Abstract
IMPORTANCE
Genetic factors contribute to risk for bipolar disorder (BP), yet its pathogenesis remains poorly understood. A focus on measuring multi-system quantitative traits that may be components of BP psychopathology may enable genetic dissection of this complex disorder, and investigation of extended pedigrees from genetically isolated populations may facilitate the detection of specific genetic variants that impact on BP as well as its component phenotypes.
OBJECTIVE
To identify quantitative neurocognitive, temperament-related, and neuroanatomic phenotypes that appear heritable and associated with severe bipolar disorder (BP-I), and therefore suitable for genetic linkage and association studies aimed at identifying variants contributing to BP-I risk.
DESIGN
Multi-generational pedigree study in two closely related, genetically isolated populations: the Central Valley of Costa Rica (CVCR) and Antioquia, Colombia (ANT).
PARTICIPANTS
738 individuals, all from CVCR and ANT pedigrees, of whom 181 are affected with BP-I.
MAIN OUTCOME MEASURE
Familial aggregation (heritability) and association with BP-I of 169 quantitative neurocognitive, temperament, magnetic resonance imaging (MRI) and diffusion tensor imaging (DTI) phenotypes.
RESULTS
Seventy-five percent (126) of the phenotypes investigated were significantly heritable, and 31% (53) were associated with BP-I. About 1/4 of the phenotypes, including measures from each phenotype domain, were both heritable and associated with BP-I. Neuroimaging phenotypes, particularly cortical thickness in prefrontal and temporal regions, and volume and microstructural integrity of the corpus callosum, represented the most promising candidate traits for genetic mapping related to BP based on strong heritability and association with disease. Analyses of phenotypic and genetic covariation identified substantial correlations among the traits, at least some of which share a common underlying genetic architecture.
CONCLUSIONS AND RELEVANCE
This is the most extensive investigation of BP-relevant component phenotypes to date. Our results identify brain and behavioral quantitative traits that appear to be genetically influenced and show a pattern of BP-I-association within families that is consistent with expectations from case-control studies. Together these phenotypes provide a basis for identifying loci contributing to BP-I risk and for genetic dissection of the disorder.
Introduction
Bipolar disorder (BP) encompasses a broad range of phenotypic features, however, most research into its etiology has focused on the overall syndrome1–6rather than on its components. Although genome wide association studies (GWAS) have identified the first replicated loci contributing to BP susceptibility3–6, the small relative risk attributed to these loci may reflect the complex genetic nature of the disorder. This possibility motivates efforts to identify heritable BP-associated quantitative traits for which the genetic basis is simpler, and for which higher impact variants may be detected7–12.
We describe here our investigation, in 26 pedigrees selected for multiple cases of severe BP (BP-I), of quantitative traits hypothesized to represent components of the biology underlying BP. Previous studies of these measures demonstrated association with BP, deficits in euthymic BP-affected individuals, and values in non-BP individuals intermediate between those of their BP-relatives and control subjects. These phenotypes assay temperament13–15; perceptual creativity16–18; neurocognitive function19–21; and neuroanatomy (via structural magnetic resonance imaging [sMRI] and diffusion tensor imaging [DTI])22–24. We also measured sleep, activity, and circadian rhythms, analyses of which are ongoing and will be reported separately.
Previously described pedigrees, including many of those evaluated here25–28, show BP segregation patterns suggesting the transmission of high-impact risk-alleles. However, linkage studies of such pedigrees have yielded equivocal results, presumably because BP is genetically complex even within these families3. The feasibility of identifying rare, high-impact variants through next-generation sequencing has stimulated renewed interest in pedigree studies; however, even with this technology the etiological complexity of BP hinders the identification of risk-variants. We hypothesize that BP results from the confluence of multiple etiologic processes, each of which alone may be simpler to unravel. Investigation of quantitative component phenotypes in pedigrees from population isolates such as the genetically related isolates of the Central Valley of Costa Rica (CVCR) and Antioquia, Colombia (ANT)29–31 from which we recruited the pedigrees investigated here, may lead to a better understanding of the heritable components of the disorder, and at the same time simplify the search for specific genetic risk factors.
We report here results from evaluations of the most extensive set of putative BP component phenotypes yet assessed within any study sample. For each measure we describe its degree of familial aggregation (an indicator of heritability (h2)), and of association with BP-I. These results suggest multiple phenotypes for genetic investigations of BP-I, across the domains of temperament, neurocognition, and neuroanatomy.
Methods
Sample
We investigated pedigrees from ANT (11) and CVCR (15), ascertained in previous genetic studies25–28,32–36through hospitals and clinics in each country, utilizing genealogic information to extend each pedigree. To prioritize pedigree branches for quantitative phenotyping we recruited nuclear families including at least one member with known BP-I (based on the Diagnostic Interview for Genetics Studies, DIGS37,38, and/or extensive medical records), available parents, and at least two non-BP-I siblings (see Supplementary Material, e1.1 for additional details). Families varied considerably in size (12 to 355 members, mean = 55), and in the number of individuals phenotyped in this study (three to 177, mean = 29; Table 1). Written informed consent was obtained from each participant. Institutional Review Boards at participating institutions approved all study procedures.
Table 1. Sample Characteristics by Country and Family.
Summary statistics for each country are shown in the first two rows with the remaining rows providing information for each family. The second column shows total size and number of BP-I cases for each pedigree and the remaining columns show the summary statistics for individuals with phenotype data. Education was assessed in years. Abbreviations; ANT, Antioquia, Colombia; CVCR, Central Valley of Costa Rica.
| Total Sample | Sample Assessed for Component Phenotypes | |||||
|---|---|---|---|---|---|---|
| Family | n (BP-I cases) |
n (BP-I cases) |
MRI (DTI) | Female | Mean Age (SD) <range> |
Mean Years of Education (SD) <range> |
| ANT All | 512 (96) | 353 (86) | 242 (225) | 58% | 47.7 (17.7) <18–85> | 8.3 (4.7) <0–23> |
| CVCR All | 918 (128) | 386 (95) | 285 (0) | 55% | 49.1 (15.6) <18–87> | 7.8 (4.9) <0–24> |
| ANT10 | 38 (6) | 24 (5) | 19 (18) | 75% | 52 (15.4) <29–75> | 11.2 (5.1) <3–19> |
| ANT13 | 24 (5) | 19 (4) | 15 (15) | 58% | 47.5 (20) <18–85> | 12.2 (3.9) <2–19> |
| ANT14 | 29 (8) | 22 (7) | 19 (19) | 50% | 46.8 (16.6) <20–78> | 7.3 (3.7) <3–16> |
| ANT15 | 27 (5) | 21 (5) | 14 (13) | 57% | 46 (19) <18–85> | 10.4 (3.6) <2–15> |
| ANT18 | 37 (6) | 25 (6) | 23 (21) | 56% | 56 (16) <30–81> | 8.2 (4.7) <2–18> |
| ANT23 | 48 (9) | 31 (8) | 16 (16) | 68% | 47 (17.4) <18–82> | 7.5 (4.8) <0–16> |
| ANT25 | 15 (4) | 13 (4) | 11 (11) | 54% | 58 (14.1) <43–82> | 3.5 (1.8) <1–6> |
| ANT27 | 58 (9) | 35 (6) | 22 (21) | 57% | 50.5 (18.6) <18–84> | 8.5 (4.7) <1–18> |
| ANT4 | 71 (10) | 43 (9) | 28 (26) | 58% | 43.3 (18.6) <18–81> | 6.5 (4.2) <1–16> |
| ANT7 | 149 (29) | 112 (27) | 71 (63) | 52% | 44.8 (16.8) <18–82> | 8 (4.4) <0–16> |
| ANT8 | 16 (5) | 8 (5) | 4 (2) | 75% | 53.1 (21.3) <25–85> | 13.2 (5.6) <3–23> |
| CVCR001 | 45 (8) | 7 (3) | 4 (0) | 43% | 55.3 (9.6) <44–68> | 14.9 (3.5) <11–20> |
| CVCR004 | 186 (23) | 45 (10) | 33 (0) | 53% | 55.2 (13) <28–83> | 8.3 (4.5) <0–18> |
| CVCR006 | 35 (4) | 8 (2) | 8 (0) | 38% | 50 (14.2) <28–67> | 13.1 (3.1) <8–17> |
| CVCR007 | 11 (2) | 6 (2) | 6 (0) | 50% | 53.2 (13.3) <39–78> | 13.3 (3.9) <6–17> |
| CVCR008 | 29 (7) | 13 (5) | 9 (0) | 46% | 42.6 (13.8) <20–66> | 7.2 (3.3) <3–14> |
| CVCR009 | 44 (9) | 34 (9) | 21 (0) | 68% | 40.6 (14.9) <20–74> | 8 (4.4) <0–17> |
| CVCR010 | 30 (4) | 12 (3) | 12 (0) | 58% | 43.8 (15.5) <22–74> | 12.2 (6) <5–24> |
| CVCR011 | 16 (3) | 12 (3) | 10 (0) | 67% | 50 (23.2) <21–87> | 11.8 (3.6) <6–18> |
| CVCR012 | 34 (5) | 22 (5) | 8 (0) | 64% | 42.6 (15) <21–68> | 8.1 (4.8) <0–16> |
| CVCR013 | 39 (4) | 8 (3) | 5 (0) | 75% | 53 (17.8) <35–76> | 13.9 (4.9) <6–19> |
| CVCR014 | 26 (5) | 3 (1) | 3 (0) | 67% | 50.3 (8.5) <44–60> | 5.7 (0.6) <5–6> |
| CVCR015 | 19 (2) | 10 (2) | 8 (0) | 70% | 52.1 (14.4) <38–72> | 6.4 (2.5) <3–13> |
| CVCR016 | 24 (4) | 19 (4) | 12 (0) | 47% | 52.2 (15.3) <20–81> | 3.6 (5) <0–20> |
| CVCR201 | 355 (44) | 177 (40) | 137 (0) | 51% | 49.6 (15.7) <18–87> | 6.5 (4.3) <0–19> |
| CVCR277 | 25 (4) | 10 (3) | 9 (0) | 60% | 49.4 (11) <37–71> | 10.8 (4.4) <4–17> |
Clinical Assessments
To establish DSM-IV diagnoses we used a best estimate (BE) process, modified from previous procedures33(Supplementary Material, section e1.2), and including diagnostic interviews using Spanish versions of the Mini International Neuropsychiatric Interview39and the DIGS. Individuals designated as BP-I had a BE diagnosis of BP-I, unipolar mania, or schizoaffective disorder, bipolar type, as in previous studies27,33,40. The Young Mania Rating Scale (YMRS)41and the 17-item Hamilton Depression Rating Scale (HDRS)42 administered at the time of assessment, identified individuals with significant mood symptoms (YMRS > 14 or HDRS ≥14), whom we excluded from analyses of temperament and neurocognitive measures.
Temperament and Neurocognitive Assessment
Temperament and neurocognitive measures, assessed in 738 subjects, had previously demonstrated heritability and association to BP13–16,22–24(Table 2). The temperament battery, 15 measures generated from seven instruments (Supplementary Material, e1.3), included multiple dimensions categorized into four subdomains: affective temperament, impulsivity/risk-taking, perceptual creativity and delusion-proneness (Table 2). The neurocognitive battery (Supplementary Material, e1.4) included a computerized neuropsychological evaluation43, and paper-and-pencil measures of verbal abilities, inhibitory control44, and declarative memory45.
Table 2. Behavioral and Neuroimaging Measures.
Summary of methods used to generate phenotypes. The upper rows of the table list the instruments and measures used to assess temperament and neurocognitive phenotypes. The lower rows list the neuroimaging regions of interest (ROIs). ROIs highlighted in bold represent measures that were derived by summing sub-region measures that are also included as traits (e.g. total brain volume is the sum of total cerebral, total cerebellar and brain stem volumes). For each cortical surface ROI, two measures were determined; surface area and average gray matter thickness. Abbreviations; FA, fractional anisotropy; AD, axial diffusivity; RD radial diffusivity.
| Subdomain | Instrument | Phenotype | Measure |
|---|---|---|---|
| Temperament | |||
| Delusion-proneness | Peters Delusion Inventory106 | Peters Delusion Inventory | Score on 40 items assessing delusional ideation and unusual perceptual experiences |
| Perceptual Creativity | Barron Welsh Art Scale16,107 | Barron Welsh Art Scale Dislike | Preference rating on simple/symmetric figures of 86 total |
| Barron Welsh Art Scale Like | Preference rating on complex/asymmetric figures of 86 total | ||
| Affective Temperament | TEMPS-A108 | TEMPS Anxiety | Total score on 3 anxiety items |
| TEMPS Cyclothymia | Total score on 12 cyclothymia items | ||
| TEMPS Depressive | Total score on 8 depressive items | ||
| TEMPS Hyperthymia | Total score on 8 hyperthymia items | ||
| TEMPS Irritability | Total score on 8 irritability items | ||
| Impulsivity/Risk-taking | Aggression Questionnaire109 | Aggression Questionnaire | Score on 12 item Likert-scale of aggressive traits/behaviors |
| Barratt Impulsivity Scale110 | Barratt Impulsivity Scale | Score on 30 item Likert-scale assessing frequency of impulsive behaviors | |
| Sensation Seeking Scale111,112 | Sensation Seeking Scale | Score on 40 items of sensory stimulation preferences | |
| Balloon Analog Risk Task113 | BART Low-risk Pumps | Number of balloon pumps on Low-risk trials | |
| BART Medium-risk Pumps | Number of balloon pumps on Medium-risk trials | ||
| BART High-risk Pumps | Number of balloon pumps on High-risk trials | ||
| BART Total Pumps | Total number of balloon pumps on all trials | ||
| Neurocognition | |||
| Long Term Memory | California Verbal Learning Test | CVLT Delayed Recall | Number of items out of 16 word list recalled after a 20 min. delay |
| CVLT Intrusions | Number of intrusions during list recollection | ||
| CVLT Recognition | Number of items out of 16 word list recognized after a 20 min delay | ||
| CVLT Repetitions | Number of repeated words during list recollection | ||
| CVLT Total Trials 1–5 | Number of items recalled over 5 repeated exposures of a 16 word list | ||
| Miscellaneous43 | Face Memory | Number of faces recalled from visual presentation after delay | |
| Wechsler Memory Scale45 | WMS Logical Memory Delay | Memory score for auditory story after 20 min. delay | |
| WMS Logical Memory Immediate | Memory score for auditory story immediately after presentation | ||
| WMS Logical Memory Recognition | Recognition score for auditory story after 20 min. delay | ||
| WMS Visual Reproduction Immediate | Score for visuospatial memory immediately after figure presentation | ||
| WMS Visual Reproduction Delay | Score for visuospatial memory after delay | ||
| Executive Function | Abstraction Inhibition and Working Memory114 | AIM Abstraction | Number of correctly matched shapes presented simultaneously |
| Wechsler Abbreviated Scale of Intelligence | Matrix Reasoning | Number of correctly completed patterns | |
| WASI Vocabulary | Number of correctly named/defined objects/words | ||
| Penn Conditional Exclusion Test115 | PCET # Correct | Number of correctly identified non-matching objects | |
| PCET Categories Achieved | Number of categories of achieved | ||
| Stop Signal Task | SST Correct Go | Number of correct go trials | |
| SST Correct Stop | Number correct stop trials | ||
| SST Inter-stimulus Interval | Response time (ms) on correct stop trials | ||
| Stroop Color-Word Interference Test44 | Stroop Color Word Test Errors | Number of errors on Color-Word test | |
| Stroop Color Word Test Time | Time needed to complete test | ||
| Test of Non-verbal Intelligence116 | TONI # Correct | Number of correctly completed progressive matrices | |
| Working Memory | Abstraction Inhibition and Working Memory114 | AIM Abstraction plus Memory | Number of correctly matched shapes after delayed target presentation |
| Identical Pairs Continuous Performance Test | IPCPT Hits | Number of correctly identified pairs on continuous performance test | |
| Spatial Capacity Delayed Response Test | SCAP # Correct 3 Dot Condition | Number of correct responses on 3-dot spatial delayed memory task | |
| SCAP Reaction Time 3 Dot Condition | Response time (ms) on 3-dot condition | ||
| SCAP # Correct 5 Dot Condition | Number of correct responses on 5-dot spatial delayed memory task | ||
| SCAP Reaction Time 5 Dot Condition | Response time (ms) on 5-dot condition | ||
| SCAP Mean # Correct All Trials | Mean number of correct responses on all trials | ||
| Miscellaneous43 | VWM Digits Forward # Correct | Correctly recalled digits strings in original order of presentation | |
| VWM Digits Backward # Correct | Correctly recalled digits strings in reverse order of presentation | ||
| VWM Letter-Number Seq. # Correct | Correctly recalled number-letter strings, in alpha-numeric sequence | ||
| Processing Speed | Miscellaneous43 | Digit Symbol Copy | Correctly identified digit-symbol pairs in 90 sec |
| Digit Symbol Recall | Number of digits recalled when presented with corresponding symbols | ||
| Digit Symbol Percent Correct | Percent correct on digit-symbol task | ||
| Trail Making Test | Trailmaking Letter Sequencing Time | Time needed to connect letters in alphabetical order | |
| Trailmaking Number-Letter Seq. Time | Time needed to connect alternating sequence of numbers and letters | ||
| Trailmaking Number Sequencing Time | Time needed to connect numbers in ascending order | ||
| Verbal Fluency | Miscellaneous43 | Verbal Letter Fluency | Words starting with a specific letter generated in 60 sec. |
| Verbal Category Fluency | Animal names generated in 60 sec. | ||
| Neuroimaging | |||
| Measure | Analysis Package | Regions of Interest (ROIs) | |
| MRI Volume | FreeSurfer46,47T1-Weighted Images | Amygdala, Anterior Corpus Callosum, Brain Stem, Caudate, Central Corpus Callosum, Cerebellar Cortex, Cerebellar Volume, Cerebellar White Matter, Cerebral Cortex, Cerebral Volume, Cerebral White Matter, Cerebrospinal Fluid, Fourth Ventricle, Hippocampus, Inferior Lateral Ventricle, Lateral Ventricle, Mid-Anterior Corpus Callosum, Mid-Posterior Corpus Callosum, non-White Matter Hypointensities, Nucleus Accumbens, Pallidum, Posterior Corpus Callosum, Putamen, Thalamus, Third Ventricle, Total Brain Volume, Total Corpus Callosum, Ventral Diencephalon, White Matter Hypointensities | |
| Cortical Surface Area | Caudal Anterior Cingulate, Caudal Middle Frontal, Cuneus, Entorhinal, Frontal Pole, Fusiform, Inferior Parietal, Inferior Temporal, Isthmus Cingulate, Lateral Occipital, Lateral Orbitofrontal, Lingual, Medial Orbitofrontal, Middle Temporal, Paracentral, Parahippocampal, Pars Opercularis, Pars Orbitalis, Pars Triangularis, Pericalcarine, Postcentral, Posterior Bank of Superior Temporal Sulcus, Posterior Cingulate, Precentral, Precuneus, Rostral Anterior Cingulate, Rostral Middle Frontal, Superior Frontal, Superior Parietal, Superior Temporal, Supramarginal, Temporal Pole, Transverse Temporal | ||
| FA, AD, RD | FSL TBSS50,51DTI | Anterior Thalamic Radiation, Genu Corpus Callosum, Inferior Fronto-Occipital Fasciculus, Inferior Longitudinal Fasciculus, Splenium Corpus Callosum, Uncinate Fasciculus | |
Neuroimaging
We acquired T1-weighted structural neuroimages on 1.5 Tesla scanners, from 527 subjects (285 from CVCR and 242 from ANT) (Supplementary Material, e1.5), implementing protocols for acquisition of diffusion tensor images (DTI) in ANT only. We used Freesurfer software46,47, with manual inspection of intermediate steps in the processing stream to correct common errors, to generate 96 sMRI phenotypes, including measures of volume, surface area, and cortical thickness (Table 2 and eTable2)48,49.
We determined DTI phenotypes (Supplementary Material, e1.5) using FSL50,51, employing the Johns Hopkins University probabilistic tractography atlas52to determine and customize ROIs, which we limited to tracts previously associated with BP53–55. In total we generated 18 DTI phenotypes across three categories, fractional anisotropy (FA), the degree of anisotropy; axial diffusivity (AD), diffusivity along the major axis of diffusion; and radial diffusivity (RD), an average of the diffusivities along the two minor axes56–59 (Table 2, eTable2).
Statistical Analyses
We assessed familial aggregation of traits using SOLAR60, which implements a variance component method to estimate the proportion of phenotypic variance due to additive genetic factors (narrow sense heritability). This model partitions total variability into polygenic and environmental components. The environmental component is unique to individuals while the polygenic component is shared between individuals as a function of their pedigree kinship. If the variance in phenotype Y due to the polygenic component is designated as σg2 and the environmental component as σe2, then in this model Var(Y) = σg2 + σe2, and the covariance between phenotype values of individuals i and j is Cov(Yi,Yj)=2 φij σg2, where φij is the kinship between individuals i and j.
Variance components analysis is sensitive to outliers and non-normal trait distributions. To guard against potential statistical artifacts induced by skewed distributions, we used, prior to analysis, a rank-based procedure61to inverse normal transform all phenotypes. This transformation, implemented within SOLAR, is standard in variance component analyses, as it does not induce correlations between relatives or lead to inflated estimates of heritability62.
We regressed all phenotypes on three covariates (sex, age and country). Additional covariates included years of education (temperament and neurocognitive measures), body weight (T1-weighted and DTI variables), intracranial volume (ICV, volume measurements from T1-weighted images), and total cortical surface area (regional surface area measures). We implemented regressions in SOLAR, using pedigree structures, employing residuals from these models in all further analyses.
We tested for difference in trait means between individuals with and without a diagnosis of BP-I (BP-I association analyses), using SOLAR to account for dependencies among relatives. We controlled family-wise error rate at the 0.05 level, using a Bonferroni-corrected threshold for each test (heritability and BP-I association; p<2.96×10−4). We used published evidence to assign each trait an expected a priori direction of change, designating them as BP-I-associated only if the difference was in the a priori assigned direction, therefore using a one-tailed test, eTable2.
We estimated phenotypic correlations for all trait pairs. Genetic correlations were estimated for all pairs in which both traits were significantly heritable using SOLAR63. Graphs of the estimated correlation structures used methods described in Supplementary Material, e1.6.
Results
Sample characteristics
Table 1 shows summary statistics for the sample, by family; eTable1 provides additional clinical characterization of the 181 subjects who met BE criteria for BP-I. We excluded five individuals with elevated YMRS or HDRS scores from analyses of neurocognitive and temperament data, and five additional individuals from BP-I association analyses (but not from heritability analyses) because a BP-I diagnosis could neither be confirmed nor excluded.
Heritability and Association with BP-I
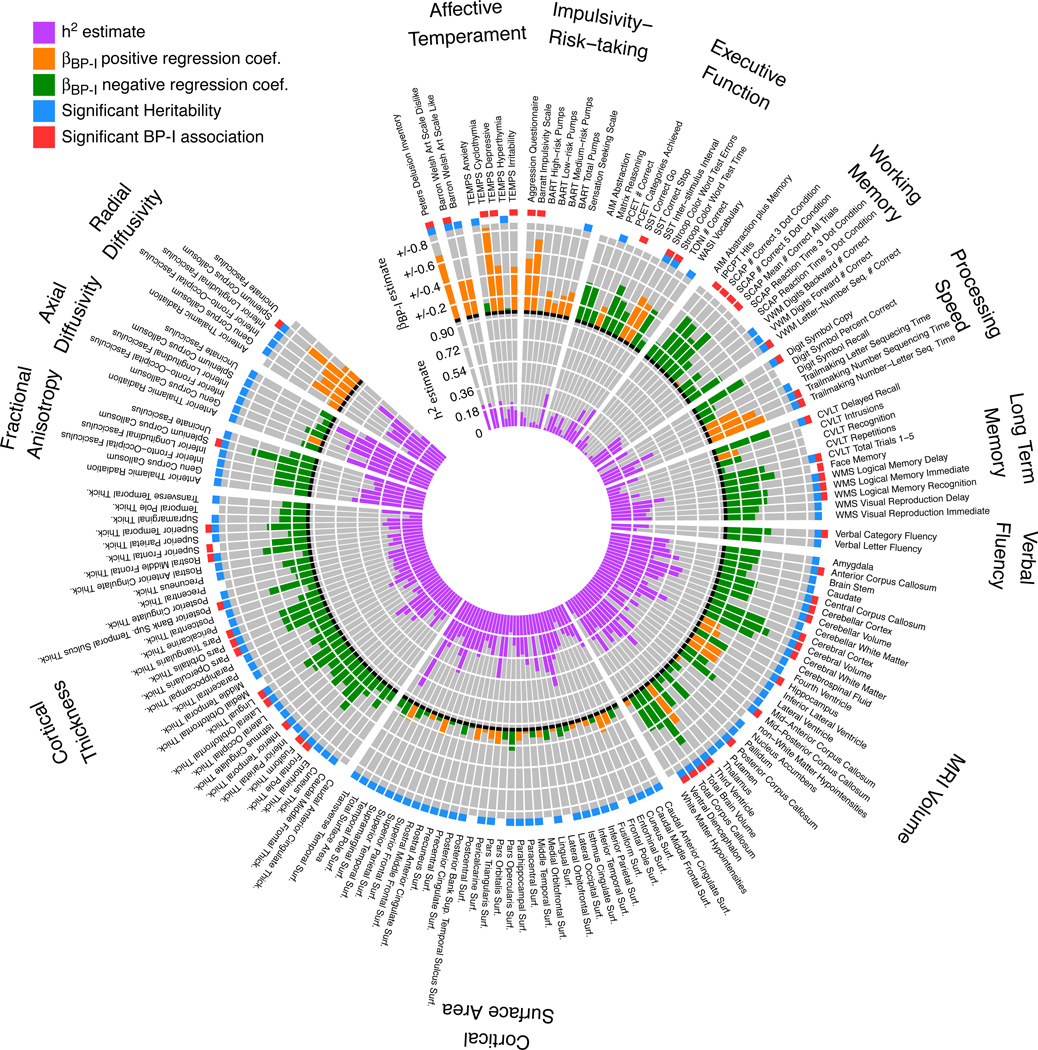
Of the 169 traits examined, 126 (74.6%) were significantly heritable, 53 (31.3%) were significantly associated with BP-I, and 41 (24.3%) were both heritable and associated with BP-I (Figure 1 and eTable2). These results were robust with respect to phenotype variations across pedigrees and countries (data not shown) and to outliers (Supplementary Material, e2 and eFigure1); for secondary analyses of the effects of medications and duration of illness on trait values see Supplementary Material, e3. Results within each domain are described below.
Figure 1. Summary of analyses of heritability and association with BP-I.
The results of analyses of heritability and of association with BP-I are shown as two histograms stacked on top of each other. Inner histogram purple bars show the magnitude of the heritability estimate for each component phenotype and the blue box next to the trait name at the outer edge of the plot indicates estimates that passed the significance threshold. Outer histogram shows the magnitude of the estimated regression coefficient for the BP-I association test. In orange are positive coefficients representing traits that are higher in BP-I subjects compared to non-BP-I family members. In green are negative coefficients representing traits that are lower in BP-I subjects. A red box at the outer edge of the circle indicates traits that exceeded the significance threshold for association with BP-I. Abbreviations; PCET; Penn Conditional Exclusion Test, SST; Stop Signal Task, TONI; Test of Nonverbal Intelligence, AIM; Abstraction Inhibition and Memory test, IPCPT; Identical Pairs Continuous Performance Test, VWM; verbal working memory, CVLT; California Verbal Learning Test, WMS; Wechsler Memory Scale, BART; Balloon Analog Risk Task; TEMPS, Temperament Evaluation of Memphis, Pisa, Paris and San Diego; WASI, Wechsler Abbreviated Scale of Intelligence; SCAP, Spatial Capacity Delayed Response Test.
Temperament
Six of the fifteen temperament measures demonstrated significant heritability, although overall this domain showed the lowest estimates of additive genetic influence (h2~0.18–0.30). In contrast, three temperament traits displayed the strongest BP-I-associations of all 169 measures: TEMPS cyclothymia scale, BIS, and PDI. Delusion-proneness (PDI) and perceptual creativity (BWAS-Dislike) were both heritable and associated with BP-I, while risk-taking propensity (BART) was neither heritable nor associated with BP-I.
Neurocognition
Some measures from all domains assessed showed significant heritability and BP-I associations. Most measures of processing speed, long-term memory and verbal fluency were significantly heritable (13/19); within this heritable subset, most were associated with BP-I (9/13). Within working memory assessments, verbal but not spatial tasks showed evidence of heritability, and BP-I subjects showed significant impairment on measures of sustained attention (IP-CPT), spatial working memory (SCAP), and verbal working memory tasks (Letter-Number Sequencing). Measures of inhibitory control (Stroop Color-Word interference and SST) showed evidence for impairment in BP-I subjects, of which the Stroop measures (Color-Word interference trials, time and number of errors) were also heritable. Nonverbal abstract reasoning measures (AIM, TONI, Matrix Reasoning) were neither significantly heritable, nor associated with BP-I.
Neuroimaging
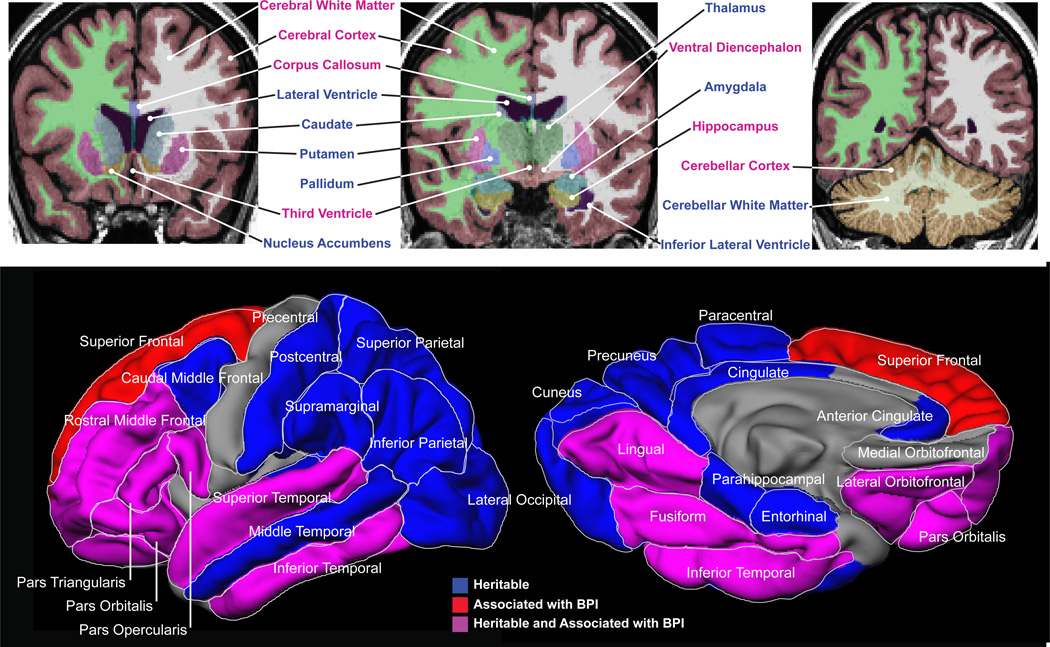
Most neuroimaging phenotypes (~88%) were significantly heritable, and a substantial number of these measures were significantly associated with BP-I. Several global measures differed between BP-I subjects and their non-BP-I relatives (decreased total cerebral gray and white matter and cerebellar volumes, with corresponding increases in third ventricle volume). Localized reductions were also observed in several structures (Figure 2), including hippocampus and ventral diencephalon (while amygdala and thalamus show a similar trend). The T1-weighted and DTI sequences provided convergent evidence for BP-I-related changes in the corpus callosum; BP-I subjects showed decreases in volume (total callosum and four of the five callosal subdivisions) and overall fractional anisotropy, while increased radial diffusivity in the splenium of the callosum indicated reduced white matter integrity.
Figure 2. Structural neuroimaging phenotypes.
Upper panel shows results of the heritability and BP-I association analyses of volumetric MRI phenotypes. The three representative T1-weighted MRI coronal images depict the results of the Freesurfer segmentation overlaid as colored masks selected to better distinguish the anatomy. Mask colors are not related to the results. The colors of the text labels indicate structures that showed significant evidence of familial aggregation (blue) and structures that were both heritable and associated with BP-I (magenta).
Lower panel depicts cortical thickness phenotypes and shows the results of the heritability and BP-I association analysis for cortical gray matter thickness. Heritable cortical regions are colored in blue, BP-I-associated regions are shown in red and regions that were both heritable and associated with BP-I are colored in magenta. The medial surface is rotated upwards by 60° to provide a view of the ventral surface.
Compared to non-BP-I relatives, BP-I subjects displayed widespread reduction of cortical thickness in heteromodal association regions in most of the prefrontal (PFC) and temporal cortex, including the superior temporal gyrus (STG), inferior temporal gyrus (IFG), fusiform and lingual regions (Figure 2, lower panel). Most lateral PFC regions, including all subregions of the inferior frontal gyrus and lateral orbitofrontal cortex, were significantly thinner in BP-I subjects. In contrast, the medial orbitofrontal region was neither heritable nor associated with BP-I. Another exception to the overall pattern of findings was the superior frontal gyrus, which showed BP-I-associated gray matter reduction but was not significantly heritable. Most measures of regional surface area were heritable but were not significantly associated with BP-I.
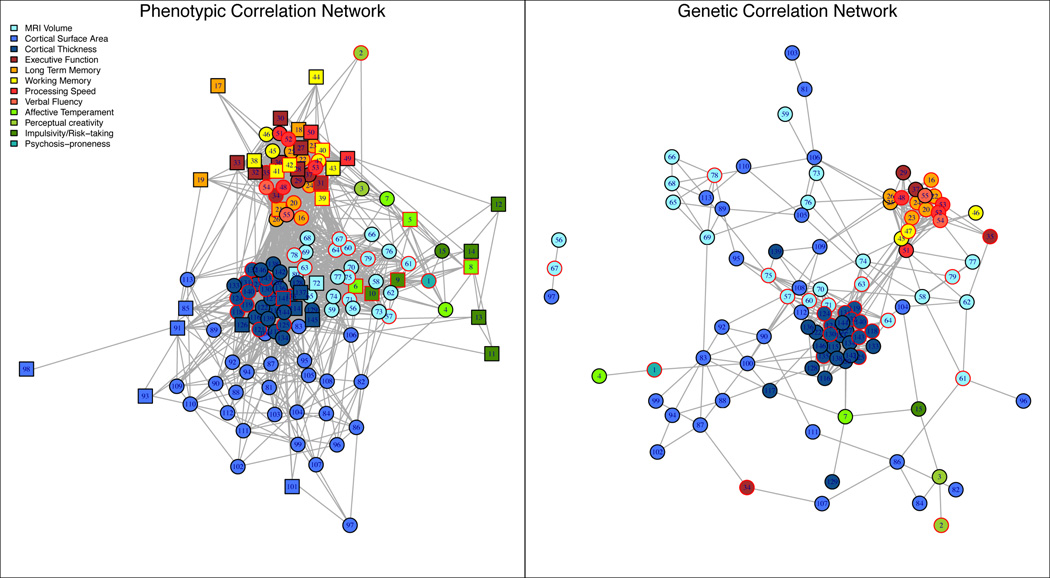
Evaluation of Between-Trait Phenotypic and Genetic Correlations
Using FDR methods we determined thresholds (t) for rejecting the null hypothesis of correlation=0; t = 2.58 standard errors (SE) from 0 for phenotypic correlations (rhop), and 2.81 SE from 0 for genetic correlations (rhog). About 20% (2117/10,585) of trait-pairs exceeded t for rhop and 9.9% (539 of 5460) of heritable pairs exceeded t for rhog. Schematic representations (Supplementary Material, e1.6) of the networks of phenotypic and genetic correlations (Figure 3) demonstrate the clustering of phenotypes by domain, showing no clear separation between heritable and non-heritable traits (circles and squares, respectively). Similarly, BP-I associated traits showed no distinct clustering (nodes with a red border). The network structure of the genetic correlations was sparser than, but qualitatively similar to, that of phenotypic correlations. Traits mainly clustered within phenotypic domains, but some genetic correlations across domains were observed, such as Stroop errors with rostral middle frontal and inferior parietal surface area (Figure 3; nodes 34, 87, and 107, right panel).
Figure 3. Network graph of correlations among phenotypes.
Network representations of pairwise phenotypic correlations are drawn in the left panel and genetic correlations are shown in the right panel. All trait pairs were included in the phenotypic correlation analysis, and only pairs in which both traits were heritable were included in the genetic correlation analysis. Nodes are colored according to their assigned subdomain (see Subdomain column in eTable2 in the Supplemental). Circular nodes represent significantly heritable phenotypes and square nodes represent non-heritable phenotypes. Traits that were significantly associated with BP-I are drawn with a red border. Nodes are connected with an edge when the hypothesis of correlation=0 was rejected using FDR-controlled thresholds. Numbers on the graph correspond to Plot ID’s for phenotypes detailed in eTable2 in the Supplemental. Examples of genetically correlated traits mentioned in the main text can be seen in the right panel and include; 1) the hippocampus (#67), amygdala (#56) and surface area of the pars opercularis (#97); and 2) Stroop Color Word Test Errors (#34) with surface area measures from the inferior parietal (#87), and rostral middle frontal (#107) ROIs.
Discussion
Through the most comprehensive evaluation to date of BP component phenotypes, we delineated measures that may help elucidate the genetic contribution to BP-I risk. Gauging the potential informativeness of traits based on their heritability and association with BP-I, we can divide them into four groups.
Measures that demonstrate both heritability and association with BP-I (Group 1) are the most promising phenotypes for identifying loci contributing to disease risk, as shown for other neuropsychiatric disorders64. Analyses at loci linked and/or associated to both BP-I and to a Group 1 phenotype will suggest the degree of BP-I genetic risk directly attributable to that measure; some loci may, of course, contribute to trait variability but not to disease risk.
All domains that we assessed include Group 1 phenotypes. Some phenotypes in this group, such as delusion-proneness65, appear broadly characteristic of the major psychoses. Others, such as perceptual creativity, appear specific to BP predisposition66–68; individuals diagnosed with BP are over-represented in creative occupations compared to individuals diagnosed with other psychiatric disorders, or to the general population67,68. Many BP-affected individuals consider heightened creativity a positive aspect of their condition69, which should fuel efforts to elucidate the mechanisms underlying this association.
Among the neurocognitive processes in Group 1, the BP-I associations reflect impairments in processing speed, verbal learning and memory, category fluency and inhibitory control, mirroring findings from previous BP and schizophrenia case-control, family and pedigree studies20,21,43,70–74. Such phenotypes could contribute to the shared risk between these disorders suggested by recent GWAS75.
Group 1 neuroimaging measures provide the first confirmation in families of BP-related anatomic variations previously identified through case-control studies76–81. Although generally in accord with sMRI findings from prior studies, our results identified larger zones of BP-I-associated gray matter reduction, which may reflect the greater size and reduced ethnic heterogeneity of the sample. We identified significant volume reduction and cortical thinning in two prefrontal systems implicated in BP pathogenesis; 1) a cortico-cognitive network anchored in the dorsolateral and ventrolateral PFC, including all subdivisions of the inferior frontal gyrus, which plays a role in attention, working memory and inhibitory control, and shows attenuated activation in fMRI studies of BP subjects82–87, and; 2) a ventral-limbic system implicated in emotional reactivity, involving the hippocampus, amygdala and orbitofrontal cortex76,78–80. Further, the reduced corpus callosum volume and white matter integrity aligns with twin studies suggesting genetically influenced alterations of this structure in BP88,89. Gray matter reduction in temporal structures, including the superior temporal sulcus (STS) and the lingual and fusiform gyri, are noteworthy given the involvement of these structures in facial emotion identification, a process impaired in BP individuals and adolescents at high-risk90–94.
Numerous phenotypes, including the majority of the neuroimaging measures, were heritable but not associated with BP-I (Group 2). The lack of difference in cortical surface area between BP-I subjects and their non-BP-I relatives supports previous evidence dissociating this measure from cortical thickness abnormalities characteristic of the disorder81. Similarly, neurocognitive traits in this category have consistently demonstrated heritability in twin and family samples73,95–102 but have shown inconsistent association with BP-I20,21,70,103.
A third set of phenotypes showed BP-I association but were not heritable (Group 3), suggesting they may be predominantly influenced by environmental or disease-specific factors. Previous studies have proposed that temperament is a key contributor to BP genetic risk104, but we found little evidence for heritability of several measures associated with emotional reactivity (cyclothymic, irritable and depressive temperament, aggression and impulsivity) that were elevated in our BP-I subjects.
Our results for neurocognitive traits are remarkably similar to those reported in the only previously published study of such traits in BP pedigrees43, with three exceptions. First, we did not find significant heritability for face memory (which was impaired in BP-I subjects in both studies). Second, we observed significant impairment in BP-I individuals on measures of sustained attention and spatial working memory. As deficits in these domains may index psychotic symptoms, regardless of diagnosis105, this discordance may reflect the larger percentage of patients in our sample with a lifetime history of psychosis. Finally, we found lower heritability for nonverbal abstract reasoning. As we report heritability estimates corrected for demographic variables, comparisons with the prior study are to its similarly corrected estimates.
We identified extensive correlation among measures within each phenotypic domain, including phenotype clusters consistently implicated in BP pathology. Some such clusters also showed evidence of shared genetic influence (e.g. limbic regions with the pars opercularis of the inferior frontal gyrus87). This analysis also suggests shared genetic influence among select measures across domains, e.g. that between Stroop test performance and surface area MRI measures.
Our ascertainment strategy emphasized close family relationships, enhancing the power for quantitative genetic analyses; however, the shared genetic and environmental backgrounds of our subjects would tend to make them more similar to each other compared to cases and independently ascertained controls and reduce power to identify phenotypic associations with BP-I. Two scenarios may explain group differences observed for some phenotypes: BP-I subjects may carry risk alleles with strong and/or non-additive phenotypic effects, and/or may have experienced different environmental exposures, either prior to illness onset, or as a consequence of the disorder. As the ascertainment of the pedigrees themselves and of the specific individuals evaluated within them were non-random with respect to clinical diagnosis, our data are not suitable for assessing the genetic relationship between these phenotypes and BP-I.
Although prior evidence supported the selection of each measure that we evaluated, the employment of alternative measures could have yielded discrepant outcomes. While such discrepancies may reflect incompatibilities in the theoretical underpinnings of different instruments (e.g., for temperament scales), identification of genetic co-associations between BP-I and specific component measures will accelerate the standardization of phenotyping.
Our findings establish a core set of measures across multiple domains as component phenotypes for identifying the genetic basis of BP-I risk. Overall, the profile of brain and behavioral impairments in these pedigrees is similar to those identified previously in case-control samples. We therefore anticipate that, while specific genetic variants contributing to these phenotypes and to BP-I risk may be distinct to the CVCR and ANT population isolates, they could suggest genes that also influence disease risk in other populations.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Health Grants R01MH075007, R01MH095454, P30NS062691 (NBF), K23MH074644-01 (CEB), and K08MH086786 (SCF), Colciencias and Codi-University Of Antioquia (CL-J). Chiara Sabatti was supported by NIH grant# R01HG006695.
Role of Funding Entitities:
The funding entities specified above played no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; nor in the preparation, review, or approval of the manuscript, or the decision to submit the manuscript for publication.
Dr. Altshuler has received past funding from Sepracor (advisory board honoraria, January 2010), Eli Lilly (consultant, September 2010), Takeda Pharmaceuticals North America, Inc., and H. Lundbeck A/S (advisory board honoraria, October 2012); and Sunovion Pharmaceuticals Inc. (advisory board honoraria, Jan 2013).
Footnotes
Nelson B. Freimer and Carrie E. Bearden had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflicts of Interest:
None of the other authors have financial conflicts of interest to report.
Author Contributions:
Study concept and design: Bearden, Freimer, Cantor, Sabatti, Risch, Service, Reus
Acquisition of data: Macaya, C. Araya, X. Araya, Castrillón, Gomez-Franco, Lopez, G. Montoya, P. Montoya, Bejarano, Luykx, Molina, Lopez-Jaramillo
Analysis and interpretation of data: Fears, Glahn, Jalbrzikowski, Altshuler, Bartzokis, Thompson, Abaryan, Al-Sharif, Ericson, Navarro, Reus, Bearden, Freimer
Drafting of the manuscript: Fears, Bearden, Freimer, Service
Critical revision of the manuscript for important intellectual content: Altshuler, Escobar, Risch, Kremeyer, Luykx, Lopez-Jaramillo, Macaya, Escobar, Ruiz-Linares, Thompson, Cantor, Reus, Sabatti
Statistical analysis: Service, Fears, Sabatti, Navarro
Administrative, technical, or material support: Teshiba, Araya, Ramirez, P. Montoya, Aldana, Tishler, Al-Sharif
Study supervision: Bearden, Freimer, Lopez-Jaramillo, Macaya, Escobar, Ospina-Duque
References
- 1.Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression: Bipolar Disorders and Recurrent Depression. USA: Oxford University Press; 2007. [Google Scholar]
- 2.McGuffin P, Rijsdijk F, Andrew M, Sham P, Katz R, Cardno A. The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch Gen Psychiatry. 2003;60:497–502. doi: 10.1001/archpsyc.60.5.497. [DOI] [PubMed] [Google Scholar]
- 3.Fears SC, Mathews CM, Freimer NF. Kaplan & Sadock's Comprehensive Textbook of Psychiatry. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2009. Genetic Linkage Analysis of Psychiatric Disorders; pp. 320–332. [Google Scholar]
- 4.Ferreira MA, O'Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008;40:1056–1058. doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sklar P, Smoller JW, Fan J, et al. Whole-genome association study of bipolar disorder. Mol Psychiatry. 2008;13:558–569. doi: 10.1038/sj.mp.4002151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan PF, Daly MJ, O'Donovan M. Genetic architectures of psychiatric disorders: the emerging picture and its implications. Nat Rev Genet. 2012;13:537–551. doi: 10.1038/nrg3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bearden CE, Freimer NB. Endophenotypes for psychiatric disorders: ready for primetime? Trends Genet. 2006;22:306–313. doi: 10.1016/j.tig.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 8.Cannon TD, Keller MC. Endophenotypes in the genetic analyses of mental disorders. Annu Rev Clin Psychol. 2006;2:267–290. doi: 10.1146/annurev.clinpsy.2.022305.095232. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 10.Kendler KS, Neale MC. Endophenotype: a conceptual analysis. Mol Psychiatry. 2010;15:789–797. doi: 10.1038/mp.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preston GA, Weinberger DR. Intermediate phenotypes in schizophrenia: a selective review. Dialogues Clin Neurosci. 2005;7:165–179. doi: 10.31887/DCNS.2005.7.2/gpreston. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walters JT, Owen MJ. Endophenotypes in psychiatric genetics. Mol Psychiatry. 2007;12:886–890. doi: 10.1038/sj.mp.4002068. [DOI] [PubMed] [Google Scholar]
- 13.Akiskal HS, Kilzieh N, Maser JD, et al. The distinct temperament profiles of bipolar I, bipolar II and unipolar patients. J Affect Disord. 2006;92:19–33. doi: 10.1016/j.jad.2005.12.033. [DOI] [PubMed] [Google Scholar]
- 14.Karam EG, Salamoun MM, Yeretzian JS, et al. The role of anxious and hyperthymic temperaments in mental disorders: a national epidemiologic study. World Psychiatry. 2010;9:103–110. doi: 10.1002/j.2051-5545.2010.tb00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vázquez GH, Kahn C, Schiavo CE, et al. Bipolar disorders and affective temperaments: a national family study testing the "endophenotype" and "subaffective" theses using the TEMPS-A Buenos Aires. J Affect Disord. 2008;108:25–32. doi: 10.1016/j.jad.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava S, Childers ME, Baek JH, et al. Toward interaction of affective and cognitive contributors to creativity in bipolar disorders: a controlled study. J Affect Disord. 2010;125:27–34. doi: 10.1016/j.jad.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 17.Santosa CM, Strong CM, Nowakowska C, Wang PW, Rennicke CM, Ketter TA. Enhanced creativity in bipolar disorder patients: a controlled study. J Affect Disord. 2007;100:31–39. doi: 10.1016/j.jad.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Simeonova DI, Chang KD, Strong C, Ketter TA. Creativity in familial bipolar disorder. J Psychiatr Res. 2005;39:623–631. doi: 10.1016/j.jpsychires.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–182. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 20.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–785. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 21.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 22.Fusar-Poli P, Howes O, Bechdolf A, Borgwardt S. Mapping vulnerability to bipolar disorder: a systematic review and meta-analysis of neuroimaging studies. J Psychiatry Neurosci. 2012;37:170–184. doi: 10.1503/jpn.110061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langan C, McDonald C. Neurobiological trait abnormalities in bipolar disorder. Mol Psychiatry. 2009;14:833–846. doi: 10.1038/mp.2009.39. [DOI] [PubMed] [Google Scholar]
- 24.Foland-Ross LC, Thompson PM, Sugar CA, et al. Investigation of cortical thickness abnormalities in lithium-free adults with bipolar I disorder using cortical pattern matching. Am J Psychiatry. 2011;168:530–539. doi: 10.1176/appi.ajp.2010.10060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McInnes LA, Escamilla MA, Service SK, et al. A complete genome screen for genes predisposing to severe bipolar disorder in two Costa Rican pedigrees. Proc Natl Acad Sci U S A. 1996;93:13060–13065. doi: 10.1073/pnas.93.23.13060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Service S, Molina J, Deyoung J, et al. Results of a SNP genome screen in a large Costa Rican pedigree segregating for severe bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B:367–373. doi: 10.1002/ajmg.b.30323. [DOI] [PubMed] [Google Scholar]
- 27.Herzberg I, Jasinska A, García J, et al. Convergent linkage evidence from two Latin-American population isolates supports the presence of a susceptibility locus for bipolar disorder in 5q31-34. Hum Mol Genet. 2006;15:3146–3153. doi: 10.1093/hmg/ddl254. [DOI] [PubMed] [Google Scholar]
- 28.Kremeyer B, García J, Müller H, et al. Genome-wide linkage scan of bipolar disorder in a Colombian population isolate replicates Loci on chromosomes 7p21-22, 1p31, 16p12 and 21q21-22 and identifies a novel locus on chromosome 12q. Hum Hered. 2010;70:255–268. doi: 10.1159/000320914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carvajal-Carmona LG, Ophoff R, Service S, et al. Genetic demography of Antioquia (Colombia) and the Central Valley of Costa Rica. Hum Genet. 2003;112:534–541. doi: 10.1007/s00439-002-0899-8. [DOI] [PubMed] [Google Scholar]
- 30.Reich D, Patterson N, Campbell D, et al. Reconstructing Native American population history. Nature. 2012;488:370–374. doi: 10.1038/nature11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Service S, DeYoung J, Karayiorgou M, et al. Magnitude and distribution of linkage disequilibrium in population isolates and implications for genome-wide association studies. Nat Genet. 2006;38:556–560. doi: 10.1038/ng1770. [DOI] [PubMed] [Google Scholar]
- 32.Jasinska AJ, Service S, Jawaheer D, et al. A narrow and highly significant linkage signal for severe bipolar disorder in the chromosome 5q33 region in Latin American pedigrees. Am J Med Genet B Neuropsychiatr Genet. 2009;150B:998–1006. doi: 10.1002/ajmg.b.30956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Freimer NB, Reus VI, Escamilla M, et al. An approach to investigating linkage for bipolar disorder using large Costa Rican pedigrees. Am J Med Genet. 1996;67:254–263. doi: 10.1002/(SICI)1096-8628(19960531)67:3<254::AID-AJMG3>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Escamilla MA, Spesny M, Reus VI, et al. Use of linkage disequilibrium approaches to map genes for bipolar disorder in the Costa Rican population. Am J Med Genet. 1996;67:244–253. doi: 10.1002/(SICI)1096-8628(19960531)67:3<244::AID-AJMG2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Freimer NB, Reus VI, Escamilla MA, et al. Genetic mapping using haplotype, association and linkage methods suggests a locus for severe bipolar disorder (BPI) at 18q22-q23. Nat Genet. 1996;12:436–441. doi: 10.1038/ng0496-436. [DOI] [PubMed] [Google Scholar]
- 36.Ophoff RA, Escamilla MA, Service SK, et al. Genomewide linkage disequilibrium mapping of severe bipolar disorder in a population isolate. Am J Hum Genet. 2002;71:565–574. doi: 10.1086/342291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nurnberger JI, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-4. [DOI] [PubMed] [Google Scholar]
- 38.Palacio CA, García J, Arbeláez MP, et al. [Validation of the Diagnostic Interview for Genetic Studies (DIGS) in Colombia] Biomedica. 2004;24:56–62. [PubMed] [Google Scholar]
- 39.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 40.Hong KS, McInnes LA, Service SK, et al. Genetic mapping using haplotype and model-free linkage analysis supports previous evidence for a locus predisposing to severe bipolar disorder at 5q31-33. Am J Med Genet B Neuropsychiatr Genet. 2004;125B:83–86. doi: 10.1002/ajmg.b.20091. [DOI] [PubMed] [Google Scholar]
- 41.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 42.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glahn DC, Almasy L, Barguil M, et al. Neurocognitive endophenotypes for bipolar disorder identified in multiplex multigenerational families. Arch Gen Psychiatry. 2010;67:168–177. doi: 10.1001/archgenpsychiatry.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology: General. 1992;121:15. [Google Scholar]
- 45.Wechsler D. Wechsler Adult Intelligence Scale® 4th Edition (WAIS®-IV) San Antonio, TX: Harcourt Assessment; 2008. [Google Scholar]
- 46.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 47.Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- 48.Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. doi: 10.1093/cercor/bhp026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raznahan A, Shaw P, Lalonde F, et al. How does your cortex grow? J Neurosci. 2011;31:7174–7177. doi: 10.1523/JNEUROSCI.0054-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SM, Jenkinson M, Woolrich MW, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 51.Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM. FSL. Neuroimage. 2012;62:782–790. doi: 10.1016/j.neuroimage.2011.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Oishi K, Zilles K, Amunts K, et al. Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter. Neuroimage. 2008;43:447–457. doi: 10.1016/j.neuroimage.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahon K, Burdick KE, Ikuta T, et al. Abnormal Temporal Lobe White Matter as a Biomarker for Genetic Risk of Bipolar Disorder. Biol Psychiatry. 2013;73:177–182. doi: 10.1016/j.biopsych.2012.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sexton CE, Mackay CE, Ebmeier KP. A systematic review of diffusion tensor imaging studies in affective disorders. Biol Psychiatry. 2009;66:814–823. doi: 10.1016/j.biopsych.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 55.Sprooten E, Sussmann JE, Clugston A, et al. White matter integrity in individuals at high genetic risk of bipolar disorder. Biol Psychiatry. 2011;70:350–356. doi: 10.1016/j.biopsych.2011.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Bartzokis G, Lu PH, Heydari P, et al. Multimodal magnetic resonance imaging assessment of white matter aging trajectories over the lifespan of healthy individuals. Biol Psychiatry. 2012;72:1026–1034. doi: 10.1016/j.biopsych.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 57.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29:2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 59.Song SK, Sun SW, Ju WK, Lin SJ, Cross AH, Neufeld AH. Diffusion tensor imaging detects and differentiates axon and myelin degeneration in mouse optic nerve after retinal ischemia. Neuroimage. 2003;20:1714–1722. doi: 10.1016/j.neuroimage.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 60.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van der Waerden BL. Order tests for the two-sample problem and their power. Indagationes Mathematicae. 1952;14:458. [Google Scholar]
- 62.Pilia G, Chen WM, Scuteri A, et al. Heritability of cardiovascular and personality traits in 6,148 Sardinians. PLoS Genet. 2006;2:e132. doi: 10.1371/journal.pgen.0020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Williams JT, Van Eerdewegh P, Almasy L, Blangero J. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet. 1999;65:1134–1147. doi: 10.1086/302570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cruchaga C, Kauwe JS, Harari O, et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer's disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schürhoff F, Szöke A, Méary A, et al. Familial aggregation of delusional proneness in schizophrenia and bipolar pedigrees. Am J Psychiatry. 2003;160:1313–1319. doi: 10.1176/appi.ajp.160.7.1313. [DOI] [PubMed] [Google Scholar]
- 66.Jamison KR. Great wits and madness: more near allied? Br J Psychiatry. 2011;199:351–352. doi: 10.1192/bjp.bp.111.100586. [DOI] [PubMed] [Google Scholar]
- 67.Kyaga S, Lichtenstein P, Boman M, Hultman C, Långström N, Landén M. Creativity and mental disorder: family study of 300,000 people with severe mental disorder. Br J Psychiatry. 2011;199:373–379. doi: 10.1192/bjp.bp.110.085316. [DOI] [PubMed] [Google Scholar]
- 68.Kyaga S, Landén M, Boman M, Hultman CM, Långström N, Lichtenstein P. Mental illness, suicide and creativity: 40-year prospective total population study. J Psychiatr Res. 2013;47:83–90. doi: 10.1016/j.jpsychires.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 69.Parker G, Paterson A, Fletcher K, Blanch B, Graham R. The 'magic button question' for those with a mood disorder--would they wish to re-live their condition? J Affect Disord. 2012;136:419–424. doi: 10.1016/j.jad.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 70.Balanzá-Martínez V, Rubio C, Selva-Vera G, et al. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: a systematic review. Neurosci Biobehav Rev. 2008;32:1426–1438. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 71.Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–115. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 72.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: a meta-analysis. Acta Psychiatr Scand Suppl. 2007:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 73.Greenwood TA, Braff DL, Light GA, et al. Initial heritability analyses of endophenotypic measures for schizophrenia: the consortium on the genetics of schizophrenia. Arch Gen Psychiatry. 2007;64:1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gur RE, Nimgaonkar VL, Almasy L, et al. Neurocognitive endophenotypes in a multiplex multigenerational family study of schizophrenia. Am J Psychiatry. 2007;164:813–819. doi: 10.1176/ajp.2007.164.5.813. [DOI] [PubMed] [Google Scholar]
- 75.Steinberg S, de Jong S, Mattheisen M, et al. Common variant at 16p11.2 conferring risk of psychosis. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- 77.Bora E, Fornito A, Yücel M, Pantelis C. Voxelwise meta-analysis of gray matter abnormalities in bipolar disorder. Biol Psychiatry. 2010;67:1097–1105. doi: 10.1016/j.biopsych.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 78.Hallahan B, Newell J, Soares JC, et al. Structural magnetic resonance imaging in bipolar disorder: an international collaborative mega-analysis of individual adult patient data. Biol Psychiatry. 2011;69:326–335. doi: 10.1016/j.biopsych.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 79.Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry. 2008;65:1017–1032. doi: 10.1001/archpsyc.65.9.1017. [DOI] [PubMed] [Google Scholar]
- 80.McDonald C, Zanelli J, Rabe-Hesketh S, et al. Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar disorder. Biol Psychiatry. 2004;56:411–417. doi: 10.1016/j.biopsych.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 81.Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- 82.Houenou J, Frommberger J, Carde S, et al. Neuroimaging-based markers of bipolar disorder: evidence from two meta-analyses. J Affect Disord. 2011;132:344–355. doi: 10.1016/j.jad.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13:1–15. doi: 10.1111/j.1399-5618.2011.00893.x. [DOI] [PubMed] [Google Scholar]
- 84.Cusi AM, Nazarov A, Holshausen K, Macqueen GM, McKinnon MC. Systematic review of the neural basis of social cognition in patients with mood disorders. J Psychiatry Neurosci. 2012;37:154–169. doi: 10.1503/jpn.100179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kupferschmidt DA, Zakzanis KK. Toward a functional neuroanatomical signature of bipolar disorder: quantitative evidence from the neuroimaging literature. Psychiatry Res. 2011;193:71–79. doi: 10.1016/j.pscychresns.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 86.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14:326–339. doi: 10.1111/j.1399-5618.2012.01021.x. [DOI] [PubMed] [Google Scholar]
- 87.Roberts G, Green MJ, Breakspear M, et al. Reduced Inferior Frontal Gyrus Activation During Response Inhibition to Emotional Stimuli in Youth at High Risk of Bipolar Disorder. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 88.Bearden CE, van Erp TG, Dutton RA, et al. Mapping corpus callosum morphology in twin pairs discordant for bipolar disorder. Cereb Cortex. 2011;21:2415–2424. doi: 10.1093/cercor/bhr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van der Schot AC, Vonk R, Brans RG, et al. Influence of genes and environment on brain volumes in twin pairs concordant and discordant for bipolar disorder. Arch Gen Psychiatry. 2009;66:142–151. doi: 10.1001/archgenpsychiatry.2008.541. [DOI] [PubMed] [Google Scholar]
- 90.Brotman MA, Guyer AE, Lawson ES, et al. Facial emotion labeling deficits in children and adolescents at risk for bipolar disorder. Am J Psychiatry. 2008;165:385–389. doi: 10.1176/appi.ajp.2007.06122050. [DOI] [PubMed] [Google Scholar]
- 91.Brotman MA, Skup M, Rich BA, et al. Risk for bipolar disorder is associated with face-processing deficits across emotions. J Am Acad Child Adolesc Psychiatry. 2008;47:1455–1461. doi: 10.1097/CHI.0b013e318188832e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mercer L, Becerra R. A unique emotional processing profile of euthymic bipolar disorder? A critical review. J Affect Disord. 2013;146:295–309. doi: 10.1016/j.jad.2012.10.030. [DOI] [PubMed] [Google Scholar]
- 93.Samamé C, Martino DJ, Strejilevich SA. Social cognition in euthymic bipolar disorder: systematic review and meta-analytic approach. Acta Psychiatr Scand. 2012;125:266–280. doi: 10.1111/j.1600-0447.2011.01808.x. [DOI] [PubMed] [Google Scholar]
- 94.Adleman NE, Kayser RR, Olsavsky AK, et al. Abnormal fusiform activation during emotional-face encoding assessed with functional magnetic resonance imaging. Psychiatry Res. 2013 doi: 10.1016/j.pscychresns.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- 96.Corvin A, Donohoe G, Hargreaves A, Gallagher L, Gill M. The cognitive genetics of neuropsychiatric disorders. Curr Top Behav Neurosci. 2012;12:579–613. doi: 10.1007/7854_2011_188. [DOI] [PubMed] [Google Scholar]
- 97.Donohoe G, Deary IJ, Glahn DC, Malhotra AK, Burdick KE. Neurocognitive phenomics: examining the genetic basis of cognitive abilities. Psychol Med. 2012:1–10. doi: 10.1017/S0033291712002656. [DOI] [PubMed] [Google Scholar]
- 98.Greenwood TA, Beeri MS, Schmeidler J, et al. Heritability of cognitive functions in families of successful cognitive aging probands from the Central Valley of Costa Rica. J Alzheimers Dis. 2011;27:897–907. doi: 10.3233/JAD-2011-110782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hervey AS, Greenfield K, Gualtieri CT. Heritability in cognitive performance: evidence using computer-based testing. J Genet Psychol. 2012;173:112–118. doi: 10.1080/00221325.2011.573025. [DOI] [PubMed] [Google Scholar]
- 100.Lee T, Mosing MA, Henry JD, et al. Genetic influences on four measures of executive functions and their covariation with general cognitive ability: the Older Australian Twins Study. Behav Genet. 2012;42:528–538. doi: 10.1007/s10519-012-9526-1. [DOI] [PubMed] [Google Scholar]
- 101.Oliver BR, Plomin R. Twins' Early Development Study (TEDS): a multivariate, longitudinal genetic investigation of language, cognition and behavior problems from childhood through adolescence. Twin Res Hum Genet. 2007;10:96–105. doi: 10.1375/twin.10.1.96. [DOI] [PubMed] [Google Scholar]
- 102.van Soelen IL, Brouwer RM, van Leeuwen M, Kahn RS, Hulshoff Pol HE, Boomsma DI. Heritability of verbal and performance intelligence in a pediatric longitudinal sample. Twin Res Hum Genet. 2011;14:119–128. doi: 10.1375/twin.14.2.119. [DOI] [PubMed] [Google Scholar]
- 103.Bearden CE, Hoffman KM, Cannon TD. The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord. 2001;3:106–50. doi: 10.1034/j.1399-5618.2001.030302.x. discussion 151-3. [DOI] [PubMed] [Google Scholar]
- 104.Evans L, Akiskal HS, Keck PE, et al. Familiality of temperament in bipolar disorder: support for a genetic spectrum. J Affect Disord. 2005;85:153–168. doi: 10.1016/j.jad.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 105.Glahn DC, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. doi: 10.1016/j.biopsych.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 106.Peters E, Joseph S, Day S, Garety P. Measuring delusional ideation: the 21-item Peters et al. Delusions Inventory (PDI) Schizophr Bull. 2004;30:1005–1022. doi: 10.1093/oxfordjournals.schbul.a007116. [DOI] [PubMed] [Google Scholar]
- 107.Barron F, Welsh GS. Artistic perception as a possible factor in personality style: Its measurement by a figure preference test. The Journal of Psychology. 1952;33:199–203. [Google Scholar]
- 108.Akiskal HS, Akiskal KK. TEMPS: Temperament Evaluation of Memphis, Pisa, Paris and San Diego. J Affect Disord. 2005;85:1. doi: 10.1016/j.jad.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 109.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 110.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 111.Kolin EA, Price L, Zoob I. Development of a Sensation-Seeking Scale. J Consult Psychol. 1964;28:477–482. doi: 10.1037/h0040995. [DOI] [PubMed] [Google Scholar]
- 112.Zuckerman M, Link K. Construct validity for the sensation-seeking scale. J Consult Clin Psychol. 1968;32:420–426. doi: 10.1037/h0026047. [DOI] [PubMed] [Google Scholar]
- 113.Lejuez CW, Read JP, Kahler CW, et al. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8:75. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- 114.Glahn DC, Cannon TD, Gur RE, Ragland JD, Gur RC. Working memory constrains abstraction in schizophrenia. Biol Psychiatry. 2000;47:34–42. doi: 10.1016/s0006-3223(99)00187-0. [DOI] [PubMed] [Google Scholar]
- 115.Kurtz MM, Ragland JD, Moberg PJ, Gur RC. The Penn Conditional Exclusion Test: a new measure of executive-function with alternate forms of repeat administration. Arch Clin Neuropsychol. 2004;19:191–201. doi: 10.1016/S0887-6177(03)00003-9. [DOI] [PubMed] [Google Scholar]
- 116.Brown L, Sherbenou RJ, Johnsen SK. Test of nonverbal intelligence. A language free measure of cognitive ability. 1997 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.