Abstract
Prenatal glucocorticoids (GCs) are routinely used for pregnant women in preterm labor to prevent respiratory distress syndrome and intraventricular hemorrhage in premature infants. However, the effect of antenatal GCs on neurogenesis in preterm neonates remains elusive. Herein, we hypothesized that prenatal GCs might suppress both glutamatergic and GABAergic neurogenesis in preterm rabbits and that this treatment would induce distinct changes in the expression of transcription factors regulating these developmental events. To test our hypotheses, we treated pregnant rabbits with betamethasone at E27 and E28, delivered the pups at E29 (term=32d), and assessed neurogenesis at birth and postnatal day 3. We quantified radial glia (Sox2+) and intermediate progenitor cells (Tbr2+) in the dorsal cortical subventricular zone to assess glutamatergic neuronal progenitors, and counted Nkx2.1+ and Dlx2+ cells in the ganglionic eminence to evaluate GABAergic neurogenesis. In addition, we assayed transcription factors regulating neurogenesis. We found that prenatal GCs did not affect the densities of radial glia and intermediate progenitors of glutamatergic or GABAergic neurons. The number of GABA+ interneurons in the ganglionic eminence was similar between the prenatal GC treated pups compared to untreated controls. Moreover, the mRNA expression of transcription factors, including Pax6, Ngn1/2, Emx1/2, Insm1, Dlx1, Nkx2.1, and Gsh2, were comparable between the two groups. However, there was a transient elevation in Mash1 protein in betamethasone treated pups relative to controls at birth. This data suggests that prenatal GC treatment does not significantly impact the balance of glutamatergic and GABAergic neurogenesis in premature infants.
Keywords: neurogenesis, betamethasone, glucocorticoid, glutamatergic, GABAergic, prematurity
Introduction
Use of prenatal glucocorticoids (GCs) in pregnant women who are in preterm labor remains the standard of care worldwide (Crowley, 2000). This treatment prevents respiratory distress syndrome and intraventricular hemorrhage in premature infants, and also enhances their survival. Indeed, the National Institute of Health Consensus Development Panel on the “Effect of corticosteroids for fetal maturation on perinatal outcomes” has recommended use of prenatal GC in premature labor (1994). Every year, about 15 million infants are born premature worldwide; and 75% of women in preterm labor (≤34 gestational weeks) are treated with GCs. Since neurogenesis continues during the third trimester (Malik et al., 2013), here we asked whether prenatal steroids would affect neurogenesis in prematurely delivered infants.
Prenatal GCs induce hippocampal degeneration and reduce brain growth in experimental animals (Uno et al., 1990, Kutzler et al., 2004). Multiple courses of prenatal GCs diminish head circumference and are associated with behavioral problems (Crowther et al., 2006, Hansen et al., 2013). In preterm infants, postnatal GCs are associated with a 35% reduction in cortical gray matter volume, abnormal frontal microstructure and a lower mental developmental index at 2 years of age (Inder et al., 2005). Therefore, it is extremely important to assess neurogenesis in premature infants exposed to prenatal GCs.
In primates, glutamatergic neurogenesis is orchestrated in the ventricular (VZ) and subventricular zones (SVZ) of the dorsal telencephalon, while GABAergic neurogenesis takes place primarily in the ventral ganglionic eminence and perhaps in the dorsal telencephalon as well ((Jakovcevski et al., 2011, Hansen et al., 2013, Ma et al., 2013). Glutamatergic neurogenesis in the dorsal telencephalon is orchestrated under the influence of graded expression of transcription factors including Pax6, Neurogenin (Ngn) 1/2, Emx1/2, and Insm1 (Englund et al., 2005, Bernardino et al., 2008, Lui et al., 2011). Likewise, interneuron neurogenesis is regulated by ventral transcription factors, including Nkx2.1, Dlx1/2, Lhx6/8, and Mash1. Controlling these transcription factor gradients are the effectors of sonic hedgehog (Shh), Notch and Wnt signaling pathways, which coordinate neurogenesis (Boku et al., 2010, Wang et al., 2011). Additionally, GC treatment induces cell cycle arrest and apoptosis of immature neurons and neuronal progenitors in the hippocampus (Crochemore et al., 2002, Yu et al., 2010). Accordingly, prenatal steroids induce apoptosis and gliosis in preterm rabbits with an associated increase in TGF-β expression (Vinukonda et al., 2010). However, a single dose of prenatal dexamethasone reduces apoptosis of non-neuronal cells at 70% gestation, but not at 90% gestation, in ovine cerebral cortex (Malaeb et al., 2009). Thus, the effect of GCs is context-dependent. Based on these considerations, we hypothesized that prenatal GCs would suppress both glutamatergic and GABAergic neurogenesis in preterm rabbits and that this might induce distinct changes in the expression of transcription factors regulating these biological events. To test our hypotheses, we used our preterm rabbit model (E29, Term=32d) because rabbits exhibit perinatal brain growth and have a gyrencephalic cerebral cortex, just as humans. Contrary to our hypotheses, we found that prenatal GCs impact neither glutamatergic nor GABAergic neurogenesis and that transcription factors regulating these processes were mostly unaffected. This study highlights that prenatal GCs do not disrupt the fine balance of glutamatergic and GABAergic neurogenesis in the cerebral cortex of preterm rabbit pups.
Materials and Methods
Animal care and betamethasone treatment
Animal protocol was approved by the Institutional Animal Care and Use Committee of New York Medical College, Valhalla, NY. Timed-pregnant New Zealand rabbits were obtained from Charles River Laboratories, Inc, Wilmington MA. Rabbits were sequentially randomized to receive either betamethasone intramuscularly (n=5) or no treatment (n=5 each). C-section was performed at E29 gestational age. Pups were dried immediately and kept warm in an infant incubator at 35°C and 80% humidity. After stabilization of their condition, we weighed the pups. The pups were fed 2–3 ml (100 ml/kg) of puppy formula (Esbilac, Petag, IL) twice daily for two days; the feed was advanced to 125 ml/kg at postnatal day 3. Live born and healthy looking pups of either sex were included in the study. Pups with postnatal complications including cardiac arrest requiring resuscitation and any apparent congenital defect were excluded from the study.
Betamethasone treatment
Pregnant rabbits received betamethasone (American Regent, Inc, Shirley, NJ) in a dose of 0.2 mg/kg/dose on E27 and E28 for a total of 2 doses 24 h apart. The dose of betamethasone administered to the pregnant rabbit was based on a) the recommended prenatal dose (12.5 mg/daily, 2 doses) for pregnant women in preterm labor to prevent respiratory distress syndrome in premature infants and b) the dose used in animal models by previous investigators (Lohninger et al., 1994, Mutafoglu et al., 2004). For these experiments, pups were sacrificed at 2 h (0d) or 72 h (3d) postnatal age.
Rabbit tissue collection and processing
Brain tissue was processed as described previously (Malik et al., 2013). Coronal slices were fixed in 4% paraformaldehyde in phosphate buffered saline (PBS; 0.1 M, pH 7.4) overnight for most immunohistochemical analysis. For GABA analysis, slices were fixed in 1% glutaraldehyde with 4% paraformaldehyde in PBS for 2 h. Tissue was then cryoprotected by incubation in PBS containing 15% sucrose, then 30% sucrose, for 24 h each. Finally, tissue was frozen after embedding into optimum cutting temperature compound (Sakura, Japan). A cryostat was used to cut frozen coronal sections of 30 μm thickness. For Western blot analyses and real time qPCR, a coronal slice of 1–2 mm thickness was collected at the level of the midseptal nucleus and snap frozen on dry ice.
Immunohistochemistry
Immunostaining was performed as described previously (Vinukonda et al., 2013). Primary antibodies used in these experiments included: goat polyclonal Sox2 (catalog #SC-6895, Santa Cruz Biotechnology), rabbit polyclonal Tbr2 (T-brain gene 2; courtesy of Robert Hevner, Seattle, WA), rabbit polyclonal Nkx2.1 (catalog #ab133638, Abcam), guinea pig polyclonal Dlx2 (Kuwajima et al., 2006), rabbit polyclonal GABA (catalog #A2052, Sigma), mouse monoclonal Ki67 (catalog #M7240; Dako). Secondary antibodies used were Alexa Fluor® 488- Donkey Anti-Mouse IgG (catalog #715-545-150), Alexa Fluor® 488-AffiniPure Donkey Anti-Guinea pig IgG (catalog #706-545-148), Alexa Fluor® 594-AffiniPure Donkey Anti-Goat IgG (catalog #705-585-147) from Jackson Immunoresearch (West Grove, PA). Briefly, fixed sections were hydrated with 0.01 M PBS, blocked in normal donkey serum in PBS with 0.01% Triton-X, and incubated with primary antibodies diluted in PBS overnight at 4°C. Sections were next washed in PBS and incubated in secondary antibody diluted in PBS containing 1% normal donkey serum. After washing in PBS, sections were mounted with Slow Fade Light Antifade reagent (Molecular Probes, Invitrogen, CA). Sections were visualized under a Confocal microscope (Nikon Instruments, Japan), and stereology was performed using a fluorescent microscope (Axioskop 2 plus, Carl Zeiss, Inc) with motorized specimen stage for automated sampling (ASI, Eugene, OR), CCD color video camera (Microfire; Optronics, Goleta, CA) and stereology software (Stereologer, SRC, Baltimore, MD).
Stereological analysis of neural progenitors
Unbiased stereology methods, with assistance from a computerized software system (Stereologer, Stereology Resource Center, Chester, MD), were used to quantify a number of parameters in two brain regions; a) dorsal VZ and SVZ underneath the corona radiata and corpus callosum and b) ganglionic eminence (forms the lateral margin of the cerebral ventricles). Briefly, coronal sections were cut on cryostat at a setting of 30 μm thickness with a section sampling interval of three (90 μm) to obtain at least 6 sections at the level of mid-septal nucleus. The sections were triple-labeled with Sox2/Tbr2/Nkx2.1/Dlx2 and Ki67 antibodies with DAPI (nuclear stain) and quantified as follows. The reference spaces (dorsal VZ and SVZ) were first outlined on the section under 5x objective. The volume of the outlined area (reference space) was quantified using a point counting probe (frame 25 μm × 25 μm; guard zone 2 μm; inter-frame interval = 300 μm). The total number of cells labeled with their respective antibodies through a defined reference space was quantified using the optical dissector probe under 60x oil lens. For the optical dissector probe (frame 25 μm × 25 μm; guard zone 1 μm, interframe interval 200 μm), the user clicked on the objects within the dissector frame. Sampling continued until the coefficient of error (CE) was less than 0.10. To assess cells co-labeled with Ki67 and Sox2, Tbr2 or other antibodies, we used a dual color filter (Filter set 74 HE GFP+mRFP shift free, Zeiss, Thornwood, NY) that visualizes both Alexa 488 and Alexa 594 labeled cells.
Quantification of GABA+ cells
GABA+ cells were quantified using a fluorescent microscope and CCD color video camera using Picture Frame software (Optronics, Goleta, CA). The counting was done in representative images taken from the ganglionic eminence of each coronal brain section in an unbiased manner using a 60x objective. We counted GABA+ neurons in approximately six to eight images per section in each brain (6–8 images × 4–6 sections × 5 brains each group). The investigator was blinded to the experimental group.
Fluorescent in situ detection of DNA fragmentation (TUNEL)
TUNEL staining was performed on fixed brain sections as described previously (Vose et al., 2013). Tissue sections were air dried on slides, hydrated in 0.01 M PBS, then permeabilized in 1:1 ethanol/acetic acid for 5 min. A commercially available ApopTag-fluorescein kit (catalog #S7110; Millipore) was used to visualize apoptotic TUNEL+ cells.
Western blot analyses
We homogenized the frozen brain tissue in sample buffer (3% SDS, 10% glycerol and 62.5 mM Tris-HCl) using a glass homogenizer and briefly sonicated the lysate prior to centrifugation. Total protein concentration of the supernatant was measured using a BCA Protein Assay Kit (Pierce, Thermo Scientifics, IL). Equal amounts of protein (10–20 μg) were loaded onto 4–15% or 4–20% gradient precast gels (BioRad, CA), based on the molecular weight of the target protein. Separated proteins were transferred onto polyvinylidene difluoride (PVDF) membrane by electro-transfer. Membranes were then incubated overnight with primary antibodies, including mouse monoclonal Dlx1 (clone L43/40, NeuroMab, CA), mouse monoclonal Gad67 (clone 1G10.2, Millipore), mouse monoclonal Mash1, (catalog #556604, BD Bioscience), and mouse polyclonal Pax6 (catalog #H00005080, Abnova). We detected target proteins with chemiluminescence ECL system (GE Healthcare) by using secondary antibodies conjugated with horseradish peroxidase (catalog #115-035-146, Jackson Immunoresearch, West Grove, PA). Membranes were stripped and reprobed with mouse monoclonal β-actin (catalog #A5316, Sigma, MO). Densitometry was performed as described previously (Ballabh et al., 2007) using ImageJ. The optical density (OD) values for each sample were normalized using the corresponding β-actin values.
Quantitative real-time polymerase chain reaction
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed as previously described (Ballabh et al., 2007). Briefly, a frozen 1–2 mm brain slice from the level of the mid-septal nucleus was used for isolation of total RNA by RNeasy Mini Kit (catalog #74104, Qiagen). cDNA was synthesized (Kit #05081955001, Roche) and analysis of mRNA expression was performed by qRT-PCR using SYBR-Green (catalog #04913850001, Roche) and an ABI PRISM 7700 Sequence Detection System (Applied Biosystems, USA). The efficiency-corrected ΔΔCT method was used for quantification. The following primers were used: Dlx1 (accession #XM_002712289.1) sense TTCCAGCAAACTCAGTACCTGGCT, antisense GCTTCTTGAACTTGGAGCGCTTGT; Emx1 (accession #BC037242.1) sense TCCAGAACCGGAGGACAAAGTACA, antisense TGATGTGATGGGAGCCCTTCTTCT; Emx2 (accession #AF301598.1) sense AAGCGCTGCTTCACCATCGAGT, antisense AGCCGTTGAGGAACGGATTTATGG; Gad67 (accession #XM_002716218.1) sense TTTGGTACATTCCACCGAGCCTCA, antisense AGCCCAACATCAGACTTCCCTTCT; Gsh2 (accession #XM_002721782.1) sense GAGTTCTCGTCCAACATGTACC, antisense GGCGGTTCTGAAACCAGATT; Insm1 (accession #NM_002196.2) sense ACTTCGAGGACGAGGTGACCA, antisense TTGCACAGCTGGCAGATGAACT; Mash1 (accession #XM_002711229.1) sense CTGGTGAACCTGGGCTTT, antisense CGTCTCCACCTTGCTCATC; Ngn1 (accession #XM_002710221.1) sense AACCGCATGCACAACCTGAA, antisense AAGCGTAGCGTCTCGATCTT; Ngn2 (accession #XM_002717014.1) sense GCATCAAGAAGACACGCAGACTGA, antisense TCTCGATCTTGGTTAGCTTGGCGT; Nkx2.1 (accession #NM_003317.3) sense GCGACGCTTCAAGCAACAGAAGTA, antisense GGTTCTGGAACCAGATCTTGAC; Pax6 (accession #NM_001082217.1) sense CCCGTCCATCTTTGCTTGGGAAAT, antisense TAGCCAGGTTGCGAAGAACTCTGT; GAPDH (accession #NM_001082253.1) sense ATCAAGAAGGTGGTGAAGCAGGCA, antisense GCTTCACAAAGTGGTCATTGAGGG.
Statistics
Data are expressed as means ± SEM. To determine differences in the density of total and cycling progenitor cells in the dorsal SVZ or ganglionic eminence between betamethasone treated pups and untreated controls at two time points, 0d and 3d, we used two-way ANOVA. Similarly, for qRT-PCR and Western blot analyses, we also used two-way ANOVA. All post hoc comparisons to test for differences between means were done using Tukey’s multiple comparison test at the 0.05 significance level.
Results
Prenatal betamethasone stunts intrauterine growth of the newborn pups
To determine the effect of prenatal GCs on the growth of preterm rabbits, we compared the weight of pups exposed to prenatal betamethasone and unexposed controls. We found that the birth weight of preterm pups exposed to betamethasone was significantly less compared to controls (mean weight: 30 ± 0.57 g vs. 41.7 ± 0.58 g; P<0.01). This data suggests that prenatal GCs reduce fetal growth.
Prenatal betamethasone does not affect glutamatergic neurogenesis
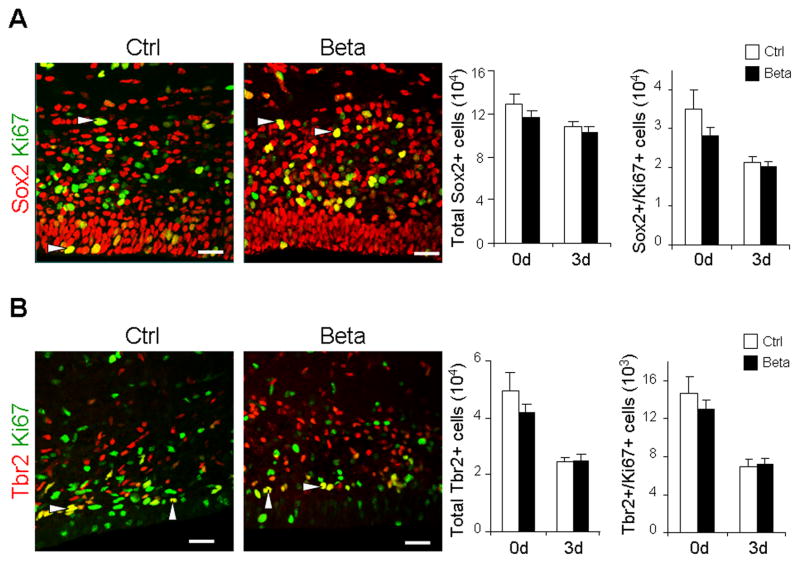
To determine whether antenatal betamethasone treatment significantly affected glutamatergic neurogenesis, we compared the density of radial glia and intermediate progenitor cells (IPC) in the dorsal SVZ of preterm rabbit pups exposed to prenatal betamethasone versus untreated controls, using a stereology protocol. Radial glia and IPC were identified by immunolabeling with Sox2 and Tbr2 antibodies, respectively, while proliferating cells were labeled with Ki67 antibodies. The number of total and proliferating Sox2+ cells was comparable between betamethasone treated pups and untreated controls at both 0d (2 hours) and 3d (72 hours; Fig. 1A). The number of total and cycling Tbr2+ cells was also similar in treated and control pups at both 0d and 3d (Fig. 1B). Together, these data suggest that prenatal betamethasone does not affect glutamatergic neurogenesis in the dorsal SVZ of preterm rabbit pups.
Fig. 1. Prenatal betamethasone treatment does not affect the density of radial glia and IPC.
Representative immunofluorescence of cryosections from dorsal SVZ of preterm pups (0d) treated with betamethasone (Beta) and controls (Ctrl) labeled with Sox2 and Ki67 (A) or Tbr2 and Ki67 (B) specific antibodies. Arrowhead indicates Sox2+Ki67+ or Tbr2+Ki67+ cells. Data are mean ± SEM (n=5 each group). Bar graphs show that both total and cycling Sox2+ or Tbr2+ cells were comparable between the groups. Scale bar, 20 μm.
Antenatal betamethasone does not influence GABAergic neurogenesis
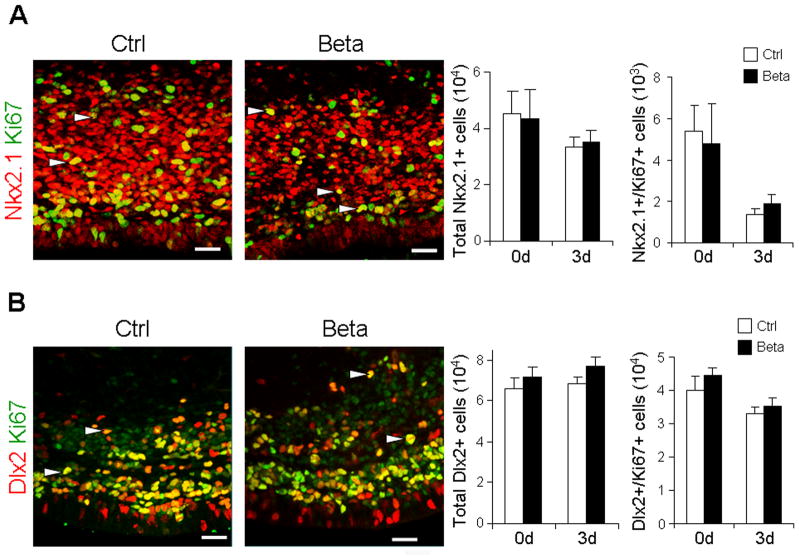
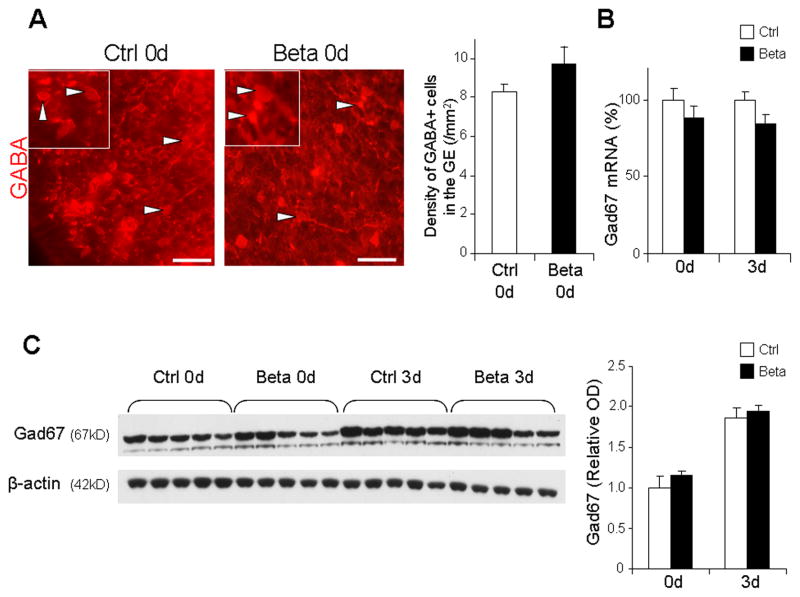
To determine whether prenatal betamethasone treatment affected GABAergic neurogenesis, we evaluated two distinct lineages of GABAergic neurons—Nkx2.1+ and Dlx2+ progenitors. Nkx2.1 and Dlx2 are major transcription factors expressed in the ganglionic eminence and play key roles in specification of interneurons (Wonders and Anderson, 2006). We quantified Nkx2.1+ and Dlx2+ interneuron progenitors in the ganglionic eminence of preterm rabbit pups exposed to prenatal betamethasone and untreated controls. Stereological analyses of immunostained coronal sections revealed that the number of total and proliferating Nkx2.1+ cells were comparable between pups treated with prenatal betamethasone and untreated controls at both at 0d and 3d (Figs. 2A). Likewise, total and cycling Dlx2+ cells were similar in number in pups exposed versus unexposed to prenatal betamethasone (Fig. 2B). To further evaluate the effect of prenatal betamethasone on GABAergic neurogenesis, we compared the density of GABA+ interneurons in the ganglionic eminence between betamethasone treated pups and untreated controls at birth. We found that the abundance of GABA+ interneurons were comparable between the two groups (Fig. 3A). Glutamate decarboxylase (Gad67) is an enzyme involved in GABA synthesis and is expressed by GABAergic neurons. Therefore, we quantified both Gad67 mRNA and protein levels. Real-time qPCR revealed that Gad67 mRNA expression was comparable between the two groups at both 0d and 3d (Fig. 3B). Consistent with mRNA levels, Western blot analysis showed that Gad67 protein concentration was similar in the forebrain homogenates of pups exposed to prenatal betamethasone and unexposed controls (Fig. 3C). Together, these data suggest that prenatal betamethasone does not affect GABAergic neurogenesis.
Fig. 2. Nkx2.1+ or Dlx2+ progenitors are unaffected by prenatal betamethasone treatment.
Representative immunofluorescence of cryosections from ganglionic eminence of 3d pups exposed (Beta) and unexposed (Ctrl) to prenatal betamethasone, labeled with Nkx2.1 and Ki67 (A) or Dlx2 and Ki67 (B). Arrowhead indicates Nkx2.1+ or Dlx2+ cells co-labeled with Ki67. Bar graphs are mean ± SEM (n=5 each group). The number of total and cycling Nkx2.1+ and Dlx2+ cells were similar between betamethasone treated pups and untreated controls. Scale bar, 20 μm.
Fig. 3. Betamethasone treatment does not affect the density of GABA+ interneurons.
A. Representative coronal sections from the ganglionic eminence of 0d pups treated with betamethasone (Beta) and untreated control (Ctrl) labeled with GABA antibodies. Inset shows GABA+ cells under high magnification. Bar graphs are mean ± SEM (n=5 each group). The density of GABA+ cells (arrowhead) was similar between the pups treated with betamethasone compared to controls. Scale bar, 25 μm. B. Gad67 mRNA expression in betamethasone treated pups (Beta) and untreated controls (Ctrl) at 0d and 3d. Bar graph shows mean ± SEM (n=5 each group). Note that Gad67 mRNA levels were comparable between groups. C. Representative Western blot analyses of Gad67 in betamethasone treated pups and untreated controls at 0d and 3d. Bar graphs are mean ± SEM (n=5 each group). Protein concentration normalized to β-actin. Gad67 protein levels were similar between the groups.
Effect of prenatal betamethasone on transcription factors regulating neurogenesis
Contrary to our expectation, prenatal betamethasone did not affect the number of glutamatergic or GABAergic progenitor cells. However, both epidemiological and neuroimaging studies have revealed that the use of postnatal steroids in preterm infants results in reduced cortical gray matter volume, abnormal frontal microstructure, and lower mental developmental index at 2 years of age (Inder et al., 2005). Moreover, multiple courses of prenatal steroids also reduce cortical growth (Crowther et al., 2006). Hence, it was important to assess the transcriptional networks of genes that play key roles in specification and maintenance of glutamatergic and GABAergic neurogenesis to further confirm that prenatal GCs do not affect neurogenesis.
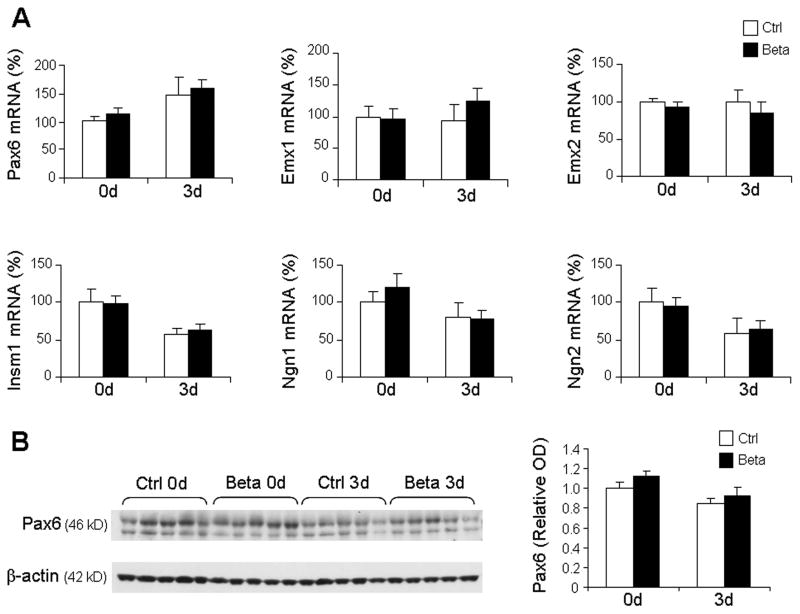
To this end, we compared mRNA and protein levels of transcription factors regulating glutamatergic neurogenesis, including Pax6, Emx1/2, Insm1, or Ngn1/2. Real time qPCR revealed that the expression of Pax6, Emx1/2, Insm1, and Ngn1/2 were comparable between betamethasone treated pups and untreated controls at 0d and 3d (Fig. 4A). Consistent with gene expression, Western blot analysis revealed that the protein levels of Pax6 were comparable between the two groups at both epochs (Fig. 4B). Western blot analyses for other transcription factors could not be successfully performed with commercially available antibodies on our rabbit brain homogenates.
Fig. 4. Prenatal betamethasone does not affect Pax6, Emx1/2, Insm1, or Ngn1/2 transcription factors.
A. mRNA levels were assayed by real time qPCR in betamethasone treated pups and untreated controls at 0d and 3d. Bar graphs show mean ± SEM (n=6 each group). Transcription factors, as indicated, were comparable between groups. B. Representative Western blot analyses of Pax6 in betamethasone treated pups and untreated controls at 0d and 3d. Bar graphs are mean ± SEM (n=5 each group). Protein concentration normalized to β-actin. Pax6 protein levels were similar between the groups.
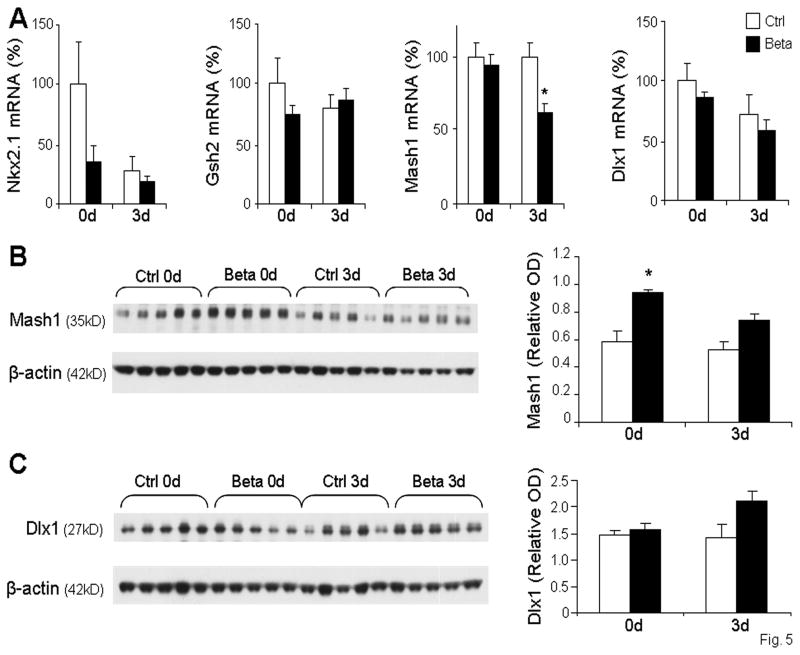
We next compared mRNA and protein levels of key transcription factors regulating GABAergic neurogenesis, including Nkx2.1, Gsh2, Mash1, and Dlx1. Real time qPCR revealed that there was no significant difference between the expression of Nkx2.1, Gsh2, or Dlx1 between rabbit pups exposed to betamethasone and untreated controls at either 0d or 3d (Fig. 5A). However, Mash1 mRNA levels were significantly reduced in betamethasone treated pups compared to controls at 3d, but not at 0d (Fig. 5A). Western blot analysis revealed significant increases in Mash1 protein in betamethasone treated pups compared to untreated controls at 0d, but not at 3d (Fig. 5B). Dlx1 protein levels showed a non-significant trend toward increase in 3d pups (p=0.052), but not at 0d (Fig. 5C). Together, these data suggest that betamethasone treatment does not significantly affect transcription factors regulating neurogenesis.
Fig. 5. Prenatal betamethasone transiently increases Mash1 protein levels, but does not affect other transcription factors regulating GABAergic neurogenesis.
mRNA levels of transcription factors were measured by qRT-PCR in betamethasone treated pups (Beta) and untreated controls (Ctrl) at 0d and 3d. Bar graphs show mean ± SEM (n=6 each group). Nkx2.1, Gsh2, and Dlx1 gene expression were comparable between treated groups and controls. However, Mash1 mRNA accumulation was decreased in betamethasone treated pups compared to controls at 3d, but not at 0d. B. Representative Western blot for Mash1, normalized to β-actin, shows an increase in protein in betamethasone treated pups versus controls at 0d, but not 3d. Bar graphs show mean ± SEM (n=5 each group). C. Representative Western blot for Dlx1, normalized to β-actin. Bar graphs show mean ± SEM (n=5 each group). *P<0.05, betamethasone treated pups vs. untreated controls.
Prenatal betamethasone does not increase apoptosis of neuronal precursors
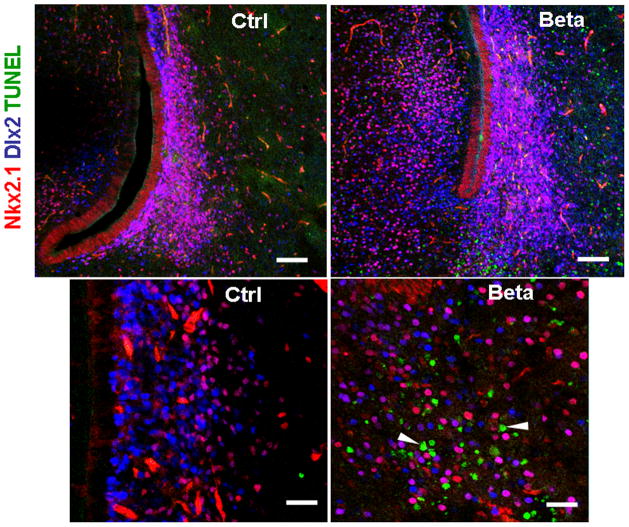
In our previous studies, we have shown that prenatal betamethasone exposure in E29 pups results in an increase in apoptosis of cells in the forebrain of treated pups compared to controls (Vinukonda et al., 2010). To determine the specific effect of prenatal betamethasone treatment on interneuron precursor cells, we triple labeled brain sections from 0d and 3d pups with TUNEL stain, Nkx2.1 and Dlx2 antibodies (n=4 each group). TUNEL+ cells were more abundant in betamethasone treated pups compared to controls, as in our previous report. However, Nkx2.1+ or Dlx2+ cells co-labeled with TUNEL were infrequent (<0.1% of all Nkx2.1+ or Dlx2+ cells, Fig. 6) in the betamethasone treated pups. In addition, TUNEL+ apoptotic cells were rare in the dorsal SVZ where Tbr2+ glutamatergic precursor cells are present. These data suggest that apoptosis caused by prenatal betamethasone treatment does not significantly reduce the population of GABAergic nor glutamatergic precursor cells.
Fig. 6. Prenatal betamethasone does not increase apoptosis of GABAergic precursor cells.
Representative image from the periventricular region of 3d betamethasone treated (Beta) and untreated (Ctrl) pups. Note the increased amount of apoptosis (arrowhead) in betamethasone treated pups. Scale bar, 50 μm (upper panel), 20 μm (lower panel)
Discussion
In the United States, the preterm birth rate is 12.5% and approximately 75% of women in labor with gestational age of less than 34 weeks are treated with GCs to prevent respiratory distress syndrome and intraventricular hemorrhage in premature infants (Meadow et al., 2003). Hence, it is enormously important to determine the effect of prenatal GCs on ongoing neurogenesis in the brain of preterm neonates. We found that antenatal GCs did not impact glutamatergic or GABAergic neurogenesis in prematurely delivered rabbit pups and that the key transcription factors controlling generation of neurons remain unaffected.
We assessed the effects of prenatal GCs on glutamatergic neurogenesis. Glutamatergic neocortical neurons are generated during embryonic development from two distinct types of progenitor cells: radial glia and IPCs. IPCs, which are generated from radial glia, proliferate for one or two mitotic cycles to produce glutamatergic neurons. Tbr2 is a well-established marker for IPC and is abundantly expressed in these progenitors. We found that the number of Tbr2+ IPCs and Sox2+ radial glia were unaltered by GC treatment. This is the first study that has directly demonstrated the effect of prenatal GCs on glutamatergic neurogenesis. Since 80% of neurons in the cerebral cortex are contributed by glutamatergic projection neurons, and repeated doses of GCs can reduce cortical growth and head circumference, it was important to assess the effect of GCs on glutamatergic neurogenesis.
Another key finding of this study is that antenatal GC did not affect GABAergic neurogenesis. GABAergic interneurons primarily originate from the ventral telencephalon, predominantly from the medial ganglionic eminence. Radial glia give rise to distinct lineages, including Dlx1/2+ and Nkx2.1+ progenitors, which mature into GABAergic interneurons. In this study, we quantified Dlx2+ and Nkx2.1+ progenitors by immunohistochemistry and also counted GABA+ cells in the ganglionic eminence to determine the effect of GCs on generation of interneurons. Our results revealed that GC treatment did not impact the density of Dlx2+, Nkx2.1+, or GABA+ cells in the ganglionic eminence. This is again the first study that has evaluated the effect of prenatal GCs on GABAergic neurogenesis. In a similar experiment on 4 day old rat pups, it has been reported that a single dose of postnatal dexamethasone does not affect the density of bromodeoxyuridine+ (proliferating) cells in the SVZ and cortex, whereas repeated doses of GCs reduces the density of proliferating cells in a dose dependent manner (Kanagawa et al., 2006). In another study, postnatal dexamethasone increases the number of GABA+ neurons in the mouse cortical plate (Baud et al., 2005). Since the effect of GC is dose, duration and preparation dependent (Uno et al., 1994, Abraham et al., 2000) and because multiple courses of prenatal GCs affect cortical growth and head circumference (Crowther et al., 2006), it is possible that multiple courses of prenatal GCs might affect neurogenesis. We cannot directly compare our data with these studies as our experiments assessed the effect of a single course of prenatal GCs in rabbits, but not postnatal or multiple doses of GCs.
We also evaluated key genes in the transcriptional network controlling neurogenesis to further confirm the effect of GCs on generation of glutamatergic neurons and GABAergic interneurons. Pax6, Ngn1/2, Emx1/2, and Insm1 are the key transcription factors regulating glutamatergic neurogenesis. These factors remain unaltered in prenatal GC treated animals compared to untreated controls, which further confirmed that prenatal GC did not affect glutamatergic neurogenesis. There is no existing data on the effect of GC on these factors to compare with our present data. Likewise, transcription factors regulating GABAergic neurogenesis were not consistently different between GC exposed and unexposed rabbit pups, except for a transient elevation in the level of Mash1. A short-lived elevation of Mash1 expression may be insufficient to affect GABAergic neurogenesis. Hence, our quantification of neuronal progenitors and their regulatory transcription factors strongly suggests that prenatal GCs do not significantly affect glutamatergic or GABAergic neurogenesis.
Even though prenatal GCs increase apoptosis in the periventricular white matter and ganglionic eminence of preterm rabbits (Vinukonda et al., 2010), we did not find an increase in programmed cell death of Dlx2+ and Nkx2.1+ cells in the ganglionic eminence of GC treated pups relative to controls. Additionally, apoptotic cells were not observed in the dorsal SVZ, which is the location of glutamatergic intermediate progenitors (Tbr2+). This suggests that these intermediate progenitors of both GABAergic and glutamatergic origin are relatively resistant to apoptosis after prenatal GC exposure. Consistent with these findings, studies in ovine fetuses have shown that prenatal dexamethasone administration at 70% gestation did not affect neuronal apoptosis, however, reduced non-neuronal apoptosis (Malaeb et al., 2009).
In this study, we evaluated the effect of prenatal betamethasone on neurogenesis at birth and 3 d, but not the long-term effects. A previous study has shown that prenatal GCs induce epigenetic changes, which play a key role in long-lasting programming of gene expression (Crudo et al., 2012). In guinea pigs, prenatal betamethasone treatment is associated with substantial changes in DNA methylation in liver, adrenal gland, kidney and placenta that persist not only in adulthood, but are also evident in the next generation (Crudo et al., 2012). A subsequent study from the same group on guinea pig hippocampus has revealed that prenatal betamethasone causes genome-wide alterations resulting in reduced DNA methylation and enhanced histone h3 lysine acetylation 24 h after GC treatment (Crudo et al., 2013). However, these changes in methylation and acetylation do not persist 14 days after GC exposure. Since epigenetic changes have been linked with adult neurogenesis (Hsieh and Eisch, 2010), it is plausible that prenatal GC might induce long-term effects on neurogenesis.
The rabbit pups delivered to betamethasone treated dams were significantly smaller in size compared to untreated controls. The present finding is consistent with previous studies in sheep, in which both single and repeated betamethasone treatment to the ewe reduced the weight of fetal liver, lungs, heart, kidney and placenta (Wonders and Anderson, 2006, Poitras et al., 2007). This reduction in weight has been attributed to the increase in fetal catabolism owing to suppression of insulin growth factor-1 (Verhaeghe et al., 2007). In a large clinical trial, it has been found that there was no difference in weight and head circumference in GC exposed infants compared to untreated controls (Crowther et al., 2006). However, some other studies suggest an association between in utero GC exposure and reduced birth size (Khan et al., 2011). Prenatal GCs are also associated with increased risk for general psychiatric disturbance and inattention in childhood and adolescence (Davis et al., 2013, Khalife et al., 2013). Although these retrospective studies on premature infants have inherent limitations including sample size, unmeasured confounders, and others, it is possible that prenatal betamethasone affects mental health in children and adolescents.
In conclusion, prenatal GC treatment does not significantly affect glutamatergic or GABAergic neurogenesis in premature rabbits. This finding is of interest to both women in preterm labor receiving GC and their physicians. However, the effect of GC on a number of neurodevelopmental processes occurring during the third trimester, including corticogenesis, oligodendrogenesis and synaptogenesis, needs careful evaluation to determine whether prenatal GCs are safe for women in preterm labor.
Highlights.
Prenatal betamethasone does not alter glutamatergic neurogenesis at birth or postnatal d3.
Prenatal betamethasone does not affect GABAergic neurogenesis at birth or postnatal d3.
Prenatal betamethasone does not change GABA+ population in ganglionic eminence.
Prenatal betamethasone does not cause apoptosis of neuronal progenitors.
Acknowledgments
The authors thank Joanne Abrahams for the assistance with images. Funding was provided by NIH/NINDS grant RO1 NS071263 (PB), and Scientist Development grant from American Heart Association (GV).
Abbreviations
- GC
glucocorticoid
- IPC
intermediate progenitor cell
- SVZ
subventricular zone
- VZ
ventricular zone
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12:1–24. [PubMed] [Google Scholar]
- Abraham I, Harkany T, Horvath KM, Veenema AH, Penke B, Nyakas C, Luiten PG. Chronic corticosterone administration dose-dependently modulates Abeta(1-42)- and NMDA-induced neurodegeneration in rat magnocellular nucleus basalis. J Neuroendocrinol. 2000;12:486–494. doi: 10.1046/j.1365-2826.2000.00475.x. [DOI] [PubMed] [Google Scholar]
- Baud O, Verney C, Evrard P, Gressens P. Injectable dexamethasone administration enhances cortical GABAergic neuronal differentiation in a novel model of postnatal steroid therapy in mice. Pediatr Res. 2005;57:149–156. doi: 10.1203/01.PDR.0000148069.03855.C4. [DOI] [PubMed] [Google Scholar]
- Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- Boku S, Nakagawa S, Koyama T. Glucocorticoids and lithium in adult hippocampal neurogenesis. Vitam Horm. 2010;82:421–431. doi: 10.1016/S0083-6729(10)82021-7. [DOI] [PubMed] [Google Scholar]
- Crochemore C, Michaelidis TM, Fischer D, Loeffler JP, Almeida OF. Enhancement of p53 activity and inhibition of neural cell proliferation by glucocorticoid receptor activation. FASEB J. 2002;16:761–770. doi: 10.1096/fj.01-0577com. [DOI] [PubMed] [Google Scholar]
- Crowley P. Prophylactic corticosteroids for preterm birth. Cochrane Database Syst Rev. 2000:CD000065. doi: 10.1002/14651858.CD000065. [DOI] [PubMed] [Google Scholar]
- Crowther CA, Haslam RR, Hiller JE, Doyle LW, Robinson JS. Neonatal respiratory distress syndrome after repeat exposure to antenatal corticosteroids: a randomised controlled trial. Lancet. 2006;367:1913–1919. doi: 10.1016/S0140-6736(06)68846-6. [DOI] [PubMed] [Google Scholar]
- Crudo A, Petropoulos S, Moisiadis VG, Iqbal M, Kostaki A, Machnes Z, Szyf M, Matthews SG. Prenatal synthetic glucocorticoid treatment changes DNA methylation states in male organ systems: multigenerational effects. Endocrinology. 2012;153:3269–3283. doi: 10.1210/en.2011-2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crudo A, Suderman M, Moisiadis VG, Petropoulos S, Kostaki A, Hallett M, Szyf M, Matthews SG. Glucocorticoid programming of the fetal male hippocampal epigenome. Endocrinology. 2013;154:1168–1180. doi: 10.1210/en.2012-1980. [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K. Fetal glucocorticoid exposure is associated with preadolescent brain development. Biological psychiatry. 2013;74:647–655. doi: 10.1016/j.biopsych.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci. 2005;25:247–251. doi: 10.1523/JNEUROSCI.2899-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DV, Lui JH, Flandin P, Yoshikawa K, Rubenstein JL, Alvarez-Buylla A, Kriegstein AR. Non-epithelial stem cells and cortical interneuron production in the human ganglionic eminences. Nat Neurosci. 2013;16:1576–1587. doi: 10.1038/nn.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J, Eisch AJ. Epigenetics, hippocampal neurogenesis, and neuropsychiatric disorders: unraveling the genome to understand the mind. Neurobiology of disease. 2010;39:73–84. doi: 10.1016/j.nbd.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder T, Neil J, Yoder B, Rees S. Patterns of cerebral injury in a primate model of preterm birth and neonatal intensive care. J Child Neurol. 2005;20:965–967. doi: 10.1177/08830738050200120601. [DOI] [PubMed] [Google Scholar]
- Jakovcevski I, Mayer N, Zecevic N. Multiple origins of human neocortical interneurons are supported by distinct expression of transcription factors. Cereb Cortex. 2011;21:1771–1782. doi: 10.1093/cercor/bhq245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagawa T, Tomimatsu T, Hayashi S, Shioji M, Fukuda H, Shimoya K, Murata Y. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am J Obstet Gynecol. 2006;194:231–238. doi: 10.1016/j.ajog.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Khalife N, Glover V, Taanila A, Ebeling H, Jarvelin MR, Rodriguez A. Prenatal glucocorticoid treatment and later mental health in children and adolescents. PloS one. 2013;8:e81394. doi: 10.1371/journal.pone.0081394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Rodriguez A, Kaakinen M, Pouta A, Hartikainen AL, Jarvelin MR. Does in utero exposure to synthetic glucocorticoids influence birthweight, head circumference and birth length? A systematic review of current evidence in humans. Paediatric and perinatal epidemiology. 2011;25:20–36. doi: 10.1111/j.1365-3016.2010.01147.x. [DOI] [PubMed] [Google Scholar]
- Kutzler MA, Coksaygan TC, Ferguson AD, Nathanielsz PW. Effects of maternally administered dexamethasone and acute hypoxemia at 0.7 gestation on blood pressure and placental perfusion in sheep. Hypertens Pregnancy. 2004;23:75–90. doi: 10.1081/PRG-120028283. [DOI] [PubMed] [Google Scholar]
- Kuwajima T, Nishimura I, Yoshikawa K. Necdin promotes GABAergic neuron differentiation in cooperation with Dlx homeodomain proteins. J Neurosci. 2006;26:5383–5392. doi: 10.1523/JNEUROSCI.1262-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohninger AK, Bock P, Salzer H, Sevelda P, Lohninger AF. Antenatal betamethasone-dose-effects on fetal rat lung morphology and surfactant. J Perinat Med. 1994;22:319–328. doi: 10.1515/jpme.1994.22.4.319. [DOI] [PubMed] [Google Scholar]
- Lui JH, Hansen DV, Kriegstein AR. Development and evolution of the human neocortex. Cell. 2011;146:18–36. doi: 10.1016/j.cell.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Wang C, Wang L, Zhou X, Tian M, Zhang Q, Zhang Y, Li J, Liu Z, Cai Y, Liu F, You Y, Chen C, Campbell K, Song H, Ma L, Rubenstein JL, Yang Z. Subcortical origins of human and monkey neocortical interneurons. Nat Neurosci. 2013;16:1588–1597. doi: 10.1038/nn.3536. [DOI] [PubMed] [Google Scholar]
- Malaeb SN, Hovanesian V, Sarasin MD, Hartmann SM, Sadowska GB, Stonestreet BS. Effects of maternal antenatal glucocorticoid treatment on apoptosis in the ovine fetal cerebral cortex. J Neurosci Res. 2009;87:179–189. doi: 10.1002/jnr.21825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik S, Vinukonda G, Vose LR, Diamond D, Bhimavarapu BB, Hu F, Zia MT, Hevner R, Zecevic N, Ballabh P. Neurogenesis continues in the third trimester of pregnancy and is suppressed by premature birth. J Neurosci. 2013;33:411–423. doi: 10.1523/JNEUROSCI.4445-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadow WL, Bell A, Sunstein CR. Statistics, not memories: what was the standard of care for administering antenatal steroids to women in preterm labor between 1985 and 2000? Obstet Gynecol. 2003;102:356–362. doi: 10.1016/s0029-7844(03)00510-6. [DOI] [PubMed] [Google Scholar]
- Mutafoglu I, Altunyurt S, Dogan E, Acar B, Koyuncuoglu M, Erten O. Effects of betamethasone and thyroid releasing hormone on fetal lung maturation: an experimental and morphometric study. Eur J Obstet Gynecol Reprod Biol. 2004;115:154–158. doi: 10.1016/j.ejogrb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- Poitras L, Ghanem N, Hatch G, Ekker M. The proneural determinant MASH1 regulates forebrain Dlx1/2 expression through the I12b intergenic enhancer. Development. 2007;134:1755–1765. doi: 10.1242/dev.02845. [DOI] [PubMed] [Google Scholar]
- Uno H, Eisele S, Sakai A, Shelton S, Baker E, DeJesus O, Holden J. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB, Farrell PM. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus macaques. I. Hippocampus. Brain Res Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Verhaeghe J, Vanstapel F, Van Bree R, Van Herck E, Coopmans W. Transient catabolic state with reduced IGF-I after antenatal glucocorticoids. Pediatric research. 2007;62:295–300. doi: 10.1203/PDR.0b013e318123f72f. [DOI] [PubMed] [Google Scholar]
- Vinukonda G, Dummula K, Malik S, Hu F, Thompson CI, Csiszar A, Ungvari Z, Ballabh P. Effect of prenatal glucocorticoids on cerebral vasculature of the developing brain. Stroke. 2010;41:1766–1773. doi: 10.1161/STROKEAHA.110.588400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinukonda G, Zia MT, Bhimavarapu BB, Hu F, Feinberg M, Bokhari A, Ungvari Z, Fried VA, Ballabh P. Intraventricular hemorrhage induces deposition of proteoglycans in premature rabbits, but their in vivo degradation with chondroitinase does not restore myelination, ventricle size and neurological recovery. Exp Neurol. 2013;247:630–644. doi: 10.1016/j.expneurol.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose LR, Vinukonda G, Jo S, Miry O, Diamond D, Korumilli R, Arshad A, Zia MT, Hu F, Kayton RJ, La Gamma EF, Bansal R, Bianco AC, Ballabh P. Treatment with thyroxine restores myelination and clinical recovery after intraventricular hemorrhage. J Neurosci. 2013;33:17232–17246. doi: 10.1523/JNEUROSCI.2713-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Barak LS, Mook RA, Jr, Chen W. Glucocorticoid hedgehog agonists in neurogenesis. Vitam Horm. 2011;87:207–215. doi: 10.1016/B978-0-12-386015-6.00030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Yu S, Patchev AV, Wu Y, Lu J, Holsboer F, Zhang JZ, Sousa N, Almeida OF. Depletion of the neural precursor cell pool by glucocorticoids. Ann Neurol. 2010;67:21–30. doi: 10.1002/ana.21812. [DOI] [PubMed] [Google Scholar]