Abstract
Formation of mutagenic heterocyclic amines (HCAs) and polycyclic aromatic hydrocarbons (PAHs) is one pathway believed to drive the association of colon cancer with meat consumption. Limited data exist on the associations of individual HCAs and PAHs in red or white meat with colon cancer. Analyzing data from a validated meat preparation questionnaire completed by 1,062 incident colon cancer cases and 1,645 population controls from an ongoing case-control study, risks of colon cancer were estimated using unconditional logistic regression models, comparing the fourth to the first quartile of mutagen estimates derived from a CHARRED based food frequency questionnaire. Total dietary intake of 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline (MeIQx) (adjusted odds ratio (aOR) = 1.88, 95% CI = 1.45–2.43, Ptrend < 0.0001), 2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline (DiMeIQx) (aOR = 1.73, 95% CI = 1.34–2.23, Ptrend < 0.0001) and meat-derived mutagenic activity (aOR = 1.84, 95% CI = 1.42–2.39, Ptrend < 0.0001) were statistically significantly associated with colon cancer risk. Meat type specific analyses revealed statistically significant associations for red meat-derived MeIQx, DiMeIQx and mutagenic activity, but not for the same mutagens derived from white meat. Our study adds evidence supporting red meat-derived, but not white-meat derived HCAs and PAHs, as an important pathway for environmental colon cancer carcinogenesis.
Keywords: colorectal cancer, diet, cooked food mutagen, case/control
INTRODUCTION
Heterocyclic amines (HCAs) and polycyclic aromatic amines (PAHs) are compounds found in the environment and in certain foods. Dietary exposure is primarily through meats cooked at high temperatures for a prolonged period of time. In meat, the high cooking temperatures turn creatine or creatinine, amino acids and sugars into HCAs (1), and incomplete combustion of organic materials creates PAHs (2). In animal studies, HCAs and PAHs have been shown to be carcinogenic (3–10). In humans, HCAs and PAHs have been inconsistently associated with a variety of cancers including colon cancer (11–23). Because there is no suitable metabolite to measure HCAs and PAHs, multiple surrogates have been proposed to estimate dietary exposure. Meat doneness, a common surrogate of dietary HCA and PAH exposure, has been inconsistently associated with colorectal cancer (12,13,15–17,19–22). Three studies reported a positive association between well-done red meat consumption and risk of colon and colorectal cancer (12,13,22). Other studies, however, found little or no association between red meat doneness (15–17,19–21) and white meat doneness (16) with colorectal cancer.
However, other surrogates like estimates of total mutagen intake and individual mutagen of several HCAs and PAHs (e.g., MeIQx (2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline), PhIP (2-amino-1methyl-6-phenyl-imidazo[4,5-b]pyridine), DiMeIQx (2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline) and BaP (benzo[a]pyrene)) of which are estimated from cooking method, preferred doneness and reported frequency of intake of meat have commonly shown not to significantly statistically increase colon and colorectal cancer risk (11,15,18,21,23). One study (14), though, found a positive association with the highest quintile of MeIQx and DiMeIQx with colorectal cancer when stratified by tumor location. Similarly, another study (22) reported a significant association between the highest quartile of total MeIQx intake with increased colorectal cancer risk. Another study (12) only noted an association for increased risk of colorectal cancer with total DiMeIQx in Whites and African Americans and a weak association with BaP in African Americans when stratified by race.
Only one previous study (17) reported separate mutagen estimates for red meat and white meat with colorectal cancer risk and noted no association for a total mutagen index for women, but found increased risk of colon cancer for white meat mutagen index. No association was found between the red meat mutagen index and colorectal cancer risk (17). The study used a total mutagen index for red meat and white meat rather than estimating individual mutagens from red and white meat.
Much of the aforementioned research has focused on meat doneness preference, total mutagen estimates or totals for individual HCAs and PAHs, and to our knowledge no study has reported associations between individual mutagen estimates for red and white meat separately in regards to colorectal cancer risk. The goal of this analysis, therefore, is to examine the associations between separately derived red meat and white meat individual mutagen estimates (MeIQx, PhIP, DiMeIQx and BaP) with colon cancer risk.
SUBJECTS & METHODS
Study subjects
The details of study design and data collection have been described elsewhere (24). In brief, participants were recruited in an ongoing population-based case-control study focusing on genetic and environmental factors and associated risks with colon cancer from 2003–2010. All participants were residents of Kentucky. The study population consisted of 1,062 newly diagnosed colon cancer cases (rectal cases excluded) identified and referred by the Kentucky Cancer Registry, a participant in the National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) Program as well as the Centers for Disease Control and Prevention’s National Program of Cancer Registries. The cancer registry was queried quarterly to identify all incident primary colon cancer cases reported within 6 months of diagnosis.
We used a randomized recruitment approach (random-digit dialing and randomly selecting phone numbers in Kentucky phone books) to recruit a population sample of controls representative of the general Kentucky population. Controls consisted of 1,645 frequency-matched individuals who needed to have never been diagnosed with any cancer except non-melanoma skin cancer and be over the age of 30, preferably ≥ 50 years old.
For cases and controls we excluded self-reported inflammatory bowel disease (e.g., Crohn’s Disease or Ulcerative Colitis), family history of familial adenomatous polyposis (FAP), and hereditary non-polyposis colorectal cancer (HNPCC). The cooperation rate for cases and controls who answered the phone and allowed eligibility determination was 70.8% and 66.7%, respectively. The study was approved by the Institutional Review Boards of the University of Kentucky, Lexington and University Hospitals Case Medical Center, and all participants provided written informed consent.
Risk factor and dietary assessment
Eligible cases and controls donated one blood sample and completed three mailed self-administered questionnaires: a lifestyle risk factor questionnaire (RFQ) developed by the National Cancer Institute Colon Cancer Familial Cancer Registry (http://epi.grants.cancer.gov/CFR/about_colon.html) and the Arizona Cancer Center Food Frequency Questionnaire (FFQ) and Meat Preparation Questionnaire (MPQ) (http://www.azcc.arizona.edu/research/shared-services/bmss/questionnaires).
The RFQ asks questions about family history of colorectal cancer, lifestyle and behavioral risk factors. Participants’ responses to the RFQ were manually entered into an electronic database for analysis. The FFQ asks questions about general dietary habits in the past year (or a year prior to diagnosis of colon cancer) for 175 food items and is used to calculate daily diet and nutrient intake.
The MPQ, a short scannable instrument adapted from a National Cancer Institute HCA concentration database and questionnaire (25,26), asks about portion sizes (e.g., small, medium and large), consumption frequency, and cooking method (e.g., baked/roasted, deep fat fried/fast food, grilled/barbecued, oven-broiled, pan-fried, and stewed) concerning 10 meat groups (e.g., sausage, hot dogs or franks, fried chicken, chicken or turkey, hamburgers or cheeseburgers, beef steaks, pork chops or ham steaks, bacon, fried fish or fish sandwich, and other fish). The MPQ, accompanied with color photographs, also asks about meat doneness for six of the meat types (hamburger, steak, pork chops, bacon, grilled or barbecued chicken, and pan-fried chicken). Participants select which picture most represents the appearance of the cooked meat that they usually consumed. Participants’ responses to portion size, consumption frequency, meat type, and meat doneness are used to calculate estimated daily intake of three HCAs (MeIQx, PhIP, DiMeIQx), one PAH (BaP) (ng/day) and total meat-derived mutagenic activity (revertant colonies/grams of daily meat intake) using the National Cancer Institute’s computerized heterocyclic amines resource for research in epidemiology of disease (CHARRED) (http://dceg.cancer.gov/neb/tools/charred) (27). The HCA and PAH estimates from the CHARRED database were derived from direct measures of cooked meats (28–30), and the meat-derived mutagenic activity was derived from meat sample extracts combined with Salmonella typhimurium strain TA98 in standard plate incorporation assays (31). The meat-derived mutagenic activity is a measure of overall mutagen potential and exposure integrating all of the classes of meat-derived mutagens. Both the FFQ and MPQ were mailed to the University of Arizona Cancer Center for scanning and electronic data output.
Dietary mutagen assessment
Meat doneness categories were self-reported categories from the RFQ. If reported eating red meat (i.e., beef), participants marked the average doneness of the outside of the meat (e.g., Lightly Browned, Medium Browned, or Heavily Browned/Blackened) and the inside of the meat (e.g., Red (rare), Pink (medium), Brown (well-done)). If reported eating white meat (i.e., chicken), participants marked the average doneness of the outside of the meat (e.g., Lightly Browned, Medium Browned, or Heavily Browned/Blackened). Dietary-intake of HCAs and PAHs were calculated as continuous variables based on responses from the MPQ. Individual mutagen estimates (MeIQx, PhIP, DiMeIQx, BaP and meat-derived mutagenic activity) from different meat type (e.g., beef and pork as red meat) were added together to generate red meat derived or white meat derived mutagen totals (Red Meat MeIQx, White Meat MeIQx, Red Meat PhIP, White Meat PhIP, etc). “White meat” for mutagen estimates was defined as chicken or turkey, and “red meat” was defined as meat other than chicken, turkey or fish. We then added the individual mutagen estimates derived from red and white meat to generate the total for individual mutagens (Total MeIQx, Total PhIP, Total DiMeIQx, Total BaP, Total Mutagenic Activity).
For the individual red meat and individual white meat derived mutagen estimates and the total mutagen estimates, quartiles were created for cases based on the distributions among the controls. Quartiles from the white meat DiMeIQx continuous variable were not generated because of too many zero values.
Other risk factors
For cases, age was defined as age at colon cancer diagnosis, and age for controls was defined as age at recruitment. Participants were sent tape measures, and participants self-reported current waist and hip measurements according to detailed instructions. Waist measurements (inches) were divided by hip measurements (inches) to calculate waist-hip ratio (WHR). Positive family history of colorectal cancer was defined as reporting colorectal cancer in one or more first-degree relatives. Regular nonsteroidal anti-inflammatory drug (NSAID) use was defined as self-reported ever usage of ibuprofen or aspirin at least twice a week for 6 months or longer. Average daily total caloric intake was assessed based on responses to the FFQ. Smoking status was coded as “never regular smoker,” “former regular smoker” or “current regular smoker.” “Regular smoker” was defined as ever smoking at least one cigarette a day for 3 months or longer. “Current” smoker for controls was defined as regularly smoking at the time of study participation, and “current” smoker for cases was defined as regularly smoking two years prior to diagnosis of colon cancer.
Statistical data analysis
Univariate analyses (Chi-square for categorical variables and t-test for continuous variables) were performed to examine differences between cases and controls. Comparing the most well-done meat doneness preference to the least done preference for red meat and chicken, odds ratios (ORs) and 95% confidence intervals (CIs) were calculated. Ptrend for the categorical meat doneness preference variables was calculated coding the doneness variable as 1 to 3. For each continuous dietary-derived mutagen variable, ORs and 95% CIs for colon cancer were then estimated from unconditional logistic regression models. Comparing the highest quartile to the lowest quartile for estimated dietary mutagen variables, ORs and 95% CIs were also calculated. Ptrend for dietary mutagen-related quartiles was based on the raw metrics of the continuous dietary-derived mutagen variables. ORs and Ptrend for white meat derived DiMeIQx were not calculated because of too many zero values to generate the quartiles. All analyses were adjusted for potential confounding by other known colon cancer risk factors including age, sex, race, WHR, average daily total caloric intake, family history of colorectal cancer, smoking status and ever regular NSAID use. We selected adjusting variables a priori based on well-known risk factors for colon cancer. When analyzing red meat doneness categories, “Chicken outside doneness” was included as an adjusting variable. When analyzing “Chicken outside doneness,” “Red meat outside doneness” was included as an adjusting variable, and in a separate model “Red meat inside doneness” was included as an adjusting variable. Similarly, in regression analysis of each of the specific meat-derived mutagens (e.g., red meat-derived MeIQx), the opposite meat type-derived mutagen (i.e., white meat-derived MeIQx) was also included as an adjusting variable.
All P values are from two-sided tests, and P values < 0.05 were considered statistically significant. All analyses were undertaken using SAS (Version 9.2, SAS Institute, Cary, NC, USA).
RESULTS
On average, cases were significantly more likely to be older, men, have a higher WHR, have a higher average daily total caloric intake, have a family history of colorectal cancer, and are typically a former or current regular smoker (P < 0.05) (Table 1). There were no significant differences between the cases and controls with regard to race and NSAID use (P ≥ 0.05). Total meat intake, however, was not statistically significantly different between cases and controls, but controls tended to consume more white meat (P = 0.01), whereas cases significantly consumed more red meat (P = 0.0002) (Table 1) that was more likely to be heavily browned on the outside (P = 0.0008) and inside (P = 0.003) (Table 2). Regarding mutagen intake, cases were significantly more likely to consume total MeIQx, red meat MeIQx, red meat PhIP, total DiMeIQx, and red meat DiMeIQx (P < 0.05) (Table 3). Controls, however, tended to consume more BaP from white meat (P = 0.02). Cases tended to have higher total meat-derived mutagenic activity exposure (P = 0.0002) and red meat-derived mutagenic activity exposure (P = 0.0001). When meat doneness preferences were compared to the least done preference, heavily browned outside doneness and well-done inside doneness for red meat were associated with increased risk of colon cancer (odds ratio (OR) = 2.24, 95% CI = 1.40–3.60, Ptrend = 0.001; OR = 2.37, 95% CI = 1.24–4.51, Ptrend = 0.001) (Figure 1). Both models for heavily browned or blackened chicken were not statistically significantly associated with increased risk of colon cancer (Ptrend ≥ 0.05). Quartile analysis (Table 4) showed an 87% increase of the risk of colon cancer among those in the highest quartile of total MeIQx compared to the lowest quartile (Ptrend < 0.0001). Similarly, those in the highest quartile of red meat MeIQx compared to those in the lowest quartile showed a 104% increase of the risk of colon cancer (Ptrend < 0.0001). A similar pattern is seen with the highest and lowest quartiles for total DiMeIQx (OR= 1.68, 95% CI = 1.29–2.17, Ptrend = 0.001) and red meat DiMeIQx (OR = 1.67, 95% CI = 1.29–2.17, Ptrend = 0.004). Red meat PhIP showed a 38% increase of colon cancer among those in the highest quartile compared to the lowest quartile (Ptrend = 0.009). None of the individual mutagen estimates for white meat was statistically significantly associated with colon cancer risk (Ptrend ≥ 0.05).
Table 1.
Descriptive Characteristics of Cases and Controls in the Kentucky Colon Cancer Study
| Variable | Cases (n=1062) N(%) |
Controls (n=1645) N(%) |
P-value1 |
|---|---|---|---|
| Age (yrs) (Mean,SD) | 62.6 (10.2) | 61.4 (10.3) | 0.003 |
| Sex (N of Men,%) | 518 (48.8) | 579 (35.2) | <0.0001 |
| Race (N,%) | 0.1 | ||
| African American | 36 (3.5) | 76 (4.8) | |
| Caucasian | 1008 (96.5) | 1520 (95.2) | |
| WHR2 (Mean,SD) | 0.918 (0.1) | 0.891 (0.1) | <0.0001 |
| Family History of Colorectal Cancer | 374 (37.7) | 430 (28.4) | <0.0001 |
| Ever Regular NSAID Use | 455 (42.8) | 741 (45.1) | 0.26 |
| Average Daily Total Caloric Intake (kcal) (Mean,SD) | 2147.1 (1230.0) | 1956 (1072.1) | 0.0001 |
| Smoking Status | 0.016 | ||
| Never Regular Smoker | 416 (39.4) | 735 (44.8) | |
| Former Regular Smoker | 448 (42.3) | 644 (39.3) | |
| Current Regular Smoker | 193 (18.3) | 260 (15.9) | |
| Average Daily Meat Intake (Mean,SD) | |||
| Total (g/day) | 77.0 (85.4) | 71.2 (78.2) | 0.08 |
| Red Meat (g/day) | 50.4 (64.8) | 41.4 (53.2) | 0.0002 |
| White Meat (g/day) | 27.4 (31.7) | 30.9 (38.2) | 0.01 |
Chi-square P-value for categorical variables; t-test P-value for continuous variables.
Waist-Hip Ratio
Table 2.
Descriptive Characteristics for Meat Doneness Variables for Cases and Controls in the Kentucky Colon Cancer Study
| Variable | Cases (n=1062) N(%) |
Controls (n=1645) N(%) |
P-value1 |
|---|---|---|---|
| Red Meat Outside Doneness | 0.0008 | ||
| Lightly Browned | 48 (5.0) | 129 (8.7) | |
| Medium Browned | 689 (72.3) | 1067 (72.1) | |
| Heavily Browned | 216 (22.7) | 283 (19.2) | |
| Red Meat Inside Doneness | 0.003 | ||
| Red (rare) | 16 (1.7) | 52 (3.5) | |
| Pink (medium) | 340 (35.5) | 580 (39.1) | |
| Brown (well-done) | 603 (62.8) | 853 (57.4) | |
| Chicken Outside Doneness | 0.08 | ||
| Lightly Browned | 53 (5.7) | 111 (7.7) | |
| Medium Browned | 673 (72.9) | 1058 (73.5) | |
| Heavily Browned or Heavily | 197 (21.4) | 270 (18.8) |
Chi-square P-value for categorical variables.
Table 3.
Descriptive Characteristics of Meat-related Mutagens for Cases and Controls in the Kentucky Colon Cancer Study
| Variable | Cases (n=1062) Mean(SD) |
Controls (n=1645) Mean(SD) |
P-value1 |
|---|---|---|---|
| MeIQx | |||
| Total (ng/day) | 75.4 (163.3) | 53.5 (101.3) | 0.0001 |
| Red Meat (ng/day) | 72.2 (162.3) | 50.5 (96.7) | 0.0001 |
| White Meat (ng/day) | 3.9 (7.0) | 4.0 (8.4) | 0.76 |
| PhIP | |||
| Total (ng/day) | 187.3 (318.7) | 169.8 (412.5) | 0.22 |
| Red Meat (ng/day) | 93.3 (192.5) | 71.3 (205.3) | 0.006 |
| White Meat (ng/day) | 96.5 (184.3) | 101.7 (243.6) | 0.54 |
| DiMeIQx | |||
| Total (ng/day) | 4.1 (7.8) | 3.1 (6.3) | 0.0003 |
| Red Meat (ng/day) | 4.0 (7.6) | 2.9 (5.9) | 0.0004 |
| White Meat (ng/day) | 0.2 (0.7) | 0.2 (0.6) | 0.3 |
| BaP | |||
| Total (ng/day) | 39.6 (69.1) | 40.7 (84.3) | 0.71 |
| Red Meat (ng/day) | 25.4 (50.4) | 22.2 (50.3) | 0.12 |
| White Meat (ng/day) | 14.7 (44.7) | 19.3 (49.5) | 0.02 |
| Meat-derived Mutagenic Activity | |||
| Total (units/day) | 9873.5 (19523.5) | 7260.1 (12960.8) | 0.0002 |
| Red Meat (units/day) | 9074.0 (19146.9) | 6437.7 (12007.4) | 0.0001 |
| White Meat (units/day) | 895.8 (1327.0) | 960.7 (1650.9) | 0.27 |
t-test P-value for continuous variables.
FIGURE 1. Meat doneness preferences, ORs and 95% CIs for doneness category by meat type.
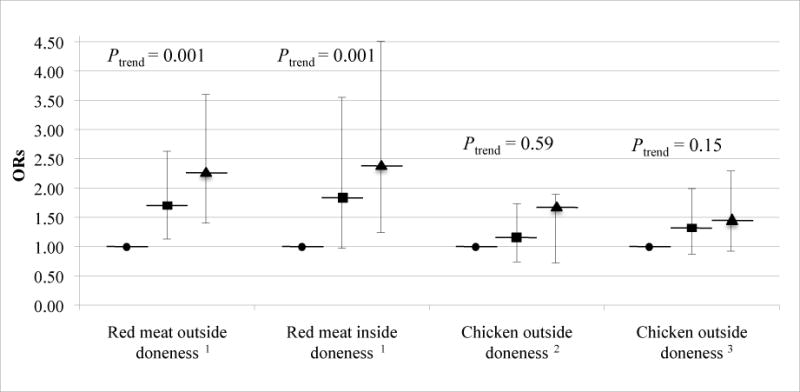
ORs (95% CI) adjusted by unconditional logistic regression for age, sex, race, WHR, average daily total caloric intake, family history of colorectal cancer and ever regular NSAID use. ● Lightly brown/rare (referent); ■ Medium brown/pink; ▲ Heavily browned or heavily browned/ blackened. Because of missing data on mutagen intake the following were excluded from all analyses in this figure: for total mutagen estimates 56 cases and 41 controls; for red meat-derived mutagen estimates 65 cases and 71 controls; for white meat-derived mutagen estimates 73 cases and 71 controls.
1 “Chicken outside doneness” was included as an adjusting variable.
2 “Red meat outside doneness” was included as an adjusting variable.
3 “Red meat inside doneness” was included as an adjusting variable.
Table 4.
Mutagen-related Compounds, ORs and 95% CIs for Specific Mutagen by Meat Subtype1
| Variable | Cases (n=1062) N(%)2 |
Controls (n=1645) N(%)2 |
OR (95% CI)3 | Ptrend4 |
|---|---|---|---|---|
| Total MeIQx | ||||
| Daily Intake (ng/day) (Mean,SD) | 75.4 (163.3) | 53.5 (101.3) | <0.0001 | |
| Quartile 15 | 168 (16.7) | 401 (25.0) | 1.00 | |
| Quartile 2 | 188 (18.7) | 401 (25.0) | 1.09 (0.84,1.42) | |
| Quartile 3 | 289 (28.7) | 401 (25.0) | 1.47 (1.14,1.90) | |
| Quartile 4 | 361 (35.9) | 401 (25.0) | 1.87 (1.44,2.44) | |
| Red Meat MeIQx | ||||
| Daily Intake (ng/day) (Mean,SD) | 72.2 (162.3) | 50.5 (96.7) | <0.0001 | |
| Quartile 15 | 165 (16.6) | 393 (25.0) | 1.00 | |
| Quartile 2 | 190 (19.1) | 394 (25.0) | 1.12 (0.86,1.47) | |
| Quartile 3 | 273 (27.4) | 394 (25.0) | 1.47 (1.13,1.91) | |
| Quartile 4 | 369 (37.0) | 393 (25.0) | 2.04 (1.55,2.68) | |
| White Meat MeIQx | ||||
| Daily Intake (ng/day) (Mean,SD) | 3.9 (7.0) | 4.0 (8.4) | 0.34 | |
| Quartile 15 | 230 (23.3) | 400 (25.4) | 1.00 | |
| Quartile 2 | 248 (25.1) | 378 (24.0) | 1.15 (0.89,1.48) | |
| Quartile 3 | 246 (24.9) | 403 (25.6) | 0.96 (0.75,1.24) | |
| Quartile 4 | 265 (26.8) | 393 (25.0) | 0.97 (0.74,1.26) | |
| Total PhIP | ||||
| Daily Intake (ng/day) (Mean,SD) | 187.3 (318.7) | 169.8 (412.5) | 0.18 | |
| Quartile 15 | 223 (22.2) | 401 (25.0) | 1.00 | |
| Quartile 2 | 225 (22.4) | 401 (25.0) | 0.95 (0.74,1.23) | |
| Quartile 3 | 268 (26.6) | 401 (25.0) | 1.08 (0.84,1.39) | |
| Quartile 4 | 290 (28.8) | 401 (25.0) | 1.18 (0.91,1.52) | |
| Red Meat PhIP | ||||
| Daily Intake (ng/day) (Mean,SD) | 93.3 (192.5) | 71.3 (205.3) | 0.009 | |
| Quartile 15 | 204 (20.5) | 394 (25.0) | 1.00 | |
| Quartile 2 | 230 (23.1) | 393 (25.0) | 1.07 (0.83,1.38) | |
| Quartile 3 | 235 (23.6) | 393 (25.0) | 0.99 (0.76,1.29) | |
| Quartile 4 | 328 (32.9) | 394 (25.0) | 1.38 (1.06,1.80) | |
| White Meat PhIP | ||||
| Daily Intake (ng/day) (Mean,SD) | 96.5 (184.3) | 101.7 (243.6) | 0.18 | |
| Quartile 15 | 271 (27.4) | 394 (25.0) | 1.00 | |
| Quartile 2 | 231 (23.4) | 401 (25.5) | 0.85 (0.67,1.09) | |
| Quartile 3 | 242 (24.5) | 385 (24.5) | 0.92 (0.72,1.19) | |
| Quartile 4 | 245 (24.8) | 394 (25.0) | 0.86 (0.67,1.11) | |
| Total DiMeIQx | ||||
| Daily Intake (ng/day) (Mean,SD) | 4.1 (7.8) | 3.1 (6.3) | 0.001 | |
| Quartile 15 | 174 (17.3) | 399 (24.9) | 1.00 | |
| Quartile 2 | 216 (21.5) | 403 (25.1) | 1.11 (0.86,1.45) | |
| Quartile 3 | 265 (26.6) | 393 (25.0) | 1.36 (1.05,1.75) | |
| Quartile 4 | 339 (34.0) | 394 (25.0) | 1.67 (1.29,2.17) | |
| White Meat DiMeIQx6 | ||||
| Daily Intake (ng/day) (Mean,SD) | 0.2 (0.7) | 0.2 (0.6) | 0.20 | |
| Total BaP | ||||
| Daily Intake (ng/day) (Mean,SD) | 39.6 (69.1) | 40.7 (84.3) | 0.47 | |
| Quartile 15 | 263 (26.1) | 401 (25.0) | 1.00 | |
| Quartile 2 | 255 (25.4) | 401 (25.0) | 1.00 (0.78,1.27) | |
| Quartile 3 | 238 (23.7) | 401 (25.0) | 0.87 (0.68,1.11) | |
| Quartile 4 | 250 (24.9) | 401 (25.0) | 0.87 (0.68,1.12) | |
| Red Meat BaP | ||||
| Daily Intake (ng/day) (Mean,SD) | 25.4 (50.4) | 22.2 (50.3) | 0.26 | |
| Quartile 15 | 256 (25.7) | 394 (25.0) | 1.00 | |
| Quartile 2 | 242 (24.3) | 393 (25.0) | 1.04 (0.81,1.33) | |
| Quartile 3 | 196 (19.7) | 393 (25.0) | 0.87 (0.67,1.12) | |
| Quartile 4 | 303 (30.4) | 394 (25.0) | 1.09 (0.85,1.40) | |
| White Meat BaP | ||||
| Daily Intake (ng/day) (Mean,SD) | 14.7 (44.7) | 19.3 (49.5) | 0.05 | |
| Quartile 15 | 268 (27.1) | 394 (25.0) | 1.00 | |
| Quartile 2 | 280 (28.3) | 393 (25.0) | 0.99 (0.78,1.26) | |
| Quartile 3 | 228 (23.1) | 394 (25.0) | 0.90 (0.70,1.16) | |
| Quartile 4 | 213 (21.5) | 393 (25.0) | 0.80 (0.62,1.04) | |
Because of missing data on mutagen intake the following were excluded from all analyses in this table: for total mutagen estimates 56 cases and 41 controls; for red meat-derived mutagen estimates 65 cases and 71 controls; for white meat-derived mutagen estimates 73 cases and 71 controls.
Values are N(%) unless otherwise notated.
ORs (95% CI) adjusted by unconditional logistic regression for age, sex, race, WHR, average daily total caloric intake, family history of colorectal cancer, smoking status and ever regular NSAID use. For each individual mutagen derived from a meat type (red or white meat), the opposite meat type for the individual mutagen was included as an adjusting variable (e.g., for Red Meat MeIQx, white meat MeIQx was included as adjusting variable).
Ptrend is for continuous variable
Referent
Because of too many zero values, quartiles were not generated for White Meat DiMeIQx.
For meat-derived mutagenic activity (a marker for all mutagens combined), quartile analysis suggests a statistically significant increase of colon cancer risk (OR = 1.77, 95% CI = 1.36–2.30, Ptrend < 0.0001) among those in the highest quartile of total meat-derived mutagenic activity compared to the lowest quartile (Figure 2). Similarly, those in the highest quartile of red meat-derived mutagenic activity compared to those in the lowest quartile shows statistically significantly increased risk of colon cancer (OR = 1.94, CI = 1.48–2.55%, Ptrend = 0.0001). White meat-derived mutagenic activity was not statistically significantly associated with colon cancer risk (Ptrend = 0.07).
FIGURE 2. Mutagen-related compounds, ORs and 95% CIs for meat-derived mutagenic activity by meat subtype.
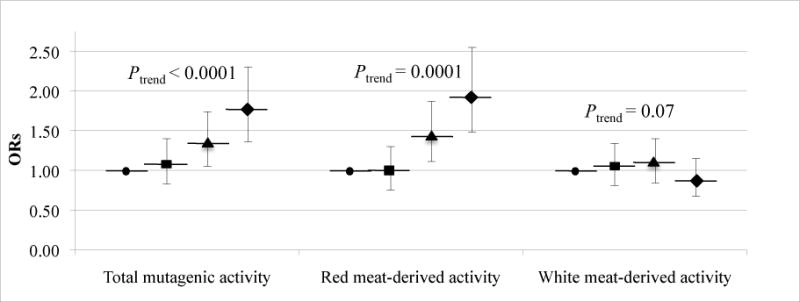
ORs (95% CI) adjusted by unconditional logistic regression for age, sex, race, WHR, average daily total caloric intake, family history of colorectal cancer and ever regular NSAID use. For each individual mutagen derived from a meat type (red or white meat), the opposite meat type for the individual mutagen was included as an adjusting variable (e.g., for red meat-derived activity, white meat-derived activity was included as adjusting variable). ● Quartile 1 (referent); ■ Quartile 2; ▲ Quartile 3; ◆ Quartile 4. Because of missing data on mutagen intake the following were excluded from all analyses in this figure: for total mutagen estimates 56 cases and 41 controls; for red meat-derived mutagen estimates 65 cases and 71 controls; for white meat-derived mutagen estimates 73 cases and 71 controls.
DISCUSSION
Surrogates for estimating dietary HCA and PAH exposure have been inconsistently associated with colorectal cancer. Total measures reflect a combination of both red and white meat, which could weaken or mask risk associations of red meat mutagen estimates with colorectal cancer. Few studies have examined the associations of total red and white meat mutagen estimates with colon cancer, or colon cancer risk association for individual mutagen estimates separately derived from red and white meat. To our knowledge this is the first study to report individual mutagen estimates separately derived from red and white meat and their association with colon cancer. Our analysis shows an increased risk of colon cancer with increased intake of specific dietary mutagens, particularly through intake of red meat and associated mutagens from red meat intake.
The one study (17) that investigated mutagen indices by red meat or white meat with colorectal cancer risk only demonstrated statistically significant associations for total white meat derived index for both men and women and for total index (red and white meat combined) for men. The study (17) used a mutagen index, but did not estimate individual mutagens. Further, the other studies (11,12,14,15,18,21–23) that analyzed individual mutagen estimates and colorectal risk identified only associations of DiMeIQx (12,14) and MeIQx (14,22) with colorectal cancer risk. In contrast, our analysis shows certain total individual mutagen estimates (MeIQx, DiMeIQx and PhIP) and red meat-derived mutagen estimates increase risk of colon cancer. We further show mutagens derived from red meat intake account for the significantly increased risk of colon cancer associated with total meat-derived mutagen estimates. Although we could not perform quartile analysis for white meat DiMeIQx because of too many of our study participants reporting zero value, the fact that total and red meat DiMeIQx values were almost identical suggests a similar effect of the red meat mutagen, supporting red meat and not white meat individual mutagen to be factor driving the statistically significant association of total individual meat-derived mutagen with colon cancer. It is thus possible that other studies that only examined overall estimates of individual mutagens could have missed significantly increased risk of colon cancer associated with specific mutagens, such as PhIP, derived from red meat but not from white meat.
Of note, there was no difference between cases and controls in total PhIP intake. Similarly, the total PhIP was not associated with colon cancer, but red meat-derived PhIP was statistically significantly associated with increased risk of colon cancer in our study. Although PhIP is generally the most abundant HCA in meat, PhIP is the least mutagenic (32,33), supporting our results.
Our finding that primarily red meat and red meat-derived mutagens increase colon cancer risk is in line with a growing epidemiologic literature (34). The mechanisms underlying the differential associations between red meat and white meat derived mutagens are still unknown. It is suggested that different constituents of white and red meat may overcome the impact of HCAs and PAHs (35). Compounds like long-chain n-3 polyunsaturated fatty acids found in fish and other compounds found in white meats could provide a protective effect for colorectal cancer, whereas other compounds largely found in red meat like heme iron could also increase colorectal cancer risk (36).
Our study is a population-based case-control study recruiting cases through a statewide surveillance network and using randomly recruited residents of Kentucky for controls. Our study uses an MPQ with color photographs that is specifically designed for estimating individual mutagens, whereas most other studies used a modified FFQ with or without photographs. The significant associations found in our analysis could be attributed to our relatively large sample size and increased power to significantly detect smaller differences of risk. Finally, the population-based study design allows for generalizability of the observed associations between red meat-derived mutagens and colon cancer risk in the wider population.
Our study has some limitations. The nature of retrospective case-control studies inherently has a possibility of information bias and recall bias with the use of average eating habits. Consumption of red meat is a known risk factor for colorectal cancer, and cases may have a tendency to recall red meat consumption differently than controls, showing a higher amount of red meat consumption. For our analysis, the amount of red meat consumed plays a factor in estimating mutagen intake, but other factors go into the calculation of mutagen intake as well, minimizing possible differential bias between cases and controls. Another limitation of our study includes modest participation of controls through our randomized recruitment methods. These methods of recruitment, though prone to selection bias because of the preference towards individuals with landlines and who are home during the day, was the most feasible method through which recruitment of a sample most representative of our case source population could be achieved. We shall point out that recruitment in our study was not restricted to only those cases or controls having landlines. Information on the status of their phone service (i.e., landline or cellular) however was not captured in the study, and sensitivity analyses restricting cases with landlines could not be performed. Despite this limitation, our analysis demonstrates that other well established risk factors for colorectal cancer exist in our study sample (e.g., age, family history of colorectal cancer, average daily total caloric intake, smoking status), lending credibility to our study.
Dietary exposure is only one possible route of exposure of HCAs and PAHs, and environmental and occupational exposure can also occur. Although the presence and amounts of the meat-derived mutagens have been relatively consistently reported by researchers using HCA and PAH surrogates (28–30,37), it is important to bear in mind that the quantities generated from the questionnaires are estimates from diet, but not total environmental exposure of the mutagens. Furthermore, the MPQ might not capture all the details for cooking techniques that influence HCA and PAH exposure. Cases and controls, therefore, could be exposed to differing levels of mutagens not captured by the MPQ.
In summary, our data add evidence supporting an increased risk of colon cancer associated specifically with dietary intake red meat-derived mutagens, rather than that from white meat or chicken. Of the many plausible mechanisms explaining red meat intake and colorectal cancer risk (38), our analysis supports estimated HCA and PAH exposure as being a possible mechanism for red meat intake to increase colon cancer risk and further supports reducing red meat intake or reducing the doneness of consumed red meat as possible interventions for colon cancer risk reduction.
Acknowledgments
We thank Carly Levin for efforts collecting data and Audrey Lynn for editorial comments on manuscript drafts. This work was supported by the Damon Runyon Cancer Research Foundation (award CI-8) and the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (grant R01-CA136726).
Abbreviations used
- BaP
benzo[a]pyrene
- CHARRED
computerized heterocyclic amines resource for research in epidemiology of disease
- DiMeIQx
2-amino-3,4,8-trimethylimidazo[4,5-f]quinoxaline
- FFQ
Food Frequency Questionnaire
- HCAs
heterocyclic amines
- MPQ
Meat Preparation Questionnaire
- MeIQx
2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline
- NSAID
nonsteroidal anti-inflammatory drug
- PAHs
polycyclic aromatic hydrocarbons
- PhIP
2-amino-1methyl-6-phenyl-imidazo[4,5-b]pyridine
- RFQ
risk factor questionnaire
References
- 1.Jagerstad M, Skog K, Grivas S, Olsson K. Formation of heterocyclic amines using model systems. Mutat Res. 1991;259:219–233. doi: 10.1016/0165-1218(91)90119-7. [DOI] [PubMed] [Google Scholar]
- 2.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 3.Canzian F, Ushijima T, Serikawa T, Wakabayashi K, Sugimura T, et al. Instability of microsatellites in rat colon tumors induced by heterocyclic amines. Cancer Res. 1994;54:6315–6317. [PubMed] [Google Scholar]
- 4.Ito N, Hasegawa R, Imaida K, Tamano S, Hagiwara A, et al. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in the rat. Mutat Res. 1997;376:107–114. doi: 10.1016/s0027-5107(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 5.Ohgaki H, Takayama S, Sugimura T. Carcinogenicities of heterocyclic amines in cooked food. Mutat Res. 1991;259:399–410. doi: 10.1016/0165-1218(91)90130-e. [DOI] [PubMed] [Google Scholar]
- 6.Shirai T, Tamano S, Sano M, Masui T, Hasegawa R, et al. Carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in rats: dose-response studies. Princess Takamatsu Symp. 1995;23:232–239. [PubMed] [Google Scholar]
- 7.Snyderwine EG, Buonarati MH, Felton JS, Turteltaub KW. Metabolism of the food-derived mutagen/carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) in nonhuman primates. Carcinogenesis. 1993;14:2517–2522. doi: 10.1093/carcin/14.12.2517. [DOI] [PubMed] [Google Scholar]
- 8.Sugimura T. Overview of carcinogenic heterocyclic amines. Mutat Res. 1997;376:211–219. doi: 10.1016/s0027-5107(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 9.Sugimura T, Wakabayashi K, Nakagama H, Nagao M. Heterocyclic amines: Mutagens/carcinogens produced during cooking of meat and fish. Cancer Sci. 2004;95:290–299. doi: 10.1111/j.1349-7006.2004.tb03205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakabayashi K, Nagao M, Esumi H, Sugimura T. Food-derived mutagens and carcinogens. Cancer Res. 1992;52(Suppl):2092S–2098S. [PubMed] [Google Scholar]
- 11.Augustsson K, Skog K, Jägerstad M, Dickman PW, Steineck G. Dietary heterocyclic amines and cancer of the colon, rectum, bladder, and kidney: a population-based study. Lancet. 1999;353:703–707. doi: 10.1016/S0140-6736(98)06099-1. [DOI] [PubMed] [Google Scholar]
- 12.Butler LM, Sinha R, Millikan RC, Martin CF, Newman B, et al. Heterocyclic amines, meat intake, and association with colon cancer in a population-based study. Am J Epidemiol. 2003;157:434–445. doi: 10.1093/aje/kwf221. [DOI] [PubMed] [Google Scholar]
- 13.Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey AB, et al. Red meat intake, doneness, polymorphisms in genes that encode carcinogen-metabolizing enzymes, and colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2008;17:3098–3107. doi: 10.1158/1055-9965.EPI-08-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Girard H, Butler LM, Villeneuve L, Millikan RC, Sinha R, et al. UGT1A and UGT1A9 functional variants, meat intake, and colon cancer, among Caucasians and African Americans. Mutat Res. 2008;644:56–63. doi: 10.1016/j.mrfmmm.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joshi AD, Corral R, Siegmund K, Haile R, Le Marchand L, et al. Red meat and poultry intake, polymorphisms in the nucleotide excision repair and mismatch repair pathways and colorectal cancer risk. Carcinogenesis. 2009;30:472–479. doi: 10.1093/carcin/bgn260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kampman E, Slattery ML, Bigler J, Leppert M, Samowitz W, et al. Meat consumption, genetic susceptibility, and colon cancer risk: a United States multicenter case-control study. Cancer Epidemiol Biomarkers Prev. 1999;8:15–24. [PubMed] [Google Scholar]
- 18.Kobayashi M, Otani T, Iwasaki M, Natsukawa S, Shaura K, et al. Association between dietary heterocyclic amine levels, genetic polymorphisms of NAT2, CYP1A1, and CYP1A2 and risk of colorectal cancer: a hospital-based case-control study in Japan. Scand J Gastroenterol. 2009;44:952–959. doi: 10.1080/00365520902964721. [DOI] [PubMed] [Google Scholar]
- 19.Murtaugh MA, Sweeney C, Ma KN, Caan BJ, Slattery ML. The CYP1A1 genotype may alter the association of meat consumption patterns and preparation with the risk of colorectal cancer in men and women. J Nutr. 2005;135:179–186. doi: 10.1093/jn/135.2.179. [DOI] [PubMed] [Google Scholar]
- 20.Muscat JE, Wynder EL. The consumption of well-done red meat and the risk of colorectal cancer. Am J Public Health. 1994;84:856–858. doi: 10.2105/ajph.84.5.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nöthlings U, Yamamoto JF, Wilkens LR, Murphy SP, Park SY, et al. Meat and heterocyclic amine intake, smoking, NAT1, and NAT2 polymorphisms, and colorectal cancer risk in the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2098–2106. doi: 10.1158/1055-9965.EPI-08-1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nowell S, Coles B, Sinha R, MacLeod S, Luke Ratnasinghe D, et al. Analysis of total meat intake and exposure to individual heterocyclic amines in a case-control study of colorectal cancer: contribution of metabolic variation to risk. Mutat Res. 2002;506–507:175–185. doi: 10.1016/s0027-5107(02)00164-1. [DOI] [PubMed] [Google Scholar]
- 23.Ollberding N, Wilkens L, Henderson B, Kolonel L, Le Marchand L. Meat consumption, heterocyclic amines and colorectal cancer risk: the multiethnic cohort study. Int J Cancer. 2012;131:E1125–E1133. doi: 10.1002/ijc.27546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Plummer SJ, Thompson CL, Tucker TC, Casey G. Association between phosphatidylinositol 3-kinase regulatory subunit p85alpha Met326lle genetic polymorphism and colon cancer risk. Clin Cancer Res. 2008;14:633–637. doi: 10.1158/1078-0432.CCR-07-1211. [DOI] [PubMed] [Google Scholar]
- 25.Sinha R, Rothman N. Exposure assessment of heterocyclic amines (HCAs) in epiemiologic studies. Mutat Res. 1997;376:195–202. doi: 10.1016/s0027-5107(97)00043-2. [DOI] [PubMed] [Google Scholar]
- 26.Sinha R, Cross A, Curtin J, Zimmerman T, McNutt S, et al. Development of a food frequency questionnaire module and databases for compounds in cooked and processed meats. Mol Nutr Food Res. 2005;49:648–655. doi: 10.1002/mnfr.200500018. [DOI] [PubMed] [Google Scholar]
- 27.Martinez ME, Jacobs ET, Ashbeck EL, Sinha R, Lance P, et al. Meat intake, preparation methods, mutagens and colorectal adenoma recurrence. Carcinogenesis. 2007;28:2019–2027. doi: 10.1093/carcin/bgm179. [DOI] [PubMed] [Google Scholar]
- 28.Sinha R, Knize MG, Salmon CP, Brown ED, Rhodes D, et al. Heterocyclic amines content of pork products cooked by different methods and to varying degrees of doneness. Food Chem Toxicol. 1998;36:289–297. doi: 10.1016/s0278-6915(97)00159-2. [DOI] [PubMed] [Google Scholar]
- 29.Sinha R, Rothman N, Salmon CP, Knize MG, Brown ED, et al. Heterocyclic amine content in beef cooked by different methods to varying degrees of doneness and gravy made from meat drippings. Food Chem Toxicol. 1998;36:279–287. doi: 10.1016/s0278-6915(97)00162-2. [DOI] [PubMed] [Google Scholar]
- 30.Kazerouni N, Sinha R, Hsu CH, Greenberg, Rothman N. Analysis of 200 food items for benzo[a]pyrene and estimation of its intake in an epidemiologic study. Food Chem Toxicol. 2001;39:423–436. doi: 10.1016/s0278-6915(00)00158-7. [DOI] [PubMed] [Google Scholar]
- 31.Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with salmonella-mammalian-microsome mutagenicity test. Mutat Res. 1975;31:347–363. doi: 10.1016/0165-1161(75)90046-1. [DOI] [PubMed] [Google Scholar]
- 32.Nakagama H, Nakanishi M, Ochiai M. Modeling human colon cancer in rodents using a food-borne carcinogen, PhIP. Cancer Sci. 2005;96:627–636. doi: 10.1111/j.1349-7006.2005.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinha R. An epidemiologic approach to studying heterocyclic amines. Mutat Res. 2002;506–507:197–204. doi: 10.1016/s0027-5107(02)00166-5. [DOI] [PubMed] [Google Scholar]
- 34.World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition, Physical Activity, and the Prevention of Cancer: A Global Perspective. Washington, DC: 2007. [Google Scholar]
- 35.Larsson S, Rafter J, Holmberg L, Bergkvist L, Wolk A. Red meat consumption and risk of cancers of the proximal colon, distal colon and rectum: the Swedish mammography cohort. Int J Cancer. 2005;113:829–834. doi: 10.1002/ijc.20658. [DOI] [PubMed] [Google Scholar]
- 36.Cross AJ, Ferrucci LM, Risch A, Graubard BI, Ward MH, et al. A large prospective study of meat consumption and colorectal cancer risk: an investigation of potential mechanisms underlying this association. Cancer Res. 2010;70:2406–2414. doi: 10.1158/0008-5472.CAN-09-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turesky RJ, Taylor J, Schnackenberg L, Freeman JP, Holland RD. Quantitation of carcinogenic heterocyclic aromatic amines and detection of novel heterocyclic aromatic amines in cooked meats and grill scrapings by HPLC/ESI-MS. J Agric Food Chem. 2005;53:3248–3258. doi: 10.1021/jf048290g. [DOI] [PubMed] [Google Scholar]
- 46.Ferguson L. Meat and cancer. Meat Sci. 2010;84:308–313. doi: 10.1016/j.meatsci.2009.06.032. [DOI] [PubMed] [Google Scholar]


