Abstract
Scope
The study aims to evaluate the status of dietary exposure to aflatoxin and fumonisin in young Tanzanian children, using previously validated biomarkers of exposure.
Methods and results
A total of 148 children aged 12 to 22 months, were recruited from three geographically distant villages in Tanzania; Nyabula, Kigwa and Kikelelwa. Plasma aflatoxin-albumin adducts (AF-alb) and urinary fumonisin B1 (UFB1) were measured by ELISA and LC-MS, respectively. AF-alb was detectable in 84% of children, was highest in fully weaned children (p<0.01) with higher levels being associated with higher maize intake (p<0.05). AF-alb geometric mean (95% CI) was 43.2 (28.7–65.0), 19.9 (13.5–29.2) and 3.6 (2.8–4.7) pg/mg albumin in children from Kigwa, Nyabula and Kikelelwa, respectively. UFB1 was detectable in 96% of children and the level was highest in children who had been fully weaned (p<0.01). The geometric UFB1 mean (95% CI) was 327.2 (217.1–493.0), 211.7 (161.1–278.1) and 82.8 (58.3–117.7) pg/ml in Kigwa, Nyabula and Kikelelwa, respectively. About 82% of all the children were exposed to both mycotoxins.
Conclusion
Young children in Tanzania are chronically exposed to both aflatoxin and fumonisin through contaminated diet, although the level of exposure varies markedly between the three villages studied.
Keywords: Aflatoxin, Biomarkers of exposure, Children, Exposure assessment, Fumonisin
1 Introduction
Aflatoxins are highly toxic metabolites of Aspergillus spp. commonly occurring in food commodities such as cereals, nuts and oil seeds [1–5]. Aflatoxin B1 is the most prevalent of the aflatoxins and has been classified by the International Agency for Research on Cancer (IARC) as a human carcinogen (Group 1) [5]. In addition to liver cancer, aflatoxin exposure has been associated with impaired child growth [6–8], immune suppression [9, 10], low birth weight [11], hepatomegaly [12], and outbreaks of acute toxicity [13, 14].
Fumonisins, which are mainly produced by Fusarium verticillioides, contaminate maize worldwide [15], with fumonisin B1 (FB1) being the most abundant of those with biological significance. FB1 has been categorised by IARC as being possibly carcinogenic in humans (Group 2B) [16]. In ecological studies, fumonisin intake has been correlated with high incidence of human oesophageal cancer in South Africa [17] and China [18]. Fumonisins have been implicated in the incidence of neural tube defects [19, 20]. Furthermore, exposure to fumonisins has been associated with growth retardation among young children in Tanzania [21]. However, epidemiological studies of this exposure are relatively weak, partly due to the previous lack of individual exposure biomarkers, which are now available [22].
Rural communities in Tanzania largely rely on maize as the staple dietary food. Maize also forms the major ingredient of children’s weaning foods (i.e. complementary food that the child eats during the breast-feeding period) in most rural households [23, 24]. This maize is mainly consumed as thin porridge (uji) as well as stiff porridge (ugali). Recent studies in Tanzania demonstrated that aflatoxins and fumonisins are frequent contaminants of maize in rural areas [25]. For example; aflatoxin contamination at levels up to 158 μg/kg was detected in 18% of maize samples in Tanzania [25]. More than half of home grown maize samples in the country were contaminated with FB1 and FB2 at levels up to 11 mg/kg [26]. Aflatoxins co-occurred with fumonisins in 10% of the maize samples, implying that the children are at risk of exposure to both mycotoxins. It was estimated [23] that fumonisin exposure in a quarter of Tanzanian infants exceeded the WHO provisional maximum tolerable daily intake of 2 μg/kg body weight per day [27].
Food based exposure estimates are not as reliable as biomarker based exposure estimates, but there are no data on biomarkers of dietary fumonisin and aflatoxin exposure in Tanzania. Aflatoxin albumin adducts (AF-alb) in blood are known to be a good biomarker of aflatoxin exposure over the preceding 2 to 3 months and have been widely used in human studies [28]. Recently, urinary free fumonisin B1 (UFB1) has been developed as a biomarker of recent exposure (1–2 days) to fumonisins and has been validated in both a Mexican population [22] and an intervention study in South Africa [29].
Exposure to mycotoxins at an early age could be particularly detrimental. Due to their immature detoxification capacity, rapid growth and higher intake relative to their body size, children could be more susceptible than adults to the effects of mycotoxins [30]. There is an urgent need for the government and the community to understand the severity and breadth of the problem in order to formulate effective interventions to prevent aflatoxin and fumonisin exposure in Tanzanian people, particularly in young children. This study has determined levels of AF-alb and UFB1 in young children in Tanzania.
2 Materials and methods
2.1 Study subjects and sample collection
This study was conducted in three villages in Tanzania; Nyabula, Kigwa, and Kikelelwa from the Iringa, Tabora, and Kilimanjaro regions, respectively. The three regions are located in different agro-ecological zones and represent different patterns of aflatoxin and fumonisin contamination in the country [25].
Fifty-six, 37 and 55 children aged between 12 and 22 months old and apparently healthy, i.e. without signs or symptoms of diseases, were surveyed from Nyabula, Kigwa and Kikelelwa, respectively. One child was selected per family, based on the birth registration at a village dispensary. Ethical approval was obtained from the National Institute of Medical Research in Tanzania and the University of Leeds, United Kingdom (HSLT/09/005), following review of the human subjects research proposal. Informed written consent was obtained from the mothers of the children.
The survey was conducted six months after the maize harvest to include exposure that may result from contamination during storage. Prior to the survey, a training session was conducted for all the field workers. A questionnaire was administered to the mothers by field workers to record the information on child age, sex, breastfeeding, maize intake and family socio-economic status (SES). Each child’s body weight (b.w.) was measured by following the standard procedure with two repeats and recorded to the nearest 0.1 kg. Diet information was recorded at two consecutive days using a 24 hour dietary recall questionnaire. For each child, maize intake per kg body weight (kg b.w.) was calculated based on the child’s maize intake per day and body weight. SES was calculated on a weight-basis score system based on family house type in terms of floor, wall and roof materials. The house scores were categorised into three groups and, based on the obtained score, families were regarded as of low, medium or high SES. House construction materials used for wall, roof and floor were considered as suitable indicators of SES because they reflect level of family income and education.
Two millilitres of venous blood were collected from each child by a qualified nurse. The plasma was separated by centrifugation at a local hospital. Morning urine was collected by the mother (who had been given pre-training on urine collection), using paediatric urine bags. Urine samples were collected on two consecutive days in order to obtain a representative estimate of exposure, given the rapid excretion of fumonisin. Samples of blood were collected on the day that the second sample of urine was collected. Both the plasma and the urine samples were kept at −20°C before being shipped in dry ice to the University of Leeds, United Kingdom for biomarker analysis.
2.2 AF-alb analysis
Plasma AF-alb levels were determined following the previously described method [31]. This procedure involved albumin extraction, digestion, purification and ELISA. For quality control purposes, three positive and one negative control samples were analyzed with each batch of samples. Samples were measured in quadruplicate on at least two occasions on separate days and coefficients of variation were less than 25%. The detection limit for the assay was 3 pg/mg of AF-alb. Any samples with AF-alb below this limit were assigned a value of 1.5 for the purpose of data analysis.
2.3 Urinary FB1 analysis
The UFB1 was measured using an HPLC/MS method as previously described [22]. Briefly, 10 ml of urine was diluted with an equal volume of distilled water and 1.6 ng of deuterium labelled FB1 (FBd6, a gift from Prof Hans-Ulrich Humpf, University of Münster, Germany) was added as an internal standard. The FB1 was isolated by solid phase extraction using a 3CC Oasis® MAX cartridge (Waters, UK) as per manufacturer’s instructions. The eluate was dried under vacuum and reconstituted in 200 μl methanol/water (1:1, v/v) before injection onto the HPLC-mass spectrometer (Waters Corp, Milford, MA, USA). Selective ion monitoring mode was set at two functions to detect FB1 (m/z 722) and FBd6 (m/z 728), respectively. One negative sample and one sample spiked with FB1 were processed together with each batch of urine samples. The limit of detection was 20 pg FB1/ml of urine. Any samples below this limit were assigned a value of 10 pg FB1/ml for the purposes of data analysis. The mean UFB1 of the two days samples was calculated to represent the exposure.
2.4 Statistical analysis of data
AF-alb and UFB1 data were natural log transformed, as they were not normally distributed. For continuous variables, differences between or among groups was compared using student’s t-test or analysis of variance. Chi-square test was performed in the case of categorical variables. Correlation and multiple regression analyses were used to assess the relationship between biomarkers and key factors. A p-value ≤0.05 was considered statistically significant. Statistical analysis was performed using the STATA® 11.1 (StataCorp LP, USA) statistical package.
3 Results
3.1 Demographic characteristics of subjects
The demographic data are presented by village in Table 1. The mean age of the children was 17 months, ranging from 12 to 22 months. The mean body weight of the children was 9.4 kg, ranging from 5.6 to 13.5 kg. The majority of mothers of the study children (88%) had received only primary education. Ninety-five percent of the participating families were subsistence farmers. Household SES in Kikelelwa was significantly higher than that of the other two villages (p<0.001).
Table 1.
Characteristics of subjects
| Variable | Description | Village
|
|||
|---|---|---|---|---|---|
| Nyabula | Kigwa | Kikelelwa | All villages | ||
| Total (n) | Subjects | 56 | 37 | 55 | 148 |
| Sex | Male:Female | 29:27 | 14:23 | 29:26 | 72:76 |
| Age (months) | Mean ± SD | 17.1±1.9 | 17.8±1.4 | 16.2±1.9 | 16.9±1.9 |
| Range | 12.8–20.2 | 15.4–21.7 | 12.4–19.5 | 12.4–21.7 | |
| Body weight (kg) | Mean ± SD | 9.1±1.1 | 9.6±1.1 | 9.6±1.4 | 9.4±1.2 |
| Range | 6.4–11.1 | 7.4–12.5 | 5.6–13.5 | 5.6–13.5 | |
| Age when weaning began (month) | Mean ± SD | 4.5±1.6 | 3.7±1.8 | 3.4±1.3 | 3.9±1.6 |
| Range | 0–6 | 0–6 | 1–6 | 0–6 | |
| Fully weaned children | % of total | 23% | 27% | 18% | 22% |
| Frequency of maize intake | Days per week | 7 | 6–7 | 2–7 | 2–7 |
| Maize intake a (g/kg b.w) | Mean ± SD | 12.1±5.8 | 8.9±5.8 | 7.9±3.6 | 9.8±5.4 |
| Range | 0–32.9 | 0–29.1 | 2.8–17.4 | 0.32.9 | |
| Family SESb level, n (%) | Low | 37 (66%) | 23 (32%) | 0 (0%) | 60 (41%) |
| Medium | 18 (32%) | 10 (27%) | 41 (75%) | 69 (47%) | |
| High | 1 (2%) | 4 (11%) | 14 (25%) | 19 (13%) | |
| Education of the mother, n (%) | Primary | 53(94%) | 27(73%) | 50(91%) | 130(88%) |
| Secondary | 1(2%) | 1(3%) | 4(7%) | 6(4%) | |
| No education | 2(4%) | 9(24%) | 1(2%) | 12(8%) | |
| Family occupation, n (%) | Peasant | 55(98%) | 34(92%) | 52(95%) | 141(95%) |
| Others | 1(2%) | 3(8%) | 3(5%) | 7(5%) | |
Maize intake as determined from two 24 hours food recall questionnaires; significant difference is between Nyabula and Kigwa (p<0.01), and between Nyabula and Kikelelwa (p<0.001)
SES: Socio-economic status. Significant difference in SES between Kikelelwa and each other village (p<0.001)
3.2 Child feeding and maize intake
At the time of the survey, 22% of children were fully weaned and 78% partially breast fed (Table 1). On average, complementary feeding started at 4 months of age (range, 0 to 6 months). Maize was the main ingredient of complementary food, normally prepared and consumed as thin or stiff porridge. The mean maize intake was 12.1, 8.9, and 7.9 g/kg b.w. at Nyabula, Kigwa, and Kikelelwa, respectively. The maize intake was statistically higher in Nyabula than in Kigwa (p<0.01) or Kikelelwa (p<0.001). The frequencies of maize consumption amongst the three villages follow the same trend (see Table 1).
3.3 Aflatoxin exposure
Plasma samples were obtained from 146 children out of 148 who were recruited. Of the 146 children, 84% were positive for AF-alb (Table 2). The prevalence of positive AF-alb was 96% in Nyabula, 97% in Kigwa and 61% in Kikelelwa, respectively. The overall geometric mean of AF-alb (95% CI) was 12.9 (9.9–16.7) pg/mg albumin. Kigwa had the highest AF-alb mean level; 43.2 (28.7–65.0) pg/mg, followed by Nyabula 19.9 (13.5–29.2) pg/mg, with Kikelelwa being the lowest at 3.6 (2.8–4.7) pg/mg. The mean levels differed significantly between villages (p<0.001). AF-alb positively correlated with the child’s age (p<0.001). The mean AF-alb level in children who were fully weaned was more than double that in the partially weaned children [24.7 (14.3–42.6) pg/mg versus 10.7 (8.0–14.3) pg/mg, (p<0.01)]. There was no difference in AF-alb levels between boys and girls.
Table 2.
Determinant factors for AF-alb and UFB1 levels
| Variable | AF-alb
|
UFB1
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | Positive % | Geometric mean (95% CI) pg/mg | p value | n | Positive % | Geometric mean (95% CI) pg/ml | p value | |
| Village | ||||||||
| Nyabula | 55 | 96% | 19.9 (13.5–29.2)a | 56 | 100% | 211.7 (161.1–278.1)a | ||
| Kigwa | 37 | 97% | 43.2 (28.7–65.0)b | p<0.001 | 37 | 100% | 327.2 (217.1–493.0)a | p<0.001 |
| Kikelelwa | 54 | 61% | 3.6 (2.8–4.7)c | 54 | 89% | 82.8 (58.3–117.7)b | ||
| All villages | 146 | 84% | 12.9 (9.9 – 16.7) | 147 | 96% | 167.3 (135.4–206.7) | ||
| Sex | ||||||||
| Male | 71 | 11.0 (7.5–16.2) | p=0.240 | 72 | 145.6 (106.5–199.1) | p=0.205 | ||
| Female | 75 | 15.0 (10.5–21.3) | 75 | 191.2 (143.1–255.5) | ||||
| Age (months) | ||||||||
| <16 | 44 | 6.1 (4.3–8.8)a | 45 | 143.2 (98.4–208.5) | ||||
| 16–18 | 56 | 16.2 (10.4–25.2)b | p<0.001 | 56 | 171.8 (120.0–245.9) | p=0.591 | ||
| >18 | 46 | 19.8 (12.3–32.0)b | 46 | 188.7 (127.9–278.1) | ||||
| Weaning status | ||||||||
| Partially weaned | 114 | 10.7 (8.0–14.3) | p<0.010 | 114 | 142.2 (112.3–180.3) | p<0.010 | ||
| Fully weaned | 32 | 24.7 (14.3–42.6) | 33 | 293.2 (189.4–453.7) | ||||
| SESd | ||||||||
| Low | 59 | 31.6 (21.7–46.2)a | 60 | 268.1 (196.9–364.9)a | ||||
| Medium | 68 | 7.8 (5.5–10.9)b | p<0.001 | 68 | 126.9 (92.4–174.3)b | p<0.001 | ||
| High | 19 | 4.9 (2.8–8.6)b | 19 | 101.6 (58.4–176.6)b | ||||
Significant difference between means marked with different letters using ANOVA with post hoc analysis.
SES: Socio-economic status.
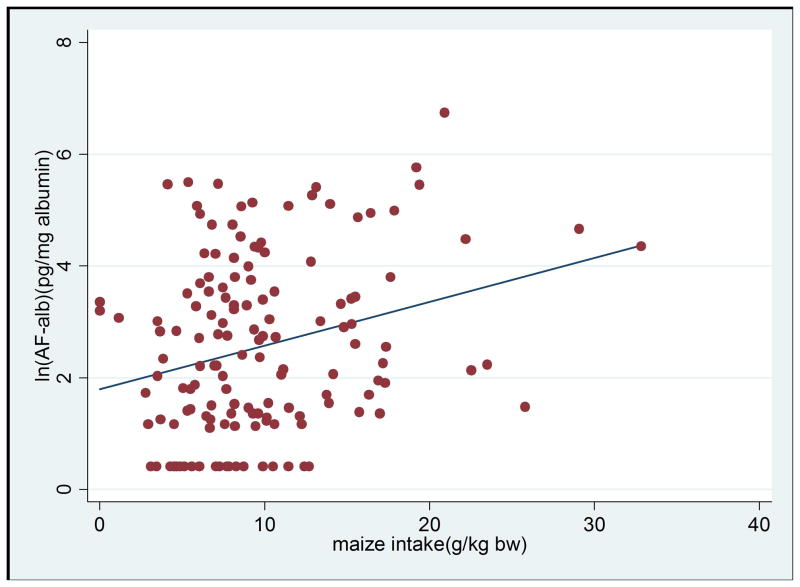
There was a positive correlation between AF-alb and maize intake (r=0.267, p=0.001) (Figure 1a). Higher levels of AF-alb were associated with higher maize intake (β=0.049, p=0.012), after adjustment for SES, child’s age and village in a multivariate regression model (Table 3). In this model, the association between age and AF-alb remained significant (β=0.122, p=0.026). Village remains the strongest determinant for AF-alb (p<0.001). SES was found to be significantly correlated with AF-alb in the univariate analysis (P<0.001), but the correlation was not significant in this multivariate regression model (Table 3).
Figure 1.
Figure 1a. Scatter plot of maize intake per kg b.w. against levels of AF-alb (AF-alb is natural log transformed), the linear regression line showing maize intake is positively correlated with blood AF-alb level (n=146; correlation coefficient =0.267; p=0.0011).
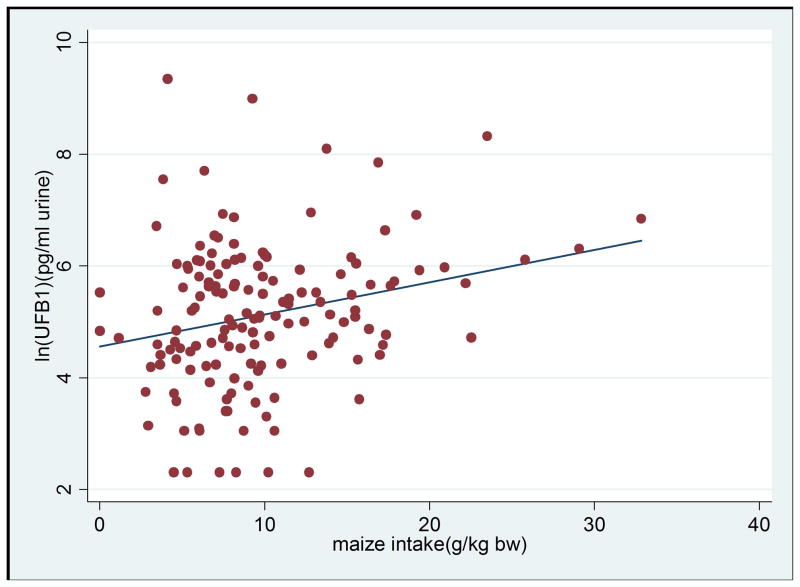
Figure 1b. Scatter plot of maize intake per kg b.w. against levels of UFB1 (UFB is natural log transformed), the linear regression line showing maize intake is positively correlated with UFB1 level (n=147; correlation coefficient=0.241; p=0.0033).
Table 3.
Multiple regression analysis for the determinants of aflatoxin and fumonisin exposure
| Predictor variables | AF-alba
|
UFB1a
|
||||
|---|---|---|---|---|---|---|
| Correlation coefficient | t-test | p-value | Correlation coefficient | t-test | p-value | |
| Maize intake (g/kg b.w) | 0.049 | 2.56 | 0.012 | 0.043 | 2.23 | 0.027 |
| SESb (low vs. medium group) | −0.406 | −1.56 | 0.120 | −0.133 | −0.52 | 0.607 |
| SES (low vs. high group) | −0.662 | −1.80 | 0.074 | −0.229 | −0.62 | 0.534 |
| Age by month | 0.122 | 2.26 | 0.026 | −0.027 | −0.51 | 0.612 |
| Village (Nyabula vs. Kikelelwa) | −1.063 | −3.58 | 0.000 | −0.668 | −2.25 | 0.026 |
| Village (Nyabula vs. Kigwa) | 0.890 | 3.42 | 0.001 | 0.610 | 2.36 | 0.020 |
dependent variables;
SES: Socio-economic status
3.4 Fumonisin exposure
One hundred and forty seven children had urine samples available and analysed, and of these, 96% were positive for UFB1 (Table 2). The overall geometric mean of UFB1 (95% CI) was 167.3 (135.4–206.7) pg/ml of urine. Kigwa had the highest UFB1 geometric mean 327.2 (217.1–493.0) pg/ml, followed by Nyabula at 211.7 (161.1–278.1) pg/ml, while Kikelelwa had the lowest at 82.8 (58.3–117.7) pg/ml. The difference between Kikelelwa and the other two villages was statistically significant (p<0.001). The UFB1 levels were significantly higher in children who were fully weaned than in those partially weaned; 293.2 (189.4–453.7) pg/ml versus 142.2 (112.3–180.3) pg/ml (p<0.01). UFB1 was positively correlated with the child’s maize intake (r=0.241, p=0.003) (Figure 1b). Multivariate regression analysis showed that village and maize intake remained significantly associated with UFB1 (p<0.05 for both) while SES was not significant (Table 3). UFB1 levels were not correlated with child’s sex or age.
3.5 Co-exposure of aflatoxin with FB1
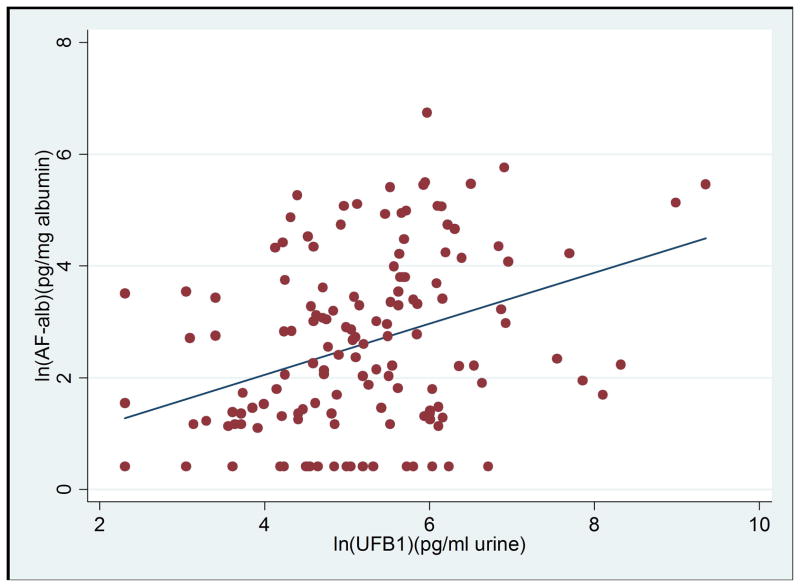
AF-alb and UFB1 were both detected in 82% of children. Kigwa village had the highest rate of co-exposed children (36/37), followed by Nyabula (53/55) then Kikelelwa (30/54). Overall, there was a significant positive correlation between levels of UFB1 and AF-alb in the children (r=0.375; p<0.001, Figure 2). Within each village, however, a significant positive correlation was only observed in Kigwa where levels of both biomarkers were highest (r=0.388, p=0.018).
Figure 2.
Scatter plot of levels of AF-alb and UFB1 in all villages (both variables are natural log transformed), the linear regression line showing blood AF-alb level is positively correlated with UFB1 level (n=145; correlation coefficient=0.375; p<0.001).
4 Discussion
4.1 Levels of exposure
The high detection rate for AF-alb in these children (84% in total) shows that aflatoxin exposure is prevalent in this population from a very early age. The overall AF-alb level found in this study is lower than those reported in children from some other parts of Africa, such as 32.8 pg/mg in Togo and Benin [32], 22.3 pg/mg in Gambia [10] and 206.5 pg/mg and 73.2 pg/mg in two neighbouring villages in Kenya [12]). This is partly because the other studies included children at an older age. Indeed younger children (<20 months old) in the Benin and Togo study (unpublished data) showed a similar level of AF-alb as the Tanzanian children, while the high AF-alb detected in Kenya was in much older children aged 6–17 years. In addition, one key determinant of the aflatoxin exposure is village location, which showed great variation in the AF-alb levels and maize consumption levels.
To date, there is no published information about levels of UFB1 in young children. In this study almost all of the children were exposed to this toxin, as revealed by the presence of the UFB1 biomarker. The overall levels of UFB1 were lower than those reported in South African adults [29] but higher than levels previously reported in adults in Mexico [22]. A few factors may contribute to this, for example, a different method of food processing and cooking, a lower level of fumonisin contamination in maize, or possibly differences in urine excretion by population or age.
A previous study in South Africa [29] reported a less than 0.1% estimated urinary excretion of FB1. However, a recent controlled study on American adults consuming maize of known concentration of FB1 contamination demonstrated that, on average, 0.5% of the toxin intake is excreted in urine [33]. It is possible that FB1 excretion in adults may not be comparable to that of young children. These variations in the UFB1 levels across populations indicate the need for further study to examine the physiological mechanism of FB1 excretion in urine among different age and ethnic groups.
4.2 Influence of village on exposure
There was variation between exposure levels to aflatoxin and FB1 in different villages, with levels of both AF-alb and UFB1 being higher in children from Kigwa, followed by Nyabula, and then Kikelelwa. There was a twelvefold difference in mean AF-alb levels between Kigwa and Kikelelwa and a fourfold difference in mean UFB1 between the same two villages. The main factors contributing to these differences are thought to be variation in food intake, or in the case of aflatoxin, maize storage and harvest practices, which could lead to different levels of fungal contamination between villages. It is notable that aflatoxin levels can increase markedly during storage, whilst fumonisins are predominantly a field problem, with little change during storage. The current biomarker data are in agreement with a previous study of foods [25], which reported on the widespread contamination of both aflatoxin and fumonisin in Kigwa compared with Nyabula and Kikelelwa. This finding of geographical location as a determinant of mycotoxin exposure is further supported by other studies in Africa [12, 32, 34, 35].
4.3 The impact of age, weaning status and maize intake on exposure
In the current study maize intake and age are found to be significant contributors to AF-alb level. Although it is possible that contaminated groundnuts could also contribute to aflatoxin intake, groundnut consumption is not as significant in Tanzania as it is in some other parts of Africa. In agreement with a previous study in Benin [32], the age of a child significantly contributes to the level of AF-alb, largely owing to increasing intake of complementary food as the child gets older, up to around three years old. This age-dependent effect is not seen in older children, for example in 6–17 year olds studied in Kenya [12]. The result confirmed the previous reasoning [32] that age is a surrogate indicator of increased maize intake and reduced breastfeeding of a child.
Maize intake but not age was found to be associated with fumonisin exposure. The association between maize intake and UFB1 shows that the biomarker is a good indicator of fumonisin intake. Consistently higher maize intake and higher levels of UFB1 were found in children from villages of Kigwa and Nyabula than in Kikelelwa. Children from Kikelelwa village on average eat less maize on a daily basis compared to the other villages due to the availability of other food options. The findings from this study were in agreement with studies on adults in Mexico [22] and China [36], which found higher mean UFB1 in participants from the high maize consumption group compared to the low consumption group. Further to previous studies in adults [22, 29, 33, 36], this data confirmed that UFB1 is a potential biomarker to assess fumonisin exposure in young children, but it is possible that the exposure biomarker relationship in young children may not be comparable to adults due to differences in physiological mechanisms that may influence FB1 excretion in urine. This needs to be addressed by more studies designed to test differences in FB1 excretion in urine in children compared to adults.
AF-alb and UFB1 in this study were significantly higher in children who were fully weaned than in those who were partially weaned and this is in good agreement with other studies on aflatoxin conducted in Benin and Togo and in Egypt [32, 37]. According to Egal and co-workers [38], the intake of solid foods and consequently aflatoxin will increase once a child stops consuming breast milk. On the other hand, it has been hypothesized that high AF-alb adduct levels in children who are not currently breastfed might be due to a possible protective effect of breast milk on the intestinal absorption of the toxin or its effect on the metabolism to active metabolites but this is as yet unsupported by experimental data [37]. Our finding that fumonisin exposure shares the same trend as aflatoxin, i.e. both increased when complementary food was being introduced, suggests that the increase in consumption of contaminated maize-based food is the driving force behind the increased exposure with age.
In conclusion, with the biomarker approach used here, this study provides for the first time detailed quantitative exposure data on aflatoxin and fumonisin in Tanzanian children, and confirms the previous findings of widespread fumonisin and aflatoxin exposure in young Tanzanian children through maize intake. Importantly, the study demonstrates the use of UFB1 as a biomarker of fumonisin exposure in young children, although further analyses to understand the biological mechanism and individual variation of FB1 excretion in urine of young children are crucial. This study highlights the need to understand the health effects of co-exposure to two toxic dietary contaminants in young children and for education and intervention for mycotoxin control in these vulnerable populations.
Acknowledgments
This work was supported by the Leverhulme-Royal Society Africa Award. Dr YY Gong and Dr CP Wild are also supported by the National Institute of Environmental Health Sciences, USA (grant N°: ES06052). The Authors would like to acknowledge the Management of Tanzania Food and Drugs Authority for facilitating this study. We wish to thank the administration authorities, subjects and field workers from all the areas where the study was conducted.
Abbreviations
- AF-alb
aflatoxin albumin adducts
- b.w
body weight
- CI
confidence interval
- FB1
fumonisin B1
- IARC
International Agency for Research on Cancer
- SES
socio-economic status
- UFB1
urinary fumonisin B1
Footnotes
The authors have declared no conflict of interest.
References
- 1.Bhat R, Rai RV, Karim AA. Mycotoxins in food and feed: present status and future concerns. Comprehensive Reviews in Food Science and Food Safety. 2010;9:57–81. doi: 10.1111/j.1541-4337.2009.00094.x. [DOI] [PubMed] [Google Scholar]
- 2.Bryden WL. Mycotoxins in the food chain: human health implications. Asia Pac J Clin Nutr. 2007;16:95–101. [PubMed] [Google Scholar]
- 3.Milićević DR, Škrinjar M, Baltić T. Real and perceived risks for mycotoxin contamination in foods and feeds: Challenges for food safety control. Toxins. 2010;2:572–592. doi: 10.3390/toxins2040572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Riordan MJ, Wilkinson MG. A survey of the incidence and level of aflatoxin contamination in a range of imported spice preparations on the Irish retail market. Food Chemistry. 2008;107:1429–1435. [Google Scholar]
- 5.IARC. Some naturally occurring substances: Food items and constituents, heterocyclic aromatic amines and mycotoxins. Vol. 56. International Agency for Reseach on Cancer Monograph; Lyon: IARC; 1993. [Google Scholar]
- 6.Gong Y, Hounsa A, Egal S, Turner PC, et al. Postweaning exposure to aflatoxin results in impaired child growth: a longitudinal study in Benin, West Africa. Environmental Health Perspectives. 2004;112:1334. doi: 10.1289/ehp.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong YY, Turner PC, Hall AJ, Wild CP. Aflatoxin exposure and impaired child growth in West Africa: an unexplored international public health burden. Mycotoxins: detection methods, management, public health and agricultural trade. 2008:53–65. [Google Scholar]
- 8.Sherif SO, Salama EE, Abdel-Wahhab MA. Mycotoxins and child health: The need for health risk assessment. Int J Hyg Environ Health. 2009;212:347–368. doi: 10.1016/j.ijheh.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Jiang Y, Jolly PE, Ellis WO, Wang JS, et al. Aflatoxin B1 albumin adduct levels and cellular immune status in Ghanaians. International immunology. 2005;17:807–814. doi: 10.1093/intimm/dxh262. [DOI] [PubMed] [Google Scholar]
- 10.Turner PC, Moore SE, Hall AJ, Prentice AM, et al. Modification of immune function through exposure to dietary aflatoxin in Gambian children. Environmental Health Perspectives. 2003;111:217. doi: 10.1289/ehp.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shuaib F, Jolly PE, Ehiri JE, Yatich N, et al. Association between birth outcomes and aflatoxin B1 biomarker blood levels in pregnant women in Kumasi, Ghana. Tropical Medicine & International Health. 2010;15:160–167. doi: 10.1111/j.1365-3156.2009.02435.x. [DOI] [PubMed] [Google Scholar]
- 12.Gong YY, Wilson S, Mwatha JK, Routledge MN, et al. Aflatoxin Exposure May Contribute to Chronic Hepatomegaly in Kenyan School Children. Environmental Health Perspectives. 2012;120:893–896. doi: 10.1289/ehp.1104357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst C, Njapau H, Cotty PJ. Outbreak of an acute aflatoxicosis in Kenya in 2004: identification of the causal agent. Applied and Environmental Microbiology. 2007;73:2762–2764. doi: 10.1128/AEM.02370-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams JH, Phillips TD, Jolly PE, Stiles JK, et al. Human aflatoxicosis in developing countries: a review of toxicology, exposure, potential health consequences, and interventions. The American journal of clinical nutrition. 2004;80:1106–1122. doi: 10.1093/ajcn/80.5.1106. [DOI] [PubMed] [Google Scholar]
- 15.Marasas WFO, Nelson PE, Toussoun TA. Toxigenic Fusarium species. Identity and mycotoxicology. Pennsylvania State University Press, University Park Pennsylvania; 1984. [Google Scholar]
- 16.IARC. IARC monographs on the evaluation of carcinogenic risks to humans/World Health Organization. Vol. 82. International Agency for Research on Cancer; 2002. Some traditional herbal medicines some mycotoxins naphthalene styrene; pp. 1–556. [PMC free article] [PubMed] [Google Scholar]
- 17.Rheeder JP, Marasas WFO, Thiel PG, Sydenham EW, et al. Fusarium moniliforme and fumonisins in corn in relation to human esophageal cancer in Transkei. Phytopathology. 1992;82:353. [Google Scholar]
- 18.Yoshizawa T, Yamashita A, Luo Y. Fumonisin occurrence in corn from high-and low-risk areas for human esophageal cancer in China. Appl Environ Microbiol. 1994;60:1626–1629. doi: 10.1128/aem.60.5.1626-1629.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Missmer SA, Suarez L, Felkner M, Wang E, et al. Exposure to fumonisins and the occurrence of neural tube defects along the Texas-Mexico border. Environmental Health Perspectives. 2006;114:237. doi: 10.1289/ehp.8221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marasas WFO, Riley RT, Hendricks KA, Stevens VL, et al. Fumonisins disrupt sphingolipid metabolism, folate transport, and neural tube development in embryo culture and in vivo: a potential risk factor for human neural tube defects among populations consuming fumonisin-contaminated maize. The Journal of nutrition. 2004;134:711–716. doi: 10.1093/jn/134.4.711. [DOI] [PubMed] [Google Scholar]
- 21.Kimanya ME, De Meulenaer B, Roberfroid D, Lachat C, et al. Fumonisin exposure through maize in complementary foods is inversely associated with linear growth of infants in Tanzania. Molecular Nutrition & Food Research. 2010;54:1659–1667. doi: 10.1002/mnfr.200900483. [DOI] [PubMed] [Google Scholar]
- 22.Gong YY, Torres-Sanchez L, Lopez-Carrillo L, Peng JH, et al. Association between tortilla consumption and human urinary fumonisin B1 levels in a Mexican population. Cancer Epidemiology Biomarkers & Prevention. 2008;17:688–694. doi: 10.1158/1055-9965.EPI-07-2534. [DOI] [PubMed] [Google Scholar]
- 23.Kimanya ME, Meulenaer BD, Baert K, Tiisekwa B, et al. Exposure of infants to fumonisins in maize based complementary foods in rural Tanzania. Molecular Nutrition & Food Research. 2009;53:667–674. doi: 10.1002/mnfr.200700488. [DOI] [PubMed] [Google Scholar]
- 24.Mamiro PS, Kolsteren PW, van Camp JH, Roberfroid DA, et al. Processed complementary food does not improve growth or hemoglobin status of rural Tanzanian infants from 6–12 months of age in Kilosa district, Tanzania. The Journal of Nutrition. 2004;134:1084–1090. doi: 10.1093/jn/134.5.1084. [DOI] [PubMed] [Google Scholar]
- 25.Kimanya ME, De Meulenaer B, Tiisekwa B, Ndomondo-Sigonda M, et al. Co-occurrence of fumonisins with aflatoxins in home-stored maize for human consumption in rural villages of Tanzania. Food Additives and Contaminants. 2008;25:1353–1364. doi: 10.1080/02652030802112601. [DOI] [PubMed] [Google Scholar]
- 26.Kimanya M, De Meulenaer B, Tiisekwa B, Ndomondo-Sigonda M, et al. Human exposure to fumonisins from home grown maize in Tanzania. World Mycotoxin Journal. 2008;1:307–313. [Google Scholar]
- 27.Bolger M, Coker RD, DiNovi M, Gaylor D, Gelderblom WC, Olsen M, et al. Fumonisins. Safety evaluation of certain mycotoxins in food. Food Additives Series No. 47, FAO Food and Nutrition Paper No. 47; Prepared for the 56th Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); Geneva (Switzerland): World Health Organization; 2001. pp. 103–279. [Google Scholar]
- 28.Wild CP, Gong YY. Mycotoxins and human disease: a largely ignored global health issue. Carcinogenesis. 2010;31:71–82. doi: 10.1093/carcin/bgp264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van der Westhuizen L, Shephard GS, Burger HM, Rheeder JP, et al. Fumonisin B1 as a urinary biomarker of exposure in a maize intervention study among South African subsistence farmers. Cancer Epidemiology Biomarkers & Prevention. 2011;20:483–489. doi: 10.1158/1055-9965.EPI-10-1002. [DOI] [PubMed] [Google Scholar]
- 30.Erkekoğlu P, Şahin G, Baydar T. A special focus on mycotoxin contamination in baby foods: Their presence and regulations. FABAD J Pharm Sci. 2008;33:51–66. [Google Scholar]
- 31.Chapot B, Wild CP. ELISA for quantification of aflatoxin-albumin adducts and their application to human exposure assessment. Techniques in diagnostic pathology. 1991;2:135–155. [Google Scholar]
- 32.Gong YY, Egal S, Hounsa A, Turner PC, et al. Determinants of aflatoxin exposure in young children from Benin and Togo, West Africa: the critical role of weaning. International journal of epidemiology. 2003;32:556–562. doi: 10.1093/ije/dyg109. [DOI] [PubMed] [Google Scholar]
- 33.Riley RT, Torres O, Showker JL, Zitomer NC, et al. The kinetics of urinary fumonisin B1 excretion in humans consuming maize-based diets. Molecular Nutrition & Food Research. 2012;56:1445–1455. doi: 10.1002/mnfr.201200166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jolly P, Jiang Y, Ellis W, Awuah R, et al. Determinants of aflatoxin levels in Ghanaians: sociodemographic factors, knowledge of aflatoxin and food handling and consumption practices. Int J Hyg Environ Health. 2006;209:345–358. doi: 10.1016/j.ijheh.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Turner PC, Sylla A, Gong YY, Diallo MS, et al. Reduction in exposure to carcinogenic aflatoxins by postharvest intervention measures in west Africa: a community-based intervention study. The Lancet. 2005;365:1950–1956. doi: 10.1016/S0140-6736(05)66661-5. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Cai Q, Tang L, Wang S, et al. Evaluation of fumonisin biomarkers in a cross-sectional study with two high-risk populations in China. Food Additives and Contaminants. 2010;27:1161–1169. doi: 10.1080/19440049.2010.481638. [DOI] [PubMed] [Google Scholar]
- 37.Shouman BO, El Morsi D, Shabaan S, Abdel-Hamid AH, et al. Aflatoxin B1 Level in Relation to Child’s Feeding and Growth. Indian Journal of Pediatrics. 2012:1–6. doi: 10.1007/s12098-011-0493-y. [DOI] [PubMed] [Google Scholar]
- 38.Egal S, Hounsa A, Gong YY, Turner PC, et al. Dietary exposure to aflatoxin from maize and groundnut in young children from Benin and Togo, West Africa. International Journal of Food Microbiology. 2005;104:215–224. doi: 10.1016/j.ijfoodmicro.2005.03.004. [DOI] [PubMed] [Google Scholar]