Abstract
The aging process alters cardiac physiology, decreases the number of cardiomyocytes and alters the energy metabolism. Mitochondrial dysfunction in aging is believed to cause these functional and phenotypic changes in the heart. Although precise understanding of alterations of mitochondrial respiration in aging is necessary to manage heart diseases in the elderly population conflicting data on the function of specific complex of electron transport chain of the heart mitochondria limits the intervention process. We have addressed these issues using the assay of mitochondrial coupling and electron flow to assess specific functional defects in mitochondria isolated from young or aged mice. Our results demonstrate that cardiac mitochondria from older mice utilize oxygen at a decreased rate via complex I, II or IV compared to younger mice. We further show that mitochondrial function decreases in young Sod2+/− mice heart compared to young wildtype mice. However, the mitochondrial function remains unchanged in older Sod2+/− mice heart compared to younger Sod2+/− mice heart. Further, the oxygen consumption remains similar in old wildtype mice and old Sod2+/− mice heart mitochondria. The expression and activity of Sod2 in young or old Sod2+/− mice heart remain unchanged. These data demonstrate that decreased oxygen utilization in older age could have resulted in decreased mitochondrial ROS-mediated oxidative damage requiring less Sod2 for protection against mitochondrial oxidative stress in older wildtype or older Sod2+/− mice.
Keywords: Mitochondria, Bioenergetics, Aging, Heart, SOD, XF24, Electron flow, Mitochondrial coupling, Complex I, Complex II
1. Introduction
Elderly populations are at a greater risk of heart disease and suffer increased myocardial damage during and following an episode of heart attack. Aged hearts are more likely to fail due to ischemia–reperfusion injury compared to younger hearts (Hare, 2001; Lesnefsky and Hoppel, 2003). Additionally, aged heart suffers greater damage during reperfusion of ischemic myocardium resulting in myocardial infarction and progressive death of the heart tissue (Lesnefsky and Hoppel, 2006; Lesnefsky et al., 2001b; Ventura-Clapier et al., 2008). Mitochondria play a vital role in cardiac energy output and are critically important in energy-demanding cardiac functions (Dai and Rabinovitch, 2009). Oxidation of biological fuels is a critical source of energy required for efficient functioning of the heart (Lesnefsky et al., 2001a). The oxidation of fuels such as NADH, pyruvate or succinate is accomplished via the mitochondrial electron transport chain (ETC). During this process energy in the form of ATP is produced due to the coupling of ETC to the proton extrusion forming the proton gradient that subsequently generates ATP via ATP synthase. However, electron leak during the passage of electrons via ETC produces superoxide anions (O2•−), which are converted to various forms of reactive oxygen species (ROS), and are detrimental to the very ETC which produces them (Brand and Nicholls, 2011). Constant burning of fuels by oxidative process over the life span of an organism is believed to oxidize the components of mitochondrial respiratory system that diminishes the function of various complexes of the mitochondrial ETC (Brand and Nicholls, 2011; Lesnefsky and Hoppel, 2006).
Superoxide dismutase-2 (Sod2) also known as manganese superoxide dismutase (MnSOD) is a mitochondrial matrix enzyme that converts O2•− (impermeable to mitochondrial membrane) to H2O2. H2O2 is permeable to mitochondrial membrane and is further degraded to molecular oxygen and water by catalase. Sod2 serves as a first line of defense to protect the mitochondria against deleterious O2•− in physiological and pathophysiological conditions. Although, free radical theory of aging (Harman, 1956) suggests that continuous generation of O2•− in the mitochondria throughout the life span of an organism initiates and accelerates the aging process, the supportive data to conclusively prove this theory remains inadequate. There has been no impact of 50% decrease in Sod2 on the life-span of mice (Jang and Remmen, 2009). Although partial decrease (~50%) in Sod2 expression in Sod2+/− mice heart decreases mitochondrial oxygen consumption in young mice, its effect on older mice has not been investigated. Taken together, it remains questionable whether accumulation of oxidative products decreases mitochondrial function in aging.
Although it is generally believed that mitochondrial function declines with age in the heart, controversies do exit due to variation in experimental procedures to assess mitochondrial function (Tatarkova et al., 2011; Van Remmen and Richardson, 2001). For example, studies have shown that aging process has no effect on cardiac mitochondrial function (Davies et al., 2001; Miro et al., 2000). In contrast, other studies have shown that mitochondrial function either declines or remains unaltered with the aging process (Kumaran et al., 2004; Rodriguez et al., 2007). Isolation procedure has been identified to be critical for functional analysis of cardiac mitochondria (Fannin et al., 1999). There are two distinct sub-populations of cardiac mitochondria; the subsarcolemmal (SSM) mitochondria that lie superficially beneath the plasma membrane, and the inter-fibrillar (IFM) mitochondria located between the myofibrils (Fannin et al., 1999; Palmer et al., 1977). Whereas only polytron homogenization releases mostly SSMs, protease digestion releases the IFMs (Lesnefsky et al., 2001a; Lesnefsky et al., 2004; Palmer et al., 1977). Studies have shown differential effect of the aging process on these two populations of mitochondria within the myocyte (Palmer et al., 1977). The assessment of integrated mitochondrial respiration by studying the maximal rate and coupling of oxidative phosphorylation uncovers age-related defects in oxidative metabolism not evident by isolated measurement of the enzyme activity of the individual electron transport chain complexes (Lesnefsky and Hoppel, 2006). Thus, the results and conclusions of experiments with aged mitochondria could be affected by the isolation procedure, specific endpoint measurement or modulation of maximal respiration, coupling and specific enzyme assays of each complex.
We sought to determine the effect of age on mouse heart mitochondrial coupling and electron flow with normal or decreased levels of Sod2 using wild-type (WT) or Sod2+/− mice. First, we optimized the bioenergetics assay with mouse heart mitochondria/well using the state-of-the art XF24 mitochondrial flux analyzer (Seahorse Bioscience, Billerica, MA). In this assay we integrated mitochondrial coupling and the electron flow experiments in the same assay plate and used uncoupler for maximal respiration determination and ADP for phosphorylating state 3 respirations to assess true mitochondrial capacity (Brand and Nicholls, 2011).
In this report we show that in aged mice a decline in function of complex I–IV occurs in the heart mitochondria. The decline is more pronounced in IFM mitochondria. Further, mitochondria isolated from the hearts of young Sod2+/− mice show diminished mitochondrial function compared to young WT mice. However, aged Sod2+/− mice do not show any change in cardiac mitochondrial function compared to young Sod2+/− mice. In addition, there was no difference in mitochondrial function between old WT and old Sod2+/− mice. The level of Sod2 expression and activity remains similar in young or old Sod2+/− mice which is about 50% of the WT mice. These studies demonstrate that whereas Sod2 levels affect mitochondrial function in young mice it does not affect mitochondrial function in older mice heart.
2. Materials and methods
2.1. Cell culture and transfections
Adenosine 5′-diphosphate sodium salt (ADP), antimycin A, oligomycin, carbonyl cyanide 4-(trifluoromethoxy)phenylhydrazone (FCCP), N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), ascorbic acid, succinate, malate, pyruvate and rotenone were purchased from Sigma Chemical Company (St. Louis, MO). Other chemicals used in the mitochondrial isolation buffer and mitochondria assay buffer (MAS) were purchased from Sigma chemical Co. Human microvascular endothelial cells (HMVEC) were purchased from Lonza CO, NJ, and were grown and propagated in endothelial basal medium with supplements (EGM-MV2; Lonza, Co., NJ). HMVEC were transfected with Sod2 siRNA (3′-GGA GCA CGC UUA CUACCUUUUdTdT-5′) or luciferase (Luc) siRNA (3′CUU ACG CUG AGU ACU UCG Att) obtained from Dharmacon, Arvada, CO, and Qiagen (Valencia, CA) using Xtremegene transfection reagent (Roche Biotech, Indianapolis, IN). After 48 h of transfection cells were sub-cultured and 25,000 cells were seeded onto v7 tissue culture plate (Seahorse Biosciences, Billerica, MA) for mitochondrial bioenergetics assay.
2.2. Animals and isolation of mitochondria
Wildtype c57BL/6 or Sod2+/− mice were purchased from Jackson laboratory (Bar Harbor, ME) and were bred and maintained in the animal facility of Texas Tech University Health Sciences Center (TTUHSC), Lubbock, TX. The protocol was approved by the institutional animal care and use committee (IACUC) of the TTUHSC. Hearts of mice (young, 2–4 months or old, 22–28 months of age) were surgically removed from anesthetized animals. Mitochondria from hearts of mice were isolated following the protocol for rats published by Rogers et al. (2011) with following modifications. One mouse heart was minced and homogenized at 4 °C using polytron homogenizer (Mini Genie, Fisher Biotech) in mitochondrial isolation buffer (70 mM sucrose, 210 mM mannitol, 5 mM HEPES, pH 7.2, 1 mM EGTA and 0.5% fatty acid free BSA). The homogenate was centrifuged at 27,000 ×g for 10 min. The pellet was resuspended in the same buffer and was centrifuged at 500 g for 5 min. The supernatant was passed once through 70 µ filters and once through 40 µ filters (BD Biosciences, CA) and then centrifuged for 5 min at 10,000 ×g. The mitochondrial pellet was suspended in mitochondrial isolation buffer without BSA and protein was estimated with bicinchoninic acid (BCA) assay (Pierce, Rockford, IL). Mitochondria were suspended at 1–2 µg/50 µl in 1× mitochondrial assay buffer (MAS; 70 mM sucrose, 220 mM mannitol, 10 mM KH2PO4, 5 mM MgCl2, 2 mM HEPES, 1 mM EGTA, and 0.2% fatty acid free BSA; pH 7.2 at room temperature) and plated into each well of the v7 assay plate of XF24 analyzer.
2.3. Isolation of subsarcolemmal (SSM) and interfibrilar (IFM) mitochondria
SSM and IFM were isolated from mice heart following the methods developed by Palmer et al. for Fisher 344 rats with modifications (Palmer et al., 1977). Briefly, mice hearts were washed and minced in ice-cold buffer A (220 mM mannitol, 70 mM sucrose, 5 mM MOPS, pH 7.4, 2 mM EGTA and 0.2% fat free BSA). The minced tissue was homogenized in a polytron homogenizer (Mini Genie, Fisher Biotech.) for 3–4 s at a setting 6. The polytron homogenate was centrifuged at 500 ×g for 10 min at 4 °C using ss34 rotor (Sorval) using a Sorval RC5 centrifuge. Buffer A was added to polytron pellet in a Potter–Elvehjem homogenizer and centrifuged at 500 ×g for 5 min. The original supernatant from the polytron homogenizing and Potter–Elvehjem supernatant were pooled and centrifuged at 3000 ×g for 10 min to obtain subsarcolemmal mitochondria (SSM). The Potter–Elvehjem pellet was resuspended in buffer B (100 mM KCl, 50 mM MOPS, pH 7.4, 2 mM EGTA, 0.2%BSA) and Peptidase (Sigma Chemical Co, St. Louis, MO) at a concentration of 5 mg/g of heart tissue and resuspended using Potter– Elvehjem homogenizer. The homogenate was diluted 2-foldwith buffer B and centrifuged 5000 ×g for 5 min. The pellet was resuspended in original volume of buffer B without peptidase and centrifuged 300 g to pellet the nuclei. The resulting supernatant was centrifuged at 3000 ×g for 10 min to obtain the IFM mitochondria. Protein was quantified using BCA (Pierce, Rockford, IL) protein assay.
2.4. XF24 instrument setup and analysis
2.4.1. Isolated mitochondria
XF24 instrument was equilibrated at 37 °C overnight. 1 µg of mouse heart mitochondria was plated in each well of the XF24 v7 plate in a volume of 50 µl containing 1× MAS with 10 mM succinate and 2 µM rotenone as substrate for coupling assay; and 10 mM pyruvate, 2 mM malate and 4 µM FCCP was added to 1× MAS for the electron flow experiment. The XF plate containing mitochondria with substrate was centrifuged for 20 min at 2000 rpm in a swinging bucket rotor (Eppendorff, CA 5810R centrifuge with microplate adaptor) using adaptors to hold the XF plate. After centrifugation, 450 µl of substrate containing 1×MAS was added to each well, and the plate was incubated in a 37 °C non-CO2 incubator for 8–10 min. While the plate was centrifuged the XF cartridge was prepared for injections for ports A, B, C and D. Stock solution of 1 M ADP was made in water. Stock solutions of oligomycin, FCCP, rotenone and antimycin A were made in DMSO. 50 µl of ADP (40 mM), 55 µl of oligomycin (25 µg/ml), 60 µl of FCCP (40 µM), and 65 µl of antimycin A (40 µM) were loaded into ports A, B, C and D respectively. The final concentrations were 4 mM ADP, 2.5 µM oligomycin, 4 mM FCCP and 4 µM antimycin A. The injections for electron flow experiment were prepared as follows: Port A, 20 µM rotenone (50 µl), Port B, 100 mM succinate (55 µl), Port C, 40 µM antimycin A (60 µl), and port D, 100 mM ascorbate with 1 mM TMPD (65 µl). The final concentrations were 2 µMrotenone, 10 mM succinate, 4 µM antimycin A and 10 mM ascorbate with 0.1 mM TMPD. The cartridge was calibrated by the XF machine, and following calibration the XF plate with mitochondria attached to the bottom was introduced into the machine and the assay continued using protocol developed by Rodgers et al. for rat heart mitochondria (Rogers et al., 2011).
2.5. XF analysis of endothelial cells
The concentrations of oligomycin, FCCP and antimycin A/rotenone were optimized using Mito stress test kit (Seahorse Biosciences, Billerica, MA). The bioenergetics assay was performed using the protocol provided in the XF24 manual with the exception of measure time of 5 min as the basal OCR of endothelial cells are below100 pmol/min for 25,000 cells that was determined in the seeding density assay. Basal respiration [(OCR with substrates) − (non-mitochondrial respiration)], ATP turnover (OCR due to oligomycin), and maximal respiration [(OCR due to uncoupling) − (non-mitochondrial respiration)] were calculated for Luc siRNA and Sod2 siRNA transfected cells as described in a recent publication (Brand and Nicholls, 2011).
2.6. MnSOD western analysis and activity assay
2.6.1. Sod2 western analysis
Sod2 protein expression was determined by western analysis using total heart mitochondrial protein prepared by radioimmunoassay precipitation buffer (RIPA) with 5% sodium deoxycholate, 1% SDS, and 1% Igepal (Sigma Co, MO) in PBS with protease inhibitors, and protein concentration was determined using Bradford protein assay (BioRad, CA). Anti-rabbit-mouse MnSOD antibody (Santa Cruz Biotech, CA) was used to probe the PVDF membrane after transfer of SDS-PAGE. The specific protein band was detected using ECL-Plus reagent (GE Biotech, NJ) using the 05-GBOX-CHEMI-XL image analyzer (Syngene, Frederick, MD).
2.6.2. Sod2 activity assay
The heart tissue was homogenized in a polytron homogenizer (Fisher Mini Gene) at speed 6 for 1 min in potassium phosphate buffer (0.05M, pH 7.8 with 10−4 EDTA) at 4 °C. The homogenates were sonicated with a Fisher sonicator for 10 s at 4 °C. The homogenate was centrifuged in a microfuge at 4 °C for 15 min. Sod2 activity was determined in the supernatant using published methods (Kuthan et al., 1986; McCord and Fridovich, 1969). A 50% inhibition of rate of reduction of ferrycytochrome c was taken as 1 unit of SOD. The final values are expressed as activity of Sod2 per mg total heart protein.
2.7. Statistical analysis
The statistical evaluations were performed with ANOVA in Graph Pad Prism software. Where necessary post-test was performed with Fisher's LSD test. The minimum number of n = 3 was employed in all experiment performed with 4–5 replicate wells in the Seahorse XF24 analyzer. Detail of numbers of animals for a specific experiment is provided in the figure legends.
3. Results
3.1. Optimization of mitochondria for XF assay
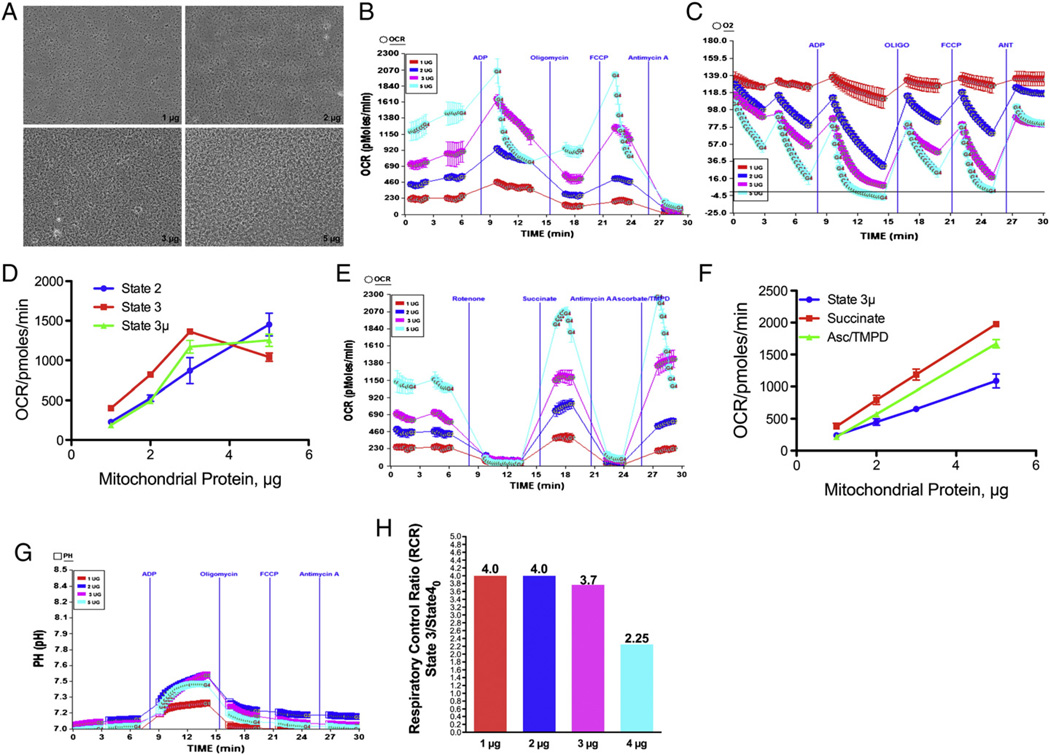
We optimized the bioenergetics assay for mouse heart mitochondria using XF24 analyzer with modified protocol of Rogers et al. (2011) as this is the first report of XF24 assay using mitochondria isolated from mouse heart. We utilized 1, 2, 3 or 5 µg of mouse heart mitochondria/ well of v7 plate (Fig. 1A) as an initial system of optimization for simultaneous determination of mitochondrial coupling and electron flow in the same XF24 microplate. As shown in Fig. 1B basal oxygen consumption rate (OCR) was increased with increased concentrations of mitochondria. However, the basal rate for mouse heart mitochondrial protein above 2 µg was non-linear (Fig. 1D). Further, at 3 or 5 µg mitochondria the absolute oxygen level fell to very low levels that could affect further measurement cycles due to decreased availability of oxygen in the micro-well (Fig. 1C). Likewise, the OCR after sequential injection of ADP or FCCP tends to be linear at 1–2 µg mitochondria (Fig. 1D). We determined that 1–2 µg of mouse heart mitochondria provides an optimal linear rate for the coupling assay. As shown in Fig. 1E, the OCR in electron flow experiment was increased as amount of mitochondria increased in a linear manner (Fig. 1E & F). Although the linearity was good the OCR was not stable for 5 µg mitochondria per well (Fig. 1E) as seen with point-to-point measurements. Further, the OCR at 1–2 µg mitochondria was stable and the injections of succinate or Asc/TMPD produced linear responses. We also evaluated the increase in the absolute pH level, and as shown in Fig. 1G the pH increased as the amount of mitochondria increased, but the increase in 2, 3 or 5 µg was not very different. These data suggest that ATP production could reach saturation level using 2 or 3 µg mitochondria/well (Rogers et al., 2011). The RCR value for 1 or 2 µg mouse heart mitochondria/well was found to be 4 (Fig. 1H), which is close to rat heart mitochondria (Lesnefsky et al., 2001a). Thus, our optimization shows that 1 µg of mouse heart mitochondria/ well of XF24 v7 plate would be the minimal amount at which the bioenergetics analysis could be performed in XF24 analyzer.
Fig. 1.
Optimization of isolated mouse heart mitochondria for bioenergetics assay with XF24 Analyzer (A) Adherence of 1, 2, 3 or 5 µg of mouse heart mitochondria to XF24 v7 cell plate in XF assay. Mouse heart mitochondria were isolated and attached to XF24 microplate as described in Materials and methods. Photomicrograph was taken before the XF assay; (B) mitochondrial coupling assay with 1, 2, 3 or 5 µg of heart mitochondria per well of XF plate as described in Materials and methods. Effect of ADP, oligomycin, FCCP and antimycin A; (C) absolute oxygen levels in the micro-chamber due to mitochondrial coupling using 1, 2, 3 or 5 µg mitochondria per well. (D) Plot of mitochondrial protein against state 2, state 3 and state 3 µ respirations. (E) Mitochondrial electron flow assay using pyruvate and malate as substrates as described in Materials and methods, and the effect of rotenone, succinate, antimycin A and ascorbate/TMPD; (F) plot of mitochondrial protein against the state 3 µ, succinate and Asc/TMPD driven respirations; (G) absolute pH level in the coupling assay. The effect of various amounts of heart mitochondria/well on RCR (state 3/state 40).
3.2. Effect of aging on mitochondrial coupling
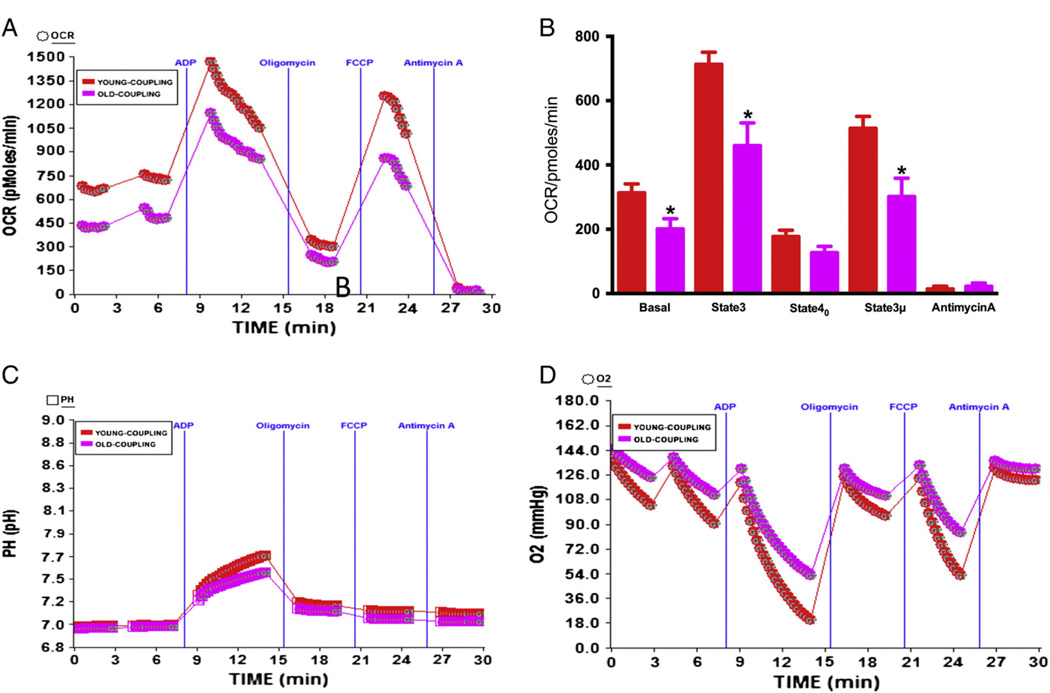
Coupling of the electron transport chain (ETC) with the oxidative phosphorylation (OXPHOS) is the major mechanism for generation of ATP utilizing oxygen. Because the oxygen demand of heart is significant, we determined whether oxygen utilization is compromised in the aging process. The effect of age on basal respiration (state 2), phosphorylating respiration in the presence of ADP (state 3), resting respirations with oligomycin (state 40), maximal uncoupling respiration in the presence of FCCP (state 3 µ), and the response to antimycin A was determined using optimal amount of heart mitochondria. It was determined that the basal OCR of young mice was significantly higher than the older mice heart using 1 µg mitochondrial protein (Fig. 2A–B). State 3 phosphorylating respiration was induced by addition of ADP that increased the OCR, which was higher in younger mice compared to older ones (Fig. 2A–B). The RCR (state3/state40) values of young mouse heart mitochondria were found to be 4.08 and for old mice mitochondria 3.76 (Table 1). There was minor non-significant difference between old or young mice in state 40 respiration induced by addition of oligomycin (Fig. 2A–B). The maximal respiration in response to uncoupling by FCCP was higher in younger mice compared to older mice (Fig. 2A–B), and the response to complex III inhibitor antimycin A was almost similar. In the coupling assay, succinate (a FADH2-linked substrate of complex II) was used with rotenone to allow the mitochondria respire via complex II as succinate without rotenone would form oxaloacetate from malate by the action of malate dehydrogenase. Oxaloacetate is impermeable to mitochondrial inner membrane and would accumulate and function as a potent inhibitor of succinate dehydrogenase, the complex II enzyme (Maklashina et al., 2004). We measured the absolute pH level following addition of ADP. The pH was increased as soon as ADP was added and continued to increase until oligomycin was injected to the wells to stop ATP synthesis due to inhibition of ATP synthase. The increase in pH in phosphorylating respiration is proportional to ATP synthesis (Rogers et al., 2011). Thus, higher level of ATP generation was noticed in young mice heart mitochondria compared to old mice mitochondria as shown by the increase in absolute pH (Fig. 2C). The absolute oxygen consumption was increased due to ADP and FCCP injections in younger mice compared to older mice heart mitochondria and decreased in oligomycin or antimycin A injections as expected (Fig. 2D).
Fig. 2.
Effect of age on coupling in mitochondria isolated from mice heart: Mitochondria were isolated from young (2–3 months old) or old (22–28 months old) mice hearts as described in Materials and methods. (A) A representative figure of XF24 coupling assay using isolated heart mitochondria, and OCR response to ADP, oligomycin, FCCP and antimycin A using point-to-point measurements. (B) Graph of basal (state 2), state 3, and state 40 respirations in young and old mice heart mitochondria (young n = 5, old n = 5); (C) effect of ADP and oligomycin on absolute pH levels; (D) absolute oxygen levels in the micro-chamber in the coupling assay of isolated young and old heart mitochondria *Significantly lower than young mice, (ANOVA: p < 0.01).
Table 1.
RCR values of isolated mitochondria from young or old mice: mitochondria were isolated from wildtype or Sod2+/− mice (young or old) as described in Materials and methods and bioenergetics assay was performed using XF24 analyzer (Seahorse Bioscience, Billerica, MA). Succinate was used as substrate, and state 3/state 40 OCR was expressed as RCR.
| Mitochondria | RCR | |
|---|---|---|
| 1 | Young WT mice | 4.087 ± 0.27 |
| 2 | Old WT mice | 3.76 ± 0.49 |
| 3 | Young WT IFM | 4.2 ± 1.0 |
| 4 | Old WT IFM | 3.99 ± 0.21 |
| 5 | Young WT SSM | 4.0 ± 0.8 |
| 6 | Old WT SSM | 4.2 ± 0.32 |
| 7 | Young Sod2+/− | 4.04 |
| 8 | Old Sod2+/− | 3.83 ± 0.21 |
| 9 | Old Sod2+/− IFM | 3.89 ± 0.12 |
3.3. Effect of age on electron flow in isolated heart mitochondria
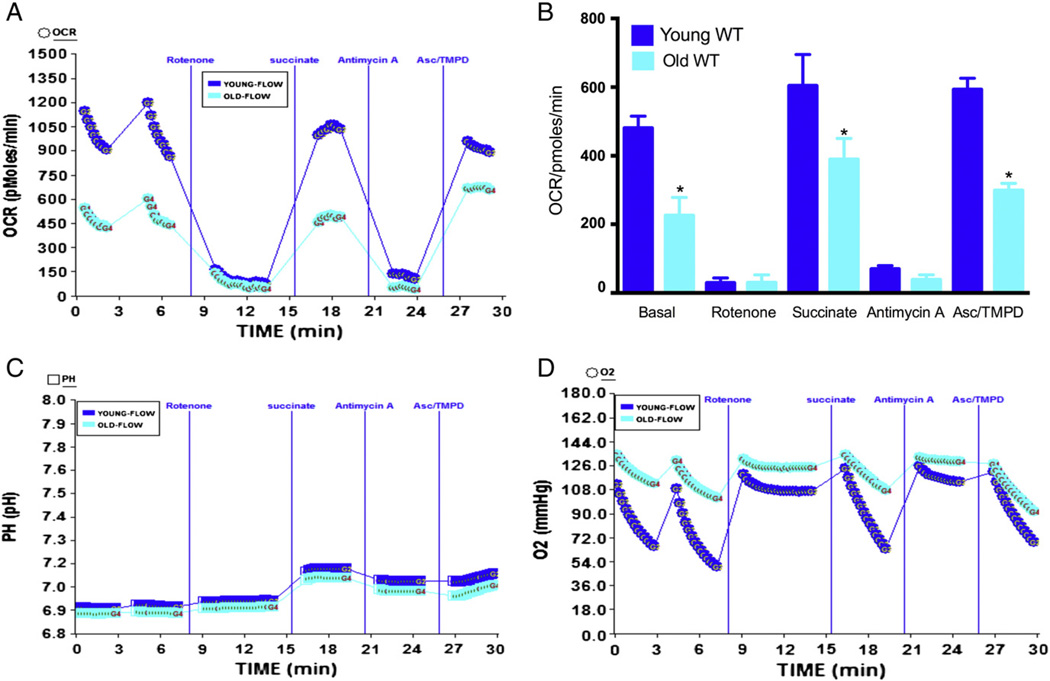
To further understand the effect of age on mitochondrial respiratory complexes, we performed the electron flow experiment using XF24 instrument. We used pyruvate and malate as substrates that drive respiration via complex I. There was a significant decrease in the OCR values of mitochondria from aged mice heart compared to younger mice (Fig. 3A–B), demonstrating significant decrease in complex I activity in the aged heart. When rotenone was injected the OCR came to baseline level indicating inhibition of complex I driven respiration (Fig. 3A–B). When complex II driven respiration was initiated by sequential addition of succinate, there was a significant increase in the OCR in young mice compared to aged mice indicating that complex II function is also significantly compromised in the aged heart (Fig. 3A–B). The function of complex IV was determined after blocking of complex III function by addition of antimycin A. The aged mice demonstrated decreased complex IV function compared to young mice when ascorbate + TMPD was used to drive complex IV-mediated oxygen consumption (Fig. 3A–B). Addition of succinate increased the absolute pH indicating accelerated level of proton production that could produce higher levels of ATP. The level of absolute oxygen consumption was significantly decreased due to succinate or ascorbate/TMPD treatment compared to older mice in mitochondria as expected (Fig. 3D).
Fig. 3.
Effect of age on electron flow in mitochondria isolated from mice heart: mitochondria were isolated from young (2–3 months) or old mice (22–28 months) heart as described in Materials and methods. (A) A representative OCR measurement figure from XF24 instrument using point-to-point data due to electron flow in the mitochondria with pyruvate and malate as substrates, and the effect of rotenone, succinate, antimycin A and Asc/TMPD as described in Materials and methods. (B) Young and old mouse mitochondria with state 3 µ, rotenone, succinate, antimycin A and Asc/TMPD mediated OCR (young n = 5, old n = 4). (C) Absolute pH levels during the electron flow; (D) absolute oxygen levels during the electron flow in the micro-chamber. *Significantly lower than young mice (ANOVA; p < 0.001).
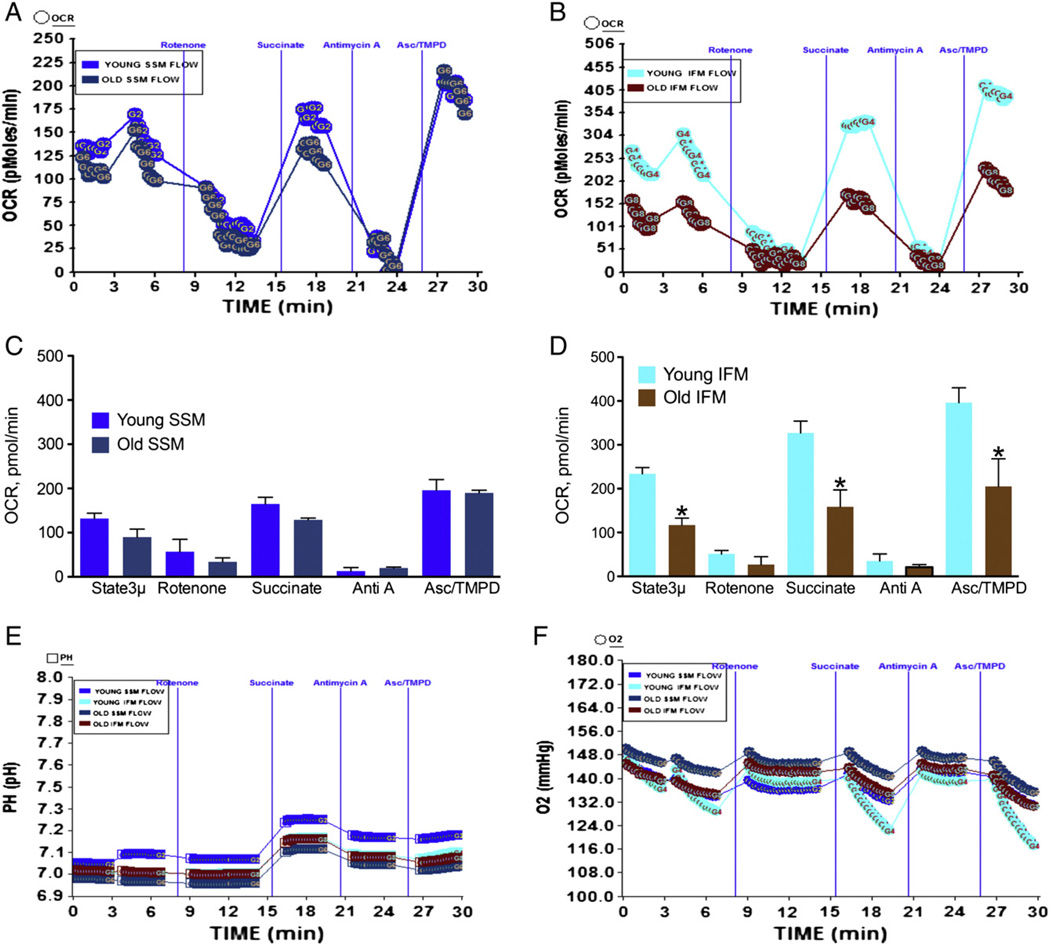
3.4. Effect of age on SSM or IFM mitochondrial coupling and electron flow
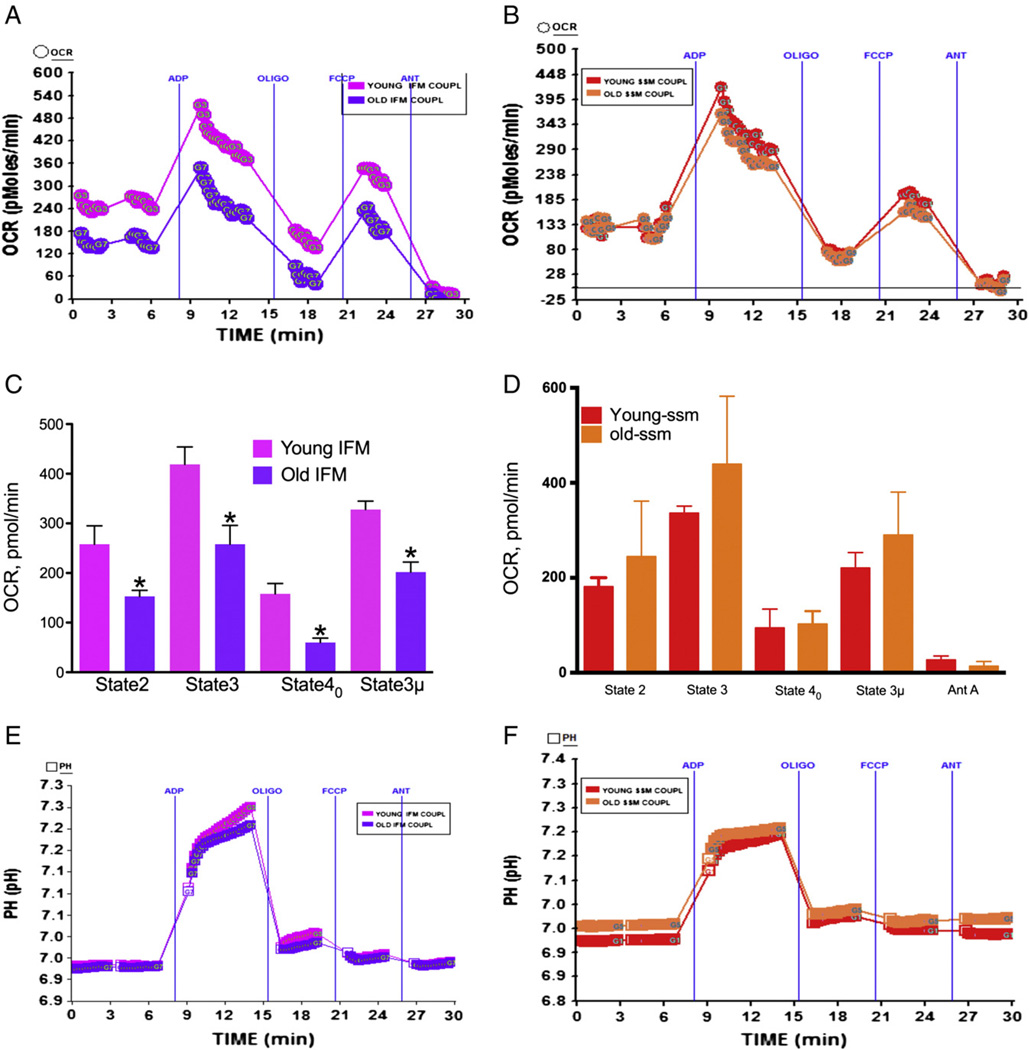
The effect age on heart mitochondrial function remains inconclusive although a number of studies have examined the oxygen consumption using Clarke's electrode and by specific enzyme assays of individual mitochondrial complexes (Choksi and Papaconstantinou, 2008; Miro et al., 2000; Sohal and Brunk, 1992). It was suggested and data show that aging differentially affects two populations of mitochondria, the SSM and IFM (Lesnefsky and Hoppel, 2006; Lesnefsky et al., 2001b; Palmer et al., 1977). Therefore, we examined the effect of age on SSM and IFM oxygen utilization using the XF assay to determine whether the differential effects are manifested in this assay. As shown in Fig. 4A–C, the IFM from aged mice showed significantly decreased state 2, state 3, and state 3 µ OCR compared to IFM from young mice heart mitochondria. However, the SSM did not show any significant decrease due to age in state 2, state 3 or state 3 µ respiratory rates (Fig. 4B–D). The RCR for young mice IFM was 4.2 whereas for old mice it was 3.99 (Table 1). For the young SSM the RCR was 4.0 and for the old SSM it was 4.2 (Table 1). The pH was increased in young IFM compared to old IFM, whereas the pH remained similar in young and old SSM (Fig. 4E & F) with minor increase in young SSM. These values are obtained using our isolation procedure and with succinate as substrate and indicate that higher level of ATP is produced in young IFM compared to old IFM or young/old SSM (Fig. 4E & F). In the electron flow experiment there was no significant difference between young or old mice heart SSM mitochondria in response to basal complex II driven respiration or in response to succinate or ascorbate/TMPD (Fig. 5A–C). In contrast, the IFM isolated from younger mice heart demonstrated significantly higher OCR in response to succinate or ascorbate/TMPD compared to IFM isolated from older mice heart (Fig. 5B–D). In addition, complex II driven basal (state 2) respiration was significantly decreased in older mice heart compared to younger mice (Fig. 5B–D). The absolute pH was increased in response to succinate and Asc/TMPD (Fig. 5E) with concomitant decrease in the level of absolute oxygen in the well (Fig. 5F).
Fig. 4.
Effect of age on mitochondrial coupling in IFM mitochondria: IFM and SSM mitochondria were isolated as described in Materials and methods. (A) A representative coupling data of isolated IFM mitochondria in young and old mice in XF24 analyzer that show the effect of ADP, oligomycin, FCCP and antimycin A; (C) basal, state 3, state 40, state 3 µ and antimycin A respiration in IFM mitochondria from young or old mice (young n = 4, and old n = 4); (E) absolute pH levels in the micro-chamber due to coupling in IFM mitochondria; (B) a representative coupling experiment of isolated SSM mitochondria in young and old mice hearts and the effect of ADP, oligomycin, FCCP and antimycin A; (D) basal, state 3, state 40, state 3 µ and antimycin A respiration in SSM mitochondria from young or old mice (young n = 4, and old n = 4); (F) absolute pH levels in the micro-chamber due to coupling in SSM mitochondria. *Significantly lower than old SSM mitochondria (ANOVA; p < 0.01).
Fig. 5.
Effect of age on mitochondrial electron flow in SSM and IFM mitochondria isolated from mice heart: SSM and IFM mitochondria were isolated as described in Materials and methods. (A) A representative figure of electron flow experiment of isolated SSM mitochondria in young (2–3 months old, n = 4) and old mice (22–28 months old, n = 3) in XF24 analyzer; (C) state 3 µ, rotenone, succinate, antimycin A and ASC/TMPD-mediated OCR in SSM mitochondria from young or old mice. (E) Absolute pH levels in the micro-chamber due to electron flow in SSM & IFM mitochondria. (B) A representative figure of electron flow of isolated IFM mitochondria in young and old mice heart. (D) State 3 µ, rotenone, succinate, antimycin A and ASC/TMPD-mediated OCR in IFM mitochondria from young or old mice. (F) Absolute oxygen levels in the micro-chamber due to electron flow in SSM & IFM mitochondria. *Significantly lower than young IFM (ANOVA; p < 0.0001).
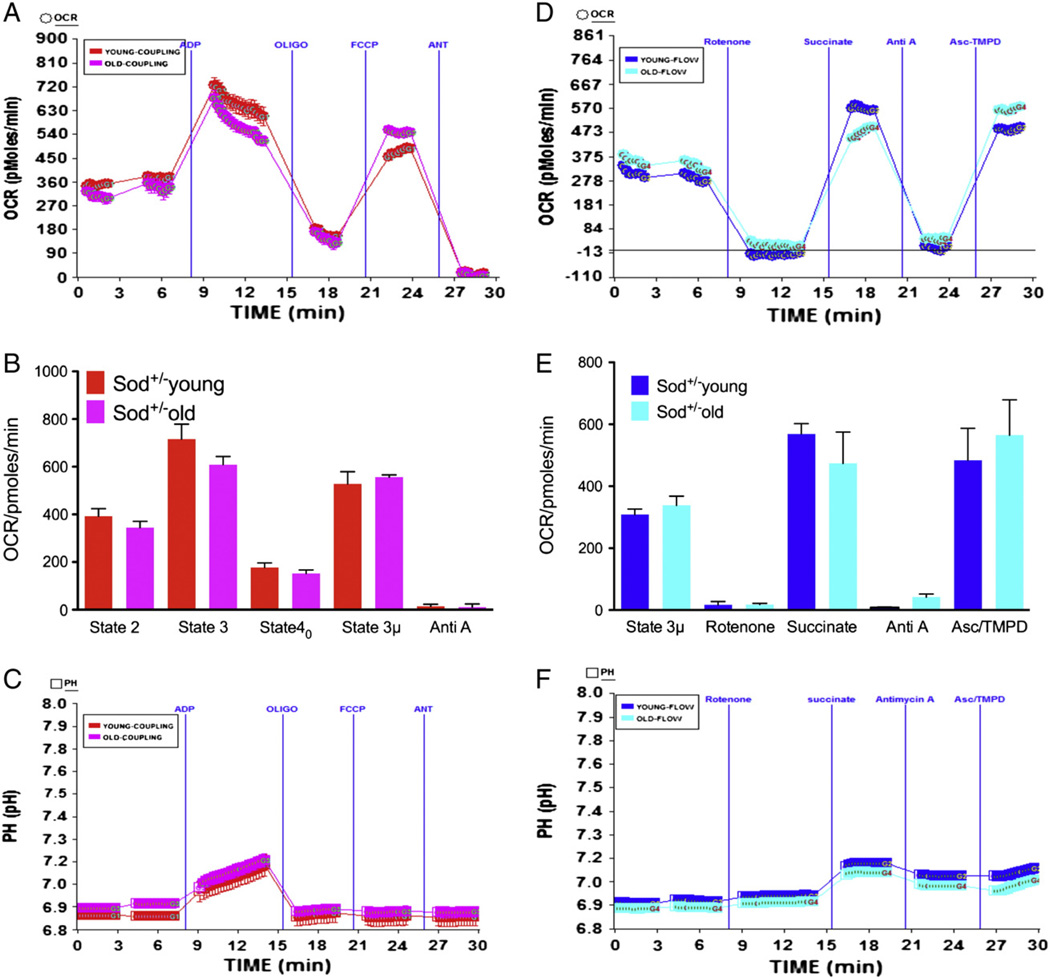
3.5. Partial loss of Sod2 does not affect mitochondrial function in young or old Sod2+/− mice
We sought to determine whether partial loss of Sod2 in the hearts of aged Sod2+/− mice would show more pronounced decrease in mitochondrial function compared to younger animals. As demonstrated in Fig. 6, isolated mitochondria from young or aged Sod2+/− mice showed no significant difference in basal, state 3, state 40 or state 3 µ respiration (Fig. 6A–B). There was no difference in the absolute pH levels in response to ADP, demonstrating that ADP utilization was similar in Sod2+/− young or old mice (Fig. 6C). The RCR values for young Sod2+/− mitochondria and for the old Sod2+/− mitochondria were 4.04 and 3.83 respectively (Table 1). We further determined the response of rotenone, succinate, antimycin A or ascorbate-TMPD on flow of electrons in Sod2+/− young or old mice. There was no difference in the electron flow in young or old mice heart mitochondria isolated from Sod2+/− mice (Fig. 6D–E). There was no significant difference in the absolute pH levels between young or old Sod2+/− mice heart mitochondria in response to succinate (Fig. 6F).
Fig. 6.
Mitochondrial coupling and electron flow in young and old Sod2+/− mice heart: Mitochondria were isolated from young (5–7 months old) or old (28 months old) Sod2+/− mice hearts as described in Materials and methods. (A) A representative coupling assay using isolated lung mitochondria and OCR response to ADP, Oligomycin, FCCP and antimycin A using point-to-point measurements in XF24. (C) Graph of basal (state 2), state 3, state 40, state 3 µ and antimycin A respiration in young and old Sod2+/− mice heart mitochondria (young n = 3, old n = 3). (E) Absolute pH levels in the coupling assay; (B) representative figure of electron flow assay using point-to-point data in the mitochondria using pyruvate and malate as substrates, and its modulation due to injections of rotenone, succinate, antimycin A and Asc/TMPD. (D) Young and old Sod2+/− mouse mitochondria with state 3 µ, succinate, antimycin A and Asc/TMPD mediated OCR; (F) absolute pH levels in the electron flow assay.
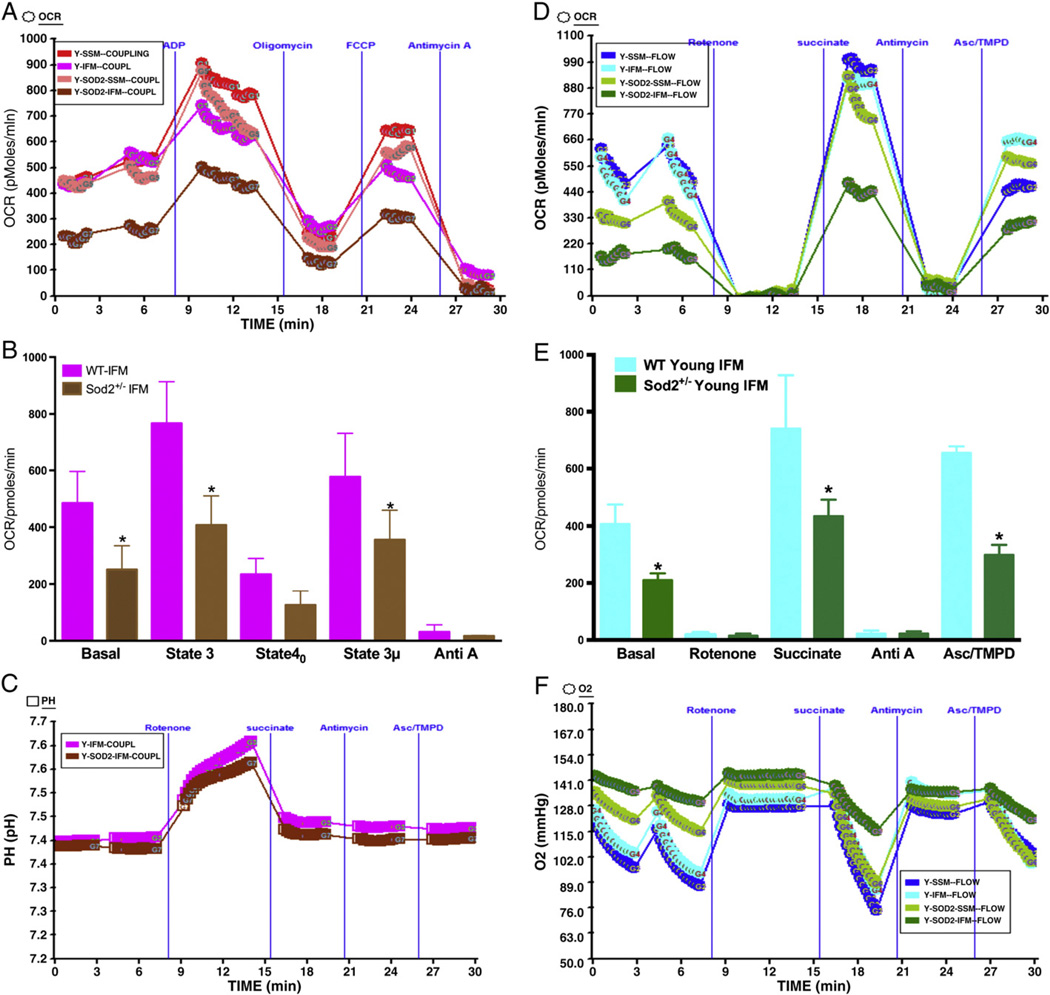
3.6. Mitochondrial function decreases in IFM mitochondria of young Sod2+/− mice compared to young WT mice
We determined whether partial loss of (~50%) of Sod2 in young Sod2+/− mice would affect mitochondrial coupling and electron flow. We isolated IFM or SSM mitochondria form young WT or young Sod2+/− mice and determined OCR of these mitochondria in coupling and flow assay. As shown in Fig. 7A & B, IFM mitochondria from young Sod2+/− mice heart showed decreased basal, state 3, and state 3 µ respiration compared to IFM mitochondria from young WT mice. The SSM mitochondria from these mice did not show any significant difference. Further, it was observed that the ATP synthesis was higher in young WT mice compared to young Sod2+/− IFM mitochondria (Fig. 7C) as shown by increase in pH during state 3 respiration in presence of ADP. In a similar manner, the OCR was significantly decreased in the IFM mitochondria isolated from young Sod2+/− mice heart in the electron flow assay compared to young WT mice (Fig. 7D & E). The absolute oxygen level was decreased significantly in young WT IFM compared to Sod2+/− IFM indicating higher oxygen consumption by WT mice mitochondria. There was no change in the oxygen level in SSM mitochondria.
Fig. 7.
Mitochondrial function in SSM and IFM of Young WT mice and Young Sod2+/− mice heart: (A) a representative figure of mitochondrial coupling; basal (state 2), state 3, state 40, state 3 µ and antimycin A respirations in young WT (3–5 months) and young Sod2+/− (6–7 months) mice heart SSM and IFM mitochondria. (B) Basal (state 2), state 3, state 40 and antimycin A respiration in young WT or young Sod2+/− mice heart mitochondria (n = 3 each). (C) The absolute pH levels in the micro-chamber due to ADP, oligomycin, FCCP and antimycin A injections; (D) a representative figure of OCR measurements in XF24 due to electron flow in the mitochondria using pyruvate and malate as substrates, and its modulation due to injections of rotenone, succinate, antimycin A and Asc/TMPD; in young WT or young Sod2+/− mice; (E) graph of OCR of young WT or young Sod2+/− mouse mitochondria with state 3 µ, succinate, antimycin A and Asc/TMPD mediated OCR (n = 3); (F) absolute oxygen levels in the micro-chamber.
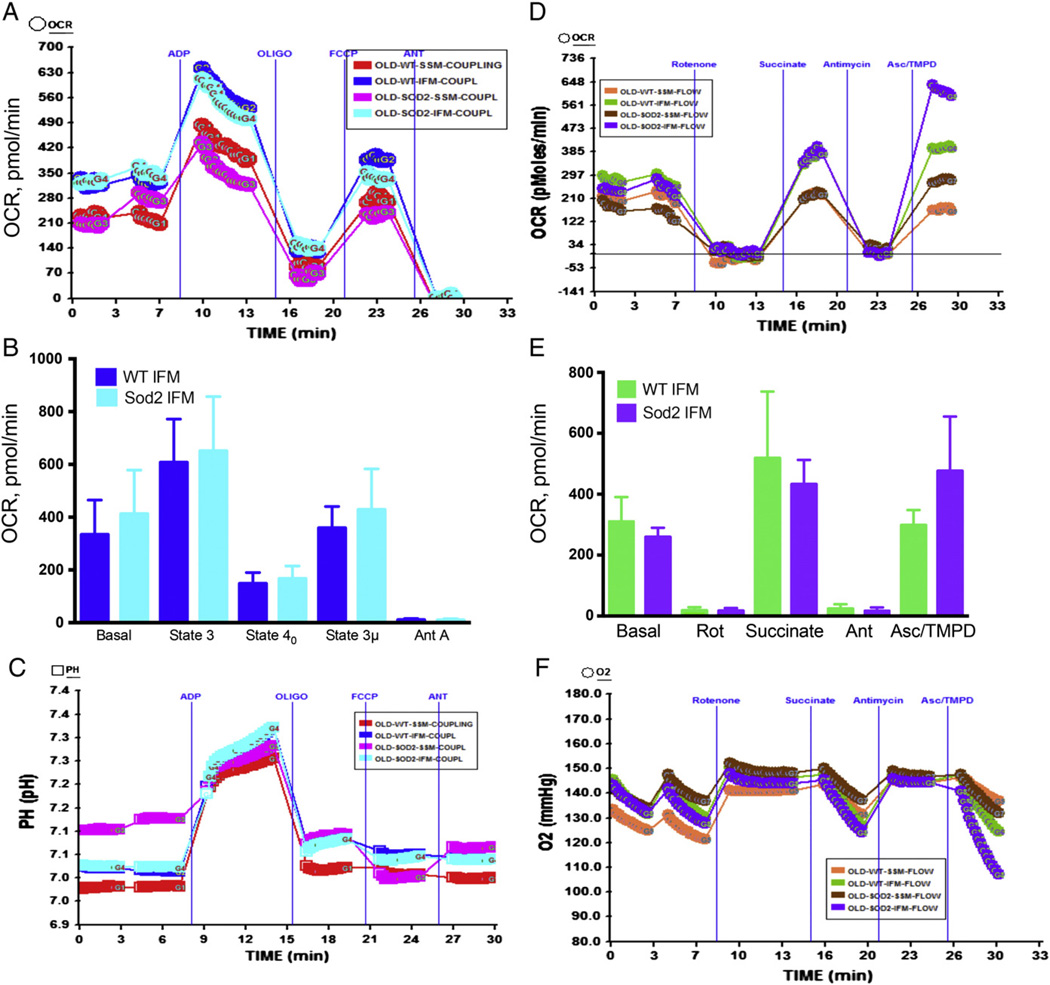
3.7. Mitochondrial function does not decline in IFM mitochondria of old Sod2+/− mice heart compared to old WT mice
If continuous generation of O2•− over the life-span of mice decreases the activity of OXPHOS and ETC components then Sod2+/− mice with decreased levels of Sod2 in the mitochondrial matrix is expected to show significantly higher magnitude of mitochondrial dysfunction compared to old WT mice mitochondria. Therefore, we determined the OCR in the IFM mitochondria from old Sod2+/− mice and old WT mice heart. To our surprise, when we compared the basal, state 3 and state 3 µ respirations in aged WT and aged Sod2+/− mice, we did not observe significant difference in the OCR (Fig. 8A–B) in the coupling assay. In addition, there was no significant change in the OCR in the electron flow experiment in aged WT or Sod2+/− mice heart IFM mitochondria (Fig. 8D–F). These data suggest that ~50% decrease in Sod2 does not affect mitochondrial function in old mice heart.
Fig. 8.
Mitochondrial function in SSM and IFM of old WT mice and old Sod2+/− mice heart: (A) A representative figure of mitochondrial coupling; basal (state 2), state 3, state 40, state 3 µ and antimycin A respirations in old WT (22 to 28 months) and old Sod2+/− (22–28 months) mice heart SSM and IFM mitochondria. (B) Basal (state 2), state 3, state 40, state 3 µ and antimycin A respiration in old WT (n = 4) or old Sod2+/− (n = 3) mice heart mitochondria. (C) The absolute pH levels in the micro-chamber due to ADP, oligomycin, FCCP and antimycin A injections; (D) a representative figure of OCR measurements in XF24 due to electron flow in the mitochondria using pyruvate and malate as substrates, and its modulation due to injections of rotenone, succinate, antimycin A and Asc/TMPD; (E) graph of OCR of old wt. or old Sod2+/− mouse mitochondria with state 3 µ, succinate, antimycin A and Asc/TMPD mediated OCR (n = 3); (F) absolute oxygen levels in the micro-chamber.
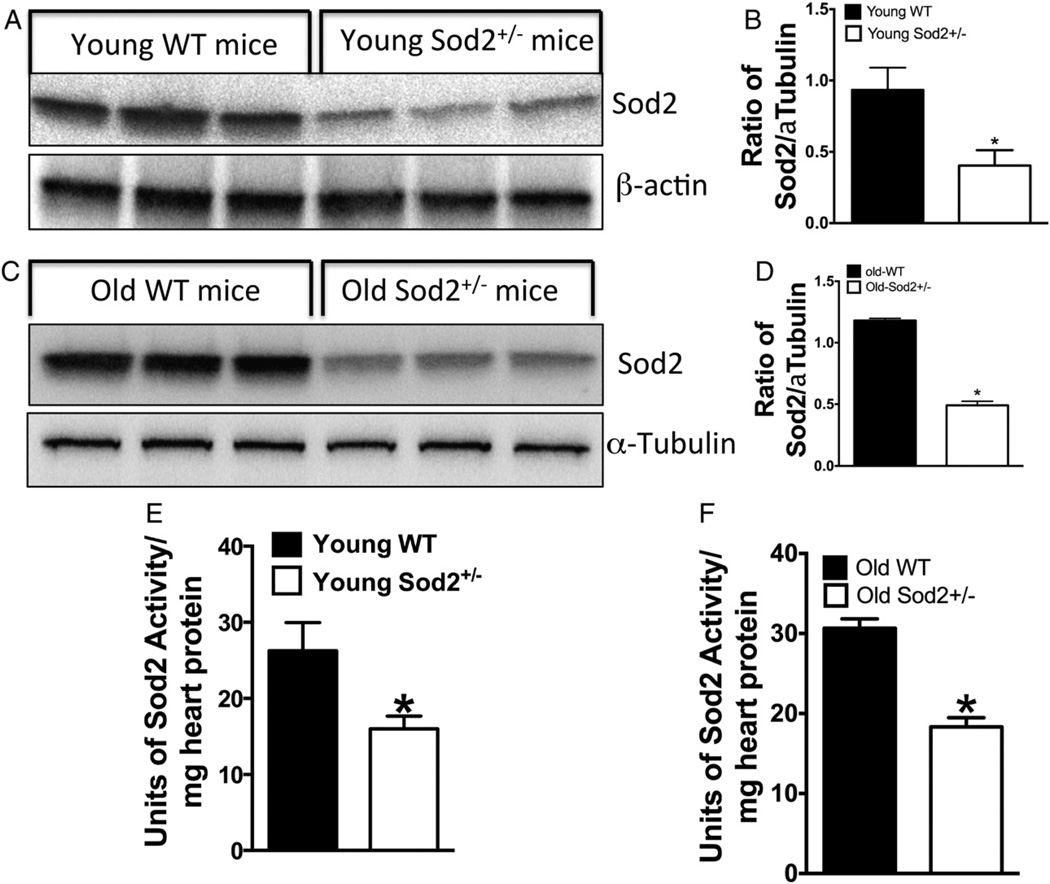
3.8. Sod2 expression level or activity does not change in young or old WT mice and young or old Sod2+/− mice
Because there was no decrease in the OCR of old WT or old Sod2+/− mitochondria in contrast to young WT or young Sod2+/− mice (Figs. 7 & 8) we determined Sod2 expression and activity in young and old mice. Although it has already been shown that young Sod2+/− mice show about 50% decrease in Sod2 level and activity compared to young WT mice, the level and activity of Sod2 in old mice heart are unknown. Our data presented in Fig. 9A, B & E show that Sod2 level and activity are about 50% lower in young Sod2+/− mice compared to WT confirming the data of others (Strassburger et al., 2005; Van Remmen et al., 2001). However, we show that the level of Sod2 in old Sod2+/− mice is also about 50% less than the old WT mice (Fig. 9C, D & F). Therefore, the level of expression or activity of Sod2 does not change in aged mice heart.
Fig. 9.
The effect of age on Sod2 expression and activity in WT or Sod2+/− heart: (A) the expression of Sod2 in western analysis of young WT and young Sod2+/− mice heart. (B) Densitometric ratio of Sod2 and tubulin. (C) The expression of Sod2 in western analysis of old WT and old Sod2+/− mice heart. (D) Densitometric ratio of Sod2 and tubulin of panel C; (E) activity of Sod2 in young WT and Sod2+/− mice heart; (E) the activity of Sod2 in old WT or old Sod2+/− heart.
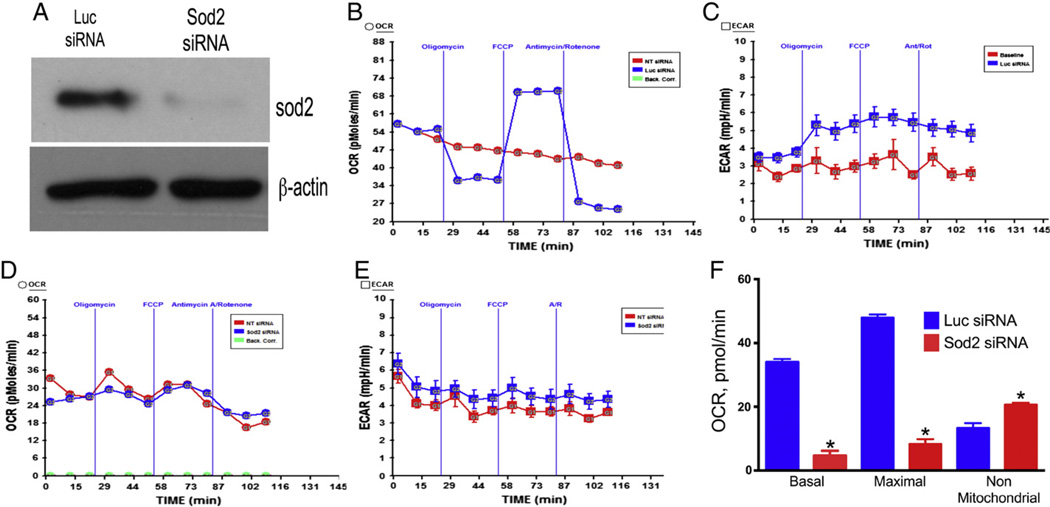
3.9. Complete loss of Sod2 by RNA interference significantly decreases basal respiration and spare respiratory capacity in endothelial cells
Because Sod2 knockout mice (Sod2−/−) die within 1–18 days of birth (Li et al., 1995), we used a cellular approach to determine the effect of complete loss of Sod2 on mitochondrial function. We down-regulated Sod2 levels by RNA interference in endothelial cells and used these cells to determine the bioenergetics profile (Fig. 10A). As demonstrated in Fig. 10B the Luc siRNA transfected cells showed about 34 pmol/min basal OCR (after subtraction of non-mitochondrial respiration) that was decreased to about 25 pmol/min due to oligomycin injection. The OCR was increased to about 50 pmol/min due to FCCP injection (after subtraction of non-mitochondrial OCR), and decreased to baseline level due to injection of rotenone/antimycin A. In contrast, the Sod2 downregulated endothelial cells respire at a rate of 8–9 pmol/min that did not decrease due to oligomycin or increased due to FCCP (Fig. 10D). However, the non-mitochondrial respiration was increased to about 22 pmol/min (compared 12 in Luc siRNA transfected cells). The ECAR of Luc siRNA HMVEC was increased in response to oligomycin due to switching of respiration to glycolysis and remains increased until injection of antimycin A/rotenone (Fig. 10C). In contrast, the ECAR was higher during the initial measurements that declined slowly over time (Fig. 10E). The basal and maximal respirations were decreased in Sod2 siRNA transfected HMVEC, but the non-mitochondrial respiration was increased in these cells compared to Luc siRNA transfected cells (Fig. 10F).
Fig. 10.
Down-regulation of Sod2 by RNA interference impairs mitochondrial function in endothelial cells. Human microvascular endothelial cells (HMVEC) were transfected with either luciferase (Luc) siRNA or Sod2 siRNA as described in Materials and methods. The cells at 25,000 s/well were seeded in XF v7 assay plate. Mitochondrial bioenergetics profile was determined in a XF24 analyzer as described in Materials and methods. (A) Downregulation of Sod2 in endothelial cells by RNA interference; (B) OCR of endothelial cells transfected with Luc siRNA and its response to oligomycin, FCCP and antimycin A/rotenone; (C) ECAR of HMVEC transfected with Luc siRNA; (D) OCR of HMVEC transfected with Sod2 siRNA; (E) ECAR of HMVEC transfected with Sod2 siRNA; (F) basal, maximal and non-mitochondrial OCR in HMVEC transfected with Luc or Sod2 siRNA.
4. Discussion
In the present report we show that mitochondrial respiration decreases in aged WT mice heart mitochondria compared to mitochondria isolated from young mice heart. The oxygen consumption by each of the complexes of mitochondrial electron transport chain is decreased in old WT heart compared to young WT heart. However, our data show that loss of ~50% Sod2 in aged Sod2+/− mice did not result in lower oxygen consumption compared to young Sod2+/− mice. In contrast, young Sod2+/− mice show decreased oxygen consumption compared to young WT mice. In addition, there was no significant difference between old WT or old Sod2+/− mice mitochondrial OCR. We show that Sod2 expression in the old Sod2+/−mice heart is about 50% less than that of WT mice similar to the reported levels in young mice (Van Remmen et al., 2001). In addition, there was no significant difference in the activity of Sod2 in young or old WT, or young or old Sod2+/− mice heart.
In the present report we have optimized the bioenergetics assay in mitochondria isolated from mouse heart using XF analyzer, and determined the effect of age on mitochondrial function. We determined the mitochondrial oxygen consumption in mouse heart SSM and IFM using XF analysis that integrates mitochondrial coupling, electron flow and maximal respiration in a single assay using ADP, uncoupler FCCP, and substrates and inhibitors of a specific complex. Rodgers et al. have optimized the bioenergetics assay of rat heart mitochondria using XF24 analyzer (Rogers et al., 2011). They found that 5 µg rat heart mitochondria is optimal for use with XF24 analysis. Our optimization assay shows that 1–2 µg of mouse heart mitochondria per well of micro titer plate is optimal. The RCR for mouse heart mitochondria using succinate as substrate was 4.087 for young mice heart and 3.76 for old mice heart using our isolation procedure which is similar to Rogers et al. (2011) with some modifications. The older IFM mitochondria showed decreased state 2, state 3, state 4 and state 3 µ respirations compared to younger IFM mitochondria. However, the state 2, state 3, state 4 and state 3 µ respiration rates were not different between young or old SSM mitochondria. These data agree with other studies utilizing Clarke's type electrodes for mitochondrial oxygen consumption analysis (Hoppel et al., 2002; Lesnefsky and Hoppel, 2003; Lesnefsky and Hoppel, 2006).
Although several studies have examined the mitochondrial function in aged rodent heart, the findings remain inconclusive (Davies et al., 2001; Fannin et al., 1999; Lesnefsky and Hoppel, 2006; Miro et al., 2000). This is largely due to experimental methodology applied to complex enzyme kinetics and substrate utilization by various complexes of the electron transport chain (Lesnefsky and Hoppel, 2006; Tatarkova et al., 2011). By using XF24 instrument and utilization of specific substrates and inhibitors of a given complex, we determined the OCR by individual complexes, which is an integrated function of an entire complex (Brand and Nicholls, 2011; Rogers et al., 2011). Additionally, the coupling and electron flow assays could be completed using one micro titer plate with various inhibitors and uncouplers in a single mitochondrial isolation. Our data show that aging decreases the integrated function of complexes I, II and IV in mouse heart mitochondria using succinate as substrate. Leflensky et al. showed that the function of complexes I and II did not decrease in aged mitochondria isolated from hearts of Fisher 344 rats, but the function of complexes III and IV were decreased using glutamate as substrate (Hoppel et al., 2002). This subtle discrepancy could be due to species difference and also due to assay methods.
Knockout mouse for Sod2 does not survive due to extensive cardiomyopathy underscoring the importance of O2•− removal in normal cardiac function (Li et al., 1995). However, heterozygote mouse for Sod2 gene expresses about 50% Sod2 in the heart and does survive without cardiomyopathy (Li et al., 1995). Sod2 is a mitochondrial matrix enzyme that protects the mitochondria from O2•− produced during normal mitochondrial metabolism. If oxidative stress was a major mechanism of age-related mitochondrial dysfunction then mitochondria from aged Sod2+/− mice should show increased mitochondrial dysfunction compared to aged WT mice. Surprisingly, our data show that mitochondrial function remains similar in old WT or old Sod2+/− mice (Fig. 8). It has been suggested that the level of antioxidant protein activity is not significantly impacted by age, but the rate of ROS increases with age (Sohal et al., 1990). Further it may be possible that mitochondrial oxygen demand decreases in aging heart requiring decreased oxygen utilization that generates decreased levels of O2•− in the mitochondria requiring low levels of Sod2 for effective protection. Our data show that OCR was decreased in young Sod2+/− mice compared to young wildtype mice similar to another study using young Sod2+/− mice and with glutamate as substrate (Van Remmen et al., 2001). Thus, it is unclear whether accumulation of oxidative products over the lifespan of the mice would have inactivated the mitochondrial components. For example, mutations in mtDNA are supposed to be increased due to enhanced oxidative stress in old mice, but there was no evidence that mtDNA mutations increase in old age (Bratic and Trifunovic, 2010). Our data suggest that in aged mice heart 50% Sod2 is adequate to maintain mitochondrial respiration. In contrast 100% Sod2 (amount of Sod2 in WT mice) is required for efficient functioning of mitochondria in young WT mice. Whereas young Sod2+/− mice with 50% Sod2 could progress onto old age without any decline in mitochondrial respiration, young WT mice with 100% Sod2 show decreased mitochondrial function in old age. Since there is no difference in the life-span of WT or Sod2+/− mice (Jang and Remmen, 2009), our data suggest that oxygen demand could decline in old age compared to young age. High oxygen demand in agile young mouse would require higher levels of Sod2 for neutralizing high O2•−. On the other hand, decreased oxygen utilization by old mice would generate less O2•− requiring less Sod2 to neutralize O2•−.
Our findings with aged and young Sod2+/− mice suggest that a ~50% reduction in Sod2 in the hearts of Sod2+/− mice is sufficient to provide protection against age-related mitochondrial dysfunction, because aged wildtype or Sod2+/− mice utilize oxygen at a similar rate. However, Sod2 is absolutely required for normal mitochondrial function as shown by our data (Fig. 10). Depletion of Sod2 by RNAi in the HMVEC almost abolished mitochondrial respiration, but increased non-mitochondrial respiration probably as a compensatory mechanism.
Acknowledgments
The work presented here is supported by NIH grants HL 1R01HL107885-01 and 1R01HL109397-01 to KCD.
Footnotes
Conflict of interest
The authors have no conflicts of interests.
References
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratic I, Trifunovic A. Mitochondrial energy metabolism and ageing. Biochim Biophys Acta. 2010;1797:961–967. doi: 10.1016/j.bbabio.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Choksi KB, Papaconstantinou J. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic Biol Med. 2008;44:1795–1805. doi: 10.1016/j.freeradbiomed.2008.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. doi: 10.1016/j.tcm.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies SM, Poljak A, Duncan MW, Smythe GA, Murphy MP. Measurements of protein carbonyls, ortho- and meta-tyrosine and oxidative phosphorylation complex activity in mitochondria from young and old rats. Free Radic Biol Med. 2001;31:181–190. doi: 10.1016/s0891-5849(01)00576-7. [DOI] [PubMed] [Google Scholar]
- Fannin SW, Lesnefsky EJ, Slabe TJ, Hassan MO, Hoppel CL. Aging selectively decreases oxidative capacity in rat heart interfibrillar mitochondria. Arch Biochem Biophys. 1999;372:399–407. doi: 10.1006/abbi.1999.1508. [DOI] [PubMed] [Google Scholar]
- Hare JM. Oxidative stress and apoptosis in heart failure progression. Circ Res. 2001;89:198–200. [PubMed] [Google Scholar]
- Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- Hoppel CL, Moghaddas S, Lesnefsky EJ. Interfibrillar cardiac mitochondrial complex III defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/a:1015251212039. [DOI] [PubMed] [Google Scholar]
- Jang YC, Remmen VH. The mitochondrial theory of aging: insight from transgenic and knockout mouse models. Exp Gerontol. 2009;44:256–260. doi: 10.1016/j.exger.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Kumaran S, Subathra M, Balu M, Panneerselvam C. Age-associated decreased activities of mitochondrial electron transport chain complexes in heart and skeletal muscle: role of l-carnitine. Chem Biol Interact. 2004;148:11–18. doi: 10.1016/j.cbi.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Kuthan H, Haussmann HJ, Werringloer J. A spectrophotometric assay for superoxide dismutase activities in crude tissue fractions. Biochem J. 1986;237:175–180. doi: 10.1042/bj2370175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Ischemia-reperfusion injury in the aged heart: role of mitochondria. Arch Biochem Biophys. 2003;420:287–297. doi: 10.1016/j.abb.2003.09.046. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Hoppel CL. Oxidative phosphorylation and aging. Ageing Res Rev. 2006;5:402–433. doi: 10.1016/j.arr.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Gudz TI, Migita CT, Ikeda-Saito M, Hassan MO, Turkaly PJ, Hoppel CL. Ischemic injury to mitochondrial electron transport in the aging heart: damage to the iron-sulfur protein subunit of electron transport complex III. Arch Biochem Biophys. 2001a;385:117–128. doi: 10.1006/abbi.2000.2066. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J Mol Cell Cardiol. 2001b;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- Lesnefsky EJ, Chen Q, Moghaddas S, Hassan MO, Tandler B, Hoppel CL. Blockade of electron transport during ischemia protects cardiac mitochondria. J Biol Chem. 2004;279:47961–47967. doi: 10.1074/jbc.M409720200. [DOI] [PubMed] [Google Scholar]
- Li Y, Huang TT, Carlson EJ, Melov S, Ursell PC, Olson JL, Noble LJ, Yoshimura MP, Berger C, Chan PH, Wallace DC, Epstein CJ. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat Genet. 1995;11:376–381. doi: 10.1038/ng1295-376. [DOI] [PubMed] [Google Scholar]
- Maklashina E, Kotlyar AB, Karliner JS, Cecchini G. Effect of oxygen on activation state of complex I and lack of oxaloacetate inhibition of complex II in Langendorff perfused rat heart. FEBS Lett. 2004;556:64–68. doi: 10.1016/s0014-5793(03)01369-3. [DOI] [PubMed] [Google Scholar]
- McCord JM, Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein) J Biol Chem. 1969;244:6049–6055. [PubMed] [Google Scholar]
- Miro O, Casademont J, Casals E, Perea M, Urbano-Marquez A, Rustin P, Cardellach F. Aging is associated with increased lipid peroxidation in human hearts, but not with mitochondrial respiratory chain enzyme defects. Cardiovasc Res. 2000;47:624–631. doi: 10.1016/s0008-6363(00)00122-x. [DOI] [PubMed] [Google Scholar]
- Palmer JW, Tandler B, Hoppel CL. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J Biol Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- Rodriguez MI, Carretero M, Escames G, Lopez LC, Maldonado MD, Tan DX, Reiter RJ, Acuna-Castroviejo D. Chronic melatonin treatment prevents age-dependent cardiac mitochondrial dysfunction in senescence-accelerated mice. Free Radic Res. 2007;41:15–24. doi: 10.1080/10715760600936359. [DOI] [PubMed] [Google Scholar]
- Rogers GW, Brand MD, Petrosyan S, Ashok D, Elorza AA, Ferrick DA, Murphy AN. High throughput microplate respiratory measurements using minimal quantities of isolated mitochondria. PLoS One. 2011;6:e21746. doi: 10.1371/journal.pone.0021746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Brunk UT. Mitochondrial production of pro-oxidants and cellular senescence. Mutat Res. 1992;275:295–304. doi: 10.1016/0921-8734(92)90033-l. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Arnold LA, Sohal BH. Age-related changes in antioxidant enzymes and prooxidant generation in tissues of the rat with special reference to parameters in two insect species. Free Radic Biol Med. 1990;9:495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- Strassburger M, Bloch W, Sulyok S, Schuller J, Keist AF, Schmidt A, Wenk J, Peters T, Wlaschek M, Lenart J, Krieg T, Hafner M, Kumin A, Werner S, Muller W, Scharffetter-Kochanek K. Heterozygous deficiency of manganese superoxide dismutase results in severe lipid peroxidation and spontaneous apoptosis in murine myocardium in vivo. Free Radic Biol Med. 2005;38:1458–1470. doi: 10.1016/j.freeradbiomed.2005.02.009. [DOI] [PubMed] [Google Scholar]
- Tatarkova Z, Kuka S, Racay P, Lehotsky J, Dobrota D, Mistuna D, Kaplan P. Effects of aging on activities of mitochondrial electron transport chain complexes and oxidative damage in rat heart. Physiol Res. 2011;60:281–289. doi: 10.33549/physiolres.932019. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Richardson A. Oxidative damage to mitochondria and aging. Exp Gerontol. 2001;36:957–968. doi: 10.1016/s0531-5565(01)00093-6. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Williams MD, Guo Z, Estlack L, Yang H, Carlson EJ, Epstein CJ, Huang TT, Richardson A. Knockout mice heterozygous for Sod2 show alterations in cardiac mitochondrial function and apoptosis. Am J Physiol Heart Circ Physiol. 2001;281:H1422–H1432. doi: 10.1152/ajpheart.2001.281.3.H1422. [DOI] [PubMed] [Google Scholar]
- Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]