Abstract
Fine particulate matter (PM2.5) in air pollution, primarily from combustion sources, is recognized as an important risk factor for cardiovascular events but studies of workplace PM2.5 exposure are rare. We conducted a prospective study of exposure to PM2.5 and incidence of ischemic heart disease (IHD) in a cohort of 11,966 US aluminum workers. Incident IHD was identified from medical claims data from 1998 to 2008. Quantitative metrics were developed for recent exposure (within the last year) and cumulative exposure; however, we emphasize recent exposure in the absence of interpretable work histories prior to follow-up. IHD was modestly associated with recent PM2.5 overall. In analysis restricted to recent exposures estimated with the highest confidence, the hazard ratio (HR) increased to 1.78 (95%CI: 1.02, 3.11) in the second quartile and remained elevated. When the analysis was stratified by work process, the HR rose monotonically to 1.5 in both smelter and fabrication facilities, though exposure was almost an order of magnitude higher in smelters. The differential exposure-response may be due to differences in exposure composition or healthy worker survivor effect. These results are consistent with the air pollution and cigarette smoke literature; recent exposure to PM2.5 in the workplace appears to increase the risk of IHD incidence.
Keywords: occupational epidemiology, particulate matter, heart disease
Introduction
Fine particulate matter (PM2.5) in air pollution, primarily from combustion sources, is recognized as an important risk factor for cardiovascular events including hypertension,1 cardiac arrhythmia,2 myocardial infarction,3 and mortality.4,5 Inhaled PM2.5 (particles with an aerodynamic diameter of less than 2.5μm) from active and passive6 cigarette smoking is also associated with increased risk of cardiovascular disease.7,8 The pathway has not been established; PM2.5 may cause cardiovascular disease secondary to pulmonary inflammation or nanoparticles may pass through the lungs into the circulatory system to cause direct damage.9 Exposure to high levels of PM2.5 is thought to have an immediate (trigger) effect on cardiovascular events, but long-term exposure likely plays a role as well.10
In a recent meta-analysis of cardiovascular mortality,11 results from studies of ambient air pollution5,12–16 and passive smoking17,18 (<1mg/day) and active cigarette smoking (>10mg/day) were presented in a single graph by transforming the exposures into a common daily dose metric. Evident in the graph is a steep rise in relative risk at low exposures followed by a plateau over the high exposure range, with a wide gap between low and high PM2.5 exposures. The range of PM2.5 exposure experienced by industrial workers neatly covers the gap; however, the contribution to this literature from the occupational arena is limited. Historically, heart disease has not been an outcome of interest in occupational epidemiology.19 Heart disease is a multi-factorial disease with well established individual-level risk factors and occupational studies are often limited by the inability to control for potential confounders.20
In this report, we describe results from a prospective study of ischemic heart disease incidence (IHD) and PM2.5 in a cohort of almost 12,000 actively employed aluminum production workers. The analysis is based on quantitative metrics of PM2.5 generated from a variety of sources in aluminum smelting, fabrication and refining facilities. We identified incident cases of IHD based on medical insurance claims in hourly workers. Smoking histories and data on other potential confounders were available from employment records and routine health exams. We focused on the association between IHD and recent occupational exposure to PM2.5. Past exposure was given less attention due to limitations in available data. We stratified by manufacturing process to explore the role of composition and job placement practices.
Methods
Cohort and outcome definition
Hourly workers enrolled in the primary insurance plan and employed for at least two years during follow up in eight U.S. aluminum facilities were considered for the cohort. Follow up began on Jan 1 1998 for six facilities and on Jan 1 2005 for two facilities subsequently acquired by the company. Actively employed workers were followed for incidence of IHD identified from health insurance claims through 2009 or until they left work (whichever occurred first). Prior to 2003, all workers were assumed to use the primary insurance plan. This assumption is supported by the fact that 97% of workers filed at least one claim in this system during this period. After 2003, insurance options increased with the acquisition of new facilities and health insurance enrollment in the primary plan was tracked on a monthly basis.
Health insurance claims for a relevant procedure (revascularization, angioplasty, or bypass), hospitalization for two or more days or a face-to-face visit with an ICD-9 code for IHD (410–414) comprised an IHD diagnosis. Admission codes only were recorded for hospitalization claims. Claims were available starting Jan 1 1996 and a two year “wash-out” was used to exclude cohort members with prevalent disease. The date of IHD incidence was the earliest date of the first applicable claim. All cohort members were therefore at work for two disease-free years after Jan 1 1996, prior to entering follow up. For example, a worker hired on Jan 1 1997 would enter follow up on Jan 1 1999 if no claims for heart disease had been filed during the two intermediate years.
Covariate data collection
Age, sex, race and job grade data were available from employment records for all workers. Additionally, smoking status, weight and height information was maintained at occupational clinics located at each facility and were made available to researchers via chart abstraction. Chart availability varied by facility with some retaining all charts and others retaining only the charts of actively employed workers. Multiple imputation was used for missing smoking and body mass index (BMI) data.
Exposure assessment
Workers in the eight study plants were primarily engaged in smelting aluminum or one of several fabricating processes involving aluminum and related products. The constituents of particulates in these divergent work environments, as well as the nature of the work environments generally, have been well characterized in the literature.21 The exposure assessment for particulate matter relied on company industrial hygiene records as well as measurements collected by the research team in 2010 and 2011. Job title and department combinations in the company industrial hygiene database did not correspond to job titles as they appeared in the work history database. To compute individual exposure histories we therefore aggregated similar jobs into distinct exposure groups within facilities and developed a mapping between the exposure and work history databases. This process incorporated changes in exposure caused by modifications in process or contaminant control (e.g., new company exposure limits).
A job exposure matrix was first constructed for arithmetic mean total particulate matter (TPM) by distinct exposure group. The company had developed an industrial hygiene database of over 300,000 samples collected over the past 25 years. We included samples at the relevant facilities collected randomly for at least 70% of an employee’s shift and analyzed using gravimetric methods.
To estimate PM2.5, side-by-side personal size-selective sampling was conducted in 2010 and 2011 in eight facilities with the traditional closed face cassette paired with a Personal Modular Impactor (PMI). The PMI measured airborne particles in three size ranges (< 2.5μm (PM2.5), 2.5–10.0 μm and >10.0 μm). The percent PM2.5 for each sample was calculated by dividing the concentration of PM2.5 (from the PMI) by the concentration of paired TPM sample (cassette). The values within each distinct exposure group at each facility were averaged to percent PM2.5, which was multiplied by TPM to create PM2.5 concentration in the job exposure matrix.
Details are provided in a separate manuscript by Noth et al (under review at Journal of Exposure Science and Environmental Epidemiology).
Exposure variable definitions
We partitioned exposure into two time-varying exposure windows: recent exposure (mg/m3) in each year and cumulative exposure (mg/m3-years). Recent exposure was the level of PM2.5 estimated for the job held on January 1 of each year. Cumulative exposure was the sum of annual average exposures computed as a weighted average of exposure from all jobs held in each year. Company job codes were not readily translated into the distinct exposure groups before 1996. Thus to extend cumulative exposure all the way back to start of employment for subjects hired before 1996, we assumed workers held the same job (with associated exposure) from hire up to 1996. Thus estimates of cumulative exposure were less reliable than those for recent exposures which only occurred during follow-up.
Respirator use was not considered, other than to address extreme exposure values. For samples over 50 mg/m3 a respirator protection factor was applied (Noth et al, under review at JESEE). In a sensitivity analysis we considered an alternative job exposure matrix in which no respirator adjustment was applied.
Given that TPM samples were collected as industrial hygiene monitoring data, not for research, jobs with potential for exposures greater than 30% of occupational exposure limits were targeted for sampling. Confidence scores were developed for exposure estimates in which higher confidence was assigned to estimates based on direct measurements rather than extrapolation algorithms (for more details, Noth et al, under review at JESEE). Some of the results presented here are restricted to recent exposures estimated by direct measurements.
Statistical analysis
Cox proportional hazard models were fit to estimate the effects of recent exposure to PM2.5 on IHD incidence. All Cox models used age in each year as the time metric. Sex, race (white/non-white), calendar year of follow-up, smoking status (ever/never), BMI, job grade (above/below median in each facility), and manufacturing process (smelter/fabrication/refinery/other) were included in all models to adjust for potential confounding. Models for recent PM2.5 were additionally adjusted for cumulative exposure up until each year to control for potential confounding by exposure history. Variables plausibly on the causal pathway between exposure and IHD, such as hypertension, were not included in the models. Robust sandwich variance estimators were used to account for clustering across person years belonging to the same person in all models.
Multiple imputation procedures in SAS were used to impute missing data for smoking status and BMI. Smoking was missing for 60% of workers and BMI was missing for 62%. The imputation proceeded in two steps, first continuous BMI was imputed using the EM algorithm to create a monotone missingness pattern, and then categorical smoking status was imputed using the logistic regression method. Case status, time since hire, hypertension, diabetes, obesity, employment termination, and all the variables used in the main models were used to impute missing information, as these variables were assumed to be sufficient to satisfy the missing at random assumption. Five imputed data sets were created and all subsequent model fits and inferences were calculated using the multivariate extension to Rubin’s rules.22
Models included recent exposure to PM2.5 defined as categorical variables. Results for recent PM2.5 are presented with two strategies for choosing cut points: 1) cases were divided equally by quintiles of exposure and 2) cases were divided equally by quartiles of exposure above the reference level of 0.05 mg/m3. The second strategy was guided by federal EPA air pollution standards; the 2006 daily standard for PM2.5 was 0.035 mg/m3. Since industrial levels of PM2.5 are higher than ambient levels and fewer exposure measurements were taken at the low levels, the lowest cut point we could set was 0.05 mg/m3. To assess the possibility that uncertainty in low exposures might introduce bias, we conducted analyses restricted to exposures with high confidence scores.
Manufacturing process (smelter or fabrication) was considered as a potential effect measure modifier. To take advantage of the continuous exposure metric, we added a penalized spline function of recent PM2.5 to stratified Cox models. Degrees of freedom were based on minimum Akaike’s Information Criterion (AIC) and biologic plausibility. R software (R Development Core Team, Vienna, Austria) was used for the spline analysis and SAS software (SAS Institute Inc, Cary, NC) was used for all other analyses.
There was less emphasis on models for cumulative exposure; exposures were less reliable prior to 1996 and we could not feasibly restrict to higher confidence estimates. Pooled and stratified models, however, were also fit to estimate the effects of cumulative exposure to PM2.5 on IHD incidence. Exposure was defined as a categorical variable with cases divided equally by quintiles of exposure. Smoking and BMI were imputed and controlled for as described above.
Results
There were 697 IHD cases identified from a cohort of 11,966 aluminum workers. The IHD cases were more likely to be male, older and hired prior to the start of follow up (Table 1). Most subjects worked in fabrication (69%); cases were slightly more likely to work in the smelters compared to the cohort as a whole. The median recent exposure was similar in the cohort and cases, however median cumulative exposures were higher for cases, consistent with their older age and earlier hire dates.
Table 1.
Demographic Characteristics of an Aluminum Manufacturing Cohort in the United States 1998–2008
| Cohort | IHD Cases | |
|---|---|---|
| No. Workers | 11,966 | 697 |
| Person-Years | 68,848 | - |
| Male no. (%) | 10,049 (84) | 655 (94) |
| White no. (%) | 9,919 (83) | 591 (85) |
| Hired ≥Follow-up no. (%) | 3,665 (31) | 81 (12) |
| Year of Hire Median (IQR) | 1984 (1974–1996) | 1977 (1970–1988) |
| Year of Birth Median (IQR) | 1955 (1948–1962) | 1948 (1944–1953) |
| Manufacturing Process | ||
| Smelter no. (%) | 2194 (18) | 152 (22) |
| Fabrication no. (%) | 8290 (69) | 449 (64) |
| Refinery no. (%) | 960 (8) | 56(8) |
| Other facility type no. (%) | 522 (5) | 40 (6) |
| Recent* PM (mg/m3) | ||
| PM2.5 median (IQR) | 0.29 (0.12–1.04) | 0.26 (0.12–1.04) |
| Cumulative PM (mg/m3-yrs) | ||
| PM2.5 median (IQR) | 5.01 (1.74–18.41) | 6.95 (2.39–26.46) |
Recent exposure is defined as exposure within the last year.
The Hazard Ratios (HRs) for incident IHD were higher when the reference level was set at 0.05 mg/m3, rather than quintiles, and even higher still when exposures were restricted to those assessed with high confidence (Table 2). The exposure-response increased to a maximum of 1.78 (95% CI 1.02, 3.11) though the pattern was non-monotonic. When cases were distributed evenly based on recent exposure quintiles, the HR was slightly elevated in the second category of exposure (1.28 (95% CI 0.92, 1.63). HRs for higher categories of recent exposure remained modestly elevated and all confidence intervals included the null. When equal quintiles were based on exposure data restricted to high confidence, the risk of IHD increased by more than 20% in the highest exposure categories, though all confidence intervals included the null.
Table 2.
Hazard Ratios for Ischemic Heart Disease According to Two Categorizations of Recent (within the last year) PM2.5, Each With All Exposures and When Restricted to Exposures Measured with High Confidence, in an Aluminum Manufacturing Cohort in the United States, 1998–2008.
| All Exposures | Only Exposures Assessed with High Confidence | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Categories by Quintiles of Exposure Among Cases | |||||||||
| Recent PM2.5 (mg/m3) | No. cases | Person- years | Hazard Ratiosa | 95% Confidence Interval | Recent PM2.5 (mg/m3) | No. cases | Person- years | Hazard Ratiosa | 95% Confidence Interval |
| ≤ 0.11 | 135 | 11,490 | 1 | ≤ 0.12 | 129 | 10,891 | 1 | ||
| >0.11–0.22 | 143 | 14,272 | 1.05 | 0.81, 1.35 | >0.12–0.23 | 102 | 10,379 | 1.05 | 0.80, 1.39 |
| >0.22–0.45 | 140 | 11,708 | 1.23 | 0.92, 1.63 | >0.23–0.50 | 114 | 10,310 | 1.04 | 0.77, 1.41 |
| >0.45–1.47 | 130 | 11,971 | 1.06 | 0.78, 1.45 | >0.50–1.63 | 109 | 8,899 | 1.29 | 0.88, 1.88 |
| >1.47 | 149 | 14,321 | 1.09 | 0.73, 1.62 | >1.63 | 123 | 11,853 | 1.21 | 0.78, 1.88 |
| Categories by Quartiles of Exposure Among Cases Above Reference Level 0.05mg/m3 | |||||||||
| Recent PM2.5 (mg/m3) | No. cases | Person- years | Hazard Ratiosa | 95% Confidence Interval | Recent PM2.5 (mg/m3) | No. cases | Person- years | Hazard Ratiosa | 95% Confidence Interval |
| ≤ 0.05 | 61 | 6,151 | 1 | ≤ 0.05 | 15 | 1,452 | 1 | ||
| >0.05–0.16 | 156 | 13,770 | 1.26 | 0.92, 1.71 | >0.05–0.16 | 132 | 11,757 | 1.58 | 0.88, 2.63 |
| >0.16–0.34 | 166 | 13,706 | 1.48 | 1.07, 2.05 | >0.16–0.37 | 148 | 12,359 | 1.78 | 1.02, 3.11 |
| >0.34–1.15 | 155 | 14,975 | 1.15 | 0.82, 1.63 | >0.37–1.47 | 139 | 13,074 | 1.48 | 0.83, 2.66 |
| >1.15 | 159 | 15,159 | 1.18 | 0.77, 1.82 | >1.47 | 143 | 13,690 | 1.48 | 0.77, 2.85 |
Hazard Ratios adjusted for age, race, gender, calendar year, smoking, facility type, bmi, job grade, and past exposure
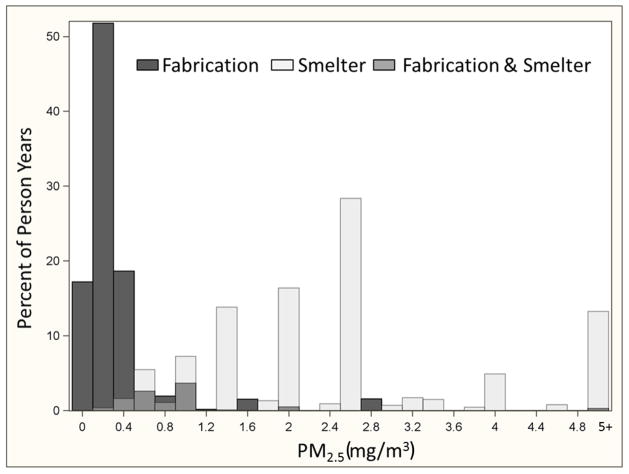
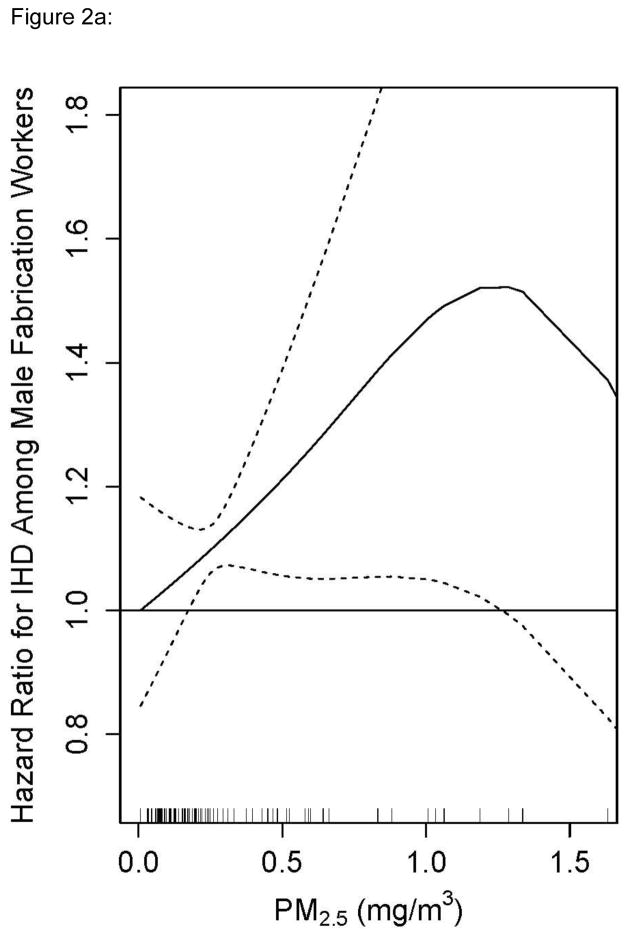
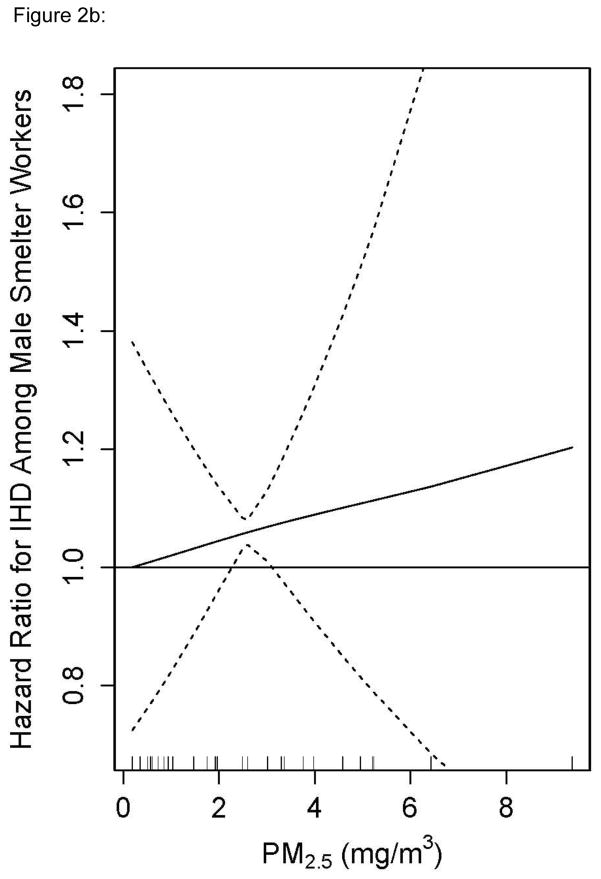
To examine effect measure modification, exposure-response was modeled as a smooth function of recent PM2.5 exposure separately in fabrication and smelters. Stratification with splines avoids the selection of a common reference group – a particular challenge in this analysis because PM2.5 exposures in the smelters were several times higher than in fabrication. The splines also allow us to take advantage of the continuous data without parametric assumptions (Figure 1). Few women were employed in the smelters and so the entire stratified analysis was restricted to males. The HR for PM2.5 and incident IHD rose in fabrication to 1.5 at 1.25 mg/m3 and was statistically significant throughout most of the exposure range (Figure 2a). The exposure response in the smelters was approximately linear and rose to an HR of 1.5 at 9 mg/m3, but was only statistically significant around the mean (Figure 2b). This stratified analysis presents evidence that the association between recent PM2.5 and IHD differs by manufacturing process.
Figure 1.
Percent of Person-years in Recent (within the last year) PM2.5 Exposure Categories shown in Overlapping Distributions for Fabrication and Smelting Processes in an Aluminum Manufacturing Cohort followed from 1998 through 2008. (Exposures above 5 mg/m3 lumped into top category.)
Figure 2.
Figure 2a: Penalized Spline of the Adjusted Hazard Ratio for Ischemic Heart Disease and Recent (within the last year) PM2.5 in a Cox Model for Males working in Fabrication Facilities in an Aluminum Manufacturing Cohort in the United States, 1998–2008. Restricted to Exposures Measured with the Highest Confidence. Graph truncated at 97th percentile of exposure.
Figure 2b: Penalized Spline of the Adjusted Hazard Ratio for Ischemic Heart Disease and Recent (within the last year) PM2.5 in a Cox Model for Males in Smelting Facilities in an Aluminum Manufacturing Cohort in the United States, 1998–2008. Restricted to Exposures \ Measured with the Highest Confidence.
In a sensitivity analysis, models with categorical exposure were fit in the each of the two work strata. In fabrication, the HR rose to 1.98 (95% CI: 1.06, 3.68) in the third exposure category and then dipped to 1.37 (95% CI 0.74, 2.51) in the top category (>0.38mg/m3) compared to a reference group of ≤ 0.05mg/m3. In smelters, the HR rose from 1.13 (95% CI 0.48, 2.65) in the first exposure category to 1.25 (95% CI 0.52, 2.99) in the top category (>5.23mg/m3) compared to a reference group of ≤ 0.075mg/m3 (data not shown).
Additional sensitivity analyses were conducted on a job exposure matrix in which no respirator adjustment was applied. The pooled and fabrication-specific results were unchanged. The spline in smelters remained linear, increased to a maximum HR of 1.4, and was statistically significant around the mean of exposure. However, without any respirator adjustment, the maximum exposure increased to 32mg/m3, thus the slope of the exposure-response curve was less steep than the slope presented in Figure 2b.
In contrast with recent exposure, there was no indication that cumulative PM2.5 exposure increased IHD risk overall; all point estimates were below one (Table 3). When we stratified analyses of categorical cumulative exposure by manufacturing process, we observed HRs from 1.13 to 0.96 in fabrication and HRs from 0.86 to 0.77 in the smelters with all confidence intervals including the null (data not shown).
Table 3.
Hazard Ratios for Ischemic Heart Disease According to Cumulative PM2.5 (mg/m3-years) in an Aluminum Manufacturing Cohort in the United States, 1998–2008.
| Cumulative PM2.5 | No. cases | Person- years | Hazard Ratiosa | 95% Confidence Interval |
|---|---|---|---|---|
| ≤ 1.89 | 140 | 16,198 | 1 | |
| >1.89–4.52 | 139 | 13,392 | 0.89 | 0.70,1.12 |
| >4.52–10.51 | 140 | 11,855 | 0.81 | 0.64,1.03 |
| >10.51–35.58 | 139 | 11,648 | 0.82 | 0.63, 1.07 |
| >35.58 | 139 | 10,669 | 0.80 | 0.59, 1.07 |
Hazard Ratios adjusted for age, race, gender, calendar year, smoking, bmi, job grade, and facility type
Discussion
Results provide evidence that recent occupational exposure to PM2.5 is associated with IHD incidence in a cohort of actively employed hourly workers. Increased risk of IHD was strongest at the relatively low PM2.5 exposure levels found in the fabricating process. By contrast, we saw no evidence that cumulative PM2.5 increases risk of IHD incidence, though the exposure estimates were less reliable and results more subject to survivor bias (as elaborated below). These findings are consistent with the literature on cardiovascular disease and ambient air pollution.
One striking feature of our PM2.5 and IHD incidence results is the shape of the exposure-response curve in the pooled analysis. Risk peaked in the second category of exposure and leveled off in higher categories. This pattern was evident in the main analysis (Table 2) especially when the 0.05 mg/m3 reference group was used. To help interpret these results, we address several factors: the reference group, workplace hire and retention practices, reliability of the exposure assessment, consistency across studies, and biological plausibility.
In choosing the lowest possible cut point for the reference group, we incorporated the wealth of existing knowledge and public health policy regarding ambient PM2.5 exposure. The ideal cut point would have been 0.015 mg/m3, the recent US EPA standard for annual ambient PM2.5. In this occupational cohort, however, no cases were exposed at or below this level. We then considered 0.035 mg/m3, the standard for daily ambient PM2.5. The daily standard can be exceeded only three days a year in the ambient environment but offered a more realistic cut-point in this industrial setting. However, only 13 cases were exposed at less than 0.035 mg/m3 and none of the exposure estimates had high confidence scores. Thus, 0.05 mg/m3 was determined to be the lowest level at which we had adequate power for categorical analysis. We also presented analysis based on a “naïve” reference group based on the 20th%-tile of the cases. Despite the increased power in the “naïve” categorization, the hazard ratios were attenuated likely due to increased baseline risk.
In categorical exposure models, there is a common reference group for all higher categories of exposure. However, the mean PM2.5 is considerably lower in fabrication than in smelters with little overlap in levels. Thus the reference group in the pooled models comprised mostly workers from fabrication whereas the higher categories comprised mostly workers from smelters. There are other relevant differences between smelters and fabrication jobs in addition to PM2.5 exposure levels and constituents. First, jobs in smelters are the most physically demanding jobs in the aluminum industry. Some smelting jobs also involve exposure to high levels of heat that can be hazardous for workers with heart disease risk factors. As a result of these hazards, there is a heart disease risk factor screening program for placement and retention in many smelter jobs. Furthermore, ongoing screening occurs for almost all smelter workers in the context of mandatory respiratory fitness and work in hot environments. Thus it is possible that the plateau in the highest two categories reflects a phenomenon analogous to the “healthy hire” effect that plagues occupational studies with external reference groups. If workers in the higher exposure categories (smelter workers) are screened and thus at less risk for heart disease than those in the reference group (fabrication workers), bias may occur even in our pooled internal analysis (Table 2) adjusting for baseline covariates.
In addition to selection bias, the plateau may also arise from misclassification of the highest exposures in the smelters. The presence of well established risk factors for occupational asthma23 and cancer24 in the smelters has driven more widespread use of respiratory protection than in fabricating jobs. Had respirators been more completely accounted for in this analysis, the result would have been to reduce the value of smelter exposures on the high end while very little would have changed on the low end. In fact, the exposure-response presented in Figure 2b was steeper than when we ignored the adjustment for values above 50mg/m3 in a sensitivity analysis. Future analyses will incorporate a more thorough evaluation of respirator use; it seems likely that such an adjustment will result in an even steeper exposure-response for smelter workers.
To explore the impact of exposure misclassification on the steep increase in risk over lower exposure categories of our pooled analysis, we restricted our analysis to recent exposures estimated with a high confidence score. Despite a marked reduction of cases and person-years in the reference group, the shape of the exposure-response was robust and the HRs increased more sharply.
There are two sources of bias that might lead to attenuation of the HRs for cumulative exposure: left-truncation25,26 and misclassification of exposure prior to the start of follow up. Attenuation from left-truncation bias occurs if those who left work prior to the start of follow up were more susceptible to the health effects of exposure than those who stayed to be included in the study. Ideally, we would restrict our analysis to workers hired after the start of follow up, however almost 70% of the cohort and 90% of the cases were already employed in 1996 when the study started. In addition, there was a lack of usable job histories prior to 1996. To estimate cumulative exposure prior to that time for workers who were already employed, we assumed that the job held at start of follow-up had been their job throughout their prior employment history. Furthermore, analyses for cumulative exposure included all exposures even those not measured with high confidence as there was no straight forward way to cumulate only the subset based on measurements. We therefore expected some attenuation, and caution against over interpreting the absence of increased risk. The impact of past exposure on IHD risk in this setting remains an open question.
By using medical claims, we were able to identify incident IHD as well as conditions such as hypertension. The use of claims data has been found to be highly specific for hypertension in a validation study for this population.27 However, the possibility remains that a worker without any diagnosis of IHD could have a fatal cardiac event without hospitalization. This rare scenario would result in misclassification of the outcome and would likely lead to attenuation.
The increased risk of IHD observed at low levels of recent exposure in this study is consistent with the existing PM2.5 literature. The cardiovascular effects of ambient PM2.5 and second hand smoke have been established at levels below our reference cut point. In many US cities PM2.5 in air pollution rarely rises above 0.03 mg/m3 and second hand smoke ranges from 0.02 to 0.05 mg/m.3 28–30
In the heart disease and PM2.5 research in occupational cohorts, internal comparisons are rare. Moreover, most studies lack detail in the exposure assessment and have limited data on potential confounders. A recent meta analysis of four studies comparing high (or any) versus low (or no) PM exposure found an RR of 1.15 (95% CI 1.06–1.14) for IHD mortality, although none of the studies included a minimally acceptable set of potential confounders.20 Of particular interest, Burstyn et al31 report a monotonic exposure – response association for both recent and cumulative PAH (benzo(a)pyrene) exposure in male asphalt workers with a 60% increased risk of IHD mortality. Individual level data for smoking was not available; however sensitivity analyses suggested a 20–40% increase in risk under realistic scenarios of confounding by smoking. Since the meta analysis, two relevant papers have been published. Costello et al32 reported increased risk of IHD mortality associated with exposure to respirable PM from straight metalworking fluids in a cohort of actively employed autoworkers. An increased risk of IHD mortality was associated with recent exposure to respirable PM before 1971 and with cumulative exposure to respirable PM after 1971, with no adjustment for smoking. The metalworking process among the autoworkers was similar to the fabrication manufacturing presented here; however, aluminum fabrication involves mostly water-based rather than oil-based (straight) fluids. Friesen et al33 reported on cumulative benzo(a)pyrene exposure in a male aluminum smelting workforce in Canada controlling for smoking status. When the cohort was restricted to actively employed workers, comparable to the smelter workers presented here, the HR for IHD mortality increased the two-fold in the highest category of cumulative exposure. Dust levels in the Canadian cohort were higher due to the time period and an older technology for smelting aluminum and no estimates for recent exposure were presented. To our knowledge, there is no occupational literature on recent exposure to respirable or fine PM and incident IHD adjusted for multiple potential confounders.
Data on many important covariates are available in this dataset, however we had to impute missing data for smoking and BMI. If the missing at random assumption was not met, then we may have residual confounding by these variables. Results presented here were similar to results from models run with a category for missingness for BMI and smoking, but slightly stronger than results unadjusted for BMI or smoking, especially in the smelters.
Three major pathophysiologic pathways by which particulate matter may cause heart disease have been proposed.34,35 First, upon entering the lungs, PM2.5 may cause oxidative stress and systemic inflammatory response36–38 which can increase concentration of blood fibrinogen,37,38 induce progression of atherosclerosis39,40 and activate cardiac myocytes and adipocytes.35 Second, PM2.5 could deposit in the upper airways and activate receptors linked to the autonomic nervous system leading to a sympathetic up-regulation and vagal withdrawal35,41 as well as hypertension.42 Third, components adsorbed on the surface of particles, or the ultrafine particles in the air mixture,43–45 could cross the lung-blood barrier and interact directly with blood cells or the endothelium. We have not measured the ultrafine component of the PM2.5 nor have we yet taken account of the composition of the particles. In fabrication, PM is likely composed of water-based metalworking fluids (soluble or synthetic fluids).32,46,47 In the smelters, the PM is likely composed of inorganic materials, i.e. fluorides,48 alumina dust, metals and related fumes,49 as well as coal tar pitch volatiles33 in some areas. Given the apparent effect modification by manufacturing process (Figures 2a and 2b), composition of PM2.5 may be as relevant to risk as concentration in this industrial setting.
Other heart disease risk factors such as noise50 and stress,51 could vary by manufacturing process and may be responsible for some of the observed differences in risk.
We did not control for hypertension in our models because it might be on the causal pathway between exposure and disease. Diagnosis with this condition might result in a change of exposure, via transferring jobs or reducing hours, thus including it in a person-year Cox model could introduce bias. We plan to apply marginal structural models to deal with time varying confounding by this condition and to address censoring due to leaving work.52
Conclusion
This is the first report of incident IHD and recent PM2.5 in an occupationally exposed US cohort of active aluminum production workers. We found evidence of increased risk of IHD even at the low end of the recent exposure range. The exposure-response was most striking when stratifying workers by manufacturing process. At the same recent exposure level, workers in the smelters have lower risk of IHD compared to the fabrication workers, possibly an unintended benefit of the workplace programs in place for heat and respiratory fitness. However, the risk does increase in both work environments, suggesting that composition of PM2.5 may be etiologically relevant for cardiovascular risk.
Acknowledgments
This work was supported by National Institutes of Health, Institute of Aging (R01 AG026291–01) and Center for Disease Control and Prevention, National Institute of Occupational Safety and Health (R01OH009939-01) and by Alcoa, Inc.
Footnotes
NIA Data Sharing
As an alternative to providing a de-identified data set to the public domain, we allow access for the purpose of re-analyses or appropriate “follow-on” analyses by any qualified investigator willing to sign a contractual covenant with the host Institution limiting use of data to a specific agreed upon purpose and observing the same restrictions as are limited in our contract with Alcoa, such as 60-day manuscript review for compliance purposes.
Conflicts of Interest: Drs Costello, Brown, Noth and Eisen do not have any conflicts of interest to declare. Ms Cantley and Ms. Tessier-Sherman receive salary support from Alcoa, Inc. through contracts with Yale University. Dr. Cullen receives salary support from Alcoa, Inc. through contracts with Stanford University. Dr. Hammond receives compensation as a member of the scientific advisory board for Alcoa, Inc and has also consulted on exposure assessment and received compensation.
References
- 1.Dong G-H, Qian ZM, Xaverius PK, et al. Association Between Long-Term Air Pollution and Increased Blood Pressure and Hypertension in China. Hypertension. 2013 doi: 10.1161/HYPERTENSIONAHA.111.00003. [DOI] [PubMed] [Google Scholar]
- 2.Peters A, Liu E, Verrier RL, et al. Air pollution and incidence of cardiac arrhythmia. Epidemiology. 2000;11(1):11–7. doi: 10.1097/00001648-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Peters A, Dockery DW, Muller JE, Mittleman MA. Increased particulate air pollution and the triggering of myocardial infarction. Circulation. 2001;103(23):2810–5. doi: 10.1161/01.cir.103.23.2810. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Rajagopalan S, Pope CA, et al. Particulate Matter Air Pollution and Cardiovascular Disease. An Update to the Scientific Statement From the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 5.Dockery DW, Pope CA, Xu X, et al. An association between air pollution and mortality in six U.S. cities. The New England journal of medicine. 1993;329(24):1753–9. doi: 10.1056/NEJM199312093292401. [DOI] [PubMed] [Google Scholar]
- 6.Law MR, Morris JK, Wald NJ. Environmental tobacco smoke exposure and ischaemic heart disease: an evaluation of the evidence. BMJ (Clinical research ed ) 1997;315(7114):973–80. doi: 10.1136/bmj.315.7114.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.U.S. Department of Health and Human Services. The Health Consequences of Smoking: Cardiovascular Disease. A Report of the Surgeon General. 1983 [Google Scholar]
- 8.U.S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General. 2004 [Google Scholar]
- 9.Mills NL, Donaldson K, Hadoke PW, et al. Adverse cardiovascular effects of air pollution. Nature clinical practice. Cardiovascular medicine. 2009;6(1):36–44. doi: 10.1038/ncpcardio1399. [DOI] [PubMed] [Google Scholar]
- 10.Gan WQ, Koehoorn M, Davies HW, Demers Pa, Tamburic L, Brauer M. Long-term exposure to traffic-related air pollution and the risk of coronary heart disease hospitalization and mortality. Environmental health perspectives. 2011;119(4):501–7. doi: 10.1289/ehp.1002511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pope CA, Burnett RT, Krewski D, et al. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure-response relationship. Circulation. 2009;120(11):941–8. doi: 10.1161/CIRCULATIONAHA.109.857888. [DOI] [PubMed] [Google Scholar]
- 12.Laden F, Schwartz J, Speizer FE, Dockery DW. Reduction in fine particulate air pollution and mortality: Extended follow-up of the Harvard Six Cities study. American journal of respiratory and critical care medicine. 2006;173(6):667–72. doi: 10.1164/rccm.200503-443OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miller KA, Siscovick DS, Sheppard L, et al. Long-term exposure to air pollution and incidence of cardiovascular events in women. The New England journal of medicine. 2007;356(5):447–58. doi: 10.1056/NEJMoa054409. [DOI] [PubMed] [Google Scholar]
- 14.Pope CA, Burnett RT, Thurston GD, et al. Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109(1):71–7. doi: 10.1161/01.CIR.0000108927.80044.7F. [DOI] [PubMed] [Google Scholar]
- 15.Pope CA, Burnett RT, Thun MJ, et al. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA : the journal of the American Medical Association. 2002;287(9):1132–41. doi: 10.1001/jama.287.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pope CA, Thun MJ, Namboodiri MM, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. American journal of respiratory and critical care medicine. 1995;151(3 Pt 1):669–74. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 17.US Department of Health and Human Services. US Department of Health and Human Services. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Atlanta, Ga: US Dept of Health and Human Services, Center for Disease Control and Prevention, Coordinating Cente; 2006. [Google Scholar]
- 18.Teo KK, Ounpuu S, Hawken S, et al. Tobacco use and risk of myocardial infarction in 52 countries in the INTERHEART study: a case-control study. Lancet. 2006;368(9536):647–58. doi: 10.1016/S0140-6736(06)69249-0. [DOI] [PubMed] [Google Scholar]
- 19.Cullen MR. Invited commentary: the search for preventable causes of cardiovascular disease--whither work? American journal of epidemiology. 2009;169(12):1422–5. doi: 10.1093/aje/kwp078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fang SC, Cassidy A, Christiani DC. A systematic review of occupational exposure to particulate matter and cardiovascular disease. International journal of environmental research and public health. 2010;7(4):1773–806. doi: 10.3390/ijerph7041773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benke G, Abramson M, Sim M. Exposures in the Alumina and Primary Aluminium Industry: an Historical Review. 1998;42(3):173–189. doi: 10.1016/s0003-4878(98)00020-9. [DOI] [PubMed] [Google Scholar]
- 22.Schafer JL. Analysis of Incomplete Multivariate Data. 1. Chapman & Hall/CRC; 1997. p. 113. [Google Scholar]
- 23.Taiwo Oa, Sircar KD, Slade MD, et al. Incidence of asthma among aluminum workers. Journal of occupational and environmental medicine/American College of Occupational and Environmental Medicine. 2006;48(3):275–82. doi: 10.1097/01.jom.0000197876.31901.f5. [DOI] [PubMed] [Google Scholar]
- 24.IARC. Monograph 34: polynuclear aromatic compounds, Part3, industrial exposures in aluminum production, coal gasification, coke production, and iron and steel founding. Genevia: World Health Organization; 1984. [Google Scholar]
- 25.Applebaum KM, Malloy EJ, Eisen EA. Reducing healthy worker survivor bias by restricting date of hire in a cohort study of Vermont granite workers. Occup Environ Med. 2007;64(10):681–687. doi: 10.1136/oem.2006.031369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Applebaum KM, Malloy EJ, Eisen EA. Left Truncation, Susceptibility, and Bias in Occupational Cohort Studies. Epidemiology. 2011;22(4):599–606. doi: 10.1097/EDE.0b013e31821d0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tessier-Sherman B, Galusha D, Taiwo OA, et al. Further validation that claims data are a useful tool for epidemiologic research on hypertension. BMC public health. 2013;13(1):51. doi: 10.1186/1471-2458-13-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenkins RA, Palausky A, Counts RW, Bayne CK, Dindal AB, Guerin MR. Exposure to environmental tobacco smoke in sixteen cities in the United States as determined by personal breathing zone air sampling. Journal of exposure analysis and environmental epidemiology. 1996;6(4):473–502. [PubMed] [Google Scholar]
- 29.Leaderer BP, Hammond SK. Evaluation of vapor-phase nicotine and respirable suspended particle mass as markers for environmental tobacco smoke. Environmental Science & Technology. 1991;25(4):770–777. [Google Scholar]
- 30.Dockery DW, Spengler JD. Personal exposure to respirable particulates and sulfates. Journal of the Air Pollution Control Association. 1981;31(2):153–9. doi: 10.1080/00022470.1981.10465205. [DOI] [PubMed] [Google Scholar]
- 31.Burstyn I, Kromhout H, Partanen T, et al. Polycyclic Aromatic Hydrocarbons and Fatal Ischemic Heart Disease. Epidemiology. 2005;16(6):744–750. doi: 10.1097/01.ede.0000181310.65043.2f. [DOI] [PubMed] [Google Scholar]
- 32.Costello S, Garcia E, Hammond SK, Eisen EA. Ischemic Heart Disease Mortality and PM 3.5 in a Cohort of Autoworkers. American journal of industrial medicine. 2012 doi: 10.1002/ajim.22152. doi: 10.10. [DOI] [PubMed] [Google Scholar]
- 33.Friesen MC, Demers PA, Spinelli JJ, Eisen EA, Lorenzi MF, Le ND. Chronic and acute effects of coal tar pitch exposure and cardiopulmonary mortality among aluminum smelter workers. American journal of epidemiology. 2010;172(7):790–9. doi: 10.1093/aje/kwq208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brook RD, Franklin B, Cascio W, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109(21):2655–71. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 35.Peters A. Ambient particulate matter and the risk for cardiovascular disease. Progress in cardiovascular diseases. 2011;53(5):327–33. doi: 10.1016/j.pcad.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 36.Salvi S, Blomberg A, Rudell B, et al. Acute inflammatory responses in the airways and peripheral blood after short-term exposure to diesel exhaust in healthy human volunteers. American journal of respiratory and critical care medicine. 1999;159(3):702–9. doi: 10.1164/ajrccm.159.3.9709083. [DOI] [PubMed] [Google Scholar]
- 37.Ghio AJ, Kim C, Devlin RB. Concentrated ambient air particles induce mild pulmonary inflammation in healthy human volunteers. American journal of respiratory and critical care medicine. 2000;162(3 Pt 1):981–8. doi: 10.1164/ajrccm.162.3.9911115. [DOI] [PubMed] [Google Scholar]
- 38.Ghio AJ, Hall A, Bassett MA, Cascio WE, Devlin RB. Exposure to concentrated ambient air particles alters hematologic indices in humans. Inhalation toxicology. 2003;15(14):1465–78. doi: 10.1080/08958370390249111. [DOI] [PubMed] [Google Scholar]
- 39.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135–43. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 40.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, Van Eeden SF. Particulate air pollution induces progression of atherosclerosis. Journal of the American College of Cardiology. 2002;39(6):935–42. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- 41.Monn C, Becker S. Cytotoxicity and induction of proinflammatory cytokines from human monocytes exposed to fine (PM2.5) and coarse particles (PM10–2.5) in outdoor and indoor air. Toxicology and applied pharmacology. 1999;155(3):245–52. doi: 10.1006/taap.1998.8591. [DOI] [PubMed] [Google Scholar]
- 42.Brook RD, Urch B, Dvonch JT, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54(3):659–67. doi: 10.1161/HYPERTENSIONAHA.109.130237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oberdörster G, Sharp Z, Atudorei V, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. Journal of toxicology and environmental health. Part A. 2002;65(20):1531–43. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 44.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. American journal of respiratory and critical care medicine. 2001;164(9):1665–8. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 45.Nemmar A, Hoet PHM, Vanquickenborne B, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105(4):411–4. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 46.Chevrier J, Picciotto S, Eisen Ea. A comparison of standard methods with g-estimation of accelerated failure-time models to address the healthy-worker survivor effect: application in a cohort of autoworkers exposed to metalworking fluids. Epidemiology. 2012;23(2):212–9. doi: 10.1097/EDE.0b013e318245fc06. [DOI] [PubMed] [Google Scholar]
- 47.Park RM. Mortality at an automotive engine foundry and machining complex. Journal of occupational and environmental medicine. 2001;43(5):483–93. doi: 10.1097/00043764-200105000-00009. [DOI] [PubMed] [Google Scholar]
- 48.Rønneberg A. Mortality and cancer morbidity in workers from an aluminium smelter with prebaked carbon anodes--Part III: Mortality from circulatory and respiratory diseases. Occupational and environmental medicine. 1995;52(4):255–61. doi: 10.1136/oem.52.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cavallari JM, Eisen EA, Fang SC, et al. PM2.5 metal exposures and nocturnal heart rate variability: a panel study of boilermaker construction workers. Environmental health. 2008;7:36. doi: 10.1186/1476-069X-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonde JP, Kolstad Ha. Noise and ischemic heart disease. Scandinavian journal of work, environment & health. 2012;38(1):1–3. doi: 10.5271/sjweh.3261. [DOI] [PubMed] [Google Scholar]
- 51.Kivimäki M, Nyberg ST, Batty GD, et al. Job strain as a risk factor for coronary heart disease: a collaborative meta-analysis of individual participant data. Lancet. 2012;380(9852):1491–7. doi: 10.1016/S0140-6736(12)60994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robins JM, Hernán Ma, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11(5):550–60. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]