Abstract
Depression and coronary heart disease (CHD) are leading contributors to disease burden in women. CHD and depression are comorbid; whether they have common etiology or depression casues CHD is unclear. The underlying pathology of CHD, coronary artery atherosclerosis (CAA), is present decades before CHD, and the temporal relationship between depression and CAA is unclear. The evidence of involvement of depression in early CAA in cynomolgus monkeys, an established model of CAA and depression, is summarized. Like people, monkeys may respond to the stress of low social status with depressive behavior accompanied by perturbations in hypothalamic-pituitary-adrenal (HPA), autonomic nervous system, lipid metabolism, ovarian, and neural serotonergic system function, all of which are associated with exacerbated CAA. The primate data are consistent with the hypothesis that depression may cause CAA, and also with the hypothesis that CAA and depression may be the result of social stress. More study is needed to discriminate between these two possibilities. The primate data paint a compelling picture of depression as a whole-body disease.
Keywords: Depression, coronary heart disease, heart rate, cholesterol, ovarian function, neural serotonergic function, platelet reactivity, inflammation
Introduction
The purpose of emotional behavior is to modulate the degree of interaction the individual seeks with its physical and social environment. High activity levels, curiosity, and aggressive or assertive behavior promote interaction, while low activity levels and a generalized lack of responsiveness to environmental stimulation reduce rates of interaction with the environment. Depression is an extreme example of a group of behaviors which result in greatly reduced interactions with the environment. Depressive-like behaviors are seen in a wide range of species. Depression is a whole-body disease. Its manifestations of sadness and cognitive dysfunction may be accompanied by perturbations of most of the major physiological systems. Depression is not a unitary disorder; rather it manifests as a continuum from depressive symptoms to clinically significant disease. Here we discuss depression in the context of its relationship to coronary heart disease (CHD). The relationship between depression and CHD appears to be graded; thus in this review depressive symptoms and disorders are considered as a continuum. First, the relationship between depression and CHD in the human population is reviewed. Next pathophysiologic changes common to both depression and CHD are reviewed. Etiological factors common to CHD and depression are discussed. A nonhuman primate model of heart disease risk and depression is presented, and the results are discussed from a comparative perspective.
I. Depression and Coronary Heart Disease in the Human Population
The Global Burden of Disease
Two of the three leading causes of the global burden of disease in 2030 are projected to be unipolar depressive disorders and CHD (Mathers and Loncar, 2006; Murray and Lopez, 1997).
CHD in Women
CHD is the leading cause of morbidity and mortality of women in the US, and most developed countries, exceeding that of all cancers combined (Heart Disease and Stroke Statistics, 2008). While CHD is decreasing in men it is not decreasing in women. Even so, CHD in women is understudied, and less well understood than in men. CHD is caused by CAA and its sequelae. The premenopausal life stage is important in determining the extent of postmenopausal CAA and CHD risk because the extent of premenopausal atherosclerosis sets the starting point and trajectory of coronary artery atherogenesis postmenopausally. Individuals at high risk for extensive postmenopausal coronary atherogenesis have well-developed plaques at the menopausal transition and display a steeper trajectory of atherogenesis than their low risk counterparts (Kaplan et al., 2002).
Depression and CHD in Women
Depressive disorders are twice as likely in women as men (Kessler et al., 1994; Gorman, 2006). Excluding suicide, major depression is associated with increased mortality, perhaps in part due to a high rate of comorbidities (Rovner et al., 1991; Kouzis et al., 1995; von Ammon et al., 2001; Bradvik and Berglund, 2001). The comorbidity of depression and CHD is particularly marked (Carney et al., 2002; Rudisch and Nemeroff, 2003; Musselman et al., 2008). Several studies demonstrate graded relative risk of CHD with depression, suggesting that milder forms of depression in addition to major depressive disorder may be clinically relevant (Musselman et al., 2008; Rudisch and Nemeroff 2003; Rugulies 2002). Since CHD is the leading cause of death of women, depression may be particularly important to the cardiovascular health of women.
Impact of Depression on CHD Risk
The results of most studies of community samples initially free of clinically detectable CHD suggest that the presence of depression increases the likelihood of developing clinically significant CHD; this effect remains after controlling for confounding factors such as smoking (Rudisch and Nemeroff, 2003). Meta-analyses suggest that the relative risk of depression for onset of CHD is 1.64 (Rugulies, 2002; Wulsin and Singal, 2003). These observations imply that depression may cause CHD. However, it is well-appreciated that individuals free of clinical CHD are not free of subclinical CAA, the development of which occurs over decades (Ross, 1993). In these prospective studies of depression and CHD, the average length of follow-up was less than 10 years. Thus, it is likely that CAA was present in most subjects who experienced cardiac events during the follow-up periods. In some studies, attempts were made to adjust for undetected CHD by eliminating subjects who developed CHD in the first two years of follow-up, or by eliminating somatic symptoms of depression (e.g. fatigue) that may indicate another disease process (Charlson and Peterson, 2002; Rudisch and Nemeroff, 2003). The effectiveness of these strategies in eliminating subclinical CHD is unclear. While these procedures sometimes reduced effect size, a relationship between depression and subsequent clinical CHD remained.
The relationship between depression and CAA is unclear. However, recent observations of associations between depression and markers of atherosclerosis including arterial calcification and endothelial dysfunction suggest that depression may be associated with CAA (Agatisa et al., 2005; Tomfohr et al., 2007). Several studies demonstrate graded relative risk of CHD with depression (Rudisch and Nemeroff, 2003; Rugulies, 2002). These observations suggest that consideration of degree of depression, rather than a dichotomous (depressed vs not depressed) experimental approach may describe this relationship more accurately. It also suggests that milder forms of depression in addition to major depressive disorder may be clinically relevant.
These studies do not discriminate between the following hypotheses: 1) Depression coincident with preclinical CAA may precipitate cardiac events; 2) depression and CHD are not causally related but may co-occur due to common underlying pathologies; and 3) depression causes CAA which results in increased CHD.
II. Pathophysiologic Changes Common to Depression and CHD
Several pathophysiologic characteristics of depression could increase CHD risk. Three commonly cited are arrhythmias, platelet reactivity, and proinflammatory processes which have been reviewed in detail previously (Musselman et al., 2008; Rudisch and Nemeroff, 2003). More recently, the impact of hypothalamic-pituitary-adrenal (HPA) function on the cardiovascular system has become better understood. The literature on associations of total plasma cholesterol (TPC) with depression is mixed, perhaps in part due to the lack of separation of suicide and depression. Suicide may be considered an impulsive behavior, and risk taking and impulsivity are associated with low total plasma cholesterol. However, two large studies have found lower high density lipoprotein cholesterol (HDLC) concentrations in depressed women only, suggesting a reevaluation sensitive to sex differences may be in order (Zhang et al., 2005; Chen et al., 2001; Diaz-Sastre et al., 2007). Finally, in females, ovarian dysfunction also deserves attention. Selective serotonin reuptake inhibitors (SSRIs), which are widely prescribed for depression, appear to affect many of these pathophysiologic processes implicating serotonin in their pathophysiology.
Autonomic Control of Cardiac Function
The autonomic nervous system responds quickly to changes in environmental demands. The HPA axis (discussed below) and the autonomic nervous system act in concert to respond to stress. The balance of sympathetic and parasympathetic inputs to the heart modulates heart rate, heart rate stability, and heart rate variability (Grippo and Johnson 2002). In coronary patients, ventricular tachycardia and premature ventricular complexes are more common in those with depression (Carney et al., 1993; Frasure-Smith et al., 1995). Depression is also a more powerful predictor of mortality in patients with cardiac arrhythmias (Frasure-Smith et al., 1995; Frasure-Smith, 1991). Depression is also a risk factor for reduced heart rate variability in cardiac patients, and reduced heart rate variability is a risk factor for mortality in post-myocardial infarction (MI) patients (Stein et al., 2000; Bigger et al., 1992; Glassman et al., 2007). Reduced complexity of beat-to-beat dynamics has also been associated with depression (Yeragani et al., 2002) and may predict arrhythmia in coronary patients (Mäkikallio et al., 1997). Depression is often accompanied by increased heart rate, which is associated with increased CHD risk in people and increased CAA in monkeys (Kannel et al., 1987; Kaplan et al., 1987). While SSRIs do not appear to alter heart rhythm or diminish arrhythmias, they may increase certain components of heart rate variability (McFarlane et al., 2001) and complexity (Yeragani et al., 2002), and decrease heart rate (Shores et al., 2001; Siepmann et al., 2003).
The Hypothalamic-Pituitary-Adrenal (HPA) Axis
In response to a stressor, hypothalamic neurons increase the synthesis and release of corticotropin-releasing hormone (CRH) into the hypothalamo-hypophyseal portal system which promotes the secretion of adrenocorticotropic hormone (ACTH) by the pituitary gland. ACTH signals the adrenal glands to secrete cortisol. Adrenal cortisol normally modulates the activity of the HPA system by providing feedback to the hippocampus, hypothalamus, and pituitary. Cortisol promotes many functions that, in the short-term, may help the body cope with a stress. However, sustained high levels of circulating cortisol concentrations, in response to repeated or sustained stress, may cause changes in physiology that have long-term detrimental effects on health including the promotion of cardiovascular risk. Perturbations in HPA axis function are common in depression and may manifest as high plasma or urinary 24 h cortisol concentrations, exaggerated cortisol responses to ACTH, impaired reduction in cortisol concentrations after dexamethasone suppression, suggesting insensitivity to glucocorticoid negative feedback, or adrenal hypertrophy (Brotman et al., 2007).
Cortisol may increase CHD risk by several means. For example, cortisol increases visceral fat deposition, and visceral obesity promotes inflammation, insulin resistance, hypertension, and hypercholesterolemia, all of which increase atherogenesis (Whitworth et al., 2005). Cortisol also promotes endothelial dysfunction, which in turn increases the likelihood of endothelial damage, contributing to atherogenesis (Broadley et al., 2006). Interactions between the HPA axis and the sympathetic nervous system may increase CHD risk in depression. Neural CRH pathways have extra-hypothalamic connections to central components of the autonomic nervous system which may alter autonomic function by stimulating sympathetic outflow, thus promoting increased heart rate and decreased heart rate variability (Grippo and Johnson, 2002). SSRIs seem to decrease cortisol concentrations, thus, SSRIs may be beneficial in slowing atherosclerosis progression by reducing HPA activity (Thakore et al., 1997; Cooney and Dinan, 2000).
Platelet Dysfunction
Increases in platelet reactivity have been observed in multiple studies of depressed patients both with and without comorbid atherosclerotic CAD. Since activated platelets are central to atherogenesis, platelet dysfunction associated with depression may contribute to early atherogenesis (Brydon et al., 2006), as well as later thrombus formation. Interestingly, antidepressants which inhibit the reuptake of serotonin into platelets and neurons, (e.g. SSRIs), decrease platelet reactivity, and may even increase risk of bleeding in susceptible individuals (Serebruany et al., 2003; Bruce and Musselman, 2005).
Inflammatory Processes
Inflammatory processes are central features of atherogenesis (Willerson and Ridker, 2004). Depressed patients who are otherwise healthy have increased circulating levels of proinflammatory cytokines, acute phase proteins, chemokines, and cellular adhesion molecules (Raison et al., 2006). Cellular adhesion molecules mediate adhesion of leukocytes to the vascular endothelium, and promote the secretion of acute phase reactants (e.g. C-reactive protein, [CRP]) which also promote cell adhesion to the vascular endothelium and coagulation (Willerson and Ridker 2004). Proinflammatory cytokines access the brain, participate in a number of neural pathways known to be involved in depression, stimulate the HPA axis, and modulate autonomic outputs that further stimulate peripheral processes promoting inflammation (Raison et al., 2006). Importantly, both obesity and cholesterol- and fat-rich diets low in omega-3 fatty acids may cause a peripheral low-grade chronic inflammatory state which may directly influence central nervous system function (Calabro and Yeh, 2008; Das 2008, Chilton et al., 2008). Thus, inflammation may initiate a cascade of changes which simultaneously promote the development of depression and CHD. SSRIs affect immune function and have been shown to reduce circulating proinflammatory cytokines (IL-1β, IL-6), CRP, and leukocyte levels (Tuglu et al., 2003; Tucker et al., 2004; Basterzi et al., 2005).
Ovarian Function
Depression is more prevalent in women than men, both in community studies and in CHD patients. Apart from its increased frequency, whether the impact of depression on CAD differs by gender is unresolved (Rudisch and Nemeroff, 2003). However, poor ovarian function increases the risk of CHD (Solomon et al., 2002). The exacerbation of risk appears to be due to reduced estradiol production (Bairey Merz et al., 2003; Hanke et al., 1997). Decreased concentrations of ovarian hormones, unaccompanied by obvious changes in menstrual cycle frequency or regularity, exacerbate CAA in nonhuman primates (Macaca fascicularis) (Kaplan and Manuck, 2004).
The function of the hypothalamic-pituitary-gonadal (HPG) axis appears perturbed in depressed women. Luteinizing hormone pulsatility is altered, and estradiol levels may be lower (Grambsch et al. 2004; Meller et al., 1997, 2001). While not clinically obvious, the changes in luteinizing hormone pulsatility are enough to disrupt ovulation, and may indicate lower sex steroid secretion throughout the menstrual cycle (Young et al., 2000). Depression has also been associated with early menopause (Harlow et al., 2003). There are three possibilities that may account for these observations. First, suppressed ovarian function might be a side effect of antidepressant pharmacotherapy. Second, premature menopause may be characterized by more abrupt or different changes in sex steroids which precipitate depression. Or finally, depression may suppress the HPG axis (Harlow and Signorello, 2000).
Studies of SSRI effects on ovarian function are sparse. In a large double-blind study, Steiner et al. (1997) demonstrated that an SSRI changed cycle length and called for more study of SSRI effects on menstrual cycles. Thus, SSRIs might have a subtle but biologically significant influence on ovarian function.
III. Etiological Factors Common to CHD and Depression
CHD and depression have etiological factors in common which could contribute to their comorbidity.
Environmental Stressors
It has long been recognized that stressful life events may precipitate a bout of depression (Riso et al., 2002). Further, individuals with a history of adverse experiences during childhood are more likely as adults to develop a depressive disorder (Brown and Harris, 1993; Brown et al., 1994, 1995; Brown and Moran, 1994). Like depression, CHD risk is increased by psychological stress (Executive Summary of the NHLBI Working Group on Cardiovascular Consequences of Chronic Stress, 2004; European Guidelines, 2007; Brotman et al., 2007).
Social Disparities in Depression and CHD
One of the most pervasive influences on health in general and specifically depressive disorders and CHD is socioeconomic status. Depressive symptoms and major depression increase with decreasing socioeconomic status in men and women (Lorant et al., 2003; Stansfeld et al., 2003; Adler and Rehkopf, 2008; Adler et al., 2008). Likewise, CHD risk factors and CHD mortality increase with decreasing socioeconomic status in men and women (Pollitt et al., 2005; Hemingway et al., 2000; Steptoe et al., 2007). Since psychological stress increases with decreasing social status (Kopp et al., 2007; Feldman and Steptoe, 2004) it is thought that the psychological stressors accompanying low socioeconomic status may account, at least in part, for increased depression and CHD risk (Adler and Rehkopf 2007).
Genetic Susceptibility
Not all individuals that experience stressful events develop a depressive disorder or CHD. Thus, there is individual susceptibility, implying the role of genetic or epigenetic influences. The heritability of major depression overall is about 37% (Sullivan et al., 2000) and appears higher in women (40–44%) than men (21–29%) (Kendler et al., 2006). CHD also has a significant heritable component, about 30–32% for left main coronary artery disease (Mayer et al., 2007). McCaffery et al. (2006) reviewed the literature to test the hypothesis that there may be a common genetic vulnerability that contributes to both depressive symptoms and CHD. They concluded that nearly 20% of variability in depressive symptoms and CHD was attributable to common genetic factors. Although several pathways are likely involved, the available evidence suggested that genes within the inflammation and serotonin pathways are probable targets to look for common genetic vulnerability to depression and CHD.
Serotonin, SSRIs, and Cardiac Risk
The serotonergic system has been implicated in the pathogenesis of depression and CHD (Jans et al., 2007; Williams et al., 2001; Muldoon et al., 2007). Serotonergic vulnerability in depression has been defined in the depression literature as a sensitivity to alterations in neural serotonergic system function (Jans et al., 2007), while in the cardiovascular literature, lower central nervous system serotonergic function is conceptualized as increasing cardiovascular risk (Williams 2007). The rapid convergence of these two literatures will no doubt result in a more sophisticated perspective on the possible role of central nervous system serotonergic function in a common etiology of depression and CHD.
SSRIs, commonly used to treat depression, have beneficial effects on processes that promote CAA and CHD. The forgoing review suggests that SSRIs may increase heart rate variability and complexity, decrease heart rate, decrease platelet reactivity, reduce circulating proinflammatory cytokines, and reduce cortisol concentrations, all factors which affect CAA and CHD. These effects are not necessarily dependent upon a change in depression. Given that SSRIs have beneficial effects on pathophysiologic promoters of CAA and CHD, it seems plausible that SSRIs might be efficacious in the prevention of CAA and treatment of CHD.
To date, no prospective clinical trial has been powered to detect changes in cardiovascular outcomes with SSRI treatment. However, reduced risk of MI in patients using SSRIs has been observed in some studies (Cohen et al., 2000; Meier et al., 2001; Sauer et al., 2001). The Sertraline Antidepressant Heart Attack Randomized Trial (SADHART) was powered to evaluate safety and efficacy for depression and demonstrated these with no significant effect on cardiovascular function or mortality in post-MI patients (Glassman et al., 2002). The Enhancing Recovery in Coronary Heart Disease Patients (ENRICHD) trial, powered to evaluate the efficacy of cognitive behavioral therapy versus usual care on mortality and recurrent infarction in post-MI patients, demonstrated no significant beneficial effect of psychotherapy but allowed concurrent SSRI treatment when necessary. Mortality and recurrent MI were lower in patients taking SSRIs, irrespective of treatment arm, underscoring the need for a controlled trial (Taylor et al., 2005). SSRI effects on the development of CAA have not been studied.
IV. Summary
Two of the three leading causes of the global burden of disease in 2030 are projected to be unipolar depressive disorders and CHD. CHD and depression have a high rate of comorbidity; yet the exact nature of the relationship between these two diseases remains unclear. Since CHD is the leading killer of women and depression is twice as common in women, understanding this comorbidity seems particularly important for the health of women. Depression appears to precede clinically detectable CHD, and several pathophysiologic characteristics of depression could increase CHD risk. However, depression and CHD have etiological factors in common which may contribute to their comorbidity. The role of neural serotonergic function appears central to the comorbidity of depression and CHD, although the specifics of this relationship are poorly understood. The best evidence to date suggests that the appearance of CHD is predated by depression, supporting the hypothesis that depression may be an independent risk factor for CHD. However, subclinical CAA, the underlying pathology of CHD, is present decades before the development of symptoms. The temporal relationship between depression and CAA is unclear; the longest prospective study of depression and CHD is no more than a decade. If depression is a major contributor to CHD in women, then a causal relationship between atherogenesis and depression should emerge premenopausally, and it should be possible to demonstrate an association between premenopausal depression and the trajectory of atherosclerosis progression leading up to and then beyond the menopausal transition. Early atherogenesis remains undetectable in human studies. Thus, the goal of the work outlined below is to determine the nature of the relationship between depression and CAA in nonhuman primates.
IV. Depressive Behavior and Associated Physiology in Adult Female Cynomolgus Monkeys
Animal Modeling Comorbidity
Animal models are helpful to our understanding of human health to the extent to which they faithfully mirror the specific aspect of the disease under study. Investigations of etiology require models in which factors which promote the development of the disease are similar to those in human beings. An animal model of comorbidity requires the model to have characteristics critical to both diseases. Accumulating evidence suggests that responses to stress are sex–specific (Kennett et al., 1986; Konkle et al., 2003; Dalla et al., 2007). Thus, we have developed a primate model of adult depression using female cynomolgus macaques (Macaca fascicularis). Primate models have several advantages. They have high levels of DNA and protein sequence homology to human beings. Commercial assays can usually be used for nucleic acid and protein immunoassay. They are large enough for imaging, biopsy, and frequent blood sampling. They are an ideal model for investigations involving the reproductive, cardiovascular, and musculo-skeletal systems, other comorbidities of cardiovascular disease including type 2 diabetes mellitus and obesity, central nervous system, behavior, and the health effects of an obligate social system. Female cynomolgus monkeys are a well-characterized model of coronary artery atherogenesis (Clarkson et al., 1995; Clarkson, 2007). The nonhuman primate nervous system is more similar to that of humans than the rodent nervous system in several respects including serotonergic system organization and function which may be critical to our understanding of depression and CHD (Azmitia et al., 1996; Amaral and Lavenex, 2007; Buckmaster and Amaral, 2001). Thus, female cynomolgus monkeys are uniquely suited for investigations of the comorbidity of depression and CHD risk.
Social Subordination Stress
When placed in social groups female cynomolgus monkeys quickly organize themselves into linear social status hierarchies which are stable for extended periods of time (Shively and Kaplan, 1991). Social status is measured as the outcomes of aggressive interactions between all pairs of monkeys in a social group. The monkey to which all other monkeys in the group direct submissive behaviors is considered the most dominant. The monkey to which all monkeys, except the most dominant monkey, direct submissive behaviors is considered the second ranking monkey, and so on. In the studies reported here, the monkeys lived in groups of 3 to 5 animals. Those that were, on average, first or second ranking were considered dominant in statistical analyses, and all others were considered subordinate (Shively, 1998). Compared to dominant monkeys, subordinates receive more aggression, are groomed less, are more vigilant, and spend more time alone (Shively, 1998). Thus, in the vernacular of human studies, subordinates appear to experience more hostility and have less social support to buffer that hostility than dominants. Subordinate monkeys respond to a standardized stressor with higher heart rates than dominants. Subordinate monkeys have higher basal cortisol levels, secrete more cortisol in response to an adrenocorticotropin challenge and have heavier adrenal glands than dominants (Shively, 1998; Shively and Kaplan, 1984). Thus, subordinate animals appear behaviorally and physiologically to be socially stressed (Shively, 1998). Subordinate females are at increased risk of various pathologies; here we focus on behavioral depression.
Definition of Depressive-Like Behavior
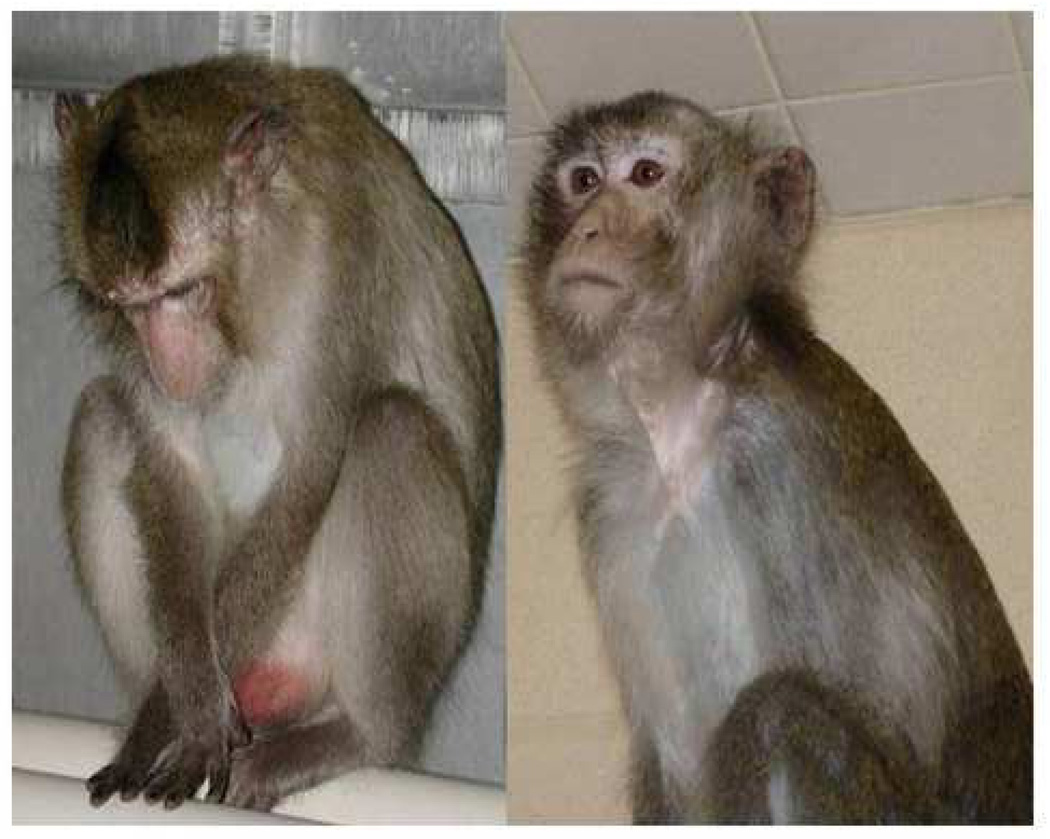
We have observed depressive behavior in two separate groups of wild-caught monkeys (78 monkeys in all) fed an atherogenic diet containing moderate amounts of fat and cholesterol. The stress of captivity may provoke depressive behavior in some individuals. In the first experiment we recorded time spent alert (attending to an object or event), resting (eyes closed), and depressed. The operational definition of “depressive behavior” was taken from Suomi et al. (1975): a slumped or collapsed body posture, with open eyes, accompanied by a lack of responsivity to environmental events. Examples of alert versus depressive behavior are shown in Figure 1.
Figure 1.
A depressed monkey and an alert monkey.
Forty-two females lived for 26 months in social groups of 4 monkeys each. Behavior was recorded during weekly observations throughout this time. Interobserver agreement in identification of depressive behavior was greater than 92%. 16 of 42 monkeys (38%) displayed depressive behavior (Shively et al., 1997). Typically, monkeys either did, or did not display the depressive behavior.
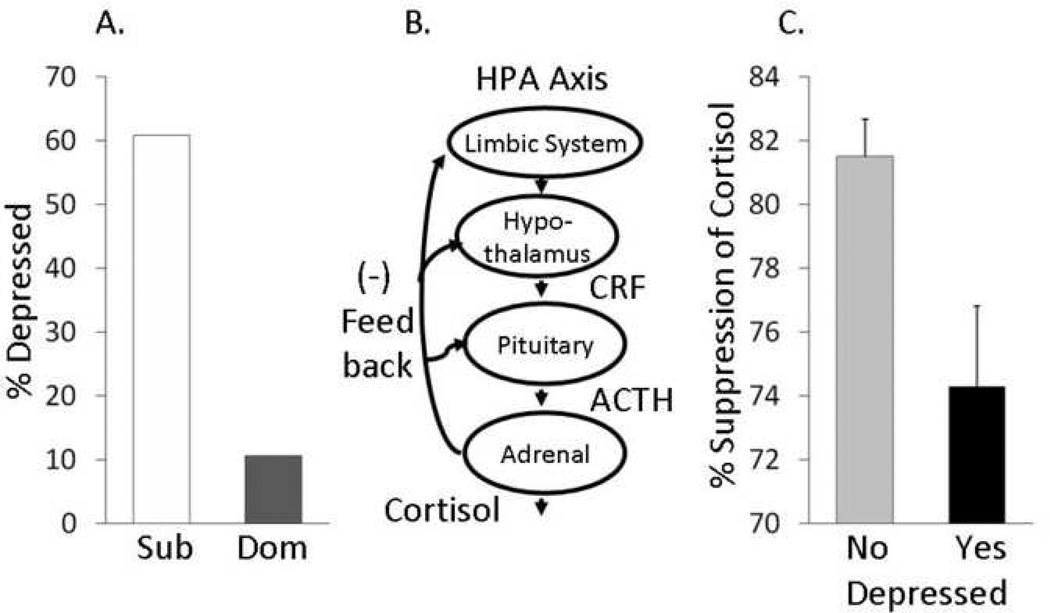
Social Status (Figure 2A)
Figure 2. Social Status, hypothalamic-pituitary-adrenal function, and depressive behavior.
A: Subordinate (Sub) female cynomolgus monkeys are more likely than dominants (Dom) to display depressive behavior; B. Schematic of the hypothalamic-pituitary-adrenal (HPA) axis (CRH= corticotrophin releasing hormone; ACTH=adrenocorticotrophic hormone). C: Dexamethasone suppression test. Females that display depressive behavior are relatively insensitive to glucocorticoid negative feedback. Adapted from Shively et al., 1997.
Depressive behavior was more commonly observed in subordinates than dominants. Sixty-one percent of subordinate females displayed depressive behavior, whereas only 10% of dominants were ever observed in this behavior (p < 0.05; Shively et al., 1997). The stress associated with low social status may increase depressive behavior.
Hypothalamic-Pituitary-Adrenal (HPA) Function (Figure 2B & C)
The bimodal nature of the expression of behavioral depression suggested that it may be useful to consider this behavior as a categorical variable. Thus, females were divided into two groups: Those that were ever observed in the depressed posture (n = 16), and those that never exhibited behavioral depression (n = 26). Females that were never observed in the depressed posture were not different from nondepressed females in baseline cortisol concentrations; however, females with behavioral depression had higher cortisol concentrations in response to dexamethasone suppression (p < 0.001), and did not suppress cortisol as well in response to dexamethasone (p = 0.006) compared to their nondepressed counterparts (Shively et al., 1997). This insensitivity to negative feedback would promote sustained high levels of cortisol. Thus, like human beings, depressive behavior in female cynomolgus monkeys is associated with low social status, and depressed individuals have perturbations in HPA axis function.
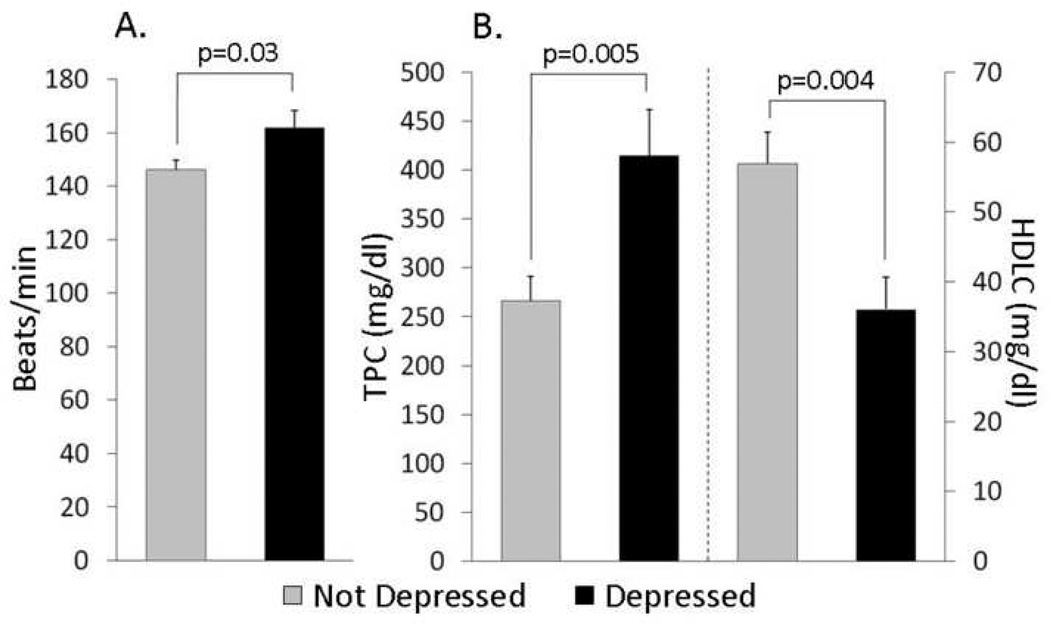
Heart Rate (Figure 3A)
Figure 3. Physiological characteristics of monkeys that display depressive behavior (n=15) compared to controls (n=21).
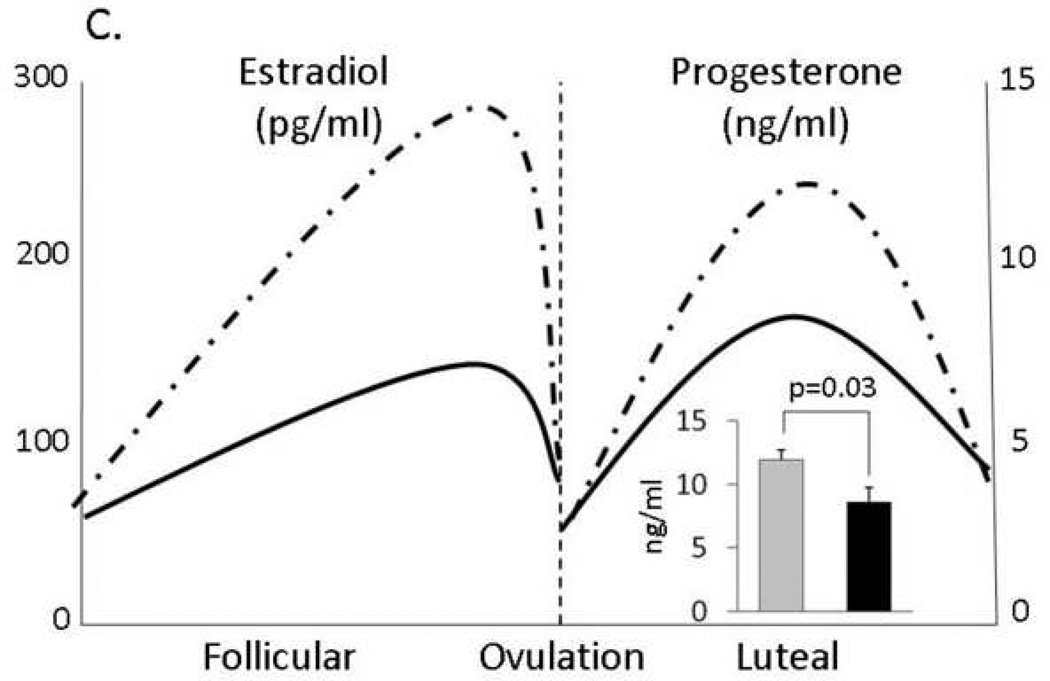
A: Average heart rates over 24 hours; B: Plasma lipid profiles of depressed and control premenopausal monkeys fed moderately atherogenic diets (TPC = total plasma cholesterol, mg/dl; HDLC = high density lipoprotein cholesterol, mg/dl); C: Ovarian function: Dotted line represent controls; black line represent depressed females. Estradiol concentrations taken from Adams et al., 1985; progesterone data taken from Shively et al., 2005.
In this experiment, heart rates (HRs) were recorded by telemetry in a randomly chosen subset of the monkeys (n = 25) while in single cages prior to social grouping. Females that later exhibited behavioral depression in social groups had higher average overnight HRs in single cages than nondepressed monkeys (p = 0.04) suggesting that inherent differences in stress responsivity (to single caging) may predict depressive behavior (Shively et al., 2002).
HR and depression were studied in a subsequent experiment of 36 females housed in social groups of four females each. After a year in social groups, HR was recorded for 24 hours using telemetry and averaged for analysis. During the preceding year depressive behavior was recorded. During this time period, depressive behavior was not significantly associated with social status, although the relationship emerged in subsequent years (Shively et al., 2005). The HRs of 15 females that displayed above average levels of behavioral depression (depressed) were compared to those of 21 females that displayed below average behavioral depression (control). Depressed females had higher overnight baseline HRs than controls (p = 0.03; Shively et al., 2005; Figure 3A). Increased HR exacerbates CAA, and HR lowering inhibits stress-induced coronary atherogenesis in cynomolgus monkeys (Kaplan et al., 1987; Manuck et al., 1997). Thus, high HR in depressed monkeys may increase CAA.
Plasma Lipid Concentrations (Figure 3B)
TPC and HDLC concentrations were also measured after a year in social groups and compared between these 15 depressed and 21 control monkeys. Depressed females had higher TPC and lower HDLC than controls (Shively et al., 2005).
Ovarian Function (Figure 3C)
These monkeys were trained to present themselves for vaginal swabbing to detect menses and for femoral venipuncture to collect blood for progesterone assay three times a week during the year that depressive behavior was quantified. Luteal-phase progesterone concentration was used as an indicator of the quality of a menstrual cycle, with high progesterone concentrations (> 4 ng/ml) indicating that ovulation had occurred, low progesterone concentrations (< 2 ng/ml) indicating an anovulatory cycle, and concentrations between 2 and 4 ng/ml suggesting luteal-phase impairment (Shively et al., 1997). The highest progesterone value found during the luteal phase was used to represent that menstrual cycle, and these peak progesterone concentrations were averaged for analysis. Depressed monkeys had lower mean peak luteal phase progesterone concentrations, indicating relatively impaired ovarian function compared to nondepressed monkeys. Low luteal phase progesterone concentrations are indicative of low follicular phase estradiol levels (Adams et al., 1985), thus depressed females may be estrogen-deficient.
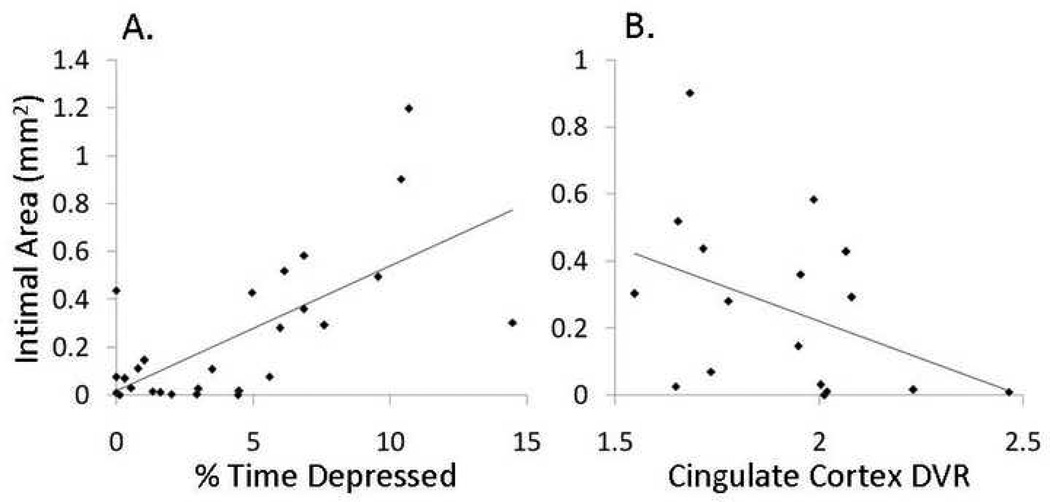
Coronary Artery Atherosclerosis (Figure 4A)
Figure 4. Coronary Artery Atherosclerosis, Depressive Behavior, and Neural Serotonergic Function.
The relationship between coronary artery atherosclerosis (CAA) measured as plaque (intimal) area and depressive behavior (A); and average serotonin (5-HT) receptor 1A binding availability in 3 ROIs of the cingulate cortex (DVR = distribution volume ratio) (B).
Because poor ovarian function, high HRs and dyslipidemia are known to exacerbate CAA, we examined CAA extent in these monkeys. Atherosclerosis extent, measured as the cross-sectional plaque area in the coronary arteries of 27 monkeys, was highly correlated with percent time spent in the depressive behavior (r = 0.67, p < 0.001, 2-sided test). This suggests that, in primates, the relationship between depression and CHD risk extends back to early atherogenesis (Shively et al., in press).
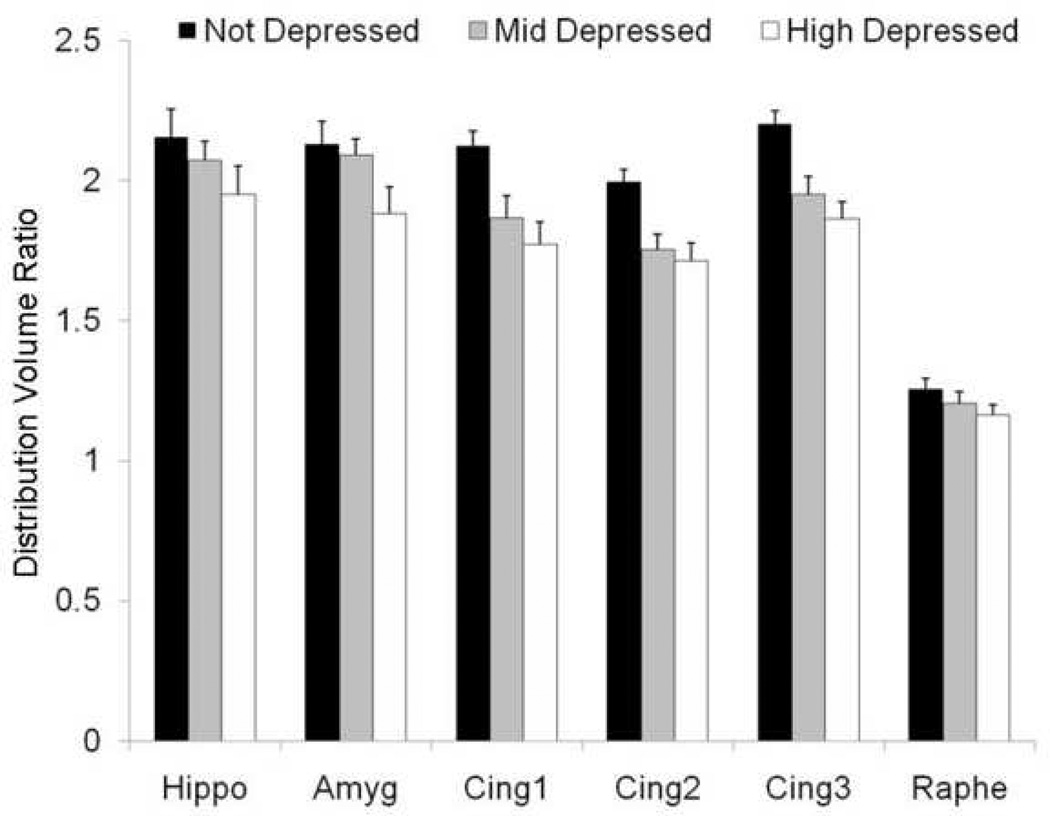
Neural Serotonergic Receptor Binding and Depressive Behavior (Figure 5)
Figure 5. Serotonin (5-HT) 1A receptor binding availability and depressive behavior.
The distribution of behavioral depression was divided into tertiles: none (n = 6), mid (n = 6), and highest amounts of time spent depressed (n = 5). Hippo = hippocampus; amyg = amygdala; cing = cingulate cortex; DVR= Distribution volume ratio [(ROI % injected dose / c.c. tissue) / (cerebellum % injected dose / c.c. tissue]. Adapted from Shively et al., 2006.
Serotonin (5-HT) 1A receptor binding potential was measured in 17 of the monkeys from the latter experiment, using positron emission tomography (PET) and the radioligand 18F-MPPF. The monkeys were rank-ordered by percent time spent in the depressed posture. Eight monkeys from the top and 9 from the bottom of the distribution of depression were scanned. Eleven regions of interest (ROI) implicated in depression were identified: amygdala, hippocampus, cingulate gyrus (three adjacent sites in area 24a just rostral to the genu of the corpus callosum), all of which were measured bilaterally, and midbrain raphe nucleus (dorsal raphe nucleus, just ventral to the aqueduct of Sylvius in the midbrain). Two ROIs were measured in the cerebellum, where 5-HT1A binding is low and not different between depressed and nondepressed monkeys, averaged, and used in the calculation of Logan values (distribution volume ratios or DVRs).
The distribution of behavioral depression was divided into tertiles: none (n = 6), mid (n = 6), and highest amounts of time spent depressed (n = 5). The relationship between behavioral depression and 5-HT1A receptor binding potential was evaluated with a 3 (levels of depression) X 11 (ROIs) ANOVA, and a significant main effect of depression was found (p = 0.003). Depressed animals had lower 5HT1A binding potential than their nondepressed counterparts across all brain areas measured. While there were differences in 5-HT1A binding among brain areas (p<0.001), there was no difference in the relationship between 5-HT1A binding potential and depression by brain areas (levels of depression X brain areas interaction p=0.44), suggesting that the relationship between 5-HT1A binding potential and depression was homogeneous across all brain areas. In most neuroanatomic regions the relationship appeared to be graded: that is, monkeys that fell in the middle tertile of depression scores also had intermediate binding potentials. Social status was not significantly associated with 5-HT1A binding potential in any brain region, and there were no significant laterality effects (Shively et al., 2006).
Decreased 5-HT1A binding potential in multiple neural areas has been reported in humans during depressive bouts and in remitted depressives, suggesting that this characteristic may be a trait, rather than a state marker of depression (Drevets et al., 1999; Sargent et al., 2000; Bhagwagar et al., 2004). These data suggest that the neurobiological substrates of depressive behavior in human and nonhuman primates may be similar (Shively et al., 2006).
Neural Serotonergic Receptor Binding and Coronary Artery Atherosclerosis Extent (Figure 4B)
The monkeys in which 5-HT1A binding potential was measured also had CAA measurements (intimal or plaque area). We examined the degree of relationship between CAA extent and 5-HT1A binding in the 11 ROIs. Atherosclerosis extent was significantly correlated with 5-HT1A binding potential in all three ROIs in the cingulate cortex (r = 0.48, p = 0.05, 2-sided test, n=17). The relationship between average cingulate cortex binding potential and atherosclerosis extent is shown in Figure 4B. This observation suggests the possibility of a direct relationship between central serotonergic function and atherogenesis.
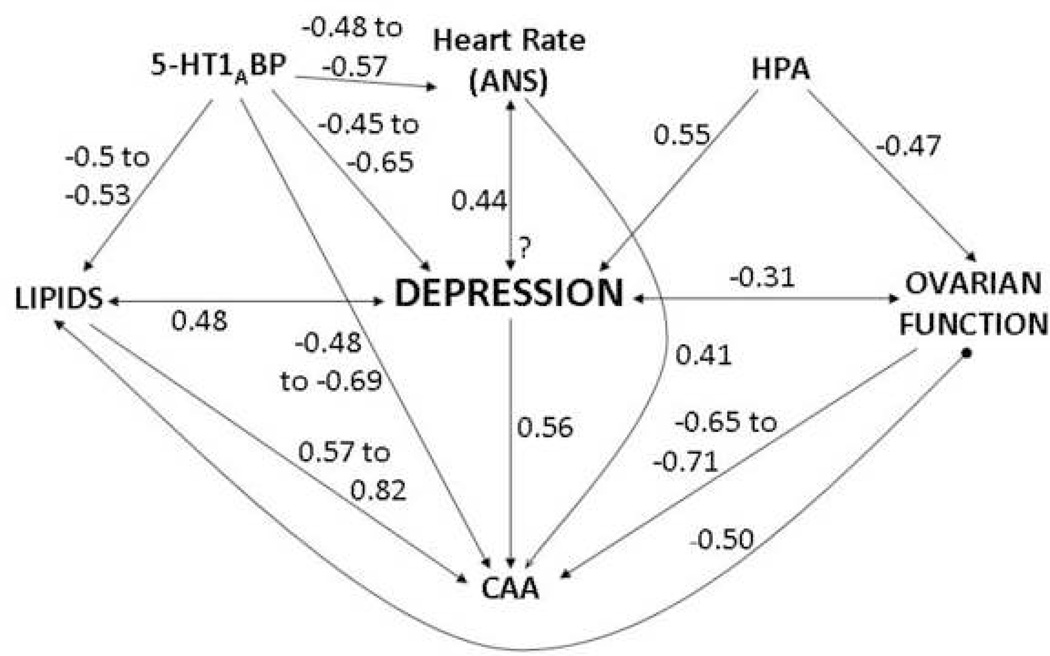
Synthesis (Figure 6)
Figure 6. Synthesis: A schematic representation of the hypothesized relationships between variables measured in our studies of coronary artery atherosclerosis (CAA) and depression.
The magnitude of the associations is approximated by univariate Pearson r correlations based on our data. Variables included had a correlation of at least 0.4 with either depression or CAA. Hypothesized directionality of relationships are based on the assumption that neural, autonomic, and HPA activity are primary precipitating factors, and depression and CAA are the disease outcomes; however, the directionality of some of these relationships is not well understood, denoted by a “?” where particularly little data are available. The strongest predictors of CAA in female primates are ovarian function, depressive behavior, circulating cholesterol concentrations, and neural serotonin function (HPA= hypothalamic-pituitary-adrenal axis; 5-HT1A BP=serotonin 1A receptor binding potential). Data taken from Shively et al., 1997; 2005; 2006; in press.
This schematic summarizes hypothesized relationships between variables that we have measured in our prior studies of CAA and depression. The magnitude of the associations is approximated by univariate Pearson r correlations based on our data. Variables included had a correlation of at least 0.4 with either depression or CAA. Hypothesized directionality of relationships are based on the assumption that neural, autonomic, and HPA activity are primary precipitating factors, and depression and CAA are the disease outcomes; however, the causality and directionality of many of these relationships are not well understood. While these are estimates, the strongest predictors of CAA in premenopausal female primates appear to be ovarian function, depressive behavior, circulating cholesterol concentrations, and neural serotonin function.
V. Conclusions
Gregarious societies, in which individuals live in groups larger than the nuclear family, are organized along hierarchical lines. When resources are plentiful, societies tend to be more egalitarian. When resources become scarce, societies become more competitive, and the stress associated with social living increases. In highly competitive situations those low in social status garner less resources that those of higher social status. However, they continue to survive albeit with greater morbidity and mortality. Social inequalities in health of human populations results in a devastating excess of disease for people of low socioeconomic status. Depression, CHD, and other pathologies are inversely related with social status in human beings and socially housed laboratory macaques. This would seem to suggest that socially housed laboratory macaques are a valid model of these aspects of human health. Since the inverse relationship between social status and health is relatively linear, the excess disease risk does not appear to be a product of poverty and deprivation. Psychological stress increases with decreasing social status in human and nonhuman primates. A growing body of evidence supports the hypothesis that the stress of low social status is a significant contributor to disease risk.
Less clear is the nature of the relationship between social stress, depression, and CHD. There are three possibilities: 1) stress may independently increase risk of depression, and of CHD, and the depression - CHD relationship may be an epiphenomena; 2) stress may increase depression which in turn increases CHD; or 3) both. The primate data currently available are consistent with the hypothesis that depression may cause CAA, and also with the hypothesis that CAA and depression may be the result of social stress. Determining which of these is the correct model may be important in designing the most efficacious interventions. Whichever model is the most accurate, however, ameliorating the detrimental effects of the stress of low social status would promote public health.
Behavior is the ultimate functional expression of neurobiological processes. Behavioral phenotyping in animal models has been undervalued in recent years to the detriment of basic and preclinical science. The primate model of depression presented here was developed based on the observation of a unique behavior pattern, which let to a series of questions concerning the importance of this behavior pattern to the health of the individual. This type of neuroethological approach has the potential to uncover novel and valuable animal models.
It is probably not coincidental that this unique behavior pattern was first recognized in studies of atherogenesis in which the monkeys were being fed a typical American diet containing significant amounts of fat and cholesterol and low in omega-3 fatty acids. Diet is a major experimental variable that is often ignored in animal modeling of human disease. Most commercial lab chows are designed to promote health and do not reflect the intake of human beings in Western societies. With increasing awareness of the impact of diet on inflammatory and neurodegenerative processes, it is a variable that should be very carefully considered in animal modeling of neurobiological processes.
The data from the monkey model paint a compelling picture of depression as a whole-body disease. Here were depicted perturbations in neural serotonergic function, autonomic function, HPA function, lipid metabolism, ovarian function, and artery wall pathology in association with depressive behavior. The fact that heart rates were higher in depressed monkeys at night suggest that sleep may be disrupted, an area in need of further study. Recently, we reported that anterior hippocampal volume was decreased in depressed female cynomolgus monkeys (Willard et al., in press). Elsewhere, we have reported lower insulin-like growth factor 1 (IGF-1), body weight, and body mass index, indicating perturbations of the somatotrophic axis and energy balance, as well as an association between depression and visceral obesity (Shively et al., 2005; Shively and Wallace 2001). A similar breadth of perturbations is characteristic of human depressives. Across the spectrum of depressive disorders mind-body dualistic thinking must finally be cast aside in order for there to be progress in understanding this disease. In order to understand depression, the neurosciences must embrace and understand the peripheral perturbations of depression as they feed back and influence central nervous system function.
A continuing quest is to understand exactly how environmental stress is transduced by the central nervous system into detrimental effects on central as well as peripheral health such as artery wall pathology. The primate data suggest that serotonin neurotransmission, particularly in cingulate cortex area 24a just rostral to the genu of the corpus callosum, may be an important link. Cingulate cortex 5-HT1A binding potential in this model is not only significantly inversely associated with depressive behavior and CAA reviewed here, but also with heart rate (Shively et al., 2006). Recent neuroimaging studies have demonstrated that sympathetic outflow to the heart is modulated by the activity of the anterior cingulate cortex (reviewed in Wong et al., 2007). Thus, in primates, a likely nexus for the depression-cardiovascular disease connection is the cingulate cortex.
This review has focused on research in female cynomolgus monkeys. However, in the human population, depression and CHD are inversely related to socioeconomic status in both genders. Depression has not been studied in male macaques to our knowledge. However, Hamm et al. (1983) observed more extensive CAA in subordinate male and female monkeys suggesting that low social status may be detrimental to the health of both sexes. Of course, social status is not the only behavioral risk factor for disease. Personality traits such as dominance, hostility, and antagonism may also contribute to disease risk independent of social status.
It is difficult to identify determinants of the earliest phases of disease progression in human studies; thus, we study the nature of the relationship between depression and coronary artery atherogenesis in a well-characterized nonhuman primate model, the cynomolgus monkey. Like human beings, some monkeys respond to social stress with depressive behavior which is accompanied by perturbations in HPA, autonomic nervous system, lipid metabolism, ovarian, and neural serotonergic system function, all of which are associated either directly or indirectly with exacerbated CAA. The observation that some individuals of a closely related species such as the macaque respond to social stress with behavior and physiology reminiscent of the human response to social stress, suggests that our sensitivity to social stress was an early adaptation to social life. Such an early adaptation becomes inculcated in every aspect of physiology; thus these responses are difficult to change. The fact that these responses appear to be well conserved suggests an adaptation historically beneficial to the species. It remains to be seen whether our stress reactive systems are adaptive in the face of the stresses imposed by modern Western society.
Acknowledgements
This work was supported by HL39789, HL14164, HL087103, MH5688, MH071580 and The John D. and Catherine T. MacArthur Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carol A. Shively, Department of Pathology, Wake Forest University School of Medicine, Winston-Salem, NC 27157
Dominique L. Musselman, Department of Psychiatry and Behavioral Sciences, Emory University School of Medicine, Atlanta, GA 30322.
Stephanie L. Willard, Integrative Neuroscience Graduate Program, Wake Forest University School of Medicine, Winston-Salem, NC 27157
References
- Adams MR, Kaplan JR, Clarkson TB, Koritnik DR. Ovariectomy, social status, and atherosclerosis in cynomolgus monkeys. Arteriosclerosis. 1985;5:192–200. doi: 10.1161/01.atv.5.2.192. [DOI] [PubMed] [Google Scholar]
- Adler N, Singh-Manoux A, Schwartz J, Stewart J, Matthews K, Marmot MG. Social status and health: a comparison of British civil servants in Whitehall-II with European- and African-Americans in CARDIA. Soc Sci Med. 2008;66(5):1034–1045. doi: 10.1016/j.socscimed.2007.11.031. [DOI] [PubMed] [Google Scholar]
- Adler NE, Rehkopf DH. U.S. disparities in health: description, causes and mechanisms. Annu Rev Public Health. 2008;29:235–252. doi: 10.1146/annurev.publhealth.29.020907.090852. [DOI] [PubMed] [Google Scholar]
- Amaral D, Lavenex P. Hippocampal neuroanatomy. In: Anderson P, Morris R, Amaral D, Bliss T, O’Keefe J, editors. The HC Book. Oxford University Press; 2007. pp. 37–114. [Google Scholar]
- Agatisa PK, Matthews KA, Bromberger JT, Edmundowicz D, Chang YF, Sutton-Tyrrell K. Coronary and aortic calcification in women with a history of major depression. Arch Intern Med. 2005;165:1229–1236. doi: 10.1001/archinte.165.11.1229. [DOI] [PubMed] [Google Scholar]
- Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5-HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- Bairey Merz CN, Johnson BD, Sharaf BL, Bittner V, Berga SL, Braunstein GD, Hodgson TK, Matthews KA, Pepine CJ, Reis SE, Reichek N, Rogers WJ, Pohost GM, Kelsey SF, Sopko G WISE Study Group. Hypoestrogenemia of hypothalamic origin and coronary artery disease in premenopausal women: a report from the NHLBI-sponsored WISE study. J Am Coll Cardiol. 2003;41:413–419. doi: 10.1016/s0735-1097(02)02763-8. [DOI] [PubMed] [Google Scholar]
- Basterzi AD, Aydemir C, Kisa C, Aksaray S, Tuzer V, Yazici K, Goka E. IL-6 levels decrease with SSRI treatment in patients with major depression. Hum Psychopharmacol. 2005;20:473–476. doi: 10.1002/hup.717. [DOI] [PubMed] [Google Scholar]
- Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation. 1992;85:164–171. doi: 10.1161/01.cir.85.1.164. [DOI] [PubMed] [Google Scholar]
- Bradvik L, Berglund M. Late mortality in severe depression. Acta Psychiatr Scand. 2001;103:111–116. doi: 10.1034/j.1600-0447.2001.00212.x. [DOI] [PubMed] [Google Scholar]
- Broadley AJ, Korszun A, Abdelaal E, Moskvina V, Deanfield J, Jones CJ, Frenneaux MP. Metyrapone improves endothelial dysfunction in patients with treated depression. J Am Coll Cardiol. 2006;48:170–175. doi: 10.1016/j.jacc.2005.12.078. [DOI] [PubMed] [Google Scholar]
- Brotman DJ, Golden SH, Wittstein IS. The cardiovascular toll of stress. Lancet. 2007;370:1089–1100. doi: 10.1016/S0140-6736(07)61305-1. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO. Aetiology of anxiety and depressive disorders in an inner-city population. 1. Early adversity. Psychol Med. 1993;23:143–154. doi: 10.1017/s0033291700038939. [DOI] [PubMed] [Google Scholar]
- Brown GW, Moran P. Clinical and psychosocial origins of chronic depressive episodes. I: A community survey. Br J Psychiatry. 1994;165:447–456. doi: 10.1192/bjp.165.4.447. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Life events and endogenous depression, a puzzle reexamined. Arch Gen Psychiatry. 1994;51:523–534. doi: 10.1001/archpsyc.1994.03950070017006. [DOI] [PubMed] [Google Scholar]
- Brown GW, Harris TO, Hepworth C. Loss, humiliation and entrapment among women developing depression: a patient and non-patient comparison. Psychol Med. 1995;25:7–21. doi: 10.1017/s003329170002804x. [DOI] [PubMed] [Google Scholar]
- Bruce EC, Musselman DL. Depression, alterations in platelet function, and ischemic heart disease. Psychosom Med. 2005;67(Suppl 1):S34–S36. doi: 10.1097/01.psy.0000164227.63647.d9. [DOI] [PubMed] [Google Scholar]
- Brydon L, Magid K, Steptoe A. Platelets, coronary heart disease, and stress. Brain Behav Immun. 2006;20:113–119. doi: 10.1016/j.bbi.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Buckmaster PS, Amaral DG. Intracellular recording and labeling of mossy cells and proximal CA3 pyramidal cells in macaque monkeys. J Comparative Neurol. 2001;430:264–281. doi: 10.1002/1096-9861(20010205)430:2<264::aid-cne1030>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Calabro P, Yeh ET. Intra-abdominal adiposity, inflammation, and cardiovascular risk: new insight into global cardiometabolic risk. Curr Hypertens Rep. 2008;10(1):32–38. doi: 10.1007/s11906-008-0008-z. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Rich MW, Smith LJ, Jaffe AS. Ventricular tachycardia and psychiatric depression in patients with coronary artery disease. Am J Med. 1993;95:23–28. doi: 10.1016/0002-9343(93)90228-h. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Miller GE, Jaffe AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res. 2002;53(4):897–902. doi: 10.1016/s0022-3999(02)00311-2. [DOI] [PubMed] [Google Scholar]
- Charlson M, Peterson JC. Medical comorbidity and late life depression: what is known and what are the unmet needs? Biol Psychiatry. 2002;52:226–235. doi: 10.1016/s0006-3223(02)01422-1. [DOI] [PubMed] [Google Scholar]
- Chen CC, Lu FH, Wu JS, Chang CJ. Correlation between serum lipid concentrations and psychological distress. Psychiatry Res. 2001;102:153–162. doi: 10.1016/s0165-1781(01)00231-1. [DOI] [PubMed] [Google Scholar]
- Chilton FH, Rudel LL, Parks JS, Arm JP, Seeds MC. Mechanisms by which botanical lipids affect inflammatory disorders. Am J Clin Nutr. 2008;87(2):498S–503S. doi: 10.1093/ajcn/87.2.498S. [DOI] [PubMed] [Google Scholar]
- Clarkson TB. Estrogen effects on arteries vary with stage of reproductive life and extent of subclinical atherosclerosis progression. Menopause. 2007;14:373–384. doi: 10.1097/GME.0b013e31803c764d. [DOI] [PubMed] [Google Scholar]
- Clarkson TB, Hughes CL, Klein KP. The nonhuman primate model of the relationship between gonadal steroids and coronary heart disease. Prog Cardiovasc Dis. 1995;38:189–198. doi: 10.1016/s0033-0620(95)80011-5. [DOI] [PubMed] [Google Scholar]
- Cohen HW, Gibson G, Alderman MH. Excess risk of myocardial infarction in patients treated with antidepressant medications: association with use of tricyclic agents. Am J Med. 2000;108:2–8. doi: 10.1016/s0002-9343(99)00301-0. [DOI] [PubMed] [Google Scholar]
- Cooney JM, Dinan TG. Hypothalamic-pituitary-adrenal axis early-feedback responses are preserved in melancholic depression: a study of sertraline treatment. Hum Psychopharmacol. 2000;15:351–356. doi: 10.1002/1099-1077(200007)15:5<351::AID-HUP193>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Dalla C, Edgecomb C, Whetstone AS, Shors TJ. Females do not express learned helplessness like males do. Neuropsychopharmacology. 2007 Aug 22; doi: 10.1038/sj.npp.1301533. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Das UN. Folic acid and polyunsaturated fatty acids improve cognitive function and prevent depression, dementia, and Alzheimer's disease--but how and why? Prostaglandins Leukot Essent Fatty Acids. 2008;78(1):11–19. doi: 10.1016/j.plefa.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Diaz-Sastre C, Baca-Garcia E, Perez-Rodriguez MM, Garcia-Resa E, Ceverino A, Saiz-Ruiz J, Oquendo MA, de Leon J. Low plasma cholesterol levels in suicidal males: a gender- and body mass index-matched case-control study of suicide attempters and nonattempters. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31(4):901–905. doi: 10.1016/j.pnpbp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, Huang Y, Gautier C, Mathis C. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Fourth Joint Task Force of the European Society of Cardiology and other societies on cardiovascular disease prevention in clinical practice (constituted by representatives of nine societies and by invited experts) European Heart Journal. 2007;28:2375–2414. doi: 10.1093/eurheartj/ehm316. [DOI] [PubMed] [Google Scholar]
- Executive Summary of the NHLBI Working Group on Cardiovascular Consequences of Chronic Stress. 2004 http://www.nhlbi.nih.gov/meetings/workshops/heart_stress.htm.
- Feldman PJ, Steptoe A. How neighborhoods and physical functioning are related: the roles of neighborhood socioeconomic status, perceived neighborhood strain, and individual health risk factors. Ann Behav Med. 2004;27:91–99. doi: 10.1207/s15324796abm2702_3. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N. In-hospital symptoms pf psychological stress as predictors of long-term outcome after acute myocardial infarction in men. Am J Cardiol. 1991;67:121–127. doi: 10.1016/0002-9149(91)90432-k. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Talajic M. Depression and 18-month prognosis after myocardial infarction. Circulation. 1995;91:999–1005. doi: 10.1161/01.cir.91.4.999. [DOI] [PubMed] [Google Scholar]
- Glassman AH, Bigger JT, Gaffney M, Van Zyl LT. Heart rate variability in acute coronary syndrome patients with major depression: influence of sertraline and mood improvement. Arch Gen Psychiatry. 2007;64:1025–1031. doi: 10.1001/archpsyc.64.9.1025. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O’Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, Jr, Krishnan KR, van Zyl LT, Swenson JR, Finkel MS, Landau C, Shapiro PA, Pepine CJ, Mardekian J, Harrison WM, Barton D, Mclvor M. Sertraline Antidepressant Heart Attack Randomized Trial (SADHEART) Group. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- Gorman JM. Gender differences in depression and response to psychotropic medication. Gend Med. 2006;3:93–109. doi: 10.1016/s1550-8579(06)80199-3. [DOI] [PubMed] [Google Scholar]
- Grambsch P, Young EA, Meller WH. Pulsatile luteinizing hormone disruption in depression. Psychoneuroendocrinology. 2004;29:825–829. doi: 10.1016/S0306-4530(03)00146-X. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–962. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Hamm TE, Jr, Kaplan JR, Clarkson TB, Bullock BC. Effects of gender and social behavior on the development of coronary artery atherosclerosis in cynomolgus macaques. Atherosclerosis. 1983;48(3):221–233. doi: 10.1016/0021-9150(83)90040-0. [DOI] [PubMed] [Google Scholar]
- Hanke H, Hanke S, Ickrath O, Lange K, Bruck B, Muck AO, Seeger H, Zwirner M, Voisard R, Haasis R, Hombach V. Estradiol concentrations in premenopausal women with coronary heart disease. Coron Artery Dis. 1997;8:511–515. [PubMed] [Google Scholar]
- Harlow BL, Signorello LB. Factors associated with early menopause. Maturitas. 2000;35(1):3–9. doi: 10.1016/s0378-5122(00)00092-x. [DOI] [PubMed] [Google Scholar]
- Harlow BL, Wise LA, Otto MW, Soares CN, Cohen LS. Depression and its influence on reproductive endocrine and menstrual cycle markers associated with perimenopause: the Harvard Study of Moods and Cycles. Arch Gen Psychiatry. 2003;60:29–36. doi: 10.1001/archpsyc.60.1.29. [DOI] [PubMed] [Google Scholar]
- Heart Disease and Stroke Statistics 2008 Update. A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. Dec 17; doi: 10.1161/CIRCULATIONAHA.107.187998. [Epub ahead of print] Jan 29, 2008 pub date. [DOI] [PubMed] [Google Scholar]
- Hemingway H, Shipley M, Macfarlane P, Marmot M. Impact of socioeconomic status on coronary mortality in people with symptoms, electrocardiographic abnormalities, both or neither: the original Whitehall study 25 year follow up. J Epidemiol Community Health. 2000;54:510–516. doi: 10.1136/jech.54.7.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans LA, Riedel WJ, Markus CR, Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol Psychiatry. 2007;12:522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Kannel WB, Kannel C, Paffenbarger RS, Jr, Cupples LA. Heart rate and cardiovascular mortality: the Framingham Study. Am Heart J. 1987;113:1489–1494. doi: 10.1016/0002-8703(87)90666-1. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction, stress, and disease: a primate continuum. ILAR J. 2004;45:89–115. doi: 10.1093/ilar.45.2.89. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Clarkson TB. The influence of heart rate on coronary artery atherosclerosis. J Cardiovasc Pharmacol. 1987;10(Suppl 2):S100–S103. [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet Gynecol. 2002;99:381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am J Psychiatry. 2006;163:109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Chaouloff F, Marcou M, Curzon G. Female rats are more vulnerable than males in an animal model of depression: the possible role of serotonin. Brain Res. 1986;382:416–421. doi: 10.1016/0006-8993(86)91355-7. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGongle KA, Zhao S, Nelson CS, Hughes M, Eshlelman S, Wittchen H, Kendler KS. Lifetime and 12-month prevalence of DSM-III-R psychiatric disorders in the United States. Arch Gen Psychiat. 1994;51:8–19. doi: 10.1001/archpsyc.1994.03950010008002. [DOI] [PubMed] [Google Scholar]
- Konkle AT, Baker SL, Kentner AC, Barbagallo LS, Merali Z, Bielajew C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: sex and strain compared. Brain Res. 2003;992:227–238. doi: 10.1016/j.brainres.2003.08.047. [DOI] [PubMed] [Google Scholar]
- Kopp MS, Skrabski A, Székely A, Stauder A, Williams R. Chronic stress and social changes: socioeconomic determination of chronic stress. Ann N Y Acad Sci. 2007;1113:325–338. doi: 10.1196/annals.1391.006. [DOI] [PubMed] [Google Scholar]
- Kouzis A, Eaton WW, Leaf PJ. Psychopathology and mortality in the general population. Soc Psychiatry Psychiatr Epidemiol. 1995;30:165–170. doi: 10.1007/BF00790655. [DOI] [PubMed] [Google Scholar]
- Lorant V, Deliège D, Eaton W, Robert A, Philippot P, Ansseau M. Socioeconomic inequalities in depression: a meta-analysis. Am J Epidemiol. 2003;157(2):98–112. doi: 10.1093/aje/kwf182. [DOI] [PubMed] [Google Scholar]
- Mäkikallio TH, Seppänen T, Airaksinen KE, Koistinen J, Tulppo MP, Peng CK, Goldberger AL, Huikuri HV. Dynamic analysis of heart rate may predict subsequent ventricular tachycardia after myocardial infarction. Am J Cardiol. 1997;80:779–783. doi: 10.1016/s0002-9149(97)00516-x. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Adams MR, McCaffery JM, Kaplan JR. Behaviorally elicited heart rate reactivity and atherosclerosis inovariectomized cynomolgus monkeys (Macaca fascicularis) Arterioscler Thromb Vasc Biol. 1997;17:1774–1779. doi: 10.1161/01.atv.17.9.1774. [DOI] [PubMed] [Google Scholar]
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer B, Erdmann J, Schunkert H. Genetics and heritability of coronary artery disease and myocardial infarction. Clin Res Cardiol. 2007;96:1–7. doi: 10.1007/s00392-006-0447-y. [DOI] [PubMed] [Google Scholar]
- McCaffery JM, Frasure-Smith N, Dubé MP, Théroux P, Rouleau GA, Duan Q, Lespérance F. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med. 2006;68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0. [DOI] [PubMed] [Google Scholar]
- McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J. 2001;142:617–623. doi: 10.1067/mhj.2001.116766. [DOI] [PubMed] [Google Scholar]
- Meier CR, Schlienger RG, Jick H. Use of selective serotonin reuptake inhibitors and risk of developing first-time acute myocardial infarction. Br J Clin Pharmacol. 2001;52:179–184. doi: 10.1046/j.0306-5251.2001.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meller WH, Zander KM, Crosby RD, Tagatz GE. Luteinizing hormone pulse characteristics in depressed women. Am J Psychiatry. 1997;154:1454–1455. doi: 10.1176/ajp.154.10.1454. [DOI] [PubMed] [Google Scholar]
- Meller WH, Grambsch PL, Bingham C, Tagatz GE. Hypothalamic pituitary gonadal axis dysregulation in depressed women. Psychoneuroendocrinology. 2001;26:253–259. doi: 10.1016/s0306-4530(00)00050-0. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Sutton-Tyrrell K, Flory JD, Pollock BG, Manuck SB. Lower central serotonergic responsivity is associated with preclinical carotid artery atherosclerosis. Stroke. 2007;38:2228–2233. doi: 10.1161/STROKEAHA.106.477638. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Lopez AD. Alternative projections of mortality and disability by cause 1990–2020: Global Burden of Disease Study. Lancet. 1997;349:1498–1504. doi: 10.1016/S0140-6736(96)07492-2. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Cowles M, McDonald W, Nemeroff CB. Effects of Mood and Anxiety Disorders on the Cardiovascular System: Implications for Treatment. In: Fuster V, Alexander RW, O’Rourke RA, Roberts R, King SB, Wellens HJJ, editors. Hurst’s The Heart. 12th Edition. New York: McGraw Hill; 2008. pp. 2169–2187. [Google Scholar]
- Pollitt RA, Rose KM, Kaufman JS. Evaluating the evidence for models of life course socioeconomic factors and cardiovascular outcomes: a systematic review. BMC Public Health. 2005;5:7. doi: 10.1186/1471-2458-5-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riso LP, Miyatake RK, Thase ME. The search for determinants of chronic depression: a review of six factors. J Affect Disord. 2002;70:103–115. doi: 10.1016/s0165-0327(01)00376-7. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990’s. Nature. 1993;362:801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Rovner BW, German PS, Brant LJ, Clark R, Burton L, Folstein MF. Depression and mortality in nursing homes. JAMA. 1991;265:993–996. doi: 10.1001/jama.265.8.993. [DOI] [PubMed] [Google Scholar]
- Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–240. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- Rugulies R. Depression as a predictor for coronary heart disease. A review and meta-analysis. Am J Prev Med. 2002;23:51–61. doi: 10.1016/s0749-3797(02)00439-7. [DOI] [PubMed] [Google Scholar]
- Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, Gunn RN, Grasby PM, Cowen PJ. Brain serotonin1A receptor binding measured by positron emission tomography with [11C]WAY-100635: effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- Sauer WH, Berlin JA, Kimmel SE. Selective serotonin reuptake inhibitors and myocardial infarction. Circulation. 2001;104:1894–1898. doi: 10.1161/hc4101.097519. [DOI] [PubMed] [Google Scholar]
- Serebruany VL, Glassman AH, Malinin AI, Sane DC, Finkel MS, Krishnan RR, Atar D, Lekht V, O’Connor CM. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul Fibrinolysis. 2003;14:563–567. doi: 10.1097/00001721-200309000-00008. [DOI] [PubMed] [Google Scholar]
- Shively CA. Social subordination stress, behavior and central monoaminergic function in female cynomolgus monkeys. Biol Psychiatry. 1998;44:882–891. doi: 10.1016/s0006-3223(97)00437-x. [DOI] [PubMed] [Google Scholar]
- Shively C, Kaplan J. Effects of social factors on adrenal weight and related physiology in Macaca fascicularis. Physiol Behav. 1984;33:777–782. doi: 10.1016/0031-9384(84)90047-7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Kaplan JR. Stability of social status rankings of female cynomolgus monkeys, of varying reproductive condition, in different social groups. Am J Primatol. 1991;23:239–245. doi: 10.1002/ajp.1350230404. [DOI] [PubMed] [Google Scholar]
- Shively CA, Wallace JM. Social status, social stress and fat distribution in primates. In: Bjorntorp P, editor. International Textbook of Obesity. Sussex, England: John Wiley & Sons, Ltd; 2001. pp. 203–211. [Google Scholar]
- Shively CA, Laber-Laird K, Anton RF. The behavior and physiology of social stress and depression in female cynomolgus monkeys. Biol Psychiatry. 1997;41:871–882. doi: 10.1016/S0006-3223(96)00185-0. [DOI] [PubMed] [Google Scholar]
- Shively CA, Williams JK, Laber-Laird K, Anton RF. Depression and coronary artery atherosclerosis and reactivity in female cynomolgus monkeys. Psychosom Med. 2002;64:699–706. doi: 10.1097/01.psy.0000021951.59258.c7. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Friedman D, Morgan T, Thompson J, Lanier T. Social stress-associated depression in adult female cynomolgus monkeys (Macaca fascicularis) Biological Psychology. 2005;69:67–84. doi: 10.1016/j.biopsycho.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Shively CA, Friedman DP, Gage HD, Bounds MC, Brown-Proctor C, Blair J, Henderson JA, Smith M, Buchheimer N. Behavioral depression and neural serotonin (5-HT1a) receptor binding potential determined by positron emission tomography in adult female cynomolgus monkeys. Arch Gen Psychiatry. 2006;63:396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Adams MR, Golden DL, Willard SL, Clarkson TB. Depressive behavior and coronary artery atherogenesis in adult female cynomolgus monkeys. Psychosomatic Medicine. doi: 10.1097/PSY.0b013e31817eaf0b. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores MM, Pascualy M, Lewis NL, Flatness D, Veith RC. Short-term sertraline treatment suppresses sympathetic nervous system activity in healthy human subjects. Psychoneuroendocrinology. 2001;26:433–439. doi: 10.1016/s0306-4530(01)00002-6. [DOI] [PubMed] [Google Scholar]
- Siepmann M, Grossmann J, Muck-Weymann M, Kirch W. Effects of sertraline on autonomic and cognitive functions in healthy volunteers. Psychopharmacology. 2003;168:293–298. doi: 10.1007/s00213-003-1448-4. [DOI] [PubMed] [Google Scholar]
- Solomon CG, Hu FB, Dunaif A, Rich-Edwards JE, Stampfer MJ, Willett WC, Speizer FE, Manson JE. Menstrual cycle irregularity and risk for future cardiovascular disease. J Clin Endocrinol Metab. 2002;87:2013–2017. doi: 10.1210/jcem.87.5.8471. [DOI] [PubMed] [Google Scholar]
- Stansfeld SA, Head J, Fuhrer R, Wardle J, Cattell V. Social inequalities in depressive symptoms and physical functioning in the Whitehall II study: Exploring a common cause explanation. J Epidemiol Community Health. 2003;57:361–367. doi: 10.1136/jech.57.5.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK, Carney RM, Freedland KE, Skala JA, Jaffe AS, Kleiger RE, Rottman JN. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res. 2000;48:317–320. doi: 10.1016/s0022-3999(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Steiner M, Lamont J, Steinberg S, Stewart D, Reid R, Streiner D. Effect of fluoxetine on menstrual cycle length in women with premenstrual dysphoria. Obstet Gynecol. 1997;90(4 Pt 1):590–595. doi: 10.1016/s0029-7844(97)00307-4. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Shamaei-Tousi A, Gylfe A, Henderson B, Bergström S, Marmot M. Socioeconomic status, pathogen burden and cardiovascular disease risk. Heart. 2007;93:1567–1570. doi: 10.1136/hrt.2006.113993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Suomi SJ, Eisele CD, Grady SA, Harlow HF. Depressive behavior in adult monkeys following separation from family environment. J Abnorm Psychol. 1975;84:576–578. doi: 10.1037/h0077066. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, Kaufmann PG, Shuster J, Mellman T, Blumenthal JA, Krishnan R, Jaffe AS ENRICHD Investigators. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Arch Gen Psychiatry. 2005;62(7):792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- Thakore JH, Barnes C, Joyce J, Medbak S, Dinan TG. Effects of antidepressant treatment on corticotropin-induced cortisol responses in patients with melancholic depression. Psychiatry Res. 1997;73(1–2):27–32. doi: 10.1016/s0165-1781(97)00106-6. [DOI] [PubMed] [Google Scholar]
- Tomfohr LM, Martin TM, Miller GE. Symptoms of depression and impaired endothelial function in healthy adolescent women. J Behav Med. 2007 Dec 29; doi: 10.1007/s10865-007-9141-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Tucker P, Ruwe WD, Masters B, Parker DE, Hossain A, Trautman RP, Wyatt DB. Neuroimmune and cortisol changes in selective serotonin reuptake inhibitor and placebo treatment of chronic posttraumatic stress disorder. Biol Psychiatry. 2004;56(2):121–128. doi: 10.1016/j.biopsych.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E. Increased serum tumor necrosis factor-alpha levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003 Dec;170:429–433. doi: 10.1007/s00213-003-1566-z. [DOI] [PubMed] [Google Scholar]
- von Ammon Cavanaugh S, Furlanetto LM, Creech SD, Powell LH. Medical illness, past depression, and present depression: a predictive triad for in-hospital mortality. Am J Psychiatry. 2001;158:43–48. doi: 10.1176/appi.ajp.158.1.43. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Williamson PM, Mangos G, Kelly JJ. Cardiovascular consequences of cortisol excess. Vasc Health Risk Manag. 2005;1:291–299. doi: 10.2147/vhrm.2005.1.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willard SL, Glover EJ, Friedman DP, Henkel CK, Shively CA. Anterior hippocampal volume is reduced in behaviorally depressed female Macaca fascicularis living in social groups. American Journal of Primatology. (in press). [Google Scholar]
- Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–II10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- Williams RB. Lower central nervous system serotonergic function and risk of cardiovascular disease: where are we, what’s next? Stroke. 2007;38(8):2213–2214. doi: 10.1161/STROKEAHA.107.494088. [DOI] [PubMed] [Google Scholar]
- Williams RB, Marchuk DA, Gadde KM, Barefoot JC, Grichnik K, Helms MJ, Kuhn CM, Lewis JG, Schanberg SM, Stafford-Smith M, Suarez EC, Clary GL, Svenson IK, Siegler IC. Central nervous system serotonin function and cardiovascular responses to stress. Psychosom Med. 2001;63(2):300–305. doi: 10.1097/00006842-200103000-00016. [DOI] [PubMed] [Google Scholar]
- Wong SW, Massé N, Kimmerly DS, Menon RS, Shoemaker JK. Ventral medial prefrontal cortex and cardiovagal control in conscious humans. Neuroimage. 2007;35(2):698–708. doi: 10.1016/j.neuroimage.2006.12.027. [DOI] [PubMed] [Google Scholar]
- Wulsin LR, Singal BM. Do depressive symptoms increase the risk for the onset of coronary disease? A systematic quantitative review. Psychosom Med. 2003;65(2):201–210. doi: 10.1097/01.psy.0000058371.50240.e3. [DOI] [PubMed] [Google Scholar]
- Yeragani VK, Roose S, Mallavarapu M, Radhakrishna RK, Pesce V. Major depression with ischemic heart disease: effects of paroxetine and nortriptyline on measures of nonlinearity and chaos of heart rate. Neuropsychobiology. 2002;46(3):125–135. doi: 10.1159/000066390. [DOI] [PubMed] [Google Scholar]
- Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitaryovarian axis in depressed women. Arch Gen Psychiatry. 2000;57(12):1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]
- Zhang J, McKeown RE, Hussey JR, Thompson SJ, Woods JR, Ainsworth BE. Low HDL cholesterol is associated with suicide attempt among young healthy women: the Third National Health and Nutrition Examination Survey. J Afffect Disord. 2005;89(1–3):25–33. doi: 10.1016/j.jad.2005.05.021. [DOI] [PubMed] [Google Scholar]