Abstract
We determined whether spinal manipulation could prevent and/or reverse the decrease and increase in paraspinal muscle spindle responsiveness caused respectively by lengthening and shortening histories of the lumbar muscles. Single unit spindle activity from multifidus and longissimus muscles was recorded in the L6 dorsal root in anesthetized cats. Muscle history was created and spinal manipulation delivered (thrust amplitude: 1.0mm, duration: 100ms) using a feedback-controlled motor attached to the L6 spinous process. Muscle spindle discharge to a fixed vertebral position (static test) and to vertebral movement (dynamic test) was evaluated following the lengthening and shortening histories. For the static test, changes in muscle spindle responsiveness were significantly less when spinal manipulation followed muscle history (p<0.01), but not when spinal manipulation preceded it (p>0.05). For the dynamic test, spinal manipulation did not significantly affect the history-induced change in muscle spindle responsiveness. Spinal manipulation may partially reverse the effects of muscle history on muscle spindle signaling of vertebral position.
Keywords: Muscle spindle, proprioception, spinal manipulation, lumbar spine, paraspinal muscle, thixotropy, muscle history, chiropractic
Abstract
Nous avons déterminé si les manipulations vertébrales pouvaient prévenir ou inverser la diminution et l’augmentation de la réactivité du fuseau musculaire paravertébral causé respectivement par les antécédents d’allongement et de raccourcissement des muscles lombaires. L’activité des fuseaux musculaires des muscles multifidus et longissimus prise isolément a été notée pour la racine dorsale de la vertèbre L6 chez des chats anesthésiés. Les muscles ont été soumis à un antécédent musculaire et la manipulation vertébrale a été effectuée (amplitude la pulsion : 1,0 mm, durée : 100 ms) au moyen d’un moteur contrôlé par rétroaction fixé à l’apophyse épineuse de L6. Les décharges du fuseau musculaire à une position vertébrale fixe (test statique) et au mouvement vertébral (test dynamique) ont été évaluées à la suite des antécédents d’allongement et de raccourcissement musculaires. Pour ce qui est du test statique, les changements dans la réactivité du fuseau musculaire étaient significativement moindres lorsque la manipulation vertébrale était effectuée après l’antécédent musculaire (p<0,01), ce qui n’était pas le cas lorsque la manipulation vertébrale la précédait (p>0,05). Pour ce qui est du test dynamique, la manipulation vertébrale n’a pas eu d’effet significatif sur le changement de la réactivité du fuseau musculaire provoqué par l’antécédent. La manipulation vertébrale peut partiellement inverser l’effet de l’antécédent musculaire sur la signalisation de la position vertébrale du fuseau musculaire.
Keywords: Fuseau musculaire, proprioception, manipulation vertébrale, colonne lombaire, muscle paravertébral, thixotropie, antécédent musculaire, chiropratique
Introduction
Spinal manipulation is often applied to correct disturbances in the mechanical behavior of spinal motion segments. Motion between facet joints is thought to become restricted or functionally asymmetric due to paraspinal muscle dysfunction, synovial meniscoids or inclusions trapped between articular surfaces of the facet joints, intra-articular or myofascial adhesions, and/or distortion of the annulus fibrosus.1–5 The disturbance, a spinal lesion, has had at least 100 synonyms used to describe it.6 Chiropractic labels it a subluxation, osteopathy labels it somatic dysfunction, and manual medicine labels it fixation or functional blockage. Regardless of professional discipline, a consensus opinion is that altered segmental motion characterizes the spinal lesion for which spinal manipulation is delivered.7,8 Controlled randomized studies indicate that spinal manipulation can induce short lasting changes in the spine’s passive range of motion and longer lasting changes in its active range of motion.9,10, but see 11
Recent findings in humans demonstrate the importance of proprioceptive input from paraspinal muscle spindles for controlling spinal motion including regional repositioning of the lumbar spine and eliciting paraspinal muscle reflex activity. In the human lumbar spine, paraspinal muscle spindles are known to contribute to conscious awareness of low back position and movement velocity.12–14 While healthy individuals can accurately reposition their lumbosacral spine, their repositioning ability is impaired when muscle spindle discharge is increased by applying vibration to the lumbar paraspinal muscles.12,15 During vibration, the correct position is consistently undershot due to the misperception of paraspinal muscle length; lumbosacral orientation is “sensed” as being flexed more than it actually is. Interestingly, lumbosacral repositioning ability is impaired in individuals with a history of low back pain even in the absence of vibration15 suggesting that abnormal proprioceptive signals can contribute to the pathophysiological mechanism of idiopathic low back pain. Additional evidence shows that simply increasing the background discharge from paraspinal muscle spindles affects paraspinal muscle reflexes. For example, vibration-induced stimulation of lumbar paraspinal muscle spindles inhibits the short latency paraspinal EMG activity normally evoked by tapping the erector spinae muscles.16
Paraspinal muscle dysfunction may arise from the history-dependence of muscle spindles in paraspinal muscles. This thixotropic property was first shown clearly for spindles in limb muscles of the anesthetized cat.17,18 A history of having stretched and held the triceps surae muscles at a relatively long length (hold-long) followed by returning them to a shorter, initial length and slowly stretching them decreases the responsiveness of their muscle spindles to both the initial length and the slow stretch when compared to a history of only having held the triceps surae muscles at the shorter, initial length. It was proposed18 that the muscle spindle apparatus stiffens at each held length. However, as the muscle is shortened following the hold-long history, the spindles kink or buckle and their ability to take up the new muscle length decreases.18 This decrease in spindle responsive following a hold-long history alters afferent inflow to the central nervous system and changes the biasing of spinal cord excitability.19 In the leg’s of humans and cats, the lengthening history alters the magnitude and timing of stretch-reflexes.20,21 In the arm’s of humans both lengthening and shortening histories relative to an intermediate length adversely affects repositioning accuracy.22
Muscle spindles in the lumbar multifidus and longissimus muscle also act thixotropically wherein the fidelity of their proprioceptive signaling is influenced by very small, maintained changes in the position of a vertebra.23–27 Maintaining a lumbar vertebra in a position that holds the attached paraspinal muscles at a relatively long versus short length compared to an intermediate length decreases or increases, respectively the subsequent responsiveness of the lumbar muscle spindles to both the intermediate position and to subsequent muscle lengthening from the intermediate position. The magnitude of the altered responsiveness is graded with the magnitude of the change in vertebral position26 and the plane in which the position occurs24. The changes are also graded with the duration over which the vertebral position is maintained.25,27 The effect is maximal by approximately 4 s of lengthening history with a time constant of 1.1 s.25 These changes in spindle behavior represent inaccuracies in the proprioceptive information they provide because the afferent inflow does not represent the actual position of the vertebra. It has been speculated that a history-induced reduction in feedback support from muscle spindles could be a causal element contributing to segmental tissue strain and injury in the low back.27
Based upon a suggestion that spinal manipulation may alter spindle sensitivity and affect muscle activity in the low back27, the aim of the present study was to determine whether spinal manipulation in an animal preparation can correct errors in muscle spindle input that may arise from the thixotropic property of muscle spindles. Specifically, we determined whether spinal manipulation prevented changes in muscle spindle discharge caused by the history of vertebral position and whether spinal manipulation reversed the changes in muscle spindle discharge caused by the history of vertebral position.
Materials and Methods
Preparation
Experiments were performed on 27 deeply anesthetized adult cats (22 males and 5 females) weighting 3.0–5.7 kg. All cats were treated in accordance with the Guiding Principles in the Care and Use of Animals approved by the American Physiological Society. All procedures were initially described by Ge et al.27 Briefly, deep anesthesia was initiated with pentobarbital sodium (35 mg/kg, iv) and maintained with additional dosages (∼5 mg/kg, iv). Cats were mechanically ventilated (model 681; Harvard Apparatus Company, Inc., Millis, MA, USA). Arterial pH, PCO2, and PO2 were measured every 90 minutes using i-STAT System (i-STAT Corporation, East Windsor, NJ, USA) and were maintained within normal range (pH 7.32–7.43; Pco2, 32–37 mm Hg; Po2, >85 mm Hg).
Paraspinal tissue dissection and a bilateral laminectomy limited to the caudal half of L4 and the entire L5 vertebra provided access to the L6 dorsal roots. The low back from L6 caudalward remained intact. To record from muscle spindle afferents from these muscles, thin filaments were teased from L6 dorsal root using sharpened forceps under a dissecting microscope until impulse activity from a single unit with a receptive field in the paraspinal muscles could be identified. Action potentials were identified using a PC-based data acquisition system (Spike 2, Cambridge Electronic Design, Cambridge, UK). Activity from a putative muscle spindle in the lumbar spine was first identified when gentle, manual compression of the lumbar paraspinal tissues evoked a high frequency discharge. Afferents whose discharge was highest in response to probing the back muscles compared with the gluteal, hip, or leg regions, and which responded to manual movement of the L6 vertebra in the dorsal-ventral directions were used. Following the experimental protocols, the back muscles were mechanically isolated by removing the lumbococcygeus muscle. That a receptive ending in the lumbar longissimus or multifidus muscles was the source of neural activity was determined using von Frey hairs (Stoelting Co, Wood Dale, Il, USA) to confirm that the most sensitive area for mechanically activating the afferent was actually located in the back muscles. Three methods were used to confirm that neural activity was from a muscle spindle as described previously:28,29 1) the afferent’s ability to follow vibration (90 Hz, 0.06 mm; Mini-Vibrator, Model NC70209, Morgan Hill, CA, USA ) applied to the muscle, 2) decreased discharge to a direct muscle twitch, and 3) sustained increase in discharge to succinylcholine injection (100–300 μg/kg, intra-arterial).
While recording afferent activity from lumbar para-spinal muscle spindles, actuation of the L6 vertebra was induced using an electronic feedback control system (Lever System Model 310; Aurora Scientific, Aurora, Ontario, Canada). A horizontally-aligned lever arm attached to the motor’s rotary drive shaft was coupled to the L6 spinous process via a pair of adjustable tissue forceps (152.4 mm long, 1 × 2 teeth) vertically aligned. The forceps were clamped tightly onto the lateral surfaces of the L6 spinous process through thin slits along either side of the vertebra. Controlled displacements of the lever arm were applied along the cat’s dorsal-ventral axis thus actuating the vertebra in a dorsal-ventral direction.
Muscle History Caused by Changes in Vertebral Position
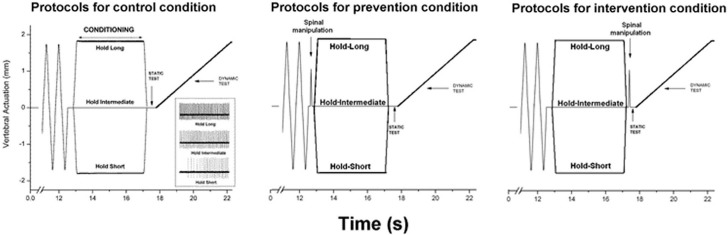
Muscle history was created by holding the L6 vertebra at an intermediate position for 4.0s (hold-intermediate), or by moving it ±1.7mm and then holding it for 4.0s at the new position. Moving the vertebra ±1.7mm maintained the attached muscles at lengths relatively shorter (hold-short) or longer (hold-long) than the hold-intermediate length (see Fig. 1). At the hold-intermediate position, paraspinal tissues exerted no force against the motor’s drive shaft. The direction that constituted hold-short was identified by a reduction in spindle discharge and hold-long by an increase in spindle discharge. Prior to creating each type of muscle history, the system was placed in a similar mechanical state by rapidly moving (10 mm/s): the L6 vertebra back and forth 10 times, stretching and shortening the attached muscles to the same magnitude as the hold amplitude (Fig. 1).
Figure 1.
Schematic of the experimental protocol during 3 spinal manipulation conditions and a representative response (inset during the control condition) of one spindle to three the 3 muscle history conditions. Loading protocol shows the change in vertebral position relative to the intermediate position.
The effects of each muscle history were assessed using a static test and a dynamic test as performed previously.23–27 The static test occurred immediately following each “hold” condition by returning the vertebra to the intermediate position for 0.5s. The dynamic test followed the static test. The vertebra was slowly moved at 0.2 mm/s to the same displacement as the hold condition (1.7mm) in a direction that stretched the paraspinal muscle. Muscle spindle discharge during each of these tests in response to the hold-intermediate history was compared with the hold-long and with the hold-short histories.
Spinal Manipulation
Spinal manipulations were delivered in a fashion similar to those described previously.30–33 Forceps were attached at the L6 vertebra to guide its motion. The forceps were positioned perpendicular to the lever arm so that force and displacement at the end of the lever arm were the same as that at the back of the cat where it was contacted by forceps. With the cat lying prone, spinal manipulation was applied in a vertical direction from dorsalward to ventralward. The displacement-time profile of the manipulation simulated that delivered clinically [discussed in 29,30]. The manipulation was always delivered with the motor in displacement control and at constant velocity (0.01m/s: thrust amplitude = 1.0mm; thrust duration = 100ms).
Experimental Design
Each cat received 3 muscle history conditions: hold-intermediate, hold-long, hold-short. Each cat received 3 manipulation conditions: no spinal manipulation (control), spinal manipulation before creating muscle history (prevention), and spinal manipulation after creating muscle history (intervention). Thus, each cat received 9 protocols and served as its own control. Each of the 9 protocols was separated by at least 5 minutes. The presentation order of the 3 manipulation conditions was randomized across cats. The presentation order of the 3 muscle history conditions was randomized within a manipulation condition.
Data Analysis
Spindle activity was quantified as mean instantaneous frequency (MIF) for the static test and mean frequency (MF) for the dynamic test.23–27 MIF was calculated by averaging the reciprocal of each time interval between consecutive action potentials. MF was calculated by dividing the number of action potentials by the dynamic test’s duration. The responsiveness was defined as the change in MIF or MF between the hold-intermediate and the hold-short (ΔMIFshort, ΔMFshort) or hold-long protocols (ΔMIFlong, ΔMFlong). A positive value indicated an increase in muscle spindle responsiveness and conversely, a negative value indicated a reduction in muscle spindle responsiveness. Values close to zero indicated that conditioning had little or no effect. Spindle responses are reported as means (lower 95% confidence limit, upper 95% confidence limit) unless otherwise indicated.
One-way ANOVA was used to compare the effects of the control, prevention and intervention conditions on muscle spindle responsiveness during the static and dynamic test. Statistical significance was set at the P < 0.05 level for the entire study. Post-hoc pairwise comparisons were performed when significance reached P < 0.05 and were adjusted for multiple comparisons using the Bonferroni method. Statistical analyses were conducted using SAS (version 9.1, SAS Institute, Cary, NC).
Results
Physiological Characteristics of the Spindles
Twenty-eight paraspinal muscle spindle afferents were studied. Receptive fields from 8 afferents were in the lumbar multifidus muscle and 20 were in the longissimus muscle. The most sensitive portion of each receptive field was located medially (i.e., either in the multifidus muscle or the medial border of the longissimus muscle) and near the L6–7 or L7-S1 facet joint. Mechanical thresholds of lumbar paraspinal muscle spindles ranged between 4.0 and 115.2 mN [35.7 (39.2) mN; mean (SD)].
The discharge of all 28 afferents increased in response to succinylcholine injection. Twenty-seven afferents were silenced by bipolar muscle stimulation; 1 afferent could not be tested because the unit was damaged by insertion of the stimulating electrode. Twenty-seven afferents were tested with vibration applied indirectly to the muscle through the thoracolumbar fascia and 28 were tested with vibration applied directly to the muscle’s exposed surface after removing the overlying fascia. During vibration through the fascia, all 27 spindle afferents were activated. Twenty-six were driven 1:1 (70 – 93 imp/s; i.e. with a discharge frequency similar to the vibration frequency) and 1 responded with a subharmonic discharge frequency (44 imp/s), however this latter unit was driven with direct muscle vibration. During vibration applied directly to the surface of the exposed muscle, 27 units were driven by direct muscle vibration. One unit could not be tested by direct muscle vibration because it died before the protocol was completed.
Responses to Conditioning and Spinal Manipulation
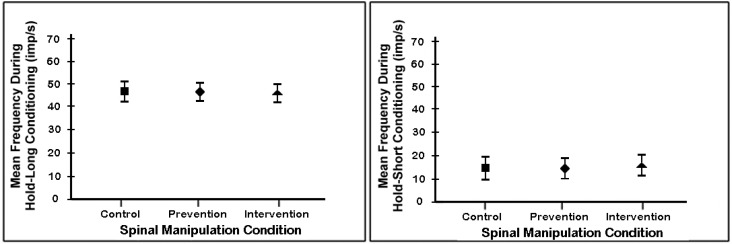
Before analyzing how the 3 spinal manipulation conditions affected the history-dependent responsiveness of muscle spindles during the static and dynamic tests, we wanted to be sure that the spinal manipulation given prior to the creation of muscle history did not differentially affect the creation of muscle history. Therefore, we compared between each of the 3 spinal manipulation conditions spindle activity during the conditioning phase (see “conditioning” label in left panel of figure 1) of both the hold-long and hold-short histories. As shown in figure 2, spindle activities regardless of manipulation condition were similar during the 4 second lengthening histories and during the 4 second shortening histories. Thus, the spinal manipulation given prior to the creation of muscle history did not affect the process of creating history in the spindles.
Figure 2.
Mean discharge frequency of paraspinal muscle spindles for each of the 3 spinal manipulation conditions during the conditioning phase used to create muscle history. Conditioning phase identified graphically in left panel of figure 1. Each symbol represents the mean ± 95% confidence interval of 28 spindles.
Static Test
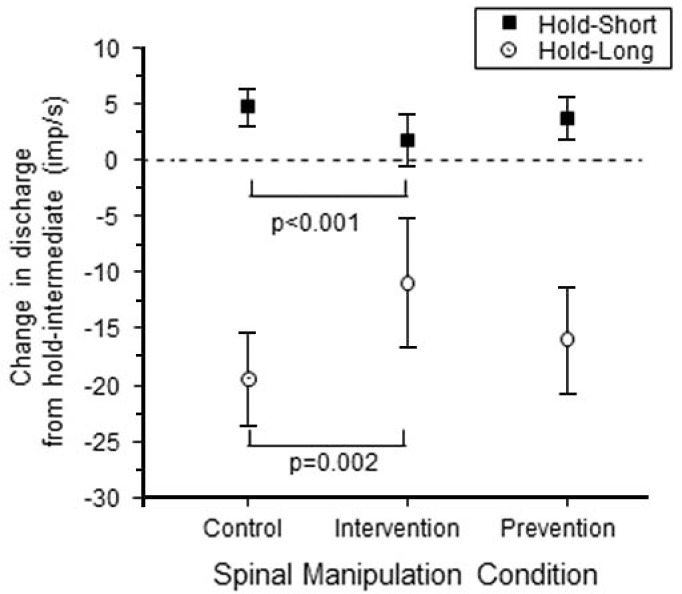
Results from the static test are summarized in figure 3. For the control condition with no spinal manipulation, hold-long compared with hold-intermediate (ΔMIFlong) decreased resting muscle spindle discharge by −19.4 (−23.6, −15.3) imp/s on average whereas hold-short compared with hold-intermediate (ΔMIFshort) increased it by 4.7 (3.0, 6.4) imp/s. This result is consistent with findings from previous studies.23–25,27 For the prevention condition where spinal manipulation was given prior to creating muscle history, ΔMIFlong decreased by −16.0 (−20.9, −11.1) imp/s and ΔMIFshort increased by 3.7 (1.8, 5.7) imp/s. There was no significant difference in the responsiveness between the control and prevention conditions for either the hold-long or hold-short condition (P = 0.33 for ΔMIFlong, P = 0.38 for ΔMIFshort). For the intervention condition where spinal manipulation was given following the creation of muscle history, ΔMIFlong decreased by −10.9 (−16.7, −5.1) imp/s and ΔMIFshort increased by 1.7 (−0.6, 4.1) imp/s. Responsiveness to the effects of muscle history during the intervention condition was significantly less than that during the control condition both for hold-long and hold-short muscle history (P = 0.002 for ΔMIFlong, P < 0.001 for ΔMIFshort).
Figure 3.
Mean change in resting spindle afferent discharge during the static test for the 3 spinal manipulation conditions. Y-axis represents the change in muscle spindle discharge following the hold-long or hold-short compared with the hold-intermediate conditionings (ΔMIFlong or ΔMIFshort). Each symbol represents the mean ± 95% confidence interval of 28 spindles.
Dynamic Test
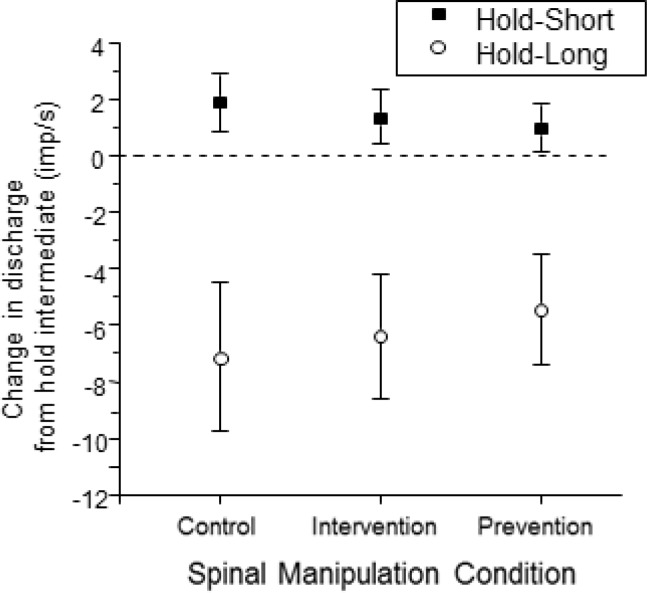
Averaged over the 8.4 s duration of the dynamic test which displaced the vertebra the same amount as the hold-long condition, ΔMFlong for the control, prevention, and intervention conditions was −7.2 (−9.8, −4.5) imp/s, −6.4 (−8.6, −4.2) imp/s, and −5.5 (−7.4, −3.5) imp/s, respectively. ΔMFshort for the three hold conditions was 1.9 (0.9, 2.9) imp/s, 1.3 (0.4, 2.3) imp/s, 1.0 (0.1, 1.9) imp/s, respectively. The magnitudes of the absolute changes in ΔMFlong and ΔMFshort were substantially larger to the lengthening compared to the shortening history. There were no significant differences in either ΔMFlong or ΔMFshort among the 3 spinal manipulation conditions (F2, 83 = 2.45, P = 0.10 and F2, 83 = 2.54, P = 0.09, respectively, Fig. 4).
Figure 4.
Mean change in spindle afferent discharge during the dynamic test for the 3 spinal manipulation conditions. Y-axis represents ΔMF averaged over the entire movement of the dynamic test. Each symbol represents the mean ± 95% confidence interval of 28 spindles.
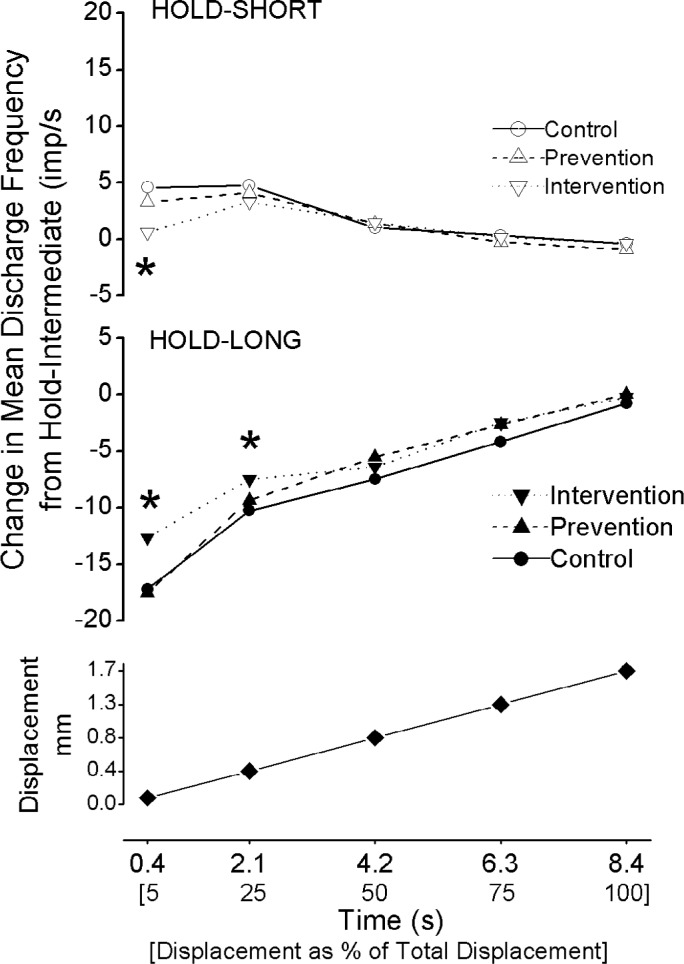
The effect of spinal manipulation on spindle responsiveness during the dynamic test was also averaged over smaller increments of the test. Because the dynamic test was always applied at the same velocity (0.2 mm/s) and to the same magnitude of vertebral movement (1.7mm), identical time points during the test represent the same magnitude of vertebral movement. Therefore responsiveness during similar amounts of vertebral movement could be compared based upon time points of the dynamic test. As shown in Figure 5 (bottom panel), comparisons were made for vertebral movement between the intermediate position and the first 0.09mm of movement (5% of total movement), between 0.09 and 0.42mm (next 20% of total movement), between 0.42 and 0.85mm (25–50 % of total movement), between 0.85 and 1.28 mm (50–75% of total movement), and between 1.28 and 1.70 mm (75–100% of total movement (Fig. 5 bottom panel). Comparisons averaging over 100% of the movement (1.70 mm) represent the average over the entire duration as described in the preceding paragraph. The spinal manipulation intervention condition returned dynamic spindle responsiveness toward normal (i.e., ΔMFlong approached zero) significantly more than either the control or prevention conditions when the vertebra was moved 5% and 25% of the full movement (F2, 83 = 6.22, P = 0.004 and F2, 83 = 3.16, 0.05, respectively, Fig. 5 middle panel). Similarly, the spinal manipulation intervention condition returned dynamic spindle responsiveness toward normal (ie ΔMFshort approached zero) significantly more than either the control or prevention conditions when the vertebra was moved 5% of the full movement (F2, 83 = 7.95, P < 0.001, Fig. 5 top panel). While the effects of the hold-short and hold-long muscle history conditions were present throughout the dynamic test, the specific effect of the spinal manipulation intervention condition was not present after 25% of the dynamic test.
Figure 5.
Time course of changes in muscle spindle discharge during the dynamic test for hold-short compared with hold-intermediate (upper panel) and for hold-long compared with hold-intermediate (middle panel). Bottom panel shows the magnitude of the vertebral movement over which the dynamic test was analyzed. * p<0.05 compared with the spinal manipulation control condition. Each symbol represents the average value between its time position and the time position of the previous data point, except for 5% which represents the average value between its time position and time 0 s.
Discussion
One clinical consequence of spinal manipulation is thought to be the normalization of paraspinal neuro-muscular dysfunction. The present study demonstrated that spinal manipulation partially reversed but did not prevent the decrease in muscle spindle responsiveness caused by the lengthening history of lumbar paraspinal muscles (i.e. by hold-long), This suggests that spinal manipulation could reduce proprioceptive errors caused by the thixotropic property of muscle spindles in paraspinal muscles. Although the nature of the paraspinal muscle dysfunction amenable to spinal manipulation is not clear, changes in proprioceptive input or processing have often been proposed as a cause.34,35 In the limbs, muscle history has been shown to disrupt neuromuscular integration by altering proprioceptive feedback from muscle spindles which creates positioning errors and modifies the timing and magnitude of reflex support.20–22 In the vertebral column, we do not know with any certainty whether para-spinal muscle history contributes to the dysfunction for which spinal manipulation is applied clinically.36
The effect of both the hold-long and hold-short history during the control condition (no spinal manipulation) was similar to our previous studies showing that the positional history of a lumbar vertebra differentially alters the responsiveness of the paraspinal muscle spindles.25,27 The discharge of spindles with a vertebra held at an intermediate position and during vertebral movement from that intermediate position decreases significantly when the intermediate position has been preceded by a vertebral position that maintains the spindle apparatus at longer length. Conversely, maintaining the spindle apparatus at shorter length relative to that at the intermediate position increases spindle responsiveness to both vertebral position and movement.
Several studies suggest that small changes in para-spinal muscle force can have a large impact on a motion segment’s biomechanical behavior and stability.37–40 These studies have contributed to the idea that damage to structures of the vertebral column and the risk of injury to the spine can be great during easy, non-demanding tasks.38 For example, in vitro experiments accompanied by a modeling approach that incorporates graded increases in the activity of 1 lumbar paraspinal muscle show an increase in vertebral stabilization.37 Graded increases in the muscle’s modeled activity decreases the interseg-mental neutral zone (range: 33%–40%) during flexion, extension, and axial rotation but not lateral bending, and decreases intersegmental range of motion (range: 7–27%) during extension and axial rotation but not flexion or lateral bending. The largest decrease in the neutral zone (hence greatest stabilization) and range of motion during these maneuvers occurs at low muscle forces (20N compared with 40N and 60N). Similarly, a very small increase (1–3% of maximal voluntary contraction) in lumbar multifidus, iliocostalis and thoracic longissimus muscle activity at L2–L4 is sufficient to restore segmental stability of the lumbar spine even when the loading moments are increased to 75% of body weight.38 When the force vectors from 5 paraspinal muscles are incorporated into the modeling approach, stabilization of an individual lumbar motion segment also increases: the interseg-mental neutral zone decreases (range: 76–83%) during flexion, extension, axial rotation, and lateral bending and intersegmental range of motion decreases (range: 55–93%) during flexion, extension, axial rotation, and lateral bending.39 Multifidus muscle accounts for 40–80% of the increased stability during sagittal flexion-extension, 45% during axial rotation, and 10–20% during lateral bending suggesting that neuromuscular mechanisms controlling multifidus muscle activity alone could functionally impact the motion segment especially during flexion-extension and axial rotation. Abnormal control of multifidus muscle may contribute to the fact that mechanical injury to the intervertebral disk occurs most often during loading moments that combine flexion, lateral bending, and axial rotation.40 We speculate that intersegmental and regional spinal postural history, when it changes the responsiveness of paraspinal muscle spindles, represents a source of inaccurate proprioceptive information from the para-spinal muscles that could affect development of low level muscle activity and compromise neuromuscular control of spinal stability.
The phenomenon of muscle history is thought to arise from the spontaneous formation of stable, non-recycling intrafusal cross-bridges between actin and myosin filaments when muscle is held at constant length.41 During voluntary muscle contraction in the limbs, co-activation of gamma- with alpha-motoneurons is thought to break these non-recycling crossbridges and return spindle afferent signaling to normal.18,42,43 However, in the spine, voluntary paraspinal extensor contractions may not be as effective at reversing the effects of muscle history44 in that forward flexion does not eliminate proprioceptive changes whose origins are consistent with a lengthening history44,45. The present study demonstrated that spinal manipulation helped reduce errors in muscle spindle signaling caused by the history of vertebral position. While passive stretching will eventually break the crossbridges as indicated in Figure 5, spinal manipulation may reduce the effects of history when voluntary movement is unable to stretch the muscles to a length that created the history in the first place. In clinical practice, spinal manipulation may have a greater influence on reducing the effects of muscle history than shown in this study because when applied manually, the practitioner typically brings a joint to its end range of motion and then moves it slightly beyond what the patient can accomplish through voluntary activity alone.46
Relevance and application
Well-designed, scientific studies using animal preparations are a means to understand neural mechanisms that contribute to the physiological effects of spinal manipulation. The knowledge gained through such studies can provide biological validation for the use of spinal manipulation, and help improve its delivery for the healthcare of patients.
In clinical practice, palpatory examination of the back identifies abnormalities in the texture and tone of para-spinal soft tissues, the presence of pain and/or tenderness in these tissues, and restrictions in spinal joint motion in or near these areas.47,48 The idea that altered proprioceptive input from paraspinal tissues can cause these abnormalities and that spinal manipulation corrects these inputs is not new. Nearly 4 decades ago Korr35,49 presented the idea the central nervous system’s ability to appropriately control and coordinate activities of the paraspinal musculature and its autonomic support requires an accurate representation of their conditions. Such assessment arises in part from reliable, coherent patterns of neural feedback from sensory receptors in the paraspinal tissues.
Korr originally proposed35 that decreased muscle spindle input from paraspinal tissues causes the central nervous system to increase gamma motoneuron activity in an effort to regain or normalize sensory feedback from these proprioceptors. One consequence of this increased gain was thought to be the change in paraspinal tissue texture and tone described above and previously measured by Denslow.50 Spinal manipulation was thought to induce a barrage of sensory input form the paraspinal muscle spindles which enabled the central nervous system to normalize gamma motoneuron activity. Although there are no data regarding spinal manipulation’s effect on gamma motoneurons, animal studies have shown that a barrage of sensory input from muscle spindles does occur during spinal manipulation when it is delivered with biomechanical characteristics similar to those used clinically.29,51 The present study confirmed that lengthening histories of paraspinal muscles reduces normal muscle spindle input, creating errors in the assessment of segmental vertebral and revealed that spinal manipulation under these conditions can return spindle input toward normal.
Acknowledgments
The authors thank Mr. Randall Sozio for technical work, Dr. Cynthia Long and Mr. Ying Cao for statistical assistance.
Footnotes
Sources of Support: Work was supported by NIH grant NS46818 to JGP and conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR15433 from the National Center for Research Resources, NIH.
References
- 1.Farfan HF. The scientific basis of manipulation procedures. In: Buchanan WW, Kahn MF, Laine V, Rodnan GP, Scott JT, Zvaifler NJ, et al., editors. Clinics in rheumatic diseases. London: W.B. Saunders Company Ltd; 1980. pp. 159–77. [Google Scholar]
- 2.Giles LGF. Anatomical Basis of Low Back Pain. Baltimore: Williams & Wilkins; 1989. [Google Scholar]
- 3.Lewit K. Manipulative Therapy in Rehabilitation of the Locomotor System. Oxford: Butterworth-Heinemann; 1991. [Google Scholar]
- 4.Haldeman S. The clinical basis for discussion of mechanisms of manipulative therapy. In: Korr IM, editor. The neurobiologic mechanisms in manipulative therapy. NY: Plenum; 1978. pp. 53–75. [Google Scholar]
- 5.Vernon H. Biological rationale for possible benefits of spinal manipulation. 1997:105–115. AHCPR Publication No. 98-N002. [Google Scholar]
- 6.Gatterman MI. What’s in a word? In: Gatterman MI, editor. Foundations of Chiropractic. St. Louis: Mosby; 1995. pp. 6–17. [Google Scholar]
- 7.Triano JJ. The Functional Spinal Lesion: An Evidence-Based Model of Subluxation. Top Clin Chiro. 2001;8(1):16–28. [Google Scholar]
- 8.Meeker WC, Haldeman S. Chiropractic:a profession at the crossroads of mainstream and alternative medicine. Ann Intern Med. 2002;136(3):216–227. doi: 10.7326/0003-4819-136-3-200202050-00010. [DOI] [PubMed] [Google Scholar]
- 9.Nansel D, Peneff A, Cremata E, Carlson J. Time course considerations for the effects of unilateral lower cervical adjustments with respect to the amelioration of cervical lateral-flexion passive end-range asymmetry. J Manipulative Physiol Ther. 1990;13(6):297–304. [PubMed] [Google Scholar]
- 10.Whittingham W, Nilsson N. Active range of motion in the cervical spine increases after spinal manipulation (toggle recoil) J Manipulative Physiol Ther. 2001;24(9):552–55. doi: 10.1067/mmt.2001.118979. [DOI] [PubMed] [Google Scholar]
- 11.Millan M, Leboeuf-Yde C, Budgell B, Descarreaux M, Amorim MA. The effect of spinal manipulative therapy on spinal range of motion: a systematic literature review. Chiropr Man Therap. 2012;20(1):23. doi: 10.1186/2045-709X-20-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brumagne S, Lysens R, Swinnen S, Verschueren S. Effect of paraspinal muscle vibration on position sense of the lumbosacral spine. Spine. 1999;24(13):1328–31. doi: 10.1097/00007632-199907010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Gade VK, Wilson SE. Position sense in the lumbar spine with torso flexion and loading. J Appl Biomech. 2007;23(2):93–102. doi: 10.1123/jab.23.2.93. [DOI] [PubMed] [Google Scholar]
- 14.Soltys JS, Wilson SE. Directional sensitivity of velocity sense in the lumbar spine. J Appl Biomech. 2008;24(3):244–51. doi: 10.1123/jab.24.3.244. [DOI] [PubMed] [Google Scholar]
- 15.Brumagne S, Cordo P, Lysens R, Verschueren S, Swinnen S. The role of paraspinal muscle spindles in lumbosacral position sense in individuals with and without low back pain. Spine. 2000;25(8):989–994. doi: 10.1097/00007632-200004150-00015. [DOI] [PubMed] [Google Scholar]
- 16.Dimitrijevic MR, Gregoric MR, Sherwood AM, Spencer WA. Reflex responses of paraspinal muscles to tapping. J Neurol Neurosurg Psychiat. 1980;43(12):1112–1118. doi: 10.1136/jnnp.43.12.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morgan DL, Prochazka A, Proske U. The after-effects of stretch and fusimotor stimulation on the responses of primary endings of cat muscle spindles. J Physiol. 1984;356:465–77. doi: 10.1113/jphysiol.1984.sp015477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles. J Neurophysiol. 1986;56(2):451–61. doi: 10.1152/jn.1986.56.2.451. [DOI] [PubMed] [Google Scholar]
- 19.Gregory JE, Morgan DL, Proske U. Changes in size of the stretch reflex of cat and man attributed to aftereffects in muscle spindles. J Neurophysiol. 1987;58(3):628–40. doi: 10.1152/jn.1987.58.3.628. [DOI] [PubMed] [Google Scholar]
- 20.Gregory JE, Mark RF, Morgan DL, Patak A, Polus B, Proske U. Effects of muscle history on the stretch reflex in cat and man. J Physiol. 1990;424:93–107. doi: 10.1113/jphysiol.1990.sp018057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wood SA, Gregory JE, Proske U. The influence of muscle spindle discharge on the human H reflex and the monosynaptic reflex in the cat. J Physiol. 1996;497:279–90. doi: 10.1113/jphysiol.1996.sp021767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregory JE, Morgan DL, Proske U. Aftereffects in the responses of cat muscle spindles and errors of limb position sense in man. J Neurophysiol. 1988;59(4):1220–30. doi: 10.1152/jn.1988.59.4.1220. [DOI] [PubMed] [Google Scholar]
- 23.Cao DY, Pickar JG. Lengthening but not shortening history of paraspinal muscle spindles in the low back alters their dynamic sensitivity. J Neurophysiol. 2011;105(1):434–41. doi: 10.1152/jn.00498.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ge W, Cao DY, Long CR, Pickar JG. Plane of vertebral movement eliciting muscle lengthening history in the low back influences the decrease in muscle spindle responsiveness of the cat. J Appl Physiol. 2011;111(6):1735–1743. doi: 10.1152/japplphysiol.00059.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge W, Pickar JG. Time course for the development of muscle history in lumbar paraspinal muscle spindles arising from changes in vertebral position. Spine J. 2008;8(2):320–28. doi: 10.1016/j.spinee.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ge W, Pickar JG. The decreased responsiveness of lumbar muscle spindles to a prior history of spinal muscle lengthening is graded with the magnitude of change in vertebral position. J Electromyogr Kinesiol. 2012;22(6):814–820. doi: 10.1016/j.jelekin.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ge W, Long CR, Pickar JG. Vertebral position alters paraspinal muscle spindle responsiveness in the feline spine: effect of positioning duration. J Physiol. 2005;569:655–665. doi: 10.1113/jphysiol.2005.095281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickar JG. An in vivo preparation for investigating neural responses to controlled loading of a lumbar vertebra in the anesthetized cat. J Neurosci Methods. 1999;89:87–96. doi: 10.1016/s0165-0270(99)00060-6. [DOI] [PubMed] [Google Scholar]
- 29.Reed WR, Cao DY, Long CR, Kawchuk GN, Pickar JG. Relationship between biomechanical characteristics of spinal manipulation and neural responses in an animal model: effect of linear control of thrust displacement versus force, thrust amplitude, thrust duration, and thrust rate. Evid Based Complement Alternat Med. 2013;2013:492039. doi: 10.1155/2013/492039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pickar JG, Sung PS, Kang YM, Ge W. Response of lumbar paraspinal muscle spindles is greater to spinal manipulative loading compared with slower loading under length control. Spine J. 2007;7(5):583–595. doi: 10.1016/j.spinee.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pickar JG, Kang YM. Paraspinal muscle spindle responses to the duration of a spinal manipulation under force control. J Manipulative Physiol Ther. 2006;29(1):22–31. doi: 10.1016/j.jmpt.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 32.Sung PS, Kang YM, Pickar JG. Effect of spinal manipulation duration on low threshold mechanoreceptors in lumbar paraspinal muscles: a preliminary report. Spine. 2005;30(1):115–22. doi: 10.1097/01.brs.0000147800.88242.48. [DOI] [PubMed] [Google Scholar]
- 33.Cao DY, Reed WR, Long CR, Kawchuk GN, Pickar JG. Effects of thrust amplitude and duration of high-velocity, low-amplitude spinal manipulation on lumbar muscle spindle responses to vertebral position and movement. J Manipulative Physiol Ther. 2013;36(2):68–77. doi: 10.1016/j.jmpt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leach RA. The Chiropractic Theories. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 35.Korr IM. Proprioceptors and somatic dysfunction. J Am Osteopath Assoc. 1975;74:638–50. [PubMed] [Google Scholar]
- 36.Owens EF, Jr, Henderson CN, Gudavalli MR, Pickar JG. Head repositioning errors in normal student volunteers: a possible tool to assess the neck’s neuromuscular system. Chiropr Osteopat. 2006;14:5. doi: 10.1186/1746-1340-14-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panjabi MM, Kuniyoshi A, Duranceau J, Oxland T. Spinal stability and intersegmental muscle forces: a biomechanical model. Spine. 1989;14(2):194–99. doi: 10.1097/00007632-198902000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Cholewicki J, McGill SM. Mechanical stability of the in vivo lumbar spine: implications for injury and chronic low back pain. Clin Biomech. 1996;11(1):1–15. doi: 10.1016/0268-0033(95)00035-6. [DOI] [PubMed] [Google Scholar]
- 39.Wilke HJ, Wolf S, Claes LE, Arand M, Weisand A. Stability increase of the lumbar spine with different muscle groups: A biomechanical in vitro study. Spine. 1995;20:192–98. doi: 10.1097/00007632-199501150-00011. [DOI] [PubMed] [Google Scholar]
- 40.Nordin M, Balagué F. Biomechanics and ergonomics in disk herniation accompanied by sciatica. In: Weinstein JN, Gordon SL, editors. Low Back Pain. Rosemont: American Academy of Orthopaedic Surgeons; 1996. pp. 23–48. [Google Scholar]
- 41.Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: a review. Prog Neurobiol. 1993;41:705–21. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- 42.Allen TJ, Ansems GE, Proske U. Evidence from proprioception of fusimotor coactivation during voluntary contractions in humans. Exp Physiol. 2008;93(3):391–98. doi: 10.1113/expphysiol.2007.040741. [DOI] [PubMed] [Google Scholar]
- 43.Wise AK, Gregory JE, Proske U. The responses of muscle spindles to small, slow movements in passive muscle and during fusimotor activity. Brain Res. 1999;821:87–94. doi: 10.1016/s0006-8993(99)01071-9. [DOI] [PubMed] [Google Scholar]
- 44.Wilson SE, Granata KP. Reposition sense of lumbar curvature with flexed and asymmetric lifting postures. Spine. 2003;28(5):513–18. doi: 10.1097/01.BRS.0000048674.75474.C4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanchez-Zuriaga D, Adams MA, Dolan P. Is activation of the back muscles impaired by creep or muscle fatigue? Spine. 2010;35(5):517–25. doi: 10.1097/BRS.0b013e3181b967ea. [DOI] [PubMed] [Google Scholar]
- 46.Grice A, Vernon H. Basic principles in the performance of chiropractic adjusting: historical review, classification, and objectives. In: Haldeman S, editor. Principles and practice of chiropractic. Norwalk: Appleton & Lange; 1992. pp. 443–58. [Google Scholar]
- 47.Kuchera WA, Kappler RE. Musculoskeletal examination for somatic dysfunction. In: Ward RC, Hruby RJ, Jerome JA, Jones JM, Kappler RE, editors. Foundations for Osteopathic Medicine. Lippincott Williams & Wilkins; 2002. pp. 633–59. [Google Scholar]
- 48.Sportelli L, Tarola G. Documentation and record keeping. In: Haldeman S, Dagenais S, Budgell B, Grunnet-Nilsson N, Hooper PD, Meeker WC, et al., editors. Principles and Practice of Chiropractic. NY: McGraw-Hill; 2005. pp. 725–41. [Google Scholar]
- 49.Korr IM. The spinal cord as organizer of disease processes:some preliminary perspectives. J Am Osteopath Assoc. 1976;76:89–99. [PubMed] [Google Scholar]
- 50.Denslow JS, Korr IM, Krems AD. Quantitative studies of chronic facilitation in human motoneuron pools. Am J Physiol. 1947;150:229–38. doi: 10.1152/ajplegacy.1947.150.2.229. [DOI] [PubMed] [Google Scholar]
- 51.Pickar JG, Wheeler JD. Response of muscle proprioceptors to spinal manipulative-like loads in the anesthetized cat. J Manipulative Physiol Ther. 2001;24(1):2–11. doi: 10.1067/mmt.2001.112017. [DOI] [PubMed] [Google Scholar]