Abstract
Objective
Aneurysms-osteoarthritis syndrome (AOS), caused by SMAD3 mutations, is a recently described autosomal-dominant syndrome characterized by arterial aneurysms, tortuosity, and aortic dissections in combination with osteoarthritis. Our objective was to evaluate the AOS-related vascular consequences in the visceral and iliac arteries and raise awareness for this aggressive syndrome among vascular specialists.
Methods
All AOS patients were monitored regularly according to our clinical AOS protocol. The study included those with one or more visceral aneurysms or tortuosity, or both. Clinical and surgical data were obtained from record abstraction.
Results
The study included 17 AOS patients (47% men) aged 47 ± 13 years. A total of 73 aneurysms were encountered, of which 46 were located in the abdomen. The common iliac artery was most commonly affected (37%), followed by the superior mesenteric artery (15%), celiac trunk (11%), and splenic artery (9%). Rapid aneurysm growth ≤1 year was found in three arteries (gastric, hepatic, and vertebral artery). Furthermore, arterial tortuosity was noted in 94% of patients. Four patients underwent six elective (endo) vascular interventions for aneurysms in the iliac, hepatic, gastric, or splenic artery, without major perioperative or postoperative complications.
Conclusions
AOS predisposes patients to widespread visceral and iliac artery aneurysms and extreme arterial tortuosity. Early elective aneurysm repair should be considered because the risk of aneurysm rupture is estimated to be very high and elective (endo) vascular interventions were not complicated by fragility of arterial tissue. Given the aggressive behavior of AOS, it is of utmost importance that vascular specialists are aware of this new syndrome.
Visceral and iliac aneurysms are relatively rare, yet potentially catastrophic when rupturing.1–4 Although most visceral and iliac aneurysms are degenerative, they can also be encountered in the setting of connective tissue disorders, such as Loeys-Dietz and vascular type Ehlers-Danlos syndrome.5–7 Recently, our group discovered a new syndrome: the aneurysms-osteoarthritis syndrome (AOS).8 This autosomal-dominant connective tissue disorder is caused by heterozygous mutations in the SMAD3 gene, located on chromosome 15q22.33 (OMIM # 613795).8 The syndrome is typically characterized by widespread arterial aneurysms and tortuosity, early-onset joint abnormalities, and mild craniofacial and cutaneous features.8–11 Penetrance is nearly 100%, and so far, AOS has only been identified in families originating from North America and Europe.10,12 The expression seems to be age-dependent and may vary from very mild (isolated bifid uvula) to severe (multiple aneurysms, dissections, or death at a young age).10
AOS is estimated to be responsible for ~2% of familial thoracic aortic aneurysms and dissections, as well as intracranial, aortic and bilateral iliac aneurysms segregating in an autosomal-dominant manner.8,12 The incidence of AOS in patients with primary visceral and iliac artery aneurysms is currently unknown and remains to be determined.
The major source of early death in AOS is aortic root dilatation, potentially leading to aortic dissection and rupture.9 In some individuals, aortic dissection occurred at relatively mildly enlarged aortic diameters.8–10 However, arterial involvement is not limited to the aorta, but can be widespread, with peripheral and intracranial aneurysms and arterial tortuosity, thereby resembling Loeys-Dietz syndrome.9,13,14 Furthermore, early-onset osteoarthritis is present in nearly all patients and is useful to discriminate AOS from other connective tissue disorders.8–10 The osteoarthritis is often the first reason the individual seeks medical advice, with a mean age at diagnosis of 42 years (youngest, 12 years old).8–10 Although the genetic background and thoracic aortic pathology of SMAD3-related AOS have been described before, so far the vascular consequences of AOS beyond the aortic root have not been highlighted. Therefore, the purpose of this study was to evaluate the AOS-related vascular abnormalities in the visceral and iliac arteries and raise awareness for this aggressive syndrome among vascular specialists.
METHODS
The study was approved by the Institutional Review Board and Ethical Committee of the Erasmus MC in Rotterdam. Written informed consent was obtained from each patient.
Patients
The medical records of the 45 identified AOS patients10 were reviewed to evaluate visceral and iliac vascular abnormalities. As previously described, aneurysmal findings included thoracic aortic aneurysms (72%), abdominal aortic aneurysms (12%), aneurysms in other thoracic or abdominal arteries (36%), and intracranial aneurysms (38%).9,10 Aortic dissection/rupture was present in 33% of patients.9,10 AOS diagnosis was confirmed by genetic analysis and clinical phenotype.10 All AOS patients were intensively monitored at regular intervals according to our clinical AOS protocol.9 Only AOS patients with one or more visceral or iliac aneurysms or tortuosity, or both, were included in this report.
Data collection
Clinical data were collected from chart abstraction and electronic patient records. Collected variables included demographics, medical history, family history, cardiovascular imaging, and (endo) vascular interventions. Computed tomography angiography (CTA) or magnetic resonance angiography (MRA) from head-to-pelvis was used to evaluate the presence of vascular abnormalities. An experienced cardiovascular radiologist evaluated presence, location, and size of aneurysms, dissections, and tortuosity. An aneurysm was defined as a dilatation of an artery by >1.5 times the expected arterial diameter. Visceral arterial tortuosity was defined as a severe (pigtail-like) curve or multiple curves in an artery. Aortic and iliac tortuosity was defined as described by Chaikof et al.15
RESULTS
The study included 17 AOS patients with visceral and iliac aneurysms (n = 15) or tortuosity (n = 9), or both. The primary reason for presentation in one patient was a symptomatic visceral or iliac artery aneurysm. This 32-year-old man presented with pain and a pulsating mass in the lower abdomen, which was caused by bilateral aneurysms in the common iliac arteries (69 and 42 mm; patient 1). In the remaining 16 patients, the visceral or iliac artery aneurysms were found when extensive imaging was performed at the time of identification of AOS because of an aortic dissection (n = 5), aortic root aneurysm (n = 6), or screening of asymptomatic family members (n = 5). Baseline characteristics are summarized in the Table.
Table.
Baseline characteristics of patients with aneurysms-osteoarthritis syndrome
| Variablea | Mean ± SD or No. (%) |
|---|---|
| Patients | 17 |
| Age, years | 47 ± 13 |
| Male sex | 8 (47) |
| Body mass index, kg/m2 | 24 ± 4 |
| Blood pressure, mm Hg | |
| Systolic | 131 ± 17 |
| Diastolic | 95 ± 8 |
| Cholesterol, mmol/L | |
| Total | 5.0 ± 1.1 |
| High-density lipoprotein | 1.5 ± 0.4 |
| Low-density lipoprotein | 3.1 ± 0.9 |
| β-blockade medication | 3 (18) |
| Smoking | |
| Never | 15 (88) |
| Current | 2 (12) |
SD, Standard deviation.
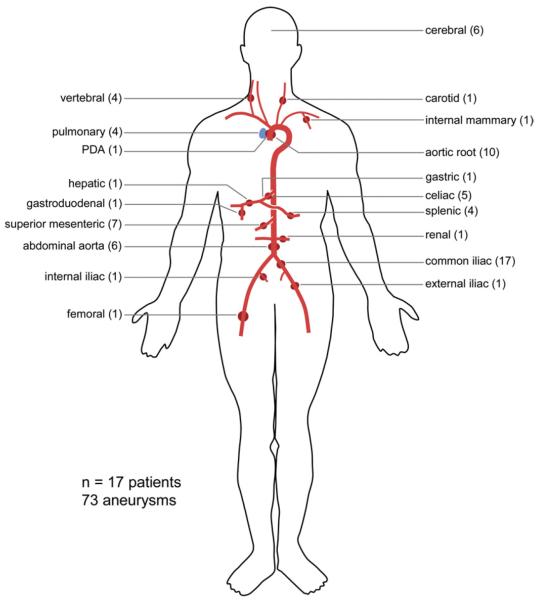
Overall, these 17 AOS patients exhibited 73 aneurysms, of which 46 were located in the abdomen (Fig 1). Detailed information about each individual patient is provided in the Supplementary Table (online only). Aneurysms were most frequently found at the common iliac artery (17 aneurysms in 10 patients), followed by the superior mesenteric artery (seven aneurysms), celiac trunk (five aneurysms), and splenic artery (four aneurysms). Additional aneurysms were found in the external iliac, internal iliac, hepatic, renal, gastric, gastroduodenal, and femoral artery. The majority of patients (59%) also had an aortic root aneurysm. In six patients the abdominal aorta was dilated (range, 23–100 mm), and four patients exhibited a type B aortic dissection. Chronic bilateral common iliac artery dissections were present in one patient at a diameter of 9 and 17 mm (none to mild dilatation). Extensive arterial tortuosity was noted throughout the arterial tree, most frequently located in the vertebral, iliac, splenic, carotid, and intracranial arteries (Fig 2; Video 1, online only).
Fig 1.
Distribution of 73 aneurysms within 17 patients with aneurysms-osteoarthritis syndrome. PDA, Patent ductus arteriosus.
Fig 2.
Arterial tortuosity in (A) aorta, visceral and iliac arteries, and (B) in the splenic artery.
Aneurysm growth
Repeated CTA or MRA scans were available in six patients, and rapid growth of an aneurysm was noted in two patients. A 30-year-old man (patient 8) had a 6-mm fusiform aneurysm in the left proximal vertebral artery, which increased to 11 mm within 10 months. An aneurysm in the right hepatic artery in a 43-year-old man (patient 7) increased in size from 11 to 18 mm in 9 months' time. Furthermore, a completely new fusiform aneurysm (15 × 11 mm) in the left gastric artery (normal diameter, 7 mm) developed in patient 7 within 11 months' time. The scans in the patients 3, 4, 11, and 15 did not show aneurysmal growth in a time period of 1 to 3 years.
Elective vascular interventions
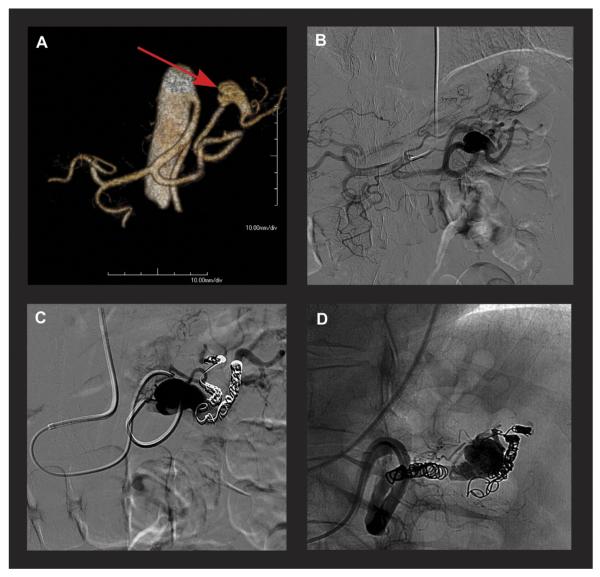
Four patients underwent six elective open or endovascular interventions for aneurysms in the iliac, hepatic, gastric, or splenic artery. Patient 1, a 32-year-old man with bilateral large iliac artery aneurysms, underwent aortobiiliac graft implantation (Gelsoft prosthesis; Vascutek, Renfrewshire, UK). No perioperative complications occurred. A follow-up MRA at 1 month showed relative stenoses of the distal anastomoses due to progressive tortuosity and elongation of the native common and external iliac arteries that were reimplanted on the prosthetic limbs (Fig 3). At 3 months, he required a mesh repair of an incisional hernia, which was likely related to the abnormal collagen composition due to AOS.
Fig 3.
A, A three-dimensional magnetic resonance angiography (MRA) shows bilateral large iliac artery aneurysms (69 and 42 mm). B, A postoperative cardiac magnetic resonance image shows relative stenoses of the distal anastomoses due to progressive tortuosity and elongation of the native iliac arteries.
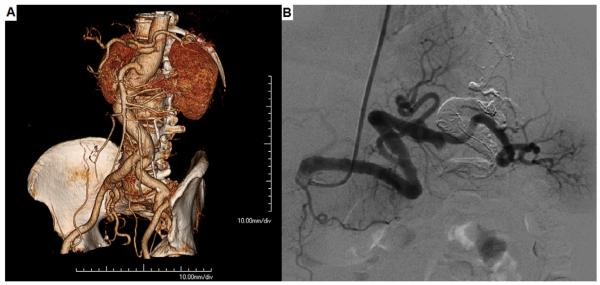
Patients 4 and 10 underwent coil embolization with occlusion of the splenic artery both proximal and distal to the aneurysm (Fig 4; Video 2, online only). Both patients exhibited abdominal pain for some days postprocedurally, which was most likely due to splenic ischemia and adequately managed with analgesics.
Fig 4.
A, A three-dimensional computed tomography angiography and (B) angiography show a splenic artery aneurysm (21 mm) and tortuosity. Coil embolization procedure to occlude of the splenic artery (C) distal and (D) proximal to the aneurysm.
Patient 7, a 35-year-old man, underwent surgical resection with end-to-end anastomosis of a splenic artery aneurysm. He presented 5 years later with abdominal pain. CTA revealed an expanding hepatic artery aneurysm for which a covered self-expandable stent graft (Viabahn; W. L. Gore, Flagstaff, Ariz) was implanted in the extrahepatic part of this aneurysm, thereby closing of a second small saccular intrahepatic artery aneurysm (Supplementary Fig, online only). This patient successfully underwent coil embolization of a fusiform aneurysm in the left gastric artery 6 months later.
After a postprocedural period of 1 month to 8 years, the four patients who underwent elective interventions were alive and asymptomatic.
DISCUSSION
To our knowledge, this is the first report describing the implications of SMAD3-related AOS in the visceral and iliac arteries. Although previous studies mainly focused on the genetic background and thoracic aortic involvement8–10; it is important to raise awareness for the vascular consequences of AOS beyond the aortic root.
General features of AOS
AOS is a recently described autosomal-dominant disorder that can predispose patients to widespread arterial aneurysms, dissections, and tortuosity.8–10 Although AOS may resemble other connective tissue disorders such as Loeys-Dietz syndrome, it can be discriminated by the presence of early onset joint anomalies such as osteoarthritis, osteochondritis dissecans, and meniscal abnormalities.10
Mutations in the SMAD3 gene have been identified as the underlying cause for AOS.8 The most likely effect of these mutations is loss of function and a paradoxic increase in transforming growth factor-β signalling in the aortic wall.10 On histology of aortic wall fragments, evident disorganization of the tunica media with fragmentation and loss of elastic fibers was observed, as well as characteristic mucoid medial degeneration and accumulation of collagen in media.8
AOS seems to be responsible for ~2% of familial poly-aneurysm disease.8,12 Because AOS has only been identified recently, data regarding the natural history of the disease are scarce, although it has become evident that aneurysmal growth can be fast and unpredictable and dissections may occur in only mildly dilated arteries.9 In addition, AOS is also associated with additional features that are common in connective-tissue disorders, such as umbilical and inguinal hernias, pelvis floor prolapse, varices, scoliosis, and velvety skin.8–10 Furthermore, mild craniofacial features, including hypertelorism (widely-spaced eyes) and uvula abnormalities (broad or bifid) might be present.8–10
Involvement of the visceral and iliac arteries in AOS
The visceral and iliac arteries in our patient group displayed widespread abnormalities, such as aneurysms and extreme tortuosity. Although aneurysms were encountered in many arteries, splenic and iliac artery aneurysms were often the largest aneurysms and therefore most frequently required treatment. Moreover, arterial tortuosity was also most prominent in the splenic and iliac arteries. Although widespread arterial tortuosity and aneurysms can also be found in Loeys-Dietz and arterial tortuosity syndrome, this is rare in patients with Marfan syndrome.13–17 Therefore, this feature can be helpful to discriminate AOS patients from Marfan patients.
It would be interesting to elucidate why the arteries of AOS patients become tortuous. Mechanical stability of arteries highly depends on elastin, which provides arterial elasticity and stiffness.18 Elastin degradation weakens the arterial wall, thereby compromising mechanical stability of arteries.18 Studies in fibulin-4– and fibulin-5–deficient mice and humans have demonstrated the association between arterial tortuosity and profound failure of elastogenesis.19 Aortic wall specimens of AOS patients also demonstrated elastin degradation.8 Therefore, we hypothesize that failure of elastogenesis is the probable cause of arterial tortuosity in AOS patients.
Endovascular treatment in AOS patients
Because AOS can be complicated by dissections at relatively small diameters, early elective aneurysm repair seems to be appropriate to avoid vascular catastrophes. Nevertheless, potential benefits should always be weighed against the risks of an intervention. Knowledge of aneurysmal growth, procedural complication rates and late postoperative outcomes is crucial. Although rate of aneurismal growth is not entirely elucidated yet, it has become clear that growth can be fast and unpredictable. The current general consensus in atherosclerotic aneurysmal disease is that (endo) vascular treatment is indicated in asymptomatic visceral artery aneurysms >2.0 cm and iliac artery aneurysms >3.0 cm.20–25 However, due to the sometimes rapid aneurysmal growth and occurrence of dissections in only mildly dilated arteries, a more aggressive treatment strategy seems to be necessary in AOS patients.
So far, (endo) vascular treatment experience in AOS patients is limited due to the recent discovery of this syndrome. Although this report only describes six (endo) vascular interventions in four AOS patients, it represents the largest cohort of abdominal (endo) vascular interventions to date. In vascular-type Ehlers-Danlos syndrome, friable vascular tissue leads to high surgical complication rates.7 In contrast, fragility of arterial tissue was not an issue in the described interventions in AOS patients nor in aortic surgery.9 Tissue handling felt the same as in patients without a connective tissue disorder; thus, elective interventions seem to be feasible and safe in AOS patients so far.
Although endovascular treatment of aortic aneurysms is generally discouraged in patients with connective disorders, little is known about open vs endovascular repair of visceral aneurysms in patients with connective-tissue disorders.26 In our opinion, the potential harmful impact of persistent radial forces of a stent graft is less of an issue in the visceral arteries than in the aorta and no issue with coil embolization. Furthermore, visceral aneurysms might be difficult to reach and treat through an open surgical procedure, and periprocedural morbidity and mortality will generally be lower in endovascular procedures. Therefore, we usually prefer an endovascular approach in AOS patients, although we strongly encourage an individualized approach weighing all potential benefits and harms, and multidisciplinary deliberation before deciding on the treatment strategy.
Clinical implications
Vascular specialists should be aware of AOS as a potential underlying cause of visceral and iliac aneurysms or tortuosity, or both, especially in patients with aortic aneurysms or dissections, joint complaints, multiple arterial aneurysms or a strong family history of aortic dissections or sudden death. Patients should be offered genetic testing and counseling for SMAD3 gene mutations when AOS is suspected. In addition, at least a transthoracic echocardiogram should be performed to look for an aortic root aneurysm. If a mutation is identified, additional counseling of family members is strongly recommended.
Because AOS is notable for unpredictable, sometimes rapid aneurysmal growth and occurrence of dissections in mildly dilated arteries, imaging of the entire arterial tree with CTA or MRA should be performed annually in confirmed AOS patients.9
To prevent aneurysm rupture or dissection, or both, elective (endo) vascular intervention in AOS patients should be considered in any visceral or iliac artery aneurysm exceeding twice the expected arterial diameter and in those that grow rapidly (>3 mm/y). Open and endovascular approaches can be used safely; however, endovascular procedures may be complicated by extreme tortuosity and should thus be performed by an experienced endovascular specialist. Imaging diagnostics using three-dimensional CTA or MRA reconstructions may be useful in planning treatment. After aneurysm repair, the entire arterial tree should be monitored because it remains at risk for aneurysm development and dissections or ruptures.
Study limitations
The retrospective nature and small sample size of this study are evident limitations. However, this study serves its most important goal, namely to raise awareness for this new, aggressive aneurysm syndrome. When AOS patients are not recognized, vascular complications that might have been prevented may occur. Larger prospective follow-up studies are warranted to elucidate the clinical course of AOS and long-term surgical outcome.
CONCLUSIONS
AOS is a recently discovered connective tissue disorder that predisposes patients to arterial aneurysms, dissections and tortuosity, and early-onset joint complaints. Extensive CTA or MRA screening frequently identified (multiple) aneurysms and extreme tortuosity within visceral and iliac arteries in these patients. Although surgical experience is limited, fragility of arterial tissue does not seem to complicate open and endovascular procedures in AOS patients. Owing to the occurrence of dissection in mildly dilated arteries, a more aggressive treatment strategy seems to be necessary. To prevent rupture, elective aneurysm repair should be considered in any visceral or iliac artery aneurysm that exceeds twice the expected arterial diameter or grows rapidly. It is paramount that vascular specialists are aware of this new syndrome and its aggressive behavior since many AOS patients are still unrecognized.
Supplementary Material
Acknowledgments
We thank the participating patients, their families, and their referring physicians. We also thank the technician assistants from the participating centers, especially René Frowijn, for his support in creating illustrations.
Footnotes
AUTHOR CONTRIBUTIONS Conception and design: DL, HV, JR
Analysis and interpretation: DL, HV, JR
Data collection: DL, HV, AM, IL, IH, JB, HD, JR
Writing the article: DL, HV, JR
Critical revision of the article: DL, HV, AM, IL, IH, JB, HD, JR
Final approval of the article: DL, HV, AM, IL, IH, JB, HD, JR
Statistical analysis: DL
Obtained funding: Not applicable
Overall responsibility: JR
Author conflict of interest: none.
REFERENCES
- 1.Stanley JC, Wakefield TW, Graham LM, Whitehouse WM, Jr, Zelenock GB, Lindenauer SM. Clinical importance and management of splanchnic artery aneurysms. J Vasc Surg. 1986;3:836–40. [PubMed] [Google Scholar]
- 2.Carr SC, Pearce WH, Vogelzang RL, McCarthy WJ, Nemcek AA, Jr, Yao JS. Current management of visceral artery aneurysms. Surgery. 1996;120:627–33. doi: 10.1016/s0039-6060(96)80009-2. [DOI] [PubMed] [Google Scholar]
- 3.Carr SC, Mahvi DM, Hoch JR, Archer CW, Turnipseed WD. Visceral artery aneurysm rupture. J Vasc Surg. 2001;33:806–11. doi: 10.1067/mva.2001.112320. [DOI] [PubMed] [Google Scholar]
- 4.Wagner WH, Allins AD, Treiman RL, Cohen JL, Foran RF, Levin PM, et al. Ruptured visceral artery aneurysms. Ann Vasc Surg. 1997;11:342–7. doi: 10.1007/s100169900058. [DOI] [PubMed] [Google Scholar]
- 5.Johnson PT, Chen JK, Loeys BL, Dietz HC, Fishman EK. Loeys-Dietz syndrome: MDCT angiography findings. AJR Am J Roentgenol. 2007;189:W29–35. doi: 10.2214/AJR.06.1316. [DOI] [PubMed] [Google Scholar]
- 6.Casey K, Zayed M, Greenberg JI, Dalman RL, Lee JT. Endovascular repair of bilateral iliac artery aneurysms in a patient with Loeys-Dietz syndrome. Ann Vasc Surg. 2012;26:e5–10. doi: 10.1016/j.avsg.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 7.Oderich GS, Panneton JM, Bower TC, Lindor NM, Cherry KJ, Noel AA, et al. The spectrum, management and clinical outcome of Ehlers-Danlos syndrome type IV: a 30-year experience. J Vasc Surg. 2005;42:98–106. doi: 10.1016/j.jvs.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 8.van de Laar IM, Oldenburg RA, Pals G, Roos-Hesselink JW, de Graaf BM, Verhagen JM, et al. Mutations in SMAD3 cause a syndromic form of aortic aneurysms and dissections with early-onset osteoarthritis. Nat Genet. 2011;43:121–6. doi: 10.1038/ng.744. [DOI] [PubMed] [Google Scholar]
- 9.Van der Linde D, Van de Laar IM, Bertoli-Avella AM, Oldenburg RA, Bekkers JA, Mattace-Raso FU, et al. Aggressive cardiovascular phenotype of aneurysms-osteoarthritis syndrome caused by pathogenic SMAD3 variants. J Am Coll Cardiol. 2012;60:397–403. doi: 10.1016/j.jacc.2011.12.052. [DOI] [PubMed] [Google Scholar]
- 10.van de Laar IM, van der Linde D, Oei EH, Bos PK, Bessems JH, Bierma-Zeinstra SM, et al. Phenotypic spectrum of the SMAD3-related aneurysms-osteoarthritis syndrome. J Med Genet. 2012;49:47–57. doi: 10.1136/jmedgenet-2011-100382. [DOI] [PubMed] [Google Scholar]
- 11.Van der Linde D, Witsenburg M, van de Laar I, Moelker A, Roos-Hesselink J. Saccular aneurysm within a persistent ductus arteriosus. Lancet. 2012;379:e33. doi: 10.1016/S0140-6736(11)61352-4. [DOI] [PubMed] [Google Scholar]
- 12.Regalado ES, Guo DC, Villamizar C, Avidan N, Gilchrist D, McGillivray B, et al. Exome sequencing identifies SMAD3 mutations as a cause of familial thoracic aortic aneurysm and dissection with intracranial and other arterial aneurysms. Circ Res. 2011;109:680–6. doi: 10.1161/CIRCRESAHA.111.248161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Hemelrijk C, Renard M, Loeys B. The Loeys-Dietz syndrome: an update for the clinician. Curr Opin Cardiol. 2010;25:546–51. doi: 10.1097/HCO.0b013e32833f0220. [DOI] [PubMed] [Google Scholar]
- 14.Loeys BL, Schwarze U, Holm T, Callewaert BL, Thomas GH, Pannu H, et al. Aneurysm syndromes caused by mutations in the TGF-beta receptor. N Engl J Med. 2006;355:788–98. doi: 10.1056/NEJMoa055695. [DOI] [PubMed] [Google Scholar]
- 15.Chaikof EL, Fillinger MF, Matsumura JS, Rutherford RB, White GH, Blankensteijn JD, et al. Identifying and grading factors that modify the outcome of endovascular aortic aneurysm repair. J Vasc Surg. 2002;35:1061–6. doi: 10.1067/mva.2002.123991. [DOI] [PubMed] [Google Scholar]
- 16.Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38:452–7. doi: 10.1038/ng1764. [DOI] [PubMed] [Google Scholar]
- 17.Judge DP, Dietz HC. Marfan's syndrome. Lancet. 2005;366:1965–76. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee AY, Han B, Lamm SD, Fierro CA, Han HC. Effects of elastin degradation and surrounding matrix support on artery stability. Am J Physiol Heart Circ Physiol. 2012;302:H873–84. doi: 10.1152/ajpheart.00463.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay ME, Dietz HC. Lessons on the pathogenesis of aneurysm from heritable conditions. Nature. 2011;473:308–16. doi: 10.1038/nature10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Petersen A, Meerwaldt R, Geelkerken R, Zeebregts C. Surgical options for the management of visceral artery aneurysms. J Cardiovasc Surg (Torino) 2011;52:333–43. [PubMed] [Google Scholar]
- 21.Pulli R, Dorigo W, Troisi N, Pratesi G, Innocenti AA, Pratesi C. Surgical treatment of visceral artery aneurysms: a 25-year experience. J Vasc Surg. 2008;48:334–42. doi: 10.1016/j.jvs.2008.03.043. [DOI] [PubMed] [Google Scholar]
- 22.Abbas MA, Fowl RJ, Stone WM, Panneton JM, Oldenburg WA, Bower TC, et al. Hepatic artery aneurysm: factors that predict complications. J Vasc Surg. 2003;38:41–5. doi: 10.1016/s0741-5214(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 23.Lakin RO, Bena JF, Sarac TP, Shahr S, Krajewski LP, Srivastava SD, et al. The contemporary management of splenic artery aneurysms. J Vasc Surg. 2011;53:958–65. doi: 10.1016/j.jvs.2010.10.055. [DOI] [PubMed] [Google Scholar]
- 24.Santilli SM, Wernsing SE, Lee ES. Expansion rates and outcomes for iliac artery aneurysms. J Vasc Surg. 2000;31:114–21. doi: 10.1016/s0741-5214(00)70073-5. [DOI] [PubMed] [Google Scholar]
- 25.Huang Y, Gloviczki P, Duncan AA, Kalra M, Hoskin TL, Oderich GS, et al. Common iliac artery aneurysm: expansion rate and results of open surgical and endovascular repair. J Vasc Surg. 2008;47:1203–11. doi: 10.1016/j.jvs.2008.01.050. [DOI] [PubMed] [Google Scholar]
- 26.Svensson LG, Kouchoukos NT, Miller DC, Bavaria JE, Coselli JS, Curi MA, et al. Expert consensus document on the treatment of descending thoracic aortic disease using endovascular stent-grafts. Ann Thorac Surg. 2008;85:S1–41. doi: 10.1016/j.athoracsur.2007.10.099. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.