MicroRNAs are required for the patterning and specification of most tissues in the Arabidopsis embryo, with the exception of the protoderm, with various regions of the embryo requiring different levels of microRNAs.
Abstract
The development of the embryo in Arabidopsis (Arabidopsis thaliana) involves a carefully controlled set of cell divisions and cell fate decisions that lead to a mature embryo containing shoot and root meristems and all basic tissue types. Over the last 20 years, a number of transcriptional regulators of embryonic patterning have been described, but little is known about the role of posttranscriptional regulators such as microRNAs (miRNAs). Previous work has centered on the study of null or very weak alleles of miRNA biosynthetic genes, but these mutants either arrest early in embryogenesis or have wild-type-looking embryos. Here, we significantly extend those analyses by characterizing embryos mutant for a strong hypomorphic allele of DICER-LIKE1 (dcl1-15). Our data demonstrate that miRNAs are required for the patterning of most regions of the embryo, with the exception of the protoderm. In mutant embryos with the most severe morphological defects, the majority of tissue identities are lost. Different levels of miRNAs appear to be required to specify cell fates in various regions of the embryo. The suspensor needs the lowest levels, followed by the root apical meristem and hypocotyl, cotyledons, and shoot apical meristem. Furthermore, we show that erecta acts as a suppressor of dcl1-15, a novel role for this signaling pathway in embryos. Our results also indicate that the regulation of the messenger RNA levels of miRNA targets involves not just the action of miRNAs but has a significant transcriptional component as well.
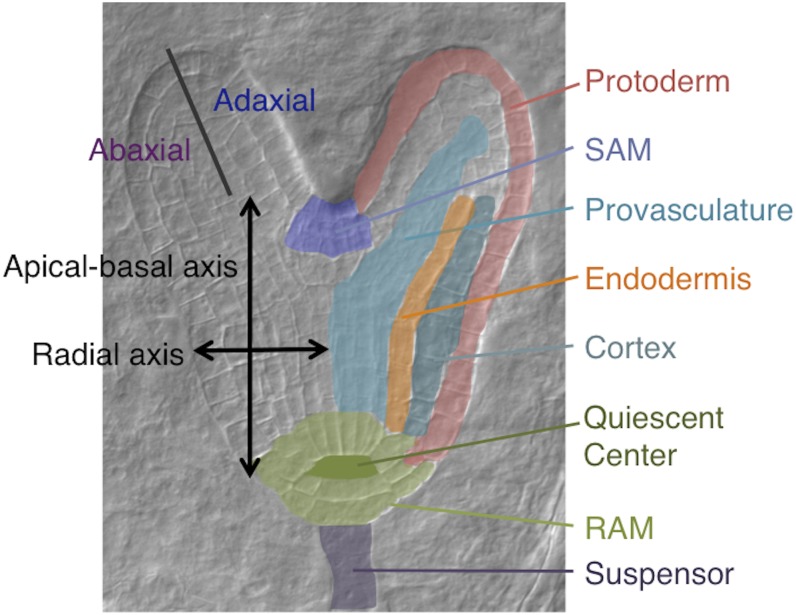
The mature plant embryo is organized around two axes: an apical-basal and a radial, or central-peripheral, axis. Groups of stem cells, or meristems, present at the apical and basal ends of the former axis (shoot apical meristem [SAM] and root apical meristem [RAM], respectively), give rise to all lateral organs postembryonically. Concentric layers of tissues are found along the radial axis, from the center to the outside: provasculature (or stele), endodermis, cortex, and protoderm. The central and peripheral domains continue as the adaxial and abaxial sides, respectively, of cotyledons and later lateral organs (Fig. 1; Jenik et al., 2007).
Figure 1.
Axes and tissues. This late heart stage embryo shows the embryonic axes (left side) and tissues (right side) mentioned in the text.
In Arabidopsis (Arabidopsis thaliana), this pattern is laid out during embryonic development through a carefully controlled and virtually invariant series of cell divisions and cell fate decisions. Embryonic stages are designated according to either the number of cells or the shape of the embryo proper (one- to 16-cell, globular, transition, heart, torpedo, bent cotyledon, and mature stages; Jürgens and Mayer, 1994). In spite of the rigid sequence of cell divisions, developmental decisions are mostly not cell autonomous (Torres-Ruiz and Jürgens, 1994). There is extensive communication between neighboring tissues, facilitated by the fact that the early embryo behaves as a single symplastic compartment (Kim et al., 2005). After fertilization, the zygote divides into a smaller apical cell and a larger basal cell (Mansfield and Briarty, 1991). The apical-basal axis is established by differential auxin levels and export abilities between the apical and basal cells and, later, between the upper and lower tiers of cells (Friml et al., 2003). The basal cell will generate the suspensor, an impermanent file of cells whose presumed function is nutrient and hormone transport (Kawashima and Goldberg, 2010), and the hypophysis. Descendants of the hypophysis will become the center of the RAM. The apical cell will generate most of the embryo proper. Distinct cell fates in the embryo proper, revealed by differential gene expression, are seen as early as the eight-cell stage (Haecker et al., 2004). The first tissue type established in the embryo is the protoderm, at the 16-cell stage. During the globular stage, signaling from the future hypocotyl helps establish the rudiments of the RAM (the quiescent center [QC] and some initials; Schlereth et al., 2010). Maintenance of the RAM, in turn, is dependent on signals from the QC (Sabatini et al., 2003). By the late globular stage, most of the radial layers are in place. The transition to the early heart stage marks the development of the SAM (which requires signals from the provasculature and the L1; Tucker et al., 2008; Knauer et al., 2013) and the establishment of bilateral symmetry in the apical domain, with the outgrowth of the cotyledons (Mansfield and Briarty, 1991; Jürgens and Mayer, 1994). By the late heart stage, the embryo body plan has been established, and the remainder of embryogenesis involves an increase in the number of cells, growth in size, and the process of maturation that will result in a full, dry seed (Raz et al., 2001).
There has been significant progress in identifying the genetic pathways involved in the determination of cell fates (tissue specification) in the different regions of the embryo (for review, see Jenik et al., 2007; Lau et al., 2012). Several principles have emerged. Many patterning genes, particularly those that specify the central and apical domains, are dynamically expressed in early embryos, with their final expression patterns not set in place until the late globular-early heart stage (Aida et al., 1999; Lynn et al., 1999; McConnell et al., 2001; Hamann et al., 2002; Emery et al., 2003). There are also many instances of mutual repression to organize and separate tissue types. For example, basal (root-specifying) genes repress apical (shoot-specifying) genes and vice versa (Grigg et al., 2009; Smith and Long, 2010), peripheral/abaxial genes limit the extent of expression of central/adaxial genes, and possibly vice versa (Eshed et al., 2004; Izhaki and Bowman, 2007), genes specifying the SAM and cotyledons negatively regulate each other (Byrne et al., 2000), and suspensor and embryo proper seem to be mutually exclusive fates (Lukowitz et al., 2004).
Whereas previous reports mainly focus on how patterning and cell specification are orchestrated at the transcriptional level in the embryo, very limited information is available about the importance of posttranscriptional regulation (and posttranscriptional regulators) in this process. MicroRNAs (miRNAs) are a class of small RNAs (20–24 nucleotides in Arabidopsis; Reinhart et al., 2002; http://mpss.udel.edu/common/web/mirAbundances.php?SITE=at_pare) that posttranscriptionally repress gene expression by binding complementary target RNAs and promoting their cleavage or inhibiting their translation. The relative importance of these two modes of actions is unclear (Brodersen et al., 2008; Pasquinelli, 2012). Mature miRNAs are generated by the cleavage of a precursor by a protein complex that includes the RNase III DICER-LIKE1 (DCL1), the double-stranded RNA-binding protein HYPONASTIC LEAVES1 (HYL1), and the C2H2-zinc finger protein SERRATE (SE). The resulting miRNAs are then 5′ methylated by HUA-ENHANCER1 (HEN1), exported from the nucleus by the EXPORTIN5 ortholog HASTY, incorporated by ARGONAUTE (AGO) proteins (such as AGO1 and AGO10/ZWILLE/PINHEAD [ZLL/PNH]) into an RNA-induced silencing complex, and brought by the RNA-induced silencing complex to their specific target mRNAs (Voinnet, 2009).
More than 400 miRNA targets are present in the embryo (Willmann et al., 2011), suggesting that they have important roles in its development. However, only a handful of them have been shown so far to regulate embryo patterning (Wu, 2013). In the apical region, miR164 represses CUP-SHAPED COTYLEDON1 (CUC1) and CUC2 to ensure proper formation of the SAM and separation of the cotyledons (Laufs et al., 2004; Mallory et al., 2004). The repression of LEAF CURLING RESPONSIVENESS by miR394 is also required for SAM stem cell maintenance (Knauer et al., 2013). The miRNA miR165/166 restricts the expression of Homeodomain-Leucine zipper (HD-Zip) III genes (PHABULOSA [PHB], PHAVOLUTA [PHV], REVOLUTA [REV], AtHB8, and INCURVATA4/CORONA [ICU4/CNA]) to the center of the hypocotyl and the adaxial side of the cotyledons, where they are required to repress RAM identities and to promote vascular, SAM, and adaxial fates (McConnell et al., 2001; Prigge et al., 2005; Grigg et al., 2009; Smith and Long, 2010). At the other end of the embryo, the differentiation of root cap cells depends on AUXIN RESPONSE FACTOR10 (ARF10) and ARF16, which are regulated by miR160 (Wang et al., 2005). Both miR165/166 and miR394 have been shown to have nonautonomous effects by moving between cells over short distances, helping coordinate tissue specification along the radial axis and in the SAM (Knauer et al., 2013; Miyashima et al., 2013). This may point to a larger role of miRNAs in cell-cell communication in the embryo.
It has been difficult to assess the roles of miRNAs during embryogenesis using null mutants in the miRNA biogenesis pathway because they either have mild or inconspicuous phenotypes (such as those of ago1, zll/pnh, hen1, and hyl1; Lynn et al., 1999; Lu and Fedoroff, 2000; Chen et al., 2002; Ronemus et al., 2006; Kurihara et al., 2009), most likely due to redundancy, or they are embryonic lethal, arresting their development early (null alleles of DCL1 [dcl1-1 to dcl1-6] or SE [se-4 and se-5]; Schwartz et al., 1994; Schauer et al., 2002; Lobbes et al., 2006; Grigg et al., 2009; Nodine and Bartel, 2010). The latter mutants produce irregularly shaped embryos that are characterized by an overproliferated suspensor, higher levels of miRNA target transcripts, and the loss of QC and central, but not protoderm, identities. The early lethality of these mutants does not allow for a good understanding of the roles of miRNAs later in embryonic development. Weak DCL1 alleles (such as dcl1-7 to dcl1-9) have pleiotropic effects on the plant, including abnormal ovules, but do not significantly affect embryogenesis (Schauer et al., 2002).
We recently identified a late embryonic lethal, hypomorphic allele of dcl1 (dcl1-15; Willmann et al., 2011) that allowed us to better probe the overall importance of miRNA-dependent regulation in the patterning process. The mutant embryos show two distinct phenotypes, which we believe are independent of each other: a precocious activation of the embryonic maturation program (described in detail in our previous article; Willmann et al., 2011) and severe patterning defects (but milder than those of null alleles). By carefully characterizing the morphological defects of a series of allelic combinations, we were able to show that different regions of the embryo require different levels of miRNAs for their patterning and that the receptor kinase ERECTA (ER) influences the hypocotyl and root meristem phenotypes. We also determined that most aspects of embryonic patterning and tissue differentiation, except for the specification of the protoderm, are dependent on the proper functioning of miRNA pathways. Surprisingly, in the complete absence of miRNAs, most regions of the embryo do not seem to be able to adopt or maintain any clear identity.
RESULTS
A Strong Hypomorphic Mutant Allele of DCL1 Results in Severe Embryonic Defects
We previously reported the isolation of an ethyl methanesulfonate-induced, missense mutation in DCL1, dcl1-15 (Willmann et al., 2011). The mutation was isolated in a mixed Wassilewskija (Ws)/Landsberg erecta (Ler) background that was er/er and then outcrossed four times to Ler before analysis. While being embryonic lethal, dcl1-15 is unlikely to be a null allele: the embryonic defects are milder than those of known null alleles (see below), and the DCL1 transcript is highly expressed in mutant embryos (Willmann et al., 2011).
To better understand the role of miRNAs in embryonic patterning, we studied not only the morphology of dcl1-15 homozygous embryos but also of trans-heterozygous combinations with the weak allele dcl1-9 and the null allele dcl1-5. The idea was to genetically generate embryos with different levels of DCL1 activity, to extend the range of observable phenotypes. dcl1-9 is in the Ler accession, and homozygous embryos are wild type looking (see below; Jacobsen et al., 1999). dcl1-5 is in the Columbia-0 accession (and therefore ER/ER), and homozygous embryos have a very severe phenotype (Fig. 2K; Schauer et al., 2002). We first describe the development of dcl1-15 homozygous embryos, mentioning other allelic combinations as necessary. We then describe the analysis of the full series of allelic combinations and the genetic interactions between alleles.
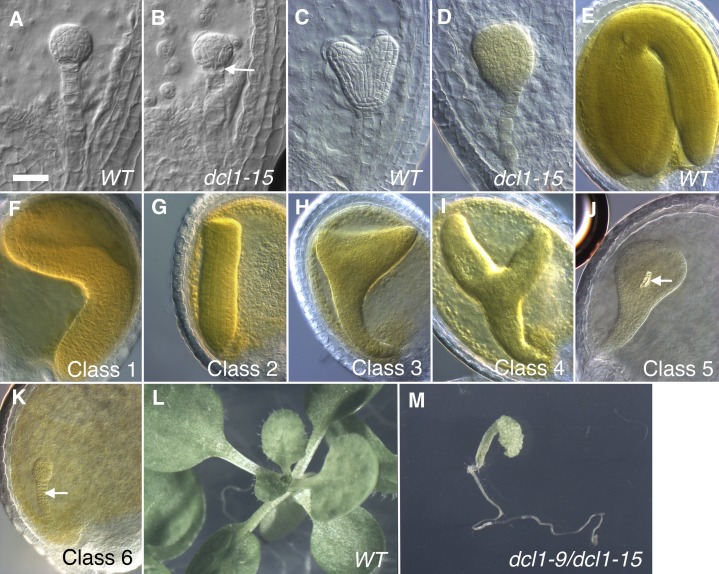
Figure 2.
Phenotypes of dcl1 embryos and seedlings. A to D, Early globular (A and B) and heart (C and D) stage wild-type (WT) and dcl1-15 embryos. The arrow in B points to the split hypophysis. E to K, Mature stage embryos showing the wild type (E) and the six different classes of dcl1 phenotypes. All embryos are dcl1-15/dcl1-15 er/er except for F (dcl1-9/dcl1-15 er/er) and K (dcl1-5/dcl1-5 ER/ER). The arrow in J points to a xylem element. The arrow in K points to the overproliferated and persistent suspensor (the suspensor has degenerated in all the other classes and the wild type). L and M, Twenty-day-old wild-type (L) and dcl1-9/dcl1-15 er/er (M) seedlings, photographed at the same magnification. Bar = 25 µm (A–D) and 50 µm (E–K).
Our initial analysis of embryonic development was done on cleared seeds from DCL1/dcl1-15 er/er self-pollinated plants. Mutant embryos were staged by referring to the wild-type embryos in the same silique. At least until the heart stages, the mutant dcl1-15 embryos and endosperm developed at the same rate as the wild-type ones. The endosperm in dcl1-15 seeds cellularized at the same time as in wild-type seeds (heart stage; data not shown). The first visible alterations in dcl1-15 embryos were abnormal divisions of the hypophysis, observed first at the 16-cell stage (17.6% of embryos; n = 108), and more consistently at the early globular stage (25.2% of embryos; n = 119; Fig. 2, A and B). At the midglobular stage, we also detected abnormal divisions in the lower tier of the embryo proper. These early phenotypes are virtually identical to those seen in embryos of null mutants, such as dcl1-5 (Schwartz et al., 1994; Nodine and Bartel, 2010). By the early heart stage, the embryos showed very aberrant patterns of cell division, distinct from those of null dcl1 embryos (Fig. 2, C and D; Schwartz et al., 1994; Nodine and Bartel, 2010). Significantly, in dcl1-15 embryos, we never saw abnormal proliferation of the suspensor (Fig. 2, C and D), commonly observed in null dcl1 mutant embryos (Fig. 2K).
When the embryos reached the later stages of embryogenesis (early bent cotyledon and later), we could classify the embryo morphological defects into six discrete phenotypic morphological classes, with our analyses (described below) suggesting that class 1 is the least abnormal and class 6 is the most abnormal (Figs. 2, E–K, and 3). Some of these classes were defined using embryos mutant for dcl1-5 or trans-heterozygous combinations. Class 1 embryos (mostly trans-heterozygotes dcl1-9/dcl1-15 er/er) had an apparently normal hypocotyl and root pole and some outgrowth of fused cotyledons (Fig. 2F). Class 2 embryos also had normal-looking hypocotyls and root poles but no cotyledons (Fig. 2G). Class 3 included cone-shaped embryos, with an unpatterned root-hypocotyl region, and a wide top where cotyledons should have developed (Fig. 2H). Class 4 embryos were similar to class 3, but the cotyledon-like organs were more rod shaped (Fig. 2I). Based on our analysis of reporter gene expression (Fig. 3; see also Fig. 8; Supplemental Fig. S1; data not shown), the cotyledon-like outgrowths do not appear to acquire actual cotyledon fate. Class 5 embryos were an unpatterned club-shaped mass of variable length (Fig. 2J) and represented the most frequent class (Fig. 3). Interestingly, all these phenotypes were reminiscent of those of the few mature embryos generated when plants homozygous for the weak mutant allele dcl1-8 are fertilized with wild-type pollen (Ray et al., 1996). In wild-type Arabidopsis, xylem tissue does not normally differentiate until after germination (Dolan et al., 1993). An unusual feature seen in 13.3% of dcl1-15 embryos (mostly class 5; n = 218) was the differentiation of one or more short xylem elements in the upper central region of the embryo (Fig. 2J). Finally, class 6 embryos (Fig. 2K) were characteristic of the null allele dcl1-5, with a small globular mass and an overproliferated, persistent suspensor. dcl1-15 embryos died late in embryogenesis but not due to desiccation intolerance (Willmann et al., 2011). At the time of abortion, large gaps in the embryos could be observed, as if the internal tissues had started to fall apart (data not shown). This phenomenon has also been observed in late stage embryos homozygous for null dcl1 alleles (Schwartz et al., 1994).
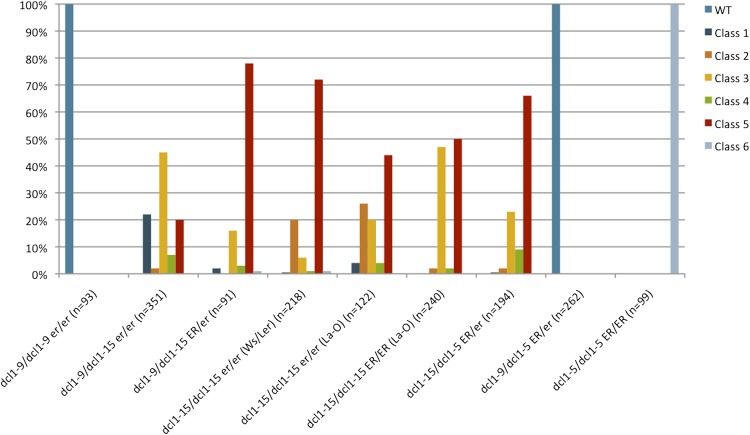
Figure 3.
Distribution of dcl1 phenotypic classes in different genetic backgrounds. WT, Wild type.
Figure 8.
Late expression patterns for reporters for the central-peripheral domains. A to G, Reporters for the central and adaxial domains. H to L, Reporters for the peripheral and abaxial domains. A to D, Bent cotyledon stage embryos expressing pSCR:GFP in the endodermis and QC in the wild type (WT). The expression domain is progressively reduced in the mutants as phenotypes increase in severity (B and C) to a dot in the center of the embryos (D). E to G, Early bent cotyledon stage embryos with the rev-9 enhancer trap expressed in the provasculature and adaxial side of the cotyledons in the wild type. Expression is progressively reduced in the mutants, depending on severity (F and G). H to J, Bent cotyledon stage embryos expressing pKAN1:GUS in peripheral tissues of the basal hypocotyl in the wild type. Expression is absent (I) or reduced (J) in the mutant. K and L, Bent cotyledon stage embryos expressing the E2023 enhancer trap in the periphery of the upper hypocotyl and the abaxial side of the cotyledons in the wild type. Very reduced expression is seen in the basal hypocotyl of the mutant (L). Magenta indicates chlorophyll autofluorescence. dcl1-15 phenotypic classes are as follows: class 2 (B and F), class 3 (C), class 5 (D, G, and L), class 4 (I and J). Bar = 100 µm (A) and 50 µm (B–L).
ER Enhances the dcl1-15 Embryo Phenotype
Previous research suggested that mutations in the receptor kinase ER enhanced the ovule defects caused by the weak allele dcl1-7 (Lang et al., 1994). However, the genotype at the ER locus does not affect the morphology of the wild-type-looking dcl1-7 seeds (data not shown). To assess whether er was also affecting dcl1-15 embryos, we crossed our original line (DCL1/dcl1-15 er/er, in Ws/Ler outcrossed to the Ler background, indicated as Ws/Ler in parentheses in Fig. 3) to Landbserg ERECTA (La-O). In the F2 generation, we selected DCL1/dcl1-15 er/er and DCL1/dcl1-15 ER/ER plants (both indicated with La-O in parentheses in Fig. 3). Since both parental lines were mostly in the Landsberg background, most differences should be attributable to the effects of the ER locus. The main difference between er/er (in the original Ws/Ler background or in the F2 of the cross) and ER/ER plants was that class 2 embryos were only apparent in the former (Fig. 3). Since class 2 embryos have a more normal-looking hypocotyl and root pole, these data suggest a suppression of the dcl1-15 embryonic phenotype by er, which is the opposite of what is seen for dcl1-7 ovules (Lang et al., 1994). This effect of er is confirmed by the fact that dcl1-9/dcl1-15 er/er embryos had milder phenotypes (including class 1) than dcl1-9/dcl1-15 ER/er embryos (Fig. 3). These data indicate a novel role for ER during embryogenesis, something that has not been apparent in previous studies (Shpak, 2013).
dcl1-15 Is a Mildly Dominant-Negative Allele
We then turned our attention to the series of allelic combinations of dcl1-5, dcl1-15, and dcl1-9 in various ER backgrounds. We hypothesized that the order of phenotypic strengths (from mildest to most severe) would be as follows: wild type = dcl1-9/dcl1-9 er/er < dcl1-9/dcl1-15 er/er < dcl1-9/dcl1-15 ER/er < dcl1-15/dcl1-15 er/er < dcl1-15/dcl1-15 ER/er ∼ dcl1-9/dcl1-5 ER/er < dcl1-15/dcl1-5 ER/er < dcl1-5/dcl1-5 ER/ER. As predicted, dcl1-15/dcl1-5 ER/ER embryos had a stronger range of phenotypes than dcl1-15/dcl1-15 ER/ER embryos (Fig. 3). Similarly, dcl1-9/dcl1-15 er/er embryos had a milder range of phenotypes than dcl1-15/dcl1-15 er/er embryos, including many more class 1 embryos, which is the mildest class of mutant embryos (Figs. 2F and 3). We tested whether dcl1-9/dcl1-15 er/er individuals were viable by germinating the dry seeds on Murashige and Skoog (MS) medium plates. Seventy-three percent (n = 26) of the trans-heterozygous seeds germinated. All the seedlings developed a viable root and hypocotyl, but the cotyledons were distorted or absent. By 20 d after germination, the mutant seedlings had not developed any true leaves and seemed arrested in their development (Fig. 2, L and M).
The surprising result came when comparing dcl1-9/dcl1-15 ER/er with dcl1-9/dcl1-5 ER/er embryos. Even though dcl1-5 is a stronger allele than dcl1-15, dcl1-9/dcl1-5 ER/er embryos had a much milder phenotype than dcl1-9/dcl1-15 ER/er embryos (Fig. 3). In fact, dcl1-9/dcl1-5 ER/er embryos were wild type in appearance. These data indicate that dcl1-9 is dominant over dcl1-5, likely because the resulting DCL1-9 enzyme retains sufficient activity to compensate for the null allele. These results also show that dcl1-15 has a dominant-negative effect on dcl1-9. The DCL1 wild-type allele is completely dominant over dcl1-15, indicating that the effects of DCL1-15 are only visible when DCL1 activity is already compromised. However, as can be seen from comparing dcl1-9/dcl1-15 ER/er with dcl1-9/dcl1-15 er/er (Fig. 3), the dominant effects of dcl1-15 over dcl1-9 can be ameliorated by the absence of ER signaling. The dominant-negative effect suggests either physical interactions between DCL1 proteins or competition for a binding partner, somehow influenced by ER signaling. This experiment gives genetic support to bimolecular fluorescence complementation data that indicated that DCL1 is part of a complex that includes dimers or multimers of the enzyme (Fang and Spector, 2007).
Embryonic Patterning Is Severely Altered in dcl1-15 Embryos
The majority of dcl1-15 embryos (classes 3–5) do not show any obvious tissue differentiation when observed with the light microscope (Fig. 2, H–J). In order to better understand how tissue identities and patterning are affected in these mutant embryos and their dynamics during development, we took three complementary approaches. First, we carried out a global transcriptional profiling experiment of wild-type and dcl1-15 torpedo stage embryos in triplicate using Affymetrix ATH1 microarrays. We chose the torpedo stage because it was the earliest stage when it was easy to distinguish mutant from wild-type embryos and isolate them from the rest of the seed tissue. This experiment was described in detail previously (Willmann et al., 2011), and we will only address here the aspects relevant to the patterning of the embryo.
In parallel, we studied the expression patterns of reporter lines for genes with specific expression patterns in the embryo (e.g. the SAM or the central/adaxial domain). Unlike transcriptional profiling, which only provides information about the mRNA levels, the analysis of reporter genes allowed us to identify the regions within the embryo in which the spatial distribution of transcripts was altered.
Lastly, we compared our transcriptional analysis of torpedo stage dcl1-15 embryos with a previously published one of early globular stage embryos of the null allele dcl1-5 (Nodine and Bartel, 2010), because it provided some additional insight into the gene expression dynamics of abnormal development in dcl1 embryos, specifically how and when tissue patterning goes astray. Our microarray analysis only provided a snapshot of torpedo stage embryos, a relatively late stage of embryo development, and we were only able to analyze a limited number of reporter genes at early stages. A couple of interesting observations from the transcriptomic analyses are that a number of miRNA targets actually show stable or reduced transcript levels in the mutants (particularly in dcl1-15) and that the set of miRNA targets that are misregulated (particularly those encoding transcription factors) is different between dcl1-5 early globular and dcl1-15 torpedo stage embryos. We have summarized these data for those who would like to explore them in more detail in Supplemental Tables S1 to S4.
The following discussion of embryonic patterning in dcl1 mutants is mostly organized by embryonic region, in part because they are patterned semiindependently (Jenik et al., 2007). However, it must be kept in mind that regions communicate with each other and that some pathways, like auxin, have effects on multiple parts of the embryo. The expression of developmentally relevant genes that are expressed broadly in the embryo is not discussed in the text, but is summarized in Table I.
Table I. Expression of region-specific and developmentally important genes in wild-type and dcl1 embryos.
Gene names in boldface indicate miRNA targets. Fold change represents log2(dcl1 expression/wild-type expression). Values that were significantly different from the wild type are in boldface, with those down-regulated underlined. The criteria for significance are as follows: for dcl1-15, an adjusted P < 0.05; for dcl1-5, at least a 1-fold change (no replicates were done for dcl1-5, and no P values are reported). For dcl1-5, genes with fewer than 20 raw reads in both the wild-type and mutant embryos were considered not expressed (Nodine and Bartel, 2010). If the gene had fewer than 20 raw reads in one genotype but more than 20 in the other, it was designated as not detected (nd) in that first genotype, and no ratio was reported.
| Name | Locus Identifier | dcl1-15 Torpedo Stage | dcl1-5 Globular Stage |
|---|---|---|---|
| fold change | |||
| Transcripts enriched in the suspensor | |||
| ARABIDOPSIS SHAGGY-RELATED PROTEIN KINASE η/BRASSINOID INSENSITIVE2 (ASKη/BIN2) | At4g18710 | 1.18 | 0.11 |
| SUCROSE TRANSPORTER3 (SUC3) | At2g02860 | 1.70 | 1.06 |
| WUSCHEL-LIKE HOMEOBOX8 (WOX8) | At5g45980 | 2.40 | 1.92 |
| WRKY2 | At5g56270 | −1.22 | 0.65 |
| Transcripts enriched in the protoderm | |||
| ARABIDOPSIS THALIANA MERISTEM LAYER1 (ATML1) | At4g21750 | 1.03 | 0.53 |
| CAPRICE (CPC) | At2g46410 | −1.85 | Not expressed |
| FIDDLEHEAD (FDH) | At2g26250 | −1.10 | 0.6 |
| GLABRA2 (GL2) | At1g79840 | 2.60 | 2.23 |
| LIPID TRANSFER PROTEIN1 (LTP1) | At2g38540 | −1.13 | 0.72 |
| PROTODERMAL FACTOR1 (PDF1) | At2G42840 | −1.18 | −0.44 |
| PROTODERMAL FACTOR2 (PDF2) | At4g04890 | 1.39 | −0.08 |
| Transcripts enriched at or around the SAM | |||
| CLAVATA1 (CLV1) | At1g75820 | −2.55 | nd wild type |
| CLAVATA3 (CLV3) | At2g27250 | −2.71 | Not expressed |
| CUP-SHAPED COTYLEDON1 (CUC1) | At3g15170 | 1.69 | nd dcl1-5 |
| CUP-SHAPED COTYLEDON2 (CUC2) | At5g53950 | −1.71 | Not expressed |
| CUP-SHAPED COTYLEDON3 (CUC3) | At1g76420 | −4.43 | nd dcl1-5 |
| KNOTTED-LIKE HOMEOBOX PROTEIN2 (KNAT2) | At1g70510 | −2.03 | Not expressed |
| PINOID (PID) | At2g34650 | −1.25 | 0.48 |
| SHOOTMERISTEMLESS (STM) | At1g62360 | −8.28 | Not expressed |
| UNUSUAL FLORAL ORGANS (UFO) | At1g30950 | −2.05 | Not expressed |
| WUSCHEL (WUS) | At2g17950 | −1.36 | Not expressed |
| Transcripts enriched in cotyledons | |||
| AINTEGUMENTA (ANT) | At4g37750 | −27.40 | Not expressed |
| ARABIDOPSIS HISTIDINE PHOSPHOTRANSFER PROTEIN6 (AHP6) | At1g80100 | −1.25 | nd dcl1-5 |
| ARABIDOPSIS THALIANA HOMEOBOX3 (HAT3) | At3g60390 | −2.09 | −1.05 |
| ARABIDOPSIS THALIANA HOMEOBOX4 (ATHB4) | At2g44910 | −2.12 | Not expressed |
| ASYMMETRIC LEAVES1 (AS1) | At2g37630 | −7.14 | 1.83 |
| BES-INTERACTING MYC-LIKE PROTEIN1 (BIM1) | At5G08130 | −1.27 | 2.00 |
| PHAVOLUTA (PHV) | At1g30490 | −1.28 | 2.95 |
| TCP4 | At3g15030 | 1.19 | 3.60 |
| YABBY3 (YAB3) | At4g00180 | −14.96 | Not expressed |
| Transcripts enriched in the RAM or initials | |||
| ARABIDOPSIS RESPONSE REGULATOR7 (ARR7) | At1g19050 | −12.47 | nd wild type |
| ARABIDOPSIS RESPONSE REGULATOR15 (ARR15) | At1g74890 | 1.14 | Not expressed |
| AUXIN RESPONSE FACTOR16 (ARF16) | At4g30080 | 2.27 | nd dcl1-5 |
| PIN-FORMED4 (PIN4) | At2g01420 | −4.20 | −1.58 |
| PIN-FORMED7 (PIN7) | At1g23080 | 3.80 | Not expressed |
| PLETHORA1 (PLT1) | At3g20840 | −7.77 | −1.27 |
| PLETHORA2 (PLT2) | At1g51190 | −4.15 | −1.30 |
| SCHIZORIZA | At1g46264 | −5.09 | Not expressed |
| TARGET OF MONOPTEROS7 (TMO7) | At1g74500 | −8.55 | Not expressed |
| WUSCHEL-LIKE HOMEOBOX5 (WOX5) | At3g11260 | Not on array | |
| Transcripts enriched in the central/adaxial domain | |||
| ARABIDOPSIS THALIANA HOMEOBOX2 (ATHB2) | At4g16780 | 2.04 | Not expressed |
| ARABIDOPSIS THALIANA HOMEOBOX8 (ATHB8) | At4g32880 | −1.18 | nd wild type |
| ARABIDOPSIS THALIANA HOMEOBOX15/CORONA (ATHB15/CNA) | At1g52150 | −7.04 | 5.28 |
| BODENLOS (BDL) | At1g04550 | −1.70 | −0.19 |
| CENTER CITY (CCT) | At4g00450 | −1.25 | −0.69 |
| COTYLEDON VASCULAR PATTERN2 (CVP2) | At1G05470 | −8.02 | Not expressed |
| GIBBERELLIN-REQUIRING1 (GA1) | At4g02780 | 5.41 | Not expressed |
| GRAND CENTRAL (GCT) | At1g55325 | −1.21 | 1.44 |
| HANABA TARANU (HAN) | At3g50870 | −9.94 | −0.35 |
| INDOLE ACETIC ACID-INDUCED PROTEIN13 (IAA13) | At2g33310 | −1.15 | 0.94 |
| INDOLE ACETIC ACID-INDUCED PROTEIN2 (IAA2) | At3g23030 | −1.15 | Not expressed |
| LONESOME HIGHWAY (LHW) | At2g27230 | −2.07 | 0.46 |
| MONOPTEROS/AUXIN RESPONSE FACTOR5 (MP/ARF5) | At1g19850 | −11.27 | −0.14 |
| PHABULOSA (PHB) | At2g34710 | −1.51 | 2.87 |
| PIN-FORMED1 (PIN1) | At1g73590 | −5.22 | 0.86 |
| PINHEAD (PNH)/ZWILLE (ZLL) | At5g43810 | −2.28 | 0.15 |
| REVOLUTA (REV) | At5g60690 | 1.41 | 1.26 |
| SCARECROW (SCR) | At3g54220 | 1.15 | −1.66 |
| SERRATE (SE) | At2g27100 | −1.77 | 0.25 |
| SHORT ROOT (SHR) | At4g37650 | 1.11 | 0.93 |
| TARGET OF MONOPTEROS5 (TMO5) | At3g25710 | −1.73 | 0.85 |
| TMO5-LIKE1 (T5L1) | At1g68810 | 1.06 | −3.12 |
| TARGET OF MONOPTEROS6 (TMO6) | At5g60200 | −12.42 | nd dcl1-5 |
| VASCULAR HIGHWAY1/BRASSINOSTEROID INSENSITIVE1-LIKE2 (VH1/BRL2) | At2g01950 | −2.09 | Not expressed |
| Transcripts enriched in the peripheral/abaxial domain | |||
| FILAMENTOUS FLOWER (FIL) | At2g45190 | −6.65 | Not expressed |
| KANADI1 (KAN1) | At5g16560 | −2.05 | Not expressed |
| KANADI3 (KAN3) | At4g17695 | 1.62 | Not expressed |
| KNOTTED-LIKE HOMEOBOX PROTEIN1 (KNAT1) | At4g08150 | −7.22 | Not expressed |
| KANADI2 (KAN2) | At1g32240 | −3.78 | Not expressed |
| Transcripts expressed broadly, developmentally important genes | |||
| ARABIDOPSIS THALIANA DEFECTIVE KERNEL1 (AtDEK1) | At1g55350 | −1.45 | −0.12 |
| CENTER CITY (CCT) | At4g00450 | −1.25 | −0.68 |
| FACKEL (FK) | At3g52940 | 1.68 | 0.41 |
| GNOM/EMBRYO DEFECTIVE30 (GN/EMB30) | At1g13980 | −1.04 | 2.38 |
| GRAND CENTRAL (GCT) | At1g55325 | −1.21 | 1.44 |
| GURKE/PASTICCINO3 (GK/PAS3) | At1g36160 | −1.01 | 1.22 |
| HOBBIT (HBT) | At2g20000 | −1.94 | 1.04 |
| HYDRA1 (HYD1) | At1g20050 | −1.05 | 0.25 |
| LEAF CURLING RESPONSIVENESS (LCR) | At1g27340 | 1.2 | 2.03 |
| NONPHOTOTROPIC HYPOCOTYL4/AUXIN RESPONSE FACTOR7 (NPH4/ARF7) | At5g20730 | −2.47 | 1.49 |
| OBERON1 (OBE1) | At3g07780 | −1.04 | 0.50 |
| OBERON2 (OBE2) | At5g48160 | Not on array | 0.66 |
| POPCORN (PCN) | At4g07410 | −1.11 | 0.79 |
| RECEPTOR-LIKE PROTEIN KINASE1 (RPK1) | At1g69270 | −2.45 | −1.33 |
| RETINOBLASTOMA-RELATED (RBR) | At3g12280 | −1.81 | 0.85 |
| ROOT-SHOOT-HYPOCOTYL DEFECTIVE (RSH) | At1g21310 | 1.67 | 2.15 |
| TITANIA1 (TTA1) | At1g14740 | 1.55 | 0.48 |
| SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE10 (SPL10) | At1g27370 | −1.16 | 7.06 |
| SQUAMOSA-PROMOTER BINDING PROTEIN-LIKE11 (SPL11) | At1g27360 | −1.07 | 2.76 |
| TITANIA2 (TTA2) | At3g63500 | 1.07 | nd wild type |
| TOADSTOOL2 (TOAD2) | At3g02130 | −2.00 | 1.10 |
| TOPLESS (TPL) | At1g15750 | −1.52 | 0.56 |
| TOPLESS-RELATED1 (TPR1) | At1g80490 | Not on array | 0.68 |
| TOPLESS-RELATED2 (TPR2) | At3g16830 | 1.08 | 0.57 |
| TOPLESS-RELATED3 (TPR3) | At5g27030 | Not on array | 2.85 |
| TOPLESS-RELATED4 (TPR4) | At3g15880 | 1.07 | 0.78 |
| TRANSPARENT TESTA GLABRA1 (TTG1) | At5g24520 | −1.10 | 1.09 |
The Suspensor
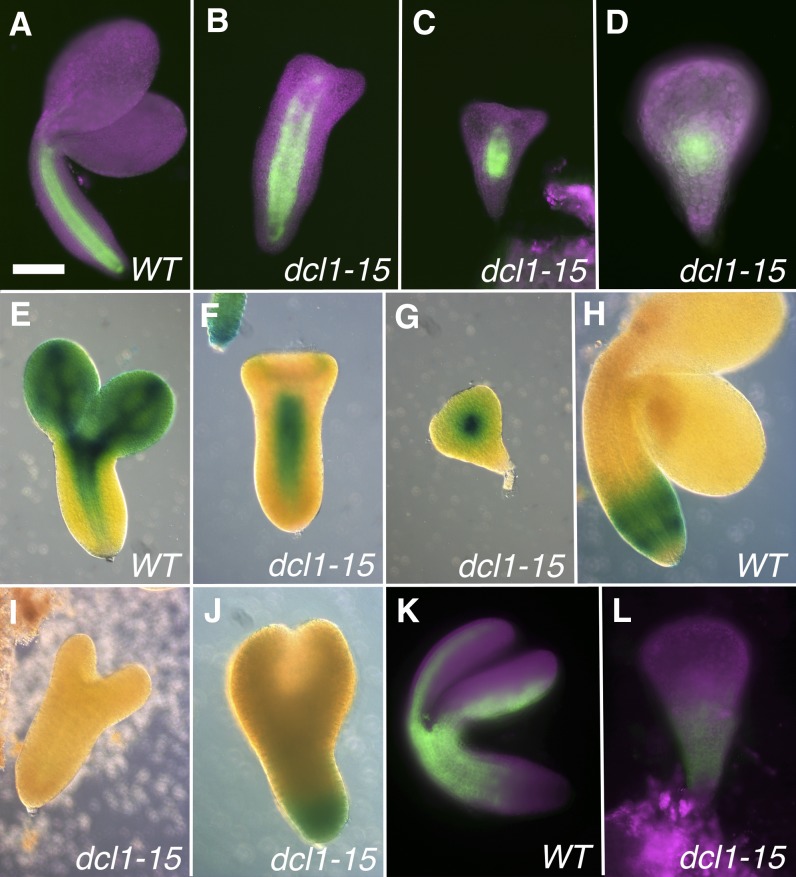
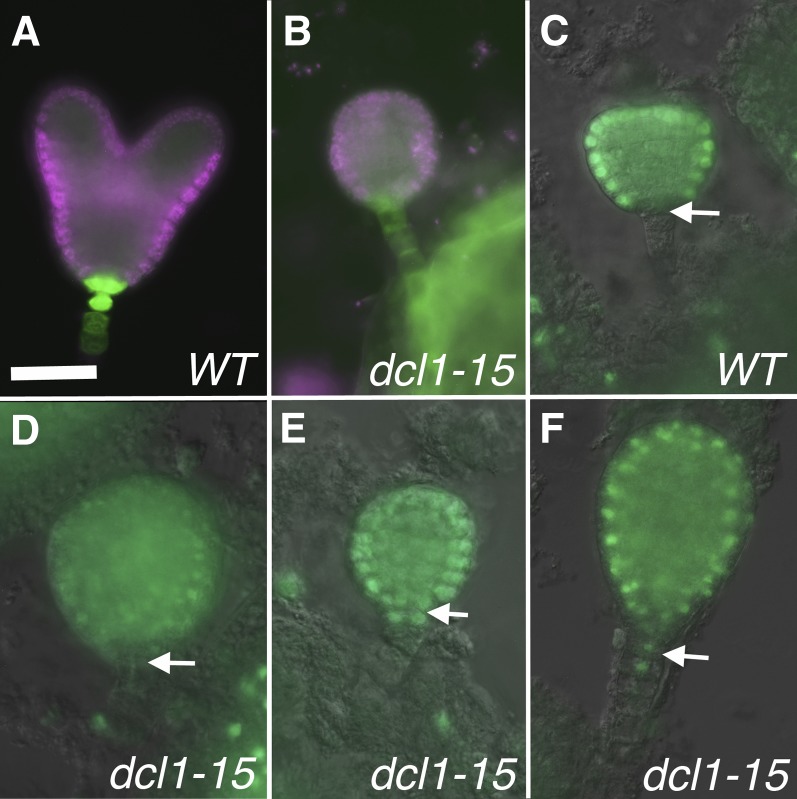
The formation of the suspensor is complete by the eight-cell stage, and it persists until the torpedo stage, when it degenerates (Mardsen and Meinke, 1985). In null dcl1 embryos, the suspensor starts developing normally, but by the globular stage it loses apparent suspensor fate, accumulating transcripts and products that are normally restricted to the embryo proper; it overproliferates and it does not degenerate (Fig. 2K; Schwartz et al., 1994; Nodine and Bartel, 2010). In contrast, in dcl1-15 embryos, the suspensor appears normal, as described above. Neither our nor other (Nodine and Bartel, 2010) transcriptomic data indicate a reduction in suspensor-expressed transcripts in the mutants (Table I). To further analyze cell fate in the suspensor, we used two reporter genes: pSUC3:GFP (for SUCROSE TRANSPORTER3; Meyer et al., 2004), which in early embryos is restricted to the suspensor and hypophysis derivatives (Fig. 4A), and pAtML1:H2B-YFP (for MERISTEM LAYER1 promoter driving HISTONE 2B fused to YFP; Roeder et al., 2010), which is expressed throughout the protoderm but is excluded from the root pole and the suspensor (Fig. 4C; Sessions et al., 1999). We only analyzed embryos up to the late heart stage, because the suspensor starts degenerating after that. All dcl1-15 embryos expressed pSUC3:GFP in the suspensor (n = 16; Fig. 4B), and 55% of mutant embryos excluded pAtML1:H2B-YFP from the suspensor and the root pole area (n = 40; Fig. 4D). However, in 25% of embryos, pAtML1:H2B-YFP expression was found in the root pole (Fig. 4E), while in the remaining 20%, the reporter had also expanded into the top of the suspensor (Fig. 4F). Since we looked at early stage embryos, we were not able to correlate these differences in expression with the severity of the terminal phenotype, which becomes apparent only later. Our morphological and gene expression data indicate that in a majority of dcl1-15 embryos, the suspensor fate is not affected. However, in some embryos, there is a partial expansion of embryo proper markers into the suspensor, but it is much milder than that observed in embryos mutant for null dcl1 alleles (Nodine and Bartel, 2010). Altogether, these observations suggests that even very reduced amounts of miRNAs are sufficient to properly maintain suspensor identities.
Figure 4.
Expression of reporters for the protoderm and suspensor. A and B, Heart stage embryos expressing pSUC3:GFP in the suspensor and derivatives of the hypophysis in both the wild type (WT) and mutant. C to F, Early heart stage embryos expressing pAtML1:H2B-YFP. The reporter is expressed in the wild type throughout the protoderm but excluded from the root pole (arrow in C) and the suspensor. In some mutants, pAtML1:H2B-YFP is excluded from the root pole (arrow) and suspensor (D). In others, it is expressed in the root pole (arrow) but not the suspensor (E) or it expands into the top of the suspensor (arrow in F). Bar = 25 µm for all.
The Protoderm
The protoderm is the first tissue type to be established in the embryo (Mansfield and Briarty, 1991), and it requires the expression of the transcription factors PROTODERMAL FACTOR1 (PDF1), PDF2, and AtML1, which also establish a positive feedback loop that reinforces this developmental decision (Abe et al., 2003). Transcriptomic data do not suggest an overall alteration in protodermal identity in dcl1 embryos (Table I). In addition, in situ localization of AtML1 and PDF1 transcripts in the null allele dcl1-5 (Nodine and Bartel, 2010) and expression of pAtML1:H2B-YFP in dcl1-15 (n = 40; Fig. 4, C–F) appear normal. These results suggest that miRNAs are not essential for the acquisition and maintenance of protodermal fate.
Auxin Transport and Response
The phytohormone auxin has been reported to play crucial roles during the patterning of the embryo. Early on, it is involved in the establishment of the suspensor and the hypophysis. Later, it is important for the establishment of the root meristem, the provasculature and possibly central fates in the hypocotyl, and the positioning and growth of the cotyledons (for review, see Möller and Weijers, 2009). Auxin is synthesized by the YUCCA (YUC) and TRYPTOPHAN AMINOTRANSFERASE OF ARABIDOPSIS1 (TAA1) families of proteins: early in embryogenesis in the suspensor by YUC3, YUC4, and YUC9 and later, mostly in the SAM and the tips of the cotyledons, by TAA1, TAA1-RELATED2, YUC1, YUC4, YUC10, and YUC11 (Cheng et al., 2007; Robert et al., 2013). Auxin is transported from its place of synthesis by the PIN-FORMED family of transporters (PIN1 in most of the embryo, PIN4 and PIN7 in the root pole), acting together with the P-glycoproteins PGP19 and PGP1 (Mravec et al., 2008). At the gene regulation level, auxin action is mediated by 23 ARF transcription factors. Among them, 15 are expressed in a variety of patterns in the embryo (Rademacher et al., 2011), with some of them predicted to be activators and some repressors of transcription (Tiwari et al., 2003; Table II). ARFs, in turn, are repressed by proteins of the AUXIN/INDOLE-3-ACETIC ACID INDUCIBLE (AUX/IAA) family (28 members in Arabidopsis; Remington et al., 2004). Auxin, acting through one of its receptors (TIR1 or AUXIN SIGNALING F-BOX1 [AFB1] to AFB3), promotes the degradation of the repressing AUX/IAA, thus freeing ARF to regulate gene expression (Dharmasiri et al., 2005). So far, seven AUX/IAA proteins have been shown to have roles during embryonic development (Hamann et al., 2002; Weijers et al., 2005; Braybrook et al., 2006; Muto et al., 2007; Ploense et al., 2009; Rademacher et al., 2012; Table II).
Table II. Expression of genes involved in auxin synthesis, transport, and response in wild-type and dcl1 embryos.
For explanations, see Table I.
| Class | Name | Locus Identifier | dcl1-15 Torpedo Stage | dcl1-5 Globular Stage |
|---|---|---|---|---|
| fold change | ||||
| Auxin synthesis | TAA1 | At1g70560 | 2.12 | 2.95 |
| TAA1-RELATED2 (TAR2) | At4g24670 | −11.78 | 2.23 | |
| YUC1 | At4g32540 | −2.04 | 0.23 | |
| YUC3 | At1g04610 | −3.76 | 0.84 | |
| YUC4 | At5g11320 | −1.19 | −2.3 | |
| YUC9 | At1g04180 | Not expressed | Not expressed | |
| YUC10 | At1g48910 | 4.45 | Not expressed | |
| YUC11 | At1g21430 | Not expressed | Not expressed | |
| Auxin transport | PIN1 | At1g73590 | −5.22 | 0.86 |
| PIN4 | At2g01420 | −4.20 | −1.58 | |
| PIN7 | At1g23080 | 3.80 | Not expressed | |
| PID | At2g34650 | −1.25 | 0.48 | |
| ENHANCER OF PINOID (ENP) | At4g31820 | −2.77 | 0.79 | |
| PGP1 | At2g36910 | 1.28 | 1.25 | |
| PGP19 | At3g28860 | −1.03 | 1.29 | |
| Auxin receptors | TIR1 | At3g62980 | −2.27 | 0.32 |
| AFB1 | At4g03190 | −9.55 | 1.26 | |
| AFB2 | At3g26810 | −1.83 | −0.89 | |
| AFB3 | At1g12820 | −1.44 | 0.20 | |
| ARFs | ARF1 (repressor) | At1g59750 | −2.11 | −0.25 |
| ARF2 (repressor) | At5g62000 | 1.21 | 0.91 | |
| ARF3/ETTIN (repressor) | At2g33860 | −2.02 | −1.43 | |
| ARF4 (repressor) | At5g60450 | −3.88 | 0.29 | |
| ARF5/MP (activator) | At1g19850 | −11.27 | −0.14 | |
| ARF6 (activator) | At1g30330 | −1.62 | 1.15 | |
| ARF7/NPH4 (activator) | At5g20730 | −2.47 | 1.49 | |
| ARF8 (activator) | At5g37020 | −2.36 | 1.44 | |
| ARF9 (repressor) | At4g23980 | −2.41 | 1.09 | |
| ARF10 (repressor) | At2g28350 | 2.24 | 2.63 | |
| ARF11 (repressor) | At2g46530 | 7.24 | −0.82 | |
| ARF13 (repressor) | At1g34170 | Not expressed | Not expressed | |
| ARF16 (repressor) | At4g30080 | 2.27 | −0.14 | |
| ARF17 (repressor) | At1g77850 | 2.36 | 3.78 | |
| ARF18 (repressor) | At3g61830 | −1.56 | 0.83 | |
| AUX/IAAs | IAA10 | At1g04100 | 1.56 | nd wild type |
| IAA11 | At4g28640 | −1.18 | Not expressed | |
| IAA12/BDL | At1g04550 | −1.70 | −0.19 | |
| IAA13/SHY2 | At2g33310 | −1.14 | 0.94 | |
| IAA18 | At1g51950 | −3.38 | 0.6 | |
| IAA19/MSG2 | At3g15540 | 1.20 | 1.95 | |
| IAA30 | At3g62100 | −2.11 | 0.29 | |
RNA sequencing data from dcl1-5 globular stage embryos indicated an overall increase in the levels of transcripts associated with auxin responses (Table II). This might be due to the elevated levels of the auxin receptors or multiple ARFs, some of which are miRNA targets. In agreement with this, the expression of DR5rev:GFP (a reporter used to visualize areas of high auxin activity, or auxin maxima; Friml et al., 2003) expanded from the hypophysis (Fig. 5A) into the center of dcl1-5 embryos (Nodine and Bartel, 2010). We looked at auxin levels and localization in dcl1-15 early embryos (late globular to heart stages) using DR5rev:GFP (n = 8) and the pPIN1:PIN1-GFP translational fusion (n = 16), which at this stage is expressed at the tips of the cotyledons and in the provasculature (Fig. 5F). We found that at these early stages, the auxin maxima had shifted to the center of the embryo, but we did not detect expression in the hypophysis (Fig. 5B). pPIN1:PIN1-GFP expression showed more variability: one-third of the embryos did not express it, while the rest still expressed it in the putative provasculature but also ectopically in the rest of the embryo (Fig. 5G). Auxin transport and response, therefore, are increased and mislocalized in dcl1-15 early embryos.
Figure 5.
Expression of auxin-related reporters. A to E, Expression of DR5rev:GFP. A and B, In transition stage wild-type (WT) embryos, the reporter is expressed in the derivatives of the hypophysis, while expression is displaced apically in the mutant. C to E, In bent cotyledon stage wild-type embryos, DR5rev:GFP is strongly expressed in the root pole, the tip of the cotyledons, the SAM where new primordia will emerge, and at lower levels in the provasculature. In less severe mutants, reporter expression is similar to that in the wild type (D), but in severe mutants, it is reduced to a central dot (arrow) and patchy root pole and epidermal expression (E). F to J, Expression of pPIN1:PIN1-GFP. F and G, In wild-type early heart stage embryos, the reporter is expressed in the tip of the cotyledons and in the provasculature, while in some mutants, it is expressed throughout the embryo. H to J, In wild-type bent cotyledon stage embryos, pPIN1:PIN1-GFP is expressed in the provasculature and in future leaf primordia (H). In less severe mutants, the reporter is still expressed in the putative provasculature (I), while in more severe mutants, it is reduced to a dot in the center (arrow in J). In C to J, magenta represents chlorophyll autofluorescence. dcl1-15 phenotypic classes are as follows: class 2 (D and I) and class 5 (E and J). Bar = 25 µm (A, B, F, and G), 50 µm (E and J), and 100 µm (C, D, H, and I).
In later stages of dcl1-15 development, we saw dramatic changes. By the torpedo stage, the levels of the transcripts of most auxin-related genes have significantly decreased (with the exception of some ARFs, PIN7, TAA1, and YUC10; Table II). Mutant embryos showed a significant reduction in the extent of expression of DR5rev:GFP (n = 56) and pPIN1:PIN1-GFP (n = 67). In wild-type embryos, auxin maxima are localized at the root pole and tips of the cotyledons, and both reporters highlight the provascular system (Fig. 5, C and H). An auxin maximum was still seen at the basal end of the mutant embryos (Fig. 5, D and E). However, expression of both reporters in the putative provasculature, while relatively normal in mild class 2 embryos, was progressively reduced to a dot in the center in the severe class 5 embryos (Fig. 5, D, E, I, and J). We also observed ectopic patchy protodermal expression of DR5rev:GFP in severe embryos (Fig. 5E). Thus, in dcl1-15 embryos, auxin localization and responses change during development: from higher and more widespread levels in early stages to a drastically reduced extent in later stages.
The Apical Domain
The apical domain of the embryo includes the SAM and the cotyledons. The first gene involved in SAM specification, WUSCHEL, is initially detected in the center of the apical region at the 16-cell stage, but the delineation of the SAM and its distinction from the cotyledons is not established until the late globular stage, with the expression of SHOOTMERISTEMLESS (STM) in the former and ASYMMETRIC LEAVES1 in the latter. The positioning of the cotyledons and their proper separation and growth also depend on auxin distribution and the CUC genes, which are regulated by miR164 (for review, see Jenik et al., 2007).
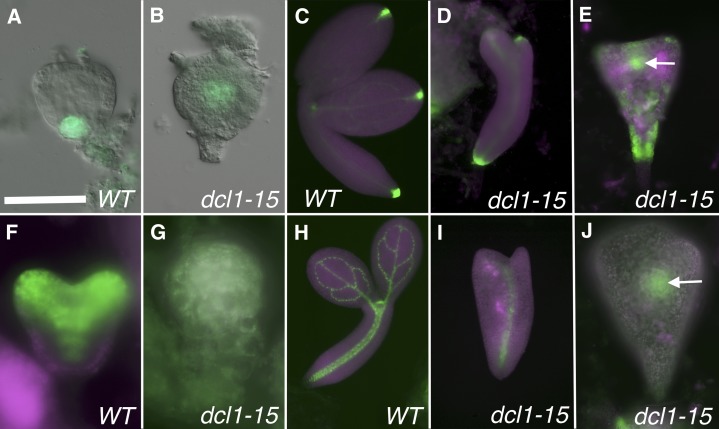
Because most apical domain-related genes are not yet expressed in early globular stage embryos, the high-throughput sequencing data from dcl1-5 were not particularly useful for studying this question (Table I). However, it is clear that in dcl1-15 torpedo stage embryos, all apical genes (with the exception of CUC1) are greatly down-regulated (Table I). dcl1 embryos do not develop an obvious SAM and, except for class 1 dcl1-9/dcl1-15 embryos, which have fused cotyledons (Fig. 2, F and M), they also lack cotyledons (Fig. 2, G–K). To confirm the absence of the SAM, we studied the expression patterns of the reporter pSTM:GUS (Jurkuta et al., 2009; n = 33) and of the enhancer trap M0233, which has been described to report CUC1 expression (Cary et al., 2002; n = 25). Neither of the reporters was expressed in the mutant embryos (classes 2, 4, and 5 observed; Fig. 6, A–D). There is a contradiction that we cannot fully explain between the observed levels of the CUC1 transcript in the microarray and the expression of the enhancer trap for the same gene. We failed to detect, in all mutant classes, reporters normally expressed in the cotyledons: rev-9 enhancer trap (n = 34; see Fig. 8, E–G), phb-6 gene trap (n = 34; Supplemental Fig. S1C), E2023 (n = 59; see Fig. 8, K and L), and pZLL:ZLL-YFP (n = 23; Supplemental Fig. S1, D–F). In summary, it is apparent that the apical region of dcl1 embryos fails to establish SAM and cotyledon fates. The identity of the cotyledon-like outgrowths in class 3 and 4 embryos remains unclear.
Figure 6.
Expression patterns of reporter genes for the meristems. A to D, Reporters for the SAM. E to H, Reporters for the RAM. A and B, Late heart stage embryos expressing pSTM:GUS in the central zone of the SAM in the wild type (WT) but not in the mutant. C and D, Early bent cotyledon stage embryos expressing the enhancer trap M0233 in the boundary between the SAM and the cotyledons in the wild type but not in the mutant. Magenta indicates chlorophyll autofluorescence. E and F, Late heart stage embryos expressing pWOX5:GFP in the QC in the wild type but not in the mutant. G and H, Bent cotyledon stage embryos expressing pPIN4:GUS in the QC and surrounding initials in both the wild type and the mutant. dcl1-15 phenotypic classes are as follows: class 5 (B and F), class 4 (D), and class 2 (H). Bar = 25 µm (A, B, E, and F) and 50 µm (C, D, G, and H).
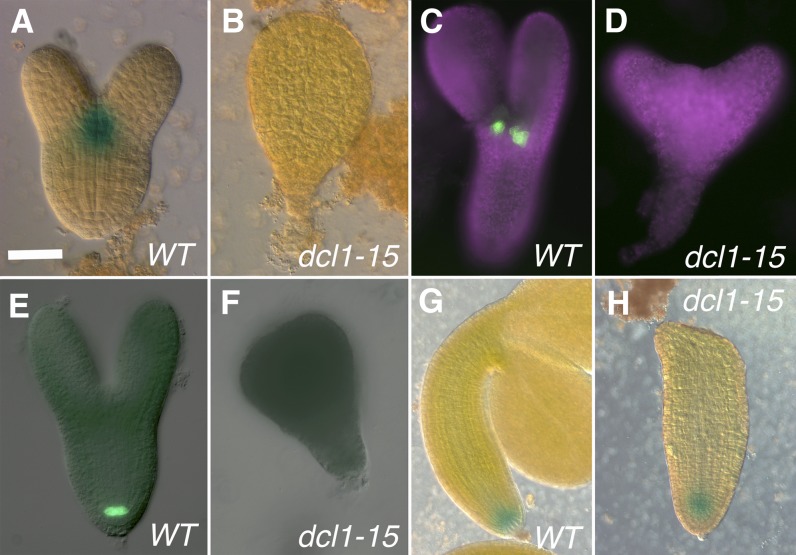
The RAM
The first step in the formation of the RAM is the auxin-dependent specification of the hypophysis during the early globular stage, followed by its division to form a lens-shaped cell, the precursor of the QC. The other initials of the RAM (cortex-endodermis, columella, and lateral root cap-epidermis) are specified between the late globular and late heart stages and maintained as such by signals from the QC. Proper QC positioning and specification depend on the intersection of auxin transport and signaling (mediated by MONOPTEROS [MP], TARGET OF MONOPTEROS7, and the PLETHORA [PLT] genes), cytokinin signaling (mediated by ARABIDOPSIS RESPONSE REGULATOR7 [ARR7] and ARR15), and signals from the stele (mediated by SCARECROW [SCR]; for review, see Jenik et al., 2007; Lau et al., 2012).
As was the case for the apical region, dcl1-5 embryos were analyzed too early by Nodine and Bartel (2010) to measure most of the players in RAM formation. However, the ones that were detectable (ARF16, PIN4, PLT1, PLT2, WUSCHEL-LIKE HOMEOBOX5 [WOX5], and SCR) were down-regulated in the mutant (Table I; Nodine and Bartel, 2010). In dcl1-15 embryos, where microarrays were done at a stage when the entire RAM would have normally been specified, most of the genes involved in RAM specification and function were also expressed at significantly lower levels than in the wild type (Table I). To confirm these data, we studied two QC reporters, pWOX5:GFP (Blilou et al., 2005; Fig. 6E) and QC25 (Sabatini et al., 1999; Supplemental Fig. S1A), a reporter more broadly expressed in the RAM (pPIN4:GUS; Friml et al., 2002; Fig. 6G), and a reporter for the QC and endodermis (pSCR:GFP; Wysocka-Diller et al., 2000; Figs. 7A and 8A). We could not detect the expression of pWOX5:GFP in early stage (up to late heart; n = 16) or late stage (n = 82; classes 2, 3, and 5) mutant embryos (Fig. 6F), with the exception of a very weak signal in two out of 14 class 2 embryos. We observed the same lack of expression in the root pole with QC25 (n = 25; including eight class 2 embryos; Supplemental Fig. S1B). In contrast, a fraction of dcl1-15 embryos did express pPIN4:GUS in its normal location, albeit at reduced levels (23 out of 24 class 2 embryos and 12 out of 54 class 5 embryos; Fig. 6H). Similarly, at the root pole, we saw the expression of pSCR:GFP in most class 2 embryos (n = 12) but not in early embryos (n = 22) or class 3 or 5 embryos (n = 69; Figs. 7B and 8, B–D). All these results suggest that, while severe dcl1 mutant embryos (dcl1-5, dcl1-15, and classes 3–5) fail to establish the QC or a RAM, weaker mutant embryos (dcl1-15 and class 2) are able to specify at least partial RAM identity. This explains the significant postembryonic root growth in the weakest of our classes (dcl1-9/dcl1-15 and class 1; Fig. 2M).
Figure 7.
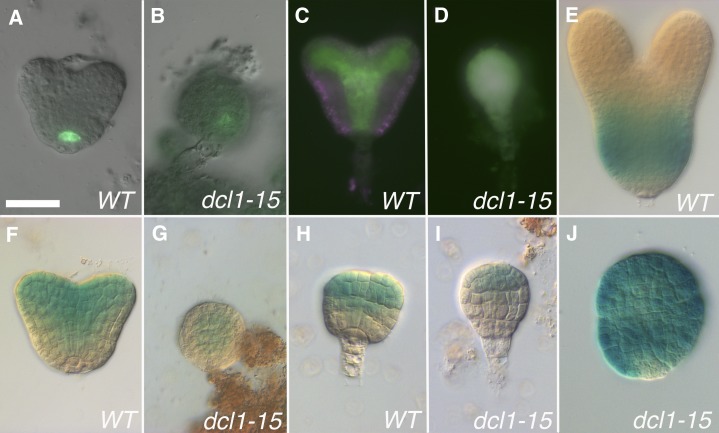
Early expression patterns for reporters for the central-peripheral domains. A to D and F to I, Reporters for the central and adaxial domains. E and J, Reporter for the peripheral domain. A and B, Early heart stage embryos expressing pSCR:GFP in the QC in the wild type (WT) and slightly displaced toward the center in the mutant. C and D, Heart stage embryos expressing pZLL:ZLL-YFP in the provasculature and adaxial side of the cotyledons in the wild type and all over the embryo in the mutant. E and J, Late heart stage embryos expressing pKAN1:GUS in peripheral tissues of the hypocotyl in the wild type and over most of the embryo in the mutant. F and G, Early heart stage embryos with the phb-6 gene trap expressed in the provasculature and adaxial side of the cotyledons in the wild type and reduced to the position of the provasculature in the mutant. H and I, Late globular stage embryos with the rev-9 enhancer trap expressed in the apical and central domains in the wild type and reduced to part of the apical domain in the mutant. Bar = 25 µm for all.
The Central and Peripheral Domains
The embryo axis is organized radially in a concentric series of tissues: provasculature, endodermis, cortex, and protoderm, the first two making up the central domain and the latter two the peripheral domain. The cotyledons arise at the boundary between the central and peripheral domains. There is continuity in gene expression between the embryo axis and the cotyledons, so that central genes are expressed on the adaxial side and peripheral genes on the abaxial side of the organs (Kerstetter et al., 2001; McConnell et al., 2001). The activity of some of the central genes (PHB, PHV, REV, ICU4/CNA, and PNH/ZLL) is also required for proper SAM specification (Lynn et al., 1999; Prigge et al., 2005). As described above, the distinction between protoderm and inner tissues is established during the passage to the 16-cell stage. From the early globular to the transition stage, a series of periclinal divisions generate the different radial tissues (Jürgens and Mayer, 1994). The first genes to be expressed only in the central domain are seen by the midglobular stage, and they are related to auxin transport and response (e.g. PIN1, MP, BDL, and TMO5; Hardtke and Berleth, 1998; Steinmann et al., 1999; Hamann et al., 2002; Kellogg, 2006; Schlereth et al., 2010). The central/peripheral pattern becomes well established around the late globular to early heart stage, when a set of genes (e.g. PHB, REV, and PNH/ZLL) become confined to the central domain (Lynn et al., 1999; McConnell et al., 2001; Emery et al., 2003) and the genes marking the peripheral domain (e.g. KANADI1 [KAN1] and KAN2) are induced (Kerstetter et al., 2001; Eshed et al., 2004). However, the mechanisms that lead to the establishment and maintenance of the central versus peripheral pattern remain unclear.
In dcl1-5 early globular stage embryos, the peripheral genes were not detected (Table I), as expected for this stage. Among the central genes, most of those that showed an increase in transcript levels were miRNA targets (Table I). By the early heart stage, several central genes (PNH/ZLL, SHR, and SCR), analyzed by in situ hybridization or using reporter genes, were not expressed (Nodine and Bartel, 2010), consistent with what we observed in severe dcl1-15 embryos (see below).
We then looked at central and peripheral genes expressed in torpedo stage dcl1-15 embryos, expecting an expansion of central fates in the absence of peripheral fates (Izhaki and Bowman, 2007). However, to our surprise, we noticed that, with very few exceptions, both were significantly down-regulated in our microarray data (Table I), as if the mutant embryos had lost both central and peripheral identities (with the exception of the protoderm; see above).
To explore this phenomenon in more detail, we looked at the expression of reporter genes for several central and peripheral genes. In early embryos (up to the heart or late heart stage), there was no consistent pattern, each gene behaving differently. As described above, the expression of the auxin-related reporter DR5rev:GFP was shifted to a central location (Fig. 5B), while pPIN1:PIN1-GFP was expanded (Fig. 5G). pSCR:GFP, first expressed in the hypophysis and later in the QC and endodermis (Fig. 7A), was not expressed in a majority of early embryos (24 out of 29), and when it was expressed (five out of 29) it was weak and in the center (Fig. 7B). pZLL:ZLL-YFP, normally found in the provasculature (Fig. 7C), was either expressed all over the embryo (six out of nine; Fig. 7D) or not at all (three out of nine). To look at the expression of HD-Zip III genes, we used a gene trap (phb-6; GUS inserted in exon 1) and an enhancer trap (rev-9; GUS inserted in the 5′ untranslated region; Hawker and Bowman, 2004). Neither reporter includes the miRNA-binding site. Therefore, they reflect the activity of the genes’ promoters, and compared with the mRNAs, expression is extended abaxially early (Fig. 7, F and H) but not late (Fig. 7E; Supplemental Fig. S1). In dcl1-15 mutant embryos, phb-6 and rev-9, in contrast to the other central reporters, were expressed in a normal pattern, but more weakly: phb-6 in the provasculature (n = 15; Fig. 7G) and rev-9 apically (n = 13; Fig. 7I). Neither reporter was found in the prospective cotyledon region in the mutants, which do not develop these organs. At the heart stage, the peripheral reporter pKAN1:GUS, which in the wild type is found in the basal region of the hypocotyl (Fig. 7E), was instead expressed all over the mutant embryo (n = 15; Fig. 7J). This ectopic expression might be due to a reduction in the levels of the HD-Zip III gene products (Izhaki and Bowman, 2007). Thus, it appears that in the earlier stages of dcl1-15 development, when the central-peripheral polarity is being established, only some genes are misregulated, and not in a consistent way. This could be interpreted as an inability in the mutant embryos to properly establish radial polarity.
Later on in the development of dcl1-15 embryos (at the stage when we did our microarray analysis and later), there is a consistent reduction in the expression of both central and peripheral reporters. For the central reporters, normally seen in the endodermis (pSCR:GFP; Fig. 8A), provasculature (DR5rev:GFP [Fig. 5C], pPIN1:PIN1-GFP [Fig. 5H], rev-9 [Fig. 8E], phb-6 [Supplemental Fig. S1C], and pZLL:ZLL-YFP [Supplemental Fig. S1D]), and/or the adaxial side of cotyledons (rev-9 [Fig. 8E], phb-6 [Supplemental Fig. S1C], and pZLL:ZLL-YFP [Supplemental Fig. S1D]), expression was absent from the shoot and root meristems but still present in the center of the hypocotyl in the milder phenotypes (class 2; Figs. 5, D and I, and 8, B and F; Supplemental Fig. S1E). As the phenotypes became more severe (class 3), the domain of expression became smaller (Fig. 8C), being reduced to a dot in class 5 embryos (Figs. 5, E and J, and 8, D and G; Supplemental Fig. S1, C and F; number of mutant embryos observed: 62 for pSCR:GFP, 21 for rev-9, 34 for phb-6, and 46 for pZLL:ZLL-YFP). Expression at these later stages was not analyzed in dcl1-5 embryos (Nodine and Bartel, 2010), but, based on what they observed at the early heart stage and the trends in dcl1-15, we hypothesize that these genes are not expressed in these embryos. For the peripheral domain, reporters pKAN1:GUS and E2023 (reflecting KAN2 expression; Gillmor et al., 2010; Fig. 8, H and K) were not expressed in a majority of embryos (48 out of 53 and 33 out of 59 embryos, respectively; Fig. 8I), but in some cases they were present, albeit with reduced expression (five out of 53 and 26 out of 59 embryos, respectively; all classes; Fig. 8, J and L).
The combination of microarray and reporter gene analysis indicates that late stage dcl1 embryos have mostly lost both central and peripheral identities, so that in very severe dcl1-15 embryos only a small region is defined as central, while little if any peripheral fates remain.
teosinte branched/cycloidea/pcf4 Does Not Suppress the Phenotype of dcl1 Embryos
Is there an miRNA target whose ectopic expression or overexpression could account for at least some of the observed dcl1-15 patterning defects? Many of the obvious candidates (HD-Zip IIIs, CUCs) were expressed either at lower levels or in smaller regions in the mutant embryos, particularly later in development (Table I; Figs. 6D and 8G; Supplemental Fig. S1C) or have already been shown to not suppress the dcl1 phenotype (phb-13 phv-11; Grigg et al., 2009). SQUAMOSA PROMOTER-BINDING PROTEIN-LIKE10 (SPL10) and SPL11 are up-regulated early but down-regulated later in mutant embryo development (Table I), and introducing spl10 and spl11 only temporarily relieves the patterning defects (Nodine and Bartel, 2010). We decided that the transcription factor TEOSINTE BRANCHED/CYCLOIDEA/PCF4 (TCP4) was a promising choice. TCP4, a target of miR319, is normally expressed in the cotyledons and leaves, where it controls cell proliferation (Palatnik et al., 2003). Seedlings with ectopically high levels of a TCP4 mRNA resistant to miRNA degradation have phenotypes reminiscent of dcl1-15 embryos (Palatnik et al., 2003), and TCP4 is overexpressed in dcl1-5 (as early as the eight-cell stage; Nodine and Bartel, 2010). We generated teosinte branched/cycloidea/pcf4 (tcp4-2)/tcp4-2 DCL1/dcl1-5 and tcp4-2/tcp4-2 DCL1/dcl1-15 plants (tcp4-2, line GK-363H08, is a null allele; Aggarwal et al., 2010). In self-pollinated plants, in both cases, we observed approximately 25% mutant embryos (n = 400 and 600, respectively). The distribution of embryo phenotypes was not different from that of DCL1/dcl1-5 or DCL1/dcl1-15 plants on their own (data not shown). This suggests that tcp4-2 does not suppress dcl1 and that the phenotypes seen in dcl1 embryos are not due to the effects of TCP4 overexpression. At least two other TCP genes are overexpressed in dcl1-5 (TCP3 and TCP24; Supplemental Table S4), however. Therefore, it is not possible to rule out a contribution of TCPs to the dcl1 phenotype, but higher order mutants are needed to test this hypothesis.
DISCUSSION
In this work, we wanted to explore the role of miRNAs in patterning and tissue specification during embryo development by using three DCL1 mutant alleles, with varying reduction in miRNA levels. Our data complemented and expanded the studies done on null DCL1 mutants (Schwartz et al., 1994; Nodine and Bartel, 2010). Our main findings are that miRNAs are required for the proper patterning of the embryo (i.e. the subdivision of undifferentiated tissues into organized domains) and for tissue specification (the establishment of cell fates), with the notable exception of the protoderm.
Different Regions of the Embryo Require Specific Levels of miRNAs for Proper Specification
Our analysis of the phenotypic series resulting from the variability of the dcl1-15 phenotype and the trans-heterozygous combinations with dcl1-5 and dcl1-9 suggests that there is regional sensitivity to the levels of miRNA. The underlying assumption for this inference is that there is a correlation between the reduction of miRNA levels and phenotypic severity. The protoderm is the only tissue type that is not affected by the absence of miRNAs. Comparing class 5 dcl1-15 and class 6 dcl1-5 embryos, it becomes clear that the first structure to recover when miRNA levels are increased is the suspensor. Next, the hypocotyl/root region proliferates to give rise to a longer embryo (classes 3 and 4). Even higher levels of miRNAs lead to a rescue of the RAM and the radial pattern of the hypocotyl (class 2 dcl1-15, influenced by the ER locus). The structures that require the highest levels of miRNA are the cotyledons and the SAM. Interestingly, this seems to reflect a basal-to-apical gradient of miRNA requirements, with most basal structures (suspensor) having the lowest requirements and most apical structures (SAM and cotyledons) having the highest requirements.
There are several possible reasons for these tissue-specific effects, all speculative at this point. One would be the existence of region-specific differences in the levels of DCL1 or other miRNA pathway factors in wild-type embryos. However, to our knowledge, there are no data to date to support this possibility, except for the broad distribution of AGO1 versus the central expression for AGO10/ZLL/PNH (Lynn et al., 1999). It is also possible that fewer miRNA targets are involved in specifying or maintaining a suspensor versus a SAM. Or perhaps the suspensor-specifying or -maintaining miRNA targets are expressed at lower levels or have a higher affinity for their inhibiting miRNA; thus, a reduction in miRNA levels might have less dramatic effects. The identification of more miRNA targets that contribute to the specification of each tissue will help answer this question.
dcl1 Embryonic Phenotypes Are Not Easily Explained by Known miRNA-Regulated Pathways
The patterning defects in dcl1 embryos likely start before they become visible at the early globular stage, since the expression of a number of miRNA target genes is altered as early as the eight-cell stage (Nodine and Bartel, 2010). Up to about the heart stage, many (but not all) miRNA targets (including transcription factors) are up-regulated (Nodine and Bartel, 2010), resulting in mutant embryos that appear to be confused in terms of patterning. This is seen graphically in the patterns of expression of reporter genes, particularly those having to do with radial and apical identities. Some of them are up, some down, some normal, reflecting an absence of appropriate tissue specification. Later on (or perhaps even at this early stage for dcl1-5), this process results in what essentially is a global absence of pattern or tissue specification, especially in the more severe classes. This includes the reduced expression of genes that normally antagonize each other (e.g. HD-Zip IIIs apically versus PLT basally or HD-Zip IIIs and auxin centrally versus KAN peripherally; Izhaki and Bowman, 2007; Grigg et al., 2009; Smith and Long, 2010), as if in the absence of miRNAs no pathway prevails. This dramatic phenotype is hard to explain in terms of known pathways, because it does not resemble the phenotype of any combination of patterning mutants described so far. For instance, both the KAN (peripheral) and the HD-Zip III (central) genes are significantly reduced in dcl1 embryos, but the kan1 kan2 kan4 phb phv rev sextuple mutant embryos look very different from dcl1 embryos (much more similar to the wild type; Izhaki and Bowman, 2007). It is also not clear whether the pathways required for earlier events in embryogenesis (establishment of pattern) are distinct from those in later stages (maintenance of pattern), making cause-and-effect relationships hard to assess with our data.
The reduced auxin activity in dcl1 embryos due to altered auxin abundance, distribution, and signaling is a promising candidate to explain the defects seen in dcl1 embryos, since auxin flow, signaling, and response are involved in patterning or specifying many tissues throughout the embryo (Möller and Weijers, 2009; Rademacher et al., 2011, 2012; Robert et al., 2013). Auxin activity and transport (as reported by DR5rev:GFP and pPIN1:PIN1-GFP and gene expression levels of other components) are significantly reduced in late-stage mutants. Some of the phenotypes observed in dcl1 embryos, such as early abnormal divisions of the hypophysis, absence of RAM, reduced hypocotyl, and fused or absent cotyledons, are consistent with auxin defects (Möller and Weijers, 2009). But even if reduced auxin can explain a number of the abnormalities observed in the mutants, it is certainly not the only contributor: unlike dcl1 mutants, severe auxin mutants such as gnom still preserve radial patterning (Wolters et al., 2011). And there is a big gap in our understanding of how miRNAs control auxin signaling and response during embryogenesis. It is clear that this regulation involves a significant amount of feedback, because even though the auxin receptors and some ARFs are miRNA targets, they are mostly down-regulated in later stage mutants.
The one tissue where we can guess the causative mechanism is the suspensor. It has recently been shown that the specification of suspensor identity and/or its maintenance, and the exclusion of embryo proper fates from it, is cell-autonomously determined by auxin. Embryos that do not synthesize auxin, or that express degradation-resistant versions of IAA proteins, have suspensors that overproliferate and show partial embryo proper fate. IAA10, specifically, is expressed in the suspensor and has been proposed to mediate this auxin response (Rademacher et al., 2012). The IAA10 transcript is up-regulated in dcl1-5 embryos, where the suspensor becomes embryo like, but not in dcl1-15, where the suspensor is almost normal (Table II). It is tempting to speculate that this difference in IAA10 expression is the cause of the suspensor abnormalities, which would suggest that IAA10 expression is regulated by an miRNA target gene. Further experiments, such as the generation of dcl1-5 iaa10 double mutants, are required to test this hypothesis.
The Precocious Maturation and Abnormal Patterning and Specification Phenotypes Are Independent
dcl1 embryos have two distinct phenotypes. On the one hand, they mature too early, indicated by changes in chloroplast morphology, accumulation of storage products, and a transcriptional profile that is characteristic of older embryos (Nodine and Bartel, 2010; Willmann et al., 2011). On the other hand, they have very abnormal division patterns and altered tissue patterning and specification (Schwartz et al., 1994; Nodine and Bartel, 2010; this work). Both phenotypes have the same age of onset (eight-cell stage at the transcript level, early globular stage microscopically). It is possible that these two phenotypes are causally connected. In particular, early maturation could lead to morphological abnormalities and embryo arrest, as hypothesized by Nodine and Bartel (2010). Our evaluation of the evidence strongly suggests that the phenotypes are independent of each other, however. The argument is 3-fold. First, all abnormal dcl1 embryos mature precociously, regardless of the severity of the terminal phenotype, from dcl1-9/dcl1-15 class 1 to dcl1-5 class 6 (Willmann et al., 2011). Second, dcl1-15 lec2-1 and dcl1-15 fus3-3 embryos do not display the early maturation phenotype, but their morphological defects are as severe as those of dcl1-15 (or even worse in the case of dcl1-15 fus3-3; Willmann et al., 2011). Finally, there are other mutant embryos that mature early (although their phenotypes are weaker than dcl1) and whose appearance is either mildly altered (miRNA-resistant SPL10 or SPL11; Nodine and Bartel, 2010) or unaffected (asil1-1 and asil2-1 single mutants or asil1-1 sil1-1 and asil2-1 sil1-1 double mutants; Gao et al., 2011; Willmann et al., 2011). The preponderance of evidence thus leads us to propose that both phenotypes have the same root cause (reduction in miRNA levels) but are genetically separable and do not influence each other. This hypothesis can only be conclusively proven if separate, independent miRNA targets can be shown to control each of these phenotypes.
miRNAs Are Required for Tissue Specification, Not for the Maintenance of an Undifferentiated, Pluripotential State
At the end of their analysis of null dcl1 mutant embryos, Nodine and Bartel (2010) proposed that the role of miRNAs during embryogenesis is to repress differentiation-promoting transcription factors (like SPL10, SPL11, and others) to maintain the potential of preglobular cells to generate diverse cell types at the subsequent globular stages and later. Therefore, the loss of miRNAs leads to the premature expression of miRNA targets, resulting in precocious differentiation and consequent loss of developmental potential.
Our data (Willmann et al., 2011; this work) suggest that there is a distinction between the effects of miRNAs on patterning and the specification of cell fates and on embryonic maturation. While patterning and cell fate specification are processes expected to be found in the embryos of all multicellular organisms, the process of maturation (accumulation of storage products [seed filling], desiccation tolerance, and dormancy) is an innovation of seed plants and imposes a pause between embryonic and vegetative development (Steeves, 1983). This pause is not required for the viability of the plant, since it can be skipped without detrimental consequences (e.g. in viviparous mutants; Meinke et al., 1994). In agreement with the hypothesis of Nodine and Bartel (2010), we found that miRNAs prevent the precocious initiation of the maturation program (at least in terms of seed filling; Willmann et al., 2011). However, our analysis suggests that, instead of promoting an undifferentiated state, miRNAs are required for correct cell and tissue type specification. When miRNAs are reduced or absent, early embryos seem confused, with genes involved in patterning expressed in ways that are not consistent with each other. Later embryos are unable to generate most elements of pattern, instead of specifying them too early. These effects are dose dependent, and as discussed above, there is a region-specific sensitivity to the loss of miRNAs.
The big challenge will be to define how many and which miRNA targets are responsible for directing embryonic patterning. The handful that have been tested either cannot suppress the dcl1 phenotypes (phb-13 phv-11 and tcp4-2; Grigg et al., 2009; this work) or suppress them temporarily (spl11-1 spl10-RNAi; Nodine and Bartel, 2010). It is very likely that there is no miRNA target that is a master regulator of patterning (although SPL10 and SPL11 may have important roles in early embryo development) and that the actions of a number of targets contribute to the dramatic defects of dcl1 embryos.
MATERIALS AND METHODS
Plant Material and Growth
Arabidopsis (Arabidopsis thaliana) plants were grown in a greenhouse in MetroMix 360 soil (SunGro Horticulture) at 20°C to 24°C with 16 h of light per day. For growth on tissue culture plates or transgenic selection, seeds were surface sterilized and grown on sterile medium containing 4.4 g L−1 MS salts, 1× Gamborg’s vitamins, and 0.5 g L−1 MES (all from Sigma), 10 g L−1 Suc (Fisher Scientific), and 7.5 g L−1 tissue culture agar (Carolina Biological), pH 5.7, plus the appropriate selective agent. All mutants and reporter lines used have been described before: dcl1-5 (Schauer et al., 2002), dcl1-7 (Lang et al., 1994), dcl1-9 (Jacobsen et al., 1999), dcl1-15 (Willmann et al., 2011), tcp4-2 (Aggarwal et al., 2010), pSUC3:GFP (Meyer et al., 2004), pAtML1:H2B-YFP (Roeder et al., 2010), pSTM:GUS (Jurkuta et al., 2009), M0233 (Cary et al., 2002), QC25 (Sabatini et al., 1999), pPIN4:GUS (Friml et al., 2002), pWOX5:GFP (Blilou et al., 2005), pSCR:GFP (Wysocka-Diller et al., 2000), pZLL:ZLL-YFP (Tucker et al., 2008), E2023 and pKAN1:GUS (Gillmor et al., 2010), rev-9 and phb-6 (Hawker and Bowman, 2004), DR5rev:GFP (Friml et al., 2003), and pPIN1:PIN1-GFP (Gordon et al., 2007). The following seeds were obtained from the Arabidopsis Biological Resource Center (ABRC): dcl1-5 (CS16069), dcl1-7 ER (CS6953), dcl1-7 er (CS3089), dcl1-9 (CS3828), La-O (CS1298), and M0233 (CS9336). The following lines were generously donated by other researchers: tcp4-2 (U. Nath), pAtML1:H2B-YFP, pWOX5:GFP, and pSUC3:GFP (W. Lukowitz), pSCR:GFP and pPIN1:PIN1-GFP (J. Long), pPIN4:GUS (S. Woody), DR5rev:GFP (J. Friml), QC25 (B. Scheres), rev-9 and phb-6 (J. Bowman), pZLL:ZLL-YFP (T. Laux), and E2023 and pKAN1:GUS (S. Gillmor).
The dcl1-15 mutation was isolated in a mixed Ws/Ler background (Willmann et al., 2011). Before analysis, the mutant was outcrossed four times to Ler. The resulting plant line was homozygous for er and was presumed to be mostly Ler, although the region of the genome surrounding dcl1-15 (top of chromosome I) was Ws, based on polymorphisms found while mapping and sequencing the mutant allele (data not shown). The mutation in the dcl1-15 allele changes a conserved Gly to Glu in one of two active sites of the RNase IIIb domain (position 1,692; Willmann et al., 2011), presumably affecting catalysis and significantly decreasing the production of miRNAs (Du et al., 2008). Plants containing dcl1-5 or dcl1-15 were genotyped by dissecting siliques, while those containing dcl1-9 were selected on MS-kanamycin plates. dcl1-15 seeds have been deposited at the ABRC (stock no. CS68113).
Optical Microscopy and Histochemistry
The dissection, clearing, and staining of seeds for morphological and reporter analyses were performed as described previously (Willmann et al., 2011). All microscopy was done on a Leica DMRB microscope (Leica Microsystems). For observation of GFP and chlorophyll fluorescence, the excitation/emission wavelengths were 480/535 nm and 560/645 nm, respectively. Images were acquired with either a ProgRes MFcool or a ProgRes C5 camera (Jenoptik). Images taken on different channels were merged with the ProgRes software. Image contrast and brightness adjustment and the assembly of figures were done with Adobe Photoshop CS5 (Adobe Systems).
Microarray Analyses
The microarray experiment was described in detail in our previous article (Willmann et al., 2011). Very briefly, 300 torpedo stage mutant embryos from siliques of DCL1/dcl1- 15 plants and 300 wild-type torpedo stage embryos from DCL1/DCL1 siblings were collected and ground in liquid nitrogen. RNA was extracted from the tissue using the RNeasy Micro kit (Qiagen) with minor modifications. Biotin-labeled complementary DNA targets were then generated for hybridization to Affymetrix Arabidopsis ATH1 microarrays at the University of Pennsylvania Core Facility. The microarrays were gcRMA (for gene chip Robust Multiarray Averaging) normalized in R, filtered using MAS5.0 presence/absence calls to remove any probe sets not expressed in at least one sample, and the probe sets were tested for differential expression in R using Limma with a Benjamini-Hochberg Multiple Test Correction ≤ 0.05.
Raw data from this article were deposited in the Gene Expression Omnibus database under accession number GSE24887. The data from the RNA sequencing experiment (Nodine and Bartel, 2010) were downloaded from the Gene Expression Omnibus (accession no. GSE25404).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Expression of other reporter genes mentioned in the text.
Supplemental Table S1. Comparison of miRNA targets expressed in both dcl1-5 and dcl1-15 embryos.
Supplemental Table S2. Expression of all miRNA targets in dcl1-15 torpedo stage embryos.
Supplemental Table S3. Expression of miRNA targets shared by dcl1-5 and dcl1-15 embryos.
Supplemental Table S4. Expression of transcription factors that are miRNA targets in dcl1-5 and dcl1-15 embryos.
Supplementary Material
Acknowledgments
We thank all the colleagues (listed in “Materials and Methods”) who donated seeds, either directly or to the ABRC; Jim Engleman (Franklin and Marshall College) for plant care; and Wolfgang Lukowitz (University of Georgia) and Juan José Ripoll (University of California, San Diego) for thoughtful comments on the article.
Glossary
- SAM
shoot apical meristem
- RAM
root apical meristem
- QC
quiescent center
- miRNA
microRNA
- Ws
Wassilewskija
- Ler
Landsberg erecta
- La-O
Landbserg ERECTA
- MS
Murashige and Skoog
- ABRC
Arabidopsis Biological Resource Center
Footnotes
This work was supported by Franklin and Marshall College, the National Institutes of Health (National Research Service Award postdoctoral fellowship to M.R.W.), and the Howard Hughes Medical Institute’s Undergraduate Science Education Program (award to Franklin and Marshall College).
The online version of this article contains Web-only data.
Articles can be viewed online without a subscription.
References
- Abe M, Katsumata H, Komeda Y, Takahashi T. (2003) Regulation of shoot epidermal cell differentiation by a pair of homeodomain proteins in Arabidopsis. Development 130: 635–643 [DOI] [PubMed] [Google Scholar]
- Aggarwal P, Das Gupta M, Joseph AP, Chatterjee N, Srinivasan N, Nath U. (2010) Identification of specific DNA binding residues in the TCP family of transcription factors in Arabidopsis. Plant Cell 22: 1174–1189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aida M, Ishida T, Tasaka M. (1999) Shoot apical meristem and cotyledon formation during Arabidopsis embryogenesis: interaction among the CUP-SHAPED COTYLEDON and SHOOT MERISTEMLESS genes. Development 126: 1563–1570 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433: 39–44 [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Stone SL, Park S, Bui AQ, Le BH, Fischer RL, Goldberg RB, Harada JJ. (2006) Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci USA 103: 3468–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P, Sakvarelidze-Achard L, Bruun-Rasmussen M, Dunoyer P, Yamamoto YY, Sieburth LE, Voinnet O. (2008) Widespread translational inhibition by plant miRNAs and siRNAs. Science 320: 1185–1190 [DOI] [PubMed] [Google Scholar]
- Byrne ME, Barley R, Curtis M, Arroyo JM, Dunham M, Hudson A, Martienssen RA. (2000) Asymmetric leaves1 mediates leaf patterning and stem cell function in Arabidopsis. Nature 408: 967–971 [DOI] [PubMed] [Google Scholar]
- Cary AJ, Che P, Howell SH. (2002) Developmental events and shoot apical meristem gene expression patterns during shoot development in Arabidopsis thaliana. Plant J 32: 867–877 [DOI] [PubMed] [Google Scholar]
- Chen X, Liu J, Cheng Y, Jia D. (2002) HEN1 functions pleiotropically in Arabidopsis development and acts in C function in the flower. Development 129: 1085–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y. (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19: 2430–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M. (2005) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9: 109–119 [DOI] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B. (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119: 71–84 [DOI] [PubMed] [Google Scholar]
- Du Z, Lee JK, Tjhen R, Stroud RM, James TL. (2008) Structural and biochemical insights into the dicing mechanism of mouse Dicer: a conserved lysine is critical for dsRNA cleavage. Proc Natl Acad Sci USA 105: 2391–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery JF, Floyd SK, Alvarez J, Eshed Y, Hawker NP, Izhaki A, Baum SF, Bowman JL. (2003) Radial patterning of Arabidopsis shoots by class III HD-ZIP and KANADI genes. Curr Biol 13: 1768–1774 [DOI] [PubMed] [Google Scholar]
- Eshed Y, Izhaki A, Baum SF, Floyd SK, Bowman JL. (2004) Asymmetric leaf development and blade expansion in Arabidopsis are mediated by KANADI and YABBY activities. Development 131: 2997–3006 [DOI] [PubMed] [Google Scholar]
- Fang Y, Spector DL. (2007) Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr Biol 17: 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friml J, Benková E, Blilou I, Wisniewska J, Hamann T, Ljung K, Woody S, Sandberg G, Scheres B, Jürgens G, et al. (2002) AtPIN4 mediates sink-driven auxin gradients and root patterning in Arabidopsis. Cell 108: 661–673 [DOI] [PubMed] [Google Scholar]
- Friml J, Vieten A, Sauer M, Weijers D, Schwarz H, Hamann T, Offringa R, Jürgens G. (2003) Efflux-dependent auxin gradients establish the apical-basal axis of Arabidopsis. Nature 426: 147–153 [DOI] [PubMed] [Google Scholar]
- Gao MJ, Li X, Lui H, Gropp GM, Lydiate DD, Wei S, Hegedus DD. (2011) ASIL1 is required for proper timing of seed filling in Arabidopsis. Plant Signal Behav 6: 1886–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillmor CS, Park MY, Smith MR, Pepitone R, Kerstetter RA, Poethig RS. (2010) The MED12-MED13 module of Mediator regulates the timing of embryo patterning in Arabidopsis. Development 137: 113–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon SP, Heisler MG, Reddy GV, Ohno C, Das P, Meyerowitz EM. (2007) Pattern formation during de novo assembly of the Arabidopsis shoot meristem. Development 134: 3539–3548 [DOI] [PubMed] [Google Scholar]
- Grigg SP, Galinha C, Kornet N, Canales C, Scheres B, Tsiantis M. (2009) Repression of apical homeobox genes is required for embryonic root development in Arabidopsis. Curr Biol 19: 1485–1490 [DOI] [PubMed] [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T. (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- Hamann T, Benkova E, Bäurle I, Kientz M, Jürgens G. (2002) The Arabidopsis BODENLOS gene encodes an auxin response protein inhibiting MONOPTEROS-mediated embryo patterning. Genes Dev 16: 1610–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardtke CS, Berleth T. (1998) The Arabidopsis gene MONOPTEROS encodes a transcription factor mediating embryo axis formation and vascular development. EMBO J 17: 1405–1411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker NP, Bowman JL. (2004) Roles for class III HD-Zip and KANADI genes in Arabidopsis root development. Plant Physiol 135: 2261–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izhaki A, Bowman JL. (2007) KANADI and class III HD-Zip gene families regulate embryo patterning and modulate auxin flow during embryogenesis in Arabidopsis. Plant Cell 19: 495–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SE, Running MP, Meyerowitz EM. (1999) Disruption of an RNA helicase/RNAse III gene in Arabidopsis causes unregulated cell division in floral meristems. Development 126: 5231–5243 [DOI] [PubMed] [Google Scholar]
- Jenik PD, Gillmor CS, Lukowitz W. (2007) Embryonic patterning in Arabidopsis thaliana. Annu Rev Cell Dev Biol 23: 207–236 [DOI] [PubMed] [Google Scholar]
- Jürgens G, Mayer U (1994) Arabidopsis. In J Bard, ed, Embryos: Color Atlas of Development. Wolfe Publishing, London, pp 7–21 [Google Scholar]
- Jurkuta RJ, Kaplinsky NJ, Spindel JE, Barton MK. (2009) Partitioning the apical domain of the Arabidopsis embryo requires the BOBBER1 NudC domain protein. Plant Cell 21: 1957–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima T, Goldberg RB. (2010) The suspensor: not just suspending the embryo. Trends Plant Sci 15: 23–30 [DOI] [PubMed] [Google Scholar]
- Kellogg EA. (2006) Progress and challenges in studies of the evolution of development. J Exp Bot 57: 3505–3516 [DOI] [PubMed] [Google Scholar]
- Kerstetter RA, Bollman K, Taylor RA, Bomblies K, Poethig RS. (2001) KANADI regulates organ polarity in Arabidopsis. Nature 411: 706–709 [DOI] [PubMed] [Google Scholar]
- Kim I, Kobayashi K, Cho E, Zambryski PC. (2005) Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc Natl Acad Sci USA 102: 11945–11950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauer S, Holt AL, Rubio-Somoza I, Tucker EJ, Hinze A, Pisch M, Javelle M, Timmermans MC, Tucker MR, Laux T. (2013) A protodermal miR394 signal defines a region of stem cell competence in the Arabidopsis shoot meristem. Dev Cell 24: 125–132 [DOI] [PubMed] [Google Scholar]
- Kurihara Y, Kaminuma E, Matsui A, Kawashima M, Tanaka M, Morosawa T, Ishida J, Mochizuki Y, Shinozaki K, Toyoda T, et al. (2009) Transcriptome analyses revealed diverse expression changes in ago1 and hyl1 Arabidopsis mutants. Plant Cell Physiol 50: 1715–1720 [DOI] [PubMed] [Google Scholar]
- Lang JD, Ray S, Ray A. (1994) sin 1, a mutation affecting female fertility in Arabidopsis, interacts with mod 1, its recessive modifier. Genetics 137: 1101–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau S, Slane D, Herud O, Kong J, Jürgens G. (2012) Early embryogenesis in flowering plants: setting up the basic body pattern. Annu Rev Plant Biol 63: 483–506 [DOI] [PubMed] [Google Scholar]
- Laufs P, Peaucelle A, Morin H, Traas J. (2004) MicroRNA regulation of the CUC genes is required for boundary size control in Arabidopsis meristems. Development 131: 4311–4322 [DOI] [PubMed] [Google Scholar]
- Lobbes D, Rallapalli G, Schmidt DD, Martin C, Clarke J. (2006) SERRATE: a new player on the plant microRNA scene. EMBO Rep 7: 1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Fedoroff N. (2000) A mutation in the Arabidopsis HYL1 gene encoding a dsRNA binding protein affects responses to abscisic acid, auxin, and cytokinin. Plant Cell 12: 2351–2366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukowitz W, Roeder A, Parmenter D, Somerville C. (2004) A MAPKK kinase gene regulates extra-embryonic cell fate in Arabidopsis. Cell 116: 109–119 [DOI] [PubMed] [Google Scholar]
- Lynn K, Fernandez A, Aida M, Sedbrook J, Tasaka M, Masson P, Barton MK. (1999) The PINHEAD/ZWILLE gene acts pleiotropically in Arabidopsis development and has overlapping functions with the ARGONAUTE1 gene. Development 126: 469–481 [DOI] [PubMed] [Google Scholar]
- Mallory AC, Dugas DV, Bartel DP, Bartel B. (2004) MicroRNA regulation of NAC-domain targets is required for proper formation and separation of adjacent embryonic, vegetative, and floral organs. Curr Biol 14: 1035–1046 [DOI] [PubMed] [Google Scholar]
- Mansfield SG, Briarty LG. (1991) Early embryogenesis in Arabidopsis thaliana. II. The developing embryo. Can J Bot 69: 461–476 [Google Scholar]
- Mardsen MPF, Meinke DW. (1985) Abnormal development of the suspensor in an embryo-lethal mutant of Arabidopsis thaliana. Am J Bot 72: 1801–1812 [Google Scholar]
- McConnell JR, Emery J, Eshed Y, Bao N, Bowman JL, Barton MK. (2001) Role of PHABULOSA and PHAVOLUTA in determining radial patterning in shoots. Nature 411: 709–713 [DOI] [PubMed] [Google Scholar]
- Meinke DW, Franzmann LH, Nickle TC, Yeung EC. (1994) leafy cotyledon mutants of Arabidopsis. Plant Cell 6: 1049–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Lauterbach C, Niedermeier M, Barth I, Sjolund RD, Sauer N. (2004) Wounding enhances expression of AtSUC3, a sucrose transporter from Arabidopsis sieve elements and sink tissues. Plant Physiol 134: 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashima S, Honda M, Hashimoto K, Tatematsu K, Hashimoto T, Sato-Nara K, Okada K, Nakajima K. (2013) A comprehensive expression analysis of the Arabidopsis MICRORNA165/6 gene family during embryogenesis reveals a conserved role in meristem specification and a non-cell-autonomous function. Plant Cell Physiol 54: 375–384 [DOI] [PubMed] [Google Scholar]
- Möller B, Weijers D. (2009) Auxin control of embryo patterning. Cold Spring Harb Perspect Biol 1: a001545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mravec J, Kubeš M, Bielach A, Gaykova V, Petrásek J, Skůpa P, Chand S, Benková E, Zazímalová E, Friml J. (2008) Interaction of PIN and PGP transport mechanisms in auxin distribution-dependent development. Development 135: 3345–3354 [DOI] [PubMed] [Google Scholar]
- Muto H, Watahiki MK, Nakamoto D, Kinjo M, Yamamoto KT. (2007) Specificity and similarity of functions of the Aux/IAA genes in auxin signaling of Arabidopsis revealed by promoter-exchange experiments among MSG2/IAA19, AXR2/IAA7, and SLR/IAA14. Plant Physiol 144: 187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodine MD, Bartel DP. (2010) MicroRNAs prevent precocious gene expression and enable pattern formation during plant embryogenesis. Genes Dev 24: 2678–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D. (2003) Control of leaf morphogenesis by microRNAs. Nature 425: 257–263 [DOI] [PubMed] [Google Scholar]
- Pasquinelli AE. (2012) MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nat Rev Genet 13: 271–282 [DOI] [PubMed] [Google Scholar]
- Ploense SE, Wu MF, Nagpal P, Reed JW. (2009) A gain-of-function mutation in IAA18 alters Arabidopsis embryonic apical patterning. Development 136: 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge MJ, Otsuga D, Alonso JM, Ecker JR, Drews GN, Clark SE. (2005) Class III homeodomain-leucine zipper gene family members have overlapping, antagonistic, and distinct roles in Arabidopsis development. Plant Cell 17: 61–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rademacher EH, Lokerse AS, Schlereth A, Llavata-Peris CI, Bayer M, Kientz M, Freire Rios A, Borst JW, Lukowitz W, Jürgens G, et al. (2012) Different auxin response machineries control distinct cell fates in the early plant embryo. Dev Cell 22: 211–222 [DOI] [PubMed] [Google Scholar]
- Rademacher EH, Möller B, Lokerse AS, Llavata-Peris CI, van den Berg W, Weijers D. (2011) A cellular expression map of the Arabidopsis AUXIN RESPONSE FACTOR gene family. Plant J 68: 597–606 [DOI] [PubMed] [Google Scholar]
- Ray S, Golden T, Ray A. (1996) Maternal effects of the short integument mutation on embryo development in Arabidopsis. Dev Biol 180: 365–369 [DOI] [PubMed] [Google Scholar]
- Raz V, Bergervoet JHW, Koornneef M. (2001) Sequential steps for developmental arrest in Arabidopsis seeds. Development 128: 243–252 [DOI] [PubMed] [Google Scholar]
- Reinhart BJ, Weinstein EG, Rhoades MW, Bartel B, Bartel DP. (2002) MicroRNAs in plants. Genes Dev 16: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington DL, Vision TJ, Guilfoyle TJ, Reed JW. (2004) Contrasting modes of diversification in the Aux/IAA and ARF gene families. Plant Physiol 135: 1738–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert HS, Grones P, Stepanova AN, Robles LM, Lokerse AS, Alonso JM, Weijers D, Friml J. (2013) Local auxin sources orient the apical-basal axis in Arabidopsis embryos. Curr Biol 23: 2506–2512 [DOI] [PubMed] [Google Scholar]
- Roeder AHK, Chickarmane V, Cunha A, Obara B, Manjunath BS, Meyerowitz EM. (2010) Variability in the control of cell division underlies sepal epidermal patterning in Arabidopsis thaliana. PLoS Biol 8: e1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronemus MJ, Vaughn MW, Martienssen RA. (2006) MicroRNA-targeted and small interfering RNA-mediated mRNA degradation is regulated by argonaute, dicer, and RNA-dependent RNA polymerase in Arabidopsis. Plant Cell 18: 1559–1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini S, Beis D, Wolkenfelt H, Murfett J, Guilfoyle T, Malamy JE, Benfey PN, Leyser O, Bechtold N, Weisbeek P, et al. (1999) An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell 99: 463–472 [DOI] [PubMed] [Google Scholar]
- Sabatini S, Heidstra R, Wildwater M, Scheres B. (2003) SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev 17: 354–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer SE, Jacobsen SE, Meinke DW, Ray A. (2002) DICER-LIKE1: blind men and elephants in Arabidopsis development. Trends Plant Sci 7: 487–491 [DOI] [PubMed] [Google Scholar]
- Schlereth A, Möller B, Liu W, Kientz M, Flipse J, Rademacher EH, Schmid M, Jürgens G, Weijers D. (2010) MONOPTEROS controls embryonic root initiation by regulating a mobile transcription factor. Nature 464: 913–916 [DOI] [PubMed] [Google Scholar]
- Schwartz BW, Yeung EC, Meinke DW. (1994) Disruption of morphogenesis and transformation of the suspensor in abnormal suspensor mutants of Arabidopsis. Development 120: 3235–3245 [DOI] [PubMed] [Google Scholar]
- Sessions A, Weigel D, Yanofsky MF. (1999) The Arabidopsis thaliana MERISTEM LAYER 1 promoter specifies epidermal expression in meristems and young primordia. Plant J 20: 259–263 [DOI] [PubMed] [Google Scholar]
- Shpak ED. (2013) Diverse roles of ERECTA family genes in plant development. J Integr Plant Biol 55: 1238–1250 [DOI] [PubMed] [Google Scholar]
- Smith ZR, Long JA. (2010) Control of Arabidopsis apical-basal embryo polarity by antagonistic transcription factors. Nature 464: 423–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeves TA. (1983) The evolution and biological significance of seeds. Can J Bot 61: 3550–3560 [Google Scholar]
- Steinmann T, Geldner N, Grebe M, Mangold S, Jackson CL, Paris S, Gälweiler L, Palme K, Jürgens G. (1999) Coordinated polar localization of auxin efflux carrier PIN1 by GNOM ARF GEF. Science 286: 316–318 [DOI] [PubMed] [Google Scholar]
- Tiwari SB, Hagen G, Guilfoyle T. (2003) The roles of auxin response factor domains in auxin-responsive transcription. Plant Cell 15: 533–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Ruiz RA, Jürgens G. (1994) Mutations in the FASS gene uncouple pattern formation and morphogenesis in Arabidopsis development. Development 120: 2967–2978 [DOI] [PubMed] [Google Scholar]
- Tucker MR, Hinze A, Tucker EJ, Takada S, Jürgens G, Laux T. (2008) Vascular signalling mediated by ZWILLE potentiates WUSCHEL function during shoot meristem stem cell development in the Arabidopsis embryo. Development 135: 2839–2843 [DOI] [PubMed] [Google Scholar]
- Voinnet O. (2009) Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687 [DOI] [PubMed] [Google Scholar]
- Wang JW, Wang LJ, Mao YB, Cai WJ, Xue HW, Chen XY. (2005) Control of root cap formation by microRNA-targeted auxin response factors in Arabidopsis. Plant Cell 17: 2204–2216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijers D, Benkova E, Jäger KE, Schlereth A, Hamann T, Kientz M, Wilmoth JC, Reed JW, Jürgens G. (2005) Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. EMBO J 24: 1874–1885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmann MR, Mehalick AJ, Packer RL, Jenik PD. (2011) MicroRNAs regulate the timing of embryo maturation in Arabidopsis. Plant Physiol 155: 1871–1884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters H, Anders N, Geldner N, Gavidia R, Jürgens G. (2011) Coordination of apical and basal embryo development revealed by tissue-specific GNOM functions. Development 138: 117–126 [DOI] [PubMed] [Google Scholar]
- Wu G. (2013) Plant microRNAs and development. J Genet Genomics 40: 217–230 [DOI] [PubMed] [Google Scholar]
- Wysocka-Diller JW, Helariutta Y, Fukaki H, Malamy JE, Benfey PN. (2000) Molecular analysis of SCARECROW function reveals a radial patterning mechanism common to root and shoot. Development 127: 595–603 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.