Highlights
-
•
Structures of Prp8 and Brr2 have been determined.
-
•
Insights into the active site cavity of the spliceosome.
-
•
New evolutionary links between spliceosomes and group II introns.
-
•
Ligands of the catalytic magnesium ions have been found.
Abstract
Spliceosomes are large, dynamic ribonucleoprotein complexes that catalyse the removal of introns from messenger RNA precursors via a two-step splicing reaction. The recent crystal structure of Prp8 has revealed Reverse Transcriptase-like, Linker and Endonuclease-like domains. The intron branch-point cross-link with the Linker domain of Prp8 in active spliceosomes and together with suppressors of 5′ and 3′ splice site mutations this unambiguously locates the active site cavity. Structural and mechanistic similarities with group II self-splicing introns have encouraged the notion that the spliceosome is at heart a ribozyme, and recently the ligands for two catalytic magnesium ions were identified within U6 snRNA. They position catalytic divalent metal ions in the same way as Domain V of group II intron RNA, suggesting that the spliceosome and group II intron use the same catalytic mechanisms.
Current Opinion in Structural Biology 2014, 25:57–66
This review comes from a themed issue on Macromolecular machines
Edited by Karl-Peter Hopfner and Tom Smith
For a complete overview see the Issue and the Editorial
Available online 28th January 2014
0959-440X/$ – see front matter, © 2014 The Authors. Published by Elsevier Ltd. All rights reserved.
Introduction
The spliceosome is a large RNA-protein complex, which catalyzes the excision of non-coding introns and ligation of exons from the precursors of messenger RNAs (pre-mRNAs) via a two-step trans-esterification reaction. Proteomic studies have revealed that the spliceosome is immensely complex and highly dynamic [1]. For each intron, the spliceosome assembles de novo from its five canonical subunits: U1, U2, U4, U5 and U6 small nuclear ribonucleoprotein particles (snRNPs) and various other non-snRNP factors [2]. The assembly process begins with the recognition of the 5′ splice site (5′SS) and the branch point sequence (BP) by U1 and U2 snRNPs, respectively, and further recruitment of the U4/U6.U5 tri-snRNP leads to the formation of the pre-catalytic complex B. A major remodeling event catalyzed by the two DExD/H box helicases Prp28 and Brr2 [3] leads to formation of the catalytically competent complex B*, in which the first step of splicing occurs.
The enormous complexity and highly dynamic nature of the spliceosome present a formidable challenge for structural and mechanistic studies. Until recently high resolution structural information was mostly restricted to small proteins or single domains of spliceosomal components [4]. At the same time, electron cryo-microscopy has provided valuable insights into the global shape of the splicing machinery, but compositional and conformational heterogeneity has limited the resolution to 20–30 Å [5]. Now substantial progress has been made towards high-resolution structure determination of large multi-component spliceosomal assemblies (Table 1). Following landmark work on the U1 snRNP [6,7] and the U4 snRNP core domain [8] the structures of the two largest spliceosomal proteins, Prp8 [9••] and Brr2 [10•,11••,12••], have recently been reported, providing valuable insights into spliceosomal activation and the architecture of the active site.
Table 1.
A list of structures of spliceosomal components reported in the last two years
| Structure | Organism | MW [kDa] | Resolution | PDB code |
|---|---|---|---|---|
| U5 snRNP components | ||||
| Prp81834–2086:Aar2 | Yeast | 70 | 1.8 | 3SBT |
| Brr2402–2125 | Human | 197 | 2.7 | 4F91a |
| Prp8885–2413:Aar2 | Yeast | 218 | 2.0 | 4I43a |
| Prp81834–2392:Aar2 | Yeast | 105 | 2.1 | 4ILG |
| Brr2442–2163:Prp82148–2395 | Yeast | 224 | 3.1 | 4BGD |
| Brr2404–2125:Prp82067–2335 | Human | 228 | 3.6 | 4KIT |
| Prp81770–1990 | Human | 23 | 1.4 | 4JK7a |
| U4 snRNP | ||||
| U4 core domain | Human | 100 | 3.6 | 2Y9Aa |
| U2 snRNP components | ||||
| Prp91–389:Prp1150–266:Prp2187–237 | Yeast | 89 | 3.1 | 4DGW |
| U2/U6 snRNA hybrid | Yeast | 36 | NMR | 2LKR |
| Other factors | ||||
| Cwc2 Cwc2 SF114–132:U2AF65375–475 SF11–145:U2AF65372–475 U2AF65148–342:oligo(U) |
Yeast Yeast Human Human Human |
26 25 26 29 24 |
2.4 1.9 2.3 NMR NMR |
3TP2 3U1Ma 4FXW 2M0Ga 2YH1 |
Multiple coordinate files deposited.
Structure and function of Prp8: pseudo-enzyme conglomerate
Prp8 is the largest and most highly conserved spliceosomal protein (280 kDa, 61% identity between yeast and human). It interacts extensively with two other integral U5 snRNP components, the EF-2-like GTPase Snu114 and the DExD/H box helicase Brr2 [13,14] and cross-links with all critical sites of chemistry in the pre-mRNA substrate (5′-SS, 3′-SS, BP) as well as with U5 and U6 snRNAs [15–18]. A number of Prp8 alleles have been shown to suppress splicing defects caused by mutations in the 5′-SS, 3′-SS and BP [19–22]. These data clearly indicate that Prp8 lies at the heart of the splicing machinery.
Despite intensive study the domain architecture of Prp8 remained elusive until recently. The first structural insights came from the C-terminal domain, which revealed similarities to the Jab1/MPN fold, known from some de-ubiquitinating metallo-enzymes [23,24]. However its putative isopeptidase active site proved to be impaired. The discovery of an RNaseH-like domain in Prp8 [25–27] has attracted much attention due to its proposed involvement in Mg2+ ion coordination as part of a composite RNA-protein spliceosomal active site [28]. In canonical RNaseH, RNA backbone cleavage proceeds via a two-metal ion mechanism [29] similar to that postulated for splicing catalysis [30]. The active site of the RNaseH-like domain of Prp8 lacks two of the four negatively charged residues involved in Mg2+ coordination, and no magnesium ions were observed in the original published crystal structures [25–27]. Recently, MacMillan and co-workers reported structures of an alternative conformation of the RNaseH-like domain of Prp8, in which a distortion of the β-finger unmasked a previously inaccessible aspartate residue, allowing magnesium ion binding under crystallization conditions [31]. Mutations promoting the second step of splicing stabilise this new (“open”) conformation. Nevertheless, as yet there is no experimental evidence to support a role for this magnesium ion at the spliceosomal active site [32].
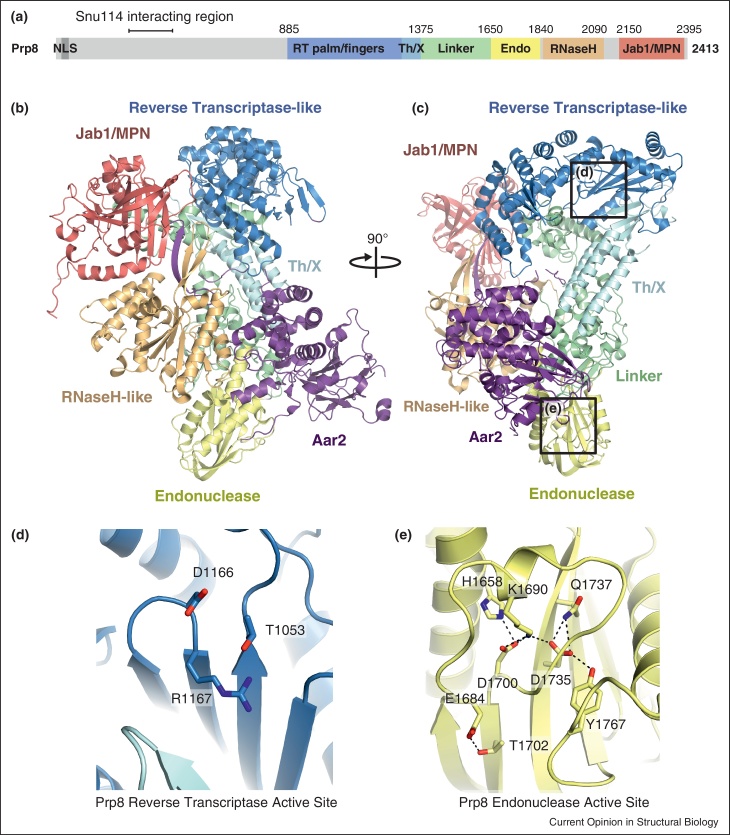
The structure of a large fragment of Prp8 (residues 885–2413) in complex with Aar2, a U5 snRNP assembly factor, revealed three novel domains in the middle part of the protein (Figure 1). Reverse Transcriptase-like (RT), Linker and type II Restriction Endonuclease-like domains interact intimately with each other to form a compact structural unit, the “large domain” [9••]. Notably, the existence of the Reverse Transcriptase-like domain in Prp8 was correctly predicted by Dlakic and Mushegian [33•]. The large domain, RNaseH-like and Jab1/MPN domains are connected by disordered linkers and represent structurally independent units (in the absence of Aar2). The active site of the Reverse Transcriptase-like domain contains only one of three aspartates involved in Mg2+ coordination at the canonical polymerase active site, and therefore it is unlikely to be involved in metal ion-dependent catalysis (Figure 1d). The endonuclease domain of Prp8 has preserved all the negatively charged residues that bind Mn2+ ions at the active site of the structurally similar PA endonuclease from influenza virus. However in Prp8 they do not bind divalent metal ions; instead they interact with a polypeptide loop lying on top of the active site [9••] (Figure 1e). This, together with the fact that these residues are not essential for yeast viability, makes it very unlikely that they function in spliceosomal catalysis.
Figure 1.
Structure of the yeast Prp8-Aar2 complex [9••]. (a) Primary domain architecture of yeast Prp8. (b) Three dimensional arrangement of the six domains of Prp8 and their interactions with Aar2. (c) Orthogonal view of the complex. (d) Impaired active site of the RT domain of Prp8. (e) the active site of Prp8 Endonuclease domain, showing conserved catalytic residues interacting with a polypeptide loop positioned on top of the active site.
The active site of the spliceosome: RNA core in a protein shell
Splicing defects caused by mutations in the 5′-SS, BP and 3′-SS can be rescued by mutations in Prp8. Over the years a number of splice site suppressor alleles of Prp8 have been isolated [19–22]. Most of these suppressor mutations map to the inner surface of the cavity formed by the RT Thumb, Linker, Endonuclease and RNaseH-like domains [9••] (Figure 2). Site-specific RNA-protein cross-linking in activated, affinity purified step 2 spliceosomes reveals contacts between BP and Prp8, which were precisely mapped to a short sequence between residues 1585-1598 (Norman and Newman, unpublished data). This region is disordered in the Prp8-Aar2 structure, however its estimated position together with suppressor mutations unambiguously locate splicing chemistry in the cavity formed by the RT Thumb, Linker, Endonuclease and RNaseH-like domains [9••] (Figure 2).
Figure 2.
The active site cavity of the spliceosome [9••]. Splice site suppressor mutations (red spheres) co-localize mostly to the inner surface of a cavity formed by RNaseH-like and Large domains of Prp8. Branch-point crosslinks (BP + 2 XL) analysed in stalled and affinity purified, yeast step 2 spliceosomes map to a short sequence (1585–1598), part of a disordered loop between residues marked with blue spheres (Norman and Newman, unpublished data). A cross-link between 5′-splice site and the RNaseH-like domain in the human system is marked with green spheres [17]. Positions of suppressor mutations together with site-specific RNA-protein cross-linking sites unambiguously locate the active site cavity of the spliceosome.
The spliceosome active site is organized around the U5 snRNA loop 1 and the U2/U6 snRNA duplex [34], which are involved in binding and correct positioning of the sites of chemistry in the pre-mRNA substrate (5′SS, 3′SS and BP). Furthermore the U6 snRNA harbours the catalytic magnesium ion binding sites [35,36,37••]. Using a combination of NMR and SAXS, Butcher and co-workers determined the structure of a protein-free yeast U2/U6 snRNA duplex [38]. The structure revealed a Y-shaped three-way junction with the U6 internal stem-loop (U6 ISL) co-axially stacked with U2/U6 helix Ib. In this arrangement the two catalytic magnesium binding sites (U6 U80 and the AGC catalytic triad), are too far apart to form a two-metal active site like that observed in mechanistically similar group II introns [39], implying that the structure would be modified in active spliceosomes.
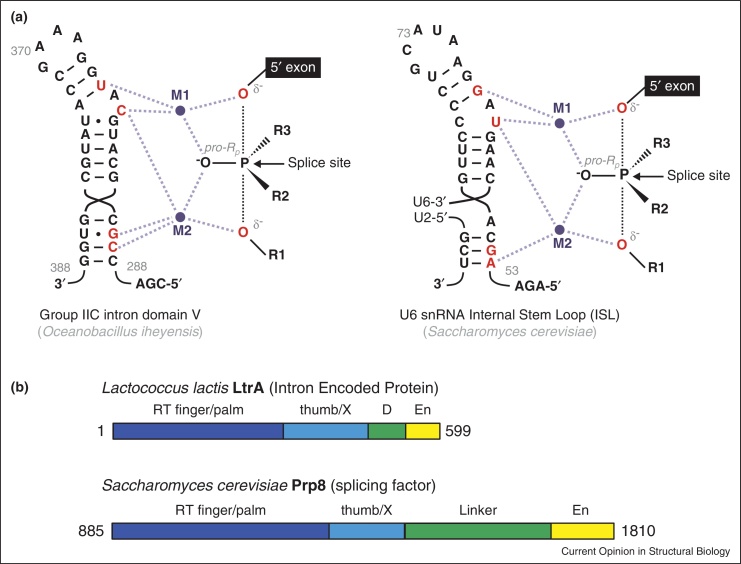
Structural and mechanistic similarities between spliceosomes and group II intron ribozymes strongly suggest they may have evolved from a common ancestor [40].The crystal structure of a self-splicing group IIC intron from Oceanobacillus iheyensis revealed an intricate architectural RNA scaffold surrounding a tightly packed catalytic core [39]. Notably, Prp8 and the group II intron scaffold have remarkably similar dimensions, so that the group II intron catalytic core can be fitted neatly into the active site cavity of the spliceosome [9••,41]. Recently Fica et al. [37••] identified ligands of two catalytic magnesium ions in the spliceosome for the first and second catalytic steps by elegant stereospecific phosphorothioate substitutions and metal rescue experiments. Strikingly, all of the catalytic metal ligands in U6 snRNA correspond to those observed to position catalytic divalent metal ions in group II intron RNA (Figure 5a; [37••,42]). This suggests that the spliceosome and the group II intron use the same catalytic mechanisms, providing further support for their common evolutionary origin.
Figure 5.
Similarities between components of group II introns and the spliceosomes. (a) Secondary structures and catalytic metal interactions in the domain V of group IIC intron [42] and spliceosomal U6 snRNA Internal Stem Loop (ISL); catalytic magnesium ions M1 and M2 are coordinated by RNA nucleotides labeled in red. For the first step reaction, R1 represents 2′ hydroxyl of branch point adenosine (or equivalent water molecule in hydrolytic group IIC intron); R2, the intron; R3, the pro-Sp oxygen. For the second step exon ligation reaction, R1 represents 3′ oxygen leaving group; R2 the pro-Sp oxygen; R3 the 3′ exon. Adapted from [37••]. (b) Comparison of the domain architecture of Prp8 and Intron Encoded Protein reveals a previously unknown evolutionary link between the two systems. RT, reverse transcriptase domain; En, endonuclease domain; D, DNA binding domain.
An NTC-related protein, Cwc2 has also been shown to contact spliceosomal RNA in Bact, B* and C complexes [43]. Cwc2 is essential for the formation of catalytically active step 1 spliceosomes and it cross-links specifically to the U6-ISL and the region upstream of the U6 ACAGAGA box. It was proposed to play a role in remodelling U6 snRNA into a catalytically active conformation, by bringing distant parts of U6 snRNA into close proximity [43]. The structure of Cwc2 revealed a unique combination of a zinc finger (ZnF) and an RNA Recognition Motif (RRM) forming a compact toroidal unit [44,45]. Two subdomains with a potential for nucleic acid binding may provide a platform for multipartite RNA recognition in the spliceosome.
Brr2 structures and implications for spliceosome activation
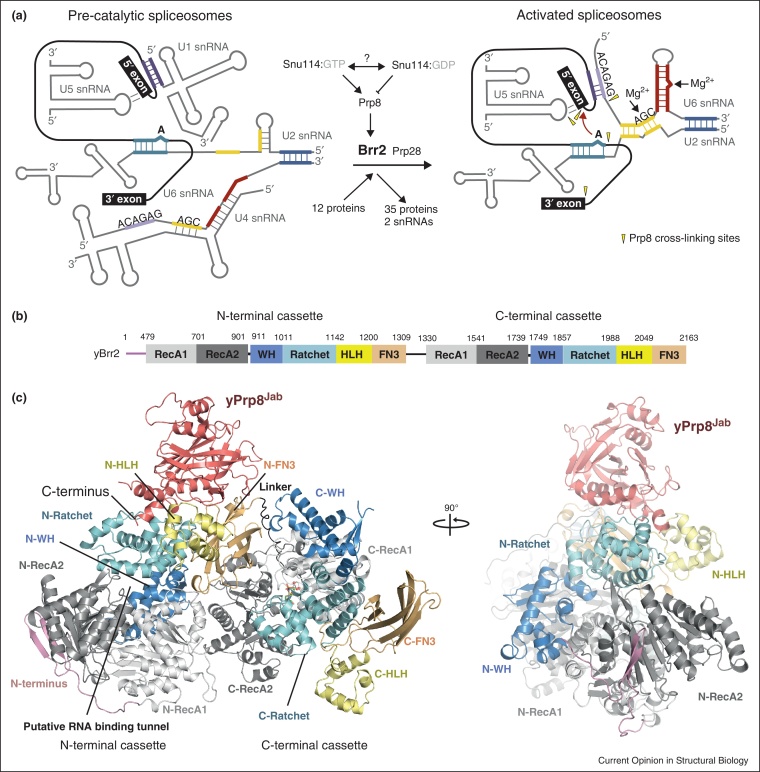
Brr2, an integral U5 snRNP component, is a large Ski2-like helicase (∼250 kDa) responsible for unwinding the U4/U6 snRNA duplex during catalytic activation of the spliceosome (Figure 3a). It consists of two consecutive helicase cassettes, but only the N-terminal cassette is functional and essential for cell-viability and U4/U6 unwinding [46]. Recently the crystal structure of a 200 kDa human Brr2 fragment [10•] revealed two Hel308-like cassettes [47] forming a compact entity with a large, functionally important inter-cassette interface (Figure 3b,c) [10•]. Previous yeast two-hybrid and pull-down studies [23,48] suggested that the C-terminal cassette might modulate N-terminal cassette activity through interactions with Snu114 and the RNaseH-like and Jab1/MPN domains of Prp8. The EF-2-like GTPase Snu114 regulates Brr2 activity in a nucleotide-dependent manner [49,50] while the Jab1/MPN domain strongly stimulates Brr2 helicase activity in vitro [51,52]. Nguyen et al. [11••] and Mozaffari-Jovin et al. [12••] recently reported the crystal structures of yeast and human Brr2, respectively, in complex with the Prp8 Jab1/MPN domain. Contrary to earlier results [23] the Jab1/MPN domain interacts exclusively with the N-terminal cassette of Brr2 (Figure 3c). Residues in Prp8 whose mutations cause type 13 retinitis pigmentosa are located at or close to the interaction surface and they are important for the modulation of yeast Brr2 unwinding in vitro [51,11••]. The main difference between the human and yeast complex structures lies in the very C-terminal region of the Jab1/MPN domain. Residues 2396–2413 (2318–2335 in human) are disordered in the yeast Jab1/MPN domain, whereas in the human structure weak, discontinuous density attributed to this region was observed in the putative RNA-binding tunnel of Brr2. This tail was proposed to prevent premature U4/U6 unwinding before activation and to inhibit Brr2 in post-activation steps of splicing [12••]. Using various truncated forms of the U4/U6 snRNA duplex for unwinding assays Mozaffari-Jovin et al. [52] concluded that Brr2 binds to the 3′ end and translocates along the U4 snRNA. Furthermore unwinding was inhibited upon addition of micromolar concentrations of the Prp8 RNaseH-like domain and the authors proposed a novel mechanism whereby Prp8 RNaseH-like domain negatively regulates the unwinding activity of Brr2.
Figure 3.
Crystal structures of the Brr2 helicase [10•,11••,12••]. (a) RNA interaction network in pre-catalytic and activated spliceosomes. Extensively based-paired U4/U6 snRNA duplex undergoes remodelling by the action of Brr2 helicase leading to the formation of the catalytically active secondary structures in the U6 snRNA. Adapted from [2]. (b) Schematic representation of the domain organizations of Brr2. Domains are colour coded: pink, N-terminal extension; light grey, RecA1; dark grey, RecA2; blue: winged-helix (WH); cyan, ratchet; yellow, helix-loop-helix (HLH) and orange, fibronectin3-like (FN3). (c) Overall structure of Brr2:Jab1/MPN complex. The structural domains are coloured as in (a) and labelled with N- and C- to indicate which helicase cassette they belong to.
Recently Hahn et al. [53] showed that the N-terminal helicase cassette of Brr2 cross-links with the 5′ and 3′ splice sites suggesting that it is closely associated with the active site cavity formed by Prp8 prior to the second step of splicing. Suppressors of U4cs-1 and brr2-1 [54] map on one face of Prp8 [9••] and this surface might interact with Brr2, although this interaction could be transient and modulated by other proteins such as Snu114. We proposed a plausible model demonstrating how Prp8 and Brr2 might collaborate to shape the active site of the spliceosome [11••]. In this model, U6 snRNA unwound from the U4 snRNA could be introduced into the active site cavity to base pair with the U2 snRNA.
U5 snRNP biogenesis: an ordered assembly pathway
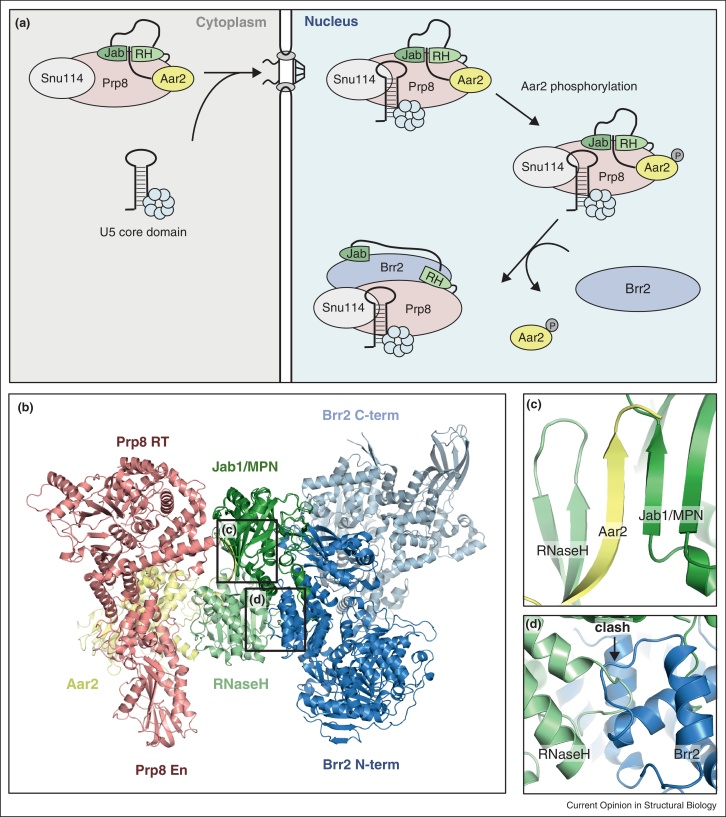
The cytoplasmic precursor of yeast U5 snRNP lacks the mature U5 snRNP component Brr2 but contains Aar2 instead. Upon nuclear import, Aar2 is replaced by Brr2 in a phosphorylation-dependent manner [55,56] (Figure 4a). The structure of the Prp8:Aar2 complex revealed a critical role for Aar2 in the organization of Prp8 domains [9••]. While the main body of Aar2 binds principally to the junction of the Linker and Endonuclease domains, the very C-terminal tail extends much further and inserts between Jab1/MPN and RNaseH-like domains forming a remarkable intermolecular beta-sheet, zipping the two domains together (Figure 4c). Biochemical experiments showed that the binding of Aar2 and Brr2 to Prp8 is mutually exclusive [55,56,11••]. Superposition of the crystal structures of yeast Brr2-Jab1/MPN [11••] and Prp8-Aar2 complexes [9••] (using the Jab1/MPN domain of Prp8, present in both structures) provided insights into the molecular basis of the competition between Aar2 and Brr2 (Figure 4b). Surprisingly, the Brr2-binding interface of the Jab1/MPN domain is still exposed in the Prp8-Aar2 complex, however the tight domain arrangement, enforced by the presence of Aar2, causes a clash between Brr2 and the RNaseH-like domain of Prp8, disabling Brr2 binding to the Jab1/MPN domain of Prp8 by a steric hindrance mechanism [11••] (Figure 4d).
Figure 4.
U5 snRNP biogenesis. (a) Schematic representation of the key stages in yeast U5 snRNP biogenesis. The precursor particle, Aar2-U5 snRNP is transported from the cytoplasm to the nucleus, where after phosphorylation Aar2 is replaced by the helicase Brr2 [55]. (b) Superposition of Jab1/MPN domain of Prp8-Aar2 and Brr2-Jab1/MPN complexes [9••,11••]. (c) Intermolecular beta sheet formed between Aar2 and Jab1/MPN and RNaseH-like domains of Prp8. (d) A steric clash between Brr2 and the RNaseH-like domain of Prp8 reveals molecular basis of the competitive binding of Brr2 and Aar2 to Prp8.
Evolutionary origin of the spliceosome
The fact that nuclear pre-mRNA splicing and group II intron self-splicing proceed via two successive trans-esterification reactions with a lariat-intron intermediate led to the far-sighted hypothesis that both systems have a common evolutionary origin [57,58]. The identification of structurally and functionally conserved counterparts of domains V, VI and the Exon Binding Sites (EBS) of group II introns in the RNA core of the spliceosome [59] together with the discovery of trans-splicing suggested that the spliceosomal snRNAs might have their origin in the domains of group II introns having become independent transcription units capable of acting in trans, in the course of evolution [60]. However, it remained unclear how ancestral spliceosomes recruited numerous proteins on the way to their present complexity. Domain IV of group II introns encodes a multi-functional protein. The thumb/X domain of this Intron Encoded Protein (IEP) facilitates self-splicing activity of group II introns whereas the Reverse Transcriptase and Endonuclease domains are involved in the intron mobility mechanism [59]. Although there is no evidence for such activities in Prp8 from modern spliceosomes the similarities in the sequence and domain architecture between IEP and Prp8 (Figure 5b) provide a new and compelling evolutionary link between group II intron self-splicing and nuclear pre-mRNA splicing [9••,33•]. This suggests that one of the most central proteins in the spliceosome may have been recruited from an ancestral IEP, that has lost its original activities and started to function as an assembly platform for snRNAs and substrate pre-mRNA in modern spliceosomes.
Conclusions and future perspectives
The last few years have seen tremendous progress in structural studies of the splicing machinery. As crystallization targets became more complex it has proved possible to reconstitute and solve the structure of entirely recombinant spliceosomal subunits (U1 snRNP [6]) as well as large, multi-domain proteins and their complexes (Prp8 [9••] and Brr2 [10•,11••,12••]). These structures have provided invaluable insights into the basic splicing mechanism and have paved the way for even larger and more challenging targets. To fully understand the biological implications of these and other emerging structures it will be necessary to combine data from different sources. A hybrid approach combining crystallography and electron microscopy may play a very important role in the future. The introduction of direct electron detectors and recent developments in image processing have, in the most favourable cases, allowed 3D reconstructions of asymmetric assemblies at close to 4 Å resolution [61]. A combination of crystallography and high-resolution electron microscopy could allow structural analysis of increasingly complex assemblies, with the ultimate goal of pseudo-atomic models of the entire spliceosome.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
Acknowledgements
We would like to acknowledge Yasushi Kondo, John Hardin, Christine Norman, Pei-Chun Lin and Israel Sanchez Fernandez for helpful comments on the manuscript. We would also like to apologize to all our colleagues whose work could not be cited here due to space limitations. This work was supported by the Medical Research Council.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Wojciech P Galej, Email: wgalej@mrc-lmb.cam.ac.uk.
Kiyoshi Nagai, Email: kn@mrc-lmb.cam.ac.uk.
References
- 1.Wahl M.C., Will C.L., Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. doi: 10.1016/j.cell.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 2.Will C.L., Lührmann R. Spliceosome structure and function. Cold Spring Harb Perspect Biol. 2011;3:1–23. doi: 10.1101/cshperspect.a003707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cordin O., Hahn D., Beggs J.D. Structure, function and regulation of spliceosomal RNA helicases. Curr Opin Cell Biol. 2012;24:431–438. doi: 10.1016/j.ceb.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Newman A.J., Nagai K. Structural studies of the spliceosome: blind men and an elephant. Curr Opin Struct Biol. 2010;20:82–89. doi: 10.1016/j.sbi.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 5.Lührmann R., Stark H. Structural mapping of spliceosomes by electron microscopy. Curr Opin Struct Biol. 2009;19:96–102. doi: 10.1016/j.sbi.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Pomeranz Krummel D.A., Oubridge C., Leung A.K.W., Li J., Nagai K. Crystal structure of human spliceosomal U1 snRNP at 5.5 A resolution. Nature. 2009;458:475–480. doi: 10.1038/nature07851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weber G., Trowitzsch S., Kastner B., Lührmann R., Wahl M.C. Functional organization of the Sm core in the crystal structure of human U1 snRNP. EMBO J. 2010;29:4172–4184. doi: 10.1038/emboj.2010.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leung A.K.W., Nagai K., Li J. Structure of the spliceosomal U4 snRNP core domain and its implication for snRNP biogenesis. Nature. 2011;473:536–539. doi: 10.1038/nature09956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9••.Galej W.P., Oubridge C., Newman A.J., Nagai K. Crystal structure of Prp8 reveals active site cavity of the spliceosome. Nature. 2013;493:638–643. doi: 10.1038/nature11843. [DOI] [PMC free article] [PubMed] [Google Scholar]; Paper describing a structure of a major part of Prp8 revealing the active site cavity of the spliceosome. It accounts for nearly two decades of biochemical and genetic experiments on Prp8 and provides new insights into the evolutionary origin of the spliceosome.
- 10•.Santos K.F., Mozaffari S., Weber G., Pena V., Lührmann R., Wahl M.C. Structural basis for functional cooperation between tandem helicase cassettes in Brr2-mediated remodeling of the spliceosome. Proc Natl Acad Sci U S A. 2012;109:17418–17423. doi: 10.1073/pnas.1208098109. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report a crystal structure of the human Brr2 helicase confirming the previously predicted tandem arrangement of helicase modules in Brr2. Accompanying biochemical data provides evidence that the inter-cassette interface is important for helicase activity in vitro.
- 11••.Nguyen T.H.D., Li J., Galej W.P., Oshikane H., Newman A.J., Nagai K. Structural basis of Brr2-Prp8 interactions and implications for U5 snRNP biogenesis and the spliceosome active site. Structure. 2013;21:910–919. doi: 10.1016/j.str.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the structure of a nearly full-length yeast Brr2 helicase in complex with Jab1/MPN domain of Prp8. The structure provides important insights into spliceosomal activation and molecular pathology of the Retinitis Pigmentosa type 13. Structure of the corresponding complex from human was reported simultaneously by Mozaffari-Jovin et al.
- 12••.Mozaffari-Jovin S., Wandersleben T., Santos K.F., Will C.L., Lührmann R., Wahl M.C. Inhibition of RNA helicase Brr2 by the C-terminal tail of the spliceosomal protein Prp8. Science. 2013;341:4–80. doi: 10.1126/science.1237515. [DOI] [PubMed] [Google Scholar]; This paper describes the structure of human Brr2 helicase in complex with Jab1/MPN domain of Prp8 providing detailed insights into Brr2–Prp8 interface. Structure of the corresponding complex from yeast was reported simultaneously by Nguyen et al.
- 13.van Nues R.W., Beggs J.D. Functional contacts with a range of splicing proteins suggest a central role for Brr2p in the dynamic control of the order of events in spliceosomes of Saccharomyces cerevisiae. Genetics. 2001;157:1451–1467. doi: 10.1093/genetics/157.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S., Rauhut R., Vornlocher H., Lu R. The network of protein–protein interactions within the human U4/U6.U5 tri-snRNP. RNA. 2006;12:1418–1430. doi: 10.1261/rna.55406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turner I., Norman C.M., Churcher M.J., Newman A.J. Dissection of Prp8 protein defines multiple interactions with crucial RNA sequences in the catalytic core of the spliceosome. RNA. 2006;12:375–386. doi: 10.1261/rna.2229706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dix I., Russell C.S., Keefe R.T., Newman A.J., Beggs J.D. Protein–RNA interactions in the U5 snRNP of Saccharomyces cerevisiae. RNA. 1998;4:1239–1250. doi: 10.1017/s1355838298981109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reyes J.L., Gustafson E.H., Luo H.R., Reyes J.L., Gustafson E.H., Luo H.R., Moore M.J., Konarska M.M. The C-terminal region of hPrp8 interacts with the conserved GU dinucleotide at the 5′ splice site. RNA. 1999;5:167–179. doi: 10.1017/s1355838299981785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vidal V.P.I., Verdone L., Mayes A.E., Beggs J.D. Characterization of U6 snRNA–protein interactions. RNA. 1999;5:1470–1481. doi: 10.1017/s1355838299991355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grainger R.J., Beggs J.D. Prp8 protein: at the heart of the spliceosome. RNA. 2005;11:533–557. doi: 10.1261/rna.2220705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Umen J.G., Guthrie C. Mutagenesis of the yeast gene PRP8 reveals domains governing the specificity and fidelity of 3′ splice site selection. Genetics. 1996;143:723–739. doi: 10.1093/genetics/143.2.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Collins C.A., Guthrie C. Allele-specific genetic interactions between Prp8 and RNA active site residues suggest a function for Prp8 at the catalytic core of the spliceosome. Genes Dev. 1999;13:1970–1982. doi: 10.1101/gad.13.15.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu L., Query C.C., Konarska M.M. Opposing classes of prp8 alleles modulate the transition between the catalytic steps of pre-mRNA splicing. Nat Struct Mol Biol. 2007;14:519–526. doi: 10.1038/nsmb1240. [DOI] [PubMed] [Google Scholar]
- 23.Pena V., Liu S., Bujnicki J.M., Lührmann R., Wahl M.C. Structure of a multipartite protein–protein interaction domain in splicing factor prp8 and its link to retinitis pigmentosa. Mol Cell. 2007;25:615–624. doi: 10.1016/j.molcel.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Shen J., Guarnieri M.T., Heroux A., Yang K., Zhao R. Crystal structure of the C-terminal domain of splicing factor Prp8 carrying retinitis pigmentosa mutants. Protein Sci. 2007;303:1024–1031. doi: 10.1110/ps.072872007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pena V., Rozov A., Fabrizio P., Lührmann R., Wahl M.C. Structure and function of an RNase H domain at the heart of the spliceosome. EMBO J. 2008;27:2929–2940. doi: 10.1038/emboj.2008.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ritchie D.B., Schellenberg M.J., Gesner E.M., Raithatha S.A., Stuart D.T., Macmillan A.M. Structural elucidation of a PRP8 core domain from the heart of the spliceosome. Nat Struct Mol Biol. 2008;15:1199–1205. doi: 10.1038/nsmb.1505. [DOI] [PubMed] [Google Scholar]
- 27.Yang K., Zhang L., Xu T., Heroux A., Zhao R. Crystal structure of the beta-finger domain of Prp8 reveals analogy to ribosomal proteins. Proc Natl Acad Sci U S A. 2008;105:13817–13822. doi: 10.1073/pnas.0805960105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abelson J. Is the spliceosome a ribonucleoprotein enzyme? Nat Struct Mol Biol. 2008;15:7–1235. doi: 10.1038/nsmb1208-1235. [DOI] [PubMed] [Google Scholar]
- 29.Nowotny M., Gaidamakov S.A., Crouch R.J., Yang W. Crystal structures of RNase H bound to an RNA/DNA hybrid: substrate specificity and metal-dependent catalysis. Cell. 2005;121:1005–1016. doi: 10.1016/j.cell.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 30.Steitz T.A., Steitz J.A. A general two-metal-ion mechanism for catalytic RNA. Proc Natl Acad Sci U S A. 1993;90:6498–6502. doi: 10.1073/pnas.90.14.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schellenberg M.J., Wu T., Ritchie D.B., Fica S., Staley J.P., Atta K.A., LaPointe P., MacMillan A.M. A conformational switch in PRP8 mediates metal ion coordination that promotes pre-mRNA exon ligation. Nat Struct Mol Biol. 2013;20:728–734. doi: 10.1038/nsmb.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abelson J. Toggling in the spliceosome. Nat Struct Mol Biol. 2013;20:645–647. doi: 10.1038/nsmb.2603. [DOI] [PubMed] [Google Scholar]
- 33•.Dlakić M., Mushegian A. Prp8, the pivotal protein of the spliceosomal catalytic center, evolved from a retroelement-encoded reverse transcriptase. RNA. 2011;17:799–808. doi: 10.1261/rna.2396011. [DOI] [PMC free article] [PubMed] [Google Scholar]; A thorough bioinformatics analysis of Prp8 sequences from different species revealing weak similarities between middle part of Prp8 and bacterial and fungal group II intron encoded proteins.
- 34.Nilsen T. RNA Structure and Function. Cold Spring Harbor Monograph Archive; 1998. RNA–RNA interactions in nuclear Pre-mRNA splicing; pp. 279–307. [Google Scholar]
- 35.Yean S.L., Wuenschell G., Termini J., Lin R.J. Metal-ion coordination by U6 small nuclear RNA contributes to catalysis in the spliceosome. Nature. 2000;408:881–884. doi: 10.1038/35048617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huppler A., Nikstad L.J., Allmann A.M., Brow D.A., Butcher S.E. Metal binding and base ionization in the U6 RNA intramolecular stem-loop structure. Nat Struct Biol. 2002;9:431–435. doi: 10.1038/nsb800. [DOI] [PubMed] [Google Scholar]
- 37••.Fica S., Tuttle N., Novak T., Li N., Lu J., Koodathingal P., Dai Q., Staley J., Piccirilli J. RNA catalyzes nuclear pre-mRNA splicing. Nature. 2013;503:229–234. doi: 10.1038/nature12734. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study employing metal rescue strategies to pinpoint residues in U6 snRNA involved in positioning of the catalytic magnesium ions at the spliceosomal active site. It provides firm evidence that the splicing chemistry is RNA-based.
- 38.Burke J.E., Sashital D.G., Zuo X., Wang Y.-X., Butcher S.E. Structure of the yeast U2/U6 snRNA complex. RNA. 2012;18:673–683. doi: 10.1261/rna.031138.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toor N., Keating K.S., Taylor S.D., Pyle A.M. Crystal structure of a self-spliced group II intron. Science. 2008;320:77–82. doi: 10.1126/science.1153803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating K.S., Toor N., Perlman P.S., Pyle A.M. A structural analysis of the group II intron active site and implications for the spliceosome. RNA. 2010;16:1–9. doi: 10.1261/rna.1791310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anokhina M., Bessonov S., Miao Z., Westhof E., Hartmuth K., Lührmann R. RNA structure analysis of human spliceosomes reveals a compact 3D arrangement of snRNAs at the catalytic core. EMBO J. 2013;32:2804–2818. doi: 10.1038/emboj.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marcia M., Pyle A.M. Visualizing group II intron catalysis through the stages of splicing. Cell. 2012;151:497–507. doi: 10.1016/j.cell.2012.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rasche N., Dybkov O., Schmitzová J., Akyildiz B., Fabrizio P., Lührmann R. Cwc2 and its human homologue RBM22 promote an active conformation of the spliceosome catalytic centre. EMBO J. 2012;31:1591–1604. doi: 10.1038/emboj.2011.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu P., Lu G., Yan C., Wang L., Li W., Yin P. Structure of the mRNA splicing complex component Cwc2: insights into RNA recognition. Biochem J. 2012;441:591–597. doi: 10.1042/BJ20111385. [DOI] [PubMed] [Google Scholar]
- 45.Schmitzová J., Rasche N., Dybkov O., Kramer K., Fabrizio P., Urlaub H., Lührmann R., Pena V. Crystal structure of Cwc2 reveals a novel architecture of a multipartite RNA-binding protein. EMBO J. 2012;31:2222–2234. doi: 10.1038/emboj.2012.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim D., Rossi J.J. The first ATPase domain of the yeast 246-kDa protein is required for in vivo unwinding of the U4/U6 duplex. RNA. 1999;5:959–971. doi: 10.1017/s135583829999012x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Büttner K., Nehring S., Hopfner K.-P. Structural basis for DNA duplex separation by a superfamily-2 helicase. Nat Struct Mol Biol. 2007;14:647–652. doi: 10.1038/nsmb1246. [DOI] [PubMed] [Google Scholar]
- 48.Zhang L., Xu T., Maeder C., Bud L.-O., Shanks J., Nix J., Guthrie C., Pleiss J.A., Zhao R. Structural evidence for consecutive Hel308-like modules in the spliceosomal ATPase Brr2. Nat Struct Mol Biol. 2009;16:731–739. doi: 10.1038/nsmb.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Small E.C., Leggett S.R., Winans A.A., Staley J.P. The EF-G-like GTPase Snu114p regulates spliceosome dynamics mediated by Brr2p, a DExD/H box ATPase. Mol Cell. 2006;23:389–399. doi: 10.1016/j.molcel.2006.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bartels C., Urlaub H., Luhrmann R., Fabrizio P. Mutagenesis suggests several roles of Snu114p in pre-mRNA splicing. J Biol Chem. 2003;278:28324–28334. doi: 10.1074/jbc.M303043200. [DOI] [PubMed] [Google Scholar]
- 51.Maeder C., Kutach A.K., Guthrie C. ATP-dependent unwinding of U4/U6 snRNAs by the Brr2 helicase requires the C terminus of Prp8. Nat Struct Mol Biol. 2009;16:42–48. doi: 10.1038/nsmb.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mozaffari-Jovin S., Santos K.F., Hsiao H.-H., Will C.L., Urlaub H., Wahl M.C., Lührmann R. The Prp8 RNase H-like domain inhibits Brr2-mediated U4/U6 snRNA unwinding by blocking Brr2 loading onto the U4 snRNA. Genes Dev. 2012;26:2422–2434. doi: 10.1101/gad.200949.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hahn D., Kudla G., Tollervey D., Beggs J.D. Brr2p-mediated conformational rearrangements in the spliceosome during activation and substrate repositioning. Genes Dev. 2012;26:2408–2421. doi: 10.1101/gad.199307.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuhn A.N., Brow D.A. Suppressors of a cold-sensitive mutation in yeast U4 RNA define five domains in the splicing factor Prp8 that influence spliceosome activation. Genetics. 2000;155:1667–1682. doi: 10.1093/genetics/155.4.1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Boon K.-L., Grainger R.J., Ehsani P., Barrass J.D., Auchynnikava T., Inglehearn C.F., Beggs J.D. prp8 mutations that cause human retinitis pigmentosa lead to a U5 snRNP maturation defect in yeast. Nat Struct Mol Biol. 2007;14:1077–1083. doi: 10.1038/nsmb1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber G., Cristão V.F., de L., Alves F., Santos K.F., Holton N., Rappsilber J., Beggs J.D., Wahl M.C. Mechanism for Aar2p function as a U5 snRNP assembly factor. Genes Dev. 2011;25:1601–1612. doi: 10.1101/gad.635911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharp P.A. On the origin of RNA splicing and introns. Cell. 1985;42:397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- 58.Cech T. The generality of self-splicing RNA: relationship to nuclear mRNA splicing. Cell. 1986;44:207–210. doi: 10.1016/0092-8674(86)90751-8. [DOI] [PubMed] [Google Scholar]
- 59.Lambowitz A.M., Zimmerly S. Group II introns: mobile ribozymes that invade DNA. Cold Spring Harb Perspect Biol. 2011;3:a003616. doi: 10.1101/cshperspect.a003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sharp P.A. Five easy pieces. Science. 1991;254:663–665. doi: 10.1126/science.1948046. [DOI] [PubMed] [Google Scholar]
- 61.Bai X.-C., Fernandez I.S., McMullan G., Scheres S.H. Ribosome structures to near-atomic resolution from thirty thousand cryo-EM particles. Elife. 2013;2:e00461. doi: 10.7554/eLife.00461. [DOI] [PMC free article] [PubMed] [Google Scholar]