Abstract
Chromosome 22q11.2 deletion syndrome (22q11DS) is the most common microdeletion in humans. Nonverbal learning disability (NLD) has been used to describe the strengths and deficits of children with 22q11DS, but the applicability of the label for this population has seldom been systematically evaluated. The goal of the current study was to address how well the NLD diagnosis characterizes children and adolescents with 22q11DS. A total of 74 children and adolescents with 22q11DS were given neurocognitive, socioemotional, and academic assessments to measure aspects of NLD. Of the cohort, 20% met at least 7 of 9 assessed criteria for NLD; 25% showed verbal skills exceeding their nonverbal skills as assessed by an IQ test; and 24% showed the good rote verbal capacity commonly associated with NLD. Hypothesizing that if the entire cohort did not show consistent NLD characteristics, the descriptor might be more accurate for a distinct subgroup, the authors used latent class analysis to divide participants into three subgroups. However, the lines along which the groups broke out were more related to general functioning level than to NLD criteria. All three groups showed a heightened risk for psychiatric illness, highlighting the importance of careful mental health monitoring for all children with 22q11DS.
Keywords: nonverbal learning disability, 22q11 deletion syndrome, velocardiofacial syndrome, latent class modeling
Chromosome 22q11.2 deletion syndrome (22q11DS), also known as DiGeorge syndrome or velocardiofacial syndrome (VCFS), is one of the most prevalent genetic disorders and the most common chromosomal microdeletion in humans, with an incidence of 1 in 2,000 (Shprintzen, 2008). This hemizygous deletion within the long arm of Chromosome 22 leads to congenital heart disease, palatal anomalies, hypocalcemia, and T-cell immunodeficiency (Robin & Shprintzen, 2005; Shprintzen, 2000). Beginning in adolescence and continuing into adulthood, patients with 22q11DS display high rates of schizophrenia, as well as bipolar disorder, major depressive disorder, and anxiety disorders (Bassett et al., 1998; Green et al., 2009; Murphy, Jones, & Owen, 1999; Papolos et al., 1996; Shprintzen, 2000; Xu et al., 2008). Anxiety disorders and depression are also prevalent in children with 22q11DS (Antshel et al., 2006; Green et al., 2009; Jolin et al., 2009). Additional childhood behavioral manifestations include attention-deficit/hyperactivity disorder (ADHD), oppositional defiant disorder (ODD; Antshel et al., 2006; Arnold, Siegel-Bartelt, Cytrynbaum, Teshima, & Schachar, 2001; Feinstein, Eliez, Blasey, & Reiss, 2002; Green et al., 2009; Jolin et al., 2009; Lewandowski, Shashi, Berry, & Kwapil, 2007; Sobin, Kiley-Brabeck, & Karayiorgou, 2005; Zagursky, Weller, Jessani, Abbas, & Weller, 2006), poor social skills (Kiley-Brabeck & Sobin, 2006), and autism spectrum symptoms (Antshel et al., 2007; Vorstman et al., 2006).
A complex academic achievement profile has been described, with relative strengths in reading, spelling, and verbal rote memory and deficits in reading comprehension and math (Antshel & Khan, 2008; Bearden et al., 2001; Lajiness-O’Neill et al., 2006; Lewandowski et al., 2007; Moss et al., 1999; van Amelsvoort et al., 2004; Woodin et al., 2001; Zinkstok & van Amelsvoort, 2005). Impairments in executive function, including cognitive flexibility, working memory, and inhibition of inappropriate responses have also been reported (Bearden et al., 2001; Lajiness-O’Neill et al., 2006; Lewandowski et al., 2007; Moss et al., 1999; Woodin et al., 2001). Taken together, these neurocognitive, psychiatric, and academic characteristics have led some to suggest the nonverbal learning disability as a phenotypic model for understanding 22q11DS (Lepach & Petermann, 2011; Rourke et al., 2002; Simon, 2008; Swillen et al., 1999).
The average full-scale IQ (FSIQ) for children with 22q11DS has frequently been reported to be in the 70s, although there is considerable variation among individuals. A study of 103 children with 22q11DS reported a mean FSIQ of 73.48 (SD = 11.73, range = 50 to 109; De Smedt et al., 2007). In a sample of 50 school-age children with 22q11DS, Woodin and colleagues (2001) found a mean FSIQ of 76 (SD = 12.70), whereas Moss and colleagues (1999) assessed 26 children and reported a mean FSIQ of 71.2 (SD = 12.8, range = 42 to 92). Jacobson et al. (2010) enrolled 31 children with 22q11DS referred for educational underachievement and found a mean IQ of 65.4 (SD = 9.7). In 2006, Shashi and colleagues reported an association between a specific polymorphism and IQ. They found a mean IQ of 62.44 (SD = 1.7) in patients with the Val allele of the COMT gene and 74.77 (SD = 11.29) in patients with the Met allele. Average IQs in the 70s have also been reported in adults with 22q11DS, including those with schizophrenia (Bassett et al., 2005; Chow, Watson, Young, & Bassett, 2006; van Amelsvoort et al., 2004).
Near-normal FSIQ is sometimes cited as a prerequisite for identification of specific learning disabilities (SLDs), including nonverbal learning disability (NLD; Forrest, 2004; Mayes & Calhoun, 2005; Petti, Voelker, Shore, & Hayman-Abello, 2003; Schiff, Bauminger, & Toledo, 2009). By this criterion, most children with 22q11DS could not be said to have an SLD. However, SLDs are also identified with no regard to FSIQ. In response to the 1999 Individuals With Disabilities Act (IDEA), schools commonly defined SLD based on a discrepancy between IQ and achievement scores, irrespective of FSIQ (Mayes & Calhoun, 2005). The 2004 revision of IDEA emphasized students’ response to intervention, again irrespective of FSIQ (Kavale, Kauffman, Bachmeier, & LeFever, 2008). Several researchers have argued that low achievement scores should be the core criterion for SLD (Brueggemann, Kamphaus, & Dombrowski, 2008). Whether children with lower IQs, including many children with 22q11DS, should be excluded from consideration as learning disabled remains a question of debate.
In 1995, Tsatsanis and Rourke listed VCFS as a disorder showing virtually all the characteristics of NLD. Since then, NLD has repeatedly been reported to describe the 22q11DS cognitive phenotype (Bearden et al., 2001; Moss et al., 1999; Swillen et al., 1999; Wang, Woodin, Kreps-Falk, & Moss, 2000; Woodin et al., 2001). The goal of the current study is to examine whether NLD is an appropriate descriptor for patients with 22q11DS.
NLD
NLD was first proposed by Johnson and Myklebust (1967) as a description of children who could read and write adequately but had problems with visuospatial tasks, arithmetic, and social skills. This description was elaborated substantially by Rourke and colleagues, who first identified a group of children with verbal IQs in the average range and well above their performance (nonverbal) IQs, then attributed to this group additional characteristics, including good rote verbal skills and verbal memory contrasted with deficits in tactile-perceptual skills, motor coordination, visual-perceptual organization, nonverbal problem solving, arithmetic, pragmatic language, and social skills (Harnadek & Rourke, 1994; Rourke & Finlayson, 1978; Rourke & Strang, 1978; Rourke & Telegdy, 1971; Rourke, Yanni, MacDonald, & Young, 1973; Rourke, Young, & Flewelling, 1971; Strang & Rourke, 1983).
Sample sizes in these studies were not large (n ≤ 30 for each NLD group), and participant descriptions suggest that the same children may have participated in several studies (Spreen, 2011). The verbal/performance IQ (VIQ/PIQ) discrepancy initially used to screen for NLD (Rourke et al., 1971; Rourke & Telegdy, 1971) was suggested to be a less clear indicator in younger children (Rourke et al., 1973) and may occur in fewer than one third of older children identified as having NLD (Drummond, Ahmad, & Rourke, 2005; Pelletier, Ahmad, & Rourke, 2001). Nonetheless, diagnostic criteria defined by Rourke and colleagues, often including a VIQ/PIQ discrepancy, are still widely used in studies of the NLD population (Bloom & Heath, 2010; Forrest, 2004; Galway & Metsala, 2011; Keller, Tillery, & McFadden, 2006; Petti et al., 2003; Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010).
NLD is not included in the current Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR; American Psychiatric Association, 2000), and some researchers have disputed its existence as a distinct subtype of learning disability (Eling, 2007; Spreen & Haaf, 1986). One cluster analysis of 159 children with learning disabilities found several subtypes, but not one that matched the NLD description (Spreen & Haaf, 1986). Some recent studies of children with learning disabilities have found NLD to be relatively uncommon, at rates of 2.8% (Hendriksen et al., 2007) and 6.8% (Grodzinsky, Forbes, & Bernstein, 2010) in learning-disabled populations.
Other researchers have suggested alterations in certain criteria. For instance, Semrud-Clikeman and colleagues, using diagnostic criteria proposed by Rourke and Tsatsanis (1996), have suggested that children with NLD do not reliably show deficits in motor coordination (Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010). A few studies have found more language difficulties than the term nonverbal would suggest (Worling, Humphries, & Tannock, 1999; Zera, 2001). Subtypes within the NLD construct have been proposed to improve description of individuals (Grodzinsky et al., 2010). However, recent, controlled studies, including some with large sample sizes, have supported the idea of a distinct subgroup with difficulties with near-normal verbal skills but deficits in visual-spatial skills (Mammarella & Pazzaglia, 2010; Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010) and social perception (Galway & Metsala, 2011; Semrud-Clikeman, Walkowiak, Wilkinson, & Minne, 2010).
NLD in 22q11DS
In 1999, based on a sample of 9 children with 22q11DS, Swillen and colleagues proposed that the neuropsychological and psychosocial profile of 22q11DS corresponded with the pattern of functioning defined by NLD. Several subsequent studies of children and adolescents with 22q11DS found a discrepancy between VIQ and PIQ, with mean VIQ 6 to 15 points higher. Of these studies, some compared group means without considering the fraction of participants with a significant discrepancy (Lepach & Petermann, 2011; Wang et al., 2000; Woodin et al., 2001). When participants were considered individually, the proportion with VIQ significantly higher than PIQ ranged from close to half (Jacobson et al., 2010) to approximately one third (Bearden et al., 2001) to less than 20% (De Smedt et al., 2007; Niklasson, Rasmussen, Oskarsdottir, & Gillberg, 2001). Other researchers found no significant difference between VIQ and nonverbal IQ subtests (Campbell et al., 2009; Sobin, Kiley-Brabeck, Daniels, et al., 2005). Antshel and Khan (2008), investigating a comprehensive list of NLD diagnostic criteria in 100 children with 22q11DS, found that 20% met at least 8 of 10 criteria. These variable results suggest that NLD may more accurately describe a subset of children with 22q11DS than the entire population.
Swillen et al. (1999) reported that 5 of 9 children studied had a VIQ > PIQ discrepancy, which in 3 participants met or exceeded 10 points, relatively good core language skills, significantly better reading and spelling than arithmetic scores, and good auditory memory, compared with deficits in tactile-perceptual, visual-perceptual, visual-spatial, visual attention, and psychomotor domains, along with problems with processing new and complex material. These characteristics are in line with NLD assets and deficits as described by Rourke et al. (2002).
In a larger sample of 50 school-age children with 22q11DS, the mean VIQ was 10 points higher than the mean PIQ (83 vs. 73). The proportion of children exhibiting a significant split was not reported. The children also showed deficits in attention, story memory, visuospatial memory, and arithmetic performance relative to other areas of achievement and psychosocial functioning (Woodin et al., 2001). Significantly better verbal than visual recall has been reported (Bearden et al., 2001; Sobin, Kiley-Brabeck, Daniels, et al., 2005), along with deficits in visual attention, working memory, and motor skills (Sobin, Kiley-Brabeck, Daniels, et al., 2005) and more general visual-spatial deficits (Bearden et al., 2001). However, Sobin, Kiley-Brabeck, Daniels, et al. (2005) found neither verbal strengths nor visual-spatial memory deficits.
Jacobson et al. (2010) corroborated previous findings by demonstrating significantly higher mean VIQ than PIQ, along with relative strengths in verbal memory and basic reading, in a sample of 31 children and adolescents with 22q11DS. In nearly half the participants (48.4%), VIQ exceeded performance by at least 11.3 points, the clinical significance level proposed in the testing guidelines. However, means for all of these measures fell below the average range, and differences between verbal and visual-spatial memory were not statistically significant. Mean FSIQ was 65.4, lower than earlier reported means for the 22q11DS population (Shashi et al., 2006; Swillen et al., 1997; Woodin et al., 2001), probably because all participants had been referred for assessment based on concerns about their educational progress. These findings may therefore not generalize to the entire 22q11DS population.
Although large studies may find a VIQ/PIQ discrepancy of only a few points to be statistically significant, the clinical significance of these findings is less clear. For instance, although one study of 103 children found that a 6-point difference between VIQ and PIQ was statistically significant, only 17% of the participants (18/103) had a clinically significant discrepancy, defined by the authors as greater than 15 points (De Smedt et al., 2007). Meanwhile, 26% of participants showed the reverse pattern, with PIQ higher than VIQ, and in 5% the difference was clinically significant. Similarly, a smaller study found 7 of 30 respondents (23%) with PIQ > VIQ, compared with only 4 (13%) who had a VIQ > PIQ discrepancy exceeding 10 points (Niklasson et al., 2001).
Not all studies of 22q11DS have demonstrated clear verbal strengths relative to nonverbal skills. Many early studies did not incorporate a control group, and later studies that did often found differences in VIQ and PIQ among controls similar to those seen in patients with 22q11DS (Campbell et al., 2009; Lajiness-O’Neill et al., 2006; Lewandowski et al., 2007). One study found significantly higher VIQ than PIQ in a group of 14 children with 22q11DS but also in their 8 siblings, suggesting that a genetic difference apart from the 22q11 deletion might lead to such discrepancies (Lajiness-O’Neill et al., 2006). In a study of more impaired children with 22q11DS with mean FSIQ 59.4, the difference between VIQ and PIQ scores was not significant (Campbell et al., 2009).
Furthermore, the types and degree of language impairment in 22q11DS are inconsistent with NLD. Although the NLD diagnosis includes content disorders of language, particularly poor pragmatics, some studies have described children with 22q11DS as having significant overall language impairment, scoring more than two standard deviations below the mean on standardized measures of language skills (Moss et al., 1999). The severity of language disorders in the 22q11DS population may be increased by either the velopharyngeal insufficiency characteristic of the syndrome or autism spectrum disorders prevalent in the population.
The Present Study
We previously reported that a subset of children with 22q11DS (20% of our cohort) manifested a significant difference between VIQ and PIQ, suggestive of NLD (Lewandowski et al., 2007). However, our cumulative clinical experience has indicated significant variability in the neurobehavioral presentation of children and adolescents with 22q11DS, suggesting that only a minority may exhibit all the symptoms of NLD. The current study was undertaken to examine whether children with 22q11DS show a pattern of deficits and assets characteristic of NLD. Our first objective was to determine how many children with 22q11DS showed neurocognitive, academic, and social-behavioral characteristics associated with NLD. We hypothesized that most would not exhibit the major features of NLD. Our second research objective was to classify children with 22q11DS into distinct subgroups based on their neurocognitive performance. We hypothesized that one of these subgroups would display NLD features, including elevated rates of the academic and social-behavioral problems common in NLD.
Method
Participants
The sample consists of 74 patients with 22q11DS recruited through the Department of Pediatrics at Wake Forest University School of Medicine (WFUSM), the Department of Pediatrics at Duke University Health Systems (DUHS), and an advertisement on the Velocardiofacial Syndrome Educational Foundation website. Mean age was 10.7 (SD = 2.9), and 56% were male. Confirmation of 22q11DS was made through fluorescence in situ hybridization or comparative genomic hybridization. Because participants were drawn from the sample population of a larger study for which autism and psychosis were exclusionary criteria, none was diagnosed with an autism spectrum or psychotic disorder. This study was approved by the Institutional Review Boards at WFUSM and DUHS, and consent was obtained from the parent or legal guardian of each participant.
Measures
Criteria for NLD were based on those described by Rourke et al. (2002). The authors named the primary deficits of NLD as tactile and visual perception, psychomotor skills, and dealing with new circumstances. They described these deficits as leading to difficulties in problem solving, pragmatic language, arithmetic, and social learning, which in turn led to social skills deficits and internalized psychopathology. Antshel and Khan (2008) used these criteria to determine which children in their sample with 22q11DS also had NLD. Table 1 shows measures used to assess each criterion. Internal reliability for these measures in our sample population was assessed using Cronbach’s alpha, for which values ranged from .67 to .89. Deficits in tactile perception, time perception, and pragmatic language were not assessed.
Table 1.
These Characteristics of NLD Were Described by Rourke et al. (2002): The Measures We Used to Assess Each Characteristic Are Listed
| NLD Characteristic | Measure | |
|---|---|---|
| 1 | Tactile-perceptual deficits | Not assessed |
| 2 | Psychomotor coordination deficits | WISC Processing Speed ≤ 85 |
| 3 | Deficits in visual-perceptual organization | WISC Performance IQ ≤ 85 |
| 4 | Difficulty managing new information | Working Memory ≤ 85 |
| 5 | Impairment in nonverbal problem-solving | WCST Perseverative Errors ≤ 85 |
| 6 | Distorted sense of time | Not assessed |
| 7 | Good rote verbal capacity | WISC Verbal IQ > 85 |
| 8 | High verbosity with deficits in pragmatic language | Not assessed |
| 9 | Relative difficulty with arithmetic | WIAT Math ≤ 85 |
| 10 | Poor reading comprehension | WIAT Reading Comprehension ≤ 85 |
| 11 | Deficits in social perception and judgment | SSRS ≤ 85 |
| 12 | Increased risk of internalizing psychopathology | CBCL Internalizing ≥ 60 |
Note: CBCL = Child Behavior Checklist; NLD = nonverbal learning disability; SSRS = Social Skills Rating System; WCST = Wisconsin Card Sorting Test; WIAT = Wechsler Individual Achievement Test; WISC = Wechsler Intelligence Scale for Children.
Additional variables described below were used in the latent class analysis. The latent class analysis considered not only measures targeting the criteria defined by Rourke et al. (2002) but also measures of other characteristics that have been associated with NLD: VIQ/PIQ discrepancy, verbal memory, visual attention, and basic reading.
Wechsler Intelligence Scale for Children, 3rd and 4th Editions (WISC-III/WISC-IV)
Since an updated version of the WISC was published during the course of our data collection, the WISC-III was administered to participants before June 2005 and the WISC-IV was administered thereafter. The WISC-III (Wechsler, 2001) consists of 10 subtests that combine to yield VIQ, PIQ, and FSIQ. Two additional subtests were administered to yield four factor scores: Verbal Comprehension, Perceptual Organization, Freedom from Distractibility, and Processing Speed. The WISC-IV (Wechsler, 2003) consists of an FSIQ score and four composite scores: Verbal Comprehension Index, Perceptual Reasoning Index, Working Memory Index, and Processing Speed Index. For this study, we employed all factor scores from each version of the WISC. Participants’ scores may be referred to collectively as including VIQ (VIQ on the WISC-III or Verbal Comprehension Index score on the WISC-IV) and PIQ (PIQ on the WISC-III or Perceptual Reasoning Index score on the WISC-IV.
Wechsler Individual Achievement Test, 2nd edition (WIAT-II)
The WIAT-II is widely used to assess academic achievement. Scores have a mean of 100 with a standard deviation of 15. This study utilized three subtests from the WIAT-II: Word Reading, Reading Comprehension, and Math.
Wisconsin Card Sorting Test (WCST)
The WCST is an executive function measure that has been used with many clinical populations (Chase-Carmichael, Ris, Weber, & Schefft, 1999; Chelune & Baer, 1986). A series of “cards” are presented with patterns that differ in number, shape, and color. To correctly match pairs of cards, the participant must determine the rule for matching. Feedback is provided after each response. After 10 consecutive correct responses, the matching rule changes without the participant’s knowledge. Perseverative errors on the WCST served as a variable in this study.
California Verbal Learning Test–Children’s Version (CVLT-C)
The CVLT-C is a test of verbal learning and memory for children ages 5–16 years (Delis, Kramer, Kaplan, & Ober, 1994). Participants are asked to recall as many words as they can from lists read aloud, and a list is repeated five times to examine learning and recall over the five trials. For this study we used the total recall variable.
Continuous Performance Test (CPT)
The CPT measures the ability to focus and sustain attention and inhibit inappropriate responses (Cornblatt, Risch, Faris, Friedman, & Erlenmeyer-Kimling, 1988). In the Identical Pairs (IP) task, a series of numbers or shapes appear on the computer screen and participants are instructed to lift their fingers off the mouse button when identical stimuli are presented on consecutive trials. In the AX task (named for a task requiring response when the letter X follows the letter A) participants are instructed to lift their fingers off the button when the number 8 is preceded by the number 2. The signal detection index used in the present study is a measure of attention regulation.
Child Behavior Checklist (CBCL)
The CBCL is a parent report of children’s social competencies and behavioral and emotional problems (Achenbach & Ruffle, 2000). The CBCL measures used in this study were Total Behavior Problems, Internalizing Problems, and Externalizing Problems for children 6 to 18 years of age.
Social Skills Rating System (SSRS)
The SSRS assesses social behaviors of children and adolescents, ages 3 years and older, based on reports by the teacher, parent, and child (Gresham & Elliot, 1990). The present study uses the total social skills and problem behavior scores from the parent rating form.
Computerized Diagnostic Interview for Children (C-DISC)
The C-DISC is a semistructured psychiatric interview administered to the child’s caregiver and is based on the DSM-IV criteria for mental disorders. Modules reported in the present study include Anxiety Disorders and ADHD, as these disorders are prevalent in children and adolescents with 22q11DS.
Procedures
Participants were assessed individually at WFUSM and DUHS. Trained graduate students in clinical psychology administered the neurocognitive tests under the supervision of a licensed clinical neuropsychologist. The CBCL and SSRS questionnaires were completed by parents during a child’s testing session or returned by mail.
Data Analysis
In this exploratory study, we approached from two different angles the question of whether NLD is a useful descriptor for children with 22q11DS. We first considered how many NLD criteria our participants met. As a second and alternate approach, we used latent class analysis to group participants based on cognitive abilities, the foundation on which academic and other skills are built. Our goal was to determine whether a subset of the sample, if not the whole sample, resembled the prototypical child with NLD.
For the criteria to address our first research question, we chose characteristics described by Rourke et al. (2002) and subsequently used by Antshel and Khan (2008) to estimate NLD prevalence in 22q11DS. These criteria were chosen to permit comparison of our results with Antshel and Khan’s conclusions about the same population. We calculated the number and percentage of participants displaying each characteristic described by Rourke et al. (2002). Antshel and Khan qualified a child for inclusion in the NLD group when he or she met at least 8 out of 10 criteria. In line with this threshold, we examined the number and percentage of participants who met 7 out of 9 assessed NLD criteria.
As implied by the term nonverbal learning disability, “good verbal capacity” (Harnadek & Rourke, 1994) or “well-developed rote verbal abilities” with “high verbosity” (Rourke et al., 2002) are characteristic of NLD. We defined good verbal capacity as a VIQ of at least 85, that is, within one standard deviation of the mean. This threshold has been used in several recent studies (Galway & Metsala, 2011; Gross-Tsur, Shalev, Manor, & Amir, 1995; Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010; Semrud-Clikeman, Walkowiak, Wilkinson, & Minne, 2010; Zera, 2001), although some earlier studies used a threshold of 79 (Casey, Rourke, & Picard, 1991; Harnadek & Rourke, 1994) and others have required near-normal FSIQ (Forrest, 2004; Petti et al., 2003; Schiff et al., 2009).
VIQ > PIQ discrepancy has also often been cited as part of NLD diagnosis (Bloom & Heath, 2010; Casey et al., 1991; Fisher & DeLuca, 1997; Galway & Metsala, 2011; Grodzinsky et al., 2010; Harnadek & Rourke, 1994; Keller et al., 2006; Pelletier et al., 2001; Petti et al., 2003). Although, as shown in Table 1, this measure was not part of our diagnostic criteria, we did calculate the number and percentage of participants with VIQ > PIQ.
We thought that looking at NLD from a different angle might confirm earlier researchers’ results by identifying a subgroup of children with 22q11DS and NLD. Because neuropsychological assets and deficits have been proposed to underlie the academic and socioemotional aspects of NLD (Harnadek & Rourke, 1999), to address our second research question we began with the neurocognitive characteristics of NLD: IQ, memory, attention, and perseveration scores derived from the WISC, CVLT, CPT, and WCST, respectively. We applied latent class analysis (LCA; Latent Gold Version 4.5) to determine the presence of homogeneous subgroups based on these neurocognitive variables, then used academic and socioemotional variables to confirm the validity of those groups. We chose LCA because this method generates fit statistics to help judge group validity.
The LCA took into consideration not only the variables used in investigating the first research question but also several additional NLD criteria described in other sources: VIQ/PIQ discrepancy (Galway & Metsala, 2011; Gross-Tsur et al., 1995; Semrud-Clikeman, Walkowiak, Wilkinson, & Christopher, 2010; Semrud-Clikeman, Walkowiak, Wilkinson, & Minne, 2010; Zera, 2001), visual attention deficits (Harnadek & Rourke, 1994), good verbal memory (Harnadek & Rourke, 1994; Liddell & Rasmussen, 2005), and good basic reading, that is, decoding (Casey et al., 1991; Galway & Metsala, 2011; Grodzinsky et al., 2010; Harnadek & Rourke, 1994; Hendriksen et al., 2007; Rourke et al., 1971). Given the variety of approaches to NLD diagnosis, we hoped that broadening our scope of inquiry for the second research question might reveal patterns not evident in our first analysis.
The analysis comprises four steps: (a) determining the number of groups, using data with complete observations; (b) “filling out” partially missing values using a multiple imputation procedure (here set # of imputed data set = 40); (c) fitting the K-group latent class model to each imputed data set; and (d) determining group membership based on the most common result from each imputed data set via LCA. Although latent class models are robust to violations of normality (Magidson & Vermunt, 2002), assumptions of normality were checked using QQ plots.
A three-group solution was chosen and judged to have clinical relevance. The imputed values were predicted from a regression model (SAS Version 9.2 PROC MI) that uses available data to predict the values of missing entries. Instead of the typically small number of imputations used (e.g., 5), we used a relatively large number of imputations because we found that the latent class membership determination began to stabilize when the number of imputed data sets reached 40. Each imputed data set was run through the LCA resulting in an array of group membership. A composite group membership value was determined by the mode of the resulting group membership array. Validity of the groups was assessed using ANOVA to compare the groups in terms of academic and socioemotional variables.
Results
Alignment of 22q11DS with the NLD Diagnosis
For this analysis 70 participants had complete data, operationally defined as data available for at least 6 of the 9 assessed criteria. Of the included participants, 9 were missing data on both social skills measures because of caregivers not returning rating scales. Other reasons for missing data included computer malfunctions during computerized tests and participants running out of time, being too tired to perform well, or performing at too low a level to complete the assessments used.
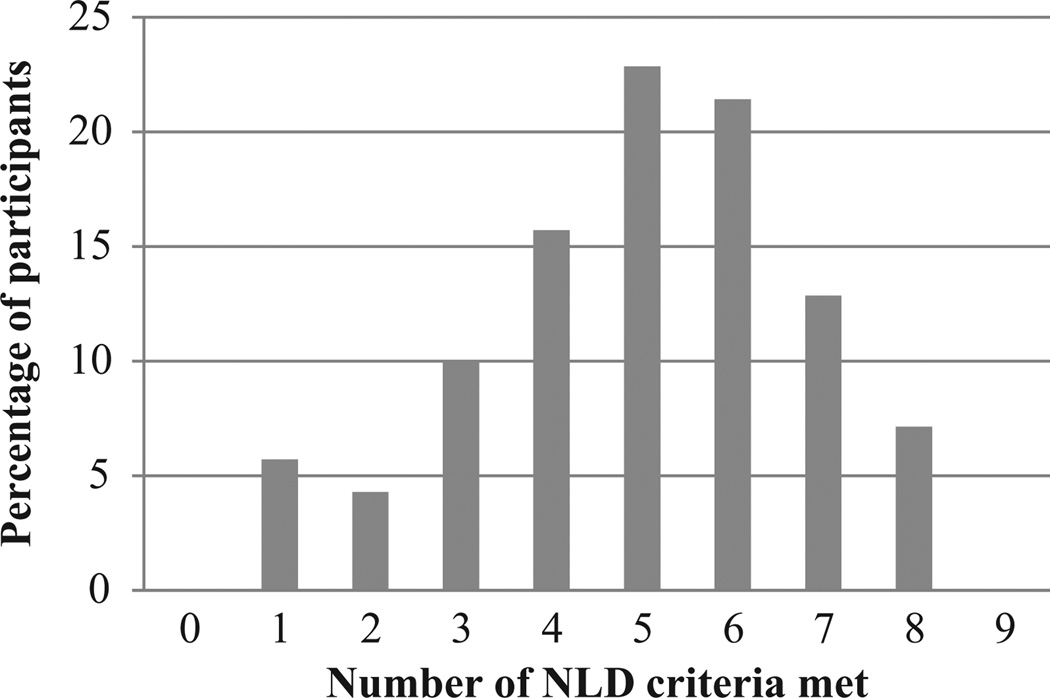
Although a total of 44% of participants with 22q11DS met either 5 or 6 of the assessed NLD criteria, only 13% met 7 criteria and 7% met 8 criteria. Figure 1 shows the percentage of participants with 22q11DS who met each possible number of NLD criteria. The average number met was 5. No participants met all 9 assessed criteria, but all met at least 1 criterion.
Figure 1.
Although a total of 44% of participants with 22q11DS met either 5 or 6 of the assessed NLD criteria, only 20% met either 7 or 8 criteria.
Note: NLD = nonverbal learning disability; 22q11DS = Chromosome 22q11.2 deletion syndrome.
Table 2 presents the percentages of individuals who met criteria for impairment on the neurocognitive, socioemotional, and academic characteristics associated with NLD as described by Rourke et al. (2002). The NLD characteristics evidenced by the largest fraction of participants were visual-perceptual organization deficits (81%) and difficulty with arithmetic (80%). Approximately two thirds of individuals showed deficits in psychomotor coordination (69%) and working memory (65%). Working memory was the only measure on which male and female participants differed in terms of mean score (81.62 for males vs. 74.56 for females, p < .05). Approximately half the sample (54%) had the expected problems with reading comprehension, and 45% of participants demonstrated impaired nonverbal problem solving, based on perseveration on the WCST. In evaluating the socioemotional problems associated with NLD, parent reports indicated that 62% of the cohort had internalizing problem behaviors and 39% showed social skills deficits. Only 24% of participants in the present study had good rote verbal capacity, making it the NLD characteristic least often evident in this cohort. Notably, of the participants with good rote verbal skills, only one (1.4%) met 7 of 9 NLD criteria.
Table 2.
The Number of Respondents With 22q11DS Who Met Each NLD Criterion Is Reported Here: Missing Data Are Reflected in Lower Denominators for Certain Measures
| NLD Characteristic | Measure | Present | % |
|---|---|---|---|
| Deficits in visual-perceptual organization | WISC Performance IQ ≤ 85 | 57/70 | 81.4 |
| Relative difficulty with arithmetic | WIAT Math ≤ 85 | 56/70 | 80.0 |
| Psychomotor coordination deficits | WISC Processing Speed ≤ 85 | 47/68 | 69.1 |
| Difficulty managing new information | Working Memory ≤ 85 | 44/68 | 64.7 |
| Increased risk of internalizing psychopathology | CBCL Internalizing ≥ 60 | 36/58 | 62.1 |
| Poor reading comprehension | WIAT Reading Comprehension ≤ 85 | 37/69 | 53.6 |
| Impairment in nonverbal problem solving | WCST Perseverative Errors ≤ 85 | 30/66 | 45.5 |
| Deficits in social perception and judgment | SSRS ≤ 85 | 24/61 | 39.3 |
| Good rote verbal capacity | WISC Verbal IQ > 85 | 17/70 | 24.3 |
| Tactile-perceptual deficits | Not assessed | ||
| Distorted sense of time | Not assessed | ||
| High verbosity with deficits in pragmatic language | Not assessed |
Note: CBCL = Child Behavior Checklist; NLD = nonverbal learning disability; SSRS = Social Skills Rating System; 22q11DS = Chromosome 22q11.2 deletion syndrome; WIAT = Wechsler Individual Achievement Test; WISC = Wechsler Intelligence Scale for Children.
Verbal/performance discrepancy based on WISC-III and WISC-IV index scores was also examined, although it was not included in our list of diagnostic criteria. A VIQ at least 10 points higher than PIQ has often been used as a measure of visual-perceptual organization deficits to screen for children with NLD (Bloom & Heath, 2010; Casey et al., 1991; Fisher & DeLuca, 1997; Galway & Metsala, 2011; Grodzinsky et al., 2010; Harnadek & Rourke, 1994; Keller et al., 2006), although it has been found to be present in a minority of children with NLD (Pelletier et al., 2001). In the present study, 25% of participants had VIQ > PIQ by 10 points or more, whereas 17% had PIQ > VIQ by the same margin. Most participants with near-normal Verbal Comprehension scores also had Perceptual scores within 10 points of their Verbal scores (65%). Mean VIQ and PIQ for the group were similar (77 vs. 75, respectively).
Latent Class Grouping
Although some of the children demonstrated assets and deficits consonant with an NLD model, as expected there was significant variability. LCA was conducted with the goal of establishing a classification system for children with 22q11DS, which might include a subgroup with NLD. All 74 participants were placed in groups based on neurocognitive variables associated with NLD, including not only the measures used in the previous analysis (assessing visual-spatial organization, verbal capacity, ability to manage new information, psychomotor coordination, and nonverbal problem solving) but also verbal memory and visual attention. Verbal memory has been described as an asset for children with NLD, visual attention as a deficit (Harnadek & Rourke, 1994).
Assumptions of normality were confirmed for most measures, and the few violations were not expected to affect the LCA since this involves creating categorical groupings. A significant latent class model was generated. The AIC scores were as follows: 3870 for one group, 3807 for two, 3792 for three, 3766 for four, and 3780 for five groups. Because in the four-group model one of the groups was quite small (comprising approximately 8% of the patients), a parsimonius three-group model was chosen. Pairwise comparisons showed that all three groups differed significantly on measures of verbal comprehension, perceptual organization, processing speed, and working memory.
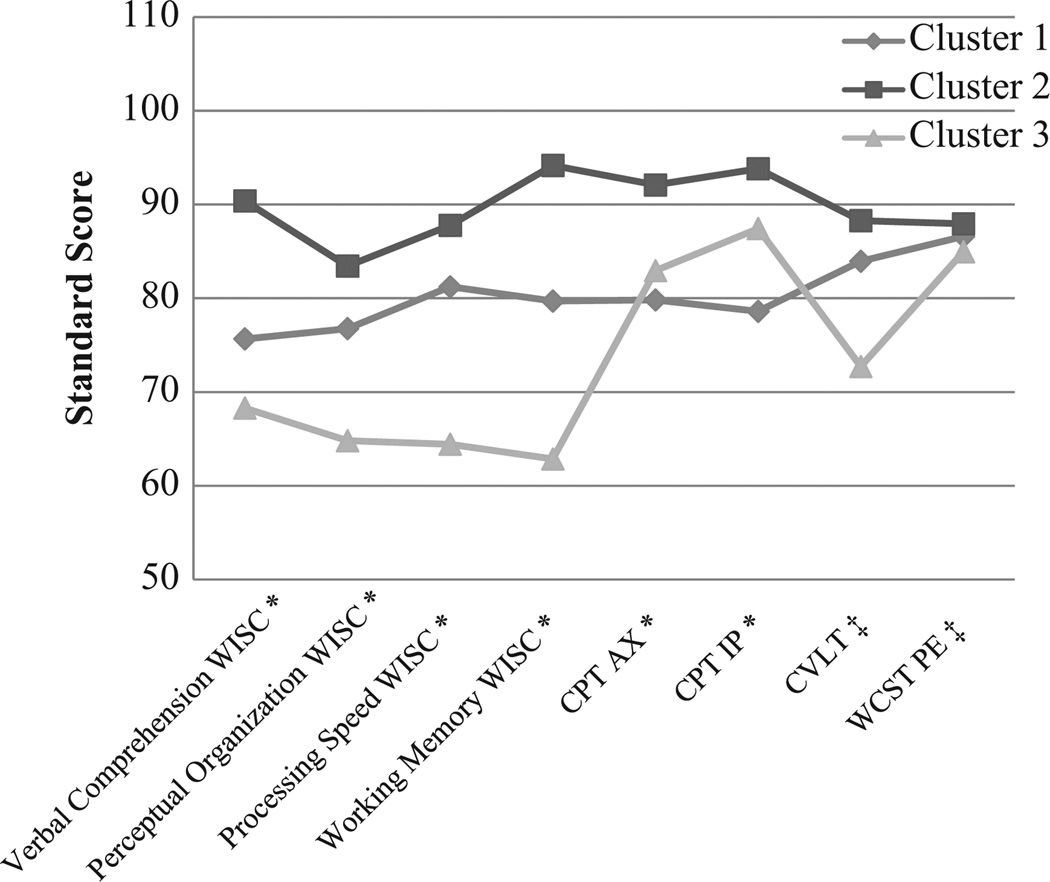
Group 1 comprised 45% of the cohort and represented members with neurocognitive functioning typical for 22q11DS, though below the average for normally achieving children. Group 2, which included 24% of participants, demonstrated the highest performance on all measures. Group 3 included 31% of participants and had the lowest scores on all measures except those of visual attention. On one visual attention measure, the CPT-IP, Group 3 earned higher scores than Group 1. Figure 2 illustrates differences in performance among the three groups.
Figure 2.
Latent class analysis produced these three clusters based on neurocognitive variables; significant differences remained after partialling out variance associated with age and socioeconomic status.
‡ns. *p < .001.
The highest functioning group had significantly higher socioeconomic status than the average- (p < .01) and low-functioning groups (p < .05). The average-functioning group had the youngest children on average, and the lowest-functioning group contained the oldest children. However, when age and socioeconomic status were partialled out, significant differences remained among all three groups.
The highest functioning group also contained more male participants (72%). Membership in the other groups was approximately evenly split between the genders, the average-functioning group being 48% male and the lowerfunctioning group 51% male.
Group Validity
Group validity was determined using socioemotional and academic characteristics of NLD rather than neurocognitive characteristics. The academic achievement variables were Broad Mathematics, Reading Comprehension, and Word Reading scores on the WIAT. The socioemotional measures, internalizing behaviors on the CBCL and social skills on the SSRS, were calculated based on ratings scales completed by the children’s parents.
Academic achievement measures clearly differentiated the three groups. Group 2, the highest functioning, showed the best reading and math skills, although their math skills were lower than their reading skills. Group 3, the lowest functioning, showed severe impairments in math skills and reading comprehension, with word reading in the borderline range. Group 1 had an intermediate profile, with borderline math skills, borderline to low-average reading comprehension, and low-average word reading. Table 3 shows scores for each group.
Table 3.
Validity (socioemotional and academic) Variables Are Analyzed by Group
| Group 1, Average |
Group 2, Relatively High |
Group 3, Relatively Low |
|||||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | Pairwise Comparisons | |
| Age at assessment | 9.28 | 1.98 | 11.01 | 2.65 | 11.94 | 2.72 | 2 > 1†, 3 > 1* |
| Overall SES | 35.00 | 11.48 | 23.50 | 13.66 | 34.65 | 14.74 | 1 > 2*, 3 > 2† |
| CBCL Externalizing Behaviors | 51.20 | 9.29 | 50.58 | 8.32 | 56.06 | 9.77 | ns |
| CBCL Internalizing Behaviors | 57.03 | 15.73 | 58.58 | 10.14 | 64.35 | 9.58 | ns |
| CBCL Total Problem Behaviors | 60.03 | 10.82 | 56.92 | 10.00 | 64.94 | 7.21 | ns |
| Parent Social Skills Rating System | 91.13 | 19.46 | 96.08 | 15.76 | 85.05 | 15.26 | ns |
| WIAT Reading Comprehension | 81.61 | 12.16 | 94.89 | 12.07 | 68.62 | 19.78 | 2 > 1*,1 > 3† |
| WIAT Broad Mathematics | 74.03 | 14.53 | 88.83 | 11.37 | 56.81 | 15.90 | 2 > 1 > 3* |
| WIAT Word Reading | 87.13 | 11.12 | 95.33 | 10.27 | 75.14 | 16.92 | 2 > 1†,1 > 3* |
| C-DISC any anxiety (%) | 43.75 | 33.33 | 57.14 | ||||
| C-DISC ADHD (%) | 43.75 | 33.33 | 52.38 | ||||
Note: There were significant differences among all three groups for the academic variables, with the lowest functioning group earning the lowest scores and the highest functioning group earning the highest scores. CBCL = Child Behavior Checklist; C-DISC = Computerized Diagnostic Interview for Children; WIAT = Wechsler Individual Achievement Test.
p < .05.
p < .001.
Social skills ratings, in contrast, were similar in highand low-functioning groups. Nor were significant differences found in internalizing behaviors, although Group 3 did have internalizing scores in the bottom 10th percentile. Although both Group 1 and Group 3 had Total Behavior Problem scores at least one standard deviation below the mean for the general population, none of the groups received ratings reflecting significant externalizing problems.
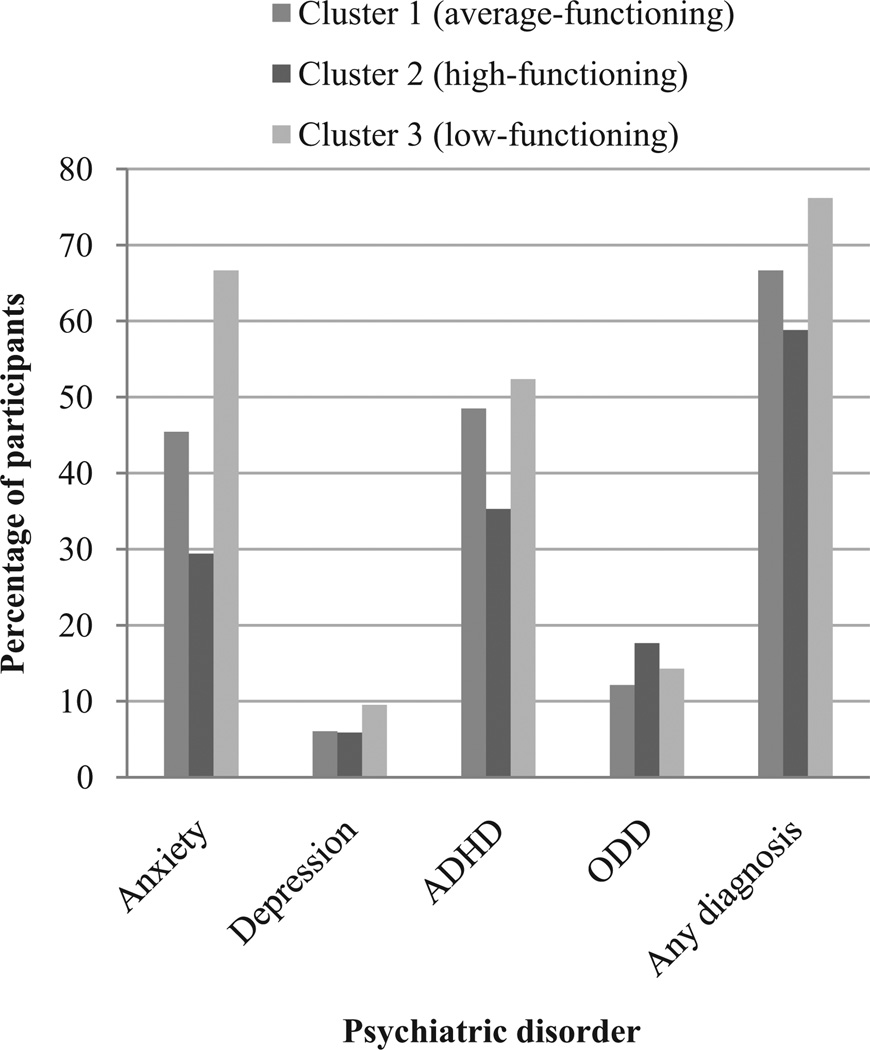
Psychiatric diagnoses were determined based on C-DISC scores or medication for a specific psychiatric disorder. Figure 3 illustrates rates in each group. As expected, anxiety disorders and ADHD were most prevalent, occurring in 46% and 45% of the sample population, respectively. ODD and depressive disorders occurred at rates of 14.0% and 6.8%, respectively. Of the sample population, 21.0% was receiving medication to treat ADHD, 8.6% to treat depression, and 7.1% to treat anxiety. Chi-square analysis found no significant differences among the three groups in terms of the frequency of any of these diagnoses, total rate of psychiatric diagnoses, or ADHD medication usage. In each group, more than 50% of members had some psychiatric diagnosis, suggesting that psychiatric disorders occur across the spectrum of individuals with 22q11DS.
Figure 3.
Although the low-functioning group had the highest rate of psychiatric disorders, these disorders were also prevalent in the average- and high-functioning groups.
Discussion
The NLD model is frequently used to describe the phenotypic presentation of individuals with 22q11 deletion syndrome. However, few studies have systematically analyzed whether these children consistently conform to the NLD diagnosis. The current study set out to determine whether the NLD diagnosis is consistently appropriate for either the entire population of children and adolescents with 22q11DS or a distinct subgroup of this population.
Because NLD diagnostic criteria reported in the literature vary, we considered several possible approaches to identifying NLD. We defined and assessed 9 characteristics of NLD based on the model presented by Rourke et al. (2002). The criteria our sample most often met were visual-perceptual organization deficits and arithmetic difficulty, both of which at least 80% of our population showed. In all, 70% met criteria for a psychiatric disorder based on C-DISC scores or medical history, and more than 60% were reported by their parents to show internalizing behaviors, which have been cited as characteristic of NLD. Nearly 70% showed poor psychomotor coordination, and 65% had working memory impairments, which would be expected to reduce their ability to manage new information. More than half had difficulty with reading comprehension. This pattern of deficits goes some way toward explaining why children with 22q11DS have been labeled as NLD.
However, the impairments of children with 22q11DS often extend beyond those expected in NLD. Notably, in the present study, only 20% of participants showed good verbal capacity, as assessed by a Verbal Comprehension score within one standard deviation of the mean, whereas 25% had Verbal scores exceeding their Perceptual scores by at least 10 points. The average Verbal Comprehension score for the group was 77. In short, verbal skills were not a demonstrable strength in most participants. If NLD is used to represent deficits of children with 22q11DS, clinicians should be aware that these children may not show assets typically associated with the learning disorder, such as normal verbal comprehension scores.
Consistent with Antshel and Khan’s (2008) application of the NLD diagnosis as described by Rourke et al. (2002) to a group of children with 22q11DS, we operationally defined the NLD label as appropriate when any 7 of our 9 criteria were met. Of our population, 20% met this many criteria. These results support the findings of Antshel and Khan, who also reported a prevalence of 20% for NLD in 22q11DS. Some earlier studies had reported higher prevalence of NLD in this population (Moss et al., 1999; Swillen et al., 1999).
When distinct subgroups were generated through LCA, using the neurocognitive deficits seen in NLD and subsequently mapping on the academic and social-behavioral manifestations, no one group showed the pattern of assets and deficits characteristic of NLD. Instead, the three groups differed in levels of neurocognitive, social, and academic functioning. They are most clearly described as high-, low-, and average-functioning groups. Mean FSIQ for the high-functioning group was closest to the average for the general population (85, vs. 100 in the general population), whereas the average-functioning group had a mean IQ similar to those usually cited for patients with 22q11DS (73). Mean IQ in the lowest functioning group was 60. As shown in Figure 2, other measures of functioning generally followed these lines.
The low-functioning group had the lowest scores on every task except the Continuous Performance Test, which measures attention regulation. The rote nature of this task may have reduced its utility for discriminating among these particular groups. Still, this relative strength was puzzling because the lowest functioning group had a high rate of ADHD and no higher rate of ADHD medication usage than any other group. Although anxiety might possibly have contributed to increased vigilance, all three groups had statistically similar anxiety rates.
Although the families of the high-functioning group had the highest socioeconomic status (SES), the other two groups did not differ in terms of SES, corroborating our previous finding that cognition and achievement are not universally associated with SES in the 22q11DS population (Shashi, Keshavan, et al., 2010). An unanticipated finding was that mean age was significantly higher in the low-functioning group compared with the others. One possible explanation is that because tests of IQ and academic achievement are scored using age-based norms, the slow rate of cognitive growth for many children with 22q11DS may cause them to diverge more from the mean as they age. Longitudinal models could be used to investigate this question.
Genotypic influences may contribute to the differences among high-, low-, and average-functioning groups. We have previously shown that 22q11DS individuals with the Met allele perform better on measures of neurocognition than those with the Val allele (Shashi, Howard, et al., 2010). However, further exploration of this possibility was beyond the scope of this article.
All three groups showed similarly high rates of psychiatric disorders, ADHD and anxiety disorders being the most common. We considered the possibility that power was insufficient to detect significant differences in anxiety and ADHD between the lowest functioning group and the other two, but this seems improbable because the effect sizes were small. This finding highlights the importance of careful mental health monitoring for all children with 22q11DS. Clinicians may associate higher neurocognitive, social, and academic functioning with lower risk for psychiatric disorders. These data demonstrate that they should remain vigilant for anxiety disorders and other psychiatric concerns in patients with relatively intact cognitive functioning.
The battery of assessments used in this study was originally designed to capture information concerning participants’ risk for developing psychosis, rather than to determine NLD diagnoses. A limitation of this study is that tactile-perceptual deficits, high verbosity, and distorted sense of time, sometimes listed as features of NLD (Rourke et al., 2002), were not assessed. Our use of working memory as a proxy for the ability to manage novel or complex information is another limitation; a more direct measure might have been more accurate. For instance, Antshel and Khan (2008) assessed this criterion by having two raters observe each child in an interpersonal situation. A future prospective study could assess NLD characteristics more comprehensively in additional populations of interest.
Rourke et al. (2002) described 22q11DS as a Level 1 manifestation of NLD, a disorder showing virtually all the NLD assets and deficits. Based on the low rates of many NLD characteristics in our sample, along with our inability to identify a distinct subgroup manifesting most features of NLD, we disagree with this assertion. We propose instead that 22q11DS be considered, like Asperger syndrome, a “similar, but basically different” disorder in relation to NLD. Spreen (2011) argued that the high rate of psychosis associated with 22q11DS makes NLD an inadequate descriptor for this population. Our study shows that even nonpsychotic children and adolescents with 22q11DS are not consistently well described by NLD. Applying the NLD label to these children could bias the expectations of teachers or caregivers and might lead to unrealistic goals or inappropriate interventions, such as interventions dependent on good verbal skills. Consequently, we recommend avoiding the term NLD for the general population of children with 22q11DS, although it may accurately describe the strengths and weaknesses of certain children with 22q11DS.
Ongoing investigation into the phenotypic profile of children and adolescents with 22q11DS may help to guide care for this population in the future. In the meantime, clinical practice should be based on a thorough examination of the neurocognitive, academic, and social functioning of each individual child, with interventions being tailored to each child’s specific developmental needs.
Acknowledgments
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by funding from the National Institute of Mental Health (grant number R01MH78015-01A1) awarded to Vandana Shashi, M.D.
Biographies
Kelly Schoch is the Clinical Research Coordinator and genetic counselor for the 22q11 Deletion Syndrome clinic at Duke Medical Center.
Waverly Harrell is a post-doctoral associate at Duke University Medical Center. She received her PhD in school psychology from the University of North Carolina at Chapel Hill.
Stephen R. Hooper is a Professor of Psychiatry and Pediatrics at the University of North Carolina School of Medicine, a Professor of Psychology and Education at the University of North Carolina-Chapel Hill, and a Professor of Psychiatry in the Department of Psychiatry and Behavioral Sciences at Duke University Medical School. Dr. Hooper is also the Director of Child and Adolescent Neuropsychology and the Director of Education and Training at the Carolina Institute for Developmental Disabilities at the University of North Carolina School of Medicine.
Edward H. Ip is Professor and Associate Chair of Research and Faculty Development, Department of Biostatistical Sciences, Wake Forest University School of Medicine. He received his PhD in statistics from Stanford University and his research interests include analytic methods for social and behavioral sciences.
Thomas R. Kwapil is a Professor in the Psychology Department and an Associate Dean for Research at the University of North Carolina at Greensboro. His research examines risk for schizophrenia and bipolar disorders.
Vandana Shashi is an Associate Professor of Pediatrics in the Duke University School of Medicine and a clinical geneticist and pediatrician with an interest in neurodevelopmental disorders, specifically Chromosome 22q11 Deletion Syndrome.
Footnotes
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Achenbach TM, Ruffle TM. The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatric Reviews. 2000;21(8):265–271. doi: 10.1542/pir.21-8-265. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Author; Washington, DC: 2000. [Google Scholar]
- Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, Kates WR. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) Journal of Autism and Developmental Disorders. 2007;37(9):1776–1786. doi: 10.1007/s10803-006-0308-6. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgens AM, Dhamoon A, Kates WR. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velocardiofacial syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [DOI] [PubMed] [Google Scholar]
- Antshel KM, Khan FM. Is there an increased familial prevalence of psychopathology in children with nonverbal learning disorders? Journal of Learning Disabilities. 2008;41(3):208–217. doi: 10.1177/0022219408317546. [DOI] [PubMed] [Google Scholar]
- Arnold PS, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R. Velo-cardio-facial syndrome: Implications of microdeletion 22q11 for schizophrenia and mood disorders. American Journal of Medical Genetics. 2001;105:354–362. doi: 10.1002/ajmg.1359. [DOI] [PubMed] [Google Scholar]
- Bassett AS, Chow EWC, Husted J, Weksberg R, Caluseriu O, Webb GD, Gatzoulis MA. Clinical features of 78 adults with 22q11 deletion syndrome. American Journal of Medical Genetics Part A. 2005;138(4):307–313. doi: 10.1002/ajmg.a.30984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AS, Hodgkinson K, Chow EW, Correia S, Scutt LE, Weksberg R. 22q11 deletion syndrome in adults with schizophrenia. American Journal of Medical Genetics. 1998;81(4):328–337. [PMC free article] [PubMed] [Google Scholar]
- Bearden CE, Woodin MF, Wang PP, Moss E, Donald-McGinn D, Zackai E, Cannon TD. The neurocognitive phenotype of the 22q11.2 deletion syndrome: Selective deficit in visual-spatial memory. Journal of Clinical and Experimental Neuropsychology. 2001;23(4):447–464. doi: 10.1076/jcen.23.4.447.1228. [DOI] [PubMed] [Google Scholar]
- Bloom E, Heath N. Recognition, expression, and understanding facial expressions of emotion in adolescents with nonverbal and general learning disabilities. Journal of Learning Disabilities. 2010;43:180–192. doi: 10.1177/0022219409345014. [DOI] [PubMed] [Google Scholar]
- Brueggemann AE, Kamphaus RW, Dombrowski SC. An impairment model of learning disability diagnosis. Professional Psychology: Research and Practice. 2008;39:424–430. [Google Scholar]
- Campbell LE, Stevens A, Daly E, Toal F, Azuma R, Karmiloff-Smith A, Murphy KC. A comparative study of cognition and brain anatomy between two neurodevelopmental disorders: 22q11.2 deletion syndrome and Williams syndrome. Neuropsychologia. 2009;47(4):1034–1044. doi: 10.1016/j.neuropsychologia.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Casey JE, Rourke BP, Picard EM. Syndrome of nonverbal learning disabilities: Age differences in neuropsychological, academic, and socioemotional functioning. Developmental Psychopathology. 1991;3:329–345. [Google Scholar]
- Chase-Carmichael CA, Ris MD, Weber AM, Schefft BK. Neurologic validity of the Wisconsin Card Sorting Test with a pediatric population. Clinical Neuropsychol-ogy. 1999;13(4):405–413. doi: 10.1076/1385-4046(199911)13:04;1-Y;FT405. [DOI] [PubMed] [Google Scholar]
- Chelune GJ, Baer RA. Developmental norms for the Wisconsin Card Sorting test. Journal of Clinical and Experimental Neuropsychology. 1986;8(3):219–228. doi: 10.1080/01688638608401314. [DOI] [PubMed] [Google Scholar]
- Chow EWC, Watson M, Young DA, Bassett AS. Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophrenia Research. 2006;87(1–3):270–278. doi: 10.1016/j.schres.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, Identical Pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Research. 1988;26(2):223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- De Smedt B, Devriendt K, Fryns JP, Vogels A, Gewillig M, Swillen A. Intellectual abilities in a large sample of children with Velo-Cardio-Facial Syndrome: An update. Journal of Intellectual Disability Research. 2007;51(Pt. 9):666–670. doi: 10.1111/j.1365-2788.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test–Second Edition(CVLT-II) Psychological Corporation; San Antonio, TX: 1994. [Google Scholar]
- Drummond CR, Ahmad SA, Rourke BP. Rules for the classification of younger children with nonverbal learning disabilities and basic phonological processing disabilities. Archives of Clinical Neuropsychology. 2005;20:171–182. doi: 10.1016/j.acn.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Eling P. Nee tegen NLD [Arguments against NLD] Psy-choloog. 2007;42(9):483–487. [Google Scholar]
- Feinstein C, Eliez S, Blasey C, Reiss AL. Psychiatric disorders and behavioral problems in children with velocardiofacial syndrome: Usefulness as phenotypic indicators of schizophrenia risk. Biological Psychiatry. 2002;51:312–318. doi: 10.1016/s0006-3223(01)01231-8. [DOI] [PubMed] [Google Scholar]
- Fisher NJ, DeLuca JW. Verbal learning strategies of adolescents and adults with the syndrome of nonverbal learning disabilities. Child Neuropsychology. 1997;3(3):192–198. [Google Scholar]
- Forrest BJ. The utility of math difficulties, internalized psychopathology, and visual-spatial deficits to identify children with the nonverbal learning disability syndrome: Evidence for a visuospatial disability. Child Neuropsychology. 2004;10:129–146. doi: 10.1080/09297040490911131. [DOI] [PubMed] [Google Scholar]
- Galway TM, Metsala JL. Social cognition and its relation to psychosocial adjustment in children with nonverbal learning disabilities. Journal of Learning Disabilities. 2011;44(1):33–49. doi: 10.1177/0022219410371680. [DOI] [PubMed] [Google Scholar]
- Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, Eliez S. Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(11):1060–1068. doi: 10.1097/CHI.0b013e3181b76683. [DOI] [PubMed] [Google Scholar]
- Gresham FM, Elliot SN. Social Skills Rating System. American Guidance Service; Circle Pines, MN: 1990. [Google Scholar]
- Grodzinsky GM, Forbes PW, Bernstein JH. A practice-based approach to group identification in nonverbal learning disorders. Child Neuropsychology. 2010;16(5):433–460. doi: 10.1080/09297041003631444. [DOI] [PubMed] [Google Scholar]
- Gross-Tsur V, Shalev RS, Manor O, Amir N. Developmental right-hemisphere syndrome: Clinical spectrum of the nonverbal learning disability. Journal of Learning Disabilities. 1995;28(2):80–86. [PubMed] [Google Scholar]
- Harnadek MC, Rourke BP. Principal identifying features of the syndrome of nonverbal learning disabilities in children. Journal of Learning Disabilities. 1994;27(3):144–154. doi: 10.1177/002221949402700303. [DOI] [PubMed] [Google Scholar]
- Hendriksen JG, Keuler EH, Feron FJ, Wassenberg R, Jolles J, Vles JS. Subtypes of learning disabilities: Neuropsychological and behavioural functioning of 495 children referred for multidisciplinary assessment. European Child and Adolescent Psychiatry. 2007;16(8):517–524. doi: 10.1007/s00787-007-0630-3. [DOI] [PubMed] [Google Scholar]
- Jacobson C, Shearer J, Habel A, Kane F, Tsakanikos E, Kravariti E. Core neuropsychological characteristics of children and adolescents with 22q11.2 deletion. Journal of Intellectual Disability Research. 2010;54(8):701–713. doi: 10.1111/j.1365-2788.2010.01298.x. [DOI] [PubMed] [Google Scholar]
- Johnson DJ, Myklebust HR. Learning disabilities: Educational principles and practices. Grune and Stratton; New York, NY: 1967. [Google Scholar]
- Jolin EM, Weller RA, Jessani NR, Zackai EH, McDonald-McGinn DM, Weller EB. Affective disorders and other psychiatric diagnoses in children and adolescents with 22q11.2 deletion syndrome. Journal of Affective Disorders. 2009;119(1–3):177–180. doi: 10.1016/j.jad.2009.02.016. [DOI] [PubMed] [Google Scholar]
- Kavale KA, Kauffman JM, Bachmeier RJ, LeFever GB. Response-to-intervention: separating the rhetoric of self-congratulation from the reality of specific learning disability identification. Learning Disability Quarterly. 2008;31:135–150. [Google Scholar]
- Keller WD, Tillery KL, McFadden SL. Auditory processing disorder in children diagnosed with nonverbal learning disability. American Journal of Audiology. 2006;15(2):108–113. doi: 10.1044/1059-0889(2006/014). [DOI] [PubMed] [Google Scholar]
- Kiley-Brabeck K, Sobin C. Social skills and executive function deficits in children with the 22q11 deletion syndrome. Applied Neuropsychology. 2006;13(4):258–268. doi: 10.1207/s15324826an1304_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajiness-O’Neill R, Beaulieu I, Asamoah A, Titus JB, Bawle E, Ahmad S, Pollack R. The neuropsychological phenotype of velocardiofacial syndrome (VCFS): Relationship to psychopathology. Archives of Clinical Neuropsychology. 2006;21(2):175–184. doi: 10.1016/j.acn.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Lepach AC, Petermann F. Nonverbal and verbal learning: A comparative study of children and adolescents with 22q11 deletion syndrome, non-syndromal nonverbal learning disorder and memory disorder. Neurocase. 2011;17:480–490. doi: 10.1080/13554794.2010.536954. [DOI] [PubMed] [Google Scholar]
- Lewandowski KE, Shashi V, Berry PM, Kwapil TR. Schizophrenic-like neurocognitive deficits in children and adolescents with 22q11 deletion syndrome. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 2007;144(1):27–36. doi: 10.1002/ajmg.b.30379. [DOI] [PubMed] [Google Scholar]
- Liddell GA, Rasmussen C. Memory profile of children with nonverbal learning disability. Learning Disabilities Research & Practice. 2005;20(3):137–141. [Google Scholar]
- Magidson J, Vermunt JK. Latent class models for clustering: A comparison with K-means. Canadian Journal of Marketing Research. 2002;20:37–44. [Google Scholar]
- Mammarella IC, Pazzaglia F. Visual perception and memory impairments in children at risk of nonverbal learning disabilities. Child Neuropsychology. 2010;16:564–576. doi: 10.1080/09297049.2010.485125. [DOI] [PubMed] [Google Scholar]
- Mayes SD, Calhoun SL. Test of the definition of learning disability based on the difference between IQ and achievement. Psychological Reports. 2005;97:109–116. doi: 10.2466/pr0.97.1.109-116. [DOI] [PubMed] [Google Scholar]
- Moss EM, Batshaw ML, Solot CB, Gerdes M, Donald-McGinn DM, Driscoll DA, Wang PP. Psychoeducational profile of the 22q11.2 microdeletion: A complex pattern. Journal of Pediatrics. 1999;134(2):193–198. doi: 10.1016/s0022-3476(99)70415-4. [DOI] [PubMed] [Google Scholar]
- Murphy KC, Jones LA, Owen MJ. High rates of schizophrenia in adults with velo-cardio-facial syndrome. Archives of General Psychiatry. 1999;56(10):940–945. doi: 10.1001/archpsyc.56.10.940. [DOI] [PubMed] [Google Scholar]
- Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genetics in Medicine. 2001;3(1):79–84. doi: 10.1097/00125817-200101000-00017. [DOI] [PubMed] [Google Scholar]
- Papolos DF, Faedda GL, Veit S, Goldberg R, Morrow B, Kucherlapati R, Shprintzen RJ. Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: Does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? American Journal of Psychiatry. 1996;153(12):1541–1547. doi: 10.1176/ajp.153.12.1541. [DOI] [PubMed] [Google Scholar]
- Pelletier PM, Ahmad SA, Rourke BP. Classification rules for basic phonological processing disabilities and nonverbal learning disabilities: Formulation and external validity. Child Neuropsychology. 2001;7(2):84–98. doi: 10.1076/chin.7.2.84.3127. [DOI] [PubMed] [Google Scholar]
- Petti VL, Voelker SL, Shore DL, Hayman-Abello SE. Perception of nonverbal emotion cues by children with nonverbal learning disabilities. Journal of Developmental and Physical Disabilities. 2003;15(1):23–36. [Google Scholar]
- Robin NH, Shprintzen RJ. Defining the clinical spectrum of deletion 22q11.2. Journal of Pediatrics. 2005;147(1):90–96. doi: 10.1016/j.jpeds.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Ahmad SA, Collins DW, Hayman-Abello BA, Hayman-Abello SE, Warriner EM. Child clinical/pediatric neuropsychology: Some recent advances. Annual Review of Psychology. 2002;53:309–339. doi: 10.1146/annurev.psych.53.100901.135204. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Finlayson AJ. Neuropsychological significance of variations in patterns of academic performance: Verbal and visuo-spatial abilities. Journal of Abnormal Child Psychology. 1978;6:121–133. doi: 10.1007/BF00915788. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Strang JD. Neuropsychological significance of variations in academic performance: Motor, psychomotor, and tactile-perceptual abilities. Journal of Pediatric Psychology. 1978;3:62–66. [Google Scholar]
- Rourke BP, Telegdy GA. Lateralizing significance of WISC verbal-performance discrepancies for older children with learning disabilities. Perceptual and Motor Skills. 1971;33:875–883. doi: 10.2466/pms.1971.33.3.875. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Tsatsanis KD. Syndrome of nonverbal learning disabilities: Psycholinguistic assets and deficits. Topics in Language Disorders. 1996;16(2):30–44. [Google Scholar]
- Rourke BP, Yanni DW, MacDonald GW, Young GC. Neuropsychological significance of lateralized deficits on the grooved pegboard test for older children with learning disabilities. Journal of Consulting and Clinical Psychology. 1973;41:128–134. doi: 10.1037/h0035613. [DOI] [PubMed] [Google Scholar]
- Rourke BP, Young GC, Flewelling RW. The relationship between WISC Verbal-Performance discrepancies and selected verbal, auditory-perceptual, and problem solving abilities in children with learning disabilities. Journal of Clinical Psychology. 1971;27:475–479. doi: 10.1002/1097-4679(197110)27:4<475::aid-jclp2270270421>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Schiff R, Bauminger N, Toledo I. Analogical problem solving in children with verbal and nonverbal learning disabilities. Journal of Learning Disabilities. 2009;42(1):3–13. doi: 10.1177/0022219408326213. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Christopher G. Neuropsychological differences among children with Asperger syndrome, nonverbal learning disabilities, attention deficit disorder, and controls. Developmental Neuropsychology. 2010;35(5):582–600. doi: 10.1080/87565641.2010.494747. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Walkowiak J, Wilkinson A, Minne EP. Direct and indirect measures of social perception, behavior, and emotional functioning in children with Asperger’s disorder, nonverbal learning disorder, or ADHD. Journal of Abnormal Child Psychology. 2010;38(4):509–519. doi: 10.1007/s10802-009-9380-7. [DOI] [PubMed] [Google Scholar]
- Shashi V, Howard TD, Keshavan MS, Kaczorowski J, Berry MN, Schoch K, Kwapil TR. COMT and anxiety and cognition in children with chromosome 22q11.2 deletion syndrome. Psychiatry Research. 2010;178(2):433–436. doi: 10.1016/j.psychres.2010.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shashi V, Keshavan MS, Howard TD, Berry MN, Basehore MJ, Lewandowski E, Kwapil TR. Cognitive correlates of a functional COMT polymorphism in children with 22q11.2 deletion syndrome. Clinical Genetics. 2006;69(3):234–238. doi: 10.1111/j.1399-0004.2006.00569.x. [DOI] [PubMed] [Google Scholar]
- Shashi V, Keshavan M, Kaczorowski J, Schoch K, Lewandowski KE, McConkie-Rosell A, Kwapil TR. Socioeconomic status and psychological function in children with chromosome 22q11.2 deletion syndrome: Implications for genetic counseling. Journal of Genetic Counseling. 2010;19(5):535–544. doi: 10.1007/s10897-010-9309-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: A distinctive behavioral phenotype. Mental Retardation and Developmental Disabilities Research Reviews. 2000;6(2):142–147. doi: 10.1002/1098-2779(2000)6:2<142::AID-MRDD9>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Shprintzen RJ. Velo-cardio-facial syndrome: 30 Years of study. Developmental Disabilities Research Reviews. 2008;14(1):3–10. doi: 10.1002/ddrr.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon TJ. A new account of the neurocognitive foundations of impairments in space, time, and number processing in children with chromosome 22q11.2 deletion syndrome. Developmental Disabilities Research Reviews. 2008;14(1):52–58. doi: 10.1002/ddrr.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Daniels S, Khuri J, Taylor L, Blundell M, Karayiorgou M. Neuropsychological characteristics of children with the 22q11 deletion syndrome: A descriptive analysis. Child Neuropsychology. 2005;11(1):39–53. doi: 10.1080/09297040590911167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobin C, Kiley-Brabeck K, Karayiorgou M. Lower prepulse inhibition in children with the 22q11 deletion syndrome. Child Neuropsychology. 2005;11(1):39–53. doi: 10.1176/appi.ajp.162.6.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O. Nonverbal learning disabilities: A critical review. Child Neuropsychology. 2011;17:418–443. doi: 10.1080/09297049.2010.546778. [DOI] [PubMed] [Google Scholar]
- Spreen O, Haaf RG. Empirically derived learning disability subtypes: A replication attempt and longitudinal patterns of 15 years. Journal of Learning Disabilities. 1986;19(3):170–180. doi: 10.1177/002221948601900308. [DOI] [PubMed] [Google Scholar]
- Strang JD, Rourke BP. Concept-formation/nonverbal reasoning abilities of children who exhibit specific academic problems with arithmetic. Journal of Clinical Child Psychology. 1983;12:33–39. [Google Scholar]
- Swillen A, Devriendt K, Legius E, Eyskens B, Dumoulin M, Gewillig M, Fryns JP. Intelligence and psychosocial adjustment in velocardiofacial syndrome: A study of 37 children and adolescents with VCFS. Journal of Medical Genetics. 1997;34(6):453–458. doi: 10.1136/jmg.34.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swillen A, Devriendt K, Legius E, Prinzie P, Vogels A, Ghesquiere P, Fryns JP. The behavioural phenotype in velo-cardio-facial syndrome (VCFS): From infancy to adolescence. Genetic Counseling. 1999;10(1):79–88. [PubMed] [Google Scholar]
- Tsatsanis KD, Rourke BP. Conclusions and future directions. In: Rourke BP, editor. Syndrome of nonverbal learning disabilities: Neurodevelopmental manifestations. Guilford; New York, NY: 1995. pp. 476–496. [Google Scholar]
- van Amelsvoort T, Henry J, Morris R, Owen M, Linszen D, Murphy K, Murphy D. Cognitive deficits associated with schizophrenia in velo-cardio-facial syndrome. Schizophrenia Research. 2004;70(2–3):223–232. doi: 10.1016/j.schres.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, van Engeland H. The 22q11.2 deletion in children: High rate of autistic disorders and early onset of psychotic symptoms. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45(9):1104–1113. doi: 10.1097/01.chi.0000228131.56956.c1. [DOI] [PubMed] [Google Scholar]
- Wang PP, Woodin MF, Kreps-Falk R, Moss EM. Research on behavioral phenotypes: Velocardiofacial syndrome (deletion 22q11.2) Developmental Medicine and Child Neurology. 2000;42(6):422–427. doi: 10.1017/s0012162200000785. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 3rd ed. Psychological Corporation; San Antonio, TX: 2001. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. Psychological Corporation; San Antonio, TX: 2003. [Google Scholar]
- Woodin M, Wang PP, Aleman D, Donald-McGinn D, Zackai E, Moss E. Neuropsychological profile of children and adolescents with the 22q11.2 microdeletion. Genetics in Medicine. 2001;3(1):34–39. doi: 10.1097/00125817-200101000-00008. [DOI] [PubMed] [Google Scholar]
- Worling DE, Humphries T, Tannock R. Spatial and emotional aspects of language inferencing in nonverbal learning disabilities. Brain and Language. 1999;70(2):220–239. doi: 10.1006/brln.1999.2156. [DOI] [PubMed] [Google Scholar]
- Xu B, Roos JL, Levy S, van Rensburg EJ, Gogos JA, Karayiorgou M. Strong association of de novo copy number mutations with sporadic schizophrenia. Nature Genetics. 2008;40(7):880–885. doi: 10.1038/ng.162. [DOI] [PubMed] [Google Scholar]
- Zagursky K, Weller RA, Jessani N, Abbas J, Weller EB. Prevalence of ADHD in children with velocardiofacial syndrome: A preliminary report. Current Psychiatry Reports. 2006;8(2):102–107. doi: 10.1007/s11920-006-0006-2. [DOI] [PubMed] [Google Scholar]
- Zera DA. A reconceptualization of learning disabilities via a self-organizing systems paradigm. Journal of Learning Disabilities. 2001;34(1):79–94. doi: 10.1177/002221940103400107. [DOI] [PubMed] [Google Scholar]
- Zinkstok J, van Amelsvoort T. Neuropsychological profile and neuroimaging in patients with 22q11.2 Deletion Syndrome: A review. Child Neuropsychology. 2005;11(1):21–37. doi: 10.1080/09297040590911194. [DOI] [PubMed] [Google Scholar]