Abstract
Avoidance of long-term immunosuppression is a desired goal in organ transplantation. Mixed chimerism offers a promising approach to tolerance induction, and we have aimed to develop low-toxicity, non-immunodepleting approaches to achieve this outcome. In a mouse model achieving fully MHC-mismatched allogeneic bone marrow engraftment with minimal conditioning (3 Gy total body irradiation followed by anti-CD154 and T cell-depleted allogeneic bone marrow cells), CD4 T cells in the recipient are required to promote tolerance of pre-existing alloreactive recipient CD8 T cells and thereby permit chimerism induction. We now demonstrate that mice devoid of CD4 T cells and NK cells reject MHC class-I deficient and class I/class II-deficient marrow in a CD8 T cell-dependent manner. This rejection is specific for donor alloantigens, since recipient hematopoiesis is not affected by donor marrow rejection and MHC class-I deficient bone marrow that is syngeneic to the recipient is not rejected. Recipient CD8 T cells are activated and develop cytotoxicity against MHC class I-deficient donor cells in association with rejection. These data implicate a novel CD8 T cell-dependent bone marrow rejection pathway, wherein recipient CD8 T cells indirectly activated by donor alloantigens promote direct killing, in a TCR-independent manner, of class I-deficient donor cells.
Keywords: Bone marrow transplantation, indirect alloreactivity, tolerance, CD8 T cells
Introduction
Alloresponses are distinct from classical immune responses to viral, tumor or self-antigens in that they involve two pathways of alloantigen recognition, termed direct and indirect. Direct recognition denotes recognition of an intact donor MHC molecule on a donor cell, while the indirect pathway of alloantigen recognition requires uptake and processing of foreign antigens (MHC and polymorphic non-MHC proteins) by recipient antigen-presenting cells (APCs) and presentation of donor-derived peptides on recipient MHC molecules. The precursor frequency of indirectly alloreactive T cells is 100-fold lower than that of directly alloreactive T cells (1). Alloreactive CD4 and CD8 T cells can independently mediate solid organ allograft rejection by either of these pathways (2–5). Cross-presentation is the ability of APCs to load peptides derived from exogenous antigens onto MHC class I molecules. This presentation pathway is able to initiate immune response by cross-primed CD8 T cells. While cross-priming and cross-presentation of antigens to CD8 T cells have been well-described (5–7), only indirectly alloreactive CD4 cells and not CD8 T cells have been historically considered to be relevant to rejection of fully MHC-mismatched allografts. This belief reflects the apparent lack of a TCR ligand for cross-primed recipient CD8+ cytotoxic T lymphocytes (CTLs) on donor grafts that lack recipient MHC class I alleles. More recently, cross-primed CD8 T cells have been shown to reject MHC class I-deficient skin grafts (5, 7, 8), and recipient endothelium responsible for graft neovascularization was suggested to be the target of this allorecognition (7). However, cross-primed CD8 T cells have not been shown to play a role in rejection of primarily vascularized organ allografts (5) or cellular allografts, and the existence of such a role would indeed be counterintuitive.
Mixed chimerism has recently enjoyed preliminary success for tolerance induction in humans (9–11). In order to minimize the level of host immunodepletion required for mixed chimerism induction, we have replaced T cell depleting mAbs with costimulatory blockade in mice. Pre-existing peripheral and intrathymic alloreactive T cells are tolerized by BMT with costimulatory blockade (12–14). Using a conditioning regimen involving 3 Gy TBI and one injection of anti-CD154 mAb that achieves durable mixed chimerism, we have previously shown that recipient CD4 T cells and recipient MHC class II are required to tolerize peripheral CD8 T cells to fully MHC-mismatched allogeneic bone marrow grafts (15, 16). Since these results suggested a role for CD4 cell-mediated indirect allorecognition in tolerizing CD8 T cells, we questioned whether the CD8 T cells requiring this “indirect” CD4 pathway were directly or indirectly alloreactive. Studies to address this question revealed that directly alloreactive CD8 T cells required these “indirectly alloreactive” CD4 cells in order to be tolerized, as CD8 T cells in mice lacking MHC class I on their APCs rejected allogeneic bone marrow when indirect class II presentation was absent (15). However, we also considered the possibility that an unexpected CD8 T cell-mediated indirect marrow rejection pathway might exist. We now describe the existence of a CD4 T cell-independent BM rejection pathway that appears to involve alloreactive CD8 T cells, which promote specific rejection of MHC class-I deficient allogeneic donor marrow without affecting host hematopoiesis.
Materials and Methods
Animals
All studies were performed under an institutionally approved protocol in accordance with the NIH Guide. B10.A (H2a), B10.S (H2s), B10.RIII (H2r) and B10.Q (H2q) mice, C57BL/6 (B6), and CD45.1 congenic C57BL/6 mice were purchased from Frederick Cancer Research Center (Frederick, MD) or from The Jackson Laboratory (Bar Harbor, ME). KbDb−/− (H-2b) C57BL/6, C56BL/10 (B10) mice were purchased from Taconic Farms (Germantown, NY). KbDb−/− CIITA−/− C57BL/6 mice were bred in our animal facility. All mice were housed in a specific pathogen-free microisolator environment.
Conditioning
Age-matched (6–8 weeks old) female mice received a low dose of TBI (3 Gy) from a 137Cesium irradiator or RS2000 X-ray irradiator on Day −1 with respect to bone marrow transplantation (BMT). When indicated, anti-CD4 mAb (GK1.5; 1.76 mg/mouse) and, where indicated anti-CD8 mAb (2.43 at doses indicated) was included in the conditioning. Anti-NK1.1 mAb PK136 (0.15 mg/mouse or 0.6mg/mouse) was administered i.p. to prevent NK cell-mediated rejection of class I-deficient marrow (17, 18). In some experiments, PK136 (0.15mg/mouse) was given twice per week for 6 weeks following transplantation to achieve long term NK cell depletion. Anti-mouse CD154 mAb (MR1; 2 mg/mouse; National Cell Culture Center) was administered i.p. on Day 0 prior to transplantation with 20–25 × 106 T cell depleted (TCD) allogeneic bone marrow cells (BMC) by tail vein injection. Donor BM was depleted of T cells using magnetic beads coated with anti-CD4 and anti-CD8 antibodies according to the manufacturer’s instructions (Miltenyi Biotec).
Multilineage chimerism among white blood cell lineages
Four-color flow cytometric analysis was performed on white blood cells to analyze the development of multilineage chimerism (19). Recipient-derived cells were identified using fluorescein isothiocyanate (FITC)-conjugated anti-H-2Ks mAb KH49 or biotin-conjugated anti-H-2Dq mAb, and donor-derived cells were identified with phycoerythrin (PE)-conjugated anti-I-Ab mAb. Cells were counterstained with (PE)-conjugated anti-CD4 (Becton Dickinson (BD)/Pharmingen, San Diego, CA), or MAC-1 (Caltag, San Francisco, CA) and with Allophycocyanin (APC)-conjugated anti-CD8 or anti-B220 mAb (BD/PharMingen), respectively. For the short-term experiments (i.e., mice sacrificed at 4, 7 or 11 days post-BMT), a mouse was considered chimeric when it demonstrated ≥ 1.5% donor chimerism in the MAC1 and B220 lineages in the blood. For the long-term experiments (i.e., chimerism checked at 2 weeks and later post-BMT), a mouse was considered chimeric when it demonstrated 5% or more donor chimerism in all lineages tested. Of note T cell chimerism, which arises from 4 to 6 weeks post-BMT, was not tested at the early time points. Negative control mAbs included HOPC1-FITC (prepared in our laboratory) and rat anti-mouse IgG2a-PE or -APC.
Direct cytotoxicity assay
Briefly, splenic CD8 T cells were isolated from B10.S animals rejecting the KbDb−/− BMCs or from conditioned but untransplanted control B10.S mice by anti-CD8 Miltenyi microbeads (purity of 94–98%). Cells in triplicate were then serially diluted and coincubated with 51Cr-labeled ConA blast target cells for 4 hours.
Complete blood counts
Complete blood count (CBC) was measured on a HEMAvet® counter (Drew Scientific Inc, Oxford CT) at indicated time points.
Skin grafting
Mice were shaved and anesthetized with ketamine/xylazine. Full thickness tail skin (0.5–1.0 cm2) from KbDb −/− (donor-specific) or B10.RIII (3rd party) mice was grafted and was considered rejected when <10% of the graft remained viable.
Statistical analysis
Statistical analyses were performed using the Kruskal-Wallis test followed by a Dumn’s multiple comparison test. T test (Mann Wihitney test) was used for comparison between two groups. Survival analysis was performed using a log-rank (Mandel-Cox) test with Prism GraphPad software.
Results
CD8 T cells can reject MHC class I-deficient BM
In our model of mixed chimerism induction with 3 Gy TBI and anti-CD154, we have previously shown that recipient CD4 T cells are needed to tolerize pre-existing alloreactive recipient CD8 T cells (12, 20). We now addressed the possibility that indirectly alloreactive CD8 T cells could reject allogeneic marrow and require recipient CD4 T cells for tolerance induction in this model. We transplanted MHC class I-deficient BM from KbDb−/− B6 donor mice into allogeneic MHC class I-positive B10.S recipients so that direct recognition of the donor by recipient CD8 T cells could not occur. To avoid BM rejection by recipient NK cells due to the lack of donor MHC class I, we depleted NK cells from all recipients using anti-NK1.1 mAb PK136 as described (17, 18).
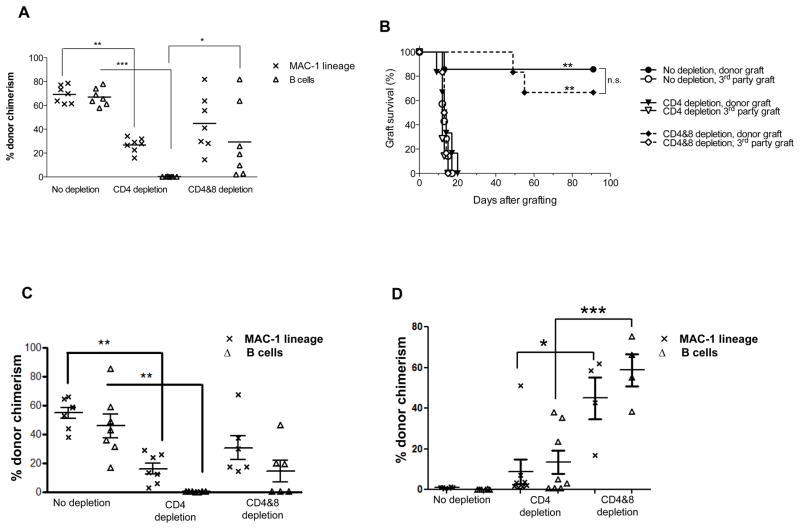
When MHC class I-deficient B6 mice were used as donors, all B10.S mice developed stable and long-lasting multilineage chimerism following conditioning with 3 Gy TBI/anti-CD154 (Figure 1A). However, when CD4 T cells were depleted in vivo, chimerism was absent in all mice by 6 weeks and at all subsequent time points (Figure 1A). In such animals, indirectly alloreactive CD8 T cells should be the only recipient lymphocyte population able to mediate allograft rejection, since directly alloreactive CD8 T cells have no target on MHC class I-negative donor marrow and CD4 T cells, NK cells and donor T cells were depleted. To test whether or not the rejection was mediated by CD8 T cells, one group that received KbDb−/− donor BM was depleted of both CD4 and CD8 T cells in vivo. In this group, stable mixed chimerism was achieved in 4 of 7 animals up to 21 weeks (Figure 1A and data not shown for 21 weeks time point). These results suggest that indirectly alloreactive CD8 T cells are able to reject allogeneic MHC class I-deficient bone marrow and that CD4 T cells are required to tolerize indirectly alloreactive CD8 T cells in our model. All three groups shown in Figure 1A were grafted with KbDb−/− donor and third party skin 20 weeks following BMT. Third party skin was rapidly rejected by all groups (Figure 1B). All except one animal in the non-depleted group accepted donor-type skin grafts permanently, consistent with their durable chimerism. In contrast, all mice initially receiving CD4 depleting mAb (at the time of BMT) rejected their donor grafts, consistent with their lack of chimerism. All chimeras initially depleted of CD4 and CD8 T cells accepted donor-type skin grafts (Figure 1B).
Figure 1. Recipient CD8 T cells reject MHC class I-deficient allogeneic BM.
B10.S or B10.Q mice were conditioned with 3 Gy TBI on Day −1, anti-NK1.1 mAb on Days −7 and −2, and anti-CD154 mAb on Day 0, with or without anti-CD4 mAb on Day-1, with or without anti-CD8 mAb (1.44 mg) on Day −5. Conditioned mice received 20×106 KbDb−/− or β2-microglobulin-deficient TCD BM cells on Day 0. (A) The level of chimerism is shown for the MAC-1 and the B cell lineage 6 weeks post-BMT. Each symbol represents an individual animal. Lines indicate mean values. ***p<0.001, **p<0.01, *p<0.05 with a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. (B) Mice presented in panel A received a donor-type KbDb−/− and 3rd party B10.RIII skin graft at 20 weeks post-BMT. Percentage of grafts surviving is shown over time for all three groups. One out of two similar experiments is shown with 6–8 mice per group per experiment. Statistical analyses were performed using a log-rank (Mandel-Cox) test and show differences: **p<0.01 for donor skin grafted animals receiving no T cell depletion versus those receiving CD4 depletion and **p<0.01 for donor skin grafted animals receiving CD4 depletion versus those receiving CD4 and 8 depletion while no difference (n.s; not significant) was observed between donor skin grafted animals receiving no depletion versus those receiving CD4 and 8 depletion. (C) The level of chimerism is shown for the MAC-1 and the B cell lineages 6 weeks post-transplantation of β2-microglobulin-deficient TCD BM cells to conditioned B10.S recipients. Each symbol represents an individual animal. Lines indicate mean values. **p<0.01 with a Kruskal-Wallis test followed by a Dunn’s multiple comparison test. (D) The level of chimerism is shown for the MAC-1 and B cell lineage 3 weeks post-transplantation of KbDb−/− TCD BMCs to conditioned B10.Q recipients. ***p<0.001; and *p<0.05 with a T test, for B10.Q recipients receiving CD4 and 8 depletion versus those receiving CD4 depletion. Each group contains 4–8 animals.
Because KbDb−/− mice express normal levels of MHC class Ib molecules, we also assessed rejection of β2-microglobulin-deficient B6 BMCs, which lack class Ib MHC expression, in conditioned B10.S recipients. As shown Figure 1C, while conditioned control B10.S mice accepted the KbDb−/− BMCs, depletion of CD4 T cells led to rejection of the MHC class I-deficient donor BM. Depletion of both CD4 and CD8 T cells reversed the rejection. These data thus indicated that rejection is not directed toward polymorphisms in class Ib MHC molecules.
To determine whether the rejection of KbDb−/− BMCs by CD8 T cells was limited to the KbDb−/−→B10.S combination, we also used B10.Q (H-2q) mice as recipients of KbDb−/− BMCs. As shown in Figure 1D, B10.Q recipients conditioned with 3 Gy TBI/anti-CD154 without any T cell depletion rejected the KbDb−/− BMCs at 3 weeks post-transplant. CD4 depletion alone permitted a low level of donor chimerism in the B cell and MAC-1 lineages. In contrast, donor chimerism in both lineages was significantly higher in CD8 and CD4 T cell-depleted recipients than in the CD4 T cell depleted group, demonstrating CD8 T cell-mediated rejection of KbDb−/− BMCs. Therefore, CD8 T cell-mediated rejection of KbDb−/− BMCs is not limited to a single donor-recipient combination.
Activation of recipient CD8 T cells by MHC class I-deficient BM
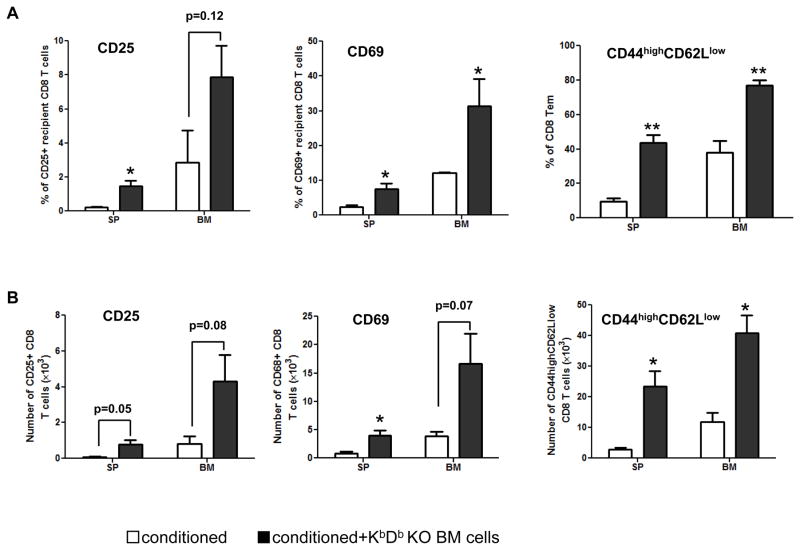
We investigated whether recipient CD8 T cells were activated in association with the rejection of KbDb−/− donor BMCs. As shown in Figure 2A and B, significantly increased levels of CD25 and CD69 on CD8 T cells and increased percentages of CD44highCD62Llow effector memory CD8 T cells were found in the spleen and bone marrow of recipients of KbDb−/− donor BMCs compared to control recipients. In addition, the absolute numbers of CD69+ CD8 T cells in the spleen and CD44highCD62Llow effector memory CD8 T cells in spleen and bone marrow were significantly increased in recipients of KbDb−/− BMCs. Collectively, these data show that recipient CD8 T cells were activated and expanded by allogeneic KbDb−/− donor BMCs.
Figure 2. Activation of recipient CD8 T cells by MHC class I-deficient allogeneic BM.
B10.Q mice were conditioned with 3 Gy TBI on Day −1, anti-NK1.1 mAb on Days −7 and −2, and anti-CD154 mAb on Day 0. Conditioned mice received 20×106 KbDb−/− TCD BM cells on Day 0. Mice in the control group were conditioned but did not receive BM cells. On Day 11 post-transplant, the expression of CD25, CD69, CD44 and CD62L on recipient CD8 T cells in the spleen and bone marrow was determined. (A) Cumulative data showing the percentages of CD25+, CD69+ and CD44highCD62Llow CD8 T cells among total recipient CD8 T cells in the spleen and bone marrow. (B) Cumulative data showing the absolute numbers of CD25+, CD69+ and CD44highCD62Llow recipient CD8 T cells in the spleen and bone marrow. Data are presented as mean ± SEM. *p<0.05 with t-test, for B10.Q recipients receiving KbDb−/− TCD BM cells versus those not receiving BM cells. p values of other groups are also shown. Each group contains 3 animals. Open bar: conditioned recipient without transplantation of KbDb−/− TCD BMCs. Black bar: conditioned recipient without transplantation of KbDb−/− TCD BMCs. SP: spleen. BM: bone marrow.
Rejection of MHC class I-deficient BM does not affect recipient hematopoiesis
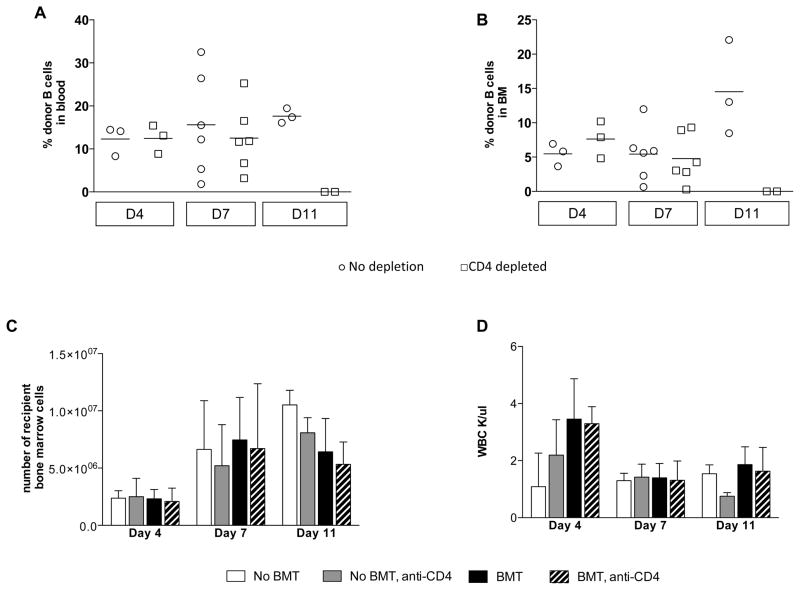
The above data strongly implicated an indirect pathway for CD8 T cell-mediated allogeneic marrow rejection. Since self MHC/donor peptide complexes recognized by indirectly alloreactive CD8 T cells could not be presented by the MHC class I-deficient donor BM, it seemed likely that donor marrow rejection by this pathway involved an indirect effector mechanism with soluble factors such as cytokines produced by CD8 T cells recognizing their donor peptide/self MHC ligands on recipient APCs. In this case, rejection of donor marrow might be associated with “bystander” destruction of recipient BM cells, so that recipient hematopoiesis would be affected as well. To test this hypothesis, B10.S mice were conditioned as described above and did or did not receive a fully allogeneic MHC class I-deficient (KbDb −/−) BMT. Groups of BMT recipients were or were not given CD4 cell-depleting mAb. In order to identify the best times to examine recipient hematopoiesis, we first evaluated the kinetics of rejection of MHC class I-deficient marrow by indirectly alloreactive CD8 T cells. We focused on the B cell lineage because it begins to show chimerism within a few days of conditioning and BMT (20). On Days 4 and 7 post-BMT, no differences in B cell chimerism were observed between groups that did or did not receive depleting anti-CD4 mAb (Figure 3A). By Day 11, no donor B cells were detected in the BM or in the blood of the CD4 T cell-depleted recipient mice (Figure 3A, B), while only a very low percentage of donor B cells remained in the spleen (data not shown).
Figure 3. Specificity of rejection of MHC class I-deficient allogeneic BM by recipient CD8 T cells.
B10.S mice were conditioned with 3 Gy TBI on Day −1, anti-NK1.1 mAb on Day −1 and anti-CD154 mAb on Day 0, with or without CD4 depleting mAb administration on Day −1. After the conditioning, mice received 20×106 KbDb−/− B6 TCD BM cells. The percentage of donor B cell chimerism is shown in recipients of class I-deficient BMT over time in blood (A) and bone marrow (B). Day 4 and Day 11, one experiment is shown with 2–3 animals per group. Two experiments are shown for Day 7 with 6 animals in total. Each symbol represents an individual animal. Lines indicate mean values. No differences were detected between the groups when analyzed by a Kruskal-Wallis test. (C) and (D) B10.S mice were conditioned as above and did or did not receive 20×106 KbDb−/− B6 TCD BM cells. Total numbers (mean ± SD) of recipient (KH49+) BM cells from two tibias per animal are shown in C and mean WBC counts are shown in D. For Day 4 and Day 11, one experiment is shown with 2–3 animals per group. Two experiments are shown for Day 7 with 6 animals per group in total (Mean, ± SD).
Next, we evaluated recipient hematopoiesis at the same time points for which donor chimerism was assessed above (Days 4, 7 and 11). We examined the total number of recipient (B10.S) BM cells (KH49+) (Figure 3C) and total WBC counts (Figure 3D). The absolute number of recipient BM cells on Day 4 was significantly lower than on Days 7 and 11 in groups receiving or not receiving allogeneic BM cells, with or without CD4 depletion (Figure 5C), likely reflecting the transient myelosuppressive effect of TBI. Importantly, no differences between CD4-depleted (chimeric) mice and non-CD4-depleted (rejecting) mice were observed in the absolute number of recipient bone marrow cells, regardless of whether or not class I-deficient allogeneic marrow was administered. It should be noted that the percentage of CD4 cells in the BM was so low that CD4 T cell depletion by itself would not affect the absolute number of KH49+ cells. No differences in total WBC counts were detected between mice receiving treatment with or without allogeneic BMT (Figure 3D). We conclude from these observations that recipient hematopoiesis was not affected by rejection of MHC class I-deficient, allogeneic BMCs, suggesting that a circulating soluble factor was unlikely to be the effector mechanism of rejection.
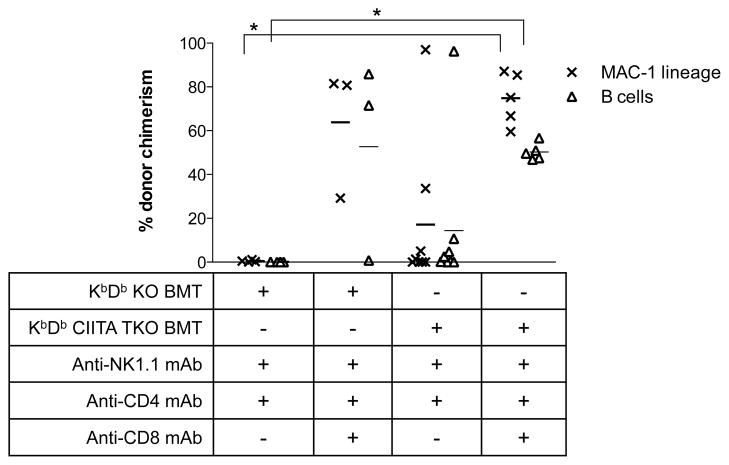
Figure 5. CD8 T cells do not reject MHC class I-deficient allogeneic BM via recognition of allogeneic MHC class II.
B10.S mice were given 3 Gy TBI on Day-1, anti-CD4 and anti-NK1.1 mAbs on Days −5, −1 and 5 and anti-CD8, 0.72 mg on each Days −5, −1 and 5 (when indicated in the table) prior to injection with 25×106 BM cells from the indicated donors. Donor chimerism in the MAC-1 and the B cell lineage 4 weeks after BMT is depicted. Each symbol represents an individual animal. Lines indicate mean values. *p<0.05 with a Kruskal-Wallis test followed by a Dunn’s multiple comparison test.
CD8 T cells do not reject MHC class I-deficient syngeneic BMCs
The apparent rejection of allogeneic marrow in vivo by indirectly alloreactive CD8 T cells (Figures 1 and 3) was surprising, so we considered possible alternative explanations. One possibility was that activating NK receptors, which have been reported to be expressed by subsets of repeatedly stimulated CD8 T cells (21) and by naïve cells after antigenic stimulation in vitro (22), might promote rejection of donor marrow in the absence of class I recognition by counterbalancing inhibitory NK cell receptors. Under such conditions, the “missing self” type of recognition used by NK cells to attack cells lacking sufficient inhibitory “self” MHC class I ligands could be applicable to CD8 T cells (23–25). To address the possibility that the rejection of MHC class I-deficient allogeneic BMCs was due to the lack of MHC class I expression, but not due to the presence of donor alloantigens. we transplanted syngeneic KbDb−/− B6 BM into CD45.1 WT B6 recipients. Recipients were depleted of CD4 T cells and NK cells, leaving only recipient CD8 T cells as possible mediators of rejection. Figure 4 shows that recipient CD8 T cells did not reject MHC class I-deficient syngeneic BM cells, demonstrating that alloantigens are required for the rejection of MHC class I-deficient allogeneic donor BMCs by recipient CD8 T cells. Therefore, our initial observation of rejection of MHC class I-deficient allogeneic BM by recipient CD8 T cells (Figure 1) is not simply due to “missing self” recognition.
Figure 4. “Missing-self” does not induce rejection of MHC class I-deficient, syngeneic BMCs by recipient CD8 T cells.
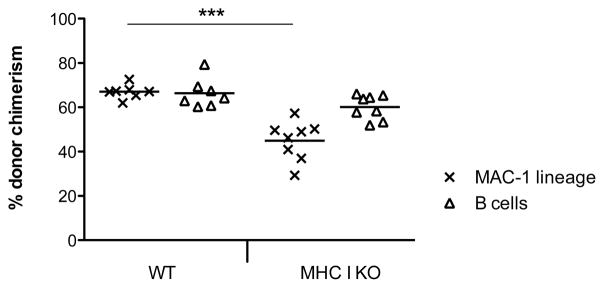
CD45.1 C57BL/6 mice were conditioned with 3 Gy TBI on Day −1 and received NK and CD4 depleting mAbs on Days −1 and 7. After the conditioning, mice received either 25×106 KbDb−/− C57BL/6 (MHC I KO) (n=8) or 25×106 CD45.2 C57BL/6 (WT) (n=7) BM cells. Donor chimerism in the MAC-1 and the B cell lineage is depicted 6 weeks after BMT. Each symbol represents an individual animal. Lines indicate mean values. ***p=0.0003 with a Mann Whitney test.
Rejection of class I-deficient marrow is not mediated by recognition of allogeneic MHC class II molecules by recipient CD8 T cells
Because MHC class II-restricted CD8 T cells have been described (26), we tested the possibility that rejection of MHC class I-deficient allogeneic BM by recipient CD8 T cells was due to recognition of allogeneic MHC class II. B10.S mice conditioned with 3 Gy TBI, anti-CD4 and anti-NK1.1 mAbs received either 25×106 KbDb−/− B6 BM cells or 25×106 KbDb CIITA triple KO (TKO), class I and II deficient, B6 BM cells, with or without CD8 T cell depletion. Two of 3 mice receiving KbDb−/− BM cells and conditioned with the full T cell depletion regimen achieved multilineage chimerism as expected (Figure 5), whereas 4 of 4 mice receiving only CD4 and NK1.1 depleting mAbs rejected the BMT. Five of five mice receiving KbDb CIITA TKO BM cells together with the full T cell depletion regimen also achieved chimerism, excluding a role for CD8−CD4− T cells in the rejection process. In contrast, 6 of 8 mice receiving KbDb CIITA TKO B6 BM cells without CD8 T cell depletion rejected the BM. Therefore, the rejection of class I-deficient marrow is not mediated by MHC class II-restricted directly alloreactive CD8 T cells. Moreover, these results suggest that donor MHC expression is not required for rejection of minor histocompatibility antigen-disparate allogeneic BMCs.
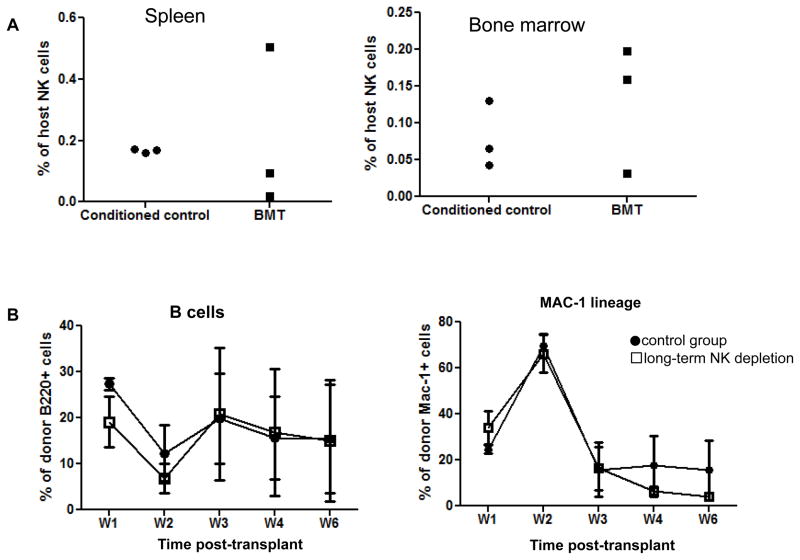
Recipient NK cells do not play a role in rejecting MHC class I-deficient BM
The above result demonstrating that activated recipient CD8 T cells specifically rejected class I-deficient allogeneic BMCs led us to consider the possibility that activated donor-reactive CD8 T cells promoted other cell types to kill the donor cells. As MHC class I-deficient donor BMCs are sensitive to NK cell-mediated killing, we investigated whether activated recipient CD8 T cells might promote rejection of MHC class I-deficient BMCs by resistant NK cells that escaped depletion with PK136 mAb. To this end, we first quantified NK cells in the spleen and bone marrow of mice rejecting the MHC class I-deficient BMCs. Eleven days post-BMT, NK cells in spleen and BM of B10.Q recipients accounted for only 0.2–0.5% of total cells (Figure 6A), making a role for NK cells in rejection unlikely. Furthermore, we investigated the impact of long-term NK cell depletion on the rejection of MHC class I-deficient BMCs. As shown in Figure 6B, long-term depletion of NK cells did not prevent the rejection of MHC class I-deficient BMCs. Therefore, the rejection of MHC class I-deficient BMCs in NK cell-depleted mice is NK cell-independent.
Figure 6. Recipient NK cells do not play a role in rejecting MHC class I-deficient BM.
In the experiment described in Figure 2, recipient NK cells were quantified by flow cytometry. B10.Q mice were conditioned with 3 Gy TBI and anti-CD4 mAb on Day −1, anti-NK1.1 mAb on Days −7 and −2, and anti-CD154 mAb on Day 0. Conditioned mice received 20×106 KbDb−/− TCD BM cells on Day 0. One group received PK136 (0.15mg/mouse) twice per week following transplantation of KbDb−/− TCD BM cells. (A) Percentages of PK136+ NK cells in the spleen and bone marrow. (B) The level of chimerism is shown for the B cell and the MAC-1 lineage at different time points post-transplantation of KbDb−/− TCD BM cells conditioned B10.S recipients that did or did not receive long term NK cell depletion. Data are presented as mean ± SEM. Each group contains 4–5 mice.
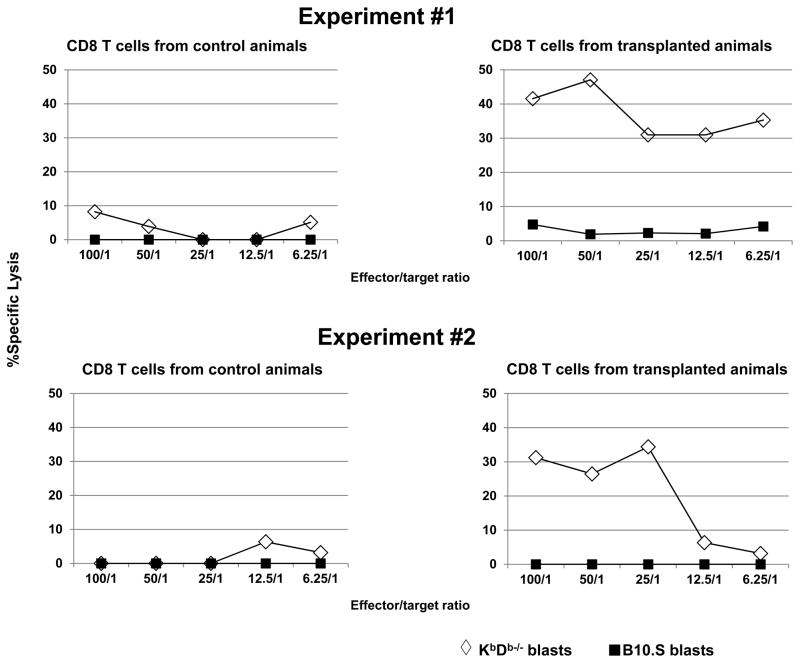
Cytotoxicity of recipient CD8 T cells against MHC class I-deficient BM
To directly assess whether activated recipient CD8 T cells could be cytotoxic effector cells that rejected KbDb−/− allogeneic donor BMCs, cytotoxicity of CD8 T cells from rejecting animals was studied in a direct cytotoxicity assay. Enriched CD8 T cells from conditioned animals that had not received KbDb−/− donor BMCs did not kill either KbDb−/− or syngeneic B10. S blasts. In contrast, CD8 T cells from conditioned, CD4 cell-depleted animals that rejected the KbDb−/− donor BMCs showed cytotoxicity against KbDb−/− blasts but not against syngeneic B10.S blasts (Figure 7). These data demonstrate that recipient CD8 T cells from animals rejecting MHC class I-deficient BM developed specific cytotoxicity against the KbDb−/− donor, strongly suggesting that recipient CD8 T cells are the effector cells that kill transplanted KbDb−/− donor BMCs in vivo.
Figure 7. Cytotoxicity of recipient CD8 T cells against MHC class I-deficient allogeneic BM.
B10.S mice were conditioned with 3 Gy TBI and anti-CD4 mAb on Day −1, anti-NK1.1 mAb on Days −7 and −2, and anti-CD154 mAb on Day 0 with or without (control) 20×106 KbDb−/− TCD BM cells on Day 0. On Day 8–9 post-transplant, 2–3 recipients in each group were euthanized and their splenocytes were pooled. Splenic CD8 T cells were then isolated by anti-CD8 microbeads. These CD8 T cells were cocultured with Cr51-labeled KbDb−/− and syngeneic B10.S ConA-blasts for 4 hours. Levels of Cr51 in the supernatant were then determined and percent specific lysis was calculated as described (20). Data from two experiments performed with similar results are shown.
Discussion
We demonstrate here that CD8 T cells can eliminate allogeneic marrow lacking MHC class I molecules in a highly specific manner that does not affect recipient hematopoiesis. Since syngeneic marrow lacking class I MHC was not rejected, this rejection mechanism is directed at alloantigens. Similar results were seen with heavy chain and β2-microglobuin-deficient donors, ruling out direct recognition of class Ib molecules. Rejection also does not require class II MHC expression by the donor and is independent of CD4 T cell-mediated help. Rejection of marrow lacking class I and class II antigens must therefore be directed against minor histocompatability differences between the donors and recipients. Our data suggest that CD8 T cells may specifically destroy cells that cannot intrinsically express the antigens they recognize via their TCR.
Our results are consistent with the possibility that rejection of class I-deficient marrow invovles “indirect” recognition by CD8 T cells and a CD8 T cell-mediated TCR-independent cytotoxic mechanism. Effector mechanisms of cellular destruction by CD8 T cells include direct cytotoxic attack and production of cytokines and other inflammatory mediators. Several cytokines produced by CD8 T cells, such as IFN-γ and TNFα, may be toxic to hematopoietic cells (27–30). Cytotoxic mechanisms lead to highly specific destruction of cells expressing target antigens, whereas cytokine-mediated destruction can theoretically affect non-antigen-bearing “bystander” cells. However, we found no impairment in recipient hematopoiesis during the process of allogeneic class I-deficient donor BM rejection by recipient CD8 T cells. The absence of a “bystander” effect on recipient hematopoiesis argues against a cytokine-mediated mechanism of rejection.
Indirectly alloreactive CD8 T cells have been previously implicated in the rejection of class I-deficient skin grafts (5, 7), and it was hypothesized that these T cells may destroy the recipient blood vessels supporting the skin allograft via cytotoxic mechanisms (7). Little is known about the microanatomy of engrafted allogeneic bone marrow, and it is possible that a similar mechanism might apply to marrow rejection by indirectly alloreactive CD8 T cells. Given the rich vascularity and sinusoidal structure of the marrow microenvironment, it seems possible that selective targeting of microvascular structures presenting donor antigens indirectly could result in specific destruction of donor cells in that micro-environment. However, transplanted hematopoietic stem cells (HSCs) migrate preferentially to the endosteal stem cell niche in irradiated mice (31), where they are immunoprotected by Tregs (32) and possibly other elements. While rejection in this scenario would more likely be directed at progenitor cells derived from these HSCs and spare HSCs in the endosteal niche, we found no evidence for persisting donor HSCs in adoptive transfer studies (supplementary Figure 1), suggesting that the class I-deficient HSCs were destroyed in a CD8 T cell-dependent process.
In contrast to the lack of evidence for cytokine-mediated marrow injury, we found evidence for direct cytotoxic destruction of donor BMCs. Recipient CD8 T cells demonstrated direct cytotoxicity against MHC class I-deficient donor cells in association with rejection of class I-deficient marrow, indicating that recipient CD8 T cells reject MHC class I-deficient BMC by directly killing them. NK cells were not required in the rejection. To our knowledge, our data are the first to demonstrate direct cytotoxicity against MHC class I-deficient allogeneic cells by CD8 T cells. Whether or not these are same cells recognizing and activated by cross-presented donor alloantigens is unclear.
CD8 T cells have been shown to express activating and inhibitory NK cell receptors that interact with MHC I molecules (see (33) for review) and may exhibit TCR-independent NK-cell-like activity following repeated stimulation (24, 25, 34). Such CD8 T cells would not receive an inhibitory signal from MHC class I-deficient donor BM via class I MHC-dependent inhibitory receptors, resulting in “specific” killing of the donor cells via a “missing self” type of recognition (33) that would leave recipient hematopoiesis unaffected. Although our data show that recipient CD8 T cells did not destroy MHC class I-deficient syngeneic BMCs, we hypothesize that cytotoxicity against allogeneic MHC class I-deficient donor cells NK cell receptors may be induced by activation of indirectly alloreactive CD8 T cells. In a parent-to-F1 bone marrow transplantation model, expression of NKG2D ligands was only detected on BMCs undergoing NK cell-mediated rejection and not on BMCs in syngeneic recipients (35). Likewise, the lack of rejection of MHC class I-deficient syngeneic bone marrow cells in our study (Figure 4) may reflect the absence of induced expression of ligands for NK cell receptors when there is no alloresponse. When MHC class I-deficient BMCs are transplanted to allogeneic recipients, the alloresponses mounted by the indirectly alloreactive recipient CD8 T cells may upregulate the expression of ligands for activating NK cell receptors on donor BMCs. These receptors may be selectively upregulated on activated indirectly alloreactive recipient CD8 T cells.
In conclusion, we demonstrate here the surprising existence of a highly specific marrow rejection process mediated by alloreactive CD8 T cells in the absence of class I ligand expression by the donor cells. This observation raises intriguing questions about allogeneic marrow rejection that warrant further investigation.
Supplementary Material
Acknowledgments
We thank Ms. Shavree Washington for expert assistance with the manuscript and Drs. Donna Farber, Gilles Benichou and Josef Kurtz for helpful comments on the paper. This work was supported by National Institutes of Health Grants R01 HL49915 and P01 HL18646. F.H. was supported by the Fondation pour la Recherche Médicale (France), and the American Society of Transplantation/American Society of Blood and Marrow Transplantation (USA). T.F. was supported by the Swiss Foundation for Medical and Biological Grants/Novartis Switzerland and the Walter and Gertrud Siegenthaler Foundation (University of Zurich). C.L.L. was supported by Government funds awarded by DoD, Air Force Office of Scientific Research, National Defense Science and Engineering Graduate (NDSEG) Fellowship, 32 CFR 168a.
Abbreviations
- BMT
bone marrow transplantation
- BM
bone marrow
- BMCs
bone marrow cells
- APC
antigen presenting cell
- CTL
cytotoxic T lymphocyte
- TBI
total body irradiation
- TKO
triple KO
- TCD
T cell depleted
- Ab
antibody
Footnotes
Disclosure: The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.Liu Z, Sun YK, Xi YP, Maffei A, Reed E, Harris P, et al. Contribution of direct and indirect recognition pathways to T cell alloreactivity. J Exp Med. 1993;177(6):1643–1650. doi: 10.1084/jem.177.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boisgérault F, Liu Y, Anosova N, Ehrlich E, Dana MR, Benichou G. Role of CD4+ and CD8+ T cells in allorecognition: lessons from corneal transplantation. J Immunol. 2001;167(4):1891–1899. doi: 10.4049/jimmunol.167.4.1891. [DOI] [PubMed] [Google Scholar]
- 3.Braun YM, McCormack A, Webb G, Batchelor JR. Mediation of acute but not chronic rejection of MHC-incompatible rat kidney grafts by alloreactive CD4 T cells activated by the direct pathway of sensitization. Transplantation. 1993;55(1):177–182. doi: 10.1097/00007890-199301000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Popov IA, Fedoseyeva EV, Orr PL, Garovoy MR, Benichou G. Direct evidence for in vivo induction of CD8+ cytotoxic T cells directed to donor MHC class I peptides following mouse allotransplantation. Transplantation. 1995;60(12):1621–1624. [PubMed] [Google Scholar]
- 5.Valujskikh A, Lantz O, Celli S, Matzinger P, Heeger PS. Cross-primed CD8+ T cells mediate graft rejection via a distinct effector pathway. Nat Immunol. 2002;3(9):844–851. doi: 10.1038/ni831. [DOI] [PubMed] [Google Scholar]
- 6.Bradley JA. A roundabout route to rejection: the contribution of cross-primed CD8 T cells. Am J Transplant. 2004;4(5):675–677. doi: 10.1111/j.1600-6143.2004.00452.x. [DOI] [PubMed] [Google Scholar]
- 7.He C, Heeger PS. CD8 T cells can reject major histocompatibility complex class I-deficient skin allografts. Am J Transplant. 2004;4(5):698–704. doi: 10.1111/j.1600-6143.2004.00416.x. [DOI] [PubMed] [Google Scholar]
- 8.Bishop DK, Wood SC, Eichwald EJ, Orosz CG. Immunobiology of allograft rejection in the absence of IFN-gamma: CD8+ effector cells develop independently of CD4+ cells and CD40-CD40 ligand interactions. J Immunol. 2001;166(5):3248–3255. doi: 10.4049/jimmunol.166.5.3248. [DOI] [PubMed] [Google Scholar]
- 9.Fudaba Y, Spitzer TR, Shaffer J, Kawai T, Fehr T, Delmonico F, et al. Myeloma responses and tolerance following combined kidney and nonmyeloablative marrow transplantation: in vivo and in vitro analyses. Am J Transplant. 2006;6(9):2121–2133. doi: 10.1111/j.1600-6143.2006.01434.x. [DOI] [PubMed] [Google Scholar]
- 10.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358(4):353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358(4):362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 12.Takeuchi Y, Ito H, Kurtz J, Wekerle T, Ho L, Sykes M. Earlier low-dose TBI or DST overcomes CD8+ T-cell-mediated alloresistance to allogeneic marrow in recipients of anti-CD40L. Am J Transplant. 2004;4(1):31–40. doi: 10.1046/j.1600-6135.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 13.Wekerle T, Kurtz J, Ito H, Ronquillo JV, Dong V, Zhao G, et al. Allogeneic bone marrow transplantation with co-stimulatory blockade induces macrochimerism and tolerance without cytoreductive host treatment. Nat Med. 2000;6(4):464–469. doi: 10.1038/74731. [DOI] [PubMed] [Google Scholar]
- 14.Wekerle T, Sayegh MH, Hill J, Zhao Y, Chandraker A, Swenson KG, et al. Extrathymic T cell deletion and allogeneic stem cell engraftment induced with costimulatory blockade is followed by central T cell tolerance. J Exp Med. 1998;187(12):2037–2044. doi: 10.1084/jem.187.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol. 2008;181(1):165–173. doi: 10.4049/jimmunol.181.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehr T, Takeuchi Y, Kurtz J, Wekerle T, Sykes M. Early regulation of CD8 T cell alloreactivity by CD4+CD25− T cells in recipients of anti-CD154 antibody and allogeneic BMT is followed by rapid peripheral deletion of donor-reactive CD8+ T cells, precluding a role for sustained regulation. Eur J Immunol. 2005;35(9):2679–2690. doi: 10.1002/eji.200526190. [DOI] [PubMed] [Google Scholar]
- 17.Bix M, Liao N-S, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349(6307):329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 18.Kiessling R, Hochman PS, Haller O, Shearer GM, Wigzell H, Cudkowicz G. Evidence for a similar or common mechanism for natural killer cell activity and resistance to hemopoietic grafts. Eur J Immunol. 1977;7(9):655–663. doi: 10.1002/eji.1830070915. [DOI] [PubMed] [Google Scholar]
- 19.Tomita Y, Sachs DH, Khan A, Sykes M. Additional monoclonal antibody (mAB) injections can replace thymic irradiation to allow induction of mixed chimerism and tolerance in mice receiving bone marrow transplantation after conditioning with anti-T cell mABs and 3-Gy whole body irradiation. Transplantation. 1996;61(3):469–477. doi: 10.1097/00007890-199602150-00027. [DOI] [PubMed] [Google Scholar]
- 20.Ito H, Kurtz J, Shaffer J, Sykes M. CD4 T cell-mediated alloresistance to fully MHC-mismatched allogeneic bone marrow engraftment is dependent on CD40-CD40 ligand interactions, and lasting T cell tolerance is induced by bone marrow transplantation with initial blockade of this pathway. J Immunol. 2001;166(5):2970–2981. doi: 10.4049/jimmunol.166.5.2970. [DOI] [PubMed] [Google Scholar]
- 21.Thimme R, Appay V, Koschella M, Panther E, Roth E, Hislop AD, et al. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79(18):12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMahon CW, Raulet DH. Expression and function of NK cell receptors in CD8+ T cells. Curr Opin Immunol. 2001;13(4):465–470. doi: 10.1016/s0952-7915(00)00242-9. [DOI] [PubMed] [Google Scholar]
- 23.Dhanji S, Teh S-J, Oble D, Priatel JJ, Teh H-S. Self-reactive memory-phenotype CD8 T cells exhibit both MHC-restricted and non-MHC-restricted cytotoxicity: a role for the T-cell receptor and natural killer cell receptors. Blood. 2004;104(7):2116–2123. doi: 10.1182/blood-2004-01-0150. [DOI] [PubMed] [Google Scholar]
- 24.Merck E, Voyle RB, MacDonald HR. Ly49D engagement on T lymphocytes induces TCR-independent activation and CD8 effector functions that control tumor growth. J Immunol. 2009;182(1):183–192. doi: 10.4049/jimmunol.182.1.183. [DOI] [PubMed] [Google Scholar]
- 25.Meresse B, Chen Z, Ciszewski C, Tretiakova M, Bhagat G, Krausz TN, et al. Coordinated induction by IL15 of a TCR-independent NKG2D signaling pathway converts CTL into lymphokine-activated killer cells in celiac disease. Immunity. 2004;21(3):357–366. doi: 10.1016/j.immuni.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 26.Pearce EL, Shedlock DJ, Shen H. Functional characterization of MHC class II-restricted CD8+CD4− and CD8−CD4− T cell responses to infection in CD4−/− mice. J Immunol. 2004;173(4):2494–2499. doi: 10.4049/jimmunol.173.4.2494. [DOI] [PubMed] [Google Scholar]
- 27.Johnson R, Waddelow T, Caro J, Oliff A, Roodman G. Chronic exposure to tumor necrosis factor in vivo preferentially inhibits erythropoiesis in nude mice. Blood. 1989;74(1):130–138. [PubMed] [Google Scholar]
- 28.Pronk CJH, Veiby OP, Bryder D, Jacobsen SEW. Tumor necrosis factor restricts hematopoietic stem cell activity in mice: involvement of two distinct receptors. J Exp Med. 2011;208(8):1563–1570. doi: 10.1084/jem.20110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Selleri C, Sato T, Anderson S, Young NS, Maciejewski JP. Interferon-γ and tumor necrosis factor-α suppress both early and late stages of hematopoiesis and induce programmed cell death. J Cell Physiol. 1995;165(3):538–546. doi: 10.1002/jcp.1041650312. [DOI] [PubMed] [Google Scholar]
- 30.Talmadge JE, Bowersox O, Tribble H, Lee SH, Shepard HM, Liggitt D. Toxicity of tumor necrosis factor is synergistic with gamma-interferon and can be reduced with cyclooxygenase inhibitors. Am J Pathol. 1987;128(3):410–425. [PMC free article] [PubMed] [Google Scholar]
- 31.Xie Y, Yin T, Wiegraebe W, He XC, Miller D, Stark D, et al. Detection of functional haematopoietic stem cell niche using real-time imaging. Nature. 2009;457(7225):97–101. doi: 10.1038/nature07639. [DOI] [PubMed] [Google Scholar]
- 32.Fujisaki J, Wu J, Carlson AL, Silberstein L, Putheti P, Larocca R, et al. In vivo imaging of Treg cells providing immune privilege to the haematopoietic stem-cell niche. Nature. 2011;474(7350):216–219. doi: 10.1038/nature10160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vivier E, Anfossi N. Inhibitory NK-cell receptors on T cells: witness of the past, actors of the future. Nat Rev Immunol. 2004;4(3):190–198. doi: 10.1038/nri1306. [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Bai F, Sokol L, Zhou J, Ren A, Painter JS, et al. A critical role for DAP10 and DAP12 in CD8+ T cell–mediated tissue damage in large granular lymphocyte leukemia. Blood. 2009;113(14):3226–3234. doi: 10.1182/blood-2008-07-168245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara K, Benjamin J, Takaki R, Phillips JH, Lanier LL. Function of NKG2D in natural killer cell-mediated rejection of mouse bone marrow grafts. Nat Immunol. 2005;6(9):938–945. doi: 10.1038/ni1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.