Two Arabidopsis mitogen-activated protein kinases, are involved in the funicular guidance phase of pollen tube growth in plant reproduction.
Abstract
Double fertilization in flowering plants requires the delivery of two immotile sperm cells to the female gametes by a pollen tube, which perceives guidance cues, modifies its tip growth direction, and eventually enters the micropyle of the ovule. In spite of the recent progress, so far, little is known about the signaling events in pollen tubes in response to the guidance cues. Here, we show that MPK3 and MPK6, two Arabidopsis (Arabidopsis thaliana) mitogen-activated protein kinases, mediate the guidance response in pollen tubes. Genetic analysis revealed that mpk3 mpk6 double mutant pollen has reduced transmission. However, direct observation of mpk3 mpk6 mutant pollen phenotype was hampered by the embryo lethality of double homozygous mpk3–/– mpk6–/– plants. Utilizing a fluorescent reporter-tagged complementation method, we showed that the mpk3 mpk6 mutant pollen had normal pollen tube growth but impaired pollen tube guidance. In vivo pollination assays revealed that the mpk3 mpk6 mutant pollen tubes were defective in the funicular guidance phase. By contrast, semi-in vitro guidance assay showed that the micropylar guidance of the double mutant pollen tube was normal. Our results provide direct evidence to support that the funicular guidance phase of the pollen tube requires an in vivo signaling mechanism distinct from the micropyle guidance. Moreover, our finding opened up the possibility that the MPK3/MPK6 signaling pathway may link common signaling networks in plant stress response and pollen-pistil interaction.
In flowering plants, successful fertilization is dependent on extensive cell-cell communication between male and female gametophytes. After landing on a compatible stigma surface, a mature pollen grain germinates to form a pollen tube, which penetrates the stigma, perceives guidance cues along the growth path, and modifies its tip growth direction toward the ovule (Hülskamp et al., 1995). In Arabidopsis (Arabidopsis thaliana), the pollen tube guidance can be divided into two phases: funicular guidance, in which the pollen tube emerges from the septum and proceeds to a funiculus, and micropylar guidance, in which the pollen tube grows toward and enters the micropyle of an ovule (Hülskamp et al., 1995).
In pollen tube, it is believed that receptors on the tube tip perceive various guidance cues and regulate downstream signaling pathways to modify tip reorientation toward the ovule (Higashiyama, 2010; Takeuchi and Higashiyama, 2011). Two receptor-like kinase genes, Lost In Pollen tube guidance1 (LIP1) and LIP2, are involved in guidance control of pollen tubes. LIP1 and LIP2 were anchored to the membrane in the pollen tube tip region via palmitoylation, which was essential for their guidance control (Liu et al., 2013). Therefore, LIP1 and LIP2 are the essential components of the receptor complex in micropylar guidance. The Glu receptor-like channels facilitate Ca2+ influx across the plasma membrane and regulate pollen tube growth and morphogenesis (Michard et al., 2011). This interesting work revealed that there is a signaling mechanism between the male gametophyte and pistil tissue that is similar to the amino acid-mediated communication in animal nervous systems (Michard et al., 2011). Recent findings also highlight the importance of the endoplasmic reticulum (ER), ion homeostasis, and protein processing in pollen tube guidance (Li et al., 2011; Lu et al., 2011; Li and Yang, 2012). Two pollen-expressed cation proton exchangers (CHXs), CHX21 and CHX23, were reported to mediate K+ transport in ER and are essential for the pollen tube to respond to directional signals from the ovule in Arabidopsis (Lu et al., 2011). POLLEN DEFECTIVE IN GUIDANCE1 plays an important role in micropylar guidance in pollen tube (Li et al., 2011). It is an ER luminal protein involved in ER protein retention and interacts with a luminal chaperone involved in Ca2+ homeostasis and ER quality control (Li et al., 2011). Therefore, the ER quality control is likely an important mechanism in surveillance of signaling factors in pollen tube guidance (Li and Yang, 2012).
In spite of the recent progresses, so far, little is known about the cytoplasmic signaling events in pollen tubes in response to the guidance cues. Mitogen-activated protein kinase (MAPK, or MPK) cascades are conserved signaling pathways that respond to extracellular stimuli and regulate various cellular activities. In Arabidopsis, MPK3 and MPK6 are induced by various biotic and abiotic stresses and collaboratively play important roles in defense response and plant development (Zhang, 2008). Here, we show that MPK3 and MPK6 are also critical to pollen tube guidance. Utilizing a fluorescent reporter-tagged complementation method, we demonstrated that mpk3 mpk6 pollen was defective in pollen tube guidance at the funicular guidance phase. Intriguingly, the micropylar guidance of mpk3 mpk6 pollen tube is not affected.
RESULTS AND DISCUSSION
Genetic Analysis Reveals Distorted Transmission of mpk3 mpk6 Pollen
In Arabidopsis, double mutation of MPK3 and MPK6 leads to embryo lethality (Wang et al., 2007). In addition, we noticed that the mpk3–/– mpk6+/– plants exhibited distorted male transmission when backcrossed to wild-type pistils (mpk3 mpk6:mpk3 MPK6 = 35:316, P < 0.0001, male transmission efficiency = 11.08%; male transmission efficiency: no. of mutant progenies/no. of wild-type progenies × 100%). Back crosses using mpk3+/–, mpk6+/–, or mpk3+/– mpk6–/– as male parents showed that mpk3 single mutant pollen had a normal transmission rate (mpk3:MPK3 = 89:80, P = 0.4887, male transmission efficiency = 111.25%), while the mpk6 single mutant pollen transmission was moderately reduced in competition with the wild-type pollen (mpk6:MPK6 = 74:156, P < 0.0001, male transmission efficiency = 47.24%). The transmission defect of mpk3 mpk6 pollen was partially suppressed when a limited number (<20) of pollen grains from mpk3–/– mpk6+/– plants were used for pollination (mpk3 mpk6:mpk3 MPK6 = 95:144, P = 0.0019, male transmission efficiency = 65.97%). This result indicated that the mpk3 mpk6 pollen is less competitive than mpk3 pollen but is mostly able to fertilize ovules in the absence of competitor pollen. MPK3 and MPK6 are both expressed in pollen, with MPK6 showing a higher expression level than MPK3 (Supplemental Fig. S1; Supplemental Materials and Methods S1). In pollen tubes, MPK3 and MPK6 protein were enriched in nuclei, as revealed by the yellow fluorescent protein (YFP) fusion (Supplemental Fig. S2; Supplemental Materials and Methods S1).
A Fluorescent Reporter-Tagging Complementation Method for Isolating mpk3 mpk6 Pollen Grains
Our previous study using mpk3+/– mpk6–/– plants in the quartet1 background demonstrated that mpk3 mpk6 pollen has no developmental defect (Wang et al., 2008). To test if mpk3 mpk6 pollen tube growth is impaired, we performed backcrosses using pollen from mpk3–/– mpk6+/– plants and wild-type pistils and collected each individual seed along the siliques. We found that mpk3+/– mpk6+/– seeds were randomly distributed, suggesting no pollen tube growth defect of the double mutant in vivo (Supplemental Table S1).
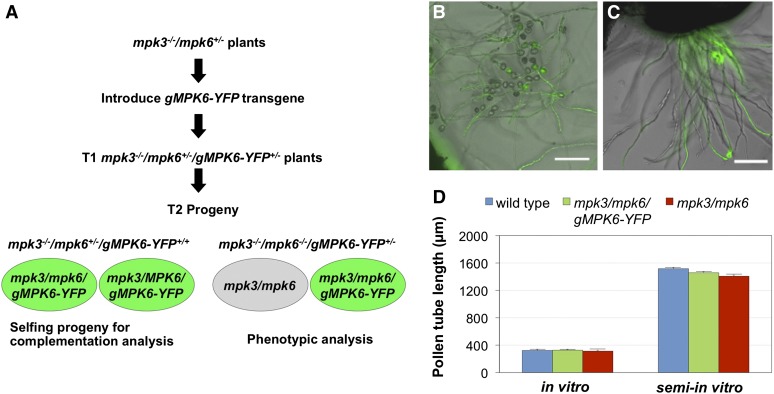
Because the mpk3–/– mpk6–/– double mutant is embryo lethal, we cannot obtain homogeneous mpk3 mpk6 pollen population. To observe directly the phenotype of mpk3 mpk6 pollen, we developed a fluorescent reporter-tagged complementation method (Fig. 1A). A construct with a genomic MPK6 fragment (gMPK6) tandemly linked to a pollen-specific Late Anther Tomato53 (LAT52) promoter-driven YFP reporter cassette (Twell et al., 1990), designated gMPK6-YFP, was introduced into mpk3–/– mpk6+/– plants. Selfing progenies of mpk3–/– mpk6+/– gMPK6-YFP+/+ plants revealed normal pollen transmission (mpk3–/– mpk6–/– gMPK6-YFP+/+:mpk3–/– mpk6+/– gMPK6-YFP+/+:mpk3–/– MPK6 gMPK6-YFP+/+ = 47:98:44, P = 0.89, expected 1:2:1), indicating successful complementation of the double mpk3 mpk6 mutant pollen function by the gMPK6-YFP transgene. Furthermore, plants in mpk3–/– mpk6–/– gMPK6-YFP+/– genotype were identified, in which the nonfluorescent mpk3 mpk6 double mutant pollen was distinguishable from the YFP-tagged-gMPK6 complemented pollen (genotype: mpk3 mpk6 gMPK6-YFP; Fig. 1A). In vitro and semi-in vitro germination of pollen of the mpk3–/– mpk6–/– gMPK6-YFP+/– plants verified that the double mutant pollen is indistinguishable from complemented pollen in germination and pollen tube growth (Fig. 1, B–D).
Figure 1.
Reduced mpk3 mpk6 pollen transmission is not associated with defective pollen germination or pollen tube growth. A, Generation of fluorescent reporter-tagged MPK6 complemented mpk3–/– mpk6–/– mutant lines. gMPK6-YFP construct was introduced into mpk3–/– mpk6+/– plants. In T2 generation, mpk3–/– mpk6+/– gMPK6-YFP+/+ plants were identified to determine the complementation of mpk3 mpk6 pollen transmission by gMPK6-YFP. Plants of mpk3–/– mpk6–/– gMPK6-YFP+/– genotype, which produce the nonfluorescent mpk3/mpk6 double mutant pollen and the YFP-tagged complemented pollen (mpk3 mpk6 gMPK6-YFP), were identified for phenotypic analysis in vivo. B and C, Double mpk3 mpk6 mutant (nonfluorescent) and complemented mpk3 mpk6 gMPK6-YFP (fluorescent) pollen tubes grew at similar rates in both in vitro pollen germination (B) and semi-in vitro pollen germination assays (C). Bar = 100 µm. D, Quantitative comparison of pollen tube length of wild-type, mpk3 mpk6 double mutant, and mpk3 mpk6 gMPK6-YFP complemented pollen. Error bar indicates se of the mean.
mpk3 mpk6 Pollen Is Defective in Pollen Tube Guidance
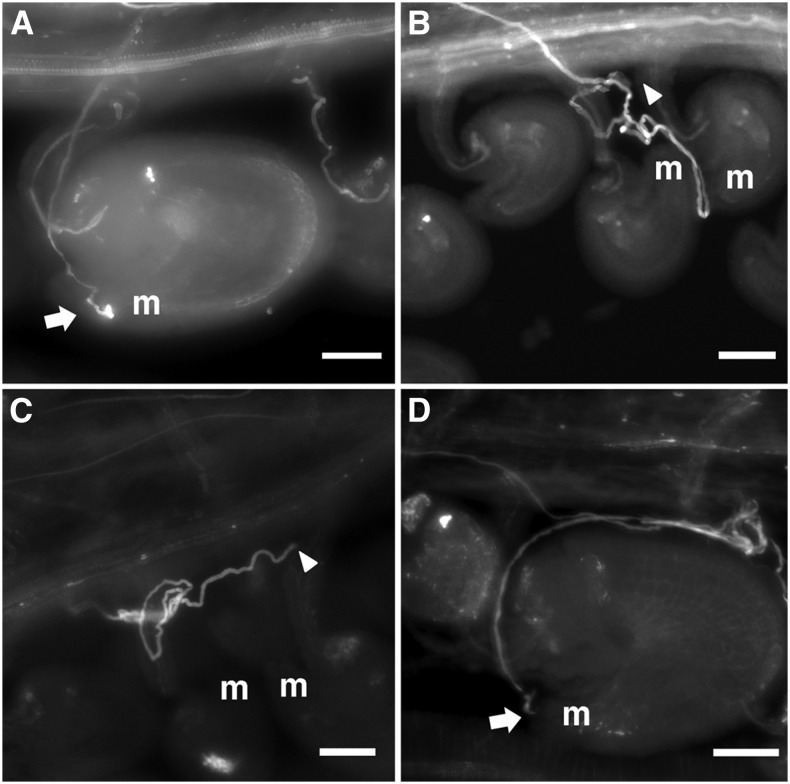
To observe the mpk3 mpk6 pollen tube growth in vivo, we isolated nonfluorescent mpk3 mp6 pollen grains from mpk3–/– mpk6–/– gMPK6-YFP+/– plants under a fluorescent dissecting microscope and performed limited pollination. In limited pollination, all wild-type pollen tubes could target and fertilize the ovules (Fig. 2A). After emerging from the transmitting tract onto the septum, wild-type pollen tubes proceeded directly onto funiculi toward micropyles for fertilization. By contrast, the majority (58%, n = 117) of the mpk3 mpk6 pollen tubes showed defects in navigation. Some of the pollen tubes were lost in the septum and elongated in random directions without growing onto the funiculus (20%, n = 117; Fig. 2, B and C). Others exhibited a wandering phenotype before eventually targeting the ovules (38%, n = 117; Fig. 2D). However, the response of the mpk3 mpk6 pollen tubes to the guidance signals was not completely blocked, because most mutant pollen tubes could eventually approach micropyle (80%, n = 117) in the absence of competing wild-type pollen. This is consistent with the genotyping result of recovered mutant transmission in limited pollination.
Figure 2.
Defective navigation of mpk3 mpk6 mutant pollen tubes in limited pollination. Pollen tube growth in wild-type pistils was visualized by aniline blue staining. A, An ovule fertilized by a wild-type pollen tube in limited pollination. B to D, Limited pollination of wild-type pistils with the nonfluorescent mpk3 mpk6 pollen grains isolated from those produced by mpk3–/– mpk6–/– gMPK6-YFP+/– plants. B and C, mpk3 mpk6 pollen tubes exhibited wandering phenotype without targeting any ovule. D, A mpk3 mpk6 pollen tube eventually targeted an ovule after wandering growth. Arrows indicate pollen tube tips that entered the micropyle. Arrowheads indicate the position of pollen tube tips that did not grow onto the ovule. The letter m indicates position of the micropyle. Bar = 100 µm.
mpk3 mpk6 Pollen Tube Is Deficient in Funicular Guidance in Vivo
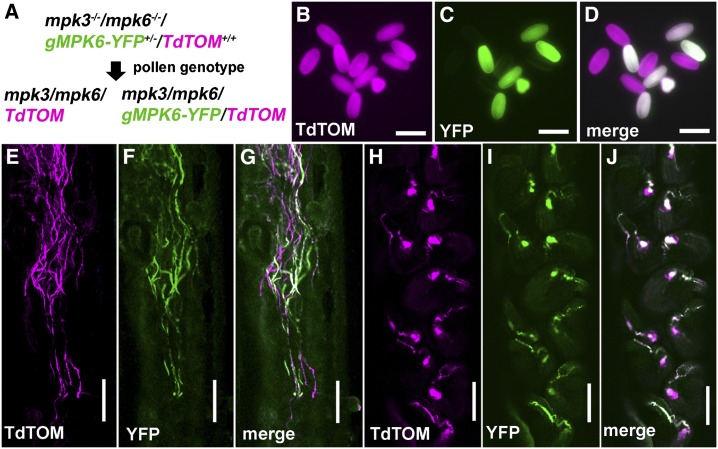
In previously reported Arabidopsis mutants with micropylar guidance defects, multiple pollen tubes were observed on one funiculus (Palanivelu et al., 2003; Kasahara et al., 2005; Shimizu et al., 2008). However, this phenotype was not observed in pistils pollinated with excessive pollen grains from mpk3–/– mpk6+/– plants (Supplemental Fig. S3). All the ovules were targeted by a single pollen tube that grew directly into the micropyle, indicating that the mpk3 mpk6 pollen tubes may be impaired in funicular guidance, i.e. the mpk3 mpk6 pollen tubes cannot find or take a longer time to find the funiculus. To verify if mpk3 mpk6 pollen tubes were defective in funicular guidance stage in vivo, we introduced a LAT52 promoter-driven tdTomato fluorescent marker (designated TdTOM) into mpk3–/– mpk6–/– gMPK6-YFP+/– plants (Fig. 3A). The T2 mpk3–/– mpk6–/– gMPK6-YFP+/– TdTOM+/+ plants produce double mutant pollen tagged by only the TdTOM marker (genotype: mpk3 mpk6 TdTOM) and the gMPK6-complemented pollen tagged by both TdTOM and YFP (genotype: mpk3 mpk6 gMPK6-YFP TdTOM; Fig. 3, B–D). In vivo observation showed that the growth of mpk3 mpk6 pollen tubes in the transmitting tract is similar to the gMPK6-complemented pollen tubes (Fig. 3, E–G). Consistent with the genetic analysis, most of the fertilized ovules were targeted by the gMPK6-complemented pollen tubes instead of mpk3 mpk6 pollen tubes (Fig. 3, H–J; 71 of 82 observed ovules, P < 0.0001; targeting efficiency of gMPK6-complemented pollen = 86.59%). The absence of mpk3 mpk6 pollen tubes on most funiculi indicated impaired funicular guidance of the mutant.
Figure 3.
Deficient funicular guidance of mpk3 mpk6 pollen tubes in vivo. A, Double fluorescent reporter-tagged complementation line. Plants of mpk3–/– mpk6–/– gMPK6-YFP+/– TdTOM+/+ genotype produce double mutant pollen tagged by TdTOM only and complemented pollen tagged by both YFP and TdTOM. B to D, Fluorescent images of TdTOM (B), YFP (C), and merged (D) channels of pollen grains from mpk3–/– mpk6–/– gMPK6-YFP+/– TdTOM+/+ plants. Pollen of mpk3 mpk6 TdTOM genotype is indicated by the cyan color in merged channel. Pollen of mpk3 mpk6 gMPK6-YFP TdTOM genotype is indicated by the white color in merged channel. Bar = 25 µm. E to G, Pistil with carpel and ovules removed at 7 h after pollination. The double mutant and complemented pollen tubes showed similar growth rates and morphology in the transmission tract. E, TdTOM. F, YFP. G, Merged. Bar = 100 µm. H to J, Pistil with carpel removed at 7 h after pollination to exhibit the ovules. In this part of the pistil, all ovules were fertilized by complemented pollen tubes. Note no double mutant pollen tubes were observed on funiculus. H, TdTOM. I, YFP. J, Merged. Bar = 100 µm.
Response of mpk3 mpk6 Pollen Tubes to Micropyle Attractants Is Normal in the Semi-in Vitro Guidance Assay
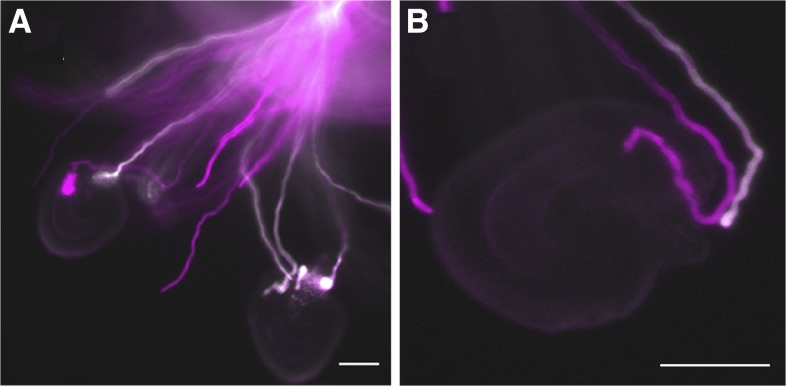
To examine if mpk3 mpk6 pollen is also defective in micropylar guidance, we performed a semi-in vitro pollen tube guidance assay, which recapitulates pollen tube navigation by micropylar attractants (Palanivelu and Preuss, 2006; Stewman et al., 2010), using pollen from mpk3–/– mpk6–/– gMPK6-YFP+/– TdTOM+/+ plants. The results showed that double mutant pollen tubes were not defective in navigation to the micropyle (ovules targeted by mpk3 mpk6 TdTOM:mpk3 mpk6 gMPK6-YFP TdTOM pollen = 43:46, expected 1:1, P = 0.75). In addition, when approaching the ovules, the double mutant pollen tubes could make sharp turns toward and enter micropyles, even in competition with gMPK6-complemented pollen tubes (Fig. 4, A and B). These results indicated that the pollen tube response to and growth toward the micropylar guidance cues are not affected in mpk3 mpk6 pollen. Therefore, MPK3/MPK6 signaling is required for in vivo pollen tube guidance response, which is distinct from the micropylar guidance.
Figure 4.
Normal micropylar guidance of the mpk3/mpk6 double mutant pollen tubes in semi-in vitro guidance assay. A and B, Double mutant pollen tubes can efficiently target micropyles in the semi-in vitro pollen tube guidance assay. Cyan color indicates mpk3 mpk6 double mutant pollen tubes (genotype: mpk3 mpk6 TdTOM), and white color indicates gMPK6-YFP complemented pollen tubes (genotype: mpk3 mpk6 gMPK6-YFP TdTOM). A, Two ovules targeted by an mpk3 mpk6 pollen tube and a gMPK6-complemented pollen tube, respectively. B, A mpk3 mpk6 pollen tube made a sharp turn toward an ovule and entered the micropyle ahead of a gMPK6-complemented pollen tube. Bar = 50 µm.
In Arabidopsis, MPK3 and MPK6 play multiple roles in signaling plant developmental processes and responses to invading pathogens and abiotic stress stimuli (Zhang and Klessig, 2001; Asai et al., 2002; Liu and Zhang, 2004; Wang et al., 2007, 2008; Beckers et al., 2009). Here, we report that the MPK3/MPK6 signaling is also required for pollen tube guidance, opening up the possibility that the MPK3/MPK6 signaling pathway may link signaling networks between stress response and reproductive development. The micropylar guidance attractants, LUREs, belong to the defensin-like super gene family of secreted Cys-rich proteins, which are an ancient class of small antimicrobial proteins (Aerts et al., 2008; Okuda et al., 2009). Intriguingly, plant antifungal defensins could activate fungal MAPK pathways to change the structural integrity of fungal membranes, which changes the influx/efflux of ion signals (such as Ca2+ and K+) and in turn affects fungal growth (Ramamoorthy et al., 2007; Aerts et al., 2008; Dresselhaus and Márton, 2009). The pollen tube response to guidance cues (such as LUREs and funicular guidance signals) might be analogous to the defensin-triggered fungal response and involve the MAPK pathway to regulate the attractant response and reorientation of pollen tube growth (Dresselhaus and Márton, 2009).
The MPK3/MPK6 signaling pathway may act downstream of pollen tube guidance signal receptors (e.g. receptor-like kinases) and is activated when the funicular guidance cues are perceived by these receptors. Previous reports showed that stress-induced activation of MPK6 or its orthologs in other species happens within one to several minutes (Zhang and Klessig, 2001). Thus, it is possible that MPK3 and MPK6 are activated quickly in response to the guidance signals (Supplemental Fig. S4). Alternatively, pollen tube growth through the stigma and pistil tissues is essential for the priming of pollen tubes for guidance response (Qin et al., 2009). We could not exclude the possibility that the MPK3/MPK6 signaling cascade may be activated during pollination to regulate expression of funicular guidance signaling genes. However, the expression of MAPK cascade components including MAPKs, their upstream MAPK kinases (MAPKKs) and MAPKK kinases (MAPKKKs) are not significantly changed in semi-in vitro pollen tubes (Qin et al., 2009). In addition, mpk3 mpk6 pollen tubes were completely competent in semi-in vitro guidance assay. These results indicated that MPK3/MPK6 downstream pathway may be specifically involved in the in vivo interactions between funiculus and pollen tube, although the underlying mechanism is still unclear. Finally, the MPK3/MPK6 signaling cascade might affect gene expression at earlier stages, such as during pollen development, and the preexisting transcripts or proteins play important roles at a later stage during pollen tube guidance (Supplemental Fig. S4).
In addition, the fluorescent reporter-tagged complementation method we developed is broadly applicable to the male gametophytic mutant study. This method allows one to confidently assign complemented and mutant genotypes to individual pollen grains under a fluorescence microscopy. Homogenous mutant pollen grains can be physically isolated. Furthermore, the method facilitates side-by-side comparison of mutant and complemented pollen in vitro and in vivo.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used as the wild type. Transfer DNA insertion alleles of MPK3 (At3g45640), mpk3-1, and MPK6 (At2g43790), mpk6-2, were described previously (Wang et al., 2007). Mutant plants with mpk3–/– mpk6+/– and mpk3+/– mpk6–/– genotypes were generated by crossing mpk3-1 and mpk6-2. Plant growth and genotyping of mutant alleles was performed as described previously (Wang et al., 2007).
Molecular Cloning and Transformation
For complementation of mpk3–/– mpk6–/– mutant, the LAT52:YFP cassette was PCR amplified from pZY90-LAT52:YFP construct (a gift from Dr. Sheila McCormick, Plant Gene Expression Center) and cloned into pGreenII-gMPK6 to generate the pGreenII-gMPK6-LAT52:YFP construct. The LAT52:TdTOM construct was prepared using a synthetic sequence (GenBank: KJ081243) encoding TdTOM (Shaner et al., 2004) that was codon optimized based on pollen-expressed genes NTP303 from tobacco (Nicotiana tabacum; Weterings et al., 1992) and LAT52 from tomato (Solanum lycopersicum; Twell et al., 1989). The LAT52 gene promoter was used for pollen-specific expression, and the sequence was cloned upstream of the nopaline synthase terminator from pBIN19 (Bevan, 1984).
Microscopy and Pollen Germination, Growth, and Guidance Assays
Pollen germination assays were performed as described (Boavida and McCormick, 2007). For pollen growth assay, the lengths of more than 100 pollen tubes per sample were measured 4 h after germination using ImageJ software (Abràmoff et al., 2004).
For in vivo fertilization assay, pistils of emasculated flowers were hand pollinated with pollen from mpk3–/– mpk6–/– gMPK6-YFP+/– TdTOM+/+ plants. At 7 h after pollination, the pistils were carefully dissected to remove carpel along one valve and to expose two rows of ovules. The dissected pistil was then placed in the pollen germination medium with exposed ovules facing up, and a cover slip was put on to mount the slide. Confocal images were taken with a Zeiss LSM 510 META NLO confocal microscope. Semi-in vitro pollination and pollen tube guidance assay was performed according to Palanivelu and Preuss (2006). Fluorescence microscopy was performed with an Olympus IX70 inverted microscope with an ORCA digital camera.
For mpk3 mpk6 mutant pollen isolation, pollen grains from newly opened flowers of mpk3–/– mpk6–/– gMPK6-YFP+/– plants were collected loosely on a slide and counted under a Leica MZFLIII dissecting microscope. Nonfluorescent mpk3 mpk6 mutant pollen grains were carefully picked up using one hair of a paintbrush and then dabbed onto the stigma surface of emasculated pistils. Each stigma was pollinated with eight pollen grains. To minimize UV damage, the pollen grains on a slide were changed when the total exposure time under UV reached 30 s. For control, the wild-type pollen was exposed under UV light for 30 s and used for pollination. Aniline blue staining of pollen tubes was performed as described previously (Boavida et al., 2009).
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers MPK3 (At3g45640) and MPK6 (At2g43790).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. MPK6 expression is higher than MPK3 in pollen.
Supplemental Figure S2. Subcellular localization of MPK3 and MPK6 fusion protein.
Supplemental Figure S3. In vivo tube growth of pollen grains from mpk3–/– mpk6+/– plants in excessive pollination.
Supplemental Figure S4. Proposed model for MPK3/MPK6 signaling in pollen tube guidance.
Supplemental Table S1. Distribution of mpk3 mpk6 fertilized seeds in mpk3–/– mpk6+/– pollinated pistils.
Supplemental Materials and Methods S1. Real-time reverse transcription-PCR quantification and subcellular localization of MPK3 and MPK6 in pollen.
Supplementary Material
Acknowledgments
We thank Weihua Tang (Institute of Plant Physiology and Ecology, Chinese Academy of Science) for helpful discussion, Sheila McCormick (Plant Gene Expression Center) for critical reading and the LAT52:eYFP plasmid, and Wolfgang Lukowitz (University of Georgia) for the pGreenII-gMPK6 plasmid.
Glossary
- ER
endoplasmic reticulum
- MAPK
mitogen-activated protein kinase
Footnotes
This work was supported by the National Science Foundation (grant no. MCB-0950519 and a Research Experiences for Undergraduates supplement to S.Z.); and a National Natural Science Foundation of China Young Investigator Award (grant no. 31300244 to J.X.).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abràmoff MD, Magalhães PJ, Ram SJ. (2004) Image processing with ImageJ. Biophoton Internatl 11: 36–78 [Google Scholar]
- Aerts AM, François IE, Cammue BP, Thevissen K. (2008) The mode of antifungal action of plant, insect and human defensins. Cell Mol Life Sci 65: 2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, Gomez-Gomez L, Boller T, Ausubel FM, Sheen J. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983 [DOI] [PubMed] [Google Scholar]
- Beckers GJ, Jaskiewicz M, Liu Y, Underwood WR, He SY, Zhang S, Conrath U. (2009) Mitogen-activated protein kinases 3 and 6 are required for full priming of stress responses in Arabidopsis thaliana. Plant Cell 21: 944–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M. (1984) Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res 12: 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boavida LC, McCormick S. (2007) Temperature as a determinant factor for increased and reproducible in vitro pollen germination in Arabidopsis thaliana. Plant J 52: 570–582 [DOI] [PubMed] [Google Scholar]
- Boavida LC, Shuai B, Yu HJ, Pagnussat GC, Sundaresan V, McCormick S. (2009) A collection of Ds insertional mutants associated with defects in male gametophyte development and function in Arabidopsis thaliana. Genetics 181: 1369–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dresselhaus T, Márton ML. (2009) Micropylar pollen tube guidance and burst: adapted from defense mechanisms? Curr Opin Plant Biol 12: 773–780 [DOI] [PubMed] [Google Scholar]
- Higashiyama T. (2010) Peptide signaling in pollen-pistil interactions. Plant Cell Physiol 51: 177–189 [DOI] [PubMed] [Google Scholar]
- Hülskamp M, Schneitz K, Pruitt RE. (1995) Genetic evidence for a long-range activity that directs pollen tube guidance in Arabidopsis. Plant Cell 7: 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara RD, Portereiko MF, Sandaklie-Nikolova L, Rabiger DS, Drews GN. (2005) MYB98 is required for pollen tube guidance and synergid cell differentiation in Arabidopsis. Plant Cell 17: 2981–2992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Xue Y, Jia DJ, Wang T, Hi DQ, Liu J, Cui F, Xie Q, Ye D, Yang WC. (2011) POD1 regulates pollen tube guidance in response to micropylar female signaling and acts in early embryo patterning in Arabidopsis. Plant Cell 23: 3288–3302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HJ, Yang WC. (2012) Emerging role of ER quality control in plant cell signal perception. Protein Cell 3: 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhong S, Guo X, Hao L, Wei X, Huang Q, Hou Y, Shi J, Wang C, Gu H, et al. (2013) Membrane-bound RLCKs LIP1 and LIP2 are essential male factors controlling male-female attraction in Arabidopsis. Curr Biol 23: 993–998 [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang S. (2004) Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16: 3386–3399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Chanroj S, Zulkifli L, Johnson MA, Uozumi N, Cheung A, Sze H. (2011) Pollen tubes lacking a pair of K+ transporters fail to target ovules in Arabidopsis. Plant Cell 23: 81–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Lima PT, Borges F, Silva AC, Portes MT, Carvalho JE, Gilliham M, Liu LH, Obermeyer G, Feijó JA. (2011) Glutamate receptor-like genes form Ca2+ channels in pollen tubes and are regulated by pistil d-serine. Science 332: 434–437 [DOI] [PubMed] [Google Scholar]
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, Kasahara RD, Hamamura Y, Mizukami A, Susaki D, et al. (2009) Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 458: 357–361 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Brass L, Edlund AF, Preuss D. (2003) Pollen tube growth and guidance is regulated by POP2, an Arabidopsis gene that controls GABA levels. Cell 114: 47–59 [DOI] [PubMed] [Google Scholar]
- Palanivelu R, Preuss D. (2006) Distinct short-range ovule signals attract or repel Arabidopsis thaliana pollen tubes in vitro. BMC Plant Biol 6: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, Vasic B, Johnson MA, Palanivelu R. (2009) Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet 5: e1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy V, Zhao X, Snyder AK, Xu JR, Shah DM. (2007) Two mitogen-activated protein kinase signalling cascades mediate basal resistance to antifungal plant defensins in Fusarium graminearum. Cell Microbiol 9: 1491–1506 [DOI] [PubMed] [Google Scholar]
- Shaner NC, Campbell RE, Steinbach PA, Giepmans BNG, Palmer AE, Tsien RY. (2004) Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol 22: 1567–1572 [DOI] [PubMed] [Google Scholar]
- Shimizu KK, Ito T, Ishiguro S, Okada K. (2008) MAA3 (MAGATAMA3) helicase gene is required for female gametophyte development and pollen tube guidance in Arabidopsis thaliana. Plant Cell Physiol 49: 1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewman SF, Jones-Rhoades M, Bhimalapuram P, Tchernookov M, Preuss D, Dinner AR. (2010) Mechanistic insights from a quantitative analysis of pollen tube guidance. BMC Plant Biol 10: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Higashiyama T. (2011) Attraction of tip-growing pollen tubes by the female gametophyte. Curr Opin Plant Biol 14: 614–621 [DOI] [PubMed] [Google Scholar]
- Twell D, Wing R, Yamaguchi J, McCormick S. (1989) Isolation and expression of an anther-specific gene from tomato. Mol Gen Genet 217: 240–245 [DOI] [PubMed] [Google Scholar]
- Twell D, Yamaguchi J, McCormick S. (1990) Pollen-specific gene expression in transgenic plants: coordinate regulation of two different tomato gene promoters during microsporogenesis. Development 109: 705–713 [DOI] [PubMed] [Google Scholar]
- Wang H, Liu Y, Bruffett K, Lee J, Hause G, Walker JC, Zhang S. (2008) Haplo-insufficiency of MPK3 in MPK6 mutant background uncovers a novel function of these two MAPKs in Arabidopsis ovule development. Plant Cell 20: 602–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ngwenyama N, Liu Y, Walker JC, Zhang S. (2007) Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19: 63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weterings K, Reijnen W, van Aarssen R, Kortstee A, Spijkers J, van Herpen M, Schrauwen J, Wullems G. (1992) Characterization of a pollen-specific cDNA clone from Nicotiana tabacum expressed during microgametogenesis and germination. Plant Mol Biol 18: 1101–1111 [DOI] [PubMed] [Google Scholar]
- Zhang S (2008). Mitogen-activated protein kinase cascades in plant intracellular signaling. In Z Yang, ed, Annual Plant Reviews, Vol 33. Wiley-Blackwell, Oxford, pp 100–136 [Google Scholar]
- Zhang S, Klessig DF. (2001) MAPK cascades in plant defense signaling. Trends Plant Sci 6: 520–527 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.