Abstract
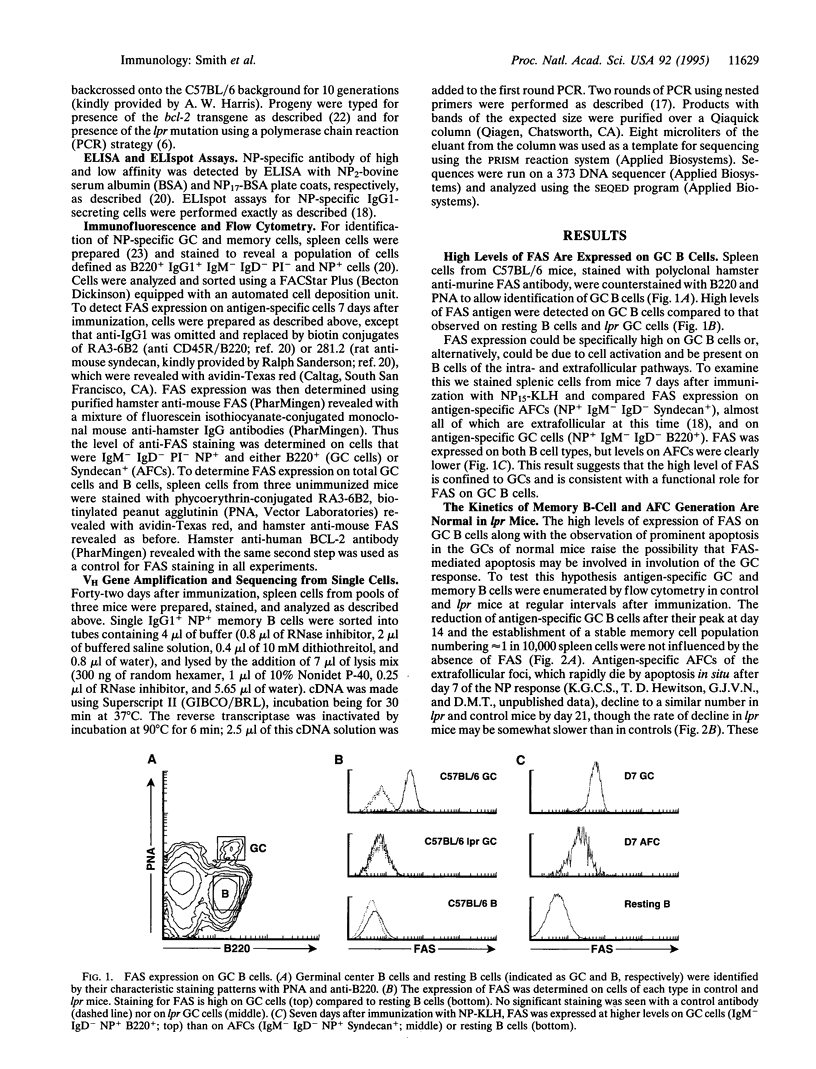
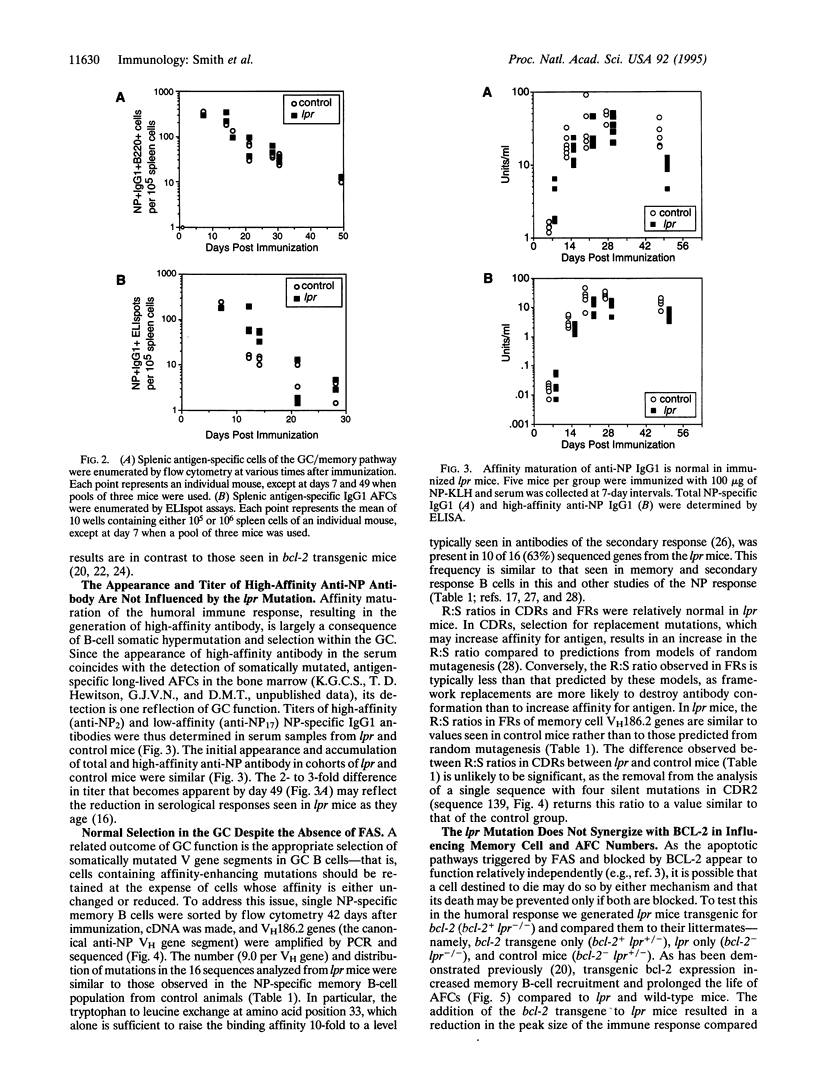
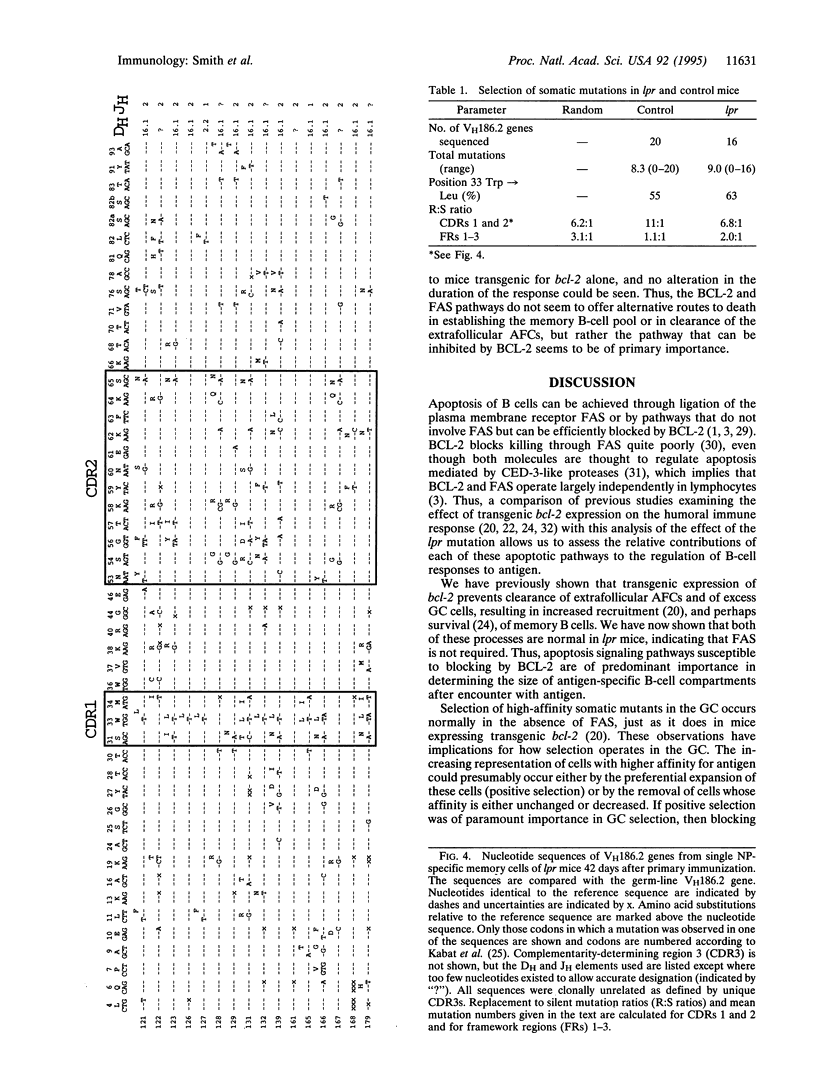
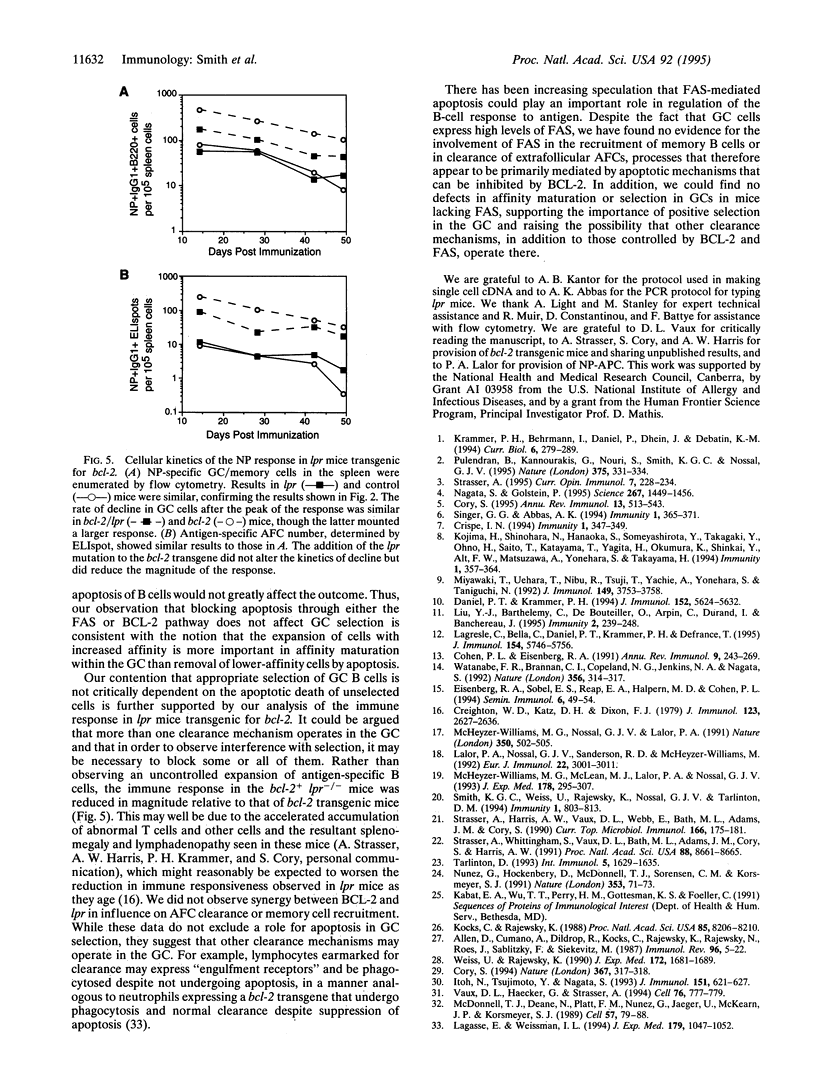
In establishing the memory B-cell population and maintaining self-tolerance during an immune response, apoptosis mediates the removal of early, low-affinity antibody-forming cells, unselected germinal center (GC) cells, and, potentially, self-reactive B cells. To address the role of the apoptosis-signaling cell surface molecule FAS in the B-cell response to antigen, we have examined the T-cell-dependent B-cell response to the carrier-conjugated hapten (4-hydroxy-3-nitrophenyl)acetyl (NP) in lpr mice in which the fas gene is mutated. High levels of FAS were expressed on normal GC B cells but the absence of FAS did not perturb the progressive decline in numbers of either GC B cells or extrafollicular antibody-forming cells. Furthermore, the rate of formation and eventual size of the NP-specific memory B-cell population in lpr mice were normal. The accumulation of cells with affinity-enhancing mutations and the appearance of high-affinity anti-NP IgG1 antibody in the serum were also normal in lpr mice. Thus, although high levels of FAS are expressed on GC B cells, FAS is not required for GC selection or for regulation of the major antigen-specific B-cell compartments. The results suggest that the size and composition of B-cell compartments in the humoral immune response are regulated by mechanisms that do not require FAS.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen D., Cumano A., Dildrop R., Kocks C., Rajewsky K., Rajewsky N., Roes J., Sablitzky F., Siekevitz M. Timing, genetic requirements and functional consequences of somatic hypermutation during B-cell development. Immunol Rev. 1987 Apr;96:5–22. doi: 10.1111/j.1600-065x.1987.tb00506.x. [DOI] [PubMed] [Google Scholar]
- Cohen P. L., Eisenberg R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu Rev Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- Creighton W. D., Katz D. H., Dixon F. J. Antigen-specific immunocompetency, B cell function, and regulatory helper and suppressor T cell activities in spontaneously autoimmune mice. J Immunol. 1979 Dec;123(6):2627–2636. [PubMed] [Google Scholar]
- Eisenberg R. A., Sobel E. S., Reap E. A., Halpern M. D., Cohen P. L. The role of B cell abnormalities in the systemic autoimmune syndromes of lpr and gld mice. Semin Immunol. 1994 Feb;6(1):49–54. doi: 10.1006/smim.1994.1008. [DOI] [PubMed] [Google Scholar]
- Kocks C., Rajewsky K. Stepwise intraclonal maturation of antibody affinity through somatic hypermutation. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8206–8210. doi: 10.1073/pnas.85.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojima H., Shinohara N., Hanaoka S., Someya-Shirota Y., Takagaki Y., Ohno H., Saito T., Katayama T., Yagita H., Okumura K. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994 Aug;1(5):357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Krammer P. H., Behrmann I., Daniel P., Dhein J., Debatin K. M. Regulation of apoptosis in the immune system. Curr Opin Immunol. 1994 Apr;6(2):279–289. doi: 10.1016/0952-7915(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Lagasse E., Weissman I. L. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994 Mar 1;179(3):1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagresle C., Bella C., Daniel P. T., Krammer P. H., Defrance T. Regulation of germinal center B cell differentiation. Role of the human APO-1/Fas (CD95) molecule. J Immunol. 1995 Jun 1;154(11):5746–5756. [PubMed] [Google Scholar]
- Lalor P. A., Nossal G. J., Sanderson R. D., McHeyzer-Williams M. G. Functional and molecular characterization of single, (4-hydroxy-3-nitrophenyl)acetyl (NP)-specific, IgG1+ B cells from antibody-secreting and memory B cell pathways in the C57BL/6 immune response to NP. Eur J Immunol. 1992 Nov;22(11):3001–3011. doi: 10.1002/eji.1830221136. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Barthélémy C., de Bouteiller O., Arpin C., Durand I., Banchereau J. Memory B cells from human tonsils colonize mucosal epithelium and directly present antigen to T cells by rapid up-regulation of B7-1 and B7-2. Immunity. 1995 Mar;2(3):239–248. doi: 10.1016/1074-7613(95)90048-9. [DOI] [PubMed] [Google Scholar]
- McDonnell T. J., Deane N., Platt F. M., Nunez G., Jaeger U., McKearn J. P., Korsmeyer S. J. bcl-2-immunoglobulin transgenic mice demonstrate extended B cell survival and follicular lymphoproliferation. Cell. 1989 Apr 7;57(1):79–88. doi: 10.1016/0092-8674(89)90174-8. [DOI] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., McLean M. J., Lalor P. A., Nossal G. J. Antigen-driven B cell differentiation in vivo. J Exp Med. 1993 Jul 1;178(1):295–307. doi: 10.1084/jem.178.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHeyzer-Williams M. G., Nossal G. J., Lalor P. A. Molecular characterization of single memory B cells. Nature. 1991 Apr 11;350(6318):502–505. doi: 10.1038/350502a0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Golstein P. The Fas death factor. Science. 1995 Mar 10;267(5203):1449–1456. doi: 10.1126/science.7533326. [DOI] [PubMed] [Google Scholar]
- Nuñez G., Hockenbery D., McDonnell T. J., Sorensen C. M., Korsmeyer S. J. Bcl-2 maintains B cell memory. Nature. 1991 Sep 5;353(6339):71–73. doi: 10.1038/353071a0. [DOI] [PubMed] [Google Scholar]
- Pulendran B., Kannourakis G., Nouri S., Smith K. G., Nossal G. J. Soluble antigen can cause enhanced apoptosis of germinal-centre B cells. Nature. 1995 May 25;375(6529):331–334. doi: 10.1038/375331a0. [DOI] [PubMed] [Google Scholar]
- Singer G. G., Abbas A. K. The fas antigen is involved in peripheral but not thymic deletion of T lymphocytes in T cell receptor transgenic mice. Immunity. 1994 Aug;1(5):365–371. doi: 10.1016/1074-7613(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Smith K. G., Weiss U., Rajewsky K., Nossal G. J., Tarlinton D. M. Bcl-2 increases memory B cell recruitment but does not perturb selection in germinal centers. Immunity. 1994 Dec;1(9):803–813. doi: 10.1016/s1074-7613(94)80022-7. [DOI] [PubMed] [Google Scholar]
- Strasser A. Life and death during lymphocyte development and function: evidence for two distinct killing mechanisms. Curr Opin Immunol. 1995 Apr;7(2):228–234. doi: 10.1016/0952-7915(95)80007-7. [DOI] [PubMed] [Google Scholar]
- Strasser A., Whittingham S., Vaux D. L., Bath M. L., Adams J. M., Cory S., Harris A. W. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux D. L., Haecker G., Strasser A. An evolutionary perspective on apoptosis. Cell. 1994 Mar 11;76(5):777–779. doi: 10.1016/0092-8674(94)90350-6. [DOI] [PubMed] [Google Scholar]
- Watanabe-Fukunaga R., Brannan C. I., Copeland N. G., Jenkins N. A., Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992 Mar 26;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]



