Abstract
Object
In this study the role of magnetic source imaging for preoperative motor mapping was evaluated by using a single-dipole localization method to analyze motor field data in 41 patients.
Methods
Data from affected and unaffected hemispheres were collected in patients performing voluntary finger flexion movements. Somatosensory evoked field (SSEF) data were also obtained using tactile stimulation. Dipole localization using motor field (MF) data was successful in only 49% of patients, whereas localization with movement evoked field (MEF) data was successful in 66% of patients. When the spatial distribution of MF and MEF dipoles in relation to SSEF dipoles was analyzed, the motor dipoles were not spatially distinct from somatosensory dipoles.
Conclusions
The findings in this study suggest that single-dipole localization for the analysis of motor data is not sufficiently sensitive and is nonspecific, and thus not clinically useful.
Keywords: magnetoencephalography, brain mapping, motor field, movement-evoked field, functional localization
The preoperative localization of functionally viable brain tissue helps to guide neurosurgical planning in optimizing the region of resection while allowing for improved postsurgical neurological function. Various neuroimaging techniques, including MS and fMR imaging, are now available to preoperatively map functional brain organization. Coregistering structural MR imaging data to functional data acquired via MS or fMR imaging allows for the intraoperative creation of a neuronavigation system.9,15,17
Magnetic source imaging has been shown to be increasingly important in preoperative planning and complements intraoperative mapping by delineating retained areas of function noninvasively and in advance, and thus reducing the time needed for intraoperative procedures. Peaks in evoked neuromagnetic field data are used to localize dipoles with the aid of source modeling algorithms. Utilizing MS imaging, relevant somatosensory, speech, and motor cortices can be mapped preoperatively to aid surgical navigation and avoid resecting the eloquent cortex. Alternatively, fMR imaging can be used in the preoperative planning, but its temporal resolution is inferior to that of MS imaging because its measured responses are not as directly correlated with evoked electrical activity. Both fMR and MS imaging have been validated as adjuncts to intraoperative mapping. 4,6,7,9,16,20,22 With the increasing use of MS imaging at clinical centers worldwide, the accuracy of functional localization has become paramount.
The utility of neuromagnetic somatosensory, auditory, and speech mapping for preoperative tumor localization has been validated previously.5,8,21,24–26 More recently, neuromagnetic MEFs have been shown to be useful in mapping motor cortex by using a single–equivalent current dipole model.19 Here, we examine the role of single–equivalent current dipole modeling of pre- and postmovement evoked responses. 3,11,13,28,29 The premovement evoked response typically peaks before the onset of movement, presumably arising from motor cortex, and this peak is referred to as the “MF.” Postmovement onset, the earliest peak activation, is referred to as the “MEF” and is presumed to reflect the sum of activity arising from motor and somatosensory cortices given the presence of both efferent activity and sensory feedback during movement. The goal of this study was to evaluate the sensitivity and specificity of MF and MEF localization, with respect to the location of somatosensory cortex, for the purpose of preoperative localization of hand motor cortex.
Clinical Material and Methods
Patient Population
We retrospectively analyzed the MS imaging data sets from 41 treated patients who had undergone surgery for intraaxial brain tumors between 2001 and 2003 (Table 1). Many of the patients were referred to the Department of Neurosurgery at the University of California, San Francisco, because of the location of the tumors near the eloquent cortex, thus prompting preoperative mapping procedures.
TABLE 1.
Clinical and radiological features in 41 patients with brain tumors
| Variable | No. (%) |
|---|---|
| affected hemisphere | |
| rt | 20 (49) |
| lt | 21 (51) |
| affected lobe | |
| frontal | 17 (41) |
| temporal | 9 (22) |
| parietal | 9 (22) |
| insular | 6 (15) |
| diagnosis | |
| astrocytoma | 19 (46) |
| glioblastoma multiforme | 9 (22) |
| oligodendroglioma | 6 (15) |
| oligoastrocytoma | 3 (7) |
| ganglioglioma | 2 (5) |
| metastasis | 1 (2) |
| glioneuronal hamartosis | 1 (2) |
Data Acquisition
Magnetic fields were recorded in a shielded room by using two 37-channel biomagnetometers with superconducting quantum interference device–based first-order gradiometer sensors (Magnes II; 4-D Neuroimaging, San Diego, CA). The sensor layout covered a circular area 144 mm in diameter. External fiducial markers (left and right preauricular points and the nasion) were recorded in reference to the sensory array. The localization error of the digitizer was less than 2 mm within 30 cm of the probe.
Somatosensory Task
Unilateral somatosensory stimulation was performed using painless pneumatic stimulation (17 psi pressure) applied as 30-msec pulses via balloon-diaphragm clips attached to the digits of both hands and the lips. Of key comparative interest was the stimulation of the distal finger pads of the affected hands. Somatosensory evoked responses were recorded at a sampling rate of 520.8 Hz and a bandwidth of 200 Hz with a high-pass filter (cutoff 1 Hz). The data epoch duration was 300 msec, including a 150-msec prestimulus baseline sample. At least 200 data epochs were recorded for each stimulus type (the interstimulus interval was randomly varied in the range of 450–550 msec), and recordings were averaged, time-locked to the stimulus onset. Finally, the averaged evoked response epoch was digitally filtered (passband 8–40 Hz) to eliminate high-frequency noise contributions. The peak of the primary somatosensory evoked response was defined as that peak in the evoked field amplitude (root mean square averaged across sensor channels) occurring in the latency range from 30 to 70 msec of the poststimulus onset and satisfying a model–data correlation threshold (r) of 0.97.
Motor Task
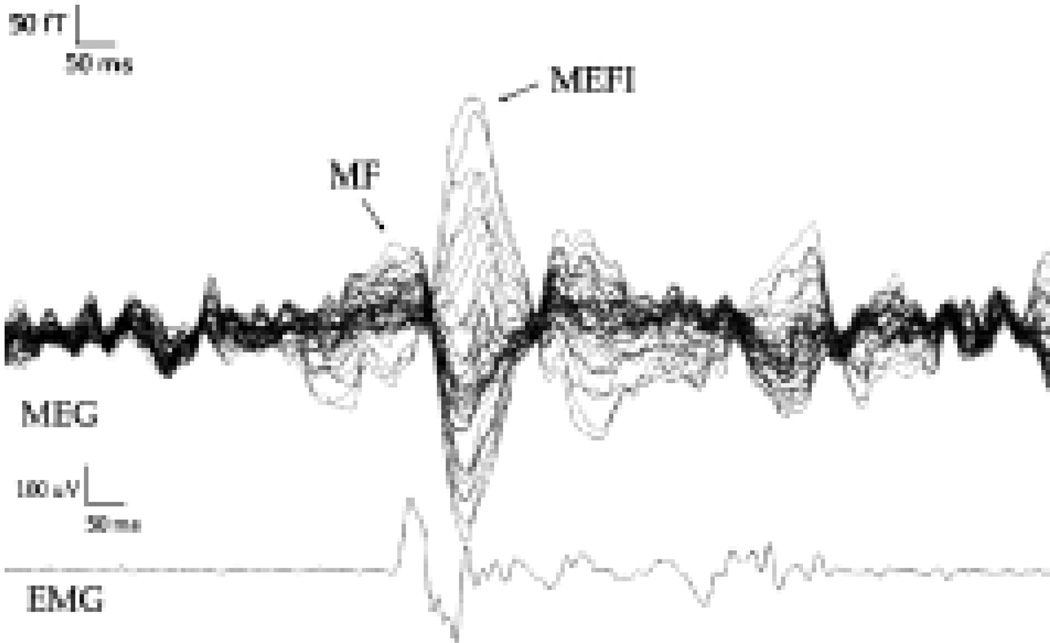
Unilateral MFs were recorded while patients performed self-paced flexion and extension movements of the involved fingers once every 3 to 4 seconds. Trials with excessive eye movements or other muscle artifacts not associated with the task were excluded. One hundred to 150 epochs were recorded for each patient. The onset of movement was manually marked for each trial by noting the EMG responses, and the magnetic fields were averaged on movement onset. The magnetic fields associated with movement have been well described.12 The most stable field has the highest amplitude of all movement fields and a latency of 100 msec after movement onset and has been labeled MEF I; this field is related to sensory input from the periphery.2 The MF occurs at the onset of movement as correlated with the onset of EMG activity and represents the corticospinal outflow. We focused on these fields in our analysis. The MF peak was defined as that peak in the evoked field amplitude (root mean square averaged across sensor channels) occurring in the latency range of 30 msec near EMG stimulus onset and satisfying a model–data correlation threshold greater than 0.95. The MEF was defined as the greatest peak occurring in the latency range of 100 msec after stimulus onset and satisfying a model–data correlation greater than 0.95. A sample waveform is demonstrated in Fig. 1.
Fig. 1.
Sample waveform showing traces obtained in a patient performing right index finger flexion.
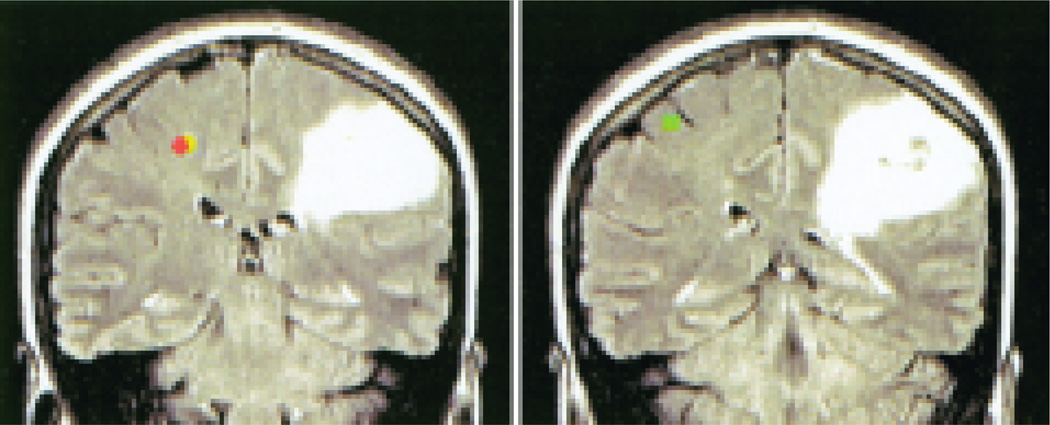
A single–equivalent current dipole model was calculated separately for each hemisphere and for each poststimulus time point, by using standard 4-D Neuroimaging software to analyze data that had been filtered between 1 and 40 Hz. The localization algorithm included an iterative least-squares minimization to compute the strength and location of a single dipole in a spherical volume of uniform conductivity that could account for the sensory and motor data. Dipole fits were accepted based on a local correlation maximum criteria of 0.95 and goodness-of-fit values greater than 0.95. The MF, MEF, and SSEF dipoles were mapped onto individual high-resolution MR images by using predefined anatomical landmarks. Sample images are featured in Fig. 2.
Fig. 2.
Coronal MS images, posterior (left) and anterior (right) views, demonstrating somatosensory and motor source localizations derived from MEG data recorded during painless tactile stimulation of the right index finger (SSEF) and self-paced index finger flexion (MF and MEF). Data are shown for the unaffected hemisphere. Orange dot represents MEF; yellow dot, MF; and green dot, SSEF.
Results
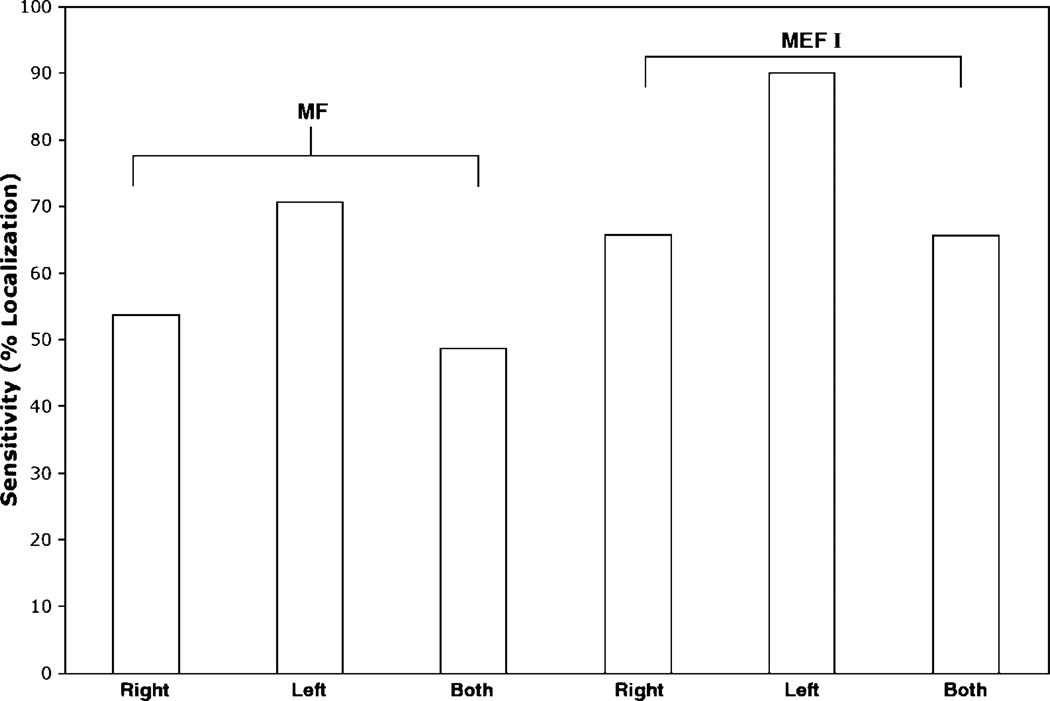
Data were collected from 41 different patients, who all were able to complete the specified movement. A set of data for a given patient was considered complete if both right- and left-hemisphere motor data were analyzable. Of these patients, only 20 had interpretable MF data sets that could be used for preoperative mapping. Successful dipole localization referred to a single dipole that met the fit criteria regardless of the validity of the localization in the three-dimensional space. Only 27 patients had MEF data sets suitable for preoperative mapping. In general, MF data were less reliable than MEF data for dipole localization. One hundred percent of the patients had a localizable SSEF dipole. Overall, there was a 49% sensitivity rate for MF data and a 66% sensitivity rate for MEF data. The sensitivity of MF data for dipole localization is shown in Table 2 and Fig. 3. For each condition dipole localization using the specified selection criteria was consistently more successful using the MEF data compared with MF data. Localization was not less successful in the affected side compared with the unaffected side.
TABLE 2.
Sensitivity of MF and MEF for dipole localization in 41 patients*
| Hemisphere |
|||||
|---|---|---|---|---|---|
| Field | Rt | Lt | Both | Unaffected | Affected |
| MF | 22 (53.7) | 29 (70.7) | 20 (48.8) | 25 (61.0) | 26 (63.4) |
| MEF | 27 (65.9) | 37 (90.2) | 27 (65.9) | 30 (73.2) | 34 (82.9) |
Values represent the number of patients (%) with successful dipole localizations.
Fig. 3.
Graph showing the sensitivity of MF and MEF I data for the right, left, and both hemispheres.
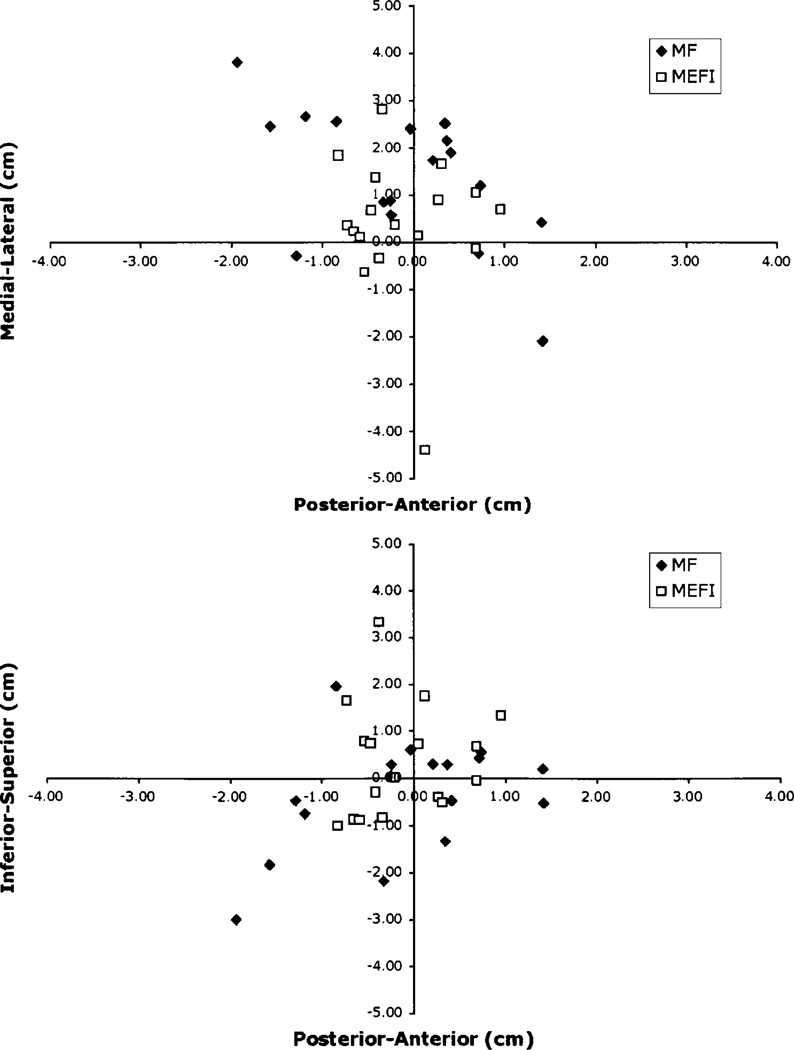
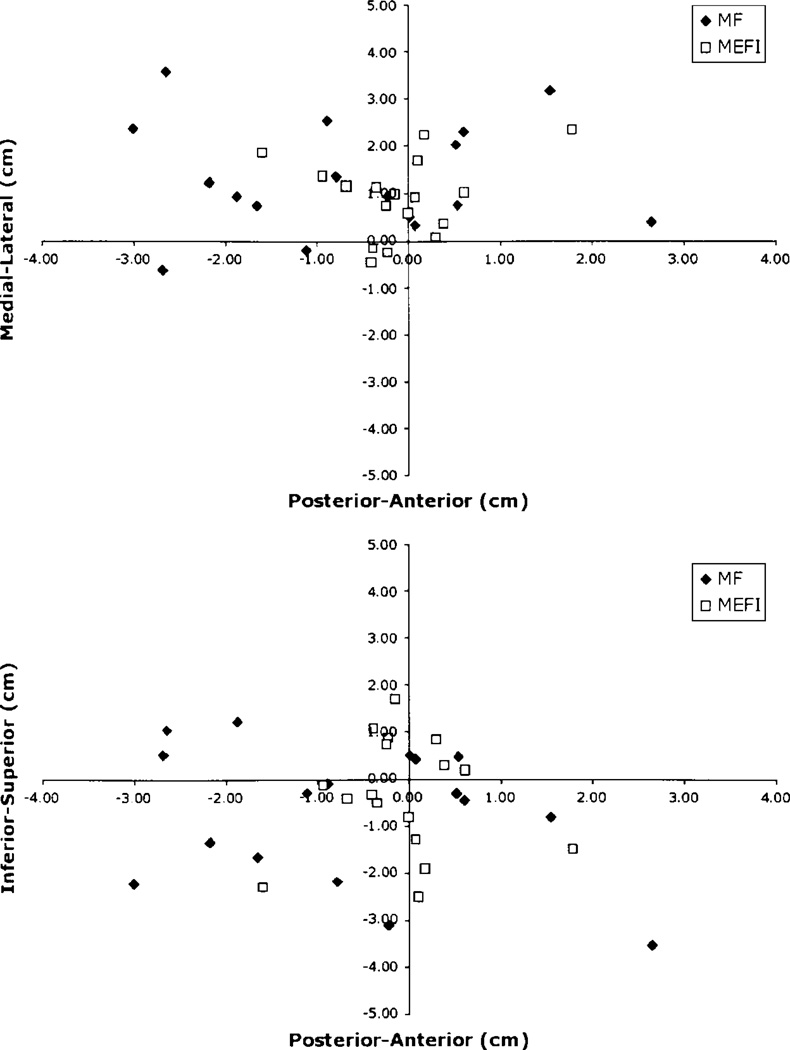
Only 17 patients (41%) had complete sets of MF and MEF data for both hemispheres. These patients underwent further analysis to determine the spatial distribution of the respective somatosensory and motor dipoles. The cartesian coordinates for the dipole localizations for the MF, MEF, and SSEF were obtained from the respective data sets. Using the SSEF as the origin, the anteroposterior, mediolateral, and inferosuperior distances were calculated for the MEF and MF dipoles by subtracting the x-, y-, and z-coordinates. The distribution of distances from the SSEF dipoles for both the unaffected and affected hemispheres are shown in Figs. 4 and 5.
Fig. 4.
Graphs depicting the location of MF and MEF I dipoles relative to SSEF dipoles in the unaffected hemisphere in individual patients with tumors.
Fig. 5.
Graphs illustrating the location of MF and MEF I dipoles relative to SSEF dipoles in the affected hemisphere in individual patients with tumors.
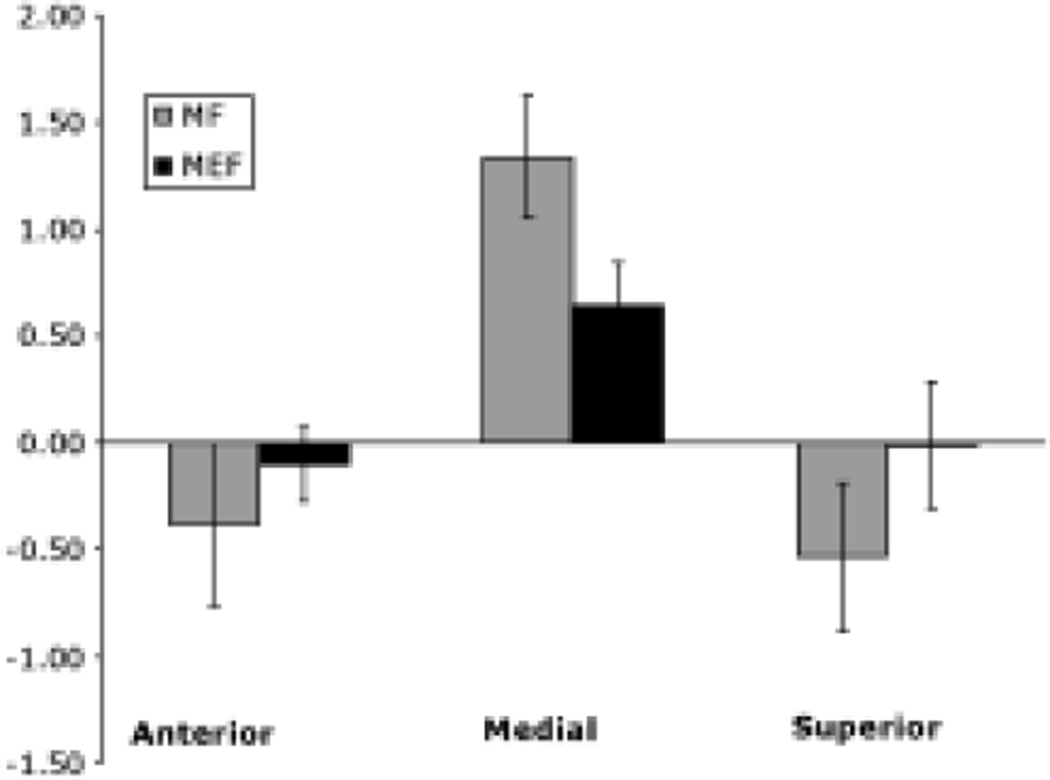
The average anteroposterior, mediolateral, and inferosuperior distances were compared between unaffected and affected hemispheres using paired t-tests. There were no statistically significant differences between the distances measured for the MF and MEF dipoles. Furthermore, the mean distance between the MF and SSEF for both hemispheres was not significantly different from 0. The average distances are shown in Fig. 6. Anteroposterior and inferosuperior distances were also not significantly different from 0 for either the MF or the MEF. In contrast, mediolateral distances were significantly different from 0 (p < 0.05) for both the MF and MEF.
Fig. 6.
Bar graph depicting the mean distances (± standard error of the mean) between SSEF, and MF and MEF I dipoles. Distances were not significantly different between affected and unaffected sides. Only mediolateral distances were significantly greater than 0 (p < 0.05).
Discussion
Motor field and MEF data are not sensitive enough for preoperative functional localization. Using the dual 74- channel MEG system (4-D Neuroimaging, Inc.), only 41% of the patients with tumors had a complete set of analyzable data allowing for successful dipole localization and mapping. There was a great deal of variability in the location of the MF and MEF dipoles in individual patients. In the affected hemisphere distances between localized dipoles for MF, SSEF, and MEF were greater than those in the corresponding unaffected hemisphere. A similar result was seen for MEF and SSEF dipole distances, suggesting that the presence of the tumor does not distort the relative location and distances between somatosensory and motor cortex. Note, however, that overall distances between MF and SSEF dipoles were not significantly different from 0, reflecting the inability of the method to distinguish these localizations. Similarly, distances between MEF and SSEF dipoles were not different from 0.
Motor field data typically are not as robust as MEF data, and authors of several studies have reported an inability to obtain data sufficient for dipole localization.3,11,13,28 Using a moving dipole model, Kristeva-Feige, et al.,13 demonstrated that the MF was often not observed in healthy persons, whereas MEF I dipole localization was more consistent. In another study the MEF dipole localization was unsuccessful in up to 21% of patients.11 These results were consistent with our findings; that is, the MF data were much less successful in dipole localization than the MEF data. In our study, localization using the MEF was more consistent in both affected and unaffected hemispheres. The robustness of MEF is attributable to the higher amplitude of the peak, which allows for easy identification and subsequent source localization given the higher signal-to-noise ratio. However, the MEF is more likely to reflect sensory activation from movement itself and is presumably derived from a source other than the motor cortex. The MF peaks are lower in amplitude and can be obscured by background noise. Furthermore, the sources responsible for the MF may be complex and bilateral and therefore not elucidated by a single-dipole model.
Several recent reports have documented the efficacy of MF dipole mapping in localizing the motor cortex in patients with tumors.1,19 Oishi and colleagues19 studied 14 patients harboring brain tumors by using MEG. In 11 of these 14 patients, an equivalent current dipole model could be applied to identify the motor cortex by using the MF; data could not be obtained in the other three patients. These authors also discussed a comparison with intraoperative stimulation, which was found to correlate with preoperative mapping. Castillo and associates1 studied six patients with brain tumors by using a combined sensorimotor paradigm and equivalent current dipole model to localize the motor cortex with the aid of MEF data. Our study includes a larger population of patients, and our results show the relatively low sensitivity of MF data in localizing motor cortex.
The spatial localizations of the MF and MEF dipoles were not distinguishable from the SSEF dipoles. Comparing anteroposterior, mediolateral, and inferosuperior distances showed that the distance from the SSEF for the MF and MEF dipoles was not significantly different from 0. Despite a significant difference from 0, the mediolateral distance showed a standard deviation of approximately 30 mm, which was consistent with the large variability. Localization uncertainty based on the registration of MS images to MR images has been reported to be 4 mm.10 Authors of previous studies have reported a standard deviation of 5 mm in 100 test-retest MS imaging measurements.6
Magnetoencephalography is an evolving technology. In the present study we utilized the dual 37-channel Magnes biomagnetometer. In previous MEG-based studies of preoperative localization, investigators have used similar magnetometers with sensor arrays containing from 37 to 204 channels.1,3–6,19,22,24,25,27 Newer models such as whole-head MEG systems combined with advanced analysis methods provide a higher spatial sampling density, which may improve the accuracy of source localization beyond dipole fitting. Nonetheless, the motor cortex in the present study was adequately covered with a high density of sensors. Concurrent advances in source localization with new dipole fitting methods could also improve results.
Other measures for preoperative motor cortex localization include fMR imaging, positron emission tomography, and diffusion tensor imaging. These modalities are more complementary than individually definitive because of problems with spatial or temporal sensitivity. Furthermore, their reliance on blood flow and metabolism, both factors that could be influenced by tumor, makes these methods suboptimal. Magnetoencephalography provides superior temporal resolution, but using single-dipole localization with MS imaging to identify functional motor cortex may not be clinically useful. Corticomuscular coherence utilizing MEG-EMG and electrocorticography-EMG has been a successful method of identifying the motor cortex and central sulcus.18,23 Recently, spatially filtered MEG was performed to explore functional activation of areas important in motor control around the central sulcus.27 In that study, MEG data were analyzed using synthetic aperture magnetometry to determine power changes in various frequency bands (event-related desynchronization). Beta event-related desynchronization was shown to localize to the contralateral sensorimotor cortex in control volunteers, whereas activation localized to the ipsilateral hemisphere of the affected hand in patients with tumors, suggesting that this paradigm may be useful in identifying not only regions of residually active cortex in the contralateral hemisphere, but also compensatory regions recruited in the ipsilateral hemisphere. However, the sensitivity and specificity of this procedure are currently unclear.
There are several potential explanations for inaccurate dipole localization beyond the limitations of source modeling. Tumor-associated edema in the affected hemisphere could lead to disturbed cortical function activity and a shift in the localization compared with the unaffected hemisphere. Tumor-induced cortical plasticity could also be a mechanism for shifting dipole localization because of motor function takeover by surrounding cortical tissue.14
The low sensitivity in the localization of MF using a single- dipole method may be a result of the distributed nature of motor cortical function. Furthermore, inaccuracies in dipole localization via this method demonstrate the inadequate specificity of motor mapping. The variability in MF data may limit its usefulness in preoperative mapping, restricting the use of MS imaging to somatosensory and auditory modalities only. A more robust source localization paradigm may be necessary for accurate preoperative motor mapping.
Conclusions
Magnetic source imaging of motor cortical activity using single–equivalent current dipole modeling methods may not be sensitive or specific enough to be clinically useful. A more robust localization paradigm may be needed to identify functionally active motor cortex for preoperative purposes.
Acknowledgments
We are indebted to Heather Graham, Suzanne Honma, Anne Findlay, and Mary Mantle for excellent technical assistance and to Timothy Roberts for help with data collection.
This work was supported by the Dana Foundation and National Institutes of Health R01 Grant No. DC004855 (S.S.N.).
Abbreviations used in this paper
- EMG
electromyography
- fMR
functional magnetic resonance
- MEF
movement-evoked field
- MEG
magnetoencephalography
- MF
motor field
- MR
magnetic resonance
- MS
magnetic source
- SSEF
somatosensory evoked field
References
- 1.Castillo EM, Simos PG, Wheless JW, Baumgartner JE, Breier JI, Billingsley RL, et al. Integrating sensory and motor mapping in a comprehensive MEG protocol: clinical validity and replicability. Neuroimage. 2004;21:973–983. doi: 10.1016/j.neuroimage.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 2.Deecke L, Weinberg H, Brickett P. Magnetic fields of the human brain accompanying voluntary movement: Bereitschaftsmagnetfeld. Exp Brain Res. 1982;48:144–148. doi: 10.1007/BF00239582. [DOI] [PubMed] [Google Scholar]
- 3.Ebmeier K, Haberland N, Steenbeck J, Hochstetter A, Kalff R. Neuronavigation and magnetic source imaging in brain tumors. Front Radiat Ther Oncol. 1999;33:78–87. doi: 10.1159/000061224. [DOI] [PubMed] [Google Scholar]
- 4.Gallen CC, Schwartz BJ, Bucholz RD, Malik G, Barkley GL, Smith J, et al. Presurgical localization of functional cortex using magnetic source imaging. J Neurosurg. 1995;82:988–994. doi: 10.3171/jns.1995.82.6.0988. [DOI] [PubMed] [Google Scholar]
- 5.Ganslandt O, Buchfelder M, Hastreiter P, Grummich P, Fahlbusch R, Nimsky C. Magnetic source imaging supports clinical decision making in glioma patients. Clin Neurol Neurosurg. 2004;107:20–26. doi: 10.1016/j.clineuro.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 6.Ganslandt O, Steinmeier R, Kober H, Vieth J, Kassubek J, Romstock J, et al. Magnetic source imaging combined with image-guided frameless stereotaxy. A new method in surgery around the motor strip. Neurosurgery. 1997;41:621–628. doi: 10.1097/00006123-199709000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Inoue T, Shimizu H, Nakasato N, Kumabe T, Yoshimoto T. Accuracy and limitation of functional magnetic resonance imaging for identification of the central sulcus: comparison with magnetoencephalography in patients with brain tumors. Neuroimage. 1999;10:738–748. doi: 10.1006/nimg.1999.0501. [DOI] [PubMed] [Google Scholar]
- 8.Ishibashi H, Morioka T, Nishio S, Shigeto H, Yamamoto T, Fukui M. Magnetoencephalographic investigation of somatosensory homunculus in patients with peri-Rolandic tumors. Neurol Res. 2001;23:29–38. doi: 10.1179/016164101101198253. [DOI] [PubMed] [Google Scholar]
- 9.Kamada K, Takeuchi F, Kuriki S, Oshiro O, Houkin K, Abe H. Functional neurosurgical simulation with brain surface magnetic resonance images and magnetoencephalography. Neurosurgery. 1993;33:269–273. doi: 10.1227/00006123-199308000-00013. [DOI] [PubMed] [Google Scholar]
- 10.Knowlton RC, Laxer KD, Aminoff MJ, Roberts TP, Wong ST, Rowley HA. Magnetoencephalography in partial epilepsy: clinical yield and localization accuracy. Ann Neurol. 1997;42:622–631. doi: 10.1002/ana.410420413. [DOI] [PubMed] [Google Scholar]
- 11.Kober H, Nimsky C, Moller M, Hastreiter P, Fahlbusch R, Ganslandt O. Correlation of sensorimotor activation with functional magnetic resonance imaging and magnetoencephalography in presurgical functional mapping: a spatial analysis. Neuroimage. 2001;14:1214–1228. doi: 10.1006/nimg.2001.0909. [DOI] [PubMed] [Google Scholar]
- 12.Kristeva-Feige R, Rossi S, Pizzella V, Tecchio F, Romani GL, Erne S, et al. Neuromagnetic fields of the brain evoked by voluntary movement and electrical stimulation of the index finger. Brain Res. 1995;682:22–28. doi: 10.1016/0006-8993(95)00313-f. [DOI] [PubMed] [Google Scholar]
- 13.Kristeva-Feige R, Walter H, Lutkenhoner B, Hampson S, Ross B, Knorr U, et al. A neuromagnetic study of the functional organization of the sensorimotor cortex. Eur J Neurosci. 1994;6:632–639. doi: 10.1111/j.1460-9568.1994.tb00308.x. [DOI] [PubMed] [Google Scholar]
- 14.Kunesch E, Classen J, Bettag M, Kahn T, Ulrich F, Bock WJ, et al. Representational cortical plasticity associated with brain tumors: evidence from laser-induced interstitial thermotherapy. Acta Neurol Scand. 2003;108:201–208. doi: 10.1034/j.1600-0404.2003.02082.x. [DOI] [PubMed] [Google Scholar]
- 15.Makela JP, Kirveskari E, Seppa M, Hamalainen M, Forss N, Avikainen S, et al. Three-dimensional integration of brain anatomy and function to facilitate intraoperative navigation around the sensorimotor strip. Hum Brain Mapp. 2001;12:180–192. doi: 10.1002/1097-0193(200103)12:3<180::AID-HBM1014>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morioka T, Yamamoto T, Mizushima A, Tombimatsu S, Shigeto H, Hasuo K, et al. Comparison of magnetoencephalography, functional MRI, and motor evoked potentials in the localization of the sensory-motor cortex. Neurol Res. 1995;17:361–367. [PubMed] [Google Scholar]
- 17.Nimsky C, Ganslandt O, Kober H, Moller M, Ulmer S, Tomandl B, et al. Integration of functional magnetic resonance imaging supported by magnetoencephalography in functional neuronavigation. Neurosurgery. 1999;44:1249–1256. doi: 10.1097/00006123-199906000-00044. [DOI] [PubMed] [Google Scholar]
- 18.Ohara S, Nagamine T, Ikeda A, Kunieda T, Matsumoto R, Taki W, et al. Electrocorticogram-electromyogram coherence during isometric contraction of hand muscle in human. Clin Neurophysiol. 2000;111:2014–2024. doi: 10.1016/s1388-2457(00)00448-x. [DOI] [PubMed] [Google Scholar]
- 19.Oishi M, Fukuda M, Kameyama S, Kawaguchi T, Masuda H, Tanaka R. Magnetoencephalographic representation of the sensorimotor hand area in cases of intracerebral tumor. J Neurol Neurosurg Psychiatry. 2003;12:1649–1654. doi: 10.1136/jnnp.74.12.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rezai AR, Hund M, Kronberg E, Zonenshayn M, Cappell J, Ribary U, et al. The interactive use of magnetoencephalography in stereotactic image-guided neurosurgery. Neurosurgery. 1996;39:92–102. doi: 10.1097/00006123-199607000-00018. [DOI] [PubMed] [Google Scholar]
- 21.Roberts TPL, Rowley HA. Magnetic source imaging as a tool for presurgical functional brain mapping. Neurosurg Clin N Am. 1997;8:421–438. [PubMed] [Google Scholar]
- 22.Roberts TPL, Zusman E, McDermott M, Barbaro N, Rowley HA. Correlation of functional magnetic source imaging with intra-operative cortical stimulation in neurosurgical patients. J Image Guid Surg. 1995;1:339–347. doi: 10.1002/(SICI)1522-712X(1995)1:6<339::AID-IGS5>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 23.Salenius S, Portin K, Kajola M, Salmelin R, Hari R. Cortical control of human motoneuron firing during isometric contraction. J Neurophysiol. 1997;77:3401–3405. doi: 10.1152/jn.1997.77.6.3401. [DOI] [PubMed] [Google Scholar]
- 24.Schiffbauer H, Berger MS, Ferrari P, Freudenstein D, Rowley HA, Roberts TP. Preoperative magnetic source imaging for brain tumor surgery: a quantitative comparison with intraoperative sensory and motor mapping. J Neurosurg. 2002;97:1333–1342. doi: 10.3171/jns.2002.97.6.1333. [DOI] [PubMed] [Google Scholar]
- 25.Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TP. Functional activity within brain tumors: a magnetic source imaging study. Neurosurgery. 2001;49:1313–1321. doi: 10.1097/00006123-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 26.Simos PG, Papanicolaou AC, Breier JI, Wheless JW, Constantinou JE, Gormley WB, et al. Localization of language-specific cortex by using magnetic source imaging and electrical stimulation mapping. J Neurosurg. 1999;91:787–796. doi: 10.3171/jns.1999.91.5.0787. [DOI] [PubMed] [Google Scholar]
- 27.Taniguchi M, Kato A, Ninomiya H, Hirata M, Cheyne D, Robinson SE, et al. Cerebral motor control in patients with gliomas around the central sulcus studied with spatially filtered magnetoencephalography. J Neurol Neurosurg Psychiatry. 2004;75:466–471. doi: 10.1136/jnnp.2002.001834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg H, Cheyne D, Crisp D. Electroencephalographic and magnetoencephalographic studies of motor function. Adv Neurol. 1990;54:193–205. [PubMed] [Google Scholar]
- 29.Woldag H, Waldmann G, Schubert M, Oertel U, Maess B, Friederici A, et al. Cortical neuromagnetic fields evoked by voluntary and passive hand movements in healthy adults. J Clin Neurophysiol. 2003;20:94–101. doi: 10.1097/00004691-200304000-00002. [DOI] [PubMed] [Google Scholar]