SUMMARY
In vertebrates, pluripotent pharyngeal mesoderm progenitors produce the cardiac precursors of the second heart field as well as the branchiomeric head muscles and associated stem cells. However, the mechanisms underlying the transition from multipotent progenitors to distinct muscle precursors remain obscured by the complexity of vertebrate embryos. Using Ciona intestinalis as a simple chordate model, we show that bipotent cardiopharyngeal progenitors are primed to activate both heart and pharyngeal muscle transcriptional programs, which progressively become restricted to corresponding precursors. The transcription factor COE (Collier/OLF/EBF) orchestrates the transition to pharyngeal muscle fate both by promoting an MRF-associated myogenic program in myoblasts and by maintaining an undifferentiated state in their sister cells through Notch-mediated lateral inhibition. The latter are stem cell-like muscle precursors that form most of the juvenile pharyngeal muscles. We discuss the implications of our findings for the development and evolution of the chordate cardiopharyngeal mesoderm.
INTRODUCTION
In vertebrates, trunk and limb skeletal muscles develop from somites (Christ and Ordahl, 1995; Scaal and Christ, 2004), whereas branchiomeric head muscles derive from the pharyngeal mesoderm, which also produces the cardiac progenitors of the second heart field (Lescroart et al., 2010; Tirosh-Finkel et al., 2006). These differences between the trunk and head muscles are reflected in distinct early regulatory networks that later converge on a core of skeletal muscle regulators and terminal differentiation genes (Harel et al., 2009; Sambasivan et al., 2009). Shared expression of the DNA-binding transcription factors Nkx2-5, Tbx1 and Islet1 between developing branchiomeric and cardiac muscles probably stems from their common clonal origin (Buckingham and Vincent, 2009; Tzahor, 2009).
Within the somitic and pharyngeal muscle lineages, muscle growth and regeneration require maintenance of muscle stem cell pools (i.e. muscle progenitors in embryo and satellite cells in adult) along with differentiating myoblasts (Gros et al., 2005; Harel et al., 2009; Relaix and Zammit, 2012; Sambasivan et al., 2009). However, the sequence of cellular and molecular events underlying the clonal continuum from early embryonic to adult muscle stem cells remains obscured by the complexity of early vertebrate embryos (Sambasivan et al., 2013).
Satellite-like cells have been identified in the basal chordate amphioxus (Somorjai et al., 2012) and Drosophila possesses transient, stem cell-like, adult muscle progenitors (Figeac et al., 2007; Ruiz Gomez and Bate, 1997). Thus, the existence of stem cell-like muscle progenitors, defined by their ability to self-renew and produce new myoblasts, predates the origin of vertebrates. However, the evolutionary origin of stem cell-like head muscle progenitors remains elusive due to the absence of clear pharyngeal muscles in amphioxus (Sambasivan et al., 2011; Tolkin and Christiaen, 2012).
Ascidians are among the closest living relatives of vertebrates (Delsuc et al., 2006) and studies in Ciona intestinalis have identified the ascidian counterpart to the vertebrate multipotent cardiopharyngeal progenitors (Stolfi et al., 2010; Tolkin and Christiaen, 2012). In early Ciona embryos, two B7.5 blastomeres express the conserved cardio-craniofacial determinant Mesp, which is essential for heart development (Figures 1A and 1B; Satou et al., 2004). During gastrulation, each B7.5 blastomere divides twice to produce two anterior tail muscle (ATM) cells and two cardiogenic progenitors called trunk ventral cells (TVCs) (Figure 1A). The TVCs are induced in late gastrula embryos by a fibroblast growth factor (FGF) signal mediated by the mitogen activated protein kinase (MAPK) pathway and the transcription factor Ets1/2 (Cooley et al., 2011; Davidson et al., 2006). Upon FGF induction, TVCs activate the transcription factor FoxF, which promotes their migration (Figures 1A and 1B; Beh et al., 2007). Each TVC then divides asymmetrically and medio-laterally to form a median first heart precursor (FHP) and a lateral secondary TVC, which divide again into a median second heart precursor (SHP) and a lateral atrial siphon muscle (ASM) founder cell (ASMF; Figure 1A; Stolfi et al., 2010; Wang et al., 2013). Bilateral pairs of ASMFs divide antero-posteriorly to form four ASM precursor cells (ASMPs), which then migrate towards the atrial siphon placode, divide and form a ring of eight cells (Figure 1A). These cells later continue to proliferate and produce two populations of body wall muscles: the ASM sensu stricto and the longitudinal muscles (LoM; Figure 1A; Sasakura et al., 2012; Stolfi et al., 2010). Ciona intestinalis juveniles possess two bilateral atrial siphons that fuse several days after metamorphosis (Chiba et al., 2004). To our knowledge, all events described herein occur symmetrically in both the left and right atrial siphon primordia.
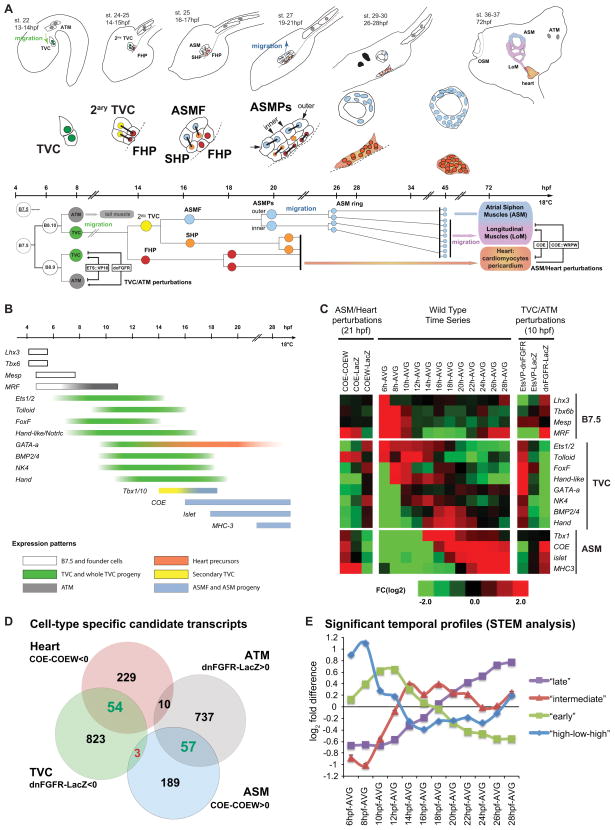
Figure 1. Schematic representation and microarray analysis of cardiopharyngeal development in Ciona intestinalis.
(A) Schematic embryos, larvae and juveniles showing the divisions, migrations (green and blue arrows), and morphogenesis of the B7.5 lineage cells (higher magnifications): Trunk ventral cells (TVC, green), anterior tail muscles (ATM, grey), secondary TVC (2ary TVC, yellow), first heart precursors (FHP, red), atrial siphon muscle founder cells (ASMF, blue), second heart precursors (SHP, orange), inner and outer ASM precursors (ASMPs, blue), the ASM ring, heart (red/orange) and longitudinal muscles (LoM). Dashed line : midline. Oral Siphon Muscles (OSM) do not derive from the B7.5 lineage. The approximate time-line applies to the diagrams and lineage tree (hpf: hours post-fertilization). The B7.5 cardiopharyngeal lineage. Same colors as above, right side is shown (B7.5: left side), only one TVC lineage is shown. Mesp>dnFGFR and Mesp>ETS::VP16 perturbations alter ATM vs TVC specification. FoxF>COE and FoxF>COE::WRPW alter ASM vs Heart fates. See the text for details. (B) Schematic representation of published spatio-temporal expression patterns of the B7.5 lineage genes. (C)Transcription profiles of B7.5, TVC, heart (GATA-a) and ASM markers in perturbations conditions and control time series from B7.5-lineage sorted cells. Colors indicate fold change in pairwise comparisons, e.g. COE-COEW, means differential expression in FoxF>COE compared to FoxF>COE::WRPW samples sorted at 21 hpf. COEW, COE::WRPW; EtsVP, Ets::VP16; AVG, average over the whole time series; FC, Fold Change. 6hpf and “TVC/ATM perturbations” datasets were previously published (Christiaen et al., 2008). (D) Euler diagrams showing the logics and numbers of “heart”, “ASM”, “TVC” and “ATM” candidate transcripts. Green or red numbers indicate significantly enriched or depleted mutual overlaps, respectively. See Table S2 for detailed statistics. (E) Significant temporal profiles determined by STEM. See also Data S1.
Following the first TVC division, the secondary TVCs activate Tbx1/10 (Figure 1A and 1B; Wang et al., 2013). After the following division, Tbx1/10 is maintained in the ASMFs where it activates the atypical helix-loop-helix DNA-binding transcription factor-coding gene COE (Figure 1; Wang et al., 2013). ASMPs later up-regulate the LIM homeobox Islet homolog and the siphon muscle differentiation marker MHC-3 (Myosin Heavy Chain-3), successively (Figure 1A and 1B; Stolfi et al., 2010; Wang et al., 2013). TVC-specific misexpression of COE using a FoxF enhancer forced the whole TVC progeny to activate Islet and MHC-3 and to assume an ASM fate at the expense of the heart (Figure 1A; Stolfi et al., 2010). Conversely, TVC-specific misexpression of the dominant repressor COE::WRPW blocked Islet and MHC-3 expressions, inhibited ASM development and caused the formation of excess heart tissue (Figure 1A; Stolfi et al., 2010).
Here, we combined fluorescence-activated cell sorting (FACS) and whole genome transcription profiling following perturbations of COE function to characterize the transcriptional dynamics underlying the specification of heart and ASM precursors in the ascidian cardiopharyngeal lineage. We present evidence that multilineage transcriptional priming defines the TVCs as pluripotent cardiopharyngeal progenitors. We show that COE orchestrates the transition from a pluripotent state to pharyngeal muscle commitment by antagonizing progenitor- and cardiac-specific gene expressions, while promoting both differentiation and the Notch-mediated maintenance of stemness among pharyngeal muscle precursors.
RESULTS
Transcription profiles recapitulate cardiopharyngeal expression dynamics
In order to characterize transcription profiles underlying heart versus pharyngeal muscle (i.e. ASM) specification, we used TVC-specific misexpression of either COE or the dominant repressor COE::WRPW to force ASM or heart specification, respectively (Figure 1). We used microarrays to profile the transcriptomes of FACS-purified cardiopharyngeal cell populations expressing FoxF>COE, FoxF>COE::WRPW or the FoxF>NLS::lacZ control and isolated from 21 hours post-fertilization (hpf) larvae, when the ASM and heart precursors have segregated and their specific transcription programs have been initiated (Figure 1). We reasoned that ASM candidate genes would be up-regulated by FoxF>COE and/or down-regulated by FoxF>COE::WRPW, while heart candidate genes would show the opposite responses given the established effects of these constructs on ASM and heart specification (Figure 1D, (Stolfi et al., 2010). Comparable logics previously identified TVC-specific expression profiles using FACS-purified samples from early embryos (Christiaen et al., 2008; Woznica et al., 2012). In these studies, Mesp enhancer-driven misexpression of a dominant negative FGF receptor (Mesp>dnFGFR) blocked TVC induction, forcing all B7.5 derivatives to form anterior tail muscles (ATM; Figure 1A). Accordingly, FACS, microarrays and in situ hybridization assays showed that genes down-regulated by Mesp>dnFGFR were expressed specifically in the TVCs. Conversely, the constitutively active form of Ets1/2, Ets::VP16, was sufficient to up-regulate TVC-specific genes and force TVC induction in the whole B7.5 lineage, while blocking ATM-specific gene expression. Thus, comparing B7.5 lineage-specific transcription profiling following electroporation of Mesp>dnFGFR, Mesp>Ets::VP16 and Mesp>LacZ identified TVC- and ATM-specific gene expression profiles (Figures 1C and 1D; Christiaen et al., 2008; Woznica et al., 2012).
To study the transcriptional dynamics underlying fate specification in the cardiopharyngeal lineage, we also profiled B7.5-lineage cells from control embryos and larvae collected every two hours from 8 to 28 hpf. This time window encompasses all developmental transitions from early TVC specification until ASM ring formation and initial differentiation (Figures 1A, 1E). This data was visualized through pairwise comparisons between each time point and the average expression values (Figure 1C). We used the STEM algorithm (Ernst and Bar-Joseph, 2006) and identified significantly over-represented temporal profiles including (1) an “high-low-high” profile with an expression peak at 8 hpf, followed by the lowest expression at 18 hpf and a second increase thereafter; 2) an “early” profile with expression peaks between 10 and 12hpf; 3) an “intermediate” profile with expression peaks between 14 and 18hpf and (4) a “late” profile with increased expression after 20hpf (Figures 1E and Data S1–2). In subsequent analyses, we used our ASM vs Heart, TVC vs ATM and temporal profiles to characterize the transcriptional signatures of cardiopharyngeal cell types, including the bipotent TVC progenitors, the cardiac and the ASM precursors.
We first determined that comprehensive transcription profiles reflected the known expression dynamics of TVC and ASM markers. The data captured the onset of COE expression at 16 hpf, as well as Islet and MHC-3 deregulation upon perturbations of COE function and their sequential activations (Figure 1A–1C; Stolfi et al., 2010; Wang et al., 2013). These patterns mirror those determined by in situ hybridization indicating that our microarray data recapitulated ASM-specific gene expression profiles.
TVC markers, including the conserved cardiac determinants NK4, GATA-a and Hand, were downregulated at 10 hpf by Mesp>dnFGFR and at 21 hpf by FoxF>COE (Figure 1C, (Christiaen et al., 2008). This is consistent with COE’s inhibitory effects on heart development and GATA-a’s heart-specific expression (Figures 1C and Data S1-1; Stolfi et al., 2010; Wang et al., 2013). The temporal profiles of cardiopharyngeal markers also recapitulated their documented expression patterns: B7.5 markers are expressed early and rapidly downregulated; the expression of successive TVC markers starts between 6 and 12hpf (Figures 1B and 1C; Beh et al., 2007; Christiaen et al., 2010; Davidson et al., 2006; Ragkousi et al., 2011; Satou et al., 2004). The Tbx1/10 profile is consistent with its transient expression in the 2ary TVCs and ASMFs between 14 and 18 hpf (Wang et al., 2013). Thus, our microarray dataset captured the gene expression dynamics underlying fates’ segregation in the cardiopharyngeal lineage.
TVCs are transcriptionally primed bipotent cardiopharyngeal progenitors
We first focused on heart candidate genes that were down-regulated by over-expression of COE and/or up-regulated by COE::WRPW. We identified 293 heart candidate genes that were significantly up-regulated upon COE::WRPW expression compared to COE misexpression (Figures 1D, 2A, Data S1-1, and Table S1). The pool of heart candidate genes was significantly enriched in TVC candidate genes, suggesting that COE activity inhibits the expression of TVC genes in the cardiopharyngeal lineage (Figures 1A–D and Data S1-1, Table S2; Christiaen et al., 2008).
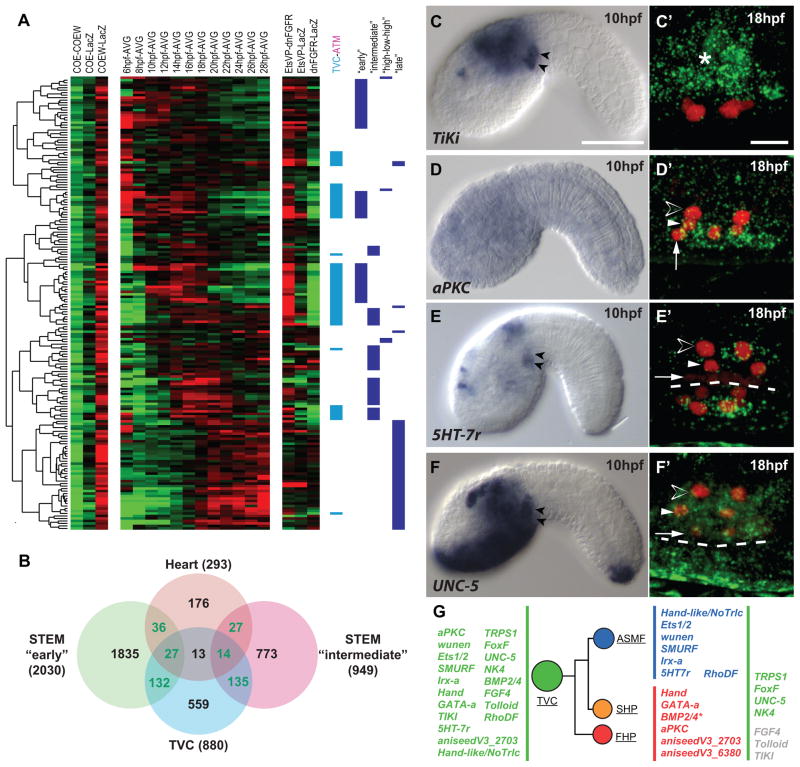
Figure 2. Multilineage priming in cardiopharyngeal progenitors.
(A) Transcription profiles of a subset of Heart candidates. Log2 fold-change scale and abbreviations as Figure 1C. TVC (cyan), ATM (magenta) and temporal profiles classifications are shown. (B) Mutual enrichments of heart and TVC candidate genes in “early” and “intermediate” temporal profiles. Green: numbers of transcripts significantly enriched in dual categories. (C–F) Expression of selected heart/TVC candidate genes. Left: chromogenic in situ hybridization on tailbud embryos. Black arrowheads: TVCs. Right: Close-ups on 18hpf TVCs expressing Mesp>NLS::lacZ (red). Asterisk in C′:TiKi expression in the endoderm. (D′) aPKC expression restricted to the heart precursors (white arrows, FHP; white arrowheads, SHP). (E′) 5HT-7r expression restricted to the ASMFs (open arrowheads). (F′) UNC-5 expression in the whole TVC progeny. (G) Lineage tree showing TVC genes’ segregation to the heart and ASM precursors between 14 and 18 hpf (BMP2/4 restricts to the heart precursors at 20 hpf). Dashed line: midline. Scale bars, 50 μm at 10 hpf, 10 μm for at 18 hpf. See also Data S1, Figure S2 and Table S1.
Since TVCs are born at ~7hpf, migrate from ~9 to 13hpf and start dividing at ~14 hpf (Figure 1A), we anticipated that the TVC candidate genes inhibited by COE would start to be expressed during the 8 to 14 hpf time window. Accordingly, the pools of heart and TVC candidate genes were significantly enriched in genes showing the “early” and “intermediate” temporal profiles (Figures 2A, 2B and Data S1–2; Table S3). These observations indicate that the pool of COE-inhibited heart candidate genes is enriched in TVC candidate genes with peak expression levels during early (10–14hpf) and intermediate (14–18hpf) time windows. Since the asymmetric cell divisions that separate the heart and ASM precursors occur during the intermediate 14–18 hpf time window, the above expression profiles evoke a dynamic whereby TVCs are transcriptionally primed for heart specification, while cardiac-specific transcripts become restricted to the heart precursors in part through ASM-specific inhibition downstream of COE.
To test whether heart candidates genes are first expressed in the TVCs and become restricted to the heart precursors following asymmetric divisions, we performed whole mount in situ hybridization on 10 hpf tailbud embryos and 18 hpf larvae. Most tested genes were conspicuously expressed in 10 hpf TVCs (58% (19/33); Figures 2C–2G and Data S1–3; Table S4). Four genes were no longer expressed at 18 hpf, maybe reflecting regulatory activities specific to early cardiopharyngeal development. 15 genes were still active at 18 hpf, in addition to 5 genes that were not detected earlier (Figures 2D–2G; S2; Table S4). 7 genes, including FoxF and NK4, remained expressed in all TVC derivatives, possibly reflecting continuous and pleiotropic requirements (Figures 2F–2G; Wang et al., 2013). 6 genes, including Hand and GATA-a, were more highly expressed in the heart precursors than in the ASMFs, supporting our prediction that TVC genes become restricted to the heart precursors. GATA-a and Hand are homologs of conserved cardiac determinants, suggesting that their restricted expression contributes to cardiac specification.
Surprisingly, seven heart candidate genes, including the transcription regulators Ets1/2 and Hand-like/NoTrlc, were more highly expressed in ASMFs (Figures 2E––2E′, 2G and Data S1–3, Table S4). We further explored the dynamics of this restriction to the ASM (Figures 3A–3D and S2). ASM-restricted TVC genes, such as Hand-like, were already restricted to the secondary TVCs and further restricted to the ASMFs following the first and second asymmetric divisions, respectively. By 20 hpf, Hand-like was down-regulated in ASMPs, presumably downstream of COE activity (Figures 1C, 3D and Data S1-1). Other ASM-restricted TVC genes showed slightly distinct but consistent dynamics (Figure S2). These observations indicate the expression of TVC genes can become restricted to either the ASM or heart precursors following asymmetric cell divisions (Figure 2G). Thus, the transcriptome of bipotent cardiopharyngeal TVC progenitors displays characteristics of multilineage priming, a phenomenon observed in hematopoietic lineages, whereby pluripotent progenitors express mixtures of cell-type specific transcripts that eventually segregate following progressive fate restrictions (Graf and Enver, 2009; Hu et al., 1997).
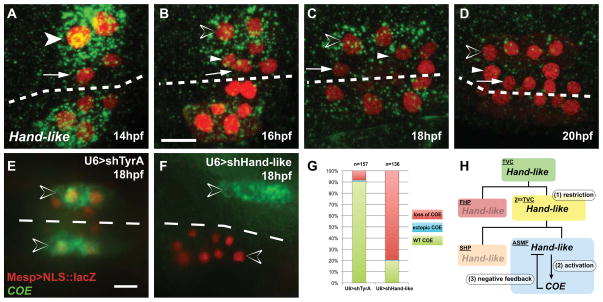
Figure 3. Hand-like/NoTrlc is restricted to the ASM founder cells and necessary for COE activation.
(A–D) Hand-like/NoTrlc expression (green) in 14 to 20 hpf TVCs marked with Mesp>NLS:LacZ (red). (A) Hand-like is first restricted to the secondary TVCs (white arrowhead). (B) Expression persists in the 16hpf SHPs (white arrowheads) and ASMFs (open arrowheads). (C, D) Expression is restricted to the 18hpf ASMFs and vanishes in the 20 hpf ASMPs. (E–G) COE expression (green) in 18 hpf TVCs (red) electroporated with U6>shTyrA (control) or combined U6>shHand-like constructs. Transfected TVCs were visualized by Mesp>NLS::lacZ (red). (E) COE is expressed in control ASMFs. (F) Hand-like knock down disrupts COE expression in electroporated ASMFs (red), but not on the non-electroporated side. (G) Proportions of COE expression phenotypes in indicated conditions, n = number of electroporated larval halves. (H) Summary model of the Hand-like and COE expression dynamics in the TVC lineage. Dashed line: midline. Anterior to the left. Scale bar, 10 μm. See also Figure S2 and Figure S3.
We then asked whether the primed and progressively restricted regulatory gene Handlike is required for ASM specification. We expressed pairs of shRNA constructs to knock down Hand-like activity by RNAi and assayed COE expression (Figures 3E–3G, S3; Nishiyama and Fujiwara, 2008; Wang et al., 2013). Hand-like knock-down inhibited COE expression in ~80% of the larvae compared to <10% in control larvae (Figure 3E–3G). This indicates that Hand-like activity is required for COE expression in the ASMFs. Our results demonstrate that (1) Hand-like is first expressed in bipotent cardiopharyngeal progenitors and progressively restricted to the ASMFs, where (2) it contributes to activating COE, which (3) feeds back negatively, probably causing Hand-like down-regulation in ASMPs (Figure 3D and 3H). This latter mechanism contributes to the regulatory transition from bipotent progenitors to pharyngeal muscle precursors.
COE activates an MRF-associated core muscle program in the ASM precursors
The above analyses indicate that COE contributes to the progression towards a committed ASM fate by inhibiting early progenitor genes as well as the cardiac program. We next investigated the ASM-specific transcriptional response to COE activation. By selecting genes that were significantly up-regulated upon COE misexpression compared to COE::WRPW, we identified 249 candidate ASM-specific COE target genes (Figures 1D, 4A and Data S1-1; Table S1).
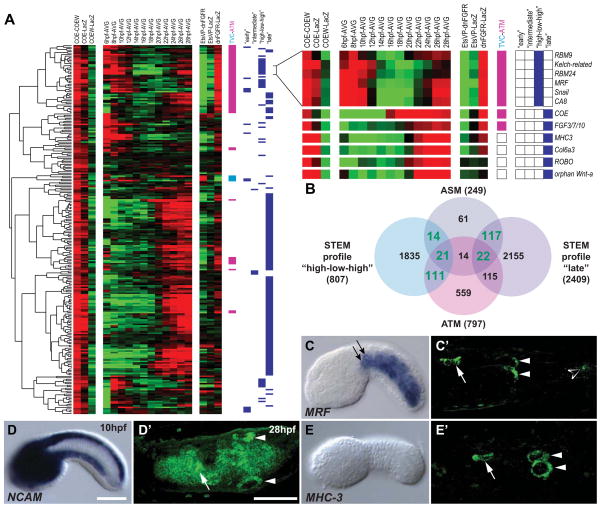
Figure 4. COE activates a core muscle program among ASM-specific genes.
(A) Experimental and temporal transcription profiles of 249 ASM candidates genes. TVC (cyan), ATM (magenta) and temporal profile classifications are shown. Selected “high-low-high” and “late” ASM candidates are shown. (B) Mutual enrichments of ASM candidate genes in “high-low-high” and “late” temporal profiles. Green: numbers of transcripts significantly enriched in dual categories. (C–E′) Expression of selected ASM candidates at 10 and 28 hpf. ASM genes are expressed in the atrial (white arrowheads) and oral (white arrows) siphon muscle rings at 28 hpf. NCAM is expressed in the endoderm and nervous system at 10 hpf and 28 hpf. MHC-3 is not expressed at 10 hpf. MRF is expressed in the ATMs (black arrows) and other tail muscles at 10 hpf and 28 hpf (open arrowhead). Anterior to the left. Scale bars, 50 μm. See also Data S1 and Tables S1–S6.
Genes showing the “high-low-high” and “late” temporal profiles were over-represented among ASM candidate genes (Figures 1D, 1E, 4A, 4B and Data S1–2). Consistent with COE-dependent activation, the expression of “late” ASM candidate started after COE turns on at 16 hpf (Figures 4A; Data S1). “Late” ASM candidates include signaling molecules (FGF3/7/10/22, Wnt-4/orphan-Wnt-a and TGFβ2), trans-membrane and extracellular matrix proteins (ROBO, NCAM, collagen6a3), and cytoskeleton regulators possibly involved in ASM migration (Figures 4, Data S1–2 and S1–5; Tables S1 and S5).
The pool of ASM candidate genes enriched in “high-low-high” expressed genes coincided with ATM candidate genes and muscle-associated GO terms were enriched among ASM and ATM candidate genes (Figures 1D, 1E, 4A, 4B, Data S1–1E; Table S6). These dual ASM/ATM candidate genes included conserved muscle-specific transcription regulators (e.g. MRF), splicing factors and effectors of terminal muscle differentiation. In situ hybridization assays confirmed that dual ATM/ASM candidates are expression in both muscle types (Figures 4D, Data S1–4, and Table S5). MRF expression starts early in the B7.5 lineage and is maintained specifically in the ATMs where it promotes muscle differentiation (Figure 1C; Christiaen et al., 2008; Meedel et al., 2007). Our data indicate that COE promotes a second, ASM-specific, activation of an MRF-associated core muscle program shared with the tail muscles. Previous studies have demonstrated a myogenic role for COE homologues upstream of MRF genes in Drosophila and Xenopus (Dubois et al., 2007; Enriquez et al., 2012; Green and Vetter, 2011). Our analyses delineate a core regulatory module for muscle differentiation that is activated twice in different branches of the same lineage. Nonetheless, muscle differentiation genes MHC-3 and myosin regulatory light chain 4 (MRLC-4) were specifically expressed in larval ASMPs (Figure 4E′; Figure S4). This suggests that COE activates ASM subtype-specific differentiation genes that complement the core myogenic program to specify pharyngeal muscles in Ciona intestinalis.
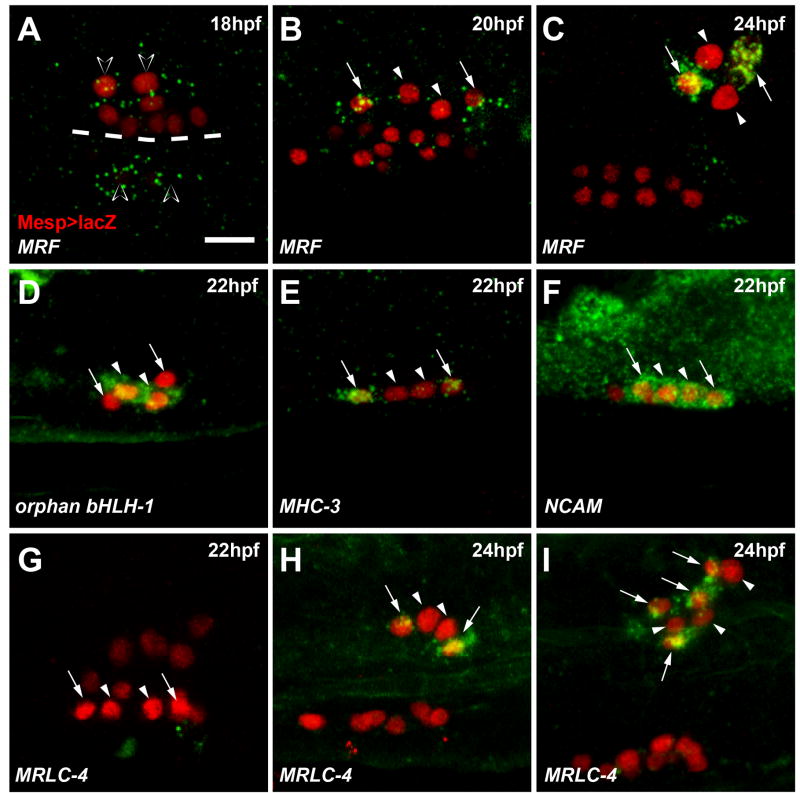
MRF expression and muscle differentiation are restricted to the outer ASM precursors
Next, we characterized the detailed transcriptional dynamics underlying ASM specification. MRF was first expressed in the two 18 hpf ASMFs (Figure 5A). Following ASMFs division, MRF expression became restricted to the two outermost cells (hereafter called outer ASM precursors, oASMPs) and excluded from their sister cells, the inner ASM precursors (iASMPs, Figure 5B). MRF expression was later maintained specifically in the oASMPs and their progeny (Figure 5C). Among ASM markers, we identified 14 pan-ASM, 12 oASMP-specific and 2 iASMP-specific genes (Figures 5D–5I; Data S1–5; Figure S4, Table S5). Muscle differentiation genes, including MRLC-4 and MHC-3, were expressed specifically in the oASMPs and their daughter cells (Figures 5E, 5G–5I, 7K, Data S1–5, and Figure S4). The iASMPs specifically expressed the regulatory genes TGFβ2 and orphan bHLH-1 (Figures 5D and Data S1–5). Expression of MRLC-4 and orphan bHLH-1 started de novo in the oASMPs and iASMPs, respectively, and were maintained in their daughter cells (Figure S4). These data revealed that ASMFs give birth to distinct precursors: oASMPs trigger a muscle differentiation program, presumably upon restricted maintenance of MRF expression, while their sister iASMPs turn off MRF and activate the specific markers orphan bHLH-1 and TGFβ2.
Figure 5. Restriction of muscle differentiation markers to a subset of ASMPs.
(A–C) MRF transcripts (green) in the 18hpf ASMFs (open arrowheads), are restricted to the oASMPs (arrows). iASMPs (arrowheads) do not express MRF. (D) iASMP-specific expression of orphan bHLH-1. (E) oASMP-specific expression of MHC-3. (F) Pan-ASM expression of NCAM. (G–I) MRLC-4 expression starts in the oASMPs at ~24 hpf (H, arrows) and is maintained in their putative daughter cells (I, arrows). MRLC-4 is not expressed in the iASMPs (G–I, arrowheads). Mesp>NLS::lacZ (red) marks the TVC progeny. Dashed line: midline. Anterior to the left. Scale bars, 10μm. See also Data S1–5 and Figure S4.
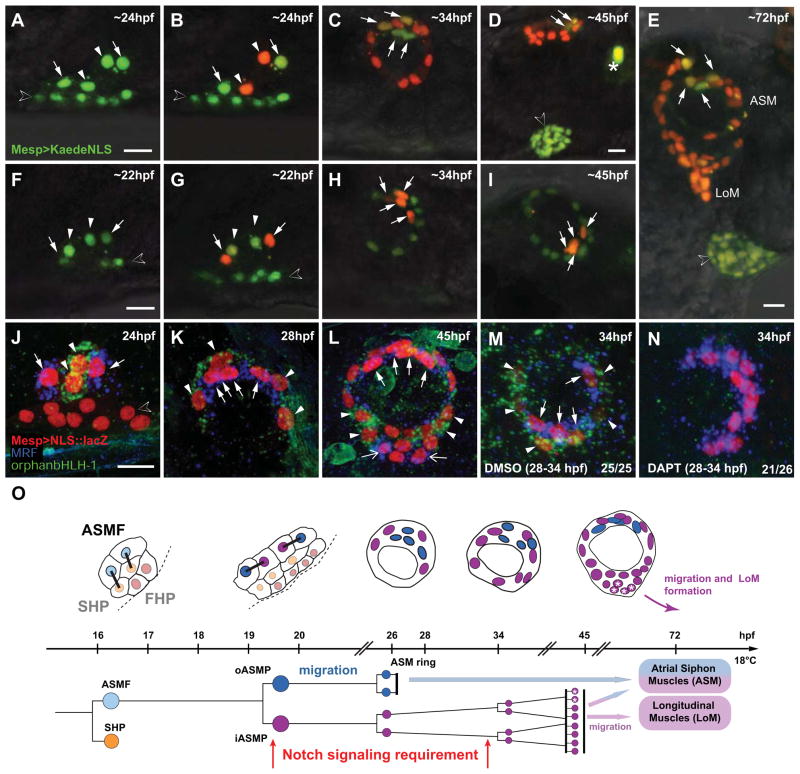
Figure 7. Inner-ASMP derivatives either self-renew or re-activate MRF to form the majority of the B7.5 derived body-wall muscles.
(A–I) Lineage tracing in individual larvae expressing Mesp>Kaede::NLS in the B7.5 lineage. (A, F) Before photoconversion: green-only Kaede::NLS fluorescence. (B) iASMPs-specific (arrowheads) green-to-red Kaede photoconversion.. Green-only fluorescence persists in the oASMPs nuclei (arrows). (F–I) oASMPs-specific Kaede photoconversion. (C, H) By 34 hpf, ASMPs form ~12-cell rings, oASMP and iASMPs have divided once and twice, respectively. (D, I) By ~45 hpf, iASMP derivatives have divided a third time and oASMP derivatives have stopped dividing. (E) iASMP-descendants (red nuclei from iASMPs in (B) form most ASMs and all nascent LoMs. Open arrowheads in D and E: heart primordium. LoM, Longitudinal Muscle. (J–N) Double in situ hybridization of MRF (blue) and orphan bHLH-1 (green) on 22 to 45 hpf Mesp>NLS::LacZ (red)-electroporated larvae. (J–L) MRF and orphan bHLH-1 are expressed in the o- (arrows) and iASMP derivatives (arrowheads), respectively. (L) At ~45hpf, iASMP-derived LoM precursors either self-renew and continue to express orphan bHLH-1 (arrowheads), or shut it off and activate MRF (open arrows, I). (M, N) 28 to 34 hpf incubation in DAPT abolishes orphan bHLH-1 and expands MRF in all ASMP derivatives. (O) B7.5-derived pharyngeal muscle development. First heart precursors (FHPs, red), second heart precursors (SHPs, orange), ASM founder cells (ASMFs, light blue), inner ASM precursors and derivatives (iASMPs, violet), outer ASM precursors and derivatives (oASMPs, dark blue). Asterisks : MRF reactivation in some iASMP derivatives. Sustained Notch signaling is required for MRF repression in the iASMPs and for renewal of their orphan bHLH-1(+) derivatives. Dashed line : midline. Anterior to the left. Scale bars, 10μm. See also Figure S6.
Notch-mediated lateral inhibition of MRF expression among ASM precursors
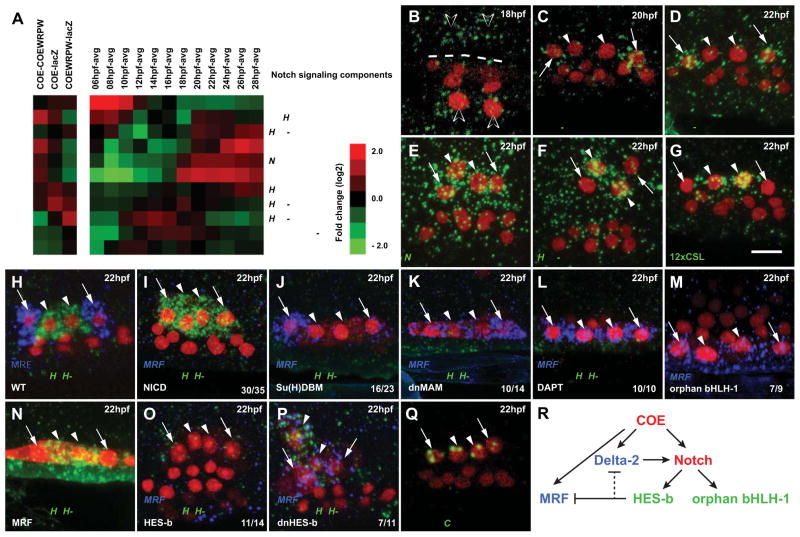
The restriction of MRF expression to the oASMPs is reminiscent of Notch-mediated lateral inhibition of muscle differentiation in Drosophila and vertebrates (Delfini et al., 2000; Kuang et al., 2007; Mourikis et al., 2012a; Ruiz Gomez and Bate, 1997). Several regulators of Notch signaling showed expression profiles typical of ASM candidate genes (Figure 6). Like MRF, the Notch ligand Delta-2 was first expressed in the ASMFs then restricted to the oASMPs (Figure 6B–6D, Figure S5F). Notch was expressed in all ASMPs (Figure 6E). The conserved Notch-activated transcriptional repressor Hairy/Enhancer-of-Split-b (HES-b; Hudson et al., 2007; Stolfi et al., 2011) was expressed specifically the iASMPs (Figure 6F). These data open the possibility that Delta-2 expression in the oASMPs activates Notch and the downstream effector HES-b in their neighboring sister cells, the iASMPs. A transcriptional reporter containing twelve copies of a CSL/SuH binding site (12xCSL; Hansson et al., 2006) showed that the Notch pathway is specifically activated in the iASMPs (Figures 6G and S5).
Figure 6. HES-b-mediated Notch signaling restricts MRF expression to the differentiating outer ASM precursors.
(A) Profiles of Notch signaling components: Delta-2, Notch, Serrate/Jagged and Su(H) show characteristics of ASM candidate genes (COE-COE::WRPW>0, increased expression after 16 hpf). HES-b expression is upregulated at ~20 hpf, when the iASMPs are born. Other components are constitutively expressed (e.g. MAM) or have different expression profiles in the B7.5 lineage (e.g. Delta). Abbreviations are as in Figure 1C. (B–D) Delta-2 expression in the ASMFs (open arrowheads, B), oASMPs (arrows, C and D) but not in the iASMPs (arrowheads, C and D). (E) Notch expression in all ASMPs. (F) iASMP-specific (arrowheads) expression of HES-b. (G) GFP mRNA (green) in larvae electroporated with 12xCSL:bpFOG>UNC-76::GFP reveal Notch signaling in the iASMPs. (H–P) Double FISH of MRF (blue) and orphan bHLH-1 (green) in control (WT) and indicated experimental conditions. Mesp>NLS::LacZ (red) marks TVC nuclei. (I) COE(-3299/-151):bpFOG-driven ASM-specific expression of NICD inhibits MRF and expands orphan bHLH-1 expression in all ASMPs. (J–L) ASM-specific expression of Su(H)DBM (J) and dnMAM (K), or incubation in DAPT (L) expands MRF and inhibits orphan bHLH-1. (M) orphan bHLH-1 misexpression expands MRF to all ASMPs. (N) MRF misexpression does not inhibit orphan bHLH-1 expression (N). (O) Islet(-3299/-151):bpFOG-driven ASM-specific expression of HES-b inhibits both MRF and orphan bHLH-1. (P) Dn-HES-b misexpression induces MRF expansion to all ASMPs without repressing orphan bHLH-1. The numbers of larvae with the indicated phenotype relative to the total are shown. Dashed line: midline. Anterior to the left. Scale bars, 10μm. (Q) Intron-specific probes show nascent COE transcripts in all ASMPs. (R) Summary model of Notch-mediated lateral inhibition of MRF through HES-b activation in the iASMPs. Red, expression in all ASMPs; green, expression in iASMPs; blue, expression in oASMPs. See also Figure S5.
To test whether Notch signaling is required for inner vs outer ASMP specification, we used an ASM-specific COE enhancer (Figure S5) to express the constitutively active intra-cellular domain of Notch (NICD), or dominant negative forms of Su(H) (Su(H)DBM) (Hudson and Yasuo, 2006) and NICD co-activator Mastermind (dnMAM) in ASMFs. We analyzed the outer and inner ASMP-specific expressions of MRF and orphan bHLH-1 by double in situ hybridization (Figure 6H). ASM-targeted expression of NICD inhibited MRF and expanded orphan bHLH-1 expression to all ASMPs (Figure 6I). Su(H)DBM and dnMAM caused the reciprocal loss of orphan bHLH-1 and expansion of MRF expression (Figures 6J and JK). Similarly, treatment with the γ-secretase inhibitor DAPT, which blocks Delta2-dependent Notch cleavage and activation in Ciona (Hudson and Yasuo, 2006), converted all ASMPs into MRF(+), orphan bHLH-1(−) oASMP-like cells (Figure 6L). These results show that localized Notch signaling is necessary and sufficient to promote orphan bHLH-1 expression and the iASMP-specific program at the expense of MRF and associated muscle differentiation.
Misexpression assays indicated that MRF and orphan bHLH-1 do not repress each other despite exclusive expression patterns in wild-type larvae (Figures 6M–N). Instead, ASM-specific misexpression of HES-b, a conserved effector of Notch-mediated repression, inhibited both MRF and orphan bHLH-1 expressions (Figure 6O). The latter effect may be due to repression of Delta-2 by HES-b, as in other cases of lateral inhibition (Kageyama et al., 2008). These data suggest that Notch is activated in each iASMP by Delta-2 from the adjacent oASMP. For the reciprocal experiment, we engineered a dominant negative form of HES-b (dnHES-b) by mutating its bHLH domain (Strom et al., 1997). DnHES-b caused ectopic MRF expression in the iASMPs without affecting orphan bHLH-1 (Figures 6O and 6P). These results further show that MRF and orphan bHLH-1 do not inhibit each other and indicate that HES-b function is required in the iASMPs for Notch-mediated repression of MRF.
Lastly, since Notch signaling has been shown to repress transcription of the COE homolog in Drosophila (Crozatier and Vincent, 1999), we used intron-specific antisense probes to test if COE is actively transcribed in wild-type iASMPs. We observed nuclear dots revealing that COE transcription is maintained in all ASMPs (Figure 6R). Thus, it is likely that COE is able to regulate all ASMP-specific gene expressions upstream of the Delta-Notch pathway.
MRF-negative inner ASMPs are stem cell-like muscle precursors
In Drosophila and vertebrates, Notch activation promotes renewal of the stem cell-like muscle precursors and prevents their precocious differentiation during development (Kuang et al., 2007; Mourikis et al., 2012a; Mourikis et al., 2012b; Ruiz Gomez and Bate, 1997; Schuster-Gossler et al., 2007; Vasyutina et al., 2007). We hypothesized that iASMPs are the B7.5-derived progenitors of body wall muscles, which grow substantially during metamorphosis (Sasakura et al., 2012; Stolfi et al., 2010). We used cell-specific photoconversion of the fluorescent protein Kaede::NLS to track the derivatives of the i- and oASMPs through metamorphosis (Figures 7A–7I and S6). ASMPs divided once and contributed equally to the 8-cell ASM rings. After 28 hpf, the oASMP derivatives stopped dividing, their four cell bodies clustered medially in contact with the atrial placode (Figures 7C, 7D, 7H, 7I and S6). Meanwhile, the iASMP derivatives divided twice more, producing 16 cells that occupied a peripheral location by ~45 hpf (Figures 7D, 7I and S6). In metamorphosing juveniles, the iASMP derivatives continued to proliferate and formed the majority of the ASM sensu stricto and all the primary longitudinal muscles (Figures 7E and S6).
We expected some iASMP derivatives to re-activate MRF upon metamorphosis. During ASMPs’ migration and in 28 hpf 8-cell rings, the oASMPs and iASMPs derivatives maintained expression of MRF and orphan bHLH-1, respectively (Figure 7J–K). During metamorphosis, in 45 hpf ASM rings, MRF expression was maintained in the four median oASMPs, while a lateral group of iASMP-derived prospective longitudinal muscle precursors began to express MRF at the expense of orphan bHLH-1 (Figure 7L). Thus, iASMP derivatives, formerly expressing orphan-bHLH-1, are able to re-activate MRF and contribute to the B7.5-derived body wall muscle development. In these 45 hpf juveniles, we still observed orphan bHLH-1(+) iASMPs derivatives, indicating that these cells can self-renew. These results indicate that iASMPs, which first remain undifferentiated in response to Notch signaling, undergo asymmetric cell divisions to both self-renew and produce MRF(+) differentiating myoblasts in a manner reminiscent of muscle stem cells. Finally, we asked whether Notch signaling is also required for the maintenance of an MRF(−), orphan bHLH-1(+) population of stem cell-like muscle precursors through self-renewal. We treated metamorphosing juveniles with DAPT from 28 to 34 hpf, i.e. after the initial separation of i- and oASMPs. This caused MRF to expand to the whole ASM progeny and abolished orphan bHLH-1 expression (Figure 7M and 7N) indicating that sustained Notch activation is required to keep a reservoir of stem cell-like MRF(−), orphan bHLH-1(+) muscle precursors.
DISCUSSION
Conserved features of the chordate cardiopharyngeal mesoderm
We presented a comprehensive characterization of the transcriptional dynamics underlying differential fate specification within the cardiopharyngeal lineage of a simple chordate. Cardiopharyngeal mesoderm progenitors in ascidians and vertebrates appear to produce heart precursors and pharyngeal muscles that segregate following a conserved clonal topology: retrospective clonal analyses in the mouse established that first heart field precursors segregate precociously from common progenitors of the second heart field and branchiomeric muscles (Lescroart et al., 2010; Meilhac et al., 2004). In the ascidian Ciona intestinalis, the TVC cardiopharyngeal progenitors produce first and second heart precursors that separate from the ASM/pharyngeal muscle precursors following stereotyped asymmetric cell divisions according to a clonal topology similar to that described in the mouse (Stolfi et al., 2010; Tolkin and Christiaen, 2012; Wang et al., 2013). Cardiopharyngeal progenitors in ascidians and vertebrates also transition through comparable regulatory states. They arise from mesoderm progenitors expressing the Mesp homologs (Saga et al., 2000; Satou et al., 2004). They activate homologs of the conserved cardiac determinants GATA4/5/6 (GATA-a), NKX2-5 (NK4) and Hand (reviewed in Davidson, 2007). The common progenitors of the second heart and pharyngeal muscle precursors activate homologs of Tbx1, which causes cardiac and craniofacial malformations when mutated in mammals (Kelly et al., 2004) and acts upstream of COE to promote pharyngeal muscle specification in Ciona (Wang et al., 2013). Finally, cardiopharyngeal precursors express the homologs of the LIM homeobox gene Islet (Nathan et al., 2008; Prall et al., 2007; Wang et al., 2013) and here we found that the ascidian ASM activate the homolog of FGF10 and HES1, which are also activated downstream of Tbx1 in the mouse cardiopharyngeal mesoderm (van Bueren et al., 2010; Watanabe et al., 2012). These observations point to an ancestral ontogenetic motif whereby homologous interactions between orthologous regulators are deployed onto a conserved clonal topology to specify cell fates in the chordate cardiopharyngeal mesoderm. Our results indicate that multipotent cardiopharyngeal progenitors are transcriptionally primed to activate both the heart and pharyngeal muscle-specific regulatory programs. This multi-lineage priming is resolved upon stereotyped asymmetric cell divisions, which segregate the pharyngeal muscle from the first and second heart precursors, successively.
Multineage priming and fates segregation among cardiopharyngeal precursors
In embryonic stem cell (ESC) models of heart differentiation, chromatin marks “poise” early cardiac regulatory genes in mesoderm progenitors for subsequent expression in cardiogenic precursors (Paige et al., 2012; Wamstad et al., 2012). “Chromatin priming” is distinct from “multilineage transcriptional priming”, which causes co-expression of active regulators. In the Ciona cardiogenic lineage, Ets1/2 is first expressed in the B7.5-derived founder cells where it contributes to TVC induction, before it becomes restricted to the TVCs and subsequently to the ASM precursors (this study and (Davidson et al., 2006). Similarly, GATA-a and RhoDF are first expressed in the TVCs, where they both contribute to migration (Christiaen et al., 2008; Ragkousi et al., 2011), while their expressions later become restricted to the heart and ASM precursors, respectively. Successive phases of expression within one lineage can reflect functional pleiotropy, but multilineage transcriptional priming is also instrumental for fates’ segregation (Graf and Enver, 2009; Ng et al., 2009). Here we showed that TVC-primed Hand-like is progressively restricted to the ASMFs and necessary for later ASM-specific activation of COE. Transient co-expression of Tbx1/10 and NK4 in Ciona secondary TVCs precedes NK4-mediated restriction of Tbx1/10 expression and COE activation to the ASMFs, permitting cardiac specification in the second heart precursors (Wang et al., 2013). Conversely, Tbx1/10 activity prevents precocious GATA-a expression in common second heart/ASM progenitors, thus presumably permitting subsequent ASM specification (Wang et al., 2013). Future studies will fully characterize the regulatory relationships connecting Hand-like, Tbx1/10 and COE during early ASM specification.
COE promotes both differentiation and Notch-mediated maintenance of stemness in pharyngeal muscle precursors
We demonstrate that COE orchestrates the transition from pluripotency to commitment in the pharyngeal muscle precursors, where it promotes both differentiation and maintenance of an undifferentiated stem cell-like state through Delta/Notch-mediated lateral inhibition. Our data indicate that MRF inhibition in the iASMPs requires the activity of the canonical Notch signaling effectors Mastermind/MAML and Su(H)/CSL-1/RBPJ and the conserved repressor HES-b. In vertebrates, a large body of evidence demonstrated that Delta-like ligands activate Notch signaling in embryonic muscle progenitors, which activate HEY/HES repressors in an Su(H)/CSL-1/RBPJ-dependent manner to inhibit MyoD expression and subsequent differentiation (Delfini et al., 2000; Hirsinger et al., 2001; Jarriault et al., 1995; Mourikis et al., 2012a; Schuster-Gossler et al., 2007; Vasyutina et al., 2007). Notch-mediated inhibition of muscle differentiation also occurs in Drosophila following asymmetric division of muscle progenitors (Corbin et al., 1991; Ruiz Gomez and Bate, 1997). These and our results indicate that Notch-mediated lateral inhibition of MRFs expression and subsequent muscle differentiation is an ancient mechanism to discriminate differentiating myoblasts from committed muscle precursors that seems to have been co-opted in the pharyngeal mesoderm of chordates.
In the mouse, conditional inactivation of Notch signaling in muscle progenitors reduces the muscle mass due to premature differentiation of embryonic muscles and interruption of proliferative self-renewal (Mourikis et al., 2012a; Schuster-Gossler et al., 2007; Vasyutina et al., 2007). In Ciona, ASMFs activate MRF before producing the stem cell-like iASMPs, where Notch activation represses MRF expression. Their sister oASMPs maintain MRF expression, activate downstream muscle differentiation genes and divide only once. By contrast, iASMPs proliferate and their progeny forms the vast majority of atrial siphon and body wall muscles and sustained Notch signaling is required to maintain the proliferating pool of undifferentiated muscle precursors. In vertebrates, adult muscle stem cells, i.e. the satellite cells, arise from a continuum of stem cell-like muscle cells originating from embryonic muscle progenitors that are primed by early expression of Myf5 or Mrf4 (Gros et al., 2005; Harel et al., 2009; Sambasivan et al., 2013; Sambasivan et al., 2009). Notch signaling is also required for the self-renewal of satellite cells, but with a different cellular outcome since it also maintains quiescence (Bjornson et al., 2012; Mourikis et al., 2012b). Therefore, the ascidian iASMPs are more similar to vertebrate stem cell-like pharyngeal muscle progenitors, which require Notch-mediated inhibition of MRF-associated differentiation to self-renew, proliferate and produce a full complement of muscle precursors for future differentiation.
We documented various regulatory activities by which COE controls pharyngeal muscle specification. We showed that COE inhibits early progenitor genes as well as determinants of the alternative cardiac fate. This activity is analogous to that of the COE homolog EBF1, which inhibits the alternative T-cell program during hematopoiesis in B-cell precursors (Nechanitzky et al., 2013; Pongubala et al., 2008).
We showed that COE acts upstream of MRF activation during ASM specification. Similarly, the Drosophila homolog of COE, Collier, cooperates with the MRF homolog Nautilus to determine muscle identity (Crozatier and Vincent, 1999; Enriquez et al., 2012). Two Xenopus homologs of COE, EBF-2 and -3, also activate the MRF paralogs, MyoD and Myf5, and regulate skeletal muscle development, including in pharyngeal mesoderm-derived jaw muscles (Green and Vetter, 2011). These and our results lead us to speculate that the evolutionary and ontogenetic origins of branchiomeric muscles and associated stem cells can be traced back to pharyngeal muscle progenitors of common ancestors of vertebrates and tunicates and developed under the regulatory influence of COE homologs.
EXPERIMENTAL PROCEDURES
Animals, electroporation, and pharmacological treatments
Gravid Ciona intestinalis adults were obtained from M-REP (San Diego, CA). The Mesp driver (Davidson et al., 2005) was used to mark the B7.5 lineage with either NLS::lacZ (50μg) or H2B::mCherry (15 μg). The minimal FoxF(TVC) enhancer (Beh et al., 2007) was used to drive COE or COE::WRPW as previously described (Stolfi et al., 2010). ASM-specific perturbation of Notch signaling used (COE-3299/-151):bpFOG>NICD, >Su(H)DBM, or >dnMAM::mCherry. (COE-3299/-151):bpFOG>mCherry was used to visualize expressing cells. The γ-secretase inhibitor IX (DAPT) (Calbiochem) was used at 10μM, larvae and juveniles were anesthetized with menthol before imaging and photoconversion with a Leica SP8 X inverted confocal microscope.
Dissociation, FACS, and RNA extraction
Embryos and larvae were dissociated essentially as previously described (Christiaen et al., 2009a). Fluorescent activated sell sorting (FACS) was performed with a BD-FACSAria cell sorter (BD Biosciences). The RNAqueous®-Micro Kit (Ambion) was used for RNA extraction. RNA quality was assayed on Agilent Bioanalyzer 2100 with RNA 6000 Pico Chips and associated kit (Agilent).
Target probe preparation and microarray hybridization
We used the Ovation® Pico WTA System (NuGen), the Encore™ Biotin Module (NuGen) and RNA 6000 Nano LabChip (Agilent) for probe preparation and quality control. Target cDNA Preparation, microarray hybridization, washes, staining, and scanning were performed according to NuGen instructions using custom-design Affymetrix GeneChip® (Christiaen et al., 2008).
Microarray data analysis
Raw expression values were normalized together with previous data (Christiaen et al., 2008; Woznica et al., 2012) to estimate probe set expression values using the robust multi-chip analysis (RMA) algorithm (Irizarry et al., 2003). Normalization, probesets-to-gene models conversion and estimation of differential expression were performed as described in the Supplemental Experimental Procedures. The microarray data is available at NCBI GEO under accession GSE54746. We used the STEM program (Ernst et al., 2005) to identify significantly over-represented temporal profiles. See the Supplemental Experimental Procedures for the details of GO term and clustering analysis.
Probe synthesis, Immunochemistry and in situ hybridizations
Whole mount in situ hybridizations and immunostaining of embryos and larvae were performed as described (Christiaen et al., 2009b) with modifications. Stained larvae were mounted in Permount following chromogenic in situ hybridization or Prolong Gold antifade reagent (Invitrogen, P10144) for fluorescent in situ hybridization.
Molecular cloning and probes synthesis
Template plasmids were obtained from the Ciona intestinalis Gene collection (Satou et al., 2002), the Gateway ORF collection (Roure et al., 2007) or cloned by RT-PCR from cDNA libraries. Oligonucleotides sequences are available in the supplemental methods.
Imaging and cell-specific photoconversion of Kaede
For imaging and Kaede (Ando et al., 2002) photoconversion, single larvae were isolated and cultured in glass-bottom 96-well plates (Thermo Scientific, Nunc, #164588). Photoconversions were carried out using the HC PL FLUOTAR 20×/0.50 objective on our Leica Microsystems inverted TCS SP8 X confocal microscope, which is equipped with a 405nm diode laser, a white light laser, an acousto-optical beam splitter, two HyD detectors and operated by the Leica LAS software. Images of the fluorescent WMISH were generated using either a TCS SP5 or the HC PL APO 63×/1.30 objective on our TCS SP8 X confocal microscope, except in Figures 3E and 3F, and Data S1, which images were acquired with a Leica DM2500 epifluorescence microscope.
Supplementary Material
Highlights.
Bipotent cardiopharyngeal progenitors display multilineage transcriptional priming
Primed muscle or heart determinants are then restricted to corresponding precursors
COE activates an MRF-associated core muscle differentiation program
Notch signaling maintains stemness among pharyngeal muscle precursors
Acknowledgments
We are grateful to Pui Leng Ip and Kenneth Birnbaum for support with the FACS. We thank R Antonio Herrera for help with microarray experiments. We thank Anna Di Gregorio and Mike Levine for providing access to cDNA libraries. We thank Farhana Salek, Emily Salmon-Denikos and Renee Marie Bogdanovic for technical assistance. We thank Maija Slaidina for comments on the manuscript. Our work is supported by Grants 10SDG4310061 from the American Heart Association, R01HL108643 from NHLBI/NIH and by the New York Cardiac Center.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beh J, Shi W, Levine M, Davidson B, Christiaen L. FoxF is essential for FGF-induced migration of heart progenitor cells in the ascidian Ciona intestinalis. Development. 2007;134:3297–3305. doi: 10.1242/dev.010140. [DOI] [PubMed] [Google Scholar]
- Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem cells. 2012;30:232–242. doi: 10.1002/stem.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M, Vincent SD. Distinct and dynamic myogenic populations in the vertebrate embryo. Current opinion in genetics & development. 2009;19:444–453. doi: 10.1016/j.gde.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Chiba S, Sasaki A, Nakayama A, Takamura K, Satoh N. Development of Ciona intestinalis juveniles (through 2nd ascidian stage) Zoological science. 2004;21:285–298. doi: 10.2108/zsj.21.285. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anatomy and embryology. 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Davidson B, Kawashima T, Powell W, Nolla H, Vranizan K, Levine M. The transcription/migration interface in heart precursors of Ciona intestinalis. Science. 2008;320:1349–1352. doi: 10.1126/science.1158170. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Stolfi A, Levine M. BMP signaling coordinates gene expression and cell migration during precardiac mesoderm development. Developmental biology. 2010;340:179–187. doi: 10.1016/j.ydbio.2009.11.006. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Wagner E, Shi W, Levine M. Isolation of individual cells and tissues from electroporated sea squirt (Ciona) embryos by fluorescence-activated cell sorting (FACS) Cold Spring Harbor protocols 2009. 2009a doi: 10.1101/pdb.prot5349. pdb prot5349. [DOI] [PubMed] [Google Scholar]
- Christiaen L, Wagner E, Shi W, Levine M. Whole-mount in situ hybridization on sea squirt (Ciona intestinalis) embryos. Cold Spring Harbor protocols. 2009b;2009 doi: 10.1101/pdb.prot5348. pdb prot5348. [DOI] [PubMed] [Google Scholar]
- Cooley J, Whitaker S, Sweeney S, Fraser S, Davidson B. Cytoskeletal polarity mediates localized induction of the heart progenitor lineage. Nature cell biology. 2011;13:952–957. doi: 10.1038/ncb2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbin V, Michelson AM, Abmayr SM, Neel V, Alcamo E, Maniatis T, Young MW. A role for the Drosophila neurogenic genes in mesoderm differentiation. Cell. 1991;67:311–323. doi: 10.1016/0092-8674(91)90183-y. [DOI] [PubMed] [Google Scholar]
- Crozatier M, Vincent A. Requirement for the Drosophila COE transcription factor Collier in formation of an embryonic muscle: transcriptional response to notch signalling. Development. 1999;126:1495–1504. doi: 10.1242/dev.126.7.1495. [DOI] [PubMed] [Google Scholar]
- Davidson B. Ciona intestinalis as a model for cardiac development. Seminars in cell & developmental biology. 2007;18:16–26. doi: 10.1016/j.semcdb.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Shi W, Beh J, Christiaen L, Levine M. FGF signaling delineates the cardiac progenitor field in the simple chordate, Ciona intestinalis. Genes & development. 2006;20:2728–2738. doi: 10.1101/gad.1467706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson B, Shi W, Levine M. Uncoupling heart cell specification and migration in the simple chordate Ciona intestinalis. Development. 2005;132:4811–4818. doi: 10.1242/dev.02051. [DOI] [PubMed] [Google Scholar]
- Delfini MC, Hirsinger E, Pourquie O, Duprez D. Delta 1-activated notch inhibits muscle differentiation without affecting Myf5 and Pax3 expression in chick limb myogenesis. Development. 2000;127:5213–5224. doi: 10.1242/dev.127.23.5213. [DOI] [PubMed] [Google Scholar]
- Delsuc F, Brinkmann H, Chourrout D, Philippe H. Tunicates and not cephalochordates are the closest living relatives of vertebrates. Nature. 2006;439:965–968. doi: 10.1038/nature04336. [DOI] [PubMed] [Google Scholar]
- Dubois L, Enriquez J, Daburon V, Crozet F, Lebreton G, Crozatier M, Vincent A. Collier transcription in a single Drosophila muscle lineage: the combinatorial control of muscle identity. Development. 2007;134:4347–4355. doi: 10.1242/dev.008409. [DOI] [PubMed] [Google Scholar]
- Enriquez J, de Taffin M, Crozatier M, Vincent A, Dubois L. Combinatorial coding of Drosophila muscle shape by Collier and Nautilus. Developmental biology. 2012;363:27–9. doi: 10.1016/j.ydbio.2011.12.018. [DOI] [PubMed] [Google Scholar]
- Ernst J, Bar-Joseph Z. STEM: a tool for the analysis of short time series gene expression data. BMC bioinformatics. 2006;7:191. doi: 10.1186/1471-2105-7-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst J, Nau GJ, Bar-Joseph Z. Clustering short time series gene expression data. Bioinformatics. 2005;21(Suppl 1):i159–168. doi: 10.1093/bioinformatics/bti1022. [DOI] [PubMed] [Google Scholar]
- Figeac N, Daczewska M, Marcelle C, Jagla K. Muscle stem cells and model systems for their investigation. Developmental dynamics : an official publication of the American Association of Anatomists. 2007;236:3332–3342. doi: 10.1002/dvdy.21345. [DOI] [PubMed] [Google Scholar]
- Graf T, Enver T. Forcing cells to change lineages. Nature. 2009;462:587–594. doi: 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- Green YS, Vetter ML. EBF proteins participate in transcriptional regulation of Xenopus muscle development. Developmental biology. 2011;358:240–250. doi: 10.1016/j.ydbio.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros J, Manceau M, Thome V, Marcelle C. A common somitic origin for embryonic muscle progenitors and satellite cells. Nature. 2005;435:954–958. doi: 10.1038/nature03572. [DOI] [PubMed] [Google Scholar]
- Hansson EM, Teixeira AI, Gustafsson MV, Dohda T, Chapman G, Meletis K, Muhr J, Lendahl U. Recording Notch signaling in real time. Developmental neuroscience. 2006;28:118–127. doi: 10.1159/000090758. [DOI] [PubMed] [Google Scholar]
- Harel I, Nathan E, Tirosh-Finkel L, Zigdon H, Guimaraes-Camboa N, Evans SM, Tzahor E. Distinct origins and genetic programs of head muscle satellite cells. Developmental cell. 2009;16:822–832. doi: 10.1016/j.devcel.2009.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsinger E, Malapert P, Dubrulle J, Delfini MC, Duprez D, Henrique D, Ish-Horowicz D, Pourquie O. Notch signalling acts in postmitotic avian myogenic cells to control MyoD activation. Development. 2001;128:107–116. doi: 10.1242/dev.128.1.107. [DOI] [PubMed] [Google Scholar]
- Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes & development. 1997;11:774–785. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- Hudson C, Lotito S, Yasuo H. Sequential and combinatorial inputs from Nodal, Delta2/Notch and FGF/MEK/ERK signalling pathways establish a grid-like organisation of distinct cell identities in the ascidian neural plate. Development. 2007;134:3527–3537. doi: 10.1242/dev.002352. [DOI] [PubMed] [Google Scholar]
- Hudson C, Yasuo H. A signalling relay involving Nodal and Delta ligands acts during secondary notochord induction in Ciona embryos. Development. 2006;133:2855–2864. doi: 10.1242/dev.02466. [DOI] [PubMed] [Google Scholar]
- Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic acids research. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch. Nature. 1995;377:355–358. doi: 10.1038/377355a0. [DOI] [PubMed] [Google Scholar]
- Kageyama R, Ohtsuka T, Shimojo H, Imayoshi I. Dynamic Notch signaling in neural progenitor cells and a revised view of lateral inhibition. Nature neuroscience. 2008;11:1247–1251. doi: 10.1038/nn.2208. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Jerome-Majewska LA, Papaioannou VE. The del22q11.2 candidate gene Tbx1 regulates branchiomeric myogenesis. Human molecular genetics. 2004;13:2829–2840. doi: 10.1093/hmg/ddh304. [DOI] [PubMed] [Google Scholar]
- Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lescroart F, Kelly RG, Le Garrec JF, Nicolas JF, Meilhac SM, Buckingham M. Clonal analysis reveals common lineage relationships between head muscles and second heart field derivatives in the mouse embryo. Development. 2010;137:3269–3279. doi: 10.1242/dev.050674. [DOI] [PubMed] [Google Scholar]
- Meedel TH, Chang P, Yasuo H. Muscle development in Ciona intestinalis requires the b-HLH myogenic regulatory factor gene Ci-MRF. Developmental biology. 2007;302:333–344. doi: 10.1016/j.ydbio.2006.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meilhac SM, Esner M, Kelly RG, Nicolas JF, Buckingham ME. The clonal origin of myocardial cells in different regions of the embryonic mouse heart. Developmental cell. 2004;6:685–698. doi: 10.1016/s1534-5807(04)00133-9. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Gopalakrishnan S, Sambasivan R, Tajbakhsh S. Cell-autonomous Notch activity maintains the temporal specification potential of skeletal muscle stem cells. Development. 2012a;139:4536–4548. doi: 10.1242/dev.084756. [DOI] [PubMed] [Google Scholar]
- Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem cells. 2012b;30:243–252. doi: 10.1002/stem.775. [DOI] [PubMed] [Google Scholar]
- Nathan E, Monovich A, Tirosh-Finkel L, Harrelson Z, Rousso T, Rinon A, Harel I, Evans SM, Tzahor E. The contribution of Islet1-expressing splanchnic mesoderm cells to distinct branchiomeric muscles reveals significant heterogeneity in head muscle development. Development. 2008;135:647–657. doi: 10.1242/dev.007989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechanitzky R, Akbas D, Scherer S, Gyory I, Hoyler T, Ramamoorthy S, Diefenbach A, Grosschedl R. Transcription factor EBF1 is essential for the maintenance of B cell identity and prevention of alternative fates in committed cells. Nature immunology. 2013;14:867–875. doi: 10.1038/ni.2641. [DOI] [PubMed] [Google Scholar]
- Ng SY, Yoshida T, Zhang J, Georgopoulos K. Genome-wide lineage-specific transcriptional networks underscore Ikaros-dependent lymphoid priming in hematopoietic stem cells. Immunity. 2009;30:493–507. doi: 10.1016/j.immuni.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama A, Fujiwara S. RNA interference by expressing short hairpin RNA in the Ciona intestinalis embryo. Development, growth & differentiation. 2008;50:521–529. doi: 10.1111/j.1440-169X.2008.01039.x. [DOI] [PubMed] [Google Scholar]
- Paige SL, Thomas S, Stoick-Cooper CL, Wang H, Maves L, Sandstrom R, Pabon L, Reinecke H, Pratt G, Keller G, et al. A temporal chromatin signature in human embryonic stem cells identifies regulators of cardiac development. Cell. 2012;151:221–232. doi: 10.1016/j.cell.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pongubala JM, Northrup DL, Lancki DW, Medina KL, Treiber T, Bertolino E, Thomas M, Grosschedl R, Allman D, Singh H. Transcription factor EBF restricts alternative lineage options and promotes B cell fate commitment independently of Pax5. Nature immunology. 2008;9:203–215. doi: 10.1038/ni1555. [DOI] [PubMed] [Google Scholar]
- Prall OW, Menon MK, Solloway MJ, Watanabe Y, Zaffran S, Bajolle F, Biben C, McBride JJ, Robertson BR, Chaulet H, et al. An Nkx2–5/Bmp2/Smad1 negative feedback loop controls heart progenitor specification and proliferation. Cell. 2007;128:947–959. doi: 10.1016/j.cell.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragkousi K, Beh J, Sweeney S, Starobinska E, Davidson B. A single GATA factor plays discrete, lineage specific roles in ascidian heart development. Developmental biology. 2011;352:154–163. doi: 10.1016/j.ydbio.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Relaix F, Zammit PS. Satellite cells are essential for skeletal muscle regeneration: the cell on the edge returns centre stage. Development. 2012;139:2845–2856. doi: 10.1242/dev.069088. [DOI] [PubMed] [Google Scholar]
- Roure A, Rothbacher U, Robin F, Kalmar E, Ferone G, Lamy C, Missero C, Mueller F, Lemaire P. A multicassette Gateway vector set for high throughput and comparative analyses in ciona and vertebrate embryos. PloS one. 2007;2:e916. doi: 10.1371/journal.pone.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz Gomez M, Bate M. Segregation of myogenic lineages in Drosophila requires numb. Development. 1997;124:4857–4866. doi: 10.1242/dev.124.23.4857. [DOI] [PubMed] [Google Scholar]
- Saga Y, Kitajima S, Miyagawa-Tomita S. Mesp1 expression is the earliest sign of cardiovascular development. Trends in cardiovascular medicine. 2000;10:345–352. doi: 10.1016/s1050-1738(01)00069-x. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Comai G, Le Roux I, Gomes D, Konge J, Dumas G, Cimper C, Tajbakhsh S. Embryonic founders of adult muscle stem cells are primed by the determination gene Mrf4. Developmental biology. 2013 doi: 10.1016/j.ydbio.2013.04.018. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Gayraud-Morel B, Dumas G, Cimper C, Paisant S, Kelly RG, Tajbakhsh S. Distinct regulatory cascades govern extraocular and pharyngeal arch muscle progenitor cell fates. Developmental cell. 2009;16:810–821. doi: 10.1016/j.devcel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Sambasivan R, Kuratani S, Tajbakhsh S. An eye on the head: the development and evolution of craniofacial muscles. Development. 2011;138:2401–2415. doi: 10.1242/dev.040972. [DOI] [PubMed] [Google Scholar]
- Sasakura Y, Kanda M, Ikeda T, Horie T, Kawai N, Ogura Y, Yoshida R, Hozumi A, Satoh N, Fujiwara S. Retinoic acid-driven Hox1 is required in the epidermis for forming the otic/atrial placodes during ascidian metamorphosis. Development. 2012;139:2156–2160. doi: 10.1242/dev.080234. [DOI] [PubMed] [Google Scholar]
- Satou Y, Imai KS, Satoh N. The ascidian Mesp gene specifies heart precursor cells. Development. 2004;131:2533–2541. doi: 10.1242/dev.01145. [DOI] [PubMed] [Google Scholar]
- Satou Y, Takatori N, Fujiwara S, Nishikata T, Saiga H, Kusakabe T, Shin-i T, Kohara Y, Satoh N. Ciona intestinalis cDNA projects: expressed sequence tag analyses and gene expression profiles during embryogenesis. Gene. 2002;287:83–96. doi: 10.1016/s0378-1119(01)00826-5. [DOI] [PubMed] [Google Scholar]
- Scaal M, Christ B. Formation and differentiation of the avian dermomyotome. Anatomy and embryology. 2004;208:411–424. doi: 10.1007/s00429-004-0417-y. [DOI] [PubMed] [Google Scholar]
- Schuster-Gossler K, Cordes R, Gossler A. Premature myogenic differentiation and depletion of progenitor cells cause severe muscle hypotrophy in Delta1 mutants. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:537–542. doi: 10.1073/pnas.0608281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somorjai IM, Somorjai RL, Garcia-Fernandez J, Escriva H. Vertebrate-like regeneration in the invertebrate chordate amphioxus. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:517–522. doi: 10.1073/pnas.1100045109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Gainous TB, Young JJ, Mori A, Levine M, Christiaen L. Early chordate origins of the vertebrate second heart field. Science. 2010;329:565–568. doi: 10.1126/science.1190181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolfi A, Wagner E, Taliaferro JM, Chou S, Levine M. Neural tube patterning by Ephrin, FGF and Notch signaling relays. Development. 2011;138:5429–5439. doi: 10.1242/dev.072108. [DOI] [PubMed] [Google Scholar]
- Strom A, Castella P, Rockwood J, Wagner J, Caudy M. Mediation of NGF signaling by post-translational inhibition of HES-1, a basic helix-loop-helix repressor of neuronal differentiation. Genes & development. 1997;11:3168–3181. doi: 10.1101/gad.11.23.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh-Finkel L, Elhanany H, Rinon A, Tzahor E. Mesoderm progenitor cells of common origin contribute to the head musculature and the cardiac outflow tract. Development. 2006;133:1943–1953. doi: 10.1242/dev.02365. [DOI] [PubMed] [Google Scholar]
- Tolkin T, Christiaen L. Development and evolution of the ascidian cardiogenic mesoderm. Current topics in developmental biology. 2012;100:107–142. doi: 10.1016/B978-0-12-387786-4.00011-7. [DOI] [PubMed] [Google Scholar]
- Tzahor E. Heart and craniofacial muscle development: a new developmental theme of distinct myogenic fields. Developmental biology. 2009;327:273–279. doi: 10.1016/j.ydbio.2008.12.035. [DOI] [PubMed] [Google Scholar]
- van Bueren KL, Papangeli I, Rochais F, Pearce K, Roberts C, Calmont A, Szumska D, Kelly RG, Bhattacharya S, Scambler PJ. Hes1 expression is reduced in Tbx1 null cells and is required for the development of structures affected in 22q11 deletion syndrome. Developmental biology. 2010;340:369–380. doi: 10.1016/j.ydbio.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasyutina E, Lenhard DC, Wende H, Erdmann B, Epstein JA, Birchmeier C. RBP-J (Rbpsuh) is essential to maintain muscle progenitor cells and to generate satellite cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:4443–4448. doi: 10.1073/pnas.0610647104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamstad JA, Alexander JM, Truty RM, Shrikumar A, Li F, Eilertson KE, Ding H, Wylie JN, Pico AR, Capra JA, et al. Dynamic and coordinated epigenetic regulation of developmental transitions in the cardiac lineage. Cell. 2012;151:206–220. doi: 10.1016/j.cell.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Razy-Krajka F, Siu E, Ketcham A, Christiaen L. NK4 Antagonizes Tbx1/10 to Promote Cardiac versus Pharyngeal Muscle Fate in the Ascidian Second Heart Field. PLoS biology. 2013;11:e1001725. doi: 10.1371/journal.pbio.1001725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Zaffran S, Kuroiwa A, Higuchi H, Ogura T, Harvey RP, Kelly RG, Buckingham M. Fibroblast growth factor 10 gene regulation in the second heart field by Tbx1, Nkx2-5, and Islet1 reveals a genetic switch for down-regulation in the myocardium. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:18273–18280. doi: 10.1073/pnas.1215360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woznica A, Haeussler M, Starobinska E, Jemmett J, Li Y, Mount D, Davidson B. Initial deployment of the cardiogenic gene regulatory network in the basal chordate, Ciona intestinalis. Developmental biology. 2012;368:127–139. doi: 10.1016/j.ydbio.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.