Abstract
Purpose
There are limited data regarding breast cancer subtypes among Hispanic women. The current study assessed the distribution and prognosis of molecular subtypes defined by joint expression of the hormone receptors (HR; estrogen and progesterone) and human epidermal growth factor receptor 2 (HER2).
Methods
Using California Cancer Registry data, we identified Hispanic women diagnosed with invasive breast cancer from 2005–2010. Breast cancer subtypes were defined as HR+/HER2−, HR+/HER2+, HR−/HER2+, and HR−/HER2− (triple negative). We estimated breast cancer subtype frequencies and used polytomous logistic regression, Kaplan Meier survival plots and Cox regression to examine differences in relation to demographic and clinical characteristics.
Results
Among 16,380 Hispanic women with breast cancer, HR+/HER− subtype was most common (63%), followed by triple negative (16%), HR+/HER2+ (14%) and HR−/HER2+ (8%). Women in lower SES neighborhoods had greater risk of triple negative and HR−/HER2+ subtypes relative to HR+/HER2− (p<0.05). Hispanic women with triple negative and HR−/HER2+ tumors experienced poorer survival than those with HR+/HER− tumors. Breast cancer-specific mortality increased with decreasing SES, relative to the highest SES quintile, from HR=1.38 for quintile 4 to HR=1.76 for quintile 1 (lowest SES level).
Conclusion
Our findings indicate that Hispanic women residing in low SES neighborhoods had significantly increased risk of developing and dying from HR− than HR+ breast cancers. Similar patterns of subtype frequency and prognosis among California Hispanic women and studies of other racial/ethnic groups underscore the need to better understand the impact of SES on risk factor exposures that increase the risk of breast cancer subtypes with poor prognosis.
Keywords: breast cancer, Hispanic, Latina, socioeconomic status, triple negative
Introduction
Hispanic women may be prone to developing breast cancer molecular subtypes (defined by estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2)) associated with poor prognosis, such as ER−/PR−, and triple negative (ER−/PR−/HER2−) breast cancers [1–3]. Studies among Hispanics point to a 20%–40% increased risk of developing triple negative and ER−/PR− breast cancers, compared to non-Hispanic white women [3,2]. Women diagnosed with ER−/PR− or triple negative tumors have significantly greater risk of death than more favorable prognosis subtypes (i.e., ER+/PR+) [4,5]. Thus, defining the population-based distribution of breast cancer subtypes among Hispanic women provides information of clinical, prognostic and therapeutic value.
However, only two studies have examined the patterns of breast cancer subtypes among Hispanic women [6,7]. Hines and colleagues assessed breast tumors of 69 Hispanic women [6], and found that the four most prevalent subtypes were ER+/PR+/HER2− (41%), triple negative (17%), ER−/PR−/HER2+ (15%) and ER+/PR+/HER2+ (13%). Ortiz et al. showed a similar subtype distribution among 663 women with breast cancer in Puerto Rico, and reported a higher risk of death for triple negative and ER+/PR+/HER2+ tumors, compared to the ER+/PR+/HER2− subtype [7]. To expand on this work, data from the California Cancer Registry (CCR) on 16,380 Hispanic women diagnosed with invasive breast cancer were used to characterize the molecular subtypes defined by joint hormone receptor (HR) and HER2 status. Our objective was to conduct a large, population-based assessment of the distribution and survival by breast cancer subtype and examine associations with demographic and clinical attributes.
Methods
Study population
We obtained data from the CCR on all Hispanic female California residents aged 25 years and older, diagnosed with a first primary invasive breast cancer between 2005–2010. Patient sociodemographic information included age at diagnosis, race/ethnicity, birthplace, insurance status, marital status and residential address at diagnosis. Race/ethnicity and birthplace data are abstracted from medical records or death certificates [8]. The North American Association of Central Cancer Registries Hispanic Identification Algorithm (NHIA) was used to improve the classification of Hispanic ethnicity [9]. Patient clinical information included American Joint Committee on Cancer (AJCC) stage, tumor grade, tumor size, lymph node involvement, tumor metastasis and first course of treatment (surgery, chemotherapy and radiation).
Nativity
Patient nativity was classified as previously described [10], based on: (1) cancer registry-based data from medical records and/or death certificates and (2) imputation using the first five digits of the patient’s social security number (SSN), for those with unknown birthplace (34.5%). SSN digits are linked to the state and year of issuance, from which nativity was imputed as follows: Women who received their SSN before age 21 years were considered United States (U.S.)-born, whereas those who received their SSN on or after age 21 years as foreign-born. The age threshold was determined and validated based on a prior cohort of Hispanic cancer patients [11].
Neighborhood socioeconomic status (SES) and Hispanic enclave
Each patient’s residential address at diagnosis was geocoded to a census block group. Participants with incomplete residential address information (7.3%) were assigned an SES value based on their county of residence. Neighborhood SES was determined based on an index that incorporates 2000 Census (for cases diagnosed in 2005) and 2006–2010 American Community Survey data (for cases diagnosed after 2005) on education, occupation, unemployment, household income, poverty, rent, and house values [22]. Hispanic enclave was based on 2000 Census variables (% linguistically isolated, % linguistically isolated who speak Spanish, % speaking limited English, % speaking limited English who spoke Spanish, % recent immigrants, % Hispanic, % foreign-born), developed via principal components analysis. Participants were assigned to a neighborhood SES quintile and Hispanic enclave quintile based on the distribution of each variable across California block groups.
Breast cancer subtype definition
A detailed description of the methods used for classification of breast cancer subtypes has been published elsewhere [12]. Based on joint tumor expression of ER, PR, and HER2, as described in the pathology record, breast cancers were classified into four distinct subtype categories: HR+/HER2− was defined as ER+ or PR+ and HER2+; HR+/HER2+ as ER+ or PR+ and HER2+; HR−/HER2+ as ER− and PR− and HER2+; and triple negative as ER−, PR− and HER2− [13,14,4,15–17]. Participants missing the tumor marker information needed to assign to a subtype were excluded (n=3,253 (16.6%)).
Statistical analysis
Associations between Breast Cancer Subtypes and Patient Attributes
Adjusted polytomous regression models were used to estimate odds ratios (ORs) and 95% confidence intervals (CIs) by sociodemographic and clinical attributes for triple negative, HR+/HER2+ and HR−/HER2+ versus HR+/HER2− subtypes. Tests for trend were considered statistically significant at p≤0.05.
Kaplan-Meier survival curves depicting time from diagnosis to death were calculated for triple negative, HR+/HER2+ and HR−/HER2+ versus HR+/HER2− subtypes. Surviving Participants were censored at the time of last known follow-up. Wilcoxon rank order test was used to test homogeneity of survival by subtype (SAS Institute v 9.3, Cary, NC).
Adjusted Cox proportional hazard modeling was performed to estimate the risk of death from breast cancer for all subtypes adjusted for patient sociodemographic and clinical characteristics. Survival time, in months, was defined as time from diagnosis to whichever of the following occurred first: death from breast cancer, last known contact, death due to other causes, or end of study follow-up (December 31, 2010). We tested the proportionality assumption using scaled Schoenfeld residuals. AJCC stage levels were included in the models as a stratifying variable, allowing the underlying hazard function to vary by stage.
Results
HR+/HER2− was the most common subtype (62.6%), followed by triple negative (15.6%), HR+/HER2+ (13.8%) and HR−/HER2+ (8.1%) (Table 1). Over 50% of breast cancers were diagnosed among foreign-born Hispanics, overall and within each subtype, compared to U.S.-born Hispanics. Women in the lowest SES and highest Hispanic enclave neighborhoods comprised the greatest proportions of participants among all breast cancer subtypes.
Table 1.
Demographic and Tumor Characteristics of the Breast Cancer Subtypes in Hispanic Women with Invasive Breast Cancer, California, 2005–2010
| Characteristics | Total n=16,380 | HR+/HER2− n=10,247(62.6%) | Triple negative n=2,549 (15.6%) | HR+/HER2+ n=2,265 (13.8%) | HR−/HER2+ n=1,319 (8.1%) |
|---|---|---|---|---|---|
| n (%) | |||||
| Nativity | |||||
| US-Born | 7633 (46.6) | 4932 (48.1) | 1193 (46.8) | 976 (43.1) | 532 (40.3) |
| Foreign-Born | 8747 (53.4) | 5315 (51.9) | 1356 (53.2) | 1289 (56.9) | 787 (59.7) |
| Socioeconomic status | |||||
| Quintile 1 (Low) | 4261 (26.0) | 2478 (24.2) | 713 (28.0) | 645 (28.5) | 425 (32.2) |
| Quintile 2 | 3723 (22.7) | 2302 (22.5) | 608 (23.9) | 511 (22.6) | 302 (22.9) |
| Quintile 3 | 3027 (18.5) | 1915 (18.7) | 485 (19.0) | 394 (17.4) | 233 (17.7) |
| Quintile 4 | 2484 (15.2) | 1625 (15.9) | 348 (13.7) | 344 (15.2) | 167 (12.7) |
| Quintile 5 (High) | 1683 (10.3) | 1170 (11.4) | 204 (8.0) | 207 (9.1) | 102 (7.7) |
| Unknown/missing | 1433 (7.3) | 757 (7.4) | 191 (7.5) | 164 (7.2) | 90 (6.8) |
| Hispanic enclave | |||||
| Quintile 1 (Low) | 1359 (8.3) | 903 (8.8) | 197 (7.7) | 162 (7.2) | 97 (7.4) |
| Quintile 2 | 2217 (13.5) | 1472 (14.4) | 333 (13.1) | 253 (11.2) | 159 (12.1) |
| Quintile 3 | 2810 (17.2) | 1793 (17.5) | 440 (17.3) | 376 (16.6) | 201 (15.2) |
| Quintile 4 | 3992 (24.4) | 2544 (24.8) | 605 (23.7) | 540 (23.8) | 303 (23.0) |
| Quintile 5 (High) | 5261 (32.1) | 3062 (29.9) | 861 (33.8) | 837 (37.0) | 501 (38.0) |
| Unknown/missing | 917 (4.5) | 473 (4.6) | 113 (4.4) | 97 (4.3) | 58 (4.4) |
| Age at diagnosis (years) | |||||
| <45 | 3633 (22.2) | 1867 (18.2) | 771 (30.2) | 641 (28.3) | 354 (26.8) |
| 45–49 | 2444 (14.9) | 1529 (14.9) | 373 (14.6) | 357 (15.8) | 185 (14.0) |
| 50–54 | 2348 (14.3) | 1380 (13.5) | 405 (15.9) | 336 (14.8) | 227 (17.2) |
| 55–59 | 2024 (12.4) | 1246 (12.2) | 308 (12.1) | 284 (12.5) | 186 (14.1) |
| 60–64 | 1720 (10.5) | 1179 (11.5) | 233 (9.1) | 197 (8.7) | 111 (8.4) |
| ≥65 | 4211 (25.7) | 3046 (29.7) | 459 (18.0) | 450 (19.9) | 256 (19.4) |
| Marital status at diagnosis | |||||
| Married | 9293 (56.7) | 5773 (56.3) | 1460 (57.3) | 1299 (57.4) | 761 (57.7) |
| Never married | 2917 (17.8) | 1771 (17.3) | 463 (18.2) | 453 (20.0) | 230 (17.4) |
| Previously married | 3598 (22.0) | 2351 (22.9) | 529 (20.8) | 453 (20.0) | 265 (20.1) |
| Unknown | 793 (3.5) | 352 (3.4) | 97 (3.8) | 60 (2.6) | 63 (4.8) |
| Insurance status† | |||||
| Private/military | 8199 (50.1) | 5260 (51.3) | 1272 (49.9) | 1058 (46.7) | 609 (46.2) |
| Public | 6825 (41.7) | 4161 (40.6) | 1062 (41.7) | 1020 (45.0) | 582 (44.1) |
| Uninsured | 235 (1.4) | 142 (1.4) | 37 (1.5) | 36 (1.6) | 20 (1.5) |
| Unknown | 1489 (6.8) | 684 (6.7) | 178 (7.0) | 151 (6.7) | 108 (8.2) |
| Clinical Characteristics | |||||
| AJCC tumor stage | |||||
| I | 6034 (36.8) | 4379 (42.7) | 680 (26.7) | 667 (29.4) | 308 (23.4) |
| II | 6186 (37.8) | 3644 (35.6) | 1170 (45.9) | 860 (38.0) | 512 (38.8) |
| III | 2768 (16.9) | 1473 (14.4) | 472 (18.5) | 492 (21.7) | 331 (25.1) |
| IV | 778 (4.7) | 409 (4.0) | 127 (5.0) | 141 (6.2) | 101 (7.7) |
| Unknown | 1137 (3.7) | 342 (3.3) | 100 (3.9) | 105 (4.6) | 67 (5.1) |
| Tumor grade | |||||
| Low | 9053 (55.3) | 7321 (71.4) | 403(15.8) | 1054 (46.5) | 275 (20.8) |
| High | 6659 (40.7) | 2501 (24.4) | 2065 (81.0) | 1115 (49.2) | 978 (74.1) |
| Unknown | 1356 (4.1) | 425 (4.1) | 81 (3.2) | 96 (4.2) | 66 (5.0) |
| Tumor size (cm) | |||||
| 0–2.00 | 8111 (49.5) | 5793 (56.5) | 910 (35.7) | 947 (41.8) | 461 (35.0) |
| 2.01–5.00 | 6065 (37.0) | 3370 (32.9) | 1188 (46.6) | 961 (42.4) | 546 (41.4) |
| >5.00 | 1493 (9.1) | 743 (7.3) | 317 (12.4) | 227 (10.0) | 206 (15.6) |
| Microinvasion | 132 (0.8) | 44 (0.4) | 32 (1.3) | 29 (1.3) | 27 (2.0) |
| Diffuse | 85 (0.5) | 45 (0.4) | 11 (0.4) | 16 (0.7) | 13 (1.0) |
| Unknown | 990 (3.0) | 252 (2.5) | 91 (3.6) | 85 (3.8) | 66 (5.0) |
| Lymph node involvement | |||||
| Negative | 9241 (56.4) | 6117 (59.7) | 1423 (55.8) | 1098 (48.5) | 603 (45.7) |
| Positive | 6840 (41.8) | 3965 (38.7) | 1083 (42.5) | 1109 (49.0) | 683 (51.8) |
| Unknown | 640 (1.8) | 165 (1.6) | 43 (1.7) | 58 (2.6) | 33 (2.5) |
| Metastasis | |||||
| Negative | 15255 (93.1) | 9644 (94.1) | 2368 (92.9) | 2055 (90.7) | 1188 (90.1) |
| Positive | 778 (4.7) | 409 (4.0) | 127 (5.0) | 141 (6.2) | 101 (7.7) |
| Unknown | 637 (2.1) | 194 (1.9) | 54 (2.1) | 69 (3.0) | 30 (2.3) |
Notes. Column percentages based on individuals with valid, non-missing information on risk factor.
Insurance status is based on payer source at diagnosis. Public insurance included Medicaid and other government-assisted programs; and private insurance included health maintenance organizations, preferred provider organizations, managed care not otherwise specified, and military care.
Association between Breast Cancer Subtypes and Patient Attributes
Foreign-born Hispanic women were significantly more likely than U.S.-born Hispanic women to be diagnosed with HR−/HER2+ versus HR+/HER2− breast cancer (OR=1.17, 95% CI:1.02–1.35); other subtypes did not differ by nativity (Table 2). Compared to women living in the highest SES neighborhoods, those in lower SES neighborhoods had a 1.32–1.42 fold greater risk of triple negative (p<0.05) and 1.17–1.43 fold greater risk of HR−/HER2+ relative to HR+/HER2− breast cancer (p<0.05). Hispanic women aged 45–49 years had a lower risk of the triple negative (OR=0.72, 95% CI:0.60–0.87) and HR−/HER2+ (OR=0.59, 95% CI:0.47–0.75) subtypes, compared to women aged 50–54 years.
Table 2.
Adjusted Odds Ratios of Sociodemographic and Tumor Characteristics associated with Breast Tumor Subtypes (vs. HR+/HER2−) in Hispanic Women, California, 2005–2010
| HR+/HER2−
|
Triple negative
|
HR+/HER2+
|
HR−/HER2+
|
||||
|---|---|---|---|---|---|---|---|
| Characteristics | % | % | OR [95% CI] | % | OR [95% CI] | % | OR [95% CI] |
| Sociodemographic | |||||||
| Nativity | |||||||
| US-Born | 48.1 | 46.8 | Ref. | 43.1 | Ref. | 40.3 | Ref. |
| Foreign-Born | 51.9 | 53.2 | 0.97 (0.87–1.09) | 56.9 | 1.06 (0.95–1.18) | 59.7 | 1.17 (1.02–1.35) |
| Socioeconomic status | |||||||
| Quintile 1 (Low) | 24.2 | 28.0 | 1.42 (1.13–1.79) | 28.5 | 0.96 (0.77–1.20) | 32.2 | 1.43 (1.07–1.92) |
| Quintile 2 | 22.5 | 23.9 | 1.40 (1.12–1.74) | 22.6 | 0.93 (0.76–1.15) | 22.9 | 1.21 (0.91–1.60) |
| Quintile 3 | 18.7 | 19.0 | 1.39 (1.12–1.72) | 17.4 | 1.01 (0.82–1.24) | 17.7 | 1.27 (0.96–1.68) |
| Quintile 4 | 15.9 | 13.7 | 1.32 (1.06–1.64) | 15.2 | 1.15 (0.94–1.41) | 12.7 | 1.17 (0.89–1.56) |
| Quintile 5 (High) | 11.4 | 8.0 | Ref.† | 9.1 | Ref. | 7.7 | Ref.† |
| Hispanic enclave | |||||||
| Quintile 1 (Low) | 8.8 | 7.7 | Ref. | 7.2 | Ref.‡ | 7.4 | Ref. |
| Quintile 2 | 14.4 | 13.1 | 0.98 (0.78–1.23) | 11.2 | 0.89 (0.70–1.12) | 12.1 | 1.04 (0.77–1.41) |
| Quintile 3 | 17.5 | 17.3 | 0.97 (0.77–1.21) | 16.6 | 1.07 (0.86–1.34) | 15.2 | 0.96 (0.71–1.29) |
| Quintile 4 | 24.8 | 23.7 | 0.88 (0.70–1.10) | 23.8 | 1.07 (0.86–1.34) | 23.0 | 0.91 (0.68–1.22) |
| Quintile 5 (High) | 29.9 | 33.8 | 0.98 (0.77–1.23) | 37.0 | 1.34 (1.06–1.68) | 38.0 | 1.12 (0.83–1.52) |
| Age at diagnosis (years) | |||||||
| <45 | 18.2 | 30.2 | 1.03 (0.87–1.22) | 28.3 | 1.15 (0.98–1.36) | 26.8 | 0.86 (0.70–1.06) |
| 45–49 | 14.9 | 14.6 | 0.72 (0.60–0.87) | 15.8 | 0.87 (0.73–1.05) | 14.0 | 0.59 (0.47–0.75) |
| 50–54 | 13.5 | 15.9 | Ref. | 14.8 | Ref. | 17.2 | Ref. |
| 55–59 | 12.2 | 12.1 | 0.93 (0.76–1.14) | 12.5 | 0.97 (0.79–1.17) | 14.1 | 0.95 (0.75–1.21) |
| 60–64 | 11.5 | 9.1 | 0.83 (0.67–1.03) | 8.7 | 0.79 (0.64–0.97) | 8.4 | 0.71 (0.54–0.93) |
| ≥65 | 29.7 | 18.0 | 0.81 (0.67–0.97) | 19.9 | 0.78 (0.65–0.94) | 19.4 | 0.78 (0.62–0.98) |
| Marital status | |||||||
| Married | 56.3 | 57.3 | Ref. | 57.4 | Ref. | 57.7 | Ref. |
| Never married | 17.3 | 18.2 | 0.91 (0.79–1.05) | 20.0 | 1.02 (0.89–1.17) | 17.4 | 0.85 (0.71–1.02) |
| Previously married | 22.9 | 20.8 | 1.10 (0.96–1.27) | 20.0 | 1.05 (0.91–1.20) | 20.1 | 1.02 (0.85–1.21) |
| Insurance status | |||||||
| Private/military | 51.3 | 49.9 | Ref. | 46.7 | Ref. | 46.2 | Ref. |
| Public | 40.6 | 41.7 | 1.06 (0.94–1.20) | 45.0 | 1.24 (1.10–1.39) | 44.1 | 1.13 (0.97–1.31) |
| Uninsured | 1.4 | 1.5 | 0.89 (0.57–1.39) | 1.6 | 1.00 (0.66–1.52) | 1.5 | 1.00 (0.59–1.69) |
| Clinical | |||||||
| Tumor grade | |||||||
| Low | 71.4 | 15.8 | Ref. | 46.5 | Ref. | 20.8 | Ref. |
| High | 24.4 | 81.0 | 12.26 (10.79–13.93) | 49.2 | 2.46 (2.21–2.74) | 74.1 | 8.43 (7.21–9.86) |
| Metastasis | |||||||
| Negative | 94.1 | 92.9 | Ref. | 90.7 | Ref. | 90.1 | Ref. |
| Positive | 4.0 | 5.0 | 0.91 (0.70–1.20) | 6.2 | 1.27 (0.99–1.64) | 7.7 | 1.00 (0.73–1.36) |
| Tumor Size (cm) | |||||||
| 0–2.00 | 56.5 | 35.7 | Ref. | 41.8 | Ref. | 35.0 | Ref. |
| 2.01–5.00 | 32.9 | 46.6 | 1.29 (1.14–1.45) | 42.4 | 1.18 (1.05–1.32) | 41.4 | 1.08 (0.92–1.26) |
| >5.00 | 7.3 | 12.4 | 1.59 (1.31–1.91) | 10.0 | 1.14 (0.94–1.38) | 15.6 | 1.64 (1.31–2.05) |
| Microinvasion | 0.4 | 1.3 | 2.38 (1.37–4.12) | 1.3 | 1.99 (1.19–3.35) | 2.0 | 3.14 (1.81–5.46) |
| Diffuse | 0.4 | 0.4 | 1.90 (0.92–3.90) | 0.7 | 2.43 (1.30–4.55) | 1.0 | 4.41 (2.24–8.71) |
| Lymph nodes involved | |||||||
| Negative | 59.7 | 55.8 | Ref. | 90.7 | Ref. | 45.7 | Ref. |
| Positive | 38.7 | 42.5 | 0.54 (0.48–0.60) | 6.2 | 0.86 (0.77–0.97) | 51.8 | 0.82 (0.71–0.94) |
Notes. Models adjusted for all variables listed in the table. Abbreviations: Ref. = Referent category; OR=odds ratio; CI=confidence interval. Bold text signifies statistically significant associations.
p for trend <0.05;
p for trend <0.01.
Breast Cancer-Specific Survival and Mortality by Breast Cancer Subtype
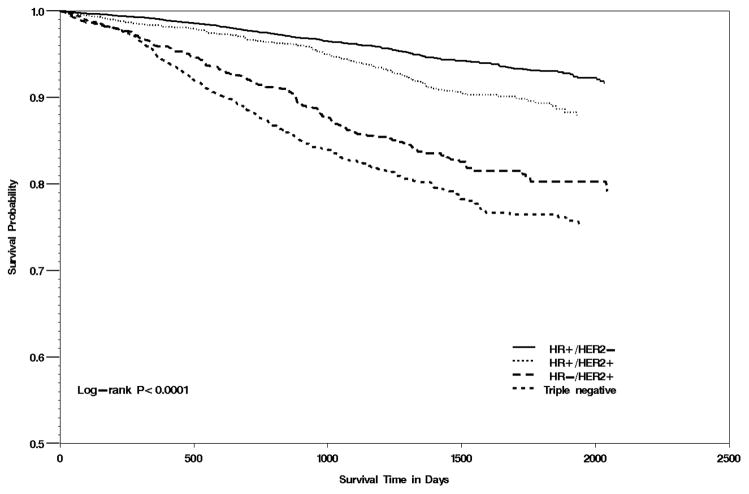
Breast cancer-specific survival significantly differed between tumor subtypes (p<0.0001; Figure 1). Over the approximately 5.5 years of follow-up, Hispanic women with triple negative and HR−/HER2+ breast cancer had the lowest probability of survival. After multivariate adjustment (Table 3), Hispanic women diagnosed with triple negative breast cancer were 4-times more likely to die from the disease compared to those diagnosed with the HR+/HER2− subtype (HR=4.05, 95% CI:3.35–4.90). Overall, the risk of death from breast cancer followed a step-wise pattern by SES, such that the risk of death from breast cancer increased as a woman’s neighborhood SES level decreased; compared to quintile 5 (highest SES), HRs were 1.38 (95%CI:0.98–1.94) for quintile 4 and 1.76 (95% CI:1.25–2.49) for quintile 1.
Figure 1.
Kaplan-Meier curve of Hispanic women by breast cancer subtype, California 2005–2010
Table 3.
Breast cancer-specific mortality, by tumor subtype, in Hispanic Women in California, 2005–2010
| All subtypes
|
HR+/HER2−
|
Triple negative
|
HR+/HER2+
|
HR−/HER2+
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Deaths (n=993) |
HR (95% CI) | Deaths (n=345) |
HR (95% CI) | Deaths (n=357) |
HR (95% CI) | Deaths (n=133) |
HR (95% CI) | Deaths (n=158) |
HR (95% CI) | |
| Nativity | ||||||||||
| US-Born | 426 | Ref. | 138 | Ref. | 153 | Ref. | 66 | Ref. | 69 | Ref. |
| Foreign-Born | 567 | 0.97 (0.83–1.13) | 207 | 1.12 (0.86–1.47) | 204 | 0.99 (0.76–1.28) | 67 | 0.68 (0.43–1.06) | 89 | 0.72 (0.48–1.08) |
| Socioeconomic status | ||||||||||
| Quintile 1 (Low) | 336 | 1.76 (1.25–2.49) | 117 | 1.62 (0.91–2.88) | 120 | 1.21 (0.69–2.13) | 41 | 2.85 (0.90–9.00) | 58 | 4.02 (1.35–11.99) |
| Quintile 2 | 230 | 1.67 (1.19–2.36) | 77 | 1.32 (0.74–2.34) | 76 | 1.14 (0.65–1.98) | 31 | 2.84 (0.91–8.86) | 46 | 4.95 (1.70–14.40) |
| Quintile 3 | 179 | 1.54 (1.10–2.15) | 58 | 1.31 (0.74–2.32) | 79 | 1.44 (0.85–2.44) | 22 | 2.34 (0.79–6.93) | 20 | 1.79 (0.59–5.38) |
| Quintile 4 | 127 | 1.38 (0.98–1.94) | 43 | 1.04 (0.59–1.83) | 40 | 1.02 (0.59–1.79) | 23 | 2.46 (0.83–7.30) | 21 | 3.01 (1.03–8.77) |
| Quintile 5 (High) | 69 | Ref.† | 29 | Ref. | 23 | Ref. | 8 | Ref. | 9 | Ref.† |
| Hispanic enclave | ||||||||||
| Quintile 1 (Low) | 63 | Ref. | 19 | Ref. | 25 | Ref. | 9 | Ref. | 10 | Ref. |
| Quintile 2 | 117 | 1.05 (0.75–1.48) | 37 | 0.79 (0.43–1.46) | 47 | 1.25 (0.73–2.13) | 15 | 0.68 (0.24–1.90) | 18 | 1.65 (0.65–4.20) |
| Quintile 3 | 161 | 0.98 (0.71–1.36) | 56 | 1.01 (0.57–1.80) | 58 | 1.05 (0.62–1.80) | 24 | 0.70 (0.27–1.78) | 23 | 1.11 (0.44–2.83) |
| Quintile 4 | 238 | 0.92 (0.66–1.27) | 88 | 1.01 (0.57–1.80) | 84 | 0.98 (0.58–1.66) | 32 | 0.83 (0.33–2.09) | 34 | 0.84 (0.33–2.14) |
| Quintile 5 (High) | 382 | 0.92 (0.66–1.28) | 133 | 0.98 (0.55–1.77) | 128 | 1.11 (0.65–1.89) | 51 | 0.59 (0.22–1.60) | 70 | 0.94 (0.37–2.40) |
| Age at diagnosis (years0 | ||||||||||
| <45 | 245 | 0.94 (0.73–1.20) | 60 | 0.65 (0.42–1.03) | 116 | 1.35 (0.88–2.08) | 29 | 0.49 (0.25–0.96) | 40 | 1.37 (0.76–2.46) |
| 45–49 | 130 | 0.96 (0.73–1.27) | 48 | 0.79 (0.49–1.27) | 53 | 1.25 (0.77–2.03) | 12 | 0.55 (0.25–1.20) | 17 | 1.33 (0.66–2.67) |
| 50–54 | 149 | Ref. | 51 | Ref. | 43 | Ref. | 28 | Ref. | 27 | Ref. |
| 55–59 | 105 | 0.94 (0.70–1.26) | 40 | 0.98 (0.59–1.60) | 36 | 1.16 (0.69–1.95) | 9 | 0.33 (0.13–0.82) | 20 | 1.02 (0.50–2.07) |
| 60–64 | 81 | 0.90 (0.65–1.24) | 28 | 0.72 (0.42–1.26) | 30 | 1.45 (0.83–2.54) | 13 | 0.71 (0.29–1.70) | 10 | 0.90 (0.40–2.07) |
| ≥65 | 283 | 1.30 (1.01–1.67) | 118 | 1.09 (0.71–1.69) | 79 | 1.55 (0.95–2.52) | 42 | 1.01 (0.54–1.90) | 44 | 1.55 (0.84–2.86) |
| Marital status at diagnosis | ||||||||||
| Married | 507 | Ref. | 155 | Ref. | 204 | Ref. | 68 | Ref. | 80 | Ref. |
| Never married | 192 | 1.06 (0.87–1.29) | 72 | 1.40 (1.00–1.96) | 64 | 0.94 (0.67–1.32) | 24 | 0.89 (0.50–1.58) | 32 | 1.16 (0.70–1.93) |
| Prev. married | 248 | 1.14 (0.95–1.37) | 93 | 1.44 (1.05–1.98) | 79 | 0.86 (0.63–1.19) | 38 | 1.16 (0.71–1.89) | 38 | 1.42 (0.86–2.34) |
| Insurance status | ||||||||||
| Private | 394 | Ref. | 139 | Ref. | 146 | Ref. | 46 | Ref. | 63 | Ref. |
| Public | 500 | 1.13 (0.96–1.34) | 170 | 0.99 (0.74–1.33) | 180 | 1.23 (0.92–1.64) | 75 | 1.25 (0.77–2.01) | 75 | 1.12 (0.73–1.70) |
| Uninsured | 24 | 1.41 (0.88–2.26) | 9 | 1.58 (0.72–3.45) | 7 | 1.53 (0.68–3.43) | <5 | 3.11 (0.91–10.65) | 5 | 0.57 (0.14–2.35) |
| Subtype | ||||||||||
| HR+/HER2− | 345 | Ref. | ||||||||
| Triple Negative | 357 | 4.05 (3.35–4.90) | ||||||||
| HR+/HER2+ | 133 | 1.20 (0.95–1.52) | ||||||||
| HR−/HER2+ | 158 | 2.26 (1.80–2.84) | ||||||||
Notes. Cox models were adjusted for all variables presented in addition to year of diagnosis, tumor size (continuous), lymph node (yes/no), and first course of treatment (surgery, chemotherapy, and radiation therapy); AJCC stage levels I–IV and unknown was included as a stratifying variable. Abbreviations: Ref.=Referent category; HR=hazard ratio; CI=confidence interval. Bold text signifies statistically significant associations.
Public insurance included Medicaid and other government-assisted programs; and private insurance included health maintenance organizations, preferred provider organizations, managed care not otherwise specified, and military care.
p for trend <0.05.
For U.S.-born Hispanic women, the risk of breast cancer-specific mortality was significantly greater for all subtypes compared to HR+/HER2− tumors (Table 4), whereas, among foreign-born Hispanic women, only those diagnosed with triple negative and HR−/HER2+ subtypes had significantly increased risk of death from breast cancer.
Table 4.
Breast cancer-specific mortality by Hispanic nativity
| Breast cancer subtype | U.S.-born Hispanic
|
Foreign-born Hispanic
|
||
|---|---|---|---|---|
| Deaths | HR [95% CI] | Deaths | HR [95% CI] | |
| HR+/HER2− | 138 | Ref. | 207 | Ref. |
| Triple negative | 153 | 4.38 (3.23–5.95) | 204 | 4.10 (3.20–5.26) |
| HR+/HER2+ | 66 | 1.57 (1.11–2.22) | 67 | 0.93 (0.67–1.31) |
| HR−/HER2+ | 69 | 3.22 (2.25–4.61) | 89 | 1.91 (1.41–2.58) |
Notes. Cox models were adjusted for socioeconomic status (quintiles), Hispanic enclave (quintiles), age at diagnosis (continuous), marital status (married, never married, previously married), insurance status (private, public, uninsured, unknown), tumor size (continuous), lymph node (yes/no), tumor grade (low/high/unknown) and first course of treatment (surgery, chemotherapy, and radiation therapy); AJCC stage levels I–IV and unknown was included as a stratifying variable. Bold text signifies statistically significant associations Abbreviations: Ref.=Referent category; HR=hazard ratio; CI=confidence interval.
Discussion
Our study is among the first, certainly the most comprehensive with 16,380 participants, population-based analyses of breast cancer subtypes among U.S. Hispanic women. These results confirm and extend emerging patterns for the molecular breast cancer subtypes. Similar to other racial/ethnic groups [18,13,19,20], residence in a low SES neighborhood was significantly associated with an increased risk of diagnosis and dying from HR−/HER2+ and triple negative breast cancers. Foreign-born Hispanics were at greater risk of diagnosis with HR−/HER2+, compared to U.S.-born Hispanics. The risk of death from triple negative breast cancer was substantial, as both U.S.- and foreign-born Hispanic women diagnosed with this subtype had an approximately 4-fold greater risk of death than those with HR+/HER2− breast cancer.
Although our case-only analyses cannot speak directly to cancer etiology or risk, these data underscore the potential impact of SES, a social determinant of health, on risk factors that may be etiologically important in increasing women’s risk of developing poor prognostic breast cancer subtypes. For instance, women of low SES status, or residing in low SES neighborhoods, may have lower access to and consume fewer healthy foods (i.e., vegetables and nutrient-rich foods), fewer opportunities to engage in physical activity, and higher levels of obesity [21,22], which studies indicate are associated with increased risk of ER− [23–25] or triple negative breast cancers [26]. Moreover, low SES may be related to younger age at first birth and lack of breastfeeding [27,28], factors associated with an elevated risk of triple negative or basal-like breast cancers [29–31].
Importantly, if socioeconomic differences play a role in the risk of developing certain tumor subtypes, then the breast cancer-specific mortality disadvantage observed among Hispanic and other racial/ethnic women may be partly due to the intrinsic aggressiveness of the tumor subtype, and the more commonly recognized factors associated with low SES or living in low SES neighborhoods [32]. Consistent with studies among other racial/ethnic groups [33,4,34,5], our study confirms the adverse characteristics of certain breast cancers, such that compared to the HR+/HER2− subtype, Hispanic women diagnosed with HR+/HER2+, HR−/HER2+, and triple negative subtypes had significantly greater risk of death.
While our study adds valuable information on breast cancer subtypes among Hispanic women, it has some limitations. Tumor subtype information was missing for approximately 16.6% of potentially eligible participants, although the magnitude and direction of biases related to missing data are unknown. We did not have individual-level data on income or education; nevertheless, neighborhood-level measures may capture information on environmental factors that occur as a result of its socioeconomic condition and deprivation [20]. The distribution of breast cancer subtypes among Hispanic women in California may not reflect distributions among other U.S. Hispanic women; accordingly, our findings may be most generalizable to women of primarily Mexican descent, the largest Hispanic subgroup in California [35]. The imputation method used to classify nativity is subject to error and may have led to some misclassification; however, most participants (about 65%) were classified based on cancer registry birthplace data, previously shown to have high accuracy [11,36]. Similarly, Hispanic ethnicity may be subject to misclassification [37,38], although classification should have improved with application of a registry-wide algorithm [9]. While Hispanics are a diverse population, we could not include information on Hispanic origin because it was missing for approximately 40% of participants. Breast cancer-specific survival analyses are subject to the accuracy of the underlying cause of death code, which has been shown to be 84–90% accurate [39,40]. Lastly, those tumor characteristics associated with aggressive breast cancer subtypes, including HR− and younger age at diagnosis, are also associated with interval-detected cancers, which may lead to length bias and lower survival than screen-derived cancers [41,42].
Conclusion
In the largest population-based study of breast cancer subtypes among U.S. Hispanic women conducted to date, we found that residing in a low SES neighborhood was significantly associated with increased risk of developing and dying from HR− breast cancers. Foreign-born women were at greater risk of HR−/HER2+ tumors, although nativity was not associated with other subtypes. Similarity in the distribution and prognosis of breast cancer subtypes among California Hispanic women and previous studies in other racial/ethnic groups underscores the need to understand how sociodemographic factors, such as SES, contribute to the distinct patterns of breast cancer subtype incidence and mortality among women of all racial/ethnic backgrounds.
Acknowledgments
MPB, SA and WFA are employees of the National Cancer Institute at the National Institutes of Health and no additional funding was provided specifically for this work. The collection of cancer incidence data used in this study was supported by the California Department of Health Services as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement #1U58 DP000807-01 awarded to the Public Health Institute. The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, the California Department of Health Services, the National Cancer Institute, or the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be inferred.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Banegas MP, Li CI. Breast cancer characteristics and outcomes among Hispanic Black and Hispanic White women. Breast Cancer Res Treat. 2012;134 (3):1297–1304. doi: 10.1007/s10549-012-2142-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ooi SL, Martinez ME, Li CI. Disparities in breast cancer characteristics and outcomes by race/ethnicity. Breast Cancer Res Treat. 2010 doi: 10.1007/s10549-010-1191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parise CA, Bauer KR, Brown MM, Caggiano V. Breast cancer subtypes as defined by the estrogen receptor (ER), progesterone receptor (PR), and the human epidermal growth factor receptor 2 (HER2) among women with invasive breast cancer in California, 1999–2004. The breast journal. 2009;15 (6):593–602. doi: 10.1111/j.1524-4741.2009.00822.x. TBJ822 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Carey LA, Perou CM, Livasy CA, Dressler LG, Cowan D, Conway K, Karaca G, Troester MA, Tse CK, Edmiston S, Deming SL, Geradts J, Cheang MC, Nielsen TO, Moorman PG, Earp HS, Millikan RC. Race, breast cancer subtypes, and survival in the Carolina Breast Cancer Study. JAMA. 2006;295 (21):2492–2502. doi: 10.1001/jama.295.21.2492. [DOI] [PubMed] [Google Scholar]
- 5.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: a prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9 (1):R6. doi: 10.1186/bcr1639. bcr1639 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hines LM, Risendal B, Byers T, Mengshol S, Lowery J, Singh M. Ethnic disparities in breast tumor phenotypic subtypes in Hispanic and non-Hispanic white women. Journal of women’s health (2002) 2011;20 (10):1543–1550. doi: 10.1089/jwh.2010.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ortiz AP, Frias O, Perez J, Cabanillas F, Martinez L, Sanchez C, Capo-Ramos DE, Gonzalez-Keelan C, Mora E, Suarez E. Breast cancer molecular subtypes and survival in a hospital-based sample in Puerto Rico. Cancer Med. 2013;2 (3):343–350. doi: 10.1002/cam4.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomez SL, Le GM, West DW, Satariano WA, O’Connor L. Hospital policy and practice regarding the collection of data on race, ethnicity, and birthplace. Am J Public Health. 2003;93 (10):1685–1688. doi: 10.2105/ajph.93.10.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.North American Association of Central Cancer Registries (NAACCR) Race and Ethnicity Work Group NAACCR. Guideline for Enhancing Hispanic/Latino Identification: Revised NAACCR Hispanic/Latino Identification Algorithm [NHIA v2.2] North American Association of Central Cancer Registries; Springfield, IL: [Google Scholar]
- 10.Gomez SL, Quach T, Horn-Ross PL, Pham JT, Cockburn M, Chang ET, Keegan TH, Glaser SL, Clarke CA. Hidden breast cancer disparities in Asian women: disaggregating incidence rates by ethnicity and migrant status. Am J Public Health. 2010;100(Suppl 1):S125–131. doi: 10.2105/AJPH.2009.163931. AJPH.2009.163931 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gomez SL, Glaser SL. Quality of cancer registry birthplace data for Hispanics living in the United States. Cancer Causes Control. 2005;16 (6):713–723. doi: 10.1007/s10552-005-0694-7. [DOI] [PubMed] [Google Scholar]
- 12.Clarke CA, Keegan TH, Yang J, Press DJ, Kurian AW, Patel AH, Lacey JV., Jr Age-specific incidence of breast cancer subtypes: understanding the black-white crossover. J Natl Cancer Inst. 2012;104 (14):1094–1101. doi: 10.1093/jnci/djs264. djs264 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bauer KR, Brown M, Cress RD, Parise CA, Caggiano V. Descriptive analysis of estrogen receptor (ER)-negative, progesterone receptor (PR)-negative, and HER2-negative invasive breast cancer, the so-called triple-negative phenotype: a population-based study from the California cancer Registry. Cancer. 2007;109 (9):1721–1728. doi: 10.1002/cncr.22618. [DOI] [PubMed] [Google Scholar]
- 14.Bernstein L, Lacey JV., Jr Receptors, associations, and risk factor differences by breast cancer subtypes: positive or negative? J Natl Cancer Inst. 2011;103 (6):451–453. doi: 10.1093/jnci/djr046. djr046 [pii] [DOI] [PubMed] [Google Scholar]
- 15.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28 (16):2784–2795. doi: 10.1200/JCO.2009.25.6529. JCO.2009.25.6529 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, Dowsett M, Fitzgibbons PL, Hanna WM, Langer A, McShane LM, Paik S, Pegram MD, Perez EA, Press MF, Rhodes A, Sturgeon C, Taube SE, Tubbs R, Vance GH, van de Vijver M, Wheeler TM, Hayes DF. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25 (1):118–145. doi: 10.1200/JCO.2006.09.2775. [DOI] [PubMed] [Google Scholar]
- 17.Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen-Dale AL, Brown PO, Botstein D. Molecular portraits of human breast tumours. Nature. 2000;406 (6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Gordon NH. Association of education and income with estrogen receptor status in primary breast cancer. Am J Epidemiol. 1995;142 (8):796–803. doi: 10.1093/oxfordjournals.aje.a117718. [DOI] [PubMed] [Google Scholar]
- 19.Andaya AA, Enewold L, Horner MJ, Jatoi I, Shriver CD, Zhu K. Socioeconomic disparities and breast cancer hormone receptor status. Cancer Causes Control. 2012;23 (6):951–958. doi: 10.1007/s10552-012-9966-1. [DOI] [PubMed] [Google Scholar]
- 20.Gordon NH. Socioeconomic factors and breast cancer in black and white Americans. Cancer Metastasis Rev. 2003;22 (1):55–65. doi: 10.1023/a:1022212018158. [DOI] [PubMed] [Google Scholar]
- 21.Drewnowski A, Specter SE. Poverty and obesity: the role of energy density and energy costs. Am J Clin Nutr. 2004;79 (1):6–16. doi: 10.1093/ajcn/79.1.6. [DOI] [PubMed] [Google Scholar]
- 22.Gordon-Larsen P, Nelson MC, Page P, Popkin BM. Inequality in the built environment underlies key health disparities in physical activity and obesity. Pediatrics. 2006;117 (2):417–424. doi: 10.1542/peds.2005-0058. 117/2/417 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Jung S, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Cerhan JR, Gaudet MM, Giles GG, Goodman G, Hakansson N, Hankinson SE, Helzlsouer K, Horn-Ross PL, Inoue M, Krogh V, Lof M, McCullough ML, Miller AB, Neuhouser ML, Palmer JR, Park Y, Robien K, Rohan TE, Scarmo S, Schairer C, Schouten LJ, Shikany JM, Sieri S, Tsugane S, Visvanathan K, Weiderpass E, Willett WC, Wolk A, Zeleniuch-Jacquotte A, Zhang SM, Zhang X, Ziegler RG, Smith-Warner SA. Fruit and vegetable intake and risk of breast cancer by hormone receptor status. J Natl Cancer Inst. 2013;105 (3):219–236. doi: 10.1093/jnci/djs635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Spiegelman D, Baglietto L, Bernstein L, Boggs DA, van den Brandt PA, Buring JE, Gapstur SM, Giles GG, Giovannucci E, Goodman G, Hankinson SE, Helzlsouer KJ, Horn-Ross PL, Inoue M, Jung S, Khudyakov P, Larsson SC, Lof M, McCullough ML, Miller AB, Neuhouser ML, Palmer JR, Park Y, Robien K, Rohan TE, Ross JA, Schouten LJ, Shikany JM, Tsugane S, Visvanathan K, Weiderpass E, Wolk A, Willett WC, Zhang SM, Ziegler RG, Smith-Warner SA. Carotenoid intakes and risk of breast cancer defined by estrogen receptor and progesterone receptor status: a pooled analysis of 18 prospective cohort studies. Am J Clin Nutr. 2012;95 (3):713–725. doi: 10.3945/ajcn.111.014415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137 (3):869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 26.Vona-Davis L, Rose DP, Hazard H, Howard-McNatt M, Adkins F, Partin J, Hobbs G. Triple-negative breast cancer and obesity in a rural Appalachian population. Cancer Epidemiol Biomarkers Prev. 2008;17 (12):3319–3324. doi: 10.1158/1055-9965.EPI-08-0544. 17/12/3319 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heck KE, Braveman P, Cubbin C, Chavez GF, Kiely JL. Socioeconomic status and breastfeeding initiation among California mothers. Public Health Rep. 2006;121 (1):51–59. doi: 10.1177/003335490612100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinez GM, Chandra A, Abma JC, Jones J, Mosher WD. Fertility, contraception, and fatherhood: data on men and women from cycle 6 (2002) of the 2002 National Survey of Family Growth. Vital Health Stat. 2006;23 (26):1–142. [PubMed] [Google Scholar]
- 29.Li CI, Beaber EF, Tang MT, Porter PL, Daling JR, Malone KE. Reproductive factors and risk of estrogen receptor positive, triple-negative, and HER2-neu overexpressing breast cancer among women 20–44 years of age. Breast Cancer Res Treat. 2013;137 (2):579–587. doi: 10.1007/s10549-012-2365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phipps AI, Chlebowski RT, Prentice R, McTiernan A, Wactawski-Wende J, Kuller LH, Adams-Campbell LL, Lane D, Stefanick ML, Vitolins M, Kabat GC, Rohan TE, Li CI. Reproductive history and oral contraceptive use in relation to risk of triple-negative breast cancer. J Natl Cancer Inst. 2011;103 (6):470–477. doi: 10.1093/jnci/djr030. djr030 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Millikan RC, Newman B, Tse CK, Moorman PG, Conway K, Dressler LG, Smith LV, Labbok MH, Geradts J, Bensen JT, Jackson S, Nyante S, Livasy C, Carey L, Earp HS, Perou CM. Epidemiology of basal-like breast cancer. Breast Cancer Res Treat. 2008;109 (1):123–139. doi: 10.1007/s10549-007-9632-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vona-Davis L, Rose DP. The influence of socioeconomic disparities on breast cancer tumor biology and prognosis: a review. Journal of women’s health. 2009;18 (6):883–893. doi: 10.1089/jwh.2008.1127. [DOI] [PubMed] [Google Scholar]
- 33.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P, Narod SA. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13 (15 Pt 1):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. 13/15/4429 [pii] [DOI] [PubMed] [Google Scholar]
- 34.Onitilo AA, Engel JM, Greenlee RT, Mukesh BN. Breast cancer subtypes based on ER/PR and Her2 expression: comparison of clinicopathologic features and survival. Clin Med Res. 2009;7 (1–2):4–13. doi: 10.3121/cmr.2009.825. 7/1-2/4 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ennis SR, Rios-Vargas M, Albert NG. Census 2010 Briefs. 2011. The Hispanic Population: 2010. [Google Scholar]
- 36.Gomez SL, Glaser SL. Quality of birthplace information obtained from death certificates for Hispanics, Asians, and Pacific Islanders. Ethn Dis. 2004;14 (2):292–295. [PubMed] [Google Scholar]
- 37.Clegg LX, Reichman ME, Hankey BF, Miller BA, Lin YD, Johnson NJ, Schwartz SM, Bernstein L, Chen VW, Goodman MT, Gomez SL, Graff JJ, Lynch CF, Lin CC, Edwards BK. Quality of race, Hispanic ethnicity, and immigrant status in population-based cancer registry data: implications for health disparity studies. Cancer Causes Control. 2007;18 (2):177–187. doi: 10.1007/s10552-006-0089-4. [DOI] [PubMed] [Google Scholar]
- 38.Gomez SL, Glaser SL. Misclassification of race/ethnicity in a population-based cancer registry (United States) Cancer Causes Control. 2006;17 (6):771–781. doi: 10.1007/s10552-006-0013-y. [DOI] [PubMed] [Google Scholar]
- 39.Ederer F, Geisser MS, Mongin SJ, Church TR, Mandel JS. Colorectal cancer deaths as determined by expert committee and from death certificate: a comparison. The Minnesota Study. J Clin Epidemiol. 1999;52(5):447–452. doi: 10.1016/s0895-4356(99)00016-5. S0895-4356(99)00016-5 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Percy C, Ries LG, Van Holten VD. The accuracy of liver cancer as the underlying cause of death on death certificates. Public Health Rep. 1990;105 (4):361–367. [PMC free article] [PubMed] [Google Scholar]
- 41.Gilliland FD, Joste N, Stauber PM, Hunt WC, Rosenberg R, Redlich G, Key CR. Biologic characteristics of interval and screen-detected breast cancers. J Natl Cancer Inst. 2000;92 (9):743–749. doi: 10.1093/jnci/92.9.743. [DOI] [PubMed] [Google Scholar]
- 42.Porter PL, El-Bastawissi AY, Mandelson MT, Lin MG, Khalid N, Watney EA, Cousens L, White D, Taplin S, White E. Breast tumor characteristics as predictors of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst. 1999;91 (23):2020–2028. doi: 10.1093/jnci/91.23.2020. [DOI] [PubMed] [Google Scholar]