renetnt&folderPath=/Prescribing%20Information/PIs/Mirapex/Mirapex.pdf. [Google Scholar]
- 24.Rechtschaffen A, Kales A. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. Los Angeles, CA: UCLA Brain Information Service/Brain Research Institute; 1968. [Google Scholar]
- 25.Iber C, Ancoli-Israel S, Chesson A, Quan SF. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specification. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 26.Scharf MB, Roth T, Vogel GW, Walsh JK. A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry. 1994;55:192–9. [PubMed] [Google Scholar]
- 27.Roth T, Roehrs TA, Vogel GW, Dement WC. Evaluation of hypnotic medications. In: Prien RF, Robinson DS, editors. Clinical evaluation of psychotropic drugs: principles and guidelines. New York: Raven Press; 1994. pp. 579–92. [Google Scholar]
- 28.Lasch KE, Abraham L, Patrick J, Piault EC, Tully SE, Treglia M. Development of a next day functioning measure to assess the impact of sleep disturbance due to restless legs syndrome: the restless legs syndrome-next day impact questionnaire. Sleep Med. 2011;12:754–61. doi: 10.1016/j.sleep.2011.03.015. [DOI] [PubMed] [Google Scholar]
- 29.Walters AS, LeBrocq C, Dhar A, et al. Validation of the International Restless Legs Syndrome Study Group rating scale for restless legs syndrome. Sleep Med. 2003;4:121–32. doi: 10.1016/s1389-9457(02)00258-7. [DOI] [PubMed] [Google Scholar]
- 30.Abetz L, Vallow SM, Kirsch J, Allen RP, Washburn T, Earley CJ. Validation of the Restless Legs Syndrome Quality of Life questionnaire. Value Health. 2005;8:157–67. doi: 10.1111/j.1524-4733.2005.03010.x. [DOI] [PubMed] [Google Scholar]
- 31.Allen RP, Kosinski M, Hill-Zabala CE, Calloway MO. Psychometric evaluation and tests of validity of the Medical Outcomes Study 12-item Sleep Scale (MOS sleep) Sleep Med. 2009;10:531–9. doi: 10.1016/j.sleep.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Roth T, Lankford DA, Bhadra P, Whalen E, Resnick EM. Effect of pregabalin on sleep in patients with fibromyalgia and sleep maintenance disturbance: A randomized, placebo-controlled, 2-way crossover polysomnography study. Arthritis Care Res (Hoboken) 2012;64:597–606. doi: 10.1002/acr.21595. [DOI] [PubMed] [Google Scholar]
- 33.Bazil CW, Dave J, Cole J, Stalvey J, Drake E. Pregabalin increases slow-wave sleep and may improve attention in patients with partial epilepsy and insomnia. Epilepsy Behav. 2012;23:422–5. doi: 10.1016/j.yebeh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 34.Hindmarch I, Dawson J, Stanley N. A double-blind study in healthy volunteers to assess the effects on sleep of pregabalin compared with alprazolam and placebo. Sleep. 2005;28:187–93. doi: 10.1093/sleep/28.2.187. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G. Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology. 2002;59:1573–9. doi: 10.1212/wnl.59.10.1573. [DOI] [PubMed] [Google Scholar]
- 36.Winkelman JW, Bogan RK, Schmidt MH, Hudson JD, DeRossett SE, Hill-Zabala CE. Randomized polysomnography study of gabapentin enacarbil in subjects with restless legs syndrome. Mov Disord. 2011;26:2065–72. doi: 10.1002/mds.23771. [DOI] [PubMed] [Google Scholar]
- 37.Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/Willis-Ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14:675–84. doi: 10.1016/j.sleep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Trenkwalder C, Garcia-Borreguero D, Montagna P, et al. Ropinirole in the treatment of restless legs syndrome: results from the TREAT RLS 1 study, a 12 week, randomised, placebo controlled study in 10 European countries. J Neurol Neurosurg Psychiatry. 2004;75:92–7. [PMC free article] [PubMed] [Google Scholar]
- 39.Oertel WH, Benes H, Garcia-Borreguero D, et al. Rotigotine transdermal patch in moderate to severe idiopathic restless legs syndrome: a randomized, placebo-controlled polysomnographic study. Sleep Med. 2010;11:848–56. doi: 10.1016/j.sleep.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 40.Trenkwalder C, Benes H, Poewe W, et al. Efficacy of rotigotine for treatment of moderate-to-severe restless legs syndrome: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2008;7:595–604. doi: 10.1016/S1474-4422(08)70112-1. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Borreguero D, Williams AM. Dopaminergic augmentation of restless legs syndrome. Sleep Med Rev. 2010;14:339–46. doi: 10.1016/j.smrv.2009.11.006. [DOI] [PubMed] [Google Scholar]