Abstract
Background
Previous studies show gene expression alterations in rat embryo hearts and cell lines that correspond to the cardio-teratogenic effects of trichloroethylene (TCE) in animal models. One potential mechanism of TCE teratogenicity may be through altered regulation of calcium homeostatic genes with a corresponding inhibition of cardiac function. It has been suggested that TCE may interfere with the folic acid/methylation pathway in liver and kidney and alter gene regulation by epigenetic mechanisms. According to this hypothesis, folate supplementation in the maternal diet should counteract TCE effects on gene expression in the embryonic heart.
Approach
To identify transcriptional targets altered in the embryonic heart after exposure to TCE, and possible protective effects of folate, we used DNA microarray technology to profile gene expression in embryonic mouse hearts with maternal TCE exposure and dietary changes in maternal folate.
Results
Exposure to low doses of TCE (10ppb) caused extensive alterations in transcripts encoding proteins involved in transport, ion channel, transcription, differentiation, cytoskeleton, cell cycle and apoptosis. Exogenous folate did not offset the effects of TCE exposure on normal gene expression and both high and low levels of folate produced additional significant changes in gene expression.
Conclusions
A mechanism where TCE induces a folate deficiency does not explain altered gene expression patterns in the embryonic mouse heart. The data further suggest that use of folate supplementation, in the presence of this toxin, may be detrimental and non-protective of the developing embryo.
Keywords: Halogenated hydrocarbons, trichloroethylene, cardiac, folate, embryonic development, gene expression, ryanodine receptor
1. Introduction
Halogenated hydrocarbons contaminate water supplies in the United States and around the world. Trichloroethylene (TCE) currently ranks 16th on the CERCLA Priority List of Hazardous Substances (ATSDR, 2007). It is generally used in industry as a degreasing agent and is found in paint removers, correction fluids, and household cleaners (Steinberg and DeSesso, 1993; Waters et al., 1977). Exposure to TCE through contaminated drinking water has been associated with increased incidence of congenital heart malformations in children born to exposed mothers and in model animals (Goldberg et al., 1990; Johnson et al., 1998b; Shaw et al., 1992; Spiegelstein et al., 2005).
TCE and Heart Development
The heart is the earliest functioning organ, first appearing as a simple linear tube that pumps in peristaltic fashion, and undergoing looping to form two chambers with the addition of primitive valves to inhibit retrograde flow (Forouhar et al., 2006; Liebling et al., 2006). The formation of the four chambered heart requires a series of septations in the atrioventricular canal, the atrium and the ventricle (Kirby, 2001). The first septation in the atrioventricular canal involves a process called epithelial-to-mesencyhmal transition (EMT) where endocardial cells migrate into the cardiac jelly, proliferate, and form valve leaflets (Armstrong and Bischoff, 2004). Proper developmental transitions are vital to heart function and embryo survival. Mutations in genes involved in these phases can lead to valve defects, disrupted blood flow and lethality (Clark et al., 2006; Joziasse et al., 2008).
Previous investigations using animal models have reported an association between TCE exposure and increased incidence of congenital heart malformations (Dawson et al., 1993; Goldberg et al., 1992; Johnson et al., 1998a; Johnson et al., 2003; Loeber et al., 1988; ). More recently several groups have reported on possible mechanisms by which TCE may affect heart development. In the chick model, Boyer et al. (2000) demonstrated that exposure to 200ppb TCE reduced by 50% EMT of valve progenitors in a collagen gel assay, while lower doses of TCE in ovo enhanced valvuloseptal hypercellularity, endocardial cell proliferation and altered hemodynamic in the embryonic heart (Drake et al., 2006a; Drake et al., 2006b; Mishima et al., 2006). Others found that TCE exposure altered expression of the endothelial nitric oxide synthase and disrupted VEGF-stimulated endothelial proliferation in myocytes, suggesting a possible mechanism for TCE-mediated heart malformations (Ou et al., 2003). Our own studies indicated that exposure to TCE disrupted calcium flux regulation in myocytes (Caldwell et al., 2008). Heart morphogenesis is a complex process in which cascading events must be precisely orchestrated, so any external factor altering one or more of these events is likely to perturb normal cardiac development. Currently, there is no unifying hypothesis that reconciles the effects of TCE on the developing embryonic heart.
Folate and Heart Development
Epidemiological studies have shown maternal use of multivitamins in early pregnancy can reduce the risk for development of CHD in the fetus (Gelineau-van Waes et al., 2008). A study by Botto et al., (2003) showed that the risk of heart defects was lower in children born to mothers who used multivitamin supplements than in children of those who did not take supplements. High levels of folate are usually included in recommended multivitamin supplements. Folate participates with vitamin B12 in the process that re-methylates homocysteine into methionine, which is the main methyl donor in form of S-adenosyl methionine (SAM) and is necessary for new synthesis of protein and nucleic acids. Folate is especially important in periods of rapid cell division and growth, as in infancy and pregnancy.
Chronic nutritional variations in folate metabolism may affect embryonic development and potentially increase incidence of cardiovascular defects because of the vital role of folate in DNA biosynthesis and amino acid metabolism (Li et al., 2005; Rosenquist et al., 1996). Only a few animal studies have been conducted on the effects of dietary folate and reproductive outcomes (Burgoon et al., 2002; Li et al., 2005; Sakanashi et al., 1996). A recent prevention trial in patients with prior colorectal polyps found an increased risk of reoccurrence when folic acid supplementation was used, suggesting a dual role of folate in carcinogenesis with potential support of tumor growth (Song et al., 2000).
It has been suggested that TCE induces folate deficiency in kidney by producing free radicals, which induce a B12 shortage, and consequently cause folate deficiency (Dow and Green, 2000). In rat, diet supplementation with methionine prevents the effects of TCE on induction of liver tumors, by reversing TCE-induced hypomethylation of c-myc and c-jun (Tao et al., 2000a; 2000b). To date, no studies have investigated whether trichloroethylene interferes with folate's metabolic pathways and, in turn, disrupts normal development of the embryo.
Previous work from our laboratory demonstrated that TCE exposure altered calcium regulation in cardiac myocytes and the expression of genes involved in calcium homeostasis (Caldwell et al., 2008; Selmin et al., 2008), suggesting a possible mechanism by which cardiac malformations occur. To identify other key contributing pathways that may be altered in the embryonic heart after exposure to TCE, we used DNA microarray technology to profile gene expression patterns in mice. Embryonic hearts were isolated from pregnant mice that had received a diet supplemented with low, normal, or high amounts of folate (0, 2, and 8mg/kg) in the presence or absence of 10ppb TCE in drinking water. The goal of this study was to measure transcriptional changes in the developing heart following exposure to low, environmentally significant doses of TCE, and to determine whether folic acid supplementation might counteract the effects of TCE. We found that high levels of folate supplementation with TCE exposure caused dramatic alterations in the expression level of genes involved in cellular pathways crucial for embryo development, such as transport, ion channels, differentiation, cytoskeleton, transcription, cell cycle and apoptosis. These findings suggest that a high folate diet does not offset low level TCE exposure and that the combination of TCE and high folate may have greater detrimental effects on development of the fetal heart.
2. Materials and Methods
All animals used in this study were maintained in a facility approved by the Association for Assessment and Accreditation of Laboratory Animal Care International and in accordance with the established guidelines of the University of Arizona's Institutional Animal Care and Use Committee, the Animal Welfare Act, and U.S. Public Health Service policy standards.
2.1. Fetal Collection and Morphological Staging
Wild type mice (129S1/SvlmJ) were obtained from Jackson Laboratories (Bar Harbor, ME). Female mice were assigned to 3 different folate diets for four weeks before mating: 0mg/kg folate, 2mg/kg folate, and 8mg/kg folate (Dyets Version of the Clifford/Koury Folate Deficient L-Amino Acid Rodent Diet Without Succinyl Sulfathiazole, Modified for Pelleting; Dyets Inc, Bethlehem, PA), and received water ad libitum. The presence of a vaginal plug was considered indicative of day 0 pregnancy. At this time, each folate group was divided in two sub-groups of control and TCE exposed mice, and maternal exposure to 10ppbTCE was started via drinking water.
TCE was prepared daily in glass bottles that had been soaked in concentrated TCE solution overnight, rinsed and dried in a chemical hood before use. Each bottle was placed in metal casing to reduce light exposure and subsequent chemical breakdown. Control animals received distilled water throughout pregnancy.
At gestational time point of day 10, corresponding to the first phase of heart development, pregnant dams were sacrificed by CO2 inhalation, the abdomen opened and the uterine contents removed. The location of all viable fetuses and reabsorption sites were recorded. Decidual capsules were transferred to a phosphate-buffered saline solution and embryos were dissected free. All embryos were examined grossly for size and developmental stage. Cardiac tissue was removed from each viable embryo and placed in RNA later for future use. Maternal organs were also harvested for additional studies. Whole fetuses were viewed under a Nikon SMZ-2T stereomicroscope.
2.2. RNA extraction
Total RNA was extracted from pooled embryo hearts (n= 35, corresponding to 4-6 litters) and purified using a custom RNA extraction method. Briefly, the RNAlater solution was removed from the merged heart pools. 100uL RLT buffer with 1% B-mercaptoethanol and 600ul Trizol (Invitrogen, Carlsbad, CA) was added and mixed. RNA extraction was performed according to manufacturer instructions. Dry RNA pellets were resolubilized in 30uL RNAase-free dionized water. RNeasy Mini Kits (Qiagen, Valencia, CA) were used for further RNA cleanup if needed.
2.3. RNA and microarray preparation
Six sample groups (control and TCE exposed from each one of the three folate diet groups) were tested, each with two biological replicates for a total of 12 sample groups. Total RNA from each group was processed by the University of Arizona microarray facility (Genomics Shared Service, Tucson, AZ). RNA purity was evaluated using an Eppendorf BioPhotometer (Eppendorf North America, Westbury, NY) to obtain values of nucleotides, organics, proteins, and contaminants in the samples. Viable RNA had 260/280 ratios between 1.8 and 2.0 or higher. RNA integrity was confirmed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) following the RNA 6000 Nano Chip Series II Assay protocol. Twelve Affymetrix Mouse Gene 1.0 ST arrays (Affymetrix, Santa Clara, CA) containing over 28,000 gene-level probe sets were used for genome wide expression profiling. The arrays were processed according to the protocol (GeneChip Whole Transcript) Sense Target Labeling Assay Manual, version4) established by Affymetrix. Briefly, 400ng of total RNA were reverse transcribed into double stranded cDNA using the Affymetrix WT cDNA Synthesis and Amplification kit. The entire cDNA was then transcribed into cRNA overnight, cleaned using the Affymetrix GeneChip Sample Cleanup Module, and quantified on a NanoDrop 1000 (Thermo Scientific, Wilmington, DE). 8ug of cRNA were reverse transcribed into ssDNA, and labeled. Aliquots of the labeled DNA were combined with the hybridization solution overnight. The arrays were scanned using the Affymetrix GeneChip Scanner 3000 with 7G upgrade. The image generated was analyzed using the Affymetrix GeneChip Operating Software (GCOS) were .DAT (image), .CEL (cell intensity data), .CHP (probe analysis data) files were generated.
2.4. Statistical analysis of DNA microarrays
The Affymetrix mouse gene ST arrays were processed with a bioconductor library (http://www.bioconductor.org) designed for this array. The arrays were then normalized using the RMA function in the oligo library of bioconductor. In order to identify poor quality arrays, Spearman correlation coefficients between all arrays were computed. A plot of the coefficients revealed that correlations between all arrays were 0.98 or greater, indicating that a relatively small number of genes were changing in response to the treatments. As a further control, the distribution of values for positive and negative control probes were examined on each array and were found to have the correct expected separation. The samples included 2 arrays representing two biological replicates for each treatment.
Since there were only two replicates due to the complexity of the sample preparation, a specific approach was used that identified probes that showed a consistent change between biological duplicates. A cutoff value for the change between treatments of 1.5 fold was chosen based on an examination of the distribution of expression values on these arrays and identified approximately 200-1000 probes that were varying to the greatest extent. Probe lists were generated as described below.
Briefly, the objective was to find genes that were changing between treatments while reducing the effects of the biological variation between duplicate samples. To compare two treatments, probe expression ratios for all 4 possible combinations of the duplicate arrays were calculated. If 2 or more of these ratios were 1.5 fold or greater, the gene was considered to be changed. These probe sets were annotated using Affymetrix data files and the affected genes, biological functions, and pathways were identified. For the biochemical pathway analysis, lists of genes involved in pathways of biological interest were obtained from the KEGG database using the BIORAG resource (http://www.biorag.org ). Genes showing expression ratios between treatments of greater than 1.5 fold were then identified.
2.5. Reverse-transcriptase and quantitative real-time PCR
Total RNA from pooled embryos was transcribed into cDNA using the ISCRIPT cDNA synthesis kit (Bio-Rad Laboratories, Hercules, CA). Gene specific primers were used to amplify mouse β-actin, Ryr2, ErbB, Sumo1, INSR, GATA3, Cubn, and Gstp2 transcripts. Primers for the six transcripts and the housekeeping gene were designed using the NCBI Primer-BLAST program. SYBR Green analysis using ITAQ SYBR Green supermix with ROX (Bio-rad Laboratories, Hercules, CA) was used according to the manufacturer's instructions and carried out using the gene specific primers to ensure that single band dissociation was acquired. Briefly, the real-time PCR reactions were run at a final volume of 25 μL consisting of the following master mix: 12.5 μL of 10× buffer supermix, 1 μL each of forward and reverse primers (10μM), 9.5 μL nuclease free double-distilled water, and 1μL cDNA (1μg/μl). The ABI 7300 Real Time PCR System and Ct values were used to quantify the relative differences in PCR product. Each sample was run a minimum of three times in duplicate using the same two pools of RNA used for the microarray analysis. Expression values for each specific gene were normalized against β-actin expression levels, and expressed in fold change. Values represent means +/− standard deviation (SD). SD was calculated as the ratio of SD of the sample pool (n = or > 6) divided by the average of n.
3. Results
3.1. Physical outcomes of Day 10 embryos
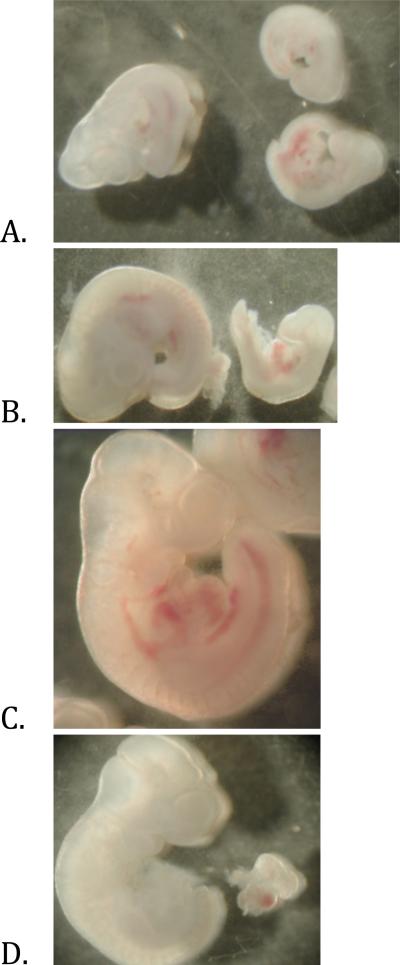
We performed a gross examination of each embryo after removal from the dam, and recorded the results in Table 2. Any empty amniotic sac was recorded as reabsorption. If no amniotic sacs were found in the womb, it was considered false pregnancy. Developmental stages were determined using mouse embryonic development charts (Kaufman, 1992) (Figure 1D). For each category, the first number represents a “per litter basis” of change, the second one is the percent change based on total number of embryos used in each group.
Figure 1. Developmental phenotype of Day 10 embryos.
A) Littermates from non-exposed dam on 0mg/kg folate. Day10 embryo shown on left with two under-developed embryos shown on right. B) Embryos from non-exposed dam on 8mg/kg folate. Day 10 embryo on left with under-developed embryo on right. C) Embryo from 10ppb TCE exposed dam on 0mg/kg folate. D) Day 10 embryo from control 2mg/kg folate group with cardiac tissue removed.
Examining the different levels of folate diet alone, without TCE exposure, both 0mg/kg (0CTL) and 8mg/kg (8CTL) folate increased the number of reabsorptions and under-developed embryos compared with the control group (2mg/kg folate, 2CTL). Normally developed embryos were reduced by 10% or more in 0CTL and 8CTL respectively, when compared against 2CTL. In the 10ppb TCE exposed embryo populations, these finding were inverted. We observed that the number of reabsorptions was decreased by ~5% in the 2mg/kg (2TCE) and 8mg/kg (8TCE) folate groups when compared against the non-exposed, control group, 2CTL. Interestingly, the percentage of reabsorptions in the 0T embryos was identical (21.3%) to 2CTL. When 0TCE and 8TCE were compared to 2TCE, we observed an increased number of reabsorptions with both low and high levels of folate in the maternal diet. In addition, there were between 7-10% less underdeveloped embryos, and 2-4% more normally developed embryos with high and low folate, respectively. Finally, the rate of false pregnancy did not change considerably across the different groups. The 0TCE and 2CTL groups had the same percentage of normally developed embryos.
3.2. Microarray Results
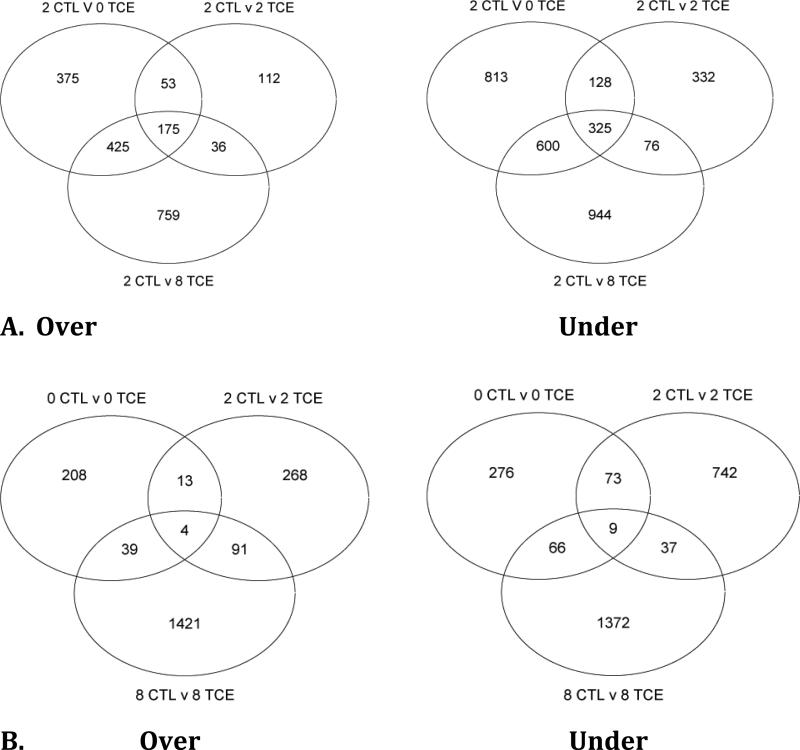
We used Venn diagrams to represent possible relations between comparison data sets (Figure 2). The numbers in the circle represents genes changed uniquely in a particular group and the overlapping sections show the number of altered genes in common between two, or all three groups. Figure 2A shows comparisons between 2CTL against all three folate levels in the presence of 10ppb TCE (0TCE, 2TCE, and 8TCE), separated into over and under-expressed transcripts. This comparison illustrates how TCE exposure in deficient, normal, and high folate groups altered transcript levels when compared to the non-exposed group at a normal folate level. Taking together the over and under-expressed diagrams, we observed that the most genes changed in the 8TCE group (2CTL v 8TCE) (3340 total), whereas 2894 transcripts were altered in 0TCE and 1226 in 2TCE. Furthermore, the overlap between 8TCE and 0TCE showed the highest number of similarly changed genes. Interestingly, all numbers in the under-expressed diagrams are much higher than in the over-expressed diagrams.
Figure 2. Venn Diagrams.
Overview of all genes altered in day 10 embryonic cardiac tissue microarrays. A) Shows comparisons between the 2mg/kg control group (2CTL) against all TCE-treated groups (OTCE, 2TCE, 8TCE). 2CTL v 0 (2, or 8) TCE include transcripts that were either under or over expressed in the TCE groups when compared with the control, 2CTL group. B) Shows comparisons between each individual TCE exposed group (OTCE, 2TCE, 8TCE) against their own folate level control group (OCTL, 2CTL, 8C).
We also compared TCE exposed versus non-exposed (CTL) groups at each of the three folate levels (Figure 2B). This diagram illustrates how TCE exposure in deficient, normal, and high folate diets altered transcript expression when compared against similar folate controls. Both over and under-expressed diagrams show a striking number of genes altered in 8TCE versus 8CTL group (1555 and 1484, respectively). 2TCE versus 2CTL is the next highest, totaling 861 under-expressed genes. In both over and under-expressed diagrams fewer than 10 genes were similarly altered by all three groups.
3.3. Gene expression changes in cellular pathways
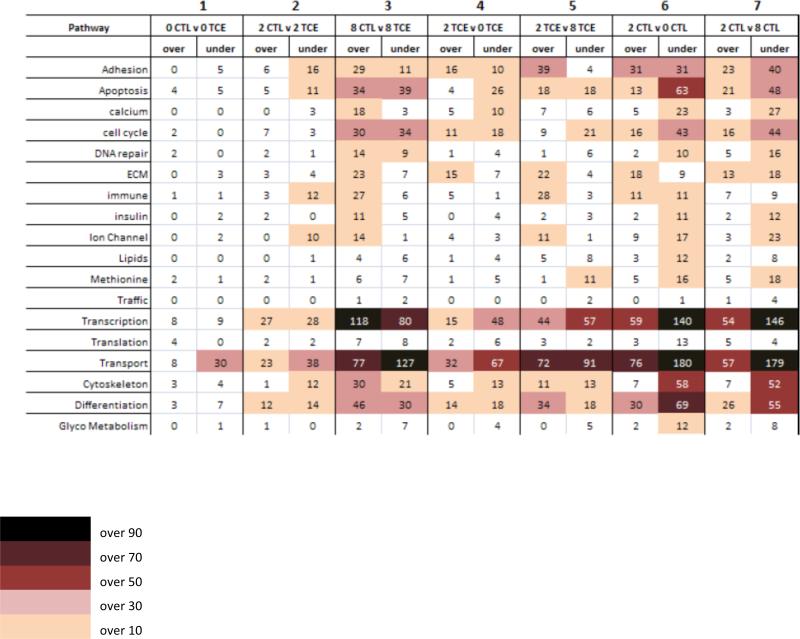
In order to better define the effects of TCE on gene expression at different levels of folate, we grouped the down- and up- regulated transcripts based on their involvement in specific cellular pathways, and then compared each group as illustrated in Figure 3. In the first three columns, TCE exposed embryos at deficient, normal, and high folate levels were compared against their own non-exposed group. Columns 4 and 5 compared 0TCE and 8TCE against the 2TCE group. These two columns illustrate the different contribution of TCE to transcriptional change in combination with different folate levels. Columns 6 and 7 compared OCTL and 8CTL against 2CTL. These columns display the effects of different levels of folate alone on gene expression.
Figure 3. Overall gene expression changes.
Numbers in each box represent genes over- or under-expressed in each one of the six comparison group, and assigned to specific Kegg pathways. In the first 3 columns, expression level of transcripts in the TCE exposed embryos at low, normal, and high folate were compared against non-exposed embryos at the same folate level. Columns four and five evaluate changes in the TCE exposed embryos at 0 and 8mg/kg folate level compared against the 2mg/kg folate level exposed to 10ppb TCE. Columns six and seven indicate the number of transcripts altered in the 0 and 8mg/kg folate controls compared against 2mg/kg folate control embryos, therefore account for folate-induced changes only.
In column 1, 0CTL v OTCE shows 107 altered transcripts representing less than 0.4% of all genes on the microarrays, with the majority involved in the transport pathway. In column 2, 2CTL v 2TCE displays 252 transcripts, corresponding to less than 0.9% of all genes represented. In particular, the majority of these changes occurred in the transcription (56 altered transcripts) and transport (61 altered transcripts) pathways. In column 3, 8CTL v 8TCE shows the most dramatic alterations, with changes in over 3% of the total transcripts: Between 20 and 30 genes were altered in the calcium, DNA repair, extra-cellular matrix, immune system, and ion channels pathways; between 30 and 60 gene changes were observed in the adhesion, apoptosis, cell cycle, cytoskeleton, and differentiation pathways, while 209 genes were altered in both transcription and transport pathways.
In columns 4 and 5, we compared the expression levels in 0TCE and 8TCE against the 2TCE group. In column 4, the adhesion, apoptosis, calcium, extra-cellular matrix, cytoskeleton, and differentiation pathways all revealed between 10 and 30 altered transcripts. The most robust alterations occurred in the transcription (48 under-expressed), and transport pathways (32 over- and 67 under-expressed). In column 5, the number of transcripts altered in 2TCE v 8TCE is lower than the number in column 3, where we compared 8CTL v 8TCE. The decrease was more pronounced in the transcription, apoptosis, cell cycle, cytoskeleton (~50%), and differentiation and transport pathway (32 and 21% fewer genes, respectively).
In columns 6 and 7, expression levels in 0CTL and 8CTL were compared to 2CTL and showed the most pronounced changes, indicating a drastic effect of folate level on gene expression. At low folate level (column 6), over 50 transcripts were altered in the apoptosis and cytoskeleton pathways, and over 70 in the differentiation pathway. At high folate level (column 7), over 80 transcripts were altered in the differentiation, over 60 in the adhesion, apoptosis and cell cycle, and over 50 in the cytoskeleton pathways.
3.4. TCE effects on individual pathways
Upon examination of individual pathways, we observed common themes of expression changes across some of the comparison columns, in particular, in the calcium and ion channel pathways. The majority of over-expressed genes in the 8CTLv 8TCE and 2CTL v 8TCE groups (Table 3A, 4A: columns 3 and 5) were actually under-expressed in 2CTL v 0CTL and 2CTL v 8CTL (Table 3B, 4B: columns 6 and 7). In both pathways we observed expression changes in genes coding for calcium, potassium, sodium and voltage-gated channels, including Ryr2, NCX, Mef2, and ErbB4. The apoptosis and cell cycle pathways (Tables 5 and 6) show the majority of changes occurring in 8CTL v 8TCE, with 30-40 transcript alterations in both the over and under-expressed tables, 15% of these with over 2-fold changes. In both 2CTL v 0CTL, and 2C TL v 8CTL (Columns 6 and 7) over 40 transcripts were under-expressed. 0CTL v 0TCE and 2CTL v 2TCE (Column 1 and 2) show minor alterations in both pathways with 5 or less genes except for 11 under-expressed genes in the 2CTL v 2TCE group.
3.5. Real-time analysis of expression changes of selected genes
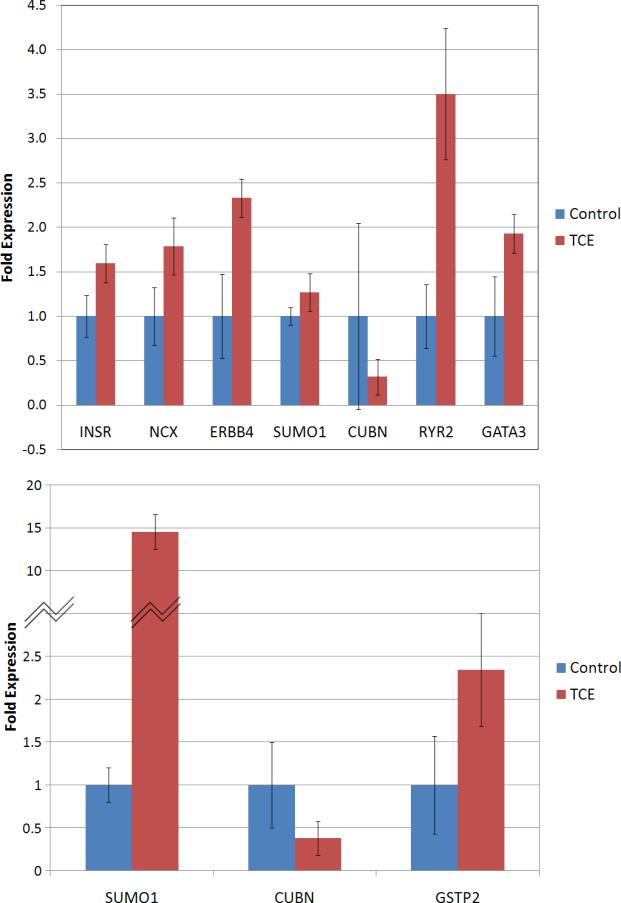
We selected eight transcripts that were altered by TCE in either 8CTL v 8TCE or 0CTL v 0TCE groups, and measured their expression levels by real-time PCR. The objective was to confirm the findings of the microarray analysis by a more sensitive technique. We chose to analyze two genes involved in calcium signaling (Ryr2 and NCX), and two genes involved in cellular growth (INSR and ERBB4). Gata3 was selected for its role during cardiomyocyte differentiation and early steps of heart tube morphogenesis (Cripps and Olson, 2002), and Glutathione-S-Transferase (GstP) for its importance in detoxification pathways. We selected the receptor Cubilin (gp280) (Sahali et al., 1988; Smith et al., 2006) and Sumo1, encoding for a protein that mediates sumoylation of histones and transcription factors, for their role in regulation of transcription during embryogenesis (Alkuraya et al., 2006).
Overall, the results obtained with real-time PCR are consistent with those from the microarray analysis. Figure 4A confirmed up-regulation of INSR, NCX, ERBB4, RYR2, and Gata3 in the 8TCE group. Sumo1 and Cubilin were found under-expressed in the microarray analysis, but no significant regulation was observed by real-time PCR. In 0TCE, the expression change in the three genes tested (Figure 4B) were similar to those in the microarray, although the difference was not significant for Cubilin and Gstp.
Figure 4. Real-time PCR analysis of transcripts selected for microarray validation.
Aliquots of RNA from the same two biological RNA pools used in the microarray analysis were run in duplicates at least three different times. Bars represent average values between different runs +/- SD.
4. Discussion
4.1. Physical outcomes of Day 10 embryos
The results from Table 2 suggest two conclusions: 1) Both high and low folate in the maternal diet leads to similar phenotypic outcomes in the embryos; and 2) Exposure to 10ppb TCE counteracted the effects of high and low folate alone. In both 0CTL and 8CTL, we observed an increased rate of reabsorbed and developmentally delayed embryos, and a decrease in normally developed embryos. However, when compared to 0TCE and 8TCE, we observed an almost perfect inversion of phenotypic outcomes (Table 2). Folate is a critical player in pathways involved in amino acid metabolism, nucleotide synthesis and methylation reactions (Wagner, 1995) so it is possible that any variation from a physiological optimum level of folate may create disturbances in one or more of these pathways. This hypothesis is corroborated by the finding that in 0TCE we observed no change in the percentage of reabsorptions and normally developed embryos when compared to 2CTL. Overall, these results suggest that TCE may facilitate the progression of these embryos through otherwise restrictive developmental checkpoints. A detailed examination of embryos is necessary to identify number and type of heart defects possibly present in each group, however, this aim will have to be addressed in future experiments.
4.2. Gene expression changes in cellular pathways
An expected finding of this study is that many changes in gene expression occurred in embryonic hearts in the 0CTL and 8CTL groups, underlying the importance of folate for development. However, TCE exposure in the presence of high folate induced the highest number of changes. This observation suggests that the effects of TCE are more dramatic and potentially detrimental to cardiac development in the presence of high folate levels in the maternal diet.
Calcium signaling is a crucial pathway for heart function (Table 3), and these findings corroborate those previously reported by our group, using in vitro models (Caldwell et al., 2008; Selmin et al., 2008). It is notable that only three out of 414 genes were altered in the 2CTL v 2TCE group and absolutely no changes were observed in the 0CTL v 0TCE level. This finding supports that notion that no further damage is caused by TCE exposure in an environment already under substantial stress due to deficient folate levels.
Ion channels are integral membrane proteins that regulate the flow of ions across the membrane and are fundamental to maintaining proper ion concentrations inside the cell (Table 4). Our data show that high folate levels in combination with TCE exposure induced over-expression of many genes in the ion channel pathway. The majority of the over-expressed genes encodes for potassium, calcium, and other cation transportation channels and corroborates previously published data from our laboratory (Caldwell et al., 2008) showing an increased calcium flux from the sarcoplasmic reticulum in cardiomyocytes exposed to 10ppb TCE. Our microarray data may be explained by TCE ability to interfere with potassium and calcium channels. Since calcium and potassium ions are very similar (both having positive charges, similar electron orbitals and atomic weights) TCE may alter the binding affinity of both channels or maintain the channels open to create a type of super-highway for calcium transport. Alternatively, TCE could alter the permeability of the cell membrane, causing an electrolyte imbalance and thus activating signaling pathways that increase synthesis of ion channels to correct the imbalance.
Significant alterations in gene expression were observed in the apoptosis and cell cycle pathways (Tables 5 and 6). Apoptosis is essential for the development of organs and structures throughout the embryonic stages, and is closely interconnected with pathways mediating the signals that direct the cell through the different phases of DNA synthesis, repair, and mitosis. Past studies have linked TCE exposure to septal and valvular malformations, hypercellularity and endocardiocyte proliferation (Drake et al., 2006a; Drake et al., 2006b; Hoffmann et al., 1994). Our results indicate that high levels of folate reduced cell cycle and apoptotic signals overall, but in the presence of 10ppb TCE, many of these signals were reversed, providing a possible explanation for the cardiac hypercellularity and induced proliferation observed by other groups.
4.3. Overall conclusions
Epidemiological and animal studies have documented the dual effect of folate on carcinogenesis: protecting normal mucosa, but enhancing progression of early lesions (Kim, 2004). Taken together, our findings also suggest that folate may have a dual effect on cardiogenesis, depending on the presence of environmental toxins, such as TCE. Additionally, our data support the notion that environmental concentrations of TCE may cause drastic changes in gene expression during critical phases of heart development. Notwithstanding the limitations associated with our study, these data suggest that the optimal dose of folate intervention needs to be determined for safe and effective prenatal care. The data also indicate that exogenous folate, whether to restore normal folate levels or raise endogenous levels does not strongly offset the effects of TCE exposure on altered gene expression. Thus, a mechanism where TCE produces a folate deficiency does not explain altered gene expression patterns in the embryonic mouse heart.
Table 1.
Sequences of the primers used for realtime PCR
| Gene | GenBank | Primer | Sequence (5′-3′) |
|---|---|---|---|
| β-actin | NM_007393 | 440F | CCAGATCATGTTTGAGACCTTCAA |
| 526R | GTGGTACGACCAGAGGCATACA | ||
| Ryr2 | NM_023868 | 4676F | ACCAAGCCAGATTACAGCACAG |
| 4899R | ACCGTCACTGTGCGCACTC | ||
| NCX | NM_011406 | 1923F | TCGATGACGAGGAGTATGAGAA |
| 2031R | CCACCAAGCTCATTCAACAA | ||
| ErbB4 | NM_010154 | 431F | AACAGCAGTACCGAGCCTTG |
| 530R | AAGGAGAGGTCCCGGTTG | ||
| Sumo1 | NM_009460 | 803F | TGCTTCACTCCTGGACTGTG |
| 1003R | TCTCTCCAGTGAAGCCACCT | ||
| INSR | NM_010568 | 1138F | AAAGTTTGCCCAACCATCTG |
| 1359R | GTGAAGGTCTTGGCAGAAGC | ||
| GATA3 | NM_00809l | 246F | GTGGTCACACTCGGATTCCT |
| 435R | GCAAAAAGGAGGGTTTAGGG | ||
| Cubn | NM_1081084 | 5921F | GAAGGGGATTCCTTCTGG |
| 6029R | AGGGGTGTCTCCTGTCAC | ||
| Gstp2 | NM_181796 | 190F | AGCCCACTTGTCTGTATGG |
| 425R | AGGGCCTTCACGTAGTCAT |
Table 2. Physical outcomes of Day 10 embryos.
Results from gross examination of every embryo removed from the dam are reported. Non-exposed controls (CTL) and 10ppb TCE exposed (TCE) embryos are sub-categorized into 0mg/kg (0CTL and 0TCE), 2mg/kg (2CTL and 2TCE), and 8mg/kg folate (8 CTL and 8TCE) groups. Developmental staging was according to Kaufman, 1992. Reabsorptions indicate empty amniotic sacs and false pregnancy denote no pregnancy development in any way. The amount of embryos are shown on a per litter basis (n) and by the percent of embryos compared to the overall total per treatment group (%).
| control water | 10ppb TCE water | |||||
|---|---|---|---|---|---|---|
| 0mg folate | 2mg folate | 8mg folate | 0mg folate | 2mg folate | 8mg folate | |
| viable embryos | 130 | 75 | 94 | 102 | 89 | 102 |
| litters | 22 | 9 | 11 | 17 | 13 | 17 |
| False Pregnancy [n (%)] | 0.18 (3.08) | 0.22 (2.67) | 0.18 (2.13) | 0.24 (3.92) | 0.08 (1.12) | 0.11 (1.96) |
| Reabsorptions/Abortions [n (%)] | 1.64 (27.69) | 1.78 (21.33) | 2.27 (26.60) | 1.29 (21.33) | 1.08 (15.73) | 0.94 (16.67) |
| Under-development [n (%)] | 0.77 (13.08) | 0.67 (8.0) | 0.82 (9.57) | 0.35 (5.88) | 1.08 (15.73) | 0.50 (8.82) |
| Normal development [n (%)] | 3.18 (53.85) | 5.67 (68.0) | 5.09 (59.57) | 4.12 (68.63) | 4.54 (66.29) | 4.0 (70.59) |
n = per litter basis
% = percent of total embryos
Table 3. Calcium pathway.
A) Over and B) under expressed genes involved in the calcium signaling pathway. Each column as described in Table 3.
| Calcium Pathway | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Over-Expressed | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| ENSMUST105420 | Adora2a | adenosine A2a receptor | |||||||
| NM_009781 | Cacna1c | calcium channel | |||||||
| NM_009783 | Cacna1g | calcium channel | |||||||
| ENSMUST31093 | Cckar | cholecystokinin A receptor | |||||||
| NM_203491 | Chrm2 | cholinergic receptor | |||||||
| ENSMUST81091 | Erbb4 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 | |||||||
| ENSMUST106091 | Gjb3 | gap junction membrane channel protein beta 3 | |||||||
| NM_010305 | Gnai1 | guanine nucleotide binding protein | |||||||
| NM_008170 | Grin2a | glutamate receptor | |||||||
| NM_001081414 | Grm5 | glutamate receptor | |||||||
| NM_001012306 | Hsd3b3 | hydroxy-delta-5-steroid dehydrogenase | |||||||
| NM_010568 | Insr | insulin receptor | |||||||
| NM_010585 | Itpr1 | inositol 1 | |||||||
| NM_019923 | Itpr2 | inositol 1 | |||||||
| ENSMUST72460 | Mef2a | myocyte enhancer factor 2A | |||||||
| NM_011058 | Pdgfra | platelet derived growth factor receptor | |||||||
| NM_008809 | Pdgfrb | platelet derived growth factor receptor | |||||||
| NM_001077495 | Pik3r1 | phosphatidylinositol 3-kinase | |||||||
| NM_013829 | Plcb4 | phospholipase C | |||||||
| NM_019588 | Plce1 | phospholipase C | |||||||
| ENSMUST27997 | Rgs5 | regulator of G-protein signaling 5 | |||||||
| NM_023868 | Ryr2 | ryanodine receptor 2 | |||||||
| NM_177652 | Ryr3 | ryanodine receptor 3 | |||||||
| ENSMUST57311 | Sfn | stratifin | |||||||
| NM_011406 | Slc8a1 | solute carrier family 8 (sodium/calcium exchanger) | |||||||
| NM_001081011 | Srgap2 | SLIT-ROBO Rho GTPase activating protein 2 | |||||||
| NM_018753 | Ywhab | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | |||||||
| Under-expressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| ENSMUST114913 | Adcy5 | adenylate cyclase 5 | |||||||
| ENSMUST109749 | Akt1 | thymoma viral proto-oncogene 1 | |||||||
| NM_009721 | Atp1b1 | ATPase | |||||||
| NM_213616 | Atp2b4 | ATPase | |||||||
| NM_021415 | Cacna1h | calcium channel | |||||||
| ENSMUST32409 | Camk1 | calcium/calmodulin-dependent protein kinase I | |||||||
| ENSMUST29454 | Casq2 | calsequestrin 2 | |||||||
| ENSMUST87104 | Cdkl5 | cyclin-dependent kinase-like 5 | |||||||
| NM_203491 | Chrm2 | cholinergic receptor | |||||||
| ENSMUST22718 | Ednrb | endothelin receptor type B | |||||||
| NM_001003817 | Erbb2 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | |||||||
| ENSMUST81091 | Erbb4 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 | |||||||
| ENSMUST59193 | F2r | coagulation factor II (thrombin) receptor | |||||||
| ENSMUST106091 | Gjb3 | gap junction membrane channel protein beta 3 | |||||||
| NM_010305 | Gnai1 | guanine nucleotide binding protein | |||||||
| NM_008139 | Gnaq | guanine nucleotide binding protein | |||||||
| NM_010312 | Gnb2 | guanine nucleotide binding protein | |||||||
| NM_138719 | Gnb5 | guanine nucleotide binding protein | |||||||
| NM_025331 | Gng11 | guanine nucleotide binding protein | |||||||
| NM_010318 | Gng5 | guanine nucleotide binding protein | |||||||
| NM_010568 | Insr | insulin receptor | |||||||
| NM_001081175 | Itpkb | inositol 1 | |||||||
| NM_019923 | Itpr2 | inositol 1 | |||||||
| NM_010605 | Kcnj5 | potassium inwardly-rectifying channel | |||||||
| ENSMUST24916 | Lhcgr | luteinizing hormone/choriogonadotropin receptor | |||||||
| BC040217 | Mef2d | myocyte enhancer factor 2D | |||||||
| ENSMUST35800 | Nfatc1 | nuclear factor of activated T-cells | |||||||
| ENSMUST33086 | Phkg2 | phosphorylase kinase | |||||||
| NM_013829 | Plcb4 | phospholipase C | |||||||
| NM_019676 | Plcd1 | phospholipase C | |||||||
| NM_019588 | Plce1 | phospholipase C | |||||||
| NM_024459 | Ppp3r1 | protein phosphatase 3 | |||||||
| NM_008854 | Prkaca | protein kinase | |||||||
| NM_008855 | Prkcb1 | protein kinase C | |||||||
| ENSMUST97275 | Prkce | protein kinase C | |||||||
| NM_008856 | Prkch | protein kinase C | |||||||
| ENSMUST27603 | Rgs18 | regulator of G-protein signaling 18 | |||||||
| NM_019769 | Rik | RIKEN cDNA 1500003O03 gene | |||||||
| NM_023868 | Ryr2 | ryanodine receptor 2 | |||||||
| NM_011406 | Slc8a1 | solute carrier family 8 (sodium/calcium exchanger) | |||||||
| ENSMUST9036 | Vdac3 | voltage-dependent anion channel 3 | |||||||
| NM_018753 | Ywhab | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | |||||||
| NM_011739 | Ywhaq | tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein | |||||||
Table 4. Ion channel pathway.
A) Over and B) under expressed genes involved in the ion channel signaling pathway. Each column as described in Table 3.
| Ion Channel Pathway | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Over-Expressed | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| NM_009781 | Cacna1c | calcium channel | |||||||
| NM_009783 | Cacna1g | calcium channel | |||||||
| NM_009784 | Cacna2d1 | calcium channel | |||||||
| NM_007582 | Cacng1 | calcium channel | |||||||
| BC051033 | Fxyd3 | FXYD domain-containing ion transport regulator 3 | |||||||
| NM_146017 | Gabrp | gamma-aminobutyric acid receptor | |||||||
| ENSMUST76349 | Gria3 | glutamate receptor | |||||||
| NM_008170 | Grin2a | glutamate receptor | |||||||
| ENSMUST72602 | Hvcn1 | hydrogen voltage-gated channel 1 | |||||||
| NM_010585 | Itpr1 | inositol 1 | |||||||
| NM_019923 | Itpr2 | inositol 1 | |||||||
| NM_010597 | Kcnab1 | potassium voltage-gated channel | |||||||
| NM_134110 | Kcne2 | potassium voltage-gated channel | |||||||
| NM_020574 | Kcne3 | potassium voltage-gated channel | |||||||
| NM_010603 | Kcnj12 | potassium inwardly-rectifying channel | |||||||
| ENSMUST46765 | Kcnk1 | potassium channel | |||||||
| NM_008431 | Kcnk4 | potassium channel | |||||||
| NM_023872 | Kcnq5 | potassium voltage-gated channel | |||||||
| NM_173417 | Kcns3 | potassium voltage-gated channel | |||||||
| ENSMUST39450 | Mcoln3 | mucolipin 3 | |||||||
| NM_023868 | Ryr2 | ryanodine receptor 2 | |||||||
| NM_001099298 | Scn2a1 | sodium channel | |||||||
| NM_153417 | Trpm6 | transient receptor potential cation channel | |||||||
| ENSMUST103224 | Trpm7 | transient receptor potential cation channel | |||||||
| NM_022413 | Trpv6 | transient receptor potential cation channel | |||||||
| Under-expressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| ENSMUST49346 | Accn3 | amiloride-sensitive cation channel 3 | |||||||
| NM_021415 | Cacna1h | calcium channel | |||||||
| NM_009784 | Cacna2d1 | calcium channel | |||||||
| NM_023116 | Cacnb2 | calcium channel | |||||||
| NM_001037099 | Cacnb4 | calcium channel | |||||||
| ENSMUST45706 | Cftr | cystic fibrosis transmembrane conductance regulator homolog | |||||||
| ENSMUST71697 | Fxyd1 | FXYD domain-containing ion transport regulator 1 | |||||||
| BC031112 | Fxyd5 | FXYD domain-containing ion transport regulator 5 | |||||||
| NM_008070 | Gabrb2 | gamma-aminobutyric acid (GABA-A) receptor | |||||||
| NM_146017 | Gabrp | gamma-aminobutyric acid (GABA-A) receptor | |||||||
| NM_019923 | Itpr2 | inositol 1 | |||||||
| NM_145983 | Kcna5 | potassium voltage-gated channel | |||||||
| NM_008424 | Kcne1 | potassium voltage-gated channel | |||||||
| NM_020574 | Kcne3 | potassium voltage-gated channel | |||||||
| NM_010600 | Kcnh1 | potassium voltage-gated channel | |||||||
| NM_013569 | Kcnh2 | potassium voltage-gated channel | |||||||
| ENSMUST39366 | Kcnh8 | potassium voltage-gated channel | |||||||
| NM_145963 | Kcnj14 | potassium inwardly-rectifying channel | |||||||
| NM_010604 | Kcnj16 | potassium inwardly-rectifying channel | |||||||
| ENSMUST42970 | Kcnj2 | potassium inwardly-rectifying channel | |||||||
| NM_010605 | Kcnj5 | potassium inwardly-rectifying channel | |||||||
| NM_001033876 | Kcnk9 | potassium channel | |||||||
| NM_010610 | Kcnma1 | potassium large conductance calcium-activated channel | |||||||
| NM_031169 | Kcnmb1 | potassium large conductance calcium-activated channel | |||||||
| NM_080465 | Kcnn2 | potassium intermediate/small conductance calcium-activated channel | |||||||
| NM_023872 | Kcnq5 | potassium voltage-gated channel | |||||||
| ENSMUST22272 | Kctd6 | potassium channel tetramerisation domain containing 6 | |||||||
| ENSMUST67951 | Kctd9 | potassium channel tetramerisation domain containing 9 | |||||||
| ENSMUST23509 | Klhl24 | kelch-like 24 (Drosophila | |||||||
| ENSMUST39450 | Mcoln3 | mucolipin 3 | |||||||
| NM_177755 | Rik | RIKEN cDNA 8230402K04 gene | |||||||
| NM_023868 | Ryr2 | ryanodine receptor 2 | |||||||
| BC039140 | Scn1b | sodium channel | |||||||
| NM_001099298 | Scn2a1 | sodium channel | |||||||
| NM_018732 | Scn3a | sodium channel | |||||||
| NM_013838 | Trpc6 | transient receptor potential cation channel | |||||||
| ENSMUST103224 | Trpm7 | transient receptor potential cation channel | |||||||
| ENSMUST9036 | Vdac3 | voltage-dependent anion channel 3 | |||||||
Table 5. Cell cycle pathway.
A) Over and B) under expressed genes involved in the cell cycle pathway. Each column as described in Table 3.
| Cell Cycle Pathway | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Over-Expressed | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| BC028526 | Anapc13 | anaphase promoting complex subunit 13 | |||||||
| ENSMUST79362 | Apc | adenomatosis polyposis coli | |||||||
| ENSMUST37440 | Atm | ataxia telangiectasia mutated homolog (human) | |||||||
| ENSMUST86248 | Aurkc | aurora kinase C | |||||||
| ENSMUST74077 | Bmp4 | bone morphogenetic protein 4 | |||||||
| NM_007561 | Bmpr2 | bone morphogenic protein receptor | |||||||
| ENSMUST107228 | Brca1 | breast cancer 1 | |||||||
| ENSMUST44620 | Brca2 | breast cancer 2 | |||||||
| ENSMUST93517 | Casp3 | caspase 3 | |||||||
| NM_001081062 | Ccno | cyclin O | |||||||
| BC005775 | Cdc26 | cell division cycle 26 | |||||||
| ENSMUST42410 | Cdk6 | cyclin-dependent kinase 6 | |||||||
| ENSMUST22009 | Cetn3 | centrin 3 | |||||||
| ENSMUST75853 | Cks2 | CDC28 protein kinase regulatory subunit 2 | |||||||
| ENSMUST49404 | Clasp1 | CLIP associating protein 1 | |||||||
| ENSMUST35089 | Clasp2 | CLIP associating protein 2 | |||||||
| ENSMUST6293 | Crkl | v-crk sarcoma virus CT10 oncogene homolog (avian)-like | |||||||
| ENSMUST5841 | Ctcf | CCCTC-binding factor | |||||||
| ENSMUST31697 | Cul1 | cullin 1 | |||||||
| ENSMUST26475 | Ddit3 | DNA-damage inducible transcript 3 | |||||||
| AF396877 | Dst | dystonin | |||||||
| NM_013507 | Eif4g2 | eukaryotic translation initiation factor 4 | |||||||
| ENSMUST81091 | Erbb4 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian) | |||||||
| BC048734 | Fgf8 | fibroblast growth factor 8 | |||||||
| ENSMUST9777 | G0s2 | G0/G1 switch gene 2 | |||||||
| ENSMUST43098 | Gadd45a | growth arrest and DNA-damage-inducible 45 alpha | |||||||
| ENSMUST32129 | Gkn1 | gastrokine 1 | |||||||
| ENSMUST21729 | Gpr132 | G protein-coupled receptor 132 | |||||||
| ENSMUST57884 | Gps2 | G protein pathway suppressor 2 | |||||||
| ENSMUST23507 | Gsk3b | glycogen synthase kinase 3 beta | |||||||
| ENSMUST80030 | Gspt1 | G1 to S phase transition 1 | |||||||
| ENSMUST38777 | Hipk2 | homeodomain interacting protein kinase 2 | |||||||
| ENSMUST28882 | Il1a | interleukin 1 alpha | |||||||
| ENSMUST12587 | Kif11 | kinesin family member 11 | |||||||
| U67204 | Macf1 | microtubule-actin crosslinking factor 1 | |||||||
| ENSMUST4986 | Mapk13 | mitogen activated protein kinase 13 | |||||||
| NM_134092 | Mtbp | Mdm2 | |||||||
| ENSMUST52965 | Nipbl | Nipped-B homolog (Drosophila) | |||||||
| NM_010151 | Nr2f1 | nuclear receptor subfamily 2 | |||||||
| ENSMUST78259 | Nsl1 | NSL1 | |||||||
| ENSMUST25204 | Pfdn1 | prefoldin 1 | |||||||
| ENSMUST18361 | Pmp22 | peripheral myelin protein | |||||||
| ENSMUST101534 | Ptn | pleiotrophin | |||||||
| ENSMUST49009 | Rad9b | RAD9 homolog B (S. cerevisiae) | |||||||
| ENSMUST28814 | Rassf2 | Ras association (RalGDS/AF-6) domain family 2 | |||||||
| ENSMUST35842 | Rassf4 | Ras association (RalGDS/AF-6) domain family 4 | |||||||
| ENSMUST22701 | Rb1 | retinoblastoma 1 | |||||||
| ENSMUST29170 | Rbl1 | retinoblastoma-like 1 (p107) | |||||||
| ENSMUST34091 | Rbl2 | retinoblastoma-like 2 | |||||||
| ENSMUST102864 | Rel | reticuloendotheliosis oncogene | |||||||
| NM_175238 | Rif1 | Rap 1 interacting factor 1 homolog (yeast) | |||||||
| ENSMUST73926 | Rps12 | ribosomal protein S12 | |||||||
| ENSMUST27495 | Sept2 | septin 2 | |||||||
| ENSMUST23095 | Sept3 | septin 3 | |||||||
| ENSMUST30724 | Sesn2 | sestrin 2 | |||||||
| ENSMUST57311 | Sfn | stratifin | |||||||
| BC086683 | Spc24 | SPC24 | |||||||
| NM_001081008 | Taf1 | TAF1 RNA polymerase II | |||||||
| ENSMUST45288 | Tgfb2 | transforming growth factor | |||||||
| NM_172664 | Tlk1 | tousled-like kinase 1 | |||||||
| AB020317 | Trp53 | transformation related protein 53 | |||||||
| ENSMUST71648 | Vegfa | vascular endothelial growth factor A | |||||||
| Under-expressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| BC028526 | Anapc13 | anaphase promoting complex subunit 13 | |||||||
| ENSMUST25561 | Anxa1 | annexin A1 | |||||||
| NM_009686 | Apbb2 | amyloid beta (A4) precursor protein-binding | |||||||
| ENSMUST29842 | Bcl10 | B-cell leukemia/lymphoma 10 | |||||||
| AF271733 | Bin3 | bridging integrator 3 | |||||||
| ENSMUST28836 | Bmp2 | bone morphogenetic protein 2 | |||||||
| NM_007561 | Bmpr2 | bone morphogenic protein receptor | |||||||
| BC061001 | Bmyc | brain expressed myelocytomatosis oncogene | |||||||
| NM_009770 | Btg3 | B-cell translocation gene 3 | |||||||
| NM_021550 | C1galt1c1 | C1GALT1-specific chaperone 1 | |||||||
| BC052789 | Cables2 | Cdk5 and Abl enzyme substrate 2 | |||||||
| ENSMUST93517 | Casp3 | caspase 3 | |||||||
| ENSMUST48192 | Ccdc5 | coiled-coil domain containing 5 | |||||||
| BC085238 | Ccnb1 | cyclin B1 | |||||||
| BC060180 | Ccng2 | cyclin G2 | |||||||
| ENSMUST114077 | Ccnyl1 | cyclin Y-like 1 | |||||||
| BC005775 | Cdc26 | cell division cycle 26 | |||||||
| ENSMUST50148 | Cdc37l1 | cell division cycle 37 homolog (S. cerevisiae)-like 1 | |||||||
| BC003893 | Cdc5l | cell division cycle 5-like (S. pombe) | |||||||
| ENSMUST42410 | Cdk6 | cyclin-dependent kinase 6 | |||||||
| ENSMUST23829 | Cdkn1a | cyclin-dependent kinase inhibitor 1A (P21) | |||||||
| ENSMUST3115 | Cdkn1b | cyclin-dependent kinase inhibitor 1B | |||||||
| BC049694 | Cdkn3 | cyclin-dependent kinase inhibitor 3 | |||||||
| ENSMUST22009 | Cetn3 | centrin 3 | |||||||
| ENSMUST68532 | Cgrrf1 | cell growth regulator with ring finger domain 1 | |||||||
| ENSMUST29679 | Cks1b | CDC28 protein kinase 1b | |||||||
| ENSMUST75853 | Cks2 | CDC28 protein kinase regulatory subunit 2 | |||||||
| ENSMUST27050 | Cops5 | COP9 (constitutive photomorphogenic) homolog | |||||||
| ENSMUST17920 | Crk | v-crk sarcoma virus CT10 oncogene homolog (avian) | |||||||
| ENSMUST4478 | Cul3 | cullin 3 | |||||||
| NM_027545 | Cwf19l2 | CWF19-like 2 | |||||||
| ENSMUST103129 | Dsn1 | DSN1 | |||||||
| ENSMUST103145 | E2f1 | E2F transcription factor 1 | |||||||
| AY957576 | E2f8 | E2F transcription factor 8 | |||||||
| ENSMUST1757 | Eef1e1 | eukaryotic translation elongation factor 1 epsilon 1 | |||||||
| NM_001003817 | Erbb2 | v-erb-b2 erythroblastic leukemia viral oncogene homolog 2 | |||||||
| ENSMUST81091 | Erbb4 | v-erb-a erythroblastic leukemia viral oncogene homolog 4 (avian) | |||||||
| NM_007951 | Erh | enhancer of rudimentary homolog (Drosophila) | |||||||
| NM_011809 | Ets2 | E26 avian leukemia oncogene 2 | |||||||
| ENSMUST81028 | Etv6 | ets variant gene 6 (TEL oncogene) | |||||||
| NM_001033244 | Fancd2 | Fanconi anemia | |||||||
| ENSMUST19907 | Fbxo5 | F-box protein 5 | |||||||
| ENSMUST187 | Fgf6 | fibroblast growth factor 6 | |||||||
| ENSMUST62292 | Foxc1 | forkhead box C1 | |||||||
| ENSMUST15456 | Gadd45b | growth arrest and DNA-damage-inducible 45 beta | |||||||
| ENSMUST32129 | Gkn1 | gastrokine 1 | |||||||
| NM_008179 | Gspt2 | G1 to S phase transition 2 | |||||||
| ENSMUST34026 | Hpgd | hydroxyprostaglandin dehydrogenase 15 (NAD) | |||||||
| BC024581 | Hrasls3 | HRAS like suppressor 3 | |||||||
| ENSMUST65537 | Jmy | junction-mediating and regulatory protein | |||||||
| ENSMUST103032 | Llgl2 | lethal giant larvae homolog 2 (Drosophila) | |||||||
| NM_010755 | Maff | v-maf musculoaponeurotic fibrosarcoma oncogene family | |||||||
| ENSMUST25078 | Map3k8 | mitogen activated protein kinase kinase kinase 8 | |||||||
| ENSMUST88827 | Mapk12 | mitogen-activated protein kinase 12 | |||||||
| ENSMUST57669 | Mapk3 | mitogen activated protein kinase 3 | |||||||
| NM_133350 | Mapre3 | microtubule-associated protein | |||||||
| ENSMUST34303 | Mphosph6 | M phase phosphoprotein 6 | |||||||
| NM_134092 | Mtbp | Mdm2 | |||||||
| ENSMUST22971 | Myc | myelocytomatosis oncogene | |||||||
| NM_144931 | Nae1 | NEDD8 activating enzyme E1 subunit 1 | |||||||
| NM_133762 | Ncapg2 | non-SMC condensin II complex | |||||||
| ENSMUST35800 | Nfatc1 | nuclear factor of activated T-cells | |||||||
| NM_010151 | Nr2f1 | nuclear receptor subfamily 2 | |||||||
| NM_001024622 | Pcnp | PEST proteolytic signal containing nuclear protein | |||||||
| ENSMUST25204 | Pfdn1 | prefoldin 1 | |||||||
| ENSMUST36374 | Phb | prohibitin | |||||||
| ENSMUST34689 | Pin1 | protein (peptidyl-prolyl cis/trans isomerase) NIMA-interacting 1 | |||||||
| ENSMUST56370 | Pmf1 | polyamine-modulated factor 1 | |||||||
| NM_198600 | Pols | polymerase (DNA directed) sigma | |||||||
| NM_008014 | Ppm1g | protein phosphatase 1G (formerly 2C) | |||||||
| NM_024209 | Ppp6c | protein phosphatase 6 | |||||||
| ENSMUST20685 | Pttg1 | pituitary tumor-transforming 1 | |||||||
| ENSMUST22136 | Rad17 | RAD17 homolog (S. pombe) | |||||||
| ENSMUST20649 | Rad50 | RAD50 homolog (S. cerevisiae) | |||||||
| ENSMUST90678 | Rap1a | RAS-related protein-1a | |||||||
| ENSMUST22701 | Rb1 | retinoblastoma 1 | |||||||
| ENSMUST27040 | Rb1cc1 | RB1-inducible coiled-coil 1 | |||||||
| ENSMUST102598 | Rbbp4 | retinoblastoma binding protein 4 | |||||||
| ENSMUST34091 | Rbl2 | retinoblastoma-like 2 | |||||||
| BC051473 | Rbx1 | ring-box 1 | |||||||
| ENSMUST84250 | Rcc1 | regulator of chromosome condensation 1 | |||||||
| NM_007483 | Rhob | ras homolog gene family | |||||||
| NM_028228 | Rik | RIKEN cDNA 2610028A01 gene | |||||||
| ENSMUST73926 | Rps12 | ribosomal protein S12 | |||||||
| NM_009101 | Rras | Harvey rat sarcoma oncogene | |||||||
| BC010774 | S100a6 | S100 calcium binding protein A6 (calcyclin) | |||||||
| ENSMUST23457 | Senp5 | SUMO/sentrin specific peptidase 5 | |||||||
| ENSMUST30724 | Sesn2 | sestrin 2 | |||||||
| BC086683 | Spc24 | SPC24 | |||||||
| NM_001005370 | Spin2 | spindlin family | |||||||
| ENSMUST29448 | Sycp1 | synaptonemal complex protein 1 | |||||||
| ENSMUST11258 | Tbrg1 | transforming growth factor beta regulated gene 1 | |||||||
| ENSMUST2678 | Tgfb1 | transforming growth factor | |||||||
| ENSMUST39562 | Trim13 | tripartite motif protein 13 | |||||||
| NM_021884 | Tsg101 | tumor susceptibility gene 101 | |||||||
| ENSMUST25914 | Vegfb | vascular endothelial growth factor B | |||||||
| ENSMUST21985 | Zfp369 | zinc finger protein 369 | |||||||
Table 6. Apoptosis pathway.
A) Over and B) under expressed genes involved in the apoptosis pathway. Each column as described in Table 3.
| Apoptosis Pathway | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Over-expressed | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| ENSMUST103020 | Aatk | apoptosis-associated tyrosine kinase | |||||||
| NM 007536 | Bcl2a1d | B-cell leukemia/lymphoma 2 related protein A1d | |||||||
| ENSMUST115094 | Birc4 | baculoviral IAP repeat-containing 4 | |||||||
| ENSMUST107228 | Brca1 | breast cancer 1 | |||||||
| ENSMUST31895 | Casp2 | caspase 2 | |||||||
| ENSMUST93517 | Casp3 | caspase 3 | |||||||
| NM 001042605 | Cd74 | CD74 antigen (invariant polypeptide of major histocompatibility complex | |||||||
| ENSMUST46506 | Clcf1 | cardiotrophin-like cytokine factor 1 | |||||||
| ENSMUST53594 | Cradd | CASP2 and RIPK1 domain containing adaptor with death domain | |||||||
| ENSMUST31697 | Cul1 | cullin 1 | |||||||
| BC092213 | Cycs | cytochrome c | |||||||
| ENSMUST26475 | Ddit3 | DNA-damage inducible transcript 3 | |||||||
| ENSMUST37907 | Ddx58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | |||||||
| ENSMUST84488 | Dock1 | dedicator of cyto-kinesis 1 | |||||||
| ENSMUST39516 | Egln3 | EGL nine homolog 3 (C. elegans) | |||||||
| ENSMUST27066 | Eya1 | eyes absent 1 homolog (Drosophila) | |||||||
| ENSMUST25691 | Fas | Fas (TNF receptor superfamily member 6) | |||||||
| NM 010185 | Fcer1g | Fc receptor | |||||||
| BC048734 | Fgf8 | fibroblast growth factor 8 | |||||||
| ENSMUST57884 | Gps2 | G protein pathway suppressor 2 | |||||||
| ENSMUST23507 | Gsk3b | glycogen synthase kinase 3 beta | |||||||
| ENSMUST30683 | Hgf | hepatocyte growth factor | |||||||
| ENSMUST38777 | Hipk2 | homeodomain interacting protein kinase 2 | |||||||
| NM 010478 | Hspa1b | heat shock protein 1B | |||||||
| NM 010414 | Htt | huntingtin | |||||||
| ENSMUST20702 | Igfbp3 | insulin-like growth factor binding protein 3 | |||||||
| ENSMUST102786 | Itgb3bp | integrin beta 3 binding protein (beta3-endonexin) | |||||||
| ENSMUST49248 | Malt1 | mucosa associated lymphoid tissue lymphoma translocation gene 1 | |||||||
| ENSMUST95806 | Map3k5 | mitogen activated protein kinase kinase kinase 5 | |||||||
| ENSMUST67429 | Mdm4 | transformed mouse 3T3 cell double minute 4 | |||||||
| AK018196 | Mitf | microphthalmia-associated transcription factor | |||||||
| ENSMUST28288 | Notch1 | Notch gene homolog 1 (Drosophila) | |||||||
| ENSMUST30154 | Nudt2 | nudix (nucleoside diphosphate linked moiety X)-type motif 2 | |||||||
| ENSMUST51209 | Peg3 | paternally expressed 3 | |||||||
| ENSMUST32573 | Pglyrp1 | peptidoglycan recognition protein 1 | |||||||
| NM 001077495 | Pik3r1 | phosphatidylinositol 3-kinase | |||||||
| NM 011159 | Prkdc | protein kinase | |||||||
| AK083198 | Prlr | prolactin receptor | |||||||
| ENSMUST59507 | Purb | purine rich element binding protein B | |||||||
| ENSMUST6851 | Qrich1 | glutamine-rich 1 | |||||||
| ENSMUST22034 | Rasa1 | RAS p21 protein activator 1 | |||||||
| NM 011279 | Rnf7 | ring finger protein 7 | |||||||
| ENSMUST115866 | Rock1 | Rho-associated coiled-coil containing protein kinase 1 | |||||||
| ENSMUST102843 | Rtn4 | reticulon 4 | |||||||
| ENSMUST101118 | Rybp | RING1 and YY1 binding protein | |||||||
| NM 001099298 | Scn2a1 | sodium channel | |||||||
| ENSMUST21728 | Siva1 | SIVA1 | |||||||
| NM 011434 | Sod1 | superoxide dismutase 1 | |||||||
| ENSMUST49931 | Spn | sialophorin | |||||||
| ENSMUST112747 | Spp1 | secreted phosphoprotein 1 | |||||||
| XM 915205 | Syngap1 | synaptic Ras GTPase activating protein 1 homolog | |||||||
| ENSMUST45288 | Tgfb2 | transforming growth factor | |||||||
| ENSMUST95753 | Tia1 | cytotoxic granule-associated RNA binding protein 1 | |||||||
| NM 178931 | Tnfrsf14 | tumor necrosis factor receptor superfamily | |||||||
| NM 026654 | Toe1 | target of EGR1 | |||||||
| AF357400 | Tpt1 | tumor protein | |||||||
| ENSMUST40312 | Trib3 | tribbles homolog 3 (Drosophila) | |||||||
| AB020317 | Trp53 | transformation related protein 53 | |||||||
| ENSMUST40231 | Trp63 | transformation related protein 63 | |||||||
| ENSMUST106236 | Unc5c | unc-5 homolog C (C. elegans) | |||||||
| ENSMUST71648 | Vegfa | vascular endothelial growth factor A | |||||||
| ENSMUST21937 | Zfp346 | zinc finger protein 346 | |||||||
| Under-expressed | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |||
| Sequence ID | Gene ID | Description | 0 CTL v 0 TCE | 2 CTL v 2 TCE | 8 CTL v 8 TCE | 2 TCE v 0 TCE | 2 TCE v 8 TCE | 2 CTL v 0 CTL | 2 CTL v 8 CTL |
| NM 001033369 | Acvr1c | activin A receptor | |||||||
| ENSMUST109749 | Akt1 | thymoma viral proto-oncogene 1 | |||||||
| NM 013467 | Aldh1a1 | aldehyde dehydrogenase family 1 | |||||||
| ENSMUST329 | Alox12 | arachidonate 12-lipoxygenase | |||||||
| NM 009686 | Apbb2 | amyloid beta (A4) precursor protein-binding | |||||||
| ENSMUST6828 | Aplp1 | amyloid beta (A4) precursor-like protein 1 | |||||||
| ENSMUST3066 | Apoe | apolipoprotein E | |||||||
| ENSMUST96119 | Asah2 | N-acylsphingosine amidohydrolase 2 | |||||||
| NM 022305 | B4galt1 | UDP-Gal:betaGlcNAc beta 1 | |||||||
| ENSMUST108089 | Bag1 | Bcl2-associated athanogene 1 | |||||||
| ENSMUST54636 | Bag5 | BCL2-associated athanogene 5 | |||||||
| ENSMUST33093 | Bax | Bcl2-associated X protein | |||||||
| ENSMUST2091 | Bcap31 | B-cell receptor-associated protein 31 | |||||||
| ENSMUST29842 | Bcl10 | B-cell leukemia/lymphoma 10 | |||||||
| ENSMUST66460 | Bcl2a1c | B-cell leukemia/lymphoma 2 related protein A1c | |||||||
| ENSMUST22806 | Bcl2l2 | Bcl2-like 2 | |||||||
| ENSMUST115094 | Birc4 | baculoviral IAP repeat-containing 4 | |||||||
| NM 172149 | Bnip1 | BCL2/adenovirus E1B interacting protein 1 | |||||||
| NM 009760 | Bnip3 | BCL2/adenovirus E1B interacting protein 1 | |||||||
| ENSMUST27499 | Bok | Bcl-2-related ovarian killer protein | |||||||
| NM 175362 | Card11 | caspase recruitment domain family | |||||||
| ENSMUST93517 | Casp3 | caspase 3 | |||||||
| ENSMUST29626 | Casp6 | caspase 6 | |||||||
| ENSMUST26062 | Casp7 | caspase 7 | |||||||
| BC055070 | Ccl2 | chemokine (C-C motif) ligand 2 | |||||||
| NM 001042605 | Cd74 | CD74 antigen (invariant polypeptide of major histocompatibility complex | |||||||
| ENSMUST23829 | Cdkn1a | cyclin-dependent kinase inhibitor 1A (P21) | |||||||
| NM 009883 | Cebpb | CCAAT/enhancer binding protein | |||||||
| ENSMUST34233 | Ciapin1 | cytokine induced apoptosis inhibitor 1 | |||||||
| NM 007702 | Cidea | cell death-inducing DNA fragmentation factor | |||||||
| ENSMUST59091 | Clca1 | chloride channel calcium activated 1 | |||||||
| NM 025680 | Ctnnbl1 | catenin | |||||||
| BC092213 | Cycs | cytochrome c | |||||||
| ENSMUST111530 | Dad1 | defender against cell death 1 | |||||||
| NM 022994 | Dap3 | death associated protein 3 | |||||||
| ENSMUST37907 | Ddx58 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | |||||||
| BC024780 | Diablo | diablo homolog (Drosophila) | |||||||
| ENSMUST103145 | E2f1 | E2F transcription factor 1 | |||||||
| ENSMUST55990 | Eef1a2 | eukaryotic translation elongation factor 1 alpha 2 | |||||||
| ENSMUST1757 | Eef1e1 | eukaryotic translation elongation factor 1 epsilon 1 | |||||||
| ENSMUST39516 | Egln3 | EGL nine homolog 3 (C. elegans) | |||||||
| ENSMUST27066 | Eya1 | eyes absent 1 homolog (Drosophila) | |||||||
| ENSMUST33394 | Fadd | Fas (TNFRSF6)-associated via death domain | |||||||
| ENSMUST35038 | Faim | Fas apoptotic inhibitory molecule | |||||||
| ENSMUST25691 | Fas | Fas (TNF receptor superfamily member 6) | |||||||
| ENSMUST73152 | Fastkd1 | FAST kinase domains 1 | |||||||
| ENSMUST27103 | Fastkd2 | FAST kinase domains 2 | |||||||
| ENSMUST19198 | Fis1 | fission 1 (mitochondrial outer membrane) homolog (yeast) | |||||||
| ENSMUST75491 | Fkbp8 | FK506 binding protein 8 | |||||||
| ENSMUST15456 | Gadd45b | growth arrest and DNA-damage-inducible 45 beta | |||||||
| NM 010295 | Gclc | glutamate-cysteine ligase | |||||||
| NM 008129 | Gclm | glutamate-cysteine ligase | |||||||
| AK005055 | Glo1 | glyoxalase 1 | |||||||
| ENSMUST82429 | Gpx1 | glutathione peroxidase 1 | |||||||
| XM_001478151 | Hbxip | hepatitis B virus × interacting protein | |||||||
| ENSMUST28600 | Hipk3 | homeodomain interacting protein kinase 3 | |||||||
| ENSMUST26572 | Hras1 | Harvey rat sarcoma virus oncogene 1 | |||||||
| NM 010478 | Hspa1b | heat shock protein 1B | |||||||
| ENSMUST89645 | Htra2 | HtrA serine peptidase 2 | |||||||
| ENSMUST20702 | Igfbp3 | insulin-like growth factor binding protein 3 | |||||||
| ENSMUST889 | Il4 | interleukin 4 | |||||||
| ENSMUST102786 | Itgb3bp | integrin beta 3 binding protein (beta3-endonexin) | |||||||
| ENSMUST47616 | Jmjd6 | jumonji domain containing 6 | |||||||
| ENSMUST65537 | Jmy | junction-mediating and regulatory protein | |||||||
| NM 023788 | Mageh1 | melanoma antigen | |||||||
| AK018196 | Mitf | microphthalmia-associated transcription factor | |||||||
| ENSMUST22971 | Myc | myelocytomatosis oncogene | |||||||
| NM 144931 | Nae1 | NEDD8 activating enzyme E1 subunit 1 | |||||||
| NM 023312 | Ndufa13 | NADH dehydrogenase (ubiquinone) 1 alpha subcomplex | |||||||
| ENSMUST5647 | Ndufs3 | NADH dehydrogenase (ubiquinone) Fe-S protein 3 | |||||||
| NM 010910 | Nefl | neurofilament | |||||||
| ENSMUST53540 | Ngfrap1 | nerve growth factor receptor (TNFRSF16) associated protein 1 | |||||||
| ENSMUST28288 | Notch1 | Notch gene homolog 1 (Drosophila) | |||||||
| ENSMUST23779 | Nr4a1 | nuclear receptor subfamily 4 | |||||||
| ENSMUST21284 | Ntn1 | netrin 1 | |||||||
| NM 028778 | Nuak2 | NUAK family | |||||||
| ENSMUST30154 | Nudt2 | nudix (nucleoside diphosphate linked moiety X)-type motif 2 | |||||||
| ENSMUST57195 | Nup62 | nucleoporin 62 | |||||||
| NM 001081170 | Pacs2 | phosphofurin acidic cluster sorting protein 2 | |||||||
| ENSMUST29424 | Pdcd10 | programmed cell death 10 | |||||||
| ENSMUST32577 | Pdcd5 | programmed cell death 5 | |||||||
| ENSMUST22060 | Pdcd6 | programmed cell death 6 | |||||||
| ENSMUST27247 | Pdcl3 | phosducin-like 3 | |||||||
| NM 022032 | Perp | PERP | |||||||
| NM 009344 | Phlda1 | pleckstrin homology-like domain | |||||||
| ENSMUST34296 | Pik3r2 | phosphatidylinositol 3-kinase | |||||||
| ENSMUST98513 | Plekhf1 | pleckstrin homology domain containing | |||||||
| ENSMUST14578 | Plg | plasminogen | |||||||
| ENSMUST33938 | Polb | polymerase (DNA directed) | |||||||
| ENSMUST27373 | Ppm1f | protein phosphatase 1F (PP2C domain containing) | |||||||
| NM 001010836 | Ppp1r13l | protein phosphatase 1 | |||||||
| BC002034 | Prdx2 | peroxiredoxin 2 | |||||||
| NM 011159 | Prkdc | protein kinase | |||||||
| NM 011871 | Prkra | protein kinase | |||||||
| ENSMUST59507 | Purb | purine rich element binding protein B | |||||||
| ENSMUST27040 | Rb1cc1 | RB1-inducible coiled-coil 1 | |||||||
| NM 007483 | Rhob | ras homolog gene family | |||||||
| NM 026443 | Rik | RIKEN cDNA 1700020C11 gene | |||||||
| BC051541 | Rik | RIKEN cDNA 2600009E05 gene | |||||||
| BC025611 | Ripk2 | receptor (TNFRSF)-interacting serine-threonine kinase 2 | |||||||
| NM 019955 | Ripk3 | receptor-interacting serine-threonine kinase 3 | |||||||
| AK220529 | Rnf130 | ring finger protein 130 | |||||||
| NM 011279 | Rnf7 | ring finger protein 7 | |||||||
| ENSMUST30399 | Rragc | Ras-related GTP binding C | |||||||
| ENSMUST101118 | Rybp | RING1 and YY1 binding protein | |||||||
| ENSMUST78481 | Scin | scinderin | |||||||
| NM 001099298 | Scn2a1 | sodium channel | |||||||
| ENSMUST21728 | Siva1 | SIVA1 | |||||||
| NM 026404 | Slc35a4 | solute carrier family 35 | |||||||
| ENSMUST25997 | Smndc1 | survival motor neuron domain containing 1 | |||||||
| ENSMUST56150 | Snrk | SNF related kinase | |||||||
| ENSMUST102774 | Sqstm1 | sequestosome 1 | |||||||
| ENSMUST27263 | Stk17b | serine/threonine kinase 17b (apoptosis-inducing) | |||||||
| ENSMUST88585 | Sulf1 | sulfatase 1 | |||||||
| XM 915205 | Syngap 1 | synaptic Ras GTPase activating protein 1 homolog (rat) | |||||||
| ENSMUST18407 | Tbx5 | T-box 5 | |||||||
| NM 025780 | Thap2 | THAP domain containing | |||||||
| NM 153552 | Thoc1 | THO complex 1 | |||||||
| ENSMUST102793 | Tm2d1 | TM2 domain containing 1 | |||||||
| ENSMUST19997 | Tnfaip3 | tumor necrosis factor | |||||||
| NM 134131 | Tnfaip8 | tumor necrosis factor | |||||||
| NM 011609 | Tnfrsf1a | tumor necrosis factor receptor superfamily | |||||||
| NM 026654 | Toe1 | target of EGR1 | |||||||
| ENSMUST21471 | Txndc1 | thioredoxin domain containing 1 | |||||||
| ENSMUST50183 | Uaca | uveal autoantigen with coiled-coil domains and ankyrin repeats | |||||||
Acknowledgements
We thank the Genomics Facility Core, especially Candace Clark and Jose Munoz-Rodriguez, and the Bioinformatics Service, especially David Mount, of the Southwest Environmental Health Sciences Center and Arizona Cancer Center at the University of Arizona for carrying out the microarray hybridization and data analysis. Thanks to P.A. Thorne and David Perkins for their technical assistance.
This work was supported by NIH, SBPR Program No P42ES04940 (O.S and R.R), and by NIH, NIEHS Grant No. ES06694 (SWEHSC)
References
- Alkuraya FS, Saadi I, Lund JJ, Turbe-Doan A, Morton CC, Maas RL. SUMO1 haploinsufficiency leads to cleft lip and palate. Science. 2006;313(5794):1751. doi: 10.1126/science.1128406. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95(5):459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR- Agency for Toxic Substances and Disease Registry . 2007 CERCLA Priority List of Hazardous Substances. Department of Health and Human Services; Atlanta: 2007. [Google Scholar]
- Botto LD, Mulinare J, Erickson JD. Do multivitamin or folic acid supplements reduce the risk for congenital heart defects? Evidence and gaps. Am J Med Genet A. 2003;121(2):95–101. doi: 10.1002/ajmg.a.20132. [DOI] [PubMed] [Google Scholar]
- Boyer AS, Finch WT, Runyan RB. Trichloroethylene inhibits development of embryonic heart valve precursors in vitro. Toxicol Sci. 2000;53(1):109–117. doi: 10.1093/toxsci/53.1.109. [DOI] [PubMed] [Google Scholar]
- Burgoon JM, Selhub J, Nadeau M, Sadler TW. Investigation of the effects of folate deficiency on embryonic development through the establishment of a folate deficient mouse model. Teratology. 2002;65(5):219–227. doi: 10.1002/tera.10040. [DOI] [PubMed] [Google Scholar]
- Caldwell PT, Thorne PA, Johnson PD, Boitano S, Runyan RB, Selmin O. Trichloroethylene disrupts cardiac gene expression and calcium homeostasis in rat myocytes. Toxicol Sci. 2008;104(1):135–143. doi: 10.1093/toxsci/kfn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark KL, Yutzey KE, Benson DW. Transcription factors and congenital heart defects. Annu Rev Physiol. 2006;68:97–121. doi: 10.1146/annurev.physiol.68.040104.113828. [DOI] [PubMed] [Google Scholar]
- Cripps RM, Olson EN. Control of cardiac development by an evolutionarily conserved transcriptional network. Dev Biol. 2002;246(1):14–28. doi: 10.1006/dbio.2002.0666. [DOI] [PubMed] [Google Scholar]
- Dawson BV, Johnson PD, Goldberg SJ, Ulreich JB. Cardiac teratogenesis of halogenated hydrocarbon-contaminated drinking water. J Am Coll Cardiol. 1993;21(6):1466–1472. doi: 10.1016/0735-1097(93)90325-u. [DOI] [PubMed] [Google Scholar]
- Dow JL, Green T. Trichloroethylene induced vitamin B(12) and folate deficiency leads to increased formic acid excretion in the rat. Toxicology. 2000;146(2-3):123–136. doi: 10.1016/s0300-483x(00)00156-6. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Hu N, Smith SM, Lough J. Cardiogenic effects of trichloroethylene and trichloroacetic acid following exposure during heart specification of avian development. Toxicol Sci. 2006a;94(1):153–162. doi: 10.1093/toxsci/kfl083. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough JW, Smith SM. Gastrulating chick embryo as a model for evaluating teratogenicity: a comparison of three approaches. Birth Defects Res A Clin Mol Teratol. 2006b;76(1):66–71. doi: 10.1002/bdra.20202. [DOI] [PubMed] [Google Scholar]
- Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai HJ, Hove JR, Fraser SE, Dickinson ME, Gharib M. The embryonic vertebrate heart tube is a dynamic suction pump. Science. 2006;312(5774):751–753. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- Gelineau-van Waes J, Heller S, Bauer LK, Wilberding J, Maddox JR, Aleman F, Rosenquist TH, Finnell RH. Embryonic development in the reduced folate carrier knockout mouse is modulated by maternal folate supplementation. Birth Defects Res A Clin Mol Teratol. 2008;82(7):494–507. doi: 10.1002/bdra.20453. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Dawson BV, Johnson PD, Hoyme HE, Ulreich JB. Cardiac teratogenicity of dichloroethylene in a chick model. Pediatr Res. 1992;32(1):23–26. doi: 10.1203/00006450-199207000-00005. [DOI] [PubMed] [Google Scholar]
- Goldberg SJ, Lebowitz MD, Graver EJ, Hicks S. An association of human congenital cardiac malformations and drinking water contaminants. J Am Coll Cardiol. 1990;16(1):155–164. doi: 10.1016/0735-1097(90)90473-3. [DOI] [PubMed] [Google Scholar]
- Hoffmann P, Heinroth K, Richards D, Plews P, Toraason M. Depression of calcium dynamics in cardiac myocytes--a common mechanism of halogenated hydrocarbon anesthetics and solvents. J Mol Cell Cardiol. 1994;26(5):579–589. doi: 10.1006/jmcc.1994.1070. [DOI] [PubMed] [Google Scholar]
- Johnson PD, Dawson BV, Goldberg SJ. Cardiac teratogenicity of trichloroethylene metabolites. J Am Coll Cardiol. 1998a;32(2):540–545. doi: 10.1016/s0735-1097(98)00232-0. [DOI] [PubMed] [Google Scholar]
- Johnson PD, Dawson BV, Goldberg SJ. A review: trichloroethylene metabolites: potential cardiac teratogens. Environ Health Perspect. 1998b;106(Suppl 4):995–999. doi: 10.1289/ehp.98106s4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson PD, Goldberg SJ, Mays MZ, Dawson BV. Threshold of trichloroethylene contamination in maternal drinking waters affecting fetal heart development in the rat. Environ Health Perspect. 2003;111(3):289–292. doi: 10.1289/ehp.5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joziasse IC, van de Smagt JJ, Smith K, Bakkers J, Sieswerda GJ, Mulder BJ, Doevendans PA. Genes in congenital heart disease: atrioventricular valve formation. Basic Res Cardiol. 2008;103(3):216–227. doi: 10.1007/s00395-008-0713-4. [DOI] [PubMed] [Google Scholar]
- Kaufman MH. The Atlas of the Mouse Development. Academic Press; London: 1992. [Google Scholar]
- Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44(1):10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- Kirby M. Getting to the heart of cardiac morphogenesis. Circ Res. 2001;(88):370–372. doi: 10.1161/01.res.88.4.370. [DOI] [PubMed] [Google Scholar]
- Li D, Pickell L, Liu Y, Wu Q, Cohn JS, Rozen R. Maternal methylenetetrahydrofolate reductase deficiency and low dietary folate lead to adverse reproductive outcomes and congenital heart defects in mice. Am J Clin Nutr. 2005;82(1):188–195. doi: 10.1093/ajcn.82.1.188. [DOI] [PubMed] [Google Scholar]
- Liebling M, Forouhar AS, Wolleschensky R, Zimmermann B, Ankerhold R, Fraser SE, Gharib M, Dickinson ME. Rapid three-dimensional imaging and analysis of the beating embryonic heart reveals functional changes during development. Dev Dyn. 2006;235(11):2940–2948. doi: 10.1002/dvdy.20926. [DOI] [PubMed] [Google Scholar]
- Loeber CP, Hendrix MJ, Diez De Pinos S, Goldberg SJ. Trichloroethylene: a cardiac teratogen in developing chick embryos. Pediatr Res. 1988;24(6):740–744. doi: 10.1203/00006450-198812000-00018. [DOI] [PubMed] [Google Scholar]
- Mishima N, Hoffman S, Hill EG, Krug EL. Chick embryos exposed to trichloroethylene in an ex ovo culture model show selective defects in early endocardial cushion tissue formation. Birth Defects Res A Clin Mol Teratol. 2006;76(7):517–527. doi: 10.1002/bdra.20283. [DOI] [PubMed] [Google Scholar]
- Ou J, Ou Z, McCarver DG, Hines RN, Oldham KT, Ackerman AW, Pritchard KA., Jr. Trichloroethylene decreases heat shock protein 90 interactions with endothelial nitric oxide synthase: implications for endothelial cell proliferation. Toxicol Sci. 2003;73(1):90–97. doi: 10.1093/toxsci/kfg062. [DOI] [PubMed] [Google Scholar]
- Rosenquist TH, Ratashak SA, Selhub J. Homocysteine induces congenital defects of the heart and neural tube: effect of folic acid. Proc Natl Acad Sci U S A. 1996;93(26):15227–15232. doi: 10.1073/pnas.93.26.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahali D, Mulliez N, Chatelet F, Dupuis R, Ronco P, Verroust P. Characterization of a 280-kD protein restricted to the coated pits of the renal brush border and the epithelial cells of the yolk sac. Teratogenic effect of the specific monoclonal antibodies. J Exp Med. 1988;167(1):213–218. doi: 10.1084/jem.167.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakanashi TM, Rogers JM, Fu SS, Connelly LE, Keen CL. Influence of maternal folate status on the developmental toxicity of methanol in the CD-1 mouse. Teratology. 1996;54(4):198–206. doi: 10.1002/(SICI)1096-9926(199610)54:4<198::AID-TERA4>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Selmin OI, Thorne PA, Caldwell PT, Taylor MR. Trichloroethylene and trichloroacetic acid regulate calcium signaling pathways in murine embryonal carcinoma cells p19. Cardiovasc Toxicol. 2008;8(2):47–56. doi: 10.1007/s12012-008-9014-2. [DOI] [PubMed] [Google Scholar]
- Shaw GM, Schulman J, Frisch JD, Cummins SK, Harris JA. Congenital malformations and birthweight in areas with potential environmental contamination. Arch Environ Health. 1992;47(2):147–154. doi: 10.1080/00039896.1992.10118769. [DOI] [PubMed] [Google Scholar]
- Smith BT, Mussell JC, Fleming PA, Barth JL, Spyropoulos DD, Cooley MA, Drake CJ, Argraves WS. Targeted disruption of cubilin reveals essential developmental roles in the structure and function of endoderm and in somite formation. BMC Dev Biol. 2006;6:30. doi: 10.1186/1471-213X-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60(19):5434–5440. [PubMed] [Google Scholar]
- Spiegelstein O, Gould A, Wlodarczyk B, Tsie M, Lu X, Le C, Troen A, Selhub J, Piedrahita JA, Salbaum JM, Kappen C, Melnyk S, James J, Finnell RH. Developmental consequences of in utero sodium arsenate exposure in mice with folate transport deficiencies. Toxicol Appl Pharmacol. 2005;203(1):18–26. doi: 10.1016/j.taap.2004.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg AD, DeSesso JM. Have animal data been used inappropriately to estimate risks to humans from environmental trichloroethylene? Regul Toxicol Pharmacol. 1993;18(2):137–153. doi: 10.1006/rtph.1993.1049. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. Effect of trichloroethylene and its metabolites, dichloroacetic acid and trichloroacetic acid, on the methylation and expression of c-Jun and c-Myc protooncogenes in mouse liver: prevention by methionine. Toxicol Sci. 2000a;54(2):399–407. doi: 10.1093/toxsci/54.2.399. [DOI] [PubMed] [Google Scholar]
- Tao L, Yang S, Xie M, Kramer PM, Pereira MA. Hypomethylation and overexpression of c-jun and c-myc protooncogenes and increased DNA methyltransferase activity in dichloroacetic and trichloroacetic acid-promoted mouse liver tumors. Cancer Lett. 2000b;158(2):185–193. doi: 10.1016/s0304-3835(00)00518-8. [DOI] [PubMed] [Google Scholar]
- Wagner C. Biochemical role of folate in cellular metabolism. Folate in Health and Disease. 1995:23–42. [Google Scholar]
- Waters EM, Gerstner HB, Huff JE. Trichloroethylene. I. An overview. J Toxicol Environ Health. 1977;2(3):671–707. doi: 10.1080/15287397709529469. [DOI] [PubMed] [Google Scholar]