Abstract
Friendship pervades the human social landscape. These bonds are so important that disrupting them leads to health problems, and difficulties forming or maintaining friendships attend neuropsychiatric disorders like autism and depression. Other animals also have friends, suggesting that friendship is not solely a human invention but is instead an evolved trait. A neuroethological approach applies behavioral, neurobiological, and molecular techniques to explain friendship in terms of its underlying mechanisms, development, evolutionary origins, and biological function. Recent studies implicate a shared suite of neural circuits and neuromodulatory pathways in the formation, maintenance, and manipulation of friendships across humans and other animals. Health consequences and reproductive advantages in mammals additionally suggest that friendship has adaptive benefits. We argue that understanding the neuroethology of friendship in humans and other animals brings us closer to knowing fully what it means to be human.
Keywords: friendship, cognition, ethology, social networks, evolution
Introduction
Friendship is a hallmark of human behavior. Friends may promote our financial success,1 health,2 and even survival.3,4 Social exclusion and the loss of social partners result in feelings akin to physical pain5, and deficits in the ability or motivation to form and maintain friendly relationships are a symptom of pathologies like autism and depression.6 Yet despite its importance, the formalized scientific study of friendship is relatively new. This may be because friendship has been deemed a human construct outside the realm of biology7 or as merely an epiphenomenon of pair bonds and parental care.8 However, the last two decades have seen major shifts in thinking, with fields as diverse as psychology, anthropology, neurobiology, and economics converging to study friendship from a scientific perspective. Work in nonhuman animals has perhaps done the most in terms of ushering in this new trend; ground-breaking results have linked social bonds with reproductive success in mammals9,10 and have shown that common neural and physiological mechanisms underlie social interactions in humans and other animals.11,12 These findings undermine the idea that we are unique in our ability to make friends and invite the hypothesis that friendship is a product of natural selection that serves an adaptive function in social animals.
Here we discuss in detail the findings of the most recent research on the neuroethology of friendship. We largely focus on humans and other primates because this is where most research has been concentrated to date, but include substantial nods to other animals. We organize these findings around Nikolaas Tinbergen’s four questions in ethology.13 This framework celebrates the 50th anniversary of Tinbergen’s publication of “On the Aims and Methods of Ethology” and draws attention to the fact that there are few topics for which the union of ethology and cognitive neuroscience has been as informative. This framework allows us to integrate research that probes friendship’s evolutionary roots (question: evolutionary history) with studies that examine its neural, molecular, and developmental bases (questions: causation, ontogeny), as well as its ultimate function (question: function). A review of friendship would not be complete without addressing the puzzle posed by the evolution of cooperation and we also examine this complex issue. We end by highlighting some of the most pressing questions that remain unanswered in this burgeoning and important field.
Defining friendship based on the quality and patterning of interactions
We must begin by defining what we mean by friendship, which we use interchangeably with the term social bond throughout. The former is more commonly used in studies on humans and the latter in studies of other animals, yet both refer to the same concept.7,14,15 People may have an explicit sense of what it means to call someone a friend, but definitions of friendship are often vague and qualitative.15,16 We follow Hinde17 and propose that, like all relationships, friendship should be defined based on the quality and patterning of interactions between individuals. Accordingly, we define friends as pairs of individuals that engage in bi-directional affiliative (nonaggressive, nonreproductive) interactions with such frequency and consistency so as to differentiate them from nonfriends. That is, compared to nonfriends, friends engage in affiliative interactions considerably more often and over greater periods of time.18 Affiliation can include spending time together, conversing, vocalizing, grooming, huddling, cooperatively foraging, and sharing food, as well as forming alliances against others (Fig. 1). We specify that friendly interactions are nonreproductive so as to include sex that occurs in a non-reproductive context, as in bonobos,19 although we acknowledge that reproductive and nonreproductive sex between heterosexual partners can be difficult to differentiate in practice. Interactions should also be consistent over time; males and females that interact when the female is sexually receptive but not otherwise are not friends. But sexual partners that consistently engage in affiliative interactions over time are friends (by this definition, married couples are often friends, which fits with folk wisdom that spouses should be best friends20).
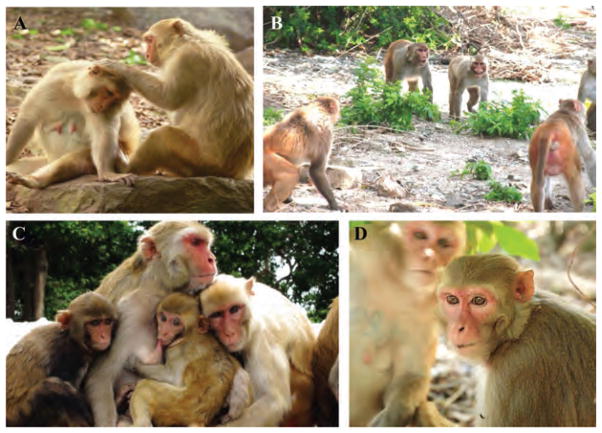
Figure 1.
In highly social animals like rhesus macaques (Macaca mulatta), (A) friends groom each other and (B) provide each other with support in agonistic encounters against other group mates. (C) Affiliative behaviors positively predict reproductive output in this species, suggesting that social bonds are adaptive.28 These bonds may function to mediate the costs of competition that arise from living in stable social groups. Friendship is underpinned by numerous neural and physiological mechanisms, and may require specific cognitive abilities, such as (D) gaze following, that allow individuals to successfully coordinate their actions with others and navigate a complex social world. Photo credits: Lauren J.N. Brent
Our definition of friendship is thus one that focuses on the phenotype. Although tempting, we believe it best to steer clear of definitions that assume the involvement of specific proximate mechanisms (e.g., reciprocity). Friendship can be based on different evolutionary strategies depending on the types of interactions involved or the identities of the social partners. Kin selection is an obvious potential explanation for affiliative interactions between relatives7,15,21 but cannot explain interactions between nonrelatives. This does not mean we should exclude affiliative relationships between kin from being defined as friends. Indeed, as we shall discuss, determining the mechanism(s) upon which cooperation between friends operates is a major line of inquiry open to much debate. We also wish to avoid definitions based on emotional engagement (e.g., love, attachment)16,22 since this is also a proximate, neurobiological mechanism that serves to promote, modify, and maintain social bonds and does not directly represent the evolved function of the bond itself.
We suspect some may disagree with our definition and we welcome this debate. Yet we suggest that disputes over definitions are somewhat moot. The scientific study of friendship is in its infancy, thus limiting this review to strict definitions would be unhelpful and we have not done so. In addition, research need not be focused explicitly on friendship (and thus reliant on a specific definition) in order to contribute to our understanding of it. Studies that improve our understanding of affiliative interactions in general, including the biological mechanisms upon which those interactions are based, are necessary components of the study of friendship.
The evolutionary history of friendship
The evolution of social groups
For friendships to form, individuals must first have access to others. In primates, the ancestral state is one of solitary living. In a landmark paper, Shultz et al.23 modeled the trajectory of primate social systems and found that stable groups composed of multiple adult males and females arose from solitary life, with harems and pair-bonded groups arising afterward. Primates are unusual in their rarity of pair bonds, which are more common in other animals, particularly birds.16,23,24 Differences in the trajectories toward social life across taxa hint at the fact that the selective pressures driving the formation of stable social groups have differed. Group living in primates is believed to have followed the shift from nocturnal to diurnal living as a means to defend against predators in a more visual world,23,25 whereas other factors, such as cooperative hunting (in carnivores) and cooperative breeding (in birds), are thought to be the selective pressures driving group living in other taxa.16 Following the formation of stable social groups, regular interactions between conspecifics became possible. Affiliative tendencies have a heritable basis in humans,26 marmots (Marmota flaviventris),27 and rhesus macaques (Macaca mulatta)28 (Fig. 2), confirming that sociality is under genetic control and is thus a trait upon which selection may act. The relationship between genes and social behavior is, of course, mediated by the nervous system. The social brain hypothesis posits that group living created selective pressures for larger and more complex brains.9,30 The last decade has been replete with evidence that brain size scales with social complexity across species,8,24,31 drawing ties between neural complexity and increased cognitive demands of social life.
Figure 2.
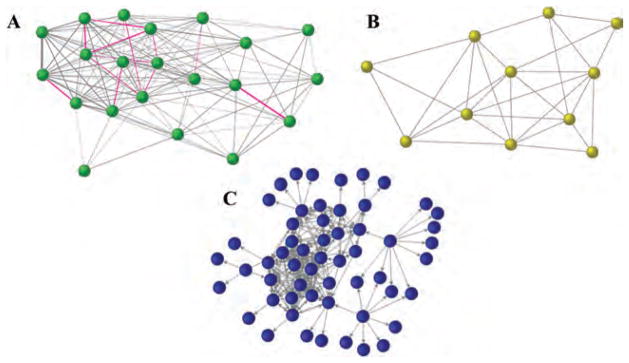
Social networks in three primate species. Networks are based on spatial proximity in (A) female rhesus macaques (n = 21), (B) coalitionary support in male chimpanzees (n = 10), and (C) named friendships in humans (n = 57). Nodes represent individuals; lines represent interactions between pairs of individuals. The thickness of the lines in (A) increase with the frequency of interaction. Arrows in (C) indicate whether named friendships were reciprocal. Individuals toward the center are more embedded in their social networks than those toward the periphery. Ties between closely related female rhesus macaques are highlighted in pink and demonstrate maternal kin bias (A). Social network position is heritable in humans26 and rhesus macaques,28 and has been associated with reproductive success in rhesus macaques28 and chimpanzees.167 Figure (A) was generated from the authors’ unpublished data, figures (B) and (C) were reproduced with permission from Refs. 167 and 26, respectively.
Friendship in primates and other animals
In a recent review, Seyfarth and Cheney14 describe the marked increase in the diversity of taxa in which friendships have been reported in the last decade. As we summarize in Table 1, social bonds exist in birds, ungulates, cetaceans, and primates. Many of these relationships are between closely related individuals. Mother–daughter pairs are the most common, followed by siblings.14 Female giraffes (Giraffa camelopardalis thornicroftii) are more likely to associate with their mothers,32 a pattern common in other herd-living mammals including red deer (Cervus elaphus),33 bison (Bison bison),34 and elephants (Loxondonta Africana).35 In many primates, females remain in their natal groups, while males disperse. In these primates, kin-biased affiliative interactions, often measured using grooming and proximity, are common.36 These include interactions between close maternal relatives (mother–daughters, maternal–siblings)37–42 and, to a lesser extent, paternal relatives38–41 (Fig. 2). Even when animals disperse from their natal groups, and are thus less likely to encounter close relatives in their lifetimes, relatives are more likely to form social bonds than nonrelatives (chimpanzees (Pan troglodytes),43 but see Ref. 44; bonobos (Pan paniscus);45 dolphins (Tursiops sp.)46). Animals that are close in age are also frequent social partners. In many species, the highest ranking male(s) sires the majority of offspring in a given year, and individuals that are close in age are often paternal siblings.47
Table 1.
Affiliative relationships in primates and other animals
| Order | Common name |
Species | Social structure |
Dispersal | Affiliative relationships† |
Kin biases | Interaction type(s) | Nonkin bonds |
3rd party recognition |
Fitness benefits |
Refs |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anseriformes | Greylag geese | Anser anser | MM–MF‡ | F | F–F | Mat, Full Sib | proximity | 179 | |||
| Artiodactyla | Bison | Bison bison | MF | M | F–F | Mat | proximity | 33 | |||
| Artiodactyla | Red deer | Cervus elphanus | MM–MF | M | F–F | Mat | proximity | 32 | |||
| Artiodactyla | Giraffe | Giraffa camelopardalis thornicroftii | MF | M | F–F | Mat | proximity | 31 | |||
| Carnivores | Spotted hyena | Crocuta crocuta | MM–MF | M | M–M, F–F | Full Sib, Mat, Pat | grooming, proximity, cooperative hunting | Yes | Yes | 180, 181, 182 | |
| Cetacea | Bottlenose dolphin | Tursiops spp. | MM–MF | M & F | M–M | mate-guarding alliances | Yes | Yes | 45, 163 | ||
| Cyprinodontiformes | Guppy | Poecilia reticulata | MM–MF | M & F | F–F | F | proximity, predator inspection | 183 | |||
| Passeriformes | Raven | Corvus corax | MM–MF‡ | M & F | M–F, M–M, F–F | Yes | contact sitting, allopreening, play, pair displays, coalitionary support | Yes | Yes | 69, 70, 93 | |
| Passeriformes | Rook | Corvus frugilegus | MM–MF‡ | M & F | M–M, M–F, F–F | food sharing, agonistic support, allopreening, bill twinning, dual food caching | Yes | Yes | Yes | 23 | |
| Perissodactyla | Donkey | Equus asinus | No data | no data | M–M, M–F, F–F | spatial proximity, nonsexual mounting | Yes | 184 | |||
| Perissodactyla | Horse | Equus caballus | UM–MF or MM–MF | M & F | F–F | No | grooming, proximity | Yes | Yes | 9 | |
| Primates | Spider monkey | Ateles belzebuth | MM–MF | M | M–F | vocalizations | 185 | ||||
| Primates | White-faced capuchin | Cebus capuchinus | MM–MF | M | F–F | Mat, Pat | grooming, proximity, coalitionary support | Yes | 41, 86 | ||
| Primates | Sooty mangabey | Cercocebus torquatus | MM–MF | M | coalitionary support | Yes | 87 | ||||
| Primates | Blue monkey | Cercopithecus mitis | MM–MF | M | F–F | No | grooming | Yes | 186 | ||
| Primates | Vervet monkey | Chlorocebus aethiops | MM–MF | M | F–F | grooming, coalitionary support | Yes | 90, 187 | |||
| Primates | Crown lemur | Eulemur coronatus | MM–MF | M | M–F, M–M | grooming | 188 | ||||
| Primates | Brown lemur | Eulemur fulvus | MM–MF | M | M–F | No | grooming | 188 | |||
| Primates | Human | Homo sapiens | MM–MF | M & F | M–M, M–F, F–F | Mat, Pat | grooming, food sharing, coalitionary support, spatial proximity | Yes | Yes | Yes | 4, 49, 145 |
| Primates | Ring-tailed lemur | Lemur catta | MM–MF | M | M–F | No? | grooming | 188 | |||
| Primates | Assamese macaque | Macaca assamensis | MM–MF | M | M–M, F–F | grooming, coalitionary support | Yes | 160, 189 | |||
| Primates | Japanese macaque | Macaca fuscata | MM–MF | M | F–F | Mat | grooming | Yes | 36 | ||
| Primates | Rhesus macaque | Macaca mulatta | MM–MF | M | F–F | Mat, Pat | grooming, proximity, coalitionary support | Yes | 27, 37, 38, 141, 142, 190 | ||
| Primates | Bonnet macaque | Macaca radiata | MM–MF | M | M–M | grooming, proximity, coalitionary support, huddle, greet | Yes | 191, 192 | |||
| Primates | Barbary macaque | Macaca sylvanus | MM–MF | M | M–M | grooming, proximity, coalitonary support, triadic interactions, body contact | Yes | 193 | |||
| Primates | Bonobo | Pan paniscus | MM–MF | F | M–F, M–M, F–F | Mat | grooming, proximity, coalitionary support | Yes | 44 | ||
| Primates | Chimpanzee | Pan troglodytes | MM–MF | F | M–M, M–F, F–F | Mat, not Pat | grooming, coalitionary support, others | Yes | Yes | 42, 167, 194–196 | |
| Primates | Hamadryas baboon | Papio hamadryas | multi-level | M | M–F, F–F | Mat | grooming, proximity | n/a | 197 | ||
| Primates | Baboon | Papio spp. | MM–MF | M | F–F, M–F | Mat, Pat | grooming, proximity, coalitionary support, greet | Yes | Yes | Yes | 10, 39, 40, 52, 67, 89, 155, 162 |
| Primates | Gelada monkey | Theropithecus geleda | multi-level | M | F–F | Yes | grooming, proximity, vocalizations(?) | 16, 169 | |||
| Proboscidea | Elephant | Loxodonta africana | MF | M | F–F | Mat | proximity | 34 | |||
| Rodentia | Degu | Octodon degus | MM–MF | M & F | F–F | proximity | Yes | 198 |
Note: Although we intend this table to be comprehensive, species for which social relationships have been documented but little studied may be absent. Additionally, we expect many species closely related to those represented also exhibit social relationships (e.g., other species of macaque) but to our knowledge no explicit study has been published for these species to date.
differentiated affiliative relationships documented to date, not necessarily adhering to a strict definition of social bonds but may simply indicate an interactional bias. MM = multi-male, MF = multi-female, UM = uni-male,
= nonbreeding flocks, M = male, F = female, Mat = bonds biased toward maternal kin (e.g., mother–offspring pairs, maternal half-siblings), Pat = bonds biased toward paternal kin (e.g., father–offspring pairs, paternal half-siblings), Full Sib = bonds biased toward full siblings. Blank cells = no available data or unknown.
But friends are not always related. Horses (Equus caballus) live in groups composed of a single stallion and several unrelated females. Yet, pairs of unrelated females form differentiated affiliative relationships.9 Unrelated spotted hyenas (Crocuta crocut), and unrelated members of many primate species also form close and enduring bonds. People may be unique in the extent to which friends are unrelated.21,48,49 This pattern could be driven by a lack of available kin, since nonkin make up the majority of hunter–gatherer groups.50
In summary, many animals form friendships with conspecifics. Social bonds are often between related individuals, but bonds between nonrelatives are not uncommon. Further research in a wider variety of taxa is required to determine whether friendship is a feature of all species that form stable social groups. Questions also remain about the impact of group composition. Apart from some exceptions, are individuals only friends with nonrelatives when kin are unavailable? To answer this question, we need comprehensive data on within-group relatedness across many taxa.
Causation: the cognitive, neural, and biochemical basis of friendship
To form friendships, animals must recognize the other members of their social group as unique individuals.51 They must track those individuals through space and time in order to coordinate their actions,16,52 and must make decisions about when to interact with others and what form those interactions should take. Social animals must also keep track of the quality of their relationships with others; that is, are they friends or foes?17,51,53 Recognizing the quality of relationships between pairs of other individuals (i.e., friends of friends, friends of foes) may also be crucial to successful navigation of the social world.53,54
Of Tinbergen’s four questions, the causation of social bonds, which encompasses their cognitive, neural, and biochemical bases, has perhaps received the greatest attention. From this research it has become apparent that the same or homologous mechanisms underlie social behaviors in a range of taxa,11,12,55 which speaks to sociality’s deep roots in our evolutionary history.
Recognizing others
In order to distinguish each other as unique individuals, animals must learn the unique recognition cues of others and use those cues to identify those individuals in the future.51 Recognition cues can include olfactory, vocal, and visual cues, which can be integrated in a multi-modal fashion.11,51 Individual recognition has been documented in both vertebrates and invertebrates.51 Hermit crabs (Pagurus longicarpus) and lobsters (Homarus americanus) recognize competitors, probably via scent.56,57 Faces are important for individual recognition in sheep (Ovis aries),58 paper wasps (Polistes fuscatus),59 and primates, including rhesus macaques,60 chimpanzees,60 and humans.61 Similar regions of the brain seem to be involved in face recognition in humans and macaques,62 where highly modular and hierarchically organized neural networks in the inferior temporal cortex known as face patches process visual information about faces, but not other objects.63,64 The presence of neural face patches in humans and monkeys strongly argues for the importance of individual recognition in the evolution of primate sociality.
Animals not only recognize their conspecifics, they also remember them. Hooded warblers (Wilsonia citrina) remember their neighbors from the previous breeding season,65 sheep differentiate former group mates after 2 years of separation,58 and dolphins remember each other’s signature whistles for up to 20 years.66 Some animals even recall the quality of past interactions. Female vervet monkeys (Chlorocebus aethiops), and chacma baboons (Papio hamadryas ursinus) discriminate the alarm calls of group mates that recently groomed them compared to those that did not.67,68 Ravens (Corvus corax) form differentiated affiliative relationships in nonbreeder flocks.69 In an experiment with captive ravens, the birds responded differently to the playback calls of former flock members compared to unfamiliar individuals.70 Fascinatingly, these corvids also differentiated amongst former group mates with whom they had an affiliative relationship compared to nonaffiliates, even in cases where social partners had been separated for as long as 3 years.70
Humans remember and also maintain friendships despite long periods of separation; young adults living long distances apart remain friends for 8 years or more.71 People also tend to be over-inclusive when differentiating kin from nonkin. These false-positive kin-recognition errors (i.e., treating nonkin as kin) appear to be more prominent in women than men, the latter of which may suffer higher costs from forming alliances with nonrelatives.21 Unrelated individuals may nonetheless be genetically similar and friends may be a kind of “functional kin.”49 This, as we will discuss, could have immense implications to our understanding of the evolution of cooperation between friends.
Obtaining social information and making social decisions
To select, acquire, and maintain friends requires information about others. But what motivates animals to obtain social information, and how do they do it? Many animals attribute reward value to social information. Both humans and other primates find social stimuli intrinsically rewarding, and certain types of social stimuli are more interesting and reinforcing than others.72–75 For instance, human infants look longer at faces than at nonface stimuli,76 while monkeys direct their gaze more often toward higher-ranking than lower-ranking animals.77,78
Consistent with these observations are findings that social information activates reward-related areas of the brain, including the anterior cingulate cortex (ACC), the orbitofrontal cortex (OFC), the nucleus accumbens, and the caudate nucleus.79–82 While some of these areas respond to social and nonsocial rewards in a similar fashion, some areas appear to be partly specialized for social information processing. For instance, when rhesus macaques were asked to choose between juice rewards and information about others, a small proportion of neurons in the OFC responded to juice rewards, while another, greater, (and nonoverlapping) proportion responded to social information.82 This finding, along with the observed relationship between OFC size and social network size in humans83 and group size across primates30 suggests that OFC is part of a specialized neural circuit involved in social behavior.
Outside the laboratory, animals are not conveniently presented with social information, but must go out and get it. Just like an animal foraging for food amongst sparsely distributed patches,84 an animal searching for social information must weigh the benefits of obtaining such information against the costs, which include missed opportunities to eat, drink, or sleep.12 In the wild, animals often interrupt their current activity to scan their surroundings. Whether this behavior requires the systematic trade-off between one type of reinforcement and another is nearly impossible for researchers to discern without knowing precisely what the animal is looking at. Yet there is evidence from the laboratory that animals take this information into account; male rhesus macaques will forgo a small amount of juice reward in order to see a picture of another monkey. Crucially, the amount of juice they forgo depends on the type of social information on offer, with pictures of female perinea garnering the highest payments, and images of low-status males garnering negative payments (i.e., they must be paid juice to look at them).72,82 This suggests that monkeys weigh the costs and benefits of their social decisions.
One type of social information that is likely to be of particular value is information about the relationships between others.85 Evidence that animals have some understanding of these third-party relationships come from studies showing, for example, that rooks redirect aggression to the social partners of their aggressors,23 and that many primates solicit help from individuals that are higher ranking than their aggressors.86–88 In an experimental setting, baboons and vervets looked longer in the direction of playback speakers when played a sequence of calls that represented monkey A winning an agonistic encounter against monkey B in cases where A was subordinate to B, compared to cases where A was dominant to B.89,90 That is, these monkeys seemed to recognize the dominance relationship between A and B and to be surprised when they heard calls that suggested it had been overturned.
In addition to recognizing the relationships between others, the ability and drive to understand the motives, intentions, and mental states of others (so-called theory of mind, or ToM) may help animals predict social challenges.85 ToM, however, is assumed to be cognitively complex and may be an ability at which animals other than humans are not very skilled. Nevertheless, some nonhuman animals express some ToM-related abilities. For instance, the ability to understand the visual perspectives of others has been demonstrated in goats,91 birds,92,93 and primates94 (Fig. 1). In one experiment, rhesus macaques showed a bias toward stealing food from experimenters whose backs were turned rather than from experimenters who could see that the food was being stolen.95 Identifying where others are looking appears to be accomplished by neurons along the superior temporal sulcus (STS),96,97 in the lateral intraparietal area,98 and in the amygdala.99 Unilateral inactivation of the STS impairs spontaneous gaze following in rhesus macaques, consistent with a role in identifying the locus of other animals’ attention.97
Understanding the relationships and intentions of others requires the brain to keep track of information that is relative not only to oneself but also to others.100 This process may be similar to the computations required to convert sensory information into a frame of reference appropriate for guiding movement.80,101,102 Consistent with this idea, a recent study found remarkable specializations in the way neurons encoded reward outcomes while rhesus macaques chose to deliver juice rewards to themselves (the subject), to a recipient monkey, or to no one. OFC neurons predominantly signaled rewards received by the actor, anterior cingulate sulcus (ACCs) neurons predominantly signaled foregone rewards, and the majority of anterior cingulate gyrus (ACCg) neurons signaled rewards delivered to the recipient or mirrored rewards delivered to either the subject or the recipient.80 Thus, ACCg neurons incorporate the experiences of others into their reward-related signals. These findings resonate with work showing that lesions in ACCg lead to social deficits,103 and that portions of the ACC are activated when people observe events happening to others or think about others’ states of mind.104,105 These observations also suggest that differences in the structure and function of the ACCg, along with other areas associated with awareness and empathy (e.g., the anterior insular cortex106,107), may underlie differences in socio-cognitive abilities between humans and other animals, as well as differences between individuals within a species.
Biochemical regulation of friendship
The hormonal and peptidergic mechanisms that modulate affiliative interactions in mammals have received extensive attention, the results of which have been summarized in a number of comprehensive reviews.5,11,55,108,109 We aim not to cover this information in detail but instead to highlight the most current findings and recent debates regarding some of the major biochemical systems that regulate friendship, namely those involving oxytocin, endorphins, dopamine, serotonin, and the hypothalamic–pituitary–adrenal (HPA) axis.
Social behavior is largely reinforcement driven. Oxytocin (OT) is a neuropeptide that stimulates lactation in mammals and is involved in bonding between mothers and infants, as well as between pair-bonded reproductive partners.110,111 OT has also been associated with social relationships outside of pair and maternal bonds. For example, OT is involved in individual recognition and social memory.112,113 Exogenous application of OT increases prosocial decisions and attention to others,114 increases feelings of trust,115 and encourages generosity.116
In addition to OT, the opioid β-endorphin is also involved in reward processes and has been associated with social behavior, especially in primates.55,117 Some researchers have proposed that while OT facilitates social interaction, it is β-endorphin that is crucial to the formation and maintenance of social bonds.55,109,118 The idea that OT facilitates social interaction, but not bonding, stems from the fact that the effects of OT are relatively short-lived119,120 and that OT reduces social vigilance,78 which may be a prerequisite for social interaction. Although not much is known about the relationship between endorphins and social interactions,118 the results of one new study support the association between endorphins and social bonds; individuals release more endorphins when rowing a boat in a social context—a prime example of behavioral synchrony, which is a key component of friendship51—compared to when rowing alone, despite exerting the same amount of physical effort in both cases.121
Regardless of the role of endorphins, new findings contradict the idea that OT merely facilitates interactions and is not also involved in bonding itself. In one study, urinary OT levels in wild male chimpanzees were elevated following social grooming.122 Crucially, increases in urinary OT were only observed in males that had groomed a chimpanzee with whom they already possessed a bond (bonded males were kin or unrelated). What mattered in terms of OT release, therefore, was not grooming in general, but grooming with a friend.122 This observation resonates with other recent findings that the positive effects of exogenously administered OT on trust-related feelings or behaviors only occur when subjects interact with people they know or with members of their in-group.123,124 Together, the results of these studies suggest that both OT and endorphins contribute to the formation and maintenance of social bonds.
Serotonin and dopamine are also ancient and potent neuromodulators. The contribution of dopamine to the formation of social memories and social preference as part of the ventral tegmental area–dopamine projection system has been well described.109,125 Much of the work on serotonin, on the other hand, has been at the phenotypic level, exploring the association between serotonin and social behaviors. For instance, administration of selective serotonin reuptake inhibitors (SSRIs) alters the rate of affiliative and aggressive interactions.126,127 Serotonin transporter binding in the midline cortex is associated with aggressive and friendly traits in rhesus macaques,128 and genetic polymorphisms in the serotonergic pathway are associated with social integration.28 The majority of research on the correlates of serotonin points to links between this neuromodulator and sensory inputs, including social stimuli.129 This has led to the proposition that serotonin modulates how individuals perceive and respond to social information.109,129 Nevertheless, the molecular processes underlying the association between serotonin and sociality are little understood and will require concerted future research efforts to disentangle.
The stress response, produced via activation of the HPA axis, warns animals that homeostasis has been disrupted and mobilizes energy to restore a homeostatic state.130 For animals for whom social relationships are crucial to success and survival,10,27 the stress response is part of the motivational system that underpins social interaction. Many animals, including humans, exhibit smaller increases in stress hormone (cortisol) levels during exposure to averse stimuli when a friend is present compared to when alone.131 In primates, social grooming reduces heart rate,132 and individuals with more tightly-knit social networks have lower baseline levels of cortisol metabolites in their feces.133,134 For animals with tightly-knit and predictable social networks, low baseline cortisol levels may be a result of these individuals being able to cope effectively with social challenges. The acute reduction of heart rate in response to social grooming can be interpreted as a response to the fulfillment of a social need (negative feedback between endorphins, OT, and the HPA axis is also likely to play a part).
Chronic activation of the stress response has well-known negative consequences for health135 and reproduction,136 both of which may negatively affect evolutionary fitness. This has led to the suggestion that stress reduction is a selective pressure in the evolution of social bonds and is, therefore, one of the ultimate functions of social bonding.7,9,14 However, it is important to remember that an association between the stress response and social behavior reflects the role of the stress response as a proximate mechanism underlying social interactions.133 To propose that stress reduction is the ultimate reason individuals make friends is akin to suggesting that thirst is the ultimate reason we drink. Clarifying the type of biological mechanism the stress response represents (proximate, not ultimate) will positively influence how research linking the HPA axis to social behavior is interpreted and, as such, will improve our understanding of the evolution of friendship.
The ontogeny of friendship
Little is known from an empirical standpoint about how friendships are initially formed. Yet systematic biases in the identities of social partners may hold clues to the establishment of friendships. Many animals prefer to be friends with close kin.36 Friends are also often characterized by homophily, the tendency to share similar characteristics,48 including age,39 and social status.137 Biases toward individuals of similar status have been proposed to result from competition for partners of the highest quality.137 Under this principle, high-ranking individuals prefer to be friends with each other to the exclusion of lower-ranking animals. This tendency results in everyone being friends with the highest ranking individual available to them—low-ranking individuals are friends with other low-ranking individuals and high-ranking individuals are friends with other high-ranking individuals.
Homophily between friends may also be a result of attraction to individuals of similar personalities or skills.48 Humans are especially predisposed toward homophily,138 with recent evidence suggesting this even extends to the genetic level; people are more likely to be friends if they have similar genotypes.139,140 Taken together, these findings advocate the need to consider not only an individual’s genome, but also their metagenome, when asking questions about the causes of friendship biases.139
There are also clear differences in the friendships formed by males and females in some species. In rhesus macaques, where males disperse from the group where they were born, while females remain, males spend significantly less time grooming and are less socially connected compared to females.28,141 In chimpanzees, on the other hand, where females disperse instead of males, there is some evidence that females are comparatively aloof.43 This patterning can be explained by attraction toward kin, as well as by maternal influence. Carol Berman et al. have shown that young rhesus macaques tend to interact with the offspring of their mothers’ friends.141,142 That is, mothers seem to introduce their infants to potential social partners. Human children, too, inherit friends from their parents.138 If parental introductions are an important step to becoming friends, it is unsurprising that individuals that disperse away from their mothers are less socially integrated.
Some human studies suggest that men have a larger number of friends than women but sacrifice quality for quantity since men tend to spend less time with each friend and rate their friendships as less important than do women.6,143 Men also tend to treat friends to whom they are unrelated in a similar fashion to how they treat strangers, whereas women treat unrelated friends as though they were kin.21 Differences have also been noted in the cognitive domain, where women are often better at empathizing and inferring the thoughts and intentions of others.144 If humans follow the typical primate pattern of male dispersal, these findings make sense; dispersing men are relatively asocial compared to women. Yet, humans have been characterized by either female dispersal145 or dispersal of both sexes.50 Friendship, it seems, is about more than dispersal, and differences in friendship between men and women require another explanation. On the other hand, it might be that gender-based differences in human friendship have been exaggerated. Meta-analyses have revealed that men and women cultivate and define friends in very similar ways, and that sex is not a very strong predictor of how much personal information people share with their friends.138 A comparative approach, whereby the causes and consequences of differences in friendship between the sexes in a range of species, cultures, and social systems are catalogued and explored, would address some of these issues.
While little is known about how friendships are initiated and solidified, research has begun to reveal the ontogenetic basis of socio-cognitive abilities. The ability to understand false beliefs, to cast moral judgments, and ToM are present in children as young as 4 years in some cases, but improve into adulthood.146–149 On the other hand, similar levels of prosociality are expressed throughout childhood (3–8 years old),150 suggesting human prosocial tendencies arise early in life. The development of socio-cognitive skills can be influenced by the environment. For instance, children that have had negative interactions with peers are less likely to perform well in ToM experimental tasks later in life.151 Similarly, social isolation in monkeys results in abnormal behaviors in both social and nonsocial domains.152 Autistic children that have an older sibling (i.e., that have consistent access to social partners) score higher in ToM tests than those without older siblings.153 These findings are consistent with a recent study that demonstrated the impact of social environment on brain anatomy; monkeys experimentally introduced to larger social groups showed an increase in grey matter volume in two brain areas implicated in social cognition, the mid-superior temporal sulcus and the rostral prefrontal cortex.154 Together, these studies demonstrate that socio-cognitive skills are present in early life but can be tuned by social interactions.
Getting by with a little help: the function of friendship
If social bonds serve a useful function and have been favored by selection, we expect them to be associated with increased survival and/or reproductive success, which are proxies of evolutionary fitness. In the first seminal paper to demonstrate such an association, Joan Silk et al. showed female baboons that spend a greater amount of time grooming and associating with others have offspring that are more likely to survive to 1 year of age.155 A similar association between affiliation and infant survival has since been found in both male and female rhesus macaques27 (Fig. 2). This is also true outside the primate order; affiliative interactions are a significant predictor of the number of foals born to female horses,9 and of lifetime reproductive success in marmots.27
In humans, research has focused on the ties between sociality and health.156 Socially isolated people are at greater risk of cardiovascular disease.157,158 infectious diseases.6 and elevated blood pressure.159 One recent meta-analysis found a 50% increased likelihood of survival for people with stronger social relationships, even after controlling for age, sex, health, and cause of death.3 The link between affiliative tendencies and fitness seems to go beyond the mere frequency of social interactions to the formation of high-quality relationships. At least this is the case in female baboons, male Assamese macaques (M. assamensis_, and dolphins, where individuals with the strongest, most enduring social bonds sire the most offspring160 and have the highest offspring survival161,162 and greatest longevity.10 Together, these findings suggest that there are adaptive benefits to social bonds. But the question remains; what causes the association between friendship and fitness?
In female horses, social integration reduces harassment from males, which has direct reproductive costs.9 Male dolphins help their alliance partners herd females away from their groups to mate.163 Most primates live in relatively stable social groups, probably to reduce predation risk.25 However, along with the benefits of group living come costs, including competition between group members for resources, such as food, space, and sex. One way to navigate a competitive world is to obtain tolerance and support from a subset of group mates. In other words, one way to cope is to make friends.
Some primates tolerate the presence at food sources of some group mates but not others, and provide those individuals with services they cannot obtain on their own, such as grooming.164 Scholars’ extensive interest in primate grooming has been fueled by the observation that many primates spend more time grooming than is likely to be necessary for hygienic purposes alone.165 As a result, grooming has been proposed to function as a type of behavioral service, or relational currency, that can be exchanged for grooming itself, or for other services, such as coalitionary support.68,137,164 Primate grooming partners are indeed more likely to support each other in fights.166 The association between grooming and coalitionary support may explain the positive relationship between grooming and reproductive success.14,155 Empirical evidence in support of this idea comes from a recent study of the social networks of wild male chimpanzees. Males that were more integrated in the coalition network were more likely to be higher ranking in the period of study that followed (a predictor of reproductive success in chimpanzees) and were also more likely to sire offspring167 (Fig. 2). For male chimpanzees, at least, grooming may lead to coalitionary support, which may translate into reproductive success.
Questions still linger about whether friendship does in fact help individuals cope with competition. According to socio-ecological theory, within-group competition is relaxed in species with low-quality and abundant food sources.168 Yet social bonds have been documented in ungulates and folivorous primates,32,33,169 whose diets predict relaxed within-group competition. In addition to behavioral services, the ultimate benefit of friendship might come from what individuals learn from their friends (social learning170), or from the flow of behaviors, affective states, or attitudes between friends (social contagion140). Additional quantitative data on the connections between social learning, social contagion, and social bonds, as well as on the types of bonds found in species with relaxed intra-group competition, are necessary to begin to address these questions.
There are also questions about variation in social tendencies between individuals of the same species. Personalities differ between members of the same social group in seemingly every species studied to date, including differences in affiliative tendencies.171 But how do we reconcile these differences with the idea that social bonds are adaptive? If friendship is the route to success, why isn’t every individual hypersocial? What other behavioral strategies and selective pressures might explain what otherwise appears to be noise in the system? The study of personality in animals is relatively new and further research is required to begin to answer these questions. Finally, one of the most pervasive questions hanging over the heads of researchers attempting to understand the neuroethology of friendship concerns the evolution of cooperation.
You scratch my back, I’ll scratch yours? Friendship and the puzzle of cooperation
If friendship is about helping one another, we need to ask how this helping behavior evolved. That is, in order to understand the patterning of interactions associated with friendship, we must understand how to frame those interactions in light of the evolution of cooperation. As cooperation is a seemingly selfless act, explaining its evolution is a classic problem. Biologists have struggled to answer how cooperation persists given the selfish nature of individuals and their genes. Kin selection and indirect fitness benefits can explain the exchange of services between close relatives.172 Cooperation between nonrelatives is often explained by the reciprocal exchange of services.173 Yet reciprocal investment has infrequently been demonstrated in naturalistic settings, leading some researchers to conclude that alternative explanations are required.174,175
Before we put reciprocity to the side, it is worth considering how the complexities of the problem might hamper our ability to uncover evidence of it. First, we must determine how best to frame cooperation between unrelated individuals in a natural setting. Let’s take the example of grooming in primates. In a typical prisoner’s dilemma game, strangers simultaneously exchange a discrete service on a one-shot basis, and cheating is clearly defined as failure to cooperate. In contrast, for grooming primates, unrelated groupmates of different social status that have past experience of each other exchange continuous (nondiscrete) goods many times over their lifetimes. These goods aren’t necessarily the same (i.e., grooming might be exchanged for coalitionary support), making it difficult for researchers to know what constitutes a defective move.174 Indeed, temporary imbalances are common in consistent social partners, suggesting that these imbalances do not constitute cheating, or are a level of cheating that is tolerated.174 In addition, individuals often do not play simultaneously, but rather take turns in an alternating fashion where one individual grooms the other first. If primate grooming were expressed as a prisoner’s dilemma game, it would therefore take the form of an iterated and continuous prisoner’s dilemma with multiple players of varying degrees of social status that play in an alternating order. This is a complicated game to play with a difficult payoff matrix to solve. It may be no wonder that the cooperative mechanism underlying the exchange of grooming in primates has been hotly debated.
Positive reciprocity has been supported as an explanation underlying primate grooming in studies that have used experimental setups to demonstrate contingency, such that services rendered are dependent upon grooming received.67,176 Unfortunately, contingent exchange is almost impossible to demonstrate using observational data alone, which may explain why most naturalistic studies have failed to do so. When alternative grooming partners are available, negative pseudoreciprocity, whereby contingency takes the form of sanctions and results in a subject switching to a new partner,174 may also explain primate grooming. Future work should continue to explore the roles of these cooperative strategies despite previous (perhaps unsurprising) failings.
Another way to evaluate strategies underlying reciprocal exchange is to examine the cognitive machinery they require. Many have argued that calculated bookkeeping must be used to keep track of past interactions, and that this is beyond the abilities of most animals.177,178 The typical response to this statement is that calculated bookkeeping is not the only solution to this problem. Animals may instead use something less cognitively demanding termed emotional bookkeeping. Where by individuals base their interactions on their attitudes toward others.164 Indeed, emotional bookkeeping resonates with evidence that social bonds are mediated by reinforcement and are associated with trust and relaxation (described in the section on the biochemical regulation of friendship).
Nevertheless, a recent study in humans may turn the discussion of exchange between nonrelatives on its head. Unrelated friends are more likely to be genetically similar, equivalent to the level of fourth cousins,140 compared to unrelated strangers. Thus friends may gain indirect fitness benefits from each other, and cooperation between friends may be explained by (a green-beard form of) kin selection. Regardless of the mechanism, the study of the neuroethology of friendship is inexorably entwined with the study of the evolution of cooperation. Advances in both areas will considerably improve our understanding of the foundations of sociality.
Acknowledgments
This work was supported by the National Institutes of Health (R01-MH-096875: LNJB, MLP; K99-MH-099093: SWCC; R01-MH-095894, R21-NS-078687, R01-MH-086712: MLP), the Fonds de la Recherche en Santédu Québec (JFG), and by a Duke Institute for Brain Sciences Incubator Award (MLP). We thank Stuart Semple for comments and discussion.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Baron RA, Markman GD. Beyond social capital: the role of entrepreneurs’ social competence in their financial success. J Business Venturing. 2003;18:41–60. [Google Scholar]
- 2.Cohen S, Doyle WJ, Turner R, et al. Sociability and susceptibility to the common cold. Psychol Sci. 2003;14:389–395. doi: 10.1111/1467-9280.01452. [DOI] [PubMed] [Google Scholar]
- 3.Holt-Lunstad J, Smith TB, Layton JB. Social relationships and mortality risk: a meta-analytic review. PLoS Med. 2010;7:e1000316. doi: 10.1371/journal.pmed.1000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steptoe A, Shankar A, Demakakos P, Wardle J. Social isolation, loneliness, and all-cause mortality in older men and women. Proc Natl Acad Sci. 2013;110:5797–5801. doi: 10.1073/pnas.1219686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberger NI. The pain of social disconnection: examining the shared neural underpinnings of physical and social pain. Nat Rev. 2012;13:421–434. doi: 10.1038/nrn3231. [DOI] [PubMed] [Google Scholar]
- 6.Baron-Cohen S, Wheelwright S. The friendship questionnaire: an investigation of adults with Asperger syndrome or high-functioning autism, and normal sex differences. J Autism Dev Dis. 2003;33:509–517. doi: 10.1023/a:1025879411971. [DOI] [PubMed] [Google Scholar]
- 7.Massen JJM, Sterck EHM, de Vos H. Close social associations in animals and humans: functions and mechanisms of friendship. Behaviour. 2010;147:1379–1412. [Google Scholar]
- 8.Dunbar RIM, Shultz S. Evolution in the social brain. Science. 2007;317:1344–1347. doi: 10.1126/science.1145463. [DOI] [PubMed] [Google Scholar]
- 9.Cameron EZ, Setsaas TH, Linklater WL. Social bonds between unrelated females increase reproductive success in feral horses. Proc Natl Acad Sci. 2009;106:13850–13853. doi: 10.1073/pnas.0900639106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silk JB, Beehner JC, Bergman TJ, et al. Strong and consistent social bonds enhance the longevity of female baboons. Curr Biol. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- 11.Broad KD, Curley JP, Keverne EB. Mother–infant bonding and the evolution of mammalian social relationships. Philos Trans R Soc. 2006;361:2199–2214. doi: 10.1098/rstb.2006.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang SWC, Brent LJN, Adams GK, et al. Neuroethology of primate social behavior. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1301213110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tinbergen N. On the aims and methods of ethology. Zeitschrift für Tierpsychologie. 1963;20:410–433. [Google Scholar]
- 14.Seyfarth RM, Cheney DL. The evolutionary origins of friendship. Annu Rev Psychol. 2012;63:153–177. doi: 10.1146/annurev-psych-120710-100337. [DOI] [PubMed] [Google Scholar]
- 15.Silk JB. Using the ‘F’-word in primatology. Behaviour. 2002;139:421–446. [Google Scholar]
- 16.Dunbar RIM, Shultz S. Bondedness and sociality. Behaviour. 2010;147:775–803. [Google Scholar]
- 17.Hinde RA. Interactions, relationships and social structure. Man. 1976;11:1–17. [Google Scholar]
- 18.Cords M. Friendships, alliances, reciprocity and repair. In: Whiten A, Byrne RW, editors. Machiavellian Intelligence II: Extensions and Evaluations. Cambridge: Cambridge University Press; 1997. pp. 24–49. [Google Scholar]
- 19.Kuroda S. Social behavior of the pygmy chimpanzees. Primates. 1980;21:181–197. [Google Scholar]
- 20.Fennell DL. Characteristics of long-term first marriages. J Mental Health Couns. 1993;15:446–460. [Google Scholar]
- 21.Ackerman JM, Kenrick DT, Schaller M. Is friendship akin to kinship. Evol Hum Behav. 2007;28:365–374. doi: 10.1016/j.evolhumbehav.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aron A, Aron EN, Smollan D. Inclusion of other in the self scale and the structure of interpersonal closeness. J Pers Soc Psychol. 1992;63:596–612. [Google Scholar]
- 23.Shultz S, Opie C, Atkinson QD. Stepwise evolution of stable sociality in primates. Nature. 2011;479:219–222. doi: 10.1038/nature10601. [DOI] [PubMed] [Google Scholar]
- 24.Emery NJ, Seed AM, von Bayern AMP, Clayton NS. Cognitive adaptations of social bonding in birds. Phil Trans R Soc. 2007;362:489–505. doi: 10.1098/rstb.2006.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Schaik CP. Why are diurnal primates living in groups? Behaviour. 1983;87:120–144. [Google Scholar]
- 26.Fowler JH, Dawes CT, Christakis NA. Model of genetic variation in human social networks. Proc Natl Acad Sci. 2009;106:1720–1724. doi: 10.1073/pnas.0806746106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lea AJ, Blumstein DT, Wey TW, Martin JGA. Heritable victimization and the benefits of agonistic relationships. Proc Natl Acad Sci. 2010;107:21587–21592. doi: 10.1073/pnas.1009882107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brent LJN, Heilbronner SR, Horvath JE, et al. Genetic origins of social networks in rhesus macaques. Scientific Rep. 2013 doi: 10.1038/srep01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Byrne RW, Whiten A. Machiavellian Intelligence. Oxford: Oxford University Press; 1988. [Google Scholar]
- 30.Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 31.Dunbar RIM. Neocortex size and group-size in primates: a test of the hypothesis. J Hum Evol. 1995;28:287–296. [Google Scholar]
- 32.Bercovitch FB, Berry PSM. Age proximity influences herd composition in wild giraffe. J Zool. 2013 doi: 10.1111/jzo.12039. [DOI] [Google Scholar]
- 33.Albon SD, Staines HJ, Guinness FE, Clutton-Brock TH. Density-dependent changes in the spacing behaviour of Female Kin in Red Deer. J Anim Ecol. 1992;61:131–137. [Google Scholar]
- 34.Green WCH, Griswold JG, Rothstein A. Post-weaning associations among bison mothers and daughters. Anim Behav. 1989;38:847–858. [Google Scholar]
- 35.Archie EA, Moss CJ, Alberts SC. The ties that bind: genetic relatedness predicts the fission and fusion of social groups in wild African elephants. Proc R Soc. 2006;273:513–522. doi: 10.1098/rspb.2005.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chapais B, Berman CM. Kinship and Behavior in Primates. Oxford: Oxford University Press; 2007. [Google Scholar]
- 37.Chapais B, Savard L, Gauthier C. Kin selection and the distribution of altruism in relation to degree of kinship in Japanese macaques (Macaca fuscata) Behav Ecol Sociobiol. 2001;49:493–502. [Google Scholar]
- 38.Schülke O, Wenzel S, Ostner J. Paternal related-ness predicts the strength of social bonds among female rhesus macaques. PLoS One. 2013;8:e59789. doi: 10.1371/journal.pone.0059789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Widdig A, Nürnberg P, Krawczak M, et al. Paternal relatedness and age proximity regulate social relationships among adult female rhesus macaques. Proc Natl Acad Sci. 2001;98:13769–13773. doi: 10.1073/pnas.241210198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith K, Alberts SC, Altmann J. Wild female baboons bias their social behaviour towards paternal half-sisters. Proc R Soc Lond Biol Sci. 2003;270:503–10. doi: 10.1098/rspb.2002.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behav Ecol Sociobiol. 2006;61:183–195. [Google Scholar]
- 42.Perry S, Manson JH, Muniz L, et al. Kin-biased social behaviour in wild adult female white-faced capuchins, Cebus capucinus. Anim Behav. 2008;76:187–199. [Google Scholar]
- 43.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Cambridge: Harvard University Press; 1986. p. 673. [Google Scholar]
- 44.Goldberg TL, Wrangham RW. Genetic correlates of social behaviour in wild chimpanzees: evidence from mitochondrial DNA. Anim Behav. 1997;54:559–70. doi: 10.1006/anbe.1996.0450. [DOI] [PubMed] [Google Scholar]
- 45.Hohmann G, Gerloff U, Tautz D, Fruth B. Social bonds and genetic ties: kinship association and affiliation in a community of bonobos (Pan paniscus) Behaviour. 1999;136:1219–1235. [Google Scholar]
- 46.Krützen M, Sherwin WB, Connor RC, et al. Contrasting relatedness patterns in Bottlenose Dolphins (Tursiops sp.) with different alliance strategies. Proc R Soc. 2003;270:497–502. doi: 10.1098/rspb.2002.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Altmann J. Age cohorts as paternal sibships. Behav Ecol Sociobiol. 1979;6:161–164. [Google Scholar]
- 48.Fu F, Nowak MA, Christakis NA, Fowler JH. The evolution of Homophily. Scientific Rep. 2012;2 doi: 10.1038/srep00845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Christakis NA, Fowler JH. Friendship and natural selection. 2013 doi: 10.1073/pnas.1400825111. ArXiv1308.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hill KR, Walker RS, Božičević M, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331:1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 51.Tibbetts EA, Dale J. Individual recognition: it is good to be different. Trends Ecol Evol. 2007;22:529–537. doi: 10.1016/j.tree.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Kummer H, Gotz W, Angst W. Triadic differentiaton: inhibitory process protecting pair bonds in baboons. Behaviour. 1974;49:62. doi: 10.1163/156853974x00408. [DOI] [PubMed] [Google Scholar]
- 53.Cheney DL, Seyfarth RM. Baboon Metaphysics: The Evolution of a Social Mind. Chicago: Chicago University Press; 2007. [Google Scholar]
- 54.Jolly A. Lemur social behavior and primate intelligence. Science. 1966;153:501–506. doi: 10.1126/science.153.3735.501. [DOI] [PubMed] [Google Scholar]
- 55.Dunbar RIM. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci Biobehav Rev. 2008;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 56.Gherardi F, Tricarico E, Atema J. Unraveling the nature of individual recognition by odor in hermit crabs. J Chem Ecol. 2005;31:2877–2896. doi: 10.1007/s10886-005-8400-5. [DOI] [PubMed] [Google Scholar]
- 57.Karavanich C, Atema J. Individual recognition and memory in lobster dominance. Anim Behav. 1998;56:1553–1560. doi: 10.1006/anbe.1998.0914. [DOI] [PubMed] [Google Scholar]
- 58.Kendrick KM, da Costa AP, Leigh AE, et al. Sheep don’t forget a face. Nature. 2001;414:165–166. doi: 10.1038/35102669. [DOI] [PubMed] [Google Scholar]
- 59.Tibbetts EA. Visual signals of individual identity in the wasp Polistes fuscatus. Proc R Soc. 2002;269:1423–1428. doi: 10.1098/rspb.2002.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parr LA, Winslow JT, Hopkins WD, de Waal FBM. Recognizing facial cues: individual discrimination by chimpanzees (Pan troglodytes) and rhesus monkeys (Macaca mulatta) J Comp Psychol. 2000;114:47–60. doi: 10.1037/0735-7036.114.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bruce V, Young A. Understanding face recognition. Br J Psychol. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- 62.Tsao DY, Moeller S, Freiwald WA. Comparing face patch systems in macaques and humans. Proc Natl Acad Sci. 2008;105:19514–19519. doi: 10.1073/pnas.0809662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grill-Spector K, Knouf N, Kanwisher N. The fusiform face area subserves face perception, not generic within-category identification. Nat Neurosci. 2004;7:555–562. doi: 10.1038/nn1224. [DOI] [PubMed] [Google Scholar]
- 64.Tsao DY, Freiwald WA, Tootell RB, Livingstone MS. A cortical region consisting entirely of face-selective cells. Science. 2006;311:670–674. doi: 10.1126/science.1119983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godard R. Long-term-memory of individual neighbors in a migratory songbird. Nature. 1991;350:228–229. [Google Scholar]
- 66.Bruck JN. Decades-long social memory in bottlenose dolphins. Proc R Soc. 2013:280. doi: 10.1098/rspb.2013.1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cheney DL, Moscovice LR, Heesen M, et al. Contingent cooperation between wild female baboons. Proc Natl Acad Sci. 2010;107:9562–9566. doi: 10.1073/pnas.1001862107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seyfarth RM, Cheney DL. Grooming, alliances and reciprocal altruism in vervet monkeys. Nature. 1984;308:541–543. doi: 10.1038/308541a0. [DOI] [PubMed] [Google Scholar]
- 69.Fraser ON, Bugnyar T. The quality of social relationships in ravens. Anim Behav. 2010;79:927–933. doi: 10.1016/j.anbehav.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boeckle M, Bugnyar T. Long-term memory for affiliates in Ravens. Curr Biol. 2012;22:801–806. doi: 10.1016/j.cub.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnson AJ, Becker JAH, Craig EA, et al. Changes in friendship commitment: comparing geographically close and long-distance young-adult friendships. Communication Quarterly. 2009;57:395–415. [Google Scholar]
- 72.Deaner RO, Khera AV, Platt ML. Monkeys pay per view: adaptive valuation of social images by rhesus macaques. Curr Biol. 2005;15:543–548. doi: 10.1016/j.cub.2005.01.044. [DOI] [PubMed] [Google Scholar]
- 73.Emery NJ. The eyes have it: the neuroethology, function and evolution of social gaze. Neurosci Biobehav Rev. 2000;24:581–604. doi: 10.1016/s0149-7634(00)00025-7. [DOI] [PubMed] [Google Scholar]
- 74.Hayden BY, Parikh PC, Deaner RO, Platt ML. Economic principles motivating social attention in humans. Proc R Soc. 2007;274:1751–1756. doi: 10.1098/rspb.2007.0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chang SW, Winecoff AA, Platt ML. Vicarious reinforcement in rhesus macaques (Macaca mulatta) Front Neurosci. 2011;5:00027. doi: 10.3389/fnins.2011.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Johnson MH, Dziurawiec S, Ellis H, Morton J. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition. 1991;40:1–19. doi: 10.1016/0010-0277(91)90045-6. [DOI] [PubMed] [Google Scholar]
- 77.McNelis NL, Boatright-Horowitz SL. Social monitoring in a primate group: the relationship between visual attention and hierarchical ranks. Ani Cogn. 1998;1:65–69. [Google Scholar]
- 78.Ebitz RB, Watson KK, Platt ML. Oxytocin blunts social vigilance in the rhesus macaque. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1305230110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Azzi JCB, Sirigu A, Duhamel JR. Modulation of value representation by social context in the primate orbitofrontal cortex. Proc Natl Acad Sci. 2012;109:2126–2131. doi: 10.1073/pnas.1111715109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chang SWC, Gariepy JF, Platt ML. Neuronal reference frames for social decisions in primate frontal cortex. Nat Neurosci. 2013;16:243–250. doi: 10.1038/nn.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Smith DV, Hayden BY, Truong TK, et al. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. J Neurosci. 2010;30:2490–2495. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Watson KK, Platt ML. Social signals in primate orbitofrontal cortex. Curr Biol. 2012;22:2268–2273. doi: 10.1016/j.cub.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lewis PA, Rezaie R, Brown R, et al. Ventromedial prefrontal volume predicts understanding of others and social network size. Neuroimage. 2011;57:1624–1629. doi: 10.1016/j.neuroimage.2011.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charnov EL. Optimal foraging, the marginal value theorem. Theor Popul Biol. 1976;9:129–136. doi: 10.1016/0040-5809(76)90040-x. [DOI] [PubMed] [Google Scholar]
- 85.Seyfarth RM, Cheney DL. Affiliation, empathy, and the origins of theory of mind. Proc Natl Acad Sci. 2013 doi: 10.1073/pnas.1301223110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Perry S, Barrett HC, Manson JH. White-faced capuchin monkeys show triadic awareness in their choice of allies. Anim Behav. 2004;67:165–170. [Google Scholar]
- 87.Range F, Noe R. Can simple rules account for the pattern of triadic interactions in juvenile and adult female sooty mangabeys? Anim Behav. 2005;69:445–452. [Google Scholar]
- 88.Schino G, Tiddi B, Di Sorrentino EP. Simultaneous classification by rank and kinship in Japanese macaques. Anim Behav. 2006;71:1069–1074. [Google Scholar]
- 89.Bergman TJ, Beehner JC, Cheney DL, Seyfarth RM. Hierarchical classification by rank and kinship in baboons. Science. 2003;302:1234–1236. doi: 10.1126/science.1087513. [DOI] [PubMed] [Google Scholar]
- 90.Borgeaud C, van de Waal E, Bshary R. d-Party Ranks Knowledge in Wild Vervet Monkeys (Chlorocebus aethiops pygerythrus) PLoS One. 2013:8. doi: 10.1371/journal.pone.0058562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kaminski J, Call J, Tomasello M. Goats’ behaviour in a competitive food paradigm: evidence for perspective taking? Behaviour. 2006;143:1341–1356. [Google Scholar]
- 92.Emery NJ, Clayton NS. Effects of experience and social context on prospective caching strategies by scrub jays. Nature. 2001;414:443–446. doi: 10.1038/35106560. [DOI] [PubMed] [Google Scholar]
- 93.Bugnyar T, Stöwe M, Heinrich B. Ravens, corvus corax, follow gaze direction of humans around obstacles. Proc R Soc. 2004;271:1331–1336. doi: 10.1098/rspb.2004.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hare B, Call J, Agnetta B, Tomasello M. Chimpanzees know what conspecifics do and do not see. Anim Behav. 2000;59:771–785. doi: 10.1006/anbe.1999.1377. [DOI] [PubMed] [Google Scholar]
- 95.Flombaum JI, Santos LR. Rhesus monkeys attribute perceptions to others. Curr Biol. 2005;15:447–452. doi: 10.1016/j.cub.2004.12.076. [DOI] [PubMed] [Google Scholar]
- 96.Hoffman EA, Haxby JV. Distinct representations of eye gaze and identity in the distributed human neural system for face perception. Nat Neurosci. 2000;3:80–84. doi: 10.1038/71152. [DOI] [PubMed] [Google Scholar]
- 97.Roy A, Shepherd SV, Platt ML. Reversible inactivation of pSTS suppresses social gaze following in the macaque (Macaca mulatta) Soc Cogn Affect Neurosci. 2012 doi: 10.1093/scan/nss123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shepherd SV, Klein JT, Deaner RO, Platt ML. Mirroring of attention by neurons in macaque parietal cortex. Proc Natl Acad Sci. 2009;106:9489–9494. doi: 10.1073/pnas.0900419106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hoffman KL, Gothard KM, Schmid MC, Logothetis NK. Facial-expression and gaze-selective responses in the monkey amygdala. Curr Biol. 2007;17:766–772. doi: 10.1016/j.cub.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 100.Rizzolatti G, Fabbri-Destro M. The mirror system and its role in social cognition. Curr Opin Neurobiol. 2008;18:179–184. doi: 10.1016/j.conb.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 101.Behrens TE, Hunt LT, Rushworth MF. The computation of social behavior. Science. 2009;324:1160–1164. doi: 10.1126/science.1169694. [DOI] [PubMed] [Google Scholar]
- 102.Chang SW. Coordinated transformation approach to social interactions. Front Neurosci. doi: 10.3389/fnins.2013.00147. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rudebeck PH, Buckley MJ, Walton ME, Rush-worth MFS. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- 104.Marsh AA, Finger EC, Fowler KA, et al. Empathic responsiveness in amygdala and anterior cingulate cortex in youths with psychopathic traits. J Child Psychol Psychiatr. 2013;54:900–910. doi: 10.1111/jcpp.12063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Waytz A, Zaki J, Mitchell JP. Response of dorsomedial prefrontal cortex predicts altruistic behavior. J Neurosci. 2012;32:7646–7650. doi: 10.1523/JNEUROSCI.6193-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 107.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Curley JP, Keverne EB. Genes, brains and mammalian social bonds. Trends Ecol Evol. 2005;20:561–567. doi: 10.1016/j.tree.2005.05.018. [DOI] [PubMed] [Google Scholar]
- 109.Depue RA, Morrone-Strupinsky JV. A neurobehavioral model of affiliative bonding: implications for conceptualizing a human trait of affiliation. Behav Brain Sci. 2005;28:313–350. doi: 10.1017/S0140525X05000063. [DOI] [PubMed] [Google Scholar]
- 110.Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- 111.Pedersen CA, Ascher JA, Monroe YL, Prange AJ., Jr Oxytocin induces maternal behavior in virgin female rats. Science. 1982;216:648–50. doi: 10.1126/science.7071605. [DOI] [PubMed] [Google Scholar]
- 112.Ferguson JN, Young LJ, Hearn EF, et al. Social amnesia in mice lacking the oxytocin gene. Nat Genet. 2000;25:284–288. doi: 10.1038/77040. [DOI] [PubMed] [Google Scholar]
- 113.Rimmele U, Hediger K, Heinrichs M, Klaver P. Oxytocin makes a face in memory familiar. J Neurosci. 2009;29:38–42. doi: 10.1523/JNEUROSCI.4260-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chang SWC, Barter JW, Ebitz RB, et al. Inhaled oxytocin amplifies both vicarious reinforcement and self reinforcement in rhesus macaques (Macaca mulatta) Proc Natl Acad Sci. 2012 doi: 10.1073/pnas.1114621109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kosfeld M, Heinrichs M, Zak PJ, et al. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- 116.Zak PJ, Stanton AA, Ahmadi S. Oxytocin increases generosity in humans. PLoS One. 2007;2:e1128. doi: 10.1371/journal.pone.0001128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 118.Dunbar RI. Bridging the bonding gap: the transition from primates to humans. Philosop Trans R Soc. 2012;367:1837–1846. doi: 10.1098/rstb.2011.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- 120.Wallner B, Dittami J, Machatschke I. Social stimuli cause changes of plasma oxytocin and behavior in guinea pigs. Biol Res. 2006;39:251–258. doi: 10.4067/s0716-97602006000200007. [DOI] [PubMed] [Google Scholar]
- 121.Cohen EE, Ejsmond-Frey R, Knight N, Dunbar RI. Rowers’ high: behavioural synchrony is correlated with elevated pain thresholds. Biol Lett. 2010;6:106–108. doi: 10.1098/rsbl.2009.0670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Crockford C, Wittig RM, Langergraber K, et al. Urinary oxytocin and social bonding in related and unrelated wild chimpanzees. Proc R Soc. 2013 doi: 10.1098/rspb.2012.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–1411. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- 124.Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones Behav. 2010;57:368–374. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 125.Scerbina T, Chatterjee D, Gerlai R. Dopamine receptor antagonism disrupts social preference in zebrafish: a strain comparison study. Amino Acids. 2012;43:2059–2072. doi: 10.1007/s00726-012-1284-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Knutson B, Wolkowitz OM, Cole SW, et al. Selective alteration of personality and social behavior by serotonergic intervention. Am J Psychiatr. 1998;155:373–9. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- 127.Raleigh MJ, Brammer GL, Yuwiler A, et al. Serotonergic influences on the social behavior of vervet monkeys (Cercopithecus aethiops sabaeus) Exper Neurol. 1980;68:322–334. doi: 10.1016/0014-4886(80)90089-8. [DOI] [PubMed] [Google Scholar]
- 128.Yokoyama C, Kawasaki A, Hayashi T, Onoe H. Linkage between the midline cortical serotonergic system and social behavior traits: positron emission tomography studies of common marmosets. Cereb Cortex. 2013;23:2136–2145. doi: 10.1093/cercor/bhs196. [DOI] [PubMed] [Google Scholar]
- 129.Homberg JR. Serotonin and decision making processes. Neurosci Biobehav Rev. 2012;36:218–236. doi: 10.1016/j.neubiorev.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 130.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- 131.deVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 132.Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): a pilot study. J Comp Psychol. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- 133.Brent LJN, Semple S, Dubuc C, et al. Social capital and physiological stress levels in free-ranging adult female rhesus macaques. Physiol Behav. 2011;102:76–83. doi: 10.1016/j.physbeh.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 134.Crockford C, Wittig RM, Whitten PL, et al. Social stressors and coping mechanisms in wild female baboons (Papio hamadryas ursinus) Hormones Behav. 2008;53:254–265. doi: 10.1016/j.yhbeh.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 135.Cohen S, Kaplan JR, Cunnick JE, et al. Chronic social stress, affiliation, and cellular immune response in nonhuman primates. Psychol Sci. 1992;3:301–304. [Google Scholar]
- 136.Cameron JL. Stress and behaviorally induced reproductive dysfunction in primates. Semin Repr Endocrinol. 1997;15:37–45. doi: 10.1055/s-2008-1067966. [DOI] [PubMed] [Google Scholar]
- 137.Seyfarth RM. A model of social grooming among adult female monkeys. J Theor Biol. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- 138.Hruschka DJ. Friendship: Development, Ecology, and Evolution of a Social Relationship. University of California Press; 2010. [Google Scholar]
- 139.Fowler JH, Settle JE, Christakis NA. Correlated genotypes in friendship networks. Proc Natl Acad Sci. 2011;108:1993–1997. doi: 10.1073/pnas.1011687108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Christakis NA, Fowler JH. Social contagion theory: examining dynamic social networks and human behavior. Stat Med. 2013;32:556–577. doi: 10.1002/sim.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berman CM. The ontogeny of social relationships with group companions among free-ranging infant rhesus monkeys I. Social networks and differentiation. Anim Behav. 1982;30:149–162. [Google Scholar]
- 142.Berman CM, Kapsalis E. Development of kin bias among rhesus monkeys: maternal transmission or individual learning? Anim Behav. 1999;58:883–894. doi: 10.1006/anbe.1999.1221. [DOI] [PubMed] [Google Scholar]
- 143.Vigil J. Asymmetries in the friendship preferences and social styles of men and women. Hum Nat. 2007;18:143–161. doi: 10.1007/s12110-007-9003-3. [DOI] [PubMed] [Google Scholar]
- 144.Baron-Cohen S. The extreme male brain theory of autism. Trends Cogn Sci. 2002;6:248–254. doi: 10.1016/s1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- 145.Seielstad MT, Minch E, Cavalli-Sforza LL. Genetic evidence for a higher female migration rate in humans. Nat Genet. 1998;20:278–80. doi: 10.1038/3088. [DOI] [PubMed] [Google Scholar]
- 146.Calero CI, Salles A, Semelman M, Sigman M. Age and gender dependent development of theory of mind in 6 to 8-years old children. Front Hum Neurosci. 2013 doi: 10.3389/fnhum.2013.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cushman F, Sheketoff R, Wharton S, Carey S. The development of intent-based moral judgment. Cognition. 2013;127:6–21. doi: 10.1016/j.cognition.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 148.Vetter NC, Leipold K, Kliegel M, et al. Ongoing development of social cognition in adolescence. Child Neuropsychol. 2012;31:31. doi: 10.1080/09297049.2012.718324. [DOI] [PubMed] [Google Scholar]
- 149.Harenski CL, Harenski KA, Shane MS, Kiehl KA. Neural development of mentalizing in moral judgment from adolescence to adulthood. Dev Cogn Neurosci. 2012;2:162–73. doi: 10.1016/j.dcn.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.House BR, Henrich J, Brosnan SF, Silk JB. The ontogeny of human prosociality: behavioral experiments with children aged 3 to 8. Evol Hum Behav. 2012;33:291–308. [Google Scholar]
- 151.Suway JG, Degnan KA, Sussman AL, Fox NA. The relations among theory of mind, behavioral inhibition, and peer interactions in early childhood. Soc Dev. 2012;21:331–342. doi: 10.1111/j.1467-9507.2011.00634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sackett G, Holm R, Ruppenthal G, Farhrenbruch C. The effects of total social isolation rearing on behavior of Rhesus and Pigtail Macaques. In: Walsh R, Greenough W, editors. Environments as Therapy for Brain Dysfunction. US: Springer; 1976. pp. 115–131. [Google Scholar]
- 153.Matthews NL, Goldberg WA, Lukowski AF. Theory of mind in children with autism spectrum disorder: do siblings matter? Autism Res. 2013 doi: 10.1002/aur.1308. [DOI] [PubMed] [Google Scholar]
- 154.Sallet J, Mars RB, Noonan MP, et al. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- 155.Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- 156.House JS, Landis KR, Umberson D. Social relationships and health. Science. 1988;241:540–545. doi: 10.1126/science.3399889. [DOI] [PubMed] [Google Scholar]
- 157.Barth J, Schneider S, von Känel R. Lack of social support in the etiology and the prognosis of coronary heart disease: a systematic review and meta-analysis. Psychosom Med. 2010;72:229–238. doi: 10.1097/PSY.0b013e3181d01611. [DOI] [PubMed] [Google Scholar]
- 158.Udell JA, Steg P, Scirica BM, et al. Living alone and cardiovascular risk in outpatients at risk of or with atherothrombosis. Arch Intern Med. 2012;172:1086–1095. doi: 10.1001/archinternmed.2012.2782. [DOI] [PubMed] [Google Scholar]
- 159.Loucks EB, Sullivan LM, D’Agostino RB, et al. Social networks and inflammatory markers in the Framingham Heart study. J Biosoc Sci. 2006;38:835–842. doi: 10.1017/S0021932005001203. [DOI] [PubMed] [Google Scholar]
- 160.Schülke O, Bhagavatula J, Vigilant L, Ostner J. Social bonds enhance reproductive success in male macaques. Curr Biol. 2010;20:1–4. doi: 10.1016/j.cub.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 161.Frere CH, Krutzen M, Mann J, et al. Social and genetic interactions drive fitness variation in a free-living dolphin population. Proc Natl Acad Sci. 2010;107:19949–19954. doi: 10.1073/pnas.1007997107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Silk JB, Beehner JC, Bergman TJ, et al. The benefits of social capital: close social bonds among female baboons enhance offspring survival. Proc R Soc. 2009;276:3099–3104. doi: 10.1098/rspb.2009.0681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Connor RC, Smolker RA, Richards AF. 2 levels of alliance formation among male bottle-nosed dolphins (Tursiops sp) Proc Natl Acad Sci. 1992;89:987–990. doi: 10.1073/pnas.89.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Schino G, Aureli F. Reciprocal altruism in primates: partner choice, cognition, and emotions. Adv Study Behav. 2009;39:45–69. [Google Scholar]
- 165.Dunbar RIM. Functional significance of social grooming in primates. Folia Primatologica. 1991;57:121–131. [Google Scholar]
- 166.Schino G. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behav Ecol. 2007;18:115–120. [Google Scholar]
- 167.Gilby IC, Brent LJN, Wroblewski EE, et al. Fitness benefits of coalitionary aggression in male chimpanzees. Behav Ecol Sociobiol. 2013;67:373–381. doi: 10.1007/s00265-012-1457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sterck EHM, Watts DP, van Schaik CP. The evolution of female social relationships in nonhuman primates. Behav Ecol Sociobiol. 1997;41:291–309. [Google Scholar]
- 169.Tinsley Johnson E, Snyder-Mackler N, Beehner JC, Bergman TJ. Kinship and dominance rank influence the strength of social bonds in female geladas. Int J Primatol In press. [Google Scholar]
- 170.van de Waal E, Borgeaud C, Whiten A. Potent social learning and conformity shape a wild primate’s foraging decisions. Science. 2013;340:483–485. doi: 10.1126/science.1232769. [DOI] [PubMed] [Google Scholar]
- 171.Brent LJN, Semple S, MacLarnon A, et al. Personality traits in rhesus macaques are heritable but do not predict reproductive output. Int J Primatol. doi: 10.1007/s10764-013-9724-6. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hamilton WD. The genetical evolution of social behaviour. J Theor Biol. 1964;7:1–51. doi: 10.1016/0022-5193(64)90038-4. [DOI] [PubMed] [Google Scholar]
- 173.Trivers RL. Evolution of reciprocal altruism. Quart Rev Biol. 1971;46:35–57. [Google Scholar]
- 174.Raihani NJ, Bshary R. Resolving the iterated prisoner’s dilemma: theory and reality. J Evol Biol. 2011;24:1628–1639. doi: 10.1111/j.1420-9101.2011.02307.x. [DOI] [PubMed] [Google Scholar]
- 175.Dugatkin LA. Cooperation Among Animals: An Evolutionary Perspective. Oxford: Oxford University Press; 1997. [Google Scholar]
- 176.Hemelrijk CK. Support for being groomed in long-tailed macaques, Macaca fascicularis. Anim Behav. 1994;48:479–481. [Google Scholar]
- 177.Stevens JR, Hauser MD. Why be nice? Psychological constraints on the evolution of cooperation. Trends Cogn Sci. 2004;8:60–65. doi: 10.1016/j.tics.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 178.Barrett L, Henzi P, Rendall D. Social brains, simple minds: does social complexity really require cognitive complexity? Phil Trans R Soc. 2007;362:561–575. doi: 10.1098/rstb.2006.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Frigerio D, Weiss B, Kotrschal K. Spatial proximity among adult siblings in greylag geese (Anser anser): evidence for female bonding? Acta Ethologica. 2001;3:121–125. [Google Scholar]
- 180.Holekamp KE, Sakai ST, Lundrigan BL. Social intelligence in the spotted hyena (Crocuta crocuta) Phil Trans R Soc. 2007;362:523–538. doi: 10.1098/rstb.2006.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Holekamp KE, Smith JE, Strelioff CC, et al. Society, demography and genetic structure in the spotted hyena. Mol Ecol. 2012;21:613–632. doi: 10.1111/j.1365-294X.2011.05240.x. [DOI] [PubMed] [Google Scholar]
- 182.Wahaj S, Van Horn R, Van Horn T, et al. Kin discrimination in the spotted hyena (Crocuta crocuta): nepotism among siblings. Behav Ecol Sociobiol. 2004;56:237–247. [Google Scholar]
- 183.Darden SK, James R, Ramnarine IW, Croft DP. Social implications of the battle of the sexes: sexual harassement disrupts female sociality and social recognition. Proc R Soc. 2009;276:2651–2656. doi: 10.1098/rspb.2009.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Murray LMA, Byrne K, D’Eath RB. Pair-bonding and companion recognition in domestic donkeys, Equus asinus. Appl Anim Behav Sci. 2013;143:67–74. [Google Scholar]
- 185.Spehar S, Fiore A. Loud calls as a mechanism of social coordination in a fission–fusion taxon, the white-bellied spider monkey (Ateles belzebuth) Behav Ecol Sociobiol. 2013;67:947–961. [Google Scholar]
- 186.Cords M. Reciprocity and partner preferences in grooming of female blue monkeys. Int J Primatol. 1991;12:319–336. [Google Scholar]
- 187.Seyfarth RM. The distribution of grooming and related behaviours among adult female vervet monkeys. Anim Behav. 1980;28:798–813. [Google Scholar]
- 188.Kappeler PM. Variation in social structure: the effects of sex and kinship on social interactions in three lemur species. Ethology. 1993;93:125–145. [Google Scholar]
- 189.Cooper MA, Bernstein IS. Social grooming in assamese macaques (Macaca assamensis) Am J Primatol. 2000;50:77–85. doi: 10.1002/(SICI)1098-2345(200001)50:1<77::AID-AJP7>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 190.Massen JJM, Sterck EHM. Stability and durability of intra- and intersex social bonds of captive rhesus macaques (Macaca mulatta) Int J Primatol. 2013;34:770–791. [Google Scholar]
- 191.Silk JB. Male bonnet macaques use information about third-party rank relationships to recruit allies. Anim Behav. 1999;58:45–51. doi: 10.1006/anbe.1999.1129. [DOI] [PubMed] [Google Scholar]
- 192.Silk JB. Social relationships of male bonnet macaques: male bonding in a matrilineal society. Behaviour. 1994;3–4:271–291. [Google Scholar]
- 193.Berghänel A, Ostner J, Schröder U, Schülke O. Social bonds predict future cooperation in male Barbary macaques, Macaca sylvanus. Anim Behav. 2011;81:1109–1116. [Google Scholar]
- 194.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci. 2007;104:7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lehmann J, Boesch C. Sociality of the dispersing sex: the nature of social bonds in West African female chimpanzees, Pan troglodytes. Anim Behav. 2009;77:377–387. [Google Scholar]
- 196.Mitani C. Male chimpanzees form enduring and equitable social bonds. Anim Behav. 2009;77:633–640. [Google Scholar]
- 197.Swedell L. Affiliation among females in wild hamadryas baboons (Papio hamadryas hamadryas) Int J Primatol. 2002;23:1205–1226. [Google Scholar]
- 198.Wey TW, Burger JR, Ebensperger LA, Hayes LD. Reproductive correlates of social network variation in plurally breeding degus (Octodon degus) Anim Behav. 2013;85:1407–1414. doi: 10.1016/j.anbehav.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]