Abstract
Radiation therapy is a widely used cancer treatment and pre-transplantation conditioning regimen that has the potential to influence anti-tumor and post-transplantation immune responses. Although conventionally fractionated radiation doses can suppress immune responses by depleting lymphocytes, single high doses of local tumor radiation can enhance immune responses. Using phospho-flow cytometry analysis of a human monocytic cell line, we identified novel radiation-induced changes in the phosphorylation state of NFκB family members known in other cell types to maintain and regulate immune function. These phosphorylation changes were p53 independent, but were strongly dependent upon ATM activation due to DNA damage. We found that radiation promotes the activation and APC functional maturation through phosphorylation of NFκB Essential Modulator (NEMO). Our results and the analytic methods are especially well suited to the study of functional changes in APC when radiation is used for immune modulation in clinical protocols.
Keywords: monocyte, macrophage, signal transduction, irradiation, cell activation, immune response
1. Introduction
It is well known that irradiation of tumors can directly kill cancer cells, as well as tumor endothelial cells and other cells within the tumor stroma, with associated secondary effects on tumor cell viability [1, 2]. The effects on tumor infiltrating lymphocytes and antigen presenting cells (APC) within tumors are not well characterized [1-12]. Radiation is known to induce innate immune stimulatory signals [12, 13], including the release of pro-inflammatory cytokines (TNF-α, IL-1β, IL-2) [14-17], and upregulation of co-stimulatory molecules, heat shock proteins, death receptors, and major histocompatibility complex molecules [1, 12, 18, 19]. These “danger signals” can lead to maturation and activation of APC, which allow APC to process and present ingested antigen to T cells [20, 21] released by damaged and dead cells in irradiated tumors.
Therefore, the goal of the study, was to identify early radiation-induced signaling changes in APC and elucidate the effects of these signaling changes on APC receptor expression and function. We used the U937 cell line [22] derived from a histocytic lymphoma, that exhibits monocyte morphology and functions as an APC. The model was selected because it provides a homogenous population of cells, arrested in a pliant state of maturation [23].
We focused on the NFκB pathway because this pathway is activated by radiation in tumors and modulates the expression of various apoptotic and anti-apoptotic genes [25], and it is a critical regulator in the development and maintenance of T cells, B cells, and APC [26]. The NFκB pathway regulates immune events, such as the cytokine transcription, microbial phagocytosis, cell differentiation, and cell proliferation [24, 27]. In the classical or canonical pathway, NFκB complexes are inactive and reside in the cytoplasm in a complex with an inhibitory protein, IκBα. NFκB activation is mediated by the IkB kinase complex (IKK), with its’ two catalytically active kinases (IKKα and IKKβ), by the regulatory scaffold protein, NFκB Essential Modulator (NEMO). Phopshorylation and activation of NEMO is critical to phosphorylation and activation of IKKα and IKKβ, and activation of this complex leads to phosphorylation of IκBα, and release of NFκB for phosphorylation and entrance into the nucleus to modulate target gene expression [27, 28]. We hypothesize that radiation-induced effects on early events in the NFκB pathway may activate or functionally modify certain immune cells, particularly radioresistant APC. The experiments described here were designed to elucidate the poorly understood role of the NFκB pathway in radiation-induced changes in APC, which are relevant to better understanding of the mechanism by which radiation can enhance tumor immunity.
2. Materials and methods
2.1 Cells and culture
Human monocytic cells U937 (obtained from Garry Nolan, Stanford University, Stanford, California, originally from ATCC repository) were cultured in RPMI-1640 medium supplemented with 5% fetal calf serum, 1mM glutamine, penicillin/streptomycin in a humidified atmosphere containing 5% CO2 at 37°C with normal oxygen content or under hypoxic conditions (2% O2).
2.2 Irradiation
Cells were irradiated with γ-rays by using a 137Cs source with a fixed dose rate of 148 or 531 cGy/min.
2.3 Monoclonal antibodies and chemical reagents
Anti-CD3-FITC (UCHT1), Anti-CD4-PE, Anti-CD80-PE-Cy5, Anti-CD86-PE, Anti-CD40-FITC, Anti-FACS stain buffer, and Anti-Mouse IgK/Negative Control (FBS) Compensation Particles Set were purchased from BD Pharmingen. Anti p-IKKγ (NEMO) (S376)-FITC, Anti-pNFkB p65 (S536)-Alexa Fluor 488, Anti pIκBγ (Ser32) Rabbit mAb, were purchased from Cell Signaling Technology. LIVE/DEAD fixable dead cell stain kit in Aqua was purchased from Invitrogen.
2.4 Flow cytometry analysis
U937 cells were harvested and washed with PBS and stained for viable cell populations using LIVE/DEAD Fixable Green Dead Cell Stain or Ethidium Monoazide (EMA) (5μg/ml; Invitrogen). U937 cells were processed for flow cytometry as previously described [29].
2.5 Wildtype p53 transfection
pCMV-Neo-Bam p53 wt and pCMV-Neo-Bam empty control plasmids were purchased from Addgene Co.. Plasmid DNA was purified by isopropanol precipitation by centrifugation using Plasmid Midi Kit (Qiagen). U937 cells were cultured in fresh medium for one day, prior suspension in Electroporation Medium (10 μl BioBrene Plus per 100 ml and 0.2% (w/v) Glucose in PBS). DNA, suspended in 0.3 ml of Electroporation Medium at room temperature, was mixed with 0.5 ml of the cell suspension (final volume was 0.8 ml) in an electroporation cuvette. The mixture was electroporated at 960 microfarads, 300 V, left in the cuvettes at room temperature for 5 min, and then transferred to 1 ml of medium in a 15 ml tube and incubated for 5 to 10 min at room temperature. Cells were then cultured in medium, which was replaced every two days.
2.6 Cytoplasmic extract preparation and western blot analysis
Cells were lysed in cell extraction buffer (Invitrogen)., supplemented with 1mM PMSF, protease inhibitor cocktail, and phosphatase inhibitor cocktail (Sigma-Aldrich). Nuclei were separated by centrifugation and sonicated cytoplasmic extracts were obtained. The protein concentration in each sample was determined using the method of Bradford Protein Assay. Immunoblotting was performed with various antibodies p21, phospho-ATM (S1981), ATM, phospho-IKK-γ (Ser376), DNA-PK, ATR, and actin) from Cell Signaling Technology Corp. (Danvers, MA). Washed blots with TBS (1X)-Tween (1%)and incubated blots with anti-mouse IgG, or anti-rabbit IgG HRP-linked antibody. Immunoreactivity of the blots was detected by ECL Western Blot Detection Reagent (Amersham Biosciences).
2.7 Assay for quantitative determination of GSH and GSSG levels
Intracellular GSH (reduced glutathione) and GSSG (glutathione disulfide) levels were measured using the GSH reductase recycling assay as previously described [30]. Concentrations of GSH and GSSG were expressed as nmol/mg of protein and the ratios of GSSG/(GSH+GSSG) were calculated.
2.8 Transient siRNA transfection of U937 cells
These experiments used HP Validated (by quantitative RT-PCR) ATM siRNA from Qiagen, AllStars Negative (nonsilencing) Control siRNA (5nmol), HiPerfect transfection reagent and RNAse free water, and were performed in an RNAse-free environment with 20μM siRNA solutions. Transfection was performed per manufacturer's instructions.
2.9 Culture for mixed lymphocyte reaction
CD4+ T cells from human peripheral blood mononuclear cells (PBMC) were purified using indirect magnetic labeling for negative isolation of CD4 T cells with CD4+ T cell isolation kits (Miltenyi Biotech). 100,000 CD4+ T cells from a healthy donor were combined and incubated 1:10 (U937:T) with unirradiated or irradiated (2 or 20 Gy) U937 cells for up to 6 days in a humidified 37°C, 5% CO2 incubator. U937 cells were irradiated and allowed to incubate for 48 hours before combining with CD4+ T cells in 96 well plates to assess cell proliferation and cytokine production.
2.10 Cell Proliferation ELISA BrdU assay
BrdU incorporation was measured using the BrdU(colorimetric) Cell Proliferation ELISA kit Roche Applied Science. In this assay, 100,000 CD4+T cells were seeded with U937 cells at a 1:10 (APC:T) ratio in 96-well culture dishes. Absorbance was measured spectrophometrically between 450 and 595nm after 4 days incubation. Background (no BrdU label) was subtracted from measured absorbances. The mean absorbance ± S.E.M. was calculated.
2.11 ELISA cytokine supernatant assay
Single Analyte ELISArray Kits (SA Biosciences) for IL-4, TNF-α and IFN-γ were used to analyze cytokine production from MLRs per manufacturer’s instructions using the standard sandwich technique. Cells were removed by centrifugation for 10 minutes at 1000×g. Supernatants were aliquoted and stored at ≤ − 20 °C until further use.
2.12 Statistical analysis tool
The median fluorescence intensity (MFI) was calculated using FlowJo software from Treestar Corp (Ashland, OR). Two-tailed Student's t-tests were used to calculate p-values by Predictive Analytics SoftWare (PASW)) and GraphPad Sofware. Standard error of the mean (S.E.M) was calculated using GraphPad Sofware, San Diego, CA. For all tests, p value of 0.05 or less was considered significant.
3. Results
3.1 Radiation induces phosphorylation of NFκB members
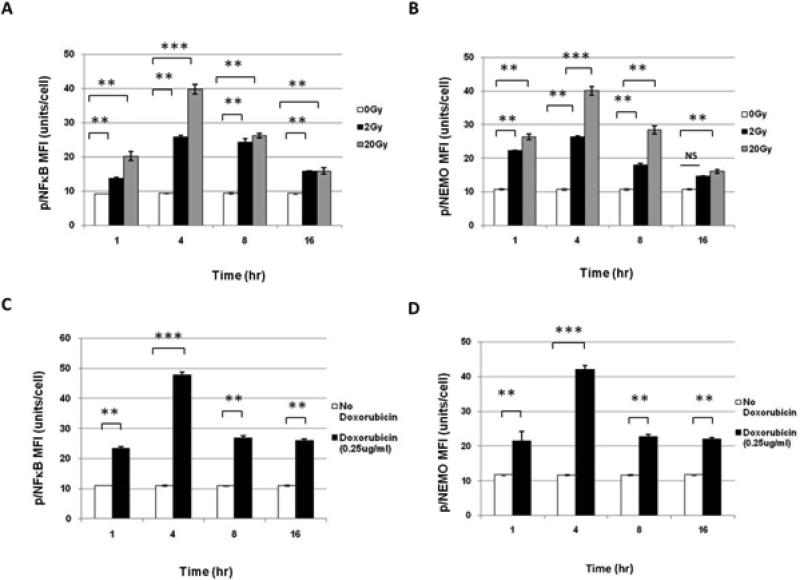
To determine how radiation affects immunologically important signaling proteins in the U937 line, cells were irradiated with 2 or 20 Gy and allowed to incubate for various timepoints. - Phosphorylated protein specific antibodies were used to identify radiation induced-changes in NFκB pathway family members. Radiation induced phosphorylation of NFκB (Figure 1A) and NFκB essential modulator (NEMO):IKKγ (Figure 1B) in a dose-dependent manner, with the greatest activation of both at 4hr after 20 Gy, which subsequently declined over 16 hrs to near baseline levels. Representative histograms are shown in Supplementary Figure 1.
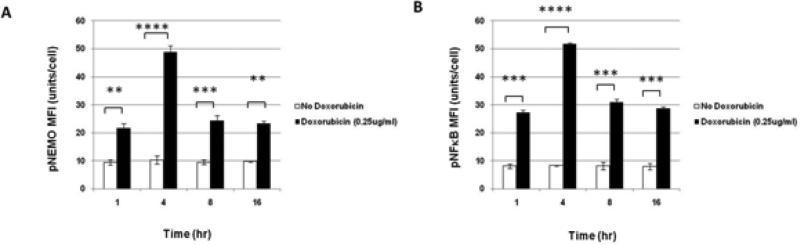
Figure 1. Phospho-flow cytometry determines Phospho-specific changes in NFκB pathway resulting from XRT and Doxorubicin in U937 cells.
(A) Treatment of U937 cells with XRT showed increased levels of NFκB phosphorylation in U937 and (B) NEMO phosphorylation 1-16 hrs after irradiation with 0, 2, and 20 Gy. (C) NFκB phosphorylation in U937 after 3 hrs with 0.25 μg/ml of Doxorubicin. (D) NEMO phosphorylation in U937 after 3 hrs with 0.25 μg/ml Doxorubicin.
We hypothesized that radiation -induced NFκB changes were due to DNA damage. To test this hypothesis, NFκB pathway phosphorylation profiles from Doxorubicin (0.1 and 0.25 μg/ml ), a known DNA damaging agent, were compared to those obtained with radiation. In U937 cells, Doxorubicin at 0.25 μg/ml, but not 0.1 μg/ml (data not shown), induced phosphorylation of NFκB and NEMO by 1 hour, with the greatest activation of both occurring at 4 hours, with subsequent decline and plateau by 16 hours (Figure 1C and 1D).
Radiation not only leads to direct DNA damage (including double stranded breaks), but also produces ROS. Since ROS can indirectly cause DNA damage and also directly activate the NFκB pathway in certain systems [31-34]. we next studied the effect of radiation on the redox status of the cell.
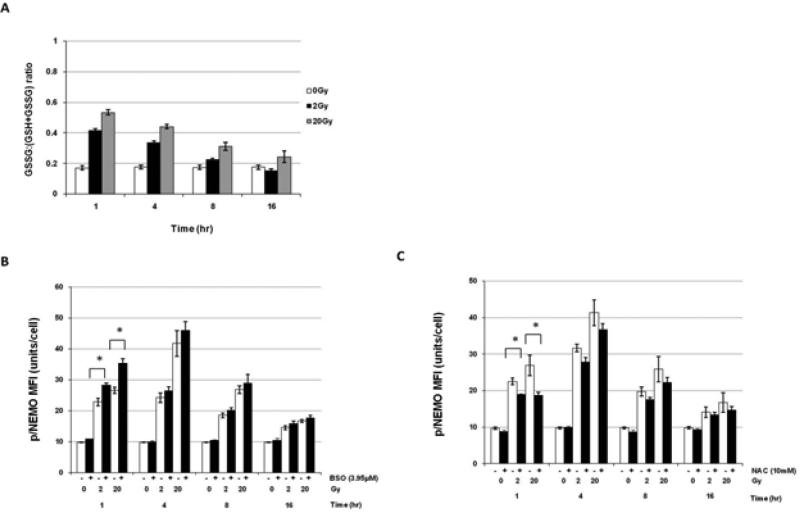
The GSSG:total GSH (GSH+GSSG)ratio in U937 cells, with an inactivated p53 gene, was the highest 1hr after irradiation with 20 Gy, compared to cells irradiated with 2 or 0 Gy, and declined over time in a dose-dependent manner to near baseline at 16 hrs(Figure 2A). L- Buthionine sulphoximine (BSO; which irreversibly inhibits γ-glutamylcysteine synthase, leading to depletion of GSH) was used to enhance ROS levels. N-acetyl cysteine (NAC; a potent antioxidant that increases the levels of GSH) was used to indirectly reduce ROS after irradiation as demonstrated in supplemental Figures 2A, 2B. Although pre-treatment of U937 cells with 1.95uM BSO prior to irradiation (0, 2, or 20 Gy) had no effect on the level of radiation-induced pNEMO (data not shown), 3.95μM BSO, significantly increased pNEMO activation at 1hr following irradiation with 2 (p=0.028) and 20 Gy (p=0.045) compared to the 0 Gy control (Figure 2B; representative histograms are shown in Supplementary Figure 2C).
Figure 2. ROS levels modestly contribute to XRT-induced phosphorylation of NEMO.
(A) Ratio of GSSG to GSH+GSSG (total GSH) in U937 cells 1-16 hrs after irradiation with 0, 2, and 20 Gy. Data are shown as the average ratio +/− standard error from three independent experiments. (B) Phosphorylation of NEMO at 1-16 hrs after (+) pre-treatment with 3.95μM BSO for 3 hrs prior to 0, 2, or 20 Gy irradiation or (-) radiation alone in U937 mutant cells (C) Phosphorylation of NEMO at 1-16 hrs after pre-treatment with 10mM NAC for 3 hrs prior to 0, 2, or 20 Gy irradiation in U937 mutant cells. (A-C) *(p=<0.05), **(p=<0.01), ***(p=<0.001); and NS, not significant. For all experiments, data is shown as normalized average MFI± S.E.M from three independent experiments unless otherwise specified.
In similar experiments, NAC resulted in significant partial inhibition of activation of pNEMO by XRT at 1 and 4 hrs (p=0.042), with the greatest inhibition occurring with 10mM NAC at 1hr after 20 Gy (p=0.041) (Figure 2C). Representative histograms of 10mM NAC+XRT changes versus XRT alone in pNEMO are shown in Supplementary Figure 2D. These results suggest that ROS may have a modest contribution to XRT induced phosphorylation of NEMO, but that other factors must play a more important role in producing the XRT induced changes described above.
3.2 Radiation-induced phosphorylation of NEMO requires ATM
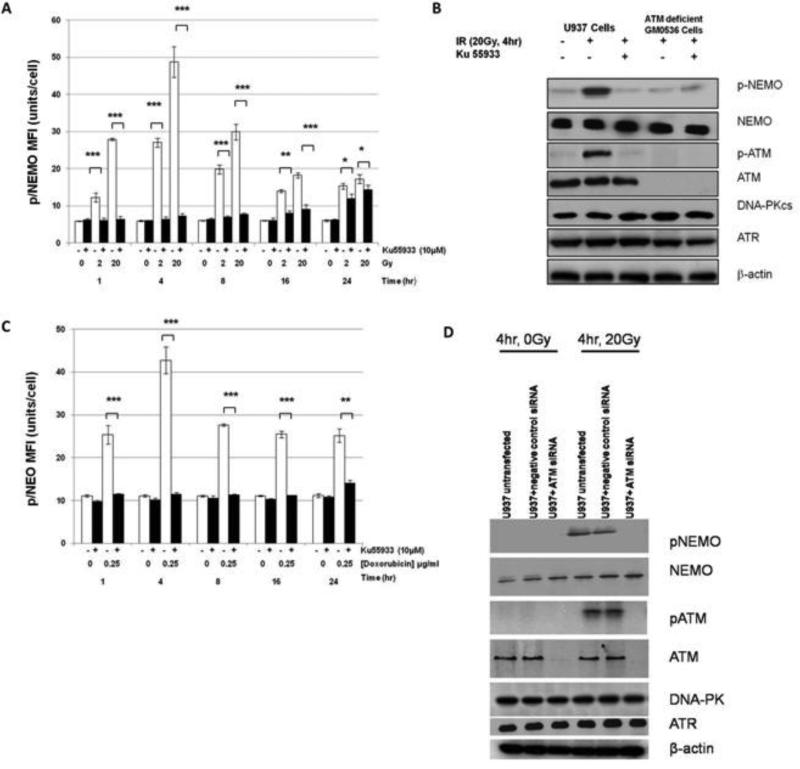
We hypothesized that radiation-induced NFκB changes were due to DNA damage. To test this hypothesis, we used an inhibitor of ATM (Ataxia Telangiectasia Mutated) kinase, Morpholin-4yl-6-thianthren-1-yl-pyran-4-one (Ku55933) (Calbiochem), that selectively inhibits ATM-dependent cellular phosphorylation following radiation (IC50=13nM), and does not inhibit DNA-Protein Kinase or ATM and rad3 related Protein Kinase. In these experiments, U937 cells were treated with 10μM Ku55933 for 60 minutes before irradiation. Ku55933 significantly inhibited (p=<0.05) radiation-mediated induction of pNEMO in U937 cells, lacking p53, at 1, 4, 8, and 16 hrs after 2 Gy and 20 Gy, with less inhibition at 24 hrs (Figure 3A). Western blots at 4hr after 20 Gy confirmed that radiation alone phosphorylated both ATM and NEMO and that Ku55933 significantly inhibited this phosphorylation. The requirement of ATM for phosphorylation of NEMO was confirmed using an ATM deficient cell line (G0536). Radiation alone phosphorylated NEMO, but not ATM, and pre-treatment with Ku55933 again significantly inhibited phosphorylation of NEMO. The amount of total NEMO, DNA-PK, and ATR, were consistent in all three treatment groups (Figure 3B). Ku55933 also significantly inhibited (p=<0.05) doxorubicin-mediated induction of pNEMO in U937 mutant cells at 1, 4, 8, and 16 and 24 hrs after doxorubicin (0.25 μg/ml) treatment (Figure 3C). U937 cells were next treated with/without siRNA for ATM or negative control siRNA. In ATM knocked down cells, NEMO and ATM were not phosphorylated after 20 Gy. There were similar amounts of total NEMO protein and ATM in all groups, except for ATM knocked-down cells that did not express ATM. ATM siRNA did not affect expression of other PIKK family kinases DNA-PKcs and ATR (Figure 3D).
Figure 3. Phosphorylation of NEMO after XRT and Doxorubicin requires ATM.
(A) U937 cells treated with 10μM Ku55933 (+) versus no inhibitor (-) prior to irradiation (0, 2, or 20 Gy) at 1-24 hrs. (B) Western blot analysis of NEMO and ATM phosphorylation in U937 and ATM deficient GM0536 cells. β-actin served as a loading control. (C) U937 cells treated with 10μM Ku55933 (+) versus no inhibitor (-) prior to 0.25μg/ml Doxorubcin at 1-24 hrs. (D)
Phosphorylation of NEMO and ATM were measured by western blot analysis in untransfected U937 and U937+negative control siRNA cells. NEMO and ATM were phosphorylated after treatment with 20 Gy but not in untreated ( 0 Gy) U937 cells. Radiation did not phosphorylate ATM or NEMO in ATM knocked down cells. β-actin served as a protein loading control. *(p=<0.05), **(p=<0.01), ***(p=<0.001); and NS: not significant.
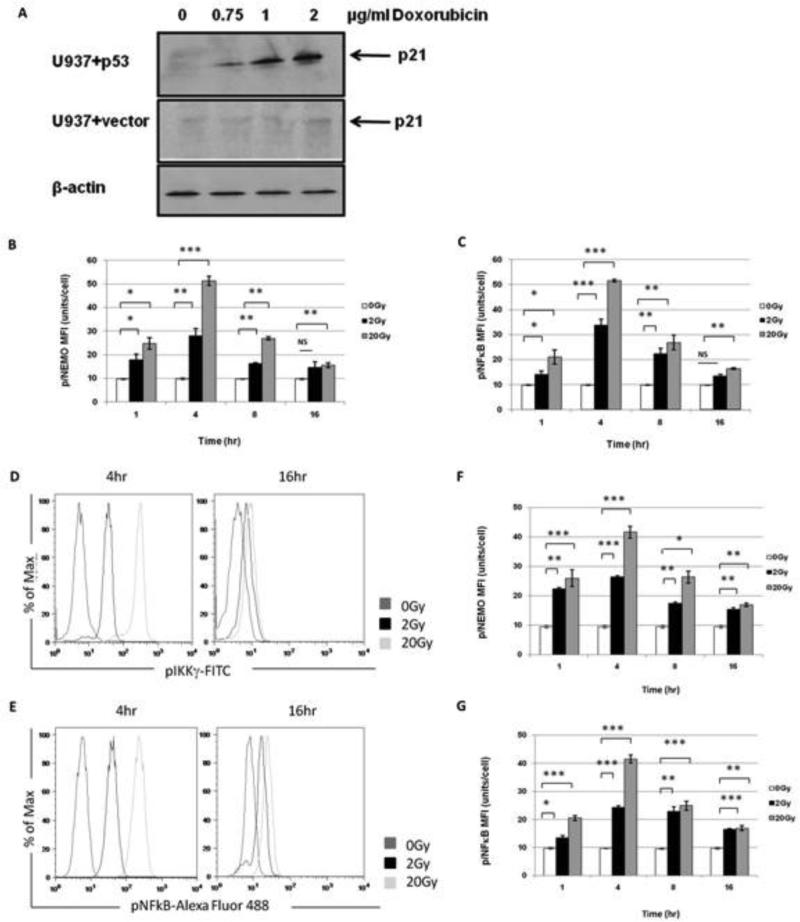
Since U937 cells possess a functionally inactive p53 gene, we transfected U937 cells with wildtype p53 to determine the role of p53 in the acute radiation- induced changes observed in U937 cells. Western blot analysis confirmed that the p53 transfected gene was functionally activated, because p21, was upregulated in a dose-dependent manner after treatment with varying doses of Doxorubicin (0.75- 2 μg/ml), a known p53 inducer. No upregulation of p21 occurred in empty vector control cells (Figure 4A).
Figure 4. XRT-induced phosphorylation of NEMO and NFκ is p53 independent.
(A) Restoration of p53 function in U937 cells. Measurement of p21 by western blot analysis in doxorubicin-treated U937 cells transfected with p53 or an empty vector. The p53 transfected U937 cells exhibited dose-dependent p21 upregulation while the empty vector control cells did not. β-actin served as a protein loading control. (B) Treatment of U937 cells with XRT showed increased levels of NEMO phosphorylation in U937 p53 wildtype cells 1-16 hrs after irradiation with 0, 2, and 20 Gy and (C) levels of NFκB phosphorylation in U937 p53 wildtype cells 1-16 hrs after irradiation with 0, 2, and 20 Gy. (B-C) NS=not significant, *(p=<0.05), **(p=<0.01), and ***(p=<0.001). (D-E) Shown are representative histograms of U937 p53 wildtype cells at 4hr and 16hr phosphorylation of (D) NEMO and (E) NFκB before and after 2 and 20 Gy. (F-G) Empty vector cells do not alter XRT-induced changes of in NFκB pathway in U937 p53 mutant cells. (F) Levels of NEMO phosphorylation in U937 empty vector cells 1-16 hrs after irradiation with 0, 2, and 20 Gy. (G) Levels of NFκB phosphorylation in U937 empty vector cells 1-16 hrs after irradiation with 0, 2, and 20 Gy. (F-G) *(p=<0.05), **(p=<0.01), ***(p=<0.001).
We found that radiation-induced phosphorylation of NEMO and NFκB was not changed by the presence of p53 (Figure 4B and 4C). Representative phosphorylation of NEMO and NFkB in U937 and p53+ U937 cells after radiation are shown in Figure 4D and 4E. Importantly, phosphorylation profiles for NEMO and NFκB were not altered by vector alone (Figure 4F and 4G). Doxorubicin induced phosphorylation changes in NEMO and NFκB in U937 cells at 1, 4, 8, and 16 hrs were striking similar whether p53 was functionally inactive or restored (Figure 5A and 5B). Furthermore, changes in the redox status of the cells after radiation were not p53 dependent (data not shown).
Figure 5. Doxorubicin-induced phosphorylation of NEMO and NFκB pathway is p53 independent.
(A) Levels of NEMO phosphorylation in wildtype p53 U937 cells 3 hrs after 0.25μg/ml Doxorubicin treatment 0, 2, and 20 Gy. (B) Levels of NFκB phosphorylation in wildtype p53 U937 cells 3 hrs after 0.25 μg/ml Doxorubicin treatment 0, 2, and 20 Gy. (A-B) **(p=<0.01), ***(p=<0.001), and ****(p=<0.0001).
3.3 NFκB pathway is necessary for maturation of U937 cells by radiation
We next studied the effect of radiation on the levels of expression of APC activation and maturation markers. Co-stimulatory molecules- CD40 and CD86, MHC Class II (HLA-DR), but not CD80, were upregulated in a dose-dependent fashion at 48 hrs. Since U937 cells do not express a basal level of CD80, it was not surprising that this costimulatory molecule was not upregulated after radiation (Figure 6A). Representative histograms of CD40, CD80, CD86, HLA-DR expression after radiation or stimulation with PMA (positive control) are shown in Supplementary Figure 3A.
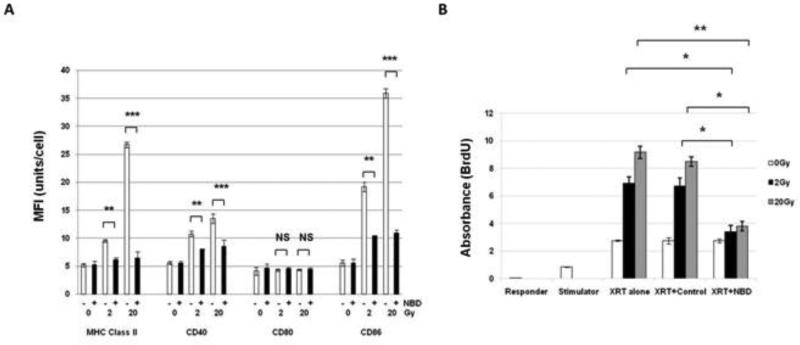
Figure 6. XRT-induced maturation and responsiveness of U937 cells requires NFκB.
(A) Inhibition of CD40, CD86, and MHC Class II phosphorylation at 48 hrs by NBD peptide (+) versus no peptide (-) after 2 or 20Gy XRT compared to CD40, CD86, and MHC Class II phosphorylation after XRT alone. NS=not significant, **(p=<0.01), and ***(p=<0.001). (B) T cell proliferation was measured following combination of CD4+ T cells with irradiated (2 or 20 Gy) U937 cells and U937 pretreated with non-specific control peptide increased T cell proliferation. Irradiated U937 cells pre-treated with NBD peptide showed reduced T cell proliferation. Responder cells alone (purified CD4+ T cells), stimulator cells alone (irradiated U937 cells), and CD4+T cells were combined with unirradiated U937 cells and served as negative controls. Shown is the average absorbance (from triplicates)±S.E.M. from three independent experiments. *(p=0.05) and **(p=<0.01).
In order to determine if the NFκB pathway is responsible for the maturation of U937 cells, a IKKγ (NEMO) Binding Domain Inhibitory Peptide (NBD) was utilized. NBD inhibits formation of the complex between NEMO and IKKα/IKKβ and thereby prevents phosphorylation of IκBα , and subsequent phosphorylation of NFκB 35. These effects were not p53 dependent (data not shown). When cells were either mock-treated with radiation, treated with radiation alone, or pre-treated with the control or NBD peptide for 12 hrs prior to radiation, NBD peptide significantly inhibited upregulation of CD40, CD86, and MHC Class II when compared to radiation +control peptide (p<0.05) and radiation alone (p≤0.009) (Figure 6A). No changes were seen in CD80 expression with radiation alone, radiation+control peptide, or radiation+NBD peptide. Control peptide values were very similar to radiation alone (data not shown).
Radiation-induced phosphorylation of IκBα occurs at 48hrs, but in irradiated cells pre-treated with the NBD peptide, phosphorylation of IκBα was inhibited at 48hrs when compared to levels in irradiated cells pre-treated with the control peptide (Supplementary Figure 3B).
3.4 NBD peptide decreases U937 cell responsiveness
It was vital to determine whether the observed changes in the NFκB pathway after XRT had any measurable effect on U937 function. In the MLR assay, U937 cells were irradiated with or without pre-incubation with the control or NBD peptide for 12 hrs. Following irradiation, cells were incubated for 48 hrs cells before adding purified CD4+ T cells (isolated from PBMC from healthy donors), in a 1(APC):10(T) ratio for 4 days. T cell proliferation was measured spectrophotometrically to quantitate the level of incorporation of BrdU. CD4+ T cells combined with irradiated U937 cells or U937 cells pre-incubated with non-specific control peptide, resulted in dose-dependent T cell proliferation when compared to CD4+ T cells incubated with unirradiated U937 cells. However, when cells were treated with the NBD peptide, T cell proliferation decreased 2 and 2.4 fold at 2 (p=0.013) and 20 Gy (p=0.002), respectively, when compared to radiation alone. T cell proliferation was decreased 2 fold and 2.3 fold at 2 (p=0.032) and 20 Gy (p=0.013), respectively, compared to cells in the control peptide treatment group (Figure 6B).
3.5 Radiation activates and enhances U937 cell response: Cytokine production increases in T cells after radiation
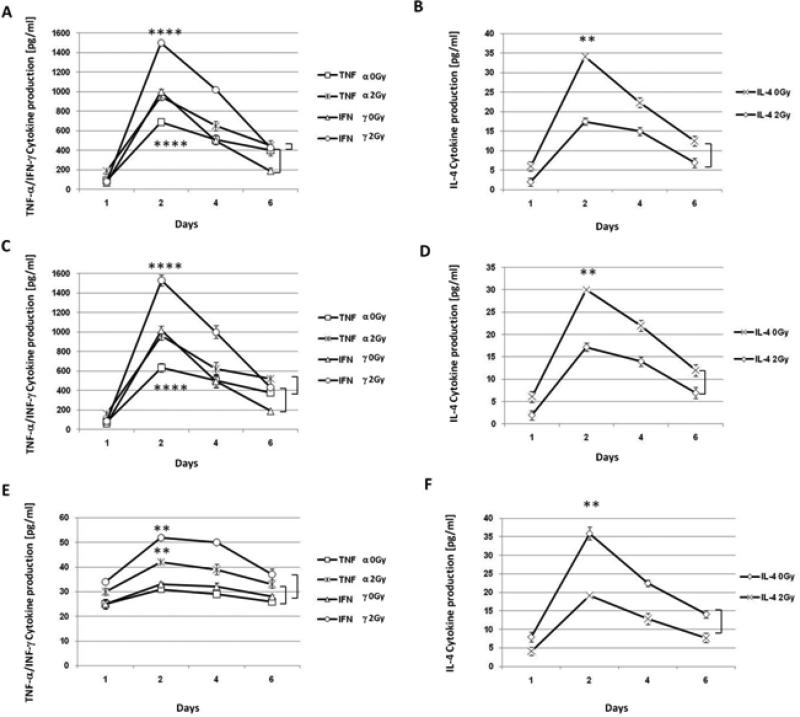
Pro-inflammatory cytokine production, another indicator of APC activation and cytokine production, was measured in MLRs using U937 cells irradiated with or without pre-incubation with the control or NBD peptide for 12 hrs. Following irradiation, cells were incubated for 48 hours before adding purified CD4+ T cells (isolated from PBMC from healthy donors), in a 1(APC):10(T) ratio and were allowed to incubate for up to 6 days. Cells were removed from MLR on days 1, 2, 4, and 6 and supernatants were collected for cytokine assays. TNF-α, and IFN-γ cytokine production peaked after incubation for 2 days in cultures of both unirradiated and irradiated cells, with a higher level of cytokine production in cultures of cells treated with 2 Gy (Figure 7A) (p≤0.0001). A similar trend in IL-4 cytokine production was seen in unirradiated cultures, but radiation did not result in a higher level of IL-4 production and in fact, dampened IL-4 cytokine production (Figure 7B). Increased levels of TNF-α and IFN-γ in cultures of irradiated cells is indicative of radiation-enhanced U937 responsiveness.
Figure 7. Supernatants from allogeneic MLR demonstrate XRT enhances TNF-α and IFN-γ cytokine production, but not IL-4.
Unirradiated and irradiated U937 cells were combined with purified CD4+ T cells, in a 1(APC):10(T) ratio and on days 1, 2, 4, and 6, supernatants were used to assay IL-4, TNF-α, and IFN-γ cytokine production via ELISA. Concentrations of cytokines were measured as pg/ml. (A) TNF-α and IFN-γ cytokine production after 0 Gy and 2 Gy. (B) IL-4 cytokine production before and after radiation (2 Gy). (C) Supernatant TNF-α and IFN-γ cytokine concentrations from MLR where U937 cells were pre-treated with control peptide before 0 or 2 Gy). (D) IL-4 cytokine concentration from supernatants of the same MLR as Figure 7C. (E) Shows inhibition of TNF-α and IFN-γ cytokine production where U937 cells were pre-treated with NBD peptide before mock-irradiation or irradiation and combining with CD4+ T cells. (F) Unchanged IL-4 cytokine production from supernatants of the same MLR as Figure 7E . (A-F) Shown is the average cytokine production (from triplicates) ±S.E.M. from three independent experiments. **(P=<0.01) and ****(p=<0.0001).
Pretreatment of U937 cells with a control peptide (non-specific to NEMO) prior to irradiation and coculture with CD4+ T cells had no effect on cytokine levels from cultures of irradiated and unirradiated cells (Figure 7C and 7D). Supernatants from similar experiments using a NBD inhibitory peptide demonstrated significant inhibition of TNF-α and IFN-γ levels (Figure 7E). The kinetics of cytokine induction were similar, but pretreatment with the inhibitor, caused more than a 30 fold decline in TNF-α levels from unirradiated and irradiated (2 Gy) cells. A significant difference between TNF-α and IFN-γ levels from unirradiated and irradiated cells was observed (p≤0.002) (Figure 7E). These results suggest that activation of NFκB is important for U937 cell function/responsiveness as measured by TNF-α and IFN-γ cytokine production and is increased by radiation, while IL-4 production appears to be independent of NFκB activation by radiation (Figure 7F).
4. Discussion
Radiation can suppress immunity by depleting lymphocytes that are highly sensitive to radiation induced apoptosis and cell death. Radiation can also augment immunity by promoting enhanced antigen presentation [1, 18], pro-inflammatory cytokine release (TNF-α, IL-1β, IL-2) [14-17], and DC activation, which can augment T cell mediated anti-tumor effects [10]. Radiation stimulates dendritic cell maturation by prostaglandin E2 (PEG-2) and cyclooxygenase-2 (COX- 2) [1, 12, 21], thereby enhancing the overall “danger milieu.” This can affect the tumor microenvironment [38] by stimulating APC homing to irradiated sites [39] via chemokine release (e.g. SDF-2) from irradiated tumor cells [19], and altering adhesion molecule expression by irradiated endothelial cells, which promote leukocyte adhesion chemotaxis [14, 40-42].
It is not well understood at the molecular level how radiation enhances tumor immunity, especially with regard to important signaling changes that occur in APC. In particular, it is not clear whether radiation in vitro can directly alter immunologically important changes in a pure population of APC. Many alterations induced by in vivo radiation are indirect and due to release of microorganisms and lipopolysaccharides from the gut or release of proinflammatory cytokines from parenchymal or stromal cells that are not APC. Therefore, the goal of the experiments described here was to identify early in vitro radiation-induced signaling changes in a pure population of APC and to elucidate the effects of these signaling changes on APC co-stimulator receptors and function.
We first examined radiation-induced signaling changes in U937 cells and the underlying mechanism (s) responsible for these changes. This cell line was chosen for initial studies because it has been widely used as a model for monocyte/macrophage differentiation in vitro [3, 8] and as a functional model for monocytic cells [9, 24-26, 28]. U937 cells display hallmark characteristics of APC, and express basal levels of major histocompatibility complex (MHC) class II and I as well as co-stimulatory molecules [43]. Differentiated U937 cells also phagocytose bacteria efficiently and are able to present bacterial antigen to CD8+ T cells [44]. In addition, we used a U937 cell line with a functionally inactive p53 gene [27], which allowed us to manipulate p53 activity in these cells to determine the role of p53 in radiation-induced changes in the NFκB signal transduction pathway. We were able to use phospho-flow cytometry (Pfcs) to accurately and rapidly assess the phosphorylation state of intracellular proteins that are known to affect complex signaling pathways.
Our results demonstrated that radiation and doxorubicin induced NFκB pathway activation in U937 cells in a p53 independent manner, since activation occurred in U937 cells with an inactivated p53 gene before and after transduction with a wild type p53 gene. In contrast, both inhibition or deficiency of ATM prevented activation of the NFκB pathway. The results indicated that control of NFκb activation is upstream of p53 control in this cell line. In contrast to the results with the U937 line, DNA damage activates the NFκB pathway in a p53 dependent manner in many tumor cells and lines such that anti-apoptotic proteins are expressed in addition to the pro-apoptotic proteins of the p53-BCL2 pathway (26, 45). The current results suggest that the role of p53 in NFκB activation after DNA damage is variable and depends on the type of cell under investigation.
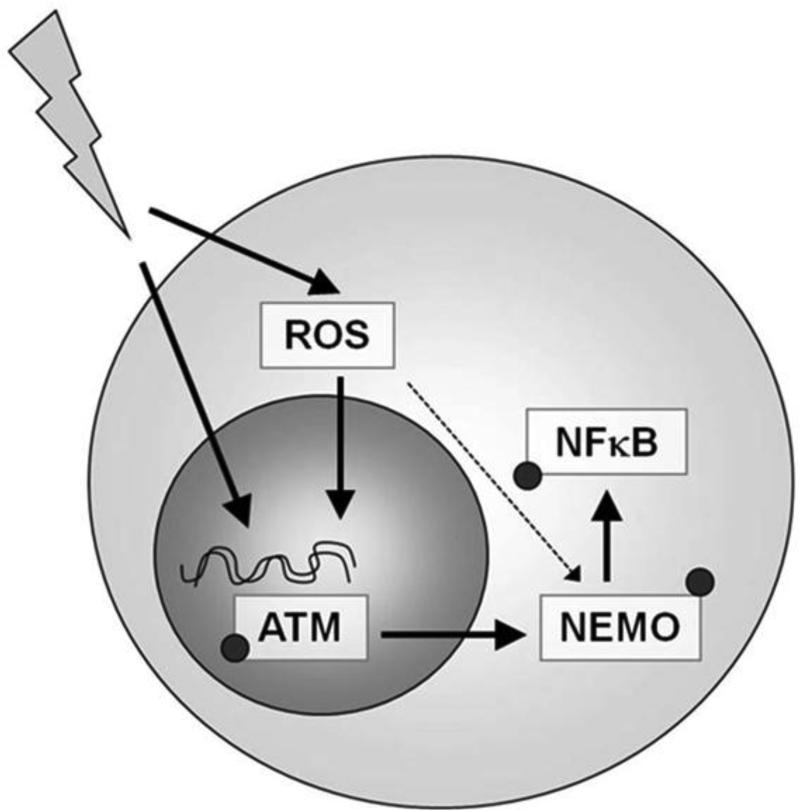
Radiation altered the redox status as indicated by changes in the GSH:GSSG ratio, and led to NFκB pathway activation. However, the impact of the redox changes was minor as compared to the ATM-dependent changes. These findings are consistent with radiation-induced double-strand breaks, resulting in phosphorylation of ATM, and subsequent activation and phosphorylation of NEMO, which activates a downstream signaling cascade that results in phosphorylation of NFκB. Radiation induced ROS can indirectly contribute to activation of the NFκB pathway in U937 cells, by damaging DNA also (e.g. double-strand breaks), and thereby phosphorylating ATM and activating the NFκB signaling pathway. ROS may directly stimulate phosphorylation of NEMO, but this would be a minor pathway compared with the double stranded break-mediated ATM pathway, as indicated by results showing little effect of redox status on radiation (ROS) –induced changes on the NFκB pathway (Figure 8).
Figure 8. XRT-induced activation of NFκB pathway requires ATM, but not ROS in U937 cells.
Schematic of the mechanism of action for XRT-induced NFκB changes in U937 cells, in which XRT induced double-strand breaks, results in phosphorylation of ATM, and subsequent activation and phosphorylation of NEMO. ROS may also directly phosphorylate NEMO, but this would be a minor pathway.
Radiation increased U937 co-stimulatory and MHC molecule expression, and the ability to stimulate T cell proliferation and cytokine production. Inhibition of NEMO, diminished U937 cell responsiveness after radiation as shown by decreased co-stimulatory and MHC molecule expression, decreased T cell proliferation in MLR, and decreased TNF-α and IFN-γ cytokine production by T cells in MLR. Decreased IL-4 cytokine production by T cells after radiation was not changed by inhibition of NEMO, which may be because IL-4 production is not regulated by NFκB and/or that IL-4 can function as an inhibitor of NFκB activation [46]. Overall, our results demonstrate that activation of the NFκB pathway is necessary for the increased antigen presenting function of the U937 cell line in response to radiation.
Although anti-tumor immunity would be enhanced by increased antigen presentation function in the tumor stroma, DNA damage of tumor cells and tumor stromal cells may also promote tumor survival via NFκB activation. A recent study (47) showed that NFκB activation in tumor stromal cells such as fibroblasts can in turn activate WNT 16B to promote survival of tumor cells and resistance to tumor killing by chemotherapy. Furthermore, p53 dependent NFκB activation in breast tumor cells results in the increased expression of IL-6, a pro-inflammatory cytokine, that can promote tumor growth (48). In addition, IL-6 can decrease MHC class II expression on dendritic cells via arginase activation, and impair anti-tumor immunity (48). Thus, DNA damage in tumors can have opposing effects that either enhance or suppress tumor immunity or tumor survival.
The current results demonstrate a novel mechanism by which radiation can enhance APC function and tumor immunity through signaling pathways such as NFκB. These findings may be clinically relevant, since adjustment of tumor radiation dose, fractionation, and duration can alter the balance of factors that increase tumor immunity while increasing tumor killing of current immunotherapy protocols that use radiation and could directly enhance tumor immunity [1-9].
Supplementary Material
Highlights.
Radiation therapy has the potential to influence anti-tumor immune responses in cancer patients.
We analyzed radiation-induced immune response of monocytic cells using phospho-flow cytometry.
We identified novel radiation-induced changes in the phosphorylation of NFκB family member.
The phosphorylation changes were dependent on ATM activation by radiation-induced DNA damage.
Radiation promotes the activation and APC functional maturation through phosphorylation of NEMO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–666. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watters D. Molecular mechanisms of ionizing radiation-induced apoptosis. Immunol Cell Biol. 1999;77:263–271. doi: 10.1046/j.1440-1711.1999.00824.x. [DOI] [PubMed] [Google Scholar]
- 3.Nikitina EY, Gabrilovich DI. Combination of gamma-irradiation and dendritic cell administration induces a potent antitumor response in tumor-bearing mice: approach to treatment of advanced stage cancer. Int J Cancer. 2001;94:825–833. doi: 10.1002/1097-0215(20011215)94:6<825::aid-ijc1545>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 4.Ganss R, Ryschich E, Klar E, Arnold B, Hammerling GJ. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 2002;62:1462–1470. [PubMed] [Google Scholar]
- 5.Larsson M, Fonteneau JF, Bhardwaj N. Dendritic cells resurrect antigens from dead cells. Trends Immunol. 2001;22:141–148. doi: 10.1016/s1471-4906(01)01860-9. [DOI] [PubMed] [Google Scholar]
- 6.Teitz-Tennenbaum S, Li Q, Rynkiewicz S, et al. Radiotherapy potentiates the therapeutic efficacy of intratumoral dendritic cell administration. Cancer Res. 2003;63:8466–8475. [PubMed] [Google Scholar]
- 7.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–825. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 8.Kim KW, Kim SH, Shin JG, et al. Direct injection of immature dendritic cells into irradiated tumor induces efficient antitumor immunity. Int J Cancer. 2004;109:685–690. doi: 10.1002/ijc.20036. [DOI] [PubMed] [Google Scholar]
- 9.Chakravarty PK, Alfieri A, Thomas EK, et al. Flt3-ligand administration after radiation therapy prolongs survival in a murine model of metastatic lung cancer. Cancer Res. 1999;59:6028–6032. [PubMed] [Google Scholar]
- 10.Paulos CM, Wrzesinski C, Kaiser A, et al. Microbial translocation augments the function of adoptively transferred self/tumor-specific CD8+ T cells via TLR4 signaling. J Clin Invest. 2007;117:2197–2204. doi: 10.1172/JCI32205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brody JD, Goldstein MJ, Czerwinski DK, Levy R. Immunotransplantation preferentially expands T-effector cells over T-regulatory cells and cures large lymphoma tumors. Blood. 2009;113:85–94. doi: 10.1182/blood-2008-05-155457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McBride WH, Chiang CS, Olson JL, et al. A sense of danger from radiation. Radiat Res. 2004;162:1–19. doi: 10.1667/rr3196. [DOI] [PubMed] [Google Scholar]
- 13.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 14.Ishihara H, Tsuneoka K, Dimchev AB, Shikita M. Induction of the expression of the interleukin-1 beta gene in mouse spleen by ionizing radiation. Radiat Res. 1993;133:321–326. [PubMed] [Google Scholar]
- 15.Hallahan DE, Spriggs DR, Beckett MA, Kufe DW, Weichselbaum RR. Increased tumor necrosis factor alpha mRNA after cellular exposure to ionizing radiation. Proc Natl Acad Sci U S A. 1989;86:10104–10107. doi: 10.1073/pnas.86.24.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hong JH, Chiang CS, Tsao CY, Lin PY, McBride WH, Wu CJ. Rapid induction of cytokine gene expression in the lung after single and fractionated doses of radiation. Int J Radiat Biol. 1999;75:1421–1427. doi: 10.1080/095530099139287. [DOI] [PubMed] [Google Scholar]
- 17.Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor alpha cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friedman EJ. Immune modulation by ionizing radiation and its implications for cancer immunotherapy. Curr Pharm Des. 2002;8:1765–1780. doi: 10.2174/1381612023394089. [DOI] [PubMed] [Google Scholar]
- 19.Teitz-Tennenbaum S, Li Q, Okuyama R, et al. Mechanisms involved in radiation enhancement of intratumoral dendritic cell therapy. J Immunother. 2008;31:345–358. doi: 10.1097/CJI.0b013e318163628c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. 2001;13:114–119. doi: 10.1016/s0952-7915(00)00191-6. [DOI] [PubMed] [Google Scholar]
- 21.McLellan AD, Brocker EB, Kampgen E. Dendritic cell activation by danger and antigen-specific T-cell signalling. Exp Dermatol. 2000;9:313–322. doi: 10.1034/j.1600-0625.2000.009005313.x. [DOI] [PubMed] [Google Scholar]
- 22.Sundstrom C, Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976;17:565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- 23.Harris P, Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985;37:407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- 24.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 25.Verheij M, Bartelink H. Radiation-induced apoptosis. Cell Tissue Res. 2000;301:133–142. doi: 10.1007/s004410000188. [DOI] [PubMed] [Google Scholar]
- 26.Magne N, Toillon RA, Bottero V, et al. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231:158–168. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 27.Scheidereit C. IkappaB kinase complexes: gateways to NF-kappaB activation and transcription. Oncogene. 2006;25:6685–6705. doi: 10.1038/sj.onc.1209934. [DOI] [PubMed] [Google Scholar]
- 28.Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 29.Schulz KR, Danna EA, Krutzik PO, Nolan GP. Single-cell phospho-protein analysis by flow cytometry. Curr Protoc Immunol. 2007 doi: 10.1002/0471142735.im0817s78. Chapter 8, Unit 8 17. [DOI] [PubMed] [Google Scholar]
- 30.Rahman I, Kode A, Biswas SK. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat Protoc. 2006;1:3159–3165. doi: 10.1038/nprot.2006.378. [DOI] [PubMed] [Google Scholar]
- 31.Nordberg J, Arner ES. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic Biol Med. 2001;31:1287–1312. doi: 10.1016/s0891-5849(01)00724-9. [DOI] [PubMed] [Google Scholar]
- 32.Ho E, Chen G, Bray TM. Supplementation of N-acetylcysteine inhibits NFkappaB activation and protects against alloxan-induced diabetes in CD-1 mice. FASEB J. 1999;13:1845–1854. [PubMed] [Google Scholar]
- 33.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-kappa B. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 34.Verhasselt V, Vanden Berghe W, Vanderheyde N, Willems F, Haegeman G, Goldman M. N-acetyl-L-cysteine inhibits primary human T cell responses at the dendritic cell level: association with NF-kappaB inhibition. J Immunol. 1999;162:2569–2574. [PubMed] [Google Scholar]
- 35.Strickland I, Ghosh S. Use of cell permeable NBD peptides for suppression of inflammation. Ann Rheum Dis. 2006;65(Suppl 3):iii75–82. doi: 10.1136/ard.2006.058438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muul LM, Silvin C, James SP, Candotti F. Measurement of proliferative responses of cultured lymphocytes. Curr Protoc Immunol. 2008 doi: 10.1002/0471142735.im0710s82. Chapter 7:Unit 7 10 11-17 10 24. [DOI] [PubMed] [Google Scholar]
- 37.Koren HS, Anderson SJ, Larrick JW. In vitro activation of a human macrophage-like cell line. Nature. 1979;279:328–331. doi: 10.1038/279328a0. [DOI] [PubMed] [Google Scholar]
- 38.Lan F, Zeng D, Higuchi M, Huie P, Higgins JP, Strober S. Predominance of NK1.1+TCR alpha beta+ or DX5+TCR alpha beta+ T cells in mice conditioned with fractionated lymphoid irradiation protects against graft-versus-host disease: “natural suppressor” cells. J Immunol. 2001;167:2087–2096. doi: 10.4049/jimmunol.167.4.2087. [DOI] [PubMed] [Google Scholar]
- 39.Steinauer KK, Gibbs I, Ning S, French JN, Armstrong J, Knox SJ. Radiation induces upregulation of cyclooxygenase-2 (COX-2) protein in PC-3 cells. Int J Radiat Oncol Biol Phys. 2000;48:325–328. doi: 10.1016/s0360-3016(00)00671-4. [DOI] [PubMed] [Google Scholar]
- 40.Zong ZW, Cheng TM, Su YP, et al. Crucial role of SDF-1/CXCR4 interaction in the recruitment of transplanted dermal multipotent cells to sublethally irradiated bone marrow. J Radiat Res (Tokyo) 2006;47:287–293. doi: 10.1269/jrr.0531. [DOI] [PubMed] [Google Scholar]
- 41.Cameron RB, Spiess PJ, Rosenberg SA. Synergistic antitumor activity of tumor-infiltrating lymphocytes, interleukin 2, and local tumor irradiation. Studies on the mechanism of action. J Exp Med. 1990;171:249–263. doi: 10.1084/jem.171.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 43.Buggins AG, Lea N, Gaken J, et al. Effect of costimulation and the microenvironment on antigen presentation by leukemic cells. Blood. 1999;94:3479–3490. [PubMed] [Google Scholar]
- 44.Passmore JS, Lukey PT, Ress SR. The human macrophage cell line U937 as an in vitro model for selective evaluation of mycobacterial antigen-specific cytotoxic T-cell function. Immunology. 2001;102:146–156. doi: 10.1046/j.1365-2567.2001.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Poltz R, Naumann M. Dynamics of p53 and NF-κB regulation in response to DNA damage and identification of target proteins suitable for therapeutic intervention. BMC Syst Biol. 2012;6:125. doi: 10.1186/1752-0509-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahn KS, Aggarwal BB. Transcription factor NF-kappaB: a sensor for smoke and stress signals. Ann N Y Acad Sci. 2005;1056:218–233. doi: 10.1196/annals.1352.026. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Campisi J, Higano C, Beer TM, Porter P, Coleman I, True L, Nelson PS. Treatment-induced damage to the tumor microenvironment promotes prostate cancer therapy resistance through WNT16B. Nat Med. 2012;18:1359–1368. doi: 10.1038/nm.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narita Y, Kitamura H, Wakita D, Sumida K, Masuko K, Terada S, Nakano K, Nishimura T. The Key Role of IL-6-Arginase Cascade for Inducing Dendritic Cell-Dependent CD4+ T Cell Dysfunction in Tumor-Bearing Mice. J Immunol. 2013;190:812–820. doi: 10.4049/jimmunol.1103797. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.