Abstract
Objectives
To evaluate the effectiveness and cost-effectiveness of strategies to treat hepatitis C virus (HCV) in HIV/HCV co-infected patients in the U.S.
Subjects
Simulated cohort of HIV/HCV genotype 1 co-infected, non-cirrhotic, HCV treatment-naïve individuals enrolled in U.S. HIV guideline-concordant care.
Design/Interventions
Monte Carlo simulation comparing 5 strategies: no treatment; “dual therapy" with pegylated-interferon (PEG) and ribavirin (RBV); starting all patients (“PEG/RBV trial”) or some patients (“IL28B triage”) on PEG/RBV and advancing those with treatment failure to PEG/RBV and telaprevir (TVR), and “triple therapy” PEG/RBV/TVR for all patients. Sensitivity analyses varied efficacies and costs and included a scenario with interferon (IFN)-free therapy.
Main Measures
SVR, life expectancy (LE), discounted quality-adjusted life expectancy (QALE) and lifetime medical cost, and incremental cost-effectiveness ratios (ICERs) in $/QALY gained.
Results
“PEG/RBV trial,” “IL28B triage,” and “triple therapy” each provided 72% sustained virologic response (SVR) and extended QALE compared to “dual therapy” by 1.12, 1.14, and 1.15 QALY respectively. The ICER of “PEG/RBV trial” compared to “dual therapy” was $37,500/QALY. “IL28B triage” and “triple therapy” provided little benefit compared to “PEG/RBV trial” and had ICERs exceeding $300,000/QALY. In sensitivity analyses, IFN-free treatment attaining 90% SVR had an ICER <$100,000/QALY compared to “PEG/RBV trial” when its cost was ≤ $109,000 (125% of the cost of PEG/RBV/TVR).
Conclusion
HCV protease inhibitors are most efficiently used in HIV/HCV co-infection after a trial of PEG/RBV, sparing protease inhibitor for those who attain RVR and SVR. The cost-effectiveness of IFN-free regimens for HIV/HCV will depend on the cost of these therapies.
Keywords: HIV/HCV co-infection, cost-effectiveness, telaprevir, interferon-free
Introduction
HCV co-infection is a leading cause of morbidity and mortality among HIV-infected individuals [1]. Newer HCV therapies utilizing HCV protease inhibitors were licensed for the treatment of HCV mono-infection in the U.S. and Europe in 2011 [2]. Phase 2 clinical trials in HIV/HCV co-infected patients demonstrate sustained virologic response (SVR) rates as high as 74% in those with HCV genotype 1 infection [3, 4]. Clinical trial results for oral interferon (IFN)-free regimens for HCV mono-infected patients have been presented at national conferences, and the first IFN-free regimen for the treatment of HCV genotypes 2 and 3 in HCV mono-infected patients was submitted to the FDA in April 2013 [5]. These regimens attain 90% or greater SVR, with little toxicity and only 12 weeks of therapy [6–9].
The improved efficacy and toxicity profiles of new treatments are accompanied by higher costs [1, 10, 11]. Because many HIV/HCV co-infected patients rely on publicly-funded health insurance (or other public payers such as the prison healthcare system), treatment for HIV/HCV co-infection often occurs in resource-constrained settings [12]. In such environments, efficient use of HCV therapy could increase the number of people treated for HCV, maximizing the population-level benefits of HCV treatment.
Genome-wide association studies have discovered that those with homozygosity at a single nucleotide polymorphism (rs12979860) related to the interleukin-28 beta subunit (IL28B) gene, the “CC” genotype, have better treatment response to peginterferon (PEG) and ribavirin (RBV) than non-CC genotypes [13–16]. Using IL28B to triage CC genotype patients to initiate PEG/RBV without an HCV protease inhibitor could control costs. Another potential strategy is to initiate all patients on PEG/RBV, adding an HCV-protease inhibitor only for those who experience virologic failure. The comparative- and cost-effectiveness of such approaches in HIV/HCV co-infection are unknown.
To inform strategies for use of new therapies for HIV/HCV co-infected patients, we investigated the cost-effectiveness of alternative treatment options and identified approaches that would efficiently use scarce budgetary resources, potentially expanding access to HCV treatment.
Methods
Analytic Overview
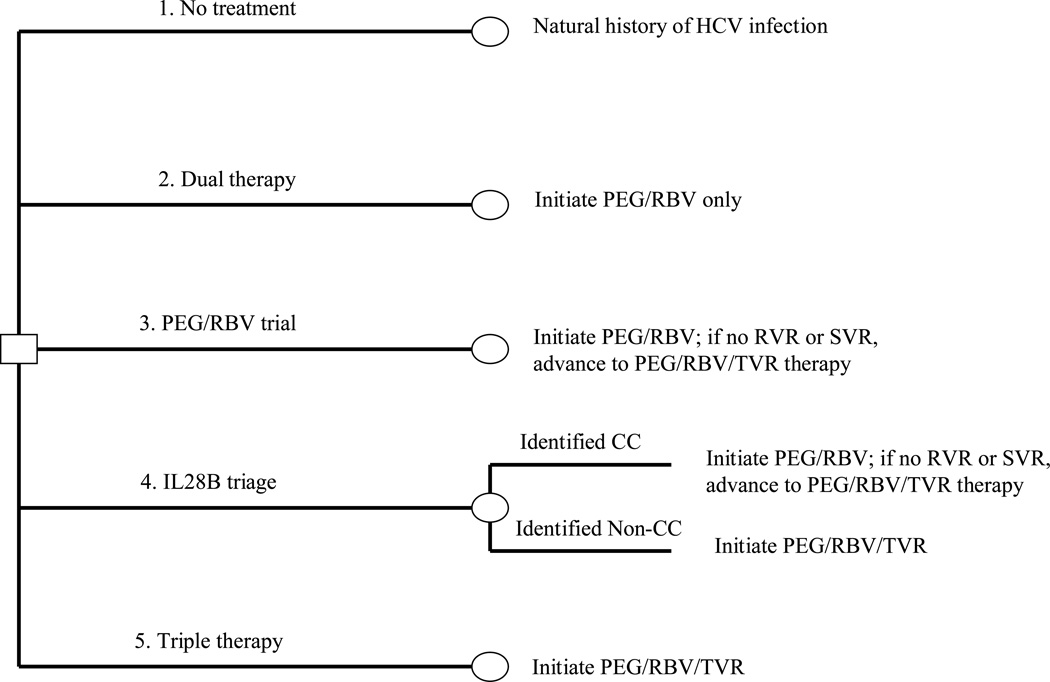
We used the Hepatitis C Cost-Effectiveness (HEP-CE) model, a Monte Carlo simulation of screening and treatment of HCV, to estimate the effectiveness and cost-effectiveness of strategies for treating HIV/HCV co-infection. The model is summarized below and details are available elsewhere [17] and in the supplemental materials. We considered 5 HCV treatment strategies (Figure 1):
No treatment
“Dual therapy” – 48 weeks of response-guided PEG/RBV.
“PEG/RBV trial” – 48 weeks of response-guided PEG/RBV. Individuals who fail PEG/RBV at any time during therapy advance to triple therapy (strategy 5).
“IL28B triage” – Individuals are triaged to commence either PEG/RBV or triple therapy (strategy 5) based on IL28B genotype. Those with “CC” alleles initiate PEG/RBV, while all others start triple therapy. Patients who fail PEG/RBV advance to triple therapy.
“Triple therapy”—Treatment with 48 weeks of PEG/RBV in combination with the HCV protease inhibitor telaprevir (TVR).
Figure 1. Treatment strategy schematic.
Simplified decision tree depicting the treatment strategies considered for treating HCV infection of HIV/HCV co-infected patients without cirrhosis. Note: figure layout was modeled after a similar figure by Liu et al. 2012 [67].
All analyses simulated a cohort of 10 million hypothetical HIV/HCV co-infected individuals chronically infected with HCV genotype 1, non-cirrhotic, HCV treatment-naïve, and enrolled in U.S. HIV guideline-concordant care. Per these guidelines, individuals were either on suppressive antiretroviral therapy (ART) or were HIV-treatment-naïve with CD4 >500/ml (Table 1).
Table 1.
Model inputs for an analysis of the cost-effectiveness of HCV therapies in HIV/HCV co-infected patients
| Variable | Base Case Value |
Range Evaluated in Sensitivity Analyses |
Source(s) |
|---|---|---|---|
| Cohort characteristics | |||
| Average age, years (S.D.)* | 45 (6) | 35–55 | [44–48] |
| Proportion male | 0.66 | 0–1.0 | [44–47] |
| Average age at HCV infection (years)* | 26 (20–30) | 16–36 | [52] |
| Prevalence of IL28B CC genotype | 0.32 | 0.26–0.39 | [14] |
| Mean CD4 count, cells/µl (S.D.)* | 520 (100) | 350–700 | [49–51] |
| Proportion with CD4 > 500 at baseline on ART | 0.756 | 0.5–1.0 | [49–51, 71] |
| Standardized mortality ratio (SMR)a | [72, 73] | ||
| Men | 4.69 | 1.00–6.01 | |
| Women | 7.80 | 1.00–14.14 | |
| IL28B test characteristics | |||
| Sensitivity | 0.99 | 0.95–1.00 | [74–76] |
| Specificity | 0.99 | 0.96–1.00 | [74–76] |
| HCV disease progression | |||
| Median years to cirrhosis from age of infection (10%–90%)* | 25 (23–27) | 10–40 | [53] |
| Median years first liver-event after developing cirrhosis (10%–90%)* | 10.8 (9.2–14.3) | 5.6–19.3 | [25, 77] |
| Liver-related mortality with cirrhosis (deaths/100 PYs) | 2.73 | 1.38–4.08 | [25, 26] |
| HIV disease progression | |||
| Rate of CD4 decline (cells/µl/month)b | 3.03–6.38 | 1.51–9.56 | [78] |
| Incidence of AIDS events (events/100 PYs)c | 0.12–0.55 | 0.06–0.83 | [79–86] |
| HCV therapy efficacy | |||
| PEG/RBV therapy | |||
| CC genotype at rs12979860 | |||
| Probability of RVR | 0.80 | 0.74–0.91 | [16, 57] |
| Probability of SVR given RVR | 0.91 | 0.77–0.94 | [16, 57] |
| Probability of withdrawal (toxicity or non-adherence) | 0.23 | 0.19–0.29 | [57] |
| Probability of withdrawal due to toxicity | 0.10 | 0.08–0.13 | [87] |
| Total probability of SVR | 0.55 | 0.40–0.69 | [16, 54–57] |
| Non-CC allele (TT or TC) | |||
| Probability of RVR | 0.42 | 0.33–0.52 | [16, 57] |
| Probability of SVR given RVR | 0.64 | 0.57–0.73 | [16, 57] |
| Probability of withdrawal (toxicity or non-adherence) | 0.23 | 0.19–0.29 | [57] |
| Probability of withdrawal due to toxicity | 0.10 | 0.08–0.13 | [87] |
| Total probability of SVR | 0.20 | 0.13–0.30 | [16, 54–57] |
| PEG/RBV/TVR | |||
| Probability of having treatment failure (HCV viremia >1,000/ml) | 0.18 | 0.02–0.21 | [88] |
| Probability of withdrawal (toxicity or non-adherence) | 0.18 | 0.06–0.23 | [3] |
| Probability of withdrawal due to toxicity | 0.08 | 0.03–0.10 | [88, 89] |
| Total probability of SVR | 0.74 | 0.65–0.86 | [3] |
| IFN-free therapy | |||
| Probability of withdrawal | 0.03 | 0.02–0.04 | [6, 7] |
| Probability of withdrawal for toxicity | 0.006 | 0.004–0.009 | See text |
| Total probability of SVR | 0.90 | 0.80–0.95 | [6, 7] |
| Probability of death due to toxicity for all strategies | 0 | 0–0.029 | [57, 88, 90] |
| HIV therapy efficacy | |||
| ART efficacy (proportion HIV RNA < 400 copies/ml at 24 weeks)d | 0.15–0.86 | 0.13–0.99 | [91–95] |
| ART efficacy (proportion HIV RNA < 50 copies/ml at 24 weeks)d | 0.15–0.65 | 0.12–0.75 | [91–95] |
| CD4 rise on suppressive ART (cells/µl/month)d | 26–90 | 13–135 | [91–95] |
| HIV loss to follow-up (rate/100 PYs)e | 24.17 | 12.09–36.26 | [96] |
| Costs | |||
| Costs of screening tests and ART | |||
| IL28B assay test | $80 | $40-$120 | [31] |
| ART costsd | $1,600-$4,800 | $800-$7,700 | [33, 34, 38] |
| Healthcare costs | |||
| Without HCV (HIV only)f | $300- $20,600 | $150-$30,900 | [31, 32, 35–37] |
| With HCVf | $370-$23,300 | $190-$35,000 | [19, 31, 39] |
| HCV therapy costs/month | |||
| TVR | $15,200 | $7,600-$23,000 | [33] |
| PEGg | $2,100 | $1,100-$3,200 | [33] |
| RBVh | $1,400 | $700-$2,100 | [33] |
| Filgrastimi | $1,900 | $900-$2,700 | [33] |
| Clobetasol propionatej | $160 | $80-$320 | [33] |
| Total costs of dual therapyl | $43,000 | $21,500-$64,500 | [31, 33] |
| Total costs of triple therapyk | $87,300 | $43,700-$131,000 | [31, 33] |
| Total costs of IFN-free therapy | $131,000 | $98,200-$196,500 | See text |
| Cost of treatment ending toxicity for triple and IFN-free therapy | $360 | $180-$540 | [30, 31, 33, 97, 98] |
| Cost of treatment ending toxicity for dual therapy | $420 | $210-$630 | [30, 31, 33, 57, 97] |
| Provider visit costsm | $120 | $60–180 | |
| Quality of life | |||
| HCV-related quality of life | |||
| No fibrosis to moderate fibrosis | 0.89 | 0.75–0.95 | [20, 22, 43] |
| Cirrhosis | 0.62 | 0.55–0.75 | [20, 22, 43] |
| Decompensated cirrhosis | 0.48 | 0.40–0.60 | [20, 22, 43] |
| On IFN (applied to appropriate HCV-attributable QoL) | 0.90 | 0.84–0.96 | [42] |
| On IFN-free therapy (applied to appropriate HCV-attributable QoL)n | 0.95 | 0.90–0.99 | See text |
| Major toxicity decrement (monthly)o | 0.16 | 0.09–0.25 | [99] |
| HIV-related quality of life (CD4 cells/µl) | |||
| >500 | 0.87 | 0.78–0.96 | [41] |
| 351–500 | 0.86 | 0.77–0.95 | [41] |
| 251–350 | 0.86 | 0.77–0.95 | [41] |
| 101–250 | 0.85 | 0.76–0.94 | [41] |
| 51–100 | 0.85 | 0.76–0.94 | [41] |
| ≤50 | 0.83 | 0.74–0.92 | [41] |
| With acute AIDS-related eventp | 0.69–0.78 | 0.69–0.78 | [40] |
SD: standard deviation; IL28B: interleukin-28B; ART: anti-retroviral therapy; PYs: person-years; PEG: peginterferon; RBV: ribavirin; TVR: telaprevir; SVR: sustained virologic response; RVR: rapid virologic response; IFN: interferon; OI: opportunistic infection; MSM: men who have sex with men; IDU: injection drug user
Note: all costs are in 2011 U.S. dollars.
These parameters are entered into the model as distributions rather than point estimates, allowing for first-order Monte Carlo variance. Numbers in parentheses next to the base case value represent either the standard deviation (if normally distributed) or the tenth and ninetieth percentile values (if non-normally distributed) of the distribution. The ranges provided in the sensitivity analysis column provide the range of central measure (mean or median) that we tested in sensitivity analyses.
The SMR captures elevated non-HIV and non-HCV mortality among those who are HIV/HCV co-infected. It reflects competing risks of death from substance use and other co-morbidities. To determine the SMR for the entire cohort, we first identified risk-group specific SMRs (MSM, IDU, heterosexual risk) by sex, and then took the weighted average of these estimates, using the proportion of each risk-factor among HIV/HCV co-infected patients.
Depending on HIV RNA.
Depending on CD4, OI history and event type.
Depending on ART regimen.
Beginning in month 18 (we assumed no HIV-related loss to follow-up during HCV treatment).
Depending on age, sex, duration of HIV infection, and CD4 count.
13% of patients received a reduced weekly dose of 135 mcg in response to non-treatment ending neutropenia [100].
Assumed to be 1,200 mg/day for a 75 kg person; 36% of patients on triple therapy and 17% of patients on dual therapy receive a reduced dose RBV = 600 mg/day in response to non-treatment ending anemia [100].
13% of patients developed non-treatment ending neutropenia (absolute neutrophil count < 750/ml) and received filgrastim 300 mcg/two times weekly [100].
28% of patients on triple therapy during the first 3 months of therapy receive 150 g per month for treating mild rash [100].
Includes an additional cost of a nursing visit for patients who have adverse events.
Depending on treatment month.
Treatment visit costs are higher in the first month compared to other months.
The multiplier is applied for 3 months instead of 12 months for IFN-free therapy, resulting in 0.5 quality-adjusted life months saved compared to being on PEG/RBV therapy.
This utility “toll” was subtracted from a patient’s health state utility during the month of a major toxicity event.
Depending on type of OI event.
We projected outcomes including the percent attaining sustained virologic response (SVR), life expectancy (LE), discounted quality-adjusted life expectancy (QALE), discounted lifetime medical costs, and the incremental cost effectiveness ratio (ICER) of each strategy compared to its next costliest alternative. We conducted one-way and multi-way sensitivity analyses on these results.
We also considered scenarios using an oral, IFN-free regimen that was more effective and less toxic than PEG/RBV/TVR. We considered a range of IFN-free regimen efficacies and costs, and we identified cost/efficacy combinations leading to IFN-free therapy having an ICER <$100,000/QALY when compared to the preferred treatment strategy without an IFN-free regimen. To explore cost-reducing strategies in cost-constrained environments, we considered scenarios similar to the base case where patients initiate a trial of PEG/RBV, but instead of switching to triple therapy upon a failed course of PEG/RBV, they switch to IFN-free therapy.
Model structure
HCV Disease Progression
The model simulates HCV disease progression through 3 stages of liver disease: mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis. Consistent with previous studies, all disease stages of HCV-infection are associated with increased resource utilization and decreased quality of life (QoL) [18–24]. When individuals become cirrhotic, they are subject to increased mortality attributable to liver disease [25, 26]. With successful treatment (SVR), HCV-related mortality, resource utilization, and QoL revert to those of HIV mono-infected individuals.
HIV Disease Progression
We used the Cost-Effectiveness of Preventing AIDS Complications (CEPAC) model to estimate the cohort’s HIV-related outcomes and costs [27]. CEPAC simulates HIV disease progression through CD4 count and HIV RNA levels. We used the CEPAC model to assess the cohort’s progression of HIV disease across a range of CD4 and viral load categories. CEPAC provided sex-stratified estimates of monthly HIV-related mortality conditional upon being alive at the beginning of the month (life table), mean monthly medical costs related to HIV-disease, and QoL related to HIV-infection. We used these CEPAC outputs as HEP-CE model inputs, such that in every month, individuals in the HEP-CE model were exposed to sex and time-dependent HIV-attributable mortality, costs, and QoL changes (see supplemental materials).
HCV Therapy
1. “Dual therapy”
All individuals initiate a planned 48-week course of weekly PEG alfa-2a 180 mcg subcutaneously in combination with twice daily oral RBV 600 mg (average cohort weight 80kg). Simulated patients undergo routine HCV RNA testing at the end of treatment week 4. Those with detectable viremia stop HCV therapy, while those with suppressed HCV RNA (rapid virologic response—RVR) continue a planned 48-week treatment course [3].
While taking HCV medications, all patients experience a monthly QoL decrement related to adverse therapy symptoms. Additionally, a proportion of patients on therapy experience non-treatment ending toxicities, including moderate anemia managed by RBV dose reduction and moderate neutropenia managed with PEG dose reduction and twice weekly filgrastim 300 mcg subcutaneously. Patients with non-treatment ending toxicities accrue cost adjustments related to dosing changes and additional therapies, but they remain on HCV treatment and are eligible to attain SVR. In every month, patients also risk treatment discontinuation due to non-adherence or major toxicity, including severe anemia or rash. Major toxicity is associated with additional costs and an additional QoL decrement.
2. “PEG/RBV trial”
All patients initiate the same PEG/RBV regimen as in the “dual therapy” strategy. Those who fail to attain virologic suppression at week 4 (RVR) subsequently add telaprevir to their regimen for 12 weeks as described below (“triple therapy”). Patients who attain RVR on PEG/RBV at week 4 but do not achieve SVR at treatment completion, are re-treated with PEG/RBV/TVR. Patients who stop PEG/RBV therapy due to non-adherence or major toxicity are ineligible to advance to PEG/RBV/TVR.
3. “IL28B triage”
IL28B genotyping is used to triage patients to start either PEG/RBV (CC genotype), or PEG/RBV/TVR (non-CC genotypes). The approach to modeling PEG/RBV therapy and the addition of TVR to failing regimens is the same as that for “PEG/RBV trial.”
The efficacy of protease-based therapy among those who fail PEG/RBV is lower than its efficacy as first-line therapy [28]. Exposure to PEG/RBV, however, does not compromise protease efficacy if the individual simply started treatment with PEG/RBV/TVR [28]. We therefore assume that non-responders to PEG/RBV are more likely to be non-responders to PEG/RBV/TVR when retreated in all strategies, and we assume that in the “PEG/RV trial” strategy exposure of patients to PEG/RBV ahead of adding a protease inhibitor does not reduce the overall percentage of the cohort who ultimately attain SVR.
4. “Triple therapy”
All patients initiate a regimen of PEG/RBV/TVR for 12 weeks followed by 36 weeks of PEG/RBV alone for a 48-week total therapy course. Patients receive 750 mg three times daily of TVR in combination with the same dosage of PEG/RBV as described above. Patients undergo routine HCV RNA monitoring at treatment weeks 4 and 12. Those with HCV RNA >1,000 copies/ml at either time point stop therapy. We did not specifically model TVR dose increases required when using efavirenz, but effectively included such dose changes in drug cost sensitivity analyses. The approach to modeling adherence, toxicity, and therapy disutility was the same as for dual therapy, but we included rash as potential treatment toxicity.
5. “Interferon-free regimen”
Patients initiate a 12-week course of an HCV protease inhibitor, a polymerase inhibitor, and RBV [6, 7, 29]. The regimen has lower toxicity and higher adherence, QoL while on therapy, and SVR rate than IFN-containing regimens. Individuals face a risk of treatment ending toxicity and non-adherence, but we assumed there are no early stopping criteria for IFN-free therapy.
Costs
We assessed costs in the model from the health system perspective. In each simulation month, individuals accrue “background costs” associated with non-HIV/HCV-related healthcare. In addition to these costs, there are HCV- and HIV-specific costs. HCV-associated costs include those of HCV medications, physician visits, laboratory tests for monitoring and safety, emergency department visits, and hospitalizations for liver-related events (Table 1). HIV-associated costs include costs of ART, laboratory monitoring, and hospital admissions associated with AIDS-related events [30–38].
To reflect increased resource utilization among those with HIV/HCV co-infection compared to HIV mono-infection, all costs except those of HIV and HCV medications and HIV-related testing are 70% greater in co-infected individuals than in HIV mono-infected [19, 39].
QoL
QoL estimates include independent effects related to HIV- and HCV-infection integrated in the model using a multiplicative assumption [20, 22, 40–43]. HIV-related QoL is a function of current CD4 count and acute AIDS-related events. HCV-related QoL is a function of fibrosis stage, HCV treatment status, and treatment-related toxicity (Table 1).
Base case parameters
The cohort was 66% male [44–47], mean age 45 years (S.D. 6 years) [44–48], mean CD4 count 520/µl (S.D. 100/µl) [49–51], and 32% CC genotype prevalent [14] (Table 1). The median time to cirrhosis from HCV infection (mean age of infection 26 years [52]) was 25 years [53], and the rate of liver-related deaths with cirrhosis was 2.73 per 100 person-years [25, 26].
The total SVR probability for PEG/RBV among those with CC genotype was 55% [16, 54–57] and 20% for CT or TT [16, 54–56]. The total SVR probability with PEG/RBV/TVR was 74% [3] and ranged from 80–100% with an IFN-free regimen [6, 7]. The probability of withdrawal due to toxicity or non-adherence was 11% for triple therapy [3], 23% for dual therapy [57], and 3% for IFN-free therapy [6, 7]. The cost of a complete course of dual and triple therapy, including the cost of managing toxicities, was $43,000 and $87,300 respectively. The cost of a complete course of IFN-free therapy ranged from $87,300-$175,000 [31, 33]. Those with mild to moderate fibrosis, cirrhosis, and decompensated cirrhosis had a QoL of 0.89, 0.62, and 0.48 respectively [20, 22, 43].
Analyses
We calculated the incremental cost-effectiveness ratio (ICER) of each treatment strategy as the additional cost divided by the additional quality-adjusted life years (QALY) gained compared to the next less expensive strategy [58, 59]. Strategies were considered inefficient and excluded from ICER calculations if they resulted in higher costs but fewer QALYs gained or had a higher ICER than a more effective strategy [59, 60]. QALYs and costs were both discounted at 3% annually [59]. We assumed a societal willingness-to-pay of $100,000 per QALY where strategies below the threshold were considered “cost-effective” [61, 62].
Results
Base case
Without HCV treatment, undiscounted LE was 13.24 years, QALE was 6.76 QALYs, and discounted lifetime medical costs were $198,700 (Table 2). “Dual therapy” yielded 30.8% attaining SVR, increased LE by 0.52 years to 13.76 years, QALE by 0.84 QALY to 7.60 QALY, and lifetime medical costs by $23,200. The ICER for treating patients with dual therapy compared to no treatment was $27,700/QALY gained.
Table 2.
Incremental cost effectiveness ratios of HIV/HCV telaprevir-based treatment strategies
| Undiscounted | Discounted | Incremental | |||||
|---|---|---|---|---|---|---|---|
| Strategy | % Attaining SVR | Life Expectancy | Cost ($) | QALE | Cost ($) | QALY | CER ($/QALY) |
| No treatment | 0 | 13.240 | 198,700 | 6.760 | --- | --- | --- |
| Dual therapy | 30.8 | 13.761 | 221,900 | 7.600 | 23,200 | 0.839 | 27,700 |
| PEG/RBV trial | 72.1 | 14.459 | 264,200 | 8.728 | 42,300 | 1.128 | 37,500 |
| IL28B triage | 72.3 | 14.463 | 269,200 | 8.743 | 5,000 | 0.016 | 319,400 |
| Triple therapy | 72.5 | 14.462 | 277,700 | 8.750 | 8,500 | 0.007 | 1,240,000 |
QALY: quality-adjusted life year; CER: cost-effectiveness ratio; PEG/RBV: Peginterferon and Ribavirin; IL28B: interleukin-28B
Note: all costs and QALYs are lifetime and discounted at an annual rate of 3%. Costs are in 2011 US dollars and rounded to the nearest $100. All life-years and QALYs are rounded to the nearest thousandth.
The “PEG/RBV trial” strategy was the least costly approach to using an HCV protease-inhibitor. “PEG/RBV trial” increased SVR to 72% and LE and QALE compared to “dual therapy” by 0.70 years and 1.13 QALY, a larger gain than that provided by “dual therapy” compared to “no treatment.” “PEG/RBV trial” increased lifetime medical cost compared to “dual therapy” by $42,300 to $264,200, resulting in an ICER for “PEG/RBV trial” compared to “dual therapy” of $37,500/QALY.
The “IL28B triage” and “triple therapy” scenarios both increased SVR by <1% compared to “PEG/RBV trial.” As a result, LE and QALE increased by less than 0.01 QALY, resulting in ICERs >$300,000/QALY (Table 2).
Sensitivity analysis
“PEG/RBV trial” remained the preferred (<$100,000/QALY) treatment strategy when we varied treatment efficacy for both PEG/RBV and PEG/RBV/TVR regimens. Across all efficacy assumptions, the ICERs of “IL28B triage” compared to “PEG/RBV trial” and of “triple therapy” compared to “IL28B triage” remained more than $250,000/QALY.
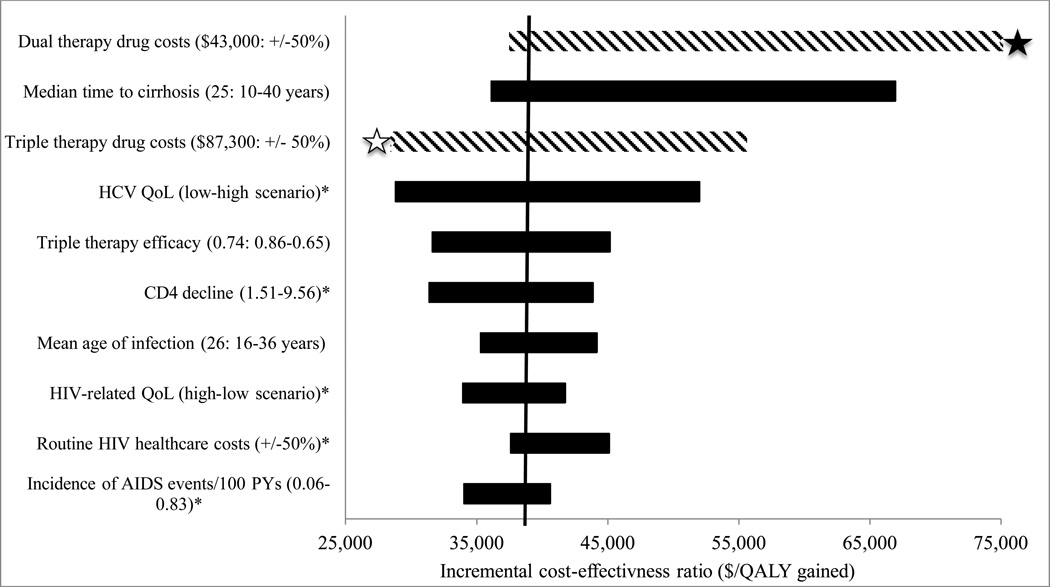
Total treatment costs had the greatest impact on cost-effectiveness conclusions (Figure 2). With a higher cost of PEG/RBV therapy, the “PEG/RBV trial” and “dual therapy” strategies became less efficient than “IL28B triage.” With higher PEG/RBV costs, “triple therapy” remained economically unattractive with an ICER >$500,000/QALY.
Figure 2. Tornado diagram “PEG/RBV trial” one-way sensitivity analyses.
Tornado diagram illustrating the incremental cost effectiveness ratio (ICER) of the “PEG/RBV trial” strategy compared to its next best alternative when varying model input parameters through plausible ranges. Long bars demonstrate parameters that have a large impact on ICERs. Bars with a striped pattern illustrate parameters that led to the “PEG/RBV trial” strategy becoming dominated by either “IL28B triage” or “triple therapy”, meaning that the other option provided greater life expectancy at a lower cost per QALY gained. The white star indicates that the “PEG/RBV trial” strategy became dominated by “triple therapy.” The black star indicates that the “PEG/RBV trial” strategy became dominated by “IL28B triage.” The asterisk refers to Table 1 for base case values. (QALY= quality-adjusted life years; QoL= quality of life).
When we reduced the cost of PEG/RBV/TVR by 50%, the “triple therapy” strategy was most efficient, with an ICER compared to no treatment of $20,500/QALY. This remained the preferred strategy at a threshold of $100,000/QALY as long as the cost of PEG/RBV/TVR was less than $50,000 (57% of base case cost). When we increased the cost of PEG/RBV/TVR by 50%, the “PEG/RBV trial” strategy was preferred with an ICER of $55,600/QALY compared to “dual therapy.”
“PEG/RBV trial” remained the preferred treatment strategy with an ICER <$100,000/QALY across a broad range of other sensitivity analyses including HIV therapy efficacy, time to cirrhosis, QoL, and costs of routine medical care, ARTs and laboratory tests (Figure 2).
IFN-free scenario
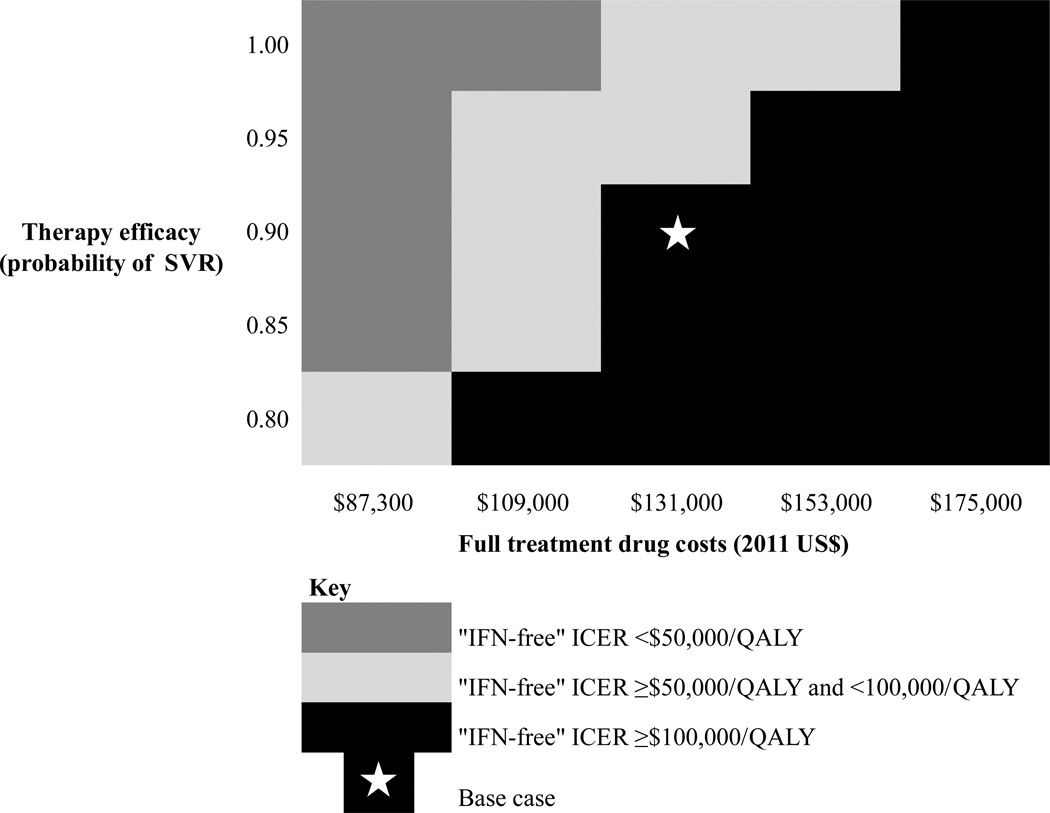
Treating individuals with an “IFN-free” regimen achieving 90% SVR extended discounted QALE by 2.56 years compared to no treatment, by 0.59 years compared to “PEG/RBV trial,” and by 0.57 years compared to “triple therapy.” In a two-way sensitivity analysis comparing “IFN-free” therapy to “PEG/RBV trial,” an IFN-free regimen that provided a 90% SVR rate had an ICER <$100,000/QALY when the cost of the IFN-free regimen was ≤ 125% of the cost of a complete course of PEG/RBV/TVR, or approximately $109,000 (Figure 3). An IFN-free regimen that attained 95% SVR had an ICER <$100,000/QALY when the cost of the IFN-free regimen was ≤ 150% of the cost of a complete course of PEG/RBV/TVR, or $131,000.
Figure 3. Two-way sensitivity analysis varying costs and efficacy of IFN-free therapy as an alternative to “PEG/RBV trial” strategy.
Two-way sensitivity analysis comparing an IFN-free regimen as an alternative to the “PEG/RBV trial” strategy at various cost multipliers of the base case cost of PEG/RBV/TVR ($87,000-$175,000) and efficacies (80% SVR rate – 100% SVR rate). The striped boxes reflect an incremental cost effectiveness ratio (ICER) of “IFN-free” compared to “PEG/RBV trial” <$100,000/QALY, making “IFN-free” the preferred strategy. In contrast, the black boxes reflect an ICER of “IFN-free” compared to “PEG/RBV trial” >$100,000/QALY, making “PEG/RBV trial” the preferred strategy.
When we considered potential intermediate strategies for using IFN-free therapy, a strategy in which all patients initiated PEG/RBV and only those with treatment failure advanced to an IFN-free regimen, was preferred with an ICER compared to “dual therapy” of $51,800/QALY (Supplemental Table 4). In this scenario, providing IFN-free therapy to all patients was cost-effective (ICER <$100,000/QALY) only when the cost of a course of IFN-free treatment was less than $88,000 (base case $131,000), or when the QoL of being on PEG/RBV was less than 0.3 (similar to having compensated cirrhosis).
Discussion
We assessed the cost-effectiveness of new therapies to treat HIV/HCV genotype 1 co-infected individuals and found that although new HCV therapies improve life expectancy in co-infected patients, they substantially increase costs and are most efficiently used after an initial trial of PEG/RBV to determine protease inhibitor necessity. Furthermore, the economic efficiency of future IFN-free regimens will depend greatly on their cost. In highly cost-constrained environments, initiating treatment with PEG/RBV, or using IL28B genotyping to triage patients to IFN-free therapy, may be economically attractive.
It is not surprising that we found that initiating all patients on “triple therapy” is not cost-effective. The REALIZE study demonstrates that using a lead-in of PEG/RBV before adding TVR is efficacious and does not compromise overall SVR [28]. Extrapolating this finding to naïve patients, in HIV/HCV co-infected patients, there is little disadvantage to the “PEG/RBV trial” approach as patients who do not attain RVR with PEG/RBV alone can add TVR to their regimen without extending the treatment course or decreasing treatment efficacy. Those who attain RVR with PEG/RBV have a >95% chance of ultimately attaining SVR [57, 63].
Several important observations explain the greater economic efficiency of the “PEG/RBV trial” strategy compared to “IL28B triage.” First, the efficacy of PEG/RBV in non-CC genotypes is approximately 20%; thus, the “IL28B triage” strategy forgoes substantial cost savings without additional clinical benefits when it assigns the 20% of patients who would have attained SVR on PEG/RBV instead to triple therapy. Second, the negative predictive value of failing to attain RVR as a predictor of attaining SVR is approximately 98% [57, 63], while that of IL28B CC is only 80% [64–66]. Therefore, the “PEG/RBV trial” strategy functions as a more specific “diagnostic test” than IL28B testing to prioritize patients to triple therapy.
A cost-effectiveness analysis in HCV mono-infection has reported that protease inhibitor-based therapy for all HCV mono-infected patients is cost-effective when compared to the “IL28B triage” strategy [67]. In the base case analysis, however, that study did not consider retreatment with a protease-based regimen for patients who were triaged to PEG/RBV. When the authors did consider re-treatment, the ICER of “triple therapy” was approximately $100,000/QALY, and for some subgroups, IL28B–triage was a dominant strategy. A critical difference between HCV mono- and co-infection is that in mono-infection, the overall treatment course of protease-based therapy is usually 6 months, while that of PEG/RBV is 12 months. As a result, strategies that assign some mono-infected patients to initiate PEG/RBV have a greater negative impact on QoL. Simultaneously, protease-based regimens are relatively less costly because using a protease inhibitor often saves the expense of 6 additional months of PEG/RBV. There may be greater disadvantage of the “PEG/RBV trial” approach in HCV-mono infection than in HIV/HCV co-infection, where response-guided therapy to shorten therapy is not yet proven. Finally, even in HCV mono-infection, the cost-effectiveness of initiating all patients on triple therapy is not entirely clear, as at least one cost-effectiveness analysis has found that “IL28B–triage” is preferred [68].
In two-way sensitivity analysis, we demonstrated the range of costs and efficacies across which future IFN-free regimens would be cost-effective compared to the “PEG/RBV trial” approach using TVR. We found that total therapy costs remained the critical factor determining cost-effectiveness. Assuming an IFN-free regimen that attains 90% SVR, treating all patients with IFN-free therapy will be cost-effective compared to protease-based regimens only if the IFN-free regimen costs are approximately $109,000 or less.
We also explored strategies to reduce the cost of IFN-free therapy by triaging such medications to a proportion of the population. In that case, initiating PEG/RBV and advancing to IFN-free treatment for failure was the preferred strategy. Importantly, a trial of PEG/RBV was cost-effective despite the fact that in the base case scenario, QoL while taking therapy was 10 times worse for an IFN-containing regimen than for IFN-free (0.10 QALY lost vs. 0.01 QALY). Only when the QoL while taking IFN was similar to that of having decompensated cirrhosis did initiating IFN-free therapy for all patients become cost-effective.
Concern that failing an initial course of PEG/RBV could compromise the efficacy of future treatment might limit enthusiasm for the “PEG/RBV trial” approach. Phase 2 trials of IFN-free regimens in HCV mono-infection demonstrate a lower efficacy among treatment-experienced patients [8, 9]. Such findings, however, likely demonstrate that failing PEG/RBV is a marker for having difficult to treat HCV. No data exist to suggest that first-line PEG/RBV itself decreases the efficacy of IFN-free treatment. Routine use of IFN-free regimens in budget-constrained settings will therefore require price negotiations for IFN-free therapy to provide acceptable value for money compared to using those funds to treat a larger number of patients with a strategy that initiates some or all patients on PEG/RBV.
There are several limitations to this analysis. First, we based efficacy estimates for protease-based therapy on phase 2 clinical trials and developed estimates for an IFN-free regimen using trials in HCV mono-infected patients. Nonetheless, the findings that the ICERs of universal triple therapy and IFN-free therapy strategies were >$100,000/QALY at base case therapy costs were consistent across a plausible range of efficacy assumptions. Second, many HCV providers may be inclined to treat HIV/HCV co-infected cirrhotic patients with currently available therapies, but wait for the improved toxicity-profile of IFN-free regimes for patients without cirrhosis [69, 70]. Given the importance of therapy costs in determining the cost-effectiveness of treatment, it might also be economically attractive to defer HCV therapy for those with early-stage fibrosis until such time that a generic HCV protease inhibitor is available. Such questions of “treat now or defer”, while interesting, are outside the scope of this analysis, as they are critically dependent on still unknown relative efficacies of current and future therapies in early-stage and cirrhotic patients, as well as on the relative prices of multiple future drugs.
In summary, this analysis informs strategies for maximizing the population-level benefits of new HCV therapies in HIV/HCV co-infected patients. We found that in the era of “triple therapy,” initiating PEG/RBV and adding TVR when patients fail to attain RVR or SVR maximizes the benefits attainable from constrained healthcare budgets. IFN-free regimens with improved efficacy may provide reasonable value, but this will depend on the cost of these regimens.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank Ms. Marion Robine for her help using the CEPAC model.
Supported by the National Institutes on Drug Abuse (R01DA031059, R01DA027379) and the National Institute of Allergy and Infectious Diseases (R37AI042006).
The project described was supported by grants from the National Institute on Drug Abuse and the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ingiliz P, Rockstroh JK. HIV-HCV co-infection facing HCV protease inhibitor licensing: implications for clinicians. Liver Int. 2012;32:1194–1199. doi: 10.1111/j.1478-3231.2012.02796.x. [DOI] [PubMed] [Google Scholar]
- 2.Food and Drug Administration. [Accessed July 10, 2013];Approval of Incivek (TVRaprevir), a direct acting antiviral drug (DAA) to treat hepatitis C (HCV) From: http://www.fda.gov/ForConsumers/ByAudience/ForPatientAdvocates/ucm256328.htm.
- 3.Sulkowski MS, Sherman KE, Dieterich DT, Bsharat M, Mahnke L, Rockstroh JK, et al. Combination Therapy With TVRaprevir for Chronic Hepatitis C Virus Genotype 1 Infection in Patients With HIV: A Randomized Trial. Ann Intern Med. 2013;159:86–96. doi: 10.7326/0003-4819-159-2-201307160-00654. [DOI] [PubMed] [Google Scholar]
- 4.Sulkowski M, Pol S, Mallolas J, Fainboim H, Cooper C, Slim J, et al. Boceprevir versus placebo with pegylated interferon alfa-2b and ribavirin for treatment of hepatitis C virus genotype 1 in patients with HIV: a randomised, double-blind, controlled phase 2 trial. Lancet Infect Dis. 2013;13:597–605. doi: 10.1016/S1473-3099(13)70149-X. [DOI] [PubMed] [Google Scholar]
- 5.Gilead Sciences. [Accessed July 10th, 2013];Gilead Submits New Drug Application to U.S. FDA for Sofosbuvir for the Treatment of Hepatitis C. from: http://investors.gilead.com/phoenix.zhtml?c=69964&p=irol-newsArticle&id=1804362.
- 6.Gane E, Hyland R, Ding X, Pang P, McHutchison J, Symonds W, et al. 100% suppression of viral load through 4 weeks’ post-treatment for sofosbuvir + ledipasvir (GS-5885) + ribavirin for 12 weeks in treatment-naïve and -experienced Hepatitis C Virus GT 1 patients; 20th Conference on Retrovirsuses and Opportunistic Infection; March 3–6; Atlanta, GA. 2013. [Google Scholar]
- 7.Lawitz E, Cohen D, Poordad F, Kowdley K, Everson G, Freilich B, et al. 12 weeks of ABT-450/ritonavir, non-nucleoside inhibitor and ribavirin achieved SVR24 in >90% of treatment naïve Hepatitis C Virus GT1 patients and 47% of prior non-responders; 20th Conference on Retroviruses and Opportunistic Infections; March 3–6, 2013; Atlanta, GA. [Google Scholar]
- 8.Jacobson IM, Gordon SC, Kowdley KV, Yoshida EM, Rodriguez-Torres M, Sulkowski MS, et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N Engl J Med. 2013;368:1867–1877. doi: 10.1056/NEJMoa1214854. [DOI] [PubMed] [Google Scholar]
- 9.Lawitz E, Mangia A, Wyles D, Rodriguez-Torres M, Hassanein T, Gordon SC, et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N Engl J Med. 2013;368:1878–1887. doi: 10.1056/NEJMoa1214853. [DOI] [PubMed] [Google Scholar]
- 10.Sulkowski MS. Hepatitis C virus-human immunodeficiency virus coinfection. Liver Int. 2012;32(Suppl 1):129–134. doi: 10.1111/j.1478-3231.2011.02719.x. [DOI] [PubMed] [Google Scholar]
- 11.Holmberg SD, Spradling PR, Moorman AC, Denniston MM. Hepatitis C in the United States. N Engl J Med. 2013;368:1859–1861. doi: 10.1056/NEJMp1302973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spaulding AS, Kim AY, Harzke AJ, Sullivan JC, Linas BP, Brewer A, et al. Impact of new therapeutics for hepatitis C virus infection in incarcerated populations. Top Antivir Med. 2013;21:27–35. [PMC free article] [PubMed] [Google Scholar]
- 13.Clark PJ, Thompson AJ, McHutchison JG. IL28B genomic-based treatment paradigms for patients with chronic hepatitis C infection: the future of personalized HCV therapies. Am J Gastroenterol. 2011;106:38–45. doi: 10.1038/ajg.2010.370. [DOI] [PubMed] [Google Scholar]
- 14.Dayyeh BK, Gupta N, Sherman KE, de Bakker PI, Chung RT. IL28B alleles exert an additive dose effect when applied to HCV-HIV coinfected persons undergoing peginterferon and ribavirin therapy. PLoS One. 2011;6:e25753. doi: 10.1371/journal.pone.0025753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindh M, Lagging M, Arnholm B, Eilard A, Nilsson S, Norkrans G, et al. IL28B polymorphisms determine early viral kinetics and treatment outcome in patients receiving peginterferon/ribavirin for chronic hepatitis C genotype 1. J Viral Hepat. 2011;18:e325–331. doi: 10.1111/j.1365-2893.2010.01425.x. [DOI] [PubMed] [Google Scholar]
- 16.Rallon NI, Naggie S, Benito JM, Medrano J, Restrepo C, Goldstein D, et al. Association of a single nucleotide polymorphism near the interleukin-28B gene with response to hepatitis C therapy in HIV/hepatitis C virus-coinfected patients. AIDS. 2010;24:F23–29. doi: 10.1097/QAD.0b013e3283391d6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linas BP, Wong AY, Schackman BR, Kim AY, Freedberg KA. Cost-effective screening for acute hepatitis C virus infection in HIV-infected men who have sex with men. Clin Infect Dis. 2012;55:279–290. doi: 10.1093/cid/cis382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McAdam-Marx C, McGarry LJ, Hane CA, Biskupiak J, Deniz B, Brixner DI. All-cause and incremental per patient per year cost associated with chronic hepatitis C virus and associated liver complications in the United States: a managed care perspective. J Manag Care Pharm. 2011;17:531–546. doi: 10.18553/jmcp.2011.17.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linas BP, Wang B, Smurzynski M, Losina E, Bosch RJ, Schackman BR, et al. The impact of HIV/HCV co-infection on health care utilization and disability: results of the ACTG Longitudinal Linked Randomized Trials (ALLRT) Cohort. J Viral Hepat. 2011;18:506–512. doi: 10.1111/j.1365-2893.2010.01325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong CA, Gulamhussein A, Heathcote EJ, Lilly L, Sherman M, Naglie G, et al. Health-state utilities and quality of life in hepatitis C patients. Am J Gastroenterol. 2003;98:630–638. doi: 10.1111/j.1572-0241.2003.07332.x. [DOI] [PubMed] [Google Scholar]
- 21.Daltro-Oliveira R, Morais-de-Jesus M, Pettersen KM, Parana R, Quarantini LC. Impact of sustained virologic response on quality of life in chronic HCV carriers. Ann Hepatol. 2013;12:399–407. [PubMed] [Google Scholar]
- 22.Grieve R, Roberts J, Wright M, Sweeting M, DeAngelis D, Rosenberg W, et al. Cost effectiveness of interferon alpha or peginterferon alpha with ribavirin for histologically mild chronic hepatitis C. Gut. 2006;55:1332–1338. doi: 10.1136/gut.2005.064774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodger AJ, Jolley D, Thompson SC, Lanigan A, Crofts N. The impact of diagnosis of hepatitis C virus on quality of life. Hepatology. 1999;30:1299–1301. doi: 10.1002/hep.510300504. [DOI] [PubMed] [Google Scholar]
- 24.Vera-Llonch M, Martin M, Aggarwal J, Donepudi M, Bayliss M, Goss T, et al. Health-related quality of life in genotype 1 treatment-naive chronic hepatitis C patients receiving TVRaprevir combination treatment in the ADVANCE study. Aliment Pharmacol Ther. 2013;38:124–133. doi: 10.1111/apt.12354. [DOI] [PubMed] [Google Scholar]
- 25.Pineda JA, Aguilar-Guisado M, Rivero A, Giron-Gonzalez JA, Ruiz-Morales J, Merino D, et al. Natural history of compensated hepatitis C virus-related cirrhosis in HIV-infected patients. Clin Infect Dis. 2009;49:1274–1282. doi: 10.1086/605676. [DOI] [PubMed] [Google Scholar]
- 26.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288. doi: 10.1016/j.cgh.2009.11.018. 288 e281. [DOI] [PubMed] [Google Scholar]
- 27.Walensky RP, Sax PE, Nakamura YM, Weinstein MC, Pei PP, Freedberg KA, et al. Economic savings versus health losses: the cost-effectiveness of generic antiretroviral therapy in the United States. Ann Intern Med. 2013;158:84–92. doi: 10.7326/0003-4819-158-2-201301150-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zeuzem S, Andreone P, Pol S, Lawitz E, Diago M, Roberts S, et al. TVRaprevir for retreatment of HCV infection. N Engl J Med. 2011;364:2417–2428. doi: 10.1056/NEJMoa1013086. [DOI] [PubMed] [Google Scholar]
- 29.Sulkowski M, Gardiner D, Rodriguez-Torres M, Reddy KR, Hassanein T, Jacobson IM, et al. European Association for the Study of the Liver, April 4th–28th. Netherlands: Amsterdam; 2013. Sustained virologic response with daclatasvir plus sofosbuvir ± ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed TVRaprevir (TVR) or boceprevir (BOC) [Google Scholar]
- 30.United States Department of Health and Human Services Center for Medicare Services. [Accessed January 15th, 2013];Physician Fee Schedule. 2011 from: http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/index.html?redirect=/PhysicianFeeSched.
- 31.United States Department of Health and Human Services Center for Medicare Services. [Accessed 1 February 2013];Clinical Diagnostic Laboratory Fee Schedule. 2011 Available: http://www.cms.gov/ClinicalLabFeesched/02_clinlab.asp.
- 32.University Health Systems Consortium. [Accessed January 30th, 2013];CDP Online Report. 2008 from: http://www.uhc.edu.
- 33. [Accessed 25 June 2013];Micromedex 2.0. Drug Topics Red Book Online. 2013 Available: http://www.micromedexsolutions.com.
- 34.United States Department of Labor Bureau of Labor Statistics. [Accessed January 15th, 2013];Consumer Price Index - All Urban Consumers. 2011 From: http://www.bls.gov/cpi/
- 35.Bamezai A, Melnick G, Nawathe A. The cost of an emergency department visit and its relationship to emergency department volume. Ann Emerg Med. 2005;45:483–490. doi: 10.1016/j.annemergmed.2004.08.029. [DOI] [PubMed] [Google Scholar]
- 36.Bozzette SA, Berry SH, Duan N, Frankel MR, Leibowitz AA, Lefkowitz D, et al. The care of HIV-infected adults in the United States. HIV Cost and Services Utilization Study Consortium. N Engl J Med. 1998;339:1897–1904. doi: 10.1056/NEJM199812243392606. [DOI] [PubMed] [Google Scholar]
- 37.Gebo KA, Fleishman JA, Conviser R, Hellinger J, Hellinger FJ, Josephs JS, et al. Contemporary costs of HIV healthcare in the HAART era. AIDS. 2010;24:2705–2715. doi: 10.1097/QAD.0b013e32833f3c14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levinson D. Medicaid drug price comparisons: Average manufacturer price to published prices; Department of Health and Human Services. 2005 from: http://www.oig.hhs.gov/oei/reports/oei-05-05-00240.pdf.
- 39.Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006;44:990–997. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- 40.Paltiel AD, Scharfstein JA, Seage GR, 3rd, Losina E, Goldie SJ, Weinstein MC, et al. A Monte Carlo simulation of advanced HIV disease: application to prevention of CMV infection. Med Decis Making. 1998;18:S93–105. doi: 10.1177/0272989X98018002S11. [DOI] [PubMed] [Google Scholar]
- 41.Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- 42.Siebert U, Sroczynski G, Rossol S, Wasem J, Ravens-Sieberer U, Kurth BM, et al. Cost effectiveness of peginterferon alpha-2b plus ribavirin versus interferon alpha-2b plus ribavirin for initial treatment of chronic hepatitis C. Gut. 2003;52:425–432. doi: 10.1136/gut.52.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stein K, Dalziel K, Walker A, McIntyre L, Jenkins B, Horne J, et al. Screening for hepatitis C among injecting drug users and in genitourinary medicine clinics: systematic reviews of effectiveness, modelling study and national survey of current practice. Health Technol Assess. 2002;6:1–122. [PubMed] [Google Scholar]
- 44.Fishbein DA, Lo Y, Reinus JF, Gourevitch MN, Klein RS. Factors associated with successful referral for clinical care of drug users with chronic hepatitis C who have or are at risk for HIV infection. J Acquir Immune Defic Syndr. 2004;37:1367–1375. doi: 10.1097/01.qai.0000131932.21612.49. [DOI] [PubMed] [Google Scholar]
- 45.Hall CS, Charlebois ED, Hahn JA, Moss AR, Bangsberg DR. Hepatitis C virus infection in San Francisco’s HIV-infected urban poor. J Gen Intern Med. 2004;19:357–365. doi: 10.1111/j.1525-1497.2004.30613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mehta SH, Lucas GM, Mirel LB, Torbenson M, Higgins Y, Moore RD, et al. Limited effectiveness of antiviral treatment for hepatitis C in an urban HIV clinic. AIDS. 2006;20:2361–2369. doi: 10.1097/QAD.0b013e32801086da. [DOI] [PubMed] [Google Scholar]
- 47.Strasfeld L, Lo Y, Netski D, Thomas DL, Klein RS. The association of hepatitis C prevalence, activity, and genotype with HIV infection in a cohort of New York City drug users. J Acquir Immune Defic Syndr. 2003;33:356–364. doi: 10.1097/00126334-200307010-00010. [DOI] [PubMed] [Google Scholar]
- 48.Fultz SL, Justice AC, Butt AA, Rabeneck L, Weissman S, Rodriguez-Barradas M. Testing, referral, and treatment patterns for hepatitis C virus coinfection in a cohort of veterans with human immunodeficiency virus infection. Clin Infect Dis. 2003;36:1039–1046. doi: 10.1086/374049. [DOI] [PubMed] [Google Scholar]
- 49.Backus LI, Boothroyd DB, Phillips BR, Mole LA. Pretreatment assessment and predictors of hepatitis C virus treatment in US veterans coinfected with HIV and hepatitis C virus. J Viral Hepat. 2006;13:799–810. doi: 10.1111/j.1365-2893.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 50.Pineda JA, Mira JA, Gil Ide L, Valera-Bestard B, Rivero A, Merino D, et al. Influence of concomitant antiretroviral therapy on the rate of sustained virological response to pegylated interferon plus ribavirin in hepatitis C virus/HIV-coinfected patients. J Antimicrob Chemother. 2007;60:1347–1354. doi: 10.1093/jac/dkm373. [DOI] [PubMed] [Google Scholar]
- 51.Zinkernagel AS, von Wyl V, Ledergerber B, Rickenbach M, Furrer H, Battegay M, et al. Eligibility for and outcome of hepatitis C treatment of HIV-coinfected individuals in clinical practice: the Swiss HIV cohort study. Antivir Ther. 2006;11:131–142. [PubMed] [Google Scholar]
- 52.Freeman AJ, Dore GJ, Law MG, Thorpe M, Von Overbeck J, Lloyd AR, et al. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatology. 2001;34:809–816. doi: 10.1053/jhep.2001.27831. [DOI] [PubMed] [Google Scholar]
- 53.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22:1979–1991. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 54.Aparicio E, Parera M, Franco S, Perez-Alvarez N, Tural C, Clotet B, et al. IL28B SNP rs8099917 is strongly associated with pegylated interferon-alpha and ribavirin therapy treatment failure in HCV/HIV-1 coinfected patients. PLoS One. 2010;5:e13771. doi: 10.1371/journal.pone.0013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pineda JA, Caruz A, Di Lello FA, Camacho A, Mesa P, Neukam K, et al. Low-density lipoprotein receptor genotyping enhances the predictive value of IL28B genotype in HIV/hepatitis C virus-coinfected patients. AIDS. 2011;25:1415–1420. doi: 10.1097/QAD.0b013e328348a7ac. [DOI] [PubMed] [Google Scholar]
- 56.Pineda JA, Caruz A, Rivero A, Neukam K, Salas I, Camacho A, et al. Prediction of response to pegylated interferon plus ribavirin by IL28B gene variation in patients coinfected with HIV and hepatitis C virus. Clin Infect Dis. 2010;51:788–795. doi: 10.1086/656235. [DOI] [PubMed] [Google Scholar]
- 57.Torriani FJ, Rodriguez-Torres M, Rockstroh JK, Lissen E, Gonzalez-Garcia J, Lazzarin A, et al. Peginterferon Alfa-2a plus ribavirin for chronic hepatitis C virus infection in HIV-infected patients. N Engl J Med. 2004;351:438–450. doi: 10.1056/NEJMoa040842. [DOI] [PubMed] [Google Scholar]
- 58.Drummond M, Sculpher M, Torrance G, O’Brien B, Stoddart G. Methods for the economic evaluation of health care programmes. Third edition. Oxford: Oxford University Press; 2005. [Google Scholar]
- 59.Gold M, Siegel J, Russell L, Weinstein M. Cost-Effectiveness in Health and Medicine. New York: Oxford University Press; 1996. [Google Scholar]
- 60.Cantor SB. Cost-effectiveness analysis, extended dominance, and ethics: a quantitative assessment. Med Decis Making. 1994;14:259–265. doi: 10.1177/0272989X9401400308. [DOI] [PubMed] [Google Scholar]
- 61.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–1641. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 62.Braithwaite RS, Meltzer DO, King JT, Jr., Leslie D, Roberts MS. What does the value of modern medicine say about the $50,000 per quality-adjusted life-year decision rule? Med Care. 2008;46:349–356. doi: 10.1097/MLR.0b013e31815c31a7. [DOI] [PubMed] [Google Scholar]
- 63.Chung RT, Andersen J, Volberding P, Robbins GK, Liu T, Sherman KE, et al. Peginterferon Alfa-2a plus ribavirin versus interferon alfa-2a plus ribavirin for chronic hepatitis C in HIV-coinfected persons. N Engl J Med. 2004;351:451–459. doi: 10.1056/NEJMoa032653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fried MW, Hadziyannis SJ, Shiffman ML, Messinger D, Zeuzem S. Rapid virological response is the most important predictor of sustained virological response across genotypes in patients with chronic hepatitis C virus infection. J Hepatol. 2011;55:69–75. doi: 10.1016/j.jhep.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 65.Martinot-Peignoux M, Maylin S, Moucari R, Ripault MP, Boyer N, Cardoso AC, et al. Virological response at 4 weeks to predict outcome of hepatitis C treatment with pegylated interferon and ribavirin. Antivir Ther. 2009;14:501–511. [PubMed] [Google Scholar]
- 66.Poordad FF. Review article: the role of rapid virological response in determining treatment duration for chronic hepatitis C. Aliment Pharmacol Ther. 2010;31:1251–1267. doi: 10.1111/j.1365-2036.2010.04300.x. [DOI] [PubMed] [Google Scholar]
- 67.Liu S, Cipriano LE, Holodniy M, Owens DK, Goldhaber-Fiebert JD. New protease inhibitors for the treatment of chronic hepatitis C: a cost-effectiveness analysis. Ann Intern Med. 2012;156:279–290. doi: 10.1059/0003-4819-156-4-201202210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gellad Z. The cost-effectiveness of a TVRaprevir-inclusive regimen as initial therapy for genotype 1 hepaticis C infection in individuals with the CC IL28B polymorphism; 62nd Annual Meeting of the American Association for the Study of Liver Diseases (AASLD); November 4–11; San Francisco. 2011. [Google Scholar]
- 69.Coffin PO, Scott JD, Golden MR, Sullivan SD. Cost-effectiveness and Population Outcomes of General Population Screening for Hepatitis C. Clin Infect Dis. 2012;54:1259–1271. doi: 10.1093/cid/cis011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hagan LM, Yang Z, Ehteshami M, Schinazi RF. All-oral, interferon-free treatment for chronic hepatitis C: cost-effectiveness analyses. Journal of Viral Hepatitis. 2013:1–11. doi: 10.1111/jvh.12111. [DOI] [PubMed] [Google Scholar]
- 71.Reekie J, GaTVRl JM, Yust I, Bakowska E, Rakhmanova A, Losso M, et al. Fatal and nonfatal AIDS and non-AIDS events in HIV-1-positive individuals with high CD4 cell counts according to viral load strata. AIDS. 2011;25:2259–2268. doi: 10.1097/QAD.0b013e32834cdb4b. [DOI] [PubMed] [Google Scholar]
- 72.Arendt M, Munk-Jorgensen P, Sher L, Jensen SO. Mortality among individuals with cannabis, cocaine, amphetamine, MDMA, and opioid use disorders: a nationwide follow-up study of Danish substance users in treatment. Drug Alcohol Depend. 2011;114:134–139. doi: 10.1016/j.drugalcdep.2010.09.013. [DOI] [PubMed] [Google Scholar]
- 73.Seage GR, 3rd, Holte SE, Metzger D, Koblin BA, Gross M, Celum C, et al. Are US populations appropriate for trials of human immunodeficiency virus vaccine? The HIVNET Vaccine Preparedness Study. Am J Epidemiol. 2001;153:619–627. doi: 10.1093/aje/153.7.619. [DOI] [PubMed] [Google Scholar]
- 74.Akkarathamrongsin S, Sugiyama M, Matsuura K, Kurbanov F, Poovorawan Y, Tanaka Y, et al. High sensitivity assay using serum sample for IL28B genotyping to predict treatment response in chronic hepatitis C patients. Hepatol Res. 2010:956–962. doi: 10.1111/j.1872-034X.2010.00702.x. [DOI] [PubMed] [Google Scholar]
- 75.Kani S, Tanaka Y, Matsuura K, Watanabe T, Yatsuhashi H, Orito E, et al. Development of new IL28B genotyping method using Invader Plus assay. Microbiol Immunol. 2012;56:318–323. doi: 10.1111/j.1348-0421.2012.00439.x. [DOI] [PubMed] [Google Scholar]
- 76.Lin M, Rouster SD, Charlton A, Sherman KE. High-resolution melting analysis: A rapid and accurate alternative method for the detection of IL28B single-nucleotide polymorphisms; Conference on Retroviruses and Opportunistic Infections; March 5–8, 2012; Seattle. [Google Scholar]
- 77.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, et al. The natural history of compensated cirrhosis due to hepatitis C virus: A 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 78.Mellors JW, Munoz A, Giorgi JV, Margolick JB, Tassoni CJ, Gupta P, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–954. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 79.Multicenter AIDS Cohort Study (MACS) Public Dataset: Release PO4. Springfield, VA: National Technical Information Service; 1995. [Google Scholar]
- 80.Bozzette SA, Larsen RA, Chiu J, Leal MA, Jacobsen J, Rothman P, et al. A placebo-controlled trial of maintenance therapy with fluconazole after treatment of cryptococcal meningitis in the acquired immunodeficiency syndrome. California Collaborative Treatment Group. N Engl J Med. 1991;324:580–584. doi: 10.1056/NEJM199102283240902. [DOI] [PubMed] [Google Scholar]
- 81.Cole SR, Hernan MA, Robins JM, Anastos K, Chmiel J, DeTVRs R, et al. Effect of highly active antiretroviral therapy on time to acquired immunodeficiency syndrome or death using marginal structural models. Am J Epidemiol. 2003;158:687–694. doi: 10.1093/aje/kwg206. [DOI] [PubMed] [Google Scholar]
- 82.Drew WL, Ives D, Lalezari JP, Crumpacker C, Follansbee SE, Spector SA, et al. Oral ganciclovir as maintenance treatment for cytomegalovirus retinitis in patients with AIDS. Syntex Cooperative Oral Ganciclovir Study Group. N Engl J Med. 1995;333:615–620. doi: 10.1056/NEJM199509073331002. [DOI] [PubMed] [Google Scholar]
- 83.Ioannidis JP, Cappelleri JC, Skolnik PR, Lau J, Sacks HS. A meta-analysis of the relative efficacy and toxicity of Pneumocystis carinii prophylactic regimens. Arch Intern Med. 1996;156:177–188. [PubMed] [Google Scholar]
- 84.Pedrol E, Gonzalez-Clemente JM, GaTVRl JM, Mallolas J, Miro JM, Graus F, et al. Central nervous system toxoplasmosis in AIDS patients: efficacy of an intermittent maintenance therapy. AIDS. 1990;4:511–517. doi: 10.1097/00002030-199006000-00003. [DOI] [PubMed] [Google Scholar]
- 85.Powderly WG, Saag MS, Cloud GA, Robinson P, Meyer RD, Jacobson JM, et al. A controlled trial of fluconazole or amphotericin B to prevent relapse of cryptococcal meningitis in patients with the acquired immunodeficiency syndrome.The NIAID AIDS Clinical Trials Group and Mycoses Study Group. N Engl J Med. 1992;326:793–798. doi: 10.1056/NEJM199203193261203. [DOI] [PubMed] [Google Scholar]
- 86.Wheat J, Hafner R, Wulfsohn M, Spencer P, Squires K, Powderly W, et al. Prevention of relapse of histoplasmosis with itraconazole in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1993;118:610–616. doi: 10.7326/0003-4819-118-8-199304150-00006. [DOI] [PubMed] [Google Scholar]
- 87.Carrat F, Bani-Sadr F, Pol S, Rosenthal E, Lunel-Fabiani F, Benzekri A, et al. Pegylated interferon alfa-2b vs standard interferon alfa-2b, plus ribavirin, for chronic hepatitis C in HIV-infected patients: a randomized controlled trial. JAMA. 2004;292:2839–2848. doi: 10.1001/jama.292.23.2839. [DOI] [PubMed] [Google Scholar]
- 88.Sherman KE, Flamm SL, Afdhal NH, Nelson DR, Sulkowski MS, Everson GT, et al. Response-guided TVRaprevir combination treatment for hepatitis C virus infection. N Engl J Med. 2011;365:1014–1024. doi: 10.1056/NEJMoa1014463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dieterich D, Soriano V, Sherman KE, Girard P-M, Rockstroth JK, Henshaw J, et al. TVRaprevir in combination with peginterferon Alfa-2a/ribavirin in HCV/HIV co-infected patients: SVR12 interim analysis; Conference for Retroviruses and Opportunistic Infections; Mar 5–8; Seattle, Washington. 2012. [Google Scholar]
- 90.Mangia A, Santoro R, Minerva N, Ricci GL, Carretta V, Persico M, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. N Engl J Med. 2005;352:2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 91.Gallant JE, DeJesus E, Arribas JR, Pozniak AL, Gazzard B, Campo RE, et al. Tenofovir DF, Emtricitabine, and Efavirenz vs. Zidovudine, Lamivudine, and Efavirenz for HIV. N Engl J Med. 2006;354:251–260. doi: 10.1056/NEJMoa051871. [DOI] [PubMed] [Google Scholar]
- 92.Grinsztejn B, Nguyen B, Katlama C, GaTVRl J, Lazzarin A, Vittecog D, et al. Safety and efficacy of the HIV-1 integrase inhibitor raltegravir (MK-0518) in treatment-experienced patients with multidrug-resistant virus: a phase II randomised controlled trial. Lancet. 2007;369:1261–1269. doi: 10.1016/S0140-6736(07)60597-2. [DOI] [PubMed] [Google Scholar]
- 93.Johnson M, Grinsztejn B, Rodriguez C, Coco J, DeJesus E, Lazzarin A, et al. Atazanavir plus ritonavir or saquinavir, and lopinavir/ritonavir in patients experiencing multiple virological failures. AIDS. 2005;19:685–694. doi: 10.1097/01.aids.0000166091.39317.99. [DOI] [PubMed] [Google Scholar]
- 94.Lalezari JP, Goodrich J, deJesus E, Lampiris H, Gulick R, Saag M, et al. Efficacy and safety of maraviroc plus optimized background therapy in viremic ART-experienced patients infected with CCR5-tropic HIV-1: 24-week results of a phase 2b/3 study in the US and Canada; Conference on Retroviruses and Opportunistic Infections; February 25–28; Los Angeles, CA. 2007. [Google Scholar]
- 95.Nelson M, Arasteh K, Clotet B, Cooper DA, Henry K, Katlama C, et al. Durable Efficacy of Enfuvirtide Over 48 Weeks in Heavily Treatment-Experienced HIV-1-Infected Patients in the T-20 Versus Optimized Background Regimen Only 1 and 2 Clinical Trials. JAIDS. 2005;40:404–412. doi: 10.1097/01.qai.0000185314.56556.c3. [DOI] [PubMed] [Google Scholar]
- 96.Flieishman J. B Linas., editor. HIV loss to follow up data from the HIV Research Network. 2013
- 97.Gao X, Stephens JM, Carter JA, Haider S, Rustgi VK. Impact of adverse events on costs and quality of life in protease inhibitor-based combination therapy for hepatitis C. Expert Rev Pharmacoecon Outcomes Res. 2012;12:335–343. doi: 10.1586/erp.12.10. [DOI] [PubMed] [Google Scholar]
- 98.McHutchison JG, Lawitz EJ, Shiffman ML, Muir AJ, Galler GW, McCone J, et al. Peginterferon alfa-2b or alfa-2a with ribavirin for treatment of hepatitis C infection. N Engl J Med. 2009;361:580–593. doi: 10.1056/NEJMoa0808010. [DOI] [PubMed] [Google Scholar]
- 99.Schackman BR, Teixeira PA, Weitzman G, Mushlin AI, Jacobson IM. Quality-of-life tradeoffs for hepatitis C treatment: do patients and providers agree? Med Decis Making. 2008;28:233–242. doi: 10.1177/0272989X07311753. [DOI] [PubMed] [Google Scholar]
- 100.Food and Drug Administration. [Accessed August 7, 2013];TVRaprevir package insert. 2012 from: http://www.fda.gov.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.