Abstract
Study Objectives:
Alcohol tolerance is a major contributor towards the development of alcohol dependence. Does alcohol intake result in rapid tolerance development to alcohol induced NREM sleep promotion? This has never been examined. Our objective was to examine whether two bouts of alcohol consumption on consecutive days results in rapid tolerance development to alcohol-induced NREM sleep promotion.
Design:
N/A.
Setting:
N/A.
Patients or Participants:
C57BL/6J mice.
Interventions:
Mice (N = 5) were implanted with sleep electrodes using standard surgical conditions. Following postoperative recovery and habituation, the experiment was begun. On baseline day, water bottle changes were performed at 10:00 (3 h after dark onset) and 14:00 to mimic conditions during alcohol consumption days. On next 2 days, (Days 1 and 2) mice were allowed to self-administer alcohol (20% v/v) for 4 h beginning at 10:00 and ending at 14:00. Sleep-wakefulness was continuously recorded from 10:00 to 18:00 (8 h; 4 h during alcohol + 4 h post-alcohol) on all 3 days.
Measurements and Results:
Although mice consumed comparable amounts of alcohol on Days 1 and 2, NREM sleep and wakefulness were significantly and differentially affected during 4 h post-alcohol period. A robust alcohol-induced NREM sleep promotion was observed on Day 1. However, no such sleep promotion was observed on Day 2, suggesting rapid tolerance development.
Conclusions:
Our study is the first to demonstrate that alcohol consumption for two consecutive days results in development of rapid tolerance to alcohol-induced sleep promotion.
Citation:
Sharma R; Sahota P; Thakkar MM. Rapid tolerance development to the NREM sleep promoting effect of alcohol. SLEEP 2014;37(4):821-824.
Keywords: Alcohol dependence, rapid tolerance, alcohol, mice, sleep
INTRODUCTION
Alcohol tolerance is defined as an accentuated reduction of its effect with the constant use of the same quantity or a need to progressively consume higher amounts of alcohol to achieve the same effect. Alcohol tolerance is a diagnostic criterion of alcohol dependence, a predictor of vulnerability to alcoholism and a major contributor toward the promotion of alcohol drinking behaviors.1–6 Functional tolerance to alcohol is the result of the brain adapting to alcohol-induced disruption and is temporarily classified as: (1) acute tolerance, which is observed during a single session of alcohol consumption; (2) rapid tolerance, which is observed between 8 to 24 hours after the effects of first alcohol administration has disappeared; and (3) chronic tolerance, which is detected after long term (days) alcohol exposure.3,7–12 Tolerance to several behavioral effects of alcohol including hypothermia, loss of righting reflex, and ataxia has been examined.8,12–15 However, rapid tolerance to the sleep promoting effects of alcohol has never been examined.
Alcohol is a potent somnogen and promotes NREM sleep. It is among the most extensively used “over-the-counter” sleep aid. Several different population-based studies suggest that approximately 10% to 40% of general population use alcohol as a sleep aid.16–21 Does alcohol intake result in rapid tolerance to the NREM sleep promoting effects? We hypothesized that alcohol consumption for two consecutive days would result in rapid tolerance to the sleep-promoting effect of alcohol.
MATERIALS AND METHODS
Animals
Adult male C57BL/6J mice (7-8 weeks; 22-26 g; Jackson Laboratories [Bar Harbor, ME]) were housed under reverse 12-12 hour light/dark cycle (light onset = 19:00) with ad lib access to standard laboratory chow and water for 8 to 10 days before experiments were begun. All experimental procedures were approved by the Institutional Animal Care and Use Committee of Harry S. Truman Memorial Veterans' Hospital.
Surgery
Under aseptic conditions and isoflurane anesthesia, mice were stereotaxically implanted with 3 screw electrodes to record brain activity (electroencephalogram [EEG]) with 2 screws implanted at posterior 2.2 mm and lateral ± 1.5 mm; and one screw at anterior 2.5 mm and lateral = 1.5 mm (all coordinates relative to bregma22). Three flexible wire electrodes were secured to the neck (nuchal) muscle to record muscle activity (electromyogram [EMG]). Two anchors were also fixed onto the skull. All EEG and EMG electrodes were connected to a multi-channel electrode pedestal (MS363, Plastics One Inc., Roanoke, VA), and the entire assembly was secured to the skull with dental cement. The wound was sutured and animals monitored until ambulatory. Subcutaneous flunixin (2.5 mg/kg/12 h for one day) was used as a postoperative analgesic.
Postoperative Recovery and Habituation
Following surgery, each animal was housed singly (with identical conditions as described previously) and allowed to recover for 3 days, with last 2 days in the sleep recording cage. The recording cage was similar to the home cage except taller (height = 10 inches) with open top, and a grommeted hole on one (shorter) side of the cage for dispensing water (and/ or alcohol) in 15 mL bottles fitted with metal sipper tubes. During the next 7 days, mice were allowed to habituate to the special lightweight cables (Plastics One Inc., Roanoke, VA) that minimally interfere with animal movements. Mice were unrestrained and were able to move freely.
Alcohol Self-Administration
We used the modified version of “drinking in the dark” protocol.23 The basic paradigm was to offer each mouse a single bottle containing 20% (v/v; in tap water) alcohol (200-proof ethanol; Fisher Scientific, Pittsburgh, PA, USA; prepared fresh) instead of its usual water bottle, for a period of 4 h, beginning 3 h after dark onset.
Baseline Day
The experiment was begun by initiation of electrographic recording of sleep-wakefulness on baseline day along with mimicking the conditions as on alcohol exposure days. The protocol in detail is as follows. Beginning at approximately 09:30, the animals were untethered from their recording cable, weighed, and re-connected. At 10:00 (3 h after dark onset) the water bottles were replaced with new water bottles. Sleep-wakefulness recording was initiated. The animals were left undisturbed for next 4 hours. At 14:00, the new water bottles were replaced with original water bottles. Sleep-wakefulness was continuously recorded until 18:00 (total 8 h; 4 h during alcohol + 4 h post-alcohol).
Alcohol Days 1 and 2
On alcohol Days 1 and 2, the same protocol, as described for baseline day was repeated, except at 10:00 water bottles were replaced with pre-weighed alcohol bottles (containing 20% alcohol), and at 14:00 alcohol bottles were replaced with original water bottles. The removed alcohol bottles were weighed to calculate the amount of alcohol consumed. Sleep-wakefulness was continuously recorded for 8 hours.
Data Acquisition and Analysis
Sleep-wakefulness data was acquired using 16-channel poly-graph and manually scored as (a) wakefulness, (b) non-rapid eye movement (NREM) and (c) rapid eye movement (REM) sleep as described previously.24,25
Blood Alcohol Concentration (BAC)
Measurement of BAC requires animal handling, nicking the tail, and removal of blood. This may disturb/stress the animal and affect sleep.25,26 Thus, BAC was measured in a separate group of animals that were not implanted with sleep electrodes but were exposed to same alcohol drinking paradigm (without sleep-wakefulness recordings) for 2 days. The BAC measurement was performed as follows: Immediately after measurement of alcohol consumption, mice were removed from their cages and a small amount (∼ 25 μL) of blood was removed from the tail vein for BAC measurement. Subsequently, mice were returned to their cages and left undisturbed until the next day (4 h post drinking) when another blood sample was collected (as described above). The collected blood sample was centrifuged to separate plasma which was used for BAC analysis using Ethanol Measurement Kit (Ethanol L3K) as per manufacturer's instructions (Sekisui Diagnostics LLC, Lexington, MA).
Statistics
One-way repeated measure ANOVA (Graphpad Prism, San Diego, CA) followed by Dunnett post hoc test was used to examine rapid tolerance development to the sleep promoting effects of alcohol.
RESULTS
Mice (N = 5) consumed comparable amounts of alcohol on Days 1 (Mean ± SEM = 3.67 ± 0.74 g/kg) and 2 (Mean ± SEM = 3.97 ± 0.67 g/kg) and amount of time spent in different states of sleep-wakefulness during 4 h of alcohol consumption was comparable (Figure 1A). However, signifi-cant effects were observed in the amount of time spent in wakefulness (F3, 14 = 5.7, P = 0.03; repeated measures ANOVA) and NREM sleep (F3, 14 = 5.8, P = 0.02; repeated measures ANOVA) during 4 h post-alcohol. There was no change in REM sleep (F3, 14 = 0.28, P = 0.8; Figure 1B).
Figure 1.
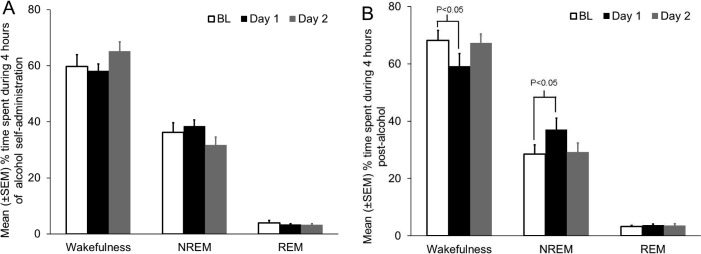
Rapid tolerance development to NREM sleep-promoting effects of alcohol. (A) The amount of time spent in wakefulness, NREM sleep and REM sleep during 4 hours of alcohol consumption was comparable across all 3 days. (B) Alcohol consumption on Day 1 resulted in a robust NREM sleep promotion during 4 hours post-alcohol. However, although the animals consumed comparable amount of alcohol on Day 1 and Day 2, no such NREM sleep promotion was observed on Day 2 suggesting the development of rapid tolerance to NREM sleep-promoting effects of alcohol (see text for details).
Alcohol consumption for 4 h on Day 1 resulted in a strong NREM sleep promotion. Mice spent significantly more time in NREM sleep (P < 0.05, Dunnett post hoc test) and significantly less time in wakefulness (P < 0.05, Dunnett post hoc test) during 4 h post-alcohol on Day 1 as compared to baseline.
However, no such NREM sleep promotion was observed on the second day suggesting rapid tolerance development. As compared to baseline, there was no change in any sleep-wakefulness state during 4 h post-alcohol.
Blood Alcohol Concentration (BAC)
BAC was measured in a separate group of mice (N = 5; no surgery or sleep recording). Mean (± SEM) alcohol consumption (g/kg) on Day 1 = 3.66 (± 0.12), and on Day 2 = 3.75 (± 0.19). Mean (± SEM) BAC (mg/dL; measured immediately after 4 h of alcohol consumption) on Day 1 = 58.3 (± 14.8) and on Day 2 = 70.8 (± 7.6).
DISCUSSION
Our study is the first to demonstrate that two episodes of alcohol consumption within 24 hours results in rapid tolerance development to the NREM sleep promoting effects of alcohol. Since tolerance to alcohol is a major contributor towards the development of alcohol dependency, our study has potential clinical implications, especially since alcohol is extensively used as a sleep aid.
In the present study, we used C57BL/6J mice to examine the development of rapid tolerance to the sleep promoting effects of alcohol because the C57BL/6J mice self-administer high amounts of alcohol in relatively short period of time for post-ingestive intoxicating effects, and not simply for taste or caloric fulfillment.27,28 Since human alcohol dependency is associated with oral self-ingestion, we preferred alcohol self-ingestion protocol rather than forced alcohol administration (intraperitoneal or intragastric administration), to examine rapid tolerance development to the NREM sleep-promoting effects of alcohol.29 In addition, forced alcohol administration requires animal handling which can be stressful and can cause pain (especially intraperitoneal injections), which may affect sleep-wakefulness.26 In our paradigm, we allowed the animals to voluntarily consume alcohol in a non-stressful environment (in their own cage). Although alcohol was the only source of fluid and no other fluid choice was offered, previous studies have shown that several other genotypes avoid alcohol or consume very little in the same paradigm. Avoidance or voluntary fluid deprivation for four hours has no major effects on mice physiologically.30–33 Finally, we used “within subject” design that offers several advantages including an increase in statistical power without increasing animal numbers and a reduction in error variance associated with individual differences.
Tolerance to alcohol can be either metabolic or functional. Metabolic tolerance results from a more rapid elimination of alcohol from the body and is associated with a specific group of alcohol metabolizing liver enzymes that are only activated after chronic drinking.34–36 Thus, tolerance development to the NREM sleep promoting effects observed within 24 hours after first episode of alcohol consumption suggests that tolerance developed to the NREM sleep promoting effects was not due to rapid elimination of alcohol. This is also supported by our BAC results, which suggested similar BAC levels after alcohol consumption on Day 1 and 2.
Temporally, functional tolerance is divided into acute tolerance, rapid tolerance, and chronic tolerance.8 Of these three, rapid tolerance development to NREM sleep promoting effects of alcohol has significant importance because rapid tolerance is an index of chronic tolerance and development of rapid tolerance promotes development of cross-tolerance to several sedative drugs including benzodiazepines, which are among the most commonly prescribed drugs for insomnia and other sleep disorders.7,8,17,37–40
Interestingly, our study is supported by the human study in which Yules and his colleagues showed tolerance development to REM sleep reducing effects of alcohol following the administration of the same dose of alcohol on the previous day.43 What are the mechanisms responsible for rapid tolerance development to the NREM sleep promoting effects of alcohol? While this is yet unclear, we have demonstrated that adenosine acting via A1 receptor plays an important role in alcohol induced NREM sleep promotion. Thus, it is likely that adenosinergic mechanisms may play a critical role in rapid tolerance development to NREM sleep promoting effects of alcohol.24,25,41 Is development of rapid tolerance, the result of reduced adenosinergic tone in the wake-promoting basal forebrain? Indeed, in vitro studies suggest that acute alcohol interacts with equilibrative nucleoside transporter 1 (ENT1) to inhibit adenosine influx resulting in increased extracellular adenosine. However, chronic alcohol exposure reduces this inhibition.42 Does rapid tolerance development involve ENT1 down-regulation in the wake-promoting basal forebrain? Further studies are required to answer these questions.
We believe our study is the first to demonstrate that alcohol consumption for two consecutive days results in tolerance development to the NREM sleep promoting effects of alcohol. Although, our study is simple and straightforward, it is relevant and timely, especially since alcohol is extensively used as a sleep aid.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by the Harry S. Truman Memorial Veterans Hospital, and funded by research grants (AA020334 & AA0174720) from National Institute of Alcohol Abuse and Alcoholism. The authors have indicated no financial conflicts of interest.
REFERENCES
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington DC: American Psychiatric Press; 1994. [Google Scholar]
- 2.World Healh Organization. International statistical classification of diseases and related health problems. 10th ed. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 3.Tabakoff B, Cornell N, Hoffman PL. Alcohol tolerance. Ann Emerg Med. 1986;15:1005–12. doi: 10.1016/s0196-0644(86)80119-6. [DOI] [PubMed] [Google Scholar]
- 4.Schuckit MA. Biological markers in alcoholism. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:191–9. doi: 10.1016/0278-5846(86)90073-4. [DOI] [PubMed] [Google Scholar]
- 5.Waller MB, McBride WJ, Lumeng L, Li TK. Initial sensitivity and acute tolerance to ethanol in the P and NP lines of rats. Pharmacol Biochem Behav. 1983;19:683–6. doi: 10.1016/0091-3057(83)90345-3. [DOI] [PubMed] [Google Scholar]
- 6.Altman J, Everitt BJ, Robbins TW, et al. The biological, social and clinical bases of drug addiction: commentary and debate. Psychopharmacology (Berl) 1996;125:285–345. doi: 10.1007/BF02246016. [DOI] [PubMed] [Google Scholar]
- 7.Khanna JM, Kalant H, Shah G, Weiner J. Rapid tolerance as an index of chronic tolerance. Pharmacol Biochem Behav. 1991;38:427–32. doi: 10.1016/0091-3057(91)90302-i. [DOI] [PubMed] [Google Scholar]
- 8.Kalant H. Research on tolerance: what can we learn from history? Alcohol Clin Exp Res. 1998;22:67–76. doi: 10.1111/j.1530-0277.1998.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 9.Pietrzykowski AZ, Treistman SN. The molecular basis of tolerance. Alchohol Res Health. 2008:298–309. [PMC free article] [PubMed] [Google Scholar]
- 10.Khanna JM, Kalant H, Le AD, LeBlanc AE. Role of serotonergic and adrenergic systems in alcohol tolerance. Prog Neuropsychopharmacol. 1981;5:459–65. doi: 10.1016/0364-7722(81)90027-8. [DOI] [PubMed] [Google Scholar]
- 11.Sakharkar AJ, Zhang H, Tang L, Shi G, Pandey SC. Histone deacetylases (HDAC)-induced histone modifications in the amygdala: a role in rapid tolerance to the anxiolytic effects of ethanol. Alcohol Clin Exp Res. 2012;36:61–71. doi: 10.1111/j.1530-0277.2011.01581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crabbe JC, Rigter H, Uijlen J, Strijbos C. Rapid development of tolerance to the hypothermic effect of ethanol in mice. J Pharmacol Exp Ther. 1979;208:128–33. [PubMed] [Google Scholar]
- 13.Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19:491–5. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- 14.Gill K, Deitrich RA. Acute tolerance to the ataxic effects of ethanol in short-sleep (SS) and long-sleep (LS) mice. Psychopharmacology (Berl) 1998;136:91–8. doi: 10.1007/s002130050543. [DOI] [PubMed] [Google Scholar]
- 15.Kurtz DL, Stewart RB, Zweifel M, Li TK, Froehlich JC. Genetic differences in tolerance and sensitization to the sedative/hypnotic effects of alcohol. Pharmacol Biochem Behav. 1996;53:585–91. doi: 10.1016/0091-3057(95)02055-1. [DOI] [PubMed] [Google Scholar]
- 16.Johnson EO, Roehrs T, Roth T, Breslau N. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21:178–86. doi: 10.1093/sleep/21.2.178. [DOI] [PubMed] [Google Scholar]
- 17.Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9:728–38. doi: 10.1007/s13311-012-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25:101–9. [PMC free article] [PubMed] [Google Scholar]
- 19.Brower KJ. Alcohol's effects on sleep in alcoholics. Alcohol Res Health. 2001;25:110–25. [PMC free article] [PubMed] [Google Scholar]
- 20.Dorrian J, Paterson J, Dawson D, Pincombe J, Grech C, Rogers AE. Sleep, stress and compensatory behaviors in Australian nurses and midwives. Rev Saude Publica. 2011;45:922–30. doi: 10.1590/s0034-89102011005000059. [DOI] [PubMed] [Google Scholar]
- 21.Gold DR, Rogacz S, Bock N, et al. Rotating shift work, sleep, and accidents related to sleepiness in hospital nurses. Am J Public Health. 1992;82:1011–4. doi: 10.2105/ajph.82.7.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. 3rd ed. New York, NY: Academic Press; 2008. [Google Scholar]
- 23.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 24.Sharma R, Engemann S, Sahota P, Thakkar MM. Role of adenosine and wake-promoting basal forebrain in insomnia and associated sleep disruptions caused by ethanol dependence. J Neurochem. 2010;115:782–94. doi: 10.1111/j.1471-4159.2010.06980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thakkar MM, Engemann SC, Sharma R, Sahota P. Role of wake-promoting basal forebrain and adenosinergic mechanisms in sleep-promoting effects of ethanol. Alcohol Clin Exp Res. 2010;34:997–1005. doi: 10.1111/j.1530-0277.2010.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longordo F, Fan J, Steimer T, Kopp C, Luthi A. Do mice habituate to “gentle handling?” A comparison of resting behavior, corticosterone levels and synaptic function in handled and undisturbed C57BL/6J mice. Sleep. 2011;34:679–81. doi: 10.1093/sleep/34.5.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sprow GM, Thiele TE. The neurobiology of binge-like ethanol drinking: Evidence from rodent models. Physiol Behav. 2012;106:325–31. doi: 10.1016/j.physbeh.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Belknap JK, Crabbe JC, Young ER. Voluntary consumption of ethanol in 15 inbred mouse strains. Psychopharmacology (Berl) 1993;112:503–10. doi: 10.1007/BF02244901. [DOI] [PubMed] [Google Scholar]
- 29.Darbra S, Prat G, Pallares M, Ferre N. Tolerance and sensitization to the hypnotic effects of alcohol induced by chronic voluntary alcohol intake in rats. J Psychopharmacol. 2002;16:79–83. doi: 10.1177/026988110201600107. [DOI] [PubMed] [Google Scholar]
- 30.Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann N Y Acad Sci. 2011;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rhodes JS, Ford MM, Yu CH, et al. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 32.Toth LA, Gardiner TW. Food and water restriction protocols: physiological and behavioral considerations. Contemp Top Lab Anim Sci. 2000;39:9–17. [PubMed] [Google Scholar]
- 33.Gupta T, Syed YM, Revis AA, et al. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–8. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- 34.Lieber CS. Metabolism of ethanol and associated hepatotoxicity. Drug Alcohol Rev. 1991;10:175–202. doi: 10.1080/09595239100185231. [DOI] [PubMed] [Google Scholar]
- 35.Lieber CS. The microsomal ethanol oxidizing system: its role in ethanol and xenobiotic metabolism. Biochem Soc Trans. 1988;16:232–9. doi: 10.1042/bst0160232. [DOI] [PubMed] [Google Scholar]
- 36.National Institute on Alcohol Abuse and Alcoholism. Alcohol and Tolerance. 1995 Report No. 28 PH 356. [Google Scholar]
- 37.Khanna JM, Chau A, Shah G. Characterization of the phenomenon of rapid tolerance to ethanol. Alcohol. 2011;13:621–8. doi: 10.1016/s0741-8329(96)00083-3. [DOI] [PubMed] [Google Scholar]
- 38.Gougos A, Khanna JM, Le AD, Kalant H. Tolerance to ethanol and cross-tolerance to pentobarbital and barbital. Pharmacol Biochem Behav. 1986;24:801–7. doi: 10.1016/0091-3057(86)90414-4. [DOI] [PubMed] [Google Scholar]
- 39.Sullivan S. Update on emerging drugs for insomnia. Expert Opin Emerg Drugs. 2012;17:295–8. doi: 10.1517/14728214.2012.693158. [DOI] [PubMed] [Google Scholar]
- 40.Morato GS, Khanna JM. N-methyl-D-aspartate receptors, nitric oxide, and ethanol tolerance. Braz J Med Biol Res. 1996;29:1415–26. [PubMed] [Google Scholar]
- 41.Sharma R, Engemann SC, Sahota P, Thakkar MM. Effects of ethanol on extracellular levels of adenosine in the basal forebrain: an in vivo microdialysis study in freely behaving rats. Alcohol Clin Exp Res. 2010;34:813–8. doi: 10.1111/j.1530-0277.2010.01153.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265:1946–51. [PubMed] [Google Scholar]
- 43.Yules RB, Freedman DX, Chandler KA. The effect of ethyl alcohol on man's electroencephalographic sleep cycle. Electroencephalogr Clin Neurophysiol. 1966;20:109–11. doi: 10.1016/0013-4694(66)90153-2. [DOI] [PubMed] [Google Scholar]


