Abstract
Introduction
Rare disease registries are a priority at European level and specific actions are being implemented by the European Commission to support their development.
In Italy, a National Registry of rare diseases has been established in 2001 as a network of regional registries. The latter have gradually been established and the full coverage of the Italian territory was attained during 2011.
Methods
Here we describe the basic features of the National Registry of rare diseases; the activities carried out to promote consistent operations in the regional registries; and the overall quality and composition of the records collected.
Results
After a validation process, including removal of duplicate records, 110,841 records of patients with rare diseases, single and with group denominations, are stored in the National Registry of rare diseases. They correspond to the overall diagnoses communicated to national registry by regional registries up to 30 June 2012.
The quality of the data collected by the the National Registry of rare diseases has been assessed with respect to completeness and consistency of procedures. Variables characterising case and diagnosis showed a very limited number of missing values. Records reported at least one case of 485 rare conditions.
Discussion
To date, the National Registry of rare diseases is a surveillance system with the main objective of producing epidemiologic evidence on rare diseases in Italy, and of supporting policy making and health services planning.
Data quality still represents a limitation for any sound epidemiological estimate of rare diseases in Italy. However, improvements of the quality of collected data and the completeness of case notifications should be strengthened.
Keywords: Italy, rare diseases, registry, public health
Introduction
Rare Diseases (RD) are a special challenge for the healthcare systems, because of their limited knowledge about their natural history, chronicity, need for long-term follow-up and potential high demand for assistance. Sound data on their prevalence and incidence are scantly available1 but, since they are as many as 7–8,000, it is estimated that, in the European Union only they affect about 29 million people2. Thus, the term “Registry” in the field of RD means more than a common epidemiologic tool for collection of secondary data related to patients with a specific RD diagnosis. Indeed, RD registries represent an effective way to achieve a sufficient sample size for epidemiological and clinical research3–5. Therefore, RD registries are key instruments for the surveillance of these diseases, with the aim of improving patient care and health care planning6. For these reasons registries are a priority at European level in the field of rare diseases2,7 and specific actions are being implemented to support the development of RD registries8.
A national institutional registry of rare disease patients, the Registro Nazionale Malattie Rare (RNMR), has been established in Italy in 2001 and is run by the National Centre for Rare Diseases (Centro Nazionale Malattie Rare, CNMR) of the Istituto Superiore di Sanità (National Institute of Health). Since then, the national and regional health authorities have cooperated for its implementation and after some years necessary for the development of the local communication networks and of the gradual establishment of the regional registries (RRs), the system achieved the full coverage of the national territory during 2011.
In consideration of the expected developments resulting from the European Union initiatives, this paper presents the main steps of the implementation of the RNMR and its main achievements, with the aim of sharing the RNMR experience with scientists and policy makers in the field of RD.
Methods
Legal basis, integration in the health system and scope of the RNMR
The RNMR has been established at the National Institute of Health by Ministerial Decree (MD) in 2001 as part of the measures devoted to improve health care for RD patients9. Indeed, it also envisaged the establishment, at regional or interregional level, of a network of formally designated centres (FDCs) with recognised expertise on RD, which could carry out the confirmatory investigations free of charge for the suspect patient, ensure a better and more effective patient management and ascertain the right of the patient to RD clinical assistance and treatments. This decree made reference to a list of 314 individual RD identified with own codes and 52 groups and subgroups of RD with group codes. Within these groups, 160 example pathologies were mentioned. The RNMR mandate was to monitor RD and inform the national and regional planning of measures for the protection of RD patients. The decree also explicitly mentioned the faculty for RNMR to liaise with international registries and to collect demographic, anamnesis, clinical, laboratory, determinant data of use for medical, biomedical and epidemiological research. Patient pseudonymization and the possibility of patient tracing in case of need were allowed in compliance with data protection legislation.
Due to the devolved organisation of the Italian health system, which gives the Regions the responsibility for the delivery of health services, the MD provided that the RNMR was functionally linked to RRs, which were to be established by regional authorities. Two agreements between the Central Government and the Regional Authorities, in 2002 and 2007, ensured the coordinated implementation of the RRs, defining the set of variables and the procedures for the data communication between the RRs and the RNMR10,11.
Establishment of regional registries
Following the MD, to ensure the delivery of RD special assistance, the regional authorities proceeded to the designation of their FDCs on the basis of criteria related to competence in the diagnosis, care and treatment of RD and to the availability of appropriate complementary services. Subsequently, the regional authorities established the RRs with regional decrees and other regulations, which ensured the necessary infrastructures as well as the legitimacy and security of the data flow from the FDCs to the corresponding RRs in compliance with the personal data protection legislation. In many cases, specific information systems have been developed at regional level with the aim of supporting the data collection and in some cases, the delivery of health care services to RD patients. The RRs may differ in aims and internal organisation: some have mainly epidemiological and public health purposes, in support of regional planning, while others also aim at evaluating health services and diagnostic procedures, or are integrated in the regional health care delivery system.
Data set and data communication
A common data set for the communication of data from the RRs to the RNMR was defined to fulfil the mandate of RNMR. This set is detailed in Table I. The diagnosis was expressed according to the exemption code attributed by the MD. The RRs sent RNMR the data batches regarding confirmed diagnoses made by their FDCs in each semester of the calendar year. Data communication took place normally during January and July. This practice is changing to an annual transfer based on whole calendar years.
Table I.
The common data set of the RNMR.
| Variables | Reason |
|---|---|
| ID (encrypted code based on given name, family name, birth date and place, sex) | Avoid multiple registrations of the same patient; conduct any type of record linkage |
| Sex | Epidemiologic analysis |
| Date of birth | Age at disease onset, diagnosis, death |
| Place of residence | Prevalence, geographical distribution, patient mobility |
| Live - dead condition | Prevalence |
| Date of death | Prevalence; age at death |
| Diagnosis of rare disease | Epidemiologic analysis |
| Date of diagnosis | Incidence; diagnosis delay |
| Centre of RD diagnosis | Patient mobility; health service utilization |
| Date of disease onset | Incidence; diagnosis delay |
| Orphan drug used | Treatment monitoring |
Training of the operators
Statisticians and computer technicians of the RNMR have held training courses from 2002 to 2009 to the Regions without a proprietary rare diseases registry to introduce the operators of the RRs to the proprietary software developed by CNMR and to standardize the methods used to collect the data throughout the country.
Quality control procedures
Experts in each RRs were responsible for the validation of the data of the corresponding FDCs. Some information systems supporting registries allow quality data control at the moment of the data entry by users. Furthermore, quality control processes are regularly carried out by RRs staff. In addition, at national level, the quality control of the common data set was carried out on the whole database in two steps by the RNMR operators. The first step consisted of a control of duplicate records, logical inconsistencies and range errors on individual data batches sent by the RRs. In case that range errors or logical inconsistencies were found, the records were sent back to the RRs for a check with the regional data sources. Inconsistencies and range errors which were not resolved after this control were considered as missing data. The second step was carried out on the database resulting from the merging of the regional data batches. In particular, this procedure checked for possible duplicates coming from different RRs and a specific protocol for the management of duplicate records was followed as shown in Table II. As shown in this table, the record management was dependent on the type of duplication and different record extractions were carried out depending on the scope of the analyses.
Table II.
Description and process management of duplicate records.
| Duplicate type 1 | Duplicate type 2 | Duplicate type 3 | |
|---|---|---|---|
|
|
|||
| Description | Records with same ID, diagnosis and diagnostic centre. | Records with same ID and diagnosis and different diagnostic centre for RDs. | Records with same ID and different rare disease diagnosis. |
| Presumed cause | Additional notifications are made of a case already notified. | The case was diagnosed in two or more different centres, which notified the case. | The case is affected by more RDs. |
|
|
|||
| Record management | |||
|
|
|||
| Aim of analysis | |||
| Analysis of the activity of diagnosis centres | Only the record with less recent diagnosis is considered. The other records are discarded. |
All records are considered. No records are discarded. |
All records are considered. No records are discarded. |
| Analysis of cases recorded in the RNMR | Only the record with less recent diagnosis is considered. The other records are discarded. |
Only the record with less recent diagnosis is considered. No records are discarded. |
Only the record with less recent diagnosis is considered. No records are discarded. |
| Analysis of RDs recorded in the RNMR | Only the record with less recent diagnosis is considered. The other records are discarded. |
Only the record with less recent diagnosis is considered. No records are discarded. |
All records are considered. No records are discarded. |
| Number of duplicate records | 3,322 | 3,393 | 2,483 |
Periodic meetings and reporting
The experts responsible for the RRs convened yearly to report on achievements and experience in the management of their registries and to discuss common issues. In 2012, the RNMR, in collaboration with the RRs, prepared its first annual report12, where the main features of the RRs and of the data quality were described.
Compliance with personal data protection Regulations
Each regional registry collects information on patients according to the national data protection law. When part of the data collected by RRs are communicated to the RNMR, an algorithm is used for the secure transfer of information. The data transmission took place by means of a temporary channel opened temporarily for this purpose and the personal data were transmitted separately from sensitive data. Personal and sensitive data were stored in separate servers of the informatics service of the National Institute of Health, protected with advanced firewall and technological systems.
Results
The geographic coverage of RNMR
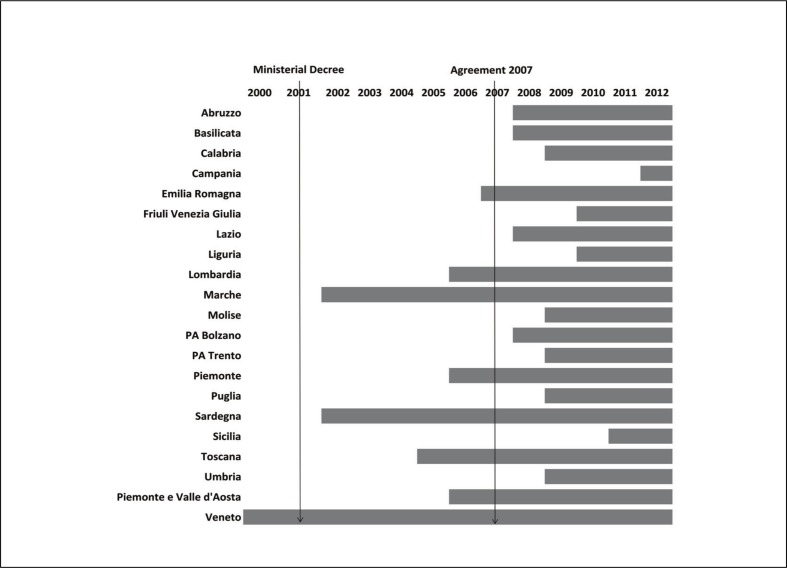
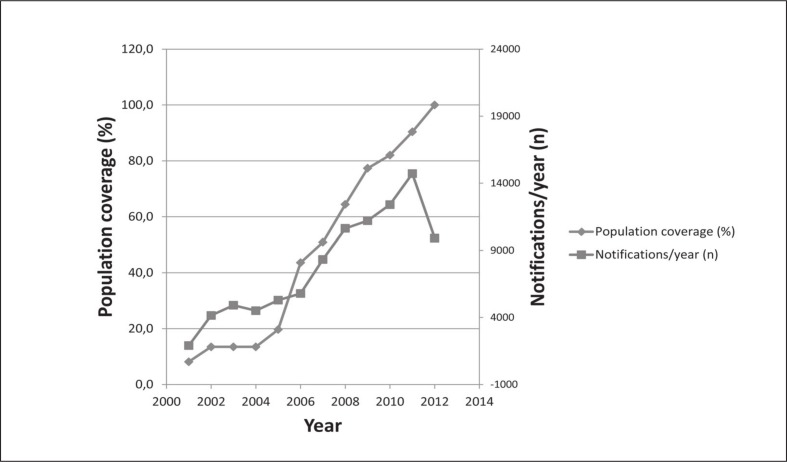
When the MD was put into effect, a RR of RD patients was in operation in the Veneto region only. Since then, RRs were progressively established and connected to the RNMR (Figure 1). The 2007 Agreement between Central Government and the Regions11, triggered a significant acceleration of the process (Figure 2) and the full coverage of the Italian territory connected with the RNMR was achieved during 2011. The establishment of the RRs resulted in a significant increase of the cases communicated to the RNMR, which approximately paralleled the increase in the nominal population coverage calculated from the official resident population of the regions.
Figure 1.
Establishment of regional RD registries in Italy.
The year indicated refers to the year of institution of the registry.
Figure 2.
Nominal population coverage of the RNMR and notification flow.
The year indicated refers to the institution of the regional registry. Notifications reported for the year 2012 refer to the first half of the year.
Quality of the common data set
Up to 30 June 2012, 112,766 records of RD patients were communicated to the RNMR. During the subsequent validation process, 25 records showed missing values for diagnosis or ID code or missing date of birth or sex. After check with the RRs, 17 records were discarded and 8 records were retained for subsequent processing. The remaining set of 112,749 records were checked for the presence of duplicate records, i.e., multiple records of a same patient received from different RRs and managed depending on the type of duplication as reported in Table II, which also shows the results of this analysis. Discarded Type 1 records, which represent true duplicates, were 1 908, corresponding to 1.7% of the total valid records. Therefore the cleaned database was made of 110,841 records.
The quality of the data collected by the RNMR has been assessed with respect to completeness and consistency of procedures. Table III summarizes the results of this control for each variable of the common data set. While completeness analysis was based on the observation of the database records, the assessment of consistency of procedures summarizes the discrepancies emerged during the preparation of the first RNMR report. The variables characterizing case and diagnosis showed a very limited number of missing values and a remarkable improvement could be observed, during time, in the completeness of recording of the centre making diagnosis. It is often the case with registries, that the date of death of patients is not collected systematically and timely by registry data providers. Although some records reported live cases with unrealistic age, there was no criterion that the RNMR could use to spot all records that did not update this information regularly. The date of disease onset is largely and persistently incomplete. An analysis of the records showed that missing data were mostly associated with some RRs. Indeed, while it is difficult to define the date of the symptoms onset in the case of patients affected by a rare diseases since a long time, or when the first signs or symptoms of a rare diseases are unspecific, some RRs do not collect this data. The completeness of data regarding the use of orphan drugs was assessed as follows. At first, the diseases were identified, for which an indication of use of orphan drugs was given; then, making reference to all the records of each of the diseases so identified, the fraction of records with missing values was calculated. This analysis indicated that, in the whole database, treatments were indicated for 56 conditions and that incomplete records (i.e. records which do not show the indication of a drug nor the explicit indication of no drug use) represented 14.9%. The lack of this data showed a marked decrease with time, indicating the steady improvement in its collection. Table 3 also summarizes the observations regarding the accuracy of the minimum set of data collected by RNMR.
Table III.
Completeness and accuracy of the common dataset.
| Common data set | Completeness (% missing)a | Accuracy | ||
|---|---|---|---|---|
|
| ||||
| Whole database | Calendar years 2010–2011 | Calendar year 2012 (6 months) | ||
| (110,841 records) | (27,114 records) | (9,913 records) | ||
| Gender | 0.0 | 0.0 | 0.0 | Data is collected consistently among data sources according to usual identification procedures |
| Date of birth | 0.0 | 0.0 | 0.0 | Data is collected consistently among data sources according to usual identification procedures |
| Region of residence | 0.1 | 0.1 | 0.0 | Data is collected consistently among data sources according to usual identification procedures |
| Diagnosis | 0.0 | 0.0 | 0.0 | Diagnoses are controlled and validated by the RRsb and their accuracy rely on the selection criteria for the centres to become FDC of the RD network |
| Date of diagnosis | 1.0 | -- | -- | Inconsistencies among regions on the identification of the relevant diagnostic event and diagnostic centre. |
| Centre of RD diagnosis | 11.9 | 1.2 | 0.4 | Inconsistencies among regions on the identification of the relevant diagnostic event and diagnostic centre. A centre may change name following reorganisation. |
| Vital status | ND | ND | ND c | Uncertainties in this variable depend on the fact that the data sources of RRsb may not be fully aware of the reason why patients are lost to follow up. In the future, date of decease will be collected from death registries: this will result in a systematic and precise, although delayed, appraisal of this condition. |
| Date of decease | ND | ND | ND | Expectedly good (when reported). In the future, date of decease will be collected from death registries: this will result in a systematic and precise, although delayed, appraisal of this condition. |
| Date of disease onset | 46.4 | 57.1 | 81.4 | Uncertainties in this variable depend on i) lack of patient’s recollection; ii) gradual appearance of unspecific symptoms over a long period. Symptoms could also not set on due to effective treatment following early diagnosis (e.g. following neonatal screening) |
| Orphan drug used | 14.9 | 10.7 | 4.6 | The name of the active substance is communicated, but no standard catalogue or coding is used. |
Data completeness is measured as the proportion (%) of records without the indication of the value of the variable; calendar years refer to the date of diagnosis;
Regional registry;
ND = not determined
Preliminary results after the start of activity with full nominal coverage of the national territory
Altogether, the valid records which are stored at present into RNMR are 110.841. This number corresponds to the overall diagnoses communicated to RNMR up to 30 June 2012 by RRs which have started their collection at different dates between 2000 and the end of 2011. The records referred to diagnoses made in the first 6 months of 2012, with RRs covering all the national territory, were 9,913. Although these features prevent, at present, any sound epidemiological estimate of rare diseases in Italy, some observations on the composition of the rare diseases notifications communicated to RNMR, may be of interest. Table IV shows the distribution, across the ICD 9-CM Chapters, of the diagnoses notified to the RNMR. The RDs under this surveillance system include conditions named individually and with group denominations. Altogether, the records reported at least one case of 485 conditions. All groups and subgroups mentioned in the MD were represented, making up 58,942 records, including 7,328 records of 137 pathologies mentioned as examples. Besides these, 296 individual RDs were represented with 51,899 records. Of these records, about 50% were made up by 15 most frequent diseases: achalasia, amyotrophic lateral sclerosis, behçet disease, bullous pemphigoid, chronic inflammatory demyelinating polyneuropathy, down syndrome, hereditary hemorrhagic telangiectasia, idiopathic central precocious puberty, keratoconus, Klinefelter syndrome, Lambert-Eaton syndrome, Marfan syndrome, mixed cryoglobulinemia, pemphigus, Turner syndrome. Twenty-nine diseases were represented with one record only.
Table IV.
Distribution, across the ICD 9-CM Chapters, of diseases notified to RNMR up to 30 June 2012.
| ICD 9-CM Chapters | Percent records | |
|---|---|---|
| 1. | Infectious and parasitic diseases | 0.1 |
| 2. | Neoplasms | 5.0 |
| 3. | Endocrine, nutritional, and metabolic diseases, and immunity disorders | 17.4 |
| 4. | Diseases of the blood and blood-forming organs | 16.6 |
| 6. | Diseases of the central nervous system and sense organs | 26.0 |
| 7. | Diseases of the circulatory system | 4.3 |
| 9. | Diseases of the digestive system | 1.3 |
| 10. | Diseases of the genitourinary system | 0.6 |
| 12. | Diseases of the skin and subcutaneous tissue | 3.3 |
| 13. | Diseases of the musculoskeletal system and connective tissue | 5.5 |
| 14. | Congenital anomalies | 19.7 |
| 15. | Certain conditions originating in the perinatal period | 0.1 |
| 16. | Symptoms, signs, and ill-defined conditions | 0.0 |
|
| ||
| Total | 100 | |
Discussion
The European Commission Communication: “Rare diseases: Europe’s challenge”2 and the subsequent Council Recommendation7 emphasize the strategic importance of Patient Registries in the field of RD.
The national network of Centres, identified at regional and interregional level, and of Regional/Interregional Registries dedicated to RD, was successfully implemented in Italy following the MD and the agreements between the Government and the Italian regions. To date, the Italian RNMR is a surveillance system, with the the main objective of producing epidemiologic evidence on RD in Italy, and of supporting policy making and HS planning. This role of the RNMR has been confirmed by the draft National Plan on RD, recently proposed for public consultation by the Ministry of Health13. Considering the complexity of building such registries in a devolved system of responsibility for healthcare delivery, substantial efforts were necessary in various steps of RNMR development, especially at regional level where the supporting infrastructures had to be established.
The strong legal base, its integration in the public health service and its connection with the dedicated national RD patient protection policy ensures the registry stability, comprehensiveness and population coverage. However, the need to cope with different levels of local resources, the slow responsiveness and limited flexibility typical of institutional processes as well as the need to rely on quality and comparable data made the implementation of the RNMR a slow and stepwise process, which is far from being concluded. The common data set agreed among the central and regional health authorities, is indeed the result of a selection of data, the collection of which could be sustainable, made comparable and be used to provide information coherent with the national scope of the RNMR, as distinct from the regional level responsibilities.
During the gradual establishment of the Regional RD registries, which achieved nominal completion during 2011, a steadily increasing flow of data on RD patients was established from the RRs to the RNMR. Moreover, we could observe also a continuously increasing involvement of the operators of the RD registry system. To achieve this result, an important role was played by the dedicated training courses in the regions which do not have a proprietary Rare Diseases Registry, conducted in a systematic way by the experts of the RNMR.
The preparation of the first Report on the RNMR activity9, in collaboration with the RRs, turned to be a powerful tool to review the operation of this complex system and to highlight the need for a number of critical improvements. The collection of the vital status and death date should be undertaken systematically. To this aim, the RNMR is seeking access to the national database of death records. Moreover, the inconsistencies in the collection of the disease onset date and the adoption of improved definitions of the diagnostic event relevant to determine the date of diagnosis and the centre making diagnosis are being discussed. Finally a more accurate list of active substances is to be adopted. The procedures used to validate records and to control data completeness also should be better distributed between the RNMR and the RRs to make the data flow a smooth process.
The actual coverage of the population depends on the quality and functions of the information systems developed at regional or interregional level. The different lengths of periods of operation of the RRs (ranging from about 1 to more than 10 years) result in different effectiveness of the registration process and only some regions collect data on RD patients using multiple data sources. Therefore, with the progressive improvement of RRs operations, the heterogeneity of the case reporting completeness will become less and less relevant. However, this issue has to be assessed and addressed appropriately.
At present, therefore, the data currently stored in the RNMR represents the baseline for a continuous improvement of the national and RRs and to start a validation process through the comparison of the RNMR results with studies in other population groups. For an overall improvement of the Italian RD surveillance system, CNMR is promoting collaborations with National statistical services, clinicians, patient associations and other data sources. Moreover, additional opportunities for improvement will come from the participation of CNMR and other experts of the Italian RD network in other European and global initiatives, such as the European Platform for rare disease registries (EPIRARE: www.epirare.eu), the International Rare Disease Research Consortium, (IRDiRC: www.irdirc.org) and the Integrated Platform connecting databases, registries, biobanks and clinical bioinformatics for Rare Disease research (RD-Connect: www.rd-connect.eu.
Footnotes
The Authors declare no conflicts of interest.
National Rare Disease Registry collaborating group
Giuseppina Annicchiarico and Ettore Attolini (Regional Coordination of Rare Diseases, Apulia Region), Antonello Antonelli (Department for Hygiene and Health and for Social Assistance of the Autonomous Region, Directorate General of Health, Sardinia Region), Rosalba Barone (Department of Health Protection and Health Policies, Calabria Region), Bruno Bembi and Laura Deroma (Regional Coordination Centre for Rare Diseases, Friuli-Venezia Giulia Region), Fabrizio Bianchi (Institute of Clinical Physiology CNR/Fondativo G. Monasterio, Pisa, Tuscany Region) and Cecilia Berni (Regional Coordination of Rare Diseases, Tuscany Region), Lucia Borsellino and Salvatore Scondotto (Department of Health and Epidemiological Observatory, Sicily Region), Francesco Benedicenti (Provincial Coordination Centre for Rare Diseases, Bolzano Autonomous Province) and Paola Zuech (Provincial Epidemiological Observatory, Bolzano Autonomous Province), Paola Casucci and Maria Concetta Patisso (Regional Directorate of Health and Social Cohesion, Umbria Region), Domenico di Lallo (Public Health Agency, Latium Region), Maria Lucia Di Nunzio (Directorate General V, Molise Region), Annunziata Di Palma (Provincial Coordination Centre for Rare Diseases, Trento Autonomous Province), Matteo Volta and Maria Vizioli (Directorate General of Health and Social Policy, Emilia Romagna Region), Paola Facchin and Monica Mazzucato (Coordination Rare Diseases, Rare Diseases Registry, Veneto Region), Orazio Gabrielli (Salesi Hospital, Clinical pediatric, Marche Region), Roberto Della Casa ed Iris Scala (Coordination Centre for Rare Diseases, Campania Region), Gedeone Baraldo (Directorate General for Health, Lombardy Region) ed Erica Daina (Coordination Centre for Rare Diseases, Institute for Pharmacological Research “Mario Negri”, Lombardy Region), Giandomenico Palka (Department of Biomedical Sciences, University Hospital of Chieti, Abruzzo Region), Dario Roccatello and Vittorio Modena (CMID, Centre of Research of Immunopathology and Rare Diseases; ASL TO2, S. Giovanni Bosco Hospital, Piedmont Region), Mirella Rossi (Regional Health Agency, Liguria Region), Domenico Tripaldi and Antonella Angione (Department of Health, Safety and Social Solidarity, Personal Services and the Community, Basilicata Region).
References
- 1.Posada de la Paz M, Villaverde-Hueso A, Alonso V, János S, et al. Rare Diseases Epidemiology Research Adv Exp Med Biol. 2010;686:17–39. doi: 10.1007/978-90-481-9485-8_2. [DOI] [PubMed] [Google Scholar]
- 2.Communication from the Commission to the European Parliament, the Council, the European economic and social committee and the committee of the regions on rare diseases: Europe challenges. Commision of the European Communities. [Accessed on 15/01/2014]. Available on line at http://ec.europa.eu/health/ph_threats/non_com/docs/rare_com_en.pdf.
- 3.Griggs RC, Batshaw M, Dunkle M, Gopal-Srivastava R, et al. Rare Diseases Clinical Research Network: Clinical research for rare disease: opportunities, challenges, and solutions. Mol Genet Metab. 2009;96:20–6. doi: 10.1016/j.ymgme.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cole JA, Taylor JS, Hangartner TN, Weinreb NJ, et al. Reducing selection bias in case-control studies from rare disease registries. Orphanet J Rare Dis. 2011;12:6, 61. doi: 10.1186/1750-1172-6-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luisetti M, Campo I, Scabini R, Zorzetto M, et al. The problems of clinical trials and registries in rare diseases. Respir Med. 2010;104(Suppl 1):S42–4. doi: 10.1016/j.rmed.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 6.Webb SM, Santos A, Valassi E. The value of a European registry for pituitary adenomas: the example of Cushing’s syndrome registry. Ann Endocrinol (Paris) 2012;73:83–9. doi: 10.1016/j.ando.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 7.Council Recommendation of 8 June 2009 on an action in the field of rare diseases. The Council of the European Union; [Accessed on 23/08/2013]. Available on line at: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:C:2009:151:0007:0010:EN:PDF. [Google Scholar]
- 8.European Commission. Supporting rare diseases registries and providing a European Platform for rare diseases registration. [Accessed on 28/08/2013]. Available on line at: http://ec.europa.eu/health/rare_diseases/policy/registries/index_en.htm.
- 9.Italian Ministry of Health. Decreto 18 maggio 2001, n. 279. Regolamento di istituzione della rete nazionale delle malattie rare e di esenzione dalla partecipazione al costo delle relative prestazioni sanitarie, ai sensi dell’articolo 5, comma 1, lettera b), del decreto legislativo 29 aprile 1998, n. 124. [Accessed on 25/03/2014]. (GU n. 160 del 12-7-2001- Suppl. Ordinario n.180/L) (in Italian). Available at: http://www.iss.it/binary/cnmr/cont/DM279-2001.1205943575.pdf.
- 10.Conferenza Stato Regioni. Accordo tra il Ministro della Salute, le Regioni e le Province Autonome di Trento e Bolzano sui criteri di individuazione e di aggiornamento dei Centri Interregionali di riferimento delle malattie rare. [Accessed on 25/03/2014]. (Repertorio Atti n 1485 dell’11 luglio 2002) (in Italian). Available at: http://www.iss.it/binary/cnmr/cont/STATOREGIONI2002.1205943700.pdf.
- 11.Conferenza Stato Regioni. Accordo, ai sensi dell’articolo 4 del Decreto Legislativo 28 Agosto 1997, n. 281, tra il Governo, le Regioni e le Province Autonome di Trento e Bolzano sul riconoscimento dei Centri di coordinamento regionali e/o interregionali, di Presidi assistenziali sovraregionali per patologie a bassa prevalenza e sull’attivazione dei registri regionali ed interregionali delle malattie rare. [Accessed on 25/03/2014]. (Repertorio 103/ CSR del 10 maggio 2007) (in Italian). Available at: http://www.iss.it/binary/cnmr/cont/STATOREGIONI2007.1205943700.pdf.
- 12.Taruscio D. Rapporto anno 2011. (Rapporti ISTISAN 11/20) Roma: Istituto Superiore di Sanità; 2011. [Accessed on 15/11/2013]. Il Registro Nazionale e i Registri Regionali/ interregionali delle Malattie Rare. (in Italian). Available on line at: http://www.iss.it/publ/index.php?lang=1&id=2529&tipo=5. [Google Scholar]
- 13.Italian Ministry of Health. Piano Nazionale Malattie Rare 2013–2016. [Accessed on 15/01/2014]. Available on line at: http://www.salute.gov.it/imgs/C_17_pagineAree_3296_listaFile_itemName_0_file.pdf.