INTRODUCTION
The biological function of proteins is directly linked to their specific three-dimensional structures; cells have several mechanisms to guarantee the structural fidelity of their proteins. Normally, incorrectly folded proteins trigger complex biological responses such as the heat shock and the unfolded protein response that assist with protein folding or protein degradation. However, overloading or failure of these systems can cause the absence of a particular protein function or overabundance and aggregation of misfolded proteins, resulting in protein misfolding disorders. In the examples of these disorders where there is a loss-of-function, the proteins are unable to fold properly, and are usually eliminated through ubiquitination and degradation in the proteasome. Loss-of-function disorders include Cystic Fibrosis (Cystic Fibrosis Transmembrane Conductance Regulator protein (CFTR)), Marfan syndrome (Fibrillin), Lysosomal storage diseases (Glucocerebrosidase), and certain types of cancer (tumor suppressor p53). The gain-of-toxic function protein misfolding diseases are characterized by the accumulation and aggregation of non-functional and toxic protein that damages cells and tissues; they present with a wide variety of phenotypes depending on the precursor protein affected by misfolding and the affected cells and tissues.
THE AMYLOIDOSES
The most well-known (although not common in all cases) protein misfolding diseases are the amyloid diseases or amyloidoses in which the pathogenic protein misfolds and ultimately aggregates as insoluble amyloid fibrils, most commonly in the extracellular space. These are highly heterogeneous diseases where a combination of biochemical, and/or environmental factors allows the normally soluble protein to sample partially folded states leading to self-association and amyloid formation in which the proteins adopt cross β-sheet structures. Amyloidoses are very complicated diseases to study and diagnose because they often involve different organ systems (in the case of the systemic diseases) and indistinct symptoms that can be easily misdiagnosed. In recent years, better understanding of their pathogenesis and molecular mechanisms has led to important advancements in diagnosis and treatment with improved prognosis for many amyloidosis patients, but there are many aspects of these diseases that still remain poorly understood.
During the past three decades, great efforts have been applied to unraveling the in vitro principles behind amyloid diseases (in vitro amyloid formation) with the long term goal of developing therapies. In vitro amyloid fibril formation reactions are generally defined as a nucleated self-assembly process that is favorable under destabilizing conditions that promote the formation and/or accumulation of non-native folding intermediates (in most cases partially unfolded states) prone to amyloid aggregation as will be discussed below. These self-polymerization reactions are characterized by three phases. The first phase is a nucleation or “lag” phase in which non-native protein conformations slowly oligomerize to form the amyloid fibril nucleus. Once a critical concentration of fibril nucleus is reached, the reaction reaches the “elongation” phase, in which it is thought that the fibril nucleus is able to either interact with native proteins and incorporate them into the fibril or grow by sequential incorporation of more non-native protein conformations. This phase proceeds until the reaction reaches a steady-state or “plateau” phase, in which no further polymerization occurs.
Human amyloid diseases are a heterogeneous group of pathologies that can be derived from 27 different proteins including β2-microglobulin (Aβ2m) in dialysis-related amyloidosis; transthyretin (ATTR) in familial amyloidosis, amyloid β peptide (Aβ) in Alzheimer's disease, and immunoglobulin light chain (AL) in light chain amyloidosis (1). Despite the differences in size, tertiary, quaternary structure, and function of their precursor proteins, the amyloid fibrils formed by all these proteins share a common morphology, adopt a cross-β structure, and form protofilaments (2-5 nm in diameter) which can either coil together or adhere laterally to form fibrils (2) (Figure 1). Amyloid fibrils show a distinctive X-ray diffraction pattern, with distinct intensities at 4.7 and 10 Å corresponding to the intra-strand and stacking distances of the β-sheets, respectively (3). These characteristic cross-β signals were first observed for the egg-stalk of the lacewing Chrysopa (4), and subsequently for amyloid deposits from amyloidosis patients (5).
Figure 1.
Amyloid fibril structure showing conversion from natively folded protein to partially unfolded intermediate to amyloid fibril. Electron microscopy images show different morphologies of amyloid fibrils.
Amyloid fibrils are distinguished from other protein aggregates because they show apple green birefringence when stained by Congo red and viewed under polarized light (histologically). Although Congo red has been the gold standard for amyloid detection for over 50 years, it is a challenging technique that requires a long staining process and skilled interpretation for accurate results. Recently, new methods of detection and typing have been developed including the use of luminescent conjugated polythiophenes/oligothiophenes (LCPs/LCOs) that preferentially bind amyloid deposits with a signature spectral shift; these fluorophores have potential diagnostic applications for systemic and localized amyloidoses (6-8). In vitro, amyloid fibril formation is generally monitored by Thioflavin T (ThT) binding. Positive ThT fluorescence is complemented by transmission electron microscopy (TEM) and/or atomic force microscopy (AFM) to confirm the presence of amyloid fibrils. In general, amyloid fibrils appear as long, unbranched fibrils that cluster together, coil, or stack laterally.
Differences between systemic and localized amyloidosis
Amyloidoses are clinically heterogeneous because one or more organs (such as heart, kidneys, liver, pancreas, peripheral, autonomic, and central nervous systems, among others) can be involved.
In the localized amyloidoses, the intracellular and/or extracellular amyloid deposits occur only in the organ or tissue of precursor protein synthesis. Some examples of localized amyloidosis are neurodegenerative disorders like Parkinson's, Alzheimer's, and Huntington's disease. Among the localized amyloidoses, Huntington's and Parkinson's disease present intracellular protein deposition. Alzheimer's disease can be described as an intracellular and extracellular disease, with the presence of intracellular Tau protein fibrils and extracellular amyloid β fibrils, although the process of oligomerization may begin intracellularly.
In contrast, all the systemic amyloidoses are extracellular. They occur when the precursor protein is expressed and secreted at one site distinct from the major sites of deposition. It is common that multiple organ systems have amyloid deposits in these diseases (9, 10). All systemic amyloidosis are listed on Table 1.
Table 1.
The systemic amyloidoses
| Disease | Precursor Protein | Cause | Organs Affected |
|---|---|---|---|
| (AL) Light Chain amyloidosis | Immunoglobulin light chain | Acquired mutation & overproduction | Heart, kidney, liver |
| (AH) Heavy Chain Amyloidosis | Immunoglobulin heavy chain | Myeloma-associated | Kidney, liver |
| (ATTR) Senile systemic amyloidosis | Transthyretin | Accumulation of WT TTR | Heart, vessels, soft tissues |
| (ATTR) Familial amyloidotic polyneuropathy | Transthyretin | Hereditary mutation of TTR | Heart, kidney, CNS |
| (AA) secondary amyloidosis | Serum amyloid A protein | Overproduction secondary to chronic inflammation | Kidney, liver, spleen |
| (Aβ2M) Dialysis-related amyloidosis | β-2 microglobulin | Chronic dialysis, Cu2+ triggers oligomerisation | Joints, heart, GI, lung |
| (ALys) Lysozyme amyloidosis | Lysozyme | Hereditary mutation | Kidney, liver, spleen |
| (ApoAI) ApoAI amyloidosis | Fragments of apolipoprotein AI | Hereditary mutation | Kidney, heart, liver |
| (ApoAII) ApoAII amyloidosis | Fragments of apolipoprotein AII | Hereditary mutation | Kidney |
| (ApoAIV) ApoAII amyloidosis | Fragments of apolipoprotein AIV | Hereditary mutation | Kidney |
| (Afib) Fibrinogen amyloidosis | Fibrinogen α-chain mutants | Hereditary mutation | Kidney |
| Finnish hereditary amyloidosis | Fragments of gesolin mutants | Hereditary mutation | Cornea, facial and peripheral nerves, skin |
| (ACys) Cystatin amyloidosis | Cystatin C | Hereditary mutation | Cerebral blood vessels |
| (ABriPP) BriPP amyloidosis | BriPP | Hereditary mutation | Microvasculature of the brain |
The systemic amyloidoses share many non-specific symptoms including fatigue, weakness, loss of appetite, and weight loss. Even the more organ-specific symptoms in systemic amyloidoses, such as swollen ankles (edema) due to kidney or heart involvement, tingling in the fingers or toes (paraesthesia) due to nerve involvement, or breathlessness due to cardiac involvement, are non-specific, making their diagnosis and typing difficult. Recent advances in amyloid deposits laser microdissection coupled with mass spectroscopy have allowed for direct diagnosis and better identification of the precursor protein (11), reducing the number of misdiagnosed amyloidosis cases and allowing for the appropriate treatment to be provided for each patient.
WHAT CHARACTERIZES SYSTEMIC AMYLOIDOSES?
There are at least five general mechanisms to generate an amyloid precursor in vivo. These mechanisms are reviewed in reference (12):
By propagation of conformational changes that are prone to amyloid aggregation which serve as a molecular template for additional misfolding and aggregation of the properly folded proteins where neither overproduction nor proteolysis has been observed (e.g. Prion diseases) (13).
Failure/disregulation of proteolytic processes of the unfolded/misfolded protein that leads to the accumulation of the aggregation-prone precursor with no overproduction of the precursor, in many cases due to mutations in either the precursor protein or the protease (e.g. Alzheimer's disease) (14).
Mutations that render the protein either kinetic or thermodynamically unstable increasing the population of partial unfolded species and then enhancing its aggregation in an energetically favorable aggregation process (e.g ATTR amyloidosis), (15-24).
Overproduction of amyloid protein precursor by abnormal expansion of secretory cells population (25) resulting in high levels of circulating protein that can overcome the extracellular protein quality control and degradation system (e.g AL amyloidosis), (25, 26).
Loss of a critical interaction that stabilizes a protein by a co-chaperoning mechanism (e.g. Transthyretin, immunoglobulin light chains).
Interestingly, clinical and experimental data suggest that the first two modes of formation of amyloid precursor are usually associated with localized amyloidosis, and the last three modes are more related to systemic amyloidosis (27).
As mentioned above, the important difference between systemic and localized amyloidosis is whether the sites of protein synthesis and release and amyloid deposition are distinct or the same; this difference is linked to the normal physiological function of each amyloidogenic protein and the type of cell that synthesizes it.
In the neurodegenerative localized amyloidoses such as Alzheimer's disease, Spongiform encephalopathies, and Parkinson's disease, the precursor proteins are synthesized by neurons which either produce proteins for their own functional needs or small peptides packaged in vesicles for synaptic transmission. Therefore, neurons are poorly adapted to deal with amyloidogenic proteins; even a small amount of misfolded amyloid prone precursors may overwhelm the neuron's compensatory mechanism with intracellular aggregation and local deposition.
In contrast, a hallmark of systemic amyloidosis such as AL amyloidosis and ATTR amyloidosis is that their amyloidogenic protein precursors are synthesized by cells (plasma cells for AL amyloidosis and hepatocytes for ATTR amyloidosis) that are specialized to synthesize and secrete large quantities of proteins into blood plasma (sometimes called professional secretory cells). As seen later, in these professional secretory cells the endoplasmic reticulum (ER) plays a crucial role in the proper folding of the large amounts of proteins synthesized by these cells.
The importance of the endoplasmic reticulum in protein folding
In complex organisms such as mammals, a considerable fraction of the proteome is secreted into the extracellular space to act far from the site of synthesis (organ or tissue). These proteins, after being synthesized by ER-bound ribosomes, are translocated to the ER lumen in an unfolded state with reduced cysteine residues to be folded and form their disulfide-bonds before being transported and modified by the Golgi apparatus for secretion. The ER in plasma cells, the β-cells of the pancreatic islets, hepatocytes and other “professional secretory cells” required to secrete large quantities of protein can reach extraordinary efficiency.
Professional secretory cells
In the last two decades of the 20th century, studies with several secreted proteins including immunoglobulin molecules (28-31) have shown that proteins that fail to fold or assemble eventually are processed by the “ER primary quality control”, and that each secretory cell has its own variation on the unfolded protein response (UPR) machinery (for a good review see references (30, 32)) .
As we have mentioned above, not all differentiated cell types have the same secretory capacity. Van Anken and Braakman have reported that the mammalian ER UPR is very diverse, allowing activation of different subsets of downstream effectors, in particular during the development of a particular tissue. The IreI pathway seems to be very important during the development of secretory tissues; for example, the Ire1a and XBP-1 transcription factors are essential for liver development and B cell development (33). The most extensively studied example of secretory cell development is the maturation of quiescent B lymphocytes into mature plasma cells. The volume of ER cisternae expands at least threefold in order to accommodate the bulk biosynthesis of immunoglobulin molecules. XBP-1 −/− B cells can only minimally secrete antibody. Transfection of the XBP-1 gene alone can trigger B cell differentiation; however, the characteristic feature of induction of the UPR in these cells involves a specific splice in the XBP-1 transcript, generating a molecule which can enhance transcription of downstream targets.
Studies of the secretion and folding of various clinically-relevant mutants of misfolding prone protein, i.e. TTR, have shown that it is a combination of the energetics of folding and the proteostatic and secretory capacities of the cell that determines the efficiency of secretion. Only the most destabilized TTR variants are subjected to ER-associated degradation (ERAD) and then only in certain tissues (34). In professional secretory cells involved in the synthesis of amyloid precursor proteins such as TTR mutants and AL proteins, even if a certain percentage of the protein being synthesized by these cells is not properly folded and is retained within the secretory pathway to refold by the action of chaperones or be degraded by ERAD, enough protein is secreted. Protein homeostasis, or “proteostasis,” and diseases related to misregulation of protein maintenance are reviewed in great detail by Powers et al (35).
TYPES OF SYSTEMIC AMYLOIDOSES
The systemic amyloidoses affect various organ systems and are caused by multiple precursor proteins as listed on Table 1.
A more “benign” amyloidosis is dialysis-related amyloidosis (DRA), one chronic complication of long-term haemodialysis that affects quality of life. Patients receiving maintenance haemodialysis for more than 10 years lose the ability to clear β2-microglobulin, the light chain of the major histocompatibility complex (HMC) class I molecule, leading to a 60-fold increase in β2m serum concentration. β2m can form amyloid deposits in the soft tissues surrounding joints causing a variety of conditions like carpal tunnel syndrome, chronic joint pain, and destructive arthropathy. It is proposed that β2m amyloid formation is mediated by low pH or the presence of cations such as Cu2+ (36). However, the incidence of DRA is now falling, possibly due to the use of more biocompatible dialysis membranes (in the past, dialysis membranes were treated with Cu2+) and cleaner dialysis fluids (37, 38).
Serum amyloid A protein (AA) Amyloidosis, also known as secondary or inflammation-associated amyloidosis, occurs as a result of chronic inflammation caused by conditions such as rheumatoid arthritis, familial Mediterranean fever, or chronic infections like tuberculosis. AA Amyloidosis is caused by the proteolytic cleavage of the serum amyloid A protein into fibril-forming fragments that deposit predominantly in the kidney, liver, or spleen.
Transthyretin (TTR) is a homotetrameric protein (15) that transports thyroxine and binds retinol. Extracellular amyloid deposition of TTR is associated with two types of amyloidosis, Familial amyloidotic polyneuropathy (FAP) (or ATTR amyloidosis, an autosomal dominant disease) with deposits formed by TTR point mutants, and Systemic Senile Amyloidosis (SSA) with WT TTR deposits (19).
More than 100 point mutations have been characterized (18) that cause dissociation of the TTR tetramer leading to the misfolding and aggregation of the monomers. V30M is the most common mutation causing FAP, and V122I is the most common clinically important mutation worldwide (16). Amyloid fibrils deposit in various organs, primarily the heart and peripheral nerves. The median survival for ATTR amyloidosis is 5-15 years depending on the organ involvement. SSA occurs more slowly, manifests in the elderly population, and is characterized by amyloid deposition in the heart, demonstrating that WT TTR also has the capability of causing organ damage.
The other less common systemic amyloidoses are hereditary, caused by destabilizing mutations or deletions in the following proteins: Lysozyme, Apolipoprotein AI, Apolipoprotein AII, Apolipoprotein AIV, Fibrinogen a-chain, Gelsolin, and Cystatin C. LECT-2 is the newly added member to this class of hereditary systemic amyloidosis (39, 40), although there is still very little known about its pathophysiology.
LIGHT CHAIN (AL) AMYLOIDOSIS: WHAT DO WE KNOW AND WHERE ARE WE GOING?
One of the most clinically interesting and perhaps the most complex systemic amyloidosis is AL amyloidosis. AL amyloidosis is the most commonly diagnosed systemic amyloidosis in the Western world, with an incidence of 10 patients per million per year (41). It is a fatal progressive disease characterized by extracellular deposition of immunoglobulin light chains into insoluble fibrillar aggregates. Most AL amyloidosis patients are over 45 years old with an average age of 67 years old. AL amyloidosis patients have a median survival of 12-40 months after diagnosis (42).
AL amyloidosis is caused by an abnormal proliferation of monoclonal plasma cells that secrete a high amount of free immunoglobulin light chains (LC) into the bloodstream. These LCs self-assemble and deposit as insoluble amyloid fibrils in various organs, ultimately causing organ failure and death (Figure 2). Current treatments target the plasma cell population and are not curative.
Figure 2.
Light chain (AL) amyloidosis pathology A) clonal expansion of plasma cells secreting light chain dimers that deposit in vital organs as amyloid fibrils B) Immunoglobulin structure showing two identical heavy chains (HC) and two identical light chains (LC) linked together via covalent interchain disulfide bonds.
Although the first amyloid to be identified and biochemically characterized was a human immunoglobulin light chain, the molecular mechanism of amyloid aggregation for AL amyloidosis remains unclear (43). This deposition can occur almost anywhere in the body; the most frequent sites of AL deposition are the kidneys, heart, peripheral nerves, gastrointestinal tract, and liver. The least common form of AL amyloidosis deposition is a tumor-like localized deposition called amyloidoma which could occur anywhere in the body, including in the brain (44, 45). Patients with clinical cardiac involvement have the worst prognosis with a median survival of less than a year. It is not known what leads to the pattern of tissue deposition in a given patient (46).
Certain light chain germline genes are over-represented in AL amyloidosis proteins (47). As we will review later, the high variability in immunoglobulin light chain sequence could result in slightly different structural and biophysical properties of the abnormal light chain proteins as well as the different propensities for amyloid formation, organ involvement, and degree of severity of the disease in every single patient with AL amyloidosis. Despite many recent advances in our understanding of the mechanisms of AL amyloidogenesis, much remains to be understood about the pathogenesis of AL amyloidosis.
Structure and Biochemistry of immunoglobulin light chains (LCs)
Human immunoglobulin molecules such as Immunoglobulin G (IgG) are composed of two identical heavy chains (HC) and two identical LCs linked together via covalent interchain disulfide bonds (Figure 3A). In an IgG molecule, the HC has a variable domain and three different constant domains. All LCs have two independent domains (Figure 3B), a high variable N-terminal domain (VL) of 110 to 120 residues and a constant C-terminal domain (CL) of 105-107 residues. The VL is linked to antigen recognition and binding, while CL has structural functions (Figure 3A).
Figure 3.
(a) X-ray structure of κIgG antibody (PDB code: 1IGT). Two heavy chains (green), and two light chains (gold) assemble in a heterotetramer. (b) Structure of full length light chain (PDB code: 1IGT, chain A). Both the CL (red) and VL (yellow) domains present a characteristic immunoglobulin fold. (c) Structural alignment of X-ray crystal structures of λVL 6aJL2 germline (gold) and κVL κ1 O18/O8JK2 (red) germline proteins (PDB code: 2W0K and 2Q20, respectively). The FR region shows a very similar structure while the CDRs (blue) show significant differences as a result of high variability in the sequence. κ and λ germline proteins present a highly conserved single tryptophan residue at position 35 (black sticks), in close proximity of the characteristic immunoglobulin disulfide bridge (gray sticks).
The LC is formed by random recombination of multiple gene segments; κ LC molecules could be constructed by using one of the 40 possible Vκ gene segments together with one of the 5 possible Jκ gene segments and the single Cκ gene, while λ LC could be constructed by using one of the 33 possible Vλ gene segments, one of the 4 possible Jλ genes and 5 of the possible Cλ genes. The random selection of individual V, J, and C genes contributes to the diversity of LCs, allowing the generation of 3000 LC variants (48) (Figure 2B). In addition, somatic mutations improve the antibody affinity leading to further sequence variation (49).
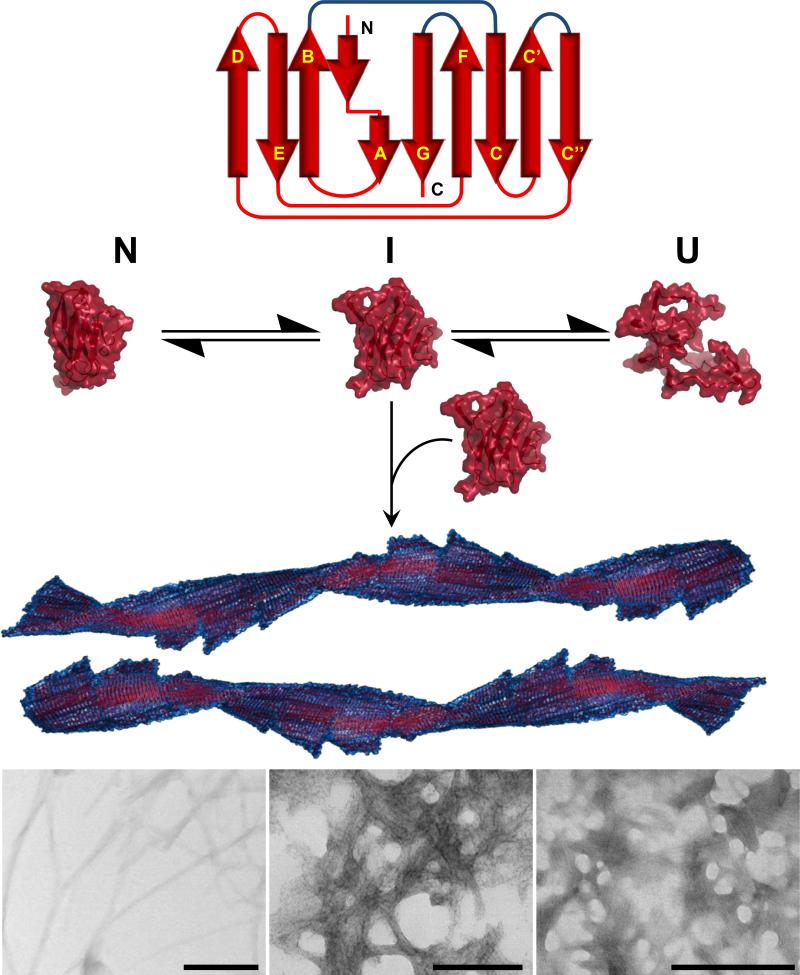
The VL domain structure consists of nine β-strands packed tightly against each other in two antiparallel β-sheets and joined together by a disulfide bridge with a completely buried central hydrophobic core forming a β-sandwich (Figure 3C) (50, 51).
The β-strands are labeled A, B, C, C’, C”, D, E, F, and G from the N- to C-termini. These strands form the framework regions (FRs). The VL domain interacts with the heavy chain variable domain (VH) through-strands C, C′, F, and G (Figure 2B and 3C). Strands B and C; C’ and C”; F, and G are connected by unstructured loops (complementarity determining regions or CDRs) that are more variable in both size and sequence than the rest of the domain and determine the specificity of the antigen-antibody interactions (Figure 3C)(50, 51). All VL domains have a highly conserved tryptophan residue at position 35 buried in the hydrophobic core of the VL in close proximity to the internal disulfide bridge. The Trp 35 fluorescence is quenched in the native state by the adjacent disulfide bond, so it can be used as a ‘reporter group’ for the conformational state of VL domains (52).
Abundant protein in plasma
In AL amyloidosis, free light chains are secreted in excess by a small plasma cell clone population in the bone marrow without evidence of multiple myeloma (in AL amyloidosis, there are <10% malignant plasma cells in bone marrow) (53).
Normal plasma cells synthesize LC independently of HC in a relatively balanced HC/LC synthesis, and subsequently combine them in the ER to assemble antibodies. However, the biosynthetic process can differ in malignant cells; some synthesize equal amounts of LCs and HCs, while others secrete excess free LCs (in a 3-to 4-fold excess) (54, 55). This overexpression could allow some free LC to escape into circulation. Occasionally, two light chains may combine to form a LC dimer by both covalent disulfide linkage and non-covalent interactions of their dimer interfaces before secretion.
Neither dimerization via disulfide bond nor non-covalent interactions necessarily lead to amyloid deposition (Figure 2A). The presence of a monoclonal free light chain in serum and/or urine is a relatively frequent event in individuals older than 50 years of age, but only a minor fraction will progress into AL amyloidosis (53, 56). The deposition of a monoclonal light chain as amyloid fibril in vivo is a consequence of the mutual influence between the biophysical and structural properties of the amyloidogenic protein and the environmental conditions of the targeted tissue(s).
Biochemical determinants for misfolding: sequence analysis of light chain sequences
As was discussed above, unlike other amyloid precursors, the LCs are highly heterogeneous in sequence (48). Studies by Comenzo, Abraham, and Prokaeva demonstrated that AL VL germline donor gene usage comprises VλI, VλII, VλIII, VλVI, and VκI. There were slight differences in the sample size, sample selection, and the frequency of use of each germline donor gene in each study. Comenzo and co-workers demonstrated that 30% of AL VL genes used Vλ6a germline donor (57). Abraham and co-workers found that 77% of the κ patients selected for their study used the VκI subgroup (48); a similar observation was made by Prokaeva and co-workers (58). The Vλ6a and Vλ3r germline donor genes were identified to be precursors of more than 50% of the AL λ light chains (48, 57, 59). In clear contrast with their high frequency in AL amyloidosis, both gene segments are expressed by approximately 2% and 8% of the peripheral blood B λ lymphocytes, respectively (48, 57, 60). These studies support the notion that certain LC germline sequences are over-represented in AL amyloidosis and may be intrinsically more prone to aggregation.
At present, the association between the above mentioned VL/J germline gene segments and AL amyloidosis is unknown. Two studies have tested the stability and amyloid formation kinetics of κ and λ germline proteins to determine if the germline sequences over-represented in AL amyloidosis are amyloidogenic.
Because the λ6a germline is expressed exclusively in AL patients and is rarely expressed in the normal LC repertoire (it is one of the last germline genes screened in the process of selection) (48, 57, 58), del Pozo Yauner et al. studied the thermodynamic stability of the λ6a germline protein and found that it was more stable than Wil, a λ6a AL light chain protein with 11 somatic mutations (61). The λ6a germline also had significantly slower fibril formation kinetics than Wil.
In the case of κI VL gene segment, Baden et al. compared AL-09, an AL light chain protein that has 7 somatic mutations, to its germline protein κI O18/O8 (62). The germline protein was more thermodynamically stable than AL-09, and although it was able to form fibrils, its fibril formation kinetics were significantly slower than AL-09. Previous studies had demonstrated that when fibril formation of κI O18/O8 AL protein BIF and MM protein GAL was tested at 37°C, only AL protein BIF formed fibrils (63).
κI O18/O8 and λ6a possess comparable thermodynamic stability, but λ6a presents faster fibril formation kinetics compared to κI O18/O8 (14 hours for λ6a compared to 216 hours for κI O18/O8 at 37°C, the latter one in the presence of seeds). This may indicate that within the amyloidogenic germline sequences, there is an increase in fibrillogenic propensity for λ6a germline proteins. This may explain the fact that λ6a is over-represented among AL amyloidosis patients (57). However, more studies are necessary to verify that additional AL-prone germline sequences (such as λ1, λ2, and λ3) are more amyloidogenic than normal immunoglobulin repertoire germline sequences.
An early sequence analysis of AL proteins showed that the VL region of amyloidogenic proteins are highly mutated compared with their parent germline genes, as expected, suggesting that these proteins have acquired somatic mutations as functional immunoglobulin molecules (64). Destabilizing somatic mutations are found in both the CDR and FR regions and appear to increase the propensity of LCs to aggregate (65-70). The role of somatic mutations in the disease pathophysiology of AL amyloidosis has been approached in different ways. Several studies have compared amino acid sequences of AL proteins, searching for patterns of common mutations or mutational regions among large numbers of sequences.
In an analysis of 121 κI light chains (37 of which were from AL amyloidosis patients), Stevens found four structural features that render a light chain protein more likely to be amyloidogenic (71). The structural features involved loss or gain of certain residues, including a mutation that introduces a putative glycosylation site, mutations of Arg61 or Ile27b and mutations of Pro residues in β-turns. A more recent analysis of 141 κ and λ light chain sequences from AL amyloidosis patients catalogued the non-conservative mutations in these proteins and modeled their positions onto known light chain structures to correlate structural regions (β-strands or loops) with potentially destabilizing mutations (47). This study showed that non-conservative mutations accumulate in specific regions of AL light chain proteins and that the location of any non-conservative mutations may be a more important amyloidogenic determinant than the total number of mutations found in these proteins.
Structural determinants of amyloidogenicity in light chain proteins
The structural rearrangements promoting amyloid fibril aggregation of the light chains has not yet been characterized at the atomic level. At present, the only reports available describe the global structural changes associated with fibrillar aggregation. Fink and co-workers studied the effect of low pH and different concentrations of guanidinium hydrochloride (GdnHCl) on the kinetics of in vitro fibril aggregation of the recombinant VL SMA, an AL light chain from the κIV germline. By spectroscopic and hydrodynamic methods, Fink and co-workers showed that SMA fibrillogenesis in vitro is promoted by conditions at which a relatively unfolded, but compact, SMA protein intermediate is populated. This SMA intermediate was characterized by a substantial loss of tertiary and secondary structures (72, 73).
Differing from the fibrillar-competent intermediate, a second intermediate, characterized by a native-like folding, was detected at less stringent conditions. It was observed that the native-like intermediate preferentially formed amorphous aggregates. These findings led the authors to postulate that a highly unfolded intermediate favored the conformational rearrangements necessary to form AL amyloid fibrils (73, 74), although the role of amorphous aggregation as a possible on- or off-pathway event in amyloid fibril formation has not been fully explored for AL amyloidosis. Another interesting observation is that the N-terminal region of SMA VL is implicated in the intermolecular contacts stabilizing the AL fibril (74). This region undergoes a characteristic rearrangement through the aggregation process that gives rise to a conformational epitope common to non-native and fibrillar light chains (75).
Structural studies have shown that most VL domains from AL amyloidosis patients crystallize as monomers or dimers with the expected antiparallel β-sheet immunoglobulin fold. The dimer observed in most cases is homologous to the dimer conformation occurring between light and heavy chains in immunoglobulin molecules.
Studies from our laboratory have shown that the germline κI O18/O8 crystallized as a canonical dimer while the amyloidogenic protein AL-09 adopted an altered dimer with a 90° rotation with respect to the canonical dimer structure (62). The three non-conservative mutations in AL-09 are in or around the dimer interface (N34I, K42Q, and Y87H). Restorative mutational analysis showed that a single mutation in AL-09 back to the germline amino acid found in position 87 (AL-09 H87Y) stabilized the protein, delayed amyloid formation, and changed its conformation from the altered dimer to the canonical dimer interface (76). Although the side chain change from Histidine to Tyrosine does not alter the local topology around residue 87, it induces a conformational change in the loops between β-strands C and C’ and β strands F and G. This mutation also induces a change in Y36 and F98 side chain spatial orientation, (residues in the dimer interface), reshaping the dimer interface topology through the creation of new inter-subunit contacts (see Figure 4, animation). These findings suggest that the residue in position 87 is critically involved in the contacts that stabilize the VL dimer interface through long-distance interactions and changing Tyrosine to Histidine causes major disruptions in these interactions.
Figure 4.
This morph animation show the transition between three unique dimer orientation present in the crystal structures of κI O18/O8 and AL-09, as well as NMR structure of κI Y87H in order to see and understand the structural differences between them. This animation does not represent genuine conformational changes. Surface representation of the residues in the monomer A involved in the κI dimer interface is colored in magenta while residues outside the dimer interface are shown in cyan. Monomer B (ribbons) of AL-09 (blue, PDB code: 2Q1E), κVL κI O18/O8 (gold, PDB code: 2Q20), κI Y87H (red, PDB code: 2KQM) show a ~90° and ~ 180° rotation to the monomer A from the canonical dimer interface respectively. In yellow ribbon is shown the differences in dimer structures as the hands on a clock moving in intervals of 90°. Interface residues Q3, Y36, F98 and Q100 (red sticks) show the biggest conformational differences between dimer interfaces.
We have also reported that the reciprocal mutant κI Y87H, in which we mutated the germline residue (Tyr) towards the residue found in AL-09 (His), crystallized as a canonical dimer. However, using solution Nuclear Magnetic Resonance (NMR) spectroscopy, we showed that this protein adopts a different dimer interface rotated 180° from the canonical dimer interface, and 90° from the AL-09 altered dimer interface (77). The different dimer structures could be compared to the hands on a clock moving in intervals of 90° (see Figure 4, animation).
Sequence alignments of 141 AL light chains revealed that non-conservative mutations on the VL dimer interface, especially Histidine mutations, are very common in AL proteins (47). Taken together, our results suggest that dynamic dimerization could occur frequently in AL proteins. Our structural studies show that light chains are able to dimerize in different conformations; the residues in the dimer interface determine whether or not a dimer conformation will be favored or if the numerous interfaces will be populated at the same time. Some of these dimer conformations are highly amyloidogenic.
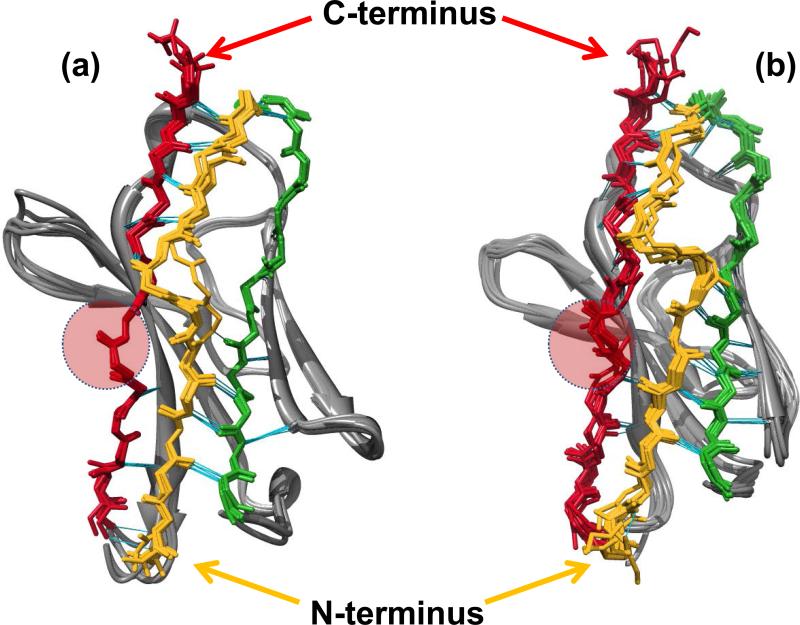
Richardson has proposed that natural β-sheet proteins may use structural motifs to avoid β-sheet-mediated aggregation. This is the type of aggregation usually observed in de novo design peptides that adopt β-sheet structure and in amyloid formation (78). For β-sandwich proteins, two strategies can be identified. The first one relates with β-edge blocking by dimerization via inter molecular H-bonds with adjacent β-strands. The second one is observed in light chains, and it is the presence of a β-bulge and/or proline residues in the edge β-strand G and the presence of a β-sheet switch, where β-strand A (residues 1-14) starts as the edge strand of one sheet, then crosses in an abrupt kink to finish as the edge strand of the opposite sheet, thereby protecting the β-edges of β-strands B and G (Figure 5 A and B), and avoiding β-sheet mediated aggregation. Del Pozo Yauner reported that substitutions of the residues that conform the β-sheet switch motif (P7S or H8S) in the λ6a germline protein, affected the thermodynamic stability and amyloidogenic properties of the mutant as well as modified the arrangement of the dimer interface in the crystal structure (Figure 5A).
Figure 5.
Structural comparison between the N-Terminal region of (a) λVL proteins and (b) κVL proteins (PDB codes: 2W0K, 2W0L, 3B5G, 1CD0, 1PW3, 1PEW, 2CD0, 2Q20, 2KQN, 3CDY, 2Q1E, 3DVI, 2KQM, 3CDC, 3DVF respectively). Significant differences in the N-terminal region are observed. κVL proteins present more H-bond (cyan lines) interactions between β-strand A (gold) and the edges of β-strands B (green) and β-strand G (red). In both proteins β-strand A adopts a characteristic conformation known as “β-sheet switch”, which has been proposed to protect the two edge β-strands B and G from non-native intermolecular interactions that lead to aggregation. The topology of the N-terminal region in both germline sequences is similar and includes a β-bulge (residues 7-8) between two segments of β-strand A. Interestingly, the size of the β-bulge in κVL is greater than in λVL proteins, and κVL presents more contacts between β-strand A and the C-terminus and β-strand B than λVL. Red circles indicate the β-bulge motif in β-strand G. CDRs are at the bottom of the models.
Protein stability and kinetics of amyloid formation – when this information is useful and when it is not
Numerous experiments have implicated thermodynamic stability as one of the major modulators of the amyloidogenicity of LCs. An empirical rule has been proposed that AL proteins are less stable than the non-amyloidogenic ones belonging to the same germline gene product (63, 68, 70). Additionally, it has been shown that conditions destabilizing light chain folding, such as high temperature, extreme pH values, and the presence of chemical denaturants as urea or GndHCl, promote the aggregation of these proteins as amyloid fibrils in vitro (63, 79-81). These data suggested that partially folded species play a key role in light chain fibrillogenesis.
Studies using VL proteins from AL patients have shown that mutations in the VL that reduce the thermodynamic stability are more prone to form amyloid fibrils (67, 82, 83). In an analysis linking mutations and stability, Hurle et al. analyzed 36 sequences (18 κ and 18 λ) in search of rare amino acid replacements that occurred in structurally significant regions of the proteins (67). They constructed single-point mutants incorporating the rare residues into a non-amyloidogenic light chain protein to determine whether the mutations destabilized the protein significantly enough to induce unfolding. Four of the six mutations were destabilizing, leading to the conclusion that some but not all the mutations play a role in amyloidogenicity.
To determine if a single mutation is enough to make a protein amyloidogenic, Davis et al. studied and compared the VL of AL protein SMA and the MM protein LEN. Only eight residues differ between these two proteins. Each SMA mutation was introduced into LEN. Of the SMA mutations tested, only P40L, located in the loop region between β-strand C and C’, was able to show ThT enhancement (84). It is likely that the P40L mutant was less stable than wild-type LEN because Pro40 is conserved among 98% of all κ and λ germline sequences.
In vitro fibril formation studies have revealed that AL proteins form fibrils under a variety of solution conditions with varying kinetics and morphology of fibrils, although most of the VL AL proteins characterized in our laboratory and belonging to the κI O18/O8 have very similar thermodynamic stability. AL-09 is unique because it forms amyloid fibrils with very similar kinetics across a wide variety of solution conditions when the removal of preformed aggregates enriches the solution for monomeric species (85). Additionally, AL-09 fibril formation kinetics are significantly faster than other AL proteins studied in our laboratory. We propose that for κI proteins, the altered dimer interface populated by AL-09 facilitates the initial misfolding events that trigger amyloid formation, while the other κI AL proteins require stochastic conformational fluctuations to populate the appropriate misfolded intermediate that leads the amyloid formation reaction. Certain conditions appear to be particularly favorable for light chains to form amyloid fibrils. AL protein SMA and MM protein LEN, both κIV light chains, have shown that amyloid formation is enhanced at low pH while amorphous aggregation occurred around neutral pH; all of these reactions populated different partially folded intermediates (72, 80, 81, 86, 87).
Another link between thermodynamic stability and fibril formation is found in the recently analyzed κI O18/O8 and λ6a germline proteins. These proteins were significantly more stable than all AL amyloidogenic proteins that have been studied to date (61, 62). The Tm values (melting temperatures, at which 50% of the proteins are unfolded) for the germline proteins were increased by 15°C and 11.6°C, respectively, with respect to their corresponding AL proteins analyzed in each study. Both κI O18/O8 and λ6a germline proteins had slower fibril formation kinetics than their amyloidogenic counterparts.
Del Pozo Yauner incorporated the R25G mutation into the λ6a germline protein, because this mutation is found in 25% of amyloidogenic λ6 LCs and probably represents an allotypic variant (61, 88, 89). This mutation caused a 6°C decrease in Tm value for the mutated protein, and the R25G mutant had faster amyloid formation kinetics than the λ6a germline protein. The R25G mutation appears to affect the structure of the CDR1, altering its conformation and increasing the amyloidogenicity of the protein (89).
Baden et al. undertook a systematic restorative mutational analysis of the non-conservative mutations of AL-09 to try to understand the correlation between thermodynamic stability and amyloid fibril formation propensities (76). As we mentioned before, AL-09 has three non-conservative restorative mutations: N34I, K42Q, and Y87H. The restorative mutant AL-09 H87Y displayed increased thermodynamic stability and delayed fibril formation kinetics similar to κI O18/O8 with significant structural alterations (previously discussed). Restoring the Asn34 residue had intermediate effects on stability and fibril formation propensity, while reintroducing Lys42 did not appear to alter the thermodynamics to any extent but delayed amyloid formation kinetics significantly.
In complementary experiments, introducing the His87 residue into κI O18/O8 (κI Y87H) destabilized the protein to an intermediate level with respect to AL-09. κI Y87H also had intermediate fibril formation kinetics between those measured for κI O18/O8 and AL-09, indicating that this mutation alone may not have been sufficient to cause the amyloidogenicity observed in AL-09. However, introducing a second mutation into κI O18/O8 (Ile34, in addition to His87) completely destabilized the protein and exhibited the same fast fibril kinetics as AL-09.
In a very recent study, members of our laboratory have found that an AL protein from the κI O18/O8 germline can be too unstable for fibrillogenesis; with a Tm of 29.8°C, AL-T03 was 70% unfolded at 37°C (the temperature of fibril formation incubation) and did not form amyloid fibrils after 1000 h incubation. Interestingly, this protein came from a patient that presented with hematologic response (reduction of free light chain in the serum) and organ response after receiving an autologous stem cell transplant treatment (N. Katoh, T. Poshusta, M. Gertz, A. Dispenzieri and M. Ramirez-Alvarado, unpublished observations). While this is a single instance, these results suggest that:
partially folded species are required for amyloid formation to occur in AL amyloidosis
the rate of amyloid formation in vitro and organ response may be inversely correlated
Other groups have studied fibril formation comparing AL and MM proteins. Jto, an MM protein, and Wil, an AL protein, are both light chain variable domain proteins from the λ6a germline that differ by 19 amino acids. Fibrils were formed with both Jto and Wil at 37°C, pH 7.5 (70). MM protein Jto displayed slower amyloid formation kinetics than AL protein Wil.
Wall et al. noted an ionic interaction between Asp29 and Arg68 in the MM protein Jto, whereas AL protein Wil has neutral amino acids in these positions (90). To test the importance of this ionic interaction, mutations were made to Jto to introduce the neutral residues (from Wil) at these sites (JtoD29A, JtoR68S). Thermodynamic stability parameters for these mutants were the same, and the rate of fibril formation for JtoD29A was the same as that for Jto. However, fibril formation kinetics was much faster for JtoR68S, and an X-ray crystal structure of this mutant revealed several side-chain differences compared to Jto and JtoD29A. These differences changed the electrostatic potential surface and increased the amount of solvent-exposed hydrophobic surface for the protein. These results highlight critical structural features such as ionic interactions that participate in the stability of AL proteins and could play critical roles delaying amyloid formation.
As we discussed above, experimental data suggest that significant changes in VL thermodynamic stability as a result of a single-mutation do not necessarily lead to changes in VL amyloidogenicity. Likewise, it has been proposed that partially folded species play a key role in VL fibrillogenesis. However, most of the VL work mentioned above follows a two-state folding pathway. A partially unfolded intermediate has been implicated in studies with five VL domains (63, 72, 91, 92) and, these intermediates have been characterized in SMA and λ6a germline (72, 91).
According to Khurana et al., partially unfolded intermediates are critical precursors in the fibrillar and amorphous aggregation of SMA. The rates of interconversion between the native conformation and the partially folded intermediates suggest a transient character of these intermediates (72).
Similarly, Blancas-Mejía et al. reported that the λ6a germline protein showed a reversible two-state unfolding on a fast time scale (3 min), but a partially unfolded intermediate accumulated on a long time scale (24 hours) at urea concentrations around the midpoint of the unfolding curve or only where native and unfolded states are equally populated; moreover, the rate of interconversion between these states is the slowest. This situation could promote the slow accumulation of a kinetic intermediate state that is prone to aggregate (91).
From these two reports, it is clear that the rate of interconversion between the native and unfolded state is the slowest and transient folding intermediates accumulate because of kinetic traps caused by partial misfolding.
In summary, it is known that some non-amyloidogenic VL domains have similar thermodynamic stability to their amyloidogenic counterparts; some mutations do not alter the thermodynamic stability while having a dramatic effect on the fibril formation kinetics; partially unfolded intermediates are not present in the folding pathway of most of the VL domains, and intermediates found in SMA and λ6a germline are transient. Taken together, this data suggests that the correlation between fibrillogenesis and low thermodynamic stability is not sufficient to explain AL amyloid fibril formation, suggesting that other factors (possibly kinetic factors such as the kinetic stability of the proteins) may be important in the molecular mechanism of AL amyloidosis.
Variable domain vs. full length protein deposition
In 1969, Glenner (43) found that the main component of human amyloid fibrils is the VL, confirmed by later studies that found that the VL and small fragments of the CL are part of the amyloid fibril (93). These findings were supported by the observation that pepsin digestion of certain light chains (also called Bence Jones proteins) can aggregate as amyloid fibrils, and the fact that aberrant synthesis of immunoglobulin light chains has been observed (94-96). Consequently, most LC biophysical studies have been conducted using recombinant VL. However, recent proteomics studies of amyloid deposits from a large group of patient samples showed that the amyloid fibrils consist of the entire LC (VL + CL) (11, 97-99).
Studies describing the properties of full length light chains from AL amyloidosis patients have been performed using for the most part urine-derived proteins. In a comparative analysis study of full length proteins isolated from urine samples from Multiple Myeloma (MM), Light Chain Deposition Disease (LCDD), and AL amyloidosis patients, aggregation reactions were followed by ThT fluorescence at the melting temperature for each protein for 72 hours. The results indicated that MM proteins formed spherical species, LCDD formed amorphous aggregates, and AL proteins formed fibrils (100).
It was only recently that the constant domain within the λ6a protein AL-01-095 was characterized (CL belongs to LC3* 04). Klimtchuk et al. found that the CL domain within AL-01-095 full length protein appears to confer great thermodynamic stability (98).
Amyloid formation reactions with full length AL-09 and κI O18/O8 show slow rates of amyloid formation with respect to the corresponding VL AL-09 and κI O18/O8. The deposits found using electron microscopy show larger degree of disorder compared to deposits made by VL proteins (DiCostanzo, Olatoye and Ramirez-Alvarado, unpublished observations). These results suggest that the presence of the constant domain affects the misfolding pathway for these proteins. In the cases where VL domain fragments are found in the amyloid deposits (such as those reported by Olsen 1989 (93)), it is clear that aberrant light chain synthesis or in vivo proteolysis could be occurring and further studies are needed to understand these processes further.
AL cellular and tissue toxicity – what we know and what we do not know
One of the great challenges in studying the amyloidoses is determining the most toxic species populated along the amyloid formation reaction. For years it was thought that the extracellular amyloid fibrils themselves were the main toxic species by creating a physical barrier around cells, blocking nutrient exchange, and by attracting macrophages that caused tissue damage. More recently, it has been demonstrated that the soluble amyloid species can be as toxic as, if not more toxic, as the insoluble fibrils for a number of amyloid diseases. In the case of AL amyloidosis, it has been shown that soluble AL proteins can induce apoptosis in cardiomyocytes in cell culture (101, 102). Interestingly, Sikkink et al. found that light chain monomers and dimers were the predominant species present at the time of maximum apoptotic activity.
Another important question is how these light chain species are internalized and whether internalization is required for toxicity. Monis et al. performed internalization experiments in fibroblasts that suggested that the amyloidogenic light chains are internalized via constitutive pinocytosis into an endosomal/lysosomal pathway mediated by microtubules (103).
Work from the Herrera group has shown that AL light chains, as well as LCDD light chains are able to bind a receptor at the membrane of primary human mesangial cells, after which they are internalized. Surprisingly, AL and LCDD proteins exhibit different intracellular trafficking patterns. AL light chains are delivered to the mature lysosomal compartment, where amyloid formation occurs (104). Notably, it was observed that AL and LCDD light chains induce divergent phenotypic transformation of human mesangial cells, correlating with the phenotypic changes observed in this cell type in patients suffering from AL and LCDD, respectively. It will be of great relevance to determine if all AL light chains share the capacity to selectively bind the non-yet identified human mesangial cell receptor and to induce the same intracellular trafficking pathway and phenotypic variation. Monis et al. argues that different cell types such as rat cardiac fibroblasts and mesangial cells may have different modes of internalization for AL proteins (103).
The internalization of full-length AL-09, AL-09 restorative mutants, and the germline κI O18/O8 into mouse cardiomyocytes has been studied (Levinson, Olatoye, Randles, Howell, DiCostanzo and Ramirez-Alvarado, under review). Although all these proteins internalized into lysosomal components, the degree of internalization of each protein correlated with the rate of amyloid formation of the corresponding VL protein. The effect of these proteins on the cardiomyocytes was variable within each condition; the lysosomal expansion profile was quite heterogeneous. Under the conditions used in this study, no light chain-induced toxicity was observed.
While cell culture studies on amyloidogenic protein-derived toxicity have been extremely useful, one of the limitations of these cell culture studies has been that they include only one type of cell, and in some cases it is not the main cell type affected by the amyloidogenic protein. This has precluded any of these studies from understanding the interplay between cellular interactions that may be happening in target tissues, the effect of tissue structure in the pathophysiology of the disease, and the specific effects that amyloidogenic light chains may have in target cellular systems.
AL Cell and Tissue Model Systems
Arendt and co-workers established the first amyloidogenic human cell lines (105). They isolated plasma cells from an AL amyloidosis patient both pre- (ALMC-1) and post- (ALMC-2) stem cell transplant. These cell lines secrete a full length λ6a LC protein called ALMC. While there is some genetic variation between ALMC-1 and ALMC-2 cells, the protein sequences from both cell lines are 100% identical. These cell lines secreted fully folded protein with a β-sheet structure that is as stable as other full length proteins (100) and has the ability to form amyloid fibrils in vitro. These cell lines are a valuable tool not only because this is the only human-derived system that secretes significant amounts of protein for biophysical studies, but also because they have tremendous potential to be used as secretion systems to understand how the plasma cell microenvironment could affect the secretion rates of different amyloidogenic light chains and how that can affect the pathogenic nature of the protein at a distance.
After the amyloidogenic light chain protein is secreted, the initial tissues affected by AL amyloidosis toxicity and deposition are the blood vessels: AL amyloidosis patients display early endothelial microcirculatory dysfunction (106); amyloid infiltration occurs in epicardial coronary arteries of most AL amyloidosis patients (107), and the majority of AL patients also display myocardial ischemia (decrease in blood supply) (108). It has been demonstrated that the infusion of AL light chains obtained from patients with cardiac amyloidosis causes diastolic dysfunction in isolated mouse heart (109). Human arterioles are an important tissue system to study the effect of amyloidogenic light chains; Migrino et al. found that arterioles display higher levels of superoxide and impaired dilation to nitroprusside when exposed to amyloidogenic light chains (110). More recently, Migrino and coworkers exposed arterioles to full length AL-09 and showed that these arterioles displayed reduced vasodilation to acethylcholine (comparable to amyloid β peptide) (S. Truran, M. Ramirez-Alvarado, A.C. DiCostanzo, D. Franco, C. Burciu, A. Maltagliati, and R. Migrino, personal communication). Clearly, more research is needed to fully understand the role of microvascular dysfunction in AL amyloidosis.
In summary, while we have increased our understanding about biochemical and structural determinants of AL proteins, including the role of mutations, dimerization structures, intermediate species, and thermodynamic stability in fibrillogenesis, there is still much to be understood about the pathogenesis of AL amyloidosis. Cellular and tissue experiments are providing important insight in the mechanisms of AL cellular internalization and tissue damage that will be useful in developing therapies targeted to the toxic species.
CURRENT ANIMAL MODELS OF THE SYSTEMIC AMYLOIDOSES
One of the great challenges in studying the systemic amyloidoses is developing an animal model that recapitulates the phenotype of the human disease given the differences between humans and laboratory animals. As we will see, murine models have been attempted for many of the systemic amyloidoses, but generally the pattern of amyloid deposition and toxicity does not mirror the human disease. We will concentrate on AL, AA, and ATTR amyloidosis models. Buxbaum has reviewed the drawbacks and challenges of amyloidosis animal models in great detail (111). We will also review here some of the most recent animal models reported for AL amyloidosis not covered in the Buxbaum review.
AL Amyloidosis Murine Models
There have been two recent animal models reported for AL amyloidosis. In 2010, Shi and co-workers reported a murine model in which wild type and dominant negative p38α transgenic mice were initially injected with amyloidogenic light chains through the tail vein followed by systemic intravenous infusion via the use of an osmotic mini-pump for 7 days. Wild type animals with fully active p38α presented an increase in the Bax/Bcl2 ratio and a very modest increase in cellular apoptosis as determined by TUNEL staining (101).
Ward and colleagues developed a transgenic mice model expressing an amyloidogenic λ6 LC using the cytomegalovirus (CMV) promoter; by 24-30 months, 83% of the transgenic mice developed amyloid deposits in the stomach (112). There was no cardiac or kidney deposition or dysfunction as is generally observed in AL amyloidosis patients. Previously, it was reported that 4’-iodo-4’-doxy doxorubicin inhibits amyloid formation and promotes reabsorption of amyloid deposits (113). Subsequently, tetracycline antibiotics have been screened for these properties based on their structural homologies (reviewed by Almeida and Saraiva (114)). Treatment with doxycycline decreased amyloid formation in these AL amyloidosis transgenic mice (23% of the treated mice had Congo red positive deposits compared to 69% of the untreated controls). The authors conclude that this transgenic model could be useful for testing modulators of fibril inhibition, although it does not recapitulate the phenotype of AL amyloidosis.
AA Amyloidosis murine models
The inflammation inducible murine AA models were the earliest models of a systemic amyloidosis as reviewed by Buxbaum in 2009. They are so far the most successful models of systemic amyloidosis and have been widely used because the murine AA model was the first in vivo demonstration of fibril seeding, in which introduction of preformed fibrils from a sick animal into a healthy one accelerated fibrillization. One challenge with these inducible models is that the inflammatory stimulus must be reintroduced to maintain the amyloid deposits or they will be slowly cleared. One successful way to get around the reintroduction of the inflammatory stimulus is by utilizing transgenic animals that express human IL-6 that spontaneously develop AA amyloidosis (115, 116).
TTR murine models
Several transgenic TTR mouse models have been developed. One of these models expressed mutant human V30M TTR and produced amyloid deposits in the tissues where mRNA was found. However, the animals did not develop autonomic or peripheral neuropathy, and no mRNA expression in liver or choroid plexus was found. The L55P TTR murine models provided useful information about the role of the murine TTR in the context of TTR amyloid deposition because endogenous murine TTR formed heterotetramers with the human TTR preventing aggregation and amyloid deposition, even upon seeding (117-119). Another approach was to cross L55P mice with heat shock factor 1 (HSF1) knockout mice (lacking chaperone response); this was the first TTR murine model to develop amyloid deposits in the sciatic nerve and dorsal root ganglia, in addition to the gastrointestinal tract (120). This implicates a role for HSF1 in TTR deposition; however, the exact mechanism is not known. Very recently, Buxbaum et al. generated transgenic mice with ~90 copies of the wild type human TTR gene expressed under the control of its own promoter. The human TTR gene shows appropriate tissue specific expression, and the animals show age dependent tissue deposition patterns similar to those in humans. Microarray analysis shows that animals with no cardiac amyloid deposits express genes in the liver encoding for molecules involved in various aspects of protein homeostasis. This liver gene expression is absent in the animals with cardiac deposition. The authors called this phenomenon “chaperoning at a distance” (23).
Back to cells or other animal models – how can we learn from the available models about the pathophysiology of disease
A successful transgenic animal model could allow better understanding of the pathophysiology of amyloid generation and deposition beyond what we can understand working with proteins in a test tube, but human diseases are distinct from those currently produced in murine models as we discussed above. Buxbaum in 2009 proposed that the challenge is to develop a model in which secretion of the pathogenic protein overcomes cellular protein quality control mechanisms leading to aggregation and amyloid deposition, so that the systemic effects of the disease can be studied. This requires understanding and manipulating the cell biology of the model organism which can be distinct from human, as it has become apparent with the different mouse models for amyloidoses. For example, in a successful AL amyloidosis model, it may be necessary to produce clonal expansion in vivo. Furthermore, the model should display the pattern of tissue deposition and progression observed in patients. The use of Caenorhabditis elegans (worms) and Drosophila melanogaster (fruit fly) to study amyloidosis has allowed the study of some disease processes. While the relationship between cellular and molecular phenotypes of these models and human disease may be limited in some instances, these alternative organismal models may facilitate the in vivo screen for inhibitors of amyloidosis (111).
TTR and Tafamidis
Despite the challenges in developing TTR animal models that successfully mimic the human pathophysiology, extensive research into the basic pathogenesis of the TTR amyloid cascade using in vitro biochemical, biophysical, and cell culture approaches has led to a successful Phase II/III trial with a kinetic stabilizer named Tafamidis (121). About 20 years ago, Kelly and colleagues first established that TTR tetramer dissociation is the rate-limiting step in TTR amyloidogenesis (122-125) and began the search for small molecules to kinetically stabilize the tetramer and prevent amyloid formation (126). The result of this research is Tafamids meglumine (Fx-1006A) which selectively binds to the two normally unoccupied thyroxine binding sites of the tetramer, stabilizing WT TTR and many pathogenic TTR variants (121). In a phase II/III clinical trial of tafamidis in V30M TTR-FAP patients, clinical efficacy over 18 months of treatment was demonstrated. Patients receiving Tafamidis had 52% less neurologic deterioration after 18 months, 55% and 84% preservation of large- and small-nerve fiber function, respectively, and improved nutritional status compared to placebo controls, showing an improved quality of life (127). This illustrates how understanding the basic biochemistry of amyloidogenesis can yield an effective treatment despite the lack of an animal model.
Perspectives and Future Directions
Despite many recent advances in our knowledge of the pathogenesis of the systemic amyloidoses, major questions remain unresolved:
What determines the apparent tissue specificity of most amyloidoses?
What are the molecular bases for the heterogeneity in survival in AL amyloidosis?
What biological, biochemical, and physicochemical processes modulate the tendency of a given amyloidogenic protein to deposit?
What is the most toxic species of the amyloid formation reaction?
What is (are) the mechanism(s) of tissue damage?
How does the cellular microenvironment affect amyloidogenesis and how does it change over the course of disease?
What are the best therapeutic strategies to target the multiple aspects involved in these diseases and which ones are the most important to target at a particular time in the disease progression?
As we move forward, multidisciplinary research involving collaboration among basic scientists and clinicians will facilitate new discoveries that in turn raise additional questions, propelling more studies of these devastating yet fascinating, complex diseases.
Acknowledgments
We would like to thank Joel Buxbaum for his critical reading of this review and his excellent suggestions. Kristi Simmons helped us with excellent editing support. The Ramirez-Alvarado team has been supported through the years by NIH R01 grants GM 071514, CA111345, AHA grant 0630077N, the Mayo Foundation, and the generous support from amyloidosis patients and their families.
References
- 1.Sipe JD, Benson MD, Buxbaum JN, Ikeda S-I, Merlini G, et al. Amyloid fibril protein nomenclature: 2010 recommendations from the nomenclature committee of the International Society of Amyloidosis. Amyloid. 2010;17:101–04. doi: 10.3109/13506129.2010.526812. [DOI] [PubMed] [Google Scholar]
- 2.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem. 2006;75:333–66. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- 3.Sunde M, Serpell LC, Bartlam M, Fraser PE, Pepys MB, Blake CC. Common core structure of amyloid fibrils by synchrotron X-ray diffraction. J Mol Biol. 1997;273:729–39. doi: 10.1006/jmbi.1997.1348. [DOI] [PubMed] [Google Scholar]
- 4.Geddes AJ, Parker KD, Atkins EDT, Beighton E. “Cross-β” conformation in proteins. Journal of Molecular Biology. 1968;32:343–58. doi: 10.1016/0022-2836(68)90014-4. [DOI] [PubMed] [Google Scholar]
- 5.Eanes ED, Glenner GG. X-ray diffraction studies on amyloid filaments. Journal of Histochemistry & Cytochemistry. 1968;16:673–77. doi: 10.1177/16.11.673. [DOI] [PubMed] [Google Scholar]
- 6.Hammarstrom P, Simon R, Nystrom S, Konradsson P, Aslund A, Nilsson KP. A fluorescent pentameric thiophene derivative detects in vitro-formed prefibrillar protein aggregates. Biochemistry. 2010;49:6838–45. doi: 10.1021/bi100922r. [DOI] [PubMed] [Google Scholar]
- 7.Nilsson KP, Hammarstrom P, Ahlgren F, Herland A, Schnell EA, et al. Conjugated polyelectrolytes--conformation-sensitive optical probes for staining and characterization of amyloid deposits. Chembiochem : a European journal of chemical biology. 2006;7:1096–104. doi: 10.1002/cbic.200500550. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson KP, Lindgren M, Hammarstrom P. A pentameric luminescent-conjugated oligothiophene for optical imaging of in vitro-formed amyloid fibrils and protein aggregates in tissue sections. Methods in molecular biology. 2012;849:425–34. doi: 10.1007/978-1-61779-551-0_29. [DOI] [PubMed] [Google Scholar]
- 9.Westermark P, Benson MD, Buxbaum JN, Cohen AS, Frangione B, et al. A primer of amyloid nomenclature. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2007;14:179–83. doi: 10.1080/13506120701460923. [DOI] [PubMed] [Google Scholar]
- 10.Falk RH, Comenzo RL, Skinner M. The systemic amyloidoses. The New England journal of medicine. 1997;337:898–909. doi: 10.1056/NEJM199709253371306. [DOI] [PubMed] [Google Scholar]
- 11.Vrana JA, Gamez JD, Madden BJ, Theis JD, Bergen HR, 3rd, Dogan A. Classification of amyloidosis by laser microdissection and mass spectrometry-based proteomic analysis in clinical biopsy specimens. Blood. 2009;114:4957–9. doi: 10.1182/blood-2009-07-230722. [DOI] [PubMed] [Google Scholar]
- 12.Buxbaum J. The Amyloidoses. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. Rheumatology. 4 ed. Mosby/Elsevier; Philadelphia: 2008. pp. 1669–80. [Google Scholar]
- 13.Prusiner S. Scrapie prions. Annual review of microbiology. 1989;43:345–74. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- 14.Haass C, Hung A, Selkoe D. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. The journal of neuroscience: the official journal of the Society for Neuroscience. 1991;11:3783–93. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serag AA, Altenbach C, Gingery M, Hubbell WL, Yeates TO. Identification of a subunit interface in transthyretin amyloid fibrils: evidence for self-assembly from oligomeric building blocks. Biochemistry. 2001;40:9089–96. doi: 10.1021/bi010655s. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya-Suzuki A, Yazaki M, Kametani F, Sekijima Y, Ikeda S. Wild-type transthyretin significantly contributes to the formation of amyloid fibrils in familial amyloid polyneuropathy patients with amyloidogenic transthyretin Val30Met. Human pathology. 2011;42:236–43. doi: 10.1016/j.humpath.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Blake CC, Geisow MJ, Oatley SJ, Rerat B, Rerat C. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 A. Journal of molecular biology. 1978;121:339–56. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 18.Connors LH, Lim A, Prokaeva T, Roskens VA, Costello CE. Tabulation of human transthyretin (TTR) variants, 2003. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2003;10:160–84. doi: 10.3109/13506120308998998. [DOI] [PubMed] [Google Scholar]
- 19.Hund E, Linke RP, Willig F, Grau A. Transthyretin-associated neuropathic amyloidosis. Pathogenesis and treatment. Neurology. 2001;56:431–5. doi: 10.1212/wnl.56.4.431. [DOI] [PubMed] [Google Scholar]
- 20.Koo EH, Lansbury PT, Jr., Kelly JW. Amyloid diseases: abnormal protein aggregation in neurodegeneration. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:9989–90. doi: 10.1073/pnas.96.18.9989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Terry CJ, Damas AM, Oliveira P, Saraiva MJ, Alves IL, et al. Structure of Met30 variant of transthyretin and its amyloidogenic implications. The EMBO journal. 1993;12:735–41. doi: 10.1002/j.1460-2075.1993.tb05707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsuzuki T, Mita S, Maeda S, Araki S, Shimada K. Structure of the human prealbumin gene. The Journal of biological chemistry. 1985;260:12224–7. [PubMed] [Google Scholar]
- 23.Buxbaum JN, Tagoe C, Gallo G, Walker JR, Kurian S, Salomon DR. Why are some amyloidoses systemic? Does hepatic “chaperoning at a distance” prevent cardiac deposition in a transgenic model of human senile systemic (transthyretin) amyloidosis? FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012 doi: 10.1096/fj.11-189571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laidman J, Forse GJ, Yeates TO. Conformational change and assembly through edge beta strands in transthyretin and other amyloid proteins. Acc Chem Res. 2006;39:576–83. doi: 10.1021/ar050017s. [DOI] [PubMed] [Google Scholar]
- 25.Gass JN, Gunn KE, Sriburi R, Brewer JW. Stressed-out B cells? Plasma-cell differentiation and the unfolded protein response. Trends in Immunology. 2004;25:17–24. doi: 10.1016/j.it.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 26.Wilson MR, Yerbury JJ, Poon S. Potential roles of abundant extracellular chaperones in the control of amyloid formation and toxicity. Mol Biosyst. 2008;4:42–52. doi: 10.1039/b712728f. [DOI] [PubMed] [Google Scholar]
- 27.Buxbaum JN, Linke RP. A Molecular History of the Amyloidoses. Journal of Molecular Biology. 2012;421:142–59. doi: 10.1016/j.jmb.2012.01.024. [DOI] [PubMed] [Google Scholar]
- 28.Bole DG, Hendershot LM, Kearney JF. Posttranslational association of immunoglobulin heavy chain binding protein with nascent heavy chains in nonsecreting and secreting hybridomas. The Journal of Cell Biology. 1986;102:1558–66. doi: 10.1083/jcb.102.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bonifacino JS, Suzuki CK, Lippincott-Schwartz J, Weissman AM, Klausner RD. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. The Journal of cell biology. 1989;109:73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sitia R, Braakman I. Quality control in the endoplasmic reticulum protein factory. Nature. 2003;426:891–94. doi: 10.1038/nature02262. [DOI] [PubMed] [Google Scholar]
- 31.Sitia R, Neuberger M, Alberini C, Bet P, Fra A, et al. Developmental regulation of IgM secretion: The role of the carboxy-terminal cysteine. Cell. 1990;60:781–90. doi: 10.1016/0092-8674(90)90092-s. [DOI] [PubMed] [Google Scholar]
- 32.Anelli T, Sitia R. Protein quality control in the early secretory pathway. EMBO J. 2008;27:315–27. doi: 10.1038/sj.emboj.7601974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Anken E, Braakman I. Endoplasmic reticulum stress and the making of a professional secretory cell. Critical reviews in biochemistry and molecular biology. 2005;40:269–83. doi: 10.1080/10409230500315352. [DOI] [PubMed] [Google Scholar]
- 34.Sekijima Y, Wiseman RL, Matteson J, Hammarström P, Miller SR, et al. The Biological and Chemical Basis for Tissue-Selective Amyloid Disease. Cell. 2005;121:73–85. doi: 10.1016/j.cell.2005.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Powers ET, Morimoto RI, Dillin A, Kelly JW, Balch WE. Biological and Chemical Approaches to Diseases of Proteostasis Deficiency. Annual Review of Biochemistry. 2009;78:959–91. doi: 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 36.Smith DP, Ashcroft AE, Radford SE. Hemodialysis-Related Amyloidosis. In: Kelly JW, Dobson CM, editors. Protein Misfolding Diseases: Current and Emerging Principles and TherapiesM Ramirez-Alvarado. Wiley; 2010. pp. 347–79. [Google Scholar]
- 37.Floege J, Ketteler M. beta2-microglobulin-derived amyloidosis: an update. Kidney international. Supplement. 2001;78:S164–71. doi: 10.1046/j.1523-1755.2001.59780164.x. [DOI] [PubMed] [Google Scholar]
- 38.Morgan CJ, Gelfand M, Atreya C, Miranker AD. Kidney dialysis-associated amyloidosis: a molecular role for copper in fiber formation. Journal of molecular biology. 2001;309:339–45. doi: 10.1006/jmbi.2001.4661. [DOI] [PubMed] [Google Scholar]
- 39.Benson MD, James S, Scott K, Liepnieks JJ, Kluve-Beckerman B. Leukocyte chemotactic factor 2: A novel renal amyloid protein. Kidney Int. 2008;74:218–22. doi: 10.1038/ki.2008.152. [DOI] [PubMed] [Google Scholar]
- 40.Murphy CL, Wang S, Kestler D, Larsen C, Benson D, et al. Leukocyte Chemotactic Factor 2 (LECT2)-Associated Renal Amyloidosis: A Case Series. American Journal of Kidney Diseases. 2010;56:1100–07. doi: 10.1053/j.ajkd.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merlini G, Palladini G. Amyloidosis: Is a cure possible? Annals of Oncology. 2008;19:63–66. doi: 10.1093/annonc/mdn200. [DOI] [PubMed] [Google Scholar]
- 42.Kumar SK, Gertz MA, Lacy MQ, Dingli D, Hayman SR, et al. Recent improvements in survival in primary systemic amyloidosis and the importance of an early mortality risk score. Mayo Clin Proc. 2011;86:12–8. doi: 10.4065/mcp.2010.0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glenner gG, Cuatrecasas p, Isersky c, Bladen hA, Eanes eD. Physical and chemical properties of amyloid fibers ii. Isolation of a unique protein constituting the major component from human splenic amyloid fibril concentrates. Journal of Histochemistry & Cytochemistry. 1969;17:769–80. doi: 10.1177/17.12.769. [DOI] [PubMed] [Google Scholar]
- 44.Gambetti P, Russo C. Human brain amyloidoses. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 1998;13(Suppl 7):33–40. doi: 10.1093/ndt/13.suppl_7.33. [DOI] [PubMed] [Google Scholar]
- 45.Schroder R, Deckert M, Linke RP. Novel isolated cerebral ALlambda amyloid angiopathy with widespread subcortical distribution and leukoencephalopathy due to atypical monoclonal plasma cell proliferation, and terminal systemic gammopathy. Journal of neuropathology and experimental neurology. 2009;68:286–99. doi: 10.1097/NEN.0b013e31819a87f9. [DOI] [PubMed] [Google Scholar]
- 46.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108:2520–30. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 47.Poshusta TL, Sikkink LA, Leung N, Clark RJ, Dispenzieri A, Ramirez-Alvarado M. Mutations in specific structural regions of immunoglobulin light chains are associated with free light chain levels in patients with AL amyloidosis. PLoS One. 2009;4:e5169. doi: 10.1371/journal.pone.0005169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham RS, Geyer SM, Price-Troska TL, Allmer C, Kyle RA, et al. Immunoglobulin light chain variable (V) region genes influence clinical presentation and outcome in light chain-associated amyloidosis (AL). Blood. 2003;101:3801–08. doi: 10.1182/blood-2002-09-2707. [DOI] [PubMed] [Google Scholar]
- 49.Janeway CA. How the immune system works to protect the host from infection: A personal view. Proceedings of the National Academy of Sciences. 2001;98:7461–68. doi: 10.1073/pnas.131202998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bork P, Holm L, Sander C. The Immunoglobulin Fold: Structural Classification, Sequence Patterns and Common Core. Journal of Molecular Biology. 1994;242:309–20. doi: 10.1006/jmbi.1994.1582. [DOI] [PubMed] [Google Scholar]
- 51.Clarke J, Cota E, Fowler SB, Hamill SJ. Folding studies of immunoglobulin-like beta-sandwich proteins suggest that they share a common folding pathway. Structure. 1999;7:1145–53. doi: 10.1016/s0969-2126(99)80181-6. [DOI] [PubMed] [Google Scholar]
- 52.Feige MJ, Hendershot LM, Buchner J. How antibodies fold. Trends in Biochemical Sciences. 2010;35:189–98. doi: 10.1016/j.tibs.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Obici L, Perfetti V, Palladini G, Moratti R, Merlini G. Clinical aspects of systemic amyloid diseases. Biochimica et biophysica acta. 2005;1753:11–22. doi: 10.1016/j.bbapap.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 54.Zolla S, Buxbaum J, Franklin EC, Scharff MD. Synthesis and assembly of immunoglobulins by malignant human plasmacytes. I. Myelomas producing gamma-chains and light chains. The Journal of experimental medicine. 1970;132:148–62. doi: 10.1084/jem.132.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baumal R, Coffino P, Bargellesi A, Buxbaum J, Laskov R, Scharff MD. The regulation of immunoglobulin synthesis and assembly. Annals of the New York Academy of Sciences. 1971;190:235–49. doi: 10.1111/j.1749-6632.1971.tb13538.x. [DOI] [PubMed] [Google Scholar]
- 56.Aguzzi F, Bergami MR, Gasparro C, Bellotti V, Merlini G. Occurrence of monoclonal components in general practice: clinical implications. European journal of haematology. 1992;48:192–5. doi: 10.1111/j.1600-0609.1992.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 57.Comenzo RL, Zhang Y, Martinez C, Osman K, Herrera GA. The tropism of organ involvement in primary systemic amyloidosis: contributions of Ig V(L) germ line gene use and clonal plasma cell burden. Blood. 2001;98:714–20. doi: 10.1182/blood.v98.3.714. [DOI] [PubMed] [Google Scholar]
- 58.Prokaeva T, Spencer B, Kaut M, Ozonoff A, Doros G, et al. Soft tissue, joint, and bone manifestations of AL amyloidosis: clinical presentation, molecular features, and survival. Arthritis Rheum. 2007;56:3858–68. doi: 10.1002/art.22959. [DOI] [PubMed] [Google Scholar]
- 59.Ozaki S, Abe M, Wolfenbarger D, Weiss DT, Solomon A. Preferential expression of human lambda-light-chain variable-region subgroups in multiple myeloma, AL amyloidosis, and Waldenstrom's macroglobulinemia. Clin Immunol Immunopathol. 1994;71:183–9. doi: 10.1006/clin.1994.1070. [DOI] [PubMed] [Google Scholar]
- 60.Perfetti V, Casarini S, Palladini G, Vignarelli MC, Klersy C, et al. Analysis of V (lambda)-J(lambda) expression in plasma cells from primary (AL) amyloidosis and normal bone marrow identifies 3r (lambda III) as a new amyloid-associated germline gene segment. Blood. 2002;100:948–53. doi: 10.1182/blood-2002-01-0114. [DOI] [PubMed] [Google Scholar]
- 61.Del Pozo Yauner L, Ortiz E, Sanchez R, Sanchez-Lopez R, Guereca L, et al. Influence of the germline sequence on the thermodynamic stability and fibrillogenicity of human lambda 6 light chains. Proteins. 2008;72:684–92. doi: 10.1002/prot.21934. [DOI] [PubMed] [Google Scholar]
- 62.Baden EM, Owen BA, Peterson FC, Volkman BF, Ramirez-Alvarado M, Thompson JR. Altered dimer interface decreases stability in an amyloidogenic protein. J Biol Chem. 2008;283:15853–60. doi: 10.1074/jbc.M705347200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim Y, Wall JS, Meyer J, Murphy C, Randolph TW, et al. Thermodynamic modulation of light chain amyloid fibril formation. J Biol Chem. 2000;275:1570–4. doi: 10.1074/jbc.275.3.1570. [DOI] [PubMed] [Google Scholar]
- 64.Perfetti V, Ubbiali P, Vignarelli MC, Diegoli M, Fasani R, et al. Evidence that amyloidogenic light chains undergo antigen-driven selection. Blood. 1998;91:2948–54. [PubMed] [Google Scholar]
- 65.Bellotti V, Mangione P, Merlini G. Review: immunoglobulin light chain amyloidosis--the archetype of structural and pathogenic variability. J Struct Biol. 2000;130:280–9. doi: 10.1006/jsbi.2000.4248. [DOI] [PubMed] [Google Scholar]
- 66.Davis DP, Gallo G, Vogen SM, Dul JL, Sciarretta KL, et al. Both the environment and somatic mutations govern the aggregation pathway of pathogenic immunoglobulin light chain. Journal of Molecular Biology. 2001;313:1021–34. doi: 10.1006/jmbi.2001.5092. [DOI] [PubMed] [Google Scholar]
- 67.Hurle MR, Helms LR, Li L, Chan W, Wetzel R. A role for destabilizing amino acid replacements in light-chain amyloidosis. Proc Natl Acad Sci U S A. 1994;91:5446–50. doi: 10.1073/pnas.91.12.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Raffen R, Dieckman LJ, Szpunar M, Wunschl C, Pokkuluri PR, et al. Physicochemical consequences of amino acid variations that contribute to fibril formation by immunoglobulin light chains. Protein Sci. 1999;8:509–17. doi: 10.1110/ps.8.3.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Stevens FJ, Argon Y. Pathogenic light chains and the B-cell repertoire. Immunology Today. 1999;20:451–57. doi: 10.1016/s0167-5699(99)01502-9. [DOI] [PubMed] [Google Scholar]
- 70.Wall J, Schell M, Murphy C, Hrncic R, Stevens FJ, Solomon A. Thermodynamic instability of human lambda 6 light chains: correlation with fibrillogenicity. Biochemistry. 1999;38:14101–8. doi: 10.1021/bi991131j. [DOI] [PubMed] [Google Scholar]
- 71.Stevens FJ. Four structural risk factors identify most fibril-forming kappa light chains. Amyloid. 2000;7:200–11. doi: 10.3109/13506120009146835. [DOI] [PubMed] [Google Scholar]
- 72.Khurana R, Gillespie JR, Talapatra A, Minert LJ, Ionescu-Zanetti C, et al. Partially folded intermediates as critical precursors of light chain amyloid fibrils and amorphous aggregates. Biochemistry. 2001;40:3525–35. doi: 10.1021/bi001782b. [DOI] [PubMed] [Google Scholar]
- 73.Qin Z, Hu D, Zhu M, Fink AL. Structural characterization of the partially folded intermediates of an immunoglobulin light chain leading to amyloid fibrillation and amorphous aggregation. Biochemistry. 2007;46:3521–31. doi: 10.1021/bi061716v. [DOI] [PubMed] [Google Scholar]
- 74.Khurana R, Souillac PO, Coats AC, Minert LJ, Ionescu-Zanetti C, et al. A model for amyloid formation in immunoglobulin light chains based on comparison of amyloidogenic and benign proteins and specific antibody binding. Amyloid. 2003;10:97–109. doi: 10.3109/13506120309041731. [DOI] [PubMed] [Google Scholar]
- 75.O'Nuallain B, Thakur AK, Williams AD, Bhattacharyya AM, Chen S, et al. Kinetics and thermodynamics of amyloid assembly using a high-performance liquid chromatography-based sedimentation assay. Methods Enzymol. 2006;413:34–74. doi: 10.1016/S0076-6879(06)13003-7. [DOI] [PubMed] [Google Scholar]
- 76.Baden EM, Randles EG, Aboagye AK, Thompson JR, Ramirez-Alvarado M. Structural insights into the role of mutations in amyloidogenesis. J Biol Chem. 2008;283:30950–6. doi: 10.1074/jbc.M804822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Peterson FC, Baden EM, Owen BA, Volkman BF, Ramirez-Alvarado M. A single mutation promotes amyloidogenicity through a highly promiscuous dimer interface. Structure. 2010;18:563–70. doi: 10.1016/j.str.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Richardson JS, Richardson DC. Natural β-sheet proteins use negative design to avoid edge-to-edge aggregation. Proceedings of the National Academy of Sciences. 2002;99:2754–59. doi: 10.1073/pnas.052706099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kim YS, Cape SP, Chi E, Raffen R, Wilkins-Stevens P, et al. Counteracting effects of renal solutes on amyloid fibril formation by immunoglobulin light chains. J Biol Chem. 2001;276:1626–33. doi: 10.1074/jbc.M007766200. [DOI] [PubMed] [Google Scholar]
- 80.Souillac PO, Uversky VN, Millett IS, Khurana R, Doniach S, Fink AL. Effect of association state and conformational stability on the kinetics of immunoglobulin light chain amyloid fibril formation at physiological pH. J Biol Chem. 2002;277:12657–65. doi: 10.1074/jbc.M109230200. [DOI] [PubMed] [Google Scholar]
- 81.Souillac PO, Uversky VN, Millett IS, Khurana R, Doniach S, Fink AL. Elucidation of the molecular mechanism during the early events in immunoglobulin light chain amyloid fibrillation. Evidence for an off- pathway oligomer at acidic pH. J Biol Chem. 2002;277:12666–79. doi: 10.1074/jbc.M109229200. [DOI] [PubMed] [Google Scholar]
- 82.Stevens PW, Raffen R, Hanson DK, Deng Y-L, Berrios-Hammond M, et al. Recombinant immunoglobulin variable domains generated from synthetic genes provide a system for in vitro characterization of light-chain amyloid proteins. Protein Sci. 1995;4:421–32. doi: 10.1002/pro.5560040309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wetzel R. Domain stability in immunoglobulin light chain deposition disorders. Adv Protein Chem. 1997;50:183–242. doi: 10.1016/s0065-3233(08)60322-8. [DOI] [PubMed] [Google Scholar]
- 84.Davis PD, Raffen R, Dul LJ, Vogen MS, Williamson KE, et al. Inhibition of amyloid fiber assembly by both BiP and its target peptide. Immunity. 2000;13:433–42. doi: 10.1016/s1074-7613(00)00043-1. [DOI] [PubMed] [Google Scholar]
- 85.Martin DJ, Ramirez-Alvarado M. Comparison of amyloid fibril formation by two closely related immunoglobulin light chain variable domains. Amyloid. 2010;17:129–36. doi: 10.3109/13506129.2010.530081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ionescu-Zanetti C, Khurana R, Gillespie JR, Petrick JS, Trabachino LC, et al. Monitoring the assembly of Ig light-chain amyloid fibrils by atomic force microscopy. Proc Natl Acad Sci U S A. 1999;96:13175–9. doi: 10.1073/pnas.96.23.13175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Souillac PO, Uversky VN, Fink AL. Structural transformations of oligomeric intermediates in the fibrillation of immunoglobulin light chain LEN. Biochemistry. 2003;42:8094–104. doi: 10.1021/bi034652m. [DOI] [PubMed] [Google Scholar]
- 88.Ch'ang LY, Yen CP, Besl L, Schell M, Solomon A. Identification and characterization of a functional human Ig V lambda VI germline gene. Mol Immunol. 1994;31:531–6. doi: 10.1016/0161-5890(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 89.del Pozo Yauner L, Ortiz E, Becerril B. The CDR1 of the human lambdaVI light chains adopts a new canonical structure. Proteins. 2006;62:122–9. doi: 10.1002/prot.20779. [DOI] [PubMed] [Google Scholar]
- 90.Wall JS, Gupta V, Wilkerson M, Schell M, Loris R, et al. Structural basis of light chain amyloidogenicity: comparison of the thermodynamic properties, fibrillogenic potential and tertiary structural features of four Vlambda6 proteins. J Mol Recognit. 2004;17:323–31. doi: 10.1002/jmr.681. [DOI] [PubMed] [Google Scholar]
- 91.Blancas-Mejia LM, Tellez LA, del Pozo-Yauner L, Becerril B, Sanchez-Ruiz JM, Fernandez-Velasco DA. Thermodynamic and Kinetic Characterization of a Germ Line Human λ6 Light-Chain Protein: The Relation between Unfolding and Fibrillogenesis. Journal of Molecular Biology. 2009;386:1153–66. doi: 10.1016/j.jmb.2008.12.069. [DOI] [PubMed] [Google Scholar]
- 92.Martsev SP, Dubnovitsky AP, Vlasov AP, Hoshino M, Hasegawa K, et al. Amyloid fibril formation of the mouse V(L) domain at acidic pH. Biochemistry. 2002;41:3389–95. doi: 10.1021/bi015894u. [DOI] [PubMed] [Google Scholar]
- 93.Olsen KE, Sletten K, Westermark P. Fragments of the constant region of immunoglobulin light chains are constituents of AL-amyloid proteins. Biochem Biophys Res Commun. 1998;251:642–7. doi: 10.1006/bbrc.1998.9508. [DOI] [PubMed] [Google Scholar]
- 94.Preud'homme JL, Morel-Maroger L, Brouet JC, Cerf M, Mignon F, et al. Synthesis of abnormal immunoglobulins in lymphoplasmacytic disorders with visceral light chain deposition. The American Journal of Medicine. 1980;69:703–10. doi: 10.1016/0002-9343(80)90421-0. [DOI] [PubMed] [Google Scholar]
- 95.Buxbaum J. Aberrant immunoglobulin synthesis in light chain amyloidosis. Free light chain and light chain fragment production by human bone marrow cells in short-term tissue culture. The Journal of clinical investigation. 1986;78:798–806. doi: 10.1172/JCI112643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Buxbaum J. Mechanisms of disease: monoclonal immunoglobulin deposition. Amyloidosis, light chain deposition disease, and light and heavy chain deposition disease. Hematology/oncology clinics of North America. 1992;6:323–46. [PubMed] [Google Scholar]
- 97.Lavatelli F, Perlman DH, Spencer B, Prokaeva T, McComb ME, et al. Amyloidogenic and associated proteins in systemic amyloidosis proteome of adipose tissue. Molecular & cellular proteomics : MCP. 2008;7:1570–83. doi: 10.1074/mcp.M700545-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Klimtchuk ES, Gursky O, Patel RS, Laporte KL, Connors LH, et al. The Critical Role of the Constant Region in Thermal Stability and Aggregation of Amyloidogenic Immunoglobulin Light Chain. Biochemistry. 2010;49:9848–57. doi: 10.1021/bi101351c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sethi S, Theis JD, Leung N, Dispenzieri A, Nasr SH, et al. Mass Spectrometry–Based Proteomic Diagnosis of Renal Immunoglobulin Heavy Chain Amyloidosis. Clinical Journal of the American Society of Nephrology. 2010;5:2180–87. doi: 10.2215/CJN.02890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sikkink LA, Ramirez-Alvarado M. Biochemical and aggregation analysis of Bence Jones proteins from different light chain diseases. Amyloid. 2008;15:29–39. doi: 10.1080/13506120701815324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shi J, Guan J, Jiang B, Brenner DA, Del Monte F, et al. Amyloidogenic light chains induce cardiomyocyte contractile dysfunction and apoptosis via a non-canonical p38alpha MAPK pathway. Proc Natl Acad Sci U S A. 2010;107:4188–93. doi: 10.1073/pnas.0912263107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sikkink LA, Ramirez-Alvarado M. Cytotoxicity of amyloidogenic immunoglobulin light chains in cell culture. Cell Death and Disease. 2010;1:e98. doi: 10.1038/cddis.2010.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Monis GF, Schultz C, Ren R, Eberhard J, Costello C, et al. Role of endocytic inhibitory drugs on internalization of amyloidogenic light chains by cardiac fibroblasts. The American journal of pathology. 2006;169:1939–52. doi: 10.2353/ajpath.2006.060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Keeling J, Teng J, Herrera GA. AL-amyloidosis and light-chain deposition disease light chains induce divergent phenotypic transformations of human mesangial cells. Lab Invest. 2004;84:1322–38. doi: 10.1038/labinvest.3700161. [DOI] [PubMed] [Google Scholar]
- 105.Arendt BK, Ramirez-Alvarado M, Sikkink LA, Keats JJ, Ahmann GJ, et al. Biologic and genetic characterization of the novel amyloidogenic lambda light chain-secreting human cell lines, ALMC-1 and ALMC-2. Blood. 2008;112:1931–41. doi: 10.1182/blood-2008-03-143040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Berghoff M, Kathpal M, Khan F, Skinner M, Falk R, Freeman R. Endothelial dysfunction precedes C-fiber abnormalities in primary (AL) amyloidosis. Ann Neurol. 2003;53:725–30. doi: 10.1002/ana.10552. [DOI] [PubMed] [Google Scholar]
- 107.Wittich CM, Neben-Wittich MA, Mueller PS, Gertz MA, Edwards WD. Deposition of amyloid proteins in the epicardial coronary arteries of 58 patients with primary systemic amyloidosis. Cardiovasc Pathol. 2007;16:75–8. doi: 10.1016/j.carpath.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 108.Neben-Wittich MA, Wittich CM, Mueller PS, Larson DR, Gertz MA, Edwards WD. Obstructive intramural coronary amyloidosis and myocardial ischemia are common in primary amyloidosis. Am J Med. 2005;118:1287. doi: 10.1016/j.amjmed.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 109.Liao R, Jain M, Teller P, Connors LH, Ngoy S, et al. Infusion of Light Chains From Patients With Cardiac Amyloidosis Causes Diastolic Dysfunction in Isolated Mouse Hearts. Circulation. 2001;104:1594–97. [PubMed] [Google Scholar]
- 110.Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, et al. Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol. 2010;145:67–8. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Buxbaum JN. Animal models of human amyloidoses: are transgenic mice worth the time and trouble? FEBS Lett. 2009;583:2663–73. doi: 10.1016/j.febslet.2009.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ward JE, Ren R, Toraldo G, Soohoo P, Guan J, et al. Doxycycline reduces fibril formation in a transgenic mouse model of AL amyloidosis. Blood. 2011;118:6610–7. doi: 10.1182/blood-2011-04-351643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Merlini G, Ascari E, Amboldi N, Bellotti V, Arbustini E, et al. Interaction of the anthracycline 4'-iodo-4'-deoxydoxorubicin with amyloid fibrils: inhibition of amyloidogenesis. Proceedings of the National Academy of Sciences. 1995;92:2959–63. doi: 10.1073/pnas.92.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Almeida MR, Saraiva MJ. Clearance of extracellular misfolded proteins in systemic amyloidosis: Experience with transthyretin. FEBS Letters. doi: 10.1016/j.febslet.2012.07.029. In press. [DOI] [PubMed] [Google Scholar]
- 115.Wall JS, Richey T, Allen A, Donnell R, Kennel SJ, Solomon A. Quantitative tomography of early-onset spontaneous AA amyloidosis in interleukin 6 transgenic mice. Comp Med. 2008;58:542–50. [PMC free article] [PubMed] [Google Scholar]
- 116.Wall JS, Richey T, Williams A, Stuckey A, Osborne D, et al. Comparative Analysis of Peptide p5 and Serum Amyloid P Component for Imaging AA Amyloid in Mice Using Dual-Isotope SPECT. Mol Imaging Biol. 2011 doi: 10.1007/s11307-011-0524-0. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tagoe CE, French D, Gallo G, Buxbaum JN. Amyloidogenesis is neither accelerated nor enhanced by injections of preformed fibrils in mice transgenic for wild-type human transthyretin: the question of infectivity. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2004;11:21–6. doi: 10.1080/13506120410001674982. [DOI] [PubMed] [Google Scholar]
- 118.Tagoe CE, Reixach N, Friske L, Mustra D, French D, et al. In vivo stabilization of mutant human transthyretin in transgenic mice. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2007;14:227–36. doi: 10.1080/13506120701464396. [DOI] [PubMed] [Google Scholar]
- 119.Wei L, Kawano H, Fu X, Cui D, Ito S, et al. Deposition of transthyretin amyloid is not accelerated by the same amyloid in vivo. Amyloid : the international journal of experimental and clinical investigation : the official journal of the International Society of Amyloidosis. 2004;11:113–20. doi: 10.1080/13506120410001726344. [DOI] [PubMed] [Google Scholar]
- 120.Santos SD, Magalhaes J, Saraiva MJ. Activation of the heat shock response in familial amyloidotic polyneuropathy. Journal of neuropathology and experimental neurology. 2008;67:449–55. doi: 10.1097/NEN.0b013e31816fd648. [DOI] [PubMed] [Google Scholar]
- 121.Bulawa CE, Connelly S, Devit M, Wang L, Weigel C, et al. Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:9629–34. doi: 10.1073/pnas.1121005109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Lai Z, McCulloch J, Lashuel HA, Kelly JW. Guanidine hydrochloride-induced denaturation and refolding of transthyretin exhibits a marked hysteresis: equilibria with high kinetic barriers. Biochemistry. 1997;36:10230–9. doi: 10.1021/bi963195p. [DOI] [PubMed] [Google Scholar]
- 123.Lashuel HA, Lai Z, Kelly JW. Characterization of the transthyretin acid denaturation pathways by analytical ultracentrifugation: implications for wild-type, V30M, and L55P amyloid fibril formation. Biochemistry. 1998;37:17851–64. doi: 10.1021/bi981876+. [DOI] [PubMed] [Google Scholar]
- 124.Miroy GJ, Lai Z, Lashuel HA, Peterson SA, Strang C, Kelly JW. Inhibiting transthyretin amyloid fibril formation via protein stabilization. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:15051–6. doi: 10.1073/pnas.93.26.15051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Colon W, Kelly JW. Partial denaturation of transthyretin is sufficient for amyloid fibril formation in vitro. Biochemistry. 1992;31:8654–60. doi: 10.1021/bi00151a036. [DOI] [PubMed] [Google Scholar]
- 126.Johnson SM, Connelly S, Fearns C, Powers ET, Kelly JW. The Transthyretin Amyloidoses: From Delineating the Molecular Mechanism of Aggregation Linked to Pathology to a Regulatory-Agency-Approved Drug. Journal of molecular biology. 2012;421:185–203. doi: 10.1016/j.jmb.2011.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Coelho T, Maia LF, Martins da Silva A, Waddington Cruz M, Planté-Bordeneuve V, et al. Tafamidis for transthyretin familial amyloid polyneuropathy. Neurology. 2012;79:785–92. doi: 10.1212/WNL.0b013e3182661eb1. [DOI] [PMC free article] [PubMed] [Google Scholar]