Abstract
Diabetic nephropathy is the most common cause of chronic kidney disease and represents a large and ominous public health problem. Patients with diabetic kidney disease have exceptionally high rates of cardiovascular morbidity and mortality. In fact, the excess mortality among patients with diabetes appears to be largely limited to the subgroup with kidney disease and explained by their high burden of cardiovascular disease. The mechanisms underlying the strong association between diabetic kidney disease and various forms of cardiovascular disease are poorly understood. Traditional risk factors for cardiovascular disease, although prevalent among those with diabetes, do not fully account for the heightened risk observed. Despite their susceptibility to cardiovascular disease, patients with chronic kidney disease are less likely to receive appropriate risk factor modification than the general population. Moreover, as patients with chronic kidney disease have commonly been excluded from major cardiovascular trials, the evidence for potential treatments remains limited. Currently, mainstays of treatment for diabetic kidney disease include blockade of the renin-angiotensin-aldosterone system, and control of hypertension, hyperglycemia and dyslipidemia. Increased awareness of the vulnerability of this patient population and more timely interventions are likely to improve outcomes, while large evidence gaps are filled with newer studies.
Keywords: coronary artery disease, diabetes mellitus, chronic kidney disease, end-stage renal disease
The prevalence of diabetes mellitus in the United States has grown steeply over the past few decades. Diabetes was recently estimated to affect 11.3% of the adult population.1 Globally, the number of people with diabetes is increasing as well and is expected to exceed 430 million by 2030.2 Diabetes has long been known to be associated with a high risk of cardiovascular (CV) morbidity and mortality.3 Patients with diabetes are more than twice as likely to die from vascular causes as those without diabetes, and cardiovascular disease (CVD) accounts for approximately 60% of the life years lost from diabetes.4 This excess in cardiovascular mortality among patients with diabetes has also been demonstrated in multiple prospective trials.5,6 It appears to be caused not only by a higher incidence of cardiovascular events, but also by worse CVD outcomes among those with diabetes.7 Patients with diabetes but no prior history of myocardial infarction (MI) appear as likely to die from coronary heart disease as patients without diabetes who have established coronary artery disease.8 Accordingly, diabetes has been considered to be a “coronary artery disease equivalent” by many. As a result, clinical guidelines have placed strong emphasis on primary and secondary prevention of CVD among patients with diabetes.
More recently, the significant impact of chronic kidney disease (CKD) on cardiovascular risk has been increasingly recognized. Patients with CKD are far more likely to die, predominantly from CVD, than to progress to end-stage renal disease (ESRD).9 Diabetes is the leading cause of CKD in the United States, and may be considered thereby to contribute to a significant proportion of the CVD associated with CKD. Patients with diabetic nephropathy have a disproportionally higher risk for CVD when compared to patients with diabetes who do not have kidney disease. This review focuses on the inter-relationships between diabetes, chronic kidney disease and cardiovascular disease.
Epidemiology of chronic kidney disease and diabetic nephropathy
CKD is defined by the Kidney Disease Improving Global Outcomes (KDIGO) Work Group as functional or structural abnormalities of the kidneys persisting for ≥ 3 months as manifested by decreased estimated glomerular filtration rate (eGFR) (≤ 60 mL/min/1.73m2) or by evidence of kidney damage (e.g., albuminuria or proteinuria).10 Similarly to diabetes, CKD represents a formidable and rapidly growing public health problem.11 Between 1988–1994 and 1999–2004, CKD increased in prevalence from 10% to 13% in the United States, however, this increase did not persist after accounting for the increase in prevalence of diabetes during the same time period.12 Based on the National Health and Nutrition Examination Surveys (NHANES), the prevalence of diabetic nephropathy rose from 2.2% to 3.3% between 1988–1994 and 2005–2008, in direct proportion to the concomitant increase in diabetes.13 From 1991 to 2010, the number of patients with ESRD more than doubled from 209,000 to 580,000, with diabetic nephropathy accounting for approximately 38% of those cases. In socioeconomic terms, the burden of CKD is enormous with Medicare expenditure on the US ESRD program alone reaching 33 billion dollars in 2010.
Associations between chronic kidney disease and cardiovascular disease
In recent years, a growing number of studies have demonstrated the exceptionally high risk of CVD in patients with CKD. In fact, death related to cardiovascular causes is the main competing outcome to development of ESRD in patients with CKD. For patients with CKD stage 3 (eGFR ≤ 60 mL/min/1.73m2), the risk of death is over ten times higher than the risk of progression to ESRD.14 In part, the high risk for death accounts for the large difference in numbers of patients with earlier stages of CKD when compared to those with ESRD. For those relatively few patients who do eventually reach ESRD, prognosis is grim with a five-year survival averaging less than 40% largely due to CVD-associated morbidity and mortality.
Both decreased eGFR and increased albuminuria have been identified as independent risk factors for all-cause and cardiovascular mortality in the general population.15–18 A meta-analysis of general population cohort studies demonstrated that hazard ratios (HR) for cardiovascular mortality, defined as death from MI, heart failure, stroke or sudden death, increased progressively with declining eGFR. Once below 60 mL/min/1.73m2, in the absence of albuminuria, the HR for CV death increased from 1.52 for patients with an eGFR of 45–59 mL/min/1.73m2 to 13.51 for patients with an eGFR of 15–29 mL/min/1.73m2. Albuminuria above 10 mg/g, in the absence of decreased eGFR, was similarly found to be associated with progressively increased risk of CV mortality. When both were present together, decreased eGFR and albuminuria were multiplicatively associated with CV mortality.19 With the establishment of CKD as a powerful predictor of CVD, clinical guidelines, including those of the American Heart Association, have begun to endorse the inclusion of patients with CKD in the highest risk group in their recommendations for prevention, detection and treatment of CVD.20
Diabetes, diabetic nephropathy and mortality
Diabetes is a strong risk factor not only for CVD, but also for the development of CKD.21 The risk of ESRD is increased 12-fold in patients with diabetes.22 Roughly 40% of patients with diabetes have evidence of CKD upon screening for decreased eGFR and albuminuria.23
The development of albuminuria has long been recognized as a poor prognostic indicator in patients with diabetes.24 Numerous studies examining the association of decreased eGFR and/or increased albuminuria with CV endpoints in patients with diabetes have made similar observations as those in general population cohorts; both eGFR and albuminuria independently predict increased CV morbidity and mortality.25–29
Given the heightened CV risk with both diabetes and CKD, three studies were recently performed to more specifically elucidate the impact of diabetic nephropathy on premature mortality in patients with diabetes. The first of these was the FinnDiane study, a national prospective study on 4,201 Finnish patients with type 1 diabetes. During a median follow-up of 7 years, study participants had 3.6-fold higher mortality compared to the age- and sex-matched members of the general population. Remarkably however, excess mortality was only observed in the subgroup of patients with diabetes who had CKD.30 The validity of these findings were strengthened when another group made similar observations in a separate study population consisting of 658 American patients with type 1 diabetes who had been followed for a median of 20 years.31 The third study examined 10-year cumulative mortality by diabetes and kidney disease status among 15,046 subjects with type 2 diabetes from the third NHANES. Patients with diabetes and kidney disease were found to have a standardized mortality of 31.1%, representing an absolute difference of 23.4% when compared to the reference group who did not have diabetes or CKD, after adjustment for age, sex, race, smoking, blood pressure and cholesterol. In fact, mortality was similar for patients with diabetes but without nephropathy when compared to the reference group.32 Taken together, these notable studies demonstrate that in the absence of nephropathy, the mortality of patients with diabetes does not significantly differ from the general population. How kidney disease is causally related to the excess mortality risk observed in these studies remains to be determined.
Mechanisms and manifestations of cardiovascular disease in chronic kidney disease
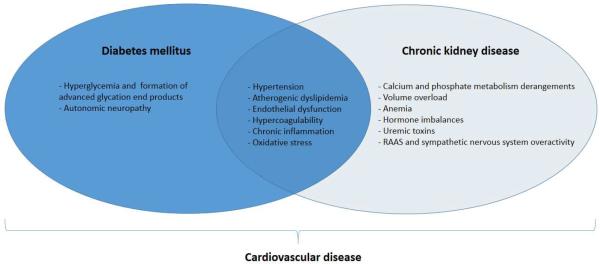
Definitive reasons for the associations between diabetes, CKD and CVD are not well known (Figure 1). A detailed discussion of the many underlying mechanisms that have been proposed is beyond the scope of this review. Although patients with CKD, particularly those with diabetic kidney disease, commonly have coexisting traditional risk factors for CVD, these do not fully explain the increased incidence of CV events and mortality.33 Moreover, some traditional risk factors such as hypertension, hyperlipidemia and obesity have actually been observed to have an inverse association with CVD in patients with advanced CKD.34 Other non-traditional factors thought to contribute to CVD in patients with CKD include derangements in calcium-phosphate homeostasis, arterial stiffening, anemia, hyperaldosteronism, chronic inflammation, abnormal nitric oxide metabolism and endothelial dysfunction.35–40 In addition, both diabetes and CKD have been shown to be hypercoagulable conditions, associated with platelet dysfunction and alterations in plasma levels of clotting factors and mediators of fibrinolysis.41–42 This becomes more pronounced when both conditions are present together, resulting heightened risk of thrombotic events.43 Paradoxically, bleeding risk is also increased in those with advanced CKD.
Figure 1.
Overview of major established and proposed mechanisms of CVD in patients with DM and CKD.
Cardiovascular complications observed in patients with CKD vary widely. Whether the manifestations of CVD vary by the underlying etiology of CKD is unclear, as studies have generally not made such comparisons directly. CKD is associated with CAD and with increased risk of death and nonfatal cardiovascular outcomes after MI.44–45 Left ventricular hypertrophy and congestive heart failure, which predominantly result from diastolic dysfunction, are highly prevalent among patients with CKD and those initiating therapy for ESRD (estimated to affect 74% and 31%, respectively).46–47 Along with heart failure, sudden cardiac death is the leading cause of mortality in patients with ESRD, accounting for approximately one quarter of all deaths in this population.48 Milder degrees of CKD have also been shown to independently increase the risk of sudden cardiac death among patients with concomitant CAD.49 Moreover, according to a recent meta-analysis, the relative risk of both ischemic and hemorrhagic stroke is increased to approximately 1.4 in patients with CKD stages 3–4 (eGFR 15–59 mL/min/1.73m2).50 For patients with ESRD, the relative risk of stroke compared to the age-matched general population is increased even further, up to five- to ten-fold, resulting in an overall stroke rate of approximately 4% per year.51 The high rate of stroke in patients with ESRD is likely in part due to the high prevalence of atrial fibrillation in this population.52 Notably, in the setting of decreased eGFR or albuminuria, the stroke risk associated with atrial fibrillation is progressively increased.53 In the dialysis population, diabetes also functions as an additional independent predictor of risk.54 Finally, peripheral artery disease is prevalent among patients with CKD, affecting about 1 in 4 adults over 40 years of age with CKD. Taken together, these various cardiovascular disease manifestations in patients with CKD contribute to their excess CVD mortality.
Prevention and management of diabetic nephropathy and reduction of cardiovascular risk
In light of the high mortality of patients with diabetic nephropathy, primary prevention of its development and efforts to hinder its progression once it is established are of utmost importance. Unfortunately, CKD frequently goes unrecognized by both patients and providers.55 Furthermore, patients with diagnosed CKD have been found to be less likely than the general population to receive appropriate CVD risk-factor modification.45,56 A large opportunity to improve outcomes in this vulnerable patient population lies in simply raising awareness of these issues, and intervening as early as possible.
Management of hypertension and albuminuria
Observational studies in the general population have demonstrated a strong and linear relationship between blood pressure and CV risk, with the risk of CV events doubling for every increase in systolic/diastolic blood pressure of 20/10 mmHg above 115/75 mmHg.57 In addition to its direct effect on CV risk, hypertension contributes to both deterioration of kidney function in diabetes and albuminuria, and is thus an important target for management. Early studies such as the UKPDS demonstrated that tight blood pressure control (<150/85 mm Hg) improved renal outcomes.58–59 Based on such trials, clinical guidelines have long recommended a goal blood pressure of less than 130/80 mmHg for patients with diabetes. However, due to lack of evidence from prospective trials, this universal treatment goal was challenged in the most recent KDOQI Clinical Practice Guidelines for Management of Blood Pressure in CKD, and a less stringent goal of less than 140/90 mmHg was recommended for those patients with diabetes who do not have significant albuminuria (<30 mg/24 hours). More intensive therapy leading to a blood pressure less than 140/90 mmHg was also not supported for hypertensive adults with diabetes or CKD, with or without proteinuria, in the recent 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults.60
By antagonizing the renin angiotensin aldosterone system (RAAS), angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARBs) lower blood pressure and decrease albuminuria. The Reduction of End Points in Non-Insulin-Dependent Diabetes With the Angiotensin II Antagonist Losartan (RENAAL) and Irbesartan Diabetic Nephropathy Trial (IDNT) studies demonstrated that losartan and irbesartan, respectively, are protective against progression of nephropathy in patients with type 2 diabetes, independent of blood pressure reduction.61–62 Renoprotective effects of ACEI-I had been reported prior to this study in patients with type 1 diabetes.63 The Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) trial, which included patients both with and without diabetes, demonstrated that although a combination of ramipril and telmisartan decreased proteinuria compared to monotherapy with either agent, time to dialysis initiation was not delayed. Although neither agent was found to be superior to the other in the monotherapy arms, the study was not powered to detect such a difference. Adequately powered head-to-head trials comparing the effectiveness of ACE-I and ARBs in this setting are limited. The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial, which compared therapy with losartan and lisinopril to losartan alone in patients with type 2 diabetes and macroalbuminuria (>300 mg/day) also failed to demonstrate benefit from combination therapy, and was in fact stopped early due to safety concerns.64 A recent meta-analysis demonstrated that RAAS inhibitors reduce progression to microalbuminuria (30–300 mg/day) in normoalbuminuric patients with type 2 diabetes, but not in type 1 diabetes. Already a cornerstone in the management of diabetic nephropathy, these results might be used to support the routine use of RAAS inhibitors in the management of patients with type 2 diabetes, regardless of baseline albuminuria.65
Management of hyperglycemia
Hyperglycemia, the hallmark of diabetes, is a fundamental therapeutic target in all patients with diabetes. As demonstrated in landmark trials such as Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) and UKPDS, tight glycemic control decreases the risk of microvascular complications, including nephropathy, in patients with both type 1 and type 2 diabetes.66–67 In UKPDS, patients with newly-diagnosed type 2 diabetes were randomized to receive either intensive glycemic control or conventional therapy achieving hemoglobin A1c (HbA1c) levels of 7.0% and 7.9%, respectively. The relative risk for development of microalbuminuria in the intensive group was 0.76 (99% confidence interval 0.62–0.91, p < 0.01). Although no significant difference was initially observed in macrovascular endpoints, a subsequent 10 year follow-up study during which time patients in both groups had reached similar HbA1c levels after approximately 1 year showed a 15% relative risk reduction in MI (p=0.01) and a 13% decrease in all-cause mortality (p=0.007).68 Despite these early findings, evidence from more recent clinical trials suggesting beneficial effects from tight glycemic control on macrovascular outcomes in patients with diabetes is minimal and this has been a matter of controversy. Several large recent randomized controlled trials including Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE), Action to Control Cardiovascular Risk in Diabetes (ACCORD) and Veterans Affairs Diabetes Trial (VADT) have failed to demonstrate a reduction in major cardiovascular events by reducing HbA1c levels aggressively to less than 7%.69–71 Moreover, patients treated to a median HbA1c of 6.4% in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial had a higher rate of mortality (HR 1.22, 95% CI 1.01–1.46, p=0.04) than the control group which had a median HbA1c of 7.5%. This excess mortality could not be explained by the frequency of hypoglycemia episodes. Combined, current evidence supports treatment to a goal HbA1c level of 7.0% to delay development of microvascular complications, including nephropathy. Notably, no prospective randomized clinical trials have evaluated the effect of glycemic control on health outcomes in patients with CKD stages 3–5. Because patients with CKD are more vulnerable to hypoglycemia than those with preserved eGFR, it remains unclear whether the same HbA1c goal is optimal for this population.
Management of dyslipidemia
Dyslipidemia, a strong modifiable risk factor for CV events in the general population, is common among patients with diabetes and CKD. Data demonstrating a reduction in CV events from lowering LDL cholesterol in patients with CKD stages 1–3 initially came from several post-hoc analyses of large randomized controlled trials that had included a sufficient number of patients with CKD to permit such estimates.72–73 Subsequently, the Study of Heart and Renal Protection (SHARP) trial evaluated the efficacy and safety of treating patients with moderate-to-severe kidney disease with a combination of simvastatin and ezetimibe.74 The study included 9270 patients of whom 3023 were on dialysis. The relative risk of major atherosclerotic events, defined as non-fatal MI, non-hemorrhagic stroke and any arterial revascularization procedure during the median follow-up of 4.9 years was 0.83 (95% CI 0.63–0.93, p=0.0036). However, as opposed to what has been demonstrated in cohorts without advanced CKD, no mortality benefit was observed. Moreover, no statistically significant reduction in CV events was observed in patients on dialysis therapy. Subgroup analyses did not demonstrate any differences among patients with and without diabetes, but such analyses were likely underpowered. Nonetheless, the lack of benefit from lipid-lowering therapy in patients with ESRD in Study of Heart and Renal Protection (SHARP) was consistent with findings from two smaller RCTs.48,75 Although some conflicting data previously existed suggesting that lipid-lowering therapy might hamper progression of CKD, this was not demonstrated in the much larger Study of Heart and Renal Protection (SHARP) trial.
Previously, lipid-lowering therapy, with statins as first-line agents, was recommended for patients with diabetes and CKD stages 1–4 to achieve serum LDL cholesterol levels of below 100 mg/dL or optionally below 70 mg/dL. However, specific LDL cholesterol treatment targets were removed from recent recommendations on managing lipids in patients with CKD in the KDIGO 2013 Clinical Practice Guidelines.76 These recommendations align with those in the recent prevention guidelines by the American Heart Association and American College of Cardiology which advised that the decision to initiate cholesterol-lowering treatment (specifically statin therapy) should be based on the absolute risk of coronary events.77 Thus, statin therapy should be considered in nearly all patients with diabetes and CKD given their high risk of CVD and the pleiotropic effects of statins. Whether patients on statins should discontinue their use once dialysis commences remains unclear.
Lifestyle changes
All patients with diabetic nephropathy should receive recommendations on lifestyle changes that may decrease their risk of progression and development of CVD. For patients who smoke, cessation of tobacco use is imperative, as this may not only significantly decrease the risk of CVD directly but also slow the progression of early diabetic nephropathy.78 In addition to adhering to a diabetic diet, patients with nephropathy have traditionally been advised to restrict both dietary salt and protein intake. The reduction of salt intake by 8.5 g/day has been shown to decrease blood pressure by 7/3 mmHg, an effect size similar to that commonly seen from single drug therapy.79 Of note however, a recent prospective observational study in patients with type 1 diabetes without ESRD demonstrated that the association between salt intake as estimated by urinary sodium excretion and mortality is non-linear, with reduced survival seen both in those with the highest and lowest levels of excretion, regardless of kidney disease.80 Clinical trials will be needed to assess the effect of dietary salt restriction on mortality in this setting. Dietary protein restriction can improve eGFR in patients with diabetic kidney disease, although the quality of evidence for this is not high.81 Finally, combining lifestyle changes with intensive medical management may significantly lower risk of CVD and impede progression of microvascular disease.82
Management of cardiovascular disease
As reviewed here, patients with CKD, especially those with diabetic nephropathy, have a very high burden of CVD. When viewed from a cardiovascular focus, patients diagnosed with heart failure and CAD are often likely to have coexisting CKD, with prevalence rates reported up to approximately 60%.83–84 Thus, it is striking that despite their large numbers and poor cardiovascular outcomes, patients with CKD have commonly been excluded from major CVD trials.85 Accordingly, the evidence base for management of CVD in patients with CKD remains limited. The pathophysiology and manifestations of CVD in patients with CKD differ from the general population, and they often have a higher risk of adverse events with interventions such as anticoagulation and administration of contrast. Although optimal strategies for the prevention, diagnosis, and management of specific cardiovascular disease complications have yet to be determined,86 general strategies that address shared risk-factors including those reviewed above may provide a reasonable starting point from which to improve outcomes while newer evidence is generated from ongoing and future trials.
Conclusions
The prevalence of diabetes and chronic kidney disease, which has already reached epidemic proportions in Western societies, continues to increase. Given the substantial morbidity and mortality associated with these conditions, the rising prevalence represents a daunting problem. In light of data demonstrating that cardiovascular disease disproportionally affects patients with diabetic nephropathy, early and aggressive interventions are needed to lessen their risk of adverse outcomes. Unfortunately, current treatment options provide only modest benefits. Further understanding of the pathogenesis of diabetic nephropathy and the mechanisms by which it is associated with cardiovascular disease are urgently needed. Alarmingly, patients with chronic kidney disease, including diabetic nephropathy, are underdiagnosed, undertreated and underrepresented in cardiovascular disease trials. Increased awareness of the tremendously high risk for cardiovascular disease in the setting of diabetic kidney disease, addressing known risk factors, as well as a focus on filling evidence gaps is critical to improving outcomes.
Clinical Summary
-
-
The prevalence of diabetes mellitus has grown steeply over the past few decades in the United States and is fueling an increase in prevalence of diabetic kidney disease.
-
-
Although patients with diabetic nephropathy have a disproportionally high risk for cardiovascular disease when compared to patients with diabetes who do not have kidney disease, the mortality of patients with diabetes does not significantly differ from the general population in the absence of nephropathy.
-
-
Cardiovascular complications observed in patients with diabetic kidney disease vary widely, ranging from heart failure to coronary artery disease to peripheral arterial disease to sudden cardiac death.
-
-
Although optimal strategies for the prevention, diagnosis, and management of specific cardiovascular disease complications have yet to be determined, addressing shared risk-factors including hypertension, elevated blood glucose, and dyslipidemia may provide a reasonable starting point from which to improve outcomes while newer evidence is generated from ongoing and future trials.
Acknowledgments
Supported in part by NIH R01DK093938 and P30DK096493 (UDP)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no disclosures or conflicts of interest (full disclosure for UDP available at https://www.dcri.org/about-us/conflict-of-interest). The authors take full responsibility for the content of this article, and this work is solely their own. This manuscript represents the authors' original work and is not under consideration for publication elsewhere. All authors meet criteria for authorship and have signed a statement attesting authorship.
References
- 1.CDC statistics Available at: http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011.pdf.
- 2.Shaw JE, Sicree R a, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010;87(1):4–14. doi: 10.1016/j.diabres.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Kannel WB. Diabetes and Cardiovascular Disease. The Framingham Study. JAMA. 1979;241(19):2035. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 4.Seshasai SRK, Kaptoge S, Thompson A, et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364(9):829–41. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ. 2006;332(7533):73–8. doi: 10.1136/bmj.38678.389583.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox CS, Matsushita K, Woodward M, et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: a meta-analysis. Lancet. 2012;380(9854):1662–73. doi: 10.1016/S0140-6736(12)61350-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun BY, Dobson AJ, Heller RF. The impact of diabetes on survival among patients with first myocardial infarction. Diabetes Care. 1997;20(5):704–8. doi: 10.2337/diacare.20.5.704. [DOI] [PubMed] [Google Scholar]
- 8.Haffner S, Lehto S. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N. Engl. J. Med. 1998;339(4):229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 9.Keith DS, Nichols G a, Gullion CM, Brown JB, Smith DH. Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch. Intern. Med. 2004;164(6):659–63. doi: 10.1001/archinte.164.6.659. [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Eckardt K, Tsukamato Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Atkins R, Coresh J, et al. Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–59. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 12.Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298(17):2038–47. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 13.De Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA. 2011;305(24):2532–9. doi: 10.1001/jama.2011.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriksen BO, Ingebretsen OC. The progression of chronic kidney disease: a 10-year population-based study of the effects of gender and age. Kidney Int. 2006;69(2):375–82. doi: 10.1038/sj.ki.5000058. [DOI] [PubMed] [Google Scholar]
- 15.Astor BC, Hallan SI, Miller ER, Yeung E, Coresh J. Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am. J. Epidemiol. 2008;167(10):1226–34. doi: 10.1093/aje/kwn033. [DOI] [PubMed] [Google Scholar]
- 16.Bello AK, Hemmelgarn B, Lloyd A, et al. Associations among estimated glomerular filtration rate, proteinuria, and adverse cardiovascular outcomes. Clin. J. Am. Soc. Nephrol. 2011;6(6):1418–26. doi: 10.2215/CJN.09741110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004;351(13):1296–305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 18.Hemmelgarn BR, Manns BJ, Lloyd A, et al. Relation between kidney function, proteinuria, and adverse outcomes. JAMA. 2010;303(5):423–9. doi: 10.1001/jama.2010.39. [DOI] [PubMed] [Google Scholar]
- 19.Matsushita K, van der Velde M, Astor BC, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010;375(9731):2073–81. doi: 10.1016/S0140-6736(10)60674-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sarnak MJ, Levey AS, Schoolwerth AC, et al. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–69. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 21.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PWF, Levy D. Predictors of new-onset kidney disease in a community-based population. JAMA. 2004;291(7):844–50. doi: 10.1001/jama.291.7.844. [DOI] [PubMed] [Google Scholar]
- 22.Brancati FL. Risk of End-stage Renal Disease in Diabetes Mellitus - A Prospective Cohort Study of Men Screened for MRFIT. JAMA. 1997;278(23):2069. [PubMed] [Google Scholar]
- 23.Garg AX, Kiberd B a, Clark WF, Haynes RB, Clase CM. Albuminuria and renal insufficiency prevalence guides population screening: results from the NHANES III. Kidney Int. 2002;61(6):2165–75. doi: 10.1046/j.1523-1755.2002.00356.x. [DOI] [PubMed] [Google Scholar]
- 24.Mogensen CE. Microalbuminuria predicts clinical proteinuria and early mortality in maturity-onset diabetes. N. Engl. J. Med. 1984;310(6):356–60. doi: 10.1056/NEJM198402093100605. [DOI] [PubMed] [Google Scholar]
- 25.Boer I de, Katz R, Cao J. Cystatin C, albuminuria, and mortality among older adults with diabetes. Diabetes Care. 2009;32(10) doi: 10.2337/dc09-0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ninomiya T, Perkovic V, de Galan BE, et al. Albuminuria and kidney function independently predict cardiovascular and renal outcomes in diabetes. J. Am. Soc. Nephrol. 2009;20(8):1813–21. doi: 10.1681/ASN.2008121270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.So WY, Kong APS, Ma RCW, et al. Glomerular filtration rate, cardiorenal end points, and all-cause mortality in type 2 diabetic patients. Diabetes Care. 2006;29(9):2046–52. doi: 10.2337/dc06-0248. [DOI] [PubMed] [Google Scholar]
- 28.Perkovic V, Verdon C, Ninomiya T, et al. The relationship between proteinuria and coronary risk: a systematic review and meta-analysis. PLoS medicine. 2008;5(10):e207. doi: 10.1371/journal.pmed.0050207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ninomiya T, Perkovic V, Verdon C, et al. Proteinuria and stroke: a meta-analysis of cohort studies. Am. J. Kidney Dis. 2009;53(3):417–25. doi: 10.1053/j.ajkd.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 30.Groop PH, Thomas MC, Moran JL, et al. The presence and severit of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes. 2009;58(7):1651–8. doi: 10.2337/db08-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orchard TJ, Secrest a M, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia. 2010;53(11):2312–9. doi: 10.1007/s00125-010-1860-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Afkarian M, Sachs MC, Kestenbaum B, et al. Kidney disease and increased mortality risk in type 2 diabetes. J. Am. Soc. Nephrol. 2013;24(2):302–8. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longenecker JC, Coresh J, Powe NR, et al. Traditional cardiovascular disease risk factors in dialysis patients compared with the general population: the CHOICE Study. J. Am. Soc. Nephrol. 2002;13(7):1918–27. doi: 10.1097/01.asn.0000019641.41496.1e. [DOI] [PubMed] [Google Scholar]
- 34.Kovesdy CP, Anderson JE. Reverse epidemiology in patients with chronic kidney disease who are not yet on dialysis. Semin. Dial. 2007;20(6):566–9. doi: 10.1111/j.1525-139X.2007.00335.x. [DOI] [PubMed] [Google Scholar]
- 35.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch. Intern. Med. 2008;168(4):397–403. doi: 10.1001/archinternmed.2007.110. [DOI] [PubMed] [Google Scholar]
- 36.Guérin AP, Pannier B, Marchais SJ, London GM. Arterial structure and function in end-stage renal disease. Curr. Hypertens. Rep. 2008;10(2):107–11. doi: 10.1007/s11906-008-0021-2. [DOI] [PubMed] [Google Scholar]
- 37.Collins AJ, Ma JZ, Ebben J. Impact of hematocrit on morbidity and mortality. Semin. Nephrol. 2000;20(4):345–9. [PubMed] [Google Scholar]
- 38.Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat. Rev. Nephrol. 2010;6(5):261–73. doi: 10.1038/nrneph.2010.30. [DOI] [PubMed] [Google Scholar]
- 39.Weiner DE, Tighiouart H, Elsayed EF, et al. Inflammation and cardiovascular events in individuals with and without chronic kidney disease. Kidney Int. 2008;73(12):1406–12. doi: 10.1038/ki.2008.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stehouwer CD a, Smulders YM. Microalbuminuria and risk for cardiovascular disease: Analysis of potential mechanisms. J. Am. Soc. Nephrol. 2006;17(8):2106–11. doi: 10.1681/ASN.2005121288. [DOI] [PubMed] [Google Scholar]
- 41.Jalal DI, Chonchol M, Targher G. Disorders of hemostasis associated with chronic kidney disease. Semin. Thromb. Hemost. 2010;36(1):34–40. doi: 10.1055/s-0030-1248722. [DOI] [PubMed] [Google Scholar]
- 42.Carr ME. Diabetes mellitus: a hypercoagulable state. J. Diabetes Complications. 2001;15(1):44–54. doi: 10.1016/s1056-8727(00)00132-x. [DOI] [PubMed] [Google Scholar]
- 43.Baber U, Bander J, Karajgikar R, et al. Combined and independent impact of diabetes mellitus and chronic kidney disease on residual platelet reactivity. Thromb. Haemost. 2013;110(1):118–23. doi: 10.1160/TH13-01-0004. [DOI] [PubMed] [Google Scholar]
- 44.Chonchol M, Whittle J, Desbien A, Orner MB, Petersen LA, Kressin NR. Chronic kidney disease is associated with angiographic coronary artery disease. Am. J. Nephrol. 2008;28(2):354–60. doi: 10.1159/000111829. [DOI] [PubMed] [Google Scholar]
- 45.Anavekar N. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N. Engl. J. Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 46.Foley RN, Parfrey PS, Harnett JD, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47(1):186–92. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 47.Cerasola G, Nardi E, Palermo A, Mulè G, Cottone S. Epidemiology and pathophysiology of left ventricular abnormalities in chronic kidney disease: a review. J. Nephrol. 24(1):1–10. doi: 10.5301/jn.2010.2030. [DOI] [PubMed] [Google Scholar]
- 48.Wanner C, Krane V, März W, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N. Engl. J. Med. 2005;353(3):238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 49.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76(6):652–8. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee M, Saver JL, Chang K-H, Liao H-W, Chang S-C, Ovbiagele B. Low glomerular filtration rate and risk of stroke: meta-analysis. BMJ. 2010;341:c4249. doi: 10.1136/bmj.c4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Seliger SL, Gillen DL, Longstreth WT, Kestenbaum B, Stehman-Breen CO. Elevated risk of stroke among patients with end-stage renal disease. Kidney Int. 2003;64(2):603–9. doi: 10.1046/j.1523-1755.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- 52.Wizemann V, Tong L, Satayathum S, et al. Atrial fibrillation in hemodialysis patients: clinical features and associations with anticoagulant therapy. Kidney Int. 2010;77(12):1098–106. doi: 10.1038/ki.2009.477. [DOI] [PubMed] [Google Scholar]
- 53.Go AS, Fang MC, Udaltsova N, et al. Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation. 2009;119(10):1363–9. doi: 10.1161/CIRCULATIONAHA.108.816082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sozio SM, Armstrong PA, Coresh J, et al. Cerebrovascular disease incidence, characteristics, and outcomes in patients initiating dialysis: the choices for healthy outcomes in caring for ESRD (CHOICE) study. Am. J. Kidney Dis. 2009;54(3):468–77. doi: 10.1053/j.ajkd.2009.01.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Plantinga L, Tuot D, Powe N. Awareness of chronic kidney disease among patients and providers. Adv. Chronic Kidney Dis. 2010;17(3):225–236. doi: 10.1053/j.ackd.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fox CS, Muntner P, Chen AY, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121(3):357–65. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–13. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 58.Turner R, Holman R, Stratton I, et al. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 59.Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258):412–9. doi: 10.1136/bmj.321.7258.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.James PA, Oparil S, Carter BL, et al. 2014 Evidence-Based Guideline for the Management of High Blood Pressure in Adults Report From the Panel Members Appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2013 doi: 10.1001/jama.2013.284427. in press; doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 61.Brenner B, Cooper M. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N. Engl. J. Med. 2001;345(12):861–869. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 62.Lewis E, Hunsicker L. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N. Engl. J. Med. 2001;345(12):851–860. 60. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 63.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N. Engl. J. Med. 1993;329(20):1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 64.Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N. Engl. J. Med. 2013;369(20):1892–903. doi: 10.1056/NEJMoa1303154. [DOI] [PubMed] [Google Scholar]
- 65.Hirst J a, Taylor KS, Stevens RJ, et al. The impact of renin-angiotensin-aldosterone system inhibitors on Type 1 and Type 2 diabetic patients with and without early diabetic nephropathy. Kidney Int. 2012;81(7):674–83. doi: 10.1038/ki.2011.413. [DOI] [PubMed] [Google Scholar]
- 66.Reichard P, Nilsson BY, Rosenqvist U. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 67.Turner RC, Holman RR, Cull CA, et al. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 68.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-year follow-up of intensive glucose control in type 2 diabetes. N. Engl. J. Med. 2008;359(15):1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 69.Patel A, MacMahon S, Chalmers J, Neal B. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008:2560–72. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 70.Wounds D. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N. Engl. J. Med. 2009;360(2):129–39. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 72.Tonelli M, Isles C, Curhan GC, et al. Effect of pravastatin on cardiovascular events in people with chronic kidney disease. Circulation. 2004;110(12):1557–63. doi: 10.1161/01.CIR.0000143892.84582.60. [DOI] [PubMed] [Google Scholar]
- 73.Colhoun HM, Betteridge DJ, Durrington PN, et al. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS) Am. J. Kidney Dis. 2009;54(5):810–9. doi: 10.1053/j.ajkd.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 74.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377(9784):2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fellström BC, Jardine AG, Schmieder RE, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N. Engl. J. Med. 2009;360(14):1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 76.Tonelli M, Wanner C, Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members Lipid Management in Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2013 Clinical Practice Guideline. Ann Intern Med. 2014;160(3):182–189. doi: 10.7326/M13-2453. [DOI] [PubMed] [Google Scholar]
- 77.Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 in press. [Google Scholar]
- 78.Phisitkul K, Hegazy K, Chuahirun T, et al. Continued smoking exacerbates but cessation ameliorates progression of early type 2 diabetic nephropathy. Am. J. Med. Sci. 2008;335(4):284–91. doi: 10.1097/MAJ.0b013e318156b799. [DOI] [PubMed] [Google Scholar]
- 79.Suckling RJ, He FJ, Macgregor GA. Altered dietary salt intake for preventing and treating diabetic kidney disease. Cochrane Database Syst. Rev. 2010;8:12. doi: 10.1002/14651858.CD006763.pub2. [DOI] [PubMed] [Google Scholar]
- 80.Nezu U, Kamiyama H, Kondo Y, Sakuma M, Morimoto T, Ueda S. Effect of low-protein diet on kidney function in diabetic nephropathy: meta-analysis of randomised controlled trials. BMJ open. 2013;3(5):1–11. doi: 10.1136/bmjopen-2013-002934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thomas MC, Moran J, Forsblom C, et al. The association between dietary sodium intake, ESRD, and all-cause mortality in patients with type 1 diabetes. Diabetes Care. 2011;34(4):861–6. doi: 10.2337/dc10-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaede P, Vedel P, Larsen N, Jensen GVH, Parving H-H, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 83.Ezekowitz J, McAlister FA, Humphries KH, et al. The association among renal insufficiency, pharmacotherapy, and outcomes in 6,427 patients with heart failure and coronary artery disease. J. Am. Coll. Cardiol. 2004;44(8):1587–92. doi: 10.1016/j.jacc.2004.06.072. [DOI] [PubMed] [Google Scholar]
- 84.McClellan WM, Langston RD, Presley R. Medicare patients with cardiovascular disease have a high prevalence of chronic kidney disease and a high rate of progression to end-stage renal disease. J. Am. Soc. Nephrol. 2004;15(7):1912–9. doi: 10.1097/01.asn.0000129982.10611.4c. [DOI] [PubMed] [Google Scholar]
- 85.Coca SG, Krumholz HM, Garg AX, Parikh CR. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296(11):1377–84. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 86.Herzog C a, Asinger RW, Berger AK, et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2011;80(6):572–86. doi: 10.1038/ki.2011.223. [DOI] [PubMed] [Google Scholar]