Abstract
Objective To determine the efficacy and safety of topically applied capsaicin for chronic pain from neuropathic or musculoskeletal disorders.
Data sources Cochrane Library, Medline, Embase, PubMed, an in-house database, and contact with manufacturers of topical capsaicin.
Study selection Randomised controlled trials comparing topically applied capsaicin with placebo or another treatment in adults with chronic pain.
Data extraction Primary outcome was dichotomous information for the number of patients with about a 50% reduction in pain. Outcomes were extracted at four weeks for musculoskeletal conditions and eight weeks for neuropathic conditions. Secondary outcomes were adverse events and withdrawals due to adverse events.
Data synthesis Six double blind placebo controlled trials (656 patients) were pooled for analysis of neuropathic conditions. The relative benefit from topical capsaicin 0.075% compared with placebo was 1.4 (95% confidence interval 1.2 to 1.7) and the number needed to treat was 5.7 (4.0 to 10.0). Three double blind placebo controlled trials (368 patients) were pooled for analysis of musculoskeletal conditions. The relative benefit from topical capsaicin 0.025% or plaster compared with placebo was 1.5 (1.1 to 2.0) and the number needed to treat was 8.1 (4.6 to 34). Around one third of patients experienced local adverse events with capsaicin, which would not have been the case with placebo.
Conclusions Although topically applied capsaicin has moderate to poor efficacy in the treatment of chronic musculoskeletal or neuropathic pain, it may be useful as an adjunct or sole therapy for a small number of patients who are unresponsive to, or intolerant of, other treatments.
Introduction
Capsaicin, the compound in chilli peppers that makes them taste “hot,” binds to nociceptors in the skin, causing an initial excitation of the neurones and a period of enhanced sensitivity. This is usually perceived as itching, pricking, or burning, with cutaneous vasodilation, and is thought to be due to selective stimulation of afferent C fibres and release of substance P. This is followed by a refractory period with reduced sensitivity and, after repeated applications, persistent desensitisation, possible due to depletion of substance P.1 Studies have also shown that the resulting hypalgesia is associated with degeneration of epidermal nerve fibres.1
Topical creams with capsaicin are used to treat pain from postherpetic neuralgia and diabetic neuropathy (0.075% cream 3-4 times daily for eight weeks), osteoarthritis (0.025% cream four times daily), and rheumatoid arthritis.2,3 Capsaicin has also been used to treat pain due to pruritus, psoriasis, mastectomy, bladder disorders, and cluster headaches.2 Capsaicin is available in the United Kingdom on prescription only, but may be present in small quantities in topical rubefacients sold through pharmacies. In England in 2002 over 120 000 prescriptions were for topical capsaicin (total cost £2.2m) out of 4.5m prescriptions for rubefacients and other topical antirheumatic drugs.4
Adverse events from capsaicin are mainly at the application site (burning, stinging, erythema), and systemic events are rare.2 Achieving double blind conditions in placebo controlled trials using capsaicin can therefore be difficult. Respiratory irritation has also been reported from inhalation of dried cream.5
We performed a meta-analysis of randomised controlled trials to determine the efficacy of topical capsaicin in the treatment of chronic pain from neuropathic and musculoskeletal disorders and adverse events and withdrawals.
Methods
Relevant studies were sought through the Cochrane Library, Medline, PreMedline, Embase, and PubMed up to April 2003 regardless of publication language, type, date, or status. We also searched an in-house database of 13 000 randomised clinical trials in pain research from 1950 identified through a refined Medline search strategy together with handsearching of 40 biomedical journals.6 Reference lists of retrieved articles and reviews were also examined.
The search strategy included capsaicin and capsicum, together with registered brand names (see bmj.com for search terms).2,3 Seventeen manufacturers of topical capsaicin in Europe and North America were contacted.
We identified randomised, active or placebo controlled trials in which treatments were in adults with chronic pain from either neuropathic conditions (diabetic neuropathy, postherpetic neuralgia, polyneuropathy, other neuropathies, or chronic postoperative pain) or musculoskeletal disorders (arthritic disorders, back pain, other chronic muscle pain, or fibromyalgia). Treatment had to be applied 3-4 times daily, with at least 10 patients in each group. Outcomes closest to four weeks (minimum three weeks) were extracted in musculoskeletal conditions and outcomes closest to eight weeks (minimum six weeks) were extracted in neuropathic conditions.
Quality and validity assessment and data abstraction
Each potentially relevant trial was assessed for quality using a validated three item scale with a maximum score of five.7 Studies had to score at least two points (randomised, double blind) to be included for efficacy analysis. Open label and single blind trials were acceptable for safety analysis. Trial validity was assessed on a 16 point scale,8 with the intention of performing a sensitivity analysis on low scoring trials.
Outcomes were extracted by one reviewer and verified by another. Assessments of quality and validity were made independently by at least two reviewers. Disputes were settled by consensus.
Outcomes
We defined clinical success as about a 50% reduction in pain.9 This was the number of patients with either a “good” or “excellent” global assessment of treatment or “none” or “slight” pain on rest or movement measured on a suitable categorical scale. A hierarchy of outcomes was used to extract information on efficacy (see bmj.com).9 We also accepted the number of patients showing undefined “improvement.” As the outcome may not have represented a 50% or more reduction in pain, we performed a separate sensitivity analysis on these trials. Secondary outcomes were the numbers of patients reporting one or more local adverse event, cough, and withdrawal due to adverse events.
Quantitative data synthesis
Analysis was based on an intention to treat. We pooled the number of patients in each trial and calculated the numbers needed to treat and 95% confidence intervals.10 The fixed effects model was used to calculate relative benefits and 95% confidence intervals.11 A statistically significant benefit of treatment over control was assumed when the lower limit of the confidence interval of the relative benefit was greater than one. A statistically significant benefit of control over active treatment was assumed when the upper limit of confidence interval was less than one. Numbers needed to harm and relative risks were calculated for adverse effects and withdrawals in the same way as for numbers needed to treat and relative benefits.
Provided there was sufficient information, we aimed to perform sensitivity analyses on pooled outcomes using the z test (P < 0.01 for a significant difference) in neuropathic compared with musculoskeletal pain and in any given pain condition (for example, diabetic neuropathy ν polyneuropathy).12 Calculations were performed with Microsoft Excel and RevMan 4.2. QUOROM guidelines were followed.13 Homogeneity of trials was assessed visually.14
Results
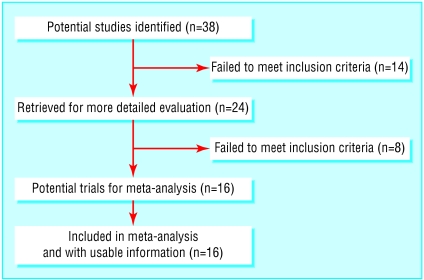
Overall, 38 potential papers were identified and 22 excluded (see table A on bmj.com). A large review included 14 trials and 991 patients.15 We excluded nine of those trials: two were duplicate publications of an included multicentre study, four were on psoriasis, two used outcomes of articular tenderness rather than direct measures of pain, and one had no information on efficacy in the abstract. None of the 17 manufacturers of topical capsaicin provided studies.
In addition to the five remaining papers from the review,16-20 we found seven with information on efficacy and four with information only for adverse events or withdrawals.21-31 We included 16 papers in this review, totalling 1556 patients aged 20 to 95 years (fig 1).
Fig 1.
Flow of papers in review
Only two trials scored fewer than three points for quality (see tables B and C on bmj.com).27,28 One, the only single blind trial, was an active controlled trial comparing different doses of capsaicin.27 Validity scores ranged from nine to 14. In 11 trials baseline pain was moderate to severe, and in five trials patients were only included if they were unresponsive or intolerant to conventional therapies. Seven trials16,19,20,23,25,26,31 allowed concomitant oral drugs for neuropathic pain without change in dose or frequency, and three trials made no mention of such therapy.17,24,30 Two trials allowed concomitant oral drugs for musculoskeletal pain without change in dose or frequency,18,28 three trials prohibited concomitant therapy,21,22,27 and one trial made no mention of such therapy.29
Efficacy and sensitivity analyses
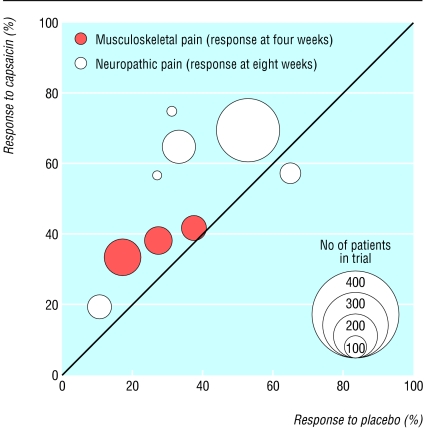
Capsaicin was significantly better than placebo for the treatment of both neuropathic and musculoskeletal pain (table and fig 2; also see bmj.com). In neuropathic conditions, the mean treatment response rate (percentage of patients with at least 50% pain relief) at four weeks for capsaicin 0.075% was 57% (range 53% to 75% in individual trials), and the mean placebo response rate was 42% (range 31% to 55%). The number needed to treat was 6.4 (95% confidence interval 3.8 to 21). The mean treatment response rate at eight weeks for capsaicin 0.075% was 60% (range 20% to 75%), and the mean placebo response rate was 42% (range 10% to 65%; fig 2). The number needed to treat was 5.7 (4.0 to 10). These effects were supplementary to unchanged oral therapy.
Table 1.
Estimates of efficacy and harm from meta-analysis of randomised controlled trials of capsaicin for treatment of chronic pain associated with neuropathic or musculoskeletal conditions
|
No (%) responding to intervention
|
||||||
|---|---|---|---|---|---|---|
| Characteristic | No of trials | No of patients | Treatment | Placebo | Relative benefit (95% CI)* | Number needed to treat (95% CI)† |
| Efficacy | ||||||
| Musculoskeletal pain: | ||||||
| At four weeks | 3 | 368 | 70/186 | 46/182 | 1.5 (1.1 to 2.0) | 8.1 (4.6 to 34) |
| Neuropathic pain: | ||||||
| At four weeks | 4 | 313 | 91/159 | 64/154 | 1.4 (1.1 to 1.7) | 6.4 (3.8 to 21) |
| At eight weeks | 6 | 656 | 197/331 | 136/325 | 1.4 (1.2 to 1.7) | 5.7 (4.0 to 10) |
| By outcome type: | ||||||
| Undefined improvement | 4 | 532 | 179/268 | 128/264 | 1.4 (1.2 to 1.6) | 5.5 (3.8 to 10) |
| Global or percentage pain reduction | 2 | 124 | 18/63 | 8/61 | 2.1 (0.99 to 4.3) | 6.5 (3.4 to 69) |
| By trial size: | ||||||
| <40 patients | 2 | 57 | 20/30 | 8/27 | 2.3 (1.2 to 4.3) | 2.7 (1.6 to 7.7) |
| ≥40 patients | 4 | 599 | 177/301 | 128/298 | 1.4 (1.2 to 1.6) | 6.3 (4.2 to 13) |
| Harm | ||||||
| Musculoskeletal pain: | ||||||
| Local adverse events at four weeks | 3 | 190 | 48/98 | 9/92 | 5.0 (2.6 to 9.6) | 2.6 (2.0 to 3.6) |
| Withdrawals at four weeks‡ | 4 | 398 | 19/203 | 6/195 | 2.5 (1.1 to 5.6) | 16.0 (9.1 to 63) |
| Neuropathic pain | ||||||
| Local adverse events at eight weeks | 4 | 300 | 89/154 | 26/146 | 3.2 (2.2 to 4.6) | 2.5 (2.0 to 3.3) |
| Withdrawals at eight weeks‡ | 5 | 503 | 40/253 | 6/250 | 5.5 (2.6 to 12) | 7.5 (5.5 to 12) |
| Combined | ||||||
| Local adverse events | 7 | 490 | 137/252 | 35/238 | 3.6 (2.6 to 5.0) | 2.5 (2.1 to 3.1) |
| Withdrawals‡ | 9 | 901 | 59/456 | 12/445 | 4.0 (2.3 to 6.8) | 9.8 (7.3 to 15) |
Relative risks (95% confidence intervals) for harm.
Numbers needed to harm (95% confidence intervals) for harm.
Related to adverse events.
Fig 2.
L'Abbé plot showing response to capsaicin and placebo in individual randomised controlled trials
In musculoskeletal conditions, the mean treatment response rate at four weeks for capsaicin 0.025% or plaster was 38% (range 34% to 42%; fig 2), and the mean placebo response rate was 25% (range 17% to 37%). The number needed to treat was 8.1 (4.6 to 34). Only one of the three trials with efficacy data allowed concomitant oral therapy.
Information on efficacy from the two active controlled trials could not be pooled. Data were insufficient from which to draw any conclusions concerning relative efficacy for alternative drugs or doses.
Sensitivity analyses of pooled information showed no significant difference between numbers needed to treat for trial size, type of pain, or outcome (table). Insufficient information prevented other sensitivity analyses.
Adverse events and withdrawals
Significantly more patients had local adverse events and adverse event related withdrawals with capsaicin than with placebo. We found no significant difference between numbers needed to harm for musculoskeletal pain and neuropathic pain (table).
Overall, 54% of patients using capsaicin had one or more local adverse events compared with 15% using placebo. The number needed to harm for one patient to have a local adverse event with capsaicin who would not have done so with placebo was 2.5 (2.1 to 3.1). Adverse event related withdrawals occurred in 13% of patients using capsaicin and 3% of patients using placebo. The number needed to harm was 9.8 (7.3 to 15.0).
Coughing was reported in 8% of patients using capsaicin 0.075% and none using capsaicin 0.025%. One active controlled trial reported coughing in one of 32 patients using 0.025% capsaicin and in seven using capsaicin 0.25%.
Discussion
Topical capsaicin is better than placebo for the treatment of chronic pain from neuropathic and musculoskeletal disorders. This finding agrees with the results of a large review published in 1994, but there are some major differences.15 Firstly, we provide numbers needed to treat rather than odds ratios, because they are easier to understand and interpret and give an absolute rather than relative measure of treatment effect.32,33
Secondly, more trials have become available since the 1994 review, and our findings are based on more studies (n = 16) and more patients (n = 1556). We excluded nine of 14 studies in the 1994 review because they were duplicate publications, concerned dermatological conditions, used outcomes that were not direct measurements of pain, or provided insufficient information on relevant outcomes. We used an intention to treat analysis and a more structured hierarchy of outcomes from which to extract information. The net effect of these differences should be a more accurate, although conservative, estimate of efficacy. Based on information supplied by the authors of the 1994 review, we were able to calculate selected numbers needed to treat: 4.2 for diabetic neuropathy (four trials, 309 patients) and 3.3 for osteoarthritis (three trials, 382 patients).33
Our review gives lower estimates of efficacy for capsaicin. In neuropathic conditions the number needed to treat at eight weeks using topical capsaicin 0.075% was 5.7—that is, that for around every six patients, one would achieve at least a 50% reduction in pain who would not have done so if given placebo. At four weeks, the number needed to treat was slightly worse (6.4). In musculoskeletal conditions, the number needed to treat at four weeks with topical capsaicin 0.025% or plaster was 8.1.
Even this may be an overestimate, due to the difficulty in creating double blind conditions because some patients will recognise a stinging or burning sensation with treatment. Both active and placebo treatments were rubbed on, precluding any effect of rubbing. Average placebo responses of 42% for neuropathic pain and 25% for musculoskeletal conditions in topical capsaicin trials are comparable with placebo responses for oral analgesics or topical NSAIDs (12%-40%) for a variety of conditions and end points.34
Although capsaicin has lower efficacy in musculoskeletal conditions, the difference was not statistically significant. Too few trials were available to be certain if the difference was due to the lower dose of capsaicin. Patients with neuropathic pain received three times the dose used in musculoskeletal pain. In addition, efficacy estimates for musculoskeletal pain were based on information from fewer trials and fewer patients than for neuropathic pain and are therefore less robust.
We only had sufficient information to pool results from placebo controlled trials, making it impossible to judge relative efficacy with other analgesics. Indirect comparisons between treatments are still valid, however.35 A substantial meta-analysis of topical NSAIDs and a review of rubefacients from limited data were undertaken.9,36 In chronic musculoskeletal conditions, capsaicin 0.025% or plaster was not as effective as topical NSAIDs (number needed to treat 3.1, 95% confidence interval 2.7 to 3.8) or rubefacients (5.3, 3.6 to 10.2), giving a rank order of efficacy of topical NSAIDs (most effective), rubefacients, then capsaicin. The efficacy of topical NSAIDs and rubefacients in neuropathic pain is unknown.
Most of the studies were of medium or good quality, and most scored well for validity. Differences between trials will be due to the variability in trial size, outcomes, scales, and quality of reporting. Although most trials had more than 40 patients, large amounts of information are needed to obtain credible results for weak analgesics.37 The outcome of undefined improvement is not useful because it does not show by how much pain has been reduced. More useful are global or categorical outcomes of pain relief for which the scales used to rate treatment effect or pain relief are defined. The outcome of relief of pain by at least half, reported in three studies in this review, is the most useful measure for deriving outcomes such as numbers needed to treat. A recent review on arthritis also found that useful outcomes were often not reported or poorly reported.38 In addition, variability between trials may arise because of differing efficacy in different neuropathic conditions or musculoskeletal pain. Data were insufficient for subgroup analysis.
Local adverse events were common when reported. The number needed to harm for local adverse events from capsaicin were similar, despite dose. Around one in three patients treated with capsaicin will experience a local adverse event who would not have done so if given placebo. The UK Department of Health guidelines state that capsaicin is poorly tolerated in the treatment of shingles and postherpetic neuralgia because of the intense burning sensation after application, but this is misleading as it implies that every patient will experience this sensation. Withdrawals related to adverse events give a better sense of tolerability, since some sensations can be mild and are less likely to discourage continuation of treatment. Combined outcomes in neuropathic and musculoskeletal pain give a number needed to harm of 9.8 for adverse event related withdrawals—that is, for every 10 patients treated with capsaicin, one will withdraw due to an adverse event who would not have done so if given placebo. Some evidence shows that side effects diminish with use.5
What is already known on this topic
A large review found that capsaicin was effective in reducing pain associated with diabetic neuropathy, osteoarthritis, and psoriasis
It was, however, less effective in reducing pain from postherpetic neuralgia
What this study adds
For every six patients with neuropathic pain using capsaicin 0.075% for eight weeks, one additional patient would benefit
For every eight patients with musculoskeletal pain using capsaicin 0.025% for four weeks, one additional patient would benefit
One in three patients using capsaicin had local adverse events; one in 10 withdrew who would not have done so with placebo
A study in healthy volunteers showed significant degeneration of epidermal nerve fibres within a few days of using capsaicin 0.075%.1 Once capsaicin is discontinued, reinnervation occurs, with almost full return of sensation (over six weeks after three weeks of treatment). It is not known what effect long term treatment may have on regeneration, and it has been questioned whether capsaicin should be used in conditions with ongoing nerve disease.39
None of the 17 manufacturers contacted supplied information. Given the relatively poor efficacy of capsaicin, does it have a part to play in pain therapy? Most of the trials stated that patients had moderate or severe chronic pain, and some recruited patients only if they were unresponsive to other treatments. For patients with chronic moderate or severe pain, even a small reduction in pain can be beneficial.
Supplementary Material
 Details of search terms and studies are on bmj.com
Details of search terms and studies are on bmj.com
Contributors: LM was involved in planning, searching, reading the papers, quality scoring, data extraction, analysis, and writing. RAM was involved in planning, reading the papers, quality scoring, analysis, and writing; he will act as guarantor for the paper. The guarantor accepts full responsibility for the conduct of the study, had access to the data, and controlled the decision to publish. SD was involved in reading the papers, quality scoring, data extraction, and commenting on the text. JE was involved in planning, analysis, and commenting on the text. HJM was involved in planning and commenting on the text.
Funding: This study was supported by funds from the Oxford Pain Relief Trust.
Competing interests: RAM and HJM have consulted for various pharmaceutical companies, but no company manufacturing capsaicin. RAM, HJM, and JE have received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. All authors have received research support from charities, government, and industry sources at various times, but no such support was received for this work. No author has any direct stockholding in any pharmaceutical company.
Ethical approval: Not required.
References
- 1.Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. Topical capsaicin in humans: parallel loss of epidermal nerve fibres and pain sensation. Pain 1999;81: 135-45. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds JEF ed. Martindale: the extra pharmacopoeia. 32nd edn. London: Royal Pharmaceutical Society, 1999.
- 3.British Medical Association. Royal Pharmaceutical Society of Great Britain. British national formulary. London: BMA, RPS, 2003. (No 45.)
- 4.Prescription cost analysis. England 2002. Department of Health, London. 2003 ISBN 1 84182 710 X. www.doh.gov.uk/prescriptionstatistics/index.htm
- 5.Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging 1995;7: 317-28. [DOI] [PubMed] [Google Scholar]
- 6.Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66: 239-46. [DOI] [PubMed] [Google Scholar]
- 7.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17: 1-12. [DOI] [PubMed] [Google Scholar]
- 8.Smith LA, Oldman AD, McQuay HJ, Moore RA. Teasing apart quality and validity in systematic reviews: an example from acupuncture trials in chronic neck and back pain. Pain 2000;86: 119-32. [DOI] [PubMed] [Google Scholar]
- 9.Moore RA, Tramer MR, Carroll D, Wiffen PJ, McQuay HJ. Quantitative systematic review of topically applied non-steroidal anti-inflammatory drugs. BMJ 1998;316: 333-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cook D, Sackett DL. On the clinically important difference. Ann Intern Med 1992;117: A16-7. [Google Scholar]
- 11.Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, eds. Statistics with confidence—confidence intervals and statistical guidelines. London: British Medical Journal, 1995: 50-63. [DOI] [PMC free article] [PubMed]
- 12.Tramer MR, Reynolds DJ, Moore RA, McQuay HJ. Impact of covert duplicate publication on meta-analysis: a case study. BMJ 1997;315: 635-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Quality of reporting of meta-analyses. Lancet 1999;354: 1896-900. [DOI] [PubMed] [Google Scholar]
- 14.L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med 1987;107: 224-33. [DOI] [PubMed] [Google Scholar]
- 15.Zhang WY, Li Wan Po A. The effectiveness of topically applied capsaicin. A meta-analysis. Eur J Clin Pharmacol 1994;46: 517-22. [DOI] [PubMed] [Google Scholar]
- 16.The Capsaicin Study Group. Treatment of painful diabetic neuropathy with topical capsaicin. A multicenter, double-blind, vehicle-controlled study. Arch Intern Med 1991;151: 2225-9. [DOI] [PubMed] [Google Scholar]
- 17.Chad DA, Aronin N, Lundstrom R, McKeon P, Ross D, Molitch M, et al. Does capsaicin relieve the pain of diabetic neuropathy? Pain 1990;42: 387-8. [DOI] [PubMed] [Google Scholar]
- 18.Deal CL, Schnitzer TJ, Lipstein E, Seibold JR, Stevens RM, Levy MD, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther 1991;13: 383-95. [PubMed] [Google Scholar]
- 19.Bernstein JE, Korman NJ, Bickers DR, Dahl MV, Millikan LE. Topical capsaicin treatment of chronic postherpetic neuralgia. J Am Acad Dermatol 1989;21: 265-70. [DOI] [PubMed] [Google Scholar]
- 20.Watson CP, Evans RJ. The postmastectomy pain syndrome and topical capsaicin: a randomized trial. Pain 1992;51: 375-9. [DOI] [PubMed] [Google Scholar]
- 21.Altman RD, Aven A, Holmburg CE, Pfeifer LM, Sack M, Young GT. Capsaicin cream 0.025% as monotherapy for osteoarthritis: a double-blind study. Semin Arthritis Rheum 1994;23: 25-33. [Google Scholar]
- 22.Keitel W, Frerick H, Kuhn U, Schmidt U, Kuhlmann M, Bredehorst A. Capsicum pain plaster in chronic non-specific low back pain. Arzneimittelforschung 2001;51: 896-903. [DOI] [PubMed] [Google Scholar]
- 23.Ellison N, Loprinzi CL, Kugler J, Hatfield AK, Miser A, Sloan JA, et al. Phase III placebo-controlled trial of capsaicin cream in the management of surgical neuropathic pain in cancer patients. J Clin Oncol 1997;15: 2974-80. [DOI] [PubMed] [Google Scholar]
- 24.Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy WJ, O'Brien PC. Double-blind, placebo-controlled study of the application of capsaicin cream in chronic distal painful polyneuropathy. Pain 1995;62: 163-8. [DOI] [PubMed] [Google Scholar]
- 25.Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther 1993;15: 510-26. [PubMed] [Google Scholar]
- 26.Biesbroeck R, Bril V, Hollander P, Kabadi U, Schwartz S, Singh SP, et al. A double-blind comparison of topical capsaicin and oral amitriptyline in painful diabetic neuropathy. Adv Ther 1995;12: 111-20. [PubMed] [Google Scholar]
- 27.Schnitzer TJ, Posner M, Lawrence ID. High strength capsaicin cream for osteoarthritis pain: rapid onset of action and improved efficacy with twice daily dosing. J Clin Rheumatol 1995;1: 268-73. [PubMed] [Google Scholar]
- 28.Schnitzer T, Morton C, Coker S. Topical capsaicin therapy for osteoarthritis pain: achieving a maintenance regimen. Semin Arthritis Rheum 1994;23: 34-40. [Google Scholar]
- 29.Winocur E, Gavish A, Halachmi M, Eli I, Gazit E. Topical application of capsaicin for the treatment of localized pain in the temporomandibular joint area. J Orofac Pain 2000;14: 31-6. [PubMed] [Google Scholar]
- 30.McCleane G. Topical application of doxepin hydrochloride, capsaicin and a combination of both produces analgesia in chronic human neuropathic pain: a randomized, double-blind, placebo-controlled study. Br J Clin Pharmacol 2000;49: 574-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paice JA, Ferrans CE, Lashley FR, Shott S, Vizgirda V, Pitrak D. Topical capsaicin in the management of HIV-associated peripheral neuropathy. J Pain Symptom Manage 2000;19: 45-52. [DOI] [PubMed] [Google Scholar]
- 32.Naylor CD, Chen E, Strauss B. Measured enthusiasm: does the method of reporting trial results alter perceptions of therapeutic effectiveness? Ann Intern Med 1992;117: 916-21. [DOI] [PubMed] [Google Scholar]
- 33.Moore R, Edwards J, Barden J, McQuay H. Bandolier's little book of pain. Oxford: Oxford University Press, 2003: 238-40.
- 34.Kalso E, Moore RA. Five easy pieces on evidence-based medicine (2). Eur J Pain 2000;4: 321-4. [DOI] [PubMed] [Google Scholar]
- 35.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ 2003;326: 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mason L, Moore RA, Edwards JE, McQuay HJ, Wiffen PJ. Systematic review of efficacy of topical rubefacients containing salicylates for the treatment of acute and chronic pain. BMJ; 2004:doi:10.1136/bmj.38040.607141. [DOI] [PMC free article] [PubMed]
- 37.Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything—large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78: 209-16. [DOI] [PubMed] [Google Scholar]
- 38.Gøtzsche PC. Reporting of outcomes in arthritis trials measured on ordinal and interval scales is inadequate in relation to meta-analysis. Ann Rheum Dis 2001;60: 349-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simone DA, Nolano M, Johnson T, Wendelschafer-Crabb G, Kennedy WR. Intradermal injection of capsaicin in humans produces degeneration and subsequent reinnervation of epidermal nerve fibres: correlation with sensory function. J Neurosci 1998;18: 8947-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.