Abstract
Dendritic cells (DCs) are the major sentinel, antigen-presenting and regulatory components of the immune system. One of the central DC functions is to rapidly sense and alert host immune system of a pathogen invasion. In the present study, we investigated the role of DC exosomes (DCex) in this sentinel function. We demonstrated that DCex could bind bacterial Toll-like-receptor ligands (TLR-Ls), and acquire their ability to strongly activate bystander DCs. Consequently, bystander DCs enhance the expression of transmembrane tumor necrosis factor, secretion of proinflammatory cytokines and cross-talk with natural killer cells leading to the elevated secretion of IFNγ. These findings newly show that DCex can bind and cross-present TLR-Ls to innate-immunity effector cells, and indicate a potent mechanism to systemically alert the host immune system of pathogen invasion. They also suggest a potential novel strategy to generate effective vaccines by binding TLR-L-immune adjuvants to DCex.
Keywords: Dendritic cells, NK cells, Exosomes, TLR ligands, transmembrane TNF, Th1 response
1. Introduction
DCs are the major sentinel, antigen-presenting and regulatory cells of the immune system that function at the interface of innate and adaptive immunity [1,2]. Central DC sentinel functions are rapid detection of invading pathogens and subsequent mobilization of the immune system. These functions are enabled by a range of pattern recognition receptors called TLRs that are expressed on DCs. TLRs recognize structurally conserved molecules named pathogen-associated molecular patterns (PAMPs), or TLR-Ls, derived from various microorganisms [3]. Using TLRs, DCs rapidly recognize and respond to pathogen-derived TLR-Ls by undergoing maturation, and enhanced secretion of proinflammatory and immunoregulatory cytokines. Thus matured DCs effectively mediate inflammation as well as polarization and enhancement of innate immune functions [3]. Due to these DC-stimulating functions, TLR-Ls are used as potent immune adjuvants to modulate the quality, and enhance the magnitude and overall effectiveness of vaccine-induced immune responses [3].
In a cancer setting, an important sentinel function of DCs is their ability to recognize and kill cancer cells. We have previously demonstrated in both humans and mice that DCs express several transmembrane tumor necrosis factor (TNF) superfamily ligands on the plasma membrane and efficiently mediate via these ligands the apoptosis of virtually all types of cancer cells. This function defines DCs as important effectors of the anticancer immune surveillance mechanism [4–6].
DCs mediate and regulate their functions by interacting and cooperating with other cells of the immune system. The central pathway of this mechanism is the DC cross-talk with natural killer (NK) cells, the major effector cells of the innate immune system that also potently mediate tumoricidal and immunoregulatory functions [7–9]. This cross-talk is mostly mediated via cell-to-cell contact and participation of secreted cytokines such as IL-12 and IL-18. It is characterized by reciprocal stimulation and regulation of DCs and NK cells leading to their enhanced Th1 polarization. Consequently, NK cells become activated and highly tumoricidal, and increase secretion of IFNγ and GM-CSF. On the other hand, DCs undergo maturation, and increase secretion of IL-1β, IL-12, and TNF [6,8,9]. We have demonstrated in both mice and humans that DC/NK-cell cross-talk mainly occurs through transmembrane TNF (tmTNF) and/or trans-presented IL-15 (transIL-15) [6,10,11]. The cross-talk is highly amplified by the NK-cell-activating cytokine IL-2 and the DC-stimulating TLR4-L bacterial lipopolysaccharide (LPS) [6,10]. Consequently, the amplified cross-talk enables development of enhanced tumor-specific T-cell responses and effective antitumor activity in vivo [6,7]. These findings suggest that DC/NK-cell interaction is a central immunoregulatory mechanism that defines the quality and magnitude of innate and adaptive immune reactions, and that immune adjuvants based on bacterial TLR-Ls may function by stimulating this potentially important immunologic mechanism.
In addition to the secreted-cytokines and plasma membrane-bound ligands, DCs can also utilize their endosome-derived secreted vesicles, exosomes, to communicate with other cells within the immune-system. DCex are composed of the cytosolic material encapsulated by a limiting membrane made of the lipid bilayer and inserted transmembrane molecules. DCex transmembrane molecules include a selection of biologically important DC plasma membrane molecules such as MHC class I and class II molecules, intercellular adhesion molecule-1 (ICAM-1), integrins, and T cell co-stimulatory molecules CD40, CD80 and CD86 [14,15]. These molecules are correctly oriented on the outer surface of the DCex limiting membrane, and are biologically active. Consequently, the DCex MHC molecules make highly immunogenic complexes with tumor antigen-derived epitopes and remotely induce strong tumor-specific T cell responses and antitumor activity in mice [16,17]. Due to these functions, DCex-tumor antigen vaccines have been tested for their anticancer therapeutic potential in stage I clinical trials. Unfortunately, the therapy failed to induce tumor-specific T-cell and clinical responses [18,19]. A possible cause of this failure could be that DCex have a limited endogenous adjuvant function and the treatments were performed without the exogenous adjuvant support.
To induce effective adaptive immune responses, DCex should not only prime antigen specific T cells, but should also strongly stimulate innate immune mechanisms that regulate the quality and magnitude of adaptive immune functions. We have recently shown that DCex, like DCs, express transmembrane TNF, FasL and TRAIL, and directly induce apoptosis in cancer cells via a cooperative activity of these ligands. In addition, DCex, like DCs, express tmTNF and directly induce the Th1 polarization and enhanced activity of NK cells via this ligand [20]. However, the latter function of DCex, which might be critical for their effective vaccine ability, is a hundred fold weaker than that of DCs.
In the present study, we examined whether DCex could indirectly induce a robust innate immunity response by binding TLR-Ls and mediating via the bound ligands an enhanced stimulation of bystander DCs. We showed that the bacterial LPS, a TLR4 ligand, and the synthetic Pam3, a TLR1/2 ligand, can bind to DCex surface. Consequently, DCex acquire the ability to activate bystander DCs as shown by their increased expression of tmTNF, augmented secretion of proinflammatory and immunoregulatory cytokines, and ability to mediate enhanced Th1 polarization and activation of NK cells. These findings define a novel function of DCex, and suggest a new strategy to improve DCex vaccines by binding TLR-ligand-immune adjuvants to DCex.
2. Materials and methods
2.1. Mice
T-cell/B-cell-deficient SCID (B6; 129S7-Rag1tm1mom/J), TNF-deficient (B6;129S-Tnftm1Gkl/J), TNF receptor 1 (TNFR1)-deficient (B6;129S-Tnfrsf1atm1Imx Il1r1tm1Imx/J), TNFR2-deficient (B6.129S2-Tnfrsf1btm1Mwm/J); CD14-deficient (B6.129S-Cd14tm1Frm/J) and TLR4-deficient (C.C3-Tlr4Lps-d/J) female mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Mice were housed at the University of Pittsburgh Cancer Institute’s Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) internationally accredited animal facility. The animal studies were performed in accordance with protocols approved by the University of Pittsburgh Institutional Animal Care and Use Committee.
2.2. Reagents
The following reagents, antibodies and kits were used in the present study: recombinant mouse GM-CSF and IL-4 (R&D Systems, Minneapolis, MN); recombinant human interleukin 2 (IL-2) (Chiron Corp., Emeryville, CA); high purity TLR4 ligand (with less than 3% impurities), Escherichia coli serotype 055:B5 lipopolysaccharide (LPS) (part No 7193, lot No GL1457; Lonza, Walkersville, MD); synthetic TLR1/2 agonist Pam3CSK4 (Pam3, InvivoGen, San Diego, CA); phycoerythrin (PE)-conjugated rat anti-mouse TNF, CD14 and TLR4 monoclonal antibodies (BD-Pharmingen, CA, USA); unconjugated rat anti-mouse TNF (XT22, Pierce-Endogen, Rockford, IL), human TNFR2-Fc fusion protein (ENBREL, etanercept; Amgen, Thousand Oaks, CA), hamster anti-mouse TNFR1 and TNFR2 (BD-Pharmingen) and isotype control monoclonal antibodies (BD-Pharmingen); dominant negative TNF constructs (DNTNF1, XPro1595; and DNTNF2, XENP550; Xencor, Monrovia, CA); Limulus Amebocyte Lysate (LAL) Chromogenic Assay kit (Thermo Fisher Scientific Inc, Pittsburgh, PA); mouse Quantikine IFN-γ enzyme-linked immunosorbent assay (ELISA) kit (R&D Systems); and mouse TNF DuoSet ELISA kits (R&D Systems).
2.3. In vitro generation of DCs
Immature myeloid DCs (iDCs) were generated by 6-day culture of SCID mouse bone marrow cells (0.1 × 106/mL) in complete cell culture medium (CM) constituted of RPMI 1640 medium, 0.1 mM nonessential amino acids, 2 mM sodium pyruvate, 1 mM L-glutamine, 100 μg/ml streptomycin, 100 U/ml penicillin, 10% fetal bovine serum (FBS) (Life Technologies, Grand Island, NY), 50 μM 2-mercaptoethanol (Bio-Rad, Hercules, CA); and supplemented with 15 ng/mL recombinant mouse GM-CSF and IL-4. iDCs were 95% lineage marker-CD11c+CD205+/−, which expressed low to intermediate levels of CD80, CD86, CD40, MHC I and MHC II molecules. Mature DCs (mDCs) were produced by an overnight stimulation of day 5 iDCs with 1 μg/mL LPS. DCs derived from TNF, CD14 and TLR4 deficient mice showed normal growth and expression of costimulatory molecules.
2.4. Activation and expansion of NK cells
Fresh SCID mouse splenocytes (0.1 × 106/mL) were cultured for 6 days in SCGM medium (CellGenix GmbH, Freiburg, Germany) supplemented with 5% FBS, 50 μM 2-mercaptoethanol and 6,000 IU/mL IL-2. NK cells expanded 15–20 fold, and were ≥98% NK1.1+NKp46+DX5+CD3−CD69+. They are referred to as cultured IL-2-activated NK (aNK) cells.
2.5. In vitro generation and purification of DCex
DCex were obtained and assessed for purity as previously described [20]. Briefly, iDCex were generated by replacing day 5 iDC-culture-conditioned medium with fresh CM supplemented with GM-CSF/IL-4, and culturing iDCs for additional 24 h. mDCex were generated by replacing day 5 iDC culture-conditioned media with fresh CM supplemented with GM-CSF/IL-4 and 1 μg/mL LPS or Pam3, and culturing DCs for additional 24 h. On day 6, the newly generated cell culture-conditioned media were collected from iDC and mDC cultures. These cell culture-conditioned media were sequentially centrifuged for 10 min at 300 g and 1000 g, and filtered through 0.45 μm pore filters, to remove cells and debris. Subsequently, cell/debris-free cell culture-conditioned media were concentrated in Centricon Plus-70 filter units (Millipore, Billerica, MA) by centrifugation at 1000 g for 45 min. DCex were separated from the cell culture-conditioned media by ultracentrifugation at 100,000 g (Optima LE-80K ultracentrifuge, 90-Ti rotor, Beckman Coulter) for 60 min. DCex were washed three times with 15 ml PBS using Amicon Ultra-15 filter units (Millipore) and centrifugation at 1000 g for 25 min. Finally, DCex were resuspended in 200 μl PBS. Quantities of the purified DCex were measured using the BCA Protein Assay Kit (Thermo Scientific, Rockford, IL). Quality of DCex was evaluated by electron microscopy (EM) and flow cytometry [20]. The purified DCex were homogenous populations of 30 to 100 nm in diameter and oval-biconcave shape micro-vesicles made of an electron-dense double-layer membrane surrounding an electron-clear material. In addition DCex expressed on their surface typical DC markers, including MHC I, MHC II, CD80, CD86, CD40 and CD14.
2.6. Binding of LPS to iDCex
iDC-conditioned media were depleted of cells and cell debris by centrifugations (10 min at 300 g and 1000 g), and filtration through a 0.45 μm pore filter. Supernatants were mixed with 1 μg/mL or 0.1 μg/mL LPS and incubated overnight at 37°C. Following the incubation, iDCex with LPS coupled to their surface (iDCex*LPS) were purified, and unbound LPS was removed by three washes with 15 ml PBS, as aforementioned for iDCex and mDCex.
2.7. Detection and quantification of DCex-bound LPS
Intact mDCex generated by 24-h culturing of iDCs in the presence of 1 μg/mL LPS (mDCex*LPS), and iDCex*LPS obtained by 24 h incubation of iDCex in the presence of 1 μg/mL or 0.1 μg/mL LPS were tested for the presence and quantity of bound LPS using LAL Chromogenic Assay kit (Thermo Fisher Scientific Inc), as recommended by the company.
2.8. Measurements of membrane-bound and soluble TNF
TNF expression on DC plasma membrane was examined by flow cytometry of intact DCs. DCs were washed once with FACS buffer (PBS, 1% v/v FCS and 0.1% w/v NaN3) and incubated with fluorochrome-conjugated anti-TNF or isotype-matched nonreactive control antibodies (20 μg/mL) for 1 h at 4°C. Subsequently, DCs were washed three times with FACS buffer and fixed with 1% paraformaldehyde solution in PBS. DCs were examined using the XL Epics Flow Cytometer (Beckman Coulter Ramsey, MN) and the obtained data were analyzed using the Beckman Coulter Expo32 Software.
Soluble TNF secreted during cultures of iDCs (100,000/200 μL/well of 96 well plate), in the absence or presence of 1 μg/mL LPS, mDCex*LPS or iDCex*LPS for 24 h, were measured in DC-conditioned media using ELISA. Data were presented as means ± standard deviation (SD) of TNF ng/0.5 × 106 DCs/mL.
2.9. Quantification of cytokines and chemokines in DCex lysates and DCex-induced DC culture-conditioned media
Both surface-bound and inside-stored cytokines and chemokines were quantified in lysates of iDCex and mDCex*LPS. In addition, secreted cytokines and chemokines were examined in culture-conditioned media of iDCs (100,000/well) cultured for 24 h in the absence or presence of 1 μg/mL LPS, iDCex or mDCex*LPS. DCex were lysed using the radioimmunoprecipitation assay (RIPA) cell-lysis buffer (Upstate, Charlottesville, VA) containing EDTA-supplemented protease inhibitor cocktail (Roche Applied Science, Indianapolis, IN). DCex lysates and DC culture-conditioned media were analyzed using a Millipore multiplex mouse cytokine kit, as recommended by the company. The amounts of cytokines per 100 μg of exosome proteins and 1 mL of DC culture-conditioned media were determined.
2.10. DCex stimulation of bystander DCs and DC/NK cell crosstalk
Two experimental models were used. In the first one, in vitro stimulated and expanded iDCs and aNK cells were co-cultured. iDCs and aNK cells were suspended in CM and plated (50,000/well/200 μL) in round bottom 96-well plates (Corning, Sunnyvale, CA). Typically, one μg/mL iDCex, mDCex*LPS, iDCex*LPS or LPS alone were added to the wells containing iDCs and aNK cells. In some experiments, titrating concentrations of 0.001 to 10 μg mDCex*LPS/mL, or 0.1 to 1,000 pg/mL bound LPS to iDCex*LPS, and 1000 to 1000000 pg/mL soluble LPS were examined. Control wells contained iDCs and aNK cells alone. To assess the role of TNF and their receptors in DC/NK cell cross-talk stimulated with DCex*LPS, wild type and TNF deficient DCs and mDCex*LPS, and wild type and TNFR1- or TNFR2-deficient aNK cells were comparatively examined. In addition, DNTNF (Xencor) that selectively sequesters soluble TNF [21], XT22 anti-TNF antibody (Pierce-Endogen) and ENBREL TNFR2-Fc fusion protein (Amgen) that neutralize both soluble and transmembrane TNF, and anti-TNFR1 and anti-TNFR2 blocking antibodies (BD-Pharmingen) (20 μg/mL) were applied to the mixture of iDCs, aNK cells and mDCex*LPS. Control wells contained a mixture of iDCs, aNK cells, mDCex*LPS and isotype control nonreactive antibodies. The plates were gently shaken for 1 h at room temperature, to ensure DCex*LPS contacts with iDC and NK cell, and then incubated at 37°C for 24 h.
In the second experimental model, freshly isolated SCID mouse splenocytes (100,000/well), containing a mixture of 50% NK1.1+NKp46+CD3− NK cells and 30% CD11c+ iDCs, were incubated in CM at 37°C for 24 h without or with 1 μg/mL LPS, Pam3, iDCex, mDCex*LPS, and DCex generated by 24-h stimulation of iDCs with 1 μg/mL Pam3 (mDCex*Pam3), in the absence or presence of IL-2 (6,000 IU/ml).
After the incubations, IFN-γ was measured in cell culture-conditioned media using the IFN-γ ELISA kit (R&D Systems). Data were presented as means ± SD of IFN-γ ng/0.5 × 106 NK cells/mL.
2.11. Statistics
All the assays were performed in triplicates. Data were statistically evaluated using the SPSS commercial program package (version 10.0 SPSS Inc., Chicago, IL). The data are reported as means of triplicates ± SD. Statistical significance of data was assessed using the Student’s t test. The results were considered significantly different with p≤0.05.
3. Results
3.1. DCex bind LPS and the resulting complex strongly increases cell-surface expression and secretion of TNF by bystander DCs
DCs express various receptors that can bind TLR-Ls [3], and DCex might do the same. If so, DCex could be able to chaperone the ligands to bystander DCs and activate them. We examined whether exosomes of wild-type mouse (wild-type) mature and immature DCs (mDCex and iDCex, respectively) can bind the TLR4-L LPS (Fig. 1). We exposed the intact mDCex and iDCex to 1 μg/mL LPS for 24 h. Unbound LPS was subsequently washed out, and DCex were assessed for bound LPS using an LAL assay. The LPS-exposed mDCex and iDCex are further referred to as mDCex*LPS and iDCex*LPS, respectively. We found that the LPS-exposed exosomes contained 10 to 25 pg of LPS per 1 ng of DCex. These data show that wild-type DCex can bind LPS.
Figure 1. DCex bind LPS.
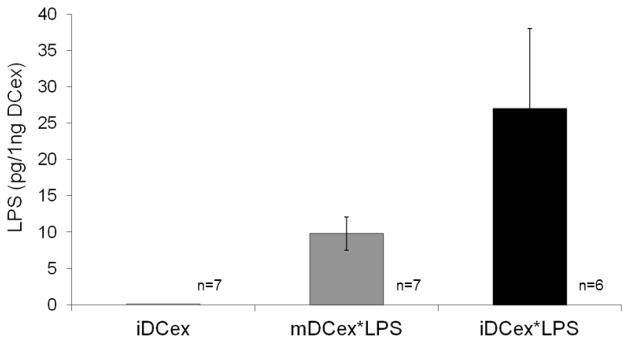
Intact mDCex*LPS and iDCex*LPS were generated in the presence of 1 μg/mL LPS as described in Materials and Methods, and tested for bound LPS using the LAL assay. Presented data are means ± SD of results obtained in 6–7 experiments performed in triplicates. Data of mDCex*LPS and iDCex*LPS are significantly different from those of iDCex (p<0.001).
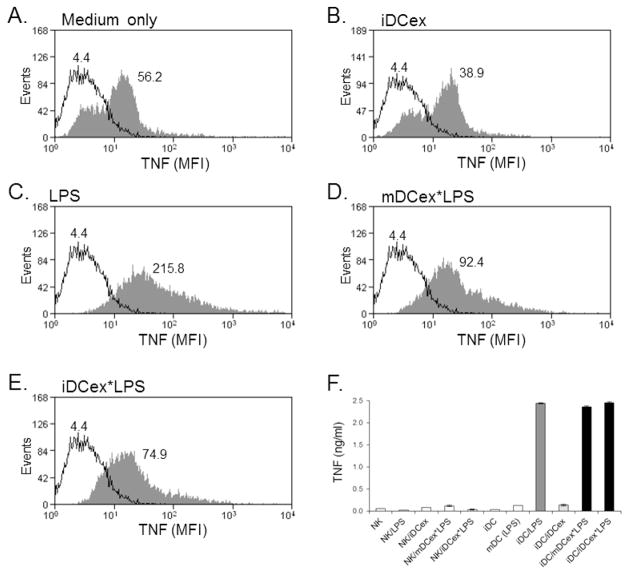
Next, we investigated whether DCex-bound LPS is functional. Because LPS strongly induces the expression of tmTNF and secretion of soluble TNF by wild-type DCs [10], we tested whether these responses could be induced by wild-type mDCex*LPS and iDCex*LPS (Fig. 2). We found that 1μg/mL of LPS, 1μg/mL of mDCex*LPS and 1μg/mL of iDCex*LPS, but not 1μg/mL of iDCex alone, similarly and highly enhanced cell-surface expression (Figs. 2A–E) and secretion (Fig. 2F) of TNF by iDCs. These data show that DCex-bound LPS has similar biological activity as its soluble counterpart.
Figure 2. DCex-bound LPS induces bystander iDCs to increase plasma-membrane expression and secretion of TNF.
iDCs were incubated for 24 h either in the culture medium alone (A) or containing 1 μg/mL iDCex (B), 1 μg/mL LPS (C), 1 μg/mL mDCex*LPS (D), and 1 μg/mL iDCex*LPS (E). The treatments were also examined for the ability to stimulate soluble TNF secretion (F). After the incubation, DCs were tested for cell surface expression of TNF using flow cytometry (A–E), and secretion of soluble TNF in DC culture-conditioned media using ELISA (F). Data in A–E are mean fluorescence intensity (MFI). Data in F are means of triplicates ± SD of TNF ng/mL. The enhancements of TNF secretion by iDCs following stimulation with LPS (iDC/LPS), mDCex*LPS (iDC/mDCex*LPS) and iDCex*LPS (iDC/iDCex*LPS) were significant (p<0.001) in comparison to that of iDCs stimulated with iDCex (iDC/iDCex) or other presented controls. Presented data are representative of two to three similar experiments performed.
DCs co-express the conventional LPS receptors CD14 and TLR4 (3). In an attempt to identify the receptors responsible for LPS coupling to DCex, we evaluated the expression of CD14 and TLR4 on wild-type DCex (Suppl. Figs. 1A–B), and the ability of wild-type and CD14 or TLR4 deficient iDCex*LPS to induce enhanced TNF secretion by wild type and CD14 or TLR4 deficient iDCs (Suppl. Figs. 1C–D). We found that wild-type both iDCex (shown) and mDCex (not shown) expressed both CD14 and TLR4. These wild-type iDCs strongly responded not only to wild-type but also to CD14 and TLR4 deficient iDCex*LPS, and released high and similar quantities of TNF. In sharp contrast to wild-type iDCs, CD14 or TLR4 deficient iDCs were unresponsive to either wild-type or CD14 and TLR4 deficient iDCex*LPS. In addition, neither wild-type nor CD14 and TLR4 deficient iDC responded to LPS-unexposed wild-type or CD14 and TLR4 deficient iDCex. These results showed that not only wild-type but also CD14 and TLR4 deficient iDCex*LPS stimulated iDCs via the conventional LPS receptors CD14 and TLR4, and that this stimulation required the expression of both receptors on responder iDCs. In these experiments, we used a high purity LPS (containing less than 3% impurities). Therefore, our data showed that LPS bound to not only wild-type but also to CD14 or TLR4 deficient iDCex, and the bound LPS, but not some potential impurities or endogenous DCex molecules, stimulated wild-type iDCs. The findings further demonstrated that the co-expression of CD14 and TLR4 on DCex wasn’t an absolute requirement for LPS binding to DCex and its cross-presentation to bystander DCs. It follows that DCex efficiently bind and cross-present LPS to iDCs not only via CD14-TLR4 receptor complex but also via either CD14 or TLR4. It is also possible that the unconventional TLR receptors such as heat shock proteins and lectin-like receptors participate in LPS binding to DCex [22,23].
3.2. DCex-bound LPS stimulates DC cross-talk with NK cells
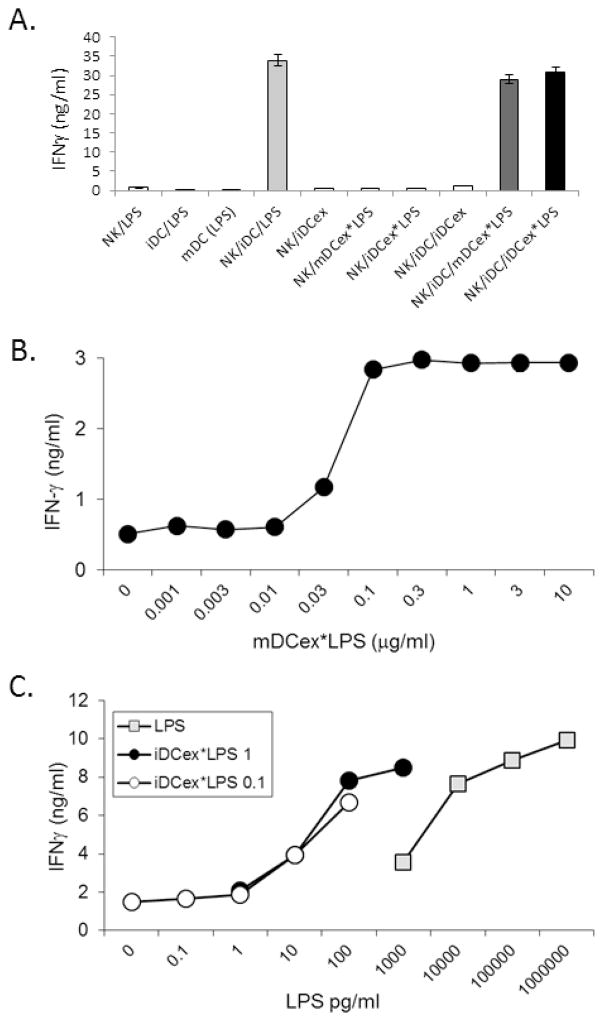
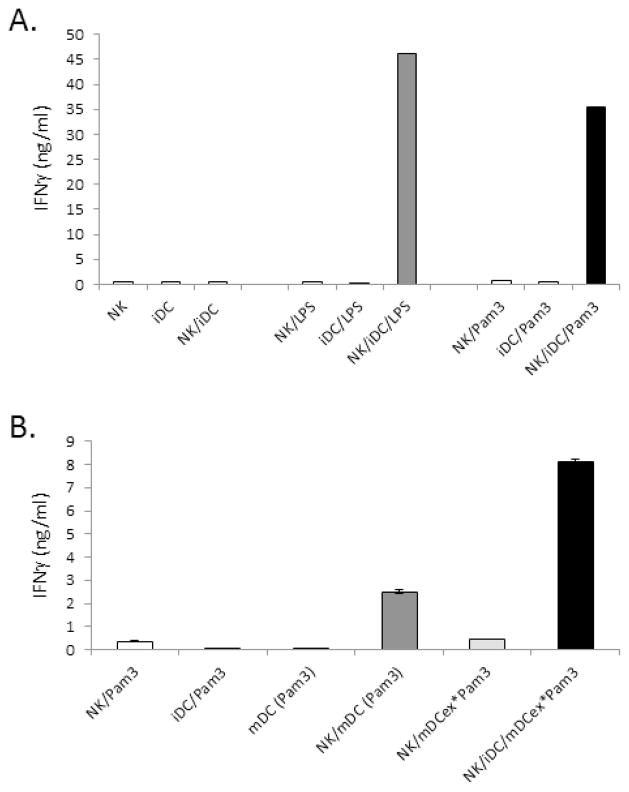
We have previously demonstrated that LPS induces the increases in expression of tmTNF by DCs, and enhances tmTNF-mediated DC/NK cell cross-talk and heightening of IFNγ secretion by NK cells [10,11]. Because both mDCex*LPS and iDCex*LPS promoted the expression of tmTNF on bystander iDCs (Fig. 2), we examined whether DCex coupled to LPS could enhance DC/NK-cell cross-talk (Fig. 3A). We found that 1 μg/mL of LPS alone, 1 μg/mL of mDCex*LPS and 1 μg/mL iDCex*LPS, but not 1 μg/mL of iDCex alone, effectively and similarly induced enhanced DC/NK cell crosstalk, as demonstrated by the presence of highly increased levels of IFNγ in LPS- or DCex*LPS-stimulated DC/NK-cell cultures.
Figure 3. DCex-bound LPS stimulates bystander iDCs to induce enhanced NK-cell IFNγ secretion.
DCs, DCex and aNK cells were obtained as described in Materials and Methods. (A) LPS bound to mDCex and iDCex enhances iDC ability to activate and Th1-polarize NK cells. aNK cells, iDCs and their mixtures were co-incubated overnight with 6,000 IU/ml IL-2 and/or 1 μg/mL LPS, iDCex, mDCex*LPS (generated by stimulation of iDCs with 1μg/mL LPS) or 1 μg/mL iDCex*LPS (obtained by incubation of iDCex with 1μg/mL LPS). (B) mDCex*LPS stimulate iDC cross-talk with NK cells in a dose dependent manner. Titrated concentrations of mDCex*LPS (1 μg contained 10 ng of LPS), were mixed with NK cells, iDCs and 6,000 IU/mL IL-2, and co-cultured overnight. (C) iDCex*LPS stimulate more potently iDC-induced IFNγ secretion by NK cells than LPS alone. Prior to this test, iDCex were pre-incubated with either 0.1 or 1 μg/mL LPS (iDCex*LPS 0.1, and iDCex*LPS 1, respectively). iDCex*LPS were purified, extensively washed to remove unbound LPS, and bound quantities of LPS on intact iDCex were determined using the LAL assay. The experiment was performed by an overnight co-culture of NK cells, iDCs, 6,000 IU/mL IL-2 and titrated concentrations of LPS either bound to iDCex (iDCex*LPS 0.1, iDCex*LPS 1) or unbound (LPS). Following these stimulations, cell-free supernatants were tested for IFNγ presence using ELISA. Data are means of triplicates ± SD ng/mL of IFNγ. Elevations in NK-cell IFNγ secretion following aNK cells/iDCs stimulations with LPS (NK/iDC/LPS), mDCex*LPS (NK/iDC/mDCex*LPS) and iDCex*LPS (NK/iDC/iDCex*LPS) were significant (p<0.001) in comparison to the data obtained by stimulation of NK cells/iDCs with iDCex (NK/iDC/iDCex) and other presented controls (A).
Considering that 1 ng of mDCex*LPS and 1 ng of iDCex*LPS contained 10 to 25 pg of bound LPS (Fig. 1) and that 1 μg of DCex*LPS had similar activity as 1 μg of free LPS (Fig. 2), the above findings indicated that DCex*LPS could be 40 to 100 fold more active than free LPS. This possibility was directly tested by the side-by-side comparison of the stimulating effects of titrated concentrations of mDCex*LPS defined by their total protein quantity containing 10 ng of bound LPS per 1 μg of exosomes (Fig. 3B), LPS bound to iDCex*LPS and free LPS (Fig. 3C) on DC/NK cell cross-talk and induction of increased NK-cell secretion of IFNγ. We found that 0.1 μg/mL of mDCex*LPS containing only 1 ng of bound LPS induced maximal DC/NK-cell cross-talk, as evidenced by the plateau-reaching highest increase in IFNγ secretion (Fig. 3B). Similar findings were obtained with two different preparations of iDCex*LPS (Fig. 3C). Strikingly, 100 pg/mL of DC-bound LPS induced the same activity as 10,000 pg/mL of LPS alone. These findings demonstrate that DCex-LPS complex mediated the enhancement of NK/DC crosstalk a 100 fold more potently than free LPS.
The strikingly greater activity of DCex-bound LPS than LPS alone could be attributed to potential co-stimulations mediated by transmembrane ligands expressed on DCex-limiting membrane and/or cytokines bound to or stored within DCex. We have previously found that iDCex and especially mDCex express biologically active tmTNF [10,11]. In the present study, we found that iDCex and especially mDCex lysates contained a number of immunoregulatory and DC-regulatory cytokines, including IL-15, IL-2, IL-12p70, IL-1α, and IL-1β (Suppl. Fig. 2A). These cytokines, with the exception of IL12p70, were secreted at higher levels by iDCs stimulated with 1 μg/mL of mDCex*LPS (10 ng of bound LPS) than with 1 μg/mL of LPS alone (Suppl. Fig. 2B). Similarly, iDCex and especially mDCex lysates were found to contain several chemokines, including IP10, MIP-1α, RANTES and CX3CL1 (Suppl. Fig. 3A). In addition, 1 μg/mL of mDCex*LPS (10 ng of bound LPS) was superior to 1 μg/mL of unbound LPS at inducing MIP-1β and especially RANTES secretion by iDCs (Suppl. Fig. 3B). These data indicate that DC cytokines and chemokines bound to or stored within DCex, as well as previously described tmTNF expressed on DCex surface [20], could provide co-stimulatory signal(s) to amplify DC stimulation by DCex-bound LPS.
3.3. DCex*LPS enhance DC/NK cell cross-talk via DC-TNF interaction with NK-cell-TNFR2
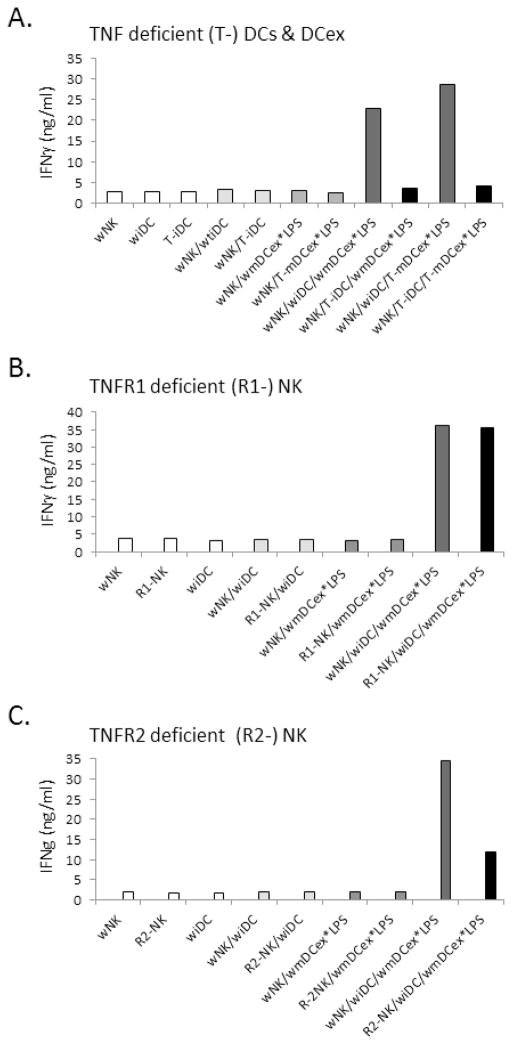
We have previously shown in mice that LPS-amplified DC/NK-cell crosstalk leading to heightened secretion of IFNγ by NK cells is mediated by the interplay of DC-expressed tmTNF with TNFR2 expressed on NK cells [10]. We examined whether DCex*LPS-mediated enhancement of DC/NK-cell cross-talk is mediated by a similar molecular mechanism (Fig. 4). One μg/mL of wild-type or TNF-deficient mouse mDCex*LPS (10 ng of bound LPS) were mixed with wild-type or TNF-deficient iDCs and combined with wild-type aNK cells (Fig. 4A). Furthermore, 1 μg/ml of wild-type mDCex*LPS were mixed with wild-type iDCs and combined with either wild-type or TNFR1-deficient (Fig. 4B) or TNFR2-deficient (Fig. 4C) aNK cells. When wild-type iDCs were combined with-wild type or TNFR1-deficient NK cells, both wild type and TNF-deficient mDCex*LPS enhanced the iDC/NK-cell crosstalk, as determined by the increased IFNγ secretion. In sharp contrast, when TNF-deficient iDCs were combined with wild-type NK cells (Figs. 4A, 4B), or wild-type iDCs were combined with TNFR2-deficient NK cells (Fig. 4C), the enhancement of iDC/NK-cell crosstalk was not induced by wild-type and/or TNF-deficient mDCex*LPS. These findings show that the interaction between DC-TNF with NK-cell-TNFR2 mediates DCex*LPS-induced enhancement of DC/NK-cell cross-talk leading to increased secretion of IFNγ by NK cells.
Figure 4. TNF of bystander DCs stimulated with DCex*LPS mediates increased NK-cell IFNγ secretion via interaction with TNFR2 of NK cells.
(A) Increasingly expressed TNF on bystander DCs stimulated with mDCex*LPS, but not TNF of mDCex*LPS, mediates the enhancement of NK-cell activation and IFNγ secretion. Wild-type aNK cells (wNK), wild-type iDC (wiDC) or TNF knockout iDCs (T-iDC) were cultured either alone or co-cultured in the absence or presence of wild-type mDCex*LPS (wmDCex*LPS) or TNF knockout mDCex*LPS (T-mDCex*LPS). (B) NK-cell TNFR1 is not required for activation of NK cells by TNF expressed by bystander DCs stimulated with mDCex*LPS. wNK cells, TNFR1 knockout aNK cells (R1-NK) and wiDCs were either cultured alone or co-cultured in the absence or presence of wmDCex*LPS. (C) NK-cell TNFR2 is required for activation of NK cells by TNF expressed by bystander DCs stimulated with mDCex*LPS. wNK cells, TNFR2 knockout aNK cells (R2-NK) and wiDCs were either cultured alone or co-cultured in the absence or presence of wmDCex*LPS. One μg/mL of mDCex*LPS was utilized to stimulate iDC cross-talk with NK cells. After the incubation, cell-free supernatants were tested for IFNγ using ELISA. The presented data are means of triplicates ± SD ng/mL of IFNγ. Data are representative of 2 experiments performed. In A, the data of wNK/T-iDC/wmDCex*LPS or wNK/T-iDC/T-mDCex*LPS are significantly lower than the data of wNK/wiDC/wmDCex*LPS or wNK/wiDC/T-mDCex*LPS (p<0.001). In C, the data of R2-NK/wiDC/wmDCex* are significantly lower than the data of wNK/wiDC/wmDCex*LPS (p<0.001).
3.4. DCex*LPS promotes DC/NK cell cross-talk via DC tmTNF
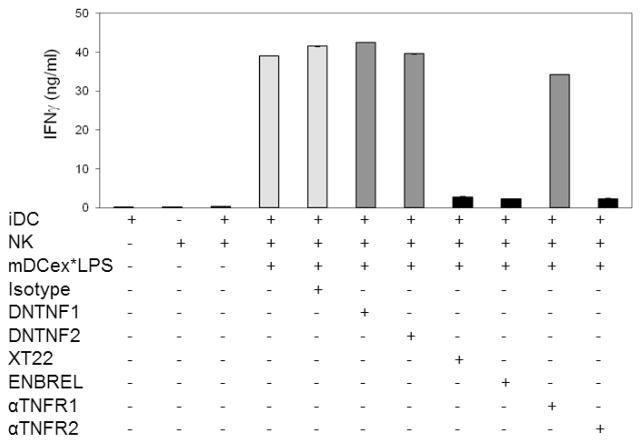
Next, we wanted to determine whether transmembrane and/or soluble TNF produced by bystander DCs are the key mediators of the DCex*LPS-induced enhancement of DC/NK-cell cross-talk. The enhancement of iDC/NK-cell cross-talk was either induced by 1 μg/mL of unbound LPS (Supplementary Fig. 4) or 1 μg/mL of mDCex*LPS having 10 ng of bound LPS (Fig. 5). Control samples were either untreated or treated with isotype control antibodies. The experimental samples were treated with dominant negative TNF (DNTNF1 and DNTNF2), to selectively sequester soluble TNF [21]; XT22 anti-TNF antibody and ENBREL (TNFR2-Fc fusion protein), to block both soluble and tmTNF; and anti-TNFR1 or anti-TNFR2 antibodies, to block the TNF binding sites of TNFR1 or TNFR2, respectively. While the two DNTNFs and anti-TNFR1 antibody did not affect either unbound LPS- or mDCex*LPS-induced enhancement of iDC/NK-cell cross-talk manifested by increased IFNγ secretion, XT22 antibody, ENBREL and anti-TNFR2 almost completely inhibited both unbound LPS- or DCex*LPS-induced enhancements of iDC/NK-cell cross-talk. These data demonstrate that DCex*LPS induce the amplification of iDC/NK-cell crosstalk similarly as unbound LPS, by the interaction of DC tmTNF and NK-cell TNFR2.
Figure 5. Elevated transmembrane TNF expressed on bystander DCs following stimulation with DCex*LPS mediates enhanced NK-cell IFNγ secretion via interaction with NK-cell-TNFR2.
Wild-type aNK cells, iDCs and aNK cell/iDCs either alone or in the presence of 1 μg/mL mDCex*LPS, and/or 20 μg/mL isotype control antibodies (Isotype), two different preparations of dominant negative TNF (DNTNF1 and DNTNF2) that selectively sequester soluble TNF, XT22 anti-TNF antibody and ENBREL (etanercept, TNFR2-Fc construct) that neutralize both soluble and transmembrane TNF, and anti-TNFR1 (αTNFR1) and anti-TNFR2 (αTNFR2) antibodies that block TNF-binding domains of the corresponding receptors were incubated for 24 h. Following the incubation, cell-culture conditioned media were assessed for IFN-γ presence using ELISA. Data are representative from 3 experiments performed. They are means of triplicates ± SD of IFNγ ng/mL. The data of iDC, NK, or NK/iDC/mDCex*LPS treated with XT22, ENBREL or αTNFR2 are significantly lower than the data of NK/iDC/mDCex*LPS untreated, or NK/iDC/mDCex*LPS treated with Isotype, DNTNF1, DNTNF2 or αTNFR1 (p<0.001).
3.5. TLR1/2 ligand Pam3 can also bind to and increase the ability of DCex to stimulate DC/NK cell cross-talk
The presented data show that DCex can effectively bind, chaperon, and cross-present LPS to and enhance the activity of bystander DCs. To determine whether the TLR ligand-binding ability of DCex is not solely restricted to LPS, we examined whether Pam3, a TLR1/2 ligand, could also bind to DCex and enhance their innate immune functions (Fig. 6). We found that 1 μg/mL of Pam3, similarly as 1 μg/mL of LPS, enhanced tmTNF expression on DCs (data not shown) and, subsequently, DC/NK cell cross-talk (Fig. 6A). Furthermore, mDCex generated by 24 h stimulation of iDCs with 1 μg/mL Pam3 (mDCex*Pam3) showed an enhanced ability to stimulate iDC/NK cell cross-talk as demonstrated by enhanced secretion of IFNγ by NK cells (Fig. 6B). The enhanced cross-talk mediated by mDCex*Pam3 was superior to that of Pam3-matured DCs [NK/mDC (Pam3)]. Overall, these findings indicate that DCex are also able to bind, chaperon and cross-present Pam3 to, and enhance cross-talk of bystander DCs with NK cells. Therefore, the TLR ligand-binding ability of DCex is not restricted to LPS.
Figure 6. Pam3 bound to DCex enhances bystander iDC cross-talk with NK cells.
(A) Pam3 stimulates similarly as LPS NK cell/DC crosstalk. aNK cells (NK), iDCs (iDC) and aNK cells/iDCs (NK/iDC) alone and/or in the presence of 1 μg/mL LPS or Pam3 were incubated for 24 h. (B) Pam3-exposed DCex enhance the cross-talk between bystander iDCs and NK cells. aNK cells and iDCs were co-incubated with 1 μg/mL Pam3 (NK/Pam3, iDC/Pam3, respectively). mDC generated by 24 h stimulation of iDCs with 1 μg/mL Pam3 were incubated either alone [mDC (Pam3)] or in the presence of aNK cells [NK/mDC (Pam3)]. aNK cells and aNK cells/iDCs were co-incubated with 1μg/mL mDCex generated in the presence of 1 μg/mL Pam3 (NK/mDCex*Pam3, NK/iDC/mDCex*Pam3, respectively). After 24 h incubation, cell-free supernatants were tested for IFNγ presence using ELISA. Data are representative of 2 experiments performed. The data are means of triplicates ± SD ng/mL of IFNγ. The data of NK/iDC/LPS, NK/iDC/Pam3 (A), NK/mDC (Pam3) or NK/iDC/mDCex*Pam3 (B) are significantly higher than those of the presented controls (p<0.001).
3.6. DCex-TLR ligand complex enhances crosstalk between in vivo produced resting NK cells and DCs
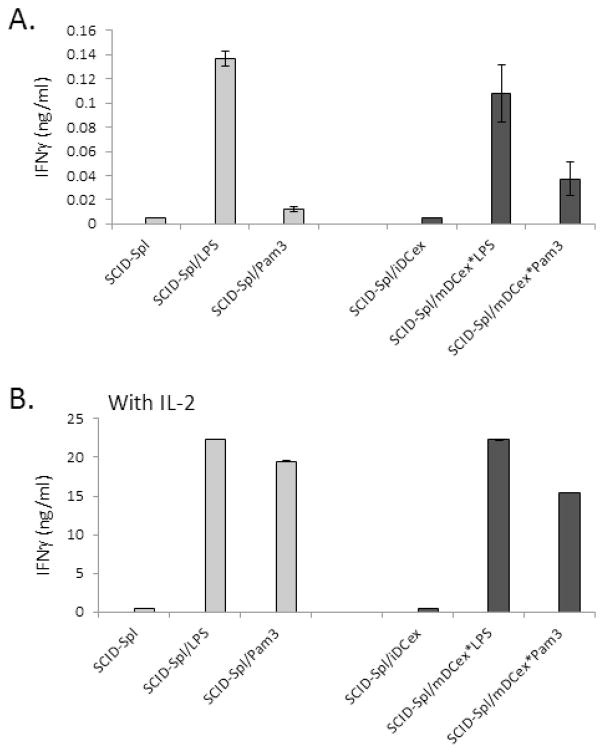
The aforementioned experiments were performed with DCs and NK cells generated and expanded in vitro with the help of cytokines. To further assess and confirm the biological relevance of our findings, we examined whether freshly isolated SCID mouse resting splenocytes, which were constituted of 50% NK1.1+NKp46+CD3− NK cells and 30% CD11c+ iDCs and lacked T and B cells, could respond to DCex*LPS or DCex*Pam3 by enhanced DC/NK cell cross-talk determined by increased NK-cell secretion of IFNγ (Fig. 7). We found that 24 h stimulation with 1 μg/mL of LPS or Pam3, and 1 μg/mL mDCex*LPS or mDCex*Pam3 amplified cross-talk between freshly isolated resting DCs and NK cells in the absence (Fig. 7A) and especially in the presence (Fig. 7B) of IL-2. These data indicate that DCex with bound TLR-Ls can activate in vivo-generated resting DCs and NK cells, and might have biologically significant function in vivo.
Figure 7. DCex-bound TLR ligands stimulate crosstalk of in vivo-generated resting NK cells and iDCs.
SCID mouse splenocytes (SCID-Spl) containing a mixture of 50% NK1.1+CD3− NK cells and 30% CD11C+ DCs were freshly isolated and incubated overnight alone (SCID-Spl) or in the presence of 1μg/mL LPS (SCID-Spl/LPS), 1μg/mL Pam3 (SCID-Spl/Pam3), 1 μg/mL mDCex generated in the presence of 1 μg/mL LPS (SCID-Spl/mDCex*LPS) or 1μg/mL Pam3 (SCID-Spl/mDCex*Pam3). SCID-Spl and TLR ligands or mDCex-binding TLR ligands were co-cultured for 24 h in the absence (A) or presence (B) of 6,000 IU IL-2/mL. After this co-culture, cell culture-conditioned media were tested for IFNγ presence using ELISA. Data are representative of 2 experiments performed. The data are means of triplicates ± SD ng/mL of IFNγ. The data of SCID-Spl/LPS, SCID-Spl/mDCex*LPS, SCID-Spl/mDCex*Pam3 (A), SCID-Spl/LPS, SCID-Spl/Pam3, SCID-Spl/mDCex*LPS or SCID-Spl/mDCex*Pam3 (B) are significantly higher than those of controls (SCID-Spl or SCID-Spl/iDCex) (p<0.001).
4. Discussion
DCex express on their limiting membrane the functional transmembrane TNF superfamily ligands TNF, Fas ligand and TRAIL, and directly mediate by the ligand-collective and TNF-individual activities two essential DC innate immunity functions, killing of tumor cells, and activation and Th1-polarization of NK cells, respectively [20]. DCex also express functional MHC molecules on their limiting membrane and indirectly induce effective adaptive immune responses via bystander DCs, which acquire and present DCex-MHC-antigenic peptide complexes to antigen-specific T cells [16,17]. In the present study, we show that DCex also indirectly promote strong pro-inflammatory innate immune responses by binding and cross-presenting TLR-Ls to bystander DCs, which undergo maturation in response to these “danger signals”. Subsequently, this maturation increases the ability of DCs to cross-talk with and mediate enhanced activation and Th1-polarizitation of NK cells.
It has been shown that the TLR-L 19-kDa lipoprotein of intracellular pathogens S. typhimurium and Mycobacterium bovis can traffic to the multivesicular bodies and associate with the exosomes of the pathogen-infected macrophages [24,25]. The question left unanswered by these studies was whether the exosome-associated TLR-Ls were incorporated inside of or bound to the surface of exosomes. We now demonstrate that DCex express the conventional TLR4-L (LPS) receptors CD14 and TLR4 and bind to their surface significant amounts of LPS. We also provide indications that DCex bind the TLR1/2-L Pam3 too.
Previous studies have also determined that exosomes released from macrophages infected with intracellular pathogens stimulate proinflammatory responses by activating uninfected bystander macrophages to secrete proinflammatory mediators such as TNF, RANTES and inducible nitric oxide synthase [24,25]. The activation of bystander macrophages required their TLR2, TLR4 and MyD88 signaling, which suggested the involvement of TLR-Ls. However, the pathogen-derived ligands remained unknown. In addition, the exosomes of macrophages stimulated with LPS failed to induce the proinflammatory response of bystander macrophages, indicating that macrophage-derived exosomes did not effectively bind LPS. In sharp contrast, in the present study, we clearly demonstrate that DCex can bind onto their surface LPS as well as Pam3, and DCex with the bound ligands can effectively stimulate a strong innate immune proinflammatory response and Th1 polarization by inducing bystander DCs to undergo maturation, increase expression of tmTNF, and enhance secretion of major proinflammatory and immunoregulatory cytokines. Subsequently, DCex strongly induce TLR-L- and bystander DC-dependent and tmTNF-mediated NK-cell activation, Th1 polarization and IFNγ secretion. Comparative analyses of the data obtained with macrophage- and DC-derived exosomes indicate that these two types of exosomes might be different in their ability to bind TLR-Ls. DCex might efficiently bind TLR-Ls, but macrophage-derived exosomes might not be as efficient. It is also possible that TLR-Ls are more efficiently presented by DCex to bystander DCs than by macrophage-derived exosomes to bystander macrophages. In support of this idea, our study shows that 1 ng DCex can bind 10 to 25 pg of LPS, and DCex-bound LPS is a hundred-fold more effective at activating bystander DCs and inducing their cross-talk with NK cells than the unbound LPS. These findings indicate that both the binding and the presentation of bound TLR-Ls by DCex are highly efficient.
DCex not only express on their limiting membrane biologically active molecules found on DC plasma membrane, such as MHC molecules, transmembrane TNF superfamily ligands, co-stimulatory and adhesion molecules, and show the ability to bind TLR-Ls, but they also have copious quantities of major DC-secreting cytokines and chemokines stored inside and/or bound to the limiting membrane of DCex. The latter findings reveal novel functions of DCex such as secretion, protection and transport of the proinflammatory and immunoregulatory mediators. Therefore, DCex might be able to chaperon, disseminate and convey multiple essential DC functions to a large number of neighboring and distant target cells, and thus induce systemic and amplified immune functions in an orchestrated fashion via the paracrine/endocrine pathway.
Due to their ability to convey MHC-antigenic peptide complexes to bystander DCs, which then efficiently present the antigen and prime antigen-specific CD4+ and CD8+ T cells [16,17], DCex have been considered as a promising novel therapeutic anticancer agent. As a result, iDCex armed with tumor specific antigens have been tested for treatment of patients with advanced-stage melanoma and lung cancer in stage-I clinical trials [18,19]. However, the therapy failed to induce tumor-specific T-cell priming and measurable clinical responses. A possible reason for the failure of this vaccine could be that the DCex treatment lacked adjuvant co-administration support. The novel findings described in the present paper that TLR-Ls with potent immune adjuvant property can bind to DCex offer a promising new strategy to generate superior DCex vaccines that can effectively induce, enhance and appropriately polarize innate and adaptive immune mechanisms. This likely potent new immunization strategy is awaiting a formal testing.
Supplementary Material
Highlights.
This study newly shows that DC exosomes can bind and cross-present TLR ligands to innate-immunity effector cells, and indicate a potent mechanism to systemically alert the host immune system of pathogen invasion. The findings also suggest a potential novel strategy to generate effective vaccines by binding TLR-ligand-immune adjuvants to DC exosomes.
Acknowledgments
We thank Dr. David Szymkowski (Xencor, Monrovia, CA) for providing us with the dominant negative TNF reagents.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nature Rev Immunol. 2003;3:984–993. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- 3.Trinchieri G, Sher A. Cooperation of Toll-like receptor signals in innate immune defense. Nature Rev Immunol. 2007;7:179–190. doi: 10.1038/nri2038. [DOI] [PubMed] [Google Scholar]
- 4.Lu G, Janjic BM, Janic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. II. Role of TNF, LT-α1β2, FasL and TRAIL. J Immunol. 2002;168:1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 5.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–30. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 6.Vujanovic NL. Role of TNF superfamily ligands in innate immunity. Immunol Res. 2011;50:159–174. doi: 10.1007/s12026-011-8228-8. [DOI] [PubMed] [Google Scholar]
- 7.Becknell B, Caligiuri MA. Natural killer cells in innate immunity and cancer. J Immunother. 1989;31:685–692. doi: 10.1097/CJI.0b013e318182de23. [DOI] [PubMed] [Google Scholar]
- 8.Moretta A, Marcenaro E, Sivori S, Chiesa MD, Vitale M, Moretta L. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. TRENDS Immunol. 2005;26:668–675. doi: 10.1016/j.it.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 9.Moretta A. Natural killer cells and dendritic cells: rendezvous in abused tissues. Nature Rev Immunol. 2002;2:957–964. doi: 10.1038/nri956. [DOI] [PubMed] [Google Scholar]
- 10.Xu J, Chakrabarti AK, Tan JA, Ge L, Gambotto A, Vujanovic NL. Essential role of the TNF-TNFR2 cognate interaction in mouse dendritic cell-natural killer cell cross-talk. Blood. 2007;109:3333–3341. doi: 10.1182/blood-2006-06-026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vujanovic L, Szymkowski DE, Vujanovic NL, Butterfield LH. Virally-infected and cytokine-matured human dendritic cells activate natural killer cells via cooperative activity of plasma membrane-bound TNF and IL-15. Blood. 2010;116:575–583. doi: 10.1182/blood-2009-08-240325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fevrier B, Raposo G. Exosomes: endosomal-derived vesicles shipping extracellular messages. Curr Opin Cell Biol. 2004;16:415–421. doi: 10.1016/j.ceb.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and function of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- 14.Chaput N, Flament C, Vlaud S, Taie J, Roux S, Spatz A, André F, LePecq JB, Boussac M, Garin J, Amigorena S, Théry C, Zitvogel L. Dendritic cell derived exosomes: biology and clinical implementations. J Leuk Biol. 2006;80:471–478. doi: 10.1189/jlb.0206094. [DOI] [PubMed] [Google Scholar]
- 15.Morelli AE, Larregina AT, Shufesky WJ, Sullivan MLG, Beer-Stolz D, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC, Falo LD, Thomson AW. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257–3266. doi: 10.1182/blood-2004-03-0824. [DOI] [PubMed] [Google Scholar]
- 16.Zitvogel L, Regnault A, Lozier A, Wolfers J, Flament C, Tenza D, Ricciardi-Castagnoli P, Raposo G, Amigorena S. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell-derived exosomes. Nature Med. 1998;4:594–600. doi: 10.1038/nm0598-594. [DOI] [PubMed] [Google Scholar]
- 17.Therry C, Duban L, Segura E, Veron P, Lantz O, Amigorena S. Indirect activation of naïve CD4+ T cells by dendritic cell-derived exosomes. Nature Immunol. 2002;3:1156–1162. doi: 10.1038/ni854. [DOI] [PubMed] [Google Scholar]
- 18.Chaput N, Andre F, Schartz NE, Flament C, Angevin E, Escudier B, Zitvogel L. Exosomes and anti-tumor immunotherapy. Bulletin du Cancer. 2003;90:695–698. [PubMed] [Google Scholar]
- 19.Viaud S, Thery C, Ploix S, Tursz T, Lapierre V, Lantz O, Zitvogel L, Chaput N. Dendritic cell-derived exosomes for cancer immunotherapy: what’s next. Cancer Res. 2010;70:1281–1285. doi: 10.1158/0008-5472.CAN-09-3276. [DOI] [PubMed] [Google Scholar]
- 20.Munich S, Sobo-Vujanovic A, Buchser WJ, Beer-Stolz D, Vujanovic NL. Dendritic cell exosomes directly kill tumor cells and activate natural killer cells via TNF superfamily ligands. OncoImmunol. 2012;1:1074–1083. doi: 10.4161/onci.20897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zalevsky J, Secher T, Ezhevsky SA, Janot L, Steed PM, O’Brien C, Eivazi A, Kung J, Nguyen DHT, Doberstein SK, Erard F, Ryffel B, Szymkowski DE. Dominant-negative inhibitors of soluble TNF attenuate experimental arthritis without suppressing innate immunity to infection. J Immunol. 2007;179:1872–1883. doi: 10.4049/jimmunol.179.3.1872. [DOI] [PubMed] [Google Scholar]
- 22.Habich C, Kempe K, van der Zee R, Rumenapf R, Akiyama H, Kolb H, Burkart V. Heat shock protein 60: specific binding of lipopolysaccharide. J Immunol. 2005;174:1298–1305. doi: 10.4049/jimmunol.174.3.1298. [DOI] [PubMed] [Google Scholar]
- 23.Couturier C, Haeffner-Cavaillon N, Caroff M, Kazatchkine MD. Binding sites for endotoxins (lipopolysaccharides) on human monocytes. J Immunol. 1991;147:1899–1904. [PubMed] [Google Scholar]
- 24.Bhatnagar S, Schorey JS. Exosomes released from infected macrophages contain Mycobacterium avium glycopeptidolipids and are proinflammatory. J Biol Chem. 2007;282:25779–25789. doi: 10.1074/jbc.M702277200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhatnagar S, Shinagawa K, Castellino FJ, Schorey JS. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.