Abstract
Diabetes is an increasing public health problem that is expected to escalate in the future due to the growing incidence of obesity in the western world.While this disease is well known for its devastating effects on the kidneys and vascular system, diabetic individuals can develop cardiac dysfunction, termed diabetic cardiomyopathy, in the absence of other cardiovascular risk factors such as hypertension or atherosclerosis. While much effort has gone into understanding the effects of elevated glucose or altered insulin sensitivity on cellular components within the heart, significant changes in the cardiac extracellular matrix (ECM) have also been noted. In this review article we highlight what is currently known regarding the effects diabetes has on both the expression and chemical modification of proteins within the ECM and how the fibrotic response often observed as a consequence of this disease can contribute to reduced cardiac function.
Keywords: diabetes, extracellular matrix, heart, advanced glycation end products (AGE), fibrosis, myofibroblast
Introduction
According to the National Institute of Diabetes and Digestive and Kidney Diseases, as of 2010 approximately 8.3% of the U.S. population, or about 25 million individuals, are affected by diabetes mellitus (DM). There are two types of diabetes: type 1 and type 2. Type 1, sometimes referred to as juvenile-onset, typically affects children or young adults and accounts for a small percentage (~5%) of all diabetes cases. In contrast, type 2 diabetes, often associated with obesity, accounts for greater than 90% of diagnosed diabetes cases. The cardiovascular complications of diabetes are well known and remain a leading cause of morbidity and mortality in individuals with this disease (Jaffe et al., 1984; Lehto et al., 1994; Shehadeh & Regan, 1995). Patients with diabetes have a higher risk for hypertension, myocardial infarction, vascular dysfunction, and heart failure (Nichols et al., 2004). In fact, over 65% of diabetic patients die due to cardiovascular complications (Ares-Carrasco et al., 2009).
The effects of diabetes on the cardiovascular system have been extensively described; however, the mechanisms of these effects have not been fully elucidated. This is in part due to the complex and multifactoral nature of diabetes. Among other things, diabetes has profound effects on the expression, organization, and modification of extracellular matrix (ECM) components in many organs (Fig. 1). As discussed in this review article, tissue remodeling and fibrosis appear to be a direct consequence of diabetes in several organs including the heart. Nonenzymatic alterations in ECM proteins due to elevated glucose levels are also a consequence of this disease. Changes in the accumulation and modification of ECM proteins impact the mechanical properties of the ECM and have deleterious consequences on cardiac function. Elevated glucose levels have also been shown to result in direct activation of several cell types transforming these cells into a contractile, myofibroblast phenotype, which can further exacerbate myocardial remodeling and fibrosis. This review article describes the alterations in the ECM resulting from diabetes and the effects these alterations can have on myocardial function.
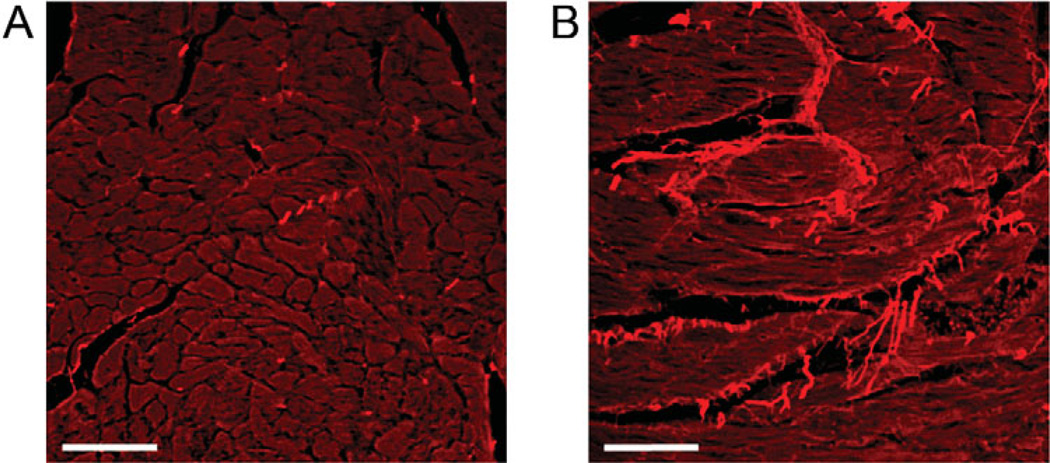
Figure 1.
Increased collagen expression in the diabetic heart. The db/db leptin receptor deficient mouse is a common model used to study the cardiovascular effects of type 2 diabetes. Picrosirus red staining of heart sections from control (A) and 12 week diabetic (B) mice illustrates the accumulation of collagen, as indicated by the bright red staining, which occurs in the diabetic heart. Scale bar = 50 µm.
Diabetes and the Extracellular Matrix
The ECM is a dynamic network comprised of structural proteins, proteoglycans, growth factors/cytokines, and enzymes that provides essential cues and scaffolding for tissue organization and structure. Alterations in ECM accumulation, composition, and organization can adversely affect organ function. Regulation of ECM homeostasis is a complex balance between biochemical factors, mechanical forces transduced via cell-ECM interactions, and cell-cell communication. During the onset and progression of DM, changes in ECM protein expression occur, leading to structural modifications in the ECM network and altered cell-ECM and cell-cell interactions (Siperstein et al., 1968). Subsequently, these changes lead to diabetes-induced organdependent diseases such as nephropathy, retinopathy, and diabetic cardiomyopathy. While organ-specific changes in ECM are summarized in Table 1, in this section we will review DM-induced structural alterations to the cardiac ECM and discuss how DM-dependent changes to the ECM of other organs (i.e., kidneys, liver, and pancreas) affect myocardial function.
Table 1.
Summary of Alterations in ECM Protein Expression During DM.*
| Cardiac | Renal | Hepatic | Pancreatic | |
|---|---|---|---|---|
| Fibrillar collagen | ↑Types I & III (myocardium and aorta) | ↑Types I & III (late glumeruloscerosis) | ↑Type I | ↑Types I & III |
| Nonfibrillar collagen | Type VI (DC) | ↑Types IV& V | ↑Type IV | ↑Type IV |
| Adhesive proteins | ↓intimal elastin ↑elastin fragments (DA) ↑FN (aorta) |
↑FN & LN (early DM) ↓LN (late DM) |
↑LN | ↑FN |
| Proteoglycans | ↓HSP & ↑chondroitin & dermatan sulfate (DA) |
↓HSP | ↔ | ↔ |
| Other changes associated with ECM proteins | ↑ advanced glycated collagen (DA) | ↑ macroalbuminuria | ↑FN (ED-B), ↑IV 7s domain (serum) ↑VI (serum) |
↔ |
Arrows indicate increased (up arrow) or decreased (down arrow) presence of listed ECM proteins. (↔) denotes no reported changes to ECM protein expression.
FN, fibronectin; LN, laminin; HSP, heparin sulfate proteoglycan; ED-B, extra binding domain splicing variant; DC, diabetic cardiomyopathy; DA, diabetic atherosclerosis.
Diabetes Mellitus and the Cardiac ECM
The cardiac ECM consists of a well-defined network of collagen (Caulfield & Borg, 1979), with fibrillar collagen types I and III localized within the myocardial interstitium and nonfibrillar collagen types IV and VI, and the glycoproteins fibronectin and laminin predominating in the myocyte basement membrane (Eghbali &Weber, 1990; Bishop & Laurent, 1995). A number of studies have examined alterations in cardiac ECM expression associated with diabetes. Previous human studies comparing diabetic and nondiabetic human patients revealed an increased presence of type I, III, and VI collagen in the hearts of both groups; however, only collagen type III expression was significantly different between the two groups. No increase in collagen IV and V was detected (Shimizu et al., 1993). As DM progresses, the left ventricle may hypertrophy and hypertension-induced diastolic dysfunction can occur (Regan et al., 1981; Shapiro et al., 1981a, 1981b). Diabetes-induced ECM remodeling may also influence the development of cardiomyopathy, atherosclerosis, carotid artery disease, and interstitial fibrosis (Seneviratne, 1977; Regan et al., 1981). For example, alloxan-induced diabetes in rats demonstrated a significant increase in collagen type VI when compared to collagen types I and IV and other ECM proteins (Spiro & Crowley, 1993). In diabetic cardiomyopathy, characterized as cardiac dysfunction in diabetic patients in the absence of other cardiovascular risk factors such as hypertension or coronary artery disease, excess collagen deposition, particularly collagens I, III, and IV, contributes to impaired LV function (Aneja et al., 2008).
DM-induced alterations to the vascular ECM are associated with several cardiovascular complications. In some diabetic models, changes in proteoglycans, notably decreases in heparan sulfate and increased levels of chondroitin and dermatan sulfate (Heickendorff et al., 1994; Tabas et al., 2007), have been observed. Tabas and colleagues (2007) suggested that changes in proteoglycan expression may lead to increased low density lipoprotein retention in the arterial wall, thus promoting atherosclerosis. In rat and pig diabetic atherosclerotic models, there is a loss of elastin in the intima associated with an increase in elastin fragmentation, yet the cause for elastin breakdown and turnover is unclear (Kwan et al., 1988; McDonald et al., 2007). Previous work has also shown an increase in collagen deposition by vascular smooth muscle cells (SMCs) during DM. The newly synthesized collagen can undergo advanced nonenzymatic glycation, which can increase low density lipoprotein binding and retention to the arterial wall, thus promoting atherosclerosis (Brownlee et al., 1985; McDonald et al., 2007). In addition, gylcation of collagen within the vascular wall contributes to decreased vascular compliance observed in diabetic models (Aronson, 2003), which can result in an increased load on the heart. Aortic SMCs isolated from type II diabetic rat models showed an increase in collagen type I mRNA levels and a decrease in transcription of fibronectin mRNA, respectively (Song & Ergul, 2006). Furthermore, aortic SMCs cultured in high glucose media showed an increase in collagen types I and III protein expression when compared to control cells cultured in normal glucose media (Bouguerra et al., 2004).
Involvement of Other Organs in Diabetes-Induced Myocardial Dysfunction
As can be seen in Table 1, diabetes is a multifactoral disease, affecting multiple organ systems whose own functional changes can impact cardiac performance. For example, during the early stages of diabetic nephropathy, acute kidney disease is associated with increased excretion of protein in the urine, a phenomenon called proteinuria (Abbate et al., 2006; Jefferson et al., 2008). Recent epidemiological studies have shown that during DM, hypertension and heart failure (HF) have a linear correlation with proteinuria, 93% and 26%, respectively (Tarnow et al., 1994;Wang et al., 1996). As diabetic nephropathy progresses, renal fibrosis and hypertrophy increase leading to a decline in glomerular function and filtration (Dalla Vestra et al., 2000; Osterby et al., 2001). During DM, the expression of normal ECM proteins in the glomerular basement membrane (GBM) and mesangium are increased, altering the structure of the diabetic kidney. The GBM and mesangium are thickened by increased synthesis of collagen types IV and V, fibronectin and laminin (Kiryu et al., 1994; Zhu et al., 1994; Osterby et al., 2001; Mason & Wahab, 2003). As the disease progresses, laminin and heparan sulfate proteoglycan expression decreases in the GBM and heparan sulfate proteoglycan is reduced in the mesangium (Falk et al., 1983; Ikeda et al., 1991; Vernier et al., 1992). Renal decline and ultimately failure does not occur until the mesangium has expanded, impeding normal kidney filtration (Steffes et al., 1989).
Renal failure and inhibition of filtration contribute to several cardiac pathologies such as hypertension, fibrosis, cardiomyopathy, hypertrophy, and atherosclerosis (Rubler et al., 1972; Anavekar et al., 2004). Rubler and colleagues (1972) reported an association between cardiac hypertrophy and fibrosis in deceased individuals diagnosed with diabetic nephropathy, while another postmortem study looked at the relationship between hypertension and diabetes and found an increase in cardiac fibrosis in hypertensive diabetic individuals compared to hypertensive or diabetic only individuals (van Hoeven & Factor, 1990). A similar study showed that cardiac fibrosis was exacerbated in hypertensive diabetic patients compared to diabetic and nondiabetic control patients (Frustaci et al., 2000). Besides the pathological alterations that occur in the myocardium during diabetic nephropathy, physiological characteristics indicative of cardiac hypertrophy and cardiomyopathy have been reported (Poirier et al., 2001). For example, echocardiograph studies showed an increase in diastolic and/or systolic dysfunction during the progression of diabetic nephropathy (Miyazato et al., 2005).
The most prevalent form of liver disease induced during DM associated with cardiovascular dysfunction is nonalcoholic fatty liver disease (NAFLD), characterized by spontaneous lipid accumulation independent of alcohol consumption (Levinthal & Tavill, 1999). Traditionally, NAFLD can promote atherosclerosic events independent of DM; however, recent studies have shown that DM further exacerbates vascular damage (Targher et al., 2005). A cohort study showed that patients diagnosed with NAFLD had decreased blood flow and increased intima thickness (Targher, 2004; Villanova et al., 2005) that positively correlated with NAFLD progression. Another population based study showed that a strong correlation existed between hepatic steatosis and atherosclerotic plaque development (Volzke et al., 2005).
Cardiometabolic syndrome (CMS) is a grouping of several cardiovascular risk factors, such as hyperglycemia, obesity, hypertension, dyslipidemia, and albumineria (Lastra & Manrique, 2007), that is often used as an indicator for cardiovascular disease, stroke, type II diabetes, and chronic kidney disease. In type II DM, there is partial loss of pancreatic β-cell function, such as glucose stimulated insulin secretion, which affects the body’s ability to remove glucose from the bloodstream, while in type I DM, the β-cell is attacked and destroyed by the host’s immune system, eliminating all insulin secretion. Therefore, β-cell function and activity is the center of DM development and progression. After DM develops, it can further damage the pancreas by inducing alterations in pancreatic ECM proteins. Fibronectin, along with collagens type I, III, and IV, have been shown to be increased and secreted into the interstitium and islet exocrine interface by pancreatic stellate cells or islet cells during diabetes (Ko et al., 2006; Hayden et al., 2008). Specifically during type II DM, fibrosis has been shown to be the main cause of pancreatic failure and CMS prevalence. Previous literature has shown that activation of pancreatic stellate and islet cells is promoted by the renin-angiotensin system (RAS) and transforming growth factor beta 1 (TGF-β1), a profibrotic cytokine (Yoshikawa et al., 2002; Ko et al., 2006). RAS inhibition, either by angiotensin converting enzyme inhibitors or angiotensin receptor blockers, has improved CMS frequency and had positive effects on preventing type II diabetes and balancing glucose metabolism (Lastra & Manrique, 2007).
Diabetes and Myofibroblast Activation
Accumulation of collagen within the ECM, or fibrosis, in a number of organs is associated with the appearance of myofibroblasts. Also referred to as contractile fibroblasts, the myofibroblast is most simply described as an “activated” fibroblast that expresses proteins and qualities characteristic of a smooth muscle cell (Fig. 2; Brown et al., 2005). Namely, they express the contractile protein α-smooth muscle actin (α-SMA) and the intermediate filament protein desmin typical of smooth muscle cells (Gabbiani, 2003; Brown et al., 2005). In this heightened state of activity, the myofibroblast exhibits increased motility, contractility, and secretion of ECM components such as collagen and fibronectin (Porter & Turner, 2009). Myofibroblasts are crucial to remodeling injured tissue, such as the wound healing response; however, the persistence of myofibroblasts has been correlated with a number of pathological fibrotic conditions (Naugle et al., 2006; Hinz, 2007). While the actions and fibrotic consequences resulting from the presence of this specific cell type may be universal throughout the body, myofibroblasts are heterogeneous in their origins. The factors that stimulate their transdifferentiation also vary and are dependent on the microenvironment, tissue, or organ system in which the cells are found (Hinz et al., 2007).
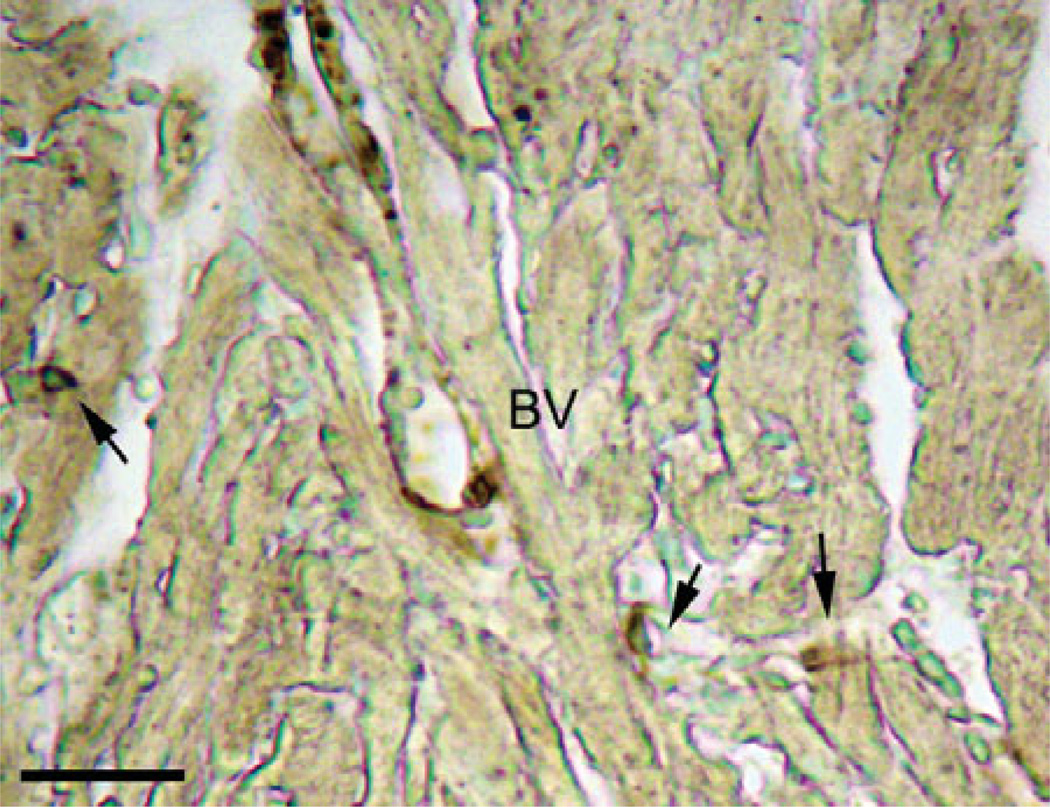
Figure 2.
Myofibroblasts in the heart. As myofibroblasts represent a contractile phenotype of the fibroblasts, staining with α-smooth muscle actin (dark brown color) reveals the location of several myofibroblasts (indicated by the arrows) in the mouse heart. Small in size, these cells reside in close proximity to neighboring cardiac myocytes and are clearly distinguishable from vascular smooth muscle cells localized around blood vessels (BV). Scale bar = 25 µm.
Cardiac Myofibroblast Origins and Activation
In the heart, the most widely accepted dogma suggests that cardiac myofibroblasts are derived from resident fibroblasts within the myocardium (Krenning et al., 2010). This argument has been disputed on the basis that myofibroblasts, both within the same and different organ systems, display significant differences in gene expression, and it has been suggested that they be considered distinct cell types (Chang et al., 2002; Krenning et al., 2010). In addition, myofibroblasts in other organs have been shown to originate from different cellular precursors. For example, in the liver and pancreas, myofibroblasts are derived from stellate cells. In the kidney, they arise, at least in part, from tubular epithelial cells via an epithelial-mesenchymal transformation (Gress et al., 1998; Yang & Liu, 2001; Cassiman et al., 2002). Myofibroblasts may also arise from other undifferentiated connective tissue cells such as pericytes or mesenchymal stem cells. Currently, there is substantial debate about the exact origin(s) of the cardiac myofibroblast (Leask, 2010). If, as is commonly proposed, myofibroblasts arise from resident cardiac fibroblasts, this could complicate comparison between model systems. Recent work has shown that the number of fibroblasts in the heart varies temporally during development and with species (Banerjee et al., 2007). This work would suggest that the rat heart may have the potential to produce more myofibroblasts compared to the mouse heart under pathological conditions and that the already elevated level of collagen within the rat heart would provide increased substrates for nonenzymatic modification associated with elevated glucose during diabetes.
Studies examining fibrosis have shown myofibroblasts to be the largest contributors of ECM in the heart and other organs (Naugle et al., 2006). Myofibroblasts have been associated with a multitude of cardiac diseases in which normally quiescent fibroblasts transdifferentiate into myofibroblasts in response to diverse signals including cardiomyocyte distress and cell death (Brown et al., 2005; Porter & Turner, 2009). Of the many factors and hormones associated with cardiac fibrosis, a subset has been shown to drive myofibroblast activation in the heart. Transforming growth factor-β has long been recognized as a profibrotic cytokine and is among the most potent mediator in the transition of fibroblasts to the myofibroblast phenotype (Gabbiani, 2003; Desmoulière et al., 2005; Leask, 2008). When TGF-β is neutralized via antibody treatment, cardiac fibroblast activation is inhibited (Kuwahara et al., 2002). Angiotensin II (Ang II), also known to stimulate profibrotic remodeling in the heart, may indirectly induce myofibroblast transdifferentiation by activating TGF-β (Gao et al., 2009; Yang et al., 2009). Downstream of TGF-β, Endothelin-1 (ET-1), a vaso-constrictive protein found in many cases of heart failure, has been shown to induce lung and cardiac fibroblasts to transition into myofibroblasts (Shi-Wen et al., 2004; Nishida et al., 2007). Cardiac myofibroblasts respond to a variety of factors and stimuli that direct their production of ECM proteins such as proinflammatory cytokines (Interleukins 1 and 6, tumor necrosis factor-a, TGF-β), vasoactive proteins (Ang II, ET-1, natriuretic peptides A and B), noradrenaline, ischemia and reperfusion, and mechanical stimuli (Porter & Turner, 2009).
Myofibroblasts and Diabetes
Since fibrosis disrupts the normal function of a number of organs in diabetic individuals, myofibroblasts, as one of the primary modulators of ECM production and turnover, are a potential therapeutic target for preventing pathological matrix remodeling. In tubulointerstitial fibrosis observed in diabetic nephropathy, increased numbers of myofibroblasts have been associated with excessive deposition of ECM (Gilbert & Cooper, 1999; Li et al., 2004). Myofibroblasts of the kidney are derived from tubular epithelial cells via the process of epithelial-mesenchymal transformation involving several mediators including TGF-β (Li et al., 2004). Interestingly, the work by Li et al. (2004) showed that myofibroblasts can be activated in the presence of advanced glycation end products (AGEs) with or without TGF-β. This response was demonstrated to involve the AGE receptor (RAGE) and ERK1/2 MAP kinase activation in vitro. Others have also shown that myofibroblast formation can occur via activation of RAGE, but not independent of TGF-β (Oldfield et al., 2001). As AGEs are a consequence of prolonged hyperglycemia, this finding provides a unique paradigm when considering therapeutic targets of fibrosis in diabetes compared to other fibrotic disease states (Goldin et al., 2006).
Excessive fibrotic scarring is also a problem in the manifestation of proliferative diabetic retinopathy and other proliferative retinal diseases where fibroblast to myofibroblast differentiation has been described (Walshe et al., 1992; Bochaton-Piallat et al., 2000). An increased presence of myofibroblasts in the diabetic eye contributes to the formation of scar-like epiretinal membranes contributing to retinal detachment. One study has shown that this myofibroblast transition is linked to TGF-β, TGF-β receptor II, and the EDA splice variant of fibronectin in proliferative diabetic retinopathy (Bochaton-Piallat et al., 2000). Another clinical study discovered reduced expression of bone morphogenetic protein 6 (BMP6) in myofibroblast progenitor cells in type 1 diabetic patients compared to healthy controls. Since BMP6 has been shown to have anti-TGF-β effects, it is hypothesized that cells lacking BMP6 may be potential contributors to disease-related tissue remodeling (Nguyen et al., 2006). Interestingly, the opposite seems to be true in dermal wounds where impairment in wound healing is observed in both diabetic humans and genetically diabetic mice (Greenhalgh et al., 1990; Brem & Tomic-Canic, 2007). In a wound healing study, Darby et al. (1997) demonstrated that nonobese diabetic mice showed reduced cell proliferation, myofibroblast transdifferentiation, procollagen I mRNA, and the presence of abnormal cell death in dermal wounds of diabetic mice compared to wild-type controls. Just as myofibroblasts are diverse in their origins, studies suggest that differences in diabetes-induced myofibroblast differentiation and mediators depend on the microenvironment and organ system.
Cardiac Myofibroblasts and Diabetes
Though patients with diabetic cardiomyopathy develop pathological cardiac fibrosis, there have been few studies investigating the role of the cardiac myofibroblast in this disease. In a study comparing the fibrotic properties of aldosterone and high levels of glucose (to simulate hyperglycemia observed in diabetes), a similar increase in cardiac myofibroblast proliferation was seen with both treatments in vitro (Neumann et al., 2002). This suggests that high glucose levels may be a stimulus of cardiac myofibroblast action and subsequent remodeling of the ECM by these cells. Conversely, in a rat streptozotocin-induced type 1 diabetic model, a reduction in α-SMA-positive cardiac myofibroblasts was reported; however, an increase in proliferation of cardiac fibroblasts in animals diabetic for 6 weeks was observed (Shamhart et al., 2009). Another in vitro study investigating human cardiac fibroblasts was carried out using methylglyoxal-modified collagen where methlglyoxal has been shown to be a component of the diabetic environment involved in carbonyl stress and impacts cell adhesion by modifying integrin binding sites on collagen (Dobler et al., 2006; Yuen et al., 2010). Findings showed increases in α-SMA and EDA-fibronectin positive myofibroblasts that was dependent upon TGF-β but not Rho-kinase. The formation of myofibroblasts by methylglyoxal-modified collagen resulted in enhanced collagen gel contraction and migration compared to cardiac fibroblasts on nontreated collagen (Yuen et al., 2010). Additionally, a decrease in adherence was observed in cells exposed to methylglyoxal treated collagen (Yuen et al., 2010). These findings support the suggestion that glycated collagen as seen in the diabetic is able to promote differentiation of fibroblasts into myofibroblasts, activating these cells independent of other biochemical factors. In agreement with this, epithelial cells have been shown to transdifferentiate into myofibroblasts under the influences of AGEs working through the RAGE receptor (Schäfer et al., 2006). While the present studies report interesting information regarding the role of myofibroblasts in the manifestation of diabetic cardiomyopathy, there is still much to be investigated to therapeutically target these cells.
Diabetes, Ages, and the Ecm
It is well established that proteins in the ECM undergo not only enzyme-mediated modification, but also modification through direct chemical reaction with other biomolecules. Of central importance to conditions related to both type I and II diabetes are the class of modification products referred to as advanced glycation end products, or AGEs. Broad in scope, the AGEs represent proteins or lipids that have been glycated via a nonenzymatic process long known to food chemists as the Maillard reaction. Found in a variety of tissues, AGEs have been correlated to normal aging (Dunn et al., 1989; Sell et al., 1996; Frey et al., 1998; Ulrich & Cerami, 2001), elevated glucose associated with diabetes (Goh & Cooper, 2008), vascular complications (Goldin et al., 2006), and cardiac disease (Tikellis et al., 2008; Boudina & Abel, 2010) among other conditions. In all of the syndromes where AGEs are now implicated, the proteins undergoing glycation are long-lived, with collagen in its different forms featured prominently.
Initially investigated in terms of varying glucose concentrations and exposure times to proteins, a more complete picture has emerged showing that metabolic pathways can, and do, also provide the starting materials necessary for formation of AGEs (Hamada et al., 1996).A variety of carbohydrates (i.e., glucose, fructose, triose phosphates, or methylglyoxal) supplies the reactive intermediates (α-hydroxy aldehydes and ketones, α, β-unsaturated aldehydes and ketones, hydroxyalkenals, and enediols), which form the final AGE adduct via a variety of mechanisms (dehydration, rearrangement, Cannizarro reaction, and Michael addition) (Baynes, 2001).When reactive carbonyl compounds, derived from carbohydrates are combined with high levels of reactive oxygen species (as would be present in tissues under oxidative stress), the result is also the formation of AGEs (Smit & Garrits, 2010). Formation of AGEs is made more complex by the fact that in many cases a single reactive intermediate can be derived from multiple carbohydrate sources, and so a focus on a single precursor, such as glucose in relation to diabetes, is unlikely to fully account for the formation of AGEs (because levels of triose phosphates and methylglyoxal will also vary). As a result, development of therapeutic agents has turned to blocking endogenous AGE receptors (Bierhaus et al., 2005, 2006) or development of agents capable of breaking the AGE inter- and intramolecular cross-links that have formed (Candido et al., 2003).
Studies aimed at determining the sites and levels of inter- versus intramolecular cross-linking have thus far focused on human serum albumin and lens tissue (Biemel et al., 2002) and ribonuclease A (Dai et al., 2008). In all three cases, glucosepane, a 7-membered ring formed from arginine and lysine residues, was the major cross-link observed. For diabetics, levels of glucosepane in collagen have been documented to be more than twice the levels found in nondiabetics (Sell et al., 2005), with results suggesting one cross-link for every two triple helical collagen molecules in diabetics (Dai et al., 2008). The rate of AGE accumulation has been correlated to not only carbohydrate concentration and protein turnover (Brownlee, 1995; Booth et al., 1997; Ferreira et al., 2003), but also to the simple process of tissue aging (Dunn et al., 1989, 1991). Under the hyperglycemic conditions common to diabetics, the rate of AGE formation is enhanced, and the impairment of cardiac function occurs at even earlier ages than found in the nondiabetic population (Nichols et al., 2004).
The impact of the formation and accumulation of AGEs has been documented in a variety of tissues. In vitro studies of cross-linking and articular collagen stiffness have correlated the decrease in instantaneous deformation that collagen experiences to increasing levels of AGEs present in the sample (Verzijl et al., 2002). In vitro results also suggest that glycation yields products that are especially stable (Fathima et al., 2004) and retain a significant degree of their intrinsic three-dimensional structure (Yamada et al., 2004). Modification of lysine and arginine residues in collagen has been found to significantly reduce its in vitro rate of degradation (Gratzer et al., 2006), further supporting the hypothesis that collagen modification by AGEs is an essentially irreversible process (Hartog et al., 2007).
In addition to the chemical modification of collagen and other proteins, which may alter their intrinsic function, AGEs also exert effects via binding to a cell surface receptor of the immunoglobulin superfamily (Basta et al., 2002). These receptors for AGEs, or RAGE, also respond to other ligands that accumulate with age and degenerative diseases such as amyloid β-peptides (Du et al., 1997) and amyloid A (Yan et al., 2000). The S100/calgranulins, polypeptides that bind calcium and accumulate in locations of inflammation, will also bind to and trigger RAGE (Hofmann et al., 1999). Discussion of the promiscuous ligand binding of the RAGE receptor and the potential signaling pathways activated by these different interactions is beyond the scope of this review; however, the reader is referred to recent a review of RAGE-ligand interactions and signaling pathways (Bierhaus et al., 2005).
At this time it appears that a strong circumstantial case can be made for AGEs contributing to heart failure. The high degree of cross-linking that accompanies AGE accumulation decreases the compliance of cardiac tissue and induces diastolic dysfunction. In assessing the effect of AGE cross-links on cardiac mechanics in volume-overload hypertrophy, Herrmann et al. (2003) demonstrated that the use of aminoguanidine, a drug that prevents AGE formation, reduced myocardial longitudinal stiffness to that of control animals. Animals with volume overload hypertrophy that did not receive aminoguanidine had myocardial longitudinal stiffness coefficients 50–100% higher at the septum and left ventricle, respectively, than control animals. More direct evidence of the involvement of RAGE and its downstream signaling pathways in heart failure has been demonstrated by Nielsen et al. (2009), who used RAGE antibodies to block the receptor in type 2 diabetic mice. In the RAGE-blocked animals, systolic function was preserved and left ventricle chamber stiffness was markedly lower compared to diabetic animals in the control group that did not receive the RAGE-blocking antibody. These studies suggest that therapeutic targeting of the AGE-RAGE interaction may provide a new tool to manage cardiovascular complications associated with diabetes.
Diabetes and Cardiovascular Function
Diabetes has long been associated with increased incidence of cardiovascular diseases including hypertension, atherosclerosis, and coronary heart disease (Tahiliani & McNeill, 1986; Candido et al., 2003). Historically, it was thought that diabetes-associated changes in cardiac structure and function were dependent on accelerated atherosclerosis and hypertension seen in diabetic patients (Poornima et al., 2006). Though still somewhat controversial, the idea that diabetes itself can lead to dysfunction of the heart, a pathology termed diabetic cardiomyopathy (Rubler et al., 1972), in the absence of other cardiovascular complications is beginning to be widely accepted. Despite the appreciation for the significance of diabetes on cardiovascular health, the full ramifications of this disease as well as the mechanisms of its pathogenesis are not fully elucidated. In fact, a number of discrepancies exist in the literature regarding the temporal progression of this disease. Some of the discrepancies noted in the pathological changes that are associated with diabetes are likely due to the different animal models utilized and the stages of diabetes examined. Indeed, there are a number of rodent models, including genetic (db/db leptin receptor deficient mice; ob/ob leptin deficient mice; Zucker diabetic rat) and toxin induced (streptozotoxin and alloxan), which recapitulate aspects of diabetes observed in the human population. The specific characteristics of these models and their phenotypes have been recently reviewed elsewhere (Poornima et al., 2006). It is also clear that diabetes-induced myocardial remodeling and pathogenesis are multifactorial and complex. Despite differences described in various studies, fibrosis, myocardial wall stiffness, and diastolic dysfunction have emerged as consistent and rather early characteristics of the diabetic heart.
Type I diabetes, often called juvenile onset diabetes, results from the autoimmune destruction of pancreatic β cells resulting in a lack of insulin production and elevated glucose levels. Type I diabetes is associated with up to a tenfold increase in microvascular disease (Retnakaran & Zinman, 2008). Despite this, a direct relationship between type I diabetes and disease of the heart and macrovasculature has been difficult to establish. The Wisconsin Epidemiological Study of Diabetic Retinopathy illustrated that elevated levels of protein glycosylation seen in diabetic individuals was only weakly correlated with increased susceptibility to myocardial infarction (Klein et al., 2004). In another epidemiological study, hyperglycemia was not significantly associated with coronary artery disease (Orchard et al., 2003). However, many of the epidemiological studies with type I diabetic patients are complicated by the fact that many of the patients are young in terms of typical cardiovascular disease onset. Several studies have indicated that atherosclerosis has an earlier onset in children with type I diabetes (Järvisalo et al., 2001; Dahl-Jørgensen et al., 2005). These individuals have increased tunica intima to tunica media ratios and decreased vascular flow velocities, indicators of early artery dysfunction. Long-term studies (Diabetes Control and Complication Trial) of type I diabetes patients illustrated that intensive insulin therapy yielded a 42% reduction in any cardiovascular events compared to patients on standard insulin therapy and a 52% reduction in nonfatal myocardial infarction, stroke or death due to a cardiovascular event (Nathan et al., 2005).
Treatment of rats with toxins of pancreatic islet cells including streptozotocin (STZ) and alloxan have been widely used as models of type I diabetes. These models rapidly develop profound hyperglycemia and modest elevation of triglycerides. Due to the effects of the toxins, these animals have reduced plasma insulin levels and have been particularly useful for examining the effects of elevated glucose in the absence of hyperinsulinemia. Studies in these models generally report induction of myocyte atrophy and death as opposed to myocyte hypertrophy seen in some other models (Dhalla et al., 1985; Depre et al., 2000). The STZ rat model also has altered myocardial mechanical properties, which correlates to prolongation of ventricular contraction and relaxation (Depre et al., 2000; Scognamiglio et al., 2004). Other studies have illustrated a correlation between increased myocardial stiffness and impaired ventricular filling in the STZ model (Vadlamudi &McNeill, 1983; Jackson et al., 1985).
Most studies in diabetic patients or in animal models of type I diabetes have illustrated that diastolic dysfunction precedes systolic alterations. Within 7 days in the STZ model, evidence of diastolic dysfunction, including increased left ventricular end-diastolic pressure and chamber stiffness, is seen (Vadlamudi & McNeill, 1983; Jackson et al., 1985). Significant decline in left ventricular systolic pressure and left ventricular dP/dt max are not seen until several weeks later. Though the literature is contradictory, these changes in systolic function often appear to occur in the absence of myocardial hypertrophy or extensive fibrosis (Poornima et al., 2006). Administration of insulin partially attenuates the systolic abnormalities, including restoration of contractile protein function (Malhotra et al., 1981).
In type 2 diabetic patients, diastolic dysfunction again appears to precede systolic dysfunction and the development of heart failure (Poirier et al., 2001; Picano, 2003). The cardiac manifestations of type 2 diabetes vary considerably depending on the duration of the disease and the degree of hyperglycemia and hyperinsulinemia. Many of the animal models of type 2 diabetes are associated with the genetic perturbation of leptin and its receptors. The leptin receptor deficient mouse (db−/db−) has been widely used as a model of type 2 diabetes as it presents with a robust systolic and diastolic dysfunction (Aasum et al., 2003; van den Bergh et al., 2006; Nielsen et al., 2009). Characteristics of this genetic model include obesity, insulin resistance, and subsequently compensatory hyperinsulinemia, as well as elevated triglyceride and glucose levels. Elevated blood glucose levels are apparent as early as 8 weeks of age in the db−/db− mice (Nielsen et al., 2009). Measures of left ventricular contractility including ejection fraction are decreased by 12 weeks of age and progressively worsen in this model (Nielsen et al., 2009). Indices of diastolic function are reduced by 16 weeks of age as the mice begin to develop a dilated phenotype. Myocardial hypertrophy becomes apparent in these animals after 3 months of age (Belke et al., 2001; Barouch et al., 2003). Systolic function appears to be preserved in these mice through 6 months of age (Barouch et al., 2003), at least when examined in vivo. Some differences have been noted in these mice when isolated working heart preparations are used for the analyses (Belke et al., 2001), including earlier onset of systolic dysfunction. This is likely due to metabolic substrate limitations in the working heart models (Poornima et al., 2006) and not real physiological differences.
Cellular Mechanisms of Diabetes-Induced Myocardial Dysfunction
Many cellular changes have been described in the diabetic heart that contribute to depressed myocardial function. Most of the work to date has focused on the effects on cardiomyocytes as the contractile cells of the heart. Multiple diabetes-associated metabolic changes have been described including hyperlipidemia or the increase of triglycerides and nonessential fatty acids (NEFAs). Increased NEFAs can alter myocardial contractility through multiple molecular mechanisms (Shulman, 2000). Accumulation of NEFAs can lead to altered cellular insulin signaling and insulin resistance via activation of atypical Protein Kinase C isoforms (Kim et al., 2001). At least in skeletal muscle cells and adipocytes, NEFAs can also inactivate Akt signaling by up-regulation of the phosphatase PTEN (phosphatase and tensin homolog; Lawlor & Alessi, 2001). Accumulation of NEFAs also appears to activate caspase 3 leading to cardiomyocyte apoptosis (Halse et al., 2001).
It is clear that elevated glucose levels have direct effects on cardiac myocytes, including the generation of reactive oxygen species, glucose oxidation, and the production of superoxide, which lead to myocyte stress and death. As discussed above, diabetes also results in activation of ECM-producing cells and subsequently tissue fibrosis. Cardiac function is inherently dependent on the biophysical properties of the myocardium, which are at least partly due to the ECM. Cardiac fibrosis associated with a number of disorders including hypertension, aortic stenosis, and myocardial infarction results in increased myocardial stiffness. This in turn alters ventricular relaxation and contributes to diastolic dysfunction in a number of disorders. Accumulation of AGEs in ECM proteins such as collagen further exacerbates the mechanical changes of the myocardium and has been shown to decrease myocardial compliance (Norton et al., 1996; Aronson, 2003; van Heerebeek et al., 2008). In addition, accumulation of intracellular AGEs contributes to decreased calcium ion transport and reduced myocyte contractility (Bidasee et al., 2003). Treatment of STZ-treated rats with aminoguanidine, which prevents AGE formation, results in decreased AGE-modified collagen in the heart and diabetes-induced myocardial stiffness (Norton et al., 1996). Studies with ALT-711, which prevents glucose-derived crosslinks in collagen and other proteins, illustrated a significant reduction in myocardial collagen content and enhanced collagen solubility (Candido et al., 2003). These studies clearly illustrate that one mechanism of diabetes-induced diastolic dysfunction is through altered ECM deposition and cross-linking, which in turn alter myocardial compliance.
Summary
Despite having profound cardiovascular consequences, many questions remain regarding the mechanisms of diabetes-induced myocardial remodeling (Table 2). Addressing these questions is critical as the incidence of diabetes is rapidly on the rise. While diabetes and the biochemical changes in the body that occur during this disease (hyperglycemia, hyperinsulinemia, hyperlipidemia) have documented impacts on cardiac myocyte function, major functional consequences of diabetes in the heart are the result of changes that occur in the myocardial ECM including increased ECM deposition and glucose-induced modification of ECM components. Fibrosis is likely promoted by elevated glucose in several ways including: (1) the direct effects of elevated glucose on fibroblast gene expression and activation, (2) indirectly through the consequences of cardiomyocyte stress and death, and (3) the stimulation of the fibroblast AGE receptor (RAGE) by AGE-modified ECM. There are likely other mechanisms of diabetes-induced myocardial damage and remodeling as diabetes is a very complex process that affects many organs simultaneously. It is imperative that the mechanisms of diabetes-induced fibrosis be identified such that novel therapeutic approaches can be developed.
Table 2.
Key Questions to Understanding the Role of the ECM in Diabetic Cardiomyopathy.
| Does AGE modification of the cardiac ECM affect collagen turnover, and does this contribute to structural changes in myocardium? |
| What is the mechanism through which elevated glucose levels trigger an increase in collagen production by the cardiac fibroblasts? |
| Based on the crystal structure and known binding information for the AGE receptor (RAGE), can RAGE-AGE specific inhibitors be designed that block this interaction but not the binding of RAGE to other ligands? |
| How might intercellular communication between myocytes and fibroblasts result in increased fibrosis due to myocyte response to hyperglycemia? |
| Does AGE modification of collagen alter myocyte-ECM interactions contributing to increased myocyte apoptosis and necrosis observed in the diabetic heart? |
| What is the role of the myofibroblast in matrix remodeling in the diabetic heart? |
| What is the temporal course of AGE-collagen modification in the heart, and is there an ideal “therapeutic window” for administration of blocking agents that could prevent/delay onset of diabetic cardiomyopathy? |
Key to developing new therapeutic strategies for controlling myocardial fibrosis observed in diabetes will be in-creased understanding of the role of the myofibroblast in diabetic cardiovascular disease. Identification of the mechanism(s) responsible for activation of fibroblasts and their transition into myofibroblasts would provide for an early intervention, likely to prevent much of the fibrosis associated with this cellular phenotype. Inhibiting the response of fibroblast/myofibroblast to AGE modifications within the matrix may also provide potential targets. Studies in diabetic mouse models have demonstrated improved cardiac function following treatment with AGE-RAGE interaction blockers (either antibodies or soluble forms of the receptor), but as RAGE binds a number of different ligands, it is unclear what other effects such approaches may have. Recently, the RAGE crystal structure and identification of ligand binding sites were published; using this information it may be possible to design an inhibitor that specifically targets the AGE-RAGE interaction permitting further regulation of cellular response to diabetes associated changes within the ECM.
Acknowledgments
The authors of this review article were supported by National Institutes of Health grants HL083441 (W.C.) and HL097214 (E.C.G.).
References
- Aasum E, Hafstad AD, Severson DL, Larsen TS. Age-dependent changes in metabolism, contractile function and ischemic sensitivity in hearts from db/db mice. Diabetes. 2003;52:434–441. doi: 10.2337/diabetes.52.2.434. [DOI] [PubMed] [Google Scholar]
- Abbate M, Zoja C, Remuzzi G. How does proteinuria cause progressive renal damage? J Am Soc Nephrol. 2006;17:2974–2984. doi: 10.1681/ASN.2006040377. [DOI] [PubMed] [Google Scholar]
- Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- Aneja A, Tang WHW, Bansilal S, Garcis MJ, Farkouh ME. Diabetic cardiomyopathy: Insights into pathogenesis, diagnostic challenges and therapeutic options. Amer J Med. 2008;121:748–757. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- Ares-Carrasco A, Picatoste B, Benito-Martin A, Zubiri I, Sanz AB, Sanchez-Nino MD, Ortiz A, Egido J, Tunon J, Lorenzo O. Myocardial fibrosis and apoptosis, but not inflammation, are present in long-term experimental diabetes. Am J Physiol Heart Circ Physiol. 2009;297:H2109–H2119. doi: 10.1152/ajpheart.00157.2009. [DOI] [PubMed] [Google Scholar]
- Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J Hypertens. 2003;21:3–12. doi: 10.1097/00004872-200301000-00002. [DOI] [PubMed] [Google Scholar]
- Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- Barouch LA, Berkowitz DE, Harrison RW, O’Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- Basta G, Lazzerini G, Massaro M, Simoncini T, Tanganelli P, Fu C, Kislinger T, Stern DM, Schmidt AM, DeCaterina R. Advanced glycation end products activate endothelium through signal-transduction receptor RAGE: A mechanism for amplification of inflammatory responses. Circulation. 2002;105:816–822. doi: 10.1161/hc0702.104183. [DOI] [PubMed] [Google Scholar]
- Baynes JW. The role of AGEs in aging: Causation or correlation. Exp Gerontol. 2001;36:1527–1537. doi: 10.1016/s0531-5565(01)00138-3. [DOI] [PubMed] [Google Scholar]
- Belke DD, Larsen TS, Severson DL. Cardiac function in perfused hearts from diabetic mice. Adv Exp Med Biol. 2001;498:241–245. doi: 10.1007/978-1-4615-1321-6_30. [DOI] [PubMed] [Google Scholar]
- Bidasee KR, Nallani K, Henry B, Dincer UD, Besch HR., Jr Chronic diabetes alters function and expression of ryanodine receptor calcium-release channels in rat hearts. Mol Cell Biochem. 2003;249:113–123. [PubMed] [Google Scholar]
- Biemel KM, Friedl DA, Lederer MO. Identification and quantification of major maillard cross-links in human serum albumin and lens protein. J Biol Chem. 2002;277:24907–24915. doi: 10.1074/jbc.M202681200. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Humpert PM, Morcos M, Wendt T, Chavakis T, Arnold B, Stern DM, Nawroth PP. Understanding RAGE, the receptor for advanced glycation end products. J MolMed. 2006;83:876–886. doi: 10.1007/s00109-005-0688-7. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: A new therapeutic target? Curr Opin Invest Drugs. 2005;7:985–991. [PubMed] [Google Scholar]
- Bishop JE, Laurent GJ. Collagen turnover and its regulation in the normal and hypertrophying heart. Eur Heart J. 1995;16:38–44. doi: 10.1093/eurheartj/16.suppl_c.38. [DOI] [PubMed] [Google Scholar]
- Bochaton-Piallat ML, Kapetanios AD, Donati G, Redard M, Gabbiani G, Pournaras CJ. TGF-b1, TGF-b receptor II and ED-A fibronectin expression in myofibroblast of vitreoretinopathy. Invest Ophthalmol Vis Sci. 2000;41:2336–2342. [PubMed] [Google Scholar]
- Booth AA, Khalifah RG, Todd P, Hudson BG. In vitro kinetic studies of formation of antigenic advanced glycation end products (AGEs) J Biol Chem. 1997;272:5430–5437. doi: 10.1074/jbc.272.9.5430. [DOI] [PubMed] [Google Scholar]
- Boudina S, Abel ED. Diabetic cardiomyopathy: Causes and effects. Rev Endocr Metab Disord. 2010;11:31–39. doi: 10.1007/s11154-010-9131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouguerra S, Benazzoug Y, Bekkhoucha F, Bourdillon MC. Effect of high glucose concentration on collagen synthesis and cholesterol level in the phenotypic modulation of aortic cultured smooth muscle cells of sand rat (Psammomys obesus) Experimental Diab Res. 2004;5:227–235. doi: 10.1080/15438600490489793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brem H, Tomic-Canic M. Cellular and molecular basis of wound healing in diabetes. J Clin Invest. 2007;117:1219–1222. doi: 10.1172/JCI32169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RD, Ambler SK, Mitchell MD, Long CS. The cardiac fibroblast: Therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. doi: 10.1146/annurev.pharmtox.45.120403.095802. [DOI] [PubMed] [Google Scholar]
- Brownlee M. Advanced protein glycosylation in diabetes and aging. Ann Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- Brownlee M, Vlassara H, Cerami A. Nonenzymatic glycosylation products on collagen covalently trap low-density lipoprotein. Diabetes. 1985;34:938–941. doi: 10.2337/diab.34.9.938. [DOI] [PubMed] [Google Scholar]
- Candido R, Forbes JM, Thomas MC, Thallas V, Dean RG, Burns WC, Tikellis C, Ritchie RH, Twigg SM, Cooper ME, Burrell LM. A breaker of advanced glycation end products attenuates diabetes-induced myocardial structural changes. Circ Res. 2003;92:785–792. doi: 10.1161/01.RES.0000065620.39919.20. [DOI] [PubMed] [Google Scholar]
- Cassiman D, Libbrecht L, Desmet V, Denef C, Roskams T. Hepatic stellate cell/myofibroblast subpopulations in fibrotic human and rat livers. J Hepatol. 2002;36:200–209. doi: 10.1016/s0168-8278(01)00260-4. [DOI] [PubMed] [Google Scholar]
- Caulfield JB, Borg TK. The collagen network of the heart. Lab Invest. 1979;40:364–372. [PubMed] [Google Scholar]
- Chang HY, Chi JT, Dudoit S, Bondre C, van de Rijn M, Botstein D, Brown PO. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci USA. 2002;99:12877–12882. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl-Jørgensen K, Larsen JR, Hanssen KF. Atherosclerosis in childhood and adolescent type 1 diabetes: Early disease, early treatment? Diabetologia. 2005;48:1445–1453. doi: 10.1007/s00125-005-1832-1. [DOI] [PubMed] [Google Scholar]
- Dai Z, Wang B, Sun G, Xingjun F, Anderson VE, Monnier VM. Identification of glucose-derived cross-linking sites in ribonuclease A. J Proteome Res. 2008;7:2756–2768. doi: 10.1021/pr700874a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla Vestra M, Saller A, Bortoloso E, Mauer M, Floretto P. Structural involvement in type I and type 2 diabetic nephropathy. Diabetes Metab. 2000;4:8–14. [PubMed] [Google Scholar]
- Darby IA, Bisucci T, Hewitson TD, MacLellan DG. Apoptosis is increased in a model of diabetes-impaired wound healing in genetically diabetic mice. Int J Biochem Cell Biol. 1997;29:191–200. doi: 10.1016/s1357-2725(96)00131-8. [DOI] [PubMed] [Google Scholar]
- Depre C, Young ME, Ying J, Ahuja HS, Han Q, Garza N, Davies PJ, Taegtmeyer H. Streptozotocininduced changes in cardiac gene expression in the absence of severe contractile dysfunction. J Mol Cell Cardiol. 2000;32:985–996. doi: 10.1006/jmcc.2000.1139. [DOI] [PubMed] [Google Scholar]
- Desmoulière A, Chaponnier C, Gabbiani G. Tissue repair, contraction, and the myofibroblast. Wound Repair Regen. 2005;13:7–12. doi: 10.1111/j.1067-1927.2005.130102.x. [DOI] [PubMed] [Google Scholar]
- Dhalla NS, Pierce GN, Innes IR, Beamish RE. Pathogenesis of cardiac dysfunction in diabetes mellitus. Can J Cardiol. 1985;1:263–281. [PubMed] [Google Scholar]
- Dobler D, Ahmed N, Song L, Eboigbodin KE, Thornalley PJ. Increased dicarbonyl metabolism in endothelial cells in hyperglycemia induces anoikis and impairs angiogenesis by RGD and GFOGER motif modification. Diabetes. 2006;55:1961–1969. doi: 10.2337/db05-1634. [DOI] [PubMed] [Google Scholar]
- Du YS, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashisth T, Chen X, Godman GC, Stern D, Schmidt AM. Amyloid-beta peptide-receptor for advanced glycation endproduct interaction elicits neuronal expression of macrophage-colony stimulating factor: A proinflammatory pathway in Alzheimer’s disease. Proc Natl Acad Sci USA. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn JA, McCance DR, Thorpe SR, Lyons TJ, Baynes JW. Age-dependent accumulation of Nε-(carboxymethyl) hydroxylysine in human skin collagen. Biochemistry. 1991;30:1205–1210. doi: 10.1021/bi00219a007. [DOI] [PubMed] [Google Scholar]
- Dunn JA, Patrick JS, Thorpe SR, Baynes JW. Oxidation of glycated proteins: Age-dependent accumulation of Nε-(carboxymethyl)lysine in len proteins. Biochemistry. 1989;28:9644–9648. doi: 10.1021/bi00450a033. [DOI] [PubMed] [Google Scholar]
- Eghbali M, Weber KT. Collagen and the myocardium: Fibrillar structure, biosynthesis and degradation in relation to hypertrophy and its regression. Mol Cell Biochem. 1990;96:1–14. doi: 10.1007/BF00228448. [DOI] [PubMed] [Google Scholar]
- Falk RJ, Scheinman JI, Mauer SM, Michael AF. Polyantigenic expansion of basement membrane constituents in diabetic nephropathy. Diabetes. 1983;32:34–39. doi: 10.2337/diab.32.2.s34. [DOI] [PubMed] [Google Scholar]
- Fathima NN, Madhan B, Rao JR, Nair BN, Ramasami T. Interaction of aAldehydes with collagen: Effect on thermal, enzymatic, and conformational stability. Int J Biol Macromol. 2004;34:241–247. doi: 10.1016/j.ijbiomac.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ferreira AEN, Ponces Freire AMJ, Voit EO. A quantitative model of the generation of Nε-(carboxylmethyl) lysine in the maillard reaction between collagen and glucose. Biochem J. 2003;376:109–121. doi: 10.1042/BJ20030496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey EB, Degenhardt TP, Thorpe SR, Baynes JW. Role of the Maillard reaction in aging of tissue proteins. J Biol Chem. 1998;273:18714–18719. doi: 10.1074/jbc.273.30.18714. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, Anversa P. Myocardial cell death in human diabetes. Circ Res. 2000;87:1123–1132. doi: 10.1161/01.res.87.12.1123. [DOI] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gao X, He X, Luo B, Peng L, Lin J, Zuo Z. Angiotensin II increases collagen I expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur J Pharmacol. 2009;606:115–120. doi: 10.1016/j.ejphar.2008.12.049. [DOI] [PubMed] [Google Scholar]
- Gilbert RE, Cooper ME. The tubulointerstitium in progressive diabetic kidney disease: More than an aftermath of glomerular injury? Kidney Int. 1999;56:1627–1637. doi: 10.1046/j.1523-1755.1999.00721.x. [DOI] [PubMed] [Google Scholar]
- Goh SY, Cooper ME. The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93:1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Gratzer PF, Santerre JP, Lee JM. The effect of chemical modification of amino acid side-chains on collagen degradation by enzymes. J Biomed Mater Res Part B. 2006;81B:1–11. doi: 10.1002/jbm.b.30629. [DOI] [PubMed] [Google Scholar]
- Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235–1246. [PMC free article] [PubMed] [Google Scholar]
- Gress TM, Menke A, Bachem M, Müller-Pillasch F, Ellenrieder V, Weidenbach H, Wagner M, Adler G. Role of extracellular matrix in pancreatic diseases. Digestion. 1998;59:625–637. doi: 10.1159/000007567. [DOI] [PubMed] [Google Scholar]
- Halse R, Pearson SL, McCormack JG, Yeaman SJ, Taylor R. Effects of tumor necrosis factor-alpha on insulin action in cultured human muscle cells. Diabetes. 2001;50:1102–1109. doi: 10.2337/diabetes.50.5.1102. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Araki N, Koh N, Horiuchi S, Hotta N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem Biophys Res Commun. 1996;228:539–543. doi: 10.1006/bbrc.1996.1695. [DOI] [PubMed] [Google Scholar]
- Hartog JW, Voors AA, Bakker SJ, Smit AJ, van Veldhuisen DJ. Advance glycation end-products (AGEs) and heart failure: Pathophysiology and clinical implications. Eur J Heart Fail. 2007;9:1146–1155. doi: 10.1016/j.ejheart.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Hayden MR, Patel K, Habibi J, Gupta D, Tekwani S, Whaley-Connell A, Sowers J. Attenuation of endocrine-exocrine pancreatic communication in type 2 diabetes: Pancreatic extracellular matrix ultrastructural abnormalities. J Cardiometab Syndr. 2008;4:234–243. doi: 10.1111/j.1559-4572.2008.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heickendorff L, Ledet T, Rasmussen LM. Glycosaminoglycans in the human aorta in diabetes-mellitus—A study of tunica media from areas with and without atherosclerotic plaque. Diabetologia. 1994;37:286–292. doi: 10.1007/BF00398056. [DOI] [PubMed] [Google Scholar]
- Herrmann KL, McCulloch AD, Omens JH. Glycated collagen cross-linking alters cardiac mechanics in volume-overload hypertrophy. Am J Physiol Heart Circ Physiol. 2003;284:H1277–H1284. doi: 10.1152/ajpheart.00168.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinz B. Formation and function of the myofibroblast during tissue repair. J Invest Dermatol. 2007;127:526–537. doi: 10.1038/sj.jid.5700613. [DOI] [PubMed] [Google Scholar]
- Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: One function, multiple origins. Am J Pathol. 2007;170:1807–1816. doi: 10.2353/ajpath.2007.070112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann MA, Drury S, Fu C, Wu Q, Taguchi A, Lu Y, Avila C, Kambham N, Slattery T, Beach D, McClary J, Nagashima M, Morser J, Bierhaus A, Neurath M, Nawroth P, Stern D, Schmidt AM. RAGE mediates a novel proinflammatory axis: The cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97:889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- Ikeda S, Makino H, Haramoto T, Shikata K, Kumagai I, Ota Z. Changes in glomerular extracellular matrices components in diabetic nephropathy. J Diabetes Complicat. 1991;5:186–188. doi: 10.1016/0891-6632(91)90068-z. [DOI] [PubMed] [Google Scholar]
- Jackson CV, McGrath GM, Tahiliani AG, Vadlamudi RV, McNeill JH. A functional and ultrastructural analysis of experimental diabetic rat myocardium. Manifestation of a cardiomyopathy. Diabetes. 1985;34:876–883. doi: 10.2337/diab.34.9.876. [DOI] [PubMed] [Google Scholar]
- Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geitman EM, Sobel BE. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J. 1984;108:31–37. doi: 10.1016/0002-8703(84)90541-6. [DOI] [PubMed] [Google Scholar]
- Järvisalo MJ, Jartti L, Näntö-Salonen K, Irjala K, Rönnemaa T, Hartiala JJ, Celermajer DS, Raitakari OT. Increased aortic intima-media thickness: A marker of preclinical atherosclerosis in high-risk children. Circulation. 2001;104:2943–2947. doi: 10.1161/hc4901.100522. [DOI] [PubMed] [Google Scholar]
- Jefferson JA, Shankland SJ, Pichler RH. Protein-uria in diabetic kidney disease: A mechanistic viewpoint. Kidney Int. 2008;74:22–36. doi: 10.1038/ki.2008.128. [DOI] [PubMed] [Google Scholar]
- Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiryu K, Morita H, Fujita Y, Kawasumi M, Shinzato T, Tsuruta Y, Nakai S, Maeda K. Phenotypic expressions of type I III, IV, V and VI collagens in patients with diabetic nephropathy: Immunohistochemical comparison between HD and non-HD patients. Nippon Jinzo Gakkai Shi. 1994;36:365–373. [PubMed] [Google Scholar]
- Klein BE, Klein R, McBride PE, Cruickshanks KJ, Palta M, Knudtson MD, Moss SE, Reinke JO. Cardiovascular disease, mortality and retinal microvascular characteristics in type 1 diabetes: Wisconsin epidemiological study of diabetic retinopathy. Arch Intern Med. 2004;164:1917–1924. doi: 10.1001/archinte.164.17.1917. [DOI] [PubMed] [Google Scholar]
- Ko SH, Hong OK, Kim JW, Ahn YB, Song KH, Cha BY, Son HY, Kim MJ, Jeong IK, Yoon KH. High glucose increases extracellular matrix production in pancreatic stellate cells by activating the renin-angiotensin system. J Cell Biochem. 2006;98:343–355. doi: 10.1002/jcb.20797. [DOI] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- Kwan CY, Wang RRJ, Beazley JS, Lee RMKW. Alterations of elastin and elastase-like activities in aortae of diabetic rats. Biochim Biophys Acta. 1988;967:322–325. doi: 10.1016/0304-4165(88)90027-x. [DOI] [PubMed] [Google Scholar]
- Lastra G, Manrique C. The expanding role of oxidative stress, rennin angiotensin system, and β-cell dysfunction in the cardiometabolic syndrome and type 2 diabetes mellitus. Antioxid Redox Sign. 2007;7:943–954. doi: 10.1089/ars.2007.1615. [DOI] [PubMed] [Google Scholar]
- Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114:2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- Leask A. Targeting the TGFbeta, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. Cell Signal. 2008;20:1409–1414. doi: 10.1016/j.cellsig.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- Lehto S, Pyorala K, Miettinen H, Ronnemaa T, Palomaki P, Tuomilehto J, Laakso M. Myocardial infarct size and mortality in patients with non-insulin-dependent diabetes mellitus. J Intern Med. 1994;236:291–297. doi: 10.1111/j.1365-2796.1994.tb00799.x. [DOI] [PubMed] [Google Scholar]
- Levinthal G, Tavill AS. Liver disease and diabetes mellitus. Clin Diabetes. 1999;17:73–93. [Google Scholar]
- Li JH, Wang W, Huang XR, Oldfield M, Schmidt AM, Cooper ME, Lan HY. Advanced glycation end products induce tubular epithelial-myofibroblast transition through the RAGE-ERK1/2 MAP kinase signaling pathway. Am J Pathol. 2004;164:1389–1397. doi: 10.1016/S0002-9440(10)63225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Penpargkul S, Fein FS, Sonnenblick EH, Scheuer J. The effect of streptozotocin-induced diabetes in rats on cardiac contractile proteins. Circ Res. 1981;49:1243–1250. doi: 10.1161/01.res.49.6.1243. [DOI] [PubMed] [Google Scholar]
- Mason RM, Wahab NA. Extracellular matrix metabolism in diabetic nephropathy. J Am Soc Nephrol. 2003;14:1358–1373. doi: 10.1097/01.asn.0000065640.77499.d7. [DOI] [PubMed] [Google Scholar]
- McDonald TO, Gerrity RG, Jen C, Chen HJ, Wark K, Wight TN. Diabetes and arterial extracellular matrix changes in a porcine model of atherosclerosis. J Histochem Cytochem. 2007;55:1149–1157. doi: 10.1369/jhc.7A7221.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazato J, Horio T, Takiuchi S, Kamide K, Sasaki O, Nakamura S, Nakahama H, Inenaga T, Takishita S, Kawano Y. Left ventricular diastolic dysfunction in patients with chronic renal failure: Impact of diabetes mellitus. Diabetes Med. 2005;22:730–736. doi: 10.1111/j.1464-5491.2005.01500.x. [DOI] [PubMed] [Google Scholar]
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. Diabetes control and complications trial/epidemiology of diabetes interventions and complications (DCCT/EDIC) study research group intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med. 2005;353:2643–2653. doi: 10.1056/NEJMoa052187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naugle JE, Olson ER, Zhang X, Mase SE, Pilati CF, Maron MB, Folkesson HG, Horne WI, Doane KJ, Meszaros JG. Type VI collagen induces cardiac myofibroblast differentiation: Implications for postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2006;290:H323–H330. doi: 10.1152/ajpheart.00321.2005. [DOI] [PubMed] [Google Scholar]
- Neumann S, Huse K, Semrau R, Diegeler A, Gebhardt R, Buniatian GH, Scholz GH. Aldosterone and D-glucose stimulate the proliferation of human cardiac myofibroblasts in vitro. Hypertension. 2002;39:756–760. doi: 10.1161/hy0302.105295. [DOI] [PubMed] [Google Scholar]
- Nguyen TQ, Chon H, van Nieuwenhoven FA, Braam B, Verhaar MC, Goldschmeding R. Myofibroblast progenitor cells are increased in number in patients with type 1 diabetes and express less bone morphogenetic protein 6: A novel clue to adverse tissue remodelling? Diabetologia. 2006;49:1039–1048. doi: 10.1007/s00125-006-0172-0. [DOI] [PubMed] [Google Scholar]
- Nichols GA, Guillon CM, Koro CE, Ephross SA, Brown JB. The incidence of congestive heart failure in type 2 diabetes: An update. Diabetes Care. 2004;27:1879–1884. doi: 10.2337/diacare.27.8.1879. [DOI] [PubMed] [Google Scholar]
- Nielsen JM, Kristiansen SB, Norregaard R, Andersen CL, Denner L, Nielsen TT, Flyvbjerg A, Botker HE. Blockage of receptor for advanced glycation end products prevents development of cardiac dysfunction in db/db type 2 diabetic mice. Eur J Heart Fail. 2009;11:638–647. doi: 10.1093/eurjhf/hfp070. [DOI] [PubMed] [Google Scholar]
- Nishida M, Onohara N, Sato Y, Suda R, Ogushi M, Tanabe S, Inoue R, Mori Y, Kurose H. Galpha12/ 13-mediated up-regulation of TRPC6 negatively regulates endothelin-1-induced cardiac myofibroblast formation and collagen synthesis through nuclear factor of activated T cells activation. J Biol Chem. 2007;282:23117–23128. doi: 10.1074/jbc.M611780200. [DOI] [PubMed] [Google Scholar]
- Norton GR, Candy G, Woodiwiss AJ. Aminoguanidine prevents the decreased myocardial compliance produced by streptozotocin-induced diabetes mellitus in rats. Circulation. 1996;93:1905–1912. doi: 10.1161/01.cir.93.10.1905. [DOI] [PubMed] [Google Scholar]
- Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, Atkins RC, Osicka T, Jerums G, Cooper ME. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orchard TJ, Olson JC, Erbey JR, Williams K, Forrest KY, Smithline Kinder L, Ellis D, Becker DJ. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care. 2003;26:1374–1379. doi: 10.2337/diacare.26.5.1374. [DOI] [PubMed] [Google Scholar]
- Osterby R, Tapia J, Nyberg G, Tencer J, Willner J, Rippe B, Torffvit O. Renal structures in type 2 diabetic patients with elevated albumin excretion rate. APMIS. 2001;109:751–761. doi: 10.1034/j.1600-0463.2001.d01-142.x. [DOI] [PubMed] [Google Scholar]
- Picano E. Diabetic cardiomyopathy. The importance of being earliest. J Am Coll Cardiol. 2003;42:454–457. doi: 10.1016/s0735-1097(03)00647-8. [DOI] [PubMed] [Google Scholar]
- Poirier P, Bogaty P, Garneau C, Marois L, Dumesnil JG. Diastolic dysfunction in normotensive men with well-controlled type 2 diabetes: Importance of maneuvers in echocardiographic screeing for preclinical diabetic cardiomyopathy. Diabetes Care. 2001;24:5–10. doi: 10.2337/diacare.24.1.5. [DOI] [PubMed] [Google Scholar]
- Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy, the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Regan TJ, Wu CF, Yeh C. Myocardial composition and function in diabetes—The effects of chronic insulin use. Circ Res. 1981;49:1268–1277. doi: 10.1161/01.res.49.6.1268. [DOI] [PubMed] [Google Scholar]
- Retnakaran R, Zinman B. Type 1 diabetes, hyperglycaemia, and the heart. Lancet. 2008;371:1790–1799. doi: 10.1016/S0140-6736(08)60767-9. [DOI] [PubMed] [Google Scholar]
- Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A .New type of cardiomyopathy associatedwith diabetic glomerulosclerosis. AmJ Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- Schäfer S, Huber J, Wihler C, Rütten H, Busch AE, Linz W. Impaired left ventricular relaxation in type 2 diabetic rats is related to myocardial accumulation of N(epsilon)-(carboxymethyl) lysine. Eur J Heart Fail. 2006;8:2–6. doi: 10.1016/j.ejheart.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Scognamiglio R, Avogaro A, Negut C, Piccolotto R, Vigili de Kreutzenberg S, Tiengo A. Early myocardial dysfunction in the diabetic heart: Current research and clinical applications. Am J Cardiol. 2004;93:17A–20A. doi: 10.1016/j.amjcard.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, Monnier VM. Glucosepane is a major protein cross-link of the senescent human extracellular matrix: Relationship with diabetes. J Biol Chem. 2005;280:12310–12315. doi: 10.1074/jbc.M500733200. [DOI] [PubMed] [Google Scholar]
- Sell DR, Lane MA, Johnson WA, Masoro EJ, Mock OB, Reiser KM, Fogarty JF, Cutler RG, Ingram DK, Roth GS, Monnier VM. Longevity and the genetic determination of collagen glycoxidation kinetics in mammalian senescence. Proc Natl Acad Sci USA. 1996;93:485–490. doi: 10.1073/pnas.93.1.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seneviratne B. Diabetic cardiomyopathy: The preclinical phase. Br Med J. 1977;1:1444–1446. doi: 10.1136/bmj.1.6074.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamhart PE, Luther DJ, Hodson BR, Koshy JC, Ohanyan V, Meszaros JG. Impact of type 1 diabetes on cardiac fibroblast activation enchanced cell cycle progression and reduced myofibroblast content in diabetic myocardium. Am J Physiol Endocrinol Metab. 2009;297:E1147–E1153. doi: 10.1152/ajpendo.00327.2009. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Howat A, Calter M. Left ventricular function in diabetes mellitus—I. Methodology and prevalence and spectrum of abnormalities. Br Heart J. 1981a;45:122–128. doi: 10.1136/hrt.45.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro L, Leatherdale B, MacKinnon J. Left ventricular function in diabetes mellitus—II.Relation between clinical features and left ventricular function. BrHeart J. 1981b;45:129–132. doi: 10.1136/hrt.45.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shehadeh A, Regan TJ. Cardiac consequences of diabetes mellitus. Clin Cardiol. 1995;18:301–305. doi: 10.1002/clc.4960180604. [DOI] [PubMed] [Google Scholar]
- Shimizu M, Umeda K, Sugihara N. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi-Wen X, Chen Y, Denton CP, Eastwood M, Renzoni EA, Bou-Gharios G, Pearson JD, Dashwood M, du Bois RM, Black CM, Leask A, Abraham DJ. Endothelin-1 promotes myofibroblast induction through the ETA receptor via a rac/phosphoinositide 3-kinase/Akt-dependent pathway and is essential for the enhanced contractile phenotype of fibrotic fibroblasts. Mol Biol Cell. 2004;15:2707–2719. doi: 10.1091/mbc.E03-12-0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman GI. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siperstein MD, Unger RH, Madison LL. Studies of muscle capillary basement membrane in normal subjects, diabetic, and prediabetic patients. J Clin Invest. 1968;47:1973–1999. doi: 10.1172/JCI105886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AJ, Garrits EG. Skin autofluorescence as a measure of advanced glycation endproduct deposition: A novel risk marker in chronic kidney disease. Curr Opin Nephrol Hypertens. 2010;19:527–533. doi: 10.1097/MNH.0b013e32833e9259. [DOI] [PubMed] [Google Scholar]
- Song W, Ergul A. Type-2 diabetes-induced changes in vascular extracellular matrix gene expression: Relation to vessel size. Cardiovasc Diabetol. 2006;5:3. doi: 10.1186/1475-2840-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiro MJ, Crowley TJ. Increased rat myocardial type VI collagen in diabetes mellitus and hypertension. Diabetologia. 1993;36:93–98. doi: 10.1007/BF00400687. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Osterby R, Chavers B, Mauer SM. Mesangial expansion as a central mechanism for loss of kidney function in diabetic patients. Diabetes. 1989;38:1077–1081. doi: 10.2337/diab.38.9.1077. [DOI] [PubMed] [Google Scholar]
- Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: Update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- Tahiliani AG, McNeill JH. Diabetes-induced abnormalities in the myocardium. Life Sci. 1986;38:959–974. doi: 10.1016/0024-3205(86)90229-8. [DOI] [PubMed] [Google Scholar]
- Targher G. Associations between liver histology and early carotid atherosclerosis in subjects with nonalcoholic fatty liver disease. Hepatology. 2004;42:974–975. doi: 10.1002/hep.20894. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Poli F, Scala L, Tessari R, Zenari L, Falezza G. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet Med. 2005;4:403–409. doi: 10.1111/j.1464-5491.2006.01817.x. [DOI] [PubMed] [Google Scholar]
- Tarnow L, Rossing P, Gall MA, Nielsen FS, Parving HH. Prevalence of arterial hypertension in diabetic patients before and after the Jnc-V. Diabetes Care. 1994;17:1247–1251. doi: 10.2337/diacare.17.11.1247. [DOI] [PubMed] [Google Scholar]
- Tikellis C, Thomas MC, Harcourt BE, Coughman MT, Pete J, Bialkowski K, Tan A, Bierhaus A, Cooper ME, Forbes JM. Cardiac inflammation associated with a western diet is mediated via activation of RAGE by AGEs. Am J Physiol Endocrinol Metab. 2008;295:E323–E330. doi: 10.1152/ajpendo.00024.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich P, Cerami A. Protein glycation, diabetes, and aging. Rec Prog Horm Res. 2001;56:1–21. doi: 10.1210/rp.56.1.1. [DOI] [PubMed] [Google Scholar]
- Vadlamudi RV, McNeill JH. Effect of alloxan- and streptozotocin-induced diabetes on isolated heart responsiveness to carbachol. J Pharmacol Exp Ther. 1983;225:410–415. [PubMed] [Google Scholar]
- van den Bergh A, Flameng W, Herijgers P. Type II diabetic mice exhibit contractile dysfunction but maintain cardiac output by favourable loading conditions. Eur J Heart Fail. 2006;8:777–783. doi: 10.1016/j.ejheart.2006.03.001. [DOI] [PubMed] [Google Scholar]
- van Heerebeek L, Hamdani N, Handoko ML, Falcao-Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJ, Schalkwijk CG, Bronzwaer JG, Diamant M, Borbély A, van der Velden J, Stienen GJ, Laarman GJ, Niessen HW, Paulus WJ. Diastolic stiffness of the failing diabetic heart: Importance of fibrosis, advanced glycation end products and myocyte resting tension. Circulation. 2008;117:43–51. doi: 10.1161/CIRCULATIONAHA.107.728550. [DOI] [PubMed] [Google Scholar]
- van Hoeven KH, Factor SM. A comparison of the pathological spectrum of hypertensive, diabetic, and hypertensive-diabetic heart disease. Circulation. 1990;82:848–855. doi: 10.1161/01.cir.82.3.848. [DOI] [PubMed] [Google Scholar]
- Vernier RL, Steffes MW, Sisson-Ross S, Mauer SM. Heparan sulfate proteoglycans in the glomerular basement membrane in type 1 diabetes mellitus. Kidney Int. 1992;41:1070–1080. doi: 10.1038/ki.1992.163. [DOI] [PubMed] [Google Scholar]
- Verzijl N, DeGroot J, Zaken CB, Braun-Benjamin O, Maroudas A, Bank RA, Mizrahi J, Schalkwijk CG, Thorpe SR, Baynes JW, Bijlsma JWJ, Lafeber FPJG, TeKoppele JM. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage. Arthrit Rheumat. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E. Endothelial dysfunction and cardiovascular risk profile in non-alcoholic fatty liver disease. Hepatology. 2005;42:473–478. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- Volzke H, Robinson DM, Kleine V, Deutscher R, Hoffmann W, Ludemann J. Hepatic steatosis is associated with an increased risk of carotid atherosclerosis. World J Gastronterol. 2005;11:1848–1853. doi: 10.3748/wjg.v11.i12.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walshe R, Esser P, Wiedemann P, Heimann K. Proliferative retinal diseases: Myofibroblasts cause chronic vitreoretinal traction. Br J Ophthalmol. 1992;76:550–552. doi: 10.1136/bjo.76.9.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Head J, Stevens L, Fuller JH. Excess mortality and its relation to hypertension and proteinuria in diabetic patients. TheWorld Health Organization multinational study of vascular disease in diabetes. Diabetes Care. 1996;19:305–312. doi: 10.2337/diacare.19.4.305. [DOI] [PubMed] [Google Scholar]
- Yamada H, Sasaki T, Niwa S, Tohoru O, Murata M, Kawakami T, Aimoto S. Intact glycation end products containing carboxymethyl-lysine and glyoxal lysine dimer obtained from synthetic collagen model peptide. Bioorg Med Chem Lett. 2004;14:5677–5680. doi: 10.1016/j.bmcl.2004.08.044. [DOI] [PubMed] [Google Scholar]
- Yan SD, Zhu H, Zhu A, Golabek A, Du H, Roher A, Yu J, Soto C, Schmidt AM, Stern D, Kindy M. Receptor-dependent cell stress and amyloid accumulation in systemic amyloidosis. Nat Med. 2000;6:643–651. doi: 10.1038/76216. [DOI] [PubMed] [Google Scholar]
- Yang F, Chung AC, Huang XR, Lan HY. Angiotensin II induces connective tissue growth factor and collagen I expression via transforming growth factor-beta-dependent and -independent Smad pathways: The role of Smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- Yang J, Liu Y. Dissection of key events in tubular epithelial to myofibroblast transition and its implications in renal interstitial fibrosis. Am J Pathol. 2001;159:1465–1475. doi: 10.1016/S0002-9440(10)62533-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H, Kihara Y, Taguchi M, Yamaguchi T, Nakamura H, Otsuki M. Role of TGF-b1 in the development of pancreatic fibrosis in Otsuka Long–Evans Tokushima fatty rats. Am J Physiol Gastrointest Liver Physiol. 2002;282:G549–G555. doi: 10.1152/ajpgi.00323.2001. [DOI] [PubMed] [Google Scholar]
- Yuen A, Laschinger C, Talior I, Lee W, Chan M, Birek J, Young EW, Sivagurunathan K, Won E, Simmons CA, McCulloch CA. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010;29:537–548. doi: 10.1016/j.matbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Zhu D, Kim Y, Steffes MW, Groppoli TJ, Butkowski RJ, Mauer SM. Application of electron microscopic immunocytochemistry to the human kidney: Distribution of type IV and type VI collagen in normal human kidney. J Histochem Cytochem. 1994;42:577–584. doi: 10.1177/42.5.8157929. [DOI] [PubMed] [Google Scholar]