Abstract
We previously identified the Arabidopsis thaliana–derived decapeptide OSIP108, which increases tolerance of plants and yeast cells to oxidative stress. As excess copper (Cu) is known to induce oxidative stress and apoptosis, and is characteristic for the human pathology Wilson disease, we investigated the effect of OSIP108 on Cu-induced toxicity in yeast. We found that OSIP108 increased yeast viability in presence of toxic Cu concentrations, and decreased the prevalence of Cu-induced apoptotic markers. Next, we translated these results to the human hepatoma HepG2 cell line, demonstrating anti-apoptotic activity of OSIP108 in this cell line. In addition, we found that OSIP108 did not affect intracellular Cu levels in HepG2 cells, but preserved HepG2 mitochondrial ultrastructure. As Cu is known to induce acid sphingomyelinase activity of HepG2 cells, we performed a sphingolipidomic analysis of OSIP108-treated HepG2 cells. We demonstrated that OSIP108 decreased the levels of several sphingoid bases and ceramide species. Moreover, exogenous addition of the sphingoid base dihydrosphingosine abolished the protective effect of OSIP108 against Cu-induced cell death in yeast. These findings indicate the potential of OSIP108 to prevent Cu-induced apoptosis, possibly via its effects on sphingolipid homeostasis.
Keywords: OSIP108, copper, apoptosis, ceramide, Saccharomyces cerevisiae
1. Introduction1
Using Tiling Array technology we recently identified a decapeptide, termed OSIP108, in the plant Arabidopsis thaliana upon treatment with the herbicide paraquat (PQ) [1]. PQ induces reactive oxygen species (ROS) [2]. We found that OSIP108 increases tolerance of plant cells to oxidative stress agents like PQ. Moreover, a significantly increased tolerance to H2O2 was also demonstrated in the model yeast Saccharomyces cerevisiae by either heterologous expression of the OSIP108-encoding gene or after exogenous application of OSIP108 to H2O2-treated yeast [13]. Hence, OSIP108 seems to protect various cell types against oxidative stress inducing agents such as PQ and H2O2
Excess copper (Cu) was shown to induce oxidative stress and apoptosis. Copper (Cu), like zinc and iron, is an essential trace element required for normal cellular functioning. For instance, Cu acts as an essential co-factor of a variety of enzymes such as cytochrome c oxidase and Cu,Zn superoxide dismutase [3, 4]. However, excess Cu can become toxic, as in the case of Wilson disease (WD), a human pathology characterized by Cu accumulation in the liver [5] resulting in acute liver failure or cirrhosis [6]. Reported mechanisms underlying Cu toxicity are related to mitochondrial dysfunction and damage, since Cu causes (i) a deficiency in the mitochondrial respiratory chain at the level of the Cu-dependent complex IV [7]; (ii) cross-linking of mitochondrial membranous proteins and subsequent contraction of the membrane [7]; (iii) oxidative stress [8–11] and (iv) increased acid sphingomyelinase (aSMase) activity [12]. The latter results in an increased production of ceramide [12], which has been shown to modulate mitochondrial outer membrane permeabilization and induce apoptosis [13, 14].
As ROS are known to induce apoptosis via both intrinsic and extrinsic apoptotic pathways [15], we investigated in the present study the potential protective effects of OSIP108 against Cu-induced oxidative stress and apoptosis. To this end, we studied the effect of OSIP108 on cell survival and apoptotic levels of either a lower and higher eukaryote (yeast and human, respectively) in the presence of toxic Cu concentrations. All data point to the anti-apoptotic potential of OSIP108 via its effect on sphingolipid homeostasis.
2. Materials and methods
2.1 Materials, yeast strains and cell lines
The yeast strains used in this study are Saccharomyces cerevisiae wild type yeast strain BY4741 (WT) and Δyca1 deletion mutant (Euroscarf, Germany) were cultured in SC (0.77 g/L complete amino acid supplement mixture (CSM) (Bio 101 Systems); 6.7 g/L yeast nitrogen base without amino acids (YNB); 20 g/L glucose) medium. HepG2, human hepatoblastoma cells were obtained from ATCC (Rockville, MD, USA) and grown in Minimal Essential Medium (MEM) supplemented with 10% fetal calf serum, 2 mM L-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Copper sulfate (CuSO4) and copper chloride (CuCl2) (Cu), were purchased from Sigma-Aldrich (St. Louis, MO, USA). OSIP108 (MLCVLQGLRE, 1161 g/mol) and OSIP3.2D (MSRRMILTQYW, 1484 g/mol) were purchased from Thermo Fisher Scientific (Ulm, Germany). Dihydrosphingosine (dhSph) was purchased from Avanti Polar Lipids, Inc (Alabama, USA). Solvent for peptides and dhSph was DMSO.
Protocols involving qRT-PCR analysis of OSIP108 treated HepG2 cells are included in the supplementary data.
2.2 Yeast Cu toxicity experiments in agar
An overnight WT yeast culture in SC was diluted 50-fold in SC growth medium containing 0.8 % agar, 0.1 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Sigma-Aldrich, St. Louis, MO, USA) and 100 μM Cu. 5μL of 100 % DMSO (vehicle control), 20 mM OSIP108 or 20 mM OSIP3.2D was spotted onto the plates. After 24h of incubation at 30°C, purple halo diameters, indicative for cell survival, were evaluated.
2.3 Yeast Cu survival in liquid media
An overnight yeast culture in SC was diluted to OD600 = 2 in fresh SC and incubated with control (distilled H2O) or 2 mM Cu upon treatment with 2 % DMSO (vehicle control) or 100 μM OSIP108. After 4 h incubation (30°C, 250 rpm) appropriate cell dilutions were plated onto YPD agar plates. Cell survival was quantified by determining CFU/ml as compared to cells receiving no Cu. As for exogenous dhSph addition, yeast cells were treated as described above in the presence or absence of dhSph (5 μg/ml – 20 μg/ml).
2.4 Detection of apoptotic markers in yeast
An overnight WT yeast culture in SC was diluted to OD600 = 2 in fresh SC and incubated with 2 mM Cu in presence of 2 % DMSO (vehicle control) or 100 μM OSIP108. After 4 h incubation (30°C, 250 rpm) 5.106 cells were washed twice with PBS and stained with 5 μg/mL dihydroethidium (Molecular Probes) (DHE) or 20 μM CaspACE FITC-VAD-FMK (Promega Benelux BV) in PBS by incubating at 30°C for 20 minutes. To detect DNA fragmentation Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed. Briefly, following Cu treatment (2 mM) in presence of vehicle control or 100 μM OSIP108, 4.107 cells were fixed with 70 % ethanol for 15' at room temperature and cell wall was digested with 30 U/ml zymolyase 20 T (Seikagaku, Tokyo, Japan) in zymolyase buffer (1 M sorbitol, 1 mM EDTA, 10 mM sodium citrate, pH 5.8) for 15 minutes at 30°C. Next, cells were incubated with permeabilization solution (0.1 % Triton X 100, 0.1 % sodium citrate) for 2 minutes on ice before incubating the cells with TUNEL reaction mixture (In Situ Cell Detection Kit, Roche Diagnostics Belgium NV, Belgium) according to manufacturer's description. Following each staining, samples were washed twice with PBS, and flow cytometry analysis was performed using BD Influx flow cytometry (BD Biosciences, New Jersey, NJ, USA). Data was analyzed using FlowJo software (Tree Star Inc., Ashland, MA, USA). 20.000 cells/sample were analyzed.
2.5 HepG2 Cu toxicity experiments
HepG2 cells were seeded at 104 cells/well in a 96-well plate in medium containing 1% DMSO (vehicle control) or OSIP108 (10 μM – 100 μM). Following 16 h preincubation, cells were treated with 0.75 mM Cu in presence of vehicle control or OSIP108 (10 μM – 100 μM) After 48 h incubation, cell viability was determined by MTT viability staining as described [16].
2.6 Detection of apoptosis and oxidative stress in HepG2 cells
HepG2 cells were seeded at 106 cells/well in a 6 well plate and incubated overnight in presence of 1% DMSO (vehicle control) or 100 μM OSIP108. Next, cells were incubated for 24 h with 0.5 mM Cu and vehicle control or 100 μM OSIP108. Cell culture supernatants and cells were collected and costained with FLUOS-labeled Annexin V (AnnV) (Roche Diagnostics NV Belgium,, Belgium) and propidium iodide (PI) (Sigma-Aldrich, St. Louis, MO, USA). ROS levels were determined using 2',7'-dichlorodihydrofluorescein diacetate (H2DCFDA; Life Technologies). Cells were incubated with Cu and the dye for 60 min at 37°C. DCF positive, viable cells were recorded. Following staining, cells were analyzed by flow cytometry (Beckman Coulter Epics XL.MCL) and data was analyzed by CXP software Version 1.0.
2.7 Cellular Cu binding
Cellular copper determination was performed according to Cater et al. [17]. Briefly, HepG2 cells were seeded at 106 cells/well into a 6-well plate and cultured for 12 h in medium containing 1 % DMSO (vehicle control) or 100 μM OSIP108. Next, the medium was replaced by fresh medium containing 0.1 mM Cu. After 24 h incubation, cells were washed with PBS and trypsinized. After centrifugation, dried cell pellets were dissolved in 65 % nitric acid (Suprapure, Merck, Germany) and incubated overnight at 65°C. Total amount of Cu was analyzed by atom absorption spectroscopy (Atomic absorption spectrophotometer Shimadzu AA6300, Japan) as described [16].
2.8 Electron microscopy
HepG2 cells were seeded at 106 cells/well in a 6-well plate in media containing 1 % DMSO or 100 μM OSIP108. After overnight incubation, cells were cultured in fresh medium containing 0.75 mM Cu and vehicle control or 100 μM OSIP108 respectively. After 16 h incubation, cells were fixed in 2 % glutaraldehyde and 0.5 % osmium tetroxide in 0.1 M PBS, dehydrated with ethanol, and embedded in Epon using standard procedures as described [18]. Thin sections were cut using an ultramicrotome and contrasted with uranyl acetate and lead citrate. Sections were examined with an EM410 electron microscope (Philips) and documented digitally (DITABIS).
2.9 Sphingolipidomics on OSIP108-treated HepG2 cells
HepG2 cells were seeded at 105 cells/plate in 60 mm plates and following 24 h incubation cells were treated with 1% DMSO (vehicle control) or 100 μM OSIP108 in serum free media. Following 48 h treatment with OSIP108, cells were washed twice with cold PBS, and lipids were extracted in 2 mL isopropanol:water:ethyl acetate (30:10:60 by vol). Cell extracts were analyzed by reverse phase high pressure liquid chromatography coupled to electrospray ionization and subsequent separation by mass spectrometry. Analysis of sphingoid bases, ceramides and sphingomyelins was performed on a Thermo Quantum Ultra mass spectrometer, operating in a multiple reaction-monitoring positive ionization mode, as described [19].
2.10 Statistical analysis
All values are presented as mean with standard error (SEM). Data were analyzed by Graphpad Prism 6 software. P < 0.05 was considered as statistically significant.
3. Results and discussion
3.1 OSIP108 increases yeast tolerance to Cu
We previously described the protective effect of the bioactive plant-derived peptide OSIP108 (MLCVLQGLRE) against H2O2-induced oxidative stress in plant and yeast cells [1]. Since Cu has been shown to induce oxidative stress and ROS [8–11], we investigated the effect OSIP108 on growth and survival of wild type S. cerevisiae BY4741 (WT) in Cu-containing media. As negative control, OSIP3.2D, a random 11 amino acid long peptide (MSRRMILTQYW) was selected from our previous study, in which in contrast to OSIP108, it failed to increase yeast tolerance to H2O2 [13]. To this end, WT yeast was inoculated in solid growth medium containing a lethal Cu concentration (100 μM) and the viability dye MTT. OSIP108 (20 mM) and OSIP3.2D (20 mM) were spotted onto the agar and after 24 h of incubation, purple halos around the spotted peptides were evaluated, indicative of viable yeast cells via conversion of the viability dye MTT. We consistently found a purple halo around the spotted OSIP108 peptide, in contrast to OSIP3.2D and solvent only (vehicle control) (Fig. 1a). In addition, we evaluated the effect of OSIP108 on yeast survival in presence of Cu in liquid media by determining colony forming units (CFU). We observed that 2 mM Cu decreased yeast survival to 26 ± 2 %, whereas survival of cells receiving 100 μM OSIP108 increased to 40 ± 4 % (Fig. 1b). OSIP108 did not affect yeast survival in absence of Cu (data not shown). These data indicate that addition of exogenous OSIP108 increases yeast tolerance to Cu.
Fig. 1.
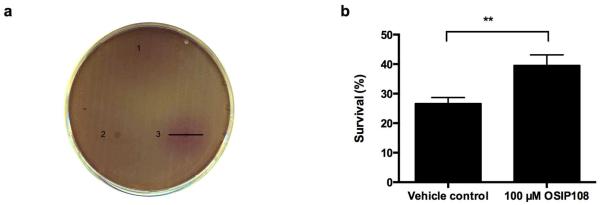
OSIP108 increases Cu tolerance of yeast cells. (a) WT yeast cells were inoculated in 100 μM Cu-containing growth medium supplemented with agar and the viability dye MTT. Vehicle control (1), OSIP3.2D (2) or OSIP108 (3) were spotted onto the agar plates. After 24 h incubation, purple halos indicative for cell survival due to conversion of the viability dye MTT, were evaluated. Bar represents halo diameter. Data are representative of 3 independent experiments. (b) WT yeast cells were incubated for 4 h with 2 mM Cu in presence of vehicle control or 100 μM OSIP108. Following incubation, cell dilutions were plated onto YPD agar plates and survival was quantified by determining colony forming units as compared to cells receiving no Cu. Experiments were performed in quadruplicate, with 4 biological repeats (**P<0.01; Student t-test)
3.2 OSIP108 prevents Cu-induced apoptosis of yeast cells
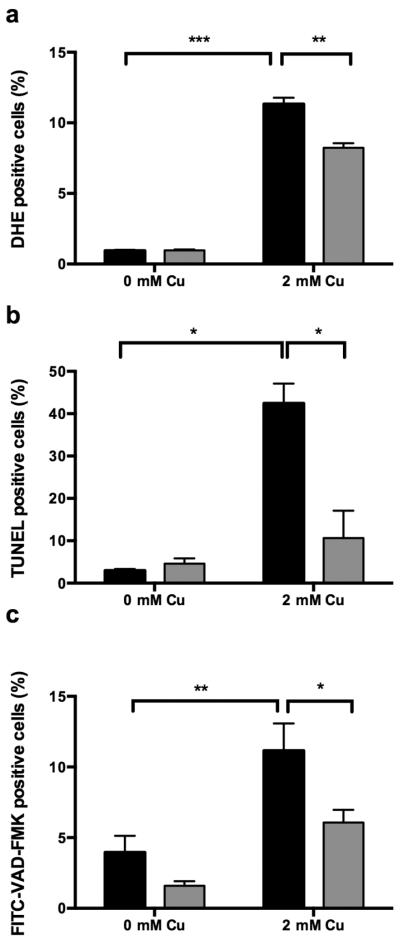
As Cu is known to induce apoptosis of yeast cells [20], we further assessed whether OSIP108 is characterized by a general anti-apoptotic activity. To this end, we investigated the prevalence of several apoptotic hallmarks by means of flow cytometry, including production of ROS, DNA fragmentation and caspase activity in yeast [21] upon Cu treatment. In a first step, we assessed the effect of different Cu doses on yeast cell survival in liquid media and the effect of OSIP108 on yeast cell survival in the presence of a toxic Cu concentration. In line with the observations described by Liang and Zhou [20], demonstrating that Cu induces apoptosis of yeast cells, we found that incubation of yeast cells with 2 mM Cu resulted in increased superoxide accumulation as documented by a significantly higher percentage of DHE positive cells as compared to cells receiving no Cu (Fig. 2a). By performing TUNEL assay, we found that Cu treatment increased the amount of fluorescent yeast cells, indicating that Cu induces DNA fragmentation in yeast (Fig. 2b). In addition, we investigated the contribution of active caspases in Cu-induced toxicity in yeast. Caspases are cysteine-aspartic specific proteases generally known to play an essential role in apoptosis. Both caspase-dependent and caspase-independent cell death pathways to apoptosis have been described in yeast [22]. By staining Cu-treated yeast cells with a fluorescent analogue of the pan caspase inhibitor (FITC-VADFMK), which binds to active caspases, and subsequent flow cytometry analysis, we found that Cu increases the amount of yeast cells with active caspases (Fig. 2c). All these data indicate that Cu induces caspase or caspase-like protease-dependent apoptosis in yeast.
Fig. 2.
OSIP108 decreases Cu-induced apoptotic hallmarks in yeast. WT yeast was incubated for 4 h with 100 μM vehicle control (black bars) or 100 μM OSIP108 (gray bars) in the presence or absence of Cu. Following incubation, cells were stained with DHE (a) to determine superoxide production, TUNEL assay (b) to detect DNA fragmentation, or FITC- labeled VAD-FMK (c) to determine active caspases or caspase-like proteases. Samples were analyzed by flow cytometry. Assays were performed in at least 3 biological repeats. (*P<0.05; **P<0.01; ***P<0.001; Student t-test)
Next, we investigated potential anti-apoptotic activity of OSIP108 using Cu-induced apoptosis in yeast as a model, by assessing the above apoptosis-related markers in presence and absence of OSIP108. We demonstrated that addition of 100 μM OSIP108 to yeast cells treated with 2 mM Cu decreased the amount of DHE positive cells (Fig. 2a) as well as the amount of cells with DNA fragmentation as determined by TUNEL assay (Fig. 2b). Moreover, we found less cells with active caspases or caspase-like proteases upon incubation with OSIP108 as compared to vehicle control (Fig. 2c). These data indicate that OSIP108 reduces all tested hallmarks of Cu-induced apoptosis in yeast, thereby increasing yeast cell survival in the presence of Cu.
Since our data indicate that active caspases or caspase-like proteases are involved in Cu-induced apoptosis in yeast, and that OSIP108 decreases the number of cells with caspase or caspase-like activity, we further investigated the Cu tolerance of Δyca1 yeast cells. The yeast-like protease Yca1p plays a major role in the caspase-dependent yeast apoptotic pathway induced by H2O2 [23], acetic acid [24] or valproic acid [25]. Previous studies by Liang and Zhou [20] and Horowitz, Lapointe and coworkers [26] reported no difference in Cu tolerance of WT and Δyca1 yeast, indicating that Cu-induced apoptosis in yeast does not require a functional metacaspase Yca1p. In line with the literature [20, 26], our results showed no significant difference in tolerance to 2 mM Cu of WT and Δyca1 yeast cells (Supplemental Fig. S1). These data indicate that the anti-apoptotic effects of OSIP108 on yeast are not mediated by the yeast metacaspase Yca1p, but probably by as yet unidentified caspases or caspase-like proteases.
3.3 OSIP108 increases Cu tolerance of the human hepatoma HepG2 cell line
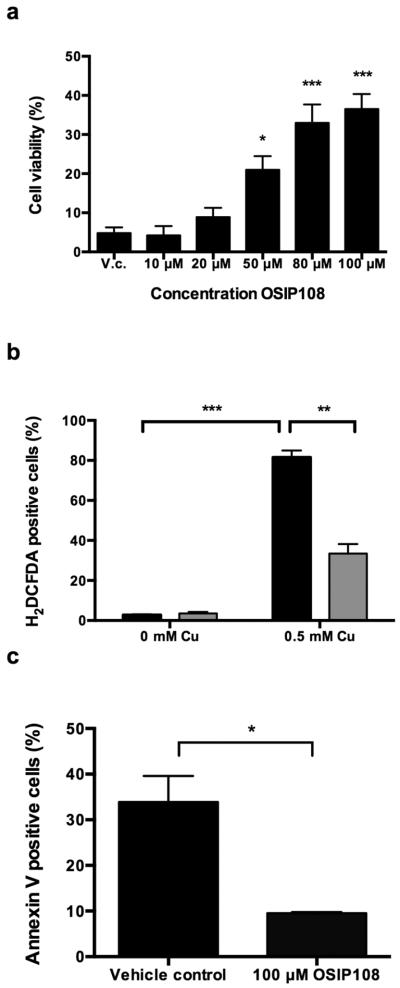
Given the conservation of copper homeostasis regulation [27–29] and apoptosis [21, 30] between yeast and human, we translated our data obtained with yeast to a higher eukaryotic HepG2 cell line. We found that incubating HepG2 cells in presence of 0.75 mM Cu decreased cell viability of cells receiving vehicle control or 100 μM OSIP108 to 4.75 ± 1.49 % and 5 ± 0.59 % respectively, while preincubation with 100 μM OSIP108 prior to simultaneous addition of 100 μM OSIP108 and 0.75 mM Cu increased cell viability to 32.75 ± 5.54 % (data not shown). In addition, titration of the OSIP108 concentration (10 μM − 100 μM) indicated a dose response relationship with regard to increase in HepG2 cell viability in the presence 0.75 mM Cu (Fig. 3a). In contrast to yeast cells, in which OSIP108 also exerts anti-apoptotic activity without OSIP108 pretreatment of the cells, we found that preincubation of HepG2 cells with OSIP108 is necessary prior to Cu addition. This could indicate that the anti-apoptotic activity of OSIP108 depends e.g. on the individual copper sensitivity of the cell type, growth characteristics and/or kinetics of OSIP108 uptake. This is, however, not the first observation of a preincubation step with a compound in order to observe anti-apoptotic effects. For instance, Guo and coworkers observed that precinbuation of PC12 cells with the antioxidant Ac-cel prior to H2O2 addition decreased H2O2-induced cytotoxicity and prevalence of apoptotic markers [31] while Lim and coworkers found that preincubation of Friedrich's ataxia patients fibroblasts with lipophilic iron chelators prevented H2O2-induced cytotoxicity [32]. Even the protective effects of the anti-apoptotic peptide Humanin (HN) against amyloid-β 1-42 (aβ 1-42)-induced apoptosis in cortical neurons is mediated by a preincubation phase prior to simultaneous addition of HN and aβ 1-42 [33].
Fig. 3.
OSIP108 reduces Cu sensitivity of mammalian cells. (a) HepG2 cells were preincubated with vehicle control (V.c.) or OSIP108 (10 μM – 100 μM) prior to further incubation with V.c. or OSIP108 (10 μM – 100 μM) and 0.75 mM Cu. Cell viability was determined by MTT staining. Mean and SEM of three experiments are shown. (*P<0.05; ***P<0.001; ANOVA test using Tukey correction). HepG2 cells were preincubated overnight with vehicle control or 100 μM OSIP108 prior to co-incubation in 0.5 mM Cu and vehicle control black bars) or 100 μM OSIP108 (gray bars) for 24 h. Following incubation, cells were stained with (b) H2DCFDA or (c) FLUOS-labeled AnnV and samples were analysed by flow cytometry. Mean and SEM of 3 biological repeats are shown. (*P<0.05; Student t-test)
3.4 OSIP108 prevents Cu-induced oxidative stress and apoptosis in HepG2 cells
Since excess Cu has been shown earlier to induce oxidative stress [8–11] and apoptosis in hepatocytes [12, 34] we further evaluated whether the observed protectant effect of OSIP108 on Cu-treated HepG2 cells could be related to its anti-apoptotic activity, similar to our experiments in yeast using flow cytometry analysis of apoptotic markers. OSIP108 treatment significantly reduced Cu-induced ROS levels in HepG2 cells (Fig. 3b). We found that a significantly lower percentage of HepG2 cells were AnnV-positive after 100 μM OSIP108 treatment as compared to vehicle-treated cells treated in presence of 0.5 mM Cu (Fig. 3c). Necrosis was also slightly reduced by OSIP108 treatment as determined by PI staining although less than 1 % PI positive cells were observed (Supplemental Fig. S2). Noteworthy here is our observation that Cu-induced alterations of cell morphology, including cell rounding and shrinkage, were reversed following OSIP108 treatment, as observed by flow cytometry analysis of cell size and granularity (data not shown). In general these data indicate that OSIP108 also prevents Cu-induced ROS formation and apoptosis of hepatocytes, similar to its effect on yeast cells.
3.5 OSIP108 does not modulate intracellular Cu levels but preserves mitochondrial ultrastructure
One of the general cellular Cu detoxification mechanisms is Cu sequestration or chelation to limit its toxicity by cysteine-rich metallothioneins, peptides or proteins, which harbor cysteinyl sulfurs that can act as ligands for Cu [35, 36]. To gain more insight into the mode of action of OSIP108, and to exclude extracellular Cu chelation by the cysteine in OSIP108, we determined the Cu-content of Cu-treated HepG2 cells in the presence of OSIP108. Atom absorbance spectroscopy analysis of HepG2 cells treated with 0.1 mM Cu showed that 100 μM OSIP108-treated cells were loaded with equal amounts of Cu as compared to the vehicle control suggesting that the peptide does not affect the Cu uptake (Fig. 4a) as is known for D-penicillamine, a Cu-chelating drug used to treat WD patients [37]. In addition, gene expression analysis of a subset of genes related to Cu transport and oxidative stress such as SOD1, encoding the superoxide dismutase 1 protein involved in detoxification of the ROS superoxide, and GSS, encoding the glutathione synthetase protein which catalyzes the second step in the synthesis of the antioxidant glutathione, indicated that OSIP108 does not affect major mechanisms of Cu homeostasis in eukaryotic cells (Supplemental Fig. S3). Furthermore, electron microscopy experiments were used to assess mitochondrial damage upon Cu treatment. We observed that 0.75 mM Cu-treated mitochondria were pleomorphic and had increased matrix density and frequently found elongated forms of mitochondria, similar to mitochondria of hepatocytes during the course of WD [7, 38] (Fig. 4b middle panel). We also we observed a high grade of vacuolization indicating advanced damage by Cu [38]. OSIP108 treatment in presence of Cu resulted in regular shaped mitochondria with normal cristae structures (Fig. 4b bottom panel) similar to cells receiving no Cu (Fig. 4b top panel). Vacuolization was absent in OSIP108 treated Cu-exposed cells, suggesting that the peptide has a high cytoprotective effect including preservation of mitochondrial ultrastructure. These data indicate that OSIP108 protects HepG2 cells against Cu-induced mitochondrial damage without chelating extracellular Cu or affecting Cu uptake. Of note, the cytoprotective effects of OSIP108 were observed at a wide range of Cu concentrations in the different assays (data not shown). However, since the rate of necrotic cells limited statistical analysis, a lower Cu concentration was assessed in some of the experiments.
Fig. 4.
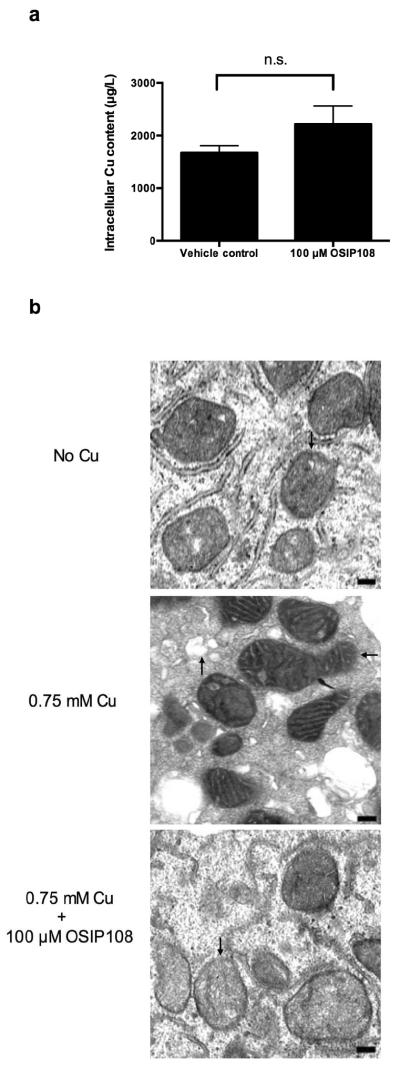
OSIP108 does not affect intracellular Cu levels and preserves mitochondrial integrity. (a) Intracellular Cu levels of 0.1 mM Cu-treated HepG2 cells pretreated with vehicle control or 100 μM OSIP108 was determined by atom absorption spectroscopy. Intracellular Cu levels were not significantly different (n.s.). Mean and SEM of 3 biological repeats is shown. (P>0.05, Student t-test). b) Electron microscopy of HepG2 cells grown in 0.75 mM Cu-containing medium in the presence or absence of 100 μM OSIP108. Regular shaped mitochondria (↓), elongated mitochondria with condensation and increased matrix density (←) and vacuolization (↑) are denoted by arrows. Bar represents 0.2 μm. Data representative for 3 biological repeats.
3.6 OSIP108 affects sphingolipid homeostasis in HepG2 cells
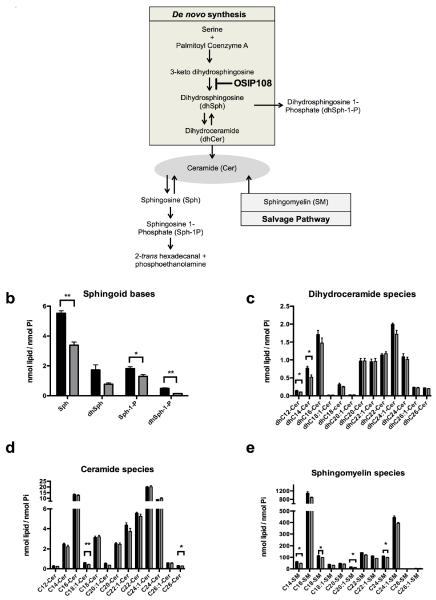
Several pathways are known to be involved in the generation of ceramides as summarized in Fig. 5a. Ceramide can be biosynthesized de novo or via the salvage pathway. The salvage pathway produces ceramide from hydrolysis of sphingomyelin. Subsequently, the ceramide produced by either the de novo or salvage pathways can be converted to sphingosine (Sph) which is then phosphorylated to produce Sphingosine-1-Phosphate (Sph-1-P). Sph-1-P is then cleaved into phosphoethanolamine and fatty acids which represent the only exit route from the sphingolipid pathway [39]. Ceramides are known to induce apoptosis [15], by altering mitochondrial function [13, 14, 40–42], and are associated with the generation of ROS [43–45]. Given the observed anti-apoptotic effect of OSIP108 in yeast and human cells, and its protective effect on Cu-induced mitochondrial damage, involving acid sphingomyelinase activation [12] we reasoned that OSIP108 might affect sphingolipid homeostasis in HepG2 cells. Based on the observation that preincubation of HepG2 cells with OSIP108 prior to Cu addition was necessary to increase HepG2 viability, we chose to perform sphingolipidomics on OSIP108-treated HepG2 cells in absence of Cu. Sphingolipidomic analysis showed that OSIP108 treatment (100 μM) decreased levels of several sphingolipid species from the three ceramide generating pathways. We found that OSIP108 decreased Sph, Sph-1P and Dihydrosphingosine-1-Phosphate (dhSph-1-P) levels (Fig. 5b), as well as several Dihydroceramide (dhCer) (C12, C14) species (Fig. 5c), ceramide (Cer) (C18:1 and C26) (Fig. 5d) and sphingomyelin (SM) species (C14, C18, C20:1, C24,) (Fig. 5e). Although dhSph levels were not significantly affected by OSIP108 treatment (P=0.0518; Student t-test), these data suggest an effect of OSIP108 on 3-ketodihydrosphingosine reductase activity, which catalyzes the reduction of 3-ketodihydrosphingosine to dhSph.
Fig. 5.
OSIP108 affects sphingolipid homeostasis in HepG2 cells. (a) Major pathways involved in ceramide generation. HepG2 cells were treated with vehicle control (black bars) or 100 μM OSIP108 (grey bars) for 48 h. Next, cells were harvested and sphingolipids were extracted. (b) Sphingoid bases, (c) Dihydroceramide species, (d) Ceramide species and (e) Sphingomyelin species were identified by tandem mass spectrometry. Mean and SEM of three biological repeats are shown. (*P<0.05; **P<0.01; Student t-test)
3.7 Exogenous dhSph addition abrogates the protective effect of OSIP108 on Cu-induced toxicity in yeast cells
Given our observation that OSIP108 affects sphingolipid homeostasis in HepG2 cells, we further investigated a putative role of sphingolipids in preventing Cu-induced cell death. Sphingolipid generating pathways are well conserved between yeast and mammalian cells and both produce dhSph as the second intermediate in de novo sphingolipid biosynthesis [46]. We chose to validate the sphingolipidomics data in yeast by investigating the effect of exogenous addition of dhSph in our yeast model for Cu-induced cell death, in the presence or absence of OSIP108, as OSIP108 apparently downregulates all sphingoid bases and sphingolipids intermediates starting from dhSph. dhSph is efficiently taken up and used in the sphingolipid metabolism in yeast upon exogenous addition [47–50]. To this end, WT yeast cells were incubated with different concentrations of dhSph (5 μg/ml – 20 μg/ml) in the presence or absence of 100 μM OSIP108, upon treatment with 2 mM Cu or control. No significant effect on yeast survival was observed with dhSph alone (Fig. 6). We observed a significant increase in survival upon incubation with OSIP108 in presence of 2 mM Cu, which was completely abolished in the presence of 20 μg/ml dhSph. A lower dhSph dose (5 μg/ml) partially abolished the protective effect of OSIP108, indicating a dose-dependent effect. Our data suggest that exogenous dhSph addition abolishes the protective effect of OSIP108 on Cu-induced cell death in a dose dependent manner, without affecting yeast viability when administered without OSIP108. Besides being incorporated into sphingolipids [50], the exogenously added dhSph could also interfere with the signaling function described for endogenous dhSph [51, 52]. Whether OSIP108 only inhibits 3-ketodihydrosphingosine reductase or also blocks sphingolipid-mediated signaling needs to be studied further.
Fig. 6.
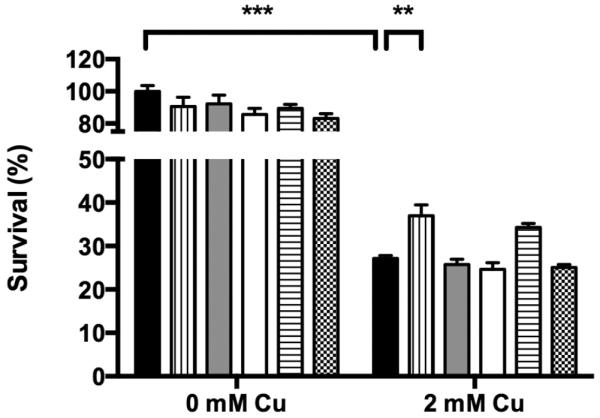
Exogenously added dhSph prevents the protective effect of OSIP108. WT yeast cells were treated with vehicle control (black bars), 100 μM OSIP108 (vertical dashed bars), 5 μg/ml dhSph (gray bars), 20 μg/ml dhSph (white bars), 5 μg/ml dhSph + 100 μM OSIP108 (horizontal dashed bars) or 20 μg/ml dhSph + 100 μM OSIP108 (chessboard pattern bars) in the presence or absence of 2 mM Cu. Following 4 h incubation, survival was quantified by determining CFU/ml as compared to vehicle control receiving no Cu. Mean and SEM of 3 biological repeats are shown. (**P<0.01; ***P<0.001; ANOVA test using Tukey corrections)
4. Conclusions and perspectives
We show here that the A. thaliana-derived peptide OSIP108, earlier selected based on its oxidative stress protectant activity [13], also protects yeast and human HepG2 cells against Cu-induced toxicity, which could further be attributed to its anti-apoptotic activity potentially mediated by an effect on sphingolipid homeostasis. Specifically regarding its protective effect on HepG2 cells, OSIP108-based therapies could thus be considered as potential new approaches in treatment of human diseases related to Cu toxicity such as WD. In this context it is worth mentioning that the natural occurrence of OSIP108 seems to be restricted to certain Arabidopsis species (Dr. Aminael Sanchez-Rodriguez, CMPG, KU Leuven, personal communication). Moreover, a BLASTP similarity search using the OSIP108 amino acid sequence via PepBank did not yield any significant hits, indicating that no OSIP108 homologues in other organisms, including lower eukaryotes (e.g. yeast) as well as higher eukaryotes (humans), are present. Hence, using OSIP108 as an anti-apoptotic compound can only be achieved via exogenous applications. In light of a putative application of OSIP108 as a drug for human pathologies linked to excessive apoptosis, the use of small peptides poses several advantages over chemical molecules, such as high specificity and low toxicity profile [53]. On the other side, their low oral bioavailability during therapeutic application, due to e.g. enzymatic degradation in the stomach and short plasma half-life [54, 55], can be problematic. However an array of approaches currently exists to improve the pharmacological properties of peptides [56, 57]. For instance, oral insulin formulations already show promising results as treatment of diabetes type 1 [58] and nasal administrations of peptides such as the polypeptide hormone calcitonin [59] have been successfully tested in clinical trials. Hence, further preclinical and clinical development of OSIP108 as a novel therapy for diseases related to excessive apoptosis such as WD or some neurodegenerative diseases, will require considerable efforts in the development of adequate OSIP108 formulations.
Supplementary Material
Highlights
OSIP108 increases yeast and HepG2 viability in presence of toxic Cu concentrations
OSIP108 decreases Cu-induced apoptotic markers of yeast and HepG2 cells
OSIP108 preserves mitochondrial ultrastructure of HepG2 cells
OSIP108 affects sphingolipid homeostasis in HepG2 cells
Exogenous dhSph blocks the effect of OSIP108 against Cu-induced toxicity in yeast
Acknowledgements
This work was supported by grants from FWO-Vlaanderen (G.A062.10N and G.0414.09), `Bijzonder Onderzoeksfonds KU Leuven' (GOA/2008/11) and also by grant CA087584 from the NIH to Y.A.H. P.S. and K.V. are supported through a PhD-grant by IWT-Vlaanderen; G.C by FP7-PEOPLE (grant 247506); D.C. by FWO-Vlaanderen as a fundamental-clinical researcher; and K.T. by `Industrial Research Fund' of KU Leuven (IOF-M). The authors acknowledge Geert Schoofs and Dr. Aminael Sanchez-Rodriguez (CMPG, KU Leuven, Belgium) for flow cytometry analysis and for homology searches of OSIP108, respectively. The authors also like to acknowledge the Lipidomics Core Facility at SUNY Stony Brook, NY for measurement of lipids.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations in order of appearance: PQ, paraquat; ROS, reactive oxygen species; Cu, copper; WD, Wilson disease; aSMase, acid sphingomyelinase; dhSph, dihydrosphingosine, MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, DHE, dihydroethidium; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; H2DCFDA, 2',7'-dichlorodihydrofluorescein diacetate, PI, propidium iodide, HN, Humanin; aβ 1-42, amyloid-β 1-42; Sph, Sphingosine; Sph-1-P, Sphingosine-1-Phosphate; dhSph-1P, Dihydrosphingosine-1-Phosphate; dhCer, Dihydroceramide; Cer, Ceramide; SM, Sphingomyelin; Des1, Dihydroceramide desaturase.
References
- [1].De Coninck B, Carron D, Tavormina P, Willem L, Craik DJ, Vos C, Thevissen K, Mathys J, Cammue BP. Mining the genome of Arabidopsis thaliana as a basis for the identification of novel bioactive peptides involved in oxidative stress tolerance. J Exp Bot. 2013;64(17):5297–5307. doi: 10.1093/jxb/ert295. [DOI] [PubMed] [Google Scholar]
- [2].Farrington JA, Ebert M, Land EJ, Fletcher K. Bipyridylium quaternary salts and related compounds. V. Pulse radiolysis studies of the reaction of paraquat radical with oxygen. Implications for the mode of action of bipyridyl herbicides. Biochim Biophys Acta. 1973;314(3):372–81. doi: 10.1016/0005-2728(73)90121-7. [DOI] [PubMed] [Google Scholar]
- [3].Tapiero H, Townsend DM, Tew KD. Trace elements in human physiology and pathology. Copper. Biomed Pharmacother. 2003;57(9):386–98. doi: 10.1016/s0753-3322(03)00012-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fraga CG. Relevance, essentiality and toxicity of trace elements in human health. Mol Aspects Med. 2005;26(4–5):235–44. doi: 10.1016/j.mam.2005.07.013. [DOI] [PubMed] [Google Scholar]
- [5].Ferenci P. Regional distribution of mutations of the ATP7B gene in patients with Wilson disease: impact on genetic testing. Hum Genet. 2006;120(2):151–9. doi: 10.1007/s00439-006-0202-5. [DOI] [PubMed] [Google Scholar]
- [6].Ala A, Walker AP, Ashkan K, Dooley JS, Schilsky ML. Wilson's disease. Lancet. 2007;369(9559):397–408. doi: 10.1016/S0140-6736(07)60196-2. [DOI] [PubMed] [Google Scholar]
- [7].Roberts EA, Robinson BH, Yang S. Mitochondrial structure and function in the untreated Jackson toxic milk (tx-j) mouse, a model for Wilson disease. Mol Genet Metab. 2008;93(1):54–65. doi: 10.1016/j.ymgme.2007.08.127. [DOI] [PubMed] [Google Scholar]
- [8].Seth R, Yang S, Choi S, Sabean M, Roberts EA. In vitro assessment of copper-induced toxicity in the human hepatoma line, Hep G2. Toxicol In Vitro. 2004;18(4):501–9. doi: 10.1016/j.tiv.2004.01.006. [DOI] [PubMed] [Google Scholar]
- [9].Sokol RJ, Twedt D, McKim JM, Jr., Devereaux MW, Karrer FM, Kam I, von Steigman G, Narkewicz MR, Bacon BR, Britton RS, et al. Oxidant injury to hepatic mitochondria in patients with Wilson's disease and Bedlington terriers with copper toxicosis. Gastroenterology. 1994;107(6):1788–98. doi: 10.1016/0016-5085(94)90822-2. [DOI] [PubMed] [Google Scholar]
- [10].Gaetke LM, Chow CK. Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology. 2003;189(1–2):147–63. doi: 10.1016/s0300-483x(03)00159-8. [DOI] [PubMed] [Google Scholar]
- [11].Arnal N, de Alaniz MJ, Marra CA. Effect of copper overload on the survival of HepG2 and A-549 human-derived cells. Hum Exp Toxicol. 2013;32(3):299–315. doi: 10.1177/0960327112456313. [DOI] [PubMed] [Google Scholar]
- [12].Lang PA, Schenck M, Nicolay JP, Becker JU, Kempe DS, Lupescu A, Koka S, Eisele K, Klarl BA, Rubben H, Schmid KW, Mann K, Hildenbrand S, Hefter H, Huber SM, Wieder T, Erhardt A, Haussinger D, Gulbins E, Lang F. Liver cell death and anemia in Wilson disease involve acid sphingomyelinase and ceramide. Nat Med. 2007;13(2):164–70. doi: 10.1038/nm1539. [DOI] [PubMed] [Google Scholar]
- [13].Rego A, Costa M, Chaves SR, Matmati N, Pereira H, Sousa MJ, Moradas-Ferreira P, Hannun YA, Costa V, Corte-Real M. Modulation of mitochondrial outer membrane permeabilization and apoptosis by ceramide metabolism. PLoS One. 2012;7(11):e48571. doi: 10.1371/journal.pone.0048571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hou Q, Jin J, Zhou H, Novgorodov SA, Bielawska A, Szulc ZM, Hannun YA, Obeid LM, Hsu YT. Mitochondrially targeted ceramides preferentially promote autophagy, retard cell growth, and induce apoptosis. J Lipid Res. 2011;52(2):278–88. doi: 10.1194/jlr.M012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thevissen K, Francois IE, Winderickx J, Pannecouque C, Cammue BP. Ceramide involvement in apoptosis and apoptotic diseases. Mini Rev Med Chem. 2006;6(6):699–709. doi: 10.2174/138955706777435643. [DOI] [PubMed] [Google Scholar]
- [16].Siaj R, Sauer V, Stoppeler S, Spiegel HU, Kohler G, Zibert A, Schmidt HH. Dietary copper triggers onset of fulminant hepatitis in the Long-Evans cinnamon rat model. World J Gastroenterol. 2012;18(39):5542–50. doi: 10.3748/wjg.v18.i39.5542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Cater MA, La Fontaine S, Shield K, Deal Y, Mercer JF. ATP7B mediates vesicular sequestration of copper: insight into biliary copper excretion. Gastroenterology. 2006;130(2):493–506. doi: 10.1053/j.gastro.2005.10.054. [DOI] [PubMed] [Google Scholar]
- [18].Robenek H, Robenek MJ, Buers I, Lorkowski S, Hofnagel O, Troyer D, Severs NJ. Lipid droplets gain PAT family proteins by interaction with specialized plasma membrane domains. J Biol Chem. 2005;280(28):26330–8. doi: 10.1074/jbc.M413312200. [DOI] [PubMed] [Google Scholar]
- [19].Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39(2):82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- [20].Liang Q, Zhou B. Copper and manganese induce yeast apoptosis via different pathways. Mol Biol Cell. 2007;18(12):4741–9. doi: 10.1091/mbc.E07-05-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carmona-Gutierrez D, Eisenberg T, Buttner S, Meisinger C, Kroemer G, Madeo F. Apoptosis in yeast: triggers, pathways, subroutines. Cell Death Differ. 2010;17(5):763–73. doi: 10.1038/cdd.2009.219. [DOI] [PubMed] [Google Scholar]
- [22].Madeo F, Carmona-Gutierrez D, Ring J, Buttner S, Eisenberg T, Kroemer G. Caspase-dependent and caspase-independent cell death pathways in yeast. Biochem Biophys Res Commun. 2009;382(2):227–31. doi: 10.1016/j.bbrc.2009.02.117. [DOI] [PubMed] [Google Scholar]
- [23].Madeo F, Herker E, Maldener C, Wissing S, Lachelt S, Herlan M, Fehr M, Lauber K, Sigrist SJ, Wesselborg S, Frohlich KU. A caspase-related protease regulates apoptosis in yeast. Mol Cell. 2002;9(4):911–7. doi: 10.1016/s1097-2765(02)00501-4. [DOI] [PubMed] [Google Scholar]
- [24].Guaragnella N, Pereira C, Sousa MJ, Antonacci L, Passarella S, Corte-Real M, Marra E, Giannattasio S. YCA1 participates in the acetic acid induced yeast programmed cell death also in a manner unrelated to its caspase-like activity. FEBS Lett. 2006;580(30):6880–4. doi: 10.1016/j.febslet.2006.11.050. [DOI] [PubMed] [Google Scholar]
- [25].Mitsui K, Nakagawa D, Nakamura M, Okamoto T, Tsurugi K. Valproic acid induces apoptosis dependent of Yca1p at concentrations that mildly affect the proliferation of yeast. FEBS Lett. 2005;579(3):723–7. doi: 10.1016/j.febslet.2004.12.051. [DOI] [PubMed] [Google Scholar]
- [26].Horowitz A, Lapointe J, Eid R, Sheibani S, Gharib N, Jones NK, Vali H, Mandato CA, Greenwood MT. The human septin7 and the yeast CDC10 septin prevent Bax and copper mediated cell death in yeast. Biochim Biophys Acta. 2013;1833(12):3186–3194. doi: 10.1016/j.bbamcr.2013.09.004. [DOI] [PubMed] [Google Scholar]
- [27].Jo WJ, Loguinov A, Chang M, Wintz H, Nislow C, Arkin AP, Giaever G, Vulpe CD. Identification of genes involved in the toxic response of Saccharomyces cerevisiae against iron and copper overload by parallel analysis of deletion mutants. Toxicol Sci. 2008;101(1):140–51. doi: 10.1093/toxsci/kfm226. [DOI] [PubMed] [Google Scholar]
- [28].Bleackley MR, Macgillivray RT. Transition metal homeostasis: from yeast to human disease. Biometals. 2011;24(5):785–809. doi: 10.1007/s10534-011-9451-4. [DOI] [PubMed] [Google Scholar]
- [29].Rustici G, van Bakel H, Lackner DH, Holstege FC, Wijmenga C, Bahler J, Brazma A. Global transcriptional responses of fission and budding yeast to changes in copper and iron levels: a comparative study. Genome Biol. 2007;8(5):R73. doi: 10.1186/gb-2007-8-5-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Madeo F, Herker E, Wissing S, Jungwirth H, Eisenberg T, Frohlich KU. Apoptosis in yeast. Curr Opin Microbiol. 2004;7(6):655–60. doi: 10.1016/j.mib.2004.10.012. [DOI] [PubMed] [Google Scholar]
- [31].Guo X, Chen Y, Liu Q, Wu J, Wang L, Tang X, Zhao W, Zhang H. Ac-cel, a novel antioxidant, protects against hydrogen peroxide-induced injury in PC12 cells via attenuation of mitochondrial dysfunction. J Mol Neurosci. 2013;50(3):453–61. doi: 10.1007/s12031-013-9955-1. [DOI] [PubMed] [Google Scholar]
- [32].Lim CK, Kalinowski DS, Richardson DR. Protection against hydrogen peroxide-mediated cytotoxicity in Friedreich's ataxia fibroblasts using novel iron chelators of the 2-pyridylcarboxaldehyde isonicotinoyl hydrazone class. Mol Pharmacol. 2008;74(1):225–35. doi: 10.1124/mol.108.046847. [DOI] [PubMed] [Google Scholar]
- [33].Hashimoto Y, Niikura T, Ito Y, Sudo H, Hata M, Arakawa E, Abe Y, Kita Y, Nishimoto I. Detailed characterization of neuroprotection by a rescue factor humanin against various Alzheimer's disease-relevant insults. J Neurosci. 2001;21(23):9235–45. doi: 10.1523/JNEUROSCI.21-23-09235.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Li YW, Wang XH, Nin Q, Luo XP. [Excessive copper induces hepatocyte apoptosis and affects Bax and Bcl-2 expression in rat liver] Zhongguo Dang Dai Er Ke Za Zhi. 2008;10(1):42–6. [PubMed] [Google Scholar]
- [35].Dameron CT, Harrison MD. Mechanisms for protection against copper toxicity. Am J Clin Nutr. 1998;67(5 Suppl):1091S–1097S. doi: 10.1093/ajcn/67.5.1091S. [DOI] [PubMed] [Google Scholar]
- [36].Sutherland DE, Stillman MJ. The “magic numbers” of metallothionein. Metallomics. 2011;3(5):444–63. doi: 10.1039/c0mt00102c. [DOI] [PubMed] [Google Scholar]
- [37].Smolarek C, Stremmel W. [Therapy of Wilson disease] Z Gastroenterol. 1999;37(4):293–300. [PubMed] [Google Scholar]
- [38].Yurkova IL, Arnhold J, Fitzl G, Huster D. Fragmentation of mitochondrial cardiolipin by copper ions in the Atp7b−/− mouse model of Wilson's disease. Chem Phys Lipids. 2011;164(5):393–400. doi: 10.1016/j.chemphyslip.2011.05.006. [DOI] [PubMed] [Google Scholar]
- [39].Gault CR, Obeid LM, Hannun YA. An overview of sphingolipid metabolism: from synthesis to breakdown. Adv Exp Med Biol. 2010;688:1–23. doi: 10.1007/978-1-4419-6741-1_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. J Bioenerg Biomembr. 2005;37(3):143–53. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6(3):118–25. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Carmona-Gutierrez D, Reisenbichler A, Heimbucher P, Bauer MA, Braun RJ, Ruckenstuhl C, Buttner S, Eisenberg T, Rockenfeller P, Frohlich KU, Kroemer G, Madeo F. Ceramide triggers metacaspase-independent mitochondrial cell death in yeast. Cell Cycle. 2011;10(22):3973–8. doi: 10.4161/cc.10.22.18212. [DOI] [PubMed] [Google Scholar]
- [43].Garcia-Ruiz C, Colell A, Mari M, Morales A, Fernandez-Checa JC. Direct effect of ceramide on the mitochondrial electron transport chain leads to generation of reactive oxygen species. Role of mitochondrial glutathione. J Biol Chem. 1997;272(17):11369–77. doi: 10.1074/jbc.272.17.11369. [DOI] [PubMed] [Google Scholar]
- [44].Rizvi F, Heimann T, Herrnreiter A, O'Brien WJ. Mitochondrial dysfunction links ceramide activated HRK expression and cell death. PLoS One. 2011;6(3):e18137. doi: 10.1371/journal.pone.0018137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Quillet-Mary A, Jaffrezou JP, Mansat V, Bordier C, Naval J, Laurent G. Implication of mitochondrial hydrogen peroxide generation in ceramide-induced apoptosis. J Biol Chem. 1997;272(34):21388–95. doi: 10.1074/jbc.272.34.21388. [DOI] [PubMed] [Google Scholar]
- [46].Rego A, Trindade D, Chaves SR, Manon S, Costa V, Sousa MJ, Corte-Real M. The yeast model system as a tool towards the understanding of apoptosis regulation by sphingolipids. FEMS Yeast Res. 2013;14(1):160–178. doi: 10.1111/1567-1364.12096. [DOI] [PubMed] [Google Scholar]
- [47].Buede R, Rinker-Schaffer C, Pinto WJ, Lester RL, Dickson RC. Cloning and characterization of LCB1, a Saccharomyces gene required for biosynthesis of the long-chain base component of sphingolipids. J Bacteriol. 1991;173(14):4325–32. doi: 10.1128/jb.173.14.4325-4332.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Nagiec MM, Baltisberger JA, Wells GB, Lester RL, Dickson RC. The LCB2 gene of Saccharomyces and the related LCB1 gene encode subunits of serine palmitoyltransferase, the initial enzyme in sphingolipid synthesis. Proc Natl Acad Sci U S A. 1994;91(17):7899–902. doi: 10.1073/pnas.91.17.7899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wells GB, Lester RL. The isolation and characterization of a mutant strain of Saccharomyces cerevisiae that requires a long chain base for growth and for synthesis of phosphosphingolipids. J Biol Chem. 1983;258(17):10200–3. [PubMed] [Google Scholar]
- [50].Hallstrom TC, Lambert L, Schorling S, Balzi E, Goffeau A, Moye-Rowley WS. Coordinate control of sphingolipid biosynthesis and multidrug resistance in Saccharomyces cerevisiae. J Biol Chem. 2001;276(26):23674–80. doi: 10.1074/jbc.M101568200. [DOI] [PubMed] [Google Scholar]
- [51].Jenkins GM, Richards A, Wahl T, Mao C, Obeid L, Hannun Y. Involvement of yeast sphingolipids in the heat stress response of Saccharomyces cerevisiae. J Biol Chem. 1997;272(51):32566–72. doi: 10.1074/jbc.272.51.32566. [DOI] [PubMed] [Google Scholar]
- [52].Ferguson-Yankey SR, Skrzypek MS, Lester RL, Dickson RC. Mutant analysis reveals complex regulation of sphingolipid long chain base phosphates and long chain bases during heat stress in yeast. Yeast. 2002;19(7):573–86. doi: 10.1002/yea.861. [DOI] [PubMed] [Google Scholar]
- [53].Otvos L., Jr. Peptide-based drug design: here and now. Methods Mol Biol. 2008;494:1–8. doi: 10.1007/978-1-59745-419-3_1. [DOI] [PubMed] [Google Scholar]
- [54].Saffran M, Kumar GS, Savariar C, Burnham JC, Williams F, Neckers DC. A new approach to the oral administration of insulin and other peptide drugs. Science. 1986;233(4768):1081–4. doi: 10.1126/science.3526553. [DOI] [PubMed] [Google Scholar]
- [55].Fix JA. Oral controlled release technology for peptides: status and future prospects. Pharm Res. 1996;13(12):1760–4. doi: 10.1023/a:1016008419367. [DOI] [PubMed] [Google Scholar]
- [56].Shaji J, Patole V. Protein and Peptide drug delivery: oral approaches. Indian J Pharm Sci. 2008;70(3):269–77. doi: 10.4103/0250-474X.42967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fjell CD, Hiss JA, Hancock RE, Schneider G. Designing antimicrobial peptides: form follows function. Nat Rev Drug Discov. 2012;11(1):37–51. doi: 10.1038/nrd3591. [DOI] [PubMed] [Google Scholar]
- [58].Clement S, Still JG, Kosutic G, McAllister RG. Oral insulin product hexyl-insulin monoconjugate 2 (HIM2) in type 1 diabetes mellitus: the glucose stabilization effects of HIM2. Diabetes Technol Ther. 2002;4(4):459–66. doi: 10.1089/152091502760306544. [DOI] [PubMed] [Google Scholar]
- [59].Peichl P, Griesmacher A, Kumpan W, Schedl R, Prosquil E, Broll H. Clinical outcome of salmon calcitonin nasal spray treatment in postmenopausal women after total hip arthroplasty. Gerontology. 2005;51(4):242–52. doi: 10.1159/000085121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.