Abstract
Recombinant 70 kDa heat shock protein (Hsp70) is an antiapoptotic protein that has a cell protective activity in stress stimuli and thus could be a useful therapeutic agent in the management of patients with acute ischemic stroke. The neuroprotective and neurotherapeutic activity of recombinant Hsp70 was explored in a model of experimental stroke in rats. Ischemia was produced by the occlusion of the middle cerebral artery for 45 minutes. To assess its neuroprotective capacity, Hsp70, at various concentrations, was intravenously injected 20 minutes prior to ischemia. Forty-eight hours after ischemia, rats were sacrificed and brain tissue sections were stained with 2% triphenyl tetrazolium chloride. Preliminary treatment with Hsp70 significantly reduced the ischemic zone (optimal response at 2.5 mg/kg). To assess Hsp70’s neurotherapeutic activity, we intravenously administered Hsp70 via the tail vein 2 hours after reperfusion (2 hours and 45 minutes after ischemia). Rats were then kept alive for 72 hours. The ischemic region was analyzed using a high-field 11 T MRI scanner. Administration of the Hsp70 decreased the infarction zone in a dose-dependent manner with an optimal (threefold) therapeutic response at 5 mg/kg. Long-term treatment of the ischemic rats with Hsp70 formulated in alginate granules with retarded release of protein further reduced the infarct volume in the brain as well as apoptotic area (annexin V staining). Due to its high neurotherapeutic potential, prolonged delivery of Hsp70 could be useful in the management of acute ischemic stroke.
Keywords: focal ischemia, stroke, neuroprotection, neurotherapy, alginate granules
Introduction
As the molecular chaperone Hsp70 is well known for its cell protective functions against stress, it could be a useful therapeutic agent in the management of patients with acute ischemic stroke.1 To date, several in vitro studies have clearly demonstrated the feasibility and efficacy of Hsp70 as a neuroprotector.2–4 Previously, our group showed the possibility that glial cells could release into the extracellular milieu an inducible form of Hsp70 that would subsequently be taken up by neurons.5 This treatment gave the neurons increased resistance to heat shock stress (44°C) and to staurosporine-induced apoptosis. Hsp70 has been shown to have neuroprotective potency in various other brain pathologies: for example, we demonstrated that Hsp70 possesses high protective activity in a model of chemically induced seizures in rats.6 After intracerebroventricular injection, the chaperone was found exerting its neuroprotective capacity in the neurons and terminals of the limbic seizure complex of the brain. Moreover, Hsp70 protected cells by removing/sequestering protein aggregates inside the cells in an in vitro model of Huntington’s disease.7
A large body of evidence indicates that Hsp70 contributes to the pathogenesis of ischemia and to cytoprotection during periods of stress. Previously, Soriano et al demonstrated that 10 minutes of focal ischemia resulted in increased Hsp70 mRNA expression throughout the middle cerebral artery (MCA) region.8 Later, Zhan et al showed that, following vessel occlusion in a model of transient ischemic attacks in rats, Hsp70 was induced in neurons throughout the entire MCA territory.9 Other authors have proposed that the region of Hsp70 expression following focal ischemia corresponds to the molecular zone of the penumbra, located outside of the infarction.10 The possibility of a correlation between Hsp70 expression and increased tolerance to ischemia has been reported in studies.11–14 Thus, increased accumulation of Hsp70 in neurons protects the cells against ischemia.10
A convincing body of published data indicates that Hsp70 exerts cerebroprotective activity, although high levels of protein are required for this to occur. Previously, Sharp et al demonstrated that, at 72 hours following reperfusion, neurons in the zone of ischemia were positive for activated caspase-3, suggesting that, at declining levels, the chaperone was not able to exert its neuroprotective activity.10 The administration of exogenous Hsp70 may provide neurons in the penumbra with the required level of protein. In a subsequent study, it was shown that intravenous (IV) administration of Fv-Hsp70 significantly decreased infarct volume by 68% and improved sensorimotor function compared to that in the saline-treated control group.15 Another therapeutic approach could involve inductors of chaperone expression (eg, flavonoids). It was demonstrated that the flavonoid baicalin, known to activate the Hsp70 cascade in neurons following reperfusion, provides effective neuroprotection in an experimental model of global ischemia.16
In the work presented here, we explored the cerebroprotective and neurotherapeutic effects of Hsp70 intravenously administered at various concentrations in a model of transient focal ischemia in rats. We also propose that, to achieve effective therapy for ischemic stroke, a high level of Hsp70 should be sustained for a long period. For this reason, Hsp70 was encapsulated into spherical alginate granules designed to release the protein slowly.
Materials and methods
Study design
We aimed to explore the neuroprotective and neurotherapeutic activity of Hsp70 in an experimental ischemic model. Three consecutive substudies were undertaken: 1) IV administration of Hsp70 prior to the onset of ischemia; 2) IV injection of Hsp70 following acute ischemia; and 3) subcutaneous implantation of alginate granules designed for slow release of Hsp70 following the onset of ischemia. All experiments were performed after the protocol of the study was approved by the ethics committee of First St Petersburg IP Pavlov State Medical University (St Petersburg, Russia) in compliance with the US Department of Health and Human Services Guide for the Care and Use of Laboratory Animals (1996).
Substudy 1: evaluation of the neuroprotective function of Hsp70
The first study assessed the cerebroprotective potency of Hsp70 administered 20 minutes prior to the onset of ischemia (Figure 1A). Following ischemia, rats were kept alive for 48 hours. The animals were then sacrificed, and their brains were extracted, sectioned, and stained by triphenyl tetrazolium chloride (TTC) for analysis of the infarct volume. The fifteen rats were divided into five groups of three animals each as follows: 1) control group treated with IV injection of bovine serum albumin (BSA) (5.0 mg/kg) in saline solution (placebo); 2) experimental group treated with single IV injection of Hsp70 at 0.5 mg/kg; 3) experimental group treated with Hsp70 1.0 mg/kg; 4) experimental group treated with Hsp70 2.5 mg/kg; and 5) experimental group treated with Hsp70 5.0 mg/kg.
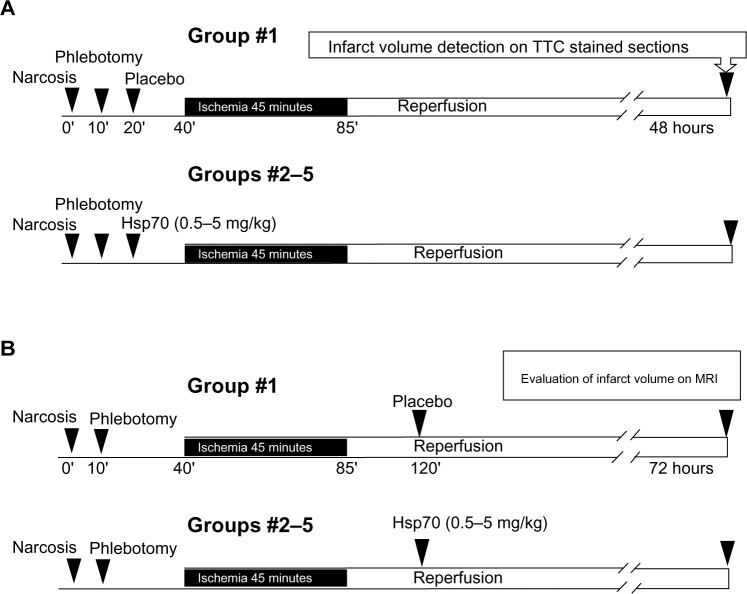
Figure 1.
Treatment scheme for the evaluation of Hsp70 neuroprotective function (A) and neurotherapeutic activity (B).
Abbreviations: MRI, magnetic resonance imaging; TTC, triphenyl tetrazolium chloride.
Substudy 2: assessment of the neurotherapeutic activity of Hsp70
The second study assessed the therapeutic activity of Hsp70 administered 2 hours following reperfusion (2 hours and 45 minutes after onset of ischemia) (Figure 1B). Animals were kept alive for 72 hours, then sacrificed and analyzed with the aid of high-field magnetic resonance imaging (MRI). The volume of brain tissue infarct was calculated. Fifteen rats were divided into five groups of three animals each as follows: 1) control group treated with IV injection of BSA (5.0 mg/kg) in saline solution (placebo); 2) experimental group treated with single IV injection of Hsp70 at 0.5 mg/kg; 3) experimental group treated with Hsp70 1.0 mg/kg; 4) experimental group treated with Hsp70 2.5 mg/kg; and 5) experimental group treated with Hsp70 5.0 mg/kg. After the MRI study, animals were sacrificed and their tissues subjected to immunohistological staining for the presence of annexin V, an early marker of apoptosis.
Substudy 3: long-term administration of Hsp70
The third study assessed the therapeutic potency of Hsp70 administered with prolonged delivery, which we achieved with alginate granules that were designed for slow release of the protein. Two hours after reperfusion (2 hours and 45 minutes after onset of ischemia), granules loaded with Hsp70 (1.0 mg/kg) or BSA (1.0 mg/kg) were implanted subcutaneously in the midscapular region. Rats were kept alive for 72 hours, then sacrificed. Rat brains were visualized on the MRI scanner to measure the ischemic zones. Animals were randomly divided into two groups of six animals each as follows: 1) control group treated with alginate granules containing BSA; and 2) experimental group treated with Hsp70-loaded granules.
Recombinant 70 kDa heat shock protein (HSP) Hsp70
Hsp70 was purified from bacteria transformed with a pMSHSP plasmid as described elsewhere.7 Briefly, Hsp70 was purified by means of anion exchange chromatography using DEAE-Sepharose (GE Healthcare UK Ltd., Little Chalfont, UK) followed by ATP-affinity chromatography on ATP-Agarose (Sigma-Aldrich, St Louis, MO, USA). Lipopolysaccharide was depleted with the help of Polymyxin B-agarose endotoxin removing gel (Sigma-Aldrich). The quantitation of endotoxin was performed using the Limulus amebocyte lysate assay (E-Toxate™ kit; Sigma-Aldrich). The resulting endotoxin content was below 0.1 EU/mg Hsp70.
For the analysis of Hsp70 penetration through the blood–brain barrier (BBB) in in vivo experiments, the chaperone was conjugated with Alexa Fluor® 555 (Invitrogen™; Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. BSA (Sigma-Aldrich) was employed as the control protein.
To achieve prolonged delivery of Hsp70, we encapsulated the chaperone into alginate granules. To produce the granules, we dissolved alginate powder (Acros Chemicals, Geel, Belgium) in phosphate-buffer solution to make a 1.2% alginate solution as described previously.17 Additionally, we added Hsp70 to the alginate solution at a concentration of 1.0 mg/mL. The alginate suspension was dropped through a 22-gauge needle into a 102 mM CaCl2 solution at a distance of 1.5 to 2 cm from the fluid surface. This caused the formation of alginate microspheres, which were allowed to harden for an additional 10 minutes. The granules were round or elliptical in appearance. To evaluate the kinetics of Hsp70 release from the granules, we used the fluorochrome-labeled protein Hsp70-Alexa Fluor® 555. The quantity of released Hsp70 was assessed using a LaserStrobe™ spectrofluorometer (Photon Technology International, Edison, NJ, USA). Nearly 80% of the protein loaded into the granules was released over 72 hours in in vitro experiments.
Rat stroke model
Male Wistar rats weighing 200–250 g were purchased from an animal nursery (Rappolovo, St Petersburg, Russia) and housed in transparent plastic cages under pathogen-free conditions, controlled temperature and humidity, and a 12-hour light–dark cycle with sterile food and water ad libitum. To create the experimental model, we applied the method of transient ligation of the right MCA as reported by Koizumi et al18 with the modifications described by Longa et al19 and Belayev et al.20 Briefly, the right common carotid artery and the internal carotid artery were exposed via a midline incision in the neck. A nylon filament suture (4-00; Ethicon, Inc., Somerville, NJ, USA) was advanced from the right external carotid artery, through the common carotid artery, and up to the internal carotid artery to block the origin of the MCA, until a mild resistance was felt. The left MCA was occluded for 45 minutes. After that, cerebral blood flow was restored by withdrawal of the nylon thread.
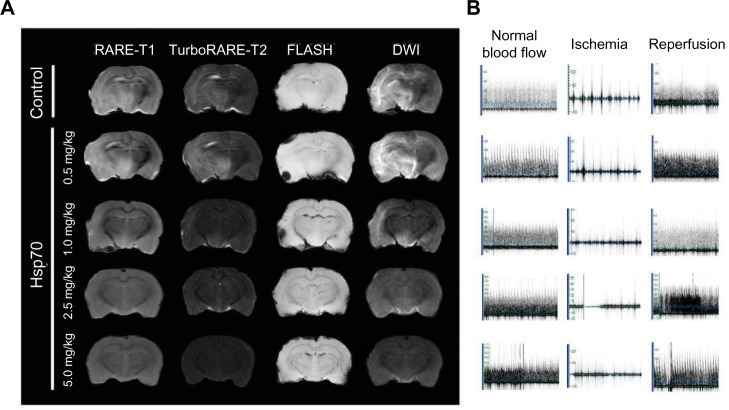
Additionally, we performed left craniotomy for arterial blood flow assessment and monitoring of the efficacy of the MCA occlusion/reperfusion. Arterial blood flow was measured in the area of left MCA vascularization by means of high-frequency ultrasound Doppler flowmetry (Minimax, Ltd., St Petersburg, Russia).21 At the cerebral surface, a standard 20 MHz probe was positioned against the wall of the artery to ensure blood flow measurement. Systolic blood flow velocity, an important parameter of cerebral perfusion, was expressed in cm/s to be measured throughout the experiments (Figure S1).
TTC staining
Brain sections 2 mm in thickness were stained with 2% TTC to assess necrosis. The slices were immersed in a 2% TTC solution for 15 minutes at 37°C (pH =7.4). Areas with no TTC staining were considered to be zones of necrosis.
MRI of experimental animals
MRI measurements were taken with an 11 T high-resolution scanner (Bruker-BioSpin, Ettlingen, Germany) using the FLASH (fast low-angle shot), RARE-T1, TurboRARE-T2, and SE diffusion-weighted imaging techniques. Pulse sequences were obtained in the coronal plane and included T2-weighted scans (TR/TE 4,200/36 ms; flip angle 180°; slice thickness 1.0 mm, interslice distance 1.2 mm; FoV 2.5×2.5 cm; matrix 256×256; in total, 20 slices), T1-weighted scans (TR/TE 1,500/7.5 ms; flip angle 180°; slice thickness 1.0 mm; FoV 2.5×2.5 cm; matrix 256×256), and FLASH scans (TR/TE 350/5.4 ms; flip angle 40°; slice thickness 1.0 mm; FoV 2.5×2.5 cm; matrix 256×256). Additionally, we obtained diffusion-weighted images (DWIs) (TR/TE 10,000/34.0 ms, flip angle 90°, slice thickness 1.0 mm; FoV 2.5×2.5 cm; matrix 256×256) in the coronal plane for analysis of the parenchymal cytotoxic edema. For the quantitative measurement of water diffusion, we applied apparent diffusion coefficient (ADC) maps. The obtained MRI scans were processed using MIPAV ([Medical Image Processing, Analysis, and Visualization] v 7.0.1) software for Microsoft Windows (Microsoft Corporation, Redmond, WA, USA).
Confocal microscopy analysis
To assess the distribution of Hsp70 labeled with Alexa Fluor® 555 in the brain following IV injection, rats were anesthetized by intraperitoneal injection with 10 mg Zoletil-100 (tiletamine hydrochloride and zolazepam; Virbac, Carros, France) and 0.2 mL 2% Rometar (xylazine hydrochloride; Bioveta, Ivanovice na Hané, Czech Republic). Following intracardial perfusion with 4% paraformaldehyde in 0.01 M phosphate-buffer solution, the brain was extracted to be examined for the presence of fluorochrome-labeled Hsp70. Each brain was embedded in TissueTek® and stored at −80°C. Serial cryosections were obtained in the coronal plane with a slice thickness of 10 μm. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Sections were mounted with DAKO mounting media (Agilent Technologies, Santa Clara, CA, USA). For analysis of the presence of annexin V in the brain tissues, we used a commercially available annexin V–fluorescein isothiocyanate kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions. Brain cryosections were stained for the presence of annexin V and nuclei were detected using DAPI. Images were captured with a Leica TCS SP5 confocal system (Leica Microsystems, Wetzlar, Germany) at 488 or 543 nm or a Leica DM IRBE microscope at 405 nm.
Statistics and analysis
The measured infarct volumes on TTC-stained brain sections or MRI scans were compared among control (BSA-treated) rats and the various groups of Hsp70-treated rats, which were given 0.5, 1.0, 2.5, and 5.0 mg/kg of Hsp70, respectively. In the substudy evaluating the therapeutic efficacy of Hsp70 with prolonged delivery, two groups were compared: control and Hsp70-treated. One-way analysis of variance tests were run to detect treatment differences. When the null hypothesis that no differences were associated with different treatment means was rejected, the Mann–Whitney U test was run to determine whether one treatment differed significantly from all others. One-way alternatives were used, since there was 0 power for a two-way test with three subjects per group. The open source software package R (v 3.0.2 [http://www.r-project.org/]; The R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis. Statistical significance was determined at the alpha =0.05 level. Observations are generally reported as mean ± standard deviation.
Results
Hsp70 penetrates through the BBB and accumulates in the brain tissues
To determine whether intravenously infused Hsp70 could pass through the BBB, we assigned three animals to receive administration of the chaperone by this means. Following occlusion of the MCA for 45 minutes, we injected Hsp70 labeled with Alexa Fluor® 555 fluorochrome (5.0 mg/kg) intravenously via the tail vein. After 24 hours, we sacrificed these rats and assessed the distribution of the Hsp70 in their brain tissues (Figure 2). Immunofluorescence images of the coronal brain sections revealed the incorporation of Hsp70 inside cells throughout the brain. Inside the cells, the chaperone appeared as red dots surrounding the nuclei. We observed a massive accumulation of Hsp70 in the ependymal cells and in the choroid plexus of the ventricles (Figure 2B and C). Hsp70 accumulated not only in the brain cortex (Figure 2A), but also in the deep brain structures (ie, the caudate nucleus, the lateral and medial septal nuclei, and the lateral preoptic area). Intriguingly, we did not observe Hsp70 in the corpus callosum (Figure 2B). Hsp70 was also dramatically accumulated in the blood vessels within the brain. It was present in the cytoplasm of the cells in all vessel layers (ie, the adventitia, media, and intima) and to a great extent in the endothelium (Figure 2G).
Figure 2.
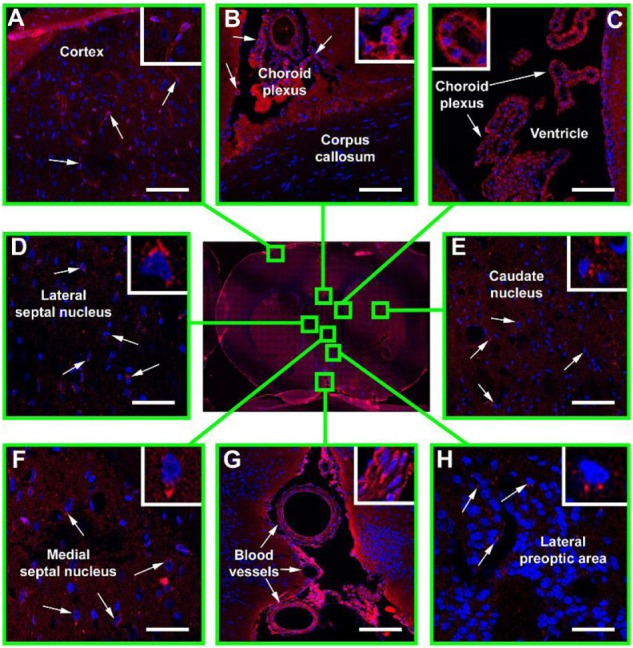
Immunofluorescence confocal microscopy of Hsp70 distribution in the ischemic rat brain.
Notes: Following transient occlusion of the right middle cerebral artery (45 minutes), Hsp70 conjugated with Alexa Fluor® 555 (Invitrogen™; Thermo Fisher Scientific, Waltham, MA, USA) (red) (5.0 mg/kg) was injected into the femoral vein. After 24 hours, the animal was sacrificed and its brain was extracted and sectioned. Nuclei were additionally stained by 4′,6-diamidino-2-phenylindole (DAPI) (blue). Shown here is a brain section obtained in the coronal plane 3 mm posterior to the bregma (stereotaxic atlas of Pelligrino, 1979). The presence of Hsp70 was assessed in the following structures: (A) brain cortex; (B) corpus callosum; (C) ventricle; (D) lateral septal nucleus; (E) caudate nucleus; (F) medial septal nucleus; (G) blood vessels; (H) lateral preoptic area. Scale bar: 25 μm.
We also analyzed the influence of transient ischemia on the permeability of the BBB to Hsp70. To determine this, we treated some rats with an IV injection of Hsp70 (5.0 mg/kg) without first inducing brain ischemia (n=3). Following 24 hours of protein IV administration, animals were sacrificed and the pattern of accumulation of Hsp70 in the ischemia-exposed brains was compared with that in the experimental group. In this assessment, we detected no protein in the brain tissues (data not shown). Additionally, we assessed the permeability of the BBB to Hsp70 administered subcutaneously in the form of alginate granules. Fluorochrome-labeled Hsp70-Alexa Fluor® 555 was loaded into alginate microspheres (1.0 mg/kg). Thirty minutes after the onset of ischemia, we located the granules subcutaneously in the midscapular area. After 24 hours, the animals were sacrificed and their brains were examined for the presence of Hsp70. The pattern of chaperone distribution corresponded to that seen when Hsp70 was infused intravenously (data not shown).
Recombinant Hsp70 exerts neuroprotective activity
To evaluate the neuroprotective activity of Hsp70, we administered Hsp70 intravenously 20 minutes prior to occlusion of the MCA. Following injection, animals were kept alive for 48 hours. They were then sacrificed and the sizes of their cerebral infarctions and penumbrae were measured in TTC-stained brain sections (Figure 3A). In the control group, when BSA (5.0 mg/kg) was administered, we observed a large area devoid of TTC staining on the cerebral artery-occluded side of the brain. The infarct zone appeared as a white area in clear contrast to the surrounding red (viable) brain tissues. In certain animals, the infarcted area was observed in the contralateral hemisphere. The ischemic zone constituted nearly 20% of the brain volume (Figure 3B). When Hsp70 was administered at a dose of 0.5 mg/kg, we did not observe any change in the volume of the infarct, but a dose of 1.0 mg/kg caused a statistically significant reduction of the ischemic area (P<0.001). Further dosage increases up to 2.5 mg/kg showed a considerable neuroprotective effect. At 2.5 mg/kg, the damaged tissue volume was less than 5% of the total brain volume. Intriguingly, a dose of 5.0 mg/kg abrogated the protective activity of Hsp70. On TTC-labeled sections, we observed a reliable delineation of ischemic brain tissue from the nonischemic white and gray matter of the brain (Figure 3A).
Figure 3.
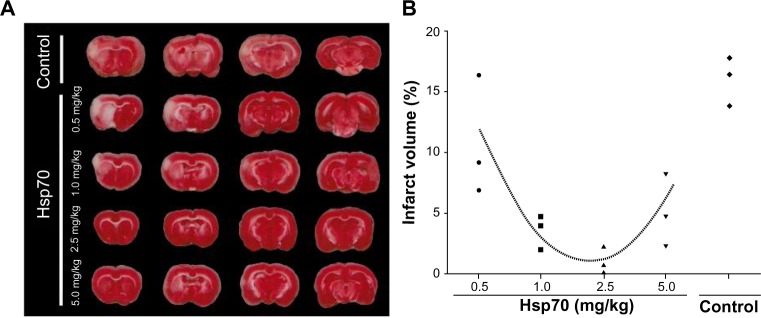
Detection of brain infarction in triphenyl tetrazolium chloride (TTC)-labeled tissue sections.
Notes: (A) Representative TTC-stained sections of rat brains from the bovine serum albumin-treated control group and the Hsp70-treated experimental groups treated with protein concentrations of 0.5, 1.0, 2.5, and 5.0 mg/kg, respectively. Ischemic tissues are visible as demarcated white areas in comparison to viable brain tissues, which appear red. (B) Diagram of the ischemic volume distribution for individual animals from control and Hsp70-treated groups. Infarct volume was quantified as a percentage (%) of total brain volume.
IV injection of Hsp70 mediates its neurotherapeutic effect in a dose-dependent manner
To explore the therapeutic potential of Hsp70, we administered it intravenously 2 hours and 45 minutes after the onset of acute occlusion of MCA. After 72 hours, MRI brain scans were recorded and analyzed to determine infarct volume and to identify the penumbra. We applied several regimens including RARE-T1, TurboRARE-T2, FLASH, and diffusion-weighted imaging in a relatively high-strength magnetic field (11 T). T1- and T2-weighted images of animals from the control group revealed massive infarcted regions representing hyperintensive zones in the right hemispheres corresponding to the animals’ occluded right MCAs (Figure 4A). Fast spin-echo T2-weighted sequences clearly revealed the areas of edema in the brain. Additionally, we applied the gradient recalled echo sequence (FLASH) to detect any blood products in the white and gray matter, but did not detect any hemorrhage. To analyze ischemic change or cytotoxic edema, we also performed DWIs. Damaged ischemic areas appeared bright on MRI diffusion imaging, clearly showing the edema in the contralateral hemisphere. IV infusion of Hsp70 at 0.5 mg/kg did not affect the infarct volume nor did it reduce the magnitude of edema on DWIs (Figure 4A). Further increasing the Hsp70 concentration to 1.0 mg/kg turned out to be beneficial for the reduction of the infarct zone. Interestingly, while the ischemic areas look like rather localized hyperintensive regions on T1- and T2-weighted scans, on DWIs, the edema appears to be more widespread and to involve more subcortical structures. Administration of Hsp70 at 2.5 or 5.0 mg/kg significantly reduced the infarcted area on T1- and T2-weighted scans. Though we clearly detected a reduction of blood flow in the right MCA using ultrasound dopplerography, we did not observe any parenchymal cytotoxic edema on DWIs, suggesting the therapeutic potency of Hsp70 at concentrations over 2.5 mg/kg (Figure 4B).
Figure 4.
Evaluation of the ischemic zone following treatment with Hsp70.
Notes: (A) Representative magnetic resonance scans of rat brains from the bovine serum albumin-treated control group and the Hsp70-treated experimental groups treated with protein concentrations of 0.5, 1.0, 2.5, and 5.0 mg/kg, respectively. Following treatment with Hsp70, magnetic resonance scans were obtained using the RARE-T1, TurboRARE-T2, FLASH and diffusion-weighted imaging (DWI) regimens. (B) Ultrasound dopplerography of the right middle cerebral artery depicting the cerebral blood flow before occlusion, during ischemia, and in the reperfusion phase. Each row corresponds to the respective magnetic resonance scan of the rat’s brain.
Long-term administration of Hsp70 using alginate granules enhances the neurotherapeutic potency of the protein
To enhance the therapeutic effect of Hsp70, we encapsulated the chaperone into alginate granules. Following the onset of focal ischemia, granules were injected subcutaneously into the midscapular region. The kinetics of sustained Hsp70 release corresponded to the observed outflow of 80% of the encapsulated protein within 72 hours. Each experimental animal was treated with granules loaded with Hsp70 at a dose of 1.0 mg/kg. Each control animal was given BSA-containing granules (1.0 mg BSA/kg); on MRI scans of these animals, the infarcted regions appear as large hyperintensive areas on T1- and T2-weighted images (Figure 5A). On DWIs, regions of cytotoxic edema in the contralateral hemisphere could also be detected.
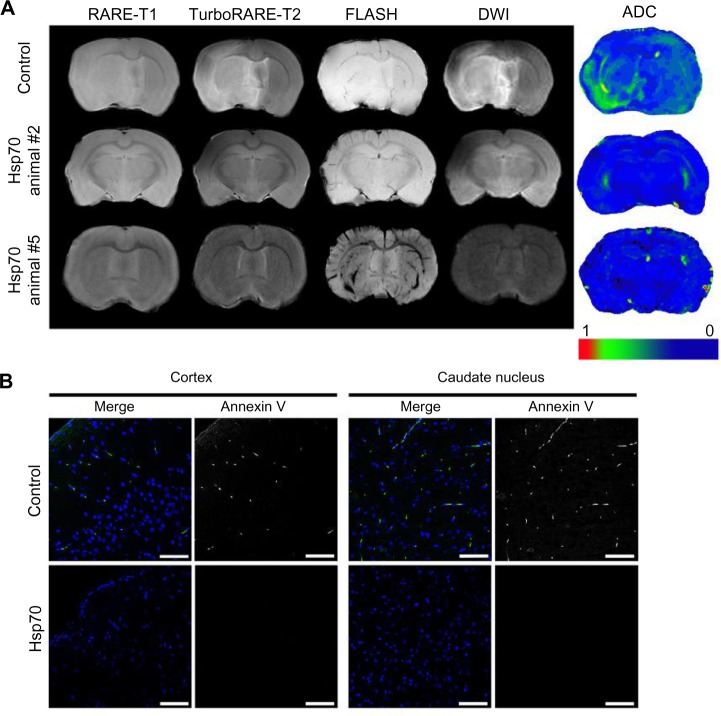
Figure 5.
Assessment of the infarcted zone following treatment with Hsp70-loaded alginate granules.
Notes: (A) Representative magnetic resonance images of rat brains from the control and Hsp70-treated groups (animals #2 and #5). Magnetic resonance imaging was performed according to the RARE-T1, TurboRARE-T2, FLASH, and diffusion-weighted imaging (DWI) regimens. Additionally, DWIs were evaluated using apparent diffusion coefficient (ADC) maps. (B) Confocal microscopic images of brain sections were obtained in the coronal plane. Apoptotic cells were detected using an annexin V kit (green). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar: 25 μm.
We quantitatively measured water diffusion in the extracellular matrix using ADC maps. In animals treated with Hsp70-loaded alginate granules, we observed a significant reduction in the volume of the infarctions in comparison to control animals. Indeed, we observed ADC normalization in the damaged areas of the brains in the experimental group (Figure 5A). This normalization was predominantly seen in the basal ganglia and white matter.
Following the MRI study, the rats’ brains were analyzed for apoptosis using an annexin V detection kit. A subsequent histological study revealed a decrease in the number of apoptotic areas in the Hsp70-treated group, whereas the control group had extensive annexin-positive zones located in the cortex and in the deep brain structures (Figure 5B).
Discussion
Currently, the only approved pharmacological therapy for ischemic stroke is the recanalization of the occluded artery with intravenously injected thrombolytics (eg, recombinant tissue plasminogen activator).22 The high risk of hemorrhage associated with this treatment emphasizes the need for additional neurotherapeutic approaches. Hsp70, a protein that is known to be upregulated in neurons following injury, might be a plausible candidate for use in the management of ischemic stroke patients.
According to our data, Hsp70 penetrates through the BBB, accumulating intracellularly throughout the brain (Figure 2). This process was observed in the ischemic brain but not in the intact brain, suggesting the influence of ischemia on the permeability of the BBB to Hsp70. Ischemic stroke as well as subsequent reperfusion injury can disrupt the BBB.23 Previously, Zhan et al reported that Hsp70 conjugated with Fv antibody (monoclonal antibody 3E10), a compound developed for the delivery of recombinant proteins to cells via equilibrative nucleoside transporter 2 (ENT2), can accumulate in the ischemic brain.15 In our study, using confocal microscopy, we found that fluorochrome-labeled Hsp70 can also penetrate the ischemic brain without any modification (Figure 2). Moreover, not only IV injection but also other routes of Hsp70 administration can be employed. For example, the subcutaneous application of Hsp70-loaded alginate granules also enabled Hsp70 to reach the brain. Intriguingly, we observed a dose-dependent accumulation of Hsp70 in the brain, as higher dosages were associated with larger quantities being retained in the brain. In a previous study, we revealed that hypoxia induces an elevated expression of CD40, a receptor for Hsp70, in glioma cells, and that the concentration of this receptor controls the preferential retention of intravenously injected Hsp70 in the tumor site.24 Presumably, the same phenomenon might contribute to the uptake of Hsp70 by the cells in ischemic brain tissues.
Hsp70 exerts a dose-dependent neuroprotective activity when administered intravenously prior to the onset of ischemia (Figure 3). The optimal effective concentration of Hsp70 was 2.5 mg/kg. Interestingly, at dosages over 5.0 mg/kg, we detected a reduction in the chaperone’s protective influence. Most probably, high dosages of Hsp70 (ie, 5.0 mg/kg) may enhance the production of proinflammatory cytokines, thus increasing ischemic injury. Asea et al demonstrated that Hsp70 can induce production of such cytokines through a CD14-dependent pathway.25 Recently, it was reported that Hsp70 at high concentrations is associated with proinflammatory cytokines (ie, TNFα, IL-1β, IL-12) in the early-onset stage of preeclampsia, subsiding to lower levels in later stages.25 Intriguingly, when Hsp70 was administered as a neurotherapeutic agent, 2 hours and 45 minutes after the onset of ischemia, we did not observe any side effects, even at a concentration of 5.0 mg/kg (Figure 4).
Hsp70 may be involved in increasing the production of whichever cytokines the body is producing at the time of administration; the types of cytokines it promotes, then, would depend on the stage of stroke. For example, in the early phase of stroke (within the first few hours), there is a many-fold increase in TNFα, IL-1, and IL-6 levels.26 This pro-inflammatory phase is followed by an increase in anti-inflammatory cytokines (eg, IL-10 and IL-1 receptor antagonist) that may reduce the intensity of the inflammatory response.27 One might suggest that an infusion of Hsp70 in the early stage further enhances the ongoing production of proinflammatory molecules, whereas the same injection in a later stage amplifies the ongoing production of anti-inflammatory cytokines. In favor of this hypothesis are the overwhelming data supporting the anti-inflammatory activity of Hsp70.28
In our experiment, we observed a significant reduction in edema in the ischemic zone on DWIs when Hsp70 was administered intravenously at concentrations over 1.0 mg/kg (Figure 4). This could be partly explained if Hsp70 suppresses the matrix metalloproteinases (MMPs). As proinflammatory agents, MMPs (eg, MMP-2, MMP-9) contribute to the disruption of the BBB and increase vascular permeability in the ischemic lesions.29,30 Hsp70 inhibits ischemia-induced MMP-2 and MMP-9, thus contributing to upregulation of the MMPs.31
Interestingly, in our analysis of MRI scans of the ischemic brain, we observed a mismatch between the implications of our T1- and T2-weighted images and those of our DWIs (Figure 4). It appears that, in certain animals, the visible changes associated with ischemia can be detected only in DWIs. Despite ambiguity in the literature concerning the interpretation of these diffusion-weighted changes, they are currently supposed to correspond to regions of permanent ischemic damage.32 Therefore, we assessed responses to Hsp70 therapy predominantly using diffusion-weighted imaging as the more sensitive detection regimen for ischemia.33,34
Encapsulation of the recombinant Hsp70 into alginate granules further increased the cerebroprotective activity of the chaperone, as was shown using high-resolution MRI (Figure 5). The therapeutic efficiency of 1.0 mg/kg Hsp70 administered with prolonged delivery was comparable to that of a single IV administration of Hsp70 at 2.5 mg/kg. The swelling of the alginate granules, enabling subsequent protein release, yields significantly higher levels of Hsp70 in comparison to a single IV injection, thus enhancing the biological influence of the chaperone. It has previously been reported by Luginbuehl et al that cellular response rates for an encapsulated protein (insulin-like growth factor I [IGF-I]) were noticeably increased due to the constant release of high concentrations of the protein from alginate capsules into the extracellular milieu.35 The importance of long-term delivery in regard to the therapeutic activity of Hsp70 has been demonstrated previously by our group in immunotherapy studies related to B16 mouse melanoma and C6 glioblastoma.36,37 Presumably, maintaining a high level of Hsp70 in the body provides a constant supply of neuroprotectors to the intracellular chaperone machinery as well as a continuous anti-inflammatory influence in the microenvironment within the ischemic zone.
Several of the mechanisms involved in Hsp70’s activity might be responsible for its neuroprotective and neurotherapeutic efficiency. It is well known that Hsp70 plays an important role in protein homeostasis (proteostasis), assisting in protein folding, protecting subunits of intracellular proteins from various stresses (eg, heat shock, hypoxia), and preventing protein aggregation or mediating disassembly.37–39 In response to stress, including ischemia, a cell’s expression level of Hsp70 increases, enhancing the cell’s stress tolerance.9,39 A recent study has suggested that the neuroprotective activity of Hsp70 might be explained not only by its role as a chaperone but also through its capability to antagonize apoptosis directly.40 Through inhibiting the translocation of Bax to mitochondria, Hsp70 prevents cytochrome c release as a result of mitochondrial membrane permeabilization, thus inhibiting caspase-dependent apoptosis.40 In another study, by Saleh et al, it was demonstrated that Hsp70 binds to apoptosis protease activating factor-1 (Apaf-1), thereby preventing the formation of the apoptosome.41 Furthermore, Hsp70 was also shown to be able to inhibit caspase-independent apoptosis through neutralizing the mitochondrial effector AIF.42 In our study, we observed a significant reduction in annexin V expression in the brains of Hsp70-treated rats in comparison to control rats, thus suggesting the antiapoptotic activity of Hsp70 as a plausible mechanism of its neurotherapeutic effect (Figure 5B). Nevertheless, other reported mechanisms of Hsp70 activity, including the possibility that it inhibits the JNK-related necrotic pathway and/or anti-inflammatory activity, might play a role in its neuroprotective capacity.43,44
Other members of the HSP family could also be applied in the management of the cerebral ischemia. Thus, 27 kDa HSP, Hsp27, is notable of its cellular protective functions.45–47 In the recent study by Teramoto et al, the therapeutic potential of Hsp27 was demonstrated in a mouse model of transient MCA occlusion.48 Animals that received an IV injection of Hsp27 following cerebral ischemia had a significantly reduced infarct size and improved neurological deficits. Other HSPs, including Hsp60 and Hsp90, have also shown antiapoptotic activity in the experimental cerebral ischemia, but are less studied.49 Promising results in brain focal ischemia were obtained with an Hsp90 antagonist, geldanamycin (benzoquinone ansamycin), which causes the release of the heat shock factor 1 (HSF1) and induces HSPs.4 Presumably, the combined application of the Hsp70 with other chaperones or their inductors might be beneficial in treatment of acute ischemia.
The therapeutic potency of Hsp70 can be further increased by combination with other cytokines known to have cerebroprotective activity (eg, IL-10 and IL-1Ra). In this manner, it has been demonstrated that treatment with the IL-1 receptor antagonist (IL-1Ra) significantly decreases infarct volume.50 Subsequent clinical trials have proven the relevance of IL-1Ra to the clinical outcomes of ischemic stroke patients.51,52 The combined application of Hsp70 and IL-1Ra might be beneficial in the treatment of ischemia.
Conclusion
Recombinant Hsp70 showed high neuroprotective and neurotherapeutic activity in this experimental model of stroke in rats, validating the possibility of its application in further clinical trials.
Supplementary material
The basic parameters of cerebral blood flow during all phases of rat stroke modeling.
Abbreviations: CCA, common carotid artery; MCA, middle cerebral artery.
Acknowledgments
We are grateful to Olga G Genbach, Nelly V Koroleva, Dmitriy N Suslov, Oleg V Galibin, Tatiana V Zakoldaeva, Irina V Kononova, and Yulia E Shevchuk for their assistance with animal experiments. We thank Olga M Suvorova and Larisa P Chokova for manufacturing the alginate granules, and Vladimir D Pautov for performing the spectrofluorometer studies. This work was supported by grants from the Russian Foundation for Basic Research (14-08-00614), a grant from the Program of Molecular and Cellular Biology (RAS), and a government grant (20.11.2012), No 14.N08.11.0001.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sharp FR, Zhan X, Liu DZ. Heat shock proteins in the brain: role of Hsp70, Hsp27, and HO-1 (Hsp32) and their therapeutic potential. Transl Stroke Res. 2013;4:685–692. doi: 10.1007/s12975-013-0271-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fink SL, Chang LK, Ho DY, Sapolsky RM. Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J Neurochem. 1997;68:961–969. doi: 10.1046/j.1471-4159.1997.68030961.x. [DOI] [PubMed] [Google Scholar]
- 3.Rajdev S, Hara K, Kokubo Y, et al. Mice overexpressing rat heat shock protein 70 are protected against cerebral infarction. Ann Neurol. 2000;47:782–791. [PubMed] [Google Scholar]
- 4.Lu A, Ran R, Parmentier-Batteur S, Nee A, Sharp FR. Geldanamycin induces heat shock proteins in brain and protects against focal cerebral ischemia. J Neurochem. 2002;81(2):355–364. doi: 10.1046/j.1471-4159.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 5.Guzhova I, Kislyakova K, Moskaliova O, et al. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/s0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- 6.Ekimova IV, Nitsinskaya LE, Romanova IV, Pastukhov YF, Margulis BA, Guzhova IV. Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem. 2010;115:1035–1044. doi: 10.1111/j.1471-4159.2010.06989.x. [DOI] [PubMed] [Google Scholar]
- 7.Guzhova IV, Lazarev VF, Kaznacheeva AV, et al. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of Huntington disease. Hum Mol Genet. 2011;20:3953–3963. doi: 10.1093/hmg/ddr314. [DOI] [PubMed] [Google Scholar]
- 8.Soriano MA, Ferrer I, Rodríguez-Farré E, Planas AM. Expression of c-fos and inducible hsp-70 mRNA following a transient episode of focal ischemia that had non-lethal effects on the rat brain. Brain Res. 1995;670:317–320. doi: 10.1016/0006-8993(94)01352-i. [DOI] [PubMed] [Google Scholar]
- 9.Zhan X, Kim C, Sharp FR. Very brief focal ischemia simulating transient ischemic attacks (TIAs) can injure brain and induce Hsp70 protein. Brain Res. 2008;1234:183–197. doi: 10.1016/j.brainres.2008.07.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharp FR, Lu A, Tang Y, Millhorn DE. Multiple molecular penumbras after focal cerebral ischemia. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Nishi S, Taki W, Uemura Y, et al. Ischemic tolerance due to the induction of HSP70 in a rat ischemic recirculation model. Brain Res. 1993;615:281–288. doi: 10.1016/0006-8993(93)90039-p. [DOI] [PubMed] [Google Scholar]
- 12.Nishino K, Nowak TS., Jr Time course and cellular distribution of hsp27 and hsp72 stress protein expression in a quantitative gerbil model of ischemic injury and tolerance: thresholds for hsp72 induction and hilar lesioning in the context of ischemic preconditioning. J Cereb Blood Flow Metab. 2004;24:167–178. doi: 10.1097/01.WCB.0000100853.67976.8B. [DOI] [PubMed] [Google Scholar]
- 13.Currie RW, Ellison JA, White RF, Feuerstein GZ, Wang X, Barone FC. Benign focal ischemic preconditioning induces neuronal Hsp70 and prolonged astrogliosis with expression of Hsp27. Brain Res. 2000;863:169–181. doi: 10.1016/s0006-8993(00)02133-8. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y, Kato H, Nakata N, Kogure K. Temporal profile of heat shock protein 70 synthesis in ischemic tolerance induced by preconditioning ischemia in rat hippocampus. Neuroscience. 1993;56:921–927. doi: 10.1016/0306-4522(93)90138-6. [DOI] [PubMed] [Google Scholar]
- 15.Zhan X, Ander BP, Liao IH, et al. Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke. 2010;41:538–543. doi: 10.1161/STROKEAHA.109.572537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dai J, Chen L, Qiu YM, et al. Activations of GABAergic signaling, HSP70 and MAPK cascades are involved in baicalin’s neuroprotection against gerbil global ischemia/reperfusion injury. Brain Res Bull. 2013;90:1–9. doi: 10.1016/j.brainresbull.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 17.Guo JF, Jourdian GW, MacCallum DK. Culture and growth characteristics of chondrocytes encapsulated in alginate beads. Connect Tissue Res. 1989;19:277–297. doi: 10.3109/03008208909043901. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi J, Yoshida Y, Nakazawa T, Ooneda G. Experimental studies of ischemic brain edema. I. A new experimental model of cerebral embolism in which recirculation can introduced into the ischemic area. Jpn J Stroke. 1986;8:1–8. [Google Scholar]
- 19.Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible muddle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 20.Belayev L, Alonso OF, Busto R, Zhao W, Ginsberg MD. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke. 1996;27:1616–1622. doi: 10.1161/01.str.27.9.1616. [DOI] [PubMed] [Google Scholar]
- 21.Shmonin A, Melnikova E, Galagudza M, Vlasov T. Characteristics of cerebral ischemia in major rat stroke models of middle cerebral artery ligation through craniectomy. Int J Stroke. 2012 Dec 4; doi: 10.1111/j.1747-4949.2012.00947.x. Epub. [DOI] [PubMed] [Google Scholar]
- 22.Bivard A, Lin L, Parsonsb MW. Review of stroke thrombolytics. J Stroke. 2013;15:90–98. doi: 10.5853/jos.2013.15.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khatri R, McKinney AM, Swenson B, Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute ischemic stroke. Neurology. 2012;79(13 Suppl 1):S52–S57. doi: 10.1212/WNL.0b013e3182697e70. [DOI] [PubMed] [Google Scholar]
- 24.Shevtsov MA, Yakovleva LY, Nikolaev BP, et al. Tumor targeting using magnetic nanoparticle Hsp70 conjugate in a model of C6 glioma. Neuro Oncol. 2014;16:38–49. doi: 10.1093/neuonc/not141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asea A, Kraeft SK, Kurt-Jones EA, et al. HSP70 stimulates cytokine production through a CD14-dependant pathway, demonstrating its dual role as a chaperone and cytokine. Nat Med. 2000;6:435–442. doi: 10.1038/74697. [DOI] [PubMed] [Google Scholar]
- 26.Peraçoli JC, Bannwart-Castro CF, Romao M, et al. High levels of heat shock protein 70 are associated with pro-inflammatory cytokines and may differentiate early- from late-onset preeclampsia. J Reprod Immunol. 2013;100:129–134. doi: 10.1016/j.jri.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Lambertsen KL, Biber K, Finsen B. Inflammatory cytokines in experimental and human stroke. J Cereb Blood Flow Metab. 2012;32:1677–1698. doi: 10.1038/jcbfm.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karikó K, Weissman D, Welsh FA. Inhibition of toll-like receptor and cytokine signaling – a unifying theme in ischemic tolerance. J Cereb Blood Flow Metab. 2004;24:1288–1304. doi: 10.1097/01.WCB.0000145666.68576.71. [DOI] [PubMed] [Google Scholar]
- 29.Borges TJ, Wieten L, van Herwijnen MJ, et al. The anti-inflammatory mechanisms of Hsp70. Front Immunol. 2012;3:95. doi: 10.3389/fimmu.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Planas AM, Solé S, Justicia C. Expression and activation of matrix metalloproteinase-2 and -9 in rat brain after transient focal cerebral ischemia. Neurobiol Dis. 2001;8:834–846. doi: 10.1006/nbdi.2001.0435. [DOI] [PubMed] [Google Scholar]
- 31.Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke. 2006;37:1399–1406. doi: 10.1161/01.STR.0000223001.06264.af. [DOI] [PubMed] [Google Scholar]
- 32.Lee JE, Kim YJ, Kim JY, Lee WT, Yenari MA, Giffard RG. The 70 kDa heat shock protein suppresses matrix metalloproteinases in astrocytes. Neuroreport. 2004;15:499502. doi: 10.1097/00001756-200403010-00023. [DOI] [PubMed] [Google Scholar]
- 33.Barber PA. Magnetic resonance imaging of ischemia viability thresholds and the neurovascular unit. Sensors (Basel) 2013;13:6981–7003. doi: 10.3390/s130606981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Macintosh BJ, Graham SJ. Magnetic resonance imaging to visualize stroke and characterize stroke recovery: a review. Front Neurol. 2013;4:60. doi: 10.3389/fneur.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luginbuehl V, Wenk E, Koch A, Gander B, Merkle HP, Meinel L. Insulin-like growth factor I-releasing alginate-tricalciumphosphate composites for bone regeneration. Pharm Res. 2005;22:940–950. doi: 10.1007/s11095-005-4589-9. [DOI] [PubMed] [Google Scholar]
- 36.Abkin SV, Pankratova KM, Komarova EY, Guzhova IV, Margulis BA. Hsp70 chaperone-based gel composition as a novel immunotherapeutic anti-tumor tool. Cell Stress Chaperones. 2013;18:391–396. doi: 10.1007/s12192-012-0391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saibil H. Chaperone machines for protein folding, unfolding and disaggregation. Nat Rev Mol Cell Biol. 2013;14:630–642. doi: 10.1038/nrm3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim YE, Hipp MS, Bracher A, Hayer-Hartl M, Hartl FU. Molecular chaperone functions in protein folding and proteostasis. Annu Rev Biochem. 2013;82:323–355. doi: 10.1146/annurev-biochem-060208-092442. [DOI] [PubMed] [Google Scholar]
- 39.Sharma SK, Christen P, Goloubinoff P. Disaggregating chaperones: an unfolding story. Curr Protein Pept Sci. 2009;10:432–446. doi: 10.2174/138920309789351930. [DOI] [PubMed] [Google Scholar]
- 40.del Zoppo GJ, Sharp FR, Heiss WD, Albers GW. Heterogeneity in the penumbra. J Cereb Blood Flow Metab. 2011;31:1836–1851. doi: 10.1038/jcbfm.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saleh A, Srinivasula SM, Balkir L, Robbins PD, Alnemri ES. Negative regulation of the Apaf-1 apoptosome by Hsp 70. Nat Cell Biol. 2000;2:476–483. doi: 10.1038/35019510. [DOI] [PubMed] [Google Scholar]
- 42.Stankiewicz AR, Lachapelle G, Foo CP, Radicioni SM, Mosser DD. Hsp70 inhibits heat-induced apoptosis upstream of mitochondria by preventing Bax translocation. J Biol Chem. 2005;280:38729–38739. doi: 10.1074/jbc.M509497200. [DOI] [PubMed] [Google Scholar]
- 43.Ravagnan L, Gurbuxani S, Susin SA, et al. Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 2001;3:839–843. doi: 10.1038/ncb0901-839. [DOI] [PubMed] [Google Scholar]
- 44.Gabai VL, Meriin AB, Yaglom JA, Wei JY, Mosser DD, Sherman MY. Suppression of stress kinase JNK is involved in HSP72-mediated protection of myogenic cells from transient energy deprivation. HSP72 alleviates the stress-induced inhibition of JNK dephosphorylation. J Biol Chem. 2000;275:38088–38094. doi: 10.1074/jbc.M006632200. [DOI] [PubMed] [Google Scholar]
- 45.Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int J Hyperthermia. 2005;21(5):379–392. doi: 10.1080/02656730500069955. [DOI] [PubMed] [Google Scholar]
- 46.Latchman DS. HSP27 and cell survival in neurones. Int J Hyperthermia. 2005;21(5):393–402. doi: 10.1080/02656730400023664. [DOI] [PubMed] [Google Scholar]
- 47.van der Weerd L, Tariq Akbar M, Aron Badin R, et al. Overexpression of heat shock protein 27 reduces cortical damage after cerebral ischemia. J Cereb Blood Flow Metab. 2010;30(4):849–856. doi: 10.1038/jcbfm.2009.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Teramoto S, Shimura H, Tanaka R, et al. Human-derived physiological heat shock protein 27 complex protects brain after focal cerebral ischemia in mice. PLoS One. 2013;8(6):e66001. doi: 10.1371/journal.pone.0066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hwang IK, Ahn HC, Yoo KY, et al. Changes in immunoreactivity of HSP60 and its neuroprotective effects in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol. 2007;208(2):247–256. doi: 10.1016/j.expneurol.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 50.Greenhalgh AD, Galea J, Dénes A, Tyrrell PJ, Rothwell NJ. Rapid brain penetration of interleukin-1 receptor antagonist in rat cerebral ischaemia: pharmacokinetics, distribution, protection. Br J Pharmacol. 2010;160:153–159. doi: 10.1111/j.1476-5381.2010.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Emsley HC, Smith CJ, Georgiou RF, et al. Acute Stroke Investigators A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76:1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh N, Hopkins SJ, Hulme S, et al. The effect of intravenous interleukin-1 receptor antagonist on inflammatory mediators in cerebrospinal fluid after subarachnoid haemorrhage: a phase II randomised controlled trial. J Neuroinflammation. 2014;11:1. doi: 10.1186/1742-2094-11-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The basic parameters of cerebral blood flow during all phases of rat stroke modeling.
Abbreviations: CCA, common carotid artery; MCA, middle cerebral artery.