Abstract
Dilated cardiomyopathy (DCM) represents the final common morphofunctional pathway of various pathological conditions in which a combination of myocyte injury and necrosis associated with tissue fibrosis results in impaired mechanical function. Recognition of the underlying aetiology of disease and accurate disease monitoring may be crucial to individually optimize therapeutic strategies and stratify patient's prognosis. In this regard, CMR has emerged as a new reference gold standard providing important information for differential diagnosis and new insight about individual risk stratification. The present review article will focus on the role of CMR in the evaluation of present condition, analysing respective strengths and limitations in the light of current literature and technological developments.
1. Introduction
The main hallmark of primary dilated cardiomyopathy (DCM) is the presence of a left or biventricular dilatation with severely impaired systolic function in the absence of abnormal loading conditions (i.e., hypertension, valve disease, etc.) or ischaemic heart disease sufficient to cause global systolic impairment [1–5].
Primary forms of disease are diagnosed in approximately 30–40% of the cases and include a series of genetic, acquired, or mixed conditions in which the pathological involvement is predominantly limited to the myocardium and associated with a strong genetic inheritance in idiopathic cases (≈30% of patients). In secondary DCM conversely, ventricular dilation occurs as the final stage of extensive myocardial damage which can be associated with an extremely heterogeneous group of systemic affections from autoimmune, cytotoxic or metabolic diseases [1, 6–13].
Recognition and differentiation of the underlying pathological substrate leading to ventricular dilatation may be crucial not only to specifically the target patient's therapy (e.g., treatment of heart failure symptoms versus revascularization versus immunosuppressive and/or antiviral) but also for better individual risk stratification because of the extremely variable prognostic implications associated with the different forms of disease [14–17].
Postischemic DCM, for example, is the consequence of postischemic ventricular remodelling leading to chronic heart failure which is usually associated with a worse prognosis [18–20] and may benefit from revascularization and/or secondary preventive pharmacotherapy with statins and aspirin; among nonischemic forms, the worst prognosis had been associated with infiltrative myocardial disease (including amyloidosis, sarcoidosis, or hemochromatosis) and toxic postchemotherapy conditions, whereas peripartum and idiopathic etiology had substantially better mid- and long-term survival as compared to those with other causes of heart failure [21–24].
For these reasons, routine diagnostic imaging workup of patients with DCM (including echocardiography, selective coronary angiography, and, when indicated, endomyocardial biopsy) has been integrated in the last few years with the use of cardiac magnetic resonance (CMR) which allows identifing and characterizing the presence and location of myocardial damage in most of the cases combining its unique tissue characterization capabilities with the assessment of biventricular regional and global function [25–35].
The present paper will review the role of CMR in the evaluation of DCM, analysing respective strengths and limitations in the light of the current literature and technological developments.
2. Epidemiology and Etiology of Disease
The true incidence of DCM is unknown and certainly underestimated in the community since strict clinical diagnostic criteria are lacking and patients may remain asymptomatic with the diagnosis being established only by screening or postmortem examination in a relevant number of cases [36–43].
It has been reported that approximately 5–8 new cases per 100 000 population per year are diagnosed representing the third cause of heart failure after ischemia and valvular heart disease corresponding to a disease prevalence of 36 cases per 100 000 in adult populations [38, 44, 45].
Epidemiology of disease was also assessed in a large longitudinal prospective cohort study of 1426 children with DCM reporting an annual incidence of 0.57 cases per 100000 per year with a statistically significant prevalence in boys versus girls, blacks versus whites, and infants (<1 year) versus children [40].
The etiology is also extremely heterogeneous; 50% the of cases are interpreted as idiopathic disease and associated with inflammatory and immunological phenomena in 20 to 30% of the patients, whereas the other half includes a broad spectrum of various conditions such as myocarditis, ischemic heart disease, peripartum disease, hypertension, HIV infection, and toxic forms [46–52].
Among toxic forms, alcoholic cardiomyopathy is the most frequent etiology of secondary DCM and accounts for approximately 4% of all cardiomyopathies, with men having a significantly worse prognosis [53, 54] and left ventricular dilation being an early finding.
Recognition of alcohol as a potential cause of cardiomyopathy is crucial since abstinence can result in an improved ejection fraction in 50% of the patients medically treated for heart failure, and continued drinking can result in further deterioration of the cardiac function. The mechanism of alcohol-induced cardiomyopathy is unclear but may involve disturbances in intracellular calcium transients, mitochondrial disruption, decreased myofibrillar proteins, and myocyte apoptosis [54–56].
Cocaine and amphetamines (including 3,4-methylenedioxymethamphetamine or “ecstasy”) can also result in DCM due to the multifactorial causes including microvascular spasms and subsequent ischemia/infarction, direct myocyte toxicity, and tachycardia-induced injury [54, 56–58].
Among chemotoxic agents, doxorubicin and anthracyclines can cause DCM which is clinically suspected in presence of increased brain-type natriuretic peptide (BNP) occurring at an early stage of this condition [59–62].
Peripartum cardiomyopathy arises in the last month of pregnancy or within 5 months postpartum with 75% of the cases manifesting in the first 2 months after delivery [63–65]; risk factors include age older than 30 years, multiparity, twin pregnancy, African American descent, and a family history of peripartum cardiomyopathy [63, 65]. This form likely depends on pregnancy-related reduced suppressor T cell activity and may result in an autoimmune type of myocardial inflammation or activation of myocarditis. Recovery, usually within 6 months, occurs in 50% of the patients [66, 67].
In pediatric population, the majority of the cases (66%) have also idiopathic forms of disease, whereas the most common known causes are myocarditis (46%) and neuromuscular disease (26%) with 1- and 5-year rates of death or transplantation of 31% and 46%, respectively [40, 68, 69].
It is likely expectable in the future that a large number of unrecognized idiopathic cases will be correctly reassigned to genetic or familial forms of diseases with subsequent changes in the epidemiology of disease [1, 36].
3. Clinical Features and Pathophysiology
Dilated cardiomyopathy represents the final common morphofunctional outcome of various biologic insults. It is a combination of myocyte injury and necrosis associated with myocardial fibrosis, which results in impaired mechanical function [14, 70, 71].
With myocyte failure and cytoskeletal uncoupling, the chambers become dilated and, according to Laplace's law, parietal stress increases inducing further mechanical disadvantage due to the increased oxygen demand and subsequent worsening of the ventricular systolic performances [72].
Thus, myocardial dysfunction can cause a vicious cycle leading to more myocardial dysfunction in a process called adverse ventricular remodelling [73–75].
Clinical manifestations are obviously more often related to signs and symptoms of congestive heart failure such as dyspnoea and effort-related fatigue [6, 76].
Major arrhythmias leading to syncope, embolic events, and even sudden cardiac death may occur at any stage of the disease although strongly dependent on the extent of replacement fibrosis and ventricular dysfunction [1, 14].
Poor contractile function and stasis can also lead to the formation of mural thrombi with symptoms related to distal embolism [77].
If right ventricle (RV) is involved, signs of right heart failure (raised jugular venous pulse, hepatomegaly, ascites, and peripheral oedema) may be also present.
4. Histological Features of DCM
Myocardial muscle in DCM may be normal or even hypertrophied primarily as a consequence of the myocyte elongation with an in-series addition of newly formed sarcomeres causing chambers enlargement with apparently normal or only slightly increased parietal wall thickness [78–80].
Histologically, typical features of DCM include substantial hypertrophy and degeneration of myocytes related to the loss of myofibrils, varying degree of interstitial fibrosis, and presence of small cluster of lymphocytes [1, 81–83].
Tissue fibrosis may be focal or diffused and induces increased left ventricular (LV) stiffness with progressively impaired diastolic and systolic function and has direct hemodynamic consequences on the remaining contractile tissue leading to changes in the chamber geometry with decompensated eccentric hypertrophy and increased parietal wall stress [84].
Fibrotic tissue, as already mentioned above, may also be a potential trigger for lethal reentrant ventricular arrhythmias representing a strong predictor of adverse cardiac outcomes [85, 86].
5. CMR Acquisition Protocol and Features
Imaging features to be assessed include the evaluation and quantification of LV dilatation and systolic dysfunction and detection of possible underlying tissue abnormalities particularly myocardial fibrosis [87, 88].
Although the acquisition technique should be tailored to the specific clinical request, a standard imaging protocol in DCM should necessarily include a four-chamber horizontal long-axis, two-chamber vertical long-axis, and short-axis views using breath-hold steady-state free precession (SSFP) cine sequences with full coverage of both ventricles to provide assessment of biventricular volumes and global and regional functions (Table 1).
Table 1.
Use and significance of different CMR sequences applied for the evaluation of primary and secondary forms of DCM.
| Sequence | Information provided | CMR imaging features |
|---|---|---|
| Cine-SSFP | Regional and global biventricular function Ventricular mass and parietal wall thickness |
Dilated left or biventricular cavities Reduced ejection fraction (<40%) Parietal wall thickness normal or slightly reduced (<5.5 mm) |
|
| ||
| T2w-STIR | Myocardial free water content increase reflecting aspecific inflammatory changes | Regional hyperintense signal subendocardial involvement to rule out ischemic versus nonischemic acute disease |
|
| ||
| IR-CE or LGE | Tissue fibrosis/scar | (i) No enhancement (59%) (ii) Subendocardial or transmural enhancement indistinguishable from patients with previous infarction (iii) Patchy or longitudinal striae of mid-wall enhancement |
|
| ||
| T1-mapping | Depiction of diffuse myocardial fibrosis | Generation of T1 maps for the quantification of decay in myocardial signal intensity |
|
| ||
| MRS (hydrogen) | Assessment of myocardial cellular triglyceride | Still few data published |
|
| ||
| MRS (phosphorous) | Measurement of myocardial energetics | Reduction in PCr (~50%) and ATP (~35%), with concomitant decrease in PCr/ATP (~25%) |
SSFP: steady-state free precession; T2w-STIR: T2-weighted short-tau Inversion recovery; IR-CE or LGE: Inversion recovery contrast-enhanced or late gadolinium enhancement; MRS: MR spectroscopy; PCr: phosphocreatine; ATP: adenosine-5-triphosphate (ATP).
At present, CMR can be considered the reference technique for the quantification of ventricular volumes and functional parameters, to measure wall thickness and ventricular mass in patients with DCM [89–95].
Buser et al. have found a significantly more heterogeneous end-diastolic LV wall thickness in patients with DCM compared to a reference normal population; furthermore, the physiological gradient in systolic wall thickening between LV basal and apical segments disappears with DCM [90, 94].
Previous studies showed that RV mass is preserved in DCM patients as compared to normal subjects, whereas LV mass is significantly greater with evidence of larger trabeculae as compared to normal subjects [91].
In advanced cases, LV dysfunction may be associated with diffuse myocardial wall thinning (diastolic wall thickness < 5.5 mm) [44].
An interesting feature of balanced cine-SSFP imaging concerns the hybrid T2/T1-weighting generated by the sequence that allows the depiction of myocardial enhancement when acquired after contrast administration due to the T1-weighted shortening induced by gadolinium (Gd) administration [96–98].
T2-weighted short-tau inversion recovery (T2w-STIR) imaging using an ECG-gated triple inversion recovery (IR) technique is also recommended to depict tissue edema when an overlapping active inflammatory process is suspected such as in acute or chronic myocarditis, sarcoidosis, Takotsubo syndrome, or acute myocardial infarction [27, 99–103].
A standard acquisition protocol should also include late enhancement imaging with T1 weighted inversion recovery images acquired 10–20 minutes after contrast administration (also called late gadolinium enhancement technique or LGE), usually obtained using a segmented 2D or 3D inversion recovery gradient-echo breath-hold approach, with inversion time optimized to null myocardial signal intensity.
It is also recommended to ensure matching section position and same slice thickness between IR-CE and cine-SSFP imaging in order to obtain direct comparison between regional wall motion abnormalities and LGE findings [104].
Use of IR-CE imaging may be helpful to characterize the myocardium and to differentiate DCM patients from LV dysfunction related to CAD [87].
LGE has been described as being present in patients with DCM in 12–35% of the cases, the most common pattern being characterized by a midwall linear distribution likely representing the intramural layer of septal fibrosis which has been observed in pathologic samples [44, 86, 87, 105].
In a previous paper, McCrohon et al. found three different LE patterns in patients with DCM: (a) no enhancement (59%; Figure 1), (b) subendocardial or transmural enhancement indistinguishable from patients with previous infarction (Figure 2), and (c) patchy or longitudinal striae of midwall enhancement clearly different from the distribution in patients with CAD (28%) (Figure 3) [87].
Figure 1.
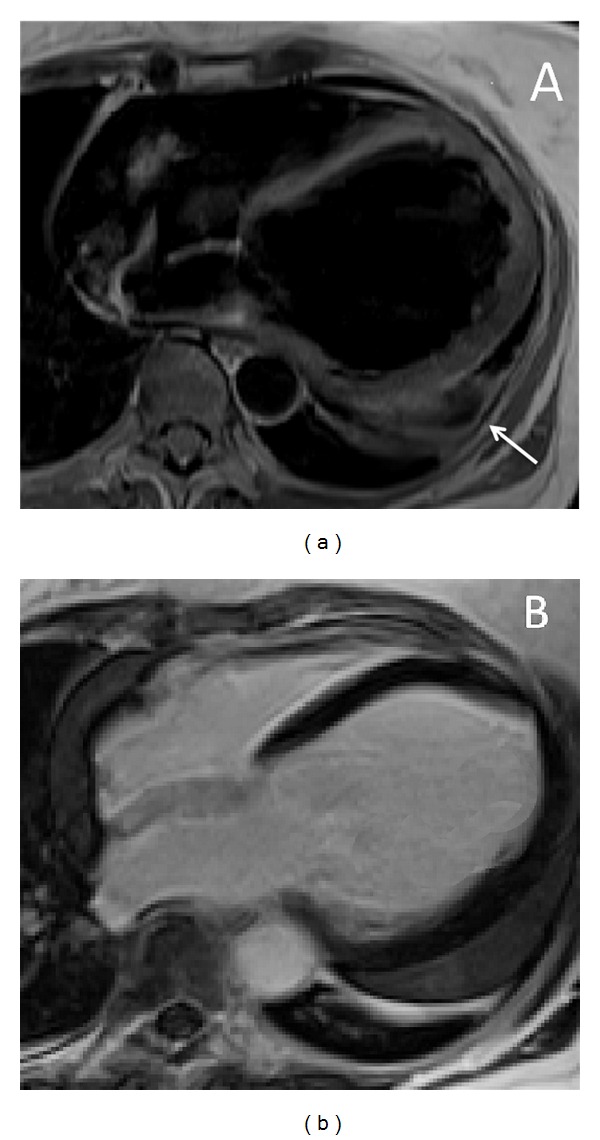
Idiopathic dilated cardiomiopathy in a 27-year-old asymptomatic patient occasionally identified during a routinary clinical screening. T1-weighted turbo spin echo 4-chamber image (a) shows a hugely dilated left ventricular chamber (end-diastolic volume 296 mL; ejection fraction 26%). Late gadolinium enhancement image acquired on the same orientation plane (b) shows absence of pathological enhancement within both ventricular chambers. There is also concomitant significant amount of pericardial effusion (arrow) located within the midbasal left ventricular lateral free wall which was initially interpreted as expression of a post pericardiomyocarditis form.
Figure 2.

Postischemic dilated cardiomyopathy in a 69-year-old patient with severe dyspnea and symptoms of systemic heart failure. Cine-SSFP short-axis images displayed on end-diastolic (a) and end-systolic (b) phases show a markedly dilated left ventricular chamber (EDV: 287 mL) with severely compromised ejection fraction (11%). LGE image (c) depicts extensive ischemic scarring within the anterior and antero-septal and lateral walls of the left ventricle with typical subendocardial distribution of myocardial enhancement. Minimal right ventricular involvement is also present and observed as a linear hyperintense rim at the level of the right interventricular septum. Selective coronary angiography confirmed the presence of a severe 3-vessel disease which was only treated with medical therapy.
Figure 3.

A specific midwall late enhancement stria in a 71-year-old patient with chronic dilated CMP. LGE image (a) acquired in a 4-chamber plane shows a linear post-Gd hyperintensity located within the midlateral wall of the left ventricle without corresponding to T2-STIR (b) signal abnormality. The present finding likely represents the expression of a postmyocarditic form of disease (inconfirmed in this patient).
Transmural or subendocardial LE pattern in DCM strongly suggests the presence of a previous myocardial infarction even in the absence of remarkable coronary angiographic abnormalities and the likely explanation of this appearance may be related to spontaneous coronary recanalization after an occlusive event or a distal embolization from minimally stenotic or unstable plaques (Figure 2).
The midwall LGE pattern, rather than being expression of focal replacement fibrosis, may also represent the morphological correlate or the exitus of an inflammatory chronic process. De Cobelli et al. found LE in 70% of patients with chronic heart failure and histologically proven chronic myocarditis and midwall LE was the most frequent pattern of distribution observed in their series suggesting that IR-CE CMR may noninvasively identify areas of myocardial damage in patients presenting with chronic heart failure and no evidence of CAD, as an expression of a myocardial inflammatory process [106] (Figure 3).
Myocardial T1-mapping techniques have also been recently proposed for the depiction of diffuse myocardial fibrosis, undetectable with conventional IR-CE CMR techniques [107]. The basic principle relies on the shortening of T1-relaxation time of myocardial tissue which directly correlated with the amount of interstitial fibrosis with collagenous replacement [31].
Fast gradient-echo sequences using multiple increasing inversion times (e.g., 50–1,000 ms) before and after contrast-medium administration at the blood/myocardium equilibrium phase are performed for this purpose [108, 109].
More recently, the use of MR spectroscopy (MRS) has been proposed in the diagnostic workup of DCM [110]. The technique allows an in vivo noninvasive evaluation of myocardial metabolism without the need for contrast agents or radionuclides [111–116]. In CMR, hydrogen spectroscopy may be useful for assessing myocardial cellular triglyceride levels, whereas phosphorus has been used to measure myocardial energetics [117].
In a previous study, Neubauer et al. assessed 39 patients with phosphorus-31 (31P) myocardial spectroscopy and found a significantly reduced cardiovascular mortality for patients with PCr/ATP > 1.6 showing that this ratio offers significantly independent prognostic information on cardiovascular mortality [118, 119].
More recently, Dr. Beer et al. assessed the beneficial effect of the training using 31P MRS in a study population of 22 subjects and found a significant improvement in LV function without adverse effects in the metabolism further supporting the use of physical exercise as an adjunct therapy in DCM [115, 120, 121].
6. Clinical Indications
CMR has evolved in the last years from an effective research tool into a clinically proven, safe, and comprehensive modality with a large spectrum of applications.
According to current guidelines and recently redefined appropriateness criteria, the use of the exam is indicated to identify the etiology of cardiac dysfunction in patients presenting with heart failure when diagnosis is unclear with conventional tools or in the evaluation of dilated cardiomyopathy in the setting of normal coronary arteries [122–127].
Current clinical indications to the exam in DCM can be schematically summarized as follows:
differential diagnosis between ischaemic and non-ischaemic forms;
preimplantation of cardiac resynchronization therapy (CRT);
detection of intracavitary thrombi;
evaluation of global biventricular function (pre-treatment and follow-up);
differential diagnosis in nonischaemic forms;
prognostic stratification which is still regarded as an investigational field of interest in this clinical setting (see the next paragraph).
6.1. Differential Diagnosis between Ischaemic and Nonischaemic Forms
Coronary MR angiography (MRA) for the evaluation of CAD was proposed in several publications mostly during the late 90s representing a potentially ideal technique for this purpose due to its complete noninvasiveness (no ionizing radiation and no need to administer IV contrast agents) [128–135].
Although various MRA sequences have been proposed, the best technique is currently based on a free-breathing, navigator-gated, 3D segmented GRE sequence; more recently a whole-heart SSFP CMR approach which utilizes an inferior in-plane spatial resolution was implemented [33, 136–138].
Regardless of the initial promising results, current limited spatial resolution of the exam and the presence of in-plane and through-plane coronary motion during the acquisition period prevent accurate quantification of lumen narrowing and impede visualization of distal segments making the detection of coronary stenoses in middistal segments problematic and not suitable for surgical planning [139, 140].
In a previous prospective study including 189 patients assessed with both MRA and MDCT for CAD exclusion, Dacher et al. found a significantly higher sensitivity of MDCT for detection of stenoses >50% using selective coronary angiography as a reference standard (82% versus 52%, resp.) and concluded that MDCT is superior to MRI for coronary assessment [139, 141].
For these reasons, already in 2004 clinical recommendations, coronary MRA was considered a level III diagnostic technique (i.e., infrequently used because the information from other imaging techniques is usually adequate) for direct evaluation of CAD, and similar conclusions were drawn by more recently published guidelines and appropriateness criteria [123–125, 138, 142].
The strength of CMR in CAD exclusion is conversely attributable to the utilization of pharmacologically inducible ischemia tests which allow the depiction of rest-stress perfusional changes (CMR-perfusion) or induced wall motion abnormalities (WMA-CMR) unmasking hidden myocardial ischemia [143–151].
If compared to direct competitors like stress-echo and SPECT, CMR-perfusion offers higher spatial resolution, permitting direct visualization of subendocardial ischemia with stress first-pass contrast-enhanced techniques and combining perfusional findings with IR-CE sequences allowing direct identification of subtle subendocardial lesions which are usually not visible with nuclear medicine modalities [152–154].
Rest-stress perfusion MR images are usually evaluated with semiquantitative approaches, such as an upslope analysis of myocardial time-intensity curve, or with a visual assessment in which perfusion defects are depicted on stress sequences (usually after adenosine or dipyridamole IV infusion) as regionally hypointense subendocardial dark rims, not visible on corresponding rest dynamic scans [155–157].
WMA-CMR relies on utilization of IV dobutamine stimulation which activates B-receptors of the myocardium inducing increased inotropism, heart rate, and stroke-volume at the cost of increased oxygen consumption resembling physical exercise [158]. While non-ischemic myocardial tissue will show progress contractility increase during stress testing, ischemic segments will exhibit WMA related to the presence of a flow-limiting stenosis [143, 159].
The sensitivity and specificity of high-dose dobutamine stress MRI for detecting significant CAD were reported to be, respectively, 83% and 83% by Hundley et al. and 86% and 86% by Nagel et al. and represent a valid diagnostic option for the detection of CAD associated with DCM [160–165].
6.2. Preimplantation of Cardiac Resynchronization Therapy (CRT)
CRT is performed in patients presenting with heart failure and DCM to provide an improvement in clinical symptoms, LV performance, and the patient's outcome. The procedure is recommended in the presence of an ejection fraction (EF) equal to or less than 35% and QRS complex duration on ECG of 120 ms or greater [166, 167] and has shown to produce a major impact on overall patient's survival resulting in risk reduction in all-cause mortality of 31% at 3-year follow-up [168].
CRT devices are biventricular pacemakers with at least two leads located in the LV cavity and coronary sinus in order to, respectively, stimulate the interventricular septum and LV lateral wall and resynchronize a heart whose walls do not contract coherently, as it happens in 25–50% of heart failure cases.
Literature however reports that there is still a significant proportion of cases (more than 30%) who do not show favourable response to device implantation and the mechanisms advocated to explain those failures included the presence of tissue scar unresponsive to cardiac pacing [169, 170]. In this regard, CMR has emerged as a valuable tool allowing simultaneously evaluating ventricular dyssynchrony using cine-SSFP or tagging techniques [171, 172] and quantifing extent and location of tissue fibrosis with IR-CE techniques as explained above. An inverse relationship between tissue scar demonstrated by CMR and CRT responses was previously shown [173] and the exam has also proved to be useful for the depiction of coronary venous anatomy to guide optimal leads placement [174].
6.3. Detection of Intracavitary Thrombi
Thrombus formation occurs in the presence of the so-called Virchow's triad which includes a hypercoagulability status, hemodynamic changes (stasis, and turbulence), and endothelial injury/dysfunction [175] and represents a common complication of DCM, usually underestimated with transthoracic echocardiography. In addition, because of the lack of diagnostic criteria, differentiating subacute thrombi from organized thrombi on echocardiography—a distinction that is important in predicting the risk of embolic complications—is challenging.
CMR is the preferred diagnostic tool to recognize its presence which is depicted as a soft-tissue intracavitary lesion, nonenhancing on postcontrast IR-CE images and with a variable signal intensity on either T1 or T2 images [176–178].
The technique has shown an excellent sensitivity and specificity for LV thrombus detection after the infarction and is superior to both transthoracic and transoesophageal echocardiography [179].
6.4. Evaluation of Global Biventricular Function (Pretreatment and Follow-Up)
Evaluation of global ventricular systolic function with cine-SSFP imaging is currently regarded as the gold standard imaging technique, not affected by the geometric assumptions used in 2D echocardiography for the left ventricle (such as the area/length method).
Furthermore, the approximation in delineating endocardial border with CMR approach is considerably less than with 2D echocardiography minimizing operator dependence and intra- and inter-observer reproducibility variability [180, 181].
In DCM, LV ejection fraction is the strongest predictor of progression to heart failure, while LV volume and mass are independently correlated with mortality and morbidity; therefore accurate quantification of all these parameters is essential for adequate patient's evaluation and also to monitor progression of disease and response to different therapeutic agents. Except for the mildly dilated forms of the disease, both ventricular chambers show a moderate to severe degree of dilatation with a severely impaired ejection fraction (e.g., lower than 20%) which requires accurate volumetric quantification and geometric follow-up changes [182, 183].
Chamber enlargement is also associated with valvular insufficiency which can be assessed with phase-contrast sequences representing an accurate technique for quantifying the severity of valve regurgitation and for providing information on diastolic function [184, 185].
6.5. Differential Diagnosis in Nonischaemic Forms
Differentiation between various forms of nonischemic DCM is still a complex and partially investigational issue of CMR in which the unique tissue characterization capability of the exams might offer a significant diagnostic added contribution as compared to conventional diagnostic tools [186–190].
CMR, in general, allows characterization of acute versus chronic injuries using T2w-imaging and T2-mapping techniques (Figure 4), quantification of intramyocardial irondeposition with T2∗ techniques in patients with hemochromatosis, and provides data regarding necrosis/fibrosis with LGE representing a valuable noninvasive alternative to endomyocardial biopsy [191, 192].
Figure 4.

Cardiac magnetic resonance (CMR) exam performed in a 45-year-old patient with acute (5th day) myocarditis presenting a cardiomyopathic onset of disease. T2 short-tau inversion recovery image (T2-STIR) acquired on mid-ventricular short axis (a) shows subepicardial edematous imbibition of the anterior and inferolateral segment of the left ventricular myocardium (arrows) with corresponding late gadolinium enhancement with the same nonischemic pattern of distribution ((b)-(c)). Left ventricle appears moderately dilated (EDV: 203 mL); systolic function was depressed (EF: 32%).
A pattern-based approach of LGE was previously reported in literature [193] relying on the concept that the location (subendocardial, transmural, subepicardial, or mesocardial) and pattern (focal or diffuse) of abnormal LGE allow not only differentiating between ischemic (infarct-related) and nonischemic cardiomyopathies but also ruling out differential diagnoses in cases of nonischemic forms.
Images analysis should also include evaluation of extracardiac abnormalities including the presence of pleural effusion of azygos-superior vena cava dilation which may be indirect signs of overlapped right heart failure.
Mid- or subepicardial striae of LGE represent the typical feature of postmyocarditic forms of disease in which ventricular dilatation either may occur acutely after the inflammation as the consequence of the direct damage of the cardiomyocytes by the etiologic agent causing extensive myocardial injury [194–196] or may be the consequence of a left or biventricular remodelling that induced a chronic inflammatory stimulus mediated by targeting T cells (very often from an autoimmune process) resulting in a higher and prolonged disease activity leading to ventricular dysfunction [197, 198]. Depiction of active myocardial inflammation in DCM is important as these patients may benefit and favourably respond to immunomodulatory therapy (Figure 4).
Midwall interventricular striae of LGE have also been described in patients with secondary forms of disease related to drug toxicity and alcohol abuse and again likely represent areas of replacement fibrosis, which have been reported in pathologic samples and may be related to subclinical foci of myocardial ischemia (Figure 5) [87].
Figure 5.
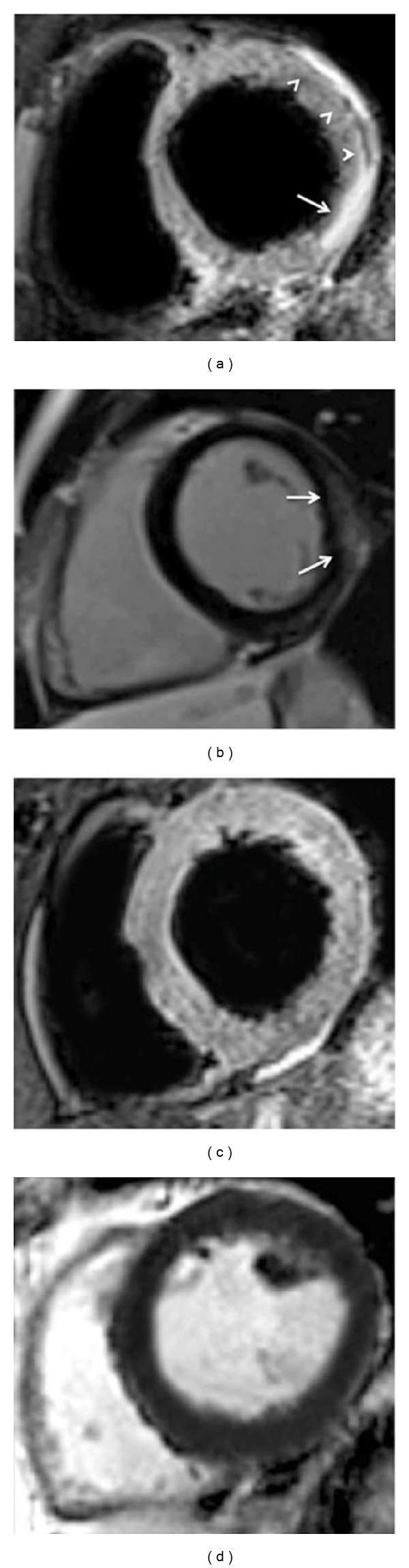
Anthracyclines induced dilated cardiomyopathy assessed with cardiac magnetic resonance at symptoms onset ((a), (b)) and eight months after drug suspension ((c), (d)). At clinical presentation, end-diastolic and end-systolic cine steady-state free-precession images show increased left ventricular volumes (EDV: 166 mL; ESV: 106 mL) with a reduced ejection fraction of 36% and a regional wall motion abnormality mostly involving apical segments. After contrast administration (b), patchy subtle areas of inhomogeneous late enhancement predominantly subepicardially distributed are depicted mostly involving the inferior and inferolateral wall of the left ventricle (arrows) and likely representing foci of replacement fibrosis related to the active inflammation associated with the drug's exposure. At follow-up ((c), (d)) after drug suspension, cine-MR shows significant recovery in global LV function (EDV 151.4 mL; ESV: 77 mL; EF: 49%) with reduced wall thickness (midseptal wall thickness from 13 mm to 10 mm). At late enhancement imaging (d), regional hyperintensity is no longer observed highly suggesting the healing of the process.
One remarkable limitation to mention regarding differential diagnosis of secondary forms of the disease concerns the widespread diffusion of tissue fibrosis which might be undepictable with CMR due to the fact that LGE sequences are designed to depict focal forms of tissue fibrosis and this explains the lack of pathological enhancement reported in most of DCM cases [87].
In this regard, T1- mapping techniques were recently proposed allowing to identify of increased interstitial accumulation of gadolinium at steady state in presence of diffuse forms of disease related to expansion of extracellular space with subsequent T1-relaxation time shortening [199–202].
Tissue fibrosis depicted with T1-mapping techniques positively correlates with the degree of ventricular dilatation, contractile, and diastolic dysfunction [31, 199, 203–205].
6.6. Prognostic Stratification
As already mentioned above, LGE is not only a marker of differentiation between primary and secondary forms of disease but may also provide intriguing information regarding patients' risk stratification [14].
There is a large series of papers in literature reporting that the presence of mid-wall striae of tissue enhancement is predictive of inducible ventricular tachycardia allowing the stratification of patient's risk and the selection of ideal candidate for ICD [75, 86, 182, 206, 207].
Assomull et al. also correlated LGE with mortality and cardiovascular events (HR, 3.4; CI, 1.4 to 8.7) and found that it was the best predictor of sudden cardiac death (HR, 5.4) [86].
The same observation was found by Wu et al. who reported that LGE predicted adverse outcomes in patients scheduled for ICD implantation with a higher event rate (heart failure, appropriate ICD discharge, and cardiac death, 44% versus 8%; P = 0.001; HR, 8.2; CI, 2.2 to 30.9; P = 0.002) [208].
More recently, however, Hombach et al. [209] did not reproduce the same results and found that midwall enhancement was not associated with an independent prognostic impact, highlighting the prominent still investigational nature of those studies requiring further clinical validation from large prospective dedicated trials.
In this regard, a large prospective longitudinal study of 472 patients with DCM with a median follow-up of 5.3 years was recently published providing evidence that the assessment of mid-wall fibrosis with LGE-CMR imaging was independent prognostic information beyond LVEF in patients with nonischemic dilated cardiomyopathy (HR, 2.43 (95% CI, 1.50–3.92); P < 0.001) [14].
7. Conclusions
CMR has progressed in the last decade from the status of an almost fully dedicated research tool into a clinically recognized diagnostic modality with clearly established guidelines and appropriateness criteria. At present, it can be considered the real gold standard technique for the evaluation of ventricular volumes and functions, the measurement of wall thickness, and quantification of ventricular masses. Although this information can be reliably obtained with echocardiography, its added value towards competing imaging modalities relies on its unique tissue characterization potentials allowing the characterization and differentiation of various forms of DCMs with potentially relevant therapeutic and prognostic implications.
Knowledge of location, extent, and distribution of tissue fibrosis also aids potential prediction of clinical outcomes with a growing prognostic evidence as shown by recent scientific literature.
Acknowledgment
Dr. Marco Francone gratefully acknowledges Gianluca De Rubeis medical student from the Sapienza University of Rome for his substantial support in the preparation of the present paper including bibliographic search and cases selection.
Conflict of Interests
The author declares that there is no conflict of interests regarding the publication of this paper.
References
- 1.Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. The New England Journal of Medicine. 1994;331(23):1564–1575. doi: 10.1056/NEJM199412083312307. [DOI] [PubMed] [Google Scholar]
- 2.Elliott P. Diagnosis and management of dilated cardiomyopathy. Heart. 2000;84(1):106–112. doi: 10.1136/heart.84.1.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Towbin JA, Bowles NE. Dilated cardiomyopathy: a tale of cytoskeletal proteins and beyond. Journal of Cardiovascular Electrophysiology. 2006;17(8):919–926. doi: 10.1111/j.1540-8167.2006.00530.x. [DOI] [PubMed] [Google Scholar]
- 4.Lakdawala NK, Winterfield JR, Funke BH. Dilated cardiomyopathy. Circulation. 2013;6:228–237. doi: 10.1161/CIRCEP.111.962050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanbe A. Dilated cardiomyopathy: a disease of the myocardium. Biological & Pharmaceutical Bulletin. 2013;36:18–22. doi: 10.1248/bpb.b212023. [DOI] [PubMed] [Google Scholar]
- 6.Jefferies JL, Towbin JA. Dilated cardiomyopathy. The Lancet. 2010;375(9716):752–762. doi: 10.1016/S0140-6736(09)62023-7. [DOI] [PubMed] [Google Scholar]
- 7.Charron P, Arad M, Arbustini E, et al. Genetic counselling and testing in cardiomyopathies: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. European Heart Journal. 2010;31(22):2715–2728. doi: 10.1093/eurheartj/ehq271. [DOI] [PubMed] [Google Scholar]
- 8.Slavich M, Florian A, Bogaert J. The emerging role of magnetic resonance imaging and multidetector computed tomography in the diagnosis of dilated cardiomyopathy. Insights into Imaging. 2011;2:453–469. doi: 10.1007/s13244-011-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stambader JD, Dorn L, Mikuz G, Sergi C. Genetic polymorphisms in dilated cardiomyopathy. Frontiers in Bioscience. 2010;2:653–676. doi: 10.2741/s92. [DOI] [PubMed] [Google Scholar]
- 10.Dellefave L, McNally EM. The genetics of dilated cardiomyopathy. Current Opinion in Cardiology. 2010;25(3):198–204. doi: 10.1097/HCO.0b013e328337ba52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNally EM, Golbus JR, Puckelwartz MJ. Genetic mutations and mechanisms in dilated cardiomyopathy. The Journal of Clinical Investigation. 2013;123(1):19–26. doi: 10.1172/JCI62862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimura A. Contribution of genetic factors to the pathogenesis of dilated cardiomyopathy: the cause of dilated cardiomyopathy: genetic or acquired? (genetic-side) Circulation Journal. 2011;75(7):1756–1765. doi: 10.1253/circj.cj-11-0368. [DOI] [PubMed] [Google Scholar]
- 13.Portig I, Wilke A, Freyland M, et al. Familial inflammatory dilated cardiomyopathy. European Journal of Heart Failure. 2006;8(8):816–825. doi: 10.1016/j.ejheart.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Gulati A, Jabbour A, Ismail TF, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. The Journal of the American Medical Association. 2013;309(9):896–908. doi: 10.1001/jama.2013.1363. [DOI] [PubMed] [Google Scholar]
- 15.Aukrust P, Yndestad A, Ueland T, Kristian Damås J, S. Frøland S, Gullestad L. The role of intravenous immunoglobulin in the treatment of chronic heart failure. International Journal of Cardiology. 2006;112(1):40–45. doi: 10.1016/j.ijcard.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Gregori D, Rosato R, Zecchin M, di Lenarda A. Use of bivariate survival curves for analyzing mortality of heart failure and sudden death in dilated cardiomiopathy. Epidemiologia e Pevenzione. 2005;29(2):106–109. [PubMed] [Google Scholar]
- 17.Arad M, Freimark D. Predicting prognosis in dilated cardiomyopathy. The Israel Medical Association Journal. 2012;14:687–689. [PubMed] [Google Scholar]
- 18.Adams KF, Jr., Dunlap SH, Sueta CA, et al. Relation between gender, etiology and survival in patients with symptomatic heart failure. Journal of the American College of Cardiology. 1997;28(7):1781–1788. doi: 10.1016/S0735-1097(96)00380-4. [DOI] [PubMed] [Google Scholar]
- 19.Bart BA, Shaw LK, McCants CB, Jr., et al. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. Journal of the American College of Cardiology. 1997;30(4):1002–1008. doi: 10.1016/s0735-1097(97)00235-0. [DOI] [PubMed] [Google Scholar]
- 20.Cooper GN., Jr. Left ventricular remodeling or restoration for congestive heart failure. Medicine and Health, Rhode Island. 2006;89(1):36–38. [PubMed] [Google Scholar]
- 21.Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. The New England Journal of Medicine. 2000;342(15):1077–1084. doi: 10.1056/NEJM200004133421502. [DOI] [PubMed] [Google Scholar]
- 22.Felker GM, Jaeger CJ, Klodas E, et al. Myocarditis and long-term survival in peripartum cardiomyopathy. American Heart Journal. 2000;140(5):785–791. doi: 10.1067/mhj.2000.110091. [DOI] [PubMed] [Google Scholar]
- 23.Itoh-Satoh M, Hayashi T, Nishi H, et al. Titin mutations as the molecular basis for dilated cardiomyopathy. Biochemical and Biophysical Research Communications. 2002;291(2):385–393. doi: 10.1006/bbrc.2002.6448. [DOI] [PubMed] [Google Scholar]
- 24.Olson TM, Illenberger S, Kishimoto NY, Huttelmaier S, Keating MT, Jockusch BM. Metavinculin mutations alter actin interaction in dilated cardiomyopathy. Circulation. 2002;105(4):431–437. doi: 10.1161/hc0402.102930. [DOI] [PubMed] [Google Scholar]
- 25.Schulz-Menger J, Friedrich MG. Magnetic resonance imaging in patients with cardiomyopathies: when and why. Herz. 2000;25(4):384–391. doi: 10.1007/s000590050030. [DOI] [PubMed] [Google Scholar]
- 26.Strohm O, Schulz-Menger J, Pilz B, Osterziel K-J, Dietz R, Friedrich MG. Measurement of left ventricular dimensions and function in patients with dilated cardiomyopathy. Journal of Magnetic Resonance Imaging. 2001;13(3):367–371. doi: 10.1002/jmri.1052. [DOI] [PubMed] [Google Scholar]
- 27.Francone M, Carbone I, Agati L, et al. Utility of T2-weighted short-tau inversion recovery (STIR) sequences in cardiac MRI: an overview of clinical applications in ischaemic and non-ischaemic heart disease. La Radiologia Medica. 2011;116(1):32–46. doi: 10.1007/s11547-010-0594-0. [DOI] [PubMed] [Google Scholar]
- 28.Bruder O, Wagner A, Jensen CJ, et al. Myocardial scar visualized by cardiovascular magnetic resonance imaging predicts major adverse events in patients with hypertrophic cardiomyopathy. Journal of the American College of Cardiology. 2010;56(11):875–887. doi: 10.1016/j.jacc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 29.Eitel I, Behrendt F, Schindler K, et al. Differential diagnosis of suspected apical ballooning syndrome using contrast-enhanced magnetic resonance imaging. European Heart Journal. 2008;29(21):2651–2659. doi: 10.1093/eurheartj/ehn433. [DOI] [PubMed] [Google Scholar]
- 30.Ordovas KG, Higgins CB. Delayed contrast enhancement on MR images of myocardium: past, present, future. Radiology. 2011;261(2):358–374. doi: 10.1148/radiol.11091882. [DOI] [PubMed] [Google Scholar]
- 31.Dass S, Suttie JJ, Piechnik SK, et al. Myocardial tissue characterization using magnetic resonance noncontrast t1 mapping in hypertrophic and dilated cardiomyopathy. Circulation. 2012;5:726–733. doi: 10.1161/CIRCIMAGING.112.976738. [DOI] [PubMed] [Google Scholar]
- 32.Leong DP, Chakrabarty A, Shipp N, et al. Effects of myocardial fibrosis and ventricular dyssynchrony on response to therapy in new-presentation idiopathic dilated cardiomyopathy: insights from cardiovascular magnetic resonance and echocardiography. European Heart Journal. 2012;33(5):640–648. doi: 10.1093/eurheartj/ehr391. [DOI] [PubMed] [Google Scholar]
- 33.Giesbrandt KJ, Bolan CW, Shapiro BP, Edwards WD, Mergo PJ. Diffuse diseases of the myocardium: MRI-pathologic review of cardiomyopathies with dilatation. American Journal of Roentgenology. 2013;200(3):W274–W282. doi: 10.2214/AJR.12.9634. [DOI] [PubMed] [Google Scholar]
- 34.Holmström M, Kivistö S, Heliö T, et al. Late gadolinium enhanced cardiovascular magnetic resonance of lamin A/C gene mutation related dilated cardiomyopathy. Journal of Cardiovascular Magnetic Resonance. 2011;13(1, article 30) doi: 10.1186/1532-429X-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masci PG, Andreini D, Francone M, et al. Prodromal angina is associated with myocardial salvage in acute ST-segment elevation myocardial infarction. European Heart Journal Cardiovascular Imaging. 2013;14(11):1041–1048. doi: 10.1093/ehjci/jet063. [DOI] [PubMed] [Google Scholar]
- 36.Codd MB, Sugrue DD, Gersh BJ, Melton LJ., III Epidemiology of idiopathic dilated and hypertrophic cardiomyopathy: a population-based study in Olmsted County, Minnesota, 1975–1984. Circulation. 1989;80(3):564–572. doi: 10.1161/01.cir.80.3.564. [DOI] [PubMed] [Google Scholar]
- 37.Dolara A, Cecchi F, Ciaccheri M. Cardiomyopathy in Italy today: extent of the problem. Giornale Italiano di Cardiologia. 1989;19(11):1074–1079. [PubMed] [Google Scholar]
- 38.Rakar S, Sinagra G, di Lenarda A, et al. Epidemiology of dilated cardiomyopathy. A prospective post-mortem study of 5252 necropsies. European Heart Journal. 1997;18(1):117–123. doi: 10.1093/oxfordjournals.eurheartj.a015092. [DOI] [PubMed] [Google Scholar]
- 39.Williams DG, Olsen EGJ. Prevalence of overt dilated cardiomyopathy in two regions of England. British Heart Journal. 1985;54(2):153–155. doi: 10.1136/hrt.54.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Towbin JA, Lowe AM, Colan SD, et al. Incidence, causes, and outcomes of dilated cardiomyopathy in children. The Journal of the American Medical Association. 2006;296(15):1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 41.Petretta M, Pirozzi F, Sasso L, Paglia A, Bonaduce D. Review and metaanalysis of the frequency of familial dilated cardiomyopathy. American Journal of Cardiology. 2011;108(8):1171–1176. doi: 10.1016/j.amjcard.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 42.Fairweather D, Cooper LT, Jr., Blauwet LA. Sex and gender differences in myocarditis and dilated cardiomyopathy. Current Problems in Cardiology. 2013;38(1):7–46. doi: 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tavazzi L. Epidemiology of dilated cardiomyopathy: a still undetermined entity. European Heart Journal. 1997;18(1):4–6. doi: 10.1093/oxfordjournals.eurheartj.a015116. [DOI] [PubMed] [Google Scholar]
- 44.Sparrow PJ, Merchant N, Provost YL, Doyle DJ, Nguyen ET, Paul NS. CT and MR imaging findings in patients with acquired heart disease at risk for sudden cardiac death. Radiographics. 2009;29(3):805–823. doi: 10.1148/rg.293085715. [DOI] [PubMed] [Google Scholar]
- 45.Taylor MRG, Carniel E, Mestroni L. Cardiomyopathy, familial dilated. Orphanet Journal of Rare Diseases. 2006;1(1, article 27) doi: 10.1186/1750-1172-1-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olbrich H-G. Epidemiology—etiology of dilated cardiomyopathy. Zeitschrift für Kardiologie. 2001;90(supplement 1):I2–I9. doi: 10.1007/s003920170052. [DOI] [PubMed] [Google Scholar]
- 47.Yoshikawa T, Baba A, Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circulation Journal. 2009;73(4):602–607. doi: 10.1253/circj.cj-08-1151. [DOI] [PubMed] [Google Scholar]
- 48.Obler D, Wu B-L, Lip V, et al. Familial dilated cardiomyopathy secondary to dystrophin splice site mutation. Journal of Cardiac Failure. 2010;16(3):194–199. doi: 10.1016/j.cardfail.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 49.Pankuweit S, Richter A, Ruppert V, Maisch B. Familial predisposition and microbial etiology in dilated cardiomyopathy. Herz. 2009;34(2):110–116. doi: 10.1007/s00059-009-3200-2. [DOI] [PubMed] [Google Scholar]
- 50.Cetta F, Michels VV. The natural history and spectrum of idiopathic dilated cardiomyopathy, including HIV and peripartum cardiomyopathy. Current Opinion in Cardiology. 1995;10(3):332–338. doi: 10.1097/00001573-199505000-00015. [DOI] [PubMed] [Google Scholar]
- 51.Yan J-R, Xie L-J, Huang M. Progress of genetics in familial dilated cardiomyopathy. Chinese Journal of Pediatrics. 2010;48(12):919–921. [PubMed] [Google Scholar]
- 52.Fatkin D, Otway R, Richmond Z. Genetics of dilated cardiomyopathy. Heart Failure Clinics. 2010;6(2):129–140. doi: 10.1016/j.hfc.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 53.Piano MR. Alcoholic cardiomyopathy: incidence, clinical characteristics, and pathophysiology. Chest. 2002;121(5):1638–1650. doi: 10.1378/chest.121.5.1638. [DOI] [PubMed] [Google Scholar]
- 54.Awtry EH, Philippides GJ. Alcoholic and cocaine-associated cardiomyopathies. Progress in Cardiovascular Diseases. 2010;52(4):289–299. doi: 10.1016/j.pcad.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 55.George A, Figueredo VM. Alcoholic cardiomyopathy: a review. Journal of Cardiac Failure. 2011;17(10):844–849. doi: 10.1016/j.cardfail.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Hens L, Dambrink JH. Alcohol and drugs: twins or evil in a young heart. Acta Cardiologica. 2012;67:469–471. doi: 10.1080/ac.67.4.2170691. [DOI] [PubMed] [Google Scholar]
- 57.Feldman J, Auer J, Berent R, et al. Cardiovascular complications of cocaine use. The New England Journal of Medicine. 2001;345(21):1575–1576. [PubMed] [Google Scholar]
- 58.Mizia-Stec K, Gasior Z, Wojnicz R, et al. Severe dilated cardiomyopathy as a consequence of Ecstasy intake. Cardiovascular Pathology. 2008;17(4):250–253. doi: 10.1016/j.carpath.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 59.Yeh ETH, Tong AT, Lenihan DJ, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004;109(25):3122–3131. doi: 10.1161/01.CIR.0000133187.74800.B9. [DOI] [PubMed] [Google Scholar]
- 60.Güler GB, Karaahmet T, Tigen K. Myocardial fibrosis detected by cardiac magnetic resonance imaging in heart failure: impact on remodeling, diastolic function and BNP levels. Anadolu Kardiyoloji Dergisi. 2011;11(1):71–76. doi: 10.5152/akd.2011.013. [DOI] [PubMed] [Google Scholar]
- 61.Kubanek M, Sramko M, Maluskova J, et al. Novel predictors of left ventricular reverse remodeling in individuals with recent-onset dilated cardiomyopathy. Journal of the American College of Cardiology. 2013;61(1):54–63. doi: 10.1016/j.jacc.2012.07.072. [DOI] [PubMed] [Google Scholar]
- 62.Nishiyama K, Tsutamoto T, Kawahara C, et al. Relationship between biological variation in B-type natriuretic peptide and plasma renin concentration in stable outpatients with dilated cardiomyopathy. Circulation Journal. 2011;75(8):1897–1904. doi: 10.1253/circj.cj-10-1083. [DOI] [PubMed] [Google Scholar]
- 63.Elkayam U, Tummala PP, Rao K, et al. Maternal and fetal outcomes of subsequent pregnancies in women with peripartum cardiomyopathy. The New England Journal of Medicine. 2001;344(21):1567–1571. doi: 10.1056/NEJM200105243442101. [DOI] [PubMed] [Google Scholar]
- 64.Capriola M. Peripartum cardiomyopathy: a review. International Journal of Women’s Health. 2013;5:1–8. doi: 10.2147/IJWH.S37137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang GY, Zhang LY, Long-Le MA, Wang L-X. Clinical characteristics and risk factors for peripartum cardiomyopathy. African Health Sciences. 2012;12(1):26–31. [PMC free article] [PubMed] [Google Scholar]
- 66.Warraich RS, Sliwa K, Damasceno A, et al. Impact of pregnancy-related heart failure on humoral immunity: clinical relevance of G3-subclass immunoglobulins in peripartum cardiomyopathy. American Heart Journal. 2005;150(2):263–269. doi: 10.1016/j.ahj.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 67.Suri V, Aggarwal N, Kalpdev A, Chopra S, Sikka P, Vijayvergia R. Pregnancy with dilated and peripartum cardiomyopathy: maternal and fetal outcome. Archives of Gynecology and Obstetrics. 2013;287(2):195–199. doi: 10.1007/s00404-012-2543-8. [DOI] [PubMed] [Google Scholar]
- 68.Hong YM. Cardiomyopathies in children. Korean Journal of Pediatrics. 2013;56(2):52–59. doi: 10.3345/kjp.2013.56.2.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hsu DT, Canter CE. Dilated cardiomyopathy and heart failure in children. Heart Failure Clinics. 2010;6(4):415–432. doi: 10.1016/j.hfc.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 70.Dorn GW., II Apoptotic and non-apoptotic programmed cardiomyocyte death in ventricular remodelling. Cardiovascular Research. 2009;81(3):465–473. doi: 10.1093/cvr/cvn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Barison A, Masci PG, Emdin M. Fibrosis and mortality in patients with dilated cardiomyopathy. The Journal of The American Medical Association. 2013;309(24):2547–2549. doi: 10.1001/jama.2013.6453. [DOI] [PubMed] [Google Scholar]
- 72.Zhong L, Ghista DN, Tan RS. Left ventricular wall stress compendium. Computer Methods in Biomechanics and Biomedical Engineering. 2012;15(10):1015–1041. doi: 10.1080/10255842.2011.569885. [DOI] [PubMed] [Google Scholar]
- 73.Goldstein S, Ali AS, Sabbah H. Ventricular remodeling: mechanisms and prevention. Cardiology Clinics. 1998;16(4):623–632. doi: 10.1016/s0733-8651(05)70039-4. [DOI] [PubMed] [Google Scholar]
- 74.Pahl E, Sleeper LA, Canter CE, et al. Incidence of and risk factors for sudden cardiac death in children with dilated cardiomyopathy: a report from the pediatric cardiomyopathy registry. Journal of the American College of Cardiology. 2012;59(6):607–615. doi: 10.1016/j.jacc.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Okutucu S, Oto A. Risk stratification in nonischemic dilated cardiomyopathy: current perspectives. Cardiology Journal. 2010;17(3):219–229. [PubMed] [Google Scholar]
- 76.Patterson JH, Adams KE., Jr. Pathophysiology of heart failure: changing perceptions. Pharmacotherapy. 1996;16(2):27S–36S. [PubMed] [Google Scholar]
- 77.Kluge J-G, Jurisch D, Tarr A, Hagendorff A, Pfeiffer D. Right atrial free-floating thrombus in dilated cardiomyopathy. European Journal of Echocardiography. 2011;12(10, article 798) doi: 10.1093/ejechocard/jer101. [DOI] [PubMed] [Google Scholar]
- 78.Beltrami CA, Finato N, Rocco M, et al. The cellular basis of dilated cardiomyopathy in humans. Journal of Molecular and Cellular Cardiology. 1995;27(1):291–305. doi: 10.1016/s0022-2828(08)80028-4. [DOI] [PubMed] [Google Scholar]
- 79.de Smet K, Verdries D, Tanaka K, de Mey J, de Maeseneer M. MRI in the assessment of non ischemic myocardial diseases. European Journal of Radiology. 2012;81(7):1546–1548. doi: 10.1016/j.ejrad.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 80.Vigliano CA, Cabeza Meckert PM, Diez M, et al. Cardiomyocyte hypertrophy, oncosis, and autophagic vacuolization predict mortality in idiopathic dilated cardiomyopathy with advanced heart failure. Journal of the American College of Cardiology. 2011;57(14):1523–1531. doi: 10.1016/j.jacc.2010.09.080. [DOI] [PubMed] [Google Scholar]
- 81.Schaper J, Froede R, Hein S, et al. Impairment of the myocardial ultrastructure and changes of the cytoskeleton in dilated cardiomyopathy. Circulation. 1991;83(2):504–514. doi: 10.1161/01.cir.83.2.504. [DOI] [PubMed] [Google Scholar]
- 82.Yarbrough WM, Mukherjee R, Stroud RE, et al. Progressive induction of left ventricular pressure overload in a large animal model elicits myocardial remodeling and a unique matrix signature. Journal of Thoracic and Cardiovascular Surgery. 2012;143(1):215–223. doi: 10.1016/j.jtcvs.2011.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Du Y, Yan L, Wang J, et al. β 1-Adrenoceptor autoantibodies from DCM patients enhance the proliferation of T lymphocytes through the beta1-AR/cAMP/PKA and p38 MAPK pathways. PLoS ONE. 2012;7(12) doi: 10.1371/journal.pone.0052911.e52911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 85.Wu T-J, Ong JJC, Hwang C, et al. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. Journal of the American College of Cardiology. 1998;32(1):187–196. doi: 10.1016/s0735-1097(98)00184-3. [DOI] [PubMed] [Google Scholar]
- 86.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. Journal of the American College of Cardiology. 2006;48(10):1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 87.McCrohon JA, Moon JCC, Prasad SK, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 88.Nanjo S, Yoshikawa K, Harada M, et al. Correlation between left ventricular diastolic function and ejection fraction in dilated cardiomyopathy using magnetic resonance imaging with late gadolinium enhancement. Circulation Journal. 2009;73(10):1939–1944. doi: 10.1253/circj.cj-08-0965. [DOI] [PubMed] [Google Scholar]
- 89.Semelka RC, Tomei E, Wagner S, et al. Normal left ventricular dimensions and function: interstudy reproducibility of measurements with cine MR imaging. Radiology. 1990;174(3):763–768. doi: 10.1148/radiology.174.3.2305059. [DOI] [PubMed] [Google Scholar]
- 90.Buser PT, Wagner S, Auffermann W, et al. Three-dimensional analysis of regional contractile performance of the normal and cardiomyopathic left ventricle using cine magnetic resonance imaging. Zeitschrift für Kardiologie. 1990;79(8):573–579. [PubMed] [Google Scholar]
- 91.Doherty NE, III, Fujita N, Caputo GR, Higgins CB. Measurement of right ventricular mass in normal and dilated cardiomyopathic ventricles using cine magnetic resonance imaging. American Journal of Cardiology. 1992;69(14):1223–1228. doi: 10.1016/0002-9149(92)90940-z. [DOI] [PubMed] [Google Scholar]
- 92.Doherty NE, III, Seelos KC, Suzuki J-I, et al. Application of cine nuclear magnetic resonance imaging for sequential evaluation of response to angiotensin-converting enzyme inhibitor therapy in dilated cardiomyopathy. Journal of the American College of Cardiology. 1992;19(6):1294–1302. doi: 10.1016/0735-1097(92)90337-m. [DOI] [PubMed] [Google Scholar]
- 93.Quarta G, Sado DM, Moon JC. Cardiomyopathies: focus on cardiovascular magnetic resonance. The British Journal of Radiology. 2011;84(3):S296–S305. doi: 10.1259/bjr/67212179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Masci PG, Dymarkowski S, Bogaert J. The role of cardiovascular magnetic resonance in the diagnosis and management of cardiomyopathies. Journal of Cardiovascular Medicine. 2008;9(5):435–449. doi: 10.2459/JCM.0b013e32827ab49f. [DOI] [PubMed] [Google Scholar]
- 95.Peacock AJ, Crawley S, McLure L, et al. Changes in right ventricular function measured by cardiac magnetic resonance imaging in patients receiving pulmonary arterial hypertension-targeted therapy: the EURO-MR study. Circulation. 2013 doi: 10.1161/CIRCIMAGING.113.000629. [DOI] [PubMed] [Google Scholar]
- 96.Sörensson P, Heiberg E, Saleh N, et al. Assessment of myocardium at risk with contrast enhanced steady-state free precession cine cardiovascular magnetic resonance compared to single-photon emission computed tomography. Journal of Cardiovascular Magnetic Resonance. 2010;12(1, article 25) doi: 10.1186/1532-429X-12-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Laissy J-P, Hyafil F, Huart V, et al. Value of contrast-enhanced, balanced cine-MR sequences in the assessment of apparent infarct size after acute myocardial infarction: a prospective comparison with delayed-enhancement sequences. Journal of Magnetic Resonance Imaging. 2005;22(6):765–771. doi: 10.1002/jmri.20443. [DOI] [PubMed] [Google Scholar]
- 98.Aletras AH, Kellman P, Derbyshire JA, Arai AE. ACUT2E TSE-SSFP: a hybrid method for T2-weighted imaging of edema in the heart. Magnetic Resonance in Medicine. 2008;59(2):229–235. doi: 10.1002/mrm.21490. [DOI] [PubMed] [Google Scholar]
- 99.Masci PG, Francone M, Desmet W, et al. Right ventricular ischemic injury in patients with acute ST-segment elevation myocardial infarction: characterization with cardiovascular magnetic resonance. Circulation. 2010;122(14):1405–1412. doi: 10.1161/CIRCULATIONAHA.110.940254. [DOI] [PubMed] [Google Scholar]
- 100.Abdel-Aty H, Boyé P, Zagrosek A, et al. Diagnostic performance of cardiovascular magnetic resonance in patients with suspected acute myocarditis: comparison of different approaches. Journal of the American College of Cardiology. 2005;45(11):1815–1822. doi: 10.1016/j.jacc.2004.11.069. [DOI] [PubMed] [Google Scholar]
- 101.Abdel-Aty H, Simonetti O, Friedrich MG. T2-weighted cardiovascular magnetic resonance imaging. Journal of Magnetic Resonance Imaging. 2007;26(3):452–459. doi: 10.1002/jmri.21028. [DOI] [PubMed] [Google Scholar]
- 102.Cocker MS, Shea SM, Strohm O, Green J, Abdel-Aty H, Friedrich MG. A New approach towards improved visualization of myocardial edema using T2-weighted imaging: a cardiovascular magnetic resonance (CMR) study. Journal of Magnetic Resonance Imaging. 2011;34(2):286–292. doi: 10.1002/jmri.22622. [DOI] [PubMed] [Google Scholar]
- 103.Iacucci I, Carbone I, Cannavale G, et al. Myocardial oedema as the sole marker of acute injury in Takotsubo cardiomyopathy: a Cardiovascular Magnetic Resonance (CMR) study. La Radiologia Medica. 2013;118(8):1309–1323. doi: 10.1007/s11547-013-0931-1. [DOI] [PubMed] [Google Scholar]
- 104.Breuckmann F, Maderwald S, Buhr C, et al. Cardiac MRI: estimation of changes in normalized myocardial gadolinium accumulation over time after contrast injection in patients with acute myocarditis and healthy volunteers. RöFo. 2011;183(10):933–938. doi: 10.1055/s-0031-1281636. [DOI] [PubMed] [Google Scholar]
- 105.Bello D, Shah DJ, Farah GM, et al. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing β-blocker therapy. Circulation. 2003;108(16):1945–1953. doi: 10.1161/01.CIR.0000095029.57483.60. [DOI] [PubMed] [Google Scholar]
- 106.De Cobelli F, Pieroni M, Esposito A, et al. Delayed gadolinium-enhanced cardiac magnetic resonance in patients with chronic myocarditis presenting with heart failure or recurrent arrhythmias. Journal of the American College of Cardiology. 2006;47(8):1649–1654. doi: 10.1016/j.jacc.2005.11.067. [DOI] [PubMed] [Google Scholar]
- 107.Liu CY, Liu YC, Wu C, et al. Evaluation of age-related interstitial myocardial fibrosis with cardiac magnetic resonance contrast-enhanced T1 mapping: MESA (Multi-Ethnic Study of Atherosclerosis) Journal of the American College of Cardiology. 2013;62(14):1280–1287. doi: 10.1016/j.jacc.2013.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Doltra A, Stawowy P, Dietrich T, Schneeweis C, Fleck E, Kelle S. Magnetic resonance imaging of cardiovascular fibrosis and inflammation: from clinical practice to animal studies and back. BioMed Research International. 2013;2013:10 pages. doi: 10.1155/2013/676489.676489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Raman FS, Kawel-Boehm N, Gai N, et al. Modified look-locker inversion recovery T1 mapping indices: assessment of accuracy and reproducibility between magnetic resonance scanners. Journal of Cardiovascular Magnetic Resonance. 2013;15, article 64 doi: 10.1186/1532-429X-15-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Neubauer S, Krahe T, Schindler R, et al. 31P magnetic resonance spectroscopy in dilated cardiomyopathy and coronary artery disease: altered cardiac high-energy phosphate metabolism in heart failure. Circulation. 1992;86(6):1810–1818. doi: 10.1161/01.cir.86.6.1810. [DOI] [PubMed] [Google Scholar]
- 111.Beer M, Buchner S, Wirbelauer J, et al. MR imaging and MR spectroscopy for characterization of cardiomyopathies in adolescents—preliminary results. RöFo. 2007;179(9):932–937. doi: 10.1055/s-2007-963302. [DOI] [PubMed] [Google Scholar]
- 112.Sardanelli F, Quarenghi M. MR spectroscopy of the heart. La Radiologia Medica. 2006;111(8):1025–1034. doi: 10.1007/s11547-006-0102-8. [DOI] [PubMed] [Google Scholar]
- 113.Nakae I, Mitsunami K, Matsuo S, Horie M. Creatine depletion and altered fatty acid metabolism in diseased human hearts: clinical investigation using 1H magnetic resonance spectroscopy and 123I BMIPP myocardial scintigraphy. Acta Radiologica. 2007;48(4):436–443. doi: 10.1080/02841850701280809. [DOI] [PubMed] [Google Scholar]
- 114.Chida K, Otani H, Saito H, et al. Feasibility of rapid-sequence 31P magnetic resonance spectroscopy in cardiac patients. Acta Radiologica. 2005;46(4):386–390. doi: 10.1080/02841850510021283. [DOI] [PubMed] [Google Scholar]
- 115.Beer M, Wagner D, Myers J, et al. Effects of exercise training on myocardial energy metabolism and ventricular function assessed by quantitative phosphorus-31 magnetic resonance spectroscopy and magnetic resonance imaging in dilated cardiomyopathy. Journal of the American College of Cardiology. 2008;51(19):1883–1891. doi: 10.1016/j.jacc.2007.09.075. [DOI] [PubMed] [Google Scholar]
- 116.Schaefer S. Magnetic resonance spectroscopy in human cardiomyopathies. Journal of Cardiovascular Magnetic Resonance. 2000;2(2):151–157. doi: 10.3109/10976640009148685. [DOI] [PubMed] [Google Scholar]
- 117.von Kienlin M. Cardiac 1H-MR spectroscopy. Magma. 1998;6(2-3):107–108. doi: 10.1016/s1352-8661(98)00030-1. [DOI] [PubMed] [Google Scholar]
- 118.Neubauer S, Horn M, Cramer M, et al. Myocardial phosphocreatine-to-ATP ratio is a predictor of mortality in patients with dilated cardiomyopathy. Circulation. 1997;96(7):2190–2196. doi: 10.1161/01.cir.96.7.2190. [DOI] [PubMed] [Google Scholar]
- 119.The next horizon in CMR: spectroscopy. Journal of Cardiovascular Magnetic Resonance. 1999;1(3):9–10. doi: 10.3109/10976649909088331. [DOI] [PubMed] [Google Scholar]
- 120.Schneider-Gold C, Beer M, Köstler H, et al. Cardiac and skeletal muscle involvement in myotonic dystrophy type 2 (DM2): a quantitative 31P-MRS and MRI study. Muscle and Nerve. 2004;30(5):636–644. doi: 10.1002/mus.20156. [DOI] [PubMed] [Google Scholar]
- 121.Löffler R, Sauter R, Kolem H, Haase A, Von Kienlin M. Localized spectroscopy from anatomically matched compartments: improved sensitivity and localization for cardiac 31P MRS in humans. Journal of Magnetic Resonance. 1998;134(2):287–299. doi: 10.1006/jmre.1998.1497. [DOI] [PubMed] [Google Scholar]
- 122.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR, 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. Journal of the American College of Cardiology. 48(3):1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 123.di Cesare E, Cademartiri F, Carbone I, et al. Clinical indications for the use of cardiac MRI. By the SIRM Study Group on Cardiac Imaging. La Radiologia Medica. 2012;118(5):752–798. doi: 10.1007/s11547-012-0899-2. [DOI] [PubMed] [Google Scholar]
- 124.Beanlands RSB, Chow BJW, Dick A, et al. CCS/CAR/CANM/CNCS/CanSCMR joint position statement on advanced noninvasive cardiac imaging using positron emission tomography, magnetic resonance imaging and multidetector computed tomographic angiography in the diagnosis and evaluation of ischemic heart disease—executive summary. Canadian Journal of Cardiology. 2007;23(2):107–119. doi: 10.1016/s0828-282x(07)70730-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hundley WG, Bluemke D, Bogaert JG, et al. Society for Cardiovascular Magnetic Resonance guidelines for reporting cardiovascular magnetic resonance examinations. Journal of Cardiovascular Magnetic Resonance. 2009;11(1, article 5) doi: 10.1186/1532-429X-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shi HW, Pu P, Deng W, et al. Prognostic value of late gadolinium enhancement in dilated cardiomyopathy patients. A meta-analysis. Saudi Medical Journal. 2013;34(7):719–726. [PubMed] [Google Scholar]
- 127.Fatkin D. Guidelines for the diagnosis and management of familial dilated cardiomyopathy. Heart Lung and Circulation. 2011;20(11):691–693. doi: 10.1016/j.hlc.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 128.di Renzi P, Gaudio C, di Cesare E, Tanzilli G, Passariello R, Campa PP. Imaging of atherosclerosis: magnetic resonance. Cardiologia. 1993;38(12):21–26. [PubMed] [Google Scholar]
- 129.Stehning C, Boernert P, Nehrke K. Advances in coronary MRA from vessel wall to whole heart imaging. Magnetic Resonance in Medical Sciences. 2007;6(3):157–170. doi: 10.2463/mrms.6.157. [DOI] [PubMed] [Google Scholar]
- 130.Stuber M, Weiss RG. Coronary magnetic resonance angiography. Journal of Magnetic Resonance Imaging. 2007;26(2):219–234. doi: 10.1002/jmri.20949. [DOI] [PubMed] [Google Scholar]
- 131.Hartnell GG. Breathhold cardiac MRI and MRA. International Journal of Cardiac Imaging. 1999;15(2):139–150. doi: 10.1023/a:1006138713163. [DOI] [PubMed] [Google Scholar]
- 132.Lorenz CH, Johansson LO. Contrast-enhanced coronary MRA. Journal of Magnetic Resonance Imaging. 1999;10:703–708. doi: 10.1002/(sici)1522-2586(199911)10:5<703::aid-jmri13>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 133.Bunce NH, Pennell DJ. Coronary MRA—a clinical experience in Europe. Journal of Magnetic Resonance Imaging. 1999;10:721–727. doi: 10.1002/(sici)1522-2586(199911)10:5<721::aid-jmri16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 134.Dirksen MS, Lamb HJ, Doornbos J, Bax JJ, Jukema JW, de Roos A. Coronary magnetic resonance angiography: technical developments and clinical applications. Journal of Cardiovascular Magnetic Resonance. 2003;5(2):365–386. doi: 10.1081/jcmr-120019419. [DOI] [PubMed] [Google Scholar]
- 135.Dirksen MS, Kaandorp TAM, Lamb HJ, Doornbos J, Corot C, de Roos A. Three-dimensional navigator coronary MRA with the aid of a blood pool agent in pigs: improved image quality with inclusion of the contrast agent first-pass. Journal of Magnetic Resonance Imaging. 2003;18(4):502–506. doi: 10.1002/jmri.10389. [DOI] [PubMed] [Google Scholar]
- 136.Sakuma H, Ichikawa Y, Suzawa N, et al. Assessment of coronary arteries with total study time of less than 30 minutes by using whole-heart coronary MR angiography. Radiology. 2005;237(1):316–321. doi: 10.1148/radiol.2371040830. [DOI] [PubMed] [Google Scholar]
- 137.D’Anastasi M, Greif M, Reiser MF, Theisen D. Magnetic resonance imaging of dilated cardiomyopathy. Radiologe. 2013;53(1):24–29. doi: 10.1007/s00117-012-2382-4. [DOI] [PubMed] [Google Scholar]
- 138.Assomull RG, Shakespeare C, Kalra PR, et al. Role of cardiovascular magnetic resonance as a gatekeeper to invasive coronary angiography in patients presenting with heart failure of unknown etiology. Circulation. 2011;124(12):1351–1360. doi: 10.1161/CIRCULATIONAHA.110.011346. [DOI] [PubMed] [Google Scholar]
- 139.Dacher J-N, Bertrand D, Gahide G, Tron C, Manrique A. Indications for MRI in coronary disease. Presse Medicale. 2008;37(4):716–723. doi: 10.1016/j.lpm.2007.07.037. [DOI] [PubMed] [Google Scholar]
- 140.Nikolaou K, Alkadhi H, Bamberg F, Leschka S, Wintersperger BJ. MRI and CT in the diagnosis of coronary artery disease: indications and applications. Insights into Imaging. 2011;2(1):9–24. doi: 10.1007/s13244-010-0049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dewey M, Teige F, Schnapauff D, et al. Noninvasive detection of coronary artery stenoses with multislice computed tomography or magnetic resonance imaging. Annals of Internal Medicine. 2006;145(6):407–415. doi: 10.7326/0003-4819-145-6-200609190-00004. [DOI] [PubMed] [Google Scholar]
- 142.Pennell DJ, Sechtem UP, Higgins CB, et al. Clinical indications for cardiovascular magnetic resonance (CMR): consensus panel report. Journal of Cardiovascular Magnetic Resonance. 2004;6(4):727–765. doi: 10.1081/jcmr-200038581. [DOI] [PubMed] [Google Scholar]
- 143.Wahl A, Paetsch I, Gollesch A, et al. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: experience in 1000 consecutive cases. European Heart Journal. 2004;25(14):1230–1236. doi: 10.1016/j.ehj.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 144.Kramer CM, Hundley WG. Steadily straining toward clinical utility: real-time quantitative CMR of myocardial deformation during stress. Journal of the American College of Cardiology. 2010;3(4):372–374. doi: 10.1016/j.jcmg.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Heilmaier C, Bruder O, Meier F, et al. Dobutamine stress cardiovascular magnetic resonance imaging in patients after invasive coronary revascularization with stent placement. Acta Radiologica. 2009;50(10):1134–1141. doi: 10.3109/02841850903216692. [DOI] [PubMed] [Google Scholar]
- 146.Bruder O, Schneider S, Nothnagel D, et al. EuroCMR (European Cardiovascular Magnetic Resonance) registry. Results of the German pilot phase. Journal of the American College of Cardiology. 2009;54(15):1457–1466. doi: 10.1016/j.jacc.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 147.Blankstein R, Shturman LD, Rogers IS, et al. Adenosine-induced stress myocardial perfusion imaging using dual-source cardiac computed tomography. Journal of the American College of Cardiology. 2009;54(12):1072–1084. doi: 10.1016/j.jacc.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 148.Bodi V, Sanchis J, Lopez-Lereu MP, et al. Prognostic and therapeutic implications of dipyridamole stress cardiovascular magnetic resonance on the basis of the ischaemic cascade. Heart. 2009;95(1):49–55. doi: 10.1136/hrt.2007.139683. [DOI] [PubMed] [Google Scholar]
- 149.Miller CD, Hwang W, Hoekstra JW, et al. Stress cardiac magnetic resonance imaging with observation unit care reduces cost for patients with emergent chest pain: a randomized trial. Annals of Emergency Medicine. 2010;56(3):209.e2–219.e2. doi: 10.1016/j.annemergmed.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Florian A, Jurcut R, Ginghina C, Bogaert J. Cardiac magnetic resonance imaging in ischemic heart disease: a clinical review. Journal of Medicine and Life. 2011;4(4):330–345. [PMC free article] [PubMed] [Google Scholar]
- 151.Schuster A, Morton G, Chiribiri A, Perera D, Vanoverschelde J-L, Nagel E. Imaging in the management of ischemic cardiomyopathy: special focus on magnetic resonance. Journal of the American College of Cardiology. 2012;59(4):359–370. doi: 10.1016/j.jacc.2011.08.076. [DOI] [PubMed] [Google Scholar]
- 152.Wagner A, Mahrholdt H, Holly TA, et al. Contrast-enhanced MRI and routine Single Photon Emission Computed Tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study. The Lancet. 2003;361(9355):374–379. doi: 10.1016/S0140-6736(03)12389-6. [DOI] [PubMed] [Google Scholar]
- 153.Wang L, Yan C, Zhao S, Fang W. Comparison of, (99m)Tc-MIBI SPECT/18F-FDG PET imaging and cardiac magnetic resonance imaging in patients with idiopathic dilated cardiomyopathy: assessment of cardiac function and myocardial injury. Clinical Nuclear Medicine. 2012;37(12):1163–1169. doi: 10.1097/RLU.0b013e3182708794. [DOI] [PubMed] [Google Scholar]
- 154.Xie B-Q, Tian Y-Q, Zhang J, et al. Evaluation of left and right ventricular ejection fraction and volumes from gated blood-pool SPECT in patients with dilated cardiomyopathy: comparison with cardiac MRI. Journal of Nuclear Medicine. 2012;53(4):584–591. doi: 10.2967/jnumed.111.096057. [DOI] [PubMed] [Google Scholar]
- 155.Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. European Heart Journal. 2008;29(4):480–489. doi: 10.1093/eurheartj/ehm617. [DOI] [PubMed] [Google Scholar]
- 156.Wolff SD, Schwitter J, Coulden R, et al. Myocardial first-pass perfusion magnetic resonance imaging: a multicenter dose-ranging study. Circulation. 2004;110(6):732–737. doi: 10.1161/01.CIR.0000138106.84335.62. [DOI] [PubMed] [Google Scholar]
- 157.Cannan C, Friedrich MG. Cardiac magnetic resonance imaging: current status and future directions. Expert Review of Cardiovascular Therapy. 2010;8(8):1175–1189. doi: 10.1586/erc.10.46. [DOI] [PubMed] [Google Scholar]
- 158.Nandalur KR, Dwamena BA, Choudhri AF, Nandalur MR, Carlos RC. Diagnostic performance of stress cardiac magnetic resonance imaging in the detection of coronary artery disease. A meta-analysis. Journal of the American College of Cardiology. 2007;50(14):1343–1353. doi: 10.1016/j.jacc.2007.06.030. [DOI] [PubMed] [Google Scholar]
- 159.Strach K, Meyer C, Schild H, Sommer T. Cardiac stress MR imaging with dobutamine. European Radiology. 2006;16(12):2728–2738. doi: 10.1007/s00330-006-0295-1. [DOI] [PubMed] [Google Scholar]
- 160.Hundley WG, Hamilton CA, Thomas MS, et al. Utility of fast cine magnetic resonance imaging and display for the detection of myocardial ischemia in patients not well suited for second harmonic stress echocardiography. Circulation. 1999;100(16):1697–1702. doi: 10.1161/01.cir.100.16.1697. [DOI] [PubMed] [Google Scholar]
- 161.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high-dose dobutamine stress MRI: comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–770. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 162.Nagel E, Lehmkuhl HB, Klein C, et al. Influence of image quality on the diagnostic accuracy of dobutamine stress magnetic resonance imaging in comparison with dobutamine stress echocardiography for the non-invasive detection of myocardial ischemia. Zeitschrift für Kardiologie. 1999;88(9):622–630. doi: 10.1007/s003920050337. [DOI] [PubMed] [Google Scholar]
- 163.Lipinski MJ, McVey CM, Berger JS, Kramer CM, Salerno M. Prognostic value of stress cardiac magnetic resonance imaging in patients with known or suspected coronary artery disease: a systematic review and meta-analysis. Journal of the American College of Cardiology. 2013;62(9):826–838. doi: 10.1016/j.jacc.2013.03.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kelle S, Nagel E, Voss A, et al. A bi-center cardiovascular magnetic resonance prognosis study focusing on dobutamine wall motion and late gadolinium enhancement in 3, 138 consecutive patients. Journal of the American College of Cardiology. 2013;61(22):2310–2312. doi: 10.1016/j.jacc.2013.02.063. [DOI] [PubMed] [Google Scholar]
- 165.Korosoglou G, Katus HA. Quantification of atherosclerotic coronary plaque: the missing link between elevated biochemical markers and adverse outcomes in the “vulnerable” patient? Journal of the American College of Cardiology. 2013;62(19):1815–1816. doi: 10.1016/j.jacc.2013.07.062. [DOI] [PubMed] [Google Scholar]
- 166.Cleland JGF, Daubert J-C, Erdmann E, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. The New England Journal of Medicine. 2005;352(15):1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 167.Leyva F, Taylor RJ, Foley PW, et al. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2012;60(17):1659–1667. doi: 10.1016/j.jacc.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 168.Moss AJ, Zareba W, Jackson Hall W, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. The New England Journal of Medicine. 2002;346(12):877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 169.Delgado V, Bax JJ. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is clinically useful. Circulation. 2011;123(6):640–655. doi: 10.1161/CIRCULATIONAHA.110.954404. [DOI] [PubMed] [Google Scholar]
- 170.Sung RK, Foster E. Assessment of systolic dyssynchrony for cardiac resynchronization therapy is not clinically useful. Circulation. 2011;123(6):656–662. doi: 10.1161/CIRCULATIONAHA.110.954420. [DOI] [PubMed] [Google Scholar]
- 171.Lardo AC, Abraham TP, Kass DA. Magnetic resonance imaging assessment of ventricular dyssynchrony: current and emerging concepts. Journal of the American College of Cardiology. 2005;46(12):2223–2228. doi: 10.1016/j.jacc.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 172.Osman NF, Sampath S, Atalar E, Prince JL. Imaging longitudinal cardiac strain on short-axis images using strain-encoded MRI. Magnetic Resonance in Medicine. 2001;46(2):324–334. doi: 10.1002/mrm.1195. [DOI] [PubMed] [Google Scholar]
- 173.Bleeker GB, Kaandorp TAM, Lamb HJ, et al. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113(7):969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 174.Younger JF, Plein S, Crean A, Ball SG, Greenwood JP. Visualization of coronary venous anatomy by cardiovascular magnetic resonance. Journal of Cardiovascular Magnetic Resonance. 2009;11(1, article 26) doi: 10.1186/1532-429X-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bagot CN, Arya R. Virchow and his triad: a question of attribution. British Journal of Haematology. 2008;143(2):180–190. doi: 10.1111/j.1365-2141.2008.07323.x. [DOI] [PubMed] [Google Scholar]
- 176.Mollet NR, Dymarkowski S, Volders W, et al. Visualization of ventricular thrombi with contrast-enhanced magnetic resonance imaging in patients with ischemic heart disease. Circulation. 2002;106(23):2873–2876. doi: 10.1161/01.cir.0000044389.51236.91. [DOI] [PubMed] [Google Scholar]
- 177.Barkhausen J, Hunold P, Eggebrecht H, et al. Detection and characterization of intracardiac thrombi on MR imaging. American Journal of Roentgenology. 2002;179(6):1539–1544. doi: 10.2214/ajr.179.6.1791539. [DOI] [PubMed] [Google Scholar]
- 178.Paydarfar D, Krieger D, Dib N, et al. In vivo magnetic resonance imaging and surgical histopathology of intracardiac masses: distinct features of subacute thrombi. Cardiology. 2001;95(1):40–47. doi: 10.1159/000047342. [DOI] [PubMed] [Google Scholar]
- 179.Srichai MB, Junor C, Rodriguez LL, et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. American Heart Journal. 2006;152(1):75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 180.Lorenz CH, Walker ES, Morgan VL, Klein SS, Graham TP., Jr. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 1999;1(1):7–21. doi: 10.3109/10976649909080829. [DOI] [PubMed] [Google Scholar]
- 181.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by echocardiography, radionuclide ventriculography and cardiovascular magnetic resonance. Are they interchangeable? European Heart Journal. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 182.Lehrke S, Lossnitzer D, Schöb M, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97(9):727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 183.Bourantas CV, Loh HP, Bragadeesh T, et al. Relationship between right ventricular volumes measured by cardiac magnetic resonance imaging and prognosis in patients with chronic heart failure. European Journal of Heart Failure. 2011;13(1):52–60. doi: 10.1093/eurjhf/hfq161. [DOI] [PubMed] [Google Scholar]
- 184.Fernandes AM, Rathi V, Biederman RW, et al. Cardiovascular magnetic resonance imaging-derived mitral valve geometry in determining mitral regurgitation severity. Arquivos Brasileiros de Cardiologia. 2013;100(6):571–578. doi: 10.5935/abc.20130103. [DOI] [PubMed] [Google Scholar]
- 185.Thavendiranathan P, Phelan D, Collier P, Thomas JD, Flamm SD, Marwick TH. Quantitative assessment of mitral regurgitation: how best to do it. Journal of the American College of Cardiology. 2012;5(11):1161–1175. doi: 10.1016/j.jcmg.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 186.Arora NP, Mohamad T, Mahajan N, et al. Cardiac magnetic resonance imaging in peripartum cardiomyopathy: a new tool to evaluate an old enigma. American Journal of the Medical Sciences. 2013 doi: 10.1097/MAJ.0b013e31828155e3. [DOI] [PubMed] [Google Scholar]
- 187.Al-Mallah MH, Shareef MN. The role of cardiac magnetic resonance imaging in the assessment of non-ischemic cardiomyopathy. Heart Failure Reviews. 2011;16(4):369–380. doi: 10.1007/s10741-010-9221-3. [DOI] [PubMed] [Google Scholar]
- 188.Crean A, Merchant N. Role of cardiac magnetic resonance imaging in identification of amyloid cardiomyopathy. Indian Heart Journal. 2004;56(6):682–683. [PubMed] [Google Scholar]
- 189.Marwick TH. Identification of diabetic cardiomyopathy with cardiac magnetic resonance imaging. International Journal of Cardiovascular Imaging. 2006;22(1):91–92. doi: 10.1007/s10554-005-8079-2. [DOI] [PubMed] [Google Scholar]
- 190.Ntusi NBA, Chin A. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. European Radiology. 2009;19(6):1324–1325. doi: 10.1007/s00330-008-1244-y. [DOI] [PubMed] [Google Scholar]
- 191.Raman SV, Basso C, Tandri H, Taylor MRG. Imaging phenotype vs genotype in nonhypertrophic heritable cardiomyopathiesdilated cardiomyopathy and arrhythmogenic right ventricular cardiomyopathy. Circulation. 2010;3(6):753–765. doi: 10.1161/CIRCIMAGING.110.957563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ptaszek LM, Price ET, Hu MY, Yang PC. Early diagnosis of hemochromatosis-related cardiomyopathy with magnetic resonance imaging. Journal of Cardiovascular Magnetic Resonance. 2005;7(4):689–692. doi: 10.1081/jcmr-65632. [DOI] [PubMed] [Google Scholar]
- 193.Cummings KW, Bhalla S, Javidan-Nejad C, Bierhals AJ, Gutierrez FR, Woodard PK. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29(1):89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 194.Mason JW. Myocarditis and dilated cardiomyopathy: an inflammatory link. Cardiovascular Research. 2003;60(1):5–10. doi: 10.1016/s0008-6363(03)00437-1. [DOI] [PubMed] [Google Scholar]
- 195.Dennert R, Crijns HJ, Heymans S. Acute viral myocarditis. European Heart Journal. 2008;29(17):2073–2082. doi: 10.1093/eurheartj/ehn296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Dennert R, Schalla S, Suylen RJV, Eurlings L, Heymans S. Giant cell myocarditis triggered by a parvovirus B19 infection. International Journal of Cardiology. 2009;134(1):115–116. doi: 10.1016/j.ijcard.2007.12.020. [DOI] [PubMed] [Google Scholar]
- 197.Basic D, Gupta S, Kwong RY. Parvovirus B19-induced myocarditis mimicking acute myocardial infarction: clarification of diagnosis by cardiac magnetic resonance imaging. Circulation. 2010;121(7):e40–e42. doi: 10.1161/CIR.0b013e3181d310ea. [DOI] [PubMed] [Google Scholar]
- 198.Myers JM, Fairweather D, Huber SA, Cunningham MW. UNIT 15.14 autoimmune myocarditis, valvulitis, and cardiomyopathy. In: Coligan EJ, Bierer BE, Margulies DH, Shevach EM, Strober W, editors. Current Protocols in Immunology. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sueyoshi E, Sakamoto I, Uetan M. Contrast-enhanced myocardial inversion time at the null point for detection of left ventricular myocardial fibrosis in patients with dilated and hypertrophic cardiomyopathy: a pilot study. American Journal of Roentgenology. 2010;194(4):W293–W298. doi: 10.2214/AJR.09.3414. [DOI] [PubMed] [Google Scholar]
- 200.Jerosch-Herold M, Sheridan DC, Kushner JD, et al. Cardiac magnetic resonance imaging of myocardial contrast uptake and blood flow in patients affected with idiopathic or familial dilated cardiomyopathy. American Journal of Physiology: Heart and Circulatory Physiology. 2008;295(3):H1234–H1242. doi: 10.1152/ajpheart.00429.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. Journal of the American College of Cardiology. 2008;52(19):1574–1580. doi: 10.1016/j.jacc.2008.06.049. [DOI] [PubMed] [Google Scholar]
- 202.Han Y, Peters DC, Dokhan B, Manning WJ. Shorter difference between myocardium and blood optimal inversion time suggests diffuse fibrosis in dilated cardiomyopathy. Journal of Magnetic Resonance Imaging. 2009;30(5):967–972. doi: 10.1002/jmri.21953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Knaapen P, van Dockum WG, Götte MJW, et al. Regional heterogeneity of resting perfusion in hypertrophic cardiomyopathy is related to delayed contrast enhancement but not to systolic function: a PET and MRI study. Journal of Nuclear Cardiology. 2006;13(5):660–667. doi: 10.1016/j.nuclcard.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 204.Knaapen P, Götte MJW, Paulus WJ, et al. Does myocardial fibrosis hinder contractile function and perfusion in idiopathic dilated cardiomyopathy? PET and MR imaging study. Radiology. 2006;240(2):380–388. doi: 10.1148/radiol.2402051038. [DOI] [PubMed] [Google Scholar]
- 205.Raman SV, Moreo A, Ambrosio G, et al. Influence of myocardial fibrosis on left ventricular diastolic function noninvasive assessment by cardiac magnetic resonance and echo. Circulation. 2009;2(6):437–443. doi: 10.1161/CIRCIMAGING.108.838367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112(18):2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Shimizu I, Iguchi N, Watanabe H, et al. Delayed enhancement cardiovascular magnetic resonance as a novel technique to predict cardiac events in dilated cardiomyopathy patients. International Journal of Cardiology. 2010;142(3):224–229. doi: 10.1016/j.ijcard.2008.12.189. [DOI] [PubMed] [Google Scholar]
- 208.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. Journal of the American College of Cardiology. 2008;51(25):2414–2421. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Hombach V, Merkle N, Torzewski J, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. European Heart Journal. 2009;30(16):2011–2018. doi: 10.1093/eurheartj/ehp293. [DOI] [PMC free article] [PubMed] [Google Scholar]


