SUMMARY
The intestinal mucosa promotes T cell responses that may be beneficial for effective mucosal vaccines. However, intestinal resident memory T (Trm) cell formation and function are poorly understood. We found that oral infection with Listeria monocytogenes induced a robust intestinal CD8 T cell response and blocking effector T cell migration showed that intestinal Trm cells were critical for secondary protection. Intestinal effector CD8 T cells were predominately composed of memory precursor effector cells (MPEC) that rapidly upregulated CD103, which was needed for T cell accumulation in the intestinal epithelium. CD103 expression, rapid MPEC formation, and maintenance in intestinal tissues were dependent on T cell intrinsic transforming growth factor-β signals. Moreover, intestinal Trm cells generated after intranasal or intravenous infection were less robust and phenotypically distinct from Trm cells generated after oral infection demonstrating the critical contribution of infection route for directing the generation of protective intestinal Trm cells.
INTRODUCTION
The intestinal mucosa sits as a dominant site for exposure to potential microbial invaders and these tissues promote the ability to rapidly respond to insults by generating robust yet regulated immunity. For most intracellular bacterial infections, generating proper T cell responses is ultimately necessary for the successful elimination of the pathogen. For Listeria monocytogenes (L. monocytogenes) infections, sterilizing immunity requires a robust T cell response capable of providing common effector functions such as interferon-γ (IFN-γ) production and lysis of infected cells. After infection, induction of a protective T cell response includes mobilization of effector cells to peripheral tissues resulting in elimination of any remaining bastions of infection. While these processes have been well defined following intravenous (i.v.) infection, the pathogen-specific CD8 T cell response has not been well characterized following oral infections.
Naïve CD8 T cells specific for a pathogen are exceedingly rare and predominately reside in secondary lymphoid tissues (Obar et al., 2008; von Andrian and Mackay, 2000). Therefore, primary CD8 T cell responses to infections require a lag period in order to mount a robust response. Following successful elimination of a pathogen, CD8 T cells establish distinct memory populations with defined migratory properties that are able to rapidly respond to challenge (Schenkel et al., 2013; Gebhardt et al., 2011; Ariotti et al., 2012). The developmental pathway a naive T cell follows to progress to memory has been widely studied. However, this process has been complicated by the identification of several distinct memory populations. One memory population resides within lymphoid tissues as central memory cells (Tcm) while effector memory cells and an associated subset, resident memory cells (Trm), reside predominately in peripheral nonlymphoid tissues. For the most part, Tcm behave similarly to naïve T cells in terms of their lymphoid microanatomical locations and trafficking patterns. However, Trm are anatomically positioned to immediately respond to antigen reexposure without an appreciable delay in mediating protective effector function (Schenkel et al., 2013; Gebhardt et al., 2009; Ariotti et al., 2012). While it is becoming clearer that TRM provide superior protection in some infection models, both lymphoid memory and Trm participate through distinct mechanisms (Gebhardt et al., 2009; Jiang et al., 2012; Mackay et al., 2012). Trm in the reproductive tract have recently been shown to provide an organizational framework to secondary immune responses by enhancing the recruitment of innate cells through rapid IFN-γ production (Schenkel et al., 2013). However, the mechanisms for generation and maintenance of Trm remain controversial and may vary depending on infection route and tissue type. In the case of the intestinal epithelium, a critical barrier tissue, a clear picture explaining the events leading to Trm development and establishment in response to oral infection has not emerged.
Here, we utilized an oral L. monocytogenes infection model to recapitulate human infection and examine the generation of intestinal TRM populations. Unexpectedly, we observed rapid formation of an intestinal CD127+ KLRG1− CD8 T cell population which resembled memory precursor effector cells (MPEC) following oral L. monocytogenes infection. These early mucosal MPEC preferentially upregulated CD103 and survived long-term, providing a novel means of identifying mucosal Trm precursors. On the contrary, KLRG1+ CD127− CD8 T cells underwent apoptosis in the intestinal epithelium consistent with short-lived effector cells (SLEC). The establishment of a rapid resident memory population was dependent on intrinsic TGFβ signals. Contrary to peripheral lymphoid tissues where long term maintenance was independent of TGFβ signals, maintenance in intestinal tissues was highly dependent on the ability to rapidly generate MPEC. Moreover, CD103 expression by infiltrating CD8 T cells promoted CD8 T cell accumulation in the epithelium, rather than retention, after oral L. monocytogenes infection. Route of infection influenced intestinal Trm as intranasal (i.n.) infection, while mucosal in nature, failed to generate comparable intestinal Trm responses. Thus, our findings identified intestinal mucosa-specific mechanisms controlling protective immunity within the intestine.
RESULTS
Protective CD8 T cell response to murinized oral L. monocytogenes infection
While i.v. and intraperitoneal (i.p.) L. monocytogenes infection has been widely utilized in murine models, inherent differences between mouse and human E-cadherin has hindered the effective examination of oral L. monocytogenes infection in mice (Bonazzi et al., 2009). The bacterial surface protein internalin A is responsible for invasion of human epithelial cells lining the intestinal mucosa through interaction with its ligand, E-cadherin. However, wild-type internalin A fails to recognize murine E-cadherin preventing invasion of murine intestinal epithelial cells. Here, we utilized a recombinant L. monocytogenes containing a mutation in the internalin A protein to facilitate invasion of murine epithelial cells (Wollert et al., 2007; Bou Ghanem et al., 2012). After oral L. monocytogenes infection, Balb/c mice generated a rapid and robust expansion of endogenous antigen-specific CD8 T cells responding to the immunodominant Kd-restricted LLO91 epitope (Figure 1A–C). This population of LLO91-specific CD8 T cells was first detected in the blood at 6 – 7 dpi and rapidly reached peak response by 9 dpi. Removal of the spleen did not impact the magnitude of the LLO91-specific CD8 T cell response suggesting that the spleen was not required as a site of T cell priming after oral infection (Figure 1C). Moreover, the integrin α4β7 was upregulated on LLO91-specific CD8 T cells located within the mesenteric lymph nodes (MLN) consistent with APC-mediated priming in intestinal tissues (Figure 1D) (Mora et al., 2003; Johansson-Lindbom et al., 2003). Together these data suggest organized intestinal lymphoid tissues such as the MLN as the principal T cell priming site following oral L. monocytogenes infection.
Figure 1. Oral L. monocytogenes infection generates a protective mucosal T cell response.
(A) The LLO91-specific CD8 T cell response was quantified in the blood after oral L. monocytogenes infection. Data are representative of at least two independent experiments with at least four mice per group (mean and s.e.m.)
(B) The LLO91-specific CD8 T cell response in tissues at 9 dpi mice. Representative contour plots are gated on CD8αβ+ T cells. The numbers within plots correspond to the percentage of cells within gates.
(C) Spleens were surgically removed (splenectomized) or mice underwent a sham surgery (control) and recovered for 2 weeks prior to oral infection. LLO91-specific CD8 T cells were enumerated at 12 dpi. Data are representative of at least two independent experiments with at least three mice per group (mean and s.e.m),
(D) Integrin α4β7 expression was determined on LLO91-specific CD8 T cells at 7 dpi. Data are representative of at least two independent experiments.
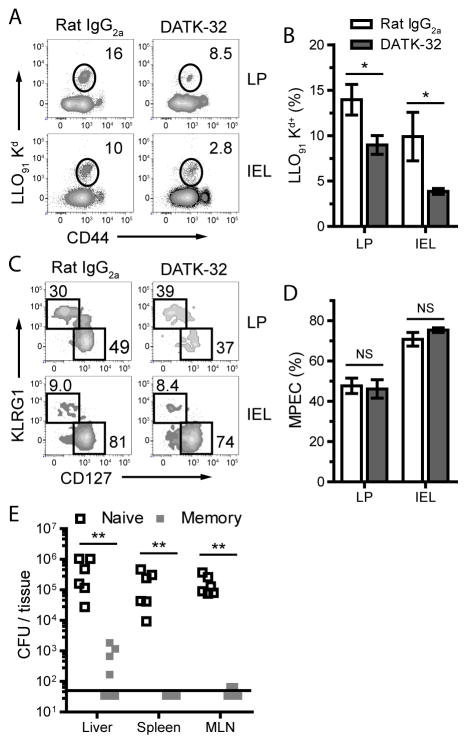
(E) Mice were orally immunized with 2×109 CFU L. monocytogenes and received 100μg DATK-32 (anti-α4β7) or Rat IgG2A injections daily for the first 14 days and every 5 days thereafter. Thirty days after primary infection, mice were rechallenged with 2×1010 CFU L. monocytogenes and the bacterial burden was determined 3 days following challenge infection. Data are pooled from two experiments with at least 12 mice per group (mean and s.e.m.). See also Figure S1.
To determine the contribution of intestinal Trm to protection following a challenge infection, mice were treated with DATK-32 (anti-α4β7) throughout the primary response and into memory homeostasis to prevent α4β7-dependent migration into the intestinal mucosa (Hamann et al., 1994; Lefrançois et al., 1999). After treatment, L. monocytogenes immune mice had reduced LLO91-specific CD8 T cells in the intestinal epithelium but not in the LP (Figure S1). No other intestinal T cell populations were significantly perturbed (Figure S1). Using this approach, pathogen-specific CD8 Trm cell numbers were reduced within the intestinal epithelium while normal circulating CD8 memory populations were maintained in the peripheral lymphoid organs, including the MLN (data not shown). Mice were then challenged with oral L. monocytogenes infection and bacterial burdens were measured. Integrin α4β7 blockade resulted in elevated bacterial burdens following challenge infection demonstrating the importance of establishing intestinal Trm populations for optimal protection against oral infections, particularly for those that reside within the intestinal epithelium (Figure 1E).
Early MPEC phenotype cells accumulate in the intestinal mucosa
The intestinal tissues are distinct from lymphoid and other nonlymphoid tissues with regard to T cell effector phenotype and function (Casey et al., 2012; Masopust et al., 2010; Masopust et al., 2006; Sheridan and Lefrancois, 2011; Masopust et al., 2001; Pope et al., 2001). Generally, cells within intestinal tissues express an activated phenotype (Masopust et al., 2006). We sought to determine how the CD8 lineage is regulated within intestinal tissues after oral L. monocytogenes infection. At 7 dpi, T cells infiltrating the intestinal LP and epithelium were heterogeneous with regard to effector differentiation based on CD127 and KLRG1 expression and were similar in phenotype to cells in the spleen and MLN (Figure 2A and B) (Joshi et al., 2007). In the spleen, antigen-specific CD8 T cells were comprised of ~50% KLRG1+ and ~20% CD127+ cells up to at least day 12 dpi (Figure 2C). In contrast, by 12 dpi, nearly the entire intestinal LLO91-specific CD8 T cell population was represented by cells with an MPEC phenotype (Figure 2). This accelerated memory phenotype formation was also evident by heightened multifunctionality with regard to cytokine production (Figure S2). However, in the spleen and lung, LLO91-specific CD8 T cells maintained a prolonged SLEC phenotype and were less multifunctional than intestinal LLO91-specific CD8 T cells (Figure 2B and D and S2).
Figure 2. Memory T cells are generated early in intestinal tissues after oral L. monocytogenes infection.
(A and B) MPEC (A) and SLEC (B) phenotype was determined for LLO91-specific CD8 T cells following oral L. monocytogenes infection. * P < 0.05, ** P < 0.01, *** P < 0.001 (unpaired two-tailed t-test). P value indicators (*) are color matched to the tissue compared to the spleen. Data are representative of at least two independent experiments with at least three mice per group (mean and s.e.m.). See also Figure S2.
(C) Representative contour plots gated on LLO91-specific CD8 T cells depicting identification of MPEC and SLEC 12 dpi. The numbers within plots correspond to the percentage of cells within gates.
(D) Quantification of SLEC and MPEC LLO91-specific CD8 T cells at 12 dpi following oral infection. ** P < 0.01 (unpaired two-tailed t-test). Data are representative of at least two independent experiments with at least three mice per group (mean and s.e.m.).
Prolonged expression of KLRG1 in the spleen suggested that preferential migration of effector subsets to the intestinal mucosa was not the underlying reason for the rapid accumulation of MPEC phenotype cells. In addition, α4β7 was comparably expressed by SLEC and MPEC phenotype cells (Figure S3A). To test whether migration of distinct subsets contributed to accelerated memory formation in intestinal tissues, DATK-32 was administered daily between 7 and 11 dpi and the intestinal tissues were harvested 1 day later to examine LLO91-specific CD8 T cells and their differentiation status. Transient α4β7 blockade reduced the size of the LP and IEL LLO91-specific CD8 T cell populations indicating that migration of effector T cells during this time period contributed to accumulation at these sites (Figure 3A and B). Despite the reduced accumulation of LLO91-specific CD8 T cells within intestinal tissues, rapid conversion to an MPEC phenotype was unaltered (Figure 3C and D). In addition, daily DATK-32 treatment or anti-CCR9 administration throughout primary infection led to reduced LLO91-specific CD8 T cells in intestinal tissues at the peak of the T cell response (Figure S3B). Even under these conditions, rapid development of a MPEC phenotype population was not hindered (Figure S3C). Taken together, these data suggest that rapid memory accumulation within intestinal tissues occurred via in situ regulation and not through differential migration of distinct T cell subsets. To determine whether rapid accumulation of intestinal MPEC occurred via proliferation or apoptosis, we examined BrdU incorporation and Annexin V reactivity, respectively. Indeed, rapid MPEC phenotype formation appeared to be predominately due to the increased apoptosis of SLEC phenotype cells and not the differential proliferation of MPEC phenotype cells in intestinal tissues (Figure S3D and E). Together, this data suggests that rapid memory development in intestinal tissues is regulated in situ by preferential SLEC apoptosis.
Figure 3. In situ events regulate MPEC accumulation within intestinal tissues.
(A – D) Balb/c mice were treated with 100μg DATK-32 or Rat IgG2a daily from 7 to 11 dpi. The LP and IEL were harvested at 12 dpi and LLO91-specific CD8 T cells were identified (A and B) and analyzed for CD127 and KLRG1 expression (C and D). Representative contour plots gated on CD8β+ T cells (A) or LLO91-specific CD8 T cells (C) are shown. The numbers within plots correspond to the percentage of cells within gates. NS, not significant; * P ≤ 0.05 (unpaired two-tailed t-test). Data represents five mice per group (mean and s.e.m.). See also Figure S3.
(E) Naïve Balb/c mice were challenged with 2×1010 CFU Lm and indicated tissues were harvested 4 days following infection. Alternatively, mice orally immunized with 2×109 CFU L. monocytogenes were challenged with 2×1010 CFU L. monocytogenes 15 days following primary infection and indicated tissues were harvested 4 days following secondary infection. Both groups were treated with 100μg DATK-32 daily following the challenge infection. ** P < 0.01 (Mann-Whitney two-tailed test). Data represents at least six mice per group (mean and s.e.m.) and is representative of two independent experiments.
We also tested whether early memory cells were capable of providing protection and reducing dissemination of bacteria in the absence of newly infiltrating intestinal T cells. Naïve or L. monocytogenes immune mice infected 15 days previously were treated with DATK-32 daily following secondary L. monocytogenes challenge and bacterial burden was quantified in the spleen, liver, and MLN after challenge infection. Bacteria were nearly undetectable in the MLN and spleen and the bacterial load in the liver was greatly reduced (Figure 3E). Thus, even very early after primary infection, protective immunity had been established in the intestinal mucosa.
MPEC phenotype cells preferentially express CD69 and CD103 in intestinal tissues
A subset of LP CD8 T cells and nearly all IEL express CD69 and the αE integrin CD103, both of which are upregulated as cells enter the mucosa (Masopust et al., 2006; Klonowski et al., 2004; Ericsson et al., 2004). As circulating memory CD8 T cells provide minimal input into the intestinal memory pool (Klonowski et al., 2004), these CD103+ memory CD8 T cells are considered resident (Gebhardt et al., 2009; Wakim et al., 2010; Mackay et al., 2012; Klonowski et al., 2004; Jiang et al., 2012; Gebhardt et al., 2011). Current evidence suggests that CD103 is involved in retention of T cells in the epithelium through interactions with E-cadherin (El Asady et al., 2005; Lee et al., 2011; Casey et al., 2012). We examined CD103 and CD69 expression on effector subsets in the mucosa twelve days after oral L. monocytogenes infection. Splenic effector cells of either MPEC or SLEC phenotypes lacked expression of CD103 and CD69, while a subset of MPEC phenotype cells in the MLN expressed CD103 (Figure 4). CD103 was exclusively expressed by MPEC phenotype cells in the LP and IEL compartments and was absent from SLEC phenotype cells (Figure 4). CD69 was also preferentially expressed by MPEC phenotype cells, with only low amounts expressed by SLEC phenotype cells. This rapid Trm development was independent of infectious dose. Mice receiving 10- or 100-fold less L. monocytogenes infection demonstrated a dose dependent expansion of LLO91-specific CD8 T cells in intestinal tissues (Figure S4A). However, the development of an MPEC phenotype population and CD103 expression at 15 dpi occurred independently of infectious dose (Figure S4B and C). These results identify CD103 and CD69 coexpression as a novel hallmark of MPEC identification in the intestinal mucosa which further supports the concept that Trm are descendants of early infiltrating MPEC.
Figure 4. CD103 expression identifies mucosal memory precursor CD8 T cells.
(A) Balb/c mice were orally infected with L. monocytogenes and LLO91-specific CD8 T cells from the indicated tissues were analyzed at 12 dpi. Representative contour plots gated on either LLO91-specific CD8 T cells with an MPEC (top row) or SLEC (bottom row) phenotypes demonstrate CD103 and CD69 staining. Numbers in plots correspond to the percentage of cells within gated quadrants.
(B) Graphical quantification of the data presented in (A). * P < 0.05, *** P < 0.001 (unpaired two-tailed t-test). Data are representative of at least two independent experiments with five mice per group (mean and s.e.m.). See also Figure S4.
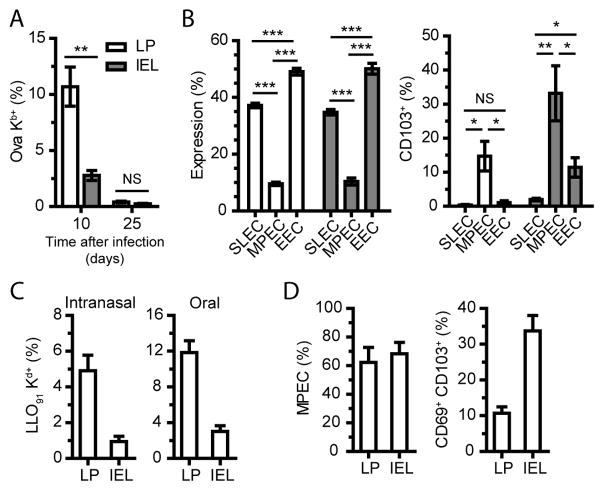
As CD8 T cells activated at remote sites traffic to the intestinal mucosa (Masopust et al., 2004), we wished to determine whether local priming was required to drive accelerated memory development in the intestinal mucosa. Just as in Balb/c mice, early accumulation of MPEC phenotype cells was evident in the intestinal mucosa of B6 mice after oral infection (Figure S5). Therefore, we performed i.n. influenza virus infection of B6 mice and tracked the resulting effectors in the intestinal mucosa. After i.n. infection with an influenza virus expressing ovalbumin, robust expansion of ova-specific CD8 T cells occurred and these cells migrated to intestinal tissues (Figure 5A). In contrast to the events following oral L. monocytogenes infection, the effector cells were largely made up of SLEC and early effector cells (EEC; KLRG1− CD127−) (Figure 5B). This lack of MPEC accumulation in the intestinal tissues resulted in poor development of long-lived memory cells and ova-specific CD8 T cells were barely detectable in the intestinal tissues at 25 dpi (Figure 5A). Despite the limited presence of MPEC in intestinal tissues following influenza virus infection, CD103 expression remained limited to MPEC phenotype cells (Figure 5B). As i.n. influenza virus infection failed to generate readily identifiable memory populations in intestinal tissues, we examined the intestinal compartment of mice infected with i.n. L. monocytogenes 30 days previously. Memory LLO91-specific CD8 T cells were readily identified in the LP and IEL, though to a lesser degree than oral infection (Figure 5C). Moreover, these cells failed to fully convert to a memory phenotype and only a small proportion expressed CD69 and CD103 (Figure 5D compared to Figure 2A and 4B). These data demonstrate that the route of priming has a dramatic impact on the process of local Trm development.
Figure 5. Intranasal infection leads to distinct Trm populations in the intestine.
(A) C57Bl/6 mice were infected with i.n. influenza virus expressing ovalbumin and the LP and IEL of the small intestine were harvested 10 and 25 days later to identify ova-specific CD8α+ TCRβ+ cells. NS, not significant; ** P < 0.01 (unpaired two-tailed t-test). See also Figure S5.
(B) Effector subset phenotype was determined for ova-specific CD8 T cells in the LP and IEL at 10 dpi (EEC - CD127neg KLRG1neg). CD103 expression was determined on ova-specific effector subsets in the LP and IEL at 10 dpi. NS, not significant; * P < 0.05, ** P < 0.01, *** P < 0.001 (unpaired two-tailed t-test).
(C) Balb/c mice were infected with i.n. or oral L. monocytogenes and the LP and IEL of the small intestine were harvested 30 – 32 days later. Cells are gated on CD8α+ TCRβ+ cells.
(D) LLO91-specific CD8 T cells from i.n. infection were examined for MPEC phenotype and CD103 and CD69 co-expression.
All data are representative of at least two independent experiments with three to four mice per group (mean and s.e.m.).
CD103 regulates accumulation but not retention within the intestinal epithelium
As oral infection induced Trm that appeared distinct from intestinal Trm primed in non-intestinal tissues, we examined the role of CD103 on CD8 T cells after oral infection. Itgae−/− (CD103 deficient) mice developed a normal T cell response within the epithelium after i.v. (Lefrançois et al., 1999) and oral infection and these cells were maintained into the memory phase (Figure S6). These findings suggest that a role for CD103 expression in the accumulation of CD8 T cells in intestinal compartments is not absolute. However, accumulation within the intestine may be a combination of multiple biologic processes including migration, proliferation, survival, and/or retention. Previous studies have co-transferred Itgae−/− and CD103-sufficient (B6) T cells into naïve recipients prior to infection to demonstrate a role for CD103 in retention of T cells in epithelial layers. Using this approach, Itgae−/− CD8 T cells are not readily maintained within the epithelium of peripheral tissues (Lee et al., 2011; Casey et al., 2012; Mackay et al., 2013). We employed this co-transfer system to test the role of CD103 expression after oral L. monocytogenes infection. At the peak of the T cell response, comparable ratios of B6 and Itgae−/− OT-I cells were present in the MLN and LP. In contrast, OT-I cells in the epithelium were heavily skewed toward B6 OT-I cells suggesting that CD103 promotes T cell accumulation in the epithelium (Figure 6A). This ratio remained unchanged at 29 dpi suggesting that CD103 was not required for further retention in the intestinal epithelium after initial establishment of the T cell population (Figure 6A). The preferential accumulation of B6 OT-I in the epithelium was apparent as early as 7 dpi but was further exaggerated at 9 dpi suggesting that CD103 provides a selective advantage for accumulation in the epithelium (Figure 6B). This selective advantage is likely due to differential migration into the epithelium as total antigen-specific CD8 T cell numbers in the LP and IEL declined after 9 dpi yet the number of CD103+ antigen-specific CD8 T cells remained constant (data not shown). Itgae−/− OT-I cells were maintained at similar proportions for at least 113 dpi with no further reductions (Figure 6B). Together, these data suggest that CD103 does not regulate retention of pathogen-specific CD8 T cells in the intestinal epithelium after oral infection (Figure 6A and B). These findings were further corroborated by examination of luminal CD8 T cells, where ratios of B6 and Itgae−/− OT-I cells were identical to those in the epithelial layer (Figure 6A). Preferential accumulation of Itgae−/− OT-I cells should occur in the lumen if CD103 was required for retention. In addition, CD103 expression appeared to have no effect on the rapid accumulation of memory in the epithelium and lumen (Figure 6C and D). This would suggest that changes in survival or proliferation of Itgae−/− cells did not contribute to the accumulation of B6 T cells in the epithelium as CD103 expression is limited to cells with an MPEC phenotype and alterations in proliferation or survival should modify the proportion of MPEC phenotype cells. Overall, these data demonstrate that CD103 promotes accumulation of CD8 T cells in the epithelium but was not required for rapid MPEC development or long-term retention of Trm in the epithelium.
Figure 6. CD103 expression provides a selective advantage for epithelial accumulation but is not required for retention.
Equal numbers of B6 (CD45.1/.2) and Itgae−/− (CD45.1) OT-I T cells were mixed and transferred into naïve B6 mice (CD45.2) prior to infection.
(A) OT-I T cells from B6 and Itgae−/− donors were quantified at 9 and 29 dpi using congenic markers in the spleen, MLN, LP, IEL, and lumen. OT-I IELs are presented as a ratio of Itgae−/− to B6 OT-I T cells and normalized to the LP ratio. NS, not significant; * P < 0.05, *** P < 0.001 (left panels, paired two-tailed t-test; right panel, unpaired two-tailed t-test). See also Figure S6.
(B) Mice orally infected with L. monocytogenes were examined at 7, 9, and 113 dpi and presented as in (A). NS, not significant; ** P < 0.005 (unpaired two-tailed t-test). Data from (A) and (B) are representative of three similar experiments with at least four mice per group (mean and s.e.m.).
(C and D) B6 or Itgae−/− OT-I cells from the intestinal epithelium (C) and lumen (D) at 9 dpi were examined for CD127 and KLRG1 expression. Representative contour plots are shown. (C) NS, not significant; ** P < 0.005, *** P < 0.001 (unpaired two-tailed t-test). Data are representative of three similar experiments with at least four mice per group (mean and s.e.m.). (D) Data represents the pooled luminal contents of at least four mice per group.
TGFβ drives rapid memory formation and subsequent maintenance of Trm cells
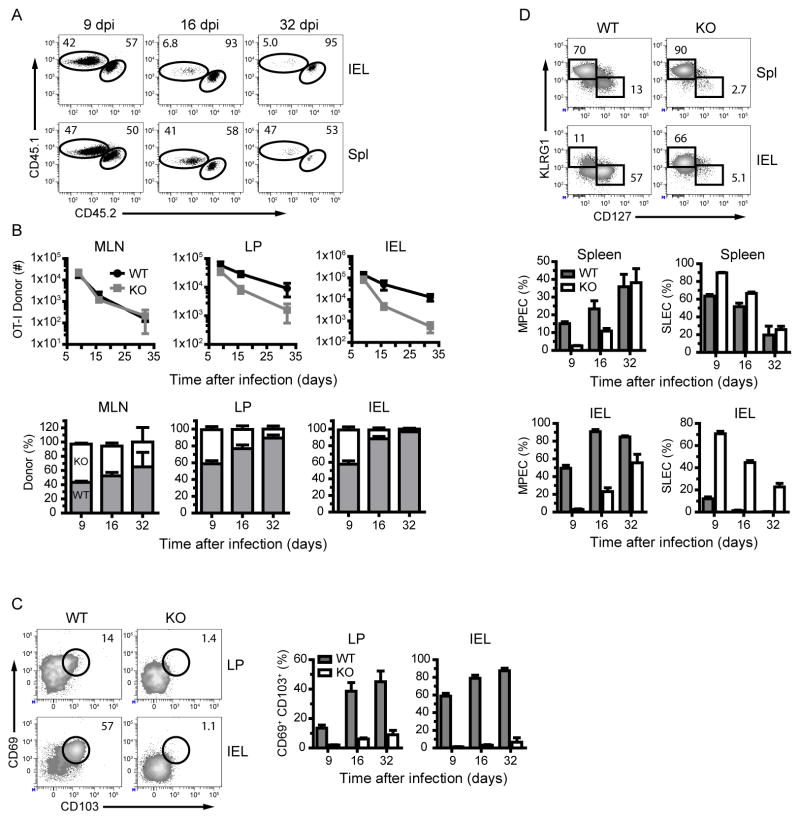
TGFβ has numerous functions that may contribute to the establishment of Trm populations. TGFβ signaling is required for upregulation of CD103 (El Asady et al., 2005) and through this action subsequently involved in retention of Trm in a number of peripheral tissues (Casey et al., 2012; Lee et al., 2011; Mackay et al., 2013). Under certain conditions TGFβ may also regulate expression of the integrin α4β7 and thereby influence intestinal homing (Zhang and Bevan, 2013). As CD103 expression was not required for T cell retention within the intestinal epithelium (Figure 6) and α4β7 expression was TGFβ-independent (Figure S7A), we asked whether impaired TGFβ signaling regulated intestinal Trm development after oral L. monocytogenes infection. We utilized a system in which TGFβ receptor II (TGFβRII) is absent and TGFβ signaling is completely abrogated (Zhang and Bevan, 2012; Zhang and Bevan, 2013; Mackay et al., 2013). Thus, we co-transferred equal numbers of CD44lo CD8 T cells from Tgfbr2fl/fl distal promoter (d) Lck-cre (TGFβRII-deficient) CD45.1 OT-I and Tgfbr2fl/fl (WT) CD45.1/.2 OT-I into CD45.2 B6 mice. At the peak of the T cell response, the overall responses and the ratios of TGFβRII-deficient to WT OT-I cells were comparable in peripheral lymphoid tissues and intestinal tissues demonstrating that the magnitude of the T cell response is unaffected by loss of TGFβ signals (Figure 7A and B). The overall responses and the ratios of TGFβRII-deficient to WT OT-I cells remained comparable in lymphoid tissues as the T cell response progressed into the memory phase. Contrary to these findings, TGFβRII-deficient T cells were not maintained in the intestinal LP and IEL as TGFβRII-deficient cells were barely detectable at 16 and 32 dpi in both tissues (Figure 7A and B). When TGFβ signaling was ablated specifically in responding OT-I T cells, CD103 upregulation did not occur at any time after infection and CD69 upregulation was initially blunted (Figure 7C and S7B). After the peak of the T cell response, CD69 expression on TGFβRII-deficient T cells was comparable to or greater than WT T cells amongst CD103− T cells suggesting that CD69 expression amongst CD103 nonexpressing cells may be TGFβ-independent (Figure S7B). However, it is unclear whether CD69+ CD103− T cells are a distinct population from or precursors to CD69+ CD103+ T cells. As CD103 expression did not mediate T cell retention in this model and a comparable loss of CD8 memory T cells was observed in the intestinal LP, a tissue were CD103 is not required for retention in any model, it is unlikely that this rapid decline was related to the inability for intestinal Trm to upregulate CD103. Collectively these data demonstrated that CD103 expression and Trm generation is TGFβ-dependent within intestinal tissues after oral L. monocytogenes infection.
Figure 7. TGFβ regulates rapid MPEC and Trm generation and is required for intestinal Trm maintenance.
Equal numbers of CD44lo CD8 T cells from Tgfbr2fl/fl distal promoter (d) Lck-cre (KO) CD45.1 OT-I and Tgfbr2fl/fl (WT) CD45.1/.2 OT-I were co-transferred into CD45.2 B6 mice and infected one day later.
(A) Representative dot plots are gated on donor cells after oral L. monocytogenes infection. The numbers within plots correspond to the percentage of cells within gates.
(B) Donor derived cells were quantified in the indicated tissues after oral L. monocytogenes infection. Absolute number and ratios of donor cells are depicted graphically with mean and s.e.m of 4 mice per group. See also Figure S7.
(C and D) Donor derived OT-I T cells were analyzed for CD69 and CD103 (C) and CD127 and KLRG1 (D) expression in the indicated tissues. Representative contour plots are gated on donor OT-I T cells as indicated at 9 dpi. The numbers within plots correspond to the percentage of cells within gates. Graphs depict the mean and s.e.m of 4 mice per group. See also Figure S7.
TGFβ is also thought to be important for regulation of CD8 T cell differentiation. TGFβ promotes SLEC apoptosis during effector responses in lymphoid tissues and thereby regulates the ratio of SLEC versus MPEC (Sanjabi et al., 2009). Whether similar effects are exerted in intestinal tissues remains unclear. To test this, we determined whether TGFβ signaling regulated early accumulation of MPEC in intestinal tissues. Indeed, while a modest increase in SLEC was observed in splenic TGFβRII-deficient cells, TGFβRII-deficient cells in the IEL lacked the rapid appearance of MPEC observed in WT T cells and did not form memory populations until 16 to 32 dpi (Figure 7D). Approximately 70% of TGFβRII-deficient cells expressed a SLEC phenotype in the intestinal epithelium at 9 dpi. This observation is consistent with a role for TGFβ in promoting the rapid apoptosis of SLEC in the intestinal epithelium. However, these data would suggest that the inability to become MPEC early in the response impairs the maintenance of this population. Moreover, IEL T cells which could not receive TGFβ signals phenocopy WT T cells in the spleen, further demonstrating that TGFβ is critical for the rapid accumulation of MPEC within intestinal tissues.
DISCUSSION
The basis of our understanding of T cell memory has relied predominantly on the role of memory T cell populations in secondary lymphoid organs. However, this centralized view of T cell memory has progressed towards recent studies highlighting the significance of memory T cells residing in peripheral tissues (Wakim et al., 2008; Gebhardt et al., 2009; Shin and Iwasaki, 2012; Jiang et al., 2012; Masopust et al., 2010). The behavior of these cell populations is distinct from their circulating counterparts and new therapeutic avenues may target enhancing long-lived resident memory populations for rational vaccine design. These populations appear to provide all the advantages of memory T cells found in secondary lymphoid organs with the added benefit of being anatomically positioned at the barriers where initial pathogenic insults occur. In this manner, Trm generated after oral L. monocytogenes challenge are ideally suited to rapidly respond to future exposures thereby limiting associated pathologies. Blockade of the α4β7 integrin after L. monocytogenes infection resulted in the establishment of a reduced Trm population within the intestinal epithelium. These mice demonstrated an enhanced susceptibility to bacterial dissemination following a challenge infection. Indeed, localized i.n. influenza infection failed to induce efficient homing of Trm to the intestinal epithelium. However, a small window of T cell migration resulted in efficient infiltration of effector cell subsets into the intestinal LP. Despite this, intestinal flu-specific CD8 T cells failed to rapidly develop a MPEC population within the intestinal tissues and were poorly maintained suggesting that i.n. routes of infection are poor inducers of intestinal residency. This was confirmed using an i.n. L. monocytogenes infection which induced a robust LP but a diminished IEL memory population. Despite their maintenance in the intestine, they did not bear resemblance to orally induced Trm populations. Therefore, approaches aimed at providing valuable protection to intestinal pathogens may utilize oral vaccination strategies to boost intestinal epithelial resident memory populations. In a similar manner, skin infections induce strong protective Trm responses in the skin (Jiang et al., 2012; Gebhardt et al., 2009) and i.n. infections induce robust Trm in the lungs (Lee et al., 2011).
It remains unclear where Trm development interfaces with our classic understanding of T cell memory generation. Even prior to Trm inclusion into this paradigm, multiple models of T cell memory could encompass the observations associated with memory T cell formation (Lefrancois and Masopust, 2009). As intestinal Trm are not populated from circulating memory T cells, it is reasonable to propose that they are generated from T cells which initially seed the site of infection during the primary response when intestinal homing receptors are expressed (Klonowski et al., 2004; Masopust et al., 2010). Surprisingly, little attention has focused on CD8 T cell differentiation into resident memory T cells within these peripheral tissues. Based on other models it is reasonable to speculate that memory development in intestinal tissues would occur gradually as it occurs in the lymphoid compartments. On the contrary, these data clearly demonstrated an extremely rapid development of memory T cells in intestinal tissues after oral L. monocytogenes infection. This rapid memory formation is driven by TGFβ-dependent in situ events. The accelerated memory formation was associated with preferential expression of CD103 and CD69 by MPEC but not by effector subsets expressing KLRG1. Clearly, both SLEC and MPEC received signals through TGFβ. In the case of SLEC, these signals interfered with their survival, whereas TGFβ signals instructed MPEC to upregulate CD103 without impacting their survival. It is unclear why CD103 expression does not occur in SLEC even though they received instructive signals through TGFβ. Similarly, cytokines like IL-15 and IL-7 may promote the maintenance of MPEC in intestinal tissues despite receiving TGFβ signals. Both KLRG1 and CD103 can recognize and bind to E-cadherin (Grundemann et al., 2006; Cepek et al., 1994) providing a potential mechanism for early E-cadherin interactions and migration of SLECs into the epithelium. Additionally, E-cadherin expression on Trm has been shown to regulate Trm accumulation in the salivary gland providing a potential mechanism for retention in epithelial layers in the absence of CD103 (Hofmann and Pircher, 2011). CD69 is also important in driving T cell migration to the lung and skin but has not been evaluated in this model (Lee et al., 2011; Mackay et al., 2013). CD8 T cells in the intestinal LP which lack CD103 in a competitive transfer model still express CD69 yet migrate into the epithelium to a lesser degree. This suggests that CD69 is not providing a selective migratory advantage or that any advantage awarded through CD69 expression is mitigated in the absence of CD103. However, as both LP and IEL subsets express high amounts of CD69 and it is reexpressed after migration into the LP, it is more likely that the CD69-S1P1 axis is regulating Trm retention within the LP and IEL after establishment (Shiow et al., 2006; Skon et al., 2013). Our studies demonstrate that Trm rapidly arise from MPECs expressing CD103 and CD69 which seed intestinal tissues early after oral infection. CD103 was involved in the accumulation of CD8 T cells into the intestinal epithelium but not in long-term retention of mucosal memory T cells. Moreover, T cells which cannot respond to TGFβ are incapable of being maintained in the lamina propria or epithelium of the intestine, even though normal numbers of antigen-specific T cells are maintained in other peripheral tissues. Collectively, these results suggest that the inability of TGFβRII-deficient T cells to be maintained in intestinal tissues is independent of TGFβ effects on CD103 expression and a result of the inability to form early MPEC populations within intestinal tissues.
METHODS
Mice
Balb/cJ and C57Bl/6J mice were purchased from The Jackson Laboratory (Bar Harbor, ME). B6-Ly5.2/Cr mice were purchased from Charles River Laboratories. Itgae−/−, Itgae−/− Rag1−/− OT-I, C57Bl/6, and Rag1−/− OT-I mice with mixed congenic backgrounds are maintained in house. Tgfbr2fl/fl dLck-cre OT-I and Tgfbr2fl/fl OT-I mice (Zhang and Bevan, 2012) were obtained from University of Washington. All mice were maintained in specific-pathogen-free conditions and 8 – 14 week old, age-matched mice were used for experiments. All animal experiments were conducted in accordance with the University of Connecticut Health Center Institutional Animal Care and Use Committee and National Institutes of Health guidelines.
Bacteria, Virus, and Infections
L. monocytogenes strain EGDe carrying a recombinant internalin A with a mutation in S192N and Y369S (InlAM) has been described previously (Wollert et al., 2007). All mice were food and water deprived for ~4 hours prior to infection, housed individually with minimal bedding, and given an approximately 0.5 cm3 piece of bread inoculated with 2×109 colony-forming units (CFU) of L. monocytogenes in PBS. In experiments designed to quantify bacterial burden, a recombinant L. monocytogenes InlAM strain 10403s, which is naturally streptomycin resistant, was used for primary (2×109 CFU) and secondary (2×1010 CFU) infections. For i.n. (i.n.) L. monocytogenes infection, mice were anesthetized by i.p. injection with 2,2,2-tribromoethanol (Avertin) before infection with L. monocytogenes InlAM strain EGDe. For oral L. monocytogenes infection of B6 mice, infection was performed with L. monocytogenes InlAM strain 10403s expressing a truncated form of ovalbumin. For i.v. L. monocytogenes infection of B6 mice, 1×103 CFU of L. monocytogenes InlAM strain 10403s expressing a truncated form of ovalbumin was injected into the tail vein. For influenza virus infection, mice were anesthetized prior to infection with 103 PFU of WSN-OVAI (Lee et al., 2011).
Tissue Preparation
Single cell suspensions were prepared from MLN, spleen, lung, LP, and IEL as previously described (Sheridan and Lefrancois, 2012; Lee et al., 2011). Briefly, MLNs, spleen, and lung were digested with 100U/ml collagenase prior to mechanical dissociation through a 70um filter. Small intestines were removed and Peyer’s patches were dissected away prior to processing. IEL, LP, and lung lymphocytes were isolated on percoll.
Flow Cytometry
Cells were stained at 4°C in the dark with combinations of directly fluorochrome-conjugated antibodies purchased from Biolegend or eBiosciences. MHC class I tetramer staining was performed at ambient temperature for 1 hour. Cells were then fixed for 20 minutes with 2% paraformaldehyde. Tetramer enrichment was used in some experiments (Obar et al., 2008). Acquisition was performed on a LSR II flow cytometer (BD) and data were analyzed with FACSDiva (BD).
In vitro stimulations
Single cell suspensions were incubated with brefeldin A in RPMI 1640 supplemented with 10% fetal bovine serum, L-glutamine, gentamycin, penicillin, and streptomycin. Cells were stimulated with 1ug/ml of LLO91–99 for 5 hours at 37°C and 5% CO2. Cells were stained intracellularly with antibodies specific for IFNγ and TNFα.
Adoptive Transfer
Equal numbers of WT OT-I and OT-I (either Itgae−/− Rag1−/− or Tgfbr2fl/fl dLck-cre) splenocytes were mixed and co-transferred into naïve B6 mice prior to infection. A total of 4×103 cells were transferred. Congenic markers were used to distinguish donors and recipients. For TGFβRII experiments CD44lo CD8α+ cells were sorted for transfer.
In vivo antibody treatments
Mice were given i.p. injections with 100–200μg anti-α4β7 (Bio X Cell; DATK32), 200μg anti-CCR9 (Biolegend; 9B1), or 100–200μg Rat IgG2A (Bio X Cell; 2A3).
Proliferation and Apoptosis
Mice were given 100μg bromodeoxyuridine (BrDU) via intraperitoneal injection. Incorporation of BrDU was determined per manufacturer’s guidelines (BD). Annexin V reactivity was performed per manufacturer’s instructions (BD)
Quantification of bacterial burden
All tissues were mechanically disassociated through a 70μm filter and incubated with 1% saponin for 1 hour at 4°C. Tissue homogenates were plated on Brain Heart Infusion agar plates supplemented with 50μg/mL streptomycin. Colonies were counted after 2 days at 37°C.
Statistical Analysis
All statistical analyses were performed using Prism 5 (GraphPad) software.
Supplementary Material
HIGHLIGHTS.
Intestinal Trm cells protect against secondary infection
Rapid Trm precursor cell generation is TGFβ dependent
CD103 regulates accumulation but not retention within intestinal epithelium
Route of infection influences intestinal Trm cell establishment
Acknowledgments
Supported by National Institutes of Health grants P01AI056172 to L.L. and L.S.C. and R01AI076457 to L.L., and by “Visualizing orally-induced T cell responses in the intestinal mucosa” reference number 2813 from the Crohn’s and Colitis Foundation of America (B.S.S.). We gratefully acknowledge the NIAID Tetramer Core Facility for providing LLO91 Kd tetramers, Wolf-Dieter Schubert (University of Pretoria) and Nancy Freitag (University of Illinois at Chicago) for providing L. monocytogenes mutants, and Michael Bevan (University of Washington) for providing Tgfbr2fl/fl dLck-cre mice. B.S.S., Q.M.P., and Y.T.L. performed experiments. L.S.C. and L.P. contributed to the study design. B.S.S. and L.L. designed the experiments, analyzed and interpreted the data, and wrote the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ariotti S, Beltman JB, Chodaczek G, Hoekstra ME, van Beek AE, Gomez-Eerland R, Ritsma L, van RJ, Maree AF, Zal T, de Boer RJ, Haanen JB, Schumacher TN. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc Natl Acad Sci U S A. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Lecuit M, Cossart P. Listeria monocytogenes internalin and E-cadherin: from bench to bedside. Cold Spring Harb Perspect Biol. 2009;1:a003087. doi: 10.1101/cshperspect.a003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Ghanem EN, Jones GS, Myers-Morales T, Patil PD, Hidayatullah AN, D’Orazio SE. InlA promotes dissemination of Listeria monocytogenes to the mesenteric lymph nodes during food borne infection of mice. PLoS Pathog. 2012;8:e1003015. doi: 10.1371/journal.ppat.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey KA, Fraser KA, Schenkel JM, Moran A, Abt MC, Beura LK, Lucas PJ, Artis D, Wherry EJ, Hogquist K, Vezys V, Masopust D. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and an integrin, αEβ7. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- El Asady R, Yuan R, Liu K, Wang D, Gress RE, Lucas PJ, Drachenberg CB, Hadley GA. TGFβ-dependent CD103 expression by CD8(+) T cells promotes selective destruction of the host intestinal epithelium during graft-versus-host disease. J Exp Med. 2005;201:1647–1657. doi: 10.1084/jem.20041044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson A, Arya A, Agace W. CCL25 enhances CD103-mediated lymphocyte adhesion to E-cadherin. Ann N Y Acad Sci. 2004;1029:334–336. doi: 10.1196/annals.1309.014. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- Grundemann C, Bauer M, Schweier O, von ON, Lassing U, Saudan P, Becker KF, Karp K, Hanke T, Bachmann MF, Pircher H. Cutting edge: identification of E-cadherin as a ligand for the murine killer cell lectin-like receptor G1. J Immunol. 2006;176:1311–1315. doi: 10.4049/jimmunol.176.3.1311. [DOI] [PubMed] [Google Scholar]
- Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of a4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc Natl Acad Sci U S A. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson-Lindbom B, Svensson M, Wurbel MA, Malissen B, Marquez G, Agace W. Selective generation of gut tropic T cells in gut-associated lymphoid tissue (GALT): requirement for GALT dendritic cells and adjuvant. J Exp Med. 2003;198:963–969. doi: 10.1084/jem.20031244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8(+) T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonowski KD, Williams KJ, Marzo AL, Blair DA, Lingenheld EG, Lefrancois L. Dynamics of blood-borne CD8 memory T cell migration in vivo. Immunity. 2004;20:551–562. doi: 10.1016/s1074-7613(04)00103-7. [DOI] [PubMed] [Google Scholar]
- Lee YT, Suarez-Ramirez JE, Wu T, Redman JM, Bouchard K, Hadley GA, Cauley LS. Environmental and antigen-receptor derived signals support sustained surveillance of the lungs by pathogen-specific CTL. J Virol. 2011;85:4085–4094. doi: 10.1128/JVI.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrancois L, Masopust D. The road not taken: memory T cell fate ‘decisions’. Nat Immunol. 2009;10:369–370. doi: 10.1038/ni0409-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefrançois L, Parker CM, Olson S, Schon MP, Muller W, Wagner N, Puddington L. The role of β7 integrins in CD8 T cell trafficking during an anti-viral immune response. J Exp Med. 1999;189:1631–1638. doi: 10.1084/jem.189.10.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon ML, Vega-Ramos J, Lauzurica P, Mueller SN, Stefanovic T, Tscharke DC, Heath WR, Inouye M, Carbone FR, Gebhardt T. The developmental pathway for CD103(+) CD8(+) tissue-resident memory T cells of skin. Nat Immunol. 2013;14:1294–1301. doi: 10.1038/ni.2744. [DOI] [PubMed] [Google Scholar]
- Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Choo D, Vezys V, Wherry EJ, Duraiswamy J, Akondy R, Wang J, Casey KA, Barber DL, Kawamura KS, Fraser KA, Webby RJ, Brinkmann V, Butcher EC, Newell KA, Ahmed R. Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Usherwood EJ, Cauley LS, Olson S, Marzo AL, Ward RL, Woodland DL, Lefrancois L. Activated primary and memory CD8 T cells migrate to nonlymphoid tissues regardless of site of activation or tissue of origin. J Immunol. 2004;172:4875–4882. doi: 10.4049/jimmunol.172.8.4875. [DOI] [PubMed] [Google Scholar]
- Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol. 2006;176:2079–2083. doi: 10.4049/jimmunol.176.4.2079. [DOI] [PubMed] [Google Scholar]
- Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, von Andrian UH. Selective imprinting of gut-homing T cells by Peyer’s patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope C, Kim SK, Marzo A, Masopust D, Williams K, Jiang J, Shen H, Lefrançois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Mosaheb MM, Flavell RA. Opposing effects of TGF-beta and IL-15 cytokines control the number of short-lived effector CD8+ T cells. Immunity. 2009;31:131–144. doi: 10.1016/j.immuni.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel JM, Fraser KA, Vezys V, Masopust D. Sensing and alarm function of resident memory CD8(+) T cells. Nat Immunol. 2013;14:509–513. doi: 10.1038/ni.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Regional and mucosal memory T cells. Nat Immunol. 2011;12:485–491. doi: 10.1038/ni.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan BS, Lefrancois L. Isolation of mouse lymphocytes from small intestine tissues. Curr Protoc Immunol. 2012;Chapter 3(Unit 3) doi: 10.1002/0471142735.im0319s99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiow LR, Rosen DB, Brdickova N, Xu Y, An J, Lanier LL, Cyster JG, Matloubian M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature. 2006;440:540–544. doi: 10.1038/nature04606. [DOI] [PubMed] [Google Scholar]
- Skon CN, Lee JY, Anderson KG, Masopust D, Hogquist KA, Jameson SC. Transcriptional downregulation of S1pr1 is required for the establishment of resident memory CD8 T cells. Nat Immunol. 2013;14:1285–1293. doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Andrian UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N Engl J Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci U S A. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollert T, Pasche B, Rochon M, Deppenmeier S, van den HJ, Gruber AD, Heinz DW, Lengeling A, Schubert WD. Extending the host range of Listeria monocytogenes by rational protein design. Cell. 2007;129:891–902. doi: 10.1016/j.cell.2007.03.049. [DOI] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. TGF-beta signaling to T cells inhibits autoimmunity during lymphopenia-driven proliferation. Nat Immunol. 2012;13:667–673. doi: 10.1038/ni.2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Bevan MJ. Transforming growth factor-beta signaling controls the formation and maintenance of gut-resident memory T cells by regulating migration and retention. Immunity. 2013;39:687–696. doi: 10.1016/j.immuni.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.