Abstract
Background
The phenotypic variance observed in panic disorder (PD) appears to be best captured by a respiratory and non-respiratory panic subtype. We compared respiratory and non-respiratory panic subtypes across a series of external validators (temporal stability, psychiatric co-morbidity, treatment response) to determine whether subtypes are best conceptualized as differing: (1) only on their symptom profiles with no other differences between them; (2) on a quantitative (i.e. severity) dimension only; or (3) qualitatively from one another.
Method
Data from a large epidemiological survey (National Epidemiologic Survey on Alcohol and Related Conditions) and a clinical trial (Cross-National Collaborative Panic Study) were used. All analytic comparisons were examined within a latent class framework.
Results
High temporal stability of panic subtypes was observed, particularly among females. Respiratory panic was associated with greater odds of lifetime major depression and a range of anxiety disorders as well as increased treatment utilization, but no demographic differences. Treatment outcome data did not suggest that the two PD subtypes were associated with differential response to either imipramine or alprazolam.
Conclusions
These data suggest that respiratory and non-respiratory panic represent valid subtypes along the PD continuum, with the respiratory variant representing a more severe form of the disorder.
Keywords: External validation, latent class, panic disorder, respiratory, stability, subtypes
Introduction
Panic disorder (PD) is a heterogeneous psychiatric syndrome characterized by unexpected, recurrent panic attacks (APA, 2000). The most recent epidemiologic data from the 2001–2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) found a 12-month prevalence of DSM-IV PD with agoraphobia (PDA) or without agoraphobia to be 2.1%, with a lifetime prevalence of 5.1% (Grant et al. 2006). The lifetime prevalence observed in the NESARC was somewhat higher than the epidemiological catchment area (ECA) survey, which observed a lifetime prevalence of 1.2% for DSM-III defined PD and 0.5% for PDA (Regier et al. 1990) as well as the National Comorbidity Study (NCS), which found a lifetime prevalence of 2.2% for DSM-III-R PD and 1.4% for PDA (Eaton et al. 1994). Differences in prevalence estimates likely reflect changes to criterion A (frequency of panic) of DSM PD criteria. Regardless of DSM version, however, females are consistently observed to be approximately two times more likely to meet criteria for PD or PDA compared to males.
Cross-national epidemiologic data also suggest that the lifetime prevalence rates for PD in other countries are generally similar to rates observed in the United States (Weissman et al. 1997). Moreover, the mean age at onset was in early to middle adulthood and PD was associated with an increased risk of agoraphobia and major depression in all countries. Among individuals seeking treatment for PD, co-morbidity rates for Axis I disorders generally range from 51% to 69%, with generalized anxiety disorder (GAD), social phobia and major depression being the most common co-morbid conditions (Sanderson et al. 1990; Brown & Barlow, 1992; Brown et al. 1995, 2001; Tsao et al. 1998, 2002). These data suggest that PD is a reliably observed psychiatric syndrome that affects females significantly more often than males and is commonly co-morbid with mood and other anxiety disorders.
In a recent comprehensive analysis, Roberson-Nay & Kendler (in press) explored heterogeneity in panic symptoms assessed in community and clinical samples with the goal of identifying clinically meaningful panic subtypes. The subtype concept is not new to the PD literature and a number of different panic subtype schemes have been proposed, ranging from diagnostic (e.g. PD with co-occurring levels of agoraphobic avoidance; Schneier et al. 1991; Starcevic et al. 1992; Uhlenhuth et al. 2006) to clinical/laboratory based models (e.g. respiratory panic; Klein, 1993). Roberson-Nay & Kendler (in press) focused on a panic symptom subtyping method versus one rooted in diagnostic covariation (e.g. PD with or without agoraphobia) or other panic-related factors (e.g. situational versus unexpected panic attacks). Moreover, the focus rested exclusively on panic symptoms in the context of PD or PDA versus panic attacks in general and no a priori predictions about the number or structure of to-be-identified panic subtypes was made. Panic symptom items from respondents participating in one of three epidemiologic surveys (ECA, NCS, NESARC-Wave 1), an adult epidemiological twin study [Virginia Adult Twin Study of Psychiatric and Substance Use Disorders (VATSPSUD)] and a clinical trial of PD [Cross-National Collaborative Panic Study (CNCPS)] were examined using latent class methods.
Robust evidence for more than one class of panic was found, with the ECA, NCS, VATSPSUD and CNCPS data suggesting a two class (i.e. subtype) model as best fitting. Most importantly, results from the ECA, VATSPSUD and the CNCPS were in agreement with each other and with those of Briggs et al. (1993), who originally found evidence for a respiratory and non-respiratory form of panic. Respiratory panic was defined as the presence of five respiratory symptoms (dyspnea, chest pain, feelings of choking, paresthesia and fear of dying) as well as elevations across most all other panic symptoms, whereas non-respiratory panic exhibited low endorsement rates of respiratory symptoms but high endorsement of all other panic symptoms (e.g. tachycardia, sweating, trembling). A two class model did not best fit the NESARC data, however. Rather, a three class model was best fitting. Among the three classes, a respiratory and non-respiratory subtype was observed as well as a milder form of respiratory panic. When analysis of NESARC data was limited to PD/PDA cases who were symptomatic in the past year and who sought treatment for their panic attacks, a three class model continued to best capture the data.
Across all epidemiologic samples, respiratory and non-respiratory panic was approximately equally prevalent. In the clinical sample (CNCPS), however, the respiratory panic subtype represented approximately 65% of the sample, suggesting that persons with this form of panic attack may seek treatment at higher rates compared with those suffering with non-respiratory panic attacks. Collectively, these results suggest that panic manifests in two primary forms, with one subgroup exhibiting respiratory-related symptoms.
The aim of the current study is to directly compare the respiratory and non-respiratory panic classes observed previously in the Roberson-Nay & Kendler (in press) study across a series of external validators. Two of Robins & Guze’s (1970) criteria for external validation will be considered, including: (1) an examination of several core clinical features of the disorder (patterns of co-morbidity, sociodemographic factors, panic features, and treatment response); (2) temporal stability of subtypes. Outcomes will help determine whether respiratory and non-respiratory panic subtypes: (1) are best understood as a single disorder that differs only on their symptom profiles with no other differences between them; (2) represent two subtypes that differ on a quantitative (i.e. severity) dimension only; or (3) represent two subtypes that differ qualitatively (i.e. distinct patterns) from one another. To show severity differences, subtypes would be predicted to differ across a range of factors with one subtype associated with increased psychiatric comorbidity, treatment utilization, longer and/or more frequent panic episodes and greater disability. To show that panic subtypes represent distinct forms with differing etiologies is more difficult. If panic is represented by two primary forms, we would predict minimal transitioning as well as distinct patterns of co-morbidity, panic features, risk factors and, possibly, differential treatment response.
Method
Data in all analyses was based on respondents in the NESARC or participants in the CNCPS (Klerman, 1988) who qualified for a current or lifetime diagnosis of PD/PDA. Although Roberson-Nay & Kendler (in press) analyzed panic symptom data from three additional epidemiological surveys (i.e. ECA, NCS and VATSPSUD), these datasets were not included in the present paper due to their small sample size, which generated model convergence issues or produced untrustworthy parameters estimates when covariates were considered in the models. Thus, the two datasets (NESARC, n=2294 and CNCPS, n=1169) with the greatest power were selected for analysis, with each representing diverse ascertainment methods (epidemiologic versus a treatment-seeking sample).
Samples
The NESARC is a longitudinal survey, with its first wave of interviews fielded between 2001 and 2002 and its second wave of interviews collected between 2004 and 2005. The NESARC is a representative sample of the United States population, with 43 093 respondents participating in the first wave. Efforts were made to re-interview all 43 093 respondents in Wave 2, with 80.4% (n=34 653) participating in Waves 1 and 2. Of persons with PD in Wave 1 (n=2294), 84.5% (n=1939) participated in Wave 2. The NESARC’s selected population includes individuals aged ≥ 18 years residing in the United States. The interview instrument used to diagnose all disorders was the NIAAA Alcohol Use Disorder and Associated Disabilities Interview Schedule, DSM-IV version.
The CNCPS was a large multi-center (n=1168) 8-week treatment trial of alprazolam, imipramine and placebo in persons with PD or PDA. The following were inclusion criteria for this study: (1) meeting modified DSM-III criteria for the diagnosis of PD, implemented via the Structured Clinical Interview for DSM-III Diagnosis, Upjohn (SCID-UP); (2) a history of at least one spontaneous panic attack with at least four symptoms; (3) having had at least one panic attack with three or more symptoms per week for the 3 weeks before study entry. Investigators underwent intensive, week-long training, with particular focus on the administration of the SCID-UP interview and other assessment instruments. Reliability studies of the SCID-UP suggest very good inter-rater reliability (κ=0.86) for diagnosis of PD (Williams et al. 1992).
Clinical change [i.e. clinician global improvement (CGI)] was assessed over 8 weeks of double-blind drug treatment. The CGI was rated on an 11-point scale, with a low rating of 0 (very bad, could not be worse), a midpoint of 5 (unchanged) and the high rating of 10 (major improvement, back to normal). All patients were rated as 5 at baseline. Patients also rated their subjective assessment of their improvement (patient global improvement); however, this measure correlated strongly and significantly with weekly CGI ratings (r range=0.77–0.84). Thus, only the CGI was examined. An additional goal of the CNCPS was to gain greater insight into the phenomenology of panic attacks including number, severity, duration and type (i.e. cued versus uncued panic attacks). Patients recorded the percentage of each day spent worrying about having a panic attack or entering a situation that might precipitate an attack. Patients also recorded intensity of anticipatory anxiety. Panic diary data were reviewed with the patient at each visit to ensure data integrity. A clinician also completed the Hamilton Anxiety Scale, a 21-item rating scale, with the patient at each visit. Percentage and intensity of anticipatory anxiety were highly correlated (r range=0.67–0.78). Thus, only percentage of anticipatory anxiety was used as an outcome measure. Finally, patients also completed the weekly Sheehan Disability Scale, which included a visual analogue scale ranging from 0 (not at all) to 10 (very severely). Patients rated their disability in three domains including social, work and family life. A total disability score was tabulated as the sum of these three domains. Selected outcomes for this paper include the CGI, Hamilton Anxiety Scale, number of uncued panic attacks per week, number of cued panic attacks per week, percentage of weekly anticipatory anxiety and Sheehan Disability Scale total score.
Medications and placebo were prescribed according to a predetermined schedule. Imipramine was increased in dosage units of 25 mg and alprazolam was increased in 1 mg units (CNCPS, Second Phase Investigators, 1992). Medication dosage was increased steadily according to the prescribed schedule, which specified a dosage of 6 mg of alprazolam, 150 of imipramine or six placebo tablets by day 19. Physicians could increase or decrease medication dose, depending upon the individual patient’s clinical state or adverse side effects, to a total of 10 identical capsules (10 mg alprazolam, 250 mg imipramine, 10 placebo capsules).
Statistical analysis
Unfortunately, not all variables were present in the NESRAC and CNCPS datasets. Thus, some analyses are based only on data from one dataset. See Table 1 for a complete variable list. Moreover, for the purpose of this paper, we used the respiratory and non-respiratory subtypes identified previously in the CNCPS study. For the NESARC, we used all three identified panic subtypes where possible (i.e. stability analysis), but for analyses in which we directly contrast the CNCPS and NESARC (i.e. lifetime comorbid diagnoses, sociodemographics, and panic-related variables), we only used the respiratory and non-respiratory subtypes observed in the NESARC, excluding the milder respiratory variant to maintain consistency within this set of analyses.
Table 1.
Variables available for analysis by dataset
| Variable list
|
Dataset
|
|
|---|---|---|
| Lifetime psychiatric disorders | NESARC | CNCPS |
| Agoraphobia | ✓ | ✓ |
| GAD | ✓ | ✓ |
| Social phobia | ✓ | ✓ |
| Specific phobia | ✓ | ✓ |
| MDD | ✓ | ✓ |
| Alcohol dependence | ✓ | – |
| Nicotine dependence | ✓ | – |
| Demographics | ||
| Gender | ✓ | ✓ |
| Age | ✓ | ✓ |
| Panic features | ||
| Age of onset | ✓ | ✓ |
| PD duration | – | ✓ |
| Pharmacological Tx | ✓ | – |
| Psychosocial Tx | ✓ | ✓ |
| Age any Tx first sought | ✓ | ✓ |
| Stability | ✓ | – |
| Treatment outcome | ||
| CGI | – | ✓ |
| Cued attacks per week (n) | – | ✓ |
| Uncued attacks per week (n) | – | ✓ |
| Anticipatory anxiety (%) | – | ✓ |
| Sheehan Disability Scale | – | ✓ |
| Hamilton Anxiety Scale | – | ✓ |
NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; CNCPS, Cross-National Collaborative Panic Study; GAD, generalized anxiety disorder; MDD, major depressive disorder; PD, panic disorder, Tx, treatment; CGI, clinician global improvement.
A check mark indicates that the variable was available for analysis.
Latent transition analysis
Latent transition analysis (LTA) is an analytic approach that explores stability/instability in latent class membership over time. The 13 DSM-IV panic symptom items assessed at Wave 1 of the NESARC and Wave 2 of the NESARC were used to determine temporal stability of panic subtypes. The LTA included only those respondents (n=376; i.e. recurrent since last interview) meeting criteria for a lifetime diagnosis of PD/PDA at Wave 1 and a separate, recurrent panic episode at Wave 2. NESARC defined a panic episode as ending if a respondent endorsed not experiencing any panic attacks for at least 2 months. Participants experiencing a continuation of the same panic episode from Wave 1 to Wave 2 were not included in the stability analysis because inclusion of these persons would represent a reliability analysis of panic symptoms versus a stability analysis. These ‘ recurrent’ criteria correspond to those defined in the NESARC-Wave 2 data notes.
The inclusion of covariates in an LTA model can describe and explain heterogeneity in the longitudinal course being examined. Two types of observed covariates appropriate for study include time-varying and time-invariant variables. The time-varying covariates are variables measured repeatedly at the same time as the outcome (e.g. depression). The time-invariant covariates are static variables that are only measured once (e.g. gender). The influence of three time-invariant covariates (gender, age at the Wave 1 assessment and age of PD/PDA onset) on transition probabilities was examined. In the context of a LTA, exploring measurement invariance involves testing the assumption that the structure of panic subtypes is similar across time and, therefore, can be considered the same. Assuming full measurement invariance of classes facilitates interpretation of transitions among panic subtypes because the subtypes (or classes) are the same across time. A χ2 difference test based on log likelihood values and scaling correction factors obtained with the maximum likelihood robust (MLR) estimator was used to assess the plausibility of measurement invariance for panic subtypes. The test indicated no significant worsening in fit when the two panic subtypes were held invariant (p>0.05). Thus, we were able to assume the same panic subtype structure across time with non-invariant transitional probabilities.
Lifetime co-morbid diagnoses, sociodemographics and panic-related variables
To determine possible differences in lifetime comorbidities, demographics and panic features, the factor mixture models described by Roberson-Nay & Kendler (in press) were re-examined with these variables entered as covariates. Two approaches were taken to generate an odds ratio (OR) between the covariates and respiratory versus non-respiratory panic. First, all models were run with each covariate entered separately to examine its unique association with panic subtypes. Next, three models were examined for each dataset, with one model containing each of three conceptual sets of covariates (lifetime co-morbidities, demographic variables and panic features). This allowed examination of covariates above and beyond the effects of other variables. Lifetime psychiatric diagnoses were coded as binary variables (0=lifetime diagnosis absent, 1=lifetime diagnosis present). For the epidemiologic surveys, duration of PD could not be calculated as current age minus age of onset because many respondents were in a remissive state, not experiencing panic for many years. To capture the most accurate measure of PD duration, illness duration was only calculated for people who were symptomatic at the time of the epidemiologic interview (age of onset–current age). Medications and therapy for panic were coded as binary variables (e.g. 0=no medication use for panic, 1=medication used to treat panic). Therapy generally reflected seeking help from psychologist, social worker or counselor.
Treatment outcome
Latent growth curve analysis (LGCA) represents an approach to repeated measurements of a dependent variable over time. The trajectory of an individual is modeled as a function of an underlying process (e.g. linear and/or non-linear change over time). LGCA was used to examine outcomes assessed over 8 weeks of treatment as part of the CNCPS.
With the exception of the CGI, the LGCAs included outcomes assessed at baseline as well as treatment weeks 1–4, week 6 and week 8, for a total of seven repeated measurements. For the CGI, the baseline was not included in the LGCA model given that all patients were rated 5 (i.e. ‘unchanged’) and, therefore, there was no variability in this data point. Thus, the intercept for the CGI represents the clinician’s rating at week 1, which reflects a week of active medication or placebo.
Before conducting LGCAs of panic outcomes, unconditional growth models (i.e. no main or interactive effects) were examined for each outcome variable to determine the best fitting function (e.g. linear, quadratic, cubic). Across all outcomes, models included an initial level (intercept), linear rate of change (slope) and differential rate (i.e. acceleration/deceleration) of change (quadratic slope). Three LGCAs were examined for each outcome variable: (1) a LGCA model containing panic class only; (2) a LGCA model containing medication treatment condition only; (3) a full model containing both main effects and their interaction. To examine the main effects of treatment, Helmert contrast coding was used to create two dichotomous treatment conditions. One contrast code reflected differences between placebo and active medication (alprazolam or imipramine) and a second contrast code compared alprazolam to imipramine. The LGCA model also included a main effect for respiratory and non-respiratory panic, using the respondent’s posterior probability of membership with respiratory class (i.e. 1 minus posterior probability in non-respiratory class). The LGCA model also included the interaction of treatment conditions and class. Four primary statistical outcomes are associated with the LGCAs; these include: (1) overall model fit; (2) significance associated with intercept (mean score at start); (3) significance of the slope (average linear rate of change); (4) significance of the quadratic function [differential (acceleration/deceleration) rate of change]. Model fit was judged using the comparative fit index (CFI), Tucker–Lewis Index (TLI) and root mean square error of approximation (RMSEA).
For the LGCAs, data collection site was used as a cluster variable, with the complex analysis type specified. The MLR estimator was used in all latent analyses, which were conducted in Mplus 5.2 (Muthen & Muthen, 2007).
Results
Temporal stability of panic subtypes
As mentioned, the NESARC-Wave 1 was best captured by a three class model including a respiratory, intermediate respiratory and non-respiratory class (Roberson-Nay & Kendler, in press). Transitional probabilities associated with a three class model (Table 2) indicated robust stability of panic subtypes (range 0.75–0.89%) from Wave 1 to Wave 2. The most significant transition occurred between respiratory and non-respiratory, with 18% of respondents transitioning from respiratory panic at Wave 1 to non-respiratory panic at Wave 2, and 11% transitioning from non-respiratory panic at Wave 1 to respiratory panic at Wave 2. Altogether, 13% of intermediate respiratory panic respondents transitioned to respiratory panic at Wave 2 while 7% of respondents identified as respiratory panic transitioned to intermediate respiratory panic at Wave 2. No respondents transitioned from non-respiratory panic at Wave 1 to intermediate respiratory panic at Wave 2 or vice versa.
Table 2.
Class membership probabilities (Δ estimates) and transitional probabilities (τ estimates) in latent class membership
| NESARC-Wave 1 | NESARC-Wave 2
|
||
|---|---|---|---|
| Respiratory | Intermediate respiratory | Non-respiratory | |
| Prevalence of classes | |||
| Wave 1 | 0.50 | 0.22 | 0.29 |
| Wave 2 | 0.43 | 0.23 | 0.34 |
| Transitional probabilities from Wave 1 (rows) to Wave 2 (columns) | |||
| Respiratory | 0.75 | 0.07 | 0.18 |
| Intermediate respiratory | 0.13 | 0.88 | 0.00 |
| Non-respiratory | 0.11 | 0.00 | 0.89 |
| Effect of covariates on transitional probabilities from Wave 1 (rows) to Wave 2 (columns) | |||
| Age | |||
| Respiratory | 0.75 | 0.07 | 0.17 |
| Intermediate respiratory | 0.13 | 0.87 | 0.00 |
| Non-respiratory | 0.11 | 0.00 | 0.89 |
| Age of onset | |||
| Respiratory | 0.75 | 0.07 | 0.18 |
| Intermediate respiratory | 0.12 | 0.88 | 0.00 |
| Non-respiratory | 0.11 | 0.00 | 0.89 |
| Gender | |||
| Respiratory | 0.75 | 0.07 | 0.19 |
| Intermediate respiratory | 0.11 | 0.89 | 0.00 |
| Non-respiratory | 0.10 | 0.00 | 0.89 |
| Transitional probabilities by gender | |||
| Males | |||
| Respiratory | 0.65 | 0.00 | 0.35 |
| Intermediate respiratory | 0.14 | 0.86 | 0.00 |
| Non-respiratory | 0.18 | 0.06 | 0.77 |
| Females | |||
| Respiratory | 0.78 | 0.09 | 0.13 |
| Intermediate respiratory | 0.11 | 0.89 | 0.00 |
| Non-respiratory | 0.08 | 0.00 | 0.92 |
The table presents probabilities of National Epidemiologic Survey on Alcohol and Related Conditions (NESARC)-Wave 1 and Wave 2 respondents transitioning between respiratory, intermediate respiratory, and non-respiratory panic classes and effects of covariates (gender, age, age of onset) on transitional probabilities.
We next examined the effect of three time-invariant covariates on transitional probabilities (Table 3). Although no covariate significantly influenced transitional rates among respondents reporting on two separate unique panic episodes, females were generally more likely to remain in the same panic class relative to males, with a greater than two-fold odds of remaining in the respiratory and intermediate respiratory classes.
Table 3.
Significance tests for predictors of latent panic class (stable) transitions (i.e. remaining in the same panic class from NESARC-Wave 1 to Wave 2)
| Covariate | OR | ℓRemoving covariate | Likelihood-ratio statistic | df | p |
|---|---|---|---|---|---|
| Gender | −5018.96 | 3.6 | 6 | 0.73 | |
| R W1→R W2 | 2.26 | ||||
| IR W1→IR W2 | 2.80 | ||||
| NR W1→NR W2 | 1.27 | ||||
| Age | −5020.74 | 4.32 | 6 | 0.63 | |
| R W1→R W2 | 0.98 | ||||
| IR W1→IR W2 | 1.00 | ||||
| NR W1→NR W2 | 1.00 | ||||
| Age of onset | −5021.20 | 0.26 | 6 | 1.00 | |
| R W1→R W2 | 1.00 | ||||
| IR W1→IR W2 | 1.00 | ||||
| NR W1→NR W2 | 1.00 |
NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; OR, odds ratio; df, degrees of freedom; R, respiratory; IR, intermediate respiratory; NR, Non-respiratory panic; W1, Wave 1; W2, Wave 2.
Lifetime co-morbidity
Results of co-morbidity analyses are presented in Table 4 with forest plots of ORs of co-morbid conditions presented in Figs 1 and 2. When lifetime co-morbid syndromes were entered separately to examine their unique association with panic subtypes, respiratory panic was associated with statistically significant increased odds of major depressive disorder (MDD) and all anxiety disorders in the NESARC and with increased odds of MDD and specific phobia in the CNCPS. When all co-morbidities were entered in the factor mixture models simultaneously to account for their covariation, respiratory panic had increased odds of only GAD in the NESARC and continued to be associated with increased odds of MDD and specific phobia in the CNCPS.
Table 4.
Results of latent class or factor mixture models with covariates entered separately and covariates entered simultaneously in three conceptual sets (lifetime psychiatric disorders, demographics and panic features)
| Covariates entered separately
|
Odds ratio (95% CI)
|
|
|---|---|---|
| Lifetime psychiatric disorders | NESARCa | CNCPS |
| Agoraphobia | 2.16 (1.51–3.11)** | 1.12 (0.30–2.34) |
| GAD | 3.31 (2.16–5.08)** | 1.47 (0.67–2.72) |
| Social phobia | 1.78 (1.29–2.49)** | 1.12 (0.32–2.37) |
| Specific phobia | 1.88 (1.40–2.54)** | 1.67 (1.15–2.44)* |
| MDD | 2.00 (1.16–3.45)* | 2.11 (1.21–3.68)* |
| Alcohol dependence | 1.34 (1.16–2.05) | – |
| Nicotine dependence | 1.71 (0.99–2.97) | – |
| Demographics | ||
| Gender | 1.07 (0.72–1.59) | 1.34 (0.91–1.96) |
| Age | 1.01 (0.98–1.05) | 1.02 (0.03–2.03) |
| Panic features | ||
| Age of onset | 1.01 (1.00–1.02) | 1.00 (0.98–1.02) |
| PD durationb | 1.00 (0.99–1.02) | 1.04 (0.05–2.05) |
| Pharmacological Tx | 2.47 (1.74–3.53)** | – |
| Psychosocial Tx | 2.09 (1.20–3.70)** | 1.18 (1.00–1.39)* |
| Age any Tx first sought | 1.00 (0.99–1.01) | 1.00 (0.98–1.02) |
| Covariates entered simultaneously | ||
| Lifetime psychiatric disorders | ||
| Agoraphobia | 1.66 (0.95–2.93) | 1.08 (0.73–1.59) |
| GAD | 2.45 (1.59–3.77)** | 1.32 (0.85–2.08) |
| Social phobia | 1.00 (0.65–1.56) | 1.33 (0.83–2.13) |
| Specific phobia | 1.23 (0.83–1.83) | 1.71 (1.16–2.52)** |
| MDD | 1.48 (0.93–2.34) | 2.09 (1.21–3.62)** |
| Alcohol dependence | 1.02 (0.68–1.54) | – |
| Nicotine dependence | 1.29 (0.89–1.87) | – |
| Demographics | ||
| Gender | 1.19 (0.87–1.63) | 1.37 (0.92–2.03) |
| Age | 1.00 (0.99–1.02) | 1.02 (1.01–1.04) |
| Panic features | ||
| Age of onset | 1.01 (0.99–1.04) | 1.03 (0.98–1.09) |
| PD durationb | – | 1.03 (0.98–1.09) |
| Pharmacological Tx | 1.14 (0.70–1.87) | – |
| Psychosocial Tx | 1.29 (0.64–2.63) | 1.10 (0.65–1.87) |
| Age any Tx first sought | 1.00 (0.98–1.02) | 1.01 (1.00–1.03) |
NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; CNCPS, Cross-National Collaborative Panic Study; GAD, generalized anxiety disorder; MDD, major depressive disorder; PD, panic disorder; Tx, treatment; −,Not assessed.
p≤0.05;
p≤0.01.
Intermediate panic not included; only respiratory versus non-respiratory comparison is presented.
PD duration was not entered with other covariates given that this variable was unknown for many respondents (i.e. age of onset was known, but age of remission was not known).
Fig. 1.
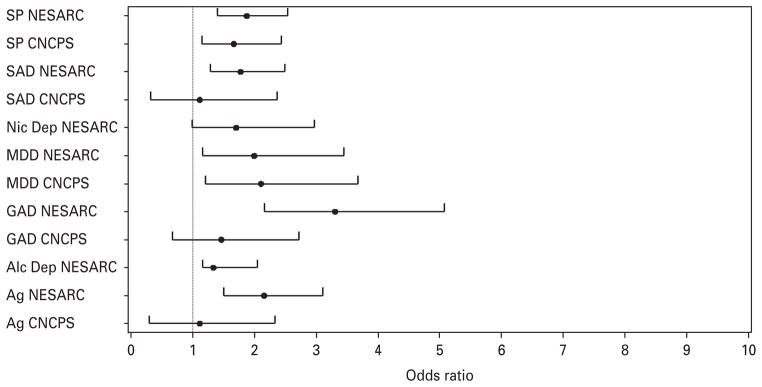
Forest plot of odds ratios (respiratory panic versus non-respiratory panic) for lifetime co-morbid diagnoses. All diagnoses were entered separately in latent class models (p values are presented in Table 4). SP, specific phobia; NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; CNCPS, Cross-National Collaborative Panic Study; SAD, social anxiety disorder/social phobia; NicDep, nicotine dependence; MDD, major depressive disorder; GAD, generalized anxiety disorder; Alc Dep, alcohol dependence; Ag, agoraphobia.
Fig. 2.
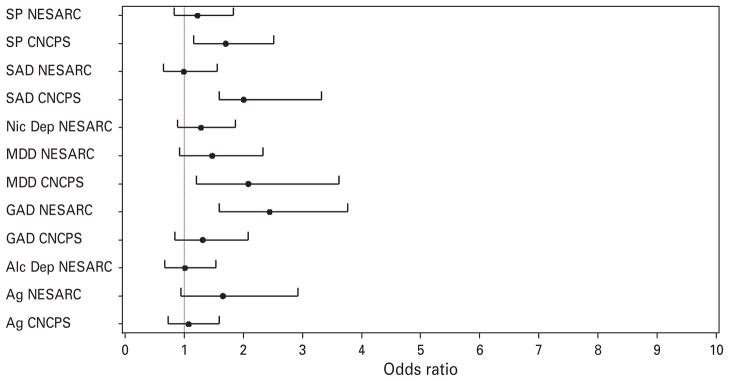
Forest plot of odds ratios (respiratory panic versus non-respiratory panic) for lifetime comorbid diagnoses. All diagnoses were entered simultaneously in latent class models (p values are presented in Table 4). SP, specific phobia; NESARC, National Epidemiologic Survey on Alcohol and Related Conditions; CNCPS, Cross-National Collaborative Panic Study; SAD, social anxiety disorder/social phobia; NicDep, nicotine dependence; MDD, major depressive disorder; GAD, generalized anxiety disorder; Alc Dep, alcohol dependence; Ag, agoraphobia.
To create greater equivalency between the NESARC and CNCPS, all covariate models were re-examined using only those NESARC respondents (n=661) meeting criteria for a current episode of PD/PDA and who also sought treatment (psychosocial or medication) for their panic attacks. When covariates were entered separately, respiratory panic continued to be associated with increased odds of agoraphobia, GAD, social phobia and specific phobia, but not MDD, alcohol dependence or nicotine dependence. When covariates were examined together, no covariates were statistically significant, but a trend (OR=1.7, p=0.052) was noted for respiratory panic having increased odds of lifetime GAD.
Panic disorder features
No class differences emerged for age of onset, illness duration or age first time treatment was sought when these covariates were examined separately or together. In the NESARC and CNCPS, the respiratory panic subtype was associated with increased odds of having sought psychosocial treatment specifically for PD and, in the NESARC, the respiratory panic subtype was associated with increased odds of using medication to alleviate symptoms associated with PD.
Demographics
No differences were detected for demographic factors including gender and respondent’s age at the time of assessment (see Table 4).
Latent growth curve analysis
Raw means for CNCPS outcome measures are presented in Table 5. Results of the latent LGCAs are presented in Table 6. All unconditional, main effect and interaction models showed good fit to the data, as indicated by CFI, TLI and RMSEA indices. The main goal of the LGCAs is to determine whether differential treatment outcome patterns are associated with respiratory and non-respiratory subtypes. Thus, we present full model results in Table 6, but interpretation of results focuses only on panic subtype (respiratory and non-respiratory) main effects and interactions involving panic subtype. Significant main effects are not interpreted in the presence of a significant interaction containing the main effect.
Table 5.
Raw means and (S.D.) for select CNCPS outcome measures
| Outcome measure | Baseline | Week 1 | Week 2 | Week 3 | Week 4 | Week 6 | Week 8 |
|---|---|---|---|---|---|---|---|
| CGI | 5.00 (0.00) | 5.83 (1.66) | 6.53 (1.58) | 6.95 (1.66) | 7.35 (1.58) | 7.72 (1.57) | 8.13 (1.53) |
| Cued | 2.89 (5.88) | 2.66 (10.64) | 1.73 (4.47) | 1.46 (4.97) | 1.12 (3.62) | 0.75 (2.95) | 0.54 (2.26) |
| Uncued | 4.14 (6.83) | 3.75 (8.93) | 2.17 (4.82) | 1.97 (5.71) | 1.33 (3.67) | 0.99 (3.35) | 0.80 (3.11) |
| Anti. Anx. % | 42.81 (32.44) | 32.71 (29.76) | 26.87 (27.75) | 23.72 (26.37) | 20.66 (24.55) | 16.19 (22.41) | 13.52 (21.04) |
| HAMA | 21.34 (8.50) | 16.65 (8.69) | 14.13 (8.11) | 12.78 (7.97) | 11.33 (7.61) | 9.78 (7.00) | 8.78 (6.87) |
| SDS | 15.52 (7.91) | 13.57 (8.34) | 11.89 (8.22) | 10.51 (8.24) | 9.34 (7.92) | 7.82 (7.65) | 6.69 (7.46) |
CNCPS, Cross-National Collaborative Panic Study; CGI, clinician global improvement; Cued, number of cued panic attacks per week; Uncued, number of uncued/spontaneous panic attacks; Anti. Anx. %, anticipatory anxiety percentage; HAMA, Hamilton Anxiety Scale; SDS, Sheehan Disability Scale.
Table 6.
Results of latent growth curve analyses (LGCA) for selected CNCPS treatment outcome measures
| Outcome measure | I | S | Q | χ2(df) | CFI | TLI | RMSEA |
|---|---|---|---|---|---|---|---|
| CGI | 5.87*** | 0.513*** | −0.035*** | 88.81 (13)*** | 0.96 | 0.96 | 0.077 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | 102.28 (16)*** | 0.96 | 0.95 | 0.071 |
| Treatment: | 100.08 (19)*** | 0.96 | 0.95 | 0.064 | |||
| (1) Placebo v. Tx | −0.66*** | N.S. | N.S. | ||||
| (2) Alpraz. v. Imipram. | 1.26*** | −0.36*** | 0.024** | ||||
| Interaction effects | 128.06 (28)*** | 0.97 | 0.96 | 0.062 | |||
| Panic subtyper × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panic subtyper × Tx (2) | N.S. | N.S. | N.S. | ||||
| Cued panic attacks per week (n) | 3.33*** | −0.527*** | 0.028*** | 65.32 (19)*** | 0.97 | 0.96 | 0.048 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | −77.44 (23)*** | 0.97 | 0.96 | 0.047 |
| Treatment: | −89.27 (27)*** | 0.97 | 0.96 | 0.047 | |||
| (1) Placebo v. Tx | N.S. | N.S. | N.S. | ||||
| (2) Alpraz. v. Imipram. | N.S. | N.S. | N.S. | ||||
| Interaction effects | −123.16 (39)*** | 0.97 | 0.95 | 0.045 | |||
| Panicr × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panicr × Tx (2) | N.S. | N.S. | N.S. | ||||
| Uncued panic attacks per week (n) | 4.60*** | −0.89*** | 0.06*** | 55.24 (19)*** | 0.92 | 0.91 | 0.043 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | 64.88 (23)*** | 0.93 | 0.91 | 0.042 |
| Treatment: | 77.32 (27)*** | 0.93 | 0.91 | 0.042 | |||
| (1) Placebo v. Tx | N.S. | N.S. | N.S. | ||||
| (2) Alpraz. v. Imipram. | N.S. | N.S. | 0.040* | ||||
| Interaction effects | 103.30 (39)*** | 0.94 | 0.91 | 0.040 | |||
| Panic subtyper × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panic subtyper × Tx (2) | N.S. | N.S. | N.S. | ||||
| Percentage anticipatory anxiety | 46.78*** | −7.18*** | 0.416*** | 111.14 (19)*** | 0.95 | 0.95 | 0.068 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | 120.75 (23)*** | 0.96 | 0.95 | 0.063 |
| Treatment: | 152.29 (27)*** | 0.95 | 0.93 | 0.066 | |||
| (1) Placebo v. Tx | N.S. | 2.792 | N.S. | ||||
| (2) Alpraz. v. Imipram. | N.S. | N.S. | N.S. | ||||
| Interaction effects | 175.32 (39)*** | 0.95 | 0.94 | 0.058 | |||
| Panic subtyper × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panic subtyper × Tx (2) | N.S. | N.S. | N.S. | ||||
| Sheehan Disability Scale | 17.16*** | −1.95*** | 0.10*** | 117.89 (19)*** | 0.97 | 0.97 | 0.07 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | 133.63 (23)*** | 0.97 | 0.97 | 0.07 |
| Treatment: | 155.11 (27)*** | 0.97 | 0.96 | 0.07 | |||
| (1) Placebo v. Tx | N.S. | 0.67* | N.S. | ||||
| (2) Alpraz. v. Imipram. | −2.10* | N.S. | 0.07* | ||||
| Interaction effects | 183.09 (39)*** | 0.97 | 0.96 | 0.06 | |||
| Panic subtyper × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panic subtyper × Tx (2) | N.S. | N.S. | N.S. | ||||
| Hamilton Anxiety Total | 23.06*** | −3.35*** | 0.21*** | 205.97 (19)*** | 0.98 | 0.98 | 0.097 |
| Main effects | |||||||
| Panic subtype | N.S. | N.S. | N.S. | 222.51 (23)*** | 0.97 | 0.96 | 0.091 |
| Treatment: | 299.06 (27)*** | 0.95 | 0.94 | 0.098 | |||
| (1) Placebo v. Tx | N.S. | 0.897** | −0.071** | ||||
| (2) Alpraz. v. Imipram. | −2.133* | N.S. | 0.117** | ||||
| Interaction effects | 337.15 (39)*** | 0.94 | 0.92 | 0.085 | |||
| Panicr × Tx (1) | N.S. | N.S. | N.S. | ||||
| Panicr × Tx (2) | N.S. | 1.255* | −0.107* |
CNCPS, Cross-National Collaborative Panic Study; I, intercept; S, slope; Q, quadratic; df, degrees of freedom; CFI, comparative fit index; TLI, Tucker–Lewis Index; RMSEA, root mean square error of approximation; CGI, clinician global improvement; Tx, treatment; N.S., not significant.
p≤0.05;
p≤0.01;
p≤0.001.
Panic (respiratory versus non-respiratory) class main effect
No statistically significant panic subtype main effects were observed.
Interaction
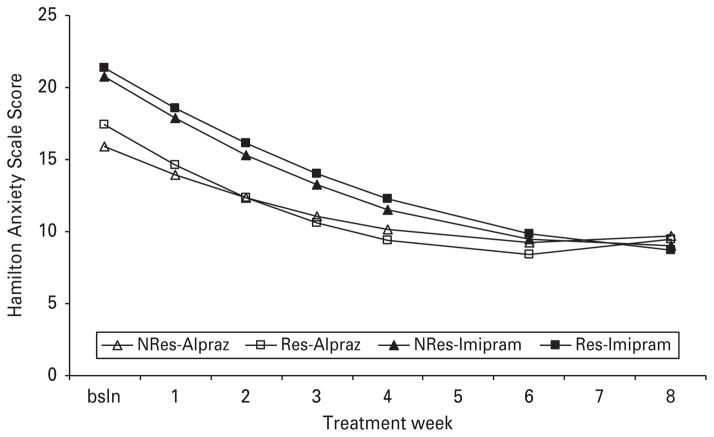
A significant interaction was observed for the slope and quadratic between panic subtype and alprazolam versus imipramine on the weekly Hamilton Anxiety Scale (see Fig. 3). The significant interactions were driven by the respiratory panic/alprazolam condition having a greater rate of change and a greater decelerating slope relative to the non-respiratory/alprazolam condition.
Fig. 3.
Interaction between active medication conditions (alprazolam versus imipramine) and panic class [respiratory (Res) versus non-respiratory (NRes)].
Discussion
A recent comprehensive examination of panic symptoms across multiple epidemiologic datasets and a treatment-seeking sample indicated that people generally tend to covary in two distinct panic subgroups as a function of symptomatic likeness (Roberson-Nay & Kendler, in press). One of the panic subgroups was characterized by a prominent respiratory component in addition to elevations on general somatic symptoms while the second subgroup presented with low endorsement rates of respiratory symptoms, but elevations on general somatic symptoms. These results were consistent with Briggs et al. (1993), who originally proposed that PD was best captured by two primary types of panic attacks. The primary goal of this paper was to determine whether respiratory and non-respiratory panic represent clinically meaningful subtypes and, if they do, do subtype differences reflect varying points along the panic continuum or qualitatively distinct forms of panic, with possible differing etiologies? We specifically addressed two of Robin and Guze’s validation criteria, including the longitudinal stability of the subtypes and features associated with the clinical presentation of each panic subtype.
First, robust temporal stability of panic subtypes was observed using longitudinal data from Wave 1 and Wave 2 of the NESARC. Given high longitudinal stability, several predictors of stability, including respondent age, gender and age of panic onset, were examined to determine whether these factors influenced rates of transition. Although no variable had a statistically significant influence on transitional rates, ORs indicated that females were more likely to remain in the same panic class relative to males.
Lifetime co-morbidity data suggested that respiratory panic tended to be associated with increased co-morbidity across a number of anxiety disorders (agoraphobia, GAD, social phobia, specific phobia) as well as major depression. Respiratory panic continued to be associated with increased co-morbidity even after controlling for co-morbidity between the lifetime psychiatric disorders. Because the NESARC indicated that respiratory panic was associated with a greater number of lifetime co-morbidities, a subset of NESARC respondents reporting presence of panic in the past year as well as a history of seeking medication or psychosocial treatment for their panic attacks were selected to more closely resemble the CNCPS. Result patterns did not change substantially when analyses were restricted to this subsample, with respiratory panic continuing to exhibit greater odds of having increased levels of lifetime psychiatric co-morbidities. Also, although not reported here, we explored the developmental course of lifetime disorders and panic subtypes, examining if subtype differences might be present as a function of whether co-morbid disorders preceded PD onset, had onset at approximately the same time as PD or developed after PD onset. No sig-nificant patterns of association were detected between the two subtypes using this approach, however. Thus, respiratory panic was associated with increased co-morbidity, with non-respiratory panic not exhibiting increased odds of any disorder and no unique patterns of co-morbidity emerging for the two panic subtypes.
Examination of panic features indicated that respiratory panic was associated with an increased tendency to seek psychosocial treatment for panic in both the NESARC and CNCPS and to be prescribed medications for panic in the NESARC. This finding is consistent with the observation in the CNCPS where a larger portion of treatment-seeking participants was identified as respiratory panic. No significant differences were observed for age of onset, illness duration or age when treatment was first sought for panic. Examination of two demographic factors (gender and age) also indicated no differences between the two panic classes.
Pharmacological treatment outcomes were examined using 8 weeks of treatment data from the CNCPS. With the exception of the Hamilton Anxiety Scale (which was statistically significant, but lacked clinical significance), no significant interactions between panic subtype and medication were detected on measures of clinician-rated global improvement, global disability or patient-rated panic variables (i.e. number of cued and uncued panic attacks per week, daily percentage of anticipatory anxiety about having panic attacks). Moreover, no panic subtype main effects were observed, suggesting that the two forms of panic responded similarly to the prescribed pharmacological interventions.
Briggs et al. (1993) analyzed CNCPS outcomes as part of their subtype validation. Unlike the present study, however, they categorically assigned patients to either a respiratory or non-respiratory group based on the results of a cluster analysis. Respiratory and non-respiratory panic groups did not differ on gender or co-morbid diagnoses, including major depression, specific phobia or social phobia. Our analyses of CNCPS measures, however, indicated that respiratory panic was associated with increased odds of having a lifetime diagnosis of major depression and specific phobia. They also observed that the respiratory condition suffered from more spontaneous panic attacks while the non-respiratory panic condition exhibited an increased number of situational cued panic attacks. We did not observe any differences between subtypes on this outcome measure or any others. Based on CGI ratings, Briggs and colleagues also maintained that respiratory panic demonstrated superior response to imipramine while non-respiratory panic showed enhanced response to alprazolam. We, however, did not find any overwhelming evidence to suggest that one subtype responded preferentially to either medication. Given that only one significant medication by panic subtype interaction was observed in Briggs’ analyses, as well as in our analyses, it seems premature to suggest that either type of medication has enhanced efficacy for one panic subtype versus the other.
The primary objective of this study was to provide additional insight into whether respiratory and non-respiratory subtypes of PD do not represent clinically meaningful different subtypes, quantitative differences only or qualitative differences only. Overall, the present results are most consistent with the respiratory and non-respiratory panic subtypes being best conceptualized as having unique symptom profiles that differ primarily in severity, with respiratory panic representing the more severe form of the disorder. This interpretation is made with the caveat that there still remains the possibility that respiratory and non-respiratory panic subtypes may vary on aspects (e.g. risk factors, genetic expression) that we were unable to examine, which would support qualitative distinctness.
Although no differences in response to imipramine or alprazolam were observed, response to psychosocial treatments may be affected by PD subtype. For example, significant evidence suggests that cognitive behavioral therapy is an effective treatment for PD (McHugh et al. 2009) and specifically targets respiration. Given that persons with PD who present with respiratory panic symptoms represent a more severe form of the disorder, these individuals may require more sessions or more sessions focusing specifically on respiratory physiology (e.g. breathing retraining, interoceptive exposure) and the ramifications associated with respiratory aberrations (e.g. respiratory alkalosis). For researchers who use Briggs and colleagues’ respiratory versus non-respiratory panic subtypes when analyzing their data, they need to be mindful that these subtypes appear to represent quantitative differences versus qualitative and this distinction will likely be reflected in their outcomes.
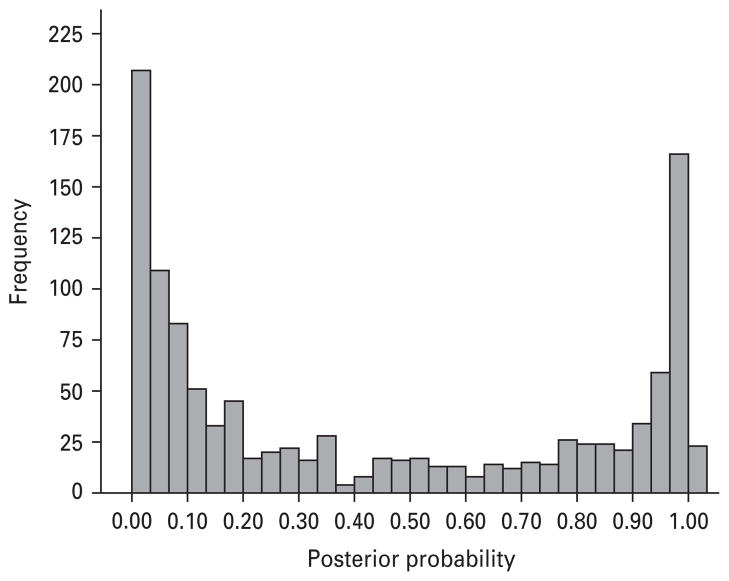
A secondary goal of the current study was to conduct all analyses within a latent class framework. This approach is undoubtedly more complicated and considerably more time-consuming, but represents a more sound statistical approach, given that error associated with latent class assignment is modeled within the analysis. We did not replicate the few differences observed by Briggs et al. (1993) and these between study differences likely stem from analyzing groups of respondents assigned to a class versus remaining within a latent class model. Fig. 4 illustrates the posterior probabilities [probability of being in respiratory (0.0–0.5) versus non-respiratory class (0.5–1.0)], which show the error rate that is associated with the latent classes. It is therefore recommended that future studies applying latent class modeling to data either conduct analyses within a latent class framework or follow the guidelines described by Goodman (2007), who offers two assignment procedures and criteria to assess when the assignment procedure is satisfactory and when it is not.
Fig. 4.
The present study includes several limitations. Some outcomes (e.g. temporal stability, treatment outcomes) were only available for one dataset. Thus, replication of result patterns was not possible for all validators. Moreover, the treatment study was not exhaustive in its review of differing types (e.g. antidepressant) and formulas of medications. Future studies should seek to examine a wider range of medications prescribed for the treatment of panic. As mentioned, this study also did not examine risk factors (e.g. childhood separation anxiety disorder) or genes (e.g. Catechol-O-methyl transferase; Hamilton et al. 2002; Woo et al. 2002; Domschke et al. 2004; Rothe et al. 2006) known to be associated with PD. Thus, additional work is needed to elucidate the pathways, course and mechanisms involved in the etiopathogenesis of PD and its subtypes.
Acknowledgments
Preparation of this manuscript was supported by grant K01-MH-080953 from the National Institutes of Health/National Institute of Mental Health to the first author (R.R.N.). The National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) was sponsored and conducted by the National Institute on Alcohol Abuse and Alcoholism (NIAAA), with supplemental support from the National Institute on Drug Abuse. The availability of data from the Cross-National Collaborative Panic Study (CNCPS) is acknowledged with gratitude.
Footnotes
Declaration of Interest
None.
References
- APA. Diagnostic and Statistical Manual of Mental Disorders. 4. American Psychiatric Association; Washington, DC: 2000. revised. [Google Scholar]
- Briggs AC, Stretch DD, Brandon S. Subtyping of panic disorder by symptom profile. British Journal of Psychiatry. 1993;163:201–209. doi: 10.1192/bjp.163.2.201. [DOI] [PubMed] [Google Scholar]
- Brown TA, Antony MM, Barlow DH. Diagnostic comorbidity in panic disorder : effect on treatment outcome and course of comorbid diagnoses following treatment. Journal of Consulting and Clinical Psychology. 1995;63:408–418. doi: 10.1037//0022-006x.63.3.408. [DOI] [PubMed] [Google Scholar]
- Brown TA, Barlow DH. Comorbidity among anxiety disorders: implications for treatment and DSM-IV. Journal of Consulting and Clinical Psychology. 1992;60:835–844. doi: 10.1037//0022-006x.60.6.835. [DOI] [PubMed] [Google Scholar]
- Brown TA, Campbell LA, Lehman CL, Grisham JR, Mancill RB. Current and lifetime comorbidity of the DSM-IV anxiety and mood disorders in a large clinical sample. Journal of Abnormal Psychology. 2001;110:585–599. doi: 10.1037//0021-843x.110.4.585. [DOI] [PubMed] [Google Scholar]
- Cross-National Collaborative Panic Study, Second Phase Investigators. Drug treatment of panic disorder: comparative efficacy of alprazolam, imipramine, and placebo. British Journal of Psychiatry. 1992;160:191–202. doi: 10.1192/bjp.160.2.191. [DOI] [PubMed] [Google Scholar]
- Domschke K, Freitag CM, Kuhlenbaumer G, Schirmacher A, Sand P, Nyhuis P, Jacob C, Fritze J, Franke P, Rietschel M, Garritsen HS, Fimmers R, Nothen MM, Lesch KP, Stogbauer F, Deckert J. Association of the functional V158M catechol-O-methyltransferase polymorphism with panic disorder in women. International Journal of Neuropsychopharmacology. 2004;7:183–188. doi: 10.1017/S146114570400416X. [DOI] [PubMed] [Google Scholar]
- Eaton WW, Kessler RC, Wittchen HU, Magee WJ. Panic and panic disorder in the United States. American Journal of Psychiatry. 1994;151:413–420. doi: 10.1176/ajp.151.3.413. [DOI] [PubMed] [Google Scholar]
- Goodman LA. On the assignment of individuals to latent classes. Sociological Methodology. 2007;37:1–22. [Google Scholar]
- Grant BF, Hasin DS, Stinson FS, Dawson DA, Goldstein RB, Smith S, Huang B, Saha TD. The epidemiology of DSM-IV panic disorder and agoraphobia in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2006;67:363–374. doi: 10.4088/jcp.v67n0305. [DOI] [PubMed] [Google Scholar]
- Hamilton SP, Slager SL, Heiman GA, Deng Z, Haghighi F, Klein DF, Hodge SE, Weissman MM, Fyer AJ, Knowles JA. Evidence for a susceptibility locus for panic disorder near the catechol-O-methyltransferase gene on chromosome 22. Biological Psychiatry. 2002;51:591–601. doi: 10.1016/s0006-3223(01)01322-1. [DOI] [PubMed] [Google Scholar]
- Klein DF. False suffocation alarms, spontaneous panics, and related conditions. An integrative hypothesis. Archives of General Psychiatry. 1993;50:306–317. doi: 10.1001/archpsyc.1993.01820160076009. [DOI] [PubMed] [Google Scholar]
- Klerman GL. Overview of the Cross-National Collaborative Panic Study. Archives of General Psychiatry. 1988;45:407–412. doi: 10.1001/archpsyc.1988.01800290021003. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Smits JA, Otto MW. Empirically supported treatments for panic disorder. Psychiatric Clinics of North America. 2009;32:593–610. doi: 10.1016/j.psc.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Muthen LK, Muthen BO. Mplus : Statistical Analysis with Latent Variables. Muthen and Muthen; Los Angeles, CA: 2007. [Google Scholar]
- Regier DA, Narrow WE, Rae DS. The epidemiology of anxiety disorders : The Epidemiologic Catchment Area (ECA) experience. Journal of Psychiatric Research. 1990;24:3–14. doi: 10.1016/0022-3956(90)90031-k. [DOI] [PubMed] [Google Scholar]
- Roberson-Nay R, Kendler KS. Panic disorder and its subtypes: a comprehensive analysis of panic symptom heterogeneity using epidemiological and treatment seeking samples. Psychological Medicine. doi: 10.1017/S0033291711000547. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins E, Guze SB. Establishment of diagnostic validity in psychiatric illness: its application to schizophrenia. American Journal of Psychiatry. 1970;126:983–987. doi: 10.1176/ajp.126.7.983. [DOI] [PubMed] [Google Scholar]
- Rothe C, Koszycki D, Bradwejn J, King N, Deluca V, Tharmalingam S, Macciardi F, Deckert J, Kennedy JL. Association of the Val158Met catechol Omethyltransferase genetic polymorphism with panic disorder. Neuropsychopharmacology. 2006;31:2237–2242. doi: 10.1038/sj.npp.1301048. [DOI] [PubMed] [Google Scholar]
- Sanderson WC, Di Nardo PA, Rapee RM, Barlow DH. Syndrome comorbidity in patients diagnosed with a DSM-III-R anxiety disorder. Journal of Abnormal Psychology. 1990;99:308–312. doi: 10.1037//0021-843x.99.3.308. [DOI] [PubMed] [Google Scholar]
- Schneier FR, Fyer AJ, Martin LY. A comparison of phobic subtypes within panic disorder. Journal of Anxiety Disorders. 1991;5:65–75. [Google Scholar]
- Starcevic V, Uhlenhuth H, Kellner R. Patterns of comorbidity in panic disorder and agoraphobia. Psychiatry Research. 1992;42:171–183. doi: 10.1016/0165-1781(92)90080-m. [DOI] [PubMed] [Google Scholar]
- Tsao JCI, Lewin MR, Craske MG. The effects of cognitive-behavior therapy for panic disorder on comorbid conditions. Journal of Anxiety Disorders. 1998;12:357–371. doi: 10.1016/s0887-6185(98)00020-6. [DOI] [PubMed] [Google Scholar]
- Tsao JCI, Mystkowski JL, Zucker BG, Craske MG. Effects of cognitive-behavior therapy for panic disorder on comorbid conditions: replication and extension. Behavior Therapy. 2002;33:493–509. [Google Scholar]
- Uhlenhuth EH, Leon AC, Matuzas W. Psychopathology of panic attacks in panic disorder. Journal of Affective Disorders. 2006;92:55–62. doi: 10.1016/j.jad.2005.12.036. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu H, Joyce PR, Karam EG, Lee C, Lellouch J, Lépine J, Newman SC, Oakley-Browne MA, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh E. The cross-national epidemiology of panic disorder. Archives of General Psychiatry. 1997;54:305–309. doi: 10.1001/archpsyc.1997.01830160021003. [DOI] [PubMed] [Google Scholar]
- Williams JB, Gibbon M, First MB, Spitzer RL, Davies M, Borus J, Howes MJ, Kane J, Pope HG, Rounsaville B, Wittchen H. The Structured Clinical Interview for DSM-III-R(SCID) : II. Multisite test-retest reliability. Archives of General Psychiatry. 1992;49:630–636. doi: 10.1001/archpsyc.1992.01820080038006. [DOI] [PubMed] [Google Scholar]
- Woo JM, Yoon KS, Yu BH. Catechol O-methyltransferase genetic polymorphism in panic disorder. American Journal of Psychiatry. 2002;159:1785–1787. doi: 10.1176/appi.ajp.159.10.1785. [DOI] [PubMed] [Google Scholar]